Abstract
Several studies suggested that staging bone marrow biopsy (BMB) could be omitted in patients with classical Hodgkin’s lymphoma (cHL) when a positron emission tomography/computed tomography (PET/CT) is performed at baseline.
To address the concordance between BMB and PET/CT in the detection of bone marrow involvement (BMI) and the BMB role in determining the Ann Arbor stage, we retrospectively collected data on 1244 consecutive patients with cHL diagnosed from January 2007 to December 2013. One thousand eighty-five patients who had undergone both BMB and PET/CT were analyzed, comparing the Ann Arbor stage assessed with PET/CT only to that resulting from PET/CT combined with BMB.
One hundred sixty-nine patients (16%) showed at least one focal skeletal lesion (FSL) at PET/CT evaluation. Only 55 patients had a positive BMB (5.1%); 34 of them presented at least one FSL at PET/CT. To the contrary, 895 out of 1030 patients with a negative BMB did not show any FSL (86.9%). Positive and negative predictive values of PET/CT for BMI were 20 and 98%, respectively; sensitivity and specificity were 62 and 87%, respectively. Fifty-four out of 55 patients with a positive BMB could have been evaluated as an advanced stage just after PET/CT; only one patient (0.1%) would have been differently treated without BMB.
Our data showed a very high negative predictive value of PET/CT for BMI and a negligible influence of BMB on treatment planning, strengthening the recent indications that BMB could be safely omitted in cHL patients staged with PET/CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Classical Hodgkin lymphoma (cHL) accounts for approximately 8% of newly diagnosed lymphomas, with a rather stable incidence [1]. It is nowadays a highly curable disease, with lasting remissions in more the 70–80% of patients after first-line therapy [2]. Treatment strategy is based on Ann Arbor stage and risk classification, so the pre-therapeutic work-up is crucial to avoid over-treatment in limited-stage patients and under-treatment of advanced-stage patients.
The Ann Arbor classification represented a cornerstone in cHL staging [3]; in 1989, staging was reviewed in the Cotswold meeting in order to limit invasive procedures, thanks to the improvement of imaging diagnostics [4]. Bone marrow involvement in cHL is not frequent, accounting for 4–11% of new patients [5] and is usually associated with the presence of B symptoms [6, 7]. Iliac crest bone marrow biopsy (BMB) is an invasive procedure for staging of patients with newly diagnosed cHL according to the 2010 ESMO guideline [8]. However, BMB investigates only a limited volume of bone marrow, and so it could miss partial, localized lymphomatous infiltration [9]. Moreover, BMB is associated with a small risk of complications, including hemorrhages and infections in the bioptic site (in up to 0.12% of patients) [10], and it may cause anxiety and pain to the patient [11, 12].
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is today recommended in the initial staging of cHL [13], response assessment after treatment [14], and for prognostic assessment as an interim scan [15]. A whole-body, non-invasive diagnostic instrument, PET/CT exhibits impressive accuracy in the identification of nodal and extranodal disease, including skeletal lesions [13, 16], leading frequently to an upstaging of patients when compared to CT scan alone.
Several studies have evaluated the role of BMB in the PET/CT era [17,18,19,20,21,22,23,24,25,26,27]. The largest one, by El-Galaly et al., has retrospectively reported data on the diagnostic and prognostic value of routine BMB in 454 treatment-naive patients with cHL undergoing PET/CT staging, showing that in light of the results of BMB, no patient was upstaged or had a change in treatment [21]. Recently, a meta-analysis evaluated the diagnostic performance of PET/CT in detecting bone marrow involvement in cHL patients, concluding that PET/CT could appropriately substitute BMB in the staging of cHL [28]. With the aim of increasing the knowledge of this issue, we performed a multi-centric, retrospective study on consecutive cHL patients referring to hematology centers of the Fondazione Italiana Linfomi, aimed at establishing the concordance between BMB and PET/CT in the detection of bone marrow involvement in cHL and to evaluate the impact of BMB in the staging of patients with cHL.
Methods
Patients
Consecutive cHL patients diagnosed from January 2007 to December 2013 referring to 16 hematological centers of the Fondazione Italiana Linfomi were included in this retrospective study. The study was approved by the ethical committee of the coordinating center (Florence, Italy) and was conducted according to the Helsinki Declaration. Informed consent was obtained from all individuals participants included in the study. Data were collected from electronic database and patient charts. All data were collected in an anonymized format, in compliance with institutional and national requirements.
