Abstract
Approximately 6 million patients are evaluated annually in emergency departments (ED) for acute chest pain and constitute up to 10% of all ED admissions in the USA (McCaig, National Hospital Ambulatory Medical Care Survey 2003: emergency department summary: advance data from vital and health statistics, No. 358. National Center for Health Statistics, 2005; Bhuiya et al., NCHS Data Brief 43:1–8, 2010). The differential diagnosis is vast and includes coronary, pulmonary, pericardial, and aortic diseases, thus posing a significant diagnostic challenge. The primary diagnostic goal is to exclude life threatening causes such as acute coronary syndrome (ACS), since an estimated 2% of these patients are inappropriately sent home and suffer higher morbidity than admitted patients (Lee and Goldman, N Engl J Med 342(16):1187–1195, 2000; Pope et al., N Engl J Med 342(16):1163–1170, 2000). Missed ACS was the number one payout per malpractice case, and accounts for 41% of claims paid. Although only a small percentage of acute chest pain patients with a normal electrocardiogram (EKG) and cardiac enzymes suffer from ACS, there is a large cost burden borne by the health-care system in evaluating these patients, estimated to be around $10–13 billion annually in the United States alone (McCaig, National Hospital Ambulatory Medical Care Survey 2003: emergency department summary: advance data from vital and health statistics, No. 358. National Center for Health Statistics, 2005).
Rapid advances in multirow detector computed tomographic (CT) technology commonly known as multislice CT (MSCT) has led to the utilization of the coronary CT angiography (CCTA) in the ED as a tool for triaging patients presenting with acute chest pain. The direct visualization of the coronary anatomy, the ability to simultaneously image the rest of the thorax to exclude aortic dissection and pulmonary embolism, and the ability to provide alternate causes of chest pain, such as pneumonia, pericardial fluid and esophageal inflammation, make this modality attractive to the practitioner. This chapter will examine the use of CCTA for the evaluation of acute chest pain.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- CT angiography
- Emergency department
- Acute coronary syndrome
- Acute chest pain
- ED triage
- Pulmonary embolism
- Aortic dissection
1 Overview of CT Technology
The advent of helical/spiral CT imaging technology and the dramatic advances in the temporal and spatial resolution of CT [3] have made it possible to visualize the coronary arteries with systems that are able to synchronize the image reconstruction with the cardiac phase [4, 5]. Images with high temporal and spiral resolution in all directions can be obtained with multiple-row detector CT scanners and expressed as isotropic spatial resolution [6]. The advances in technology have considerably decreased the gantry rotation time to as low as 330–370 ms, and based on the acquisition mode, the temporal resolution can range from 80 to 250 ms [6]. Improved detector and collimator hardware now provide submillimeter image resolution (0.4–0.5 mm). The high resolution sixty-four slice scanners have become standard for CCTA [7]. Such scanners decrease breath-hold time and reduce cardiac motion artifacts which have increased the overall percentage of “interpretable” scans, and allowed imaging without the need for beta blockade in many patients. Challenges remain in imaging patients with heavily calcified coronary arteries, coronary artery stents, and markedly obese patients. Although 320-slice machines are now available, these are not widely diffused and the existing published evidence is specific to 64-slice scanners [8].
2 Accuracy of Coronary CT Angiography
The main requirement for CCTA to be an acceptable tool for evaluation of patients suspected for coronary artery disease (CAD) includes the complete visualization of all therapeutic relevant coronary arteries [9]. The need to exclude life threatening causes such as acute coronary syndrome (ACS) among patients presenting to the ED with chest pain (CP) is crucial, since an estimated 2% of these patients are inappropriately sent home and suffer higher morbidity than admitted patients [10, 11]. The ability of CCTA to quantitate coronary artery lesion severity correlates well with invasive coronary angiography (Pearson correlation, r = 0.72) [7, 12,13,14]. The considerable standard deviation in these early studies, however, limits its quantitative accuracy (see Fig. 1).
Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. Adapted from Raff et al. [14]
Figure recreated from a diagnostic accuracy study of 64-slice coronary computed tomography angiography (CCTA) in 70 consecutive patients who underwent elective invasive coronary angiography for suspected coronary artery disease [7]. Bland–Altman analysis of the differences of percent diameter stenosis measured by CCTA versus quantitative coronary angiography (QCA) during invasive catheterization, compared to the average percent diameter stenosis by the two methods. The mean difference was 1.3 ± 14.0% (central line). A total of 94% of the values lie within 1.96 standard deviations of the mean (outer lines). There was no significant correlation between stenosis difference and stenosis severity (Spearmen correlation = −0.07, p = 0.59).
