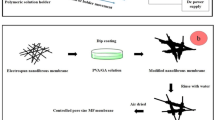
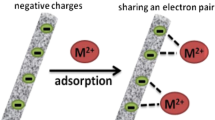
Abstract
Nanofibrous membranes created by electrospinning are nanotechnology-based technologies for developing new separating membranes, electrospun nanofibrous membranes (ENM). These can be used because of their particular characteristics to make multifunctional water treatment materials. On the other hand, its extremely porous construction with large pores reduces the capacity of NaCl to desalinize dissolved salts. As a result, it is only suited as a prefilter before delicate reverse osmosis operations to separate large/microparticles from MF applications. These membranes can be enlarged to provide water treatment by changing them to take out more intricate colloidal solutions, including oil/water suspensions, requiring an organic solution to be rejected. When an ENM surface has been changed and crosslinked with thin, selective coating layers, a combination membrane with smaller pores can be produced that can be used in UF separation. A nonporous composite membrane can be generated by adding an interfacial polymerizing layer which can be employed for nanofiltration and reverse osmosis applications. The current research on electrospun polymer membranes is covered in this chapter, emphasising progress, issues and prospective improvements in water treatment applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The scarcity of clean drinking water is currently the world's most significant issue (Ramakrishna and Shirazi 2015). Every year, water pollution and other water-related issues kill millions (Montgomery and Elimelech 2007; Warsinger et al. 2018). As the world's population grows, so do concerns about water availability, particularly in arid regions like Africa and the Middle East. With rising population and consequent industrialization, water-related issues are expected to worsen alarmingly in the coming years. These factors highlight the need for extensive research into energy-efficient and cost-effective beneficial water treatment methods (Warsinger et al. 2018).
A recent study suggests that nanotechnology could help develop the next generation of water treatment systems. Experiments with engineered nanomaterials are increasingly being conducted (Warsinger et al. 2018; Daer et al. 2015; Bethi et al. 2016). Water supply and quality can be improved through the use of nanotechnology. Aquatic treatment necessitates the development of novel membranes, particularly nanoengineered membranes such as RO and NF (Fane 2018).
Because it is a separation barrier, it allows for lower working pressures. 2–100 nm membranes can remove colloids and macromolecules (Anand et al. 2018; Yang et al. 2018; Gao et al. 2011). Modern membrane-based liquid filtration technology. Used to eliminate microparticles and germs for years. As a good particle separator, MF operates at low pressures due to the significantly larger pore width of the MF membrane, which typically ranges between 0.1 and 10 m (Rayess et al. 2011; Badruzzaman et al. 2019).
NF membranes are less expensive than RO membranes for water purification. From groundwater and surface sources, they can remove a wide range of minerals, salts, cations, and pathogens (fungi, viruses and bacteria) (Mohammad et al. 2015). Aside from the benefits mentioned above, traditional membrane techniques have several drawbacks. Traditional membranes are restricted by osmotic pressure, fouling, scaling and low porosity (80%). The flaws derive from the production methods employed by conventional membranes. It is worth noting. For MF and UF membranes, the inverting phase method works well. This is a common technique for pore membranes. This method has two main flaws: the limited size of cheap pores and contamination by solvents. The only commercially viable method to produce NF membranes is interfacial polymerization. The top selective layer's thickness can be modified, albeit not well. MF membranes can employ depth-and-trace methods similar to MD. Pore distributions and pores can vary in size (Warsinger et al. 2018; Fane 2018; Yang et al. 2018; Mohammad et al. 2015; Ang et al. 2015; Ray et al. 2016). Development and track membranes are mechanically variable. Sedimentation, flocculation, coagulation, and carbon active adsorption have proven ineffectual, making membrane technology vital in international health standards (Ang et al. 2015). Therefore, a new generation of membranes is necessary, which can transcend these limits. “Electrospun nanofibrous membranes” (ENM) have aided recent developments in water treatment.
Nanofibric nonwovens originating from basic nanofibers are created by electrospinning. Nanofibers can be produced from laboratory to pilot and industrial levels. Electrospinning has brought new possibilities in four critical areas (Ray et al. 2016; Persano et al. 2013). Some instances of energy, catalysts and health (Ray et al. 2016; Persano et al. 2013). The primary uses of electrospun nanofibers are shown in Fig. 1. Other uses for electric nanofibers include electrode materials, batteries (Jung et al. 2016) and solar power devices (Hou et al. 2019). Tissue, food and agriculture technology are useful as well. This broadens the scope of electrospun nanofiber uses. Electrospinning is the most promising and adaptable approach for producing nanofibers today.
Scheme of electrospinning plausible nanofibers formation (Reproduced with permission from Yang et al. (2018).
TFC 4921 (Fluid Systems), TFC 4820-ULPT (Fluid Systems), AG 4040 (DESAL), 4040LSA-CPA2 (Hydranautics), DK2540F (DESAL), Osmonic cell unit (SEPA CF II, General electrics -USA) (Jang et al. 2020; Brandhuber and Amy 1998, 2001; Amy et al. nd) etc. membranes have been widely used for the conventional water treatment researches. But, such membranes come with fixed structured modules and tailor-making is not allowed. Nanofibrous membranes are frequently prepared by adopting methodologies involving sintering, stretching, track-etching, template leaching and phase inversion, where the electrospinning method is quite recently developed. The electrospinning methodology delivers the salient advantages of the high level of versatility to tailor-make the pore radius, nanopore structure, porosity (>90%) and ease of incorporation of additive materials (Teychene et al. 2013). Nanometric polymer fibres are undeniably more important than in other applications as filter media. They have distinct characteristics of liquid and air filtration. The adjustment of the operating variables and dope solution content (Ahmed et al. 2015) allows the manufacture of nanofibrous structured membranes. Electrospun membranes can efficiently remove contaminants into water and wastewater treatment with their small pore size and their narrow distribution (Khulbe and Matsuura 2019). Nanofibrous membranes can absorb heavy metals and other pollutants better due to their large surface area (Huang et al. 2014; Sarbatly et al. 2016). Electrospinning has recently seen a significant increase in use for fabricating filtration membranes, which is a significant advancement. Nanofibrous membranes’ properties, structures, and functions have been gradually improved for filtration applications, allowing for new applications. New materials and functionalization methods have increased interest in electrospinning for membrane fabrication, as have advanced characterization techniques (Bassyouni et al. 2019).
This article gives an overview of recent developments in water treatment electrospun polymer nanofibrous membranes. The different modification strategies are discussed and illustrated concerning multifunctional nanofibrous composite membranes. The next section discusses the specific applications for the water treatment of these compositional nanofibrous membranes, emphasizing their past achievements in developing electrospun membranes. This paper closes with a short perspective on the role of electrospun nanofibrous membranes in future research on water treatment. The application documents for water treatment are explained in detail in this paper to encourage readers to look for new and more effective water treatment applications electrospun nanofibrous membranes through the detailed description.
2 Details of Elctrospinng Process and Its Parameters for Nanofibrous Membranes Synthesis
Nanotechnology experienced a tremendous rise in the mid-1990s due to advancements in electrospinning and nanofibre formation, which promoted modern analytical methods (Formhals 1934; Morton 1902). Electrospinning of membranes has opened up several avenues for their use, including the creation of membranes and the application of biotechnology. One major advantage of electrospinning over competing nanofibers, like gas jet technology and melting fibrillation, is that the latter two have lower and higher values (Iwamoto et al. 2007; Liao et al. 2018a). Details about the different parameters in the electrospinning process are discussed here. These extrinsic properties, operational parameters, and environmental conditions affect the ENMs’ properties.
2.1 Electrospinning Process
As demonstrated in Fig. 1, an electrospinning setup must include four components: a high voltage, a system (typically a syringe pump) that drives the dope, a spinneret, and a base metal collector. A technique called electrospinning is used to create polymer nanofibers from a liquid throttle. The charged fluid Jet is subject to various types of force, such as surface tension, Coulombic repulsion force, and electrostatic force, as shown in Fig. 1 (Liao et al. 2018a). The thinner nanofibers and increased production rate can be achieved in coaxial spinners through the air drag force applied on the outer channel (Zhmayev et al. 2010). According to the general rules, the electric spinning process follows these three phases: (1) development of rectilinear jets and spiralling trajectories, followed by bending distortion with solvent evaporation; and (2) formation of nanofibers from the nanofibers (Reneker and Fong nd).
An electric potential difference is applied primarily on a floor collector before the electrospinning process. The droplet's shape is changed to a cone-shaped Taylor Cone under the impact of applied tension. A fluid jet comes from this conical form. This cone-form structure may be maintained as long as there are sufficient solutions and each droplet is replaced during the electrospinning process. Nanofibers are not formed if the dope solution has more surface tension than the fibre voltage. All of the variables that affect nanofiber creation are the range of spinneret, the distance of the tip of a Spinneret from the grounded collection and the doping concentration.
For a predetermined distance after the start of the process of electrospinning, the polymer solution jet travels almost straight away from the orifice. The density and current of the liquid passing through the jet are inversely proportional to the distance. Electrical forces can be used to extend the jet beyond the right segment.
