Abstract
Diabetes mellitus is a chronic metabolic disease with serious health consequences for a modern civilization that often lead to premature death. With the rapid increase in the number of people diagnosed with type 2 diabetes, early identification of those individuals at higher risk of progression to diabetes is a key criterion enabling the timely intervention or treatment. In recent years, omics-based technologies have given us unprecedented insight into circulating biomarkers in common diseases. Branched-chain amino acids: valine, leucine, isoleucine, and aromatic amino acids, that is, tyrosine and phenylalanine, have been demonstrated as the most consistent metabolite biomarkers for diabetes, in particular type 2.
Therefore, amino acids quantification in biological material, primarily in plasma could be a valuable prognostic tool for determining metabolic abnormalities leading to this disease. Revealing these interactions and possible mechanisms may prove beneficial for the prediction and treatment.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Amino acids
- Branched-chain amino acids
- Insulin resistance
- Prediabetic state
- Diabetes
- Type 2 diabetes mellitus
- Metabolic disease
- Biomarker
- Metabolomics
Introduction
Amino acids are defined as organic compounds containing amino(–NH2) and carboxyl(–COOH) functional groups along with a side chain (R group) specific to each amino acid (AA). They are referred as the building blocks of proteins needed for animal nutrition and basic units for synthesis of hormones and neurotransmitters (Lopez and Mohiuddin 2021). Human proteins are made up of 20 AAs, 9 of which are considered “essential” because they cannot be synthesized from other metabolites in the human body (White and Newgard 2019). AAs play a key role in many metabolic pathways, and quantification of free amino acids (FAAs) in biological fluids and tissues has historically provided nutritional information used in the diagnosis of various diseases, particularly metabolic impairments (Nagao and Kimura 2020).
During the last decade, many studies have consistently reported the positive association of plasma or serum FAAs with insulin resistance (IR) and diabetes in individuals from different ethnic groups and with varying degrees of obesity in large prospective and cross-sectional human studies (Newgard et al. 2009; (Giesbertz and Daniel 2016; Chen et al. 2019; Bi and Henry 2017). Most of these publications demonstrated that increased levels of branched-chain amino acids in plasma, serum, and also in urine (Branched-chain amino acids (BCAAs); valine, leucine, isoleucine) are associated with obesity, IR, and diabetes, in particular type 2 diabetes mellitus (T2DM) (Newgard et al. 2009; Shah et al. 2012; McCormack et al. 2013; (Würtz et al. 2013; Bi and Henry 2017; Nie et al., 2018; Yousri et al. 2015).
Hyperaminoacidemia observed in obesity may be related to increased IR. Insulin resistance is believed to reduce the BCAA catabolism by suppressing the enzymatic activity of branched-chain alpha-keto acid dehydrogenase complex (BCKDC), which is considered as the plausible mechanism explaining the increased BCAA levels in obese or diabetic individuals (Bi and Henry 2017). Elevated BCAA levels have often been shown to predict the development of T2DM well in advance of its actual occurrence, what is very interesting from a diagnostics standpoint (Wang et al. 2011; Yamakado et al. 2015; McCormack et al. 2013; Ianni et al. 2017).
In addition, there is a body of evidence suggesting the correlation between IR and changes in aromatic amino acids (AAAs), that is tyrosine and phenylalanine, as well several other AAs (Bi and Henry 2017; Chen et al. 2019). Therefore, AAs quantification in biological material, primarily in plasma, may be a useful indicator of the presence of metabolic abnormalities leading to diabetes. This chapter reviews molecular and clinical associations between altered AA levels and diabetes development and interprets underlying biochemical mechanisms.
Diabetes Mellitus
Diabetes mellitus (DM) is a highly prevalent chronic metabolic disease with major health implications for a modern civilization (Freitas et al. 2017; Gan et al. 2020), and the eighth major mainstay of mortalities around the globe (Hameed et al. 2020). It is characterized by an increase in blood glucose level and inability to utilize glucose in adipose and muscle tissues (Zhao et al. 2017; Al-Abbasi 2012). Among the three main types of DM, i.e.,: type 1 (T1DM); type 2 (T2DM); and gestational (GDM), the T2DM is most commonly diagnosed (about 90% of cases) (“Diagnosis and Classification of Diabetes Mellitus,” 2004; Gan et al. 2020).
T2DM is characterized by abnormal glucose and lipid metabolism, resulting from resistance to the effects of insulin and insufficient response to the secretion of this hormone. As the disease progresses, there may also occur a partial β-cell insufficiency and deficiency in insulin production. (“Diagnosis and Classification of Diabetes Mellitus,” 2004; Gan et al. 2020; Chen et al. 2019).
According to the International Diabetes Federation (IDF) data, the global diabetes incidence has continuously increased each year, with an estimated 578 million cases by 2030 and 700 million people diagnosed with diabetes by the year 2045. Moreover, in 2019 one in two (50.1%) people living with diabetes were undiagnosed (Saeedi et al. 2019).
Chronic hyperglycemia is a common feature related to all DM subtypes that may lead to long-term damages (Association 2016; Freitas et al. 2017) and several vascular, neurological, immunological, and biochemical pathological changes (Al-Abbasi 2012). As the disease progresses, it is accompanied by multiple complications and dysfunction of various organs (Association 2016). Most commonly occurring is microvascular complications, e.g., diabetic retinopathy, nephropathy, and neuropathy; macrovascular complications, including coronary atherosclerotic heart disease with increased risk of cardiovascular events (Rawshani et al., 2017), and vascular disease of the lower extremities (peripheral artery disease), which is a leading cause of nontraumatic limb amputations (Vamos et al. 2010), as well as diabetic nephropathy, hypertension, and cerebrovascular disease, (Zhao et al. 2017). It is also associated with hypercholesterolemia, obesity, and other nutritional disorders (Al-Abbasi 2012).
Diabetes, particularly type 2, imposes a heavy financial strain on health care systems everywhere and shortens the life expectancy of diabetic patients (International Diabetes Federation (IDF) (2015) Diabetes Atlas. 7th Edition, International Diabetes Federation, Brussels, Belgium. – References – Scientific Research Publishing, no date), (Association 2016; Freitas et al. 2017; Zhao et al. 2017). The global T2DM prevalence is rising rapidly, particularly among those living in low- and middle-income countries (Ahola-Olli et al. 2019; Gan et al. 2020). T2DM is associated with increased mortality risk and reduced health-related quality of life, causing an immense social costs burden (Ahola-Olli et al. 2019; Chen and Gerszten 2020). The still high global incidence of T2DM and the accompanying increase in the number of its complications require earlier diagnosis and more effective treatment of this disease (Zhao et al. 2017).
The Need for New Biomarkers for Diabetes
Metabolic disorders are often present for years before becoming clinically apparent. In the early stages of T2DM, patients may have difficulties to recognize any symptoms of the disease (Zhao et al. 2017). By the time that relative insulin deficiency manifests as hyperglycemia and a T2DM is diagnosed, significant pancreatic β-cell failure already occurs. Early identification of individuals at higher risk of progression to diabetes allows the timely intervention to delay or prevent diabetes onset (Wang et al. 2011; Ahola-Olli et al. 2019).
Currently, most early screening and diagnostic methods for DM are directly related to glucose levels (Gan et al. 2020), which include fasting plasma glucose (FPG) measurement or 2-h plasma glucose (2-h PG) measurement in 75-g oral glucose tolerance (OGT) test (Association 2018; Gan et al. 2020). Moreover, the glycated hemoglobin (HbA1c) test is also often used to diagnose diabetes, providing an overall picture of average blood glucose levels over a period of past 3 months. In addition, several other clinical and laboratory predictors could be used in gauging diabetic status, such as body mass index and c-peptide measurement (Wang et al. 2011; Al-Abbasi 2012).
