Abstract
PET/CT has a key role in final response assessment after treatment in most types of malignant lymphomas, as well as in baseline staging and interim evaluation. Its application is widely established in Hodgkin lymphoma and aggressive B cell lymphomas. Moreover, recent recommendations suggest the use of PET/CT for initial staging and response evaluation in other subtypes of lymphomas (FL, MCL, MZL) as well as in the setting of auto-SCT and novel agents (PD-1 inhibitors and CAR-T cells). However, the accumulated clinical experience with the latter is considerably less. Although some answers have already been obtained, especially in the mid-treatment setting, evidence-based data on the appropriate use of PET in lymphomas are expected to be available shortly. The present chapter is a useful clinical guide since it includes an updated review of current recommendations and a summary of evidence from large clinical trials in the field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
PET/CT has a key role in final response assessment after treatment in most types of malignant lymphomas, as well as in baseline staging and interim (mid-treatment) evaluation [1, 2]. Its application is widely established in Hodgkin lymphoma (HL) and aggressive B cell lymphomas, including diffuse large B cell lymphoma (DLBCL), primary mediastinal large B cell lymphoma (PMLBCL), and other related subtypes. Although recent recommendations suggest the use of PET/CT for baseline staging and response assessment in follicular lymphomas, mantle cell lymphoma (MCL), Burkitt lymphoma, and “nodal” T cell lymphomas [anaplastic large cell (ALCL), peripheral T cell (PTCL), and angioimmunoblastic T cell lymphoma (AITL)], the accumulated clinical experience with these subtypes is considerably less [1,2,3,4,5,6,7]. The role of PET/CT is much more controversial in non-follicular low-grade lymphomas and primary extranodal lymphomas other than DLBCL [6, 8].
The various lymphoma subtypes are not equally FDG-avid and this mainly depends on their histologic features, aggressiveness, and biologic characteristics. “Routinely FDG-avid lymphomas” include HL, DLBCL, and other aggressive B cell lymphomas, lymphoblastic and Burkitt lymphoma, follicular and MCL, nodal marginal zone lymphoma, and systemic ALCL, since they are almost invariably 18-FDG-avid (>90% and usually >95–100% of the cases) [1, 2, 9, 10]. Other aggressive T cell lymphomas, mainly the non-ALCL “nodal” types, such as PTCL and AITL as well as extranodal NK/T cell lymphomas, are typically but not invariably 18-FDG-avid (>80–100% of the cases in various studies) [1, 2, 9, 10]. In contrast, other indolent lymphomas are even more “variably 18-FDG-avid.” Thus, several forms of extranodal lymphomas, including MALT and cutaneous B and T cell lymphomas, small lymphocytic, splenic marginal zone lymphoma as well as some rare lymphoma subtypes, may not be satisfactorily evaluated by PET/CT, displaying frequencies of FDG avidity between 50% and 80% [1].
14.2 PET/CT in Initial Staging
The rationale of using FDG-PET in the initial staging of lymphomas is based on its improved accuracy in determining disease extent, as compared to conventional imaging [1, 2]. PET/CT is more sensitive than CT, mainly because it can detect disease in normal-sized lymph nodes or facilitate the evaluation of extranodal disease [1, 2]. The extent of disease upstaging or—less frequently—downstaging varies according to histology and will be discussed later. Further to more accurate staging, baseline PET/CT can facilitate the interpretation of the end-of-treatment (EOT) PET/CT response assessment serving as a basis for comparison. Finally, baseline PET/CT may provide new prognostic factors related to tumor burden and metabolic activity, which are increasingly evaluated in detail, although they have not yet become standard prognostication tools.
14.2.1 Role of PET in the Initial Staging of Lymphomas
Baseline PET/CT is strongly recommended for initial staging of the routinely FDG-avid lymphomas [1, 2] (Figs. 14.1a, 14.2a, 14.3a, 14.4a, 14.5a, 14.7a). In HL, the number and density of Hodgkin-Reed-Sternberg cells in the tumor vary and FDG uptake occurs mainly by the inflammatory tumor microenvironment. PET/CT identifies 25–30% more lesions and leads to upstaging an average of 18% of patients in various studies compared to conventional staging [2]. Conversely, up to 10% of the patients (average 4% in various studies) [2] can be downstaged [1, 2, 11, 12]. Such changes might lead to major treatment modification in up to 1/4 of the patients (average 11% in the studies reviewed by Barrington et al.) [2]. In a more common scenario, the identification of more disease sites may affect radiotherapy (RT) fields, even in the absence of stage shift [12]. However, most of the knowledge on treatment approaches is based on conventional staging [11, 12]. Thus, it is not yet clearly proven that stage shift according to PET/CT should guide treatment decisions in HL. In addition, the clinical benefit to be gained from the widening of the RT fields to include anatomically subclinical disease sites may be of concern with respect to potential long-term sequelae. This is becoming particularly relevant, given the trend to adopt smaller RT fields and doses or even omit RT in appropriately selected patients.
(a) Baseline staging in a patient with Hodgkin lymphoma. Intense FDG uptake is shown in a bulky mediastinal mass. Right cervical and right epiphrenic nodal involvement is also shown. (b) Post chemotherapy evaluation revealed a residual mediastinal abnormality with FDG uptake higher than the mediastinal blood pool but not exceeding that of the liver. This would have been interpreted as positive, i.e., suggestive of residual active disease based on the 2007 IHP criteria. However, interpreted as Deauville 5-point scale score 3 (Table 14.1), it is now considered compatible with complete metabolic response based on the 2014 Lugano criteria (Table 14.2)
(a) Baseline staging in a patient with diffuse large B-cell lymphoma. Disseminated lymphadenopathy including a left pelvic mass and multiple focal osseous/bone marrow lesions suggestive of bone marrow involvement are consistent with stage IV disease. (b) Interim PET after two cycles of R-CHOP is completely negative. (c) Post R-CHOP evaluation is also negative, as correctly predicted by the negative interim examination
(a) Baseline staging in a patient with Hodgkin lymphoma, indicating cervical and mediastinal involvement. Conventional staging had revealed mildly enlarged paraortic nodes, which were not demonstrable by PET/ CT. Thus, the patient was downstaged from clinical stage IIIA to PET-stage IIA. (b) PET/CT at the time of relapse in the same patient. PET/CT had been normalized following ABVD × 6. Three months after the completion of involved field radiotherapy the patient presented with lumbar pain and elevated ESR and C-Reactive Protein levels. MRI revealed osseous abnormalities, which were confirmed by PET/CT. PET/CT normalized again after IGEV salvage chemotherapy and BEAM with autologous stem cell support
(a) Baseline staging in a patient with Hodgkin lymphoma. The patient had disseminated nodal disease, including a mass at the hepatogastric junction, and a positive bone marrow biopsy (stage IVB). (b) Interim PET after two cycles of ABVD revealed complete resolution of FDG uptake except of the hepatogastric mass, which was reduced in size and had residual FDG uptake just above that of the liver. Interim PET was interpreted as positive, Deauville score 4. The patient received intensified chemotherapy with six cycles of BEACOPP-escalated. (c) Negative end-of-treatment PET in the same patient. He remains in complete remission 8.5 years after the positive interim PET/CT (Courtesy of Drs Datseris I and Rondogianni Ph, Department of Nuclear Medicine and PET/CT, Evangelismos General Hospital, Athens, Greece)
(a) Baseline staging in a patient with Hodgkin lymphoma demonstrating stage IIB disease with extensive supradiaphragmatic nodal involvement. (b) Interim PET revealed a residual left axillary abnormality with FDG uptake above the surrounding background but below the mediastinal blood pool. Interim PET was interpreted as negative, Deauville score 2. The patient continued on ABVD. Posttreatment PET/CT was negative. Following involved field radiotherapy, the patient remains in complete remission 8 years after the negative interim PET/CT (Courtesy of Drs Datseris I and Rondogianni Ph, Department of Nuclear Medicine and PET/CT, Evangelismos General Hospital, Athens, Greece)
The situation is similar in DLBCL, the commonest form of aggressive B cell lymphomas, and PMLBCL, in which PET/CT is also strongly recommended for initial staging [1, 2]. However, the effect on treatment decisions with standard rituximab-based chemoimmunotherapy may be less important, with the potential exception of abbreviated immunochemotherapy regimens in localized DLBCL. The effect on potential RT fields may not be so relevant in DLBCL, since RT is not routinely applied in the majority of patients in many centers.
In other routinely FDG-avid lymphomas, especially follicular lymphomas and MCL, PET/CT is also recommended for initial staging [1, 2]. However, a meaningful impact on treatment strategy is not expected, since the disease is already disseminated in the vast majority of cases. In the unusual cases of early stage disease, mainly seen in a minority of patients with follicular lymphoma (less frequently in NMZL and even more rarely in MCL), PET/CT may confirm that the disease is indeed localized and potentially curable with involved field or regional RT. Baseline PET evaluation is generally not recommended in lymphoma subtypes which are not routinely FDG-avid (Fig. 14.6) [1, 2].
PET/CT may also contribute to the identification and histologic confirmation of transformed disease in patients with known indolent lymphomas. The degree of FDG uptake has been proposed to be correlated with tumor grade, proliferative activity, and aggressiveness and to be of prognostic value [9]. Studies using semiquantitative measurements based on SUVmax suggest that SUVmax >10 is usually seen in aggressive or transformed indolent lymphomas [9]. The optimal threshold to detect Richter transformation in chronic lymphocytic leukemia (CLL) may range between 5 and 10 with varying effects on sensitivity, specificity, positive and negative prognostic value and may differ in the era of novel agents [13, 14].
Finally, baseline PET/CT may be used to determine the metabolic tumor volume (MTV) and total lesion glycolysis (TLG), which is a combined evaluation of both tumor burden and metabolic activity. These parameters—and other radiomic markers—can be of prognostic significance, as described later.
14.2.2 PET in the Assessment of Bone Marrow Involvement
Numerous studies have investigated the role of PET in the assessment of bone marrow (BM) involvement. The comparative accuracy of PET/CT and bone marrow biopsy (BMb) highly depends on the specific lymphoma subtype under evaluation.
14.2.2.1 Hodgkin Lymphoma
According to current recommendations, bone marrow biopsy (BMb) can be omitted in HL, if baseline PET/CT is performed [1, 2]. The omission of BMb in this setting is also proposed by the latest version of the ESMO guidelines at a level of evidence III (from prospective cohort studies) and grade B strength of recommendation (generally recommended) [15]. However, a BMb still remains necessary in cases with no baseline PET/CT available. Indeed, PET/CT uncovers more cases of BM involvement [11, 16,17,18,19] while treatment decisions are not typically affected in the rare cases with a positive BMb but a negative PET/CT, as analyzed below. Patients with BM involvement by PET/CT may have similarly poor outcomes irrespective of BMb status, but this information is still based on rather limited data [16, 18]. Notably, PET/CT is suggestive of BM involvement only if focal lesions are present. In contrast, diffuse increased uptake, even with intensity >liver, is due to reactive BM changes caused by the cytokine milieu present in HL and should not be confused with BM involvement [1, 2, 16,17,18, 20].
In a large study of 454 HL patients, who were staged by both PET/CT and bone marrow biopsy [16] (Fig. 14.4a), 6% (27 patients) had BM involvement. However, more than twice (13% or 59 patients) had multi- (n = 31), bi- (n = 9), or unifocal (n = 19) PET/CT bone lesions and a negative BMb. No cases of BM involvement were detected among patients with diffusely increased 18-FDG uptake. Only 4/454 patients (<1%) had a positive BMb in the absence of PET/CT evidence of BM disease, and BMb did not lead to treatment modification, since all of them had already advanced disease (stage shift from III to IV). The experience of the German Hodgkin Study Group (GHSG) in the HD16–18 trials was similar [19]. Only 20/832 (2.4%) patients had a positive BMb but five fold more patients (n = 110) had a PET/CT evidence of BM disease. The negative predictive value was 99.9% as only 1/703 patients without BM disease on PET/CT had a positive BMb. In both studies, patients with both positive PET/CT and BM biopsy had much more frequently multifocal lesions, suggesting that among patients with PET/CT-based evidence of BM involvement, those who also have positive BMb have more extensive BM disease. Similarly, only 1.1% of patients with HL and a negative BM PET/CT had a positive BMb in a meta-analysis of 955 patients, including the first previously mentioned study [21] while the overall frequency of a positive BMb in the presence of a negative PET/CT was 1.9% in a study of 1085 patients [22]. Our experience, based on 172 patients, is very similar, further demonstrating that there is not even a small high-risk subgroup [17, 23], in which BMb could offer additional information. Furthermore, it appears that the outcomes of patients with positive BMb and those with PET/CT evidence of BM involvement but negative BMb are equally poor, but this should be further confirmed [16,17,18]. Thus, the biologic and prognostic significance of BM involvement detected by means of PET/CT only appears to be similar to that of histologically proven BM disease in HL [16,17,18]. Finally, PET/CT might facilitate the identification of foci of increased uptake in order to guide bone marrow biopsy, since bone marrow involvement can be patchy and incremental information could be lost.
14.2.2.2 Diffuse Large B Cell and Primary Mediastinal Large B Cell Lymphoma [24,25,26,27,28,29,30,31,32,33]
In DLBCL the frequency of BM involvement is 10–15% and PET/CT is again suggestive of BM involvement only if focal lesions with increased uptake are present (Fig. 14.2a). BM involvement may be either concordant (large cell) or discordant (small cell) compared to lymph node histology with an almost equal frequency [24]. This phenomenon, which is of prognostic significance, cannot be effectively demonstrated by PET/CT [25].
According to the 2014 Lugano recommendations, BMb could be safely omitted in DLBCL staged by PET/CT, because the probability of a positive BMb is low in the absence of focal BM lesions on PET/CT and, even in such cases, treatment strategy is not typically affected [1, 2]. However, a BMb was still indicated for the detection of discordant histology in DLBCL, if this was relevant for patient management or required by a clinical trial [1, 2]. Extending these thoughts, current ESMO and NCCN guidelines propose to omit BMb if PET/CT is suggestive of BM involvement but keep BMb in staging procedure in case of a negative PET/CT in order to detect discordant or low-volume (<10–20%) BM involvement [34, 35]. The scientific basis for these recommendations is analyzed below.
Although BMb might be omitted in the majority of DLBCL patients, it is more informative compared to HL, because more patients may have positive biopsies with negative PET/CT. PET/CT reveals on average twice more cases of BM involvement than BMb in DLBCL [26,27,28,29,30,31,32].
However, in contrast to HL, approximately 1/3 of patients with positive BMb (range, 14–50%) have a negative PET/CT, accounting for 1.5–8% of the total DLBCL population in various studies [26,27,28,29,30,31, 33, 36]. If BMb is omitted, several cases of BM involvement may be overlooked, but most of them have already features of advanced disease and management is not affected (see Lugano recommendations [1, 2]). This was recently shown clearly in a combined analysis of the PETAL and OPTIMAL trials [33]. However, PET+/BMb- cases may have a better prognosis than BMb + cases, so that BM involvement could be an adverse prognostic factor only if demonstrated at the histologic level [27, 29,30,31,32]. Thus, although of limited value, the exact role of BMB in DLBCL remains to be further investigated [31, 32, 37]. Special caution should be taken in patients with no evidence of BM disease on PET/CT and apparently limited stage, who are scheduled for abbreviated immunochemotherapy regimens, in whom a BMb would be most useful [15, 35].
In PMLBCL, the baseline probability of BM involvement is extremely low and it would be reasonable to omit BMb in the absence of relevant findings in PET/CT, especially because a positive result would not alter treatment strategy [32, 38, 39]. However, there is no formal recommendation on this for the time being.
14.2.2.3 Other Lymphoma Subtypes
In indolent lymphomas, including follicular lymphomas and MCL, BM biopsy remains the gold standard for the evaluation of BM disease, which is much more prevalent than in HL and DLBCL. PET/CT may not reveal bone marrow involvement by low-grade lymphoma [9] and BMb cannot be omitted [1, 2].
14.2.3 Potential Prognostic Impact of Baseline PET Parameters
The calculation of total metabolic tumor volume (TMTV) by baseline PET/CT may provide a better estimation of the true tumor burden compared to conventional imaging. Furthermore, total lesion glycolysis (TLG) provides a combined evaluation of both TMTV and intensity of metabolic activity (SUV mean of each lesion) [40]. These parameters, which are derived from baseline PET/CT, may provide important prognostic information in individual lymphoma subtypes.
Further to rather small studies in which TMTV was demonstrated as an independent prognostic factor for PFS [41] and OS [41, 42] in HL, the impact of TMTV and TLG has been significant in the context of randomized trials or larger patient series as well, both for early stage disease [43, 44] and for advanced disease, where TMTV may stratify patients with a negative interim PET into distinct prognostic subgroups [45,46,47,48].
