Abstract
Triple rule-out (TRO) CT simultaneously evaluates the coronary arteries, aorta and pulmonary arteries for the patient presenting with acute chest pain in the emergency department. Compared to dedicated coronary CT angiography (cCTA), TRO CT requires slightly more intravenous contrast and a higher radiation dose. Appropriate patient selection is essential. TRO CT is most appropriate for patients at low to intermediate risk for acute coronary syndrome and in whom alternative diagnoses, such as pulmonary embolism or acute aortic pathology, are being considered. Adequate patient selection, preparation, premedication, and monitoring ensure a high-quality diagnostic study. The major differences between TRO CT and dedicated cCTA are scan length and injection technique. Compared to cCTA, where images are obtained between the carina and diaphragm, TRO CT must include the entire thoracic aorta and the pulmonary arteries. Injection protocols are tailored to provide high levels of arterial enhancement in the left- (coronary arteries and aorta) and right-sided circulations (pulmonary arteries). Different strategies may be employed to limit radiation exposure, including electrocardiogram (ECG) tube current modulation and prospective ECG gating. When performed with careful attention to technique, TRO CT provides high-quality diagnostic opacification of the coronary arteries, aorta and pulmonary arteries equal to that of dedicated CT angiography. A negative study allows for the safe and rapid discharge from the emergency department and a reduction in subsequent testing.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction to Triple Rule-Out CT
Acute chest pain is a common presenting complaint in the emergency department in the United States, accounting for 5.5 million visits in 2007–2008 or 9 % of all emergency department visits (Bhuiya et al. 2010). Acute coronary syndrome (ACS) is a serious cause of acute chest pain with high morbidity and mortality, as well as an estimated annual cost of $150 billion (Kolansky 2009). Approximately 3–33 % of patients with ACS die from their ACS, and up to 30 % of discharged ACS patients are rehospitalized within 6 months (Kolansky 2009; Ropp et al. 2015). Morbidity and mortality are reduced with accurate and rapid diagnosis. However, ACS accounts for a minority of acute chest pain visits, only 13 % in 2007–2008 (Bhuiya et al. 2010). Patient presentation is often not straightforward, and various other diagnoses are often considered. The workup of acute chest pain can be expensive and time consuming and may include electrocardiogram (ECG) evaluation, laboratory tests, diagnostic imaging studies, and observation or hospital admission. The goal of diagnostic imaging is to triage patients in the emergency department and allow for safe and rapid discharge directly from the emergency department after life-threatening conditions have been excluded. The use of advanced diagnostic imaging for acute chest pain evaluation increased from 3.4 % of all emergency department visits in 1999–2000 to 15.9 % in 2007–2008 (Bhuiya et al. 2010).
Triple rule-out (TRO) CT is an attractive option for acute chest pain assessment, simultaneously examining the coronaries, aorta, and pulmonary arteries as well as the adjacent extravascular structures (Fig. 1). TRO CT excludes the potentially fatal diagnoses of pulmonary embolism and aortic dissection, along with ACS. When used in the appropriate patient population, TRO CT can be safe, efficient, and cost-effective. When performed with appropriate technique, TRO CT image quality is equivalent to dedicated coronary CT angiography (cCTA) for evaluation of the coronary arteries but with greater enhancement of the distal thoracic aorta and adequate enhancement of the pulmonary arteries for diagnosis of pulmonary embolism (Halpern et al. 2009; Shapiro et al. 2009; Rahmani et al. 2009; Johnson et al. 2007; Litmanovich et al. 2008). Note that comprehensive cardiac CT imaging may include perfusion imaging or CT fractional flow reserve calculation, but these topics are covered in other chapters of this text.
Images from a normal TRO CT. Oblique sagittal slab maximum intensity projection (MIP) image of the thoracic aorta (a) and coronal slab MIP images of the right (b) and left (c) pulmonary arteries show normal vascular opacification without acute aortic pathology or pulmonary embolism. Both right- and left-sided circulations are opacified adequately. The scan length includes the aortic arch (a) allowing for complete evaluation of the thoracic aorta. Note adequate opacification to the level of the subsegmental pulmonary artery branches (b, c). Volume-rendered images of the coronary arteries (d, e) demonstrate opacification of all coronary arteries without stenosis or segmental occlusion. Slab MIP images allow evaluation of each vessel individually to exclude disease (f left anterior descending (LAD)
Multiple studies have been performed comparing dedicated cCTA to invasive coronary angiography, demonstrating a high negative predictive value of 83–99 % (Budoff et al. 2008; Miller et al. 2008; Meijboom et al. 2008). As discussed in other chapters, dedicated cCTA may be superior to myocardial perfusion imaging in the acute chest pain setting, (Gallagher et al. 2007) with decreased time to diagnosis, with decreased cost for the acute care episode, and without increase in major cardiac adverse events (Goldstein et al. 2011). The main limitation of cCTA in the acute setting is the relatively low specificity, as patients with intermediate severity lesions often require further physiological testing to confirm presence of clinically significant disease (Goldstein et al. 2007; Chen et al. 2012). The major strength of cCTA is in its high negative predictive value in low- to intermediate-risk patients, allowing for the safe, rapid discharge of these patients directly from the emergency department after a negative evaluation (Goldstein et al. 2007; Hoffmann et al. 2012; Litt et al. 2012; Chang et al. 2008a, b). Patients discharged after a negative cCTA have very low risk of adverse cardiovascular events at 1–2 years (Rubinshtein et al. 2007; Hollander et al. 2009; Schlett et al. 2011). In patients admitted for further workup, cCTA results in decreased length of stay compared to standard evaluation. Although cCTA reduces repeat evaluations for recurrent chest pain, (Goldstein et al. 2007) at least one study links cCTA to increased downstream testing and higher radiation exposure (Hoffmann et al. 2012).
