Abstract
Objectives
Grape seed proanthocyanidin extract (GSPE) is a potent antioxidant and a free radical scavenger. This study was designed to determine whether GSPE could protect against dysfunction and oxidative stress induced by torsion–detorsion injury in rat testis.
Methods
A total of 45 male Wistar albino rats were divided into five groups: control group, sham group, torsion–detorsion (T/D) group, T/D + GSPE group, GSPE group. GSPE was administrated 100 mg/kg/day with oral gavage over seven days before torsion. Testicular torsion was performed for 2 h, and afterward, detorsion was performed for 2 h. The rats were decapitated under ketamine anesthesia, and their testes tissues were removed. Tissue malondialdehyde, advanced oxidation protein products levels, eNOS expression, apoptosis and histopathological damage scores were then compared.
Results
Testicular torsion–detorsion caused significant increases in malondialdehyde level, apoptosis and eNOS expression level and caused a significant decrease in advanced oxidation protein product levels and testicular spermatogenesis in ipsilateral testes. GSPE prevented the rise in malondialdehyde, apoptosis and eNOS expression and improved testicular morphology and Johnsen’s score.
Conclusions
As a result, testicular torsion gives rise to serious damage in testes and GSPE is a potent antioxidant agent in preventing testicular injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Testicular torsion is a urologic emergency. It requires early diagnosis and surgical intervention to prevent subfertility and infertility. The primary pathophysiologic event in testicular torsion is ischemia followed by reperfusion. Therefore, testicular torsion–detorsion (T/D) is an ischemia–reperfusion (I/R) injury to the testis [1]. I/R injury produces the yield of numerous toxic substances in the microcirculation of various types of tissue. In addition, vascular endothelial cell injury and microcirculation disorders can occur during reperfusion and can cause organ disorder. The production of free radicals such as reactive oxygen species (ROS) or nitric oxide (NO) is associated with as factors that can cause I/R injury [2].
Given this pathophysiology of testicular torsion, some research has shown that pretreatment of antioxidants and free radical scavengers prevent testicular T/D-induced testicular injury and male infertility [3]. Grape seed proanthocyanidin extract (GSPE) have been shown to exhibit a broad spectrum of pharmacological, therapeutic and chemoprotective properties. GSPE is known to have greater antioxidant activity than several well-known antioxidants, including vitamin C, vitamin E and gallic acid [4]. GSPE has been shown in animal models to prevent the development of many diseases such as diabetic nephropathy, drug-induced renal toxicity and ischemic cardiomyopathy [5, 6]. However, its precise role in T/D-induced testicular damage and therapeutic potential has not been thoroughly investigated.
In this study, we investigated the effects of GSPE on T/D-induced testicular damage, malondialdehyde (MDA) and advanced oxidation protein product (AOPP) levels, eNOS expression and germ cell apoptosis in rat. We demonstrate for the first time that GSPE protects rats from T/D-induced germ cell apoptosis in testes.
Materials and methods
Animals
Sexually mature male Wistar rats obtained from the Hakan Çetinsaya Experimental and Clinic Research Center, Erciyes University, Kayseri, Turkey, were used for this study. They were housed in plastic cages placed in a well-ventilated rat house and allowed ad libitum access to rat chow and water and subjected to natural photoperiod of 12-h light/dark cycle. All the animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Science and published by the National Institute of Health. Ethical approval for study was obtained from Erciyes University Animal Researches Local Ethics Committee, and the ethic regulations have been followed in accordance with national and institutional guidelines for the protection of animal welfare during experiments (Public Health Service (PHS), 1996). The rats were randomly assigned to five groups of nine rats per group. Group 1(control): served as control; Group 2 (sham): sham-operated; Group 3 (T/D): Torsion/Detorsion; Group 4 (T/D + GSPE): Torsion/Detorsion plus 100 mg/kg/day GSPE (oral gavage)-administrated rats; Group 5 (GSPE): 100 mg/kg/day GSPE (oral gavage)-administrated rats [7]. GSPE was dissolved in saline.