Similarly, small or medium-sized studies (<200 patients) have shown the prognostic impact of TMTV [49,50,51,52,53] and TLG [54, 55] in DLBCL, which appears to be independent from conventional prognostic systems and molecular profiling. In addition, a very recent large study of >1000 patients clearly demonstrated the additive impact of TMTV to the IPI in DLBCL in the form of International Metabolic Prognostic Index [56]. TMTV and TLG were also independent prognostic factors after adjustment for IPI and cell-of-origin within the large population (>1000 patients with DLBCL) enrolled in the GOYA trial comparing CHOP plus rituximab or obinutuzumab [57]. Furthermore, within the REMARC randomized clinical trial which included only patients with a response to R-CHOP (and consequently more favorable prognosis), baseline TMTV still remained a strong independent prognostic factor and this was independent from the administration of lenalidomide maintenance or not [58].
In PMLBCL, for which established and reproducible prognostic factors are generally lacking, baseline PET parameters may also be valuable: Within the IELSG26 study, 103 patients were treated predominantly with R-MACOP-B (84%) or R-CHOP (16%), both followed by RT [59]. Baseline SUVmax, MTV, and TLG of the mediastinal disease were associated with outcome, but only high TLG, observed in 1/3 of patients, was an independent prognostic factor, overcoming the significance of the other PET parameters, bulky disease, and other conventional prognostic factors. The 5-year PFS and OS for patients with low vs. high TLG were 99% vs. 64% (p < 0.0001) and 100% vs. 80% respectively (p = 0.0001) [59]. The prognostic significance of TMTV was confirmed by a LySA study as well as by MD Anderson and Dana Farber data under R-da-EPOCH chemotherapy [60, 61].
Other baseline PET-derived metabolic parameters may also provide important prognostic information in HL and aggressive B cell lymphomas. The distance between the 2 lesions that are farthest apart (Dmax or lesion dissemination), a measure of tumor dissemination, may add to the prognostic significance of MTV or even overcome it in DLBCL and cHL [62,63,64]. Metabolic heterogeneity refers to the intratumoral distribution of 18FDG uptake, which reflects the glucose metabolism of both the tumor cells and their microenvironment as well as other processes, such as necrosis, apoptosis, proliferation, and angiogenesis. High metabolic heterogeneity confers adverse prognosis in PMLBCL in addition to TLG [65]. In DLBCL, high metabolic heterogeneity does not correlate with TMTV and may also confer an adverse impact on prognosis [66, 67].
Baseline PET parameters have also been evaluated in other lymphoma subtypes. A high MTV predicted the outcome of high-tumor burden follicular lymphomas independently from the well-established FLIPI2 prognosticator in a pooled analysis of 3 multicenter studies [68], while it predicted outcomes independently from cell-free DNA in another study [69]. In contrast, neither baseline MTV nor TLG or SUVmax predicted the outcome of follicular lymphoma patients treated within the GALLIUM study with Obinutuzumab or rituximab plus chemotherapy (predominantly bendamustine) followed by antibody maintenance [70]. In MCL, baseline MTV and TLG—but not SUVmax—were independent predictors of PFS in a series of 87 patients [71]. Baseline MTV also predicted PFS and OS in “nodal” T cell lymphomas independently from other clinical factors and had a synergistic prognostic impact with the T cell prognostic index (PIT) [72], while it was subsequently shown to offer prognostic information independent from interim PET as well [73]. Similar data were recently published for TLG in peripheral T cell lymphomas [74]. Finally, a similar prognostic effect for TLG (and SUVmax) was shown in patients with extranodal NK/T cell lymphomas [75].
Although interesting, all this information deserves further prospective evaluation in large-scale studies along with many established clinical and biological prognostic factors before implemented in clinical practice. Standardization of the procedures is also essential for reliable clinical application.
14.3 PET/CT in Response Assessment After Completion of Therapy
14.3.1 Criteria for Response Assessment and Definitions of PET Positivity
The most important information provided by PET, as far as response evaluation is concerned, is the differentiation between viable lymphomatous tissue and necrotic or fibrotic tissue within residual masses, which are apparent on CT. Furthermore, EOT-PET/CT may uncover occult disease in normal-sized lymph nodes or bone marrow disease, which may not be demonstrable by trephine biopsy. In 2005, Juweid et al. published a retrospective study in patients with aggressive NHL, predominantly DLBCL, who underwent PET and CT after 4–8 cycles of chemotherapy [76]. They noticed that patients otherwise categorized as CRu (Complete Remission unconfirmed) based on Cheson’s 1999 criteria were usually PET-negative, and, overall, had a favorable outcome with PFS similar to that of the CR group. Patients in partial remission (PR) had strikingly different outcomes when PET was negative or positive. In the “early” PET era, response assessment had been traditionally based on the International Harmonization Project (IHP) criteria described in 2007 [77, 78]. According to that set of criteria, a positive PET at the EOT was defined in relation to the size of the residual lesion: For residuals <2 cm, any focal or diffuse FDG uptake above the background in a location not compatible with normal anatomy/physiology was considered positive. However, for residuals ≥2 cm a mild uptake above background was still compatible with CR, i.e., PET positivity was defined as FDG uptake exceeding that of the mediastinal blood pool structures (Figs. 14.1b, 14.2c, 14.4c, 14.7c).
(a) Baseline staging in a patient with Hodgkin lymphoma: Extensive supradiaphragmatic as well as infradiaphragmatic involvement consistent with stage IIIB disease. (b) Interim PET after two cycles of ABVD revealed persistence of multiple nodal sites on both sides of the diaphragm with FDG uptake markedly greater than that of the liver. A new focal osseous lesion is also seen. Interim PET was interpreted as positive, Deauville score 5. The patient continued on ABVD. (c) End-of-treatment PET after a total of six ABVD cycles demonstrated further progression. The patient had progressive disease by conventional restaging as well. (d) Further progression later on, during disease course in the same patient. Multiple focal splenic lesions are noted
More recently, the EOT response criteria were revised, adopting the Deauville 5-point scale (D5PS), which had been initially used for interim response assessment (Table 14.1). The D5PS was incorporated in the currently used Lugano criteria [1, 2]. According to current recommendations any FDG uptake up to that of the mediastinal blood pool (corresponding to D5PS 1–2) is considered compatible with CR irrespective of the size of the residual mass. Furthermore, a low-grade positivity, higher than the mediastinal blood pool and up to the uptake of the liver (D5PS 3; Table 14.1), is also considered as a favorable response. Thus, clear PET positivity at the EOT is defined as any uptake above that of the liver, corresponding to D5PS 4 or 5 (Figs. 14.1b, 14.2c, 14.4c, 14.7c). It should be noted that the D5PS score should be determined visually; the classification should not be relied on simple SUVmax comparisons between the uptake of the lesion and that of the liver or the mediastinal blood pool.
The currently used set of criteria for the evaluation of response in malignant lymphomas incorporating both PET/CT and anatomic findings are summarized in Table 14.1 [1, 2, 32] (Figs. 14.1b, 14.2c, 14.4c, 14.5c, 14.7c).
14.3.2 Who Should Have an EOT-PET-Based Response Assessment and When?
PET/CT is routinely used for final response assessment in patients with HL and aggressive B cell lymphomas. It is also currently recommended as the optimal tool for final response assessment in all other FDG-avid subtypes, especially in follicular lymphomas. However, the accuracy parameters related to EOT-PET depends on the precise histologic subtype, being highest for HL but lower for aggressive non-Hodgkin lymphomas. Although clearly recommended for final response assessment, PET/CT may not be so informative in low-grade follicular lymphomas and MCL, since these diseases are incurable and a negative PET/CT is merely reflecting an improved PFS and prolonged survival but not “true” disease eradication. When used in variably 18-FDG-avid histologic subtypes, which is not recommended as a general rule, it is essential to have a baseline PET/CT available in order to confirm that the tumor is 18-FDG-avid (Fig. 14.6).
EOT-PET/CT evaluation should preferably be performed 4–6 weeks (and at least 3 weeks) after chemotherapy and immunotherapy and 8–12 weeks after RT, in order to avoid false positive findings due to inflammatory processes and false negative due to stunning from cytostatic drugs [1, 2, 77, 78]. As far as interim PET is concerned it should better be performed as close to the next chemotherapeutic cycle as possible (see next topic).
14.3.3 Clinical Data in Individual Lymphoma Subtypes
As already stated, accuracy parameters, i.e., positive and negative predictive value (PPV, NPV) of EOT-PET/CT, depends on the histologic subtype (Hodgkin lymphoma vs. individual subtypes of non-Hodgkin lymphomas), but also on the chemotherapy regimen applied (standard or intensive) and the a priori probability of relapse, as reflected by clinical stage or other prognostic factors.
14.3.3.1 Hodgkin Lymphoma
The long-term outcome of patients with HL who achieve a PET-negative status at the end of first-line chemotherapy, depends on stage, chemotherapy regimen, and use of RT, as summarized in Table 14.2 [79,80,81,82,83,84,85,86,87,88]. Α negative PET/CT after standard ABVD chemotherapy predicts a 5-year relapse-free survival (RFS) of ~95% in stages I/II, where ABVD is typically followed by RT (Fig. 14.5c), and ~ 80% in stages III/IV, in which only few patients are irradiated (Fig. 14.4c) [79].
Within the RAPID trial, patients with non-bulky clinical stage I/IIA and a strictly negative PET (D5PS 1 or 2) after ABVD×3 were randomized to receive 30 Gy involved field (IF)-RT or no further treatment, achieving a 3-year PFS of 97% versus 91% respectively (p = 0.026) [83]. In the German Hodgkin Study Group (GHSG) HD16 trial of patients with localized, favorable HL (early stages) treated with ABVD×2, the 5-year PFS was 93% versus 86% who received consolidative IF-RT or not, if EOT-PET was strictly negative (D5PS 1 or 2) [84]. Within the GHSG HD17 trial of patients with localized, unfavorable HL (intermediate stages) treated with intensified therapy (BEACOPP-escalated ×2 plus ABVD×2) consolidative RT could be omitted without clinically meaningful loss of efficacy, if EOT-PET was strictly negative (D5PS 1 or 2). Even among patients with bulky disease, the 5-year PFS was 97% regardless of the administration of consolidative RT. [86] These data may have important implications for the design of follow-up strategies [89].
Regarding advanced HL treated with ABVD, the HD607 trial demonstrated that RT can be omitted in patients who achieve a negative PET status, defined as D5PS score 1–3, both at the interim and EOT evaluation despite the presence of bulky disease ≥5 cm [90]. The 6-year PFS was 92% versus 90% for irradiated and non-irradiated patients and the difference was not significant whatever the definition of bulk (5, 7, or 10 cm) [91]. If advanced stage patients are treated with more aggressive chemotherapy such as BEACOPP-escalated or variants, the 5-year RFS for patients with a residual mass of >2.5 cm and a negative post-chemotherapy PET/CT is approximately 90% without RT [80], falling to 88% at 10 years [87]. Within the HD15 study of the GHSG, this was comparable to the 85% observed as 10-year PFS in patients with conventional CR or residual masses <2.5 cm, who did not undergo EOT-PET/CT evaluation [87].
Despite additional RT, early stage patients who remain PET/CT-positive after ABVD chemotherapy have a 5-year RFS of 40–65% (Fig. 14.1b) [81, 82, 88, 92]. Higher 18-FDG uptake is predictive of treatment failure in this setting and could have an impact on therapeutic strategies, but this needs further clarification [88, 92]. In the above-mentioned RAPID trial, patients with favorable (defined as non-bulky) stage I/IIA HL who remained PET-positive (D5PS 3, 4 or 5) after ABVD×3 received one more ABVD cycle and IF-RT. The outcome was favorable for those with D5PS 3–4, but it was dismal for those with D5PS 5: The 5-year PFS was 95%, 88%, and 62% for patients with D5PS score 3, 4, and 5 respectively [88]. This difference was translated to overall survival difference as well. In the GHSG HD16 trials of early (favorable) stages, among patients receiving ABVD×2 plus IF-RT, the 5-year PFS was 93%, 88%, and 81% for patients with D5PS score 1–2, 3–4, or 4, respectively, while the corresponding figures were 98%, 94%, and 82% within the HD17 trial after BEACOPP-escalated ×2 plus ABVD×2 plus consolidative RT. [84, 86] This suggests that D5PS score ≥ 4 is an unfavorable prognostic factor despite additional RT (caution to be exercised to the definition of score 4; cases with conventional D5PS score 5 may have been included in the absence of new lesions).
In advanced stages, the figures are similar to early stages after ABVD, but it appears that, after more intensive chemotherapy such as BEACOPP-escalated, RT to >2.5 cm PET-positive residuals may be much more efficient for disease control with long-term RFS just below 85% [87]. The degree of conventional radiographic response appears to correlate with disease control after BEACOPP-based therapy and RT: Patients whose residual masses had been reduced by >40% in their largest diameter compared to baseline had similar outcomes with PET-negative patients with 4-year PFS of 92%. The prognosis was worse for patients with reductions ≤40%, who had a 4-year PFS of 73% [93].
14.3.3.2 Primary Mediastinal Large B Cell Lymphoma
A negative PET/CT after R-CHOP, R-MACOP-B, or R-da-EPOCH is associated with 90–95% cure rates in PMLBCL, even when RT is omitted in many patients [38, 39, 94,95,96,97,98]. According to the Vancouver experience, even patients with D5PS score 3 after R-CHOP may enjoy a > 90% long-term disease control rate without consolidative RT [97], which is similar to the outcomes achieved with RT in all these patients [98].
If irradiated, PET/CT-positive residual masses are effectively controlled in 65–70% of cases provided that the disease is responsive by conventional imaging [39, 95, 96, 98, 99]. In particular the rate of long-term disease control in patients with D5PS score 4 following R-CHOP (or R-MACOP-B) is exceptionally high in the range of 80–87% [39, 97, 98]. Among the latter patients, those with D5PS score 4 and “lower” FDG uptake may have equally favorable outcomes compared to PET-negative patients with long-term PFS >90%, while those with higher SUVmax (for example ≥5) probably have significantly inferior outcomes [99, 100]. Although patients with D5PS score 5 have inferior outcomes [39, 98,99,100,101] >40% of them can achieve long-term disease control with consolidative RT if they have achieved PR by conventional imaging [98]. However, salvage chemotherapy intending to autologous transplant is preferrable for patients with D5PS score 5 and conventionally defined stable or progressive disease [98].
If patients are treated with the more intensive combination R-dose adjusted-EPOCH (R-da-EPOCH), EOT-PET/CT can be interpreted more conservatively. Patients with D5PS scores 1–2 are not candidates for consolidative RT, but RT is also omitted in patients with D5PS 3 and 4. Serial PET/CT evaluation typically shows regression or stability even in D5PS score 4 during follow-up with no further intervention [102,103,104]. The few patients with D5PS score 5 after R-da-EPOCH can be effectively salvaged with RT if they have achieved PR by conventional methods but should be again forwarded to salvage chemotherapy intending to autologous transplant if they have conventionally defined stable or progressive disease [105].
Because of the considerable curative potential of RT, patients with PMLBCL should not be referred for high-dose therapy and autologous transplantation solely based on a positive PET/CT after immunochemotherapy and this is especially true if the uptake is not marked [94]. It should be also noted that certain patients who have low-grade positivity after immunochemotherapy remain PET/CT positive at a similar degree after RT as well without experiencing disease progression, suggesting that mild positivity (at the lower range of D5PS score 4) may be compatible with cure in this entity [99, 101, 102]. Finally, the question whether RT could be safely omitted in PET-negative patients after immunochemotherapy (other than R-da-EPOCH) is currently evaluated by the IELSG-37 randomized trial.
14.3.3.3 Diffuse Large B Cell Lymphoma
A negative PET/CT after R-CHOP carries a lower NPV in DLBCL compared with HL and PMLBCL [106,107,108,109,110,111,112,113]. The long-term event-free survival (EFS) in these patients after a negative PET/CT post R-chemotherapy is roughly 70–85% (Table 14.3) and the probability of relapse may depend on their baseline relapse risk, as reflected by the International Prognostic Index (IPI) and its components [106, 109, 110], the cell of origin [106] as well as on the depth of conventional radiographic response (CR versus PR) (Fig. 14.2c), the size of the residual mass, and the number of residual lesions [108].
