Abstract
The surface tension of the spinning solution is an important parameter in the electrospinning process. Surfactants can change the surface tension of the solution. In this paper, four different kinds of surfactants were added into 10 wt% polyvinyl alcohol/water solution. The effect of different surfactants on the solution properties, the morphology of the resulting mats, the thermal performance, and the inner structure of nanofibers were investigated. The results showed that the surface tension of the spinning solution decreased significantly when the surfactant content was less than 1 %. The viscosity and electric conductivity of the solution increased with the increasing of cationic and anionic surfactant content. The fiber diameter of poly(vinyl alcohol) mats remarkably decreased from 405 to 100 nm as the non-ionic surfactant content within the range of 1 % (v/v) increased. Besides that, the surfactant content also had some influence on the thermal performance and inner structure of nanofibers. With the surfactant content increasing, both the heat of fusions and crystallinity increased significantly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The contracting force per unit length around the perimeter of a surface is usually referred to as surface tension if the surface separates gas from liquid or solid phases and interfacial tension if the surface separated two non-gaseous phases. Surface tension can also be expressed in units of energy per unit surface area. For practical purposes, surface tension is frequently taken to reflect the change in surface-free energy per unit increase in surface area [1]. In electrospinning, the charges on the polymer solution must be high enough to overcome the surface tension of the solution. As the solution jet accelerates from the tip of the source to the collector, the solution is stretched, while surface tension of the solution may cause the solution to breakup into droplets [2–4]. When droplets are collected, a different process called electrospraying [5] is taking place rather than electrospinning, where fibers are collected instead. Surface tension has also been attributed to the formation of beads on the electrospun fibers. Thus, it is important to understand the role of surface tension in a fluid.
Surface tension has the effect of decreasing the surface area per unit mass of a fluid. In this case, when there is a high concentration of free solvent molecules, there is a greater tendency for the solvent molecules to congregate and adopt a spherical shape due to surface tension. A higher viscosity means that there is greater interaction between the solvent and polymer molecules, hence when the solution is stretched under the influence of the charges, the solvent molecules will tend to spread over the entangled polymer molecules, then prevent solvent molecules to come together under the influence of surface tension.
Fong et al. [6] found that a solvent, such as ethanol, had low surface tension. It could be added to encourage the formation of smooth fibers. Another way to reduce the surface tension is to add surfactant to the solution. The addition of surfactant was found to yield more uniform fibers. Even when insoluble surfactant is dispersed in a solution as fine powders, the fiber morphology is improved [7].
The surfactant is a molecule that lowers surface tension with a hydrophilic head that sticks to water and a hydrophobic end that sticks to organic substance. The surfactant usually contains both a hydrophobic and a hydrophilic part within a molecule [8, 9] and acts as a surface-active agent that can greatly influence the chemical properties at the interface. The driven force for a surfactant to adsorb at an interface is to decrease the free energy of the phase boundary [10, 11]. Therefore, when the boundary is covered with surfactant molecules, the surface tension is reduced. The primary classification of surfactants is made on the basis of the charge of the polar head group. Traditionally, there are four types: anionic surfactant, cationic surfactant, amphoteric surfactant with polarity (negative and the positive charge), and non-ionic surfactant with no electrical net charge [12].
Although many researchers had studied the polymer–surfactant aggregation [13–15], surface tension of the polymer–surfactant solution [16, 17], there was little research on the effect of surfactants or surface tension on the electrospinning process. The surface tension of the solution is an important parameter and has a great influence on the electrospinning process and the nanofibers. Hence, the objectives of this study are to prepare the PVA solution with different kinds of surfactants and different surfactant concentration; to research the effect of different surfactants on the solution properties including solution viscosity, solution conductivity, and surface tension of the solution; to prepare nanofibrous mats from the different PVA-surfactant solutions via electrospinning; to measure the diameter of the resulting nanofibers and observe the morphological change of the resulting mats; and then to study the effect of the different surfactants on the diameters and performance of the nanofibers.
