Abstract
Hyaluronic acid is one of the most valuable polysaccharides due to its enormous and unique biofunctionality in the human body, which renders it of highest interest for pharmaceutical and cosmeceutical applications. Within this chapter, we will give an overview of the development of hyaluronic acid as a commercial product, including the various origins, recombinant production, optimization of the production process as well as purification strategies. The main scope will be on microbial production of hyaluronic acid, the biosynthetic pathway, the different fermentation processes and strategies for overproduction, as well as optimized downstream processing. We will also give an overview of the commercial producers and their current production processes as well as the patents which are currently active in that field. In addition, we will present and discuss applications in the field of cosmetics, pharmaceuticals as well as material science. In sum, we will give a current and comprehensive overview of hyaluronic acid production in the year 2021.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microbial polysaccharides
- Hyaluronic acid
- Metabolic engineering
- Heterologous production
- Purification process
- History of hyaluronic acid
- Biosynthesis
- Hyaluronan
- In vitro production
- Rheology
1 Introduction
As native polysaccharide, hyaluronan or hyaluronic acid (HA) is present in various parts of the animal and human body, but can also be found in the surrounding environment of microbial strains. Based on its nowadays known enormous health-promoting properties and biofunctionality, such as the unique water-binding capacity, it arose a high interest even from the beginning on. From the historical point of view, hyaluronic acid was first mentioned as an unusual uronic acid-containing carbohydrate polymer with an extremely high molecular weight as extracted from the vitreous of bovine eyes in the year 1934, which also resulted in the name, based on hyalos (stands for glass in the Greek language) and uran (an abbreviation for uronic acid) (Meyer and Palmer 1934). Soon after its discovery, the unique properties of this new biopolymer were described to be different from other glycosaminoglycans (GAGs), and its monomer composition was described to be composed of uronic acids and amino sugars, as well as traces of pentose (Meyer and Palmer 1934).
Over the next 10 years, HA was isolated from various animal organs, such as joint fluid, the umbilical cord, and nowadays it is known that HA can be extracted from almost all vertebrate tissues (Cowman et al. 2015a). In the year 1937, HA was for the first time extracted from the capsules of Streptococci groups A and C, which proved that it is also a microbial polysaccharide (Kendall et al. 1937). In the year 1948, the first report of the kinetics of enzymatic hydrolysis of HA was published (Dorfman 1948) and 3 years later, the first data about the structure of HA in aqueous solutions was published, describing the relationship between viscosity and velocity gradients to be dependent on higher concentrations of HA (Ogston and Stanier 1951). A random coil (irregular helical) confirmation of HA was determined via light scattering in the year 1955 for the first time (Laurent and Gergely 1955). In 1954, the disaccharide repeating unit of HA was reported, based on enzymatic cleavage experiments by the use of hyaluronidase from the Streptococcus, revealing that the disaccharide unit is composed of glucuronic acid and N-acetyl-glucosamine, without the presence of a pentose (Linker and Meyer 1954). In addition, the molecular weight (Mw) of the repeating disaccharide unit was determined to consist of 397 Da, which reinforced the formerly hypothesized disaccharide structure. Unlike sulfated polysaccharides, some of the initial proof of HA’s ability to interact with living cells came from the observation that HA accelerates cell growth. It has also been observed that HA initiates some cell aggregation. This was the first indication of a unique binding of the polysaccharide to the cell surface and thus its highly valuable pharmacological as well as cosmeceutical properties. In addition, the different observed viscosities of HA solutions in presence of different inorganic salts caused scientific interest. In contrast to many other polysaccharides, the highest viscosity was observed in distilled water, and that phenomenon was proposed to be related to pH and ionic strength of the solution. This kind of behavior has been described for the first time by Fuoss et al. for polyelectrolytes (Fuoss 1948) and is still now a state of common knowledge. Fundamental research on the physicochemical properties of HA has already begun in the year 1951 with the study of Balazs et al. (Balazs 1979; Balazs and Laurent 1951). It also has been observed quite early that the high viscosity of HA solutions can be completely destroyed by illumination with UV light (Hvidberg et al. 1959) or exposure to X-rays in 1957 (Caputo 1957), indicating the quite unstable chemical structure of HA towards various external influences or stresses. Complete degradation of the chemical structure of HA has, later on, been described for gamma radiation exposure as well as low initial levels of ionization radiation in general (Kim et al. 2008b).
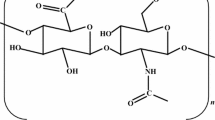
The native function as a highly valuable GAG in various parts of the human and animal body was elucidated to play diverse functions in structural and physiological maintenance of tissues, as well as the mediation of cell behaviors. It was shown to contribute to keeping tissue homeostasis and to function as a cell-signaling molecule via interaction with a variety of binding proteins. Based on its remarkable viscoelastic behavior, it also functions as a lubricant for the joints (Abatangelo et al. 2020; Dicker et al. 2014; Laurent and Fraser 1992). By that, this polysaccharide shows health-promoting properties and a high biofunctionality as well as an enormous water-binding capacity. Based on these highly valuable properties, HA is applied in medical as well as cosmetical applications, making it one of the most valuable and costly cosmeceutical polysaccharides. From the molecular structure (Donati et al. 2001), HA is a high molecular weight polymer of linear glycosaminoglycan which consists of glucuronic acid (GlcA) and N-acetylglucosamine (GlcNAc) repeats linked via β-(1–4) and β-(1–3)-glycosidic bonds (Fig. 1). By that, it is highly hydrophilic and charged, which renders its remarkable osmotic swelling capacity (Chong et al. 2005). At the beginning of its commercial production, HA was mainly obtained from the extraction of animal tissues and fluids, such as rooster combs, cattle vitreous humor, and bovine synovial fluid (Boeriu et al. 2013). Amongst most natural resources, rooster combs contain the highest content of HA with about 7.5 g/L (Laurent and Fraser 1992). Despite the high yields, animal-derived HA raised and experienced some concerns due to the risk of contaminations that can occur in the form of protein, nucleic acid, bacterial, or viral material. By that, intensive purification is essential to achieve a certain level of HA purity and, as consequence, HA production costs are much higher compared to many other microbial polysaccharides and polymers (Freitas et al. 2011). Therefore, the development of safer and more sustainable sources and production routes is of high importance. Over the years, HA production has gradually shifted towards animal-free methods by means of microbial cell factories. Microbially, HA is naturally produced by some pathogens like Streptococcus sp. and Pasteurella multocida (DeAngelis and Achyuthan 1996; Sze et al. 2016). These bacteria use HA for encapsulating their cells by what they could escape the host’s immune system, as HA masquerades the cells and protects them from inducing the immune response (Cress et al. 2014). From an industrial point of view, commercial HA production from these bacteria is complicated due to their pathogenic nature, which categorizes them as risk class 2 organisms. By that, large-scale industrial production is hampered by high regulatory hurdles and cost-intensive safety issues as well as purification procedures, which results in the high price of currently available HA products (Freitas et al. 2011).