Meanwhile, many overweight to moderately obese people are found to have completely normal fasting plasma glucose and hemoglobin A1c levels, making them undiagnosed as prediabetic despite underlying metabolic abnormalities (Bi and Henry 2017). Therefore, there is a great need for biomarkers allowing an early diagnosis of prediabetic or diabetic patients (Wang et al. 2011).
In recent years, omics-based technologies have given us unprecedented insight into circulating biomarkers of common diseases. Application of metabolomics allowed identification of biochemical changes occurring prior to the onset of diabetes and provided additional information about pathophysiological mechanisms leading to DM. Amino acids and other metabolites were proposed as predictive markers indicating early metabolic perturbances. Diagnosis of patients at risk of diabetes onset is crucial to introduce changes in life style and prevention of disease development (Würtz et al. 2013; Guasch-Ferré et al. 2016; Ahola-Olli et al. 2019; Wang et al. 2011).
Amino Acids in Metabolic Signaling and Insulin Resistance
Despite the relevant role of the glucose-related pathways, here we are concentrating mostly on the protein metabolism influence on IR development. Many amino acids, especially BCAAs, are important nutritional signals that possess direct and indirect effects (Lynch and Adams 2014). The BCAA metabolic pathway crosses with the mechanism for IR (George 2017).
Evidence that BCAAs may not only have a "reporter quality" but may also contribute to IR and T2DM comes from cell culture and animal studies that propose sustained activation of complex 1 (mTORC1) (Newgard et al. 2009; Giesbertz and Daniel 2016). Proposed mechanisms (explaining how increased levels of BCAAs might be linked to metabolic disease) involve stimulation of the mTOR/p70S6K pathway and phosphorylation of IRS-1 at multiple serine sites (Nie et al. 2018). In this context, leucine is known to activate the nutrient sensing complex, mTORC1, which results in uncoupling of insulin signaling at an early stage of IR and other metabolic disorders. However, numerous observations indicate that BCAA-mediated mTORC1 activation is not necessary or sufficient to induce IR, and subsequent metabolic dysfunction, (Lynch and Adams 2014; Yoon 2016). Recently, in a rat model of diabetes (University of California-Davis T2DM rat model, UCD-T2D) an untargeted metabolomics study was performed showing that elevated plasma BCAA levels were not observed until 6 months after the onset of diabetes. This rules out the causal role of BCAAs in the occurrence of T2DM in the studied model (Yoon 2016; Biswas et al. 2019).
Zhao et al. (2020) worked with high-fat diet-induced obese (DIO) mice. Supplementation of these animals with BCAAs leads to heavy hepatic metabolic disorders, such as suppressed lipogenesis and increased glucose production. They also found that this impairs hepatic AKT2 signaling. BCAA supplementation stops AKT2 activation through mTorc1- and mTorc2-dependent pathways and promotes AKT2 degradation. As a matter of fact, the signaling pathways are other key elements in the development of IR. AKT is responsible for the transcription factors Forkhead box O (FOXO) activation, regulating the energy metabolism. FOXOs modulate the adipogenesis process in the adipose tissue, they retain the beta cells function during oxidative stress, avoiding their replication, and they are a candidate as regulators of glucose production in the liver (Gross et al. 2009). Tyrosine kinase receptor (TKR) is an insulin receptor (IR), whose first targets are Insulin Receptor Substrate (IRS) 1, 2, 3, and 4. To better understand the most important targets, scientists knocked down these receptors in mice. While the knockdown of IRS3 or 4 showed no or limited effects on mice, knocked down IRS1 or 2 developed, respectively, IR and T2DM (Kubota et al. 2017).
An alternative mechanism referred to the BCAAs dysmetabolism suggests that deficiencies in BCAA metabolism are linked to IR and T2DM by the accumulation of BCAA levels in plasma, as well possibly toxic intermediates (Yoon 2016). BCAAs inefficient metabolism or incomplete oxidation, especially isoleucine and valine can result in anaplerotic stress and an imbalance between anaplerosis and cataplerosis that might cause suboptimal mitochondrial function in states of T2DM. For example, reduced mitochondrial branched-chain α-ketoacid dehydrogenase (BCKDH) activity results in the accumulation of branched-chain α-ketoacid (BCKA) and α-ketobutyrate (α-KB), which result in the restriction of propionyl- Coenzyme A (CoA)-derived metabolites to tricarboxylic acid (TCA) cycles, inducing anaplerotic stress and decreased amino acid fuel delivery to mitochondria (Fiehn et al. 2010; Adams et al. 2009; Yoon 2016).
Incomplete mitochondrial oxidation is thought to be the major cause for increased BCAA concentrations, independently of established IR. In obese subjects, the adipose tissue is characterized by an accumulation of fat, an increased pro-inflammatory state, and alterations in hormone and cytokine secretion that may strongly affect the mitochondrial function in peripheral tissues (Ianni et al. 2017).
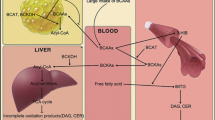
In the adipose tissue of obese and T2DM patients with IR, as well in models of obesity in rodents, the expression of genes encoding BCAAs-metabolizing enzymes is significantly downregulated by an undefined mechanism compared to metabolically healthy controls – leading to elevated plasma BCAA levels (Lackey et al. 2013; Lynch and Adams 2014; Yoon 2016). Indeed, defective BCAA oxidation results in consequent accumulation of branched-chain keto acids and branched-chain fatty acids in peripheral tissues (Ianni et al. 2017). A high level of free fatty acids (FFAs) drives tissues, such as liver and skeletal muscles, toward IR, promoting the accumulation of such bioactive lipids as diacylglycerol (DAG) and ceramide (Zabielski et al. 2019). DAG accumulation impairs insulin signaling in liver (Fig. 1). Impaired insulin signaling in muscle results in increased proteolysis, which contributes to the release of BCAAs into the circulation and further supply of substrates for mitochondrial oxidation (Ianni et al. 2017).
Diacylglycerol (DAG) accumulation impairs insulin signaling in the liver. In physiological conditions, insulin arrives at the liver, activating the insulin receptor (IR) and starting a signaling cascade. This leads to the sequential activation of insulin receptor substrate 2 (IRS2), phosphatidylinositol-3-kinase (PI3K), and protein kinase B beta (AKT2). AKT2, inhibiting glycogen synthase kinase 3 (GSK-3), promotes the activation of glycogen synthase, increasing glycogenesis. AKT2 inhibition of Forkhead box protein O1 (FOXO1) suppresses the transcription of glucose 6-phosphatase, lowering gluconeogenesis. In the presence of a nonregulated amount of NEFAs in the plasma, they enter the liver through the fatty acids transport protein 5. This brings to an accumulation of DAG in the liver. DAG promotes the membrane translocation of PKCε, an isoform of PKC responsible for the phosphorylation of the Thr1160 on the insulin receptor. The conformational changes in the kinase active site impair its signaling function, leading to a loss of regulation on glycogenesis and gluconeogenesis
However, the metabolism of BCAAs throughout the body is highly dependent on other organs; the expression of these enzymes in other organs such as the liver and muscle must be considered. Growing experimental evidence has posited that these impairments in the ability to metabolize BCAAs in adipose tissue may extend to other tissues (Lynch and Adams 2014). Expression of genes encoding enzymes of BCAA metabolism was downregulated in muscle and liver tissue of T2DM patients. Similar results were obtained in rats (Shin et al. 2014; Yoon 2016).
Zhou et al. (2019), using an integrative pathway analysis in human and mouse populations, showed that IR induced by obesity is connected with the modulations of genes of the BCAA catabolism. They demonstrated that the BCAA catabolism is impaired in the obese state. In obese mice, restoring the catabolism of BCAAs lowers their levels and improves insulin sensitivity.
On the other hand, this defective enzymatic activity in adipose tissue could be compensated for by increased BCKDC activity in the liver (Lynch and Adams 2014; Yoon 2016). As a result, some individuals may be characterized by a more global reduction in the capacity to metabolize BCAAs, which may contribute to an increase in circulating BCAA concentrations to higher ranges that is related to the development of future T2DM and IR (Shin et al. 2014; Lynch and Adams 2014).
