Abstract
Purpose
Diffuse large B-cell lymphoma (DLBCL) is a pathologically heterogeneous disease with different prognoses according to its molecular profiles. Despite the broad usage of 18F-fluoro-2-dexoxy-d-glucose (FDG) positron emission tomography/computed tomography (PET/CT), previous studies that have investigated the value of interim 18F-FDG PET/CT in DLBCL have given the controversial results. The purpose of this study was to evaluate the prognostic value of interim 18F-FDG PET/CT in DLBCL according to germinal center B cell-like (GCB) and non-GCB molecular profiling.
Methods
We enrolled 118 newly diagnosed DLBCL patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP). Interim 18F-FDG PET/CT scans performed after 2 or 3 cycles of R-CHOP treatment were evaluated based on the Lugano response criteria. Patients were grouped as GCB or non-GCB molecular subtypes according to immunohistochemistry results of CD10, BCL6, and MUM1, based on Hans’ algorithm.
Results
In total 118 DLBCL patients, 35 % were classified as GCB, and 65 % were classified as non-GCB. Interim PET/CT was negative in 70 %, and positive in 30 %. During the median follow-up period of 23 months, the positive interim 18F-FDG PET/CT group showed significantly inferior progression free survival (PFS) compared to the negative interim 18F-FDG PET/CT group (P = 0.0004) in entire patients. A subgroup analysis according to molecular profiling demonstrated significant difference of PFS between the positive and negative interim 18F-FDG PET groups in GCB subtype of DLBCL (P = 0.0001), but there was no significant difference of PFS between the positive and negative interim 18F-FDG PET groups in non-GCB subtype of DLBCL.
Conclusions
Interim 18F-FDG PET/CT scanning had a significant predictive value for disease progression in patients with the GCB subtype of DLBCL treated with R-CHOP, but not in those with the non-GCB subtype. Therefore, molecular profiles of DLBCL should be considered for interim 18F-FDG PET/CT practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of lymphoma and comprises approximately one-third of Non-Hodgkin lymphoma (NHL) in adults [1].
DLBCL is clinically, morphologically, and molecularly heterogeneous [2], and numerous studies have challenged to find effective prognostic factors for treatment. However, the exclusive diagnosis of DLBCL despite its heterogeneity has left some inconclusiveness for clinical practice, treatment response evaluation, and prognosis prediction. Therefore, further studies are needed, by delineating more homogenous groups of DLBCL. Recently, gene expression profiling (GEP) as a new diagnostic technology has provided new insights by subdividing DLBCL into 3 subtypes, the germinal center B-cell-like (GCB), the activated B-cell-like (ABC) and the type 3 subtypes [3, 4]. These 3 subtypes of DLBCL have different pathogenetic mechanisms that could benefit differently from therapy [5, 6]. However, the type 3 group is heterogeneous, with a poor outcome similar to the ABC group [7]. Therefore, DLBCL is classified as GCB and non-GCB immunohistochemistry (IHC) subgroups based on Hans’ algorithm as a routine clinical test. Studies reported that patients with GCB molecular subtype have prognostically favorable outcomes compared to those with non-GCB molecular subtypes in patients with DLBCL treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or other CHOP-like regimens [3, 8, 9].
18F-fluoro-2-dexoxy-d-glucose (FDG) positron emission tomography/computed tomography (PET/CT) is an important non-invasive diagnostic tool for the management of patients with FDG-avid lymphomas including DLBCL. It is the standard imaging modality for staging and determining the remission status at the conclusion of therapy for DLBCL [10, 11]. However, in the case of interim 18F-FDG PET/CT, there is the lack of conclusive agreement upon the prognostic value in the previous studies. Even though it is frequently performed in clinical practice, results have been various in previous trials that have investigated the predictive value of 18F-FDG PET/CT in treatment. Some studies have shown that interim 18F-FDG PET/CT is a strong prognostic indicator in DLBCL showing the potential of using interim 18F-FDG PET/CT to response-adapted therapy [12–14], whereas some studies have failed to prove the prognostic value of interim 18F-FDG PET/CT [15, 16].
In this study, we analyzed newly diagnosed DLBCL patients according to the subtypes of molecular profiling treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) who underwent interim 18F-FDG PET/CT scan during treatment. The aim of our study was to evaluate the prognostic value of interim 18F-FDG PET/CT in DLBCL according to GCB and non-GCB molecular profiling.