2 Biosynthesis Pathway
Despite the unique properties, HA itself is a more “simple” polymer in regard to its molecular structure when compared to the other highly diverse microbial polysaccharides. Its biosynthesis follows the synthase-based pathway, which normally is used for the production of homopolysaccharides, which only consist of one type of carbohydrate monomers in their polymeric structure, such as cellulose or alginate (Schmid et al. 2015). Cellulose is a real homopolysaccharide that only consists of β-(1–4) linked glucose units and can be produced by eukaryotes (algae, plants) as well as prokaryotic bacteria via the synthase-based biosynthetic pathway (Rehm 2010). In the case of alginate biosynthesis, the initial polysaccharide is formed by polymerization of solely mannuronic acid (M) residues via the alginate synthase (Alg8 in Pseudomonas aeruginosa), from which some of them are converted towards guluronic acid (G) by the action of various epimerases, thus finally resulting in the typical M:G ratio of the different alginate types (Rehm and Valla 1997). In the case of HA synthesis, the HA synthase directly uses the two building blocks β-d-glucuronic acid and β-d-N-acetyl-glucosamine and alternating links them via β-(1–4) and β-(1–3)-glycosidic bonds.
Two classes of HA synthase (HAS) have been described today (Table 1). Most of the known HAS belong to Class I (Weigel 2015), while the HAS from P. multocida is the only member of the Class II (Weigel and DeAngelis 2007). Furthermore, the latter is the only known HAS coming from Gram-negative bacteria. In contrast to the Class I, Class II HAS is not an integral membrane protein but a membrane-anchored protein instead and has two protein domains which each has a glycosyltransferase activity. On the other hand, The Class I HAS is defined as a single domain integral membrane protein (Weigel 2015). Class I bacterial HAS belongs to the glycosyltransferases and is integrated into the membrane polymerizing the precursor molecules by adding new moieties to the reducing end of the polysaccharide chain (Weigel and DeAngelis 2007). It contain a core of four transmembrane helices which are connected to at least one intracellular loop, in which the consensus sequence of processive glycosyltransferases is included. The same structure is found in cellulose and curdlan synthases (Heldermon et al. 2001; Saxena et al. 1995). A combined glycosyltransferase and translocase activity (Thomas and Brown 2010; Tlapak-Simmons et al. 1999; Weigel and DeAngelis 2007) or inclusion of an HA secreting ABC transporter (Ouskova et al. 2004; Schulz et al. 2007) is hypothesized as a reaction mechanism. But in contrast, cellulose synthase polymerizes at the non-reducing end, which is the main difference to the bacterial HAS. However, the HAS from the frog claw Xenopus laevis was shown to catalyze the polymerization at the non-reducing end (Bodevin-Authelet et al. 2005). The Streptococcal HAS is described to contain six membrane regions, of which four are integral and two are amphipathic, with an additional cytoplasmic domain (Heldermon et al. 2001). The integral membrane regions translocate the growing HA strands by forming a pore in the membrane, and the cytosolic part has the function of a glycosyltransferase domain that is expected to have several activities. These activities are described to include UDP-substrate binding and binding of the disaccharide repeating unit (HA-GlcA and HA-GlcNAc) as well as the two transferase activities to add GlcA and GlcNAc to the growing polymer chain (Weigel 2015).
For the microbial production strains, Streptococcus zooepidemicus represents the best examined organism which provided the most important insights on HA biosynthesis (Sze et al. 2016). The corresponding operon, namely has, consists of five genes: hasA, hasB, hasC, hasD, and hasE. The hasA gene encodes for HAS, the key enzyme in HA biosynthesis. The hasB and hasC genes encode for UDP-glucose dehydrogenase and UDP-glucose pyrophosphorylase, which generate UDP-GlcA. The hasD and hasE genes encode for pyrophosphorylase and phosphoglucoisomerase, which play an essential role in the generation of UDP-GlcNAc (Blank et al. 2008). Different compositions of the has-operon in bacteria have been described. For example, only the hasA, hasB, hasC genes are present in the has-operon of Streptococcus pyogenes, while the precursor encoding genes for UDP-GlcNAc is located in another genomic region (DeAngelis et al. 1993). Streptococcus uberis, for example, carries only hasA and hasB located in the operon while a hasC homolog is present at a distinct locus (Ward et al. 2001). Overall, the biosynthesis of HA follows the synthase-dependent pathway in which the HAS catalyzes not only polymerization, but also translocation and secretion (Weigel 2015). Up to now the complete mechanism of HAS is not clarified based on the complex structure and the integral membrane domains. Just recently, the first three-dimensional atomic-scale model was presented, by which it was able to identify nine HAS-specific sub-structural elements and to elucidate their roles in HA biosynthesis (Agarwal et al. 2019). A combination of in silico modelling and mutation experiments suggested a three-step molecular mechanism for the growing HA chain from the reducing end in combination with overlapping binding sites of UDP-GlcNAc and UDP-GlcA. This 3-step mechanism can be briefly summarized to involve (a) Release of the bound UDP-substrate from the polymer (results in a glycosyl enzyme intermediate), (b) Release of the two bases with catalytic activity caused by the conformational change from UDP-release, (c) Glycosyltransfer reaction ending up in a glycosidic linkage and complete release of the second catalytic base 2. By that insights, a targeted engineering of HAS concerning substrate composition and the Mw comes very close (Fig. 2).