Recently, two large studies including patients with predominantly advanced DLBCL evaluated EOT-PET after R-CHOP (or obinutuzumab-CHOP) using the D5PS [112, 113]. Both demonstrated a 3-year disease control rate of 82–83% without consolidative RT. Within the GOYA trial including >1000 patients, a positive EOT-PET (D5PS score 4–5) was an independent prognostic factor after adjustment for IPI or the cell-of-origin. Unexpectedly, among patients with complete metabolic response (D5PS score 1–3), those with IPI 0–2 had inferior 2.5-year PFS to those with IPI 3–5 (77% versus 88%, p < 0.0001), while ABC DLBCL expectedly fared worse than their GCB counterparts (2.5-year PFS 80% versus 89%, p < 0.05) [112]. According to the British Columbia experience on 723 patients, the 3-year disease control was 83% and inferior outcomes were predicted independently by baseline B-symptoms and BM involvement. The individual IPI factors were not independently associated with the outcome but the IPI per se and the cell-of-origin were not assessed. Interestingly, the outcome of patients with a negative EOT-PET was the same in the presence of bulky disease or not and independently of the presence of skeletal or craniofacial involvement, which were traditionally irradiated in some institutions [113]. The feasibility to omit RT in patients with bulky disease who achieve a PET-negative status following R-CHOP-based therapy was also confirmed in the setting of the OPTIMAL randomized trial, which was limited to elderly DLBCL patients [114].
Patients with DLBCL who remain PET/CT-positive after R-CHOP have a < 40–50% probability to remain disease-free [107,108,109,110], but even this figure suggests that false positive findings are not infrequent (Table 14.3). Within the GOYA trial only 12% of the 1092 patients had a positive EOT-PET defined as D5PS score 4–5 and still enjoyed a 3-year PFS of 49% [112]. IPI was not predictive in this subgroup, but ABC DLBCL remained worse than GCB (44% versus 63% disease control). Unfortunately, there was no mention of the potential impact of the exact D5PS score (4 versus 5), which may be critical for the outcome.
The British Columbia group also focused on the EOT-PET-positive subgroup and the role of RT. Among 723 patients with advanced DLBCL (stage III/IV or I/IIB or bulky) treated with R-CHOP, the rate of EOT-PET positivity was much higher reaching 25% (178/723) [113]. The study was focused on patients who had not experienced disease progression until the time of EOT-PET, so that they were considered as responders with residual disease. Among the 178 patients with a positive EOT-PET defined as D5PS score 4–5, 86 received RT and achieved a 3-year time-to-progression (TTP) of 69%, which was only slightly inferior to EOT-PET-negative patients (83%). This impressive success rate should be further confirmed, since it depends on optimal patient selection. In contrast, the 3-year TTP for the 92 patients that had disease not amenable to RT was only 33%. However, even in this unfavorable setting, 1/3 of the patients remain without disease progression. Notably, despite some overlap, the median SUVmax of progressors was substantially higher to that of the minority of patients who remain in remission [16.3 (up to 36.0) versus 4.5 (up to 18.1)] [113].
14.3.3.4 Follicular Lymphoma
EOT-PET carries prognostic significance for patients with FL. Dupuis et al. reported that a positive EOT-PET after 6 cycles of R-CHOP significantly affected PFS regardless of iPET status and FLIPI score [115]. A pooled analysis using EOT-PET/CT scans from 439 patients enrolled in three landmark studies (PRIMA, PET-FL, and FOLL05) showed that D5PS >4 was associated with significantly lower PFS (16.9 vs. 74 months for EOT-PET-positive and -negative patients respectively) [116]. Also, the secondary analysis of PET results from GALLIUM study reported that patients who achieved complete metabolic response had better PFS and OS irrespective of whether they received rituximab- or obinutuzumab-based treatment, or whether they achieved CR in conventional imaging [117]. Currently, restaging with EOT-PET is recommended for prognostication, but not for treatment modification decisions or patient surveillance.
14.3.3.5 Mantle Cell Lymphoma
EOT-PET is considered optional in patients with MCL and its role remains unsettled, as treatment strategies in patients with MCL are heterogenous. A study of 32 cases treated with Rituximab-Bendamustine demonstrated that patients who achieved complete metabolic response by D5PS had significantly higher PFS [118]. Similarly, in a study of 72 patients treated with alternating R-CHOP/R-high-dose cytarabine, a positive EOT-PET (D5PS score 4–5) was associated with worse PFS [119]. The LyMA-PET project demonstrated that SUVmax and D5PSS in iPET and EOT-PET had not prognostic significance; however SUVmax in iPET and ΔSUVmax (reduction of SUVmax between iPET and EOT-PET) in EOT-PET were associated with OS and PFS, respectively [120].
14.3.3.6 T Cell Lymphomas
The utility of EOT-PET in T cell lymphomas remains rather poorly defined, as T cell lymphomas consist of various histological subtypes with diverse clinical and biological characteristics and heterogenous treatment approaches. In a study of 114 patients with PTCL, iPET had not prognostic significance but a positive EOT-PET (D5PS score 4–5) was significantly associated with worse PFS and OS [121]. In another study of 140 patients with PTCL treated mainly with CHOP or CHOP-like regimens, the authors aimed to explore the role of interim (after 2 or 3–4 cycles of chemotherapy) and EOT-PET/CT. PET positivity was again defined as D5PS score 4–5. Patients with positive interim PET had significantly compromised 2-year PFS and OS. EOT-PET was also predictive as the 2-year PFS and OS were 83% and 94% vs. 6% and 27% for EOT-PET-negative and -positive patients, respectively.
14.4 Interim Response Assessment
Early prediction of response to therapy is of major importance, not only as a powerful prognostic factor but also as a potential basis for early treatment modification. Functional changes that precede the anatomic ones could potentially be more accurate in predicting treatment response early in the course of therapy.
14.4.1 Who Might Benefit from Interim PET-Based Early Response Assessment?
Early response assessment has provided a major prognostic clue for patients with advanced HL or localized HL with adverse prognostic factors [122, 123] and provides a useful tool for early treatment intensification. The prognostic effect of interim PET (iPET) is less marked, although still significant, for patients with DLBCL, but it cannot be used for early treatment modification in the absence of effective alternative chemotherapy. In the specific setting of PMLBCL, the outcomes according to iPET appear to be conflicting [124]. Data on other lymphoma subtypes, including T cell lymphomas, are sparse. Relevant studies regarding iPET are discussed below.
The D5PS was initially described for the evaluation of iPET and remains the standard tool for this purpose in HL [1, 2]. In DLBCL, the D5PS is less reproducible and is associated with inferior prognostic discrimination. An approach based on the reduction of SUVmax between baseline and iPET (ΔSUVmax at a cutoff of 66%) is prognostically superior and sufficiently reproducible. Thus, the calculation of ΔSUVmax is the recommended approach for iPET interpretation in the specific setting of DLBCL (see below) [1, 2].
14.4.2 Clinical Data in Individual Lymphoma Subtypes
14.4.2.1 Hodgkin Lymphoma
According to the D5PS (Table 14.1), a negative iPET may not be nominally negative: Any positivity in previously involved sites with 18-FDG uptake up to that of the liver is acceptable as a favorable interim response (D5PS scores 1,2,3) and this assessment should be made visually (Fig. 14.5b). Any uptake higher than the liver is considered positive (scores 4,5) (Figs. 14.4b, 14.7b). Using the D5PS, the International Validation Study demonstrated that, under ABVD chemotherapy, the 3-year PFS for patients with negative and positive iPET was 95% vs. 28% [125]. Such figures may apply not only to advanced HL, but also to intermediate stage HL (localized stages with ≥1 unfavorable features). However, the outcome of iPET-positive patients with localized disease and no adverse factors, especially no bulky disease, may be much better than the ~30% reported above [82, 122, 126]. Furthermore, the excellent outcome for iPET-negative patients with intermediate, and particularly advanced, stages has not been reproduced in subsequent studies using ABVD, as discussed below.
Under BEACOPP-escalated, the NPV of iPET is also very high, with >90% of iPET-negative patients achieving continuous CR. Nevertheless, the PPV is much lower compared with ABVD-treated patients: Large datasets within the context of randomized trials have recently revealed PFS rates between 70% and > 90% with continued BEACOPP-escalated for a total of 6 cycles in case of iPET positivity after the second cycle [127,128,129,130]. In the HD18 trial of the GHSG, 46% of advanced stage patients remained iPET-positive after BEACOPP-escalated ×2 (defined loosely as D5PS ≥3) and ~ 54% of them were still positive by the current definition of D5PS ≥4 [131]. The 3-year PFS for all these patients was ~93–94%; it was 91% for patients with D5PS ≥4.
14.4.3 Is It Reasonable to Modify Treatment of HL in Response to Interim PET Results?
In order to justify treatment modification in response to iPET result, two conditions must be met: Firstly, the outcome of iPET-positive patients could be possible to improve by an alternative therapy, and, secondly, the NPV should be sufficiently high to avoid relapses in the vast majority of iPET-negative patients.
Regarding the first condition, there are overwhelming data indicating that treatment intensification may produce long-term PFS rates of ~65% (vs. ~30% expected based on historical data with continued ABVD) in patients with advanced or even intermediate (early unfavorable) stage HL, who remain PET/CT-positive after 2 ABVD cycles (Fig. 14.4b) [90, 91, 132,133,134,135,136,137]. This is typically achieved by switching chemotherapy from ABVD to BEACOPP-escalated for at least 4 cycles [90, 91, 132,133,134,135,136, 138] but early salvage therapy and autologous stem cell transplantation may also produce similar results [139]. These data are summarized in reference #138.
Although clinical trials are mainly investigating PET-adapted therapy for advanced disease, the only randomized evidence for the superiority of treatment intensification with BEACOPP-escalated in HL comes from the H10 trial for localized stages [140]. In H10, 361/1925 (19%) of patients had persistent PET/CT positivity after ABVD×2, loosely defined according to the IHP criteria and roughly corresponding to D5PS score ≥ 3. Only 97/361 (27%) had no adverse factors, while 264 (73%) had ≥1 risk factor. These 361 patients were randomized to receive: (1) 1 or 2 further ABVD cycles (according to the absence or presence of risk factors) plus 30 Gy involved node RT (standard arm) or (2) 2 cycles of BEACOPP-escalated plus 30 Gy involved node RT (experimental arm) irrespective of risk factor classification. PFS was improved by only 2 cycles of BEACOPP-escalated with 5-year rates of 91% versus 77% in the experimental and the standard arm respectively (p = 0.002). Importantly, a marginally significant but clinically meaningful improvement was noted for 5-year OS as well (96% versus 89%, p = 0.062) [140]. However, in a subsequent analysis presented in abstract form, it became evident that the benefit of switching to BEACOPP-escalated was limited only to patients with D5PS score 4–5, while those with D5PS score 3 were effectively treated by ABVD×2 plus RT. [141]
In the specific setting that first-line therapy is based on BEACOPP-escalated instead of ABVD, the long-term PFS of iPET-positive patients may be in the range of 70 to >90%, as stated above [128, 131]. In this setting, improvement of the outcome of iPET-positive patients appears difficult. For example, the addition of rituximab was not successful in improving the outcome of iPET+ patients after BEACOPP-escalated ×2, in the GHSG HD18 trial [131].
While the first of the required conditions is partially fulfilled, the second one is becoming particularly important for the final success of iPET-directed therapy: Although major early studies had shown that the NPV of iPET could be >90% under ABVD, more recent studies and maturing data suggest that it may not be so perfect as initially thought in patients with truly advanced HL: In the US S0816 trial, the 2-year PFS of 271 patients with stage III/IV HL treated with ABVD×6 was 82% (and was projected to be reduced to ~−70–75% at 4–5 years!) and did not differ according to the D5PS score (80%, 84%, and 81% for scores 1, 2, or 3) [134, 137]. In the RATHL trial, the 3-year PFS for iPET-negative patients with continued ABVD or AVD was 84–86%, but it was only 82% for younger (<60 years old) stage III/IV patients [133]. Within the HD0801 trial (81% stage III/IV), the 2-year PFS was 81% [139], while the 3-year PFS was 87% in the HD607 trial, which included only 67% stage III/IV patients [90]. Smaller studies also provided similar results with long-term PFS for iPET-negative patients clearly <90% and as low as 71–77% [135, 136, 142]. These data have been extensively reviewed in reference #138. It is not currently known if there is a reproducible subgroup of iPET-negative patients after ABVD who remain at a high risk of failure.
The a priori risk of failure as reflected by stage IV (or extranodal involvement) or other prognostic factors may affect the NPV of iPET and should be further investigated [133, 136, 143]. A large mediastinal mass ≥ 7 cm was predictive of relapse in iPET-negative patients in another retrospective study [144]. The significance of any bulk ≥5 cm was also demonstrated in strictly iPET-negative, non-bulky stage I/IIA patients in the RAPID trial [145]. Serum lactate dehydrogenase elevation was the only predictor of conversion from iPET-negative to EOT-PET-positive and anemia was modestly associated with PFS in the HD0801 study [146, 147], while only the IPS predicted—albeit loosely—treatment failure in iPET-negative patients of the HD0607 trial [148]. Within the latter trial, baseline TMTV and IPS could define 3 groups of iPET-negative patients with highly diverse outcomes: TMTV<471 mL and IPS 0–1 (7% of patients), either elevated (80% of patients) and both elevated (TMTV≥471 mL and IPS >1; 13% of patients) with 3-year PFS of 98%, 85%, and 56% [46]. Similarly, within the H10 trial for localized HL, TMTV >147 mL (observed in only 16% of iPET-negative patients) was associated with significantly, but numerically slightly inferior outcome with 5-year PFS of 82% versus 95% for those with lower TMTV [43]. Biological prognostic factors may also be relevant, such as high content of CD68+ tumor-associated macrophages plus diffuse or rosetting PD1+ cells in the microenvironment or STAT1 negativity of tumor cells [149]. Persistence of residual TARC levels >800 pg/mL after ABVD×2 may also discriminate a rather small subgroup (19% of iPET-negative patients) with inferior outcome (4-year PFS 74% versus 89%) [150]. Despite all these data there is no evidence that any prognostic factor or combination can define a sizeable subgroup of iPET-negative patients with sufficiently poor outcome to justify a different approach from the beginning.
Apart from starting with ABVD and escalating to BEACOPP, an alternative iPET-driven strategy can be starting with BEACOPP-escalated and de-escalating chemotherapy in case of a negative iPET.
Very promising results have been reported by the LySA 2011 trial, in which this reverse iPET-driven strategy was applied to 823 patients with advanced stage HL according to the GHSG definition, i.e., stage III/IV or IIB with mediastinal bulk and/or extranodal involvement [127, 128]. The standard arm consisted of fixed treatment with BEACOPP-escalated ×6 and the experimental arm consisted of BEACOPP-escalated ×6 in case of a positive iPET after 2 cycles or BEACOPP-escalated ×2 plus ABVD ×4 if iPET was negative. The study had a non-inferiority design with a margin of 10%. The experimental arm was not inferior to the standard one with 5-year PFS rates of 86.7% versus 87.5% [128]. The 5-year PFS for iPET-negative patients was similar for the two arms reaching ~90%. For iPET-positive patients it was ~71%. It should be noted that iPET positivity was defined as metabolic activity exceeding 140% of the liver activity and only 12–13% of the patients remained iPET-positive after BEACOPP-escalated ×2 [127].
In the GHSG HD18 trial (again advanced stages according to the GHSG definition) the standard arm consisted of fixed treatment with BEACOPP-escalated (×8 or ×6 in a subsequent amendment) and the experimental arm consisted of BEACOPP-escalated (×8 or ×6 in a subsequent amendment) in case of a positive iPET after 2 cycles or BEACOPP-escalated ×4 (total cycles) if iPET was negative [130]. The definition of iPET positivity was D5PS score ≥ 3 and 48% of the patients remained iPET-positive. The 5-year PFS after BEACOPP-escalated ×4 or ×6 in patients with D5PS score 1–2 (iPET-negative) was ~91% in both arms and overall survival was numerically better with the abbreviated 4-cycle regimen [129]. The 5-year PFS for the iPET-positive population was ~88% (numerically higher in case of D5PS score 3 compared to ≥4) in sharp contrast with the 71% observed in the AHL 2011 trial with the same treatment. However, the rate of iPET positivity was 48% versus 12–13% in the two trials due to the different thresholds used and this is the possible explanation for this large discrepancy.
14.4.3.1 Diffuse Large B Cell Lymphoma
In DLBCL, iPET is also predictive of the outcome after R-CHOP or similar immunochemotherapy, but differences are not so marked compared with HL. The use of iPET to guide treatment decisions is not currently recommended [1, 2] because there is still no proven salvage therapy capable of improving the outcome of patients with a positive iPET, while the NPV of iPET is rather low.