Rotating bending spools with increasing radius (Taylor 1969; He et al. 2005; Reneker et al. 2000) develop as a result. To reduce the jet diameter from microns to nanometers, the electrically driven non-axisymmetric bending of the jet is required (Yarin et al. 2001; Shin et al. 2001a, b; Fong et al. 1999a). While the nanofibers are being stretched and tiled, the solvents are also expelled, solidifying the nanofibers. A grounded apparatus will be used to collect nanofibers and them be analyzed and characterized once they have been bent and the solvent has been evaporated. The collection devices and design choices are important in shaping the morphologies and structures of the nanofibers (Huebner and Chu 1971; Park et al. 2007).
2.2 Operating Conditions Required in the Electrospinning Process
Electrospinning can create polymeric and inorganic nanofibrous membranes. This method can produce membranes quickly and cheaply using a wide range of materials. Define pore size, fibre diameter, and layout precisely with this method. The electrospinning process considerably impacts fibre morphologies, topography, and structure. Overall, electrospinning parameters can be categorized into three types (Kenry 2017; Haider et al. 2018).
-
Process variables
-
Dope (polymer–solvent solution) variables
-
Environmental variables
2.2.1 Process Variables
Nanofibrous membranes can be developed utilizing polymeric as well as inorganic electrospinning technologies. With this quick and cost-effective process, you can use many membrane synthesizing materials. This approach also allows the careful regulation of pore dimensions, fibre diameter and fibre characteristics. The conditions under which they have spun can have a major impact on the results of fibre morphology, topography and microstructure. In general, operating parameters may be divided into three categories for electrospinning (Kenry 2017; Deitzel et al. 2001).
2.2.2 Dope (Polymer–solvent Solution) Variables
Using nanotechnology and membrane science to develop membrane-based technologies and reduce energy costs is a good strategy. MF, UF, RDO, and Membrane Distillation (MD). As RO is the most often used press-driven strategy, membrane distillation is the preferred thermal processing method (MD). Since both RO and MD systems have been extensively investigated, they are the most widely employed approaches. Nano-lters are used to reduce salt content (20–80% NaCl) and remove brackish water (Elimelech and Phillip 2011; Essalhi and Khayet 2013; Khayet et al. 2018; Lee et al. 2011). Desalination's viability and affordability have improved over the previous decade, as has research into membrane-based desalination systems. The membrane's permeability (water permeability) is vital (selectivity or salt rejection). Membranes consume less energy than thermal processes due to ongoing improvements in membrane characteristics. Electrospinning has recently been used to create nano-branches for diaper membrane manufacture (ENMs). ENMs are distinct due to their multifunctionality as water purification products. The porous architecture contains big pores (micron-sized pores), limiting its ability to reject salt-bound water. In these cases, only coarse particles or liquids with large/microparticles can be employed in MF separation or as prefilters before delicate RO processes.
Membranes can purify water by removing colloidal solutions like oil/water suspensions. An ENM membrane crosslinked with selective coating layers can separate UF. PTFE coatings can be employed on both NF and RO composite membranes. Interfacial polymerization allows for separating an ultra-lamine surface layer by RO and MD. Adding nanoparticles (NPs) to the TFNC-membrane coated layer increases permeability and salt repulsion (NPs). Desalination (hence ux and salt efficiency) can be improved by adding NPs to ENMs, making nanotechnologies more suitable for existing desalination systems.
2.2.3 Environmental Variables
The effect on the morphology of nanofibres was proven to be ambient variables such as temperature and humidity (Vrieze et al. 2009; Hardick et al. 2011). The high temperatures can be used to manufacture thinner fibres according to the literature, which is determined to be possible. Low humidity can also speed the evaporation of solvents, whereas high moisture can lead to the development of larger fibers (Hardick et al. 2011; Pelipenko et al. 2013). As previously noted, environmental variables might affect the performance of the nanofibrous membrane resulting as well as the morphology of nanofibers (Pelipenko et al. 2013). For example, if you use a high-humidity environment, it can operate as a polystyrene fibre that is a helpful strategy for producing pores. In this scenario, the use of nanofibers for the filtration of oil spillage (Hajra et al. 2003; Kim et al. 2005) offers an important benefit.
3 Applications of ENMs in Different Pressure Driven Membrane Systems for Water Treatment
The pressure applied to the feed side of the MF, UF, NF, and RO separates water into two streams: permeate and the retentate. In many cases, permeate is used as the return solution, while retentate is thrown away and concentrated. This section studies the current state of research into electrochemically induced membrane (ENM) pressurized membrane processes.
3.1 Applications of ENMs in Microfiltration System
The nanospinning-prepared membranes of nanofibrous membranes have higher porosities than the membranes of other methods and can be qualitatively improved from submicron levels to several micrometres in size (Gopal et al. 2006). As a result, both the typical MF and cartridge MF (Wang et al. 2012) are good candidates. Electrospun nanofibrous mats were used in liquid separation and particle removal as described in Gopal et al. In a similar fashion, PVDC nanofibrous membranes performed the same way as PVDM nanofibrous membranes, rejecting 1, 5, and 10 micron polystyrene particles of liquids with greater than 90% effectiveness. Nanofibrous PAN, PSU, PES and nylon-6 membranes were made to better understand the effects on membrane performance of the electrospun nanofibrous structure (Barhate et al. 2006; Gopal et al. 2007; Aussawasathien et al. 2008; Homaeigohar et al. 2010; Yoon et al. 2009a). Its performance has been investigated with different diameters of nanofibers and membrane thicknesses. The results showed that the filtration properties are affected by ENM structures.
A chemical change can also improve the characteristics of ENM. For instance, PES nanofibreous membranes became more hydrophilic and wettable immediately (Liao et al. 2013) due to a short-term oxidation treatment. Pure water flows improved with modified PES ENMs. Nanofibrous membranes outperform typical MF membranes due to their high porosity, interconnected pores, small holes, and wettability. The nanofibric membrane rejects microparticles well (Homaeigohar et al. 2010). However, the structure of electrospun membranes can create fouling. Nanofibers can be washed away under high flux and pressure, producing mechanical failure. So, before large-scale ENM uses in water, nanofibers must be mechanically strengthened and physically integrated. Heat treatment, inter-nanofibral bonding with induced solvents and crosslinking agents were all used to knit nanofibers into mats (Homaeigohar et al. 2012; Homaeigohar and Elbahri 2012). Individual reinforcement of nanofibers also enhances compact pressure resistance. For example, zirkonia nanofibers have increased filtration efficiency in PES NPs compared with neat PES NPs.
3.2 Applications of ENMs in Ultrafiltration System
Ultrafiltration is the name given to the membrane filtration technology that uses hydrostatic pressure. It is necessary to pump liquid through the membrane and feed it into the membrane with a length range between 0.01 and 0.1 m (10–100 nm) (Fig. 2) to complete the procedure. While the UF travels across the membrane, it traps both water and low-molecular solutes. The University of Florida is a vital component in water and wastewater, which is utilized to remove bacteria, colloids, and viruses from the water. Water treatment before RO desalination, treatment to recover and MBR, and post-treatment following RO desalination are all examples of applications.
Schematics of membrane water treatment system (Reproduced with permission from Yang et al. (2018).
The present application of the reversal stage is in the production of traditional polymer UF membrane, with low to moderate flow and significant fouling rates. Pore distribution seems to be inherent in the inversion stage. Partial retention is worsened because of the numerous pores in the distribution network, making the dispersion less effective. A major focus in UF membrane development is the development of surface pores isoporosity and high porosity. Thin, selective layers can be built from assembling itself or through a phase reversal. In the meantime, nanofibrous fabrics are required for use as hydrophilic and highly porous substrates. Increasing flux and maintaining a strong rejection, nanofibrous UF-composite membranes have outperformed conventional flux-restricted membranes for filtration of oil-fired wastewater.
Cover materials like PVA and chitosan were employed to create surface barrier layers on ENMs, giving them nanofibrous UF composite membranes. For an oil-in-water emulsion, splitting the emulsion into two phases revealed a very high refuse-efficiency (>99.5%) for the composite nanofibrous membrane while simultaneously permitting high wastewater flows (>130 L/m2h). Chitosan is a common coating substance due to its hydrophilicity. A hydrophobic PVDF membrane with a chitosan ultrathin selective layer was successfully tested. This composite membrane has a flow rate of 70.5 L/m2h and a BSA rejection of roughly 98.8% at 0.2 MPa after adding surface modified glutaraldehyde (GA) and terephthaloyl chloride (TPC). The membrane also shown good antifouling properties over 24 h. Finally, the adaptable electropsun was employed as a porous base material for both hydrophobic and hydrophilic polymers. Hydrophilic substrates assist resist fouling in many applications. More study is needed to maximize the network's water permeability, hydrophilicity, and antifouling qualities.