Materials and methods
Patients
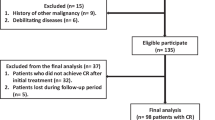
A total of 118 patients who visited Seoul National University Bundang Hospital between March 2009 and January 2015 were retrospectively enrolled. Patients were included in this study who were pathologically diagnosed with DLBCL, received R-CHOP as the first-line treatment with or without consolidative therapy, underwent interim 18F-FDG PET/CT scans after 2 or 3 cycles of R-CHOP treatment, and had IHC results, including CD10, BCL6, and MUM1. Patients were excluded if they had undergone 18F-FDG PET/CT scan after surgical resection of a lymphoma lesion, or had primary central nervous system lymphoma. Age, sex, Ann Arbor stage, Eastern Cooperative Oncology Group (ECOG) performance score (PS), International Prognostic Index (IPI) [17], revised IPI (R-IPI) [18], serum lactate dehydrogenase (LDH) level, presence of B symptoms, bulky disease (≥10 cm), extranodal involvement, and bone marrow involvement were evaluated on initial admission, prior to treatment. This study has been approved by the Institutional Review Board for review of medical records of the patients and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Acquisition of informed consents was exempted due to the retrospective character of the study.
Treatment and follow-up
Patients were treated with standard R-CHOP chemotherapy as the first-line treatment with a treatment interval of 3 weeks. Ann Arbor stage I/II patients received 4–6 cycles and stage III/IV patients received 6–8 cycles of R-CHOP chemotherapy. Depending on the stage and site of presentations, patients were given either R-CHOP alone or a combination of R-CHOP and radiotherapy. The patients underwent the following standard evaluations: a complete history and physical examination, complete blood cell count, and serum chemistries, including LDH, bone marrow aspiration and biopsy, 18F-FDG PET/CT, CT, or MR (if necessary).
Molecular profiling
Histologic slides with IHC staining and pathologic reports were reviewed. IHC testing was performed on an automated IHC stainer (Benchmark XT, Ventana Medical Systems, Inc., Tuscon, AZ, USA) using the following monoclonal antibodies for CD10 (clone SP67; Ventana Medical Systems, Inc., Tuscon, AZ, USA), BCL6 (clone GI191E/A8; Ventana Medical Systems, Inc., Tuscon, AZ, USA), MUM1 (clone MuM1p; Dako Inc., Carpinteria, CA, USA), and bcl-2 (clone 124; Dako Inc., Carpinteria, CA, USA). Patients were classified into 2 subgroups, GCB or non-GCB phenotype [3, 9] according to IHC results based on Hans’ algorithm [7]. IHC results were interpreted as positive or negative, with a uniform cutoff value of 30 % based on prior studies [7, 19].
18F-FDG PET/CT scan protocol
18F-FDG PET/CT images were obtained after 2 or 3 cycles of R-CHOP treatment, using a PET/CT scanner (Discovery VCT, GE Medical Systems, Milwaukee, WI, USA). Patients were fasted for at least 6 h. Images were obtained 50 min after 5.18 MBq/kg 18F-FDG injections. CT images were acquired from the base of cerebellum to upper thigh (120 kVp, 3.75 mm slice thickness). PET images were acquired in a three-dimensional acquisition mode (5–6 bed position, 2.5 min/bed), and were reconstructed on a 128 × 128 matrices using an iterative algorithm (ordered subset expectation maximization, 2 iterations and 8 subsets), with CT-based attenuation correction.
Image analysis based on revised response criteria
All interim 18F-FDG PET/CT scans were visually interpreted based on the 5-point scale by two nuclear medicine physicians (YSS and JHK, 11 and 6 years of experience, respectively), who were blinded to patients’ clinical information [10, 11, 20]. The achievement of a complete metabolic response (CMR) at interim restaging was defined according to the Lugano response criteria for non-Hodgkin’s lymphoma [20]. In brief, a score of 1–3 on the 5-point scale was regarded as negative (CMR) and 4 or 5 as positive (non-CMR) [10, 11].
Statistical analysis
Factors between subgroups of patients were evaluated with Chi-square test, or Fisher’s exact test. End point was progression free survival (PFS) from the start of treatment to disease progression, recurrence or death. The variables associated with PFS were evaluated with univariate/multivariate Cox proportional hazards regression model. PFS curves were derived from Kaplan–Meier survival analysis. A commercial software package (MedCalc, Version 12.2.1.0, MedCalc Software, Belgium) was used for the analyses. P values less than 0.05 were regarded as significant.