There have been several studies that have evaluated the safety and diagnostic accuracy of 64-slice CCTA for triage of ED patients with acute chest pain. Overall, these studies suggest that CCTA can identify a subset of ED chest pain patients who can be safely discharged home on the basis of CT findings [15,16,17]. In the study by Goldstein et al., a randomized control trial of 197 low-risk acute chest pain patients was evaluated by either early CCTA (n = 99) or a standard diagnostic protocol (n = 98) [18]. Patients randomized to early CCTA were eligible for discharge with normal or minimally abnormal results (<25% stenosis), patients with severe stenosis (>70%) were referred for immediate invasive angiography, whereas patients with intermediate-grade stenosis underwent additional stress testing. The two groups were compared for safety, diagnostic accuracy and efficiency. Among patients randomized to CCTA, 75% had decisive triage by CCTA alone (67% immediately discharged and 8% referred for immediate catheterization, which revealed significant disease in 7 of 8 referred cases). Importantly, CCTA alone was not considered adequate for diagnosis in 24 of 99 cases, owing either to lesions of unclear hemodynamic significance (stenosis = 26–70%) in 13 of these patients or to nondiagnostic quality scans in 11 patients (all 24 underwent noninvasive stress testing) (see Fig. 2). Among the patients discharged immediately, none had a major adverse cardiac event (MACE) or subsequent diagnosis of CAD over a 6-month follow-up period (see Table 1). The overall diagnostic accuracy of CCTA was 94%, and the negative predictive value (NPV) was 100%. Diagnostic efficiency, defined as time from randomization to definitive diagnosis, showed that the CCTA approach was more rapid (3.4 vs. 15.0 h) and reduced costs by 15%. The American College of Cardiology Foundation in their 2006 guidelines mentioned that the clinical application of CTCA for acute chest pain can only be considered “appropriate” when its application is limited to patients with intermediate pretest probability without EKG and serial biomarker changes [16].
A randomized controlled trial of multislice coronary computed tomography for evaluation of acute chest pain. Adapted from Goldstein et al. [17]
In this Diagnostic algorithm, patients in the multislice computed tomography group with normal scans were eligible for immediate discharge. Patients with severe stenosis on MSCT (over 70%) were referred for invasive angiography, whereas those with intermediate lesions or radio diagnostic scans were referred for nuclear stress scans. Patients in the standard diagnostic group underwent nuclear stress scans and were eligible for discharge if normal or refused for invasive angiography if abnormal. SOC = standard of care diagnostic evaluation.
In the blinded observational trial: rule out myocardial infarction using computer assisted tomography (ROMICAT), 368 chest pain patients with normal troponin and nonischemic EKGs were enrolled. The results showed that 50% of patients who presented with acute chest pain to the ED and were at low to intermediate likelihood of ACS had no CAD by coronary CTA, a finding that had a 100% NPV but limited positive predictive value (PPV) for the subsequent diagnosis of ACS and MACE. In addition, the results indicate that while the NPV remains excellent (98%), the exclusion of significant coronary stenosis by coronary CTA (>50%) had a limited sensitivity (77%) for the detection of ACS [19]. Kim et al., in their prospective observational study of 296 “CCTA eligible” acute chest pain patients presenting to the ED with “low to intermediate clinical risk profile,” showed the overall accuracy of CCTA for ACS was 88.5% (sensitivity), 85.1% (specificity), 60.7% (positive predictive value), and 96.6% (negative predictive value) [17].
3 Cost-Effectiveness of Coronary CT Angiography
There is some concern that injudicious use of CCTA may result in increased health-care cost. A study by Otero et al. evaluated the cost-effectiveness of CCTA, stress echocardiography, and myocardial single-photon emission computerized tomography (SPECT) for 10,000 simulated patients. Using reported imaging test characteristics, prevalence and risk of CAD, and Medicare reimbursement schedules, the study reported that the clinical application of CCTA may significantly reduce the overall observation period and total health-care cost [20].
4 Safety Concerns of Coronary CT Angiography
The primary safety concern associated with CCTA is the potential carcinogenicity from radiation exposure. The effective radiation dose of a scan is calculated as the dose-length product (measured and displayed by the scanner on each patient) multiplied by the European Commission thoracic conversion factor (0.017) to yield the effective dose in milliSieverts (mSv). Thus, the radiation dose is directly proportional to the scan length in centimeters. The dose estimates from CCTA have been found to range from 7 to 13 mSv, while dose estimates from coronary angiogram have been found to be 3–25 mSv. The cancer risk from 100 mSv was estimated to be six out of 1000 people by the International Commission on Radiation Protection. Using appropriate CCTA protocol and techniques has been found to substantially reduce patient radiation dose [8].