Patients more than 14 years old were included in the study if both PET/CT and BMB had been performed at baseline. The exclusion criteria were (i) diagnosis of nodular lymphocyte-predominant HL, (ii) HIV positivity, (iii) other previous or concomitant malignancy, (iv) and a follow-up of less than 3 months after the end of therapy.
All patients were evaluated according to the Ann Arbor staging system by the combination of both PET/CT and BMB and by the use of PET/TC only [2]. Risk stratification was performed according to the categories identified by the German Hodgkin Study Group (GHSG) and, for patients in advanced stage, to the Hasenclever score [29, 30]. Response to treatment was defined according to the International Working Group criteria [31].
Evaluation of BMB
BMB had been performed unilaterally or bilaterally according to local policies and were evaluated by local pathologists without a central review. Minimal requirements for immunohistochemical staining were pan-B, pan-T, CD30, and CD15. BMB was repeated at the end of the treatment if positive at baseline [31].
Evaluation of PET/CT
PET/CT were performed according to local protocols and scanner procedures. PET/CT were performed by co-registration of PET and multislice CT in a single, whole-body session; a low-dose CT was employed in most cases. Scans were performed from skull base to mid-tights and were registered 60–90 min after the intravenous injection of 18F-FDG; different doses of 18F-FDG were administered depending on the type of PET/CT scanner. PET/CT was repeated at the end of treatment [31] and, in most cases, after two cycles of treatment with ABVD as prognostic interim evaluation [14].
Interpretation of skeletal lesions
Due to the retrospective nature of this study, investigators could decide to further evaluate focal skeletal lesions according to their internal policies. In absence of a histological verification via guided biopsies or supplementary radiological studies, focal skeletal lesions were considered as a disease involvement if a focal 18F-FDG uptake above liver 18F-FDG uptake disappeared or persisted, according to other involved sites, in PET/CT studies performed after chemotherapy [32]. Focal skeletal lesions were categorized as unifocal (a single PET-positive site), bifocal (two PET-positive sites), and multifocal (three or more PET-positive lesions). A widespread marrow 18F-FDG uptake above liver 18F-FDG uptake in absence of focal skeletal lesions defined as above was not considered as a disease involvement. PET/CT scans of patients with focal skeletal uptakes were locally reviewed by a different nuclear medicine physician; no central review was performed.
Statistical analysis
Measures of diagnostic performance, such as positive predictive value (PPV), negative predictive value (NPV), sensitivity, and specificity, were estimated with the corresponding confidence intervals, based on the obtained results and on the number of false negatives (FN) and false positives (FP). Lastly, the agreement in the definition of the Ann Arbor stage between PET/CT plus BMB and PET/CT alone was assessed, evaluating also whether the omission of BMB could modify the patient management.
Results
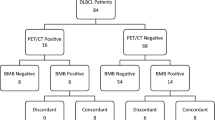
We retrospectively collected data from 1244 patients with cHL diagnosed in 16 hematology centers of the Fondazione Italiana Linfomi; 159 patients were excluded because of a lack of baseline BMB or PET/CT. Patient characteristics are reported in Table 1. The median age of the 1085 evaluable patients was 33 years (range 14–89 years), and 567 patients (52.3%) were male. Only 47 patients were less than 18 years old, with a median age of 17 years. As expected, nodular sclerosis was the most common histological subtype (769 patients, 70.9%); 455 patients (41.9%) reported B-symptoms at presentation. One hundred ninety-one patients (17.6%) underwent a bilateral BMB, while in the large majority of patients (890 patients, 82%), BMB was unilateral. A positive BMB was reported in only 55 patients (5.1%), without differences between patients who underwent unilateral or bilateral BMB. All analyzed patients reached the minimum required follow-up of 3 months.
The analysis of skeletal 18F-FDG uptake is summarized in Table 2. Overall, the PET/CT scans featured at least a focal skeletal lesion in 169 patients (15.6%); in particular, 56 patients showed a single focal lesion, 32 had two focal lesions, and 81 presented three or more focal lesions. Considering the patients with a negative BMB, 48 (4.7%) showed a single bone lesion, 30 (2.9%) had two lesions, and 57 patients (5.5%) displayed three or more lesions. Of the 55 patients with a positive BMB, eight (14.6%) had a unifocal skeletal 18F-FDG uptake, two (3.6%) a bifocal involvement, and 24 (43.6%) a multifocal localization. A diffuse and homogeneous marrow 18F-FDG uptake was observed in 53 patients (5.1%) with a negative BMB and in nine patients (16.3%) with a positive BMB. Nonetheless, all of them have been considered a stage IV with PET/CT only because of other extranodal 18F-FDG uptake sites (seven lung lesions, two liver lesions).