Results
Patient characteristics
Clinical and demographic characteristics of the 118 patients (mean age 60.0 ± 13.6 years) included in the present analysis are summarized in Table 1. Interim 18F-FDG PET/CT was performed after 2 cycles of R-CHOP in 91 (77 %) patients, or 3 cycles in 27 (23 %) patients. 28 (31 %) patients who underwent interim PET/CT after 2 cycles were relapsed and 6 (22 %) patients who underwent interim PET/CT after 3 cycles were relapsed. The timing of interim PET/CT after 2 or 3 cycles of R-CHOP was not associated with the progression rate (P = 0.39). 41(35 %) patients were classified as the GCB subtype and 77 (65 %) patients as the non-GCB molecular subtype. Bcl-2 was positive in 20 (49 %) patients of the GCB group and 56 (73 %) patients of the non-GCB group (P = 0.004). Out of 118 analyzed patients, 65 (55 %) patients achieved CMR according to the Lugano response criteria showing negative interim PET/CT results based on the 5-point scale.
Cox regression models and patient outcome
Age, sex, B symptom, Ann Arbor stage (I or II vs. III or IV), ≥2 extranodal sites, BM involvement, bulky disease, ≥2 ECOG PS, elevated LDH, high IPI, high R-IPI, interim PET/CT result (positive vs. negative), IHC subgroup (GCB vs. non-GCB), and bcl-2 overexpression were included in the univariate and multivariate Cox regression models for evaluating significant risk factors for patient outcomes and summarized in Table 2. Significant variables in the univariate analysis in total patients included elevated LDH (hazard ration (HR) = 8.076, 95 % confidence interval (CI) = 2.465–26.459, P = 0.0006), Ann Arbor stage III or IV (HR = 9.602, 95 % CI = 2.930–31.460, P = 0.0002), ≥2 ECOG PS (HR = 3.027, 95 % CI = 1.312–6.985, P = 0.009), ≥2 extranodal sites (HR = 2.418, 95 % CI = 1.218–4.803, P = 0.012), high IPI (HR = 6.790, 95 % CI = 2.800–16.463, P < 0.0001), high R-IPI (HR = 4.981, 95 % CI = 2.247–11.042, P = 0.0001), bulky disease (HR = 2.959, 95 % CI = 1.501–5.832, P = 0.002), and positive interim PET result (HR = 3.178, 95 % CI = 1.618–6.243, P = 0.001). Only significant variables in the univariate analysis were included in the multivariate Cox model. After adjustment for these covariates, Ann Arbor stage III or IV (HR = 5.285, 95 % CI = 1.464–19.083, P = 0.011) and positive interim PET/CT (HR = 3.350, 95 % CI = 1.377–8.152, P = 0.008) were the only variables significantly associated with PFS along with IPI factors. In multivariate analysis in each DLBCL subgroup along with IPI factors, Bcl-2 overexpression (HR = 8.304, 95 % CI = 1.108–62.235, P = 0.039) and positive interim PET/CT (HR = 6.023, 95 % CI = 1.006–36.048, P = 0.049) were significantly associated with PFS in the GCB DLBCL subgroup. Ann Arbor stage III or IV (HR = 10.969, 95 % CI = 1.424–84.469, P = 0.0022) was the only variable associated with PFS in the non-GCB subgroup.
Patient characteristics of GCB and non-GCB groups according to interim PET/CT results
The different patient characteristics of GCB and non-GCB subgroups between positive interim PET/CT and negative interim PET/CT results are listed in Tables 3 and 4. Only the presence of bulky disease (P = 0.009), ≥2 extranodal sites (P = 0.020), and bcl-2 overexpression (P = 0.031) in the GCB subgroup, and ≥2 ECOG PS (P = 0.018) and IPI (P = 0.011) in the non-GCB subgroup were different between interim PET/CT positive and negative patients.