Surgical procedure
All surgical procedures were performed under xylazine/ketamine (10/90 mg/kg, i.p.) anesthesia using sterile conditions. The scrotum was entered through a midline incision. The tunica vaginalis was opened, and the right testis was delivered to the surgical field. The right testis was rotated 720° in a clockwise direction and maintained in this torsion position by fixing the testicle to the scrotum with a 4–0 silk suture [8]. The ischemia period was 2 h, and orchiectomy was performed after 2 h of detorsion. GSPE (GNC, Pittsburgh, PA 15222, USA) was freshly prepared and administered daily once, a week prior to torsion/detorsion and presented at the same time of the day (10:00 am).
At the end of experimental period, animals were killed by decapitation under intraperitoneal ketamine (75 mg/kg) + xylazine (10 mg/kg) anesthesia. After decapitation, testes tissues were quickly removed. Some of the testes tissues were used for biochemical analyses, and the other tissues were used for histological procedures.
Measurement of malondialdehyde (MDA) level
Malondialdehyde in testis was measured by using the thiobarbituric acid–reactive substance assay, as described by Ohkawa et al. [9]. The principle of the method is based on the measurement of the concentration of pink chromogen compound that forms when malondialdehyde reacts with thiobarbituric acid. Malondialdehyde levels were expressed as nanomoles per gram of protein.
Measurement of advanced oxidation protein products (AOPP) level
After homogenization, the homogenate was centrifuged at 5000g for 10 min and supernatant was obtained. Advanced oxidation protein product (AOPP) levels were measured for only the supernatant fraction, using a spectrophotometric method (UV1601 Spectrophotometer; Shimadzu, Tokyo, Japan) [10]. The values are expressed as nmole/g of protein in testes tissue.
Histopathologic evaluation
The testicular tissue was examined and evaluated in random order under blindfold conditions with standard light microscopy by a histologist. Mean seminiferous tubule diameter (MSTD) was measured in micrometers (Analysis LS Research Program). More than 20 seminiferous tubular sections per testis were each given a Johnsen’s score (MTBS) from 1 to 10 as described previously [11]. In this system of classification, all tubular sections in each section of the testicular biopsy are evaluated systematically and each is given a score from 1 to 10. Complete spermatogenesis with many spermatozoa present is evaluated as score 10.
TUNEL assay
In situ detection of apoptosis was performed in the liver by terminal deoxynucleotide-transferase (TdT)-mediated dUTP nick end labeling (TUNEL) using “In situ Cell Death Detection Kit, AP” (Roche, UT, USA), according to manufacturer’s instructions. Briefly, the paraffin embedded sections of testes tissues were deparaffinized, rehydrated and permeabilized with proteinase K (S3020, DAKO, Glostrup DENMARK) that was added on each section and the slides were incubated in a humidified atmosphere for 30 min at 37 °C in the dark. The specimens were washed in phosphate-buffered saline (PBS) two times (5 min each time) and slides of added 50-μl TUNEL reaction mixture were incubated in a humidified atmosphere for 60 min at 37 °C in the dark. The sections were then washed in PBS two times and incubated with Converter-AP humidified chamber for 30 min at 37 °C. Then, sections incubate with Fast Red (F4648, Sigma, Taufkirchen, Germany) for four mins. Finally, the sections were rinsed in PBS and mounted with glycerol. In order to estimate the apoptotic index, TUNEL-positive cells in seminiferous tubules in 20 randomly chosen fields were counted. The apoptotic index was calculated as the percentage of TUNEL-positive cells.