3.3 Applications of ENMs in Nanofiltration System
The gap between Uf and the reverse (RO) (Sutherland 2008) is filled by nanofiltration (NF). (Sutherland 2008). Based on the molecular weight reduction, conventional FM-separation sizes are 100 to 1000D (MWCO). It softens, disinfects and eliminates organic pollutants and divalent ions, and NF may be widely used to clean water. NF. High transmembrane pressure causes steric and electrostatic effects of Donnan, which result in separation. While both NF and RO can reject monovalent ions like sodium chloride, NF maintains multivalent salts like sodium sulphates better.
The most widely utilized TFC membranes are non-ionizing fluid membranes made of ultrapine skin layer IP on a porous substrate. The high surface and interior porosity of nanofibrous scaffolds make the construction of TFC membranes appealing to many researchers. It is especially true in NF due to the low pressure and compressive force. Contrast this with a laboratory-made TFC membrane's 2.4-fold higher permeate flux and near-identical rejection rate (98%). Higher TFNC membrane flux (due to increased pore and porosity of the substrate) is attained as the nanofibre pore diameter increases (Fig. 3). The nanofibrous membrane could not sustain the barrier layer due to increased surface diagram and nanofiber holes. Researchers revealed that nanofibres can reduce rejection while improving penetration. However, reducing the nanofibrous membrane thickness reduces its hydraulic resistance.
a Schematic of nanofiltration (NF) with a thin film nanofibrous composite (TFNC) membrane; b Typical surface and cross-section (inserted images) morphologies of a TFNC membrane (Reproduced with permission from Yang et al. (2018).
Electrospinning is a method that provides high surface porosity and linked pores in a pressured membrane process. Because electrospinning cannot manufacture dense membranes with nanometer-length pores in a single step, research focused on developing TFNC membranes. The long-term sustainability of these water treatment microorganisms was hampered by their under-pressure stability, antifouling capabilities, and mass replication. The substrate is required for high-level TFC membrane development because it defines the flow channel across the membrane.
4 Applications of ENMs for Wastewater Treatment
4.1 Applications of ENMs for Heavy Metal Removal from Contaminated Water
As mineral adsorbents and inorganic ion fixators, hydroxyapatite NPs (HAp nPs) have been shown to accommodate a wide variety of cationic and anionic replacements. Heavy metal extraction from wastewater has recently become a focus of research. Heavy metal ions from water can be bound by HAp (Subramanian and Seeram 2013). Recently, HAp-based composite materials have gotten a lot of buzzes. A modification in humic acid HAp NPs improves Cu (II) adsorption significantly. Comparing bare HAp to ion exchange mechanisms with Ca (II) ions showed it to be greatly enhanced. A solution that adsorbs Co was found in the HAp/Zeolite composite (II). This compound (63%) outperformed HAp (58%) and zeolite (63%). (47%). It was used to calculate the Pb (II) rejection effectiveness from watery solutions using C-HAp and granular activated carbon (Fernando et al. 2015). Aqueous solutions were tested for their ability to exclude Pb (II) ions by using HAp NPs synthesized with granular activated carbon. According to the researchers, the adsorption potency of the HAp NPs was 138–83 mg/g, whereas C-HAp adsorption was 9–14 mg/g. The researchers claim that a polymeric Hap Nanocrystal and polymer composite can assist offset lower yields and longer manufacturing times for HAp NPs, respectively. Conclusions (Aliabadi et al. 2014) Plum, copper, and nickel ions are removed by a chitosan/HAp composite nanofiber. This increased the membrane's prospects for industrial application after five desorption-absorption cycles. In wastewater treatment, it is a powerful adsorbent for heavy metals like lead, mercury, and cadmium. For example, increased porosity and gas permiability.
Toxic heavy metals can be removed efficiently by electrospinning nano fibres with a high volume to the aspect ratio, specific surface area (10–100 m2/g), and porosity (80–85%) (Aadil et al. 2018). That's because they have a lot of porosity and a lot of volume-to-aspect ratios. Less need to filter wastewater after adsorption reduces costs and reduces contaminants. Adsorbent polymer (cellulose) is produced by plant cells. Dense adsorbents have been used for pollution management for decades, but little is known about their economic application. Producing submicron cellulose fibre is difficult because cellulose does not dissolve in conventional solvents. Promotes cellulose ester production (cellulose acetate) (CA). Other uses for textiles are cigarette filters, floor coverings, inks, and film. In its major application, Ca is utilized to make membrane bi-products. Non-pole solvents are easy to dissolve and treat, and they are good for electrospinning due to their strength retention when made in membranes, as shown. Making ultrafine CA porous fibres and testing their adsorption capabilities on Cu2+ were both successful.
By contrast, the greatest removal effectiveness of Cu2+ from porous ultrafine fibres was 88.6%. For CA polymer backbones without reactive function groups, the absence of reactive functional groups reduces the membranes’ separation performance. Adsorbent separation using affinity principles is not suggested for this polymer. These acetyl derivatives might be employed to treat acidic chloride solutions as adsorbents, with a maximum capacity of 110 mg/g for the selective recovery of Au, to determine the optimal cellulose to acetyl derivative conversion process (III). Auxiliary metal ions, such as Pd (IV) and Pt (Pt), can also be rejected by the CA fibre (II). Filters such as silica can improve the material's chemical and physical properties while adding new and interesting capabilities. This is done by mixing inorganic fillers. CA is an inorganic filler matrix. One investigation reported an 81.1% efficacy rate for a 0.78 nm pore-sized chitosan/CA formulation in conventional wastewater treatment plants. The use of a cellulosic polymer, such as a composite material with HAP, can assist in the purification of the environment of harmful bisphenols as the technique allows for the manufacture of ultra-thin particles with a diameter of 92 nm or less. A new method for detecting Bisphenol A in infant food samples is patentable due to new chromatography technologies being developed. It is difficult to make hybrid nanocomposites because the ceramic powder does not dissolve well in the CA. Electrospinning hybride nanofiber composites can increase the extraordinary adsorption characteristics of heavy metals due to the affinity and/or electrostatic contact between functional groups.
According to a recent study, composite CA/HAp nanofibers with a composite of 3% (wt%) CA/HAp are highly effective in repulsion of heavy metal ions from wastewater. In this composite show, one element outperforms the other. Different factors for finding the ideal conditions for ion separation of plumes (II) and iron (III) have been optimized. The ideal conditions for wastewater are pH 6, room temperature and 0.1 g V. 0.1 grammes V, pH 6, ambient temperature and lead (II) 35 min (99.7 and 95.47%). The pseudo-kinetic model and Freundlich isotherm best illustrate this process. For example, M10(XO4)6Y2 indicates a bivalent cation, while PO43 represents XO4 and OOH. HAp, for instance, Due to its wide spectrum of anions and cations interactions in an adsorption/ion exchange process, this anion exchanger (HAp) was intensively studied for a long period, leading to the discovery of several water pollutants. Due to their similar radii and the distance between the calcium ion (0,99) and both radii, Pb (1,19) and Fe (0,645) are projected to fit into the HAp grid. If the cations do not enter the apatite structure correctly, hydroxyl, phosphate, or calcium groups must be substituted. Electrodeposited Microcapsules (tortiline nickel) made of polyester/gelatin (vinyl alcohol). However, electrocatalytic activity was more influenced by nanofibers than NPs. However, when compared to NPs of the same composition, nanofibers outperform them. Contrast this with NPs or a catalyst that was initially clean and later impregnated with W, and you get a 28% increase in current density. In the metals, tungsten presence affects electrocatalytic activity dramatically. The best performance was achieved with nanofibers electrospun from tungsten precursor solution (35% wt). There is evidence that calcines temperature affects yield. There were no differences between 1000 and 700 °C in these investigations.
4.2 Applications of ENMs for Oil Spill Removal from Contaminated Water
Much emphasis has been paid to the separation and recycling of oil/water streams utilizing energy-efficient and environmentally acceptable technology. Demusification of oil/water emulsions can be accomplished via coalescence filtration. The coalescence filter separates emulsions into three basic phases, as shown in Fig. 4a. The filter medium removes solid particles from the fluid stream first. Second, droplets are captured by the fibrous bed, which then agglomerate within the fibrous network. The coalescence system combines small oil/water droplets into larger ones by passing through numerous filter layers. Finally, gravity separates the stream into oil and water layers. When water-in-oil emulsions are handled, big water droplets sink, and giant oil droplets rise to the surface. All influences the performance of the coalescence by flow, depth of the bed, surface characteristics and droplet size. Glass nanofibre-coated fibres have been found to work well with separation between oil and water (Shin and Chase 2006, 2004; Ma et al. 2010). It was discovered that even a few nanofibers increased the filter media's capture effectiveness. It did, however, exacerbate the pressure decrease (Shin and Chase 2006). The diameter and wettability of the nanofibers were also shown to influence the performance of coalescing filter medium. By reducing nanofibers, the total efficiency of the separation was increased. Materials with better weighting enabled the coalescence of water in oils. In the investigation, the filter's performance was improved by the electrospinning nylon 6 nanofibers, which measured 250 nm in diameter on the surface of the glass filter. Furthermore, micro-sized glass fiber-coated nanofibers with a diameter of 150 nm increased separation efficiency from 71 to 80% (Shin and Chase 2004).
a Filtration scheme for coalescence; b Transverse view of the TFNC membrane based on cellulose and c Superweighing membrane surface morphology fabricated for separation of water/oil. (Reproduced with permission from Yang et al. (2018).