The sensitivity and specificity of focal skeletal PET/CT lesions for detection of positive versus negative BMB were 61.8% (CI 95%, 47.7–74.6) and 86.9% (CI 95%, 84.7–88.9), respectively. PPV and NPV of focal skeletal PET/CT lesions for detection of positive BMB were 20.1% (CI 95%, 14.3–27.0) and 97.7% (CI 95%, 96.5–98.6), respectively.
We also evaluated the sensitivity, NPV, and the diagnostic accuracy of both BMB and PET/CT under the assumption that both of them are separately sufficient to reveal true bone or marrow disease. For BMB, the sensitivity was 28.9% (CI 95%, 26.3–31.8), NPV was 86.9% (CI 95%, 84.7–88.8), and the diagnostic accuracy was 88% (CI 95%, 84.5–89.0%). For PET/CT, the sensitivity was 86.5% (CI 95%, 84.3–88.5), NPV was 97.8% (CI 95%, 96.7–98.5), and the diagnostic accuracy was 98% (CI 95%, 96.0–98.5%).
A surgical- or CT-guided bone biopsy was performed in only four out of 169 patients with at least a focal skeletal lesion (2.4%); in three of them, the biopsy confirmed a disease involvement, while one patient underwent a CT-guided needle biopsy of a rib, which did not reveal a lymphomatous localization. This patient was considered a stage III according to the results of PET/CT.
The results of the staging considering the PET/CT scan alone or the PET/CT and BMB are represented in Table 3. With the PET/CT scan alone, 55 patients (5.1%) were in stage I, while 532 (49%), 258 (23.7%), and 240 (22.1%) resulted in stages II, III, and IV, respectively. The addition of BMB to PET/CT led to the upstaging of nine patients (0.8%). In particular, none of the patients in stage I with PET/CT alone was upstaged, while one out of 532 patients in stage II (0.1%; CI 95%, <0.0001–0.0117)) and eight out of 258 in stage III (3.1%; CI 95%, 0.0099–0.0521) with PET/CT alone were upstaged to stage IV with the addition of BMB.
In the 1059 patients assessed according to the GHSG risk score, none of the 184 patients in the early group and only one out of the 291 patients in the intermediate group (3.4%; CI 95%, <0.0001–0.0212) shifted to the advanced group after the incorporation of BMB to PET/CT.
Discussion
In the last years, new imaging techniques have become available in routine clinical practice, greatly modifying the diagnostic approach of many hematological diseases. PET/CT is currently employed in the baseline assessment of cHL as well as in the treatment response evaluation [13, 14] and for prognostic assessment as an interim scan [15].
With the widespread availability of high-quality PET/CT scanners, which co-registrate PET and multislice CT scans in a single whole-body examination, the possibility to detect extranodal sites of disease has become realistic, thanks to the high sensitivity of PET/CT in the detection of bone marrow involvement in cHL, as reported in many series [17,18,19,20,21]. BMB is the classical procedure to detect bone marrow involvement in lymphomas, but it is an invasive, possibly painful procedure, which samples only a limited part of bone marrow. In the past years, efforts were done in order to develop scores constituted of laboratory and clinical data to evaluate the probability of marrow involvement in cHL [5], but they never entered the routine daily practice. Recently, considering the very low positivity rate of BMB in patients with cHL [9] and the astonishing results of PET/CT, the role of BMB as a mandatory staging procedure has been debated. Several authors reported their data in relatively small cohorts of patients [21,22,23,24,25,26,27]; the largest population was described by El-Galaly et al. [21] who reported data on 454 newly diagnosed patients with cHL. With a negative predictive value of focal skeletal lesions of 99% and no patient upstaged with the addition of BMB, the authors considered that omission of BMB would have not affected the treatment choice [21]. This issue was further evaluated in a meta-analysis of nine studies performed by Adams et al., which included 955 patients from 9 different studies. Pooled sensitivity and specificity of PET/CT ranged were 96.9 and 99.7%, respectively, and even with considering the moderate quality of the studies included, the evidence was that PET/CT may replace BMB in the staging of cHL [28].