Immunohistochemistry
The expression of eNOS was detected immunohistochemically in the testes using a rabbit polyclonal antibody (sc.654 Santa Cruz Biotechnology, CA, USA) and the streptavidin–biotin peroxidase technique. The procedure was performed under identical conditions for all sections. Paraffin sections (5 μm) were deparaffinized in xylene. The sections were rehydrated, rinsed in deionized water, and antigen retrieval was carried out by microwave treatment in 0.01 M sodium citrate buffer (pH 6.0) at 95 °C for 5 min, and then, the slides were cooled rapidly at room temperature for 20 min. After sections washed with phosphate-buffered saline (PBS), endogenous peroxidase activity was inhibited 3 % H2O2 in methanol for 10 min. Five percent serum blocking was used to block the nonspecific staining. The histological sections were then incubated with the polyclonal antibody for eNOS (sc.654 Santa Cruz Biotechnology, CA, USA) at a dilution of 2.5 μg/ml in 5 % serum blocking overnight at 4 °C. After washing with PBS, sections were incubated with the biotinylated secondary antibodies. Then, the immunoreaction was amplified with streptavidin–avidin–peroxidase complex, and the sections were visualized by using 3,3P-diaminobenzidine tetrahydrochloride (DAB) and lightly counter-stained with hematoxylin.
Immunohistochemical staining was scored in a semiquantitative manner to determine the differences between the control group and the experimental groups. The intensity of the staining was recorded as weak (±), mild (+), moderate (++) and strong (+++). This analysis was performed in at least ten tubuli per testicular section, in five sections from each animal at 400 magnifications.
Statistical analysis
One-way analysis of variance (ANOVA) and post hoc Tukey’s test were used to determine differences between groups. Results are presented as mean ± SEM. Values were considered statistically significant if p < 0.05. The SPSS/PC program (Version 15.0; SPSS, Chicago, IL) was used for the statistical analysis.
Results
Histopathological findings
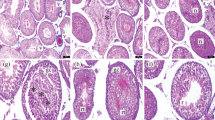
Control (Fig. 1a), sham (Fig. 1b) and only GSPE-administrated (Fig. 1c) testes showed the presence of normal testicular architecture and regular seminiferous tubular morphology with normal spermatogenesis and the presence of primary and secondary spermatocytes, spermatids and spermatozoa. Congestion of vessels and hemorrhage (Fig. 1d), desquamation of epithelial cells in the lumen, formation of the vacuoles in epithelial cells, disorder of seminiferous tubule germinal epithelium (Fig. 1e) and necrosis of some seminiferous tubules (Fig. 1f) were determined in T/D group. GSPE-pretreated group showed lesser atrophic and degenerative changes to the tubular epithelium when compared to the T/D group. Interestingly, GSPE apparently alleviated torsion/detorsion-induced histopathological damage in the testes (Fig. 2a, b).
Light microscopy of testicular tissue in different groups. In a controls, b sham and c only GSPE-administered group, normal testicular architecture was observed; d after T/D, congestion of vessels and hemorrhage (asterisk) were shown; e after T/D, formation of the vacuoles (small arrow) in epithelial cells and desquamation of the germinal epithelium of the seminiferous tubules were exhibited; f necrosis of some seminiferous tubules (n) were observed after T/D. Testicular cross-sections were stained with H&E (a, d, e, f) and Masson’s Trichome (b, c). (Scale bar 200 μm, ×200)
Light microscopy of testicular tissue in different groups. a, b GSPE pretreatment prevented testicular damage (×200); c after T/D, TUNEL-positive germ cells (arrow) were observed in the seminiferous epithelium (×400); d negative staining control is also illustrated to ensure that the staining method is working well (×200). Note that there were no detectable signals in the negative control
The MSTD and Johnsen’s MTBS values for testes in each group are shown in Table 1. It was observed that the MTBS of the T/D testes was statistically significantly lower compared to that of the control rats. A decrease was observed in MSTD value but this was not statistically significant. However, curative effect was determined with the administration of GSPE in the T/D group for MTBS. The MSTD and Johnsen’s MTBS in the GSPE-alone group were similar to that in the control group (Table 1).
Apoptotic findings
Table 1 and Fig. 2c, d illustrate apoptosis, demonstrated by TUNEL staining. The apoptotic index in the testis of control rats and sham-operated rats was found to be 0.28 ± 0.11 %, 0.32 ± 0.08 %, respectively. T/D resulted in increases in the number of TUNEL-positive cells and the apoptotic index was 1.67 ± 0.27 %. The increase in the apoptotic index was statistically significant in T/D group compared to control group (p < 0.05). GSPE administration with T/D resulted in decreases in TUNEL-positive cells, and the apoptotic index was 0.51 ± 0.09 %. The decrease in the apoptotic index was statistically significant in T/D with GSPE compared to T/D (p < 0.05). The apoptotic index in the testes of GSPE-alone-administrated rats was found to be 0.18 ± 0.07 %, and it was similar to the control.