In contrast to dedicated cCTA, TRO CT simultaneously evaluates the pulmonary arteries, entire thoracic aorta, and additional portions of the adjacent lung zones. TRO CT can be helpful to evaluate patients with acute chest pain in whom additional diagnoses other than ACS are considered. Because the image quality of TRO CT is equivalent to dedicated cCTA (Halpern et al. 2009; Shapiro et al. 2009; Rahmani et al. 2009; Johnson et al. 2007; Litmanovich et al. 2008), we expect the same diagnostic accuracy and negative predictive value for TRO CT as cCTA with respect to coronary artery evaluation. Published reports suggest that TRO CT has a sensitivity of 94.3 % and specificity of 97.4 % for the diagnosis of coronary disease (Ayaram et al. 2013) with a negative predictive value similar to dedicated cCTA of 99 % (Takakuwa and Halpern 2008), allowing for safe discharge of patients with negative evaluations (Gruettner et al. 2013). Unlike cCTA, there are only a few studies directly comparing TRO CT to invasive coronary angiography. These demonstrate 100 % sensitivity and negative predictive value for coronary disease (Johnson et al. 2008) and high agreement with invasive coronary angiography (Litmanovich et al. 2008). TRO CT reduces length of hospitalization and cost compared to invasive coronary angiography (Savino et al. 2006). Compared to nuclear stress imaging, TRO CT results in decreased length of stay and mean imaging time but with higher mean imaging cost related to the higher charge for TRO CT as compared with dedicated cCTA (Takakuwa et al. 2011).
The main advantage of TRO CT over cCTA is in the evaluation of noncoronary diagnoses (Figs. 2, 3, and 4). In a previous study at our institution, TRO CT identified a noncoronary diagnosis to explain the presenting complaint in 11 % of patients and eliminated the need for further diagnostic testing in over 75 % of patients (Takakuwa and Halpern 2008). A more recent review of our 9-year experience performing TRO CT demonstrated a significant noncoronary diagnosis that may explain the chest pain presentation in 8.9 % of patients. These most commonly included pulmonary embolism, aortic aneurysm, and other aortic pathology. Approximately one third of noncoronary diagnoses would not have been identified on a dedicated cCTA (Wnorowski and Halpern 2016) because of unopacified right-sided circulation or limited z-axis coverage in cCTA (Figs. 5 and 6).
Acute aortic pathology. Oblique sagittal slab MIP image from TRO CT (a) demonstrates a type B aortic dissection extending from the distal aortic arch (arrow) to the descending aorta. Inclusion of the aortic arch in the scan length of TRO CT allows for more complete evaluation in cases of aortic dissection. Oblique sagittal slab MIP image from TRO CT in a second patient (b) shows diffuse atherosclerotic changes with a penetrating ulcer of the mid descending thoracic aorta (arrow)
Acute pulmonary embolism. Axial slab MIP image (a) demonstrates a central filling defect (arrow) spanning the right and left main pulmonary arteries, consistent with an acute saddle pulmonary embolus. Oblique coronal slab MIP image (b) in a second patient shows acute pulmonary emboli within right upper and middle lobe segmental and subsegmental pulmonary artery branches (arrowheads)
Pneumonia. Axial CT image from a TRO CT in a soft tissue window (a) in a patient presenting with acute chest pain shows normal coronary arteries. The same axial CT image displayed in a lung window (b) demonstrates multifocal pneumonia with airspace opacities in the right middle and lower lobes (arrowheads)
Penetrating aortic ulcer of the distal aortic arch. Axial (a) and oblique sagittal (b) slab MIP images demonstrate diffuse atherosclerotic changes of the thoracic aorta with a penetrating ulcer of the distal aortic arch (arrowheads). This ulcer would not have been included within the typical z-axis coverage of a dedicated coronary CTA (cCTA)
Isolated acute upper lobe pulmonary emboli. Axial slab MIP image (a) from a TRO CT demonstrates an isolated left upper lobe pulmonary embolus extending into an anterior segmental branch (arrowheads). Oblique coronal slab MIP image (b) in a second patient shows an isolated right upper lobe pulmonary embolus involving a segmental branch (arrowhead). Opacification of right-sided circulation and extended z-axis coverage allow for identification of pulmonary emboli that would not be diagnosed on dedicated cCTA. Because of decreased z-axis coverage, isolated upper lobe pulmonary emboli would likely be missed even if dedicated cCTA opacified both right- and left-sided circulations, especially in the case of isolated upper lobe segmental branch emboli (b)
The relatively low prevalence of pulmonary embolism and aortic dissection in the TRO CT population has largely precluded calculation of sensitivity for these conditions (Ayaram et al. 2013). Diagnostic accuracy of dedicated CT angiography for acute aortic dissection (Willoteaux et al. 2004; Yoshida et al. 2003; Hamada et al. 1992; Shiga et al. 2006; Sebastia et al. 1999) and pulmonary embolism (Quiroz et al. 2005; Ghanima et al. 2005; Prologo et al. 2004; Ghaye et al. 2002) has been well established. As there is no difference in vascular enhancement and image quality in properly performed TRO CT compared to dedicated CT angiography, we can expect similar diagnostic accuracy. The high quality that is routinely obtained for aortic and pulmonary evaluation with our TRO CT studies is illustrated in the previous figures (Figs. 2, 3, 5, and 6). Overall sensitivity, specificity, and positive and negative predictive value of TRO CT for the diagnosis of all vascular pathology is 100 %, 98 %, 95 %, and 100 %, respectively (Schertler et al. 2009). Other authors who have evaluated patients presenting with suspected pulmonary embolism have also demonstrated a significant number of non-pulmonary embolism diagnoses that were well evaluated with TRO CT (Schertler et al. 2009).