We present in this paper the results of a large retrospective, multi-center study including consecutive cHL patients referring to centers of the Fondazione Italiana Linfomi. To our knowledge, this represents the largest cohort of consecutive patients analyzed with the aim to address these issues. In our large population, a histological confirmation of disease involvement trough BMB was observed in 55 out of 1085 patients (5.1%), while 169 patients (15.5%) featured at least one focal skeletal lesion at PET/CT.
A diffuse, homogeneous marrow 18F-FDG uptake was observed in 62 out of 1085 patients (5.7%), a similar proportion when compared to other populations [21]. Nine of these 62 patients had a positive BMB, a different finding compared to El-Galaly’s data, which otherwise showed an absence of BMB positivity in patients with a diffuse marrow 18F-FDG uptake. However, these nine patients could already be considered as stage IV after PET/CT alone because of other extranodal 18-F FDG uptake sites at PET/CT.
The sensitivity and specificity of focal skeletal lesions at PET/CT for identification of positive versus negative BMB were 61.8 and 86.9%, respectively. Most importantly, despite the low PPV of focal skeletal lesions (20.1%), we found a very high NPV (97.7%), confirming that the absence of focal skeletal lesions at PET/CT could be roughly associated to a negative BMB. These results are even more meaningful considering that a thorough bioptic evaluation of extranodal and skeletal sites of 18F-FDG uptake is unfeasible, either for technical and ethical reasons and to avoid a treatment delay. The lower sensitivity showed in our study compared to El-Galaly’s data [21] could possibly be explained with the different reference level used to determine the disease involvement of bone lesions, which were considered positive if showed a focal 18F-FDG uptake above liver 18F-FDG uptake, while in the El-Galaly’s study, the mediastinal blood pool was used as reference. We chose a more selective reference in order to minimize the number of false positives; considering the lower sensitivity, the NPV was particularly high, and we could then consider the liver reference as a suitable reference to evaluate bone lesions.
A striking finding in our study, however, is than even if the addition of BMB to PET/CT led to an upstage in nine patients, only one patient (0.1%) had a change in the risk group allocation and treatment choice, while the remaining eight patients could have been already considered as an advanced stage with PET/CT only.
Undoubtedly, the major limits of our study are represented by the lack of a central review of the PET/CT scans and the absence of a standardization method for the evaluation of PET/CT scans, which were performed according to local procedures. Despite their importance, these limitations give a “real-life” perspective to our results and could be applied to the majority of patients treated outside a clinical trial.
Notwithstanding a partial level of evidence, recent recommendations indicate that a BMB is no longer required for the routine evaluation of patients with cHL undergoing a PET/CT at baseline [33], as well as the latest ESMO guidelines, which suggested with a grade IIIB level of evidence that BMB could be omitted if patients are staged with PET/CT [34].
Our data strengthen the aforementioned evidence on safely omitting BMB as a staging procedure in the initial assessment of cHL patients when undergoing a PET/CT scan. However, BMB could provide information not only about disease involvement but also on marrow cellularity and the presence of dysplastic features; these data could help the treating physicians to evaluate the hematopoietic reserve in a more accurate fashion, which may be useful in elderly patients and in those patients who present with cytopenias or abnormalities of blood smear. Although BMB does not add information about the actual stage, we therefore suggest evaluating clinical and laboratory data for single patients before definitely discarding BMB (or a marrow aspiration, which could also give information about hematopoietic lineages) from the diagnostic work-up.