Immunohistochemical findings
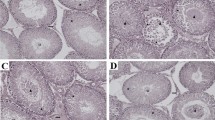
Table 2 showed eNOS expression. Immunohistochemical studies demonstrated the presence of slight eNOS immunostaining in the germinal cells of the seminiferous tubules and moderate immunostaining Leydig cells and vascular endothelial cells of the testes in the control group (Fig. 3a). eNOS immunoreactivity was considerably increased, especially in germinal cells in T/D group (Fig. 3b). GSPE treatment prevented testicular damage and the intense eNOS immunoreactivity in the T/D group (Fig. 3c). The eNOS expression in the testes of GSPE-alone-administrated rats (Fig. 3d) and sham group (Fig. 3e) was similar to that in the control. Negative controls where incubation with the primary antisera was omitted were completely unlabeled, as seen in (Fig. 3f).
Representative photomicrographs of testicular immunostaining for eNOS in different groups. In a normal controls, b sham and c only GSPE-administered group, weak eNOS immunostaining in germinal cells in the seminiferous tubules and moderate eNOS immunostaining of Leydig cells (arrow) were noted; d after T/D, eNOS expression was increased in the germ cells (arrow) of the tubules e GSPE treatment, weak eNOS immunostaining in germinal cells in the seminiferous tubules and moderate eNOS immunostaining of Leydig cells (arrow) was exhibited (d) Note that there were no detectable signals in the negative control. İmmunoperoxidase, hematoxylin counterstain. (Scale bar 50 μm, ×400)
MDA results
The MDA levels were significantly increased in the T/D group versus the control group (p < 0.05). The MDA levels in the T/D + GSPE group were significantly lower than that in the T/D group (p < 0.05), and there was no significant difference in MDA levels among the sham, GSPE and control groups (p > 0.05) (Table 3).
AOPP results
The AOPP levels were significantly decreased in the T/D group versus the control group (p < 0.05). The AOPP levels of the T/D + GSPE group had increased compared with the T/D group, but this increase was not statistically significant (p > 0.05), and there was no significant difference in AOPP levels among the sham, GSPE and control groups (p > 0.05) (Table 3).
Discussion
The major purposes of our study were to investigate whether GSPE reverses I/R injuries after testicular T/D and to compare them using histopathological, immunohistochemical and biochemical techniques. According to our results, significant I/R-associated histopathological injury developed when T/D was administered, and GSPE exhibited a protective effect against that injury.
Testicular torsion results in infertility and testicular damage. It has been demonstrated that one hour of minimum time causes testicular damage after experimental testicular torsion in the rat [12]. Thus, we formed a two-hour testicular torsion model rotating right testis 720° to constitute a severe testicular damage. In our study, we examined the effect of T/D on the testicular histology of the rats. Our results clearly showed that T/D severely damaged the seminiferous tubules and interstitium, including congestion of vessels and hemorrhage, formation of the vacuoles in epithelial cells, desquamation of epithelial cells in the lumen, disorder of seminiferous tubule germinal epithelium and necrosis of some seminiferous tubules. In the testicular tissue, MTBS is used to assess histopathological damage. It is based on the evaluation of progressive degeneration of the germinal epithelium [11, 13]. In our study, the lowest MTBS values in testes were found in T/D group. Our findings are consistent with other studies and further support their observations [8, 14]. Another histopathological criterion of testicular damage used in the present study was the MSTD value, which is also used in the scientific literature to estimate testicular tissue damage [8, 13, 14]. In the present study, the low MSTD value was also observed in T/D group compared to control group, but these decreases were not significant statistically. Earlier studies have shown significantly reduced MSTD level as a result of the T/D; however, in our work, MSTD level is not statistically significantly reduced, which could be due to short time of experimental period.