Contribution of BCAAs to IR is a complex process in which multiple, not fully understood, mechanisms are involved. A schematic presentation of possible mechanisms by which BCAAs contribute to development of IR is depicted on Fig. 2.
A schematic view of possible mechanisms by which BCAAs contribute to development of insulin resistance. The first of proposed mechanisms involves stimulation of the mTOR/p70S6K pathway. High BCAA levels therefore inhibit IRS-1 and may impair insulin signaling. This causes cells to become less responsive to the secretion of insulin, resulting in insulin resistance
Altered Amino Acid Profiles in Insulin Resistance and Diabetes
In 1969, Felig et al. (1969) found that of 20 plasma amino acids measured, the concentrations of the three BCAAs: valine, leucine, isoleucine, and aromatic amino acids, phenylalanine and tyrosine, were increased, and glycine was lowered in plasma of obese subjects compared with age- and sex-matched lean individuals. The concentration of each of the amino acids elevated in obesity correlated directly with serum insulin, suggesting that this increase was a manifestation of IR. In 2009, Newgard et al. (2009) replicated these findings of increased circulating BCAAs, tyrosine, and phenylalanine, and decreased glycine in obese insulin-resistant subjects compared to lean insulin-sensitive individuals. Broader investigations of a role of AA concentrations in the context of various pathophysiological processes have coincided with the advent of metabolomics as a tool for studying human diseases (White and Newgard 2019). Metabolomics provides a snapshot of physiological/patophysiological processes (Zhao et al. 2017).
Since then, an overwhelming number of published data have repeatedly described several changes in metabolites, including increases in BCAA and other AA levels associated with visceral obesity (Yamakado et al. 2012) and IR (Palmer et al. 2015; Seibert et al. 2015; Nakamura et al. 2014; Nagao and Kimura 2020). A summary of current human studies reporting association of amino acids with insulin resistance and T2DM is presented in Table 1. Systemic BCAAs are strongly associated with IR and further T2DM development (Felig et al. 1969; Ferrannini et al. 2013; Wang et al. 2011; Würtz et al. 2013; Biswas et al. 2019). Moreover, numerous human studies have consistently demonstrated that concentrations of BCAAs in plasma as well as in urine have the quality to predict diabetes development (Yousri et al. 2015; Giesbertz and Daniel 2016). The correlation between IR and elevated circulating BCAA levels has been confirmed in several studies involving different ethnic groups and degrees of obesity (Newgard et al. 2009; Shah et al. 2012; McCormack et al. 2013; Würtz et al. 2013; Bi and Henry 2017). FAA profiles, particularly BCAA levels, are altered prior to the development of T2DM and are significantly associated with future diabetes diagnosis (Wang et al. 2011). These changes in plasma-free amino acids (PFAAs) can predominantly result from a metabolic shift caused by early diabetes pathogenesis (Nie et al. 2018) and may serve as a better indicator of impaired IR in prediabetic state than plasma glucose levels (Allam-Ndoul et al. 2015).
In addition, the dietary patterns of protein and BCAAs supplementation also significantly influence the association between BCAAs and IR (Zheng et al. 2016; Nagata et al. 2013; Biswas et al. 2019). Infusion of BCAAs or leucine in humans, as well as dietary intake of BCAAs, reportedly worsened insulin sensitivity (Zheng et al. 2016; Nagata et al. 2013; Harris et al. 2017; Shah et al. 2012; Biswas et al. 2019), while low BCAAs consumption has been correlated with improvement in metabolic health and alleviating IR (amelioration of IR) (Biswas et al. 2019). High dietary consumption of BCAAs is suggested to increase the risk of incident IR and may accelerate the progression of metabolic disorders, such as metabolic syndrome, and diabetes, and is not associated with the pancreatic β-cells dysfunction and hyperinsulinemia in adults. Higher total dietary BCAA intake was associated with an increased risk of T2DM in three prospective cohort studies (Nagata et al. 2013; Zheng et al. 2016; Nie et al. 2018). Moreover, these results were similar in Asian population (Nie et al. 2018).
The circulating BCAAs (valine, leucine, isoleucine) and AAAs (tyrosine and phenylalanine) have been identified as risk factors for the development of T2DM (Guasch-Ferré et al. 2016; Wang et al. 2011; Floegel et al. 2013; Palmer et al. 2015; Tillin et al. 2015; Chen et al. 2016; Stančáková et al. 2012; Wang-Sattler et al. 2012; Chen et al. 2019). Evidence concerning other amino acids is inconsistent (Chen et al. 2019). For example, an inverse correlation of glutamine with T2DM risk has been shown in some (Stančáková et al. 2012; Cheng et al., 2012) but not all of the performed studies (Floegel et al., 2013; Tillin et al. 2015; Wang-Sattler et al. 2012). Glutamate was positively associated with the occurrence of T2DM in cohort studies of Finnish from the Botnia Prospective Study (Ferrannini et al. 2013) and American adults from the Framingham Heart Study (FHS) cohort (Cheng et al. 2012), as well as Swedish cohort of the Västerbotten prospective population-based intervention program (Shi et al. 2018), whereas relations of glutamate were nonsignificant in the Malmö Diet and Cancer Study (MDC) cohort free of diabetes at baseline Swedish individuals (Cheng et al. 2012).
Glycine was reported to be inversely associated with the incidence of T2DM in Germany and West coast of Finland (Floegel et al. 2013; Wang-Sattler et al. 2012; Ferrannini et al. 2013). It was also negatively correlated with incident diabetes in European but not in South Asian men (Tillin et al. 2015). Moreover, limited evidence suggests a positive association with T2DM in the case of alanine (Ala) and histidine as well as ornithine (Guasch-Ferré et al. 2016; Chen et al. 2019). Stronger correlation between BCAA concentrations and insulin sensitivity was observed in men, with clear differences associated with age and ethnicity (Ianni et al. 2017). Consistent with this view, the population-based Cardiovascular Risk in Young Finns Study demonstrated that BCAAs, along with the aromatic amino acids: phenylalanine and tyrosine, were associated with IR selectively in men (Würtz et al. 2013). Xie et al. (2014) demonstrated that the serum metabolite profiles, verified with two independent groups of participants (Chinese, n = 105 and American, n = 72) of the obese population are gender-dependent. BCAA levels were correlated with IR and differentially expressed in obese men, but not in obese women (Xie et al. 2014). Another study found that 14 out of 24 measured AA levels were significantly higher in males than females; the only glycine was lower in males. Glutamic acid, isoleucine, leucine, and tyrosine levels had the strongest correlation with steady-state plasma glucose. This association was similar in women and men, independently of obesity, and similar to traditional markers of IR. In comparison to women, men tended to have a more unfavorable AA profile with higher AA levels associated with IR and less glycine. However, the degree of association between a direct measurement of IR and AA levels were similar between sexes and equivalent to several traditional markers of IR (Seibert et al. 2015).
The correlation between BCAA concentrations and T2DM development is also significantly modified by ethnicity, with the association in Caucasians and Hispanics while it does not appear in African Americans (Lee et al. 2016).
The cohort studies on the Asian population found BCAAs as a valid of the future risk of T2DM (Tillin et al. 2015; Tai et al. 2010), while another study of a predictive model in American Indians did not develop a reporter concept of BCAAs (Zhao et al. 2015).