In summary, TRO CT provides a single imaging study to evaluate major vascular pathology in the aorta, pulmonary arteries, and coronary circulation. Evaluation with either cCTA or TRO CT reduces total healthcare costs as compared to standard of care evaluation with a nuclear stress study (Henzler et al. 2013). Although there is data suggesting TRO CT to be equivalent to dedicated coronary, aortic, or pulmonary CTA, several studies have failed to demonstrate superior clinical outcomes with TRO CT, such as improved diagnostic yield, decreased length of stay, reduced diagnostic time, reduced adverse events, or diminished downstream resource use (Rogers et al. 2011; Madder et al. 2011). The major objections to TRO CT as compared to dedicated CT angiography are higher radiation dose and increased iodinated contrast exposure, (Ayaram et al. 2013) making appropriate patient selection for this protocol of utmost importance.
2 Patient Selection
Appropriate patient selection is crucial to the diagnostic accuracy and effective application of TRO CT. The ideal patient for TRO CT presents to the emergency department with acute chest pain where an alternative diagnosis to ACS, such as pulmonary embolism or acute aortic syndrome, is a real diagnostic concern in addition to the possibility of ACS. Some centers limit TRO CT to patients with a positive d-dimer in whom pulmonary embolism needs to be excluded (Gruettner et al. 2013). At our institution, positive d-dimer, while often a factor in the decision to order a TRO CT, is not a requirement. If the suspected diagnosis is truly limited to ACS, cCTA is more appropriate because of decreased radiation dose and iodinated contrast load. Similarly, if pulmonary embolism or acute aortic syndrome is the only diagnosis considered, dedicated CT angiography of the pulmonary arteries or aorta may be preferred, as there is no justification for the additional premedication, radiation dose, and interpretation time that is required for cCTA.
Importantly, TRO CT, like cCTA, is most effective in patients at low to intermediate risk for ACS, which ensures a high negative predictive value. A high negative predictive value is critical for safe discharge of patients from the emergency department after a negative evaluation. Therefore, patients with few risk factors, negative initial cardiac biomarkers (myoglobin and troponin I), and normal or nonspecific ECG are most appropriate. Patients with positive biomarkers or dynamic ECG changes are considered high risk and are more appropriate for cardiac catheterization. Some patients may have low-level cardiac biomarkers, but the diagnosis of ACS is not certain, and other diagnoses are still being considered. In this population, TRO CT is appropriate.
There is reduced diagnostic accuracy of cCTA or TRO CT in high-risk patients or patients with known coronary artery disease for several reasons. First, high calcified plaque burden limits coronary evaluation and often leads to overestimation of disease burden due to blooming artifact. Extensive coronary calcifications are more likely in older patients with more coronary risk factors (Wexler et al. 1996). Patients with elevated calcium scores (>400–1000) are more likely to have indeterminate results (Hecht and Bhatti 2008), and the performance of calcium scoring may be considered prior to the study to determine if a patient is an appropriate candidate for cCTA or TRO CT. Second, the presence of coronary stents limits coronary evaluation due to resultant artifact, increasing the probability of indeterminate results. TRO CT is not recommended in these patient populations. Similarly, TRO CT is of limited utility in patients with a history of coronary artery bypass grafting because of the heavily calcified native vessels. Lastly, high-risk patients are more likely to have evidence of coronary disease which will require additional physiological evaluation, such as with stress testing and/or cardiac catheterization with evaluation of fractional flow reserve.
Patients selected for TRO CT must be able to tolerate the CT and cooperate with breathing instructions. Cardiac rate and rhythm must be compatible with ECG gating to ensure adequate image quality. The preferred heart rate is sinus bradycardia. While abnormal cardiac rate or rhythm is not an absolute contraindication, it can make obtaining diagnostic quality images challenging. New CT technology with increased temporal resolution has reduced the impact of arrhythmias on image quality. Ultimately, the decision of whether to proceed depends on the severity of the arrhythmia and the capability of the CT scanner. Lastly, the patient must be able to receive intravenous iodinated contrast. Patients with contrast allergy or renal impairment may not be appropriate candidates for TRO CT.
3 Patient Preparation
Careful attention to patient preparation increases the likelihood of an efficient and high-quality diagnostic study. Although frequently not possible in the emergency population, it is ideal to withhold cardiac stimulants, including caffeine, in order to reduce ectopy. Patients must have adequate intravenous access (18–20 gauge) in a large vein, such as the antecubital fossa. The intravenous line should be tested with rapid saline injection to ensure no extravasation or pain at the site. Pain with injection can affect the patient’s heart rate during the study, impacting image quality. In order to avoid compression of the subclavian vein during contrast injection and to facilitate a tight contrast bolus, the arm with the intravenous line should not be held in full abduction above the patient’s head. We have found it helpful to position the arm directly anterior to the patient, resting on the CT gantry. Correct placement of ECG leads is needed to identify clear R waves. Leads should be positioned above and below the level of the scan to reduce streak artifact. The leads should be tested during table motion to ensure they do not detach when the table moves for the scan. Lastly, a 15 s breath-hold should be practiced with the patient. The patient should be instructed to take in a slow, small breath, as a large breath will increase the amount of unopacified blood drawn into the right heart from the inferior vena cava and result in suboptimal pulmonary arterial opacification (Wittram and Yoo 2007).