As stated above, the D5PS is not so widely accepted in this setting, because of their moderate reproducibility and prognostic capacity [151] (Fig. 14.2b). Alternatively, a satisfactory iPET response can be defined by a > 66% reduction in SUVmax between baseline and interim assessment [1, 2, 151]. In the NHL International Validation Study, based on 114 DLBCL patients treated with standard R-CHOP-21 or intensified R-CHOP-14 or R-ACVBP-14, where no PET-driven treatment modification was made, the 3-year PFS was 79% vs. 44% in patients with >66% and ≤ 66% SUVmax reductions after 2 cycles of immunochemotherapy [151]. The 66% SUVmax reduction criterion was superior to the D5PS in a subanalysis of the CALGB 50303 trial (R-CHOP-21 or R-da-EPOCH), as well as in the UKCRN-ID 1760 and the SAKK 38/07 trials (both adopting R-CHOP-21 or R-CHOP-14), in which no treatment modification was made according to iPET results [152,153,154].
In the LNH-2007-3B trial, higher risk, young DLBCL patients randomly received either R-CHOP-14 or R-ACVBP-14 and underwent iPET assessments after 2 and 4 cycles, which modified subsequent treatment strategy. The study confirmed that visual analysis was not accurate enough. The cutoff for SUVmax reduction was set at 66% for PET-2 and 70% for PET-4 [155]. The 4-year PFS according to PET-2 was 80% vs. 56%, while it was 84% vs. 35% according to PET-4 [156]. The prognostic significance of iPET using the 66% SUVmax reduction criterion was also confirmed in the PETAL randomized trial and a GELTAMO phase 2 trial, both of which included treatment intensification for iPET-positive patients [157]. In the PETAL trial, 2-year PFS was 79% for iPET-negative versus 46% for iPET-positive patients despite treatment intensification in the latter (p < 0.0001). Using the D5PS the difference was much less marked and the corresponding figures were 79% versus 71% (p = 0.0068). Neither the addition of 2 rituximab infusions in iPET-negative patients nor treatment intensification in the form of a Burkitt protocol resulted in any improvement in the outcome of patients with aggressive lymphomas [158]. The corresponding 2-year OS rates were 88% versus 59% (p < 0.0001). The prognostic significance of iPET was independent from that of IPI [158, 159].
Overall, the use of the SUVmax reduction criterion over the D5PS score in DLBCL is supported by the results of studies of fixed or PET-driven modified treatment as well as by expert opinions [151,152,153,154,155,156,157,158,159,160,161].
The prognostic significance of iPET in DLBCL in various studies using either or both of the above criteria, either under the same continued treatment or after treatment escalation, is summarized in Table 14.4 [111, 124, 151, 153,154,155,156,157,158,159, 162,163,164,165,166,167].
14.4.3.2 Primary Mediastinal Large B Cell Lymphoma
At present, existing data are too limited to support the recommendation of interim response assessment and iPET-based treatment modification in PMLBCL [168,169,170]. However, data on iPET under R-CHOP-21 in PMLBCL are only derived from a small subgroup analysis of the PETAL trial [158]. A study from Memorial Sloan Kettering Cancer Center failed to show any impact of iPET on the outcome of PMLBCL, when treatment was modified in patients with positive findings: In detail, 51 patients received 4 cycles of accelerated R-C1000HOP-14 and underwent iPET, which was negative in 53% of them [169]. A significant number of patients underwent biopsies of the iPET-positive mass, which were always negative [124]. Patients subsequently received non-cross resistant therapy with 3 cycles of ICE with or without rituximab and no additional RT. No difference in PFS emerged according to iPET result irrespective of the criterion used to define positivity. Similar results were reported by Lazarovici et al. in a study of 36 patients, in which 16/17 patients with positive iPET had negative biopsies [171]. However, none of the iPET-positive patients had D5PS score 5 and treatment (mostly R- or G-ACVBP) was modified according to iPET result.
In a retrospective study of 30 patients, R-VACOP-B (n = 19) or 11 R-CHOP (n = 11) was continued irrespective of iPET result without consolidative RT. [170] A positive iPET was observed in 47% of patients. Their 3-year PFS was 57% versus 94% for those with a negative iPET (p = 0.015). However, there was a trend towards inferior prognostic performance of iPET after R-VACOP-B. In another Chinese retrospective study of 49 patients treated with R-da-EPOCH or R-CHOP, the rate of iPET positivity was 37% and 10/18 iPET-positive patients had D5PS score 5. Treatment was modified in 7/10 patients with score 5 and 1/8 with score 4. The 2-year PFS rate was 93% versus 69% versus 20% for patients with D5PS score 1–3, 4, and 5 respectively, with only score 5 conferring a clearly inferior outcome despite frequent treatment modification [172].
Finally, the previously described PETAL trial included a small subgroup of 42 patients with PMLBCL. Using the ΔSUVmax criterion, only 12% remained iPET-positive after R-CHOP×2. The 2-year FFTF was clearly superior for iPET-negative patients (89% versus 40%) despite treatment intensification in case of iPET positivity; however, the 2-year OS was virtually the same (97% versus 100%) [168]. Obviously, the effect of Burkitt-like treatment intensification could not be adequately evaluated with only 5 iPET-positive patients [168].
14.4.3.3 T Cell Lymphomas
Interim PET positivity by the ΔSUVmax criterion is also a strong prognostic factor in patients with T cell lymphomas. The rarity of these subtypes has not permitted the development of separate trials. In a subgroup analysis of 76 patients with peripheral T cell lymphomas (ALCL, AITL, or PTCL-NOS) enrolled in the PETAL trial, 25% remained iPET-positive after CHOP×2 by the ΔSUVmax criterion. This percentage was 33% for PTCL, AILT, and ALK-ALCL combined, but only 1/21 patients with ALK+ ALCL had a positive iPET [173]. In the subgroup of 55 patients with PTCL, AILT, and ALK-ALCL, the SUVmax reduction criterion provided the best discrimination in terms of PFS at the cutoff of 50% (and not 66%) with 4-year PFS rates of 50% versus 0%. The same criterion at the cutoff of 66% and the D5PS (5 versus 1–4) provided very good, but slightly inferior discriminative capacity [173]. The extremely small number of events precluded an analysis in the 21 patients with ALK+ ALCL. However, treatment intensification with a Burkitt protocol failed to improve the outcome of iPET-positive patients, but this conclusion was based on the analysis of less than 20 patients and should be interpreted with caution [173, 174]. In extranodal NK/T cell lymphomas, iPET is also prognostically relevant [75], but data on potential treatment modification are lacking.
14.5 Impact of Interim and EOT-PET on Clinical Practice: Randomized Trials
Although the prognostic significance and the diagnostic accuracy of EOT-PET/CT have already been firmly established, studies evaluating PET-guided treatment decisions are only few [83,84,85,86, 90, 91, 129,130,131, 140, 158, 175]. Evidence-based strategies for the implementation of iPET and/or EOT-PET are available only for HL and aggressive B cell lymphomas.
14.5.1 Hodgkin Lymphoma
14.5.1.1 Radiotherapy Questions
Four recent randomized trials have focused on the possibility of omitting RT in localized stage HL after a negative PET/CT. The non-inferiority EORTC H10 has been the most informative of them [140, 175]. The published results of H10 suggest that RT cannot be safely spared after ABVD×2 in patients with stage I/II HL, who become strictly PET/CT-negative by the IHP criteria [77, 78] (roughly corresponding to D5PS scores 1–2), especially in those without adverse risk factors: In H10, patients who became PET/CT-negative after ABVD×2 were randomized to receive: (1) 1 or 2 further ABVD cycles (according to the absence or presence of risk factors) plus 30 Gy involved node RT (standard arm) or (2) 2 or 4 further ABVD cycles (according to the absence or presence of risk factors) without RT (experimental arm). The study was prematurely terminated due to excess relapses in the no-RT arms [175]. More mature results revealed a clear difference in terms of 5-year PFS for patients without adverse risk factors (99% versus 87%, hazard ratio 15.8 with 95% CI 3.8–66.1), but a non-significant one for those with ≥1 adverse factors, including bulky mediastinal masses (92% versus 90%, hazard ratio 1.45 with 95% CI 0.84–2.50). In any case however, non-inferiority of ABVD compared with combined modality could not be demonstrated for patients with a negative PET after ABVD×2 [140]. The design of the other 3 trials, namely RAPID, HD16, and HD17, rather resembled to an interim PET- than an EOT-PET-driven trial [83,84,85,86].
Overall, all published trials suggest that RT cannot be omitted after 2, 3, or 4 cycles of ABVD in patients with early favorable disease (stage I/II—no adverse prognostic factors) and a strictly negative PET after 2 or 3 ABVD cycles without relevant loss in disease control (generally 10–15% at 5 years; Table 14.2), although overall survival is not affected at all. Furthermore, it is not clear if omission of RT will be associated with an increased rate of very late relapses observed >5 years from initial diagnosis, which are typically not captured during the mid-term follow-up of randomized clinical trials [176]. On the contrary, in patients with early unfavorable disease (stage I/II and ≥ 1 adverse factors) the benefit from consolidative RT in case of a strictly negative iPET (D5PS scores 1–2) appears to be minimal. Thus, surprisingly, RT can be omitted in this unfavorable subgroup—even in patients with bulky disease—with minimal loss in disease control and no impact on overall survival, provided that a total of 6 ABVD cycles are given. After more intensive chemotherapy consisting of BEACOPP-escalated ×2 plus ABVD×2, RT can be omitted if EOT-PET is strictly negative without any loss even in disease control [85].
Whether RT can be omitted in patients with an iPET D5PS score 3 is unclear, since these patients were irradiated in the above randomized trials. However, both the RATHL [133] and the HD607 randomized trial [90, 91] included a considerable percentage of patients with early unfavorable stages and the outcomes without consolidative RT appeared to be comparable in the D5PS score 1–3 (iPET-negative) subgroup irrespective of the exact classification as 1, 2, or 3, again provided that 6 A(B)VD cycles were given. However, in the CALGB 50604 trial, after only 4 ABVD cycles and no consolidative RT, patients with non-bulky stage I/II HL with an iPET D5PS score 3 had numerically lower 3-year PFS compared to D5PS score 1–2 (77% versus 94%) [177].
In advanced stage HL, RT is not considered in EOT-PET-negative patients without bulky disease, while it can be omitted in case of iPET and EOT-PET negativity (D5PS score 1–3) after ABVD ×6 in patients with bulky disease defined as dmax ≥5 cm [90, 91]. It appears that this is independent from the size of bulky disease (even if ≥10 cm) although HD607 was not powered enough to address this specific subgroup question. As already mentioned, RT can be spared irrespective of the initial bulk in advanced HL patients with a negative PET and > 2.5 cm residual abnormalities (and obviously in those with smaller or no abnormalities), if this response has been achieved by intensive chemotherapy with BEACOPP-escalated or similar regimens, because >88% of them remain disease-free at 10 years [80, 87].
14.5.2 Chemotherapy Questions
Treatment intensification in the form of BEACOPP-escalated is recommended in patients with a positive iPET after ABVD ×2. There is clear evidence from the H10 trial that BEACOPP-escalated ×2 improves PFS and marginally overall survival in early unfavorable HL compared to ABVD ×2 plus the same consolidative RT. [140] Although not tested in any randomized trial of advanced HL, switching to 4–8 cycles of BEACOPP-escalated clearly improves PFS in iPET-positive patients with stages III/IV (or unfavorable II) [90, 91, 132,133,134,135,136,137,138]. The RATHL approach using BEACOPP-escalated ×4 or BEACOPP-14 ×6 appears reasonable in terms of preserved efficacy with the least possible toxicity.
Apart from starting with ABVD, another iPET-driven strategy involves starting with BEACOPP-escalated and de-escalating chemotherapy in case of a negative iPET. However, 2 different methods of de-escalation have been tested, as described above (sect. 14.4). Collectively, both the AHL 2011 and the HD18 trial suggest that, if BEACOPP-escalated is the initial choice, 6 cycles should be given to strictly iPET-positive patients, while de-escalation can be either a total of BEACOPP-escalated ×2 plus ABVD ×4 or a total of BEACOPP-escalated ×4. The optimal threshold to define iPET positivity (more strict or looser) needs to be defined further.
Overall, iPET-adapted therapy appears attractive and has been adopted in everyday practice in many institutions. Randomized trials are not yet mature enough to examine the presence of a potential overall survival benefit over ABVD. Whether an ABVD-first and escalation or BEACOPP-first and de-escalation is preferable is not clear [138].
14.5.3 Aggressive B Cell Lymphomas
14.5.3.1 Radiotherapy Questions
Few prospective trials have evaluated the omission of RT after abbreviated chemoimmunotherapy regimens in DLBCL. Lamy et al. reported that RT can be omitted in patients with non-bulky (<7 cm), localized (stage I/II) DLBCL if a strictly negative PET was achieved after R-CHOP-14 ×4. Overall patients received 4 or 6 cycles of R-CHOP-14 according to baseline risk classification. The 5-year EFS was 92% vs. 89% for patients who were randomized to receive RT or not and relapse rates were similar [178]. Interestingly, PET positivity was defined visually as “18F-FDG uptake above the mediastinum or surrounding background in a location incompatible with normal anatomy or physiology,” which is a very strict criterion for PET-negative status. In another prospective trial of 132 patients with non-bulky (<10 cm), localized (stage I/II) aggressive B cell lymphoma (mostly DLBCL), the rate of PET positivity, defined as D5PS score ≥ 4, was 11%. RT was omitted in patients with a negative iPET after R-CHOP-21 ×3 (D5PS score 1–3), who received R-CHOP ×4 in total without RT, while patients with positive iPET received involved field RT and subsequent radioimmunotherapy with ibritumomab tiuxetan. The 5-year PFS was 89% versus 86% for patients with iPET-negative or positive respectively after R-CHOP-21 ×3 [179]. Similarly, indirect comparison of the OPTIMAL>60 and the RICOVER60 trials suggests that RT can be spared in elderly patients with bulky DLBCL (>7.5 cm) who achieve a PET-negative status after R-CHOP-14-based immunochemotherapy with a 2-year PFS for all bulky patients of 79% (irrespective of PET status or RT) [114].
All the above data suggest that RT can be spared in localized, non-bulky DLBCL even after abbreviated immunochemotherapy in case of a negative PET after 3–4 cycles of immunochemotherapy. It can also be probably spared in bulky, PET-negative patients irrespective of stage, as has been suggested by the British Columbia retrospective experience as well [113].
In PMLBCL RT can be spared after R-da-EPOCH in patients with D5PS score 1–4 after R-da-EPOCH based on the excellent results of a prospective trial [102, 103]. The results are also very encouraging after R-CHOP if RT is spared in patients with D5PS score 1–2 [97,98,99] or even score 3 [97]. However, there is still no evidence on this RT question from a randomized trial. The IELSG-37 trial is expected to shed light on this issue [180].
14.5.3.2 Chemotherapy Questions
The only randomized trial in the field of aggressive lymphomas is PETAL, which failed to demonstrate any impact of intensified treatment in iPET-positive patients with aggressive lymphomas under initial treatment with R-CHOP [158]. In a recently published GAINED trial treatment was modified according to PET-2 and PET-4 results. The outcomes were similar for PET-2-negative/PET-4-negative patients who received intensive conventional immunochemotherapy and PET-2-positive/PET-4-negative patients, who received consolidation autologous stem cell transplantation, with 2-year PFS 90% versus 84%. The 2-year PFS was 62% for PET-4-positive patients. Although the above results suggest some improvement based on PET-driven therapy, this is not a randomized comparison of treatment strategies [181]. Thus, there is no established “more effective” treatment for iPET-positive patients with DLBCL, who have inferior outcomes.
14.6 PET in the Setting of Autologous Stem Cell Transplantation (ASCT)
The evaluation of PET in patients with lymphoma undergoing ASCT was introduced early in the course of utilization of PET in clinical practice. Generally, published studies have included mixed (HL and NHL) patient populations: Patients with positive pretransplant PET have inferior outcomes than those with negative studies. Pretransplant PET appears to be an independent predictor from established clinical risk scores at the time of relapse/progression [182]. In a meta-analysis of 12 studies, incorporating 630 patients with HL and aggressive NHL who underwent ASCT and had been evaluated with pre-high-dose chemotherapy PET examination, Terasawa et al. reported a summary sensitivity of 69%, summary specificity 81%, similar prognostic accuracy among studies, and shorter PFS for patients with positive PET scan [183]. Another meta-analysis reported hazard ratios of 3.2 (for disease progression) and 4.5 (for death) for patients with positive vs. negative pretransplant PET [184].
In relapsed/refractory HL, patients who become PET-negative with salvage chemotherapy and undergo ASCT have a long-term remission rate of 80–85% vs. 40–50% for those who remain PET-positive [185, 186], although the range for these figures among several published studies is much wider, as suggested in a another, more recent meta-analysis [187] (Fig. 14.8). These results demonstrate that failure to achieve a PET-negative status does not preclude ASCT in patients with HL, especially if they are chemosensitive by conventional imaging. However, more standardized protocols are required for evaluation of pretransplant PET/CT in patients undergoing ASCT: It is not clear whether pretransplant PET should be evaluated by the D5PS, SUVmax-based, or other criteria. As a general rule, the decision to proceed to ASCT in relapsed/refractory HL should be based rather on conventional chemosensitivity criteria than on PET evaluation.