Experimental
Materials
The poly(vinyl alcohol) (PVA) with a molecular mass (M m ) of 88,000 was purchased from J&K Chemical Co. (China). Cationic surfactants (Gemini quaternary), anionic surfactants (Rosin acid sodium), Non-ionic surfactants (Heterogeneous polyoxyethylene polyoxypropylene ether), and Amphoteric surfactants (Lauryl betaine) were obtained from Henan Titaning Chemical Co. (China). The characteristics for four kinds of surfactants are shown in Table 1.
Gemini quaternary is a new-style quaternary ammonium cationic surfactant. It has lower critical micelle concentration, but higher surface activity compared with conventional single quaternary ammonium surfactant, gemini quaternary. The formula is:
Rosin acid sodium is an anionic surfactant with three benzene rings' structure. It is widely used as high-performance solid emulsifier due to its amphiphilic structure. It has excellent emulsifying properties and perfect resistance to hard water and lime soap dispersion properties. The formula is:
Heterogeneous polyoxyethylene polyoxypropylene ether (a biodegradable and low foaming, high efficiency, environmentally friendly non-ionic surfactant) is made by a reaction of heterogeneous alcohol, ethylene oxide, and propylene oxide. The formula is:
Lauryl betaine, which can dissolve in water and polar organic solvents, is an amphoteric surfactant. Under acidic conditions, the aqueous solution is cationic, while under alkaline conditions, it shows non-ionic properties. Besides that, Lauryl betaine has a good thickening, anti-static, soft, foaming, and decontamination performance. The formula is:
Preparation of polymer solution
The 10 % (mass/mass) (m/m) PVA solution was prepared by dissolving in distilled water and gently stirring at 80 °C until complete dissolution of polymer occurred. Then, each surfactant was incorporated into the PVA solution. The ratio of the PVA solution/surfactant was 100/0, 99.9/0.2, 99.5/0.5, 99.2/0.8, 99/1 % 98.8/1.2 (m/m), respectively. PVA solutions prepared were stirred for 1 h at room temperature and then used for electrospinning.
Electrospinning setup
The experimental set-up device used for electrospinning process is shown in Fig. 1. The variable high voltage power supply was used for the electrospinning. It was used to produce voltages ranging from 0 to 50 kV. The anode was directly inserted into the polymer solution in the syringe. The cathode was connected to the collector. The voltage used in the experiment was 20 kV and the current was adjusted to be constant. PVA solution was poured in a syringe attached with a capillary tip of 1 mm diameter, the flow rate was 0.5 mL h−1, and the distance between the tip and the collector was fixed at 12 cm.
Measurement of the solution properties
The surface tension of polymer solution was measured by the Wilhelmy ring method using a tension meter (DCAT11, Krüss Co., Germany) at 25 °C. The platinum plate was cleaned with an alcohol torch. The viscosity of polymer solution was measured using a digital viscometer (NDJ-8SN, Hangping Co., China) with spindle No. 62 at 100 rpm at room temperature. Electric conductivity of polymer solution was also analyzed using an electric conductivity meter (FG3 series, Mettler-Toledo Co., Ltd., Germany) at room temperature.
Measurement of the nanofibers' properties
The morphology of the resulting mats was examined by means of scanning electron microscopy (SEM: JSM-5600LV, Jeol Co., Japan) after gold sputtering. The average diameter and the diameter distribution were obtained from an image analyzer (Photoshop CS). The thermal behavior of PVA electrospun fibers prepared with or without different surfactants was determined with a differential scanning calorimeter (DSC) (Pyris DSC, PE Instruments, USA) using approx. 5.0 mg samples at a heating rate (V h = 20 °C min–1) in nitrogen at a gas flow rate of 25 ml min−1. The DSC data were compiled and analyzed by the software associated with the DSC instrument. The melting point (T m) was recorded and was determined at the onset of the melting endotherm. The microstructure changes of PVA nanofibers with or without different surfactants were performed by X-ray diffraction. The X-ray diffraction in this paper was tested by D/max-2550 PC X-ray diffractometer which had software to evaluate the crystallinity of the samples.