Interim PET/CT result and Kaplan–Meier survival analysis
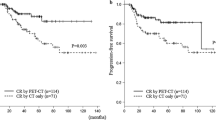
In total 118 patients, 83 (70 %) had negative interim PET/CT and 35 (30 %) had positive interim PET/CT results. During a median follow-up period of 23 months, the 2-year PFS rate was 71 % in entire patients (Fig. 1). There was no difference of PFS between patients with GCB subtype and those with non-GCB subtype in entire patients (Fig. 2a). However, the Kaplan–Meier analysis of entire patients showed a significant difference in PFS between patients with positive interim PET/CT result and those with negative interim PET/CT result (HR = 3.16, CI = 1.44–6.92, P = 0.0004) (Fig. 2b). The 2-year PFS rate for patients with negative interim PET/CT scans was 79 % compared with 51 % for patients with positive interim PET/CT scans. To evaluate the prognostic role of interim PET/CT according to molecular subtype, we analyzed PFS in GCB (n = 41) and non-GCB (n = 77) subgroups separately. A subgroup analysis demonstrated a significant difference in PFS between patients with positive interim PET/CT and those with negative interim PET/CT in the GCB subgroup (HR = 8.80, CI = 2.17–35.63, P = 0.0001) (Fig. 3a), whereas there was no difference of PFS between patients with positive interim PET/CT and negative interim PET/CT in the non-GCB subgroup (P = 0.105) (Fig. 3b). Kaplan–Meier analysis of patients who underwent interim PET/CT after 2 cycles of R-CHOP showed consistent results in the whole group (HR = 2.60, CI = 1.37–5.94, P = 0.0086), the GCB group (HR = 8.31, CI = 2.21–31.20, P = 0.0013), and the non-GCB group (HR = 1.49, CI = 0.51–4.33, P = 0.42).
Discussion
In our study, we investigated the usefulness of interim 18F-FDG PET/CT according to molecular profiles. There were significant differences of PFS between GCB subtype DLBCL patients with positive interim PET/CT and negative interim PET/CT, whereas there were no significant differences of PFS in between non-GCB subtype DLBCL patients with positive interim PET/CT and negative interim PET/CT. Numerous previous studies have attempted to prove the predictive value of interim PET or PET/CT during the chemotherapy in identifying patients likely to relapse [12, 15, 16, 21–25]. However, previous studies for interim PET or PET/CT have yielded conflicting results. Some studies reported good prognostic value of interim PET or PET/CT [12, 22, 25], whereas others reported either no or relatively poor prognostic value of interim PET or PET/CT in DLBCL [15, 16, 21, 23, 24]. Although clinicians perform a routine interim 18F-FDG PET/CT during chemotherapy in DLBCL patients to distinguish early responders from non-responders, the use of interim 18F-FDG PET/CT is still investigational due to the previous conflicting results. Recently, World Health Organization classification (WHO) introduced the revised WHO classification of lymphoid neoplasms. The revised classification refined the diagnostic criteria based on genetic or molecular information, and subdivided DLBCL into 2 subtypes as GCB and ABC [26]. However, studies about the prognosis of DLBCL according to the subtypes showed controversial results [1, 27–30], and application of this information into clinical practice awaits further studies. In this situation, our result suggests that the value of interim 18F-FDG PET/CT differs between different DLBCL subtypes. The application of interim 18F-FDG PET/CT in clinical practice should be considered differently according to the molecular profiles of DLBCL, and we anticipate that this approach will be useful for the future investigation in more specific therapeutic strategies of patients with DLBCL into high- and low-risk subgroups within GCB subtypes.
Bcl-2 is an antiapoptotic protein frequently dysregulated in DLBCL [31]. The prognostic value of bcl-2 overexpression in patients with DLBCL has been reported in several previous studies [31–33]. In concordance with previous results [33], bcl-2 overexpression rate was higher in the non-GCB group than the GCB group in our study. Bcl-2 overexpression and positive interim PET/CT were the only variables significantly associated with PFS in the GCB DLBCL subgroup. The bcl-2 overexpression was associated with a poor outcome in the GCB DLBCL (P = 0.010, data not shown) but not in the non-GCB group, as previously reported [32, 33]. One well-known mechanism of bcl-2 overexpression is the t(14;18)(q32;q31) in GCB subtype of DLBCL, whereas the mechanism of bcl-2 overexpression is associated with NF-κB activation in the non-GCB type [6, 33–35]. However, bcl-2 overexpression has not been a prognostic risk factor for non-GCB subtypes after the introduction of rituximab [30, 33], since rituximab targets NF-κB and its target bcl-2 resulting in increased susceptibility to chemotherapy by reducing the bcl-2 expression [36]. In addition, the activation of NF-κB is known to cause not only the overexpression of bcl-2 [6, 33–35], but also glucose transporters (GLUT) [37–40]. Therefore, since NF-κB mediated pathogenesis is not likely to be a significant risk factor in non-GCB subtype of DLBCL, GLUT-dependent 18F-FDG PET/CT is also not likely to have a predictive prognostic value during R-CHOP chemotherapy as in our study.