Overview of HA biosynthesis pathway in S. zooepidemicus. In the cell, glucose is converted to glucose-6-phosphate by hexokinase activity, then finally into UDP-glucuronic acid by reaction steps which involve phosphoglucomutase as encoded by the pgm gene, UDP-glucose pyrophosphorylase (hasC) and UDP-glucose dehydrogenase (hasB). On the other hand, phosphoglucoisomerase (hasE) converts the glucose-6-phosphate to fructose-6-phosphate, which finally will be converted into UDP-N-acetyl glucosamine by the amidotransferase (glmS), mutase (glmM), and acetyltransferase-pyrophosphorylase (hasD). The two precursors will be polymerized by the hyaluronic acid synthase (hasA) to finally form hyaluronic acid
3 Rheological Properties
The structure of HA (Fig. 1) allows the formation of several hydrogen bonds within the molecule, which leads to a rod-like extended structure with high rigidity (Khabarov et al. 2014; Scott and Heatley 1999). These structures may form secondary and tertiary structures like twisted ribbons and double helices with other molecule strands (Scott et al. 1991; Scott and Heatley 1999). Depending on both concentration and Mw, these molecules in solution exhibit different viscoelastic properties, from solutions with low viscosity to high viscosity solutions with shear thinning behavior. These effects can be simply explained by the overlapping of the molecules, to form structural networks, leading to these properties. By that, it becomes evident, that smaller molecule chains (low Mw) require higher concentrations for overlapping compared to larger molecules (high Mw) (Dodero et al. 2019). The concentrations at which polymer chains interactions may just occur are called critical overlap concentration c*. For hyaluronan with Mw between 0.9 and 6 MDa values between 10.9 and 0.32 mg mL−1 are reported (Cowman et al. 2015b). These effects lead to great variations in the flow behavior of HA solutions. For example, a 1% solution of high Mw HA (1.1–4.3 MDa) is described as shear thinning with zero shear viscosities of 1500–220,000 mPa s, while these values increase 10.5–12-fold by doubling the concentration to 2% (Bothner and Wik 1987). A more recent study shows similar zero shear viscosities of 900–117,000 mPa s of 1% solutions of HA between 1.1 and 4.0 MDa, while the concentration dependency increases with increasing Mw (Dodero et al. 2019). On the other hand, low Mw HA (150 kDa) exhibits almost Newtonian (i.e., shear rate independent) flow behavior with viscosities of about 40–100 mPa s (Ambrosio et al. 1999). As a viscoelastic material, however, the viscoelastic properties of HA solutions are more important compared to its flow behavior. Due to the entanglements as already described, above certain Mw and concentrations, HA forms pseudo-gel structures, which differ from real gel structures, where the molecules interact via stronger intermolecular interactions, such as ion-mediated interactions or covalent bonds. Considering the properties of pure HA in solution, some studies have been conducted in the form of oscillatory shear experiments, starting as early as 1968. HA shows the behavior of a Maxwell fluid, exhibiting liquid behavior at lower frequencies and gel-like properties at high frequencies, which are separated by a crossover frequency (Gibbs et al. 1968). These first investigations by custom-designed oscillatory Couette type rheometers in 1968 showed a pH-dependency of this crossover frequency, where the lowest crossover frequency was observed at pH 2.5 and attributed to increased chain stiffness at this pH and was recently extended by combining modern oscillatory shear experiments with micro-rheology (dynamic light scattering. This allowed an extension of the initial frequency range of 10−1–101 rad s−1 to 10−2–105 rad s−1, and the observation of the intermediate rubbery region and a second crossover frequency, which are typical for non-crosslinked entangled polymer solutions (Dodero et al. 2019). Despite the importance of understanding the rheological properties of HA at different concentrations and Mw and the availability of highly sensitive and versatile rheometry, there is still a lack of further rheological behaviors like time- and temperature-dependent behavior as most studies focus on the frequency dependency. Another cause of this lack of basic characterization might be found in the recent focus on the characterization of modified HA, HA blends and formulations, as research of HA gets more application-focused and product-driven.
4 Fermentative Production
4.1 Natural Producers
Based on the history of HA production, the native microbial producers are of high interest to replace animal-derived HA production. Most research focuses on their exploitation to produce HA in high yields with certain purity level and Mw, which highly affects its rheological properties and thus applications. Major microbial HA production is obtained from the genus Streptococcus. Since they are known pathogens, high safety measures are required for handling the bacteria, and the HA obtained thereof during fermentation and downstream processing.
Streptococci are facultative anaerobic bacteria that mainly produce lactate as the main fermentation product. The composition of fermentation products changes depending on the conditions applied and therefore optimization of process parameters is essential to intensify HA production (Chong et al. 2005). For this, the effect of different process parameters like aeration, agitation, pH, and temperature have been intensively investigated (Liu et al. 2018). HA biosynthesis, as most exopolysaccharides (EPS) pathways, is a metabolical energy-intensive process where five ATPs are required to form one disaccharide unit (Armstrong et al. 1997). By that, aerobic conditions are much more favorable than microaerophilic or anaerobic conditions since more energy for HA synthesis can be provided. Chong and Nielsen (2003) reported that aerobic conditions resulted in 50% increase of HA productivity and doubled the Mw (Fong Chong and Nielsen 2003). On the other hand, HA titer and Mw require different optimal pH and temperature therefore highest titer and Mw might not be realized at the same time. Highest HA titer was achieved at pH 7.0, while highest Mw at pH 8.0. Similarly, highest HA titer was obtained at 37 °C, but lower temperature contributed to higher Mw. For this reason, a two-stage fermentation represents a promising strategy for the optimal production of HA with high Mw (Liu et al. 2018). Furthermore, as for most microbial EPS production processes, one of the main issues of HA production is the increasing viscosity of the fermentation broth, which finally limits the oxygen and mass transfer rates in the bioreactor. The titers of microbial HA production vary depending on different factors such as the production strain, media composition as well as bioreactor design, but the achievable titers from Streptococcus sp. fermentation are up to 6–7 g/L (Lu et al. 2016; Zhang et al. 2006). While various carbon sources can be used, glucose is the typical substrate and delivers the highest HA yields (Im et al. 2009). The product titer and Mw can be increased by different approaches like temperature switches as well as the addition of intermediates and precursors for HA biosynthesis (Jagannath and Ramachandran 2010). In addition, the process variant can also strongly affect HA production and batch fermentation is commonly used due to its simplicity and product flexibility for industrial producers, but fed-batch processes are revealed to increase yields of HA production in combination with reduced process times (Liu et al. 2011). Continuous processes for microbial EPS production on an industrial scale are quite difficult to realize due to the high dilution rates which are required for cell removal and, in general, can cause genetic instability of the production strain what renders them unfavorable for commercial application on microbial processes (Blank et al. 2005; Chong et al. 2005).
From the molecular biology point of view, most studies suggested that HAS is the key enzyme as well as the limiting factor in HA production. Overexpression of the endogenous hasA is described to increase HA titers of Streptococcus sp. (Izawa et al. 2011; Zakeri et al. 2017). Competition of HA biosynthesis and biomass formation for precursors needs fine-tuning of cell growth and HA production to obtain maximal product titers. Elimination of competing pathways is a pivotal approach for optimizing HA production. For this, typical overflow metabolites of Streptococcus sp. such as lactate, acetate, formate, and ethanol were successfully targeted to channel the carbon flux towards HA biosynthesis (Chong and Nielsen 2003). Furthermore, inactivation of the hyaluronidase-encoding gene is important to prevent degradation of the HA produced. Deletion of hyaluronidase encoding gene in S. zooepidemicus was successfully applied to improve the titer and Mw of produced HA (Pourzardosht and Rasaee 2017). In general, most research for HA production by Streptococcus sp. focus on optimization of the fermentation process much more than on engineering of the production strain. This, on the one hand, can be attributed to the limited genetic tools which are available for Streptococci and, on the other hand, to the customers demand for green, biobased and not “genetically engineered” products. But next to process optimization, strain engineering is an essential and highly efficient way to further boost HA production towards economic production, which gets more and more accepted by the customers and end-users.