Large, longitudinal studies confirmed that PFAAs analysis can predict the future susceptibility of lifestyle-related diseases (Wang et al. 2011; Yamakado et al. 2015), which is a significant strength of these investigations. Various prospective, case-controlled and nested studies on the subjects of different ethnic origins have shown elevated levels of BCAAs and other amino acids are associated with the prediabetic state, IR, and T2DM. Elevations in PFAA concentrations may independently predict the future development of diabetes, metabolic syndrome, dyslipidemia, or hypertension over a 4-year period, even after adjusting for commonly accepted risk factors such as age, sex, body mass index, fasting plasma glucose, IR, waist circumference, blood pressure, and lipid variables. From 2,984 Japanese subjects in the cohort study, 2,729 individuals without DM were included in the follow-up study to investigate the ability to predict the 4-year risk of developing new-onset DM. For single PFAA levels, BCAAs (isoleucine, leucine) and AAAs (tyrosine and phenylalanine) were significantly related to the development of DM over the 4-year time period after adjusting for age, gender, BMI, fasting plasma glucose (FPG), and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR). Multiple linear regression analysis with variable selection models were constructed between PFAA levels and the visceral fat area (VFA) and 2-h post-challenge insulin (Ins120 min) values for predicting 4-year risk of developing new-onset lifestyle-related diseases. The correlation coefficients of the obtained PFAA models against VFA or Ins120 min were higher than single PFAA levels, suggesting their usefulness as versatile markers for health monitoring for future risk prediction (Yamakado et al. 2015).
The positive associations of BCAAs and aromatic amino acids with T2DM risk have subsequently been replicated in multiple other cohorts. Similarly, the Framingham Offspring Study, conducted among 2,422 normoglycemic individuals over a 12-year period, showed that BCAAs (isoleucine, leucine, valine) and AAAs (phenylalanine and tyrosine, and also tryptophan) levels have highly significant associations with future diabetes. The others were aromatic amino acids (tryptophan, phenylalanine, and tyrosine). A combination of three amino acids predicted future diabetes (with a more than fivefold higher risk for individuals in top quartile), highlighting the potentially crucial role of amino acid metabolism in early diabetes pathogenesis (Wang et al. 2011).
Chen and his colleagues verified the close correlation of valine, leucine, isoleucine, tyrosine, and phenylalanine with IR and the future development of diabetes in Chinese populations after 10 years of follow-up, suggesting an important role of these metabolites as early markers of diabetes (highlighting the predictive value of these markers for the future development of diabetes) (Chen et al. 2016).
Cheng et al. (2012) performed one of the most promising investigations to determine the plasma concentrations of 45 distinct metabolites and examine their relation to cardiometabolic risk among 1,761 individuals from two large, well-characterized clinical cohorts in the Framingham Heart Study (FHS; N=1015) and the Malmö Diet and Cancer Study (MDC; N=746). Metabolic risk factors, such as obesity, IR, high blood pressure, dyslipidemia were associated with multiple metabolites including branched-chain amino acids, other hydrophobic amino acids, tryptophan breakdown products, and nucleotide metabolites. Moreover, a particularly strong association of IR traits with decreased glutamine and increased glutamate was observed. High glutamine-glutamate ratio was associated with lower risk of incident diabetes in FHS, but not in MDC (Cheng et al. 2012). The prospective roles of circulating amino acids as reporter molecules of insulin sensitivity and diabetes were further investigated in 1,680 individuals from the population-based Cardiovascular Risk in Young Finns Study. Circulating BCAAs (isoleucine, leucine, valine) and aromatic amino acids (phenylalanine and tyrosine) from fasting serum were shown as predictors of the insulin resistance index, but not of glycemia, at 6-year follow-up in young, normoglycemic adults, with most pronounced associations for men. They were associated with HOMA-IR at baseline and for men at 6-year follow-up, while for women only leucine, valine, and phenylalanine predicted 6-year HOMA-IR (P < 0.05). These observations suggest that altered metabolism of BCAAs and AAAs precedes the development of IR in early adulthood, before the onset of impaired fasting glucose levels, which at least partially explains how these amino acids are associated with the risk of future type 2 diabetes (Würtz et al. 2013).
Tillin et al. performed the cross-sectional and prospective analyses of ethnicity, amino acids level, and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) study. The study was performed on 801 European and 643 South Asian participants with 19 years follow-up period. The authors concluded that branched-chain and aromatic amino acids may contribute to excess risk of diabetes development (Tillin et al. 2015).
Stančáková et al. investigated amino acid levels with proton nuclear magnetic resonance spectroscopy in the population-based Metabolic Syndrome in Men (METSIM) Study, including 9,369 nondiabetic or newly diagnosed T2DM Finnish men. The levels of leucine, isoleucine, tyrosine, and alanine increased, and the levels of glutamine and histidine decreased with increasing glycemia, reflecting, at least in part, insulin resistance (Stančáková et al. 2012).
In a prospective nested case-control study, conducted among Japanese employees during a 5-year follow-up, fasting serum concentrations of several amino acids, including valine, leucine, isoleucine, phenylalanine, tyrosine, alanine, glutamate, ornithine, and lysine were associated with an increased risk of incident T2DM. High glutamine levels were associated with a decreased risk of incident T2DM (Chen et al. 2019).
In another study performed among Chinese population, GC-MS-based metabolic profiling showed increased levels of several amino acids, involving BCAAs (leucine, isoleucine) as well as alanine, and serine while substantial lower levels of 2-ketoisocaproic acid as early biomarkers of T2DM. Lower concentrations of 2-ketoisocaproic acid, a product of leucine deamination, may indicate a reduced rate of conversion of leucine to 2-ketoisocaproic acid in T2DM (Zeng et al. 2009). Similarly, Fiehn et al. observed an increase in certain AA levels and their derivatives (i.e., leucine, 2-ketoisocaproate, valine, cystine, and histidine). It is noteworthly, that plasma leucine concentration was significantly increased by ∼50%, and its initial catabolic metabolite, 2-ketoisocaproic acid (α-ketoisocaproate), was significantly increased by ∼27%. Mean plasma valine level was ∼20% higher in T2DM subjects vs. nondiabetic weight/age matched African-American women, but this difference was not statistically significant. In addition, leucine and valine concentrations rose with increasing HbA1c, and significantly correlated with plasma acetylcarnitine concentrations (Fiehn et al. 2010).
Several other studies have observed the predictive ability of PFAA analysis in evaluating the risk of developing lifestyle-related diseases and associated cardiovascular diseases. Magnusson et al. (2013) investigated the metabolite profiles among 4577 subjects among whom in case of 253 first-incident of cardiovascular disease (CVD) (myocardial infarction or stroke) occurred during a mean follow-up time of 12.2 years. Fasting plasma levels of isoleucine, tyrosine, and phenylalanine were shown to predict diabetes development with a four- to sixfold increased risk for participants in the top quartile. The combination of these three amino acids may also predict future cardiovascular events during long-term follow-up. McCormack et al. reported that elevations in BCAA concentrations are significantly associated with obesity in children and adolescents, and may independently predict future IR. Elevated BCAA levels significantly correlated with BMI in the cross-sectional cohort. In the subset of participants followed longitudinally, baseline BCAA levels were positively associated with HOMA-IR measured 18 months later (McCormack et al. 2013). Metabolic profiling performed in baseline and after 6 months in plasma samples from 500 participants after at least 4 kg of weight loss in phase I revealed a cluster of metabolites comprising BCAA levels and related analytes that could predict improvement in HOMA-IR independently of the amount of weight lost (Shah et al. 2012). Nakamura et al. (2014) recruited 51 Japanese subjects diagnosed with T2DM and measured their PFAA profiles. Several amino acids: BCAAs, glutamate, tyrosine, alanine, and proline were strongly correlated with the insulin-related variables such as C-peptide, insulin, and HOMA-IR. They also observed that the levels of BCAAs, glutamate, alanine, and tryptophan were negatively correlated with adiponectin concentrations. Adiponectin plays a pivotal role in the regulation of insulin sensitivity and metabolism. Adiponectin concentrations have shown to be decreased in obese people or diabetic patients and are strongly related to IR and hyperinsulinemia in humans (Ziemke and Mantzoros 2010). These results indicated the significant relationship between PFAA profiles, adiponectin levels, and IR (Nakamura et al. 2014).