4 Patient Monitoring and Medication Administration
Patients must have vital signs monitored (heart rate and blood pressure) before, during, and after administration of medications that impact the cardiovascular system. A baseline blood pressure and heart rate determines if medications are needed or can be safely administered. Sinus bradycardia at 50–60 beats per minute is ideal (Ferencik et al. 2006). A regular heart rate and rhythm optimizes image quality and reduces radiation dose. Heart rate can be reduced with beta-blocker administration. Beta-blocker administration is generally very safe but may be contraindicated in patients with asthma, acute congestive heart failure, severe cardiomyopathy, hypotension, or recent cocaine use. Oral beta-blockers may be administered in the emergency department at least 1 h prior to the study. However, intravenous administration upon arrival to the CT suite may be needed if the heart rate is not adequately controlled with oral agents or if oral agents have not been administered. Intravenous beta-blockers may be administered while the patient is on the CT table, during performance of the scout topogram and during bolus tracking setup without a substantial increase in overall scan time. Intravenous metoprolol, what is primarily used at our institution, has an onset of action within minutes. Dose is determined by baseline heart rate; 2.5 mg intravenous metoprolol is given for a heart rate of 60–65 beats per minute, and 5 mg is given for higher heart rates. An additional 5 mg may be administered every 3–5 min until the target heart rate is achieved. Attention to blood pressure is necessary during titration. No further dose should be administered if systolic blood pressure falls below 100 mmHg. Our maximum dose of metoprolol is 30 mg. However, patients with no response after 10–20 mg are unlikely to respond with any further increased dose. In the setting of acute pulmonary embolism with tachycardia, beta-blocker administration is unlikely to result in adequate heart rate control; when pulmonary embolism is strongly suspected but a TRO CT is requested, we tend to limit the dose of metoprolol to 10 mg.
Sublingual nitroglycerin is administered prior to TRO CT for coronary vasodilation, which may improve diagnostic accuracy (Chun et al. 2008). Up to 800 mcg of sublingual nitroglycerin is administered 2–3 min prior to the scan as long as systolic blood pressure is greater than 100 mmHg. Nitroglycerin can usually be safely administered with beta-blockers, but blood pressure should be carefully monitored. Beta-blockers may actually be helpful in reducing the reflex tachycardia that may be seen after nitroglycerin administration. Contraindications to nitroglycerin administration include hypovolemia, idiopathic hypertrophic subaortic stenosis, severe aortic stenosis, and recent use of a phosphodiesterase inhibitor.
5 TRO CT Technique
5.1 CT Hardware
The advent of multi-slice, helical CT technology and ECG gating has facilitated the development of cardiac imaging with high temporal and spatial resolution. TRO CT has larger anatomic coverage compared to dedicated cCTA and thus requires at least 64 detector rows to cover the entire scan length in a single breath-hold. Today, scans may be performed more quickly and with decreased motion artifact using 256- or 320-slice scanners or high-pitch scan techniques.
5.2 Scanning Technique
The standard technique for TRO CT discussed in this section is based upon a helical, low-pitch (0.2–0.3) acquisition. The reader should be aware that with recent advances in helical CT technology, high-pitch subsecond CTA of the chest may alter the scan protocol, as will the use of prospective ECG gating with axial “step-and-shoot” technology (see below).
The major differences between TRO CT and dedicated cCTA are scan length and injection technique. Compared to cCTA, where images are obtained between the carina and diaphragm, TRO CT must include the entire thoracic aorta and the pulmonary arteries (with the exception of the small upper lobe pulmonary arteries above the aortic arch – see below). The scan is started approximately 1–2 cm above the level of the aortic arch, which is usually at the level of the inferior margins of the clavicular heads on the scout topogram. Although it is relatively uncommon to have isolated pulmonary embolism or aortic pathology outside the z-axis coverage of dedicated cCTA (Lee et al. 2011), limiting the scan length to the level of the heart alone will not definitively exclude life-threatening pathology in the aorta and pulmonary arteries (Figs. 5 and 6). In the review of almost 1200 cases in our 9-year experience of TRO CT, approximately one third of all noncoronary diagnoses would likely have been missed on a dedicated cCTA study due to unopacified right-sided circulation or limited z-axis coverage (manuscript in preparation). Furthermore, even if dedicated cCTA opacified both the right and left circulations, we found four cases of isolated segmental upper lobe pulmonary emboli that would not have been included in the z-axis coverage of the dedicated cCTA (Fig. 6). At our institution, the lung apices above the level of the aortic arch are not included in order to reduce radiation exposure. Excluding the lung apices above the arch decreases scan length by 4–5 cm, thus reducing radiation dose by 15–20 %. We are comfortable excluding the extreme lung apex from the scan, as the subsegmental pulmonary arteries at this level are generally not adequately opacified to identify embolic disease by CTA and because isolated subsegmental pulmonary embolism above the level of the aortic arch is extremely uncommon (Oser et al. 1996). With low-pitch TRO CT acquisition, it may be helpful to monitor the scan in real time and to terminate the scan as soon as the base of the heart is reached. This method may decrease scan length by 1.5–2 cm, further decreasing radiation dose by approximately 7–10 %.
The patient is positioned so that the heart is in the center of the gantry, as spatial resolution is highest at the center of the gantry due to the geometry of the x-ray beam. Using a conventional low-pitch ECG-gated helical scan, images are acquired in a cranial-to-caudal direction. The cranial-to-caudal scan direction adds a few seconds between the initiation of breath-hold and the cardiac portion of the scan. This delay provides for greater reduction and stability of the heart rate, which generally occur approximately 5–15 s after initiation of breath-hold. Furthermore, a cranial-to-caudal scan direction images the upper pulmonary artery branches prior to the heart, which is a reasonable approach as pulmonary opacification is achieved prior to coronary opacification since blood must first pass through the right-sided circulation prior to arriving on the left. Proponents of a caudal-to-cranial scan direction argue that scanning at the lung bases first results in decreased respiratory motion artifact at the lung bases (Wittram 2007); we have not found respiratory motion to be a major issue in our studies.
TRO CT is typically performed with a mean effective tube current of 600 mAs/slice and tube voltage of 100 kVp. Heavier patients may necessitate an increase in tube current (800–1000 mA) and/or voltage (120–140 kVp) to maintain diagnostic quality. Smaller patients may require less tube current. The vast majority of our studies are performed with a helical scan acquisition, and most often tube current modulation is employed to reduce radiation dose. An additional technique to reduce radiation dose is prospective ECG gating using the “step-and-shoot” axial mode. This technique may be employed only in patients with stable heart rates as any arrhythmia will substantially degrade image quality. Prospective ECG gating can decrease radiation dose by approximately 80 % relative to a retrospectively gated helical study without dose modulation. It is important to understand that prospective ECG gating does not obtain images throughout the entire cardiac cycle and therefore does not provide information about cardiac function and regional wall motion. Nonetheless, we often employ prospective ECG gating with an axial technique in younger female patients where there is greater concern about radiation exposure.