5 Calcium Scoring in Addition to the CCTA
The inclusion of the calcium score into the chest pain protocol is controversial. CT calcium scoring allows detection and quantification of coronary artery calcification. The prospective multicenter Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography (ACCURACY) trial by Budoff et al. conducted at 16 centers enrolling 230 patients compared the diagnostic accuracy of coronary arterial calcium (CAC) by 64-row CT to invasive coronary angiography, and concluded that CAC demonstrates a high sensitivity and low specificity for the presence of coronary artery stenosis [21]. Abnormal levels of calcium may place patients into a higher risk group, but does not always help with the clinical diagnosis, particularly in the presence of diffuse moderate coronary atheroma. A zero-calcium score (ZCS) is associated with an excellent prognosis in healthy patients [22]. As the calcium score rises above zero and patients have symptoms of ACS, so does the prevalence of CAD and risk of death.
A recent study by Hulten et al. reported up to a 2% prevalence rate among symptomatic patients with ZCS using CCTA [23]. Prior studies have demonstrated high sensitivity but poor specificity of positive CAC to detect obstructive CAD (≥50% stenosis by invasive coronary angiography) among patients with stable chest pain. Conversely, a CAC of 0 provides high specificity but poor sensitivity to identify obstructive CAD among patients with acute chest pain. In this regard, CAC might be a gatekeeper to identify low risk patients; however, CAC cannot reliably exclude obstructive CAD in subjects with acute chest pain seen in the ED [24].
6 Coronary CTA and Identification of Unstable Plaques
Goldstein et al., in their study, also showed that CCTA has the ability to recognize vulnerable plaques and provide additional relevant information beyond angiography alone [18]. Complex plaque morphology is the angiographic hallmark of unstable coronary lesions. Invasive, complex lesions are characterized by haziness, border irregularity, frank ulceration, intraplaque contrast persistence, and luminal filling defect. CCTA features of plaques in patients with ACS are just being identified. However, the CCTA correlates of angiographically diagnosed, complex unstable coronary lesions have not been fully delineated. The CCTA-documented lesion morphology is strikingly similar to invasive angiographic features indicative of plaque disruption, including lesion haziness, irregularity, ulceration, and intra-plaque contrast penetration (See Fig. 3). On CCTA images, complex lesions typically appeared bulky, hypodense, eccentric, and positively remodeled with features similar to complex ruptured plaque seen by intravascular ultrasound (see Fig. 4). Given the increasing use of CCTA to evaluate acute chest pain, characterization of plaque instability has considerable clinical implications [25].
A very concerning unstable plaque. A 50-year-old male patient with unstable (resting) chest pain, and normal ECG × 2 and troponin panel. CCTA shows subtotal (99%) mostly soft plaque-formed Distal LCX stenosis (white arrows). Personal history of poorly controlled diabetes × 10 years and family history of CAD. (Images obtained courtesy of Dr. Aiden Abidov)
CCTA can identify more than 1 complex plaque. A 46-year-old male patient with progressive exertional angina, normal ECG and biomarkers and evidence of obstructive predominantly calcified (white arrow) proximal LAD plaque, obstructive mixed (calcified and noncalcified proximal-mid LAD plaque (green arrow) and high grade (95%) soft mid LAD plaque (blue arrow). The patient has strong family history of CAD, and personal history of heavy smoking (40 pack/years) and untreated hyperlipidemia. (Images obtained courtesy of Dr. Aiden Abidov)
The CCTA can identify more than 1 complex plaque apart from the angiogram-identified culprit vessel. A fair amount of data shows that patients with ACS may have multiple complex plaques this may be detectable by CCTA (see Fig. 4), but significant plaque extension is underappreciated by invasive angiography.
7 CT for Diagnosis of Pulmonary Embolism
The 64-slice CT scan reduces examination time, collimation, and partial-volume averaging, and increases total volume scanned. There are several acquisition protocols for CT scanning in pulmonary embolism (PE) where breath holding is required between 4 and 40 s based on the clinical status and CT technology. Using MSCT, almost every patient is able to maintain a strict breath holding spell of a minimum 5 s which is sufficient to comply with the fastest protocol. Different protocols of contrast medium administration are available and can be either low concentration–high volume or high concentration–low volume, thus creating a balance between quality of vascular enhancement and total amount of iodine injected.
Direct demonstration can be made using vascular signs of acute PE on spiral CT pulmonary angiography (SCTPA) and these include (1) central partial intravascular defect surrounded by contrast medium, (2) eccentric partial filling defect or mural defect surrounded by contrast medium presenting an acute angle with the vessel wall, and (3) complete filling defect not surrounded by contrast medium and occupying the entire arterial vessel section (see Fig. 5). Ancillary findings related to PE included wedge-shaped pleural-based consolidation, “vascular sign” which is thickened vessel leading to the apex of the consolidation and usually indicating an infarction, oligemia, and dilatation of central arteries [26]. The acute dilatation of the right ventricle (RV) can be a useful sign to assess the severity of PE though RV strain or failure and is best detected by echocardiography; however, SCTPA can quantify some morphological abnormalities [27]. Positive results for PE on SCTPA are widely accepted as a valid demonstration of PE, but a negative result has been viewed with skepticism by many physicians. The clinical validity of a negative CT scan in a patient suspected of PE was evaluated in a systematic review of fifteen studies that showed an overall negative likelihood ratio of a venous thromboembolism after a negative SCTPA was 0.07 (95% confidence interval [CI], 0.05–0.11) and the NPV was 99.1% (95% CI, 98.7–99.5%) [28]. These results clinically validated that the use of SCTPA to rule out PE is similar to that reported for conventional pulmonary angiography, and patients with negative results need no further evaluation or treatment.