A cross-sectional and longitudinal study conducted at the Yale Pediatric Obesity Clinic showed that BCAA and α-hydroxybutyrate concentrations during an oral glucose tolerance test (OGTT) characterize insulin-resistant youth and predict worsening of glycemic control (Tricò et al. 2017). Furthermore, in a nested case-control study within the Swedish cohort of the Västerbotten prospective population-based intervention program, an untargeted metabolomics of plasma samples from 503 case-control pairs at baseline (median time 7 years before diagnosis) and samples from a subset of 187 case-control pairs at 10-year follow-up was performed. As a result 46 metabolites allowing T2DM prediction were reported (Shi et al., 2018). Identified metabolites strongly correlated with IR and/or beta cell dysfunction. Among diabetes cases, changes in phosphatidylcholines (PCs) with odd-chain fatty acids, branched-chain amino acids, 3-methyl-2-oxovaleric acid, and glutamate were observed over time along with disease progression. Prospective associations between plasma BCAA levels and T2DM risk were also established in a population-based Prevention of renal and vascular end-stage disease (PREVEND) cohort. BCAA concentrations were determined by nuclear magnetic resonance spectroscopy in 6244 subjects, among whom 301 cases of T2DM were ascertained during a mean follow-up period of 7.5 years. High levels of BCAAs were confirmed as previously identified predictive biomarkers of IR and T2DM. This association was independent of multiple risk factors, HOMA-IR and β cell function (Flores-Guerrero et al. 2018)
The association between the level of circulating BCAAs, insulin resistant obesity, and T2DM prompted consideration of BCAA levels as a predictor for future IR or T2DM in order to develop screening PFAA-based tests. Analysis of general health check-up data from 8070 subjects revealed that the amino acid balance of precipitants that developed diabetes within 4 years was similar to that of individuals with diabetes, suggesting that changes in amino acid metabolism may occur prior to the onset of diabetes. Based on these findings, an index known as the AminoIndex LifeStyle diseases (AILS) test was developed. The obtained model was created as a multivariate formula using plasma AA levels, which correlate to visceral fat area as an indicator of prediabetic visceral fat accumulation. Plasma AA levels of asparagine, glycine, alanine, valine, tyrosine, and tryptophan levels were included in the AILS (risk of diabetes) formula derived in this study. Alanine, valine, tyrosine, and tryptophan levels were significantly higher in individuals who developed diabetes within 4 years compared with levels in those who were not diagnosed with diabetes during this period, while levels of glycine were significantly lower. The AILS test was investigated whether its values normalize with such interventions as dietary and exercise counseling for 3 months. AILS values decreased significantly in individuals who managed to reduce body weight and waist circumference (Tochikubo et al. 2016), suggesting that early risk assessment using AILS could support early interventions in at-risk populations (Nagao and Kimura 2020). After commercialization in 2011, tests based on PFAAs were adopted in over 1500 clinics and hospitals in Japan, and numerous clinician-led studies have been performed to validate these tests. Evidence is accumulating that the features of AILS include its ability to assess the risk for diabetes, suggesting it can be used for diabetes prediction (Yamakado 2018).
Amino Acids Metabolic Intermediates As Markers for Insulin Resistance and Diabetes
Many recent studies suggest that not only BCAAs, but also BCAA catabolic enzymes and metabolic intermediates may play a key role in determining the relationship between BCAAs and other amino acids, and IR (Biswas et al. 2019). Obesity-related increase in BCAA levels may be the result of changes in amino acid metabolism, particularly decreased rates of their oxidation in adipose tissue (She et al. 2007; Lackey et al. 2013; Nagao and Kimura 2020; White and Newgard 2019). Recent metabolomics studies on diabetes have identified changes in plasma concentrations of BCAA-derived branched-chain keto acids, short branched-chain fatty acids, and various acylcarnitines as new entities with predictive qualities (Giesbertz and Daniel 2016).
Altered levels of BCAA-catabolic intermediates was shown to influence metabolic maladaptations during obesity and IR (Gall et al. 2010). Circulating BCKA and its association with heightened fasting plasma glucose and IR have been established by the Relationship of Insulin Sensitivity to Cardiovascular Risk (RISC) study group (Gall et al. 2010). Elevated plasma BCKAs were also confirmed by a few studies on mice (Lian et al. 2015).
Other intermediates related to BCAA catabolism are long-chain monomethyl and odd-numbered fatty acids. They can be derived from an odd-chain starter CoA with chain elongation in the fatty acid synthase complex. Monomethyl branched-chain fatty acids (mmBCFA) content in adipose tissue was reduced in obese compared to lean subjects, increased after weight loss in obese individuals, and correlated positively with insulin sensitivity (Su et al. 2015).
Val-derived metabolite, 3-hydroxyisobutyrate (3-HIB), is produced in skeletal muscle in response to forced expression of Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α). 3-HIB, secreted from muscle cells is shown to regulate trans-endothelial fatty acid transport (White and Newgard 2019). Circulating 3-HIB is positively associated with blood glucose levels in diabetic patients, suggesting that this metabolite may promote excessive lipid accumulation and impaired insulin action in skeletal muscle (White and Newgard 2019). In overweight and obese subjects, lower circulating 3-HIB levels, but not BCAA, were associated with metabolic improvement after weight loss (Haufe et al. 2017; Biswas et al. 2019) and were also correlated with insulin-stimulated glucose utilization in older obese women (Harris et al. 2017; Biswas et al. 2019). These results indicate that 3-HIB may serve as a signaling metabolite in IR and T2DM.
Interestingly, elevated plasma levels of β-aminoisobutyric acid (BAIBA) due to enhanced valine catabolism have also been shown to increase with exercise and are inversely correlated with such metabolic risk factors as fasting glucose, insulin, HOMA-IR, triglycerides, and total cholesterol in a randomized large human cohort enrolled in the longitudinal Framingham Heart Study (Roberts et al. 2014). BAIBA is identified as a novel small molecule myokine that increases brown adipocyte-specific genes expression in white adipose tissue and fatty acid β-oxidation in hepatocytes, both in vitro and in vivo, through a PPARα-mediated mechanism. Moreover it was shown to induce a brown adipose-like phenotype in human pluripotent stem cells, and improve glucose homeostasis in mice (Roberts et al. 2014; Biswas et al. 2019). Thus, BAIBA may contribute to exercise-induced protection against metabolic diseases (Roberts et al. 2014).
Newgard et al. showed that BCAAs-derived odd numbered 3- and 5-carbon acylcarnitines have a predictive value for the development of diabetes (Newgard et al. 2009). Acylcarnitines present in plasma and urine may reflect defective BCAAs mitochondrial oxidation. Various acylcarnitines derived from BCAA catabolism may be associated with IR and T2DM (Giesbertz and Daniel 2016). Mai et al. reported that alterations in serum levels of several acylcarnitines, in particular tetradecenoylcarnitine (C14:1), tetradecadienylcarnitine (C14:2), octadecenoylcarnitine (C18:1), and malonylcarnitine/hydroxybutyrylcarnitine (C3DC+C4OH) are correlated not only with T2DM but also with prediabetic states (Mai et al. 2013).
Lysine degradation product, 2-aminoadipic acid (2-AAA) was most strongly associated with the risk of diabetes development in the Framingham Heart Study (Wang et al. 2013). Participants with 2-AAA levels in the top quartile had greater than a fourfold risk of developing diabetes during the mean 12 years follow-up period. Administration of 2-AAA led to a reduction in fasting plasma glucose levels in mice fed both the standard chow and high fat diets. Moreover, treatment with 2-AAA increased insulin secretion from the pancreatic β cell line as well as murine and human islets. Our results suggest that 2-AAA is a diabetes risk marker and a potential modulator of glucose homeostasis in humans (Wang et al. 2013; Chen and Gerszten 2020).
A disadvantage of these new reporter molecules compared to BCAAs is the lower plasma and tissue levels of several species, resulting in higher analytical variation, with some compounds also less stable (Giesbertz and Daniel 2016).