In the context of mineral adsorbents and inorganic ion fixators, it has been established that hydroxyapatite NPs (HAp nPs) can accept a wide range of cationic and anionic replacements in their structural composition and function. Over the last few years, researchers have concentrated their efforts on using HAp NPs to remove heavy metals from wastewater. The ability of HAp to bind heavy metal ions from water has been proven in studies. The utilization of HAp-based composite materials has recently attracted a significant deal of attention. Increased adsorption of Cu (II) ions occurs due to the modification of humic acid HAp NPs. Comparing bare HAp to ion exchange processes with Ca (II) ions was demonstrated to be greatly improved. One of the HAp/Zeolite composite components that has been found is a solution that adsorbs Co. (II). They wanted to see if this composite (63%), HAp (58%), and zeolite would outperform HAp in terms of aiding in the elimination of toxins (47%). To calculate the Pb (II) rejection efficiency from watery solutions (Fernando et al. 2015), C-HAp has been produced in the presence of granular activated carbon. A prior study had used granular activated carbon to synthesize HAp NPs, which were then used to create C-HAp, which was used to evaluate the efficiency of aqueous solutions at excluding Pb (II). Their research revealed that, when using HAp NPs, they could increase the amount of C-HAp that could be adsorbed at the same time by a factor of 138 to 83. They also observed that the C-HAp potency could be increased by a factor of 9 to 14 mg/g. According to the researchers, a polymeric Hap Nanocrystal and polymer composite can be used to compensate for lower yields and longer manufacturing periods associated with HAp NPs. Findings from the study (Aliabadi et al. 2014) The development of a chitosan/HAp composite nanofiber to remove Plum, copper, and nickel ions has been accomplished. The membrane's chances for use in the industry significantly increased after five consecutive desorption-absorption cycles. Adsorption of poisonous pigments and heavy metals such as lead, mercury, and cadmium from wastewater is made possible by using this adsorbent. Reasons for this assumption include the permiability of gases and a material surface area with a larger porosity.
The high volume to the aspect ratio, the high specific area (10–100 m2/g), and the high porosity (up to 80%) (Aadil et al. 2018) of electrospun nanofibers (Reneker and Yarin 2008) all contribute to its excellent adsorption capacity for removing toxic heavy metals, and electrospinning for nanofiber synthesis has attracted a great deal of interest. With a standard sample volume of around 10–100 m2, this is due to a high volume-to-aspect ratio, resulting in a high specific area and high porosity. Aside from economic savings from not having to filter wastewater after adsorption, other advantages of nanofibre membranes include reducing contaminants that could have been present in the wastewater. Cells extract cellulose from plants, which is a natural polymer adsorbent. Despite this, there is a continual interest in learning more about the practical application of these adsorbents in pollution control to progress the field of pollution control research. Cellulose submicron fibre production is complex because cellulose does not dissolve in conventional solvents. Acetate, for example, aids in the production of cellulose ester compounds (CA). Aside from textiles, cigarette filters, surface coatings, inks, and motion picture film, other applications include microfilm, audiotape, microfiche, ink, and optical disc media, to name a few examples. In the manufacturing of membrane bi-products, which is the primary application for calcium, it is the most often used element globally.
With regard to non-pole solvents, it has been established that they are simple to dissolve and process, and they are particularly well suited for electrospinning due to their ability to maintain strength even when made in membranes. Preparation and analysis of Cu2+ adsorption qualities were effective in fabricating ultrafine CA porous fibres while researching the adsorption properties of Cu2+. According to Kusworo et al. (2014), the maximal Cu2+ removal efficiency of ultrafine fibres and porous ultrafine fibres was 19.1, 70.8, and 88.6%, respectively.
A fundamental disadvantage of CA polymer backbones is that they lack reactive function groups, resulting in the membranes’ separation performance being severely hampered. In addition, it should be highlighted that this polymer is not well suited for adsorbent separation based on the affinity principle [particularly, adsorbent separation based on affinity principles] and is therefore not suggested for this application. As part of their efforts to determine the most efficient method of converting wood pulp into acetyl derivatives, the researchers investigated whether the acetyl derivatives produced by cellulose synthesizers could be used to treat acidic chloride solutions as adsorbents, with a maximum capability of 110 mg/g for the selective recovery of gold. They found that they could (III). Thus, the CA fibre (AU(III) rejection efficiency) is active in the rejection of other metal ions, such as the metal ions Pd(IV) and Pt, which are present in the environment (II). Different inorganic fillers, such as silica, can be used to improve the chemical and physical properties of the material while also providing new and different functionalities to the end product. CA fibre connections are maintained by using a variety of inorganic fillers. A soft matrix for inorganic fillers, CA, is utilized as a binder for the fillers. One such formulation, with pores of 0.78 microns in size, was evaluated in conventional wastewater treatment plants and found to be 81.1% efficient at a pressure of 506.5 kilopascals (kPa). A cellulosic polymer, such as a composite material with HAP, can be used to clear the environment of hazardous bisphenol. There has been an interaction between hap and cellulosic polymers, and the technology now allows for the production of exceedingly thin particles with a diameter of 92 nm or smaller. A new approach for identifying Bisphenol A in baby food samples has been created due to the development of new chromatography methods. This method has been granted patent protection. The poor dispersion of ceramic powder in the CA, which occurs when the polymer matrix that holds the ceramic powder together is created, is a significant problem in the creation of hybrid nanocomposites. The amazing adsorption characteristics of heavy metals can be enhanced by using electrospinning to generate hybride nanofiber composites, which is a result of the affinity and/or electrostatic contact between the various functional groups.
It has recently been demonstrated that the use of CA/HAp composite nanofibers with 3% CA/HAp composite (wt%) as an exceptionally low-cost adsorbent, which is exceptionally effective at repelling heavy metal ions from wastewater, is extremely effective at repelling heavy metal ions from wastewater. The study involved participants from various universities and community organizations throughout the United States. The performance of this composite show outperforms the performance of either of the individual components or the combined show. Following considerable evaluation of numerous parameters, the optimal conditions for lead (II) and iron (III) ion separation in wastewater were discovered. Specifically, pH 6 wastewater, room temperature, and 0.1 grammes of V are the most favourable circumstances for this procedure. For the separation of lead (II) and iron (III), these conditions must be established in less than 35 and 40 min, respectively. They must be achieved under pH 6, at room temperature, with 0.1 grammes of V. These conditions must be established in less than 35 and 40 min, respectively. They must be achieved under pH 6, at room temperature, with 0.1 grammes of V. (99.7 and 95.47%) (Although it has been demonstrated that this technique is capable of conducting rejection using two models, the pseudo-kinetic second-order model and the Freundlich isotherm model are the most accurate representations of the process.) The general formula M10(XO4)6Y2 denotes a bivalent cation, whereas the compound PO4 3 denotes XO4 and OOH.
In contrast, HAp denotes a bivalent cation, and the compound PO4 3 denotes XO4 and OOH. Consider HAp as an illustration: Since it interacted in an adsorption/ion exchange process with a wide range of anions and cations for a while, this particular anion exchanger (HAp) was extensively studied for a while; as a result, it was discovered that numerous water contaminants had a connection to this chemical. Due to their similar radii and the approximate distance between the calcium ion (0,99) and both radii, it is expected that Pb (1,19) and Fe (0,645) will fit into the HAp grid. If the cations are not effectively incorporated into the apatite structure, the addition of substitute hydroxyl, phosphate, or calcium groups may be necessary.
Nitric acid, tungsten chloride, and microcapsules (tortiline nickel) made from a polymer combination of polyester and gelatin are used in this process (vinyl alcohol). When NPs and nanofibrous materials were compared, it was discovered that the latter had a stronger impact on electrocatalytic activity. When the nanofibers are combined with NPs of the same composition, which was made by calcining a solution containing 10% WCl2 in weight at 850 °C, the efficiency of the nanofibers improves. Compared with NPs, the nanofibrous morphology resulted in an increase in current density from 11.5 to 16 mA/cm2 (a 28% increase) and a 42% increase in current density from 22 to 37.75 mA/cm2 (a 42% increase), with the improvement in performance being realized in comparison to both a catalyst that started as pristine and then was further impregnated with W. A considerable impact on electrocatalytic activity was found to be caused by altering the tungsten content of the metals. Nanofibers displayed the highest performance when electrospun from a precursor tungsten solution containing 35 weight percent of the metal. According to the findings of several prior research, the temperature of the calcine has an impact on yield. 1000 degrees Celsius was shown to be the optimal temperature in these experiments, with 700 and 850 degrees Celsius equivalent.