(a, b) Bilateral pulmonary embolism in a patient presenting with circulatory collapse and chest pain (a) Reconstructed axial CT angiograms (1.25 mm thick) obtained with Multislice CT demonstrate multiple clots. Acute clots (yellow arrows) in the left and right pulmonary arteries present as filling defects in the column of contrast material, that form an acute angle with the vessel wall. (b) Coronal oblique view with large pulmonary embolism in left pulmonary artery (yellow arrow) (images obtained courtesy of Dr. Samuel Johnson)
8 CT for Diagnosis of Aortic Dissection
Aortic dissection (AD) is the most frequent and fatal aortic emergency, and with the newer imaging modalities including the MSCT scan, we are able to identify entry tears and involvement of visceral branches, making it helpful to make management decisions in the ED. A better understanding of the complex mechanisms involved with dissection and the development of endovascular techniques has made the management of AD more likely to be successful [29].
When AD is suspected, the examination must explore the entire thoracic and abdominal aorta together with the iliac and common femoral arteries. The MSCT scanners permit this extended exploration with multiplanar reformatting to clarify certain details that are difficult to analyze in the conventional transverse and axial sections [30]. The cardinal sign of AD is the appearance of a detached intimal flap in the form of a fine, hypodense band in the opacified aortic lumen. This indicates the extent of dissection in the aortic wall and thus distinguishes the true channel (true aortic lumen) from the false channel (blood circulating in the wall of the aorta) [31, 32].
“Cobwebs” are residual ribbons of media between the damaged aortic wall and the intima; these structures thus identify the false channel. In CT angiography, they appear as fine, hypodense, linear strips attached to the damaged aortic wall and may or may not rejoin the intima (see Fig. 6). The abdominal branches of the descending aorta and iliac arteries may trigger malperfusion of the organs due to the dissection during the acute phase or on follow-up (see Fig. 7) [33]. Although transesophageal echocardiography (TEE) is still useful in AD, the rapid availability of MSCT scan, nonreliability on an operator, and ability to detect malperfusion syndrome makes MSCT a much better option to be used in the ED.
Chronic aortic dissection. CT-scan obtained in descending aorta showing web like intimal flap from chronic dissection. Outer wall calcification surrounding the true lumen (F). Visualization of an aortic cobweb between the intimal flap and the outer wall (yellow arrow). (Images obtained courtesy of Dr. Samuel Johnson)
(a–c) Acute dissection with extension along the abdominal aorta. (a) Axial CT scan section at the level of the left renal artery shows dissection extend into abdominal aorta (Yellow arrow) with both renal arteries supplied by true lumen (T: true lumen) with normal perfusion. (b) Transverse image shows normal ascending aorta (white arrow) and dissection in descending with lower enhancement in the false lumen (F false lumen). (c) Coronal view shows communication (yellow double end arrow) between true (T true lumen) and false (F false lumen) lumen. (Images obtained courtesy of Dr. Samuel Johnson)
9 The “Triple Rule Out” CT Protocol
CCTA has shown diagnostic accuracy in excluding ACS in ED patients and proven clinical accuracy for diagnosis of AD [33,34,35,36] and PE [26, 28, 37,38,39]; therefore, a “triple rule-out” (TRO) scan protocol to simultaneously exclude all three potentially fatal causes of acute chest pain with a single scan has come to be an attractive option. Once 64-slice CT scanners became widely available, the technical limitations of combined simultaneous evaluation of all three vascular areas have been largely overcome. A conventional CCTA “field of view” already includes the anatomy between the carina and the diaphragm. The technical challenge of a TRO scan protocol is to obtain high and consistent contrast intensity in all three vascular beds. A combined simultaneous evaluation for the pulmonary and coronary vessels and thoracic aorta requires a carefully tailored imaging and injection protocol (see Fig. 8). In evaluating one such protocol, Vrachliotis et al. prospectively imaged 50 ED chest pain patients who underwent single-acquisition 64-slice CT angiography to evaluate the enhancement of the coronary, pulmonary, and thoracic vasculature [40]. A “triphasic” injection protocol was used that delivered the standard 100 mL of iodinated contrast at 5 mL/sec typical for CCTA examinations, followed by an additional 30 mL at 3 mL/s to maintain pulmonary artery opacification, followed by a standard saline flush injection. This protocol is easy to achieve with commercially available radiographic injectors. Importantly, a caudal–cranial scan acquisition was used (as opposed to the standard CCTA cranial–caudal technique) to scan the distal pulmonary arteries at the base of the lung earlier, as these are the most subject to problems with low contrast intensity. Mean coronary artery, pulmonary artery, and aortic enhancement values were consistently higher than 250 Hounsfield Units, and right atrial enhancement did not interfere with interpretation of the coronary arteries [40].