Conclusions
Beyond their contribution as fundamental building blocks of life, amino acids play a key role in various physiological as well as pathological processes. Recent evidences demonstrate that elevated amino acids, especially BCAA levels, are associated with a number of pathologies, including: obesity, IR, T2DM, and CVD. Therefore, the measurement and monitoring of circulating AA levels in biological fluids could represent a promising method for early detection of the disease and may provide a means of preventing its development. This chapter reviews the latest findings regarding the use of plasma/serum FAAs profiles as novel useful biomarkers in the identification of people at risk of T2DM before overt symptoms as well as focuses on potential targets relating to their signaling pathways, and metabolism that broadens our understanding of their role in insulin resistance and diabetes.
Applications to Prognosis, Other Diseases, or Conditions
In this chapter changes in circulating AAs levels and their correlations with IR, prediabetic state, and T2DM have been reviewed. Several studies have been explored in order to identify and integrate AAs metabolite biomarkers proposed so far. A large body of evidence shows that AAs, especially BCAAs, are associated with obesity, IR, prediabetic state, and T2DM as well other pathological conditions, including cancers, CVD, liver disease, chronic kidney disease (CVD), and ischemic stroke, highlighting the potential use of AAs profiling as a valuable diagnostic tool to access the risk of disease development. The use of AAs as novel disease biomarkers in clinical practice may improve treatment strategies.
Mini Dictionary of Terms
-
HOMA-IR – Homeostatic Model Assessment (HOMA) is a method for assessing β-cell function and insulin IR from basal (fasting) glucose and insulin or C-peptide concentrations. It approximates insulin resistance.
-
INSULIN RESISTANCE – A pathological condition in which cells fail to respond normally to the insulin hormone.
-
BIOMARKER – A measurable biological feature that can distinguish normal condition from pathological condition or indicate a response to an administered therapeutic drug.
-
METABOLOMICS – A scientific discipline devoted to study changes in metabolome by measurement of small molecule metabolites. Nuclear magnetic resonance (NMR) spectroscopy or mass spectrometry (MS), coupled with liquid chromatography (LC-MS), gas chromatography (GC-MS), or capillary electrophoresis (CE-MS), is usually used to measure metabolites. Not only primary metabolites, but also the substrates and products of metabolism are measured. Metabolites can be measured in a targeted (metabolic profiling) or an untargeted (metabolic fingerprinting) manner.
-
T2DM – Most commonly diagnosed type of diabetes, characterized by abnormal glucose and lipid metabolism, resulting from resistance to the effects of insulin and insufficient response to the secretion of this hormone. As this disease progresses, there may also occur a partial β-cell insufficiency and deficiency in insulin production.
Key Facts of Amino acids
A number of studies suggest that BCAAs supplementation or BCAA-rich diets has potential benefits for promoting lean body mass in obesity or catabolic disorders. Elevated BCAAs levels have been reported to improve body composition, glycemia levels, and to increase satiety for weight loss (Lynch and Adams 2014).
-
In contrast to the potential health-promoting effects of BCAAs under conditions of negative energy balance, higher BCAAs intake may lead to adverse effects on the development of IR (Nie et al. 2018). Increased levels of branched-chain amino acids in plasma, serum, and also in urine are associated with obesity, IR, and diabetes, in particular T2DM.
-
Branched-chain amino acids: valine, leucine, isoleucine, and aromatic amino acids, that is tyrosine and phenylalanine, have been demonstrated as the most consistent metabolite biomarkers for diabetes, in particular T2DM.
-
Elevated BCAA levels have often been shown to predict the development of T2DM well in advance of its actual occurrence, what is very interesting from a diagnostics standpoint.
-
BCAAs may not only have a “reporter quality” but may also contribute to the development of IR and T2DM.
Summary Points
-
In the current medical situation, DM is a worldwide epidemic. Diabetes, particularly type 2 portends a poor prognosis and shortens the life expectancy of diabetic patients. Moreover, it imposes a heavy financial strain on health care systems everywhere.
-
Early screening and testing of people at risk is the best approach to control the increasing numbers of diabetes occurrences, and it is most effective to recognize the early stages of DM before major systematic damage occurs.
-
Various prospective, case-controlled and nested studies on the subjects of different ethnic origins shown that elevated levels of BCAAs and other AAs are associated with the prediabetic state, IR, and T2DM.
-
Amino acids quantification in biological material, primarily in plasma, could be a valuable prognostic tool for determining metabolic abnormalities leading to diabetes.
-
The activation of mTORC1 by BCAAs has been suggested to trigger IR, and subsequent metabolic disorders.
-
Deficiencies in BCAAs metabolism referred as dysmetabolism lead to increased BCAA levels in obesity and/or diabetes. It can also induce the accumulation of possibly toxic intermediates, such as branched-chain keto acids, branched-chain fatty acids, and various acylcarnitines that impair cellular function(s) and may induce IR.
-
Various intermediates of BCAAs are now considered as predictive markers that reflect IR and diabetes development.
The second possible mechanism is referred as BCAAs . Deficiency in BCAAs metabolism may induce the accumulation of toxic intermediates, such as branched-chain keto acids, branched-chain fatty acids, and various acylcarnitines that promotes cellular dysfunction, which leads to poorer insulin sensitivity of skeletal muscles.
Accumulation of BCAA break-down products may also interfere with proper mitochondrial function. Overloading of mitochondria with lipid substrates leads to oxidative stress and impaired insulin action contributing to insulin resistance.
Abbreviations
- 2-AAA:
-
2-aminoadipic acid
- 2-h PG:
-
2-h plasma glucose test
- 3-HIB:
-
3-hydroxyisobutyrate
- AAAs:
-
Aromatic amino acids
- AAs:
-
Amino acids
- AILS:
-
AminoIndex LifeStyle diseases test
- AKT:
-
Protein kinase B, PKB
- Ala:
-
Alanine
- Arg:
-
Arginine
- Asn:
-
Asparagine
- Asp:
-
Aspartic acid
- BAIBA:
-
β-aminoisobutyric acid
- BCAAs:
-
Branched-chain amino acids
- BCKA:
-
Branched-chain α-ketoacid
- BCKDC:
-
Branched-chain α-ketoacid dehydrogenase complex
- BCKDH:
-
Branched-chain α-ketoacid dehydrogenase
- BHBA:
-
3-hydroxybutyrate
- BMI:
-
Body Mass Index
- Cit:
-
Citrulline
- CoA:
-
Coenzyme A
- CVD:
-
Cardiovascular disease
- Cys:
-
Cysteine
- DAG:
-
Diacylglycerol
- DM:
-
Diabetes mellitus
- FAAs:
-
Free amino acids
- FFAs:
-
Free fatty acids
- FGF21:
-
Fibroblast growth factor 21
- FHS:
-
Framingham Heart Study
- FOXO:
-
Forkhead Box O transcription factor
- FPG:
-
Fasting plasma glucose
- GC:
-
Gas chromatography
- GDM:
-
Gestational Diabetes mellitus
- GDR:
-
Glucose disposal rate
- Gln:
-
Glutamine
- Glu:
-
Glutamic acid
- Gly:
-
Glycine
- GSK-3:
-
Glycogen synthase kinase-3
- HbA1c:
-
Glycated hemoglobin
- HECP:
-
Hperinsulinemic-euglycemic clamp procedure
- His:
-
Histidine
- HOMA-IR:
-
Homeostatic Model Assessment of Insulin Resistance
- IDF:
-
International Diabetes Federation
- IGF:
-
Insulin-like growth factor
- IGT:
-
Impaired glucose tolerance
- Ile:
-
Isoleucine
- Ins120 min:
-
2-h post-challenge insulin
- IR:
-
Insulin Resistance
- IRAS:
-
Insulin Resistance Atherosclerosis Study
- IRS-1:
-
Insulin receptor substrate 1
- JNK:
-
c-Jun N-terminal kinase
- LC:
-
Liquid chromatography
- Leu:
-
Leucine
- L-GPC:
-
linoleoyl- glycerophosphocholine
- LPC:
-
Lysophosphatidylcholine
- MDC:
-
Malmö Diet and Cancer Study
- MetS:
-
Metabolic syndrome
- METSIM:
-
Metabolic Syndrome in Men Study
- mmBCFA:
-
Monomethyl branched-chain fatty acids
- MS:
-
Mass spectrometry
- mTORC1:
-
Mammalian target of rapamycin complex 1
- NEFAs:
-
Non-esterified fatty acids
- NGT:
-
Normal glucose tolerance
- NMR:
-
Nuclear magnetic resonance
- OGTT:
-
Oral glucose tolerance test
- Orn:
-
Ornithine
- PCs:
-
Phosphatidylcholines
- PFAAs:
-
Plasma-free amino acids
- Phe:
-
Phenylalanine
- PPARα:
-
Peroxisome proliferator-activated receptor α
- Pro:
-
Proline
- QMDiab:
-
Qatar Metabolomics Study on Diabetes
- RISC:
-
Relationship of Insulin Sensitivity to Cardiovascular Risk study
- ROS:
-
Reactive oxygen species
- RQ:
-
Resting respiratory quotient
- SABRE:
-
Southall And Brent REvisited Study
- Ser:
-
Serine
- T1DM:
-
Type 1 Diabetes mellitus
- T2DM:
-
Type 2 Diabetes mellitus
- TCA:
-
Tricarboxylic acid cycle
- TKR:
-
Tyrosine kinase receptor
- Trp:
-
Tryptophan
- TüF:
-
Tübingen Family study for T2DM
- Tyr:
-
Tyrosine
- UCD-T2D:
-
University of California-Davis T2DM rat model
- Val:
-
Valine
- VFA:
-
Visceral fat area
- α-HB:
-
α-hydroxybutyrate
- α-KB:
-
α-ketobutyrate
References
Adams SH, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid β-Oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American Women. J Nutr. 2009;139(6):1073–81. https://doi.org/10.3945/JN.108.103754.