(a) 18 FDG-PET before autologous stem cell transplantation in a patient with relapsed Hodgkin lymphoma: hypermetabolic lymph nodes at the upper mediastinum. (b) 18 FDG-PET 4 weeks after autologous stem cell transplantation: negative. (c) Relapsing disease 6 months later. (d) The patient received additional radiation therapy and reached CR (PET negative)
The D5PS has been evaluated in several studies with patients with scores 4—and particularly those with score 5—carrying a worse prognosis [185, 188,189,190,191]. In addition the baseline MTV at initiation of salvage therapy is a strong prognostic factor [189], while the residual pretransplant MTV may also provide independent prognostic information within the unfavorable group of pretransplant PET-positive patients [188, 191]. The outcomes of PET-positive patients with low residual MTV are closer to those with a negative pretransplant PET than to patients with more bulky residuals [188, 191]. Although such sophisticated methods may provide very promising risk stratification, they are not yet applicable in clinical practice and need further standardization and validation.
The probability of further progression after ASCT remains high in relapsed/refractory HL. As shown in the AETHERA trial, the probability of progression in high-risk patients can be significantly decreased by consolidative treatment with brentuximab vedotin, an anti-CD30 monoclonal antibody linked to a microtubule poisson. Brentuximab vedotin consolidation was highly beneficial in patients with a positive PET prior to ASCT but had minimal or no effect on those who had already achieved a PET-negative status [192]. These data should be interpreted with caution because pre-ASCT PET was not required by the protocol and, therefore, it was not performed in all patients and was not centrally and uniformly evaluated. Although this information was derived from an unplanned subgroup analysis, pre-ASCT PET might provide a clue to the optimal use of post-ASCT consolidation and should be further evaluated.
14.7 PET in the Era of Novel Agents
14.7.1 Programmed Death-1 (PD-1) Inhibitors
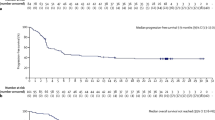
The introduction of Programmed Death-1 (PD-1) inhibitors nivolumab and pembrolizumab has provided very promising results in heavily pretreated patients with relapsed/refractory HL during the last few years [193,194,195,196,197,198]. Promising results have also been reported for PMLBCL [199, 200]. Apart from producing a high objective response rate with several durable remissions (>5 years) [197, 198] (Fig. 14.9a, b), PD-1 inhibitors may result in a transient tumor flare or pseudoprogression. For this reason, an attempt was made to modify the criteria for response assessment to PD-1 inhibitors by describing the LYRIC classification [201]. The description of these revised criteria is beyond the scope of this review. However, pseudoprogression appears to be a rather rare problem in relapsed/refractory HL, while new lesions may be also due to immune-related adverse events [202, 203].
(a) Fifty-two year old male with stage IIA classical Hodgkin lymphoma diagnosed on 2006, 10 years prior to this image, had a slow progression after ABVD × 6 + RT. He achieved a PR to IGEV salvage chemotherapy but declined ASCT approximately 5 years ago. Further ESHAP was too toxic and discontinued after 1 cycle. Brentuximab Vedotin was then instituted but the disease remained stable. The patient was initially unwilling and later ineligible for ASCT and remained on palliative therapy awaiting for a clinical trial with PD-1 inhibitor. Prior to PD-1 inhibitor initiation he had a very unusual disease localization with extensive esophagealgastric hypermetabolic mass (SUVmax 17.2), which caused dysphagia (left panel), regional small hypermetabolic lymph nodes (SUVmax 4.6) and hypermetabolic osseous/bone marrow involvement of the L4 vertebra. After the fourth PD-1 inhibitor infusion (pembrolizumab every 3 weeks) the patient had achieved a complete remission with a negative PET (second image from left, 3/2016). The patient remains in complete metabolic response (CMR) 27 months after the introduction of pembrolizumab (third and fourth images on the right, dated 6/2016 and 3/2017). (b) A 32 year old female with classical Hodgkin lymphoma, stage IIA, was diagnosed on 2012, 3 years prior to this image. After ABVD × 6 + RT she achieved a PR and subsequently relapsed. Although she did not respond to ESHAP salvage chemotherapy, she underwent ASCT, but progressed rapidly thereafter. She further received brentuximab vedotin, bendamustine and gemcitabine-vinorelbine with rapidly progressive disease after each modality. Prior to PD-1 inhibitor initiation she had very extensive disease with generalized hypermetabolic lymphadenopathy, bulky mediastinal (SUVmax 9.6) and left lung localization (SUVmax 6.6) and multiple bone marrow hypermetabolic foci (SUVmax 7.2) associated with a positive bone marrow biopsy and B-symptoms (left panel). After the fourth PD-1 inhibitor infusion (pembrolizumab every 3 weeks) the patient had achieved a complete metabolic response (CMR) with a negative PET (right panel). The patient remains in CMR 27 months after the introduction of anti-PD-1 therapy with pembrolizumab. (c) A 62 year old female with stage IIISXB nodular sclerosing classical Hodgkin lymphoma, was diagnosed approximately 2 years prior to this image. Despite a negative interim PET after ABVD × 2, she developed progressive disease after the seventh ABVD cycle. She failed to respond to IGEV and ESHAP salvage chemotherapy, always developing progressive disease with B-symptoms, pruritus and worsening anatomic findings, thus being unable to undergo ASCT. She also failed to respond to Brentuximab Vedotin and rapidly developed symptomatic progressive disease after 2 cycles of BEACOPP chemotherapy. On March 2016 she was started with the PD-1 inhibitor Nivolumab (3 mg/kg every 2 weeks). Serial CT and PET evaluations demonstrated lack of response, with some anatomic sites responding and others enlarging. However, the patient is asymptomatic and inflammatory markers (ESR, CRP and thrombocytosis) have been completely normalized. This type of sustained clinical response had never been achieved with conventional chemotherapy and Brentuximab Vedotin. It is now increasingly recognized that PD-1 inhibitors may induce relatively durable periods of meaningful clinical benefit in patients who have anatomically stable or slowly progressive disease [195]
As expected, PET/CT is superior to CT for response assessment during PD1 inhibitor treatment for relapsed/refractory HL uncovering more patients with complete metabolic responses [204, 205]. Responses are evident during the initial 2–3 months and correlate with PFS and overall survival [193, 195,196,197,198]. Based on the analysis of the KEYNOTE-087 trial for pembrolizumab the classification of responses appears to be similar irrespective of the use of the Cheson 2007 or the Lugano 2014 criteria [206]. Finally, it should be noted that some patients do not achieve an objective radiographic or PET-based response, but continue to receive clinical benefit for variable time periods despite episodes of disease progression [195]. This strategy of “treatment beyond progression” was formally assessed within the protocol of the CHECKMATE-205 trial of nivolumab.
14.7.2 Chimeric Antigen Receptor (CAR) T cells
CAR-T cells are autologous T cells, modified ex vivo to express receptors which combine antigen-binding and T cell activation properties. Currently CAR-T cell therapy is the most innovative and promising treatment approach for relapsed/refractory DLBCL and related aggressive lymphomas. Several studies have evaluated the prognostic role of PET/CT in the CAR-T cell treatment setting. Vercellino et al. highlighted the prognostic significance of TMTV, and aimed to create a prognostic model for early progression after CAR-T cell infusion by combining TMTV (>80 cm3) and clinical characteristics related to tumor burden, at the time of treatment [207]. Another study aiming to explore the prognostic role of different baseline quantitative metrics also showed that high TMTV (≥25 cm3) was associated with inferior PFS, whereas SUVmax was not of prognostic significance [208]. More studies are needed to define the optimal time point and prognostic role of PET/CT in patients treated with CAR-T cells.
14.8 Artificial Intelligence in F-FDG-PET/CT Scan
The recent applications of Artificial Intelligence (AI) in the field of medical imaging have created great expectations in cancer diagnostics and personalized treatment approaches. Machine Learning (ML) is a branch of AI that creates applications which learn “on their own” by recognizing patterns in input datasets. AI/ML in medical imaging encompasses a variety of applications (e.g., Convolutional Neural Networks—CNN) which aim to eliminate the various biases that may affect image interpretation by humans and produce results comparable to expert radiologists. As extensively discussed in this chapter, F-FDG-PET/CT imaging has been broadly used in staging and response assessment in malignant lymphomas. Until today, just a few studies related to the applications of AI in PET imaging of lymphoma have been published, but definitely, significant progress will be made during the forthcoming years.
Different methods and quantitative metrics (e.g., SUVmean, TMTV, TLG, etc.) in PET/CT have been used in order to quantify tumor burden, evaluate treatment response, and estimate prognosis. AI is expected to better manage these tasks, as its applications may permit automatic quantification and register multiple parts of the body at the same time [209]. The main tasks of AI applications in PET/CT image processing are detection, segmentation, and classification. Detection refers to localization of an area within a medical image which contains an object of interest [210]. Examples include automatically characterizing lymphoma lesions or defining areas of High Normal Activity (Hina) such as bladder and kidneys [211]. Furthermore, the use of radiomics in PET-CT scan has shown great results in separating sites with HiNA, inflammatory nonmalignant lesions, and malignant lesions as shown from Anunziata and Lartizien’s results [212, 213]. Segmentation is the process of demarcation and specific detection of the margins of an object of interest. Segmentation improves the ability of precise estimation of quantitative parameters and improves the methods of exact specification of tumor burden. It is very important to estimate the risk stratification of each patient and predict the therapy response. In order to accomplish this, radiomic features such as standardized uptake value (SUV) and total metabolic tumor volume (TMTV) are used [58, 214]. For example, TMTV and TLG could possibly be better estimated and in less time, through this procedure [215, 216]. Classification refers to the assignment of medical images into diagnostic or prognostic groups. Radiomics are usually used for this purpose in order to characterize lesions as normal or abnormal, or defining different histologic subtypes of lymphomas [216]. Classification could also lead to better prognostication, especially in procedures which highly depend on individual viewer’s experience, such as D5PS estimation [212].
The application of Delta radiomics, which compare the changes of a lesion before and after treatment may be a useful tool in order to estimate the therapy response and the prognosis of the patient [217]. It is crucial for the world’s institutions to contribute to everyday clinical practice’s amplification with AI algorithms in PET imaging [218].
Concluding, AI may provide promising and innovative tools for image processing, medical decision making, and prognostication. It is sure that several subjective and time-consuming procedures will be held automatically in the future. However, more time is needed to evaluate AI applications and produce reliable and robust results. Currently physician’s critical thinking remains invaluable beyond any doubt.
14.9 The Role of PET/CT in the Follow-Up of Lymphomas
Once a negative PET/CT has been achieved, routine follow-up of patients with HL and aggressive B cell lymphomas with PET/CT is not recommended, because it does not affect survival and the risk of false positive findings outweighs any potential benefit of “earlier” identification of relapse and will lead to many unnecessary invasive procedures to exclude relapses. There is also no role for PET/CT in the follow-up of other lymphoma subtypes.
Information regarding PET/CT restaging for relapsing or refractory lymphoma is rather limited (Fig. 14.3b). PET/CT may have a particular role in patients, mainly those with HL, who could be candidates for local treatment with curative intent.
14.10 Conclusions
FDG-PET is a unique tool for the assessment of malignant lymphomas, demonstrating high accuracy and strong prognostic significance. The implementation of PET/CT has altered the definition of response to treatment and has already a major impact on staging and the design of treatment strategies. Although data are rapidly accumulating, the exact role of PET/CT in guiding treatment decisions, especially in the mid-treatment (interim) setting, needs to be defined by randomized trials, many of which are ongoing. Questions under investigation include the role of PET to decide about consolidation radiotherapy, the potential of improving outcomes by early treatment intensification in interim PET-positive patients, or conversely, the possibility of treatment reduction in patients with negative interim PET. Although some answers have already been obtained, evidence-based data on the appropriate use of PET in lymphomas are expected to be available shortly.
References
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol. 2014;32(27):3059–67.
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of Imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32(27):3048.
d’Amore F, Gaulard P, Trümper L, Corradini P, Kim WS, Specht L, et al. Peripheral T-cell lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v108–15.
Dreyling M, Campo E, Hermine O, Jerkeman M, Le Gouill S, Rule S, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv62–71.
Dreyling M, Ghielmini M, Rule S, Salles G, Ladetto M, Tonino SH, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(3):298–308.
Zelenetz A, Gordon L, Chang J, Christian B, Abramson J, Advani R, et al. NCCN guidelines insights: B-cell lymphomas, version 3.2022. J Natl Compr Cancer Netw. 2022;17(6):650–61.
Horwitz SM, Ansell S, Ai WZ, Barnes J, Barta SK, Brammer J, et al. T-cell lymphomas, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20(3):285–308.
Zucca E, Arcaini L, Buske C, Johnson PW, Ponzoni M, Raderer M, et al. Marginal zone lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(1):17–29.
Ngeow JYY, Quek RHH, Ng DCE, Hee SW, Tao M, Lim LC, et al. High SUV uptake on FDG-PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG-PET/CT staging in lymphoma. Ann Oncol. 2009;20(9):1543–7.
Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, et al. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010;51(1):25–30.
Barrington SF, Kirkwood AA, Franceschetto A, Fulham MJ, Roberts TH, Almquist H, et al. PET-CT for staging and early response: results from the response-adapted therapy in advanced Hodgkin lymphoma study. Blood. 2016;127(12):1531–8.
Angelopoulou M, Mosa E, Pangalis G, Rondogianni P, Chatziioannou S, Prassopoulos V, et al. The significance of PET/CT in the initial staging of Hodgkin lymphoma: experience outside clinical trials. Anticancer Res. 2017;37:5727–36.
Michallet AS, Sesques P, Rabe KG, Itti E, Tordot J, Tychyj-Pinel C, et al. An 18F-FDG-PET maximum standardized uptake value > 10 represents a novel valid marker for discerning Richter’s syndrome. Leuk Lymphoma. 2016;57(6):1474–7.
Wang Y, Rabe KG, Bold MS, Shi M, Hanson CA, Schwager SM, et al. The role of 18F-FDG-PET in detecting Richter’s transformation of chronic lymphocytic leukemia in patients receiving therapy with a B-cell receptor inhibitor. Haematologica. 2020;105(11):2675–8.
Eichenauer DA, Aleman BMP, André M, Federico M, Hutchings M, Illidge T, et al. Hodgkin lymphoma: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv19–29.
El-Galaly TC, D’Amore F, Mylam KJ, Brown PDN, Bøgsted M, Bukh A, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol. 2012;30(36):4508–14.
Vassilakopoulos TP, Rondogianni P, Prassopoulos V, Chatziioannou S, Moschogiannis M, Poziopoulos C et al. Comparative assessment of bone marrow involvement (BMI) by bone marrow biopsy (BMB) or positron emission tomography/computed tomography (PET/CT) in Hodgkin lymphoma (HL). Haematol Hematol J. 2014.
Zwarthoed C, El-Galaly TC, Canepari M, Ouvrier MJ, Viotti J, Ettaiche M, et al. Prognostic value of bone marrow tracer uptake pattern in baseline PET scans in Hodgkin lymphoma: results from an international collaborative study. J Nucl Med. 2017;58(8):1249–54.
Voltin CA, Goergen H, Baues C, Fuchs M, Mettler J, Kreissl S, et al. Value of bone marrow biopsy in Hodgkin lymphoma patients staged by FDG PET: results from the German Hodgkin study group trials HD16, HD17, and HD18. Ann Oncol. 2018;29(9):1926–31.
Pedersen MA, Gormsen LC, Kamper P, Wassberg C, Andersen MD, D’Amore AL, et al. Focal skeletal FDG uptake indicates poor prognosis in cHL regardless of extent and first-line chemotherapy. Br J Haematol. 2019;186(3):431–9. https://onlinelibrary.wiley.com/doi/10.1111/bjh.15933.
Adams HJA, Kwee TC, de Keizer B, Fijnheer R, de Klerk JMH, Littooij AS, et al. Systematic review and meta-analysis on the diagnostic performance of FDG-PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: is bone marrow biopsy still necessary? Ann Oncol. 2014;25(5):921–7.
Puccini B, Nassi L, Minoia C, Volpetti S, Ciancia R, Riccomagno PC, et al. Role of bone marrow biopsy in staging of patients with classical Hodgkin’s lymphoma undergoing positron emission tomography/computed tomography. Ann Hematol. 2017;96(7):1147–53.
Vassilakopoulos TP, Angelopoulou MK, Constantinou N, Karmiris T, Repoussis P, Roussou P, et al. Development and validation of a clinical prediction rule for bone marrow involvement in patients with Hodgkin lymphoma. Blood. 2005;105(5):1875–80.