References
Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS (2006) Lymphoma incidence patterns by WHO subtypes in the United States, 1992-2001. Blood 107:265–276
Stein H (2001) WHO histological classification of Hodgkin lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds) Tumors of haematopoietic and lymphoid tissues. IARC Press, Lyon, pp 237–253
Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M (1971) Report of the committee on Hodgkin’s disease staging classification. Cancer Res 31:1860–1861
Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA, Tubiana M (1989) Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 7:1630–1636
Vassilakopoulos TP, Angelopoulou MK, Constantinou N, Karmiris T, Repoussis P, Roussou P, Siakantaris MP, Korkolopoulou P, Kyrtsonis MC, Kokoris SI, Dimopoulou MN, Variamis E, Viniou NA, Konstantopoulos K, Dimitriadou EM, Androulaki A, Patsouris E, Doussis-Anagnostopoulou IA, Panayiotidis P, Boussiotis VA, Kittas C, Pangalis GA (2005) Development and validation of a clinical prediction rule for bone marrow involvement in patients with Hodgkin lymphoma. Blood 105:1875–1880
Levis A, Pietrasanta D, Godio L, Vitolo U, Ciravegna G, Di Vito F, Gavarotti P, Guglielmelli T, Orsucci L, Raviolo E, Rota Scalabrini D, Salvi F, Tonso A, Aglietta M, Boccadoro M, Gallamini A, Saglio G, Scassa E, Gallo E (2004) A large scale of bone marrow involvement in patients with Hodgkin’s lymphoma. Clin Lymphoma 5:50–55
Connors JM (2005) State-of-the-art therapeutics: Hodgkin’s lymphoma. J Clin Oncol 23:6400–6408
Engert A, Eichenauer DA, Dreyling M (2010) Hodgkin’s lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(Suppl 5):v168–v171
Zhang QY, Foucar K (2009) Bone marrow involvement by Hodgkin and non-Hodgkin lymphomas. Hematol Oncol Clin North Am 23:873–902
Bain BJ (2004) Bone marrow biopsy morbidity and mortality: 2002 data. Clin Lab Haematol 26:315–318
Brunetti GA, Tendas A, Meloni E, Mancini D, Maggiore P, Scaramucci L, Giovannini M, Niscola P, Cartoni C, Alimena G (2011) Pain and anxiety associated with bone marrow aspiration and biopsy: a prospective study on 152 Italian patients with hematological malignancies. Ann Hematol 90:1233–1235
Hjortholm N, Jaddini E, Halaburda K, Snarski E (2013) Strategies of pain reduction during the bone marrow biopsy. Ann Hematol 92:145–149
Hutchings M, Loft A, Hansen M, Pedersen LM, Berthelsen AK, Keiding S, D’Amore F, Boesen AM, Roemer L, Specht L (2006) Position emission tomography with or without computed tomography in the primary staging of Hodgkin’s lymphoma. Haematologica 91:482–489
Juweid ME, Strobaants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, Wiseman GA, Kostakoglu L, Scheidhauer K, Buck A, Naumann R, Spaepen K, Hicks RJ, Weber WA, Reske SN, Schwaiger M, Schwartz LH, Zijlstra JM, Siegel BA, Cheson BD, Imaging Subcommittee of International Harmonization Project in Lymphoma (2007) Use of positron emission tomography for response assessment of lymphoma: consensus of the imaging subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 25:571–578
Gallamini A, Barrington SF, Biggi A, Chauvie S, Kostakoglu L, Gregianin M, Meignan M, Mikhaeel GN, Loft A, Zaucha JM, Seymour JF, Hofman MS, Rigacci L, Pulsoni A, Coleman M, Dann EJ, Trentin L, Casasnovas O, Rusconi C, Brice P, Bolis S, Viviani S, Salvi F, Luminari S, Hutchings M (2014) The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of Deauville five-point scale. Haematologica 99:1107–1113
Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, Ben-Barak A, Ben-Arie Y, Bar-Shalom R, Israel O (2010) (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med 51:25–30
Carr R, Barrington SF, Madan B, O’Doherty MJ, Saunders CA, van der Walt J, Timothy AR (1998) Detection of lymphoma in bone marrow by whole-body positron emission tomography. Blood 91:3340–3346
Moog F, Bangerter M, Kotzerke J, Guhlmann A, Frickhofen N, Reske SN (1998) 18-F-fluorodeoxyglucose-positron emission tomography as a new approach to detect lymphomatous bone marrow. J Clin Oncol 16:603–609
Moulin-Romsee G, Hindié E, Cuenca X, Brice P, Decaudin D, Bénamor M, Brière J, Anitei M, Filmont JE, Sibon D, de Kerviler E, Moretti JL (2010) (18)F-FDG PET/CT bone/bone marrow findings in Hodgkin’s lymphoma may circumvent the use of bone marrow trephine biopsy at diagnosis staging. Eur J Nucl Med Mol Imaging 37:1095–1105