4.2 Recombinant Production
Today, the majority of commercial HA is produced by the natural producer Streptococcus sp. However, the pathogenic nature of Streptococci also raises some concerns based on the presence of endotoxins which requires extensive purification and limits the applications of HA in medical sectors (Liu et al. 2011). By that, many efforts have been conducted to realize heterologous HA production by non-pathogenic microorganisms to reduce the complexity of the production process. However, initial recombinant expression resulted in quite low product titers, which rendered native HA production the most successful process for a quite long time (Liu et al. 2011). By means of sophisticated metabolic engineering approaches and especially synthetic biology-driven engineering, recombinant HA production reached a competitive and even superior approach during the last years. Compared to other important EPS, the HA biosynthesis comprises a minimalistic encoding operon. For instance, the size of the has-operon in S. zooepidemicus is 6.8 kb which is much smaller than the xanthan operon of Xanthomonas campestris, which is 14.5 kb in size, and even much less in size compared to the welan operon of Sphingomonas sp. with 30.5 kb (Schmid et al. 2015). Interestingly, only hasA is missing while the homologs of other has genes are readily available in the genome of the recombinant hosts. By that, heterologous expression of hasA, supported by overexpression of the native has homologs, is usually sufficient to direct the engineered strain into HA production.
Recombinant HA production has been demonstrated in microorganisms, such as Escherichia coli (Mao et al. 2009; Woo et al. 2019), Corynebacterium glutamicum (Cheng et al. 2019; Hoffmann and Altenbuchner 2014; Wang et al. 2020), Lactococcus lactis (Jeeva et al. 2019), Bacillus subtilis (Li et al. 2019; Widner et al. 2005), and Pichia pastoris (Jeong et al. 2014). For this, the key enzyme of HA biosynthesis, HAS, is isolated from the natural producer and heterologously expressed along with overexpression of the native genes encoding for the precursors formation of the selected host. Most studies utilized the Class I HAS from Streptococcus sp., whereas other studies utilized the Class II HAS from P. multocida. The product titer and Mw of the resulting HA vary depending on the engineered strains and process parameters (Table 2). Early recombinant production often suffered from low product titers, but just recently C. glutamicum was developed towards a highly promising recombinant HA producer. By a series of metabolic engineering approaches, Wang et al. have successfully designed an outstanding production strain with HA titers of 74 g/L (Wang et al. 2020). This is much higher compared to native production in Streptococcus sp. with 6–7 g/L (Lu et al. 2016; Zhang et al. 2006). However, such a high titer has to be compensated with low Mw. The average Mw of HA produced by the engineered strain was 53 kDa, which is significantly lower in comparison to the one produced by Streptococcus sp. with 2 MDa. It is hypothesized that a high HA titer could be achieved by rapid polymerization by the HAS (Cheng et al. 2019).
Today, industrial-scale HA production by means of recombinant cell factories has been conducted by Novozyme via an engineered B. subtilis strain. The related study described the introduction of the hasA gene from S. equisimilis together with overexpression of the endogenous tuaD, gcaD, and gtaB genes, which are homologs of hasB, hasC, and hasD, respectively. Overexpression of tuaD resulted in significantly increased HA yields, while additional overexpression of gcaD or gtaB showed only a minor impact on the production, indicating that UDP-GlcUA as a precursor is the limiting factor for HA biosynthesis in B. subtilis. This is in contrast to S. zooepidemicus where it was suggested that UDP-GlcNAc was the limiting precursor (Chen et al. 2009). Finally, the recombinant HA had a Mw in the range of 1.1–1.2 MDa with a polydispersity index of 1.5 (Widner et al. 2005). Recombinant HA production in eukaryotic microbes is rarely described and has only been demonstrated for Pichia Pastoris (Jeong et al. 2014). By that approach, a HA titer up to 1.7 g/L with a Mw of 1.2 MDa by heterologous expression of hasA gene from X. laevis along with the native hasC, hasD, hasE homologs could be achieved. Similar to B. subtilis, UDP-GlcA is also the limiting precursor in P. pastoris. Furthermore, it was demonstrated that expression of hasA under the regulation of a weak promoter resulted in HA with higher Mw in comparison to expression by the use of a strong promoter. This was due to high level of HAS resulted in the rapid exhaustion of the intracellular HA precusors, which led to a low ratio between the precursors and HAS and might demonstrate the high processivity of the HAS. For this, fine-tuning of the expression level of the HAS as well as the precursors genes are essential to achieve optimal titers and desired Mw.
4.3 In Vitro Production
Apart from microbial fermentation, several studies have also explored the potential of HA production via in vitro synthesis. This method is relatively new developed and some studies reported the production of HA in vitro in small-scale reactions. In principle, HA synthesis can be achieved by mixing the sugar nucleotide precursors, cofactors, and the HAS protein. Due to the nature of membrane-spanning Class I HAS, the research was mostly conducted on membrane fractions (Boeriu et al. 2013). Although in vitro HA production is possible, high costs of membranes, sugar nucleotides and cofactors hampers its feasibility at a commercial scale (Sze et al. 2016). For this, utilization of Class II HAS from P. multocida (PmHAS) is a better option since, unlike the class I HAS from Streptococcus, PmHAS is a peripheral protein and therefore must not be bound to a membrane to perform its function (DeAngelis 1996; Sze et al. 2016). In the year 2000, Jing and De Angelis created a mutant of the peripheral PmHAS1–703, which showed high solubility in combination with high activity (Jing and DeAngelis 2000). One-pot synthesis of in vitro HA production includes the six enzymes: glucuronic acid kinase, UDP–sugar pyrophosphorylase and pyrophosphatase for generation of UDP-GlcA; GlcNAc-1-phosphate kinase, UDP–GlcNAc pyrophosphorylases and pyrophosphatase for UDP-GlcNAc; as well as HAS for polymerization and resulted in a titer of 2.7 g/L by use of GlcA and GlcNAc as substrates (Gottschalk et al. 2019). Furthermore, Eisele et al. (2018) demonstrated in vitro HA synthesis, which utilized sucrose and GlcNAc as the main substrates (Eisele et al. 2018). The strategy involved several enzyme module systems which allowed in situ regeneration of nucleotide sugars. For this, different recombinant enzymes have to be cloned, produced, and purified before being used in the enzymatic synthesis. In addition to the substrates and enzymes, additional components like NAD+ and UDP also have to be added to the reaction mixtures. Finally, this approach achieved a final titer of 4 g/L with Mw of 2.3 MDa. Further research is required to analyze the feasibility of in vitro HA synthesis, especially in scaling up experiments. Efficient production of the enzymes, substrates reutilization, as well as product separation, need to be addressed to produce HA with high yield and purity (Boeriu et al. 2013).