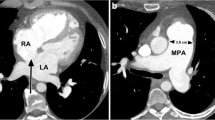
(a, b) “Triple rule out” scan acquisition in a patient with acute chest pain (a) Bilateral large pulmonary emboli are seen, as well as (b) right heart strain, RV > LV (RV = right ventricle, LV = left ventricle); pulmonary embolism in left lower lobar branches (yellow arrows). The patient was also noted to have >50% mixed calcified and noncalcified plaque in the proximal left anterior descending coronary artery). (Images obtained courtesy of Dr. Samuel Johnson)
10 Dedicated Coronary Vs. “Triple Rule Out” Scan Protocol: Radiation Dose Considerations
In spite of these technical advances, important radiation safety concerns remain that should limit indiscriminate application of a TRO scan protocol. Compared to the usual radiation dose of a standard CCTA (generally ranging from 7 to 13 mSv, depending on body habitus, gender, and scan protocol), the effective radiation dose of a TRO CT scan is often increased by 50%, simply due to the greater anatomic coverage and thus increased field of view [41]. Further, among patients who undergo CCTA as a primary triage test in the ED, there is a subset who also require a noninvasive stress test (often a radionuclide test), followed in some cases by diagnostic and interventional invasive angiographic procedures. This combined radiation dose is a cause for concern; however, changing the 0.6 mm high-resolution used for CCTA to 2 mm for scanning the upper lung fields (since pulmonary angiography does not require submillimeter resolution) in theory can significantly reduce radiation dosage. Innovative imaging protocols involving tight heart rate control and “prospective gating” can drastically reduce radiation exposure (to under 5 mSV) but these are difficult to apply in ED patients and not currently being utilized in these patients.
In addition, the TRO CT also has a slightly higher intravenous contrast load to opacify both the right and left sided circulations [41, 42]. A recently conducted systematic review and meta-analysis of eleven studies showed that TRO CT had comparable image quality to CCTA and is highly accurate in detecting CAD, but the low prevalence of PE and AD (<1% in the studies) and the increased risk of radiation and contrast exposure make TRO CT not recommendable at the moment for this indication [42].
11 Assessment for Noncardiac, Extravascular Pathology
Well over 50% of acute chest pain is caused by noncardiac conditions [43]. In patients who undergo a dedicated CCTA, images of noncardiac thoracic structures are contained in the field of view and therefore available to the expert reader. Diseases that can be detected (in addition to aortic and pulmonary arterial pathology) include pericardial thickening and/or effusions, esophageal pathology, pneumonia, pulmonary nodules, pneumothoraces, mediastinal masses, pleural effusions and masses, as well as chest-wall abnormalities. Previous studies have demonstrated that up to 1 in 6 patients without coronary abnormalities detected on CT were diagnosed with noncardiac findings that could explain their presenting symptoms [44]. These findings suggest that for patients with acute chest pain, a comprehensive review of the thoracic, cardiac, and noncardiac structures should be undertaken.
There has been an ongoing debate about whether to use a more limited approach to imaging so that it raises fewer false alarms or a broader approach so that serious pathological conditions are not missed. In the study by Johnson et al. comparing the two approaches, it was shown that almost one-fourth of all patients who underwent CCTA had extra cardiac findings [45]. When CCTA were viewed in a limited, or focused way, the result was substantially reduced sensitivity for pathologic findings outside the mediastinum and heart. Serious pathologic conditions were missed, but many false-positive diagnoses were avoided. Use of the broader view approach led to downstream workup of 10.2% of the findings, and a later follow-up of 50.6% of the patients demonstrated little or no new clinical consequence to the patient. After small hiatal hernia, lung nodules were the most common extra cardiac finding (6.2% of the patients). In another study by Lee et al., most extra cardiac findings were indeterminate pulmonary nodules [46]. Until more studies clarify the benefits and risks of identifying early lung neoplasms, we cannot say for certain whether it is prudent to report incidental extra cardiac findings on CCTA. A conservative approach of careful comparison of incidental findings with prior studies, providing clear follow-up recommendations, and proper interphysician communication will be key to preventing unnecessary utilization of medical resources.