Pakos EE, Fotopoulos AD, Ioannidis JP (2005) 18F-FDG PET for evaluation of bone marrow infiltration in staging of lymphoma: a meta-analysis. J Nucl Med 46:958–963
El-Galaly TC, D’Amore F, Mylam KJ, de Nully BP, Bøgsted M, Bukh A, Specht L, Loft A, Iyer V, Hjorthaug K, Nielsen AL, Christiansen I, Madsen C, Johnsen HE, Hutchings M (2012) Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol 30:4508–4514
Cortés-Romera M, Sabaté-Llobera A, Mercadal-Vilchez S, Climent-Esteller F, Serrano-Maestro A, Gámez-Cenzano C, González-Barca E (2014) Bone marrow evaluation in initial staging of lymphoma: 18F-FDG PET/CT versus bone marrow biopsy. Clin Nucl Med 39:e46–e52
Pelosi E, Penna D, Deandreis D, Chiappella A, Skanjeti A, Vitolo U, Bisi G (2008) FDG-PET in the detection of bone marrow disease in Hodgkin’s disease and aggressive non-Hodgkin’s lymphoma and its impact on clinical management. Q J Nucl Med Mol Imaging 52:9–16
Muzahir S, Mian M, Munir I, Nawaz MK, Faruqui ZS, Mufti KA, Bashir H, Uddin N, Siddiqui N, Maaz AU, Mahmood MT (2012) Clinical utility of 18F FDG-PET/CT in the detection of bone marrow disease in Hodgkin’s lymphoma. Br J Radiol 85:e490–e496
Pelosi E, Penna D, Douroukas A, Bellò M, Amati A, Arena V, Passera R, Bisi G (2011) Bone marrow disease detection with FDG-PET/CT and bone marrow biopsy during the staging of malignant lymphoma: results from a large multicentre study. Q J Nucl Med Mol Imaging 55:469–475
Mittal BR, Manohar K, Malhotra P, Das R, Kashyap R, Bhattacharya A, Varma N, Varma S (2011) Can fluorodeoxyglucose positron emission tomography/computed tomography avoid negative iliac crest biopsies in evaluation of marrow involvement by lymphoma at time of initial staging? Leuk Lymphoma 52:2111–2116
Cheng G, Chen W, Chamroonrat W, Torigian DA, Zhuang H, Alavi A (2011) Biopsy versus FDG PET/CT in the initial evaluation of bone marrow involvement in pediatric lymphoma patients. Eur J Nucl Med Mol Imaging 38:1469–1476
Adams HJ, Kwee TC, de Keizer B, Fijnheer R, de Klerk JM, Littooij AS, Nievelstein RA (2014) Systematic review and meta-analysis on the diagnostic performance of FDG-PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: is bone marrow biopsy still necessary? Ann Oncol 25:921–927
Engert A, Schiller P, Josting A, Herrmann R, Koch P, Sieber M, Boissevain F, De Wit M, Mezger J, Duhmke E, Willich N, Muller RP, Schmidt BF, Renner H, Muller-Hermelink HK, Pfistner B, Wolf J, Hasenclever D, Loffler M, Diehl V, German Hodgkin’s Lymphoma Study Group (2003) Involved field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s lymphoma study group. J Clin Oncol 21:3601–3108
Hasenclever D, Diehl V (1998) A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med 339:1506–1514
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V, International Harmonization Project on Lymphoma (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Weiler-Sagie M, Kagna O, Dann EJ, Ben-Barak A, Israel O (2014) Characterizing bone marrow involvement in Hodgkin’s lymphoma by FDG-PET/CT. Eur J Nucl Med Mol Imaging 41:1133–1140
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068
Eichenauer DA, Engert A, André M, Federico M, Illidge T, Hutchings M, Ladetto M (2014) Hodgkin’s lymphoma: ESMO clinical practice guidelines guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii70–iii75
Acknowledgements
The authors would like to thank C. Oakley (National Cancer Research Centre “Giovanni Paolo II”, Bari, Italy) for the linguistic revision of the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The study was approved by the ethical committee of the coordinating center (Florence, Italy) and was conducted according to the Helsinki Declaration
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Puccini, B..., Nassi, L., Minoia, C. et al. Role of bone marrow biopsy in staging of patients with classical Hodgkin’s lymphoma undergoing positron emission tomography/computed tomography. Ann Hematol 96, 1147–1153 (2017). https://doi.org/10.1007/s00277-017-2996-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-2996-8