5 Applications of Elctrospun Nanofibrous Membranes for Desalination
Seawater desalination is a widely mentioned issue. While alternative technology research is early, seawater treatment has better reliability. Desalination can be performed on inland bodies of water where it is more difficult to treat brackish water than seawater.
Desalination is the process of removing salt, minerals, and other pollutants from seawater to create freshwater that can be used for drinking and other purposes. Seawater has a high concentration of salt, making desalination costly and impractical. Salinity in saltwater is primarily dependent on the percentage of salt in the water: 3.5% (or 35 g/L) NaCl equivalent to 3500 ppm. One would like to keep NaCl (sodium chloride) levels under 280 ppm in drinking water (Feng et al. 2008; Elimelech and Phillip 2011). To desalinate seawater, we can use several different processes that utilize both kinetic and thermodynamic energy and mechanical, electrical, and chemical. The multiple-stage effect, MSF, a distillation of multi-effects, MED, mechanical vapour pressure, MVC, diaphragm distillation, MD, RO, electrodialysis, and nanoltration are a few of the technologies for desalination (NF). Second-generation membrane-based technologies utilize thermal-based technology, the former generation of which has a thermal base. Nanotechnology and membrane science are cost-efficient approaches for improving membrane technology and reducing energy expenses due to lower energy usage. Ultrafluorometry (UF), Distillate Recirculation (RDO) and Distillation of the Membrane (MD). The RO process is the most frequently used press-driven approach, and the process of membrane distillation is the most sought in thermal treatment (MD). RO and MD water processes have been thoroughly explored, which means that the most common techniques are RO and MD systems. Nano-lters (Elimelech and Phillip 2011; Essalhi and Khayet 2013; Khayet et al. 2018; Lee et al. 2011) often involve additional procedures of decreasing the salt content to 20%–80% NaCl and removing brackish water.
The viability and cost of desalination and research into membrane-based desalination systems have progressively increased in the recent decade. Durability (water permeability) is an important attribute of the membrane (selectivity or salt rejection). Due to ongoing membrane characteristics advances, membranes utilize less energy than processes that are heat propelled. Electrospinning is used to produce nanotech-enabled diaper membranes in a recent application (ENMs). ENMs have unique qualities since water purification solutions might have multifunctionality. The porous architecture includes relatively big pores (micron-sized pores), limiting the capacity of poring water to reject salt-like chloride, for example. In certain cases, in the MF separation or prefilters before sensitive RO procedures, only coarse particles and liquids with large/microparticles can be employed that are suspended in them.
Diversified membranes may be used to remove colloidal solutions, such as suspensions of water and oil, for water treatment. For UF separation, the membrane that consists of an ENM and is connected to selective coating layers can be used. In the manufacture of the NF and RO composite membranes, PTFE or polytetrafluoroethylene (PTFE) coatings may be employed. The RO and MD separation of an ultra-selective ultra-lamine surface layer are feasible through interfacial polymerization. By integrating NPs in the TFNC membrane coated layer, the durability and repellence of salt can be further enhanced by (NPs). Adding NPs to ENMs can improve desalination (and, in turn, salt and ux-efficiency) and boost the application of nanotechnology to existing desalination systems.
6 Application of ENMs for Dye Removal
With their large surface area, electrospun nanofibers can remove greater amounts of dye from wastewater by surface adsorption. Xu et al. (2012) investigated the adsorption capacity of vinyl-modified mesoporous poly(acrylic acid)/SiO2 composite nanofiber membranes for the adsorption of malachite green, a triarylmethane dye used in the production of silk, leather, and paper, according to the results of their research. The removal of cetyltrimethylammonium bromide (CTAB) from the electrospun fibres results in the formation of pores in the material. The fibrous membrane has an adsorption capacity of 240.49 mg/g and a satisfactory clearance rate for the first three regeneration cycles after being degraded. Nonetheless, after six regeneration cycles, the clearance rate was lowered to approximately 44% (Green Processing and Synthesis nd). In another study, Polyethylenimine (m-PEI) and polyvinyl chloride (PVDF) blend (m-PEI/PVDF) adsorption efficiency for anionic dyes was investigated by Ma et al. (2016) utilizing methyl orange (MO) as the model dye in their study.
In neutral pH, a nanofibrous mix comprising 49.5% m-PEI had a maximum adsorption capacity of 633.3 mg/g, significantly higher than previously reported adsorbents, according to the research (Xu et al. 2012). Using electrospun nylon-6 (PA-6) membranes, Yu et al. (2018) demonstrated that the membranes could remove indigo dye solution without the need for any other alterations to their chemistry. Although 10 layers of the produced membranes were hot-pressed together for filtration, this was not sufficient to completely remove the dye. An electrospun membrane with a single layer had a thickness of around 0.03 mm, while ten layers of hot-pressed membrane had a thickness of approximately 0.12. It was discovered that cake had formed on the membrane's upper surface after being subjected to dead-end filtering. Due to the solute concentration on the membrane surface being greater than its saturation solubility, a cake formed, resulting in the precipitation of the dye on the membrane surface. Despite the fact that the membrane's pores were not entirely blocked, the flow declined dramatically in the first 200 min and stabilized at greater than 15 L m2 h−1 after 500 min of experimentation (Ma et al. nd). Recently, Jang et al. (2020) investigated the dye adsorption capability of exfoliated graphene oxide (GO) fibres loaded with cetyltrimethylammonium chloride (CTAC) modified exfoliated graphene oxide (GO) in an aqueous system. The dyes tested were methylene blue (MB) and methyl red (MR). When combined with its different oxygenated functional groups and phenyl backbone, GO can induce attractive forces between MB and MR dye molecules, leading to an improvement in adsorption effectiveness for both dyes at higher concentrations of the compound. By employing CTAC to modify the surface of GO, researchers were able to boost the loading capacity of PAN solution from less than 10 weight percent to more than 30 weight percent of GO without affecting the electrospinning or fibre formation processes. Because the electrospun PAN membrane loaded with 30 wt% cGO was excessively brittle, 20 weight percent cGO was employed in place of the 30 weight percent cGO in the original experiment. With respect to adsorption efficiencies, the electrospun PAN/cGO membrane displayed good performance for both MB and MR dyes, with the MR dyes exhibiting the highest efficiency. According to the researchers, this is because the MR dye has a more compatible polarity with membranes and because its molecular size is smaller, which allows for greater diffusion of the solution into the depths of the electrospun membrane (Yu et al. nd).
7 Conclusion
Electrospinning nanofibers are now standard. They are ideal for encapsulating a wide range of functional nanomaterials. Even on a large scale, basic understanding and modelling of the complex spinning process have improved. As a result, the production of novel water treatment membranes has benefited. These studies have been examined and categorized. Water treatment with nanofibrous composite membranes. Developing ultrafine nanofiber membranes with high porosity is now a priority. Most unaltered ENMs feature 1 m surface pores and 100 nm nanofibers. Micro- to submicron membrane surface pores and nanofiber diameters of 100 nm to several nanometers must be optimized. Because nanofibric substrates are porous, highly fine nanofibrous membranes may efficiently filter particles. Fine nanofibers can successfully sustain grafted or polymerized cutaneous layers and prevent obtrusive substrata. Dope conductivity, voltage, and viscosity control Micron-sized nanofibers are still challenging to mass manufacture. Improved membrane substrates improve selective layer adhesion. The impacts on substrate surface roughness, surface poresity, and charges require more research. In membrane operations, membrane fouling reduces flow capacity. Electrified antifouling membranes fascinate me. Deterring membrane fouling requires more coating methods. Oriented and sized nanotubes are now possible.
Electrospinning has become a widely accepted, simple process for creating nanofibers in the last few decades. Nanofibers have unique properties for academic and industrial use such as a large specific surface area, high porosity (up to 90%), one-dimensional arrangement, easy inclusion of functional nanomaterials, and a wide range of architectural styles. Because of the high porosity of nanofibrous substrates, the filtration effectiveness of smaller particles can be improved by utilizing ultrafine nanofibrous membranes. TFNC membrane applications in high-pressure processes are made easier by ultrafine nanofibers, which efficiently support grafted or polymerized skin layers and reduce their penetration into substrates even at high pressure. The ultrafine nanofibers can be made by manipulating the dope's conductivity, surface tension, and viscosity. Even still, mass production of nanofibers smaller than 100 nm is difficult at this point. Despite these efforts, more research is needed on nanofibrous membrane optimization, the development of robust and ultrathin selective layers on nanofibrous substrates, the fabrication of complicated and multifunctional nanostructures, and the mass production of these advanced technologies nanofibrous membranes. Optimized membrane substrates not only support surface coating layers but also reduce the amount of intrusion they cause. They also improve adhesion between specific layers and substrates. Researchers aim to carry out more research on how the properties of the nanofibrous substrates interact with other properties like substrate material and roughness, surface porosity, and charge to better understand how the two materials link together. Membrane processes also face the difficulty of reducing flux below the theoretical capacity due to membrane fouling, which is a significant challenge. It's intriguing to think of electrospinning and additional modification as a way to create antifouling membranes. To prevent the formation of fouling layers on membrane surfaces, further coating techniques should be investigated.