Sehn LH, Scott DW, Chhanabhai M, Berry B, Ruskova A, Berkahn L, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2011;29(11):1452–7.
Paone G, Itti E, Haioun C, Gaulard P, Dupuis J, Lin C, et al. Bone marrow involvement in diffuse large B-cell lymphoma: correlation between FDG-PET uptake and type of cellular infiltrate. Eur J Nucl Med Mol Imaging. 2009;36(5):745–50.
Hong J, Lee Y, Park Y, Kim SG, Hwang KH, Park SHS, et al. Role of FDG-PET/CT in detecting lymphomatous bone marrow involvement in patients with newly diagnosed diffuse large B-cell lymphoma. Ann Hematol. 2012;91(5):687–95.
Cerci JJ, Györke T, Fanti S, Paez D, Meneghetti JC, Redondo F, et al. Combined PET and biopsy evidence of marrow involvement improves prognostic prediction in diffuse large B-cell lymphoma. J Nucl Med. 2014;55(10):1591–7.
Berthet L, Cochet A, Kanoun S, Berriolo-Riedinger A, Humbert O, Toubeau M, et al. In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med. 2013;54(8):1244–50.
Adams HJA, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RAJ, de Klerk JMH. Bone marrow 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography cannot replace bone marrow biopsy in diffuse large B-cell lymphoma. Am J Hematol. 2014;89(7):726–31.
Khan AB, Barrington SF, Mikhaeel NG, Hunt AA, Cameron L, Morris T, et al. PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood. 2013;122(1):61–7.
Adams HJA, Kwee TC. Do not abandon the bone marrow biopsy yet in diffuse large B-cell lymphoma. J Clin Oncol. 2015;33(10):1217.
Andreou JA, Kosmidis PA, Gouliamos AD, Prassopoulos V, Vassilakopoulos TPVE. PET/CT in lymphomas: a case-based Atlas. Cham: Springer International; 2016.
Kaddu-Mulindwa D, Altmann B, Held G, Angel S, Stilgenbauer S, Thurner L, et al. FDG PET/CT to detect bone marrow involvement in the initial staging of patients with aggressive non-Hodgkin lymphoma: results from the prospective, multicenter PETAL and OPTIMAL>60 trials. Eur J Nucl Med Mol Imaging. 2021;48(11):3550–9.
Zelenetz AD, Gordon LI, Abramson JS, Advani RH, Bartlett NL, Caimi PF, et al. NCCN guidelines insights: B-cell lymphomas, version 3.2019. J Natl Compr Cancer Netw. 2019;17(6):650–61.
Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:vii78–82.
Alzahrani M, El-Galaly TC, Hutchings M, Hansen JW, Loft A, Johnsen HE, et al. The value of routine bone marrow biopsy in patients with diffuse large B-cell lymphoma staged with PET/CT: a Danish-Canadian study. Ann Oncol. 2016;27(6):1095–9.
Vassilakopoulos TP, Prassopoulos V, Rondogianni P, Chatziioannou S, Konstantopoulos K, Angelopoulou MK. Role of FDG-PET/CT in staging and first-line treatment of Hodgkin and aggressive B-cell lymphomas. Mag Eur Med Oncol. 2015;2(8):105–14.
Vassilakopoulos TP, Pangalis GA, Katsigiannis A, Papageorgiou SG, Constantinou N, Terpos E, et al. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone with or without radiotherapy in primary mediastinal large B-cell lymphoma: the emerging standard of care. Oncologist. 2012;17(2):239–49.
Martelli M, Ceriani L, Zucca E, Zinzani PL, Ferreri AJM, Vitolo U, et al. [18F]fluorodeoxyglucose positron emission tomography predicts survival after chemoimmunotherapy for primary mediastinal large B-cell lymphoma: results of the International Extranodal Lymphoma Study Group IELSG-26 Study. J Clin Oncol. 2014;32(17):1769–75.
Meignan M, Itti E, Gallamini A, Younes A. FDG PET/CT imaging as a biomarker in lymphoma. Eur J Nucl Med Mol Imaging. 2015;42(4):623–33.
Kanoun S, Rossi C, Berriolo-Riedinger A, Dygai-Cochet I, Cochet A, Humbert O, et al. Baseline metabolic tumour volume is an independent prognostic factor in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2014;41(9):1735–43.
Song MK, Chung JS, Lee JJ, Jeong SY, Lee SM, Hong JS, et al. Metabolic tumor volume by positron emission tomography/computed tomography as a clinical parameter to determine therapeutic modality for early stage Hodgkin’s lymphoma. Cancer Sci. 2013;104(12):1656–61.
Cottereau AS, Versari A, Loft A, Casasnovas O, Bellei M, Ricci R, et al. Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood. 2018;131(13):1456–63.
Akhtari M, Milgrom SA, Pinnix CC, Reddy JP, Dong W, Smith GL, et al. Reclassifying patients with early-stage Hodgkin lymphoma based on functional radiographic markers at presentation. Blood. 2018;131(1):84–94.
Barrington SF, Kirkwood AA, Pike LC, Guezennec C, Li H, Blanc M, et al. New prognostic score incorporating MTV predicts treatment failure in advanced Hodgkin lymphoma. Hematol Oncol. 2021;39(S2)
Gallamini A, Rambaldi A, Patti C, Romano A, Viviani S, Bolis S, et al. Baseline metabolic tumor volume and IPS predict ABVD in advanced- stage Hodgkin lymphoma with a negative interim PET scan after 2 chemotherapy cycles. A retrospective analysis from the GITIL/FIL HD0607 TRIAL. Hematol Oncol. 2021;39(S2):47–8.
Pinochet P, Texte E, Stamatoullas-Bastard A, Vera P, Mihailescu SD, Becker S. Prognostic value of baseline metabolic tumour volume in advanced-stage Hodgkin’s lymphoma. Sci Rep. 2021;11(1):23195.
Pike LC, Kirkwood AA, Patrick P, Radford J, Burton C, Stevens L, et al. Can baseline PET-CT features predict outcomes in advanced Hodgkin lymphoma? A prospective evaluation of UK patients in the RATHL trial (CRUK/07/033). Hematol Oncol. 2017;9:37–8.
Song MK, Chung JS, Shin HJ, Lee SM, Lee SE, Lee HS, et al. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol. 2012;91(5):697–703.
Sasanelli M, Meignan M, Haioun C, Berriolo-Riedinger A, Casasnovas RO, Biggi A, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2014;41(11):2017–22.
Mikhaeel NG, Smith D, Dunn JT, Phillips M, Møller H, Fields PA, et al. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging. 2016;43(7):1209.
Song MK, Yang DH, Lee GW, Lim SN, Shin S, Pak KJ, et al. High total metabolic tumor volume in PET/CT predicts worse prognosis in diffuse large B cell lymphoma patients with bone marrow involvement in rituximab era. Leuk Res. 2016;42:1–6.
Cottereau AS, Lanic H, Mareschal S, Meignan M, Vera P, Tilly H, et al. Molecular profile and FDG-PET/CT total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2016;22(15):3801–9.
Kim TM, Paeng JC, Chun IK, Keam B, Jeon YK, Lee SH, et al. Total lesion glycolysis in positron emission tomography is a better predictor of outcome than the international prognostic index for patients with diffuse large B cell lymphoma. Cancer. 2013;119(6):1195–202.
Zhou M, Chen Y, Huang H, Zhou X, Liu J, Huang G. Prognostic value of total lesion glycolysis of baseline 18F-fluorodeoxyglucose positron emission tomography/computed tomography in diffuse large B-cell lymphoma. Oncotarget. 2016;7(50):83544.
Mikhaeel NG, Heymans MW, Eertink JJ, de Vet HCW, Boellaard R, Dührsen U, et al. Proposed New dynamic prognostic index for diffuse large B-cell lymphoma: international metabolic prognostic index. J Clin Oncol. 2022;40:JCO2102063.
Kostakoglu L, Mattiello F, Martelli M, Sehn LH, Belada D, Ghiggi C, et al. Total metabolic tumor volume as a survival predictor for patients with diffuse large B-cell lymphoma in the GOYA study. Haematologica. 2020;107(7):1633–42.
Vercellino L, Cottereau AS, Casasnovas O, Tilly H, Feugier P, Chartier L, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood. 2020;135(16):1396–405.
Ceriani L, Martelli M, Zinzani PL, Ferreri AJM, Botto B, Stelitano C, et al. Utility of baseline 18FDG-PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood. 2015;126(8):950–6.
Camus V, Rossi C, Sesques P, Lequesne J, Tonnelet D, Haioun C, et al. Outcomes after first-line immunochemotherapy for primary mediastinal B-cell lymphoma: a LYSA study. Blood Adv. 2021;5(19):3862–72.
Pinnix CC, Ng AK, Dabaja BS, Milgrom SA, Gunther JR, David Fuller C, et al. Positron emission tomography-computed tomography predictors of progression after DA-R-EPOCH for PMBCL. Blood Adv. 2018;2(11):1334–43.
Cottereau AS, Nioche C, Dirand AS, Clerc J, Morschhauser F, Casasnovas O, et al. 18 F-FDG PET dissemination features in diffuse large B-cell lymphoma are predictive of outcome. J Nucl Med. 2020;61(1):40–5.
Gallamini A, Rambaldi A, Patti C, Romano A, Viviani S, Silvia B, et al. Lesion dissemination in baseline PET/CT (D-MAX) and IPS score predict ABVD treatment outcome in PET-2 negative advanced-stage Hodgkin lymphoma patients enrolled in the prospective GITIL/FIL HD0607 trial. Blood. 2021;138:2443.
Cottereau AS, Meignan M, Nioche C, Capobianco N, Clerc J, Chartier L, et al. Risk stratification in diffuse large B-cell lymphoma using lesion dissemination and metabolic tumor burden calculated from baseline PET/CT. Ann Oncol. 2021;32(3):404–11.
Ceriani L, Milan L, Martelli M, Ferreri AJM, Cascione L, Zinzani PL, et al. Metabolic heterogeneity on baseline 18FDG-PET/CT scan is a predictor of outcome in primary mediastinal B-cell lymphoma. Blood. 2018;132(2):179–86.
Senjo H, Hirata K, Izumiyama K, Minauchi K, Tsukamoto E, Itoh K, et al. High metabolic heterogeneity on baseline 18FDG-PET/CT scan as a poor prognostic factor for newly diagnosed diffuse large B-cell lymphoma. Blood Adv. 2020;4(10):2286–96.
Genta S, Ghilardi G, Cascione L, Juskevicius D, Tzankov A, Schär S, et al. Integration of baseline metabolic parameters and mutational profiles predicts long-term response to first-line therapy in DLBCL patients: a post Hoc analysis of the SAKK38/07 study. Cancers (Basel). 2022;14(4):1018.
Meignan M, Cottereau AS, Versari A, Chartier L, Dupuis J, Boussetta S, et al. Baseline metabolic tumor volume predicts outcome in high-tumor-burden follicular lymphoma: a pooled analysis of three multicenter studies. J Clin Oncol. 2016;34(30):3618–26.
Delfau-Larue MH, Van Der Gucht A, Dupuis J, Jais JP, Nel I, Beldi-Ferchiou A, et al. Total metabolic tumor volume, circulating tumor cells, cell-free DNA: distinct prognostic value in follicular lymphoma. Blood Adv. 2018;2(7):807–16.
Barrington SF, Trotman J, Sahin D, Belada D, Davies A, MacEwan R, et al. Baseline PET-derived metabolic tumor volume metrics did not predict outcomes in follicular lymphoma patients treated with first-line immunochemotherapy and antibody maintenance in the phase III GALLIUM study. Blood. 2018;132(Supplement 1):2882.
Albano D, Bosio G, Bianchetti N, Pagani C, Re A, Tucci A, et al. Prognostic role of baseline 18F-FDG PET/CT metabolic parameters in mantle cell lymphoma. Ann Nucl Med. 2019;33(7):449–58.
Cottereau BS, Broussais F, Casasnovas O, Kanoun S, Roques M, et al. Prognostic value of baseline total metabolic tumor volume (TMTV0) measured on FDG-PET/CT in patients with peripheral T-cell lymphoma (PTCL). Ann Oncol. 2016;27(4):719–24.
Cottereau AS, El-Galaly TC, Becker S, Broussais F, Petersen LJ, Bonnet C, et al. Predictive value of PET response combined with baseline metabolic tumor volume in peripheral T-cell lymphoma patients. J Nucl Med. 2018;59(4):589–95.
Kitadate A, Narita K, Fukumoto K, Terao T, Tsushima T, Kobayashi H, et al. Baseline total lesion glycolysis combined with interim positron emission tomography-computed tomography is a robust predictor of outcome in patients with peripheral T-cell lymphoma. Cancer Med. 2020;9(15):5509–18.
Chang Y, Fu X, Sun Z, Xie X, Wang R, Li Z, et al. Utility of baseline, interim and end-of-treatment 18F-FDG PET/CT in extranodal natural killer/T-cell lymphoma patients treated with L-asparaginase/pegaspargase. Sci Rep. 2017;7(1):1–12.
Juweid ME, Wiseman GA, Vose JM, Ritchie JM, Menda Y, Wooldridge JE, et al. Response assessment of aggressive non-Hodgkin’s lymphoma by integrated international workshop criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2005;23(21):4652–61.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86.
Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the imaging subcommittee of international harmonization project in lymphoma. J Clin Oncol. 2007;25(5):571–8.
Vassilakopoulos T, Pangalis GA, Boutsikas G, Rontogianni P, Masouridis S, Prassopoulos V, et al. Prognostic factors in patients with Hodgkin lymphoma (HL) and a negative PET/CT after ABVD chemotherapy: potential applications for the design of follow-up strategies. Haematol Hematol J. 2012;97(Suppl 1):218.
Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379(9828):1791–9.
Barnes JA, LaCasce AS, Zukotynski K, Israel D, Feng Y, Neuberg D, et al. End-of-treatment but not interim PET scan predicts outcome in nonbulky limited-stage Hodgkin’s lymphoma. Ann Oncol. 2011;22(4):910–5.
Sher DJ, Mauch PM, Van Den Abbeele A, LaCasce AS, Czerminski J, Ng AK. Prognostic significance of mid- and post-ABVD PET imaging in Hodgkin’s lymphoma: the importance of involved-field radiotherapy. Ann Oncol. 2009;20(11):1848–53.
Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372(17):1598–607.
Fuchs M, Goergen H, Kobe C, Kuhnert G, Lohri A, Greil R, et al. Positron emission tomography-guided treatment in early-stage favorable Hodgkin lymphoma: final results of the international, randomized phase III HD16 trial by the German Hodgkin study group. J Clin Oncol. 2019;37(31):2835–45.
Borchmann P, Plütschow A, Görgen H, Kobe C, Greil R, Meissner J, et al. PET-guided omission of radiotherapy in early-stage unfavourable Hodgkin lymphoma (GHSG HD17): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:223–34.
Borchmann P, Plütschow A, Görgen H. PET-guided omission of radiotherapy in Hodgkin lymphoma: authors’ reply. Lancet Oncol. 2021;22(5):e182.
Engert A, Goergen H, Markova J, Pabst T, Meissner J, Zijlstra JM, et al. Reduced-intensity chemotherapy in patients with advanced-stage Hodgkin lymphoma: updated results of the open-label, international, randomised phase 3 HD15 trial by the German Hodgkin study group. HemaSphere. 2017;1(1):e5.
Barrington SF, Phillips EH, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Positron emission tomography score has greater prognostic significance than pretreatment risk stratification in early-stage Hodgkin lymphoma in the UK RAPID Study. J Clin Oncol. 2019;37(20):1732–41.
Hartridge-Lambert SK, Schöder H, Lim RC, Maragulia JC, Portlock CS. ABVD alone and a PET scan complete remission negates the need for radiologic surveillance in early-stage, nonbulky Hodgkin lymphoma. Cancer. 2013;119(6):1203–9.
Gallamini A, Tarella C, Viviani S, Rossi A, Patti C, Mule A, et al. Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: long-term results of the GITIL/FIL HD 0607 tria. J Clin Oncol. 2018;36(5):454–62.
Gallamini A, Rossi A, Patti C, Picardi M, Romano A, Cantonetti M, et al. Consolidation radiotherapy could be safely omitted in advanced Hodgkin lymphoma with large nodal mass in complete metabolic response after ABVD: final analysis of the randomized GITIL/FIL HD0607 trial. J Clin Oncol. 2020;38(33):3905–13.
Vassilakopoulos TP, Rontogianni P, Pangalis G, Boutsikas G, Prassopoulos V, Masouridis S, et al. Outcome and prognostic factors in patients with Hodgkin lymphoma (HL) who remain PET/CT-positive after ABVD combination chemotherapy: potential applications for the design of subsequent treatment. Haematologica. 2012;97(s1):562.