Testicular T/D is an I/R injury for the testis. Surgical intervention is the most effective method in the prevention of or reduction in testicular damage. It should be performed at once avoid loss of testicular function, thus affecting fertility [3]. The I/R injury is associated with overgeneration of ROS, such as hydrogen peroxide, hydroxyl radicals and nitric oxide. Outstretched torsion leads to testicular ischemia and high levels of oxidative stress in the testis [15]. In addition, reperfusion of the ischemic tissue elevates the generation of ROS and has destructive effects on a range of cellular functions [3]. The ROS are difficult to determine directly in tissue on account of their high reactivity and short half-life. MDA, a stable end product of lipid peroxidation generated by ROS, usually is used as an indirect indicator of ROS [16]. In our study, an elevated level of MDA in ipsilateral testes of the T/D group indicates increased oxidative stress. The rise in enzyme activity provides an explanation for ROS overgeneration in testicular tissue.
In general, formed ROS are eliminated by enzymatic and nonenzymatic antioxidants. In this situation, the administration of antioxidants may have a potential benefit in neutralizing ROS [17]. Several anti-inflammatory drugs or antioxidants (ibuprofen, sildenafil citrate, dehydroepiandrosterone, propofol, etc.) have to date been tested to prevent I/R injury in the testis [1, 17–19].
In this study, GSPE was employed as a novel antioxidant, and its protective effect was evaluated. In a recent study, grape seed extract effects were investigated on ischemia–reperfusion injury of the liver. Authors have identified that grape seed extract application has well-preservative effects on liver parenchyma [20]. Su et al. [7] in their study investigated the effect of grape seed extract on testicular tissue which was already damaged with nickel sulfate. In this study, it has been identified that grape seed extract corrects the sperm motility and reduce the oxidative damage and blocks apoptosis. Oxidative stress seems to be crucial in the etiology of T/D-induced male reproductive toxicity in humans and animals. Thus, antioxidant therapy is considered to be an important approach for the intervention of T/D-induced damage. For this reason, in this study, we have implemented GSPE for protective purposes. When T/D and grape seed extract were applied together to the rats, the big portion of the seminiferous tubules of the testis was seen in normal. Seminiferous tubular diameters, tubule and biopsy score were determined close to the controls with the implementation of the grape seed extract and T/D. Our results are compatible with earlier studies made with grape seed extracts, and it shows its protective effects too.
Oxidant-mediated protein damage can be determined by the level of advanced oxidation protein products. According to our research, there is not any study found investigating AOPP level in testicular tissue. We could say that this is the first study ever done so far in testicular tissue. Our results showed surprisingly a significant decrease in AOPP level in the T/D group compared to controls. However, an AOPP level is not statistically significantly increased in the T/D + GSPE group compared to T/D group. In other studies that have done on the other tissues, AOPP levels have increased as a result of ischemic tissue damage [21, 22]. The reason why we found lower level of AOPP values in patients compared to control could be testicular tissue protein that is more sensitive to protein damage, and thus, it might protect itself against protein destruction by increasing the antioxidant agents fast with unknown mechanism, for now.
Apoptosis is a highly regulated process used to eliminate unwanted or damaged cells from multicellular organisms. Apoptosis of germ cells plays an important role in a wide variety of physiological processes during the development of the fetal and adult testicular tissue. It is required for normal spermatogenesis [23]. Several studies showed that I/R injury in adult rodents resulted in germ cell apoptosis in testes [1, 8]. In the present study, we investigated the effects of GSPE on T/D-induced germ cell apoptosis in testes. As anticipated, I/R clearly elevated the percentage of tubules with apoptotic germ cells. In addition, the number of apoptotic germ cells per tubule was significantly increased in testes of T/D group. Pretreatment with GSPE significantly alleviated T/D-induced germ cell apoptosis in testes. These results are in agreement with those from a recent study, in which GSPE protected against T/D-induced germ cell apoptosis in rat testes [7].