Commercializing electrospinning technology requires more research. Besides water treatment, new ENM should be investigated. A multifunctional membrane that can disinfect, decontaminate and separate water may be developed with ENMs. The development of electrospun nanofiber membranes for water purification. Research on electrospunic membrane water treatment has advanced significantly in recent years. These issues will require more effort for new electrospunic membranes.
References
Aadil KR, Mussatto SI, Jha H (2018) Synthesis and characterization of silver nanoparticles loaded poly (vinyl alcohol)-lignin electrospun nanofibers and their antimicrobial activity. Int J Biol Macromol 120:763–767
Ahmed FE, Lalia BS, Hashaikeh R (2015) A review on electrospinning for membrane fabrication: challenges and applications. Desalination 356:15–30
Aliabadi M, Irani M, Ismaeili J, Najafzadeh S (2014) Design and evaluation of chitosan/hydroxyapatite composite nanofiber membrane for the removal of heavy metal ions from aqueous solution. J Taiwan Inst Chem Eng 45:518–526
Amy GL, Edwards M, Benjamin M, Carlson K, Chwirka J, Brandhuber P, McNeill L, Vagliasindi F (1998) Draft Report, AWWARF
Anand A, Unnikrishnan B, Mao JY, Lin HJ, Huang CC (2018) Graphene-based nanofiltration membranes for improving salt rejection, water flux and antifouling—a review. Desalination 429:119–133
Ang WL, Mohammad AW, Hilal N, Leo CP (2015) A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants. Desalination 363:2–18
Aussawasathien D, Teerawattananon C, Vongachariya A (2008) Separation of micron to sub-micron particles from water: electrospun nylon-6 nanofifibrous membranes as pre-fifilters. J Membr Sci 315:11–19
Azzaoui K, Lamhamdi A, Mejdoubi E, Berrabah M, Hammouti B, Elidrissi A, Fouda M, Al-Deyab S (2014) Synthesis and characterization of composite based on cellulose acetate and hydroxyapatite application to the absorption of harmful substances. Carbohydr Polym 111:41–46
Badruzzaman M, Voutchkov N, Weinrich L, Jacangelo JG (2019) Selection of pretreatment technologies for seawater reverse osmosis plants: a review. Desalination 449:78–91
Barhate RS, Loong CK, Ramakrishna S (2006) Preparation and characterization of nanofifibrous fifiltering media. J Membr Sci 283:209–218
Bassyouni M, Abdel-Aziz MH, Zoromba MS, Abdel-Hamid SMS, Drioli E (2019) A review of polymeric nanocomposite membranes for water purification. J Ind Eng Chem 73:19–46
Beachley V, Wen X (2009) Effect of electrospinning parameters on the nanofiber diameter and length. Mater Sci Eng C 29:663–668
Bethi B, Sonawane SH, Bhanvase BA, Gumfekar SP (2016) Nanomaterials-based advanced oxidation processes for wastewater treatment: a review. Chem Eng Process 109:178–189
Brandhuber P, Amy G (1998) Alternative methods for membrane filtration of arsenic from drinking water. Desalination 117:1–10
Brandhuber P, Amy G (2001) Arsenic removal by a charged ultrafiltration membrane influences of membrane operating conditions and water quality on arsenic rejection. Desalination 140:1–14
Buchko CJ, Chen LC, Shen Y, Martin DC (1999) Processing and microstruactural characterization of porous biocompatible protein polymer thin films. Polymer 40:7397–7407
Buchko CJ, Kozloff KM, Martin DC (2001) Surface characterization of porous, biocompatible protein polymer thin films. Biomaterials 22:1289–1300
Candido RG, Godoy GG, Goncalves AR (2017) Characterization and application of cellulose acetate synthesized from sugarcane bagasse. Carbohydr Polym 167:280–289
Che H, Huo M, Peng L, Fang T, Liu N, Feng L et al (2015) CO2-Responsive nanofibrous membranes with switchable oil/water wettability. Angew Chem Int Ed 54:8934–8938
Choong LT, Lin YM, Rutledge GC (2015) Separation of oil-in-water emulsions using electrospun fifiber membranes and modeling of the fouling mechanism. J Membr Sci 486:229–238
Daer S, Kharraz J, Giwa A, Hasan SW (2015) Recent applications of nanomaterials in water desalination: a critical review and future opportunities. Desalination 367:37–48
De Vrieze S, Van Camp T, Velvig A, Hagstrom B, Westbroek P, De Clerck K (2009) The effect of temperature and humidity on electrospinning. J Mater Sci 44:1357–1362
Deitzel JM, Kleinmeyer J, Harris D, Beck Tan NC (2001) The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 42:261–272
Drew C, Wang X, Samuelson LA, Kumar J (2003) The effect of viscosity and filler on electrospun fiber morphology. J Macromol Sci Part A Pure Appl Chem 40:1415–1422
El Rayess Y, Albasi C, Bacchin P, Taillandier P, Raynal J, Mietton-Peuchot M et al (2011) Cross-flow microfiltration applied to oenology: a review. J Membr Sci 382:1–19
Elimelech M, Phillip, WA (2011) The future of seawater desalination: energy, technology, and the environment. science, 333(6043), pp 712–717.
Elkady M, Shokry H, Hamad H (2016) Effect of superparamagnetic nanoparticles on the physicochemical properties of nano hydroxyapatite for ground water treatment: adsorption mechanism of Fe (II) and Mn (II). RSC Adv 6:82244–82259
Elkady M, Shokry H, Hamad H (2018) Microwave-assisted synthesis of magnetic hydroxyapatite for removal of heavy metals from groundwater. Chem Eng Technol 41:553–562
Essalhi M, Khayet M (2013) Self-sustained webs of polyvinylidene fluoride electrospun nanofibers at different electrospinning times: 1. Desalination by direct contact membrane distillation. J Membr Sci, 433:167–179
Fane AG, Wang R, Hu MX (2015) Synthetic membranes for water purifification: status and future. Angew Chem Int Ed 54:3368–86
Fan L, Xu Y, Zhou X, Chen F, Fu Q (2018) Effect of salt concentration in spinning solution on fiber diameter and mechanical property of electrospun styrene-butadiene-styrene tri-block 29 Electrospun Nanofibrous Membranes for Water Treatment https://doi.org/10.5772/intechopen.87948copolymer membrane. Polymer 153:61–69
Fane AG (2018) A grand challenge for membrane desalination: more water, less carbon. Desalination 426:155–163
Fashandi H, Karimi M (2012) Pore formation in polystyrene fiber by superimposing temperature and relative humidity of electrospinning atmosphere. Polymer 53:5832–5849
Feng C, Khulbe KC, Matsuura T, Gopal R, Kaur S, Ramakrishna S, Khayet M (2008) J Membr Sci 311:1–6
Fernando MS, Silva RM, Silva KM (2015) Synthesis, characterization, and application of nano hydroxyapatite and nanocomposite of hydroxyapatite with granular activated carbon for the removal of Pb2+ from aqueous solutions. Appl Surf Sci 351:95–103
Fong H (ed) (2006) Polymeric nanofifibers. American Chemical Society, Washington, DC, pp 1–6
Fong H, Reneker DH (2000) Electrospinning and the formation of nanofifibers. In: Salem D (ed) Structure formation in polymeric fifibers. Hanser Publishing, Cincinnati, Ohio, pp 269–288
Fong H, Chun I, Reneker DH (1999) Beaded nanofifibers formed during electrospinning. Polymer 40:4585–4592
Formhals A (1934) Process and apparatus for preparing artifificial threads. US 1975504
Gao W, Liang H, Ma J, Han M, Chen ZL, Han ZS et al (2011) Membrane fouling control in ultrafiltration technology for drinking water production: a review. Desalination 272:1–8
Ghaee A, Shariaty-Niassar M, Barzin J, Matsuura T, Ismail AF (2016) Preparation of chitosan/cellulose acetate composite nanofiltration membrane for wastewater treatment. Desalin Water Treat 57:14453–14460
Gopal R, Kaur S, Ma Z, Chan C, Ramakrishna S, Matsuura T (2006) Electrospun nanofifibrous fifiltration membrane. J Membr Sci 281:581–586
Gopal R, Kaur S, Feng CY, Chan C, Ramakrishna S, Tabe S et al (2007) Electrospun nanofifibrous polysulfone membranes as pre-fifilters: particulate removal. J Membr Sci 289:210–219
Gugliuzza A, Drioli E (2013) A review on membrane engineering for innovation in wearable fabrics and protective textiles. J Membr Sci 446:350–375
Gupta N, Kushwaha AK, Chattopadhyaya MC (2011) Adsorption of cobalt (II) from aqueous solution onto hydroxyapatite/zeolite composite. Adv Mater Lett 2:309–312
Hamad AA, Hassouna MS, Shalaby TI, Elkady MF, Abd Elkawi MA, Hamad HA (2020) Electrospun cellulose acetate nanofiber incorporated with hydroxyapatite for removal of heavy metals. Int J Biol Macromolecules 151:1299–1313
Haider A, Haider S, Kang IK (2018) A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab J Chem 11:1165–1188
Hajra MG, Mehta K, Chase GG (2003) Effects of humidity, temperature, and nanofibers on drop coalescence in glass fiber media. Sep Purif Technol 30:79–88
Hardick O, Stevens B, Bracewell DG (2011) Nanofibre fabrication in a temperature and humidity controlled environment for improved fibre consistency. J Mater Sci 46:3890–3898
He JH, Wu Y, Zuo WW (2005) Critical length of straight jet in electrospinning. Polymer 46:12637–12640
He JH, Wan YQ, Yu JY (2008) Effect of concentration on electrospun polyacrylonitrile (PAN) nanofibers. Fibers and Polymers. 9:140–142
Homaeigohar SS, Elbahri M (2012) Novel compaction resistant and ductile nanocomposite nanofifibrous microfifiltration membranes. J Colloid Interface Sci 372:6–15
Homaeigohar SS, Buhr K, Ebert K (2010) Polyethersulfone electrospun nanofifibrous composite membrane for liquid fifiltration. J Membr Sci 365:68–77