Kobe C, Kuhnert G, Kahraman D, Haverkamp H, Eich HT, Franke M, et al. Assessment of tumor size reduction improves outcome prediction of positron emission tomography/computed tomography after chemotherapy in advanced-stage Hodgkin lymphoma. J Clin Oncol. 2014;32(17):1776–81.
Vassilakopoulos TP, Pangalis GA, Polliack A. A “PET” topic in primary mediastinal large B-cell lymphoma: positive or negative, and how to handle it in the end. Leuk Lymphoma. 2015;56(1):3–5.
Pinnix CC, Dabaja B, Ahmed MA, Chuang HH, Costelloe C, Wogan CF, et al. Single-institution experience in the treatment of primary mediastinal B cell lymphoma treated with immunochemotherapy in the setting of response assessment by 18fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys. 2015;92(1):113–21.
Filippi AR, Piva C, Giunta F, Bellò M, Chiappella A, Caracciolo D, et al. Radiation therapy in primary mediastinal B-cell lymphoma with positron emission tomography positivity after rituximab chemotherapy. Int J Radiat Oncol Biol Phys. 2013;87(2):311–6.
Hayden AR, Tonseth P, Lee DG, Villa D, Gerrie AS, Scott DW, et al. Outcome of primary mediastinal large B-cell lymphoma using R-CHOP: impact of a PET-adapted approach. Blood. 2020;136(24):2803–11.
Vassilakopoulos TP, Papageorgiou SG, Angelopoulou MK, Chatziioannou S, Prassopoulos V, Karakatsanis S, et al. Positron emission tomography after response to rituximab-CHOP in primary mediastinal large B-cell lymphoma: impact on outcomes and radiotherapy strategies. Ann Hematol. 2021;100(9):2279–92.
Vassilakopoulos TP, Pangalis GA, Chatziioannou S, Papageorgiou S, Angelopoulou MK, Galani Z, et al. PET/CT in primary mediastinal large B-cell lymphoma responding to rituximab-CHOP: An analysis of 106 patients regarding prognostic significance and implications for subsequent radiotherapy. Leukemia. 2016;30(1):238–42.
Filippi AR, Piva C, Levis M, Chiappella A, Caracciolo D, Bellò M, et al. Prognostic role of pre-radiation therapy (18)F-fluorodeoxyglucose positron emission tomography for primary mediastinal B-cell lymphomas treated with R-CHOP or R-CHOP-like chemotherapy plus radiation. Int J Radiat Oncol Biol Phys. 2016;95(4):1239–43.
Ceriani L, Martelli M, Gospodarowicz MK, Ricardi U, Ferreri AJM, Chiappella A, et al. Positron emission tomography/computed tomography assessment after immunochemotherapy and irradiation using the lugano classification criteria in the IELSG-26 study of primary mediastinal B-cell lymphoma. Int J Radiat Oncol Biol Phys. 2017;97(1):42–9.
Dunleavy K, Pittaluga S, Maeda LS, Advani R, Chen CC, Hessler J, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408–16.
Melani C, Advani R, Roschewski M, Walters KM, Chen CC, Baratto L, et al. End-of-treatment and serial PET imaging in primary mediastinal B-cell lymphoma following dose-adjusted EPOCH-R: a paradigm shift in clinical decision making. Haematologica. 2018;103(8):1337–44.
Vassilakopoulos T, Chatzidimitriou C, Mellios Z, Verigou E, Papageorgiou S, Giatra H, et al. PET-scan for response assessment after Rituximab-dose-Adjusted-EPOCH (R-DA-EPOCH) in Primary Mediastinal Large B-Cell Lymphoma (PMLBCL): clinical and prognostic significance. HemaSphere. 2019;3(S1):494.
Vassilakopoulos T, Piperidou A, Mellios Z, Verigou E, Kalpadakis C, Katodritou E, et al. PET/CT imaging scan for response assessment and treatment guidance after Rituximab-Dose-Adjusted-EPOCH (R-DA-EPOCH) in Primary Mediastinal Large B-Cell Lymphoma (PMLBCL): the hellenic experience on 107 patients. HemaSphere. 2022;6:1115–6.
Kanemasa Y, Shimoyama T, Sasaki Y, Tamura M, Sawada T, Omuro Y, et al. Analysis of prognostic value of complete response by PET-CT and further stratification by clinical and biological markers in DLBCL patients. Med Oncol. 2017;34(2):29.
Thomas A, Gingrich RD, Smith BJ, Jacobus L, Ristow K, Allmer C, et al. 18-Fluoro-deoxyglucose positron emission tomography report interpretation as predictor of outcome in diffuse large B-cell lymphoma including analysis of “indeterminate” reports. Leuk Lymphoma. 2010;51(3):439–46.
Dabaja BS, Phan J, Mawlawi O, Medeiros LJ, Etzel C, Liang FW, et al. Clinical implications of positron emission tomography-negative residual computed tomography masses after chemotherapy for diffuse large B-cell lymphoma. Leuk Lymphoma. 2013;54(12):2631–8.
Vassilakopoulos T, Kanellopoulos A, Papageorgiou S, Pangalis G, Anastasopoulou A, Moschogiannis M, et al. Clinical implications and prognostic significance of positron emission tomography (PET/CT) in patients with diffuse large B-cell lymphoma (DLBCL) after R-CHOP chemoimmunotherapy. Haematol Hematol J. 2014;99(Suppl 1):Abstract PB1831.
Witzig TE, Tobinai K, Rigacci L, Ikeda T, Vanazzi A, Hino M, et al. Adjuvant everolimus in high-risk diffuse large B-cell lymphoma: final results from the PILLAR-2 randomized phase III trial. Ann Oncol. 2018;29(3):707–14.
Mamot C, Klingbiel D, Hitz F, Renner C, Pabst T, Driessen C, et al. Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large b-cell lymphoma treated with R-CHOP-14 (SAKK 38/07). J Clin Oncol. 2015;33(23):2523–9.
Kostakoglu L, Martelli M, Sehn LH, Belada D, Carella AM, Chua N, et al. End-of-treatment PET/CT predicts PFS and OS in DLBCL after first-line treatment: results from GOYA. Blood Adv. 2021;5(5):1283–90.
Freeman CL, Savage KJ, Villa DR, Scott DW, Srour L, Gerrie AS, et al. Long-term results of PET-guided radiation in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2021;137(7):929–38.
Pfreundschuh M, Christofyllakis K, Altmann B, Ziepert M, Haenel M, Viardot A, et al. Radiotherapy to bulky disease PET-negative after immunochemotherapy in elderly DLBCL patients: results of a planned interim analysis of the first 187 patients with bulky disease treated in the OPTIMAL>60 study of the DSHNHL. J Clin Oncol. 2017;35:7506.
Dupuis J, Berriolo-Riedinger A, Julian A, Brice P, Tychyj-Pinel C, Tilly H, et al. Impact of [(18)F]fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunochemotherapy: a prospective study from the Groupe d’Etudes des Lymphomes de l’Adulte and GOELAMS. J Clin Oncol. 2012;30(35):4317–22.
Trotman J, Luminari S, Boussetta S, Versari A, Dupuis J, Tychyj C, et al. Prognostic value of PET-CT after first-line therapy in patients with follicular lymphoma: a pooled analysis of central scan review in three multicentre studies. Lancet Haematol. 2014;1(1):e17–27.
Trotman J, Barrington SF, Belada D, Meignan M, MacEwan R, Owen C, et al. Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): secondary analysis of a randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1530–42.
Lamonica D, Graf DA, Munteanu MC, Czuczman MS. 18F-FDG PET for measurement of response and prediction of outcome to relapsed or refractory mantle cell lymphoma therapy with bendamustine-rituximab. J Nucl Med. 2017;58(1):62–8.
Klener P, Fronkova E, Belada D, Forsterova K, Pytlik R, Kalinova M, et al. Alternating R-CHOP and R-cytarabine is a safe and effective regimen for transplant-ineligible patients with a newly diagnosed mantle cell lymphoma. Hematol Oncol. 2018;36(1):110–5.
Bailly C, Carlier T, Berriolo-Riedinger A, Casasnovas O, Gyan E, Meignan M, et al. Prognostic value of FDG-PET in patients with mantle cell lymphoma: results from the LyMa-PET project. Haematologica. 2020;105(1):e33–6.
El-Galaly TC, Pedersen MB, Hutchings M, Mylam KJ, Madsen J, Gang AO, et al. Utility of interim and end-of-treatment PET/CT in peripheral T-cell lymphomas: A review of 124 patients. Am J Hematol. 2015;90(11):975–80.
Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107(1):52–9.
Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25(24):3746–52.
Moskowitz CH, Schöder H, Teruya-Feldstein J, Sima C, Iasonos A, Portlock CS, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol. 2010;28(11):1896–903.
Gallamini A, Barrington SF, Biggi A, Chauvie S, Kostakoglu L, Gregianin M, et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica. 2014;99(6):1107–13.
Kostakoglu L, Schöder H, Johnson JL, Hall NC, Schwartz LH, Straus DJ, et al. Interim FDG PET imaging in stage I/II non-bulky Hodgkin lymphoma: would using combined PET and CT criteria better predict response than each test alone? Leuk Lymphoma. 2012;53(11):2143–50.
Casasnovas RO, Bouabdallah R, Brice P, Lazarovici J, Ghesquieres H, Stamatoullas A, et al. PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): a randomised, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2019;20(2):202–15.
Casasnovas R-O, Bouabdallah R, Brice P, Lazarovici J, Ghesquieres H, Stamatoullas A, et al. Positron emission tomography–driven strategy in advanced Hodgkin lymphoma: prolonged follow-up of the AHL2011 phase III lymphoma study association study. J Clin Oncol. 2022;40(10):1091–101.
Kreissl S, Goergen H, Buehnen I, Kobe C, Moccia A, Greil R, et al. PET-guided eBEACOPP treatment of advanced-stage Hodgkin lymphoma (HD18): follow-up analysis of an international, open-label, randomised, phase 3 trial. Lancet Haematol. 2021;8(6):e398–409.
Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin study group. Lancet. 2017;390(10114):2790–802.
Borchmann P, Haverkamp H, Lohri A, Mey U, Kreissl S, Greil R, et al. Progression-free survival of early interim PET-positive patients with advanced stage Hodgkin’s lymphoma treated with BEACOPP escalated alone or in combination with rituximab (HD18): an open-label, international, randomised phase 3 study by the German Hodg. Lancet Oncol. 2017;18(4):454–63.
Gallamini A, Patti C, Viviani S, Rossi A, Fiore F, Di Raimondo F, et al. Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with a interim-PET positive after two ABVD courses. Br J Haematol. 2011;152(5):551–60.
Johnson P, Federico M, Kirkwood A, Fosså A, Berkahn L, Carella A, et al. Adapted treatment guided by interim PET-CT Scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374(25):2419–29.
Press OW, Li H, Schöder H, Straus DJ, Moskowitz CH, LeBlanc M, et al. US intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: Southwest oncology group S0816. J Clin Oncol. 2016;34(17):2020–7.
Ganesan P, Rajendranath R, Kannan K, Radhakrishnan V, Ganesan TS, Udupa K, et al. Phase II study of interim PET-CT-guided response-adapted therapy in advanced Hodgkin’s lymphoma. Ann Oncol. 2015;26(6):1170–4.
Vassilakopoulos T, Rontogianni F, Boutsikas G, Assimakopoulos I, Chatziioannou S, Moschogiannis M, et al. PET/CT for the early interim evaluation of response in advanced Hodgkin lymphoma after ABVDx2: effective salvage with BEACOPP but low negative predictive value for stage IV. Hematol Oncol. 2015;33(s1):abstr 463.
Stephens DM, Li H, Schoder H, Straus DJ, Moskowitz CH, LeBlanc M, et al. Five-year follow-up of SWOG S0816: limitations and values of a PET-adapted approach with stage III/IV Hodgkin lymphoma. Blood. 2019;134(15):1238–46.
Vassilakopoulos TP, Johnson PWM. Treatment of advanced-stage Hodgkin lymphoma. Semin Hematol. 2016;53(3):171–9.
Zinzani PL, Broccoli A, Gioia DM, Castagnoli A, Ciccone G, Evangelista A, et al. Interim positron emission tomography response-adapted therapy in advanced-stage Hodgkin Lymphoma: final results of the phase II part of the HD0801 study. J Clin Oncol. 2016;34(12):1376–85.
André MPE, Girinsky T, Federico M, Reman O, Fortpied C, Gotti M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35(16):1786–96.
Cottereau AS, Versari A, Loft A, Casasnovas O, et al. Deuville score evaluation of PET2 positive patients included in the H10 trial. In: 7th International Workshop on PET in Lymphoma and Myeloma; 2018.
Oki Y, Chuang H, Chasen B, Jessop A, Pan T, Fanale M, et al. The prognostic value of interim positron emission tomography scan in patients with classical Hodgkin lymphoma. Br J Haematol. 2014;165(1):112–6.
Cimino G, Zaucha JM, Cirillo S, Saviolo C, Hutchings M, El-Galaly TC, et al. The complementary prognostic role of baseline and interim PET in predicting treatment outcome in advanced-stage Hodgkin lymphoma. Blood. 2014;124(21):4405.
Lopez-Alonso R, Qi S, Mashiach T, Weiler-Sagie M, Yahalom J, Dann EJ. The presence of a bulky mediastinal mass of 7 cm or greater in diameter confers an adverse prognosis to patients with advanced Hodgkin lymphoma in case of negative interim PET/CT. Leuk Lymphoma. 2021;62(6):1313–24.
Illidge TM, Phillips EH, Counsell N, Pettengell R, Johnson PWM, Culligan DJ, et al. Maximum tumor diameter is associated with event-free survival in PET-negative patients with stage I/IIA Hodgkin lymphoma. Blood Adv. 2020;4(1):203–6.
Rigacci L, Puccini B, Broccoli A, Dona M, Gotti M, Evangelista A, et al. Clinical characteristics of interim-PET negative patients with a positive end PET from the prospective HD08-01 FIL study. Ann Hematol. 2020;99(2):283–91.
Bari A, Marcheselli R, Sacchi S, Re A, Pagani C, Tucci A, et al. The classic prognostic factors in advanced Hodgkin’s lymphoma patients are losing their meaning at the time of Pet-guided treatments. Ann Hematol. 2020;99(2):277–82.
Romano A, Pavoni C, Di Raimondo F, Tarella C, Viviani S, Rossi A, et al. The neutrophil to lymphocyte ratio (NLR) and the presence of large nodal mass are independent predictors of early response: a subanalysis of the prospective phase II PET-2-adapted HD0607 trial. Cancer Med. 2020;9(23):8735–46.
Agostinelli C, Gallamini A, Stracqualursi L, Agati P, Tripodo C, Fuligni F, et al. The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin’s lymphoma: a retrospective, European, multicentre cohort study. Lancet Haematol. 2016;3(10):e467–79.
Viviani S, Mazzocchi A, Pavoni C, Taverna F, Rossi A, Patti C, et al. Early serum TARC reduction predicts prognosis in advanced-stage Hodgkin lymphoma patients treated with a PET-adapted strategy. Hematol Oncol. 2020;38(4):501–8.
Itti E, Meignan M, Berriolo-Riedinger A, Biggi A, Cashen AF, Véra P, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur J Nucl Med Mol Imaging. 2013;40(9):1312–20.
Schöder H, Polley MYC, Knopp MV, Hall N, Kostakoglu L, Zhang J, et al. Prognostic value of interim FDG-PET in diffuse large cell lymphoma: results from the CALGB 50303 clinical trial. Blood. 2020;135(25):2224–34.
Mikhaeel NG, Cunningham D, Counsell N, McMillan A, Radford JA, Ardeshna KM, et al. FDG-PET/CT after two cycles of R-CHOP in DLBCL predicts complete remission but has limited value in identifying patients with poor outcome–final result of a UK National Cancer Research Institute prospective study. Br J Haematol. 2021;192(3):504–13.
Zucca E, Cascione L, Ruberto T, Facchinelli D, Schär S, Hayoz S, et al. Prognostic models integrating quantitative parameters from baseline and interim positron emission computed tomography in patients with diffuse large B-cell lymphoma: post-hoc analysis from the SAKK38/07 clinical trial. Hematol Oncol. 2020;38(5):715–25.
Casasnovas RO, Meignan M, Berriolo-Riedinger A, Bardet S, Julian A, Thieblemont C, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood. 2011;118(1):37–43.
Casasnovas RO, Ysebaert L, Thieblemont C, Bachy E, Feugier P, Delmer A, et al. FDG-PET-driven consolidation strategy in diffuse large B-cell lymphoma: final results of a randomized phase 2 study. Blood. 2017;130(11):1315–26.
Pardal E, Coronado M, Martín A, Grande C, Marín-Niebla A, Panizo C, et al. Intensification treatment based on early FDG-PET in patients with high-risk diffuse large B-cell lymphoma: a phase II GELTAMO trial. Br J Haematol. 2014;167(3):327–36.