NO, the end product of the enzyme NOS, is a potent biological mediator that functions at low concentrations as a signal in many diverse physiological processes but at high concentrations may cause DNA damage and cell death. NOS catalyzes the conversion of l-arginine to NO and l-citrulline. Three isoforms of NOS, namely nNOS, iNOS and eNOS, are found in the testis and are known to be associated with infertility, spermatogenesis and sperm maturation. In the testes, eNOS is expressed in the Leydig cell, Sertoli cells, spermatocytes, spermatids and endothelial cells [24]. The increased expression of eNOS in ischemia and reperfusion injury of testis has been reported previously [25]. Endothelial NOS constitutively generates a physiological level of NO and is involved in vasorelaxation [26]. Clamping of the testicular artery induces a rapid increase in NO release, and removing the clip induces a gradual decrease in NO release in testis [27, 28]. It is stated that NO produced from eNOS regulates the vasomotor function in the contralateral testis possibly through testis-specific reflex arc [26]. Therefore, we aimed to demonstrate the immunoexpression values of eNOS in the testicular tissue in the experimental model of the present study. We found significant elevations in eNOS levels after testicular IR injury. At the same time, we found that the pretreatment of GSPE decreased eNOS immunoreactivity in I/R testis of the rats and our findings are correlated with previously reported data [29, 30].
In conclusion, we have demonstrated the protective effect of GSPE on testicular ischemia–reperfusion injury for the first time. The administration of GSPE, improved the histopathological and biochemical parameters, decreased immunoexpression of T/D-induced testicular eNOS and decreased germ cell apoptosis in I/R testes. Thus, GSPE, novel antioxidants may be useful as pharmacological agents to protect against T/D-induced testicular injury.
References
Dokmeci D, Kanter M, Inan M, Aydogdu N, Basaran UN, Yalcin O, Turan FN (2007) Protective effects of ibuprofen on testicular torsion/detorsion-induced ischemia/reperfusion injury in rats. Arch Toxicol 81(9):655–663
Tamamura M, Saito M, Kinoshita Y, Shimizu S, Satoh I, Shomori K, Dimitriadis F, Satoh K (2010) Protective effect of edaravone, a free-radical scavenger, on ischaemia-reperfusion injury in the rat testis. BJU Int 105(6):870–876
Turkmen S, Mentese A, Karaguzel E, Karaca Y, Kucuk A, Uzun A, Yulug E, Turedi S (2012) A comparison of the effects of N-acetylcysteine and ethyl pyruvate on experimental testicular ischemia-reperfusion injury. Fertil Steril 98(3):626–631
Ariga T (2004) The antioxidative function, preventive action on disease and utilization of proanthocyanidins. BioFactors 21(1–4):197–201
Guler A, Sahin MA, Yucel O, Yokusoglu M, Gamsizkan M, Ozal E, Demirkilic U, Arslan M (2011) Proanthocyanidin prevents myocardial ischemic injury in adult rats. Med Sci Monit 17(11):BR326–BR331
Ulusoy S, Ozkan G, Yucesan FB, Ersoz S, Orem A, Alkanat M, Yulug E, Kaynar K, Al S (2012) Anti-apoptotic and anti-oxidant effects of grape seed proanthocyanidin extract in preventing cyclosporine A-induced nephropathy. Nephrology (Carlton) 17(4):372–379
Su L, Deng Y, Zhang Y, Li C, Zhang R, Sun Y, Zhang K, Li J, Yao S (2011) Protective effects of grape seed procyanidin extract against nickel sulfate-induced apoptosis and oxidative stress in rat testes. Toxicol Mech Methods 21(6):487–494
Kanter M (2010) Protective effects of melatonin on testicular torsion/detorsion-induced ischemia-reperfusion injury in rats. Exp Mol Pathol 89(3):314–320
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B (1996) Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49(5):1304–1313
Johnsen SG (1970) Testicular biopsy score count—a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1(1):2–25
Turner TT, Brown KJ (1993) Spermatic cord torsion: loss of spermatogenesis despite return of blood flow. Biol Reprod 49(2):401–407