Results and discussion
It is well known that various parameters such as solution concentration, ionic salts content, and applied electric field strength affect the morphology of electrospun mats, the thermal performance, and the inner structure of nanofibers [18–24].
Surface tension of the solution
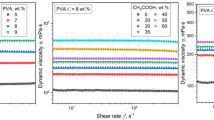
Figure 2 shows dynamic surface tension (mN m−1) of PVA solution prepared by tuning the surfactant content of 0.2, 0.5, 0.8, 1.0, 1.2 % (m/m) for each surfactant (anionic, cationic, amphoteric, and non-ionic surfactant, respectively) at room temperature. The surface tension of PVA solution alone was about 44 mN m−1. The surface tension in a mixture of PVA and cationic surfactant as a function of surfactant content decreased from 38.2 to 25.4 mN m−1. When the concentration of the anionic surfactant were less than 1 %, the surface tension of PVA solution with anionic surfactant decreased from 26.3 to 11.7 mN m−1; however, the surface tension of PVA solution with 1.2 % anionic surfactant was 38.1 mN m−1. When adding non-ionic surfactant into the PVA solution, the surface tension force decreased greatly from 43.4 to 4.6 mN m−1; however, when the concentration of non-surfactant was 1.2 %, the surface tension increased to 32.6 mN m−1. But, the surface tension force was not decreasing significantly in the PVA solution with amphoteric surfactant and the surface tension force was about 33 mN m−1.
As expected, the mixtures of PVA and surfactant had lower surface tension than the PVA solution itself in all cases, and the non-ionic and anionic surfactant can decrease the surface tension force of PVA solution significantly; however, when the concentration of the surfactant were more than 1 %, the surface tension force increased greatly. This surface behavior was regarded as evidence for the polymer–surfactant interaction, which would change with the surfactant content increase. Generally, when the surfactant content was at less critical micelle concentration, the surfactant existed in the solution as single molecule, and hardly had interaction with the polymer. When the surfactant content reached critical aggregation concentration, the surfactant began to interact with the polymer. Then, as the surfactant concentration continued to increase, the free micelles of surfactant began to form, the polymer–surfactant interaction saturated; at this time, the increasing of surfactant content had no effect on the polymer–surfactant interaction [12]. Different surfactants had different interaction saturated concentrations in the PVA solution; in the PVA/non-ionic surfactant solution, when the surfactant content was 0.5 %, the polymer–surfactant interaction saturated; then, it continued to increase the surfactant content, the surface tension of the solution did not change, and when the non-ionic surfactant content was 1.2 %, the surface tension increased due to too many free micelles. Lin et al. [25] electrospun polystyrene nanofibers with the inclusion of cationic surfactants, dodecyltrimethylammonium bromide (DTAB). He also found that the surface tension of polystyrene solution decreased slightly with an increase in the concentration of DTAB.
Viscosity of the solution
The viscosity in the mixture of PVA/surfactant as a function of surfactant content at room temperature is shown in Fig. 3. The viscosity of the PVA solution alone was about 1,076 cPs. The viscosity of the solution with different contents of non-ionic and amphoteric surfactants was constant. In addition, the viscosity of the solution in both a mixture of PVA/anionic surfactant and PVA/cationic surfactant increased with surfactant content increasing.
Keishiro et al. [26] and Cabane [13, 15] proposed that the strength of the surfactant–polymer interaction is dependent upon the chemical properties of the polymer and surfactant. The results may be explained by the fact that above the critical aggregation concentration, some ionic surfactants could bind cooperatively to non-ionic polymers. This cooperative binding, which is driven by electrical or hydrophobic interactions, resulted in the formation of polymer–surfactant complexes in the solution. The added cationic and anionic surfactants interacted strongly with the hydrophobic groups (Alkyl groups) of the polymer, leading to a strengthened association between polymer chains and thus to an increased viscosity. While for non-ionic surfactants, there was no cooperative binding between the surfactant and polymer as there was no free ion in the solution. For amphoteric surfactant, there was no cooperative binding between the surfactant and polymer due to the amphiphilic characters of the surfactant. Hence, the viscosity of the solution in both a mixture of PVA/anionic surfactant and PVA/cationic surfactant increased with the surfactant content increasing, while the viscosity of the solution in both a mixture of PVA/amphoteric surfactant and PVA/non-ionic surfactant solution was constant. The interaction of sodium dodecyl sulfate with polyethylene oxide has been investigated by Malcom. He studied the conductance, surface tension, viscosity of the solution, and got a similar result [27].