5.3 Injection Technique
Injection technique is another major difference between cCTA and TRO CT. Contrast injection in cCTA aims to completely opacify the left-sided circulation (coronary arteries and left heart) while minimizing right-sided enhancement to decrease potential artifact. Dedicated cCTA technique does not adequately opacify the pulmonary arteries for evaluation of pulmonary embolism (Dodd et al. 2008). In contrast, the goal of TRO CT is to obtain high-contrast opacification of both the right- (pulmonary arteries) and left-sided (coronary arteries and aorta) circulations. Typical enhancement is greater than 200 Hounsfield units in the pulmonary arteries and greater than 300 Hounsfield units in the coronary arteries and aorta. This is accomplished by adjusting the volume, rate, and timing of contrast injection to extend the contrast bolus to opacify the right-sided circulation. However, it is important not to have too much contrast in the superior vena cava as streak artifact degrades image quality. TRO CT necessitates more contrast material than cCTA, approximately 95 mL compared to 60–70 mL. A carefully tailored injection technique with less than 100 mL of iodinated contrast material can adequately opacify all three vascular beds.
There are several different injection strategies that may be employed. We employ a biphasic injection protocol as we have found more homogenous enhancement with such a technique. With a biphasic protocol, the first phase opacifies the left-sided circulation (coronaries and aorta), and the second phase opacifies the right-sided circulation (pulmonary arteries). Our typical protocol is 60 mL of I-350 mg/mL, followed by 60 mL dilute contrast (30 mL I-350 mg/mL and 30 mL saline). The second phase of contrast administration opacifies the right-sided circulation adequately for diagnosis of pulmonary embolism, but because it is diluted with saline, it does not result in streak artifact in the superior vena cava. The entire injection is administered at 5.5 mL/s. This rapid flow rate is maintained throughout the injection to minimize the effect of unopacified venous return through the inferior vena cava. Heating the contrast to 37° prior to administration, which decreases the viscosity, may facilitate the rapid flow rate (Cademartiri et al. 2005). The volume and rate in this biphasic protocol are optimized for a 14–15 scan time.
Rarely there may be a need for an extended TRO CT protocol of greater than 14–15 s, most likely when using an older 16 slice system with a tall patient. The biphasic technique described above can be adjusted to 80 mL I-350 mg/mL, followed by 70 mL dilute contrast (35 mL I-350 mg/mL and 35 mL saline), and the injection rate may be slowed to 5.0 mL/s (Halpern et al. 2009). This protocol is adequate for an 18–20 s scan time. As compared to cCTA studies, we do not use a saline flush for TRO CT because the saline flush may result in complete washout of contrast from the right-sided circulation, leading to nondiagnostic evaluation of the pulmonary arteries. Imaging is initiated by bolus tracking and is triggered off of the left atrium, which opacifies 2–3 s earlier than the descending aorta. If the scan is started 5 s after contrast enters the left atrium, the coronary arteries and aorta will be in the plateau phase of peak enhancement.
Other variations in injection technique have been described. One example of a triphasic technique uses undiluted contrast material (I-320 mg/mL) with a flow rate of 5 mL/s for the first phase, followed by 3 mL/s for the second phase and a saline flush for the third phase (Vrachliotis et al. 2007; Haidary et al. 2007). Even though the contrast is not diluted, the reduced flow rate in the second phase reduces the streak artifact in the superior vena cava. However, we have found that a faster flow rate of diluted contrast for the second phase is preferred as it results in less unopacified blood return from the inferior vena cava. When using a high-pitch acquisition, scan time is reduced, allowing for a potential reduction in the amount of contrast. For example, a total of 90 mL I-370 mg/mL with a flow rate of 6 mL/s may be used with automated bolus tracking and scan initiation 5 s after opacification of the ascending aorta (Bamberg et al. 2012).
6 Image Interpretation
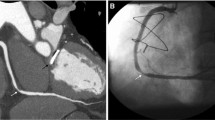
Interpretation of images from a TRO CT requires evaluation of the coronary arteries, aorta, pulmonary arteries, and adjacent lung and soft tissues. Evaluation of the coronary arteries is no different from a cCTA examination. Evaluation of the coronaries is performed on 0.6–0.8 mm axial images with 5 mm slab maximum intensity projection (MIP) reconstructions. These may be reconstructed in real time during interpretation. Cardiac post-processing software with vessel tracking may be helpful for problem solving, especially in the case of diseased vessels. Each segment of each coronary artery should be examined in multiple projections. At our institution, ACS is ruled out if there are normal coronaries (Fig. 1) or lesions with less than 25 % stenosis (Fig. 7). A stenosis of less than 50 % is unlikely to be related to chest pain symptoms and generally allows for expedited discharge with outpatient follow-up for management of coronary disease (Fig. 8). However, a stenosis of greater than 70 % or a segmental occlusion (Fig. 9) raises suspicion for ACS. Moderate lesions with narrowing in the range of 50–70 % (Fig. 10) often require additional evaluation, such as with physiologic stress testing or invasive angiography. If retrospective ECG gating is employed, multiphase reconstruction at 10 % increments throughout the cardiac cycle can be performed for functional assessment. Wall motion is assessed in standard echocardiogram projections, and regional wall motion abnormalities are correlated with anatomic data to confirm physiologic significance of any stenoses.