In this study, GCB subtype patients with positive interim PET/CT scans had a higher proportion of bcl-2 overexpression, compared to those with negative interim PET/CT scans. On the contrary, there were no differences of bcl-2 overexpression between non-GCB patients with positive and negative interim PET/CT. As previously mentioned above, bcl-2 overexpression is a risk factor in GCB subtype only. This suggests that bcl-2 may be the factor that associates positive interim PET/CT with poor prognosis in GCB patients, while interim PET/CT result is not associated with prognosis in non-GCB patients. We assume that there would be other pathogeneses than NF-κB, accounting for 18F-FDG uptake in bcl-2 overexpressing GCB patients. The association of interim PET/CT response and pathogenesis are remained to be investigated in further studies.
There are several study limitations in our study. First, this was a retrospective study. Second, Hans’ algorithm was adopted for molecular subtype classification instead of a cDNA microarray, since a cDNA microarray is still not a routine clinical test. Choi et al. reported that Hans’ algorithm has 81 % sensitivity and 92 % specificity for detecting GCB subtype [8], therefore leading to the possibility of grouping bias. Third, we did not have enough data for bcl-2 and MYC rearrangement analysis due to the retrospective character of this study. Concurrent chromosomal rearrangement of MYC plus bcl-2 possibly may have synergic clinical effect and is called double hit lymphoma which occurs in 7–10 % of DLBCL [41–43].
Conclusion
Our study shows that interim 18F-FDG PET/CT scanning has a significant predictive value for disease progression in patients with GCB molecular subtype of DLBCL, but not in those with non-GCB subtype of DLBCL treated with R-CHOP. Therefore, molecular profiles of DLBCL should be considered in interim 18F-FDG PET/CT practice.
References
A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–18.
Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;362:1417–29.
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11.
Lossos IS. Molecular pathogenesis of diffuse large B-cell lymphoma. J Clin Oncol. 2005;23:6351–7.
Dunleavy K, Wilson WH. Appropriate management of molecular subtypes of diffuse large B-cell lymphoma. Oncology (Williston Park). 2014;28:326–34.
Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125:22–32.
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82.
Choi WW, Weisenburger DD, Greiner TC, Piris MA, Banham AH, Delabie J, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15:5494–502.
Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–47.
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–58.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Yang DH, Min JJ, Song HC, Jeong YY, Chung WK, Bae SY, et al. Prognostic significance of interim (1)(8)F-FDG PET/CT after three or four cycles of R-CHOP chemotherapy in the treatment of diffuse large B-cell lymphoma. Eur J Cancer. 2011;47:1312–8.
Safar V, Dupuis J, Itti E, Jardin F, Fruchart C, Bardet S, et al. Interim [18F]fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. J Clin Oncol. 2012;30:184–90.
Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, et al. [18F]fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–81.
Pregno P, Chiappella A, Bello M, Botto B, Ferrero S, Franceschetti S, et al. Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood. 2012;119:2066–73.
Yang DH, Ahn JS, Byun BH, Min JJ, Kweon SS, Chae YS, et al. Interim PET/CT-based prognostic model for the treatment of diffuse large B cell lymphoma in the post-rituximab era. Ann Hematol. 2013;92:471–9.
A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–94.
Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–61.
Hedvat CV, Hegde A, Chaganti RS, Chen B, Qin J, Filippa DA, et al. Application of tissue microarray technology to the study of non-Hodgkin’s and Hodgkin’s lymphoma. Hum Pathol. 2002;33:968–74.
Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, et al. Diffuse large B-cell lymphoma version 1.2016. J Natl Compr Canc Netw. 2016;14:196–231.
Cashen AF, Dehdashti F, Luo J, Homb A, Siegel BA, Bartlett NL. 18F-FDG PET/CT for early response assessment in diffuse large B-cell lymphoma: poor predictive value of international harmonization project interpretation. J Nucl Med. 2011;52:386–92.
Itti E, Lin C, Dupuis J, Paone G, Capacchione D, Rahmouni A, et al. Prognostic value of interim 18F-FDG PET in patients with diffuse large B-cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl Med. 2009;50:527–33.
Moskowitz CH, Schoder H, Teruya-Feldstein J, Sima C, Iasonos A, Portlock CS, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in Advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol. 2010;28:1896–903.