Homaeigohar S, Koll J, Lilleodden ET, Elbahri M (2012) The solvent induced interfifiber adhesion and its inflfluence on the mechanical and fifiltration properties of polyethersulfone electrospun nanofifibrous microfifiltration membranes. Sep Purif Technol 98:456–463
Homaeigohar S, Zillohu AU, Abdelaziz R, Hedayati MK, Elbahri M (2016) A novel nanohybrid nanofibrous adsorbent for water purification from dye pollutants. Materials 9:848
Homayoni H, Hosseini Ravandi SA, Valizadeh M (2009) Electrospinning of chitosan nanofibers: Processing optimization. Carbohyd Polym 77:656–661
Hou W, Xiao Y, Han G, Lin JY (2019) The applications of polymers in solar cells: a review. Polymers 11:143–189
Huang Y, Miao YE, Liu T (2014) Electrospun fibrous membranes for efficient heavy metal removal. J Appl Polym Sci 131:40864–40876
Huebner AL, Chu HN (1971) Instability and breakup of charged liquid jets. J Fluid Mech 49:361–372
Iwamoto S, Nakagaito AN, Yano H (2007) Nano-fifibrillation of pulp fifibers for the processing of transparent nanocomposites. Appl Phys A 89:461–466
Jang W, Yun J, Seo Y, Byun H, Hou J, Kim JH (2020) Mixed dye removal efficiency of Electrospun Polyacrylonitrile-graphene oxide composite membranes. Polymers (Basel). 12(9):. Open Access
Jarusuwannapoom T, Hongrojjanawiwat W, Jitjaicham S, Wannatong L, Nithitanakul M, Pattamaprom C et al (2005) Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur Polymer J 41:409–421
Jia L, Qin XH (2013) The effect of different surfactants on the electrospinning poly(vinyl alcohol) (PVA) nanofibers. J Therm Anal Calorim 112:595–605
Jiang S, Chen Y, Duan G, Mei C, Greiner A, Agarwal S (2018) Electrospun nanofiber reinforced composites: a 26, advances in membrane technologies review. Polym Chem 9:2685–2720
Jung JW, Lee CL, Yu S, Kim ID (2016) Electrospun nanofibers as a platform for advanced secondary batteries: a comprehensive review. J Mater Chem A 4:703–750
Kaur S, Sundarrajan S, Rana D, Matsuura T, Ramakrishna S (2012) Inflfluence of electrospun fiber size on the separation effificiency of thin fifilm nanofifiltration composite membrane. J Membr Sci 392–393:101–111
Kenry LCT (2017) Nanofiber technology: Current status and emerging developments. Prog Polym Sci 70:1–17
Khayet M, Garc´ıa-Payo MC, Garc´ıa-Fernandez L, Contreras-Mart´ınez J (2018) Desalination 426:174–184
Khulbe KC, Matsuura T (2019) Art to use electrospun nanofiber/nanofiber based membrane in waste water treatment, chiral separation and desalination. J Membr Sci Res 5:100–125
Kim GT, Lee JS, Shin JH, Ahn YC, Hwang YJ, Shin HS et al (2005) Investigation of pore formation for polystyrene electrospun fiber: Effect of relative humidity. Korean J Chem Eng 22:783–788
Koski A, Shivkumar YS (2004) Effect of molecular weight on fibrous PVA produced by electrospinning. Mater Lett 58:493–497
Kusworo TD, Wibowo AI, Harjanto GD, Yudisthira AD, Iswanto FB (2014) Cellulose acetate membrane with improved perm-selectivity through modification dope composition and solvent evaporation for water softening. Res J App Sci Eng Techn 7:3852–3859
Lee KP, Arnot, TC, Mattia D (2011) A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J Membr Sci 370(1–2), pp 1–22.
Liao Y, Wang R, Tian M, Qiu C, Fane AG (2013) Fabrication of polyvinylidene fluoride (PVDF) nanofifiber membranes by electrospinning for direct contact membrane distillation. J Membr Sci 425–426:30–39
Liao Y, Tian M, Wang R (2017) A high-performance and robust membrane with switchable super-wettability for oil/water separation under ultralow pressure. J Membr Sci 543:123–132
Liao Y, Loh C-H, Tian M, Wang R, Fane AG (2018a) Progress in electrospun polymeric nanofifibrous membranes for water treatment: fabrication, modifification and applications. Prog Polym Sci 77:69–94
Liao Y, Loh C-H, Tian M, Wang R, Fane AG (2018b) Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog Polym Sci 77:69–94
Liu Y, He JH, Yu JY, Zeng HM (2008) Controlling numbers and sizes of beads in electrospun nanofibers. Polym Int 57:632–636
Liu RL, Tang CY, Zhao JY, Liu HQ (2015) Electrospun membranes of nanoporous structure cellulose acetate and its adsorptive behaviors using copper (II) as models. Desalin Water Treat 56:1768–1775
Ma H, Yoon K, Rong L, Mao Y, Mo Z, Fang D et al (2010) High-flflux thin-fifilm nanofifibrous composite ultrafifiltration membranes containing cellulose barrier layer. J Mater Chem 20:4692–4704
Ma Y, Zhang B, Ma H, Yu M, Li L, Li J (2016) Polyethylenimine nanofibrous adsorbent for highly effective removal of anionic dyes from aqueous solution. Sci China Mater 59:38. Open Access
Macossay J, Marruffo A, Rincon R, Eubanks T, Kuang A (2007) Effect of needle diameter on nanofiber diameter and thermal properties of electrospun poly (methyl methacrylate). Polym Adv Technol 18:180–183
Mit-uppatham C, Nithitanakul M, Supaphol P (2004) Ultrafine electrospun polyamide-6 fibers: Effect of solution conditions on morphology and average fiber diameter. Macromol Chem Phys 205:2327–2338
Mo XM, Xu CY, Kotaki M, Ramakrishna S (2004) Electrospun P(LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothetial cell proliferation. Biomaterials 25:1883–1890
Mohammad AW, Teow YH, Ang WL, Chung YT, Oatley-Radcliffe DL, Hilal N (2015) Nanofiltration membranes review: recent advances and future prospects. Desalination 356:226–254
Montgomery MA, Elimelech M (2007) Water and sanitation in developing countries including health in the equation. Environ Sci Technol 41:17–24
Morton WJ (1902) Method of dispersing fluids. US 706691
Noruzi M (2016) Electrospun nanofibers in agriculture and the food industry: a review. J Sci Food Agr 96:4663–4678
Obaid M, Tolba GMK, Motlak M, Fadali OA, Khalil KA, Almajid AA et al (2015) Effective polysulfone-amorphous SiO2 NPs electrospun nanofifiber membrane for high flflux oil/water separation. Chem Eng J 279:631–638
Park S, Park K, Yoon H, Son J, Min T, Kim G (2007) Apparatus for preparing electrospun nanofifibers: designing an electrospinning process for nanofifiber fabrication. Polym Int 56:1361–1366
Pelipenko J, Kristl J, Jankovic B, Baumgartner S, Kocbek P (2013) The impact of relative humidity during electrospinning on the morphology and mechanical properties of nanofibers. Int J Pharm 456:125–134
Persano L, Camposeo A, Tekmen C, Pisignano D (2013) Industrial upscaling of electrospinning and applications of polymer nanofibers: a review. Macromol Mater Eng 298:504–520
Qin XH, Yang EL, Li N, Wang SY (2007) Effect of different salts on electrospinning of polyacrylonitrile (PAN) polymer solution. J Appl Polym Sci 103:3865–3870
Ramakrishna S, Shirazi MMA (2015) Electrospun membranes: next generation membranes for desalination and water/wastewater treatment. J Membr Sci Res 1:46–47
Ray SS, Chen SS, Li CW, Nguyen NC, Nguyen HT (2016) A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv 6:85495–85514
Reneker DH, Yarin AL (2008) Electrospinning jets and polymer nanofibers. Polymer 49:2387–2425
Reneker DH, Yarin AL, Fong H, Koombhongse S (2000) Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J Appl Phys 87:4531–4547
Reneker DH, Kataphinan W, Theron A, Zussman E, Yarin AL (2002) Nanofifiber garlands of polycaprolactone by electrospinning. Polymer 43:6785–6794
Reneker DH, Fong H (2006) Polymeric nanofibers: Introduction.in ACS Symposium Series; American Chemical Society: Washington, DC
Sainudeen SS, Asok LB, Varghese A, Sreekumaran Nair A, Krishnan G (2017) Surfactant-driven direct synthesis of a hierarchical hollow MgO nanofibernanoparticle composite by electrospinning. RSC Adv 7:35160–35168
Sarbatly R, Krishnaiah D, Kamin Z (2016) A review of polymer nanofibers by electrospinning and their application in oil-water separation for cleaning up marine oil spills. Mar Pollut Bull 106:8–16
Shang Y, Si Y, Raza A, Yang L, Mao X, Ding B et al (2012) An in situ polymerization approach for the synthesis of superhydrophobic and superoleophilic nanofifibrous membranes for oil-water separation. Nanoscale 4:7847–7854
Shawon J, Sung C (2004) Electrospinning of polycarbonate nanofibers with solvent mixtures THF and DMF. J Mater Sci 39:4605–4613
Shin C, Chase GG (2004) Water-in-oil coalescence in micro-nanofifiber composite filters. AlChE J 50:343–350
Shin C, Chase GG (2006) Separation of water-in-oil emulsions using glass fifiber media augmented with polymer nanofifibers. J Disper Sci Technol 27:517–522
Shin YM, Hohman MM, Brenner MP, Rutledge GC (2001a) Experimental characterization of electrospinning: the electrically forced jet and instabilities. Polymer 42:09955–09967
Shin YM, Hohman MM, Brenner MP, Rutledge GC (2001b) Electrospinning: a whipping flfluid jet generates submicron polymer fifibers. Appl Phys Lett 78:1149–1151
Shin C, Chase GG, Reneker DH (2005) The effect of nanofifibers on liquid–liquid coalescence fifilter performance. AlChE J 51:3109–3113
Son WK, Youk JH, Lee TS, Park WH (2004) The effects of solution properties and polyelectrolyte on electrospinning of ultrafine poly(ethylene oxide) fibers. Polymer 45:2959–2966
Subramanian S, Seeram R (2013) New directions in nanofiltration applications—are nanofibers the right materials as membranes in desalination?. Desalination, 308, pp198–208.