Segmental occlusion of the second obtuse marginal branch (OM2) is the etiology of ACS in this patient who presented with chest pain, negative troponins, and a normal ECG. Volume-rendered image (a) and globe MIP image (b) from TRO CT demonstrate segmental occlusion of OM2 (arrowheads), raising suspicion for ACS. This patient proceeded to make positive cardiac enzymes a few hours after the TRO CT study
Up to one in six patients without coronary abnormalities are diagnosed with noncoronary findings that can explain the presenting chest pain (Onuma et al. 2006). Noncoronary diagnoses include aortic and pulmonary arterial pathology, pneumonia, valvular disease including endocarditis, pericardial effusion, lung or mediastinal masses, pneumothorax, osseous lesions, esophageal pathology, and upper abdominal pathology. In our patient population, the most frequent diagnoses seen that could explain the presenting complaint are pulmonary embolism and pneumonia. Aortic dissection is rarely encountered. It is also common to identify incidental noncoronary findings that are significant, but may not explain the presenting complaint (Gil et al. 2007). Many of these findings may require immediate action, further evaluation, or continued follow-up. In our population, the most common incidental lesions encountered are lung nodules or masses, abdominal lesions, lymphadenopathy, and additional cardiac findings such as valvular disease, shunt lesions, or wall motion abnormalities. Noncoronary structures are best evaluated using 3–5 mm thick axial images. Thinner sections can be evaluated if abnormalities are noted on the thicker slices.
7 Patient Disposition
A negative TRO study frequently obviates the need for further workup in the emergency department and allows for direct discharge. The majority of these patients do not require additional testing (Takakuwa and Halpern 2008). If a study is positive for a coronary lesion, the patient is triaged appropriately for admission or observation for further workup depending on the severity of the lesion and the clinical scenario. Any noncoronary finding that may explain the patient’s presentation is managed appropriately. Any incidental finding requiring additional evaluation is communicated to the emergency department to ensure proper follow-up is obtained after discharge.
8 Radiation Dose Considerations
A limitation of a TRO CT protocol compared to cCTA is the higher effective radiation dose, which is directly proportional to scan length. TRO CT may have increased radiation dose by up to 50 % relative to dedicated cCTA (Gallagher and Raff 2008). Furthermore, patients with indeterminate results or intermediate severity disease may require additional evaluation with myocardial perfusion imaging or invasive angiography, which leads to an even larger combined total radiation dose. As discussed earlier, tube current modulation and prospective ECG gating can significantly reduce radiation dose. Tube current modulation automatically lowers the tube current of a helical acquisition during phases of the cardiac cycle that are less useful for diagnosis, typically systole. Alternatively, prospective ECG gating is an axial acquisition limited to diastole which may further reduce radiation dose. However, prospective ECG gating can only be used in patients with stable heart rates and does not allow for functional assessment. Additionally, in smaller patients, tube voltage can be reduced, and, as dual-source CT technology is becoming increasingly available, high-pitch helical scanning may allow for adequate quality TRO CT at a fraction of the dose (<2 mSv) (Kligerman and White 2014). Finally, new model-based iterative reconstruction techniques are constantly improving; model-based iterative reconstruction can provide high-quality images with reduced radiation exposure to the patient (Halpern et al. 2014).
In our initial experience with TRO CT, the mean effective dose for a helical scan at 120 kVp without tube current modulation was typically as high as 18 mSv and was reduced to 8.75 mSv with tube current modulation (Takakuwa et al. 2009). Prospective ECG gating at 120 kVp generally reduces effective radiation dose even further to 4–5 mSv. Reducing tube voltage to 100 kVp and lowering tube current, we now often obtain prospective ECG-gated studies with doses of 2–3 mSv. One study of 256-slice TRO CT using prospective ECG gating with step-and-shoot mode documented a mean effective dose of 6.5 mSv for females, compared to 3.8 mSv for males (Perisinakis et al. 2012). This resulted in mean lifetime attributable risk of radiation-induced cancer of 41 per 105 female and 17 per 105 male patients and increased intrinsic risk of lung or breast cancer less than 0.5 % and 0.1 %, respectively. Following the principle of ALARA (as low as reasonably achievable), imaging should only be obtained when clinically appropriate, especially in highly radiosensitive populations, such as young females. TRO CT remains most appropriate in a population where there is strong consideration of alternative diagnoses in addition to ACS.
Conclusions
TRO CT is a useful technique to triage patients presenting to the emergency department with low to moderate risk of ACS and in whom alternative diagnoses, such as pulmonary embolism or aortic dissection, are also being considered. An optimized study requires careful attention to appropriate patient selection, patient preparation, and injection/scanning technique. Major objections to TRO CT as compared to dedicated cCTA are the higher radiation exposure and increased iodinated contrast dose related to the longer scan length. New CT technologies, including tube current modulation, prospective ECG gating, high-pitch helical acquisition, and model-based iterative reconstruction, have decreased radiation and contrast dose such that these issues will be less of a concern in the near future. When performed correctly, TRO CT provides high-quality diagnostic opacification of the coronary arteries, aorta, and pulmonary arteries and also allows for evaluation of adjacent extravascular pathology. A negative study allows for the safe and rapid discharge from the emergency department and a reduction in subsequent testing.