Electric conductivity of the solution
Figure 4 shows the electric conductivity in mixtures of PVA and each type of surfactant. The electric conductivity of PVA solution alone was 0.745 mS cm−1. For the PVA solvents containing the cationic, anionic, and amphoteric surfactants, the electric conductivity of polymer solutions increased with the increasing of surfactant content. However, for the PVA solution with the non-ionic surfactant, since the surface-active portion of the molecule bears no apparent ionic charge, the non-ionic surfactant had no effects on the electric conductivity of the PVA solution.
The morphology of electrospun nanofibers
The aqueous characteristics of PVA-surfactant solution were discussed in the previous section. Aqueous characteristics of PVA such as viscosity of polymer solution, electric conductivity, and dynamic surface tension depended upon the content and types of surfactants. It is obvious that some of the PVA solution in the presence of surfactants had an influence upon morphological properties of PVA non-woven mats during the electrospinning.
Figure 5a–e shows SEM images of PVA non-woven mats prepared from a mixture of PVA/surfactant via electrospinning. The diameter of electrospun fibers decreased when surfactants were incorporated into PVA solution. As clearly shown in Fig. 5, the PVA non-woven mats produced from the mixture of PVA and amphoteric had much more junctions and bundles of fibers than those produced from pure PVA solution, and the bundles of fibers increased as the surfactant content increased. In the case of non-ionic surfactant, with the increasing of the surfactant content, the diameters of the nanofibers decreased significantly, and the uniformity of the nanofibers also reduced. Figure 5d–e also shows that nanofibers prepared from PVA/non-ionic surfactant solution and PVA/amphoteric surfactant solution were uneven. Especially for the PVA solution with 0.5 % non-ionic surfactant, the diameter distribution was relatively large.
SEM images of the electrospun PVA non-woven mats prepared from various PVA-surfactant systems: a PVA solution; b PVA-cationic surfactant solution; c PVA-anionic surfactant solution; d PVA-amphoteric surfactant solution; e PVA non-ionic surfactant solution (the content of surfactant (w/w): (1): 0.2 %, (2): 0.5 %, (3): 1 %)
The average diameter of fibers from PVA solution alone was about 405 nm. The diameter of nanofibers from PVA solution with four surfactants all decreased. Many researchers had found a similar result [28–30]; they added different surfactants to different polymer solutions, the diameter of fibers all reduced because of the lower surface tension and higher conductivity. The fiber diameter of the electrospun non-woven mats prepared from a mixture of PVA/non-ionic surfactant (Fig. 5(d(1), d(2), d(3))) was much thinner when compared to the others. The average diameter of fibers from PVA/non-ionic and PVA/anionic surfactant decreased from 405 to 100 nm due to the lower surface tension and higher conductivity, while the average diameter of fibers slightly decreased as the cationic and amphoteric surfactant content increased (see Fig. 6).
Figure 6 also shows that the diameter of nanofibers from PVA/non-ionic surfactant solution and PVA/amphoteric surfactant solution was uneven, the error bar of the fiber diameter was larger.