5 Extraction and Purification
As HA is often applied in medical or pharmaceutical applications, the purification process must guarantee that the final product is free from endotoxins and proteins. In the case of microbial production by S. zooepidemicus, the removal of streptolysin and proteins is of high importance, as these compounds might lead to lysis of red blood cells or cause strong immune responses, respectively (Bitterman-Deutsch et al. 2015; Franz et al. 2011). HA from animal tissue must be purified from protein and carries the inherent risk of contaminations with animal viruses or prions. Among other regulatory criteria of medically applied HA (Huerta-Angeles et al. 2016), recent sources state a required purity of a protein content below 0.3–0.1%, depending on the application (Ferreira et al. 2021; Hyaluronate 2016). An ultrapure HA with a protein content below 0.5% was described to be non-inflammatory at a concentration of 1% (Balazs 1979). Endotoxin levels must be below 0.05 I.U. mg−1, and residual ethanol or other organic solvents ≤0.5% and bacterial contamination ≤100 cfu mg−1 (Ferreira et al. 2021; Hyaluronate 2016). These high demands on purity, especially for injection purposes, require extensive purification processes compared to other commercial EPS, resulting in the high market prices of HA.
In the case of HA production from animal sources, extraction steps precede the purification process. The first described extraction was done by acetone precipitation from bovine vitreous humor (Meyer and Palmer 1934). Later developed methods include extraction using organic solvents, isopropanol or sodium acetate, as well as quaternary ammonium salts. Enzymatic treatments using Alcalase, Papain, Pronase (a mixture of endo- and exopeptidases), and Trypsin have been described as well (Abdallah et al. 2020).
Following extraction from animal tissue or cell separation in the case of microbial production, several purification methods and combinations thereof have been investigated to obtain the required purities (≥99.5%) for pharmaceutical and medical applications. Purification methods include precipitation with ethanol or 2-propanol (isopropanol), tangential flow filtration and diafiltration, silica gel adsorption, protein electrodeposition, dialysis, and anion exchange chromatography (Abdallah et al. 2020; Cavalcanti et al. 2020). The main goal of the HA purification process is the removal of protein and other compounds causing inflammation. From microbially produced HA, the protein content after precipitation ranges from 12 to 14%, which requires further purification steps. Mainly purification is applied by filtration, as this is quite cheap compared to other methods such as enzymatic treatment, adsorption methods or ion exchange chromatography (Ferreira et al. 2021).
Highest reported theoretical purities from S. zooepidemicus fermentations are described for protein concentration as low as 0.06% by combining precipitation, silica gel adsorption, charcoal filtration followed by 5× diafiltration and sterile filtration with a yield of 50% in respect to the unpurified precipitate (Rangaswamy and Jain 2008). Another study reports an equally pure product with a protein content of 0.07% by a combination of microfiltration and ultrafiltration in a diafiltration setup, yielding 89% starting from a 1 g/L HA solution. However, these purification steps require either very long treatment times (up to 74 h) or multiple processing steps, which renders HA production very expensive or impractical for industrial scale. A recently published study based on process simulation suggests an increase in production costs of 30–78% for the production of ultrapure HA suitable for injection compared to HA for topological applications, based on fermentative production by Streptococcus sp. (Ferreira et al. 2021). Their approach, however, targets the simultaneous production of both lower and higher purity grades with 90% and 10% production ratios, respectively. A comprehensive overview of sources, processing methods and purities of HA is shown in Table 3.
6 Commercial Producers
According to Grand View Research, the worldwide HA market size will reach USD 16.6 billion by 2027 and is projected to grow by an average annual growth rate of 8.1% for the coming years (Grand View Research 2020). HA market is anticipated to rise with an increasing aging population. The global pandemic Covid-19 also has a positive impact on the market, especially for injectable HA. Most HA product is commercialized as sodium hyaluronate, but other derivatives such as hyaluronan oligosaccharides or oxidized hyaluronic acid are also available. Contipro, one of the key players in HA market, provides HA in different forms, including fibers, hydrogen, films, or micelles. Shiseido is the first company to conduct large-scale HA production from non-animal sources. Today, most companies produce HA from microbial fermentations, by means of S. zooepidemicus (Table 4). Novozymes is the only company that is running commercial scale recombinant HA production from non-Streptococci strain, by utilizing B. subtilis. Founded in 2000, Hyalose is a USA-based company that focuses on the commercialization of technologies for HA production, from conventional animal extraction, microbial fermentation, to enzymatic HA synthesis.
7 Patent
Following its first discovery in the 1930s (Meyer and Palmer 1934), HA has garnered much attentions due to its impressive characteristics. Since then, many investigations focused on the exploitation of HA and its derivatives which results in an increasing number of patents concerning HA. First patent applications on HA were filled in the 1940s, and the number of patents keep growing over the years, notably in the past 20 years (Fig. 3). According to the European Patent Office, more than 100,000 HA-relevant patents have been submitted that cover various fields of synthesis, processing, and applications. The USA is the country with the most contribution in patent applications, followed by China and Japan. Shiseido holds most numbers of the patents with more than 1841 patents. Pharmaceutical companies like Sanofi, Pfizer, and Rohto Pharma also have ample numbers of HA patents. Furthermore, many universities are also among the major patent holders. More recent patents encompass HA formulations with other substances for applications in pharmaceutical, medical, and cosmetics fields. For example, WO2021020950A1 describes hydrogels that comprised of HA and pluronic for prevention and treatment of articular and cartilage injury.
8 Conclusion and Future Outlook
In conclusion, HA is a highly valuable polysaccharide with a broad range of medical applications. Based on relatively low stability of HA compared to other polysaccharides, the downstream processing is the main cost driving factor and must be optimized in the future. As seen by the progress in heterologous expression, much higher product titer can be obtained than native production strains or HA of animal origins. By that, it can be expected that future large-scale production will be carried out via optimized production strains to improve yields and thus the economy of the whole production process. The in vitro synthesis of HA is not yet ready for large-scale and economical production due to the high cost of the substrates and enzymes production and purification. However, the progress in understanding and modelling enzymes by new and highly sophisticated tools such as AlphaFold in combination with targeted enzyme engineering might also improve the activity and stability of the HAS soon. By that, the in vitro approach might be massively improved.
Nevertheless, the production of the various enzymes still has to be considered when comparing the economics of the different process variants. An essential aspect that might define the future production processes is the desired Mw and purity for the different applications. Here, the selection of the production strain, in vitro synthesis by engineered enzymes or adapted downstream processing might be applied for HA production with specific Mw. In sum, the production of HA is of growing interest for the cosmeceutical industry, and many reports on the progress in HA production can be expected within the next years. Especially, the utilization of the latest molecular biology tools and approaches based on synthetic biology will massively accelerate the progress in HA research, thus making it a valuable polysaccharide of high functionality.