Dührsen U, Müller S, Hertenstein B, Thomssen H, Kotzerke J, Mesters R, et al. Positron emission tomography-guided therapy of aggressive Non-Hodgkin lymphomas (PETAL): a multicenter, randomized phase III trial. J Clin Oncol. 2018;36(20):2024–34.
Kurch L, Hüttmann A, Georgi TW, Rekowski J, Sabri O, Schmitz C, et al. Interim PET in diffuse large B-cell lymphoma. J Nucl Med. 2021;62(8):1068–74.
Schmitz C, Hüttmann A, Müller SP, Hanoun M, Boellaard R, Brinkmann M, et al. Dynamic risk assessment based on positron emission tomography scanning in diffuse large B-cell lymphoma: post-hoc analysis from the PETAL trial. Eur J Cancer. 2020;124:25–36.
Meignan M, Gallamini A. ΔSUVmax for interim PET in DLBCL: old is new. Blood. 2020;135(25):2202–3.
Yim SK, Yhim HY, Han YH, Jeon SY, Lee NR, Song EK, et al. Early risk stratification for diffuse large B-cell lymphoma integrating interim Deauville score and international prognostic index. Ann Hematol. 2019;98(12):2739–48.
Wight J, Wai SH, Shen E, Lee ST, Berlangieri S, Fancourt T, et al. Predicting primary treatment failure using interim FDG-PET scanning in diffuse large B-cell lymphoma. Eur J Haematol. 2021;107(4):475–83.
Safar V, Dupuis J, Itti E, Jardin F, Fruchart C, Bardet S, et al. Interim [18F]fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. J Clin Oncol. 2012;30(2):184–90.
Hertzberg M, Gandhi MK, Trotman J, Butcher B, Taper J, Johnston A, et al. Early treatment intensification with R-ICE and 90Y-ibritumomab tiuxetan (Zevalin)-BEAM stem cell transplantation in patients with high-risk diffuse large B-cell lymphoma patients and positive interim PET after 4 cycles of R-CHOP-14. Haematologica. 2017;102(2):356–63.
Swinnen LJ, Li H, Quon A, Gascoyne R, Hong F, Ranheim EA, et al. Response-adapted therapy for aggressive non-Hodgkin’s lymphomas based on early [18F] FDG-PET scanning: ECOG-ACRIN cancer research group study (E3404). Br J Haematol. 2015;170(1):56–65.
Sehn LH, Hardy ELG, Gill KK, Al-Tourah AJ, Shustik J, Macpherson NA, et al. Phase 2 trial of interim PET scan-tailored therapy in patients with advanced stage Diffuse Large B-Cell Lymphoma (DLBCL) in British Columbia (BC). Blood. 2014;124(21):392.
Duehrsen U, Müller SP, Rekowski J, Hertenstein B, Franzius C, Mesters R, et al. Positron Emission Tomography (PET) guided therapy of Aggressive lymphomas-interim PET-based outcome prediction and treatment changes in patients with B cell lymphomas participating in the PETAL trial. Blood. 2016;128(22):1857.
Moskowitz C, Hamlin PA, Maragulia J, Meikle J, Zelenetz AD. Sequential dose-dense RCHOP followed by ICE consolidation (MSKCC protocol 01–142) without radiotherapy for patients with primary mediastinal large B cell lymphoma. Blood. 2010;116(21):420.
Avigdor A, Sirotkin T, Kedmi M, Ribakovsy E, Berkowicz M, Davidovitz Y, et al. The impact of R-VACOP-B and interim FDG-PET/CT on outcome in primary mediastinal large B cell lymphoma. Ann Hematol. 2014;93(8):1297–304.
Lazarovici J, Terroir M, Arfi-Rouche J, Michot JM, Mussot S, Florea V, et al. Poor predictive value of positive interim FDG-PET/CT in primary mediastinal large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2017;44(12):2018–24.
Qin W, Jiang X, You J, Guo R, Shi Q, Dong L, et al. Deauville score evaluation of interim PET/CT in primary mediastinal large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2021;48(11):3347–50.
Schmitz C, Rekowski J, Müller SP, Hertenstein B, Franzius C, Ganser A, et al. Baseline and interim PET-based outcome prediction in peripheral T-cell lymphoma: a subgroup analysis of the PETAL trial. Hematol Oncol. 2020;38(3):244–56.
Huttmann A, Müller SP, Rekowski J, Hertenstein B, Franzius C, Franzke A, et al. Positron emission tomography (PET) guided therapy of aggressive lymphomas–interim PET-based outcome prediction and treatment changes in patients with T cell lymphomas participating in the PETAL trial. Blood. 2016;128:185.
Raemaekers JMM, André MPE, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32(12):1188–94.
Vassilakopoulos TP, Kravvariti E, Panitsas F, Angelopoulou MK, Liaskas A, Kontopidou FN, et al. Very late relapses in Hodgkin lymphoma treated with chemotherapy with or without radiotherapy: linear pattern and distinct prognostic factors. Blood Cancer J. 2022;12(7):102.
Straus DJ, Jung SH, Pitcher B, Kostakoglu L, Grecula JC, Hsi ED, et al. CALGB 50604: risk-adapted treatment of nonbulky early-stage Hodgkin lymphoma based on interim PET. Blood. 2018;132(10):1013–21.
Lamy T, Damaj G, Soubeyran P, Gyan E, Cartron G, Bouabdallah K, et al. R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood. 2018;131(2):174–81.
Persky DO, Li H, Stephens DM, Park SI, Bartlett NL, Swinnen LJ, et al. Positron emission tomography-directed therapy for patients with limited-stage diffuse large B-cell lymphoma: results of intergroup national clinical trials network study S1001. J Clin Oncol. 2020;38(26):3003–11.
Martelli M, Zucca E, Gospodarowicz M, Johnson PWM, Ricardi U, et al. A randomized, multicentre, two-arm phase III comparative study assessing the role of mediastinal radiotherapy after rituximab-containing chemotherapy regimens to patients with newly diagnosed primary mediastinal large B-cell lymphoma (PMLBCL): The IELSG-3. Hematol Oncol. 2013;31(S1):140.
Le Gouill S, Ghesquières H, Oberic L, Morschhauser F, Tilly H, Ribrag V, et al. Obinutuzumab vs rituximab for advanced DLBCL: a PET-guided and randomized phase 3 study by LYSA. Blood. 2021;137(17):2307–20.
Schot BW, Zijlstra JM, Sluiter WJ, Van Imhoff GW, Pruim J, Vaalburg W, et al. Early FDG-PET assessment in combination with clinical risk scores determines prognosis in recurring lymphoma. Blood. 2007;109(2):486–91.
Terasawa T, Lau J, Bardet S, Couturier O, Hotta T, Hutchings M, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin’s lymphoma and diffuse large B-cell lymphoma: a systematic review. J Clin Oncol. 2009;27(11):1906–14.
Poulou LS, Thanos L, Ziakas PD. Unifying the predictive value of pretransplant FDG PET in patients with lymphoma: a review and meta-analysis of published trials. Eur J Nucl Med Mol Imaging. 2010;37(1):156–62.
Angelopoulou M, Moschogiannis M, Rondogianni P, Tsirkinidis P, Nikaki A, Chatziioannou S, et al. PET/CT in the setting of Autologous Stem Cell Transplantation (ASCT) for relapsed/refractory Hodgkin Lymphoma (HL): performance of various interpretation systems. Haematol Hematol J. 2013;98(s2):Abstract P119.
Moskowitz AJ, Yahalom J, Kewalramani T, Maragulia JC, Vanak JM, Zelenetz AD, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116(23):4934–7.
Adams HJA, Kwee TC. Prognostic value of pretransplant FDG-PET in refractory/relapsed Hodgkin lymphoma treated with autologous stem cell transplantation: systematic review and meta-analysis. Ann Hematol. 2016;95(5):695–706.
Yhim HY, Eshet Y, Metser U, Lajkosz K, Cooper M, Prica A, et al. Risk stratification for relapsed/refractory classical Hodgkin lymphoma integrating pretransplant Deauville score and residual metabolic tumor volume. Am J Hematol. 2022;97(5):583–91.
Moskowitz AJ, Schöder H, Gavane S, Thoren KL, Fleisher M, Yahalom J, et al. Prognostic significance of baseline metabolic tumor volume in relapsed and refractory Hodgkin lymphoma. Blood. 2017;130(20):2196–203.
Damlaj M, Ghazi S, Syed G, Pasha T, Gmati G, Salama H, et al. Pre-autologous transplantation PET/CT using Deauville criteria is an independent predictor of progression in relapsed refractory classical Hodgkin lymphoma. Bone Marrow Transplant. 2017;52(9):1342–4.
Procházka V, Gawande RS, Cayci Z, Froelich JW, Cao Q, Wilke C, et al. Positron emission tomography-based assessment of metabolic tumor volume predicts survival after autologous hematopoietic cell transplantation for Hodgkin lymphoma. Biol Blood Marrow Transplant. 2018;24(1):64–70.
Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–62.
Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–94.
Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II Study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35(19):2125–32.
Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 Trial. J Clin Oncol. 2018;36(14):1428–39.
Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134(14):1144–53.
Ansell SM, Bröckelmann PJ, Von KG, Lee HJ, et al. Nivolumab for Relapsed or Refractory(R/R) Classical Hodgkin Lymphoma(CHL) after autologous transplantation: 5-year overall survival from phase 2 CHECKMATE 205 study. In: International Conference on Malignant Lymphoma; 2021. p. 122–5.
Armand P, Pier Luigi Zinzani M, Lee HJ, Johnson N, Al E. Five-year follow-up of keynote-087: pembrolizumab monotherapy in Relapsed/Refractory classical Hodgkin lymphoma (R/R cHL). Blood. 2021;138(Supplement 1):1366.
Zinzani PL, Santoro A, Gritti G, Brice P, Barr PM, Kuruvilla J, et al. Nivolumab combined With brentuximab vedotin for relapsed/refractory primary mediastinal large B-cell lymphoma: efficacy and safety from the phase II CheckMate 436 study. J Clin Oncol. 2019;37(33):3081–9.
Armand P, Rodig S, Melnichenko V, Thieblemont C, Bouabdallah K, Tumyan G, et al. Pembrolizumab in relapsed or refractory primary Mediastinal large B-cell lymphoma. J Clin Oncol. 2019;37(34):3291–9.
Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R, Jacene HA, et al. Refinement of the lugano classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128(21):2489–96.
Dercle L, Seban RD, Lazarovici J, Schwartz LH, Houot R, Ammari S, et al. 18F-FDG PET and CT scans detect new imaging patterns of response and progression in patients with Hodgkin lymphoma treated by anti–programmed death 1 immune checkpoint inhibitor. J Nucl Med. 2018;59(1):15–24.
Dercle L, Mokrane FZ, Schiano de Colella JM, Stamatoullas A, Morschhauser F, Brice P, et al. Unconventional immune-related phenomena observed using 18F-FDG PET/CT in Hodgkin lymphoma treated with anti PD-1 monoclonal antibodies. Eur J Nucl Med Mol Imaging. 2019;46(6):1391–2.
Mokrane FZ, Chen A, Schwartz LH, Morschhauser F, Stamatoullas A, De Colella JMS, et al. Performance of CT compared with 18F-FDG PET in predicting the efficacy of nivolumab in relapsed or Refractory Hodgkin lymphoma. Radiology. 2020;295(3):651–61.
Chen A, Mokrane FZ, Schwartz LH, Morschhauser F, Stamatoullas A, Schiano de Colella JM, et al. early 18 f-fdg pet/ct response predicts survival in relapsed or refractory Hodgkin lymphoma treated with Nivolumab. J Nucl Med. 2020;61(5):649–54.
Moskowitz CH, Chen RW, Armand P, Zinzani PL, Vassilakopoulos TP, Goldmacher GV, et al. Pembrolizumab antitumor activity in relapsed/refractory Classical Hodgkin lymphoma in keynote-087: revised response criteria for malignant lymphoma 2007 criteria versus Lugano 2014 classification. Blood. 2017;130(Supplement 1):4085.
Vercellino L, Di Blasi R, Kanoun S, Tessoulin B, Rossi C, D’Aveni-Piney M, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(22):5607–15.
Iacoboni G, Simó M, Villacampa G, Catalá E, Carpio C, Díaz-Lagares C, et al. Prognostic impact of total metabolic tumor volume in large B-cell lymphoma patients receiving CAR T-cell therapy. Ann Hematol. 2021;100(9):2303–10.
Bi L, Kim J, Kumar A, Wen L, Feng D, Fulham M. Automatic detection and classification of regions of FDG uptake in whole-body PET-CT lymphoma studies. Comput Med Imaging Graph. 2017;60:3–10.
Barrington SF, Meignan M. Time to prepare for risk adaptation in lymphoma by standardizing measurement of metabolic tumor burden. J Nucl Med. 2019;60(8):1096–102.
Sibille L, Seifert R, Avramovic N, Vehren T, Spottiswoode B, Zuehlsdorff S, et al. 18 F-FDG PET/CT Uptake classification in lymphoma and lung cancer by Using deep convolutional neural networks. Radiology. 2020;294(2):445–52.
Annunziata S, Pelliccioni A, Hohaus S, Maiolo E, Cuccaro A, Giordano A. The prognostic role of end-of-treatment FDG-PET/CT in diffuse large B cell lymphoma: a pilot study application of neural networks to predict time-to-event. Ann Nucl Med. 2021;35(1):102–10.
Lartizien C, Rogez M, Niaf E, Ricard F. Computer-aided staging of lymphoma patients with FDG PET/CT imaging based on textural information. IEEE J Biomed Heal inform. 2014;18(3):946–55.
Guo B, Tan X, Ke Q, Cen H. Prognostic value of baseline metabolic tumor volume and total lesion glycolysis in patients with lymphoma: a meta-analysis. PLoS One. 2019;14(1):e0210224.
Yu Y, Decazes P, Lapuyade-Lahorgue J, Gardin I, Vera P, Ruan S. Semi-automatic lymphoma detection and segmentation using fully conditional random fields. Comput Med Imaging Graph. 2018;70:1–7.
Kostakoglu L, Chauvie S. PET-derived quantitative metrics for response and prognosis in lymphoma. PET Clin. 2019;14(3):317–29.
Alahmari SS, Cherezov D, Goldgof DB, Hall LO, Gillies RJ, Schabath MB. Delta radiomics improves pulmonary nodule malignancy prediction in lung cancer screening. IEEE Access. 2018;6:77796–806.
Hasani N, Paravastu SS, Farhadi F, Yousefirizi F, Morris MA, Rahmim A, et al. Artificial Intelligence in lymphoma PET imaging: a scoping review (current trends and future directions). PET Clin. 2022;17(1):145–74.
De Oliveira CR, Neto AH, Siqueira S, De Padua Covas Lage LA, De Paula HM, Coutinho AM, et al. Interim fluorine-18 fluorodeoxyglucose PET-computed tomography and cell of origin by immunohistochemistry predicts progression-free and overall survival in diffuse large B-cell lymphoma patients in the rituximab era. Nucl Med Commun. 2016;37(10):1095–101.
Jiang M, Chen P, Ruan X, Xu W, Li T, Wu L, et al. Interim 18F-FDG PET/CT and BCL2 for predicting the prognosis of patients with diffuse large B-cell lymphoma in the rituximab era. Nucl Med Commun. 2018;39(2):147–53.
Kim J, Lee JO, Paik JH, Lee WW, Kim SE, Song YS. Different predictive values of interim 18 F-FDG PET/CT in germinal center like and non-germinal center like diffuse large B-cell lymphoma. Ann Nucl Med. 2017;31(1):1–11.
Li X, Sun X, Li J, Liu Z, Mi M, Zhu F, et al. Interim PET/CT based on visual and semiquantitative analysis predicts survival in patients with diffuse large B-cell lymphoma. Cancer Med. 2019;8(11):5012–22.
Zhang X, Fan W, Xia ZJ, Hu YY, Lin XP, Zhang YR, et al. Use of subsequent PET/CT in diffuse large B-cell lymphoma patients in complete remission following primary therapy. Chin J Cancer. 2015;34(2):70–8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vassilakopoulos, T.P., Liaskas, A., Piperidou, A., Ioakim, M., Prassopoulos, V. (2022). The Role of 18FDG-PET/CT in Malignant Lymphomas Clinical Implications. In: Andreou, J.A., Kosmidis, P.A., Gouliamos, A.D. (eds) Artificial Intelligence in PET/CT Oncologic Imaging. Springer, Cham. https://doi.org/10.1007/978-3-031-10090-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-10090-1_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10089-5
Online ISBN: 978-3-031-10090-1
eBook Packages: MedicineMedicine (R0)