12 Coronary CTA Limitations and Protocol Considerations
Coronary CTA has several important limitations that affect its usefulness in the triage of ED patients with acute chest pain. There are two major limitations for reliable assessment of all coronary segments: motion artifacts and severe coronary calcifications [9]. It has been convincingly shown that the heart rate and regularity of the rhythm is closely related to motion artifact, image quality, and thus accuracy of coronary stenosis estimation [7]. It is common practice to premedicate patients who have resting heart rates >65 beats/min with beta blocking drugs, and to administer sublingual nitroglycerin to patients to enhance image quality. If available, dual-source CCTA obviates the need for beta blocker administration in most patients. At this time, most facilities do not perform CCTA in patients with irregular rhythms; however, recent hardware and software improvements allow for imaging of patients with irregular rhythms including atrial fibrillation [47, 48]. A second major limitation is that CCTA presently provides data regarding anatomical lesions only, not their physiologic impact on coronary blood flow.
It is also essential to screen patients in the ED for a history of iodine allergy and to avoid administration of contrast in patients with diminished creatinine clearance. Finally, the importance of a team approach to implementation of a CCTA ED triage protocol cannot be overstated. Emergency physicians, radiologists and cardiologists must be well educated regarding the application and inherent limitations of CCTA, and a complete review of cardiac and adjacent structures available from the CT data should be performed by physicians with appropriate backgrounds and level of experience.
There are now over 30 published studies comparing CCTA to quantitative invasive coronary angiography, encompassing over 2000 patients [7, 12, 49,50,51,52]. Among the 18 studies in which per-patient analyses are available (involving 1329 patients, using either 16 or 64-slice CT), the mean subject-weighted sensitivity and specificity for the detection of obstructive CAD was 97% and 84% respectively [7]. An analysis of just the 64-slice studies revealed a sensitivity and specificity of 98% and 93%, respectively. Importantly, the combined results from all 18 studies demonstrated a mean per-patient NPV of 97%. These data support the hypothesis that a low risk CCTA may obviate the need for invasive angiography in properly selected clinical circumstances. These studies validate that patients at the opposite ends of the disease spectrum (i.e. those with <25% vs. >70% maximal luminal stenosis) can be accurately triaged by CCTA alone, while patients with lesions of intermediate severity (25–70% stenosis) may require functional testing.
13 Conclusions
Computed tomography has evolved over the past three decades into a powerful imaging tool that has proven clinical accuracy for the diagnosis of AD and PE. CCTA has been recently validated as a highly sensitive and reliable technique to confirm or exclude significant coronary stenosis in patients with suspected CAD. Initial experience suggests that CCTA is an accurate and efficient test for the triage of appropriately selected acute chest pain patients to early discharge or further inpatient diagnosis and treatment. Patients presenting the ED with a low to intermediate pretest likelihood of CAD and nonsignificant cardiac biomarkers and EKGs are best suited for CCTA-based triage. Technical advances now permit acquisition of well-opacified images of the coronary arteries, thoracic aorta, and pulmonary arteries from a single CCTA scan protocol. While this TRO technique can potentially exclude fatal causes of chest pain in all three vascular beds, the resultant higher radiation dose of this method precludes its routine use, except when there is sufficient support for the diagnosis of either AD or PE. Having good interphysician communication about the incidental findings on the CCTA scan is vital in making sure that important findings are not missed and there is appropriate utilization of medical resources.
References
McCaig LF. National Hospital Ambulatory Medical Care Survey 2003: emergency department summary: advance data from vital and health statistics, No. 358. National Center for Health Statistics; 2005.
Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief. 2010;43:1–8.
Kalender WA, Seissler W, Klotz E, Vock P. Spiral volumetric CT with single-breath-hold technique, continuous transport, and continuous scanner rotation. Radiology. 1990;176(1):181–3.
Ohnesorge B, Flohr T, Becker C, Kopp AF, Schoepf UJ, Baum U, et al. Cardiac imaging by means of electrocardiographically gated multisection spiral CT: initial experience. Radiology. 2000;217(2):564–71.
Achenbach S, Ulzheimer S, Baum U, Kachelriess M, Ropers D, Giesler T, et al. Noninvasive coronary angiography by retrospectively ECG-gated multislice spiral CT. Circulation. 2000;102(23):2823–8.
Mahesh M. Search for isotropic resolution in CT from conventional through multiple-row detector. Radiographics. 2002;22(4):949–62.
Raff GL, Goldstein JA. Coronary angiography by computed tomography: coronary imaging evolves. J Am Coll Cardiol. 2007;49(18):1830–3.
Medical AS. 64-slice computed tomographic angiography for the diagnosis of intermediate risk coronary artery disease: an evidence-based analysis. Ont Health Technol Assess Ser. 2010;10(11):1–44.
Mollet NR, Cademartiri F, Nieman K, Saia F, Lemos PA, McFadden EP, et al. Multislice spiral computed tomography coronary angiography in patients with stable angina pectoris. J Am Coll Cardiol. 2004;43(12):2265–70.
Lee TH, Goldman L. Evaluation of the patient with acute chest pain. N Engl J Med. 2000;342(16):1187–95.
Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163–70.