References
Ayaram D, Bellolio MF, Murad MH et al (2013) Triple rule-out computed tomographic angiography for chest pain: a diagnostic systematic review and meta-analysis. Acad Emerg Med 20:861–871
Bamberg F, Marcus R, Sommer W et al (2012) Diagnostic image quality of a comprehensive high-pitch dual-spiral cardiothoracic CT protocol in patients with undifferentiated acute chest pain. Eur J Radiol 81:3697–3702
Bhuiya FA, Pitts SR, McCaig LF (2010) Emergency department visits for chest pain and abdominal pain: United States, 1999–2008. NCHS Data Brief 43:1–8
Budoff MJ, Dowe D, Jollis JG et al (2008) Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 52:1724–1732
Cademartiri F, Mollet NR, van der Lugt A et al (2005) Intravenous contrast material administration at helical 16-detector row CT coronary angiography: effect of iodine concentration on vascular attenuation. Radiology 236:661–665
Chang SA, Choi SI, Choi EK et al (2008a) Usefulness of 64-slice multidetector computed tomography as an initial diagnostic approach in patients with acute chest pain. Am Heart J 156:375–383
Chang AM, Shofer FS, Weiner MG et al (2008b) Actual financial comparison of four strategies to evaluate patients with potential acute coronary syndromes. Acad Emerg Med 15:649–655
Chen ML, Mo YH, Wang YC et al (2012) 64-slice CT angiography for the detection of functionally significant coronary stenoses: comparison with stress myocardial perfusion imaging. Br J Radiol 85:368–376
Chun EJ, Lee W, Choi YH et al (2008) Effects of nitroglycerin on the diagnostic accuracy of electrocardiogram-gated coronary computed tomography angiography. J Comput Assist Tomogr 32:86–92
Dodd JD, Kalva S, Pena A et al (2008) Emergency cardiac CT for suspected acute coronary syndrome: qualitative and quantitative assessment of coronary, pulmonary and aortic image quality. AJR Am J Roentgenol 191:870–877
Ferencik M, Nomura CH, Maurovich-Horvat P et al (2006) Quantitative parameters of image quality in 64-slice computed tomography angiography of the coronary arteries. Eur J Radiol 57:373–379
Gallagher MJ, Raff GL (2008) Use of multislice CT for the evaluation of emergency room patients with chest pain: the so-called “triple rule-out”. Catheter Cardiovasc Interv 71:92–99
Gallagher MJ, Ross MA, Raff GL, Goldstein JA, O’Neill WW, O’Neil B (2007) The diagnostic accuracy of 64-slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med 49:125–136
Ghanima W, Almaas V, Aballi S et al (2005) Management of suspected pulmonary embolism (PE) by D-dimer and multi-slice computed tomography in outpatients: an outcome study. J Thromb Haemost 3:1926–1932
Ghaye B, Remy J, Remy-Jardin M (2002) Non-traumatic thoracic emergencies: CT diagnosis of acute pulmonary embolism: the first 10 years. Eur Radiol 12:1886–1905
Gil BN, Ran K, Tamar G, Shmuell F, Eli A (2007) Prevalence of significant noncardiac findings on coronary multidetector computed tomography angiography in asymptomatic patients. J Comput Assist Tomogr 31:1–4
Goldstein JA, Gallagher MJ, O’Neill WW, Ross MA, O’Neil BJ, Raff GL (2007) A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol 49:863–871
Goldstein JA, Chinnaiyan KM, Abidov A et al (2011) The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol 58:1414–1422
Gruettner J, Fink C, Walter T et al (2013) Coronary computed tomography and triple rule out CT in patients with acute chest pain and an intermediate cardiac risk profile. Part 1: impact on patient management. Eur J Radiol 82:100–105
Haidary A, Bis K, Vrachliotis T, Kosuri R, Balasubramaniam M (2007) Enhancement performance of a 64-slice triple rule-out protocol vs 16-slice and 10-slice multidetector CT-angiography protocols for evaluation of aortic and pulmonary vasculature. J Comput Assist Tomogr 31:917–923
Halpern EJ, Levin DC, Zhang S, Takakuwa KM (2009) Comparison of image quality and arterial enhancement with a dedicated coronary CTA protocol versus a triple rule-out coronary CTA protocol. Acad Radiol 16:1039–1048
Halpern EJ, Gingold EL, WHite H, Read K (2014) Evaluation of coronary artery image quality with knowledge-based iterative model reconstruction. Acad Radiol 21:805–811
Hamada S, Takamiya M, Kimura K, Imakita S, Nakajima N, Naito H (1992) Type A aortic dissection: evaluation with ultrafast CT. Radiology 183:155–158
Hecht HS, Bhatti T (2008) How much calcium is too much calcium for coronary computerized tomographic angiography? J Cardiovasc Comput Tomogr 2:183–187
Henzler T, Gruettner J, Meyer M et al (2013) Coronary computed tomography and triple rule out CT in patients with acute chest pain and an intermediate cardiac risk for acute coronary syndrome: part 2: economic aspects. Eur J Radiol 82:106–111
Hoffmann U, Truong QA, Schoenfeld DA et al (2012) Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 367:299–308
Hollander JE, Chang AM, Shofer FS et al (2009) One-year outcomes following coronary computerized tomographic angiography for evaluation of emergency department patients with potential acute coronary syndrome. Acad Emerg Med 16:693–698
Johnson TR, Nikolaou K, Wintersperger BJ et al (2007) ECG-gated 64-MDCT angiography in the differential diagnosis of acute chest pain. AJR Am J Roentgenol 188:76–82
Johnson TR, Nikolaou K, Becker A et al (2008) Dual-source CT for chest pain assessment. Eur J Radiol 18:773–780
Kligerman SJ, White CS (2014) Image quality and feasibility of an ultralow-dose high-pitch helical triple-rule-out computed tomography angiography acquired in the caudocranial direction. J Thorac Imaging 29:50–59
Kolansky DM (2009) Acute coronary syndromes: morbidity, mortality, and pharmacoeconomic burden. Am J Manag Care 15:S36–S41
Lee HY, Song IS, Yoo SM et al (2011) Rarity of isolated pulmonary embolism and acute aortic syndrome occurring outside of the field of view of dedicated coronary CT angiography. Acta Radiol 52:378–384
Litmanovich D, Zamboni GA, Hauser TH, Lin PJ, Clouse ME, Raptopoulos V (2008) ECG-gated chest CT angiography with 64-MDCT and tri-phasic IV contrast administration regimen in patients with acute non-specific chest pain. Eur Radiol 18:308–317