DSC characterization of PVA nanofibers
Figure 7 shows DSC curves of PVA electrospun nanofibers produced from 10 % PVA/water with no surfactant, 0.2 % cationic surfactant, 0.2 % anionic surfactant, 0.2 % non-ionic surfactant, 0.2 % amphoteric surfactant; and the melting peak and the heat of fusion were observed in five curves. As DSC curves showed that the PVA nanofibers with or without surfactants appear at approximately the same T m around 190 °C, the heat of fusion of PVA nanofibers with no surfactant, 0.2 % cationic surfactant, 0.2 % anionic surfactant was about 36 J g−1. However, for the nanofibers with 0.2 % non-ionic surfactant, the heat of fusion increased to 43.8954 J g−1, while for the nanofibers with 0.2 % amphoteric surfactant, the heat of fusion decreased to 32.2637 J g−1; the results indicated that the non-ionic and amphoteric surfactants can influence the thermal performance of the nanofibers, the thermal stability of the nanofibers with non-ionic surfactant was the best, while the thermal stability of the nanofibers with amphoteric surfactant was the worst. From the Figs. 5 and 6, we knew that the nanofibers produced from PVA/non-ionic solution were smallest, which indicate that the nanofibers got the greatest stretching in the electrospinning process. Hence, the molecular chains of the nanofibers were more regular, leading to the heat of fusion increase. However, adding amphoteric surfactant into the polymer solution, because both positive and negative charges were present in the surface-active portion, positive and negative charges both acted on the molecular chains and led to irregular alignment of the polymer chain, which resulted in the heat of fusion decrease.
From the DSC curves of PVA nanofibers with or without surfactant shown in Fig. 7, it is clear that pure PVA nanofibers (sample A) showed a broad T g at 94.38 °C; when adding 0.2 % surfactant into the PVA solution, the T g peak maximums increased, except the cationic surfactant; the T g peak maximums increased to 99.42 °C for the anionic surfactant, to 107.89 °C for the non-ionic surfactant, to 95.84 °C for the amphoteric surfactant. As a general rule, any structural features that reduce segmental mobility or free volume will increase the T g when adding surfactant into the PVA solution. The polymer–surfactant interaction could introduce restrictions on segmental mobility and enhanced T g. While for the cationic surfactant, although the T g decreased when adding 0.2 % cationic surfactant, when adding more (0.5 or 1 %) cationic surfactant, the T g increased (see Table 2; Fig. 8). Hence, adding bits of surfactant could enhance T g.
Table 2 shows the effect of different surfactants and surfactant contents on the T g of PVA nanofibers. Figure 8 shows the effect of surfactant content on the T g of PVA nanofibers. They both clearly show that the T g of nanofibers increased with the increasing of surfactant content. PVA is a linear aliphatic hydroxyl polymer containing secondary hydroxyl groups in every alternate carbon, and the concentration of hydroxyl groups has a significant impact on T g. In the case of adding more non-ionic and amphoteric surfactant into the PVA solution, it introduced more hydroxyl groups and enhanced hydrogen bonding, which increased the T g peak maximum because PVA, non-ionic and amphoteric surfactant all had hydroxyl groups. While for the cationic and anionic surfactants, they also had hydrophilic groups which could react with the hydroxyl groups of PVA, which could also increase the T g peak maximums.
From the DSC curves of PVA nanofibers with different surfactant contents shown in Fig. 8, it is clear that the PVA nanofibers with different surfactant contents showed approximately the same T m around 190 °C, while the heat of fusion changed significantly. For the solution with cationic and anionic surfactants, with the increasing of surfactant content, the heat of fusions all increased considerably. The results may be explained by the fact that the surface tension decreased, electric conductivity increased with the surfactant content increase. Both lower surface tension and higher conductivity could lead to higher electrostatic force. Then, in the electrospinning, the polymer solution got more stretching, the molecule chains of the polymer were more regular, the crystallinity of the nanofibers increased. Hence, the heat of fusion increased. While for the solution with non-ionic and amphoteric surfactants, with the increasing of surfactant content, the heat of fusions increased first and then decreased, reached the maximum when the content of surfactant were 0.5 %. The reason was that the polymer–surfactant interaction saturated when adding 0.5 % non-ionic and amphoteric surfactants. Then, adding more surfactant, the surface tension would increase as well as the heat of fusions. On the other hand, Figs. 5 and 6 show that with the content of surfactant increasing, the diameter of nanofibers from PVA solution with non-ionic surfactant and amphoteric surfactant decreased first and then increased, the diameter of PVA solution with 0.5 % non-ionic and amphoteric surfactant was smallest. The results of heat of fusion were in conformity with the results of surface tension and nanofibers' diameter.