Ahola-Olli AV, et al. Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia. 2019;62(12):2298–309. https://doi.org/10.1007/s00125-019-05001-w.
Al-Abbasi FA. Trend analysis of the correlation of amino acid plasma profile with glycemic status in Saudi diabetic patients. J Adv Res. 2012;3(4):305–13. https://doi.org/10.1016/j.jare.2011.10.001.
Allam-Ndoul, B. et al. Associations between branched chain amino acid levels, obesity and cardiometabolic complications. Integr Obes Diab. 2015;1(6). https://doi.org/10.15761/IOD.1000134.
Association AD. Standards of medical care in diabetes – 2016 abridged for primary care providers. Clin Diabetes. 2016;34(1):3–21. https://doi.org/10.2337/diaclin.34.1.3.
Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes – 2018. Diabetes Care. 2018;41(Supplement 1):S13–27. https://doi.org/10.2337/DC18-S002.
Bi X, Henry CJ. Plasma-free amino acid profiles are predictors of cancer and diabetes development. Nutr Diabetes. 2017;7(3):e249:1–9. https://doi.org/10.1038/nutd.2016.55.
Biswas D, Duffley L, Pulinilkunnil T. Role of branched-chain amino acid–catabolizing enzymes in intertissue signaling, metabolic remodeling, and energy homeostasis. FASEB J Wiley and Sons Inc. 2019:8711–31. https://doi.org/10.1096/fj.201802842RR.
Chen ZZ, Gerszten RE. Metabolomics and proteomics in type 2 diabetes. Circ Res. 2020;126(11):1613–27. https://doi.org/10.1161/CIRCRESAHA.120.315898.
Chen T, et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci Rep. 2016;6(1):1–8. https://doi.org/10.1038/srep20594.
Chen S, et al. Serum amino acid profiles and risk of type 2 diabetes among Japanese adults in the Hitachi Health Study. Sci Rep. 2019;9(1):1–9. https://doi.org/10.1038/s41598-019-43431-z.
Cheng S, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125(18):2222. https://doi.org/10.1161/CIRCULATIONAHA.111.067827.
“Diagnosis and Classification of Diabetes Mellitus”. Diabetes Care. American Diabetes Association Inc.; 2004. p. s5–s10. https://doi.org/10.2337/diacare.27.2007.s5.
Felig P, Marliss E, Cahill GF Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281(15):811–6. https://doi.org/10.1056/NEJM196910092811503.
Ferrannini E, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62(5):1730. https://doi.org/10.2337/DB12-0707.
Fiehn O, et al. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American Women. PLoS One. 2010;5(12):1–10. https://doi.org/10.1371/JOURNAL.PONE.0015234.
Floegel A, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62(2):639. https://doi.org/10.2337/DB12-0495.
Flores-Guerrero JL, et al. Plasma branched-chain amino acids and risk of incident type 2 diabetes: results from the PREVEND Prospective Cohort Study. J Clin Med. 2018;7(12):513. https://doi.org/10.3390/JCM7120513.
Freitas PAC, Ehlert LR, Camargo JL. Glycated albumin: a potential biomarker in diabetes. Arch Endocrinol Metabol. Sociedade Brasileira de Endocrinologia e Metabologia; 2017;61:296–304. https://doi.org/10.1590/2359-3997000000272.
Gall WE, et al. α-Hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5(5) https://doi.org/10.1371/JOURNAL.PONE.0010883.
Gan WZ, et al. Omics-based biomarkers in the diagnosis of diabetes. J Basic Clin Physiol Pharmacol De Gruyter. 2020; https://doi.org/10.1515/jbcpp-2019-0120.
George J. Branched chain amino acids: causal or predictive of type 2 diabetes. undefined [Preprint]. 2017.
Giesbertz P, Daniel H. Branched-chain amino acids as biomarkers in diabetes. Curr Opin Clin Nutr Metab Care Lippincott Williams and Wilkins. 2016:48–54. https://doi.org/10.1097/MCO.0000000000000235.
Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diabetes Rep. 2009;9(3):208–14. https://doi.org/10.1007/S11892-009-0034-5.
Guasch-Ferré M, et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care. 2016;39(5):833–46. https://doi.org/10.2337/DC15-2251.
Hameed A, et al. Altered metabolome of lipids and amino acids species: a source of early signature biomarkers of T2DM. J Clin Med. 2020;9(7):2257. https://doi.org/10.3390/jcm9072257.
Harris L-ALS, et al. Alterations in 3-Hydroxyisobutyrate and FGF21 metabolism are associated with protein ingestion–induced insulin resistance. Diabetes. 2017;66(7):1871. https://doi.org/10.2337/DB16-1475.
Haufe S, et al. Branched-chain amino acid catabolism rather than amino acids plasma concentrations is associated with diet-induced changes in insulin resistance in overweight to obese individuals. Nutr Metab Cardiovasc Dis. 2017;27(10):858–64. https://doi.org/10.1016/J.NUMECD.2017.07.001.
Ianni, F. et al. Branched-chain amino acids as potential diagnostic and prognostic disease biomarkers. Int J Clin Res Trials. 2017;2(1). https://doi.org/10.15344/2456-8007/2017/112.
International Diabetes Federation (IDF). Diabetes Atlas. 7th Edition, International Diabetes Federation, Brussels, Belgium. – References – Scientific Research Publishing (no date). 2015. https://www.scirp.org/(S(vtj3fa45qm1ean45vvffcz55))/reference/ReferencesPapers.aspx?ReferenceID=2085700. Accessed 15 June 2021.
Kubota T, Kubota N, Kadowaki T. Imbalanced insulin actions in obesity and type 2 diabetes: key mouse models of insulin signaling pathway. Cell Metab. 2017;25(4):797–810. https://doi.org/10.1016/J.CMET.2017.03.004.
Lackey DE, et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304(11):E1175. https://doi.org/10.1152/AJPENDO.00630.2012.