References
Abatangelo G, Vindigni V, Avruscio G, Pandis L, Brun P (2020) Hyaluronic acid: redefining its role. Cells 9:1–19
Abdallah MM, Fernández N, Matias AA, Bronze MR (2020) Hyaluronic acid and chondroitin sulfate from marine and terrestrial sources: extraction and purification methods. Carbohydr Polym 243:116441
Agarwal G, Krishnan KV, Prasad SB, Bhaduri A, Jayaraman G (2019) Biosynthesis of hyaluronic acid polymer: dissecting the role of sub structural elements of hyaluronan synthase. Sci Rep 9:1–12
Amagai I, Tashiro Y, Ogawa H (2009) Improvement of the extraction procedure for hyaluronan from fish eyeball and the molecular characterization. Fish Sci 75:805–810
Ambrosio L, Bortacchiello A, Netti PA, Nicolais L (1999) Rheological study on hyaluronic acid and its derivative solutions. J Macromol Sci A 36:991–1000
Armstrong DC, Cooney MJ, Johns MR (1997) Growth and amino acid requirements of hyaluronic-acid-producing Streptococcus zooepidemicus. Appl Microbiol Biotechnol 47:309–312
Balazs EA (1979) Ultrapure hyaluronic acid and the use thereof. US Patent US4141973A
Balazs EA, Laurent TC (1951) Viscosity function of hyaluronic acid as a polyelectrolyte. J Polym Sci 6:665–667. https://doi.org/10.1002/pol.1951.120060517
Bitterman-Deutsch O, Kogan L, Nasser F (2015) Delayed immune mediated adverse effects to hyaluronic acid fillers: report of five cases and review of the literature. Dermatol Rep 7:5851
Blank LM, McLaughlin RL, Nielsen LK (2005) Stable production of hyaluronic acid in Streptococcus zooepidemicus chemostats operated at high dilution rate. Biotechnol Bioeng 90:685–693
Blank LM, Hugenholtz P, Nielsen LK (2008) Evolution of the hyaluronic acid synthesis (has) operon in Streptococcus zooepidemicus and other pathogenic Streptococci. J Mol Evol 67:13–22
Bodevin-Authelet S, Kusche-Gullberg M, Pummill PE, DeAngelis PL, Lindahl U (2005) Biosynthesis of hyaluronan. J Biol Chem 280:8813–8818
Boeriu CG, Springer J, Kooy FK, van den Broek LAM, Eggink G (2013) Production methods for hyaluronan. Int J Carbohydr Chem 2013:1–14
Bothner H, Wik O (1987) Rheology of hyaluronate. Acta Otolaryngol Suppl 442:25–30
Bracke JW, Thacker K (1985) Hyaluronic acid from bacterial culture. US Patent 4517295
Brown KK, Ruiz LLC, van de Wijn I (1994) Ultrapure hyaluronic acid and method of making it. US Patent 4782046A
Caputo A (1957) Depolymerization of hyaluronic acid by X-rays. Nature 179:1133–1134. https://doi.org/10.1038/1791133a0
Cavalcanti ADD, Melo BAG, Ferreira BAM, Santana MHA (2020) Performance of the main downstream operations on hyaluronic acid purification. Process Biochem 99:160–170
Chen S-J, Chen J-L, Huang W-C, Chen H-L (2009) Fermentation process development for hyaluronic acid production by Streptococcus zooepidemicus ATCC 39920. Korean J Chem Eng 26:428–432
Chong B, Nielsen L (2003) Amplifying the cellular reduction potential of Streptococcus zooepidemicus. J Biotechnol 100:33–41. https://doi.org/10.1016/s0168-1656(02)00239-0
Chen YH, Li J, Liu L, Liu HZ, Wang Q (2012) Optimization of flask culture medium and conditions for hyaluronic acid production by a Streptococcus equisimilis mutant NC2168. Braz J Microbiol 43:1553–1561
Cheng F, Yu H, Stephanopoulos G (2019) Engineering Corynebacterium glutamicum for high-titer biosynthesis of hyaluronic acid. Metab Eng 55:276–289
Chong BF, Blank LM, Mclaughlin R, Nielsen LK (2005) Microbial hyaluronic acid production. Appl Microbiol Biotechnol 66:341–351
Cowman MK, Lee HG, Schwertfeger KL, McCarthy JB, Turley EA (2015a) The content and size of hyaluronan in biological fluids and tissues. Front Immunol 6:1–8
Cowman MK, Schmidt TA, Raghavan P, Stecco A (2015b) Viscoelastic properties of hyaluronan in physiological conditions. F1000Res 4:622
Cress BF, Englaender JA, He W, Kasper D, Linhardt RJ, Koffas MAG (2014) Masquerading microbial pathogens: capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol Rev 38:660–697
Cullis-Hill D (1989) Preparation of hyaluronic acid from synovial fluid. US Patent 4879375
DeAngelis PL (1996) Enzymological characterization of the Pasteurella multocida hyaluronic acid synthase. Biochemistry 35:9768–9771
DeAngelis PL, Achyuthan AM (1996) Yeast-derived recombinant DG42 protein of Xenopus can synthesize hyaluronan in vitro. J Biol Chem 271:23657–23660
DeAngelis PL, Papaconstantinou J, Weigel PH (1993) Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. J Biol Chem 268:19181–19184
Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X (2014) Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater 10:1558–1570
Dodero A, Williams R, Gagliardi S, Vicini S, Alloisio M, Castellano M (2019) A micro-rheological and rheological study of biopolymers solutions: hyaluronic acid. Carbohydr Polym 203:349–355
Donati A, Magnani A, Bonechi C, Barbucci R, Rossi C (2001) Solution structure of hyaluronic acid oligomers by experimental and theoretical NMR, and molecular dynamics simulation. Biopolymers 59:434–445
Dorfman A (1948) The kinetics of the enzymatic hydrolysis of hyaluronic acid. J Biol Chem 172:377–387
Eisele A, Zaun H, Kuballa J, Elling L (2018) In vitro one-pot enzymatic synthesis of hyaluronic acid from sucrose and N-acetylglucosamine: optimization of the enzyme module system and nucleotide sugar regeneration. ChemCatChem 10:2969–2981
Ferreira RG, Azzoni AR, Santana MHA, Petrides D (2021) Techno-economic analysis of a hyaluronic acid production process utilizing Streptococcal fermentation. Processes 9:241
Fong Chong B, Nielsen LK (2003) Aerobic cultivation of Streptococcus zooepidemicus and the role of NADH oxidase. Biochem Eng J 16:153–162
Franz S, Rammelt S, Scharnweber D, Simon JC (2011) Immune responses to implants—a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 32:6692–6709
Freitas F, Alves VD, Reis MAM (2011) Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol 29:388–398
Fuoss RM (1948) Viscosity function for polyelectrolytes. J Polym Sci 3:603–604. https://doi.org/10.1002/pol.1948.120030414