Sutherland K (2008) Developments in fifiltration: what is nanofifiltration. Filtr Separat 45:32–35
Tan SH, Inai R, Kotaki M, Ramakrishna S (2005) Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer 46:6128–6134
Tang Z, Wei J, Yung L, Ji B, Ma H, Qiu C et al (2009) UV-cured poly(vinyl alcohol) ultrafifiltration nanofifibrous membrane based on electrospun nanofifiber scaffolds. J Membr Sci 328:1–5
Taylor G (1964) Disintegration of water drops in an electric fifield. Proc R Soc Lond Ser A 280:383–397
Taylor G (1969) Electrically driven jets. Proc R Soc Lond Ser A 313:453–475
Teychene B, Collet G, Gallard H, Croue JP (2013) A comparative study of boron and arsenic (III) rejection from brackish water by reverse osmosis membranes. Desalination 310:109–114
Thom NT, Thanh DTM, Nam PT, Phuong NT, Buess-Herman C, Adsorption behavior of Cd2+ ions using hydroxyapatite (HAp) powder. Green Process Synthesis. https://doi.org/10.1515/gps-2018-0031
Uyar T, Besenbacher F (2008) Electrospinning of uniform polystyrene fibers: the effect of solvent conductivity. Polymer 49:5336–5343
Wang XL, Zhang C, Ouyang P (2002) The possibility of separating saccharides from a NaCl solution by using nanofifiltration in diafifiltration mode. J Membr Sci 204:271–281
Wang X, Fang D, Yoon K, Hsiao BS, Chu B (2006) High performance ultrafifiltration composite membranes based on poly(vinyl alcohol) hydrogel coating on crosslinked nanofifibrous poly(vinyl alcohol) scaffold. J Membr Sci 278:261–268
Wang R, Liu Y, Li B, Hsiao BS, Chu B (2012) Electrospun nanofifibrous membranes for high flflux microfifiltration. J Membr Sci 392–393:167–174
Wannatong L, Sirivat A, Supaphol P (2004) Effects of solvents on electrospun polymeric fibers: preliminary study on polystyrene. Polym Int 53:1851–1859
Warsinger DM, Chakraborty S, Tow EM, Plumlee MH, Bellona C, Loutatidou S et al (2018) A review of polymeric membranes and processes for potable water reuse. Prog Polym Sci 81:209–237
Xu R, Jia M, Zhang Y, Li F (2012) Sorption of malachite green on vinyl-modified mesoporous poly(acrylic acid)/SiO2 composite nanofiber membranes. Microporous Mesoporous Mater 149:111
Yang J, Kubota F, Baba Y, Kamiya N, Goto M (2014) Application of cellulose acetate to the selective adsorption and recovery of Au (III). Carbohydr Polym 111:768–774
Yang L, Wei Z, Zhong W, Cui J, Wei W (2016) Modifying hydroxyapatite nanoparticles with humic acid for highly efficient removal of Cu (II) from aqueous solution. Colloids Surf A 490:9–21
Yang Z, Ma XH, Tang CY (2018) Recent development of novel membranes for desalination. Desalination 434:37–59
Yarin AL, Koombhongse S, Reneker DH (2001) Bending instability in electrospinning of nanofifibers. J Appl Phys 89:3018–3026
Yoon K, Kim K, Wang X, Fang D, Hsiao BS, Chu B (2006) High flflux ultrafifiltration membranes based on electrospun nanofifibrous PAN scaffolds and chitosan coating. Polymer 47:2434–2441
Yoon K, Hsiao BS, Chu B (2009a) Formation of functional polyethersulfone electrospun membrane for water purifification by mixed solvent and oxidation processes. Polymer 50:2893–2899
Yoon K, Hsiao BS, Chu B (2009b) High flflux nanofifiltration membranes based on interfacially polymerized polyamide barrier layer on polyacrylonitrile nanofifibrous scaffolds. J Membr Sci 326:484–492
Yoon K, Hsiao BS, Chu B (2009c) High flflux ultrafifiltration nanofifibrous membranes based on polyacrylonitrile electrospun scaffolds and crosslinked polyvinyl alcohol coating. J Membr Sci 338:145–152
Yordem OS, Papila M, Menceloglu YZ (2008) Effects of electrospinning parameters on polyacrylonitrile nanofiber diameter: An investigation by response surface methodology. Mater Des 29:34–44
Yu Y, Ma R, Yan S, Fang J (2018) Preparation of multi-layer nylon-6 nanofibrous membranes by electrospinning and hot pressing methods for dye filtration. RSC Adv 8:12173. Open Access
Zaher A, El Rouby WMA, Barakat NAM (2020) Influences of tungsten incorporation, morphology and calcination temperature on the electrocatalytic activity of Ni/C nanostructures toward urea elimination from wastewaters. Int J Hydrogen Energy 45(15):8082–8093
Zargham S, Bazgir S, Tavakoli A, Rashidi AS, Damerchely R (2012) The effect of flow rate on morophology and deposition area of electrospun nylon 6 nanofiber. J Eng Fibers Fabr 7:42–49
Zhang C, Yuan X, Wu L, Han Y, Sheng J (2005) Study on morphology of electrospun poly(vinyl alcohol) mats. Eur Polymer J 41:423–432
Zhang S, Shim WS, Kim J (2009) Design of ultra-fine nonwowens via electrospinning of nylon 6: Spinning parameters and filtration efficiency. 28 Adv Membr Technol Mater Design 30:3659–3666
Zhao G, Zhang X, Lu TJ, Xu F (2015) Recent advances in electropsun nanofibrous scaffolds for cardiac tissue engineering. Adv Func Mater 25:5726–5738
Zhmayev E, Cho D, Joo YL (2010) Nanofifibers from gas-assisted polymer melt electrospinning. Polymer 51:4140–4144
Zhou W, He J, Cui S, Gao W (2011) Preparation of electrospun silk fibroin/cellulose acetate blend nanofibers and their applications to heavy metal ions adsorption. Fibers Polym. 12:431–437
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chakrabortty, S., Nayak, J., Chakraborty, P. (2022). Application of Electrospun Polymeric Nanofibrous Membranes for Water Treatment. In: Karchiyappan, T., Karri, R.R., Dehghani, M.H. (eds) Industrial Wastewater Treatment . Water Science and Technology Library, vol 106. Springer, Cham. https://doi.org/10.1007/978-3-030-98202-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-98202-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-98201-0
Online ISBN: 978-3-030-98202-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)