Uguralp S, Bay Karabulut A, Mizrak B, Kaymaz F, Kiziltay A, Hasirci N (2004) The effect of sustained and local administration of epidermal growth factor on improving bilateral testicular tissue after torsion. Urol Res 32(5):323–331
Dokmeci D, Inan M, Basaran UN, Yalcin O, Aydogdu N, Turan FN, Uz YH (2007) Protective effect of l-carnitine on testicular ischaemia-reperfusion injury in rats. Cell Biochem Funct 25(6):611–618
Aitken RJ, Roman SD (2008) Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev 1(1):15–24
Turner TT, Tung KS, Tomomasa H, Wilson LW (1997) Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod 57(6):1267–1274
Aksoy H, Yapanoglu T, Aksoy Y, Ozbey I, Turhan H, Gursan N (2007) Dehydroepiandrosterone treatment attenuates reperfusion injury after testicular torsion and detorsion in rats. J Pediatr Surg 42(10):1740–1744
Yildiz H, Durmus AS, Simsek H, Yaman M (2012) Dose-dependent protective effect of sildenafil citrate on testicular injury after torsion/detorsion in rats. Andrologia 44(Suppl 1):300–306
Taskara E, Gor A, Kutlu O, Karaguzel E, Cobanoglu U, Topbas M, Senel AC (2011) Does propofol prevent testicular ischemia-reperfusion injury due to torsion in the long term? Pediatr Surg Int 27(9):1003–1007
Sehirli O, Ozel Y, Dulundu E, Topaloglu U, Ercan F, Sener G (2008) Grape seed extract treatment reduces hepatic ischemia-reperfusion injury in rats. Phytother Res 22(1):43–48
Guven C, Borcek AO, Cemil B, Kurt G, Yildirim Z, Ucankus NL, Kilic N, Ceviker N (2010) Neuroprotective effects of infliximab in experimental spinal cord ischemic injury. J Clin Neurosci 17(12):1563–1567
Skvarilova M, Bulava A, Stejskal D, Adamovska S, Bartek J (2005) Increased level of advanced oxidation products (AOPP) as a marker of oxidative stress in patients with acute coronary syndrome. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 149(1):83–87
Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hsueh AJ (1995) Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology 136(1):5–12
Shiraishi K, Naito K, Yoshida K (2001) Nitric oxide promotes germ cell necrosis in the delayed phase after experimental testicular torsion of rat. Biol Reprod 65(2):514–521
Aktoz T, Kanter M, Aktas C (2010) Protective effects of quercetin on testicular torsion/detorsion-induced ischaemia-reperfusion injury in rats. Andrologia 42(6):376–383
Moon C, Ahn M, Kim S, Yasuzumi F, Shin T (2005) Increased expression of both constitutive and inducible forms of nitric oxide synthase in the delayed phase of acute experimental testicular torsion. J Vet Med Sci 67(4):453–456
Kono T, Saito M, Kinoshita Y, Satoh I, Shinbori C, Satoh K (2006) Real-time monitoring of nitric oxide and blood flow during ischemia-reperfusion in the rat testis. Mol Cell Biochem 286(1–2):139–145
Shiraishi K, Yoshida K, Naito K (2003) Activation of endothelial nitric oxide synthase in contralateral testis during unilateral testicular torsion in rats. Arch Androl 49(3):179–190
Akgul T, Karaguzel E, Surer H, Yagmurdur H, Ayyildiz A, Ustun H, Germiyanoglu C (2009) Ginkgo biloba (EGB 761) affects apoptosis and nitric-oxide synthases in testicular torsion: an experimental study. Int Urol Nephrol 41(3):531–536
Kanter M (2011) Protective effects of Ginkgo biloba (EGb 761) on testicular torsion/detorsion-induced ischemia-reperfusion injury in rats. Exp Mol Pathol 91(3):708–713
Acknowledgments
This work was supported by a research grant from the Erciyes University Scientific Research Projects Unit (EUBAP, TSY-10-2974).
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayatli, F., Akkuş, D., Kilic, E. et al. The protective effects of grape seed extract on MDA, AOPP, apoptosis and eNOS expression in testicular torsion: an experimental study. World J Urol 31, 615–622 (2013). https://doi.org/10.1007/s00345-013-1049-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-013-1049-8