Mollet NR, Cademartiri F, van Mieghem CA, Runza G, McFadden EP, Baks T, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112(15):2318–23.
Leber AW, Knez A, von Ziegler F, Becker A, Nikolaou K, Paul S, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46(1):147–54.
Raff GL, Gallagher MJ, O'Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46(3):552–7.
Gallagher MJ, Raff GL. Use of multislice CT for the evaluation of emergency room patients with chest pain: the so-called “triple rule-out”. Catheter Cardiovasc Interv. 2008;71(1):92–9.
Hollander JE, Litt HI, Chase M, Brown AM, Kim W, Baxt WG. Computed tomography coronary angiography for rapid disposition of low-risk emergency department patients with chest pain syndromes. Acad Emerg Med. 2007;14(2):112–6.
Kim J, Lee H, Song S, Park J, Jae H, Lee W, et al. Efficacy and safety of the computed tomography coronary angiography based approach for patients with acute chest pain at an emergency department: one month clinical follow-up study. J Korean Med Sci. 2010;25(3):466–71.
Goldstein JA, Gallagher MJ, O'Neill WW, Ross MA, O'Neil BJ, Raff GL. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49(8):863–71.
Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (rule out myocardial infarction using computer assisted tomography) trial. J Am Coll Cardiol. 2009;53(18):1642–50.
Otero HJ, Rybicki FJ. Reimbursement for chest-pain CT: estimates based on current imaging strategies. Emerg Radiol. 2007;13(5):237–42.
Budoff MJ, Jollis JG, Dowe D, Min J. Diagnostic accuracy of coronary artery calcium for obstructive disease: results from the accuracy trial. Int J Cardiol. 2013;166(2):505–8.
Shaw LJ, Giambrone AE, Blaha MJ, Knapper JT, Berman DS, Bellam N, et al. Long-term prognosis after coronary artery calcification testing in asymptomatic patients: a cohort study. Ann Intern Med. 2015;163(1):14–21.
Hulten E, Bittencourt MS, Ghoshhajra B, O'Leary D, Christman MP, Blaha MJ, et al. Incremental prognostic value of coronary artery calcium score versus CT angiography among symptomatic patients without known coronary artery disease. Atherosclerosis. 2014;233(1):190–5.
Rubinshtein R, Gaspar T, Halon DA, Goldstein J, Peled N, Lewis BS. Prevalence and extent of obstructive coronary artery disease in patients with zero or low calcium score undergoing 64-slice cardiac multidetector computed tomography for evaluation of a chest pain syndrome. Am J Cardiol. 2007;99(4):472–5.
Goldstein JA, Dixon S, Safian RD, Hanzel G, Grines CL, Raff GL. Computed tomographic angiographic morphology of invasively proven complex coronary plaques. JACC Cardiovasc Imaging. 2008;1(2):249–51.
Ghaye B, Remy J, Remy-Jardin M. Non-traumatic thoracic emergencies: CT diagnosis of acute pulmonary embolism: the first 10 years. Eur Radiol. 2002;12(8):1886–905.
Reid JH, Murchison JT. Acute right ventricular dilatation: a new helical CT sign of massive pulmonary embolism. Clin Radiol. 1998;53(9):694–8.
Quiroz R, Kucher N, Zou KH, Kipfmueller F, Costello P, Goldhaber SZ, et al. Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review. JAMA. 2005;293(16):2012–7.
Khayat M, Cooper KJ, Khaja MS, Gandhi R, Bryce YC, Williams DM. Endovascular management of acute aortic dissection. Cardiovasc Diagn Ther. 2018;8(1):97–107.
Novelline RA, Rhea JT, Rao PM, Stuk JL. Helical CT in emergency radiology. Radiology. 1999;213(2):321–39.
Williams DM, Joshi A, Dake MD, Deeb GM, Miller DC, Abrams GD. Aortic cobwebs: an anatomic marker identifying the false lumen in aortic dissection–imaging and pathologic correlation. Radiology. 1994;190(1):167–74.
LePage MA, Quint LE, Sonnad SS, Deeb GM, Williams DM. Aortic dissection: CT features that distinguish true lumen from false lumen. AJR Am J Roentgenol. 2001;177(1):207–11.
Willoteaux S, Lions C, Gaxotte V, Negaiwi Z, Beregi JP. Imaging of aortic dissection by helical computed tomography (CT). Eur Radiol. 2004;14(11):1999–2008.
Yoshida S, Akiba H, Tamakawa M, Yama N, Hareyama M, Morishita K, et al. Thoracic involvement of type A aortic dissection and intramural hematoma: diagnostic accuracy–comparison of emergency helical CT and surgical findings. Radiology. 2003;228(2):430–5.
Hamada S, Takamiya M, Kimura K, Imakita S, Nakajima N, Naito H. Type A aortic dissection: evaluation with ultrafast CT. Radiology. 1992;183(1):155–8.