Yoo C, Lee DH, Kim JE, Jo J, Yoon DH, Sohn BS, et al. Limited role of interim PET/CT in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol. 2011;90:797–802.
Zinzani PL, Gandolfi L, Broccoli A, Argnani L, Fanti S, Pellegrini C, et al. Midtreatment 18F-fluorodeoxyglucose positron-emission tomography in aggressive non-Hodgkin lymphoma. Cancer. 2011;117:1010–8.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90.
Fu K, Weisenburger DD, Choi WW, Perry KD, Smith LM, Shi X, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol. 2008;26:4587–94.
Ilic I, Mitrovic Z, Aurer I, Basic-Kinda S, Radman I, Ajdukovic R, et al. Lack of prognostic significance of the germinal-center phenotype in diffuse large B-cell lymphoma patients treated with CHOP-like chemotherapy with and without rituximab. Int J Hematol. 2009;90:74–80.
Nyman H, Adde M, Karjalainen-Lindsberg ML, Taskinen M, Berglund M, Amini RM, et al. Prognostic impact of immunohistochemically defined germinal center phenotype in diffuse large B-cell lymphoma patients treated with immunochemotherapy. Blood. 2007;109:4930–5.
Seki R, Ohshima K, Fujisaki T, Uike N, Kawano F, Gondo H, et al. Prognostic impact of immunohistochemical biomarkers in diffuse large B-cell lymphoma in the rituximab era. Cancer Sci. 2009;100:1842–7.
Song MK, Chung JS, Shin DH, Seol YM, Shin HJ, Choi YJ, et al. Prognostic significance of the Bcl-2 negative germinal centre in patients with diffuse large B cell lymphoma treated with R-CHOP. Leuk Lymphoma. 2009;50:54–61.
Iqbal J, Meyer PN, Smith LM, Johnson NA, Vose JM, Greiner TC, et al. BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin Cancer Res. 2011;17:7785–95.
Visco C, Tzankov A, Xu-Monette ZY, Miranda RN, Tai YC, Li Y, et al. Patients with diffuse large B-cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: a report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica. 2013;98:255–63.
Roschewski M, Staudt LM, Wilson WH. Diffuse large B-cell lymphoma-treatment approaches in the molecular era. Nat Rev Clin Oncol. 2014;11:12–23.
Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–74.
Jazirehi AR, Huerta-Yepez S, Cheng G, Bonavida B. Rituximab (chimeric anti-CD20 monoclonal antibody) inhibits the constitutive nuclear factor-{kappa}B signaling pathway in non-Hodgkin’s lymphoma B-cell lines: role in sensitization to chemotherapeutic drug-induced apoptosis. Cancer Res. 2005;65:264–76.
Kawauchi K, Araki K, Tobiume K, Tanaka N. p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nat Cell Biol. 2008;10:611–8.
Kao YS, Fong JC. Endothelin-1 induces glut1 transcription through enhanced interaction between Sp1 and NF-kappaB transcription factors. Cell Signal. 2008;20:771–8.
Sommermann TG, O’Neill K, Plas DR, Cahir-McFarland E. IKKbeta and NF-kappaB transcription govern lymphoma cell survival through AKT-induced plasma membrane trafficking of GLUT1. Cancer Res. 2011;71:7291–300.
Nishikori M. Classical and alternative NF-κB activation pathways and their roles in lymphoid malignancies. J Clin Exp Hematopathol. 2005;45:15–24.
Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol. 2010;28:3360–5.
Johnson NA, Savage KJ, Ludkovski O, Ben-Neriah S, Woods R, Steidl C, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114:2273–9.
Kramer MH, Hermans J, Wijburg E, Philippo K, Geelen E, van Krieken JH, et al. Clinical relevance of BCL2, BCL6, and MYC rearrangements in diffuse large B-cell lymphoma. Blood. 1998;92:3152–62.
Acknowledgments
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1072). No other potential conflict of interest relevant to this article was reported.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was approved by an institutional review board and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Acquisition of the informed consents was waived by the institutional review board.
Rights and permissions
About this article
Cite this article
Kim, J., Lee, JO., Paik, J.H. et al. Different predictive values of interim 18F-FDG PET/CT in germinal center like and non-germinal center like diffuse large B-cell lymphoma. Ann Nucl Med 31, 1–11 (2017). https://doi.org/10.1007/s12149-016-1123-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-016-1123-6