X-ray diffraction of PVA nanofibers
Figure 9 shows X-ray diffraction patterns of PVA nanofibers electrospun from 10 % PVA/water with no surfactant, 0.2 % cationic surfactant, 0.2 % anionic surfactant, 0.2 % non-ionic surfactant 0.2 % amphoteric surfactant. These five kinds of samples all exhibited one equatorial peaks with a diffuse meridian peak that indicated rather poor orientation. The diffraction patterns of these five samples were similar, but peak heights and peak sharpness were all different. For the nanofibers from PVA solution with non-ionic surfactants and anionic surfactants, the peak height was higher than the pure PVA solution and the peak was sharper, while for the nanofibers from PVA solution with cationic and amphoteric surfactant, the peak height was shorter than the pure PVA solution. The peak height and peak sharpness were in relation to the crystallinity of nanofibers; hence, the result of the X-ray was in conformity with the results of heat of fusion.
Figure 10 shows X-ray diffraction patterns of PVA nanofibers with different contents surfactants. The diffraction patterns of these samples were all similar. However, with the increasing of the surfactant content, the height of the peaks increased. Because the crystallinity of nanofibers was different, the software MDI jade was used to calculate the samples’ crystallinity (shown in Fig. 11).
In the Fig. 11, we could see from the crystallinity of nanofibers that the PVA solution with surfactants was larger than the pure PVA nanofibers, except the amphoteric surfactant. For the nanofibers from PVA solution with cationic and anionic surfactants, with the increasing of surfactant content, the crystallinity increased significantly; the results were in accord with the DSC results. There were two reasons for these results. On the one hand, the surface tension of the PVA solution with surfactants decreased; furthermore, the electric conductivity of polymer solutions with surfactant increased significantly. Both lower surface tension and higher conductivity could lead to higher electrostatic force, the electrospinning jet, and the polymer molecules suffered more stretching; hence, the crystallinity increased. Qin [31, 32] electrospun PAN nanofibers and found that the stretching of electrostatic force is propitious to form a crystal of PAN nanofibers.
On the other hand, Cabane and Duplessix [33, 34] found that adding ionic surfactant into polymer solution, the polymer would form aggregates. The polymer–surfactant aggregate could be described as a mixed micelle. The polymer was wrapped around this micelle; some macromolecules of the polymer were directly adsorbed at the hydrocarbon/water interface, but most of them formed loops in the surrounding water. There were so many mixed micelles, and in the electrospinning process, the micelle acted as a crystal nucleus and led to the forming of a crystal of PVA nanofibers. So, the increasing of surfactant content and the mixed micelle increase could make the crystallinity increase significantly.
While for the nanofibers produced from PVA solution with non-ionic surfactant and amphoteric surfactant, the crystallinity of nanofibers increased firstly and then decreased with the increasing of surfactant content. Although the two kinds of surfactant showed the similar tendency to the crystallinity of nanofibers, the reason for the two phenomena was different. For the nanofibers produced from the PVA solution with non-ionic surfactant, adding a small quality of surfactant can reduce the nanofibers’ diameter significantly; however, the polymer–surfactant interaction saturated when the content of surfactant reached 0.5 %; then, adding more surfactant could influence the polymer–surfactant interaction, resulting in the increasing of surface tension; the nanofibers’ diameter also increased. The nanofibers from the PVA solution with 0.5 % non-ionic surfactant undertook more stretching in the electrospinning process, so the crystallinity was the maximum. For the nanofibers produced from the PVA solution with amphoteric surfactant, the interaction between PVA and amphoteric surfactant and the solution properties was complicated due to the amphiphilic characters of the surfactant, which resulted in the instability of electrospinning process, the jet undertook unstable stretching in the electrospinning process. Figure 5e(1–3) shows that there were more junctions and bundles of fibers, indicating that the nanofibers undertook less stretching. Hence, the crystallinity of nanofibers produced from PVA solution with amphoteric surfactant was less than the pure PVA solution. On the other hand, adding amphoteric surfactant into the polymer solution, because both positive and negative charges were present in the surface-active portion, positive and negative charges both acted on the molecular chains and lead to irregular alignment of the polymer chain, which resulted in the decreasing of crystallinity.