Gherezghiher T, Koss MC, Nordquist RE, Wilkinson CP (1987) Analysis of vitreous and aqueous levels of hyaluronic acid: application of high-performance liquid chromatography. Exp Eye Res 45:347–349
Gibbs DA, Merrill EW, Smith KA, Balazs EA (1968) Rheology of hyaluronic acid. Biopolymers 6:777–791
Gomes AMV, Netto JHCM, Carvalho LS, Parachin NS (2019) Heterologous hyaluronic acid production in Kluyveromyces lactis. Microorganisms 7:294
Gottschalk J, Zaun H, Eisele A, Kuballa J, Elling L (2019) Key factors for a one-pot enzyme cascade synthesis of high molecular weight hyaluronic acid. Int J Mol Sci 20(22):5664
Grand View Research (2020) Hyaluronic acid market size worth 16.6 billion by 2027 | CAGR: 8.1%. https://www.grandviewresearch.com/press-release/global-hyaluronic-acid-market
Güngör G, Gedikli S, Toptaş Y, Akgün DE, Demirbilek M, Yazıhan N, Aytar Çelik P, Denkbaş EB, Çabuk A (2019) Bacterial hyaluronic acid production through an alternative extraction method and its characterization. J Chem Technol Biotechnol 94:1843–1852
Han HY, Jang SH, Kim EC, Park JK, Han YJ, Lee CC, Park SH, Kim YC, Park HJ (2004) Microorganism producing hyaluronic acid and purification method of hyaluronic acid. Patent WO2004016771
Heldermon C, DeAngelis PL, Weigel PH (2001) Topological organization of the hyaluronan synthase from Streptococcus pyogenes. J Biol Chem 276:2037–2046
Hemant PN, Sonal T, Bondalakunta R (2013) Process for the purification of hyaluronic acid salts (HA) from fermentation broth. Patent WO2013132506
Hoffmann J, Altenbuchner J (2014) Hyaluronic acid production with Corynebacterium glutamicum: effect of media composition on yield and molecular weight. J Appl Microbiol 117:663–678
Huerta-Angeles G, Brandejsová M, Kulhánek J, Pavlík V, Šmejkalová D, Vágnerová H, Velebný V (2016) Linolenic acid grafted hyaluronan: process development, structural characterization, biological assessing, and stability studies. Carbohydr Polym 152:815–824
Hvidberg E, Kvorning SA, Schmidt A, Schou J (1959) Effect of ultraviolet irradiation on hyaluronic acid in vitro. Acta Pharmacol Toxicol 15:356–364. https://doi.org/10.1111/j.1600-0773.1959.tb00304.x
Hyaluronate S (2016) European pharmacopoeia, vol 2, 9th edn. European Directorate for Quality of Medicines & Health Care, Strasbourg
Im J-H, Song J-M, Kang J-H, Kang D-J (2009) Optimization of medium components for high-molecular-weight hyaluronic acid production by Streptococcus sp. ID9102 via a statistical approach. J Ind Microbiol Biotechnol 36:1337–1344
Izawa N, Serata M, Sone T, Omasa T, Ohtake H (2011) Hyaluronic acid production by recombinant Streptococcus thermophilus. J Biosci Bioeng 111:665–670
Jagannath S, Ramachandran KB (2010) Influence of competing metabolic processes on the molecular weight of hyaluronic acid synthesized by Streptococcus zooepidemicus. Biochem Eng J 48:148–158
Jeeva P, Shanmuga Doss S, Sundaram V, Jayaraman G (2019) Production of controlled molecular weight hyaluronic acid by glucostat strategy using recombinant Lactococcus lactis cultures. Appl Microbiol Biotechnol 103:4363–4375
Jeong E, Shim WY, Kim JH (2014) Metabolic engineering of Pichia pastoris for production of hyaluronic acid with high molecular weight. J Biotechnol 185:28–36
Jing W, DeAngelis PL (2000) Dissection of the two transferase activities of the Pasteurella multocida hyaluronan synthase: two active sites exist in one polypeptide. Glycobiology 10:883–889
Kang DY, Kim WS, Heo IS, Park YH, Lee S (2010) Extraction of hyaluronic acid (HA) from rooster comb and characterization using flow field-flow fractionation (FlFFF) coupled with multiangle light scattering (MALS). J Sep Sci 33:3530–3536
Kendall FE, Heidelberger M, Dawson MH (1937) A serologically inactive polysaccharide elaborated by mucoid strains of group A hemolytic Streptococcus. J Biol Chem 118:61–69
Khabarov VN, Polyak F, Boykov PY, Selyanin MA (2014) Hyaluronic acid: production, properties, application in biology and medicine, 1st edn. Wiley, Chichester
Khanmohammadi M, Khoshfetrat AB, Eskandarnezhad S, Sani NF, Ebrahimi S (2014) Sequential optimization strategy for hyaluronic acid extraction from eggshell and its partial characterization. J Ind Eng Chem 20:4371–4376
Kim TH, Kim HL, Park SY, Jang DK (2008a) Method for purifying hyaluronic acid using calcium salt and phosphate salt, or calcium phosphate salt. Patent US20080194810
Kim JK, Srinivasan P, Kim JH, Choi JI, Park HJ, Byun MW, Lee JW (2008b) Structural and antioxidant properties of gamma irradiated hyaluronic acid. Food Chem 109:763–770. https://doi.org/10.1016/j.foodchem.2008.01.038
Lago G, Oruña L, Cremata JA, Pérez C, Coto G, Lauzan E, Kennedy JF (2005) Isolation, purification and characterization of hyaluronan from human umbilical cord residues. Carbohydr Polym 62:321–326
Laurent TC, Fraser JRE (1992) Hyaluronan 1. FASEB J 6:2397–2404
Laurent TC, Gergely J (1955) Light scattering studies on hyaluronic acid. J Biol Chem 212:325–333
Li Y, Li G, Zhao X, Shao Y, Wu M, Ma T (2019) Regulation of hyaluronic acid molecular weight and titer by temperature in engineered Bacillus subtilis. 3 Biotech 9:1–9
Linker A, Meyer K (1954) Production of unsaturated uronides by bacterial hyaluronidases. Nature 174:1192–1194
Liu L, Liu Y, Li J, Du G, Chen J (2011) Microbial production of hyaluronic acid: current state, challenges, and perspectives. Microb Cell Fact 10:1–9
Liu J, Wang Y, Li Z, Ren Y, Zhao Y, Zhao G (2018) Efficient production of high-molecular-weight hyaluronic acid with a two-stage fermentation. RSC Adv 8:36167–36171
Lu JF, Zhu Y, Sun HL, Liang S, Leng FF, Li HY (2016) Highly efficient production of hyaluronic acid by Streptococcus zooepidemicus R42 derived from heterologous expression of bacterial haemoglobin and mutant selection. Lett Appl Microbiol 62:316–322
Mao Z, Chen RR (2007) Recombinant synthesis of hyaluronan by Agrobacterium sp. Biotechnol Prog 23:1038–1042
Mao Z, Shin H-D, Chen R (2009) A recombinant E. coli bioprocess for hyaluronan synthesis. Appl Microbiol Biotechnol 84:63–69
Matsumura G, de Salegui M, Herp A, Pigman W (1963) The preparation of hyaluronic acid from bovine synovial fluid. Biochim Biophys Acta 69:574–576