Shiga T, Wajima Z, Apfel CC, Inoue T, Ohe Y. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med. 2006;166(13):1350–6.
Anderson DR, Kovacs MJ, Dennie C, Kovacs G, Stiell I, Dreyer J, et al. Use of spiral computed tomography contrast angiography and ultrasonography to exclude the diagnosis of pulmonary embolism in the emergency department. J Emerg Med. 2005;29(4):399–404.
Prologo JD, Gilkeson RC, Diaz M, Asaad J. CT pulmonary angiography: a comparative analysis of the utilization patterns in emergency department and hospitalized patients between 1998 and 2003. AJR Am J Roentgenol. 2004;183(4):1093–6.
Ghanima W, Almaas V, Aballi S, Dorje C, Nielssen BE, Holmen LO, et al. Management of suspected pulmonary embolism (PE) by D-dimer and multi-slice computed tomography in outpatients: an outcome study. J Thromb Haemost. 2005;3(9):1926–32.
Vrachliotis TG, Bis KG, Haidary A, Kosuri R, Balasubramaniam M, Gallagher M, et al. Atypical chest pain: coronary, aortic, and pulmonary vasculature enhancement at biphasic single-injection 64-section CT angiography. Radiology. 2007;243(2):368–76.
Burris AC, Boura JA, Raff GL, Chinnaiyan KM. Triple rule out versus coronary CT angiography in patients with acute chest pain: results from the ACIC Consortium. JACC Cardiovasc Imaging. 2015;8(7):817–25.
Ayaram D, Bellolio MF, Murad MH, Laack TA, Sadosty AT, Erwin PJ, et al. Triple rule-out computed tomographic angiography for chest pain: a diagnostic systematic review and meta-analysis. Acad Emerg Med. 2013;20(9):861–71.
Gallagher MJ, Ross MA, Raff GL, Goldstein JA, O'Neill WW, O'Neil B. The diagnostic accuracy of 64-slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med. 2007;49(2):125–36.
Onuma Y, Tanabe K, Nakazawa G, Aoki J, Nakajima H, Ibukuro K, et al. Noncardiac findings in cardiac imaging with multidetector computed tomography. J Am Coll Cardiol. 2006;48(2):402–6.
Johnson KM, Dennis JM, Dowe DA. Extracardiac findings on coronary CT angiograms: limited versus complete image review. AJR Am J Roentgenol. 2010;195(1):143–8.
Lee CI, Tsai EB, Sigal BM, Plevritis SK, Garber AM, Rubin GD. Incidental extracardiac findings at coronary CT: clinical and economic impact. AJR Am J Roentgenol. 2010;194(6):1531–8.
Andreini D, Pontone G, Mushtaq S, Conte E, Perchinunno M, Guglielmo M, et al. Atrial fibrillation: diagnostic accuracy of coronary CT angiography performed with a whole-heart 230-microm spatial resolution CT scanner. Radiology. 2017;284(3):676–84.
Mushtaq S, Conte E, Melotti E, Andreini D. Coronary CT angiography in challenging patients: high heart rate and atrial fibrillation. A review. Acad Radiol. 2019;26(11):1544–9.
Leschka S, Alkadhi H, Plass A, Desbiolles L, Grunenfelder J, Marincek B, et al. Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J. 2005;26(15):1482–7.
Pugliese F, Mollet NR, Runza G, van Mieghem C, Meijboom WB, Malagutti P, et al. Diagnostic accuracy of non-invasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol. 2006;16(3):575–82.
Ropers D, Rixe J, Anders K, Kuttner A, Baum U, Bautz W, et al. Usefulness of multidetector row spiral computed tomography with 64- × 0.6-mm collimation and 330-ms rotation for the noninvasive detection of significant coronary artery stenoses. Am J Cardiol. 2006;97(3):343–8.
Hamon M, Morello R, Riddell JW, Hamon M. Coronary arteries: diagnostic performance of 16- versus 64-section spiral CT compared with invasive coronary angiography–meta-analysis. Radiology. 2007;245(3):720–31.
Acknowledgements
Images obtained courtesy Samuel Johnson MD (Interim Chair, Department of Radiology, Wayne State University, Detroit, Michigan, USA) and Aiden Abidov MD PhD (Section Chief, Cardiology, VA Hospital Detroit, Michigan, USA. Department of Medicine/ Division of Cardiology, Wayne State University, Detroit, Michigan, USA).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kumar, V.A., O’Neil, B. (2022). Use of Multislice CT for the Evaluation of Patients with Chest Pain. In: Pena, M., Osborne, A., Peacock, W.F. (eds) Short Stay Management of Chest Pain. Contemporary Cardiology. Humana, Cham. https://doi.org/10.1007/978-3-031-05520-1_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-05520-1_15
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-031-05519-5
Online ISBN: 978-3-031-05520-1
eBook Packages: MedicineMedicine (R0)