Conclusions
In the present work, four different surfactants were added into the PVA solution. The results showed that the surfactants had different influences on the solution properties, the morphology, microstructure, and thermal performances of the nanofibers.
-
(1)
Surface tension of the polymer solution decreased significantly when the surfactant content was less than 1 %. The viscosity of the solution increased with the increasing of cationic and anionic surfactant content; non-ionic and amphoteric surfactant had no influence on the solution viscosity. In addition, the electric conductivity of polymer solutions increased with surfactant content increasing, except for the PVA solvent with non-ionic surfactant.
-
(2)
The nanofibrous non-woven mats were successfully prepared from PVA-surfactant solutions via electrospinning. The fiber diameter of PVA mats remarkably decreased from 405 to 100 nm as the non-ionic surfactant content within the range of 1 % (v/v) increased, while the average diameter of fibers slightly decreased as the cationic and amphoteric surfactant content increased.
-
(3)
Differential scanning calorimetry indicated that the surfactant content had a great effect on the heat of fusion, and with the increasing of surfactant content, the T g and heat of fusions all increased considerably. The crystallinity of nanofibers prepared from PVA solution with surfactants was larger than the pure PVA nanofibers, except the amphoteric surfactant, and with the increasing of surfactant content, the crystallinity increased significantly.
References
Ramakrishna S, Fujihara K, Teo WE, Lim TC. An introduction to electrospinning and nanofubers. Singapore: World Scientific Publishing; 2005.
Christanti Y, Walker LM. Surface tension driven jet break up of strain hardening polymer solutions. J Non-Newton Fluid. 2001;100:9–26.
Qin XH, Jia L, Lu WY, Shou DH, Fan JT. Stretching of the steady jet in electrospinning: theoretical analysis and experimental verification. Text Res J. 2011;8:388–97.
Qin XH, Yang EL, Li N, Wang SY. Effect of different salts on electrospinning of polyacrylonitrile (PAN) polymer solution. J Appl Polym Sci. 2007;103:3865–70.
Morozov VN, Morozova TY, Kallenbach NR. Atomic force microscopy of structures produced by electrospraying polymer solutions. Int J Mass Spectrom. 1998;178:143–59.
Fong H, Chun I, Reneker DH. Beaded nanofibers formed during electrospinning. Polymer. 1999;40:4585–92.
Zeng J, Xu X, Chen X, Liang Q, Bian X, Yang L, ling L. Biodegradable electrospun fibers for drug delivery. J Controlled Release. 2003;92:227–31.
Manglik RM, Wasekar VM, Zhang J. Dynamic and equilibrium surface tension of aqueous surfactant and polymeric solutions Exp. Therm Fluid Sci. 2001;25:55–64.
Tarahomjoo S, Alemzadeh I. Surfactant production by an enzymatic method enzyme. Microb Tech. 2003;33:33–7.
Robert DG. Mesoscopic simulation of polymer–surfactant aggregation. Langmuir. 2000;16:7493–502.
Fainerman VB, Miller R, Aksenenko EV. Simple model for prediction of surface tension of mixed surfactant solutions. Adv Colloid Interface Sci. 2002;96:339–59.
Milton JR. Surfactants and interfacial phenomena. 3rd ed. New Jersey: John Wiley & Sons, Inc.; 2004.
Cabane B. Structure of some polymer–detergent aggregates in water. J Phys Chem. 1977;81:1639–45.
Lay TL. Polymer–surfactant interactions: neutron scattering and reflectivity. Curr Opin Colloid Interface Sci. 1999;4:205–13.
Cabane B, Duplessix R. Organization of surfactant micelles adsorbed on a polymer molecule in water : a neutron scattering study. J Phys. 1982;43:1529–42.
Lay TL, Bernard C. Effects of surfactants on thermally collapsed poly(n-isopropylacrylamide) macromolecules. Macromolecules. 1997;30:6559–66.