Litt HI, Gatsonis C, Snyder B et al (2012) CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 366:1393–1403
Madder RD, Raff GL, Hickman L et al (2011) Comparative diagnostic yield and 3-month outcomes of “triple rule-out” and standard protocol coronary CT angiography in the evaluation of acute chest pain. J Cardiovasc Comput Tomogr 5:165–171
Meijboom WB, Meijs MF, Schuijf JD et al (2008) Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 52:2135–2144
Miller JM, Rochitte CE, Dewey M et al (2008) Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 359:2324–2336
Onuma Y, Tanabe K, Nakazawa G et al (2006) Noncardiac findings in cardiac imaging with multidetector computed tomography. J Am Coll Cardiol 48:402–406
Oser RF, Zuckerman DA, Gutierrez FR, Brink JA (1996) Anatomic distribution of pulmonary emboli at pulmonary angiography: implications for cross-sectional imaging. Radiology 199:31–35
Perisinakis K, Seimenis I, Tzedakis A, Papadakis AE, Damilakis J (2012) Triple-rule-out computed tomography angiography with 256-slice computed tomography scanners: patient-specific assessment of radiation burden and associated cancer risk. Invest Radiol 47:109–115
Prologo JD, Gilkeson RC, Diaz M, Asaad J (2004) CT pulmonary angiography: a comparative analysis of the utilization patterns in emergency department and hospitalized patients between 1998 and 2003. AJR Am J Roentgenol 183:1093–1096
Quiroz R, Kucher N, Zou KH et al (2005) Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review. JAMA 293:2012–2017
Rahmani N, Jeudy J, White CS (2009) Triple rule-out and dedicated coronary artery CTA: comparison of coronary artery image quality. Acad Radiol 16:604–609
Rogers IS, Banerji D, Siegel EL et al (2011) Usefulness of comprehensive cardiothoracic computed tomography in the evaluation of acute undifferentiated chest discomfort in the emergency department (CAPTURE). Am J Cardiol 107:643–650
Ropp A, Lin CT, White CS (2015) Coronary computed tomography angiography for the assessment of acute chest pain in the emergency department: evidence, guidelines, and tips for implementation. J Thorac Imaging
Rubinshtein R, Halon DA, Gaspar T et al (2007) Impact of 64-slice cardiac computed tomographic angiography on clinical decision-making in emergency department patients with chest pain of possible myocardial ischemic origin. Am J Cardiol 100:1522–1526
Savino G, Herzog C, Costello P, Schoepf UJ (2006) 64 slice cardiovascular CT in the emergency department: concepts and first experiences. Radiol Med 111:481–496
Schertler T, Frauenfelder T, Stolzmann P et al (2009) Triple rule-out CT in patients with suspicion of acute pulmonary embolism: findings and accuracy. Acad Radiol 16:708–717
Schlett CL, Banerji D, Siegel E et al (2011) Prognostic value of CT angiography for major adverse cardiac events in patients with acute chest pain from the emergency department: 2-year outcomes of the ROMICAT trial. JACC Cardiovasc Imaging 4:481–491
Sebastia C, Pallisa E, Quiroga S, Alvarez-Castells A, Dominguez R, Evangelista A (1999) Aortic dissection: diagnosis and follow-up with helical CT. Radiographics 19:45–60; quiz 149–150
Shapiro MD, Dodd JD, Kalva S et al (2009) A comprehensive electrocardiogram-gated 64-slice multidetector computed tomography imaging protocol to visualize the coronary arteries, thoracic aorta, and pulmonary vasculature in a single breath hold. J Comput Assist Tomogr 33:225–232
Shiga T, Wajima Z, Apfel CC, Inoue T, Ohe Y (2006) Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med 166:1350–1356
Takakuwa KM, Halpern EJ (2008) Evaluation of a “triple rule-out” coronary CT angiography protocol: use of 64-Section CT in low-to-moderate risk emergency department patients suspected of having acute coronary syndrome. Radiology 248:438–446
Takakuwa KM, Halpern EJ, Gingold EL, Levin DC, Shofer FS (2009) Radiation dose in a “triple rule-out” coronary CT angiography protocol of emergency department patients using 64-MDCT: the impact of ECG-based tube current modulation on age, sex, and body mass index. AJR Am J Roentgenol 192:866–872
Takakuwa KM, Halpern EJ, Shofer FS (2011) A time and imaging cost analysis of low-risk ED observation patients: a conservative 64-section computed tomography coronary angiography “triple rule-out” compared to nuclear stress test strategy. Am J Emerg Med 29:187–195
Vrachliotis TG, Bis KG, Haidary A et al (2007) Atypical chest pain: coronary, aortic, and pulmonary vasculature enhancement at biphasic single-injection 64-section CT angiography. Radiology 243:368–376
Wexler L, Brundage B, Crouse J et al (1996) Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation 94:1175–1192
Willoteaux S, Lions C, Gaxotte V, Negaiwi Z, Beregi JP (2004) Imaging of aortic dissection by helical computed tomography (CT). Eur Radiol 14:1999–2008
Wittram C (2007) How I, do it: CT pulmonary angiography. AJR Am J Roentgenol 188:1255–1261
Wittram C, Yoo AJ (2007) Transient interruption of contrast on CT pulmonary angiography: proof of mechanism. J Thorac Imaging 22:125–129
Wnorowski AM, Halpern EJ (2016) Diagnostic Yield of Triple-Rule-Out CT in an Emergency Setting. AJR Am J Roentgenol. W1–W7. [Epub ahead of print]
Yoshida S, Akiba H, Tamakawa M et al (2003) Thoracic involvement of type A aortic dissection and intramural hematoma: diagnostic accuracy–comparison of emergency helical CT and surgical findings. Radiology 228:430–435
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing
About this chapter
Cite this chapter
Wnorowski, A.M., Halpern, E.J. (2016). Comprehensive CT Imaging in Acute Chest Pain. In: Schoepf, U., Meinel, F. (eds) Multidetector-Row CT of the Thorax. Medical Radiology(). Springer, Cham. https://doi.org/10.1007/978-3-319-30355-0_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-30355-0_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-30353-6
Online ISBN: 978-3-319-30355-0
eBook Packages: MedicineMedicine (R0)