Meyer K, Palmer JW (1934) The polysaccharide of the vitreous humor. J Biol Chem 107:629–634
Mizuno H, Iso N, Saito T, Ogawa H, Sawairi H, Saito M (1991) Characterization of hyaluronic acid of yellowfin tuna eyeball. Nippon Suisan Gakkaishi 57:517–519
Murado MA, Montemayor MI, Cabo ML, Vázquez JA, González MP (2012) Optimization of extraction and purification process of hyaluronic acid from fish eyeball. Food Bioprod Process 90:491–498
Nakano T, Nakano K, Sim JS (1994) A simple rapid method to estimate hyaluronic acid concentrations in rooster comb and wattle using cellulose acetate electrophoresis. J Agric Food Chem 42:2766–2768
Nimrod A, Greenman B, Kanner D, Landsberg M, Beck Y (1988) Method of producing high molecular weight sodium hyaluronate by fermentation of Streptococcus. US Patent 4780414
Ogston AG, Stanier JI (1951) The dimensions of the particle of hyaluronic acid complex in sinovial fluid. Biochem J 49:585–599
Ouskova G, Spellerberg B, Prehm P (2004) Hyaluronan release from Streptococcus pyogenes: export by an ABC transporter. Glycobiology 14:931–938
Pourzardosht N, Rasaee MJ (2017) Improved yield of high molecular weight hyaluronic acid production in a stable strain of Streptococcus zooepidemicus via the elimination of the hyaluronidase-encoding gene. Mol Biotechnol 59:192–199
Rangaswamy V, Jain D (2008) An efficient process for production and purification of hyaluronic acid from Streptococcus equi subsp. zooepidemicus. Biotechnol Lett 30:493–496
Reddy J (2013) Purification and characterization of hyaluronic acid produced by Streptococcus zooepidemicus strain 3523-7. J Biosci Biotechnol Discov 2:173
Rehm BHA (2010) Bacterial polymers: biosynthesis, modifications and applications. Nat Rev Microbiol 8:578–592
Rehm BHA, Valla S (1997) Bacterial alginates: biosynthesis and applications. Appl Microbiol Biotechnol 48:281–288
Rosa CS, Tovar AF, Mourão P, Pereira R, Barreto P, Beirão LH (2012) Purification and characterization of hyaluronic acid from chicken combs. Cienc Rural 42:1682–1687
Sadhasivam G, Muthuvel A, Pachaiyappan A, Thangavel B (2013) Isolation and characterization of hyaluronic acid from the liver of marine stingray Aetobatus narinari. Int J Biol Macromol 54:84–89
Saxena IM, Brown RM, Fevre M, Geremia RA, Henrissat B (1995) Multidomain architecture of beta-glycosyl transferases: implications for mechanism of action. J Bacteriol 177:1419–1424
Schmid J, Sieber V, Rehm B (2015) Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol 6:1–24
Schulz T, Schumacher U, Prehm P (2007) Hyaluronan export by the ABC transporter MRP5 and its modulation by intracellular cGMP. J Biol Chem 282:20999–21004
Scott JE, Heatley F (1999) Hyaluronan forms specific stable tertiary structures in aqueous solution: a 13C NMR study. Proc Natl Acad Sci U S A 96:4850–4855
Scott JE, Cummings C, Brass A, Chen Y (1991) Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network-forming polymer. Biochem J 274:699–705
Swann DA (1968) Studies on hyaluronic acid. Biochim Biophys Acta Gen Subj 156:17–30
Sze JH, Brownlie JC, Love CA (2016) Biotechnological production of hyaluronic acid: a mini review. 3 Biotech 6:67
Thomas NK, Brown TJ (2010) ABC transporters do not contribute to extracellular translocation of hyaluronan in human breast cancer in vitro. Exp Cell Res 316:1241–1253
Tlapak-Simmons VL, Baggenstoss BA, Kumari K, Heldermon C, Weigel PH (1999) Kinetic characterization of the recombinant hyaluronan synthases from Streptococcus pyogenes and Streptococcus equisimilis. J Biol Chem 274:4246–4253
Volpi N, Maccari F (2003) Purification and characterization of hyaluronic acid from the mollusc bivalve Mytilus galloprovincialis. Biochimie 85:619–625
Wang Y, Hu L, Huang H, Wang H, Zhang T, Chen J, Du G, Kang Z (2020) Eliminating the capsule-like layer to promote glucose uptake for hyaluronan production by engineered Corynebacterium glutamicum. Nat Commun 11:3120
Ward PN, Field TR, Ditcham WGF, Maguin E, Leigh JA (2001) Identification and disruption of two discrete loci encoding hyaluronic acid capsule biosynthesis genes hasA, hasB, and hasC in Streptococcus uberis. Infect Immun 69:392–399
Weigel PH (2015) Hyaluronan synthase: the mechanism of initiation at the reducing end and a pendulum model for polysaccharide translocation to the cell exterior. Int J Cell Biol 2015:367579
Weigel PH, DeAngelis PL (2007) Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem 282:36777–36781
Widner B, Behr R, von Dollen S, Tang M, Heu T, Sloma A, Sternberg D, DeAngelis PL, Weigel PH, Brown S (2005) Hyaluronic acid production in Bacillus subtilis. Appl Environ Microbiol 71:3747–3752
Won TY, Lee C, Seo SH (2008) Method for purifying hyaluronic acid. Patent WO2008062998
Woo JE, Seong HJ, Lee SY, Jang Y-S (2019) Metabolic engineering of Escherichia coli for the production of hyaluronic acid from glucose and galactose. Front Bioeng Biotechnol 7:351
Yoshimura T, Shibata N, Hamano Y, Yamanaka K (2015) Heterologous production of hyaluronic acid in an epsilon-poly-l-lysine producer, Streptomyces albulus. Appl Environ Microbiol 81:3631–3640
Zakeri A, Rasaee MJ, Pourzardosht N (2017) Enhanced hyluronic acid production in Streptococcus zooepidemicus by over expressing HasA and molecular weight control with niscin and glucose. Biotechnol Rep (Amst) 16:65–70
Zhang J, Ding X, Yang L, Kong Z (2006) A serum-free medium for colony growth and hyaluronic acid production by Streptococcus zooepidemicus NJUST01. Appl Microbiol Biotechnol 72:168–172
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Meliawati, M., Gansbiller, M., Schmid, J. (2022). Hyaluronic Acid (Hyaluronan). In: Rehm, B.H.A., Wibowo, D. (eds) Microbial Production of High-Value Products. Microbiology Monographs, vol 37. Springer, Cham. https://doi.org/10.1007/978-3-031-06600-9_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-06600-9_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06599-6
Online ISBN: 978-3-031-06600-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)