Cabane B, Duplessix R, Zemb T. High resolution neutron scattering on ionic surfactant micelles : SDS in water. J Phys France. 1985;46:2161–78.
Zong XH, Kim K, Fang DF, Ran XF. Structure and process relationship of electrospun bioabsorbable nanofiber membrane. Polymer. 2002;43:4403–12.
Kim SJ, Lee CK, Kim SI. Effect of ionic salts on the processing of poly(2-acrylamido-2-methyl-1-propane sulfonic acid) nanofibers. J Polym Sci. 2005;96:1388–93.
Vince B, Wen XJ. Effect of electrospinning parameters on the nanofiber diameter and length. Mater Sci Eng C. 2008;29:1–6.
Kadomae Y, Amagasa M, Sugimoto M. Effect of electric current on beads formation in electrospinning of poly(vinyl alcohol). Int Polym Process. 2008;23:377–84.
Qin XH, Wu DQ. Effect of different solvents on poly(-caprolactone) (PCL) electrospun nonwoven membranes. J Therm Anal Calorim. 2012;107:1007–13.
Ma Q, Cebe P. Phase structure of electrospun poly(trimethylene terephthalate) composite nanofibers containing carbon nanotubes. J Therm Anal Calorim. 2010;102:425–34.
Imre MS, Eero S, Mikko H, Marianna K, Timur N, Leonid K. Thermal study on electrospun polyvinylpyrrolidone/ammonium metatungstate nanofibers: optimising the annealing conditions for obtaining WO3 nanofibers. J Therm Anal Calorim. 2011;105:73–81.
Lin T, Wang HX, Wang HM. The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology. 2004;15:1375–81.
Keishiro S, Kaoru T, Toshio T. Fee-boundary electrophoresis of sodium dodecyl sulfate-protein polypeptide complexes with special reference to DS-polyacrylamide gel electrophoresis. J Biochem. 1974;75:309–19.
Malcom NJ. The interaction of sodium dodecyl sulfate with polyethylene oxide. J Colloid Interface Sci. 1967;23:36–42.
Jung YH, Kim HY, Lee DR. Characterization of PVOH nonwoven mats prepared from surfactant-polymer system via electrospinning. Macromol Res. 2005;13:385–90.
Kenneth L, Chua KN, Gregory T. Reducing electrospun nanofiber diameter and variability using cationic amphiphiles. Polymer. 2007;48:6384–94.
Teeradech J, Walaiporn H, Sujinda J. Effect of solvents on electrospinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur Polym J. 2005;41:409–21.
Qin XH, Wang SY. Interior structure of polyacrylonitrile(PAN) nanofibers with LiCl. Mater Lett. 2008;62:1325–7.
Qin XH. Structure and property of electrospinning PAN nanofibers by different preoxidation temperature. J Therm Anal Calorim. 2010;99:571–5.
Cabane B, Duplessix R. Neutron scattering study of water-soluble polymers adsorbed on surfactant micelles. Colloids Surf. 1985;13:19–33.
Cabane B, Duplessix R. Decoration of semidilute polymer solutions with surfactant micelles. J Phys. 1987;48:651–62.
Acknowledgements
This work was partly supported by grants (50973014 and 11172064) from the National Natural Science Foundation of China and from the Foundation for the Author of National Excellent Doctoral Dissertation of P. R. China (200961), as well as sponsored by the Shanghai Rising-Star Program in China (10QA1400100) and the Fok Ying Tong Education Foundation (121071) for Prof. Xiaohong Qin. Furthermore, it was also supported by the Program for New Century Excellent Talents in University (NCET-10-0322) and the Fundamental Research Funds for the Central Universities as well as the “Shu Guang” (11SG33) project supported by the Shanghai Municipal Education Commission and Shanghai Education Development Foundation for Prof. Xiaohong Qin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, L., Qin, Xh. The effect of different surfactants on the electrospinning poly(vinyl alcohol) (PVA) nanofibers. J Therm Anal Calorim 112, 595–605 (2013). https://doi.org/10.1007/s10973-012-2607-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2607-9