Lee CC, et al. Branched-Chain amino acids and insulin metabolism: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care. 2016;39(4):582. https://doi.org/10.2337/DC15-2284.
Lian K, et al. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes. 2015;64(1):49–59. https://doi.org/10.2337/DB14-0312.
Lopez MJ, Mohiuddin SS. Biochemistry, essential amino acids. StatPearls [Preprint]. 2021.
Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10(12):723–36. https://doi.org/10.1038/nrendo.2014.171.
Magnusson M, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. 2013;34(26):1982–9. https://doi.org/10.1093/EURHEARTJ/EHS424.
Mai M, et al. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS One. 2013;8(12):e82459. https://doi.org/10.1371/JOURNAL.PONE.0082459.
McCormack SE, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr obes. 2013;8(1):52. https://doi.org/10.1111/J.2047-6310.2012.00087.X.
Nagao K, Kimura T. Use of plasma-free amino acids as biomarkers for detecting and predicting disease risk. Nutr Rev. 2020;78(Supplement_3):79–85. https://doi.org/10.1093/NUTRIT/NUAA086.
Nagata C, et al. Branched-chain amino acid intake and the risk of diabetes in a Japanese Community The Takayama Study. Am J Epidemiol. 2013;178(8):1226–32. https://doi.org/10.1093/AJE/KWT112.
Nakamura H, et al. Plasma amino acid profiles are associated with insulin, C-peptide and adiponectin levels in type 2 diabetic patients. Nutr Diabetes. 2014;4:133. https://doi.org/10.1038/nutd.2014.32.
Newgard CB, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311. https://doi.org/10.1016/J.CMET.2009.02.002.
Nie C, et al. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci MDPI AG. 2018; https://doi.org/10.3390/ijms19040954.
Palmer ND, et al. Metabolomic profile associated with insulin resistance and conversion to diabetes in the Insulin Resistance Atherosclerosis Study. J Clin Endocrinol Metab. 2015;100(3):E463. https://doi.org/10.1210/JC.2014-2357.
Rawshani A, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376(15):1407–18. https://doi.org/10.1056/NEJMOA1608664.
Roberts LD, et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19(1):96. https://doi.org/10.1016/J.CMET.2013.12.003.
Saeedi P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. https://doi.org/10.1016/j.diabres.2019.107843.
Seibert R, et al. Relationship between insulin resistance and amino acids in women and men. Phys Rep. 2015;3(5):e12392. https://doi.org/10.14814/PHY2.12392.
Shah SH, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55(2):321. https://doi.org/10.1007/S00125-011-2356-5.
She P, et al. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. 2007;293(6):1552–63. https://doi.org/10.1152/AJPENDO.00134.2007.
Shi L, et al. Plasma metabolites associated with type 2 diabetes in a Swedish population: a case–control study nested in a prospective cohort. Diabetologia. 2018;61(4):849. https://doi.org/10.1007/S00125-017-4521-Y.
Shin AC, et al. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab. 2014;20(5):898. https://doi.org/10.1016/J.CMET.2014.09.003.
Stančáková A, et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish Men. Diabetes. 2012;61(7):1895. https://doi.org/10.2337/DB11-1378.
Su X, et al. Adipose tissue monomethyl branched chain fatty acids and insulin sensitivity: effects of obesity and weight loss. Obesity (Silver Spring, Md). 2015;23(2):329. https://doi.org/10.1002/OBY.20923.
Tai ES, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53(4):757. https://doi.org/10.1007/S00125-009-1637-8.
Tillin T, et al. Diabetes risk and amino acid profiles: cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia. 2015;58(5):968. https://doi.org/10.1007/S00125-015-3517-8.
Tochikubo O, et al. Weight loss is associated with plasma free amino acid alterations in subjects with metabolic syndrome. Nutr Diabetes. 2016;6(2):e197. https://doi.org/10.1038/NUTD.2016.5.
Tricò D, et al. Elevated α-Hydroxybutyrate and branched-chain amino acid levels predict deterioration of glycemic control in adolescents. J Clin Endocrinol Metabol. 2017;102(7):2473–81. https://doi.org/10.1210/JC.2017-00475.
Vamos EP, et al. Changes in the incidence of lower extremity amputations in individuals with and without diabetes in England between 2004 and 2008. Diabetes Care. 2010;33(12):2592. https://doi.org/10.2337/DC10-0989.
Wang TJ, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–53. https://doi.org/10.1038/nm.2307.
Wang TJ, et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Investig. 2013;123(10):4309–17. https://doi.org/10.1172/JCI64801.
Wang-Sattler R, et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol. 2012;8(1):615. https://doi.org/10.1038/MSB.2012.43.
White PJ, Newgard CB. Branched-chain amino acids in disease. Science. 2019;363(6427):582–3. https://doi.org/10.1126/SCIENCE.AAV0558.
Würtz P, et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. 2013;36(3):648–55. https://doi.org/10.2337/DC12-0895.
Xie G, et al. The metabolite profiles of the obese population are gender-dependent. J Proteome Res. 2014;13(9):4062–73. https://doi.org/10.1021/PR500434S.
Yamakado M. Technology for lifestyle-related disease risk screening. Ningen Dock Int. 2018;5(1):3–14.
Yamakado M, et al. Plasma amino acid profile is associated with visceral fat accumulation in obese Japanese subjects. Clin Obesity. 2012;2(1–2):29–40. https://doi.org/10.1111/J.1758-8111.2012.00039.X.
Yamakado M. et al. Plasma free amino acid profiles predict four-year risk of developing diabetes, metabolic syndrome, dyslipidemia, and hypertension in Japanese Population. Sci Rep. 2015;5. https://doi.org/10.1038/SREP11918.
Yoon M.-S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients. 2016;8(7). https://doi.org/10.3390/NU8070405.
Yousri NA, et al. A systems view of type 2 diabetes-associated metabolic perturbations in saliva, blood and urine at different timescales of glycaemic control. Diabetologia. 2015;58(8):1855. https://doi.org/10.1007/S00125-015-3636-2.
Zabielski P, et al. The effect of high-fat diet and inhibition of ceramide production on insulin action in liver. J Cell Physiol. 2019;234(2):1851–61. https://doi.org/10.1002/JCP.27058.
Zeng M, et al. GC–MS based plasma metabolic profiling of type 2 Diabetes Mellitus. Chromatographia. 2009;69(9):941–8. https://doi.org/10.1365/S10337-009-1040-0.
Zhao J, et al. Novel metabolic markers for the risk of diabetes development in American Indians. Diabetes Care. 2015;38(2):220. https://doi.org/10.2337/DC14-2033.
Zhao Q, et al. Exploring potential biomarkers and determining the metabolic mechanism of type 2 diabetes mellitus using liquid chromatography coupled to high-resolution mass spectrometry. RSC Adv. 2017;7(70):44186–98. https://doi.org/10.1039/c7ra05722a.
Zhao H, et al. Branched-chain amino acids exacerbate obesity-related hepatic glucose and lipid metabolic disorders via attenuating Akt2 signaling. Diabetes. 2020;69(6):1164–77. https://doi.org/10.2337/DB19-0920.
Zheng Y, et al. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol. 2016;45(5):1482–92. https://doi.org/10.1093/IJE/DYW143.
Zhou M, et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes. 2019;68(9):1730–46. https://doi.org/10.2337/db18-0927.
Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91(1):258S–61S. https://doi.org/10.3945/AJCN.2009.28449C.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this entry
Cite this entry
Czajkowska, A., Hameed, A., Galli, M., Ijaz, M.U., Kretowski, A., Ciborowski, M. (2023). Altered Metabolome of Amino Acids Species: A Source of Signature Early Biomarkers of T2DM. In: Patel, V.B., Preedy, V.R. (eds) Biomarkers in Diabetes. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Cham. https://doi.org/10.1007/978-3-031-08014-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-08014-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-08013-5
Online ISBN: 978-3-031-08014-2
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences