Abstract
Ohmic heating is a highly promising alternative to conventional thermal processing of foods and food ingredients. Direct heating via the application of an electric field offers benefits in regard to process efficiency and food quality. Increased heating rates, improved homogeneity of the product temperature distribution and suitability for treatment of particle-rich foods contribute to avoidance of thermal over-processing and thus prevent valuable food compounds like bioactives from thermal degradation. Additional non-thermal effects on biological cells and tissue can enhance inactivation rates for undesired microorganisms and enzymes as well as the extraction efficiency of cellular bioactive components. Applicability and effectiveness of ohmic heating are determined by the equipment design, process parameters including applied voltage gradient, frequency of the alternating current, chosen temperature profiles and product properties such as conductivity, viscosity, concentration and size distribution of particles, inclusion of non-conductive parts, and heat sensitivity of the components. Special attention has to be devoted to the avoidance of undesired reactions at the electrode surface in form of electrolysis and metal corrosion that might enhance the degradation of bioactive components in food. Usage of adequate electrochemically inert electrode materials and careful process design are required not to compromise food quality and safety.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ohmic heating
- Moderate electric fields
- Food bioactives
- Vitamins
- Polyphenols
- Retention of bioactives
- Extraction of bioactives
- Degradation of bioactives
1 Ohmic Heating: A Promising Alternative to Conventional Heating
Heating belongs to the most important and most common process steps in food manufacturing. It serves the extension of shelf-life by inactivation of undesired microorganisms and enzymes, the induction of textural and structural alterations via tissue softening, starch gelatinization or protein denaturation as well as the preparation for and efficiency enhancement of subsequent processing steps. Besides these desired effects, there may also be adverse effects. Sensorial quality may be decreased by the loss of aroma and flavor or nutritionally detrimental effects may occur via degradation and loss of nutrients (Varghese et al. 2014). Especially bioactive ingredients such as vitamins or polyphenols often possess high temperature sensitivity and thus are strongly affected by intense thermal processing. Conventional heating is realized via external heat generation and subsequent heat transfer to the product by conduction, convection or radiation (de Alwis and Fryer 1992; Goullieux and Pain 2005; Varghese et al. 2014). Conduction of heat is often a slow process leading to inhomogeneous temperature distributions in the product. This results in over-processing in some parts of the product connected to higher quality losses (de Alwis and Fryer 1992). Thus, technologies are required that meet both the requirements for food safety or structure changes as well as sufficient product quality. Besides non-thermal technologies such as high isostatic pressure or pulsed electric fields, alternative heating methods are of interest achieving rapid and uniform heating of the product (Varghese et al. 2014).
One of these methods is ohmic heating where an electric current is passed through a material with electrical resistance with the purpose of heating it (Vicente et al. 2006). Similar to other volumetric heating methods, e.g. microwave or inductive heating, heat is generated inside the food and therefore the dependency on thermal conduction and convection is low (Goullieux and Pain 2005). This leads to fewer temperature gradients in the product. The name ohmic heating is derived from the relation between current, voltage, and resistance known as Ohm’s law (Icier 2012):
where R is the electrical resistance in ohms, V is the voltage applied in volts and I is the current in amps (Fellows 2009).In literature, the technology is also referred to as Joule heating, electric resistance heating, direct electric resistance heating, electro heating, and electro conductive heating (Bengston et al. 2006; Varghese et al. 2014; Chen 2015). The food material takes up the position of the resistance in the electric circuit (Icier 2012). Most foods contain sufficient water and ions from salts and acids to be electrical conductive and function thus as a resistance in the process. This enables an electric current to pass through it when a voltage gradient is applied (Halden et al. 1990; Icier 2012). The resistance of food material to the electrical current causes heating by converting electric energy into heat energy (Sastry 1992; Sastry and Barach 2000). In contrast to metals, in which a current is transported via movement of electrons, current flow in foods occurs due to the movement of ions. Depending on their net charge ions move within the electric field towards the electrode with opposite charge. Collision and friction between ions and other molecules during these movements lead to energy dissipation in form of heat. The amount of heat is directly related to the current flow caused by the voltage gradients in the food and the electrical conductivity of the food material (Sastry and Li 1996). Therefore, ohmic heating can be regarded as a technique for internal thermal energy generation and not as a method for thermal energy transfer (Knirsch et al. 2010).
This direct heating provides some advantages compared to conventional indirect heating methods. One requirement in food preservation is that each part of the food has been subjected to sufficient treatment intensity to reach the desired level of sterility. In conventional thermal processing, there is usually a marked time lag between the outer and inner parts of the material reaching critical temperature and experiencing sufficient thermal preservation. In consequence, a huge part of the food needs to be over-processed to guarantee complete pasteurization or sterilization and consider the whole product as safe (de Alwis and Fryer 1992). One main indication for good process design of conventional heating is therefore a narrow temperature and residence time distribution (Goullieux and Pain 2005). Uniform internal heat generation may avoid over-processing due to the absence of marked temperature gradients in the product leading to a more homogeneous treatment and a better preservation of temperature sensitive nutrients, color and aroma (Wang and Sastry 2002; Castro et al. 2004a, b; Icier and Ilicali 2005; Leizerson and Shimoni 2005a, b; Vikram et al. 2005). The absence of hot surfaces required for conventional heat transfer reduces the risk of local overheating and food burning onto the equipment (Ayadi et al. 2005; Bengston et al. 2006; Fellows 2009; Sakr and Liu 2014). Beside fewer effects on product quality, this also brings reductions in costs for clean-up and maintenance of the equipment (Reznick 1996; Varghese et al. 2014). Target temperatures can be reached very fast with ohmic heating (Sastry 2005; Bengston et al. 2006; Fellows 2009) reducing the contribution of heating time on the thermal load of the product, which also helps to preserve nutritional and sensorial quality. Due to the homogeneity of heating, there is no need for intensive mixing, which protects shear-sensitive materials (Icier 2012). The energy conversion efficiency of ohmic heating is very high (Bengston et al. 2006; Icier 2012). Around 90% of the energy is converted to heat in the food (Fellows 2009), which might save energy and costs to the processors (Varghese et al. 2014) and can be considered as a green technology. The capital costs for the equipment are relatively low compared to other direct heating methods (Bengston et al. 2006; Varghese et al. 2014).
The technology is also suitable to heat mixtures of liquid and particles that are difficult to process with conventional methods (Varghese et al. 2014). If the two phases possess an identical electrical resistance, they can be heated homogeneously (de Alwis and Fryer 1992; Icier 2012) avoiding cold spots in the solid phase and reducing the need for overcrossing the liquid. Using ohmic heating, it is thus possible to use High Temperature Short Time (HTST) and Ultra-high Temperature (UHT) techniques on particulate food materials (Imai et al. 1995). These process designs are considered to be more gently for some heat sensitive compounds compared to longer heating at lower temperatures. In case of a high electrical conductivity of the solid phase it is even possible to obtain higher temperatures in the particles than in the liquid phase, which is impossible to achieve with conventional indirect heating (Chen 2015). Particles with diameters up to 2.5 cm may be treated (Bengston et al. 2006; Chen 2015). As the food should have sufficient fluidity to be pumped and treated continuously, particle concentration is limited to approximately 60% (Bengston et al. 2006; Fellows 2009; Chen 2015). In conventional aseptic processing the particle size is limited to 15 mm maximum and 30–40% particle concentration (Varghese et al. 2014). Ohmic heating is also feasible to evenly process high viscous foods (Fellows 2009; Sakr and Liu 2014) or to pasteurize protein-rich materials such as egg white and whey without inducing their thermal coagulation (Icier 2010; Icier and Bozkurt 2011).
2 Applications, Equipment and Process Parameters
First patents for the use of direct resistance heating were already filed in the nineteenth century and first industrial use was reported in the 1930s for milk pasteurization (Goullieux and Pain 2005; Bengston et al. 2006; Chen 2015). Due to insufficient process control and deficiencies in inert electrode materials, commercial usage was limited in the following decades. In the 1980s, research on ohmic heating reawakened with the demand for adequate sterilization techniques for foods containing large particles (Bengston et al. 2006; Chen 2015). Since the FDA approval to process stable low acid food in 1993 commercial usage of ohmic heating took place in Europe, the USA and Japan along with improvements in process control and electrode materials (Goullieux and Pain 2005).
Ohmic heating can be applied in different areas of food processing including pasteurization and sterilization, blanching, cooking, evaporation and dehydration, thawing, extraction and fermentation (Goullieux and Pain 2005; Bengston et al. 2006; Icier 2012). Applications of ohmic heating are more determined by the type and characteristics of the food material, rather than the processing area. In food preservation, ohmic heating provides the aforementioned advantages in regard to heating rate and homogeneity, feasibility for certain product characteristics and preservation of food quality. Inactivation kinetics show similar results for ohmic and conventional heating in terms of D, z and activation energy value (Sastry and Barach 2000). Some authors also report additional benefits due to a higher inactivation rate of microorganism and a potential decrease in processing time (Pereira et al. 2007; Sun et al. 2008; Baysal and Icier 2010; Somavat et al. 2012a, b, 2013). Ohmic heating systems are suited to be adapted to aseptic food-processing lines (Kim et al. 1996) and are considered a useful addition for the hurdle concept (Sastry 2008). The technology may also bring advantages in the blanching of fruits and vegetables. Due to the ability to homogeneously treat larger pieces, there is no need for previous dicing. This reduces leaching of solutes during blanching due to the lower surface to volume ratio (Mizrahi 1996). Ohmic heating can as well be applied to improve thawing of foods with large sizes where conventional thawing is very time consuming. As the electrical conductivity markedly increases with phase transition and temperature, the temperature distribution of the food has to be controlled well to avoid over-processing in some parts of the product (Varghese et al. 2014). Enhanced mass transfer due to higher diffusion rates at elevated temperature and cell disintegration by thermal and electric effects can be used to improve the recovery of food compounds and the removal of water (Bengston et al. 2006). Wang and Chu (2003) found higher evaporation rates and improved results in quality analysis using ohmic heating during vacuum evaporation of orange juice. Ohmic heating is commercially used in the United States to process liquid egg and in the United Kingdom and Japan for processing of whole fruits (Bengston et al. 2006). Laboratory trials include applications on fruits and vegetables, juices, stews, meats, seafood, pasta and soups (Bengston et al. 2006). Ohmic heating is even investigated in terms of its suitability for heating and sterilizing food and waste during long term space missions (Somavat et al. 2012a, b).
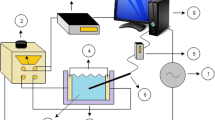
The basic elements of an ohmic heating system are an AC power source to deliver electrical energy to the system, a variable power supply to adjust the desired voltage, a treatment cell as well as measurement units to control voltage, current and temperature (Bengston et al. 2006; Icier 2012). An illustration of a pilot scale system is given in Fig. 13.1. Ohmic heaters can be designed for discontinuous or continuous processing. The typical batch treatment cell consists of a cuboid or cylindrical box with two plate electrodes at opposite sides (Chen 2015). The electrodes are generally separated by a tube or plate space that is electrically insulated (Goullieux and Pain 2005).
Schematic illustration of a pilot plant scale ohmic heating system, redrawn according to Salari and Jafari (2020): (1) AC power supply, (2) adjustable voltage transformer, (3) treatment cell, (4) electrodes, (5) thermocouple with (6) sensor, (7) sample, (8) data logger, (9) computer
The total resistance in ohmic heater determines the current flow in the product. If the resistance is too high, the current will be too low, even when maximum voltage is reached. If the resistance is too low, the maximum limiting current is reached at a low voltage and the heating power will be too low as well (Fellows 2009). The total resistance of the heater can be calculated using the following equation:
where R is the total resistance of the heater in ohms, Rs is the specific resistance of the product in ohms/m, L is the distance between the electrodes in m and A is the area of the electrodes in m2 (Fellows 2009). As the specific resistance of a food is given by its composition and recipe, the geometry of the treatment cell has to be adapted to the food properties. Every material has a critical current density which exceedance may cause arcing in the heater. The current density is given by
where Id is the current density in amps/m2, I is the current in amps and A is the area of the electrodes in m2. When the critical current density of a food material is known, the electrode area can be calculated accordingly (Fellows 2009). Industrial ohmic heating units are usually run continuously and are connected to other unit operations such as pumps, holding tubes etc. (Bengston et al. 2006). There are many different designs of commercial ohmic heating units available depending on the manufacturer (Vicente et al. 2005; Icier 2012; Varghese et al. 2014). Important equipment parameters to consider when constructing an ohmic heating system are the electrode configuration, the electrode distance, heater geometry, the frequency of the alternating current, current density, applied voltage, product velocity and velocity profile (Bengston et al. 2006). Two possible electrode configurations exist for continuous processing – transverse and collinear configuration (Goullieux and Pain 2005; Varghese et al. 2014). In transverse mode, the food is transported parallel to the electrodes and the current flow and electric field are perpendicular to the product flow (Goullieux and Pain 2005). The construction is rather simple with plane or coaxial electrodes (Varghese et al. 2014). Problems may occur due to leakage currents through the product and inhomogeneities in the current density close to the electrode edges (Varghese et al. 2014). This may lead to local overheating of the food and erosion of the electrode material. Further advanced heating systems and units used for particulate foods thus follow a collinear configuration. In collinear configuration the product food flows from one electrode to the other and parallel to the current flow and the electric field (Goullieux and Pain 2005). Electrode housings and space tuber are alternated in the treatment cell. Due to the high voltages present, tighter security precautions need to be implemented (Varghese et al. 2014).
It can further be distinguished in in-line and cross-field systems depending on the positons of the electrodes along the product flow path (Varghese et al. 2014). Often several ohmic heater columns are combined in one unit (Icier 2012). This can increase the product throughput without increasing the voltage necessary to generate the requested electric field strength. Control systems are usually connected to the equipment to monitor process parameters including temperature, flow rate and specific heat of the product (Icier 2012). Based on these data the treatment intensity can be adjusted to achieve the desired heating profile. Detailed descriptions of available designs of commercial ohmic heaters can be found in Varghese et al. (2014) and Chen (2015).
Besides equipment design and process parameters used, characteristics of the food material markedly influence the ohmic heating process. Critical factors are the electrical conductivity of each food component, viscosity, density, pH-value, where there exist, the size and concentration of particles, thermal conductivity and the specific heat capacities of each component (Fellows 2009; Chen 2015).
The electrical conductivity is the main parameter affecting heating rate in an ohmic heating process. It is the inverse of the specific electrical resistance of a material (Fellows 2009) and can be measured by the quantity of electricity transferred across a unit area, per unit potential gradient and per unit time (Goullieux and Pain 2005). Assuming negligible heat losses, the temperature rise during a continuous ohmic heating process can be calculated using the following equation:
where ΔT is the temperature rise in K, σa is the average product conductivity throughout temperature rise in S/m, A is the cross sectional area in m2, L is the distance between electrodes in m, m is the mass flow in kg/s and cP is the specific heat capacity of the product in J/kg/K (Fellows 2009).The electrical conductivity differs for different foods (Sarang et al. 2008; Chen 2015) and varies to a much greater extent than their thermal conductivities (Fellows 2009). The conductivity of a complex material is typically a sum contribution of individual ions, molar equivalent concentrations of individual ions and molar equivalent conductivity (Goullieux and Pain 2005). Ions from salts and acids and moisture increase the electrical conductivity, lipids and alcohol decrease it (Bengston et al. 2006). Analogue to the overall resistance of a heater, an optimum area can be found for the specific electrical conductivity of a product. This limits the range of foods feasible for the application of ohmic heating technology (de Alwis and Fryer 1992).
The electrical conductivity of foods is as well dependent on temperature. Usually, the conductivity increases linearly with temperature due to the enhanced ionic mobility at higher temperatures (Palaniappan and Sastry 1991; Reznick 1996; Wang and Sastry 1997a, b; Goullieux and Pain 2005). Non-linear, sigmoidal temperature-dependencies were determined at lower field strengths below 60 V/cm. Conductivity is furthermore a function of the food structure (Halden et al. 1990). Phase transitions such as starch gelatinization, protein denaturation or melting of lipids may alter the conductivity; inter alia due to the decrease in water availability (Halden et al. 1990; Wang and Sastry 1997a, b).
Increased heating rates were observed for plant tissue after reaching a critical temperature range (Goullieux and Pain 2005). The cell membranes in plant tissue are electrical insulators and current flow is usually restricted to the intercellular fluid. High temperatures may affect the cell integrity via denaturation of membrane proteins and breakdown of pectic cell wall components leading to higher ion mobility and a higher electrical conductivity (Halden et al. 1990; Palaniappan and Sastry 1991). Cyclic ohmic heating trials (Wang and Sastry 1997a, b) and previous cell disintegration using other techniques (Imai et al. 1995) confirmed the increased conductivity after cellular breakdown. The orientation of vascular bundles in the electric field and the shape of parenchyma cells can also influence conductivity and may lead to different heating rates for the same food material (Wang et al. 2001).
For multiphase systems, the electrical conductivities of all phases have to be considered. This makes prediction and controlling of heating characteristics very complex (Fellows 2009; Chen et al. 2010; Chen 2015). Beside differences in the conductivity, differences in density and moisture content may also affect the heating of the different phases (Fellows 2009). Thus particles may heat faster than the surrounding liquid even when their conductivity is lower (Fellows 2009). Thermal diffusivity and surface heat transfer coefficient between carrier fluid and particles were found to have weak effects on the process temperature (Chen et al. 2010). Frequency and waveform of the alternating current are additional factors influencing electrical conductivity and thus heating rate (Lima et al. 1999a,b; Bengston et al. 2006). In some cases it might be reasonable to increase the conductivity of the particle via salt infusion to increase heating rate and minimize overall processing time (Goullieux and Pain 2005). Salt infusion at different salt concentratons and soaking times was used to increase conductivity and heating rate of potato tissue (Wang and Sastry 1993). Due to the slow salt diffusion from the particle surface inwards suitability of salt infusion is determined by particle sizes. Obviously, a balance between process optimization and sensorial and nutritional requirements has to be achieved (Palaniappan and Sastry 1991).
Besides all benefits, there are some limitations and open questions that have to be mentioned. As mentioned before, the presence of parts with very high or low conductivity remain a problem. Non-food materials such as metals, wood or plastic possess such extreme conductivities, but they are removed from food before processing and therefore constitute no actual issue (de Alwis and Fryer 1992). Presence of fat globules in the food may create an actual problem as the current may bypass them, when conductivity of the surrounding material is high enough. This may lead to less heat treatment and insufficient microbial inactivation in these product parts (Icier 2012). Thermal conductivities of solids vary by three orders of magnitude, whereas electrical conductivity may vary by many orders of magnitude from effectively zero to infinity. Therefore, non-conductive inclusions have a much higher impact in ohmic heating compared to conventional treatments (de Alwis and Fryer 1992).
Although there is no theoretical limit for the size of particles in ohmic heating, there are some practical limitations. While heating can be performed very fast with direct methods, cooling of the product occurs via thermal conduction (de Alwis and Fryer 1992). Although very low temperatures at the heat transfer surface are considered as a minor problem in regard to food quality compared to very high temperatures, slow cooling rates may lead to over-processing in the particle center. Furthermore, particles need to be pumpable and fit through the aseptic packing system (de Alwis and Fryer 1992).
Due to the direct contact between electrodes and food material, undesired reactions such as corrosion or electrolysis may occur (Goullieux and Pain 2005; Sastry 2005) affecting food quality and safety. Extent of the reactions is affected by frequency and density of the current, temperature, electrode material and aggressiveness of the product composition (Goullieux and Pain 2005). Approaches to prevent electrolysis include application of higher frequencies up to 100 Hz, usage of titanium electrodes coated with platinum, limitations in the current density and addition of electrolyte layers between product and electrodes (Goullieux and Pain 2005). Nevertheless, control of electrode reactions is a very important aspect that needs to be considered during ohmic heating.
In addition, there are some non-technological issues affecting ohmic heating application. Although the process costs of ohmic heating are comparable to conventional ones, investment costs for the systems may deter companies from switching their processing lines (Icier 2012), especially in case of small and medium enterprises. In addition, ohmic heating systems need well-trained personnel and availability of adequate safety and quality-assurance protocols. Furthermore, consumer constraints to electrically processed products limit industrial use of the technology (Icier 2012).
3 Improved Retention of Bioactives During Ohmic Heating
As mentioned before, the usage of ohmic heating provides interesting advantages regarding temperature homogeneity and heating rate. Faster heating to process temperature and thus lower overall thermal load are considered to bring as well benefits in terms of temperature sensitive valuable food components. Several research groups addressed this issue in their publications.
Vikram et al. (2005) compared ohmic, microwave, infrared and conventional heating in regard to the degradation of nutrients in orange juice. Samples were treated with different process temperatures and holding times. In the tested temperature range of 50–90 °C ohmic heating showed the best values for the retention of vitamin C. Ohmic heating led to the fastest heating of the samples whereby overall processing time was shorter compared to conventional and infrared heating. Heating up with microwaves was rather uncontrolled due to lack of adequate temperature control and was thus of limited comparability.
Achir et al. (2016) determined the carotenoid profiles of grapefruit and blood orange juices treated with ohmic or conventional heating. Pasteurization values of both technologies were matched and amounted 50 and 150 min at a reference temperature of 70 °C using a z-value of 10 °C. Contents of lycopene and β-carotene were not affected by both heating techniques. Degradation of xanthophylls was much more pronounced for both heating techniques. The presence of oxygen in the xanthophyll structure leads to a higher heat sensitivity compared to carotenes (Salari and Jafari 2020). Losses in epoxyxanthophylls and hydroxyxanthophylls amounted up to 70% and 40%, respectively, for conventional heating, whereas reduction up to 30% and 20% were observed for ohmic heating (Achir et al. 2016). This can be traced back to the differences in temperature profiles. A temperature of 95 °C was reached after 48 s with ohmic heating whereas it took 24 min to obtain a sample temperature of 80 °C in an oil bath.
Abedelmaksoud et al. (2018) applied surface response methodology to compare the effects of ohmic and conventional heating on quality parameters of apple juice. Ohmic heating was performed in a batch cell with titanium electrodes applying voltage of 30, 35 and 40 V/cm and a sinusoidal current of 60 Hz until temperatures of 60, 70 and 80 °C were obtained. Conventional heating was performed in a shaker water bath at 90 °C. Holding time for all samples was 60 s. Ascorbic acid and carotenoid contents in ohmic heated samples were higher than those of conventionally treated ones. The improved retention of these bioactive components can again be traced back to faster heating and a lower total thermal load. Only slight differences were found for polyphenol content, total soluble solids, titratable acidity, and viscosity of the juices. Cloud value strongly increased for both heat treatments. Only slight color changes compared to the untreated sample occurred during ohmic heating that still fulfil sensorial product requirements.
Farahnaky et al. (2018) compared ohmic heating of high and low intensity with microwave and conventional cooking. Kohlrabi, turnip, potato and radish were cut in tubes and treated at a frequency of 50 Hz and voltages of 4.3 and 7.4 V/cm. Ohmic heating showed the highest rate in softening the vegetable tissue and thus possessed the shortest cooking time. The degradation of vitamin C after cooking was lower in kohlrabi, potato and radish after ohmic heating. Ohmic heating was also superior in preservation of total phenols and flavonoids as well as leaching of iron for all vegetables tested which can be explained by the shorter overall processing time.
A further advantage of ohmic heating is the opportunity to increase shelf-life of foods containing solids without the need to accept marked overprocessing of the liquid phase. Wattanayon et al. (2021) simulated vitamin C degradation in beverages with particles by adding alginate particles to orange juice. Ohmic heating of the sample in a conductive packaging was compared to conventional treatment. A pasteurization value of 5D was required for the inactivation of E. coli in the alginate particles. The total heating time to reach the pasteurization value amounted 2.35 min for ohmic and 6.03 min for conventional processing. Vitamin C content of the ohmic heating sample was similar to that of the untreated control while conventional treatment reduced the vitamin C content by more than 13%. This confirms the high potential of ohmic heating as a preservation method for solid liquid mixtures.
Furthermore, enhanced microbial inactivation due to the application of an electric field is discussed by several authors (Sastry and Barach 2000; Goullieux and Pain 2005; Knirsch et al. 2010). Lower D values were reported for Streptococcus thermophilus, Escherichia coli, Bacillus licheniformis and total aerobes (Pereira et al. 2007; Sun et al. 2008), which were traced back to pore formation in the cell membranes within the electric field. This is confirmed by results of Yoon et al. (2002) who reported a greater amount of intracellular material in ohmic heated samples compared to conventionally heated samples with similar time-temperature-history indicating electroporation of microbial cells. In addition, inactivation may be caused by local hot spots or formation of toxic substances such as free chlorine or hydrogenperoxide (Palaniappan et al. 1990). Significant lower D values were as well found for spore forming organisms, including Geobacillus stearothermophilus, Bacillus coagulans and Alicyclobacillus acidoterrestris (Baysal and Icier 2010; Somavat et al. 2012a, b; Somavat et al. 2013). This was attributed to effects on spores’ proteins and dipicolinic acid molecules (Somavat et al. 2012a, b). Cho et al. (1999) compared single versus double stage heating for ohmic and conventional inactivation of Bacillus subtilis spores. A higher lethality and a greater tyndallization effect were observed for ohmic heating.
An improved inactivation was as well reported for several enzymes. Ohmic blanching of artichoke heads at 24 V/cm and 80 °C led to higher enzyme inactivation rates compared to hot water blanching at 100 °C. Total inactivation times for peroxidase and polyphenoloxidase were 360 s and 480 s for ohmic and conventional treatment, respectively (Guida et al. 2013). Ohmic blanching of pea puree also resulted in a faster inactivation of peroxidase compared to conventional processing in a boiling water bath when voltage gradients of 30 V/cm or higher were used (Icier et al. 2006). Saxena et al. (2016) compared inactivation of polyphenoloxidase in sugarcane juice at 80 °C with and without application of an electric field. Applying a voltage gradient of 32 V/cm and keeping a process temperature of 80 °C for 1 min led to a reduction of enzyme activity to 10.07%. Conventional heating to 80 °C and maintenance of the temperature for 10 min achieved a residual activity of 6.47%. Higher inactivation rates of polyphenoloxidase were as well found by (Makroo et al. 2017) in watermelon juice comparing ohmic heating at 24 V/cm and 50 Hz to 90 °C and a water bath treatment at the same temperature. Castro et al. (2004a, b) investigated enzyme inactivation applying ohmic and conventional heating with matched temperature profiles. Inactivation of alkaline phosphatase, pectinase, and β-galactosidase was not affected by the electric field. Enhanced inactivation due to ohmic heating was determined for lipoxygenase and polyphenoloxidase.
The enhanced inactivation of microorganisms and enzymes may serve a further reduction in processing time and thus help as well to maintain valuable food components. The degradation of many heat sensitive compounds such as ascorbic acid, thiamine, riboflavin or anthocyanins follows first order kinetics (Van den Broeck et al. 1998; Vikram et al. 2005; Mercali et al. 2013, 2015; Kadakal et al. 2018). Thus a reduction in processing time is directly related to a marked benefit for the retention of food quality.
Demirdoven and Baysal (2014) compared the quality of orange juices after ohmic heating at 42 V/cm to 69 °C, 44 V/cm to 70 °C and conventional heating to 95 °C with a subsequent holding time of 60 s. Thermal treatments led to inactivations of pectin methylesterase of 96%, 95.5% and 88.3%, respectively. A slightly better retention of ascorbic acid was determined for the juice processed with the ohmic system. This was explained by the high temperature sensitivity of ascorbic acid and the lower temperatures required for enzyme inactivation using ohmic heating.
The individual temperature sensitivity of bioactives markedly influences their retention after ohmic heating. High degradation rates were especially reported for heat sensitive ascorbic acids while better retention or even higher concentrations were observed for other compounds.
Hashemi et al. (2019) compared ohmic, microwave and conventional heating for their potential to pasteurize cantaloupe juice. Ohmic heating and microwave heating were performed at 100 and 200 V and 400 and 800 W for 110 s, respectively. Conventional treatments were conducted in a hot water bath. All heating methods led to a reduction in microbial load as well as contents of vitamin C, β-carotene and phenolics. Effects increased with increasing voltage, microwave power and temperature in the conventional sample. Direct heating led to a faster inactivation of microorganisms and a higher degradation of vitamin C, but to a better preservation of β-carotene and phenolics compared to water bath heating.
Yildiz et al. (2010) applied ohmic heating to puree of blanched spinach leaves and compared the results to heating in a water bath. Lab scale equipment was used at 50 Hz and applied voltages varied between 10 and 40 V/cm. Depending on the voltage gradient heating to temperatures of 60–90 °C occurred up to four times faster than in a water bath. Slightly higher contents of β-carotene could be found in samples treated with ohmic heating compared to the untreated spinach puree. A holding time of 600 s at temperatures of 60–80 °C increases this effect. Conventional treatment led to slightly decreased contents of β-carotene indicating that ohmic heating provides better retention of carotenoids. There were no significant changes in chlorophyll content for both heating methods.
Ramnath et al. (2018) investigated the effect of electric field strength, type and concentration of lye salt on bioactive compounds in tomato puree. A laboratory unit with stainless steel electrodes was used and electric field strength varied from 928 to 1214 V/cm. Temperature development was influenced by the type and concentration of salt used. Higher temperatures were obtained with sodium chloride compared to potassium hydroxide and sodium hydroxide, respectively. Higher concentrations of vitamin A were determined in the puree when higher temperatures were reached due to use of higher electric field strength, salt type or concentration. On the contrary, the vitamin C content decreased with increasing process temperature, which was explained by the higher temperature sensitivity of vitamin C compared to vitamin A.
Similar results were obtained by Somavat (2011) who explained minor changes in carotenoid content of tomato juice after ohmic heating to temperatures between 95 and 110 °C with the high heat stability of these compounds. The content of total phenols was as well not markedly affected in this study, which was considered as a potential advantage of the ohmic technology compared to conventional heating.
Ohmic heating of rice bran was performed in lab-scale with titanium electrodes at electric field strengths of 75, 150, 225 V/cm and a frequency of 50 Hz adjusting different moisture levels (Loypimai et al. 2009). Temperature profiles were recorded with a data logger and final product temperatures after 10 min of treatment varied between 60 and 124 °C. Higher moisture contents and higher electric field strength led to higher heating rates. Higher contents of total phenolics, α-tocopherol and γ-oryzanol could be detected with highest values for 40% of moisture or 30% moisture and electric field strengths of 150 and 225 V/cm. In regard to antioxidant activity, best results were obtained with a treatment of 30% moisture and 150 V/cm electric field strength.
Rinaldi et al. (2020) pretreated peach cubes in syrup with ohmic heating and investigated the quality impact of subsequent processing with ohmic heating, high pressure and conventional pasteurization. Treatment intensities of ohmic and conventional heating were equal to a pasteurization of 100 s at 98 °C; high pressure treatment was performed at 600 MPa for 3 min. The content of previously added ascorbic acid in the samples was reduced by 6%, 16% and 22% for high pressure, ohmic and conventional heating, respectively. The total phenol content was not affected by high pressure, but increased by about 50% for both thermal treatments. This was traced back to observed tissue disintegration and connected release of phenolic compounds.
Ohmic blanching of artichoke heads at 24 V/cm at 80 °C also led to an increase in total polyphenol content of 29% compared to fresh samples, whereas hot water blanching decreased the content by 27% (Guida et al. 2013). The authors mention different potential reasons for the increased phenol detection. High temperatures could lead to a release of bound phenols from cellular tissue and chemical complexes and thus to their improved interaction in the assay. Inactivation of food endogenous enzymes during thermal treatment may impede oxidation and complexation of phenolics. Higher inactivation rates obtained with ohmic heating might lead to a better retention of monomeric phenols. Furthermore, release of cellular substances due to electroporation of cell membranes may lead to higher phenol contents in the samples after ohmic processing.
Bioactive proteins are formed during food processing in dependence of the process conditions applied. Costa et al. (2018) processed sweet whey with ohmic heating of 60 Hz and voltage gradients from 2 to 9 V/cm. A higher number of bioactive peptides were found compared to a conventional treatment at 75 °C for 15 s. The positive effect was more pronounced at lower electric field strengths. Ferreira et al. (2019) investigated the impact of different voltage gradients and current frequencies on bioactive peptide formation in whey beverages. Lower voltage gradients and frequencies resulted in higher antioxidant activities compared to higher treatment intensities. Ohmic heating led to lower anthocyanin contents and higher formation of bioactive peptides in relation to a conventional processing.
4 The Potential of Ohmic Heating for the Extraction of Bioactive Compounds
Several authors reported positive effects of ohmic heating on the extraction efficiency of plant compounds. Raw materials investigated include fruits, vegetables, herbs, oilseeds and algae. A detailed overview on the impact of moderate electric fields on extraction of food compounds is given by Gavahian et al. (2018). Improved mass transfer during ohmic heating is traced back to a combination of thermal and non-thermal effects. High temperatures induced by electrical energy dissipation may affect solubility and diffusion characteristics of compounds to be extracted. Reaching critical temperature ranges causes a thermobreak of the cell membrane via protein denaturation and leads to degradation of the cell wall by β-elimination and solubilization of pectins. This enables diffusion of intracellular components into the extraction medium. In addition, there were also non-thermal effects on cell tissue reported for processes involving application of an electric field.
Imai et al. (1995) applied electric fields of 40 V/cm for treatment times of 10, 30 and 50 s. to Japanese white radish. Temperature increase after 50 s was only 2.1 K so that the overall process temperature remained beneath 20 °C. Therefore, thermal effects on plant tissue could be neglected. Electrical impedance of the radish decreased with increasing treatment time indicating cell permeabilization and improved mobility of ions. This finding was confirmed by nuclear magnetic resonance imaging analysis of the plant tissue. Lebovka et al. (2005) as well reported a strong increase in cell disintegration index of potato and apple tissue after applying electric fields. A voltage gradient of 40 V/cm for approximately 100 s and a temperature range of 20–50 °C were used in this experiment. Pootao and Kanjanapongkul (2016) investigated changes in oil palm tissue on a cellular level. Light microscopic images revealed cell disordering and disruption of the cell wall after ohmic heating at 60 °C whereas the sample without a voltage gradient appeared intact. Diffusion of beet juice from beet cubes was enhanced by ohmic heating compared to conventional heating in the temperature range from 42 to 58 °C (Lima et al. 2001). At a temperature of 72 °C, only small diffusion differences between the both technologies were detected. Probably, the temperature for thermal cell disintegration in beet tissue was reached enabling diffusion as well in the control samples. This supports the hypothesis that tissue alterations at lower temperature can be traced back to non-thermal electric effects.
It is assumed that electroporation is the main mechanism of these non-thermal effects on tissue integrity and mass transfer. Cell electroporation implies the formation of pores in the cell membrane due to application of an electric field (Knirsch et al. 2010). In intact plant tissue, the phospholipid bilayers of the cell membranes function as a barrier for ion movement in the electric field. Accumulation of ions at the cell membrane may lead to exceedance of the critical membrane potential followed by pore formation in the phospholipid bilayer. This effect may either be reversible or irreversible. Electro-osmosis, the movement of charged molecules in a liquid in dependence of the direction of the electric field, has shown to positively affect mass transfer steps (Bazhal and Vorobiev 2000) and might contribute to improved extraction using ohmic heating as well.
Process parameters applied markedly influence the improvement of mass transfer and as well the dominant mechanism affecting cellular tissue. Thermal or non-thermal effects or a combination of both may occur during ohmic heating depending on the process temperature applied. At temperatures below the denaturation temperature of the cell membrane non-thermal effects occur whereas at temperatures markedly exceeding this temperature area thermal effects dominate (Gavahian et al. 2018).
Lima et al. (2001) observed an ohmic-enhanced diffusion of beet dye. This effect increased with increasing electric field strength. Similar results were obtained by Schreier et al. (1993) who determined increased betanin diffusion from beetroot with increasing electric field strength. Extraction yields of palm oil and sesame oil were affected by the voltage gradient applied during ohmic heating as well (Kumari et al. 2016; Pootao and Kanjanapongkul 2016). This can be explained by the higher attraction forces between ions appearing at higher field strength which lead to a higher heating rate and a pronounced electroporation effect. Onwuka and Ejikeme (2005) investigated the effect of voltage type and electrode material on yield and quality of fruit juices. Using alternating current at a treatment intensity of 110 V, juice yield of orange and tomato pulp increased with increasing treatment time. A much smaller impact on juice yield was obtained for treatments with direct current of 9 V. This can be traced back to much higher temperatures obtained at 110 V AC leading to disintegration of the tissue. Higher voltages and alternating electric fields both lead to a more intense movement of ions in the fruit pulp. Decreased juice extraction was observed after ohmic heating for pawpaw pulp. There was no marked influence of electrode material on temperature profile and juice yield.
Frequency of the applied voltage significantly affected drying rate of yam and juice yield of apples (Lima and Sastry 1999). Sinus waves of 60 Hz were compared to sawtooth waves of 4 Hz. Improvements in mass transfer were markedly greater using 4 Hz for both processes. Extraction yields from fresh mint leaves were much higher for a frequency of 50 Hz than for 500 and 5000 Hz (Sensoy and Sastry 2004). Low frequencies allow ions to accumulate at the cell wall and build up sufficient charge for electroporation. At high frequencies the rapid alteration of the electric field direction does not allow sufficient charge build-up (Icier 2012).
Benefits of ohmic-assisted extraction are the synergistic effects of temperature and electric field on cell tissue, lower temperatures needed for cell permeabilization compared to conventional heating and simpler requirements regarding the equipment when compared to other electric treatments such as pulsed electric fields or high voltage electrical discharges (Vorobiev and Lebovka 2010). The non-thermal effects on cell disintegration are of special interest for the processing of foods containing high amounts of heat sensitive components. Some research activities were done on the improved extraction of bioactives during or after ohmic heating.
El Darra et al. (2013) investigated the effect of pulsed ohmic heating on the extraction of polyphenols from grape pomace using a batch cell with stainless steel electrodes. Approximately 20 to 50 series with 300 pulses of field strengths up to 800 V/cm were applied. Pulse duration was about 100 μs. Cell disintegration index increased with temperature and electric field strength. Using a voltage gradient of 100 V/cm, even with a temperature of 60 °C a cell permeabilization index below 0.4 was determined, while with electric fields strengths of 300 and 400 V/cm values above 0.6 were reached at temperatures around 40 °C. This indicates a high impact of non-thermal cell rupture at high electric field strength. Calculating the energy consumption needed to achieve a certain degree of cell permeabilization, higher electric field strength showed to be more beneficial. Polyphenol extraction in water and 30% ethanol was improved by ohmic heating at 400 and 800 V/cm.
For wheat bran extraction, ohmic heating with electric field strengths of 14, 20 and 44 V/cm led to a faster heating up to a process temperature of 80 °C than conventional heating (Al-Hilphy et al. 2015). Despite the shorter processing time, slightly higher phenol contents and antioxidants activities were measured in the extracts.
Pereira et al. (2016) investigated the influence of voltage gradient, temperature and extraction time using as well a Box-Behnken design. Temperature profiles at voltages of 0, 15 and 30 V/cm during heating up to 90 °C were matched to figure out the impact of the electric field. Extraction of total phenols and anthocyanins from colored potatoes was affected by all three parameters. Temperature and time as well as their interactions were the main factors affecting the extraction of solutes. The yield of total phenols also increased with increasing voltage gradient whereas for anthocyanins an optimum yield at an electric field strength of 15 V/cm was observed. Combining high field strength and temperatures above 70 °C a decreased anthocyanin content which could be a result of degradation into the constituent phenolic acids.
Fraccola et al. (2016) extracted pigments from the microalgae Chlorella vulgaris using electric fields of 25 kHz and 50 V/cm. The temperature increased from 22 to 45 °C. Compared to a conventional extraction process without heating, the concentration of extracted pigments, carotenoids, chlorophyll a and b, was 15 times higher when applying ohmic heating.
Aamir and Jittanit (2017) compared extraction efficiency of ohmic heating for unpolar compounds using a water-hexane mixture with conventional hexane extraction supported by conductive heating. Both extractions were performed with increasing hexane to gac aril powder ratios at a temperature of 50 °C. Ohmic heating led to a higher extraction efficiency of gac aril oil and markedly higher contents of lycopene and β-carotene in the oil. Scanning electron micrographs revealed a rupture of cell walls in the ohmic heated samples whereas conventionally heated and untreated cells were intact and closely packed.
Bhat et al. (2017) investigated the effect of ohmic pretreatment on the phenol content of subsequently recovered gourd juice. Cubes of bottle gourd were blanched using ohmic and conventional water-bath heating and the juice extracted afterwards was analyzed on total phenols and color. An ohmic heating cell with stainless steel electrodes was used at 220 V and a frequency of 50 Hz leading to an electric field strength of approximately 30 V/cm. All thermal treatments increased the extraction of phenols into the juice. Higher total phenolic contents were detected in samples pretreated with ohmic heating to temperatures of 70 °C and above while no marked differences could be observed between temperatures of 60 to 90 °C for conventional treatments and ohmic heating of 60 °C. Optimum ohmic heating conditions in this study were 80 °C and 4 min, while prolonged heating and higher temperatures reduced the benefit of the treatment. This was traced back to an overlap of positive effects of temperature on polyphenol extraction and thermal degradation effects. LC-MS analysis revealed an increased content of gallic acid and theaflavin 3-gallate in the ohmic heating samples. Color changes were temperature dependent and did not differ markedly for ohmic heating and water bath.
Coelho et al. (2017) performed experiments on the impact of process parameters on the ohmic assisted extraction from by-products of tomato processing. A Box-Behnken design with three levels was used and temperatures of 40, 55, and 70 °C, extraction times of 0, 15 and 30 min and ethanol in water concentrations of 0, 35 and 70% were applied. Extraction yields of phenolics and carotenoids as well as the antioxidant activity of the extracts increased with increasing temperature, extraction time and ethanol concentration.
Ferreira-Santos et al. (2019) compared extraction efficiency of phenols from pine bark for ohmic heating and thermal treatment in a water bath. Polyphenol contents were markedly higher after ohmic heating when 50% ethanol was used for extraction. A slight increase in the extraction yield could be observed increasing the electrical conductivity of the medium. Extraction yields were much lower using water as an extraction medium, but still a slight improvement by ohmic heating could be observed. Results for antioxidant activity measurements were in accordance with the higher polyphenol contents. The composition of phenolic compounds was influenced by the extraction method as well as by the medium conductivity.
Moongngarm et al. (2019) studied the effect of an ohmic pretreatment on the hexane extraction of oil from rice bran and its phytochemical composition. Higher extraction yields of tocopherols, γ-oryzanol and total phenols were obtained for all ohmic heating intensities tested. Analysis of antioxidant activities resulted in positive or negative impact of ohmic heating depending on the assay used.
Mannozzi et al. (2019) compared the potential of ohmic heating as a pretreatment to juice recovery with that of pulsed electric fields. Various temperature-process combinations were investigated and the influence on juice quality determined. Conclusions on bioactive components in apple and carrot juice were drawn by means of the antioxidant activity. Results showed best results for treatments where temperatures of 80 °C were reached. This was explained by the inactivation of peroxidase and polyphenoloxidase that prevented reaction of valuable plant compounds. Additional benefits of pulsed electric fields and ohmic heating were considered the facilitated release of plant metabolites as well as the reduction in thermal load due to rapid heating.
Extraction of phenols from grape pruning residue was carried out at low and high electric field strength, 496 and 840 V/cm respectively, and compared to conventional heating and extraction at ambient temperature (Jesus et al. 2020). Increasing the temperature to 80 °C led to a higher phenol extraction independent from the heating method used. Ohmic heating provided a better antioxidant activity of the extracts, especially when the high electric field strength was used. The extracts differed as well in their polyphenolic composition.
The results presented highlight the great potential of ohmic heating to improve the mass transfer of bioactives. Improved extraction of phenols and carotenoids from plant materials may enhance recovery efficiency by increasing product yield or by reducing processing times and energy required. Higher contents of these plant metabolites in fruit and vegetable juices and sauces may contribute to a higher product quality in regard to sensorial and nutritional perspective,
5 Adverse Effects of Ohmic Heating on Bioactive Compounds
While many positive effects of ohmic heating on the retention and extractability can be found in literature, some research revealed negative impacts of electroheating on food material compared to conventional processing. This can be traced back to the electrodes in direct contact with the food system and reactions occurring during application of electric fields on their surfaces.
Electrolysis of water may occur at the electrodes leading to formation of hydrogen and oxygen at cathode and anode, respectively (Assiry et al. 2003):
The formation of oxygen may enhance oxidation reactions in the food. Arcing during the treatment may even lead to formation of singlet oxygen strongly increasing reaction rate (Assiry et al. 2003). The intensity of electrolysis is directly affected by the height of the voltage gradient. As usually alternating current is used and thus the direction of the electric field is periodically changing, both reactions may occur on each side of the treatment cell. Oxygen may interact with the food compounds until it ascends from the sample or reacts with the hydrogen formed. The frequency used is thus strongly affecting the electrode hydrolysis.
Corrosion of the electrode material may occur via direct oxidation or electrochemical formation of corroding species (Assiry et al. 2003). The generated metal ions may further oxidize, undergo secondary reactions or complex formation with food constituents or function as a catalyzer for their reaction among each other. The corrosion affinity of electrode materials markedly differs. Samaranayake and Sastry (2005) performed experiments with different electrode materials and identified titanium and platinized titanium as less prone to corrosion compared to graphite and stainless steel, who exhibit much higher corrosion rates at the tested pH values of 3.5, 5 and 6.5. Lima et al. (1999a, b) determined rust formation and bubble formation at stainless steel electrodes, while no such observations were made using titanium electrodes.
Due to the differences in material costs, stainless steel is often used in practice. Treatment parameters thus have to be optimized in regard to preservation of food quality and safety. Several research groups investigated the impact of ohmic heating on the degradation of bioactive compounds.
Onwuka and Ejikeme (2005) applied ohmic heating as a pre-treatment for the recovery of orange and tomato juice. Voltage type and electrode material were varied. Copper/copper and copper/aluminum electrodes were used to generate direct current of 9 V or alternating current of 110 V. All treatments led to a degradation of vitamin C which was more pronounced applying higher voltages and temperatures. Even in samples treated with direct current that reached a maximum temperature of 30 °C vitamin C degradation was observed. Considering the low process temperatures, this was traced back to electrochemical reactions of the electrode material. This effect was more pronounced for copper/copper electrodes than for copper/aluminum electrodes. Visual observations also indicated stronger electrolysis at the copper/copper electrodes.
On the contrary, Leizerson and Shimoni (2005a, b) did not find significant differences in the vitamin C content of orange juice treated in a continuous ohmic heater or a plate heat exchanger. In this experiment an electroheater with graphite electrodes and a frequency of 50 Hz were used. Differences between effects of ohmic heating temperatures of 90, 120 and 150 °C and varied product flow rates were as well not significant, probably due to the low overall treatment time.
Mercali et al. (2012) compared the degradation of ascorbic acid in acerola pulp during ohmic and conventional heating. An ohmic heating setup with a batch Pyrex glass cell and titanium electrodes was used. The applied voltages amounted 120–200 V and the current frequency was 60 Hz. Ohmic and conventional samples were heated to 85 °C and kept at this temperature for 3 min. Lower voltage gradients applied influenced ascorbic acid in a same magnitude as conventional heating. Higher electric field strengths led to an increased degradation of this bioactive compound. The same experimental setup was used to evaluate the impact of ohmic heating on anthocyanins in acerola pulp (Mercali et al. 2013). There were no significant differences between the degradation rates of ohmic and conventional heating in the studied temperature range of 75–90 °C.
Sarkis et al. (2013) investigated the impact of voltage gradient and solid content on anthocyanin degradation in blueberry pulp. Both parameters were positively correlated to the degradation rate. At lower voltage gradients similar or higher anthocyanin retention as for conventional treatments were obtained. Higher voltage gradients led to an increased degradation of this plant metabolite.
Saberian et al. (2015) compared ohmic heating and conventional heating for the pasteurization of aloe vera gel juice. An ohmic heating unit with stainless steel electrodes and a frequency of 60 Hz was used. Samples were heated up top 90 °C and the temperature was kept for 1 min. Conventional heating was performed in a 90 °C water bath for a holding time of 1 min. Markedly lower contents in vitamin C were found for ohmic heated samples than in conventionally treated and raw juices. This was traced back to synergistic effects of temperature, oxygen and metal ions. The contents of total phenols were vice versa and the highest content was found after ohmic heating.
Athmaselvi et al. (2017) investigated the effect of electrode material on ascorbic acid content of tropical fruit pulp. Pulp from guava, sapota and papaya was treated in a batch system with either stainless steel or titanium electrodes. Voltages of 10 and 23.33 V/cm were applied at a frequency of 50 Hz. Target temperatures amounted 70, 80, 90 and 100 °C and were kept for a holding time of 5 min. Ascorbic acid concentration in all samples decreased with increasing voltage, temperature and holding time. Usage of titanium electrodes led to a better ascorbic acid retention. This was traced back to a higher heating rate using the electrode material.
Sabanci et al. (2019) concentrated pomegranate juice via conventional and ohmic heating assisted vacuum evaporation. Titanium electrodes and voltage gradients of 7.5, 10 and 12.5 V/cm were used. Samples concentrated using ohmic heating gave lower values for their anthocyanin and total phenolic contents as well as in their antioxidant activity compared to the conventional samples. This was traced back to electrochemical reactions at the electrode surface. The use of more electrochemically inert electrode materials is suggested by the authors. The same equipment was used for the concentration of sour cherry juice (Sabanci and Icier 2019). Higher retention of total phenols and anthocyanins compared to conventional evaporation was observed. Electric fields of 10, 12 and 14 V/cm were applied and the positive effect on retention of bioactive compounds increased with the voltage gradient. This can be traced back to shorter processing times needed. Antioxidant activities in a similar range were determined for both concentration techniques.
Detrimental effects of ohmic heating were mainly observed for ascorbic acid/vitamin C and anthocyanins. Ascorbic acid degradation can occur via an oxidative or an anaerobic pathway (Assiry et al. 2003). In conventional food processing ascorbic acid losses are primarily a consequence of chemical oxidation. Chemical degradation via the anaerobic pathway is less important in food processing as usually a sufficient amount of oxygen is present. Release of oxygen by electrolysis might directly affect degradation rate by the higher (local) concentration of a reaction substrate. Presence of metal ions, especially Fe3+ and Cu2+ may accelerate the reaction by several orders of magnitude (Assiry et al. 2003). Therefore, liberation of metal ions due to corrosion reactions may lead to enhanced ascorbic acid degradation. These reinforcing factors in the electric field are considered as a third electrochemical degradation pathway (Assiry et al. 2003). Similar explanations can be found regarding the degradation of anthocyanins although the reaction pathways are less well understood. Degradation of anthocyanins is mainly caused by oxidation and is thus strongly affected by the presence of oxygen (Patras et al. 2010). Sinela et al. (2017) reported reaction path ways of metal-catalyzed oxidation and a molecule scission that are both influenced by the nature and concentration of metals present. In contrast, a stabilization of anthocyanins due to complexations with metal ions is as well reported (Liu et al. 2018).
These findings highlight the importance to separately regard individual impact factors of ohmic heating for different food systems. Some systematic research work is performed comparing ohmic and conventional heating with matched temperature profile to intensively study the influence of electric field characteristics. Results are summarized in Table 13.1.
Lima et al. (1999a, b) did not find significant differences in ascorbic acid degradation in orange juices for ohmic and conventional heating with same thermal history. Although gas production and dissolution appeared at stainless steel electrodes, there was no difference comparing this electrode material with specially coated titanium in regard to ascorbic acid concentration.
Assiry et al. (2003) studied degradation kinetics of ascorbic acid during ohmic heating with thermal holding time. Ohmic heating with uncoated stainless-steel electrodes and a frequency of 60 Hz and intensities of 0, 100, 150 and 300 W were used in a temperature range from 40 to 80 °C. Buffer solutions with pH 3.5 and different NaCl contents of 0.25, 0.5 and 1% served as a model for acidic food systems and ascorbic acid was added to the buffer after reaching a constant desired temperature to exclude the impact of heating rate. No significant differences between ohmic and conventional heating were found for ascorbic acid degradation, except for a power of 150 W, a salt concentration of 1% and a temperature of 40 °C. At the highest power and salt content, citrate complexation and a significant loss of buffering capacity were noted, resulting in an increased pH value.
Castro et al. (2004a, b) compared ohmic heating and conventional heating with matched temperature profiles in regard to ascorbic acid retention in strawberry pulp. The pulp was heated to temperatures up to 100 °C using an ohmic system with titanium electrodes and electric field strengths of 25–100 V/cm. Conventional treatments at the same temperatures were performed in industrial scale with a scraped-surface heat exchanger. Ascorbic acid degradation followed first order kinetics for both conventional and ohmic heating treatments. The electric field did not affect the rate of ascorbic acid degradation.
Yildiz et al. (2009) investigated the ohmic heating potential for preservation of pomegranate juice. The same thermal history for the conventional and ohmic heating was applied to figure out the impact of the electric field on juice quality. Therefore the electric field was varied between 10 and 40 V/cm at a frequency of 50 Hz to achieve desired heating rate. Statistical analyses revealed no significant difference in the phenol content of juices treated with ohmic heating and conventional heating in a water bath in a holding time of 12 min.
Similar degradation of vitamin C in orange and pineapple juice was detected by Tumpanuvatr and Jittanit (2012) for ohmic heating with stainless steel electrodes when performed with the same heating rate as conventional processing.
Mercali et al. (2014) performed trials on ascorbic acid concentration and color changes in acerola pulp. A batch unit with titanium electrodes was used. The effect of current frequency and process time at constant temperature was evaluated and compared to thermal treatment in a water bath. The effect of heating-up on quality changes was subtracted to eliminate the effect of different heating rates. Ascorbic acid degradation was measured for all samples during thermal treatment.
After 120 min at 85 °C values were reduced by 13–17% for ohmic heating and by 14% for the conventional treatment. The highest degradations were found in samples ohmically treated with 10 Hz, indicating that electrochemical reactions were stronger at the low frequency. Color changes were in accordance with these results.
The same research group investigated anthocyanin degradation in jaboticaba juice applying ohmic and conventional heating with same temperature profiles (Mercali et al. 2015). Ohmic processing conditions were a voltage of 25 V, an electrode distance between 5.5 and 7 cm, a frequency of 60 Hz and a treatment time of 20 min. Rate constants were in a similar range for ohmic and conventional heating in the considered temperature range of 60–90 °C.
Jaeschke et al. (2016) studied non-thermal effects of ohmic heating on quality of acerola pulp. Ascorbic acid and carotenoid degradation were similar for ohmic and conventional heated and probably prevented by limited oxygen availability. In this study titanium electrodes and a frequency of 60 Hz at 30 V were used. Temperatures and treatment time for both heating methods were 80, 85, 90 and 95 °C and 60 min, respectively.
Higher inactivation rates of polyphenoloxidase in watermelon juice were obtained by ohmic heating at 24 V/cm and 50 Hz to 90 °C compared to water bath treatment at the same temperature (Makroo et al. 2017). Similar polyphenol degradation occurred in both technologies and only slight changes in the lycopene content were found.
The effects of ohmic heating and steam injection on quality of liquid infant formula were compared using the same conditions of pre-heating and holding (Roux et al. 2016). Electric fields were applied in a continuous coaxial chamber with titanium electrodes. Currents of 25 kHz and a power of 15 kW were used to achieve heating up to 140 °C. Slightly better preservation of vitamin C and color was observed after ohmic heating leading to the conclusion that ohmic heating is suited as an equivalent sterilization technique.
No significant differences in the vitamin C content of grapefruit and orange pulp during to conventional and ohmic heating assisted drying were reported by Stojceska et al. (2019). A voltage gradient of 30 V/cm, a frequency of 80 Hz and temperatures of 70 and 100 °C were used in this study.
Sarkis et al. (2019) also applied electric fields only during temperature holding time to eliminate the impact of sample heating up. Treatment intensities of 60 Hz and 4.1 V/cm showed no differences in anthocyanin degradation in blackberry pulp compared to conventional treatments with the same temperature profile. Higher voltage gradients of 22.3 V/cm led to an increased degradation at a temperature of 80 °C.
These findings show that additional degradation of bioactives due to the electric field can be prevented choosing an adequate process design with electrochemically inert electrode materials and process parameters. Low frequencies and high voltage gradients should be avoided when processing foods containing components sensitive to oxidation or metal catalyzed reactions to reduce electrode reactions to a minimum. Regarding the summarized literature data in Table 13.1, a safe process window in regard to field strength and frequency can hardly be identified. The data suggests that especially the application of long treatment duration of 60 min or more leads to additional degradation by the electric field. It is therefore required to spend more research activity on the influence of ohmic heating parameters on electrode reaction in dependence of the food matrix processed.
6 Conclusions and Future Perspective
Preservation and recovery of bioactives to obtain high nutritional quality for foods is an important issue for the food industry. Ohmic heating offers great potential to deliver a valuable contribution to both areas – gentle thermal processing of foods and enhanced extraction of food components. Improved homogeneity and increased rates of heating as well as additional inactivation effects on microorganisms and enzymes may lead to a decrease of required processing time and overall thermal load preventing heat sensitive compounds from thermal degradation. Combination of thermal and non-thermal effects on tissue integrity and molecule diffusion might lead to higher extraction yield and decreased extraction times or solvent usage. Nevertheless, there are still several knowledge gaps that need to be filled.
Adverse effects on product quality due to the electric field have been mainly investigated for ascorbic acid/vitamin C. There is lack of research data on the influence of electrolysis and corrosion on the degradation of other vitamins, secondary metabolites, essential fatty acids, bioactive peptides or minerals (Salari and Jafari 2020). Literature findings on adequate levels for electric field strength and process intensity were not consistent and safe process windows for individual food systems need to be identified. Especially, when non-thermal effects of the electric field are desired to improve mass transfer, high voltages and low frequencies have shown to provide better results. These parameter recommendations are in contrast to those made to avoid electrode reactions and specific attention should be paid to a potential overlap of higher extractability and degradation of bioactive compounds. Further research is as well needed to rank the potential of ohmic heating in comparison to other cell disintegration techniques in regard to extraction yield, energy efficiency and product quality. Knowledge is as well required in regard to bioactive retention for a wider range of valuable compounds and products. Especially in terms of particle-rich foods and products with inhomogeneous conductivity distribution, effects on bioactive compounds are insufficiently documented.
Conductivity differences are also an issue in regard to food safety. The DFG Senate Commission on Food Safety (German Research Foundation, DFG) identified research needs for ohmic heating in different areas to guarantee product safety (SKLM 2015). Uniformity of heating and avoidance of cold spots during preservation due to different conductivities of food elements shall be assured and need a more detailed knowledge on material properties and possibilities to influence them. A more detailed investigation of microbial inactivation kinetics in the individual fractions of a heterogeneous product is also essential as well a distinction between thermal and non-thermal effects in the electric field. A more detailed analysis of potential chemical changes, especially due to electrochemical reactions, is also recommended. This includes possible effects on product allergenicity. A satisfying clarification of these safety issues will lay the foundations for maximizing the retention of bioactive substances as it sets the framework within a nutritional optimization is feasible.
To promote industrial uptake of the ohmic technology, factors related to upscaling have to be studied more intensively. Beside the higher throughputs and the requirement for continuous processing systems, higher inhomogeneities regarding the raw material properties may occur due to regional and seasonal variation. This is especially important in terms of electrical conductivity as a crucial factor for thermal and non-thermal process effects. Respective guidelines for different process targets and food systems need to be published. Process equipment with treatment chambers and electrode designs suitable for different food matrices must be available together with a reliable and easy to handle process control systems. Due to the higher energy conversion efficiency, ohmic heating can be regarded as a sustainable way of heat processing of foods. Energy footprints of developed process designs shall be considered as well during process optimization to connect healthy foods with sustainable processing.
References
Aamir M, Jittanit W (2017) Ohmic heating treatment for Gac aril oil extraction: effects on extraction efficiency, physical properties and some bioactive compounds. Innov Food Sci Emerg Technol 41:224–234
Abedelmaksoud TG, Mohsen SM, Duedahl-Olesen L et al (2018) Optimization of ohmic heating parameters for polyphenoloxidase inactivation in not-from-concentrate elstar apple juice using RSM. J Food Sci Technol-Mysore 55(7):2420–2428
Achir N, Dhuique-Mayer C, Hadjal T et al (2016) Pasteurization of citrus juices with ohmic heating to preserve the carotenoid profile. Innov Food Sci Emerg Technol 33:397–404
Al-Hilphy ARS, AlRikabi AKJ, Al-Salim AM (2015) Extraction of phenolic compounds from wheat bran using Ohmic heating. Food Sci Qual Manage 43:21–28
Assiry A, Sastry SK, Samaranayake C (2003) Degradation kinetics of ascorbic acid during ohmic heating with stainless steel electrodes. J Appl Electrochem 33(2):187–196
Athmaselvi KA, Kumar C, Poojitha P (2017) Influence of temperature, voltage gradient and electrode on ascorbic acid degradation kinetics during ohmic heating of tropical fruit pulp. J Food Meas Charact 11(1):144–155
Ayadi MA, Leuliet JC, Chopard F et al (2005) Experimental study of hydrodynamics in a flat ohmic cell—impact on fouling by dairy products. J Food Eng 70(4):489–498
Baysal AH, Icier F (2010) Inactivation kinetics of Alicyclobacillus acidoterrestris spores in orange juice by Ohmic heating: effects of voltage gradient and temperature on inactivation. J Food Prot 73(2):299–304
Bazhal M, Vorobiev E (2000) Electrical treatment of apple cossettes for intensifying juice pressing. J Sci Food Agric 80(11):1668–1674
Bengston R, Birdsall E, Feilden S et al (2006) Ohmic and inductive heating. In: Hui YH (ed) Handbook of food science, technology and engineering. CRC Press, Taylor & Francis Group, Boca Raton, FL, pp 1–8. 120-121-120-128
Bhat S, Saini CS, Sharma HK (2017) Changes in total phenolic content and color of bottle gourd (Lagenaria siceraria) juice upon conventional and ohmic blanching. Food Sci Biotechnol 26(1):29–36
Castro I, Macedo B, Teixeira JA et al (2004a) The effect of electric field on important food-processing enzymes: comparison of inactivation kinetics under conventional and ohmic heating. J Food Sci 69(9):C696–C701
Castro I, Texeira JA, Salenge S et al (2004b) Ohmic heating of strawberry products: electrical conductivity measurements and ascorbic acid degradation kinetics. Innov Food Sci Emerg Technol 5:27–36
Chen C (2015) Ohmic heating. In: Bhattacharya S (ed) Conventional and advanced food processing technologies. John Wiley & Sons, Ltd, Chichester, West Sussex, UK, pp 673–690
Chen C, Abdelrahim K, Beckerich I (2010) Sensitivity analysis of continuous ohmic heating process for multiphase foods. J Food Eng 98(2):257–265
Cho HY, Yousef AE, Sastry SK (1999) Kinetics of inactivation of Bacillus subtilis spores by continuous or intermittent ohmic and conventional heating. Biotechnol Bioeng 62(3):368–372
Coelho M, Pereira R, Texerira JA et al (2017) Valorization of tomato wastes: influence of ohmic heating process on polyphenols extraction time. Extended Abstract of Vienna Polyphenols 2017 5(2):37–40
Costa NR, Cappato LP, Ferreira MVS et al (2018) Ohmic heating: a potential technology for sweet whey processing. Food Res Int 106:771–779
de Alwis AAP, Fryer PJ (1992) Operability of the ohmic heating process - electrical conductivity effects. J Food Eng 15(1):21–48
Demirdoven A, Baysal T (2014) Optimization of ohmic heating applications for pectin methylesterase inactivation in orange juice. J Food Sci Technol-Mysore 51(9):1817–1826
El Darra N, Grimi N, Vorobiev E et al (2013) Extraction of polyphenols from red grape pomace assisted by pulsed Ohmic heating. Food Bioproc Tech 6(5):1281–1289
Farahnaky A, Kamali E, Golmakani MT et al (2018) Effect of ohmic and microwave cooking on some bioactive compounds of kohlrabi, turnip, potato, and radish. J Food Meas Charact 12(4):2561–2569
Fellows PJ (2009) Dielectric, ohmic and infrared heating. In: Food processing technology: principles and practice, 3rd edn. Woodhead Publ Ltd, Cambridge, pp 581–609
Ferreira MVS, Cappato LP, Silva R et al (2019) Ohmic heating for processing of whey-raspberry flavored beverage. Food Chem 297:8
Ferreira-Santos P, Genisheva Z, Pereira RN et al (2019) Moderate electric fields as a potential tool for sustainable recovery of phenolic compounds from Pinus pinaster Bark. ACS Sustain Chem Eng 7(9):8816–8826
Fraccola GD, Fernandes B, Geada P et al (2016) Enhancing extraction of food-grade pigments from the microalgae chlorella vulgaris through application of Ohmic heating. IFT Book of Abstracts, Chicago, USA
Gavahian M, Chu YH, Sastry S (2018) Extraction from food and natural products by moderate electric field: mechanisms, benefits, and potential industrial applications. Compr Rev Food Sci Food Saf 17(4):1040–1052
Goullieux A, Pain JP (2005) Ohmic heating. Elsevier Science Bv, Amsterdam
Guida V, Ferrari G, Pataro G et al (2013) The effects of ohmic and conventional blanching on the nutritional, bioactive compounds and quality parameters of artichoke heads. LWT-Food Sci Technol 53(2):569–579
Halden K, Dealwis AAP, Fryer PJ (1990) Changes in the electrical conductivity of foods during ohmic heating. Int J Food Sci Technol 25(1):9–25
Hashemi SMB, Gholamhosseinpour A, Niakousari M (2019) Application of microwave and ohmic heating for pasteurization of cantaloupe juice: microbial inactivation and chemical properties. J Sci Food Agric 99(9):4276–4286
Icier F (2010) Ohmic blanching effects on drying of vegetable byproduct. J Food Process Eng 33(4):661–683
Icier F (2012) Ohmic heating of fluid foods. Elsevier Academic Press Inc, San Diego
Icier F, Bozkurt H (2011) Ohmic heating of liquid whole egg: rheological behaviour and fluid dynamics. Food Bioproc Tech 4(7):1253–1263
Icier F, Ilicali C (2005) Temperature dependent electrical conductivities of fruit purees during ohmic heating. Food Res Int 38(10):1135–1142
Icier F, Yildiz H, Baysal T (2006) Peroxidase inactivation and colour changes during ohmic blanching of pea puree. J Food Eng 74(3):424–429
Imai T, Uemura K, Ishida N et al (1995) Ohmic heating of Japanese white radish Rhaphanus sativus L. Int J Food Sci Technol 30(4):461–472
Jaeschke DP, Marczak LDF, Mercali GD (2016) Evaluation of non-thermal effects of electricity on ascorbic acid and carotenoid degradation in acerola pulp during ohmic heating. Food Chem 199:128–134
Jesus MS, Ballesteros LF, Pereira RN et al (2020) Ohmic heating polyphenolic extracts from vine pruning residue with enhanced biological activity. Food Chem 316:126298
Kadakal C, Duman T, Ekinci R (2018) Thermal degradation kinetics of ascorbic acid, thiamine and riboflavin in rosehip (Rosa canina L) nectar. Food Sci Technol 38(4):667–673
Kim HJ, Choi YM, Yang TCS et al (1996) Validation of ohmic heating for quality enhancement of food products. Food Technol 50(5):253
Knirsch MC, dos Santos CA, Vicente A et al (2010) Ohmic heating—a review. Trends Food Sci Technol 21(9):436–441
Kumari K, Mudgal VD, Viswasrao G et al (2016) Studies on the effect of ohmic heating on oil recovery and quality of sesame seeds. J Food Sci Technol-Mysore 53(4):2009–2016
Lebovka NI, Praporscic I, Ghnimi S et al (2005) Does electroporation occur during the ohmic heating of food? J Food Sci 70(5):E308–E311
Leizerson S, Shimoni E (2005a) Effect of ultrahigh-temperature continuous ohmic heating treatment on fresh orange juice. J Agric Food Chem 53(9):3519–3524
Leizerson S, Shimoni E (2005b) Stability and sensory shelf life of orange juice pasteurized by continuous ohmic heating. J Agric Food Chem 53(10):4012–4018
Lima M, Sastry SK (1999) The effects of ohmic heating frequency on hot-air drying rate and juice yield. J Food Eng 41(2):115–119
Lima M, Heskitt B, Sastry SK (1999a) The effect of frequency and wave form on the electrical conductivity-temperature profiles of turnip tissue. J Food Process Eng 22:41–54
Lima M, Heskitt BF, Burianek LL et al (1999b) Ascorbic acid degradation kinetics during conventional and ohmic heating. J Food Process Preserv 23(5):421–434
Lima M, Heskitt BF, Sastry SK (2001) Diffusion of beet dye during electrical and conventional heating at steady-state temperature. J Food Process Eng 24(5):331–340
Liu Y, Tikunov Y, Schouten RE et al (2018) Anthocyanin biosynthesis and degradation mechanisms in solanaceous vegetables: a review. Front Chem 6:52
Loypimai P, Moonggarm A, Chottanom P (2009) Effects of Ohmic heating on lipase activity, bioactive compounds and antioxidant activity of rice bran. Aust J Basic Appl Sci 3(4):3642–3652
Makroo HA, Saxena J, Rastogi NK et al (2017) Ohmic heating assisted polyphenol oxidase inactivation of watermelon juice: effects of the treatment on pH, lycopene, total phenolic content, and color of the juice. J Food Process Preserv 41(6):e13271
Mannozzi C, Rompoonpol K, Fauster T et al (2019) Influence of pulsed electric field and ohmic heating pretreatments on enzyme and antioxidant activity of fruit and vegetable juices. Foods 8(7):12
Mercali GD, Jaeschke DP, Tessaro IC et al (2012) Study of vitamin C degradation in acerola pulp during ohmic and conventional heat treatment. LWT-Food Sci Technol 47(1):91–95
Mercali GD, Jaeschke DP, Tessaro IC et al (2013) Degradation kinetics of anthocyanins in acerola pulp: comparison between ohmic and conventional heat treatment. Food Chem 136(2):853–857
Mercali GD, Schwartz S, Marczak LDF et al (2014) Ascorbic acid degradation and color changes in acerola pulp during ohmic heating: effect of electric field frequency. J Food Eng 123:1–7
Mercali GD, Gurak PD, Schmitz F et al (2015) Evaluation of non-thermal effects of electricity on anthocyanin degradation during ohmic heating of jaboticaba (Myrciaria cauliflora) juice. Food Chem 171:200–205
Mizrahi S (1996) Leaching of soluble solids during blanching of vegetables by ohmic heating. J Food Eng 29(2):153–166
Moongngarm A, Loypimai P, Fitriati A et al (2019) Ohmic heating assisted extraction improves the concentrations of phytochemicals in rice bran oil and unsaponifiable matter. Int Food Res J 26(4):1389–1396
Onwuka UN, Ejikeme C (2005) Influence of voltage and electrode type on the yield and quality of fruit juice extracted by ohmic heating. Fruits 60:341–349
Palaniappan S, Sastry SK (1991) Electrical conductivities of selected solid foods during ohmic heating. J Food Process Eng 14:221–236
Palaniappan S, Sastry SK, Richter ER (1990) Effects of electricity on microorganisms: a review. J Food Process Preserv 14:393–414
Patras A, Brunton NP, O'Donnell C et al (2010) Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci Technol 21(1):3–11
Pereira R, Martins J, Mateus C et al (2007) Death kinetics of Escherichia coli in goat milk and Bacillus licheniformis in cloudberry jam treated by ohmic heating. Chem Pap 61(2):121–126
Pereira RN, Rodrigues RM, Genisheva Z et al (2016) Effects of ohmic heating on extraction of food-grade phytochemicals from colored potato. LWT-Food Sci Technol 74:493–503
Pootao S, Kanjanapongkul K (2016) Effects of ohmic pretreatment on crude palm oil yield and key qualities. J Food Eng 190:94–100
Ramnath SS, Prakesh PJ, Singh AK et al (2018) Effect of Ohmic heating and lye-salt concentrations on quality characteristics of tomato puree. Int Res J Pure Appl Chem 17(2):1–10
Reznick D (1996) Ohmic heating of fluid foods. Food Technol 50(5):250–251
Rinaldi M, Littardi P, Paciulli M et al (2020) Impact of Ohmic heating and high pressure processing on qualitative attributes of ohmic treated peach cubes in syrup. Foods 9(8):1093
Roux S, Courel M, Birlouez-Aragon I et al (2016) Comparative thermal impact of two UHT technologies, continuous ohmic heating and direct steam injection, on the nutritional properties of liquid infant formula. J Food Eng 179:36–43
Sabanci S, Icier F (2019) Effects of vacuum ohmic evaporation on some quality properties of sour cherry juice concentrates. Int J Food Eng 15(9). https://doi.org/10.1515/ijfe-2019-0055
Sabanci S, Cevik M, Cokgezme OF et al (2019) Quality characteristics of pomegranate juice concentrates produced by ohmic heating assisted vacuum evaporation. J Sci Food Agric 99(5):2589–2595
Saberian H, Esfahani ZH, Abbasi S (2015) Effect of conventional and ohmic pasteurization on some bioactive components of aloe Vera gel juice. Iran J Chem Chem Eng-Int English Ed 34(3):99–108
Sakr M, Liu S (2014) A comprehensive review on applications of ohmic heating (OH). Renew Sustain Energy Rev 39:262–269
Salari S, Jafari SM (2020) the influence of ohmic heating on degradation of food bioactive ingredients. Food Eng Rev 12(2):191–208
Samaranayake CP, Sastry SK (2005) Electrode and pH effects on electrochemical reactions during ohmic heating. J Electroanal Chem 577(1):125–135
Sarang S, Sastry SK, Knipe L (2008) Electrical conductivity of fruits and meats during ohmic heating. J Food Eng 87(3):351–356
Sarkis JR, Jaeschke DP, Tessaro IC et al (2013) Effects of ohmic and conventional heating on anthocyanin degradation during the processing of blueberry pulp. LWT-Food Sci Technol 51(1):79–85
Sarkis JR, Jaeschke DP, Mercali GD et al (2019) Degradation kinetics of anthocyanins in blackberry pulp during ohmic and conventional heating. Int Food Res J 26(1):87–97
Sastry SK (1992) A model for heating of liquid-particle mixtures in a continuous flow ohmic heater. J Food Process Eng 15(4):263–278
Sastry SK (2005) Advances in ohmic heating and moderate electric field (MEF) processing. In: Barbosa-Cánovas GV, Tapia MS, Cano MP (eds) Novel food processing technologies. CRC Press, Taylor and Francis Group, Boca Raton, FL, pp 491–499
Sastry SK (2008) Ohmic heating and moderate electric field processing. Food Sci Technol Int 14(5):419–422
Sastry SK, Barach JT (2000) Ohmic and inductive heating. J Food Sci Suppl 65(4):42–46
Sastry SK, Li Q (1996) Modeling the ohmic heating of foods. Food Technol 50(5):246–248
Saxena J, Makroo HA, Srivastava B (2016) Optimization of time-electric field combination for PPO inactivation in sugarcane juice by ohmic heating and its shelf life assessment. LWT-Food Sci Technol 71:329–338
Schreier PJR, Reid DG, Fryer PJ (1993) Enhanced diffusion during the electrical heating of foods. Int J Food Sci Technol 28(3):249–260
Sensoy I, Sastry SK (2004) Extraction using moderate electric fields. J Food Sci 69(1):E7–E13
Sinela AM, Mertz C, Achir N et al (2017) Exploration of reaction mechanisms of anthocyanin degradation in a roselle extract through kinetic studies on formulated model media. Food Chem 235:67–75
SKLM (2015). Opinion on the use of ohmic heating for the treatment of foods. DFG Senate Commission Food Safety
Somavat R (2011) Applications and effects of ohmic heating: sterilization, influence on bacterial spores, enzymes, bioactive components and quality factors in food. The Ohio State University
Somavat R, Kamonpatana P, Mohamed HMH et al (2012a) Ohmic sterilization inside a multi-layered laminate pouch for long-duration space missions. J Food Eng 112(3):134–143
Somavat R, Mohamed HMH, Chung YK et al (2012b) Accelerated inactivation of Geobacillus stearothermophilus spores by ohmic heating. J Food Eng 108(1):69–76
Somavat R, Mohamed HMH, Sastry SK (2013) Inactivation kinetics of Bacillus coagulants spores under ohmic and conventional heating. LWT-Food Sci Technol 54(1):194–198
Stojceska V, Atuonwu J and Tassou SA (2019). Ohmic and conventional drying of citrus products: energy efficiency, greenhouse gas emissions and nutritional properties. In: Proceedings of the 2nd International Conference on Sustainable Energy and Resource Use in Food Chains Including Workshop on Energy Recovery Conversion and Management; ICSEF; 2018, vol. 161. pp. 165–173
Sun HX, Kawamura S, Himoto JI et al (2008) Effects of ohmic heating on microbial counts and denaturation of proteins in milk. Food Sci Technol Res 14(2):117–123
Tumpanuvatr T, Jittanit W (2012) The temperature prediction of some botanical beverages, concentrated juices and purees of orange and pineapple during ohmic heating. J Food Eng 113(2):226–233
Van den Broeck I, Ludikhuyze L, Weemaes C et al (1998) Kinetics for isobaric-isothermal degradation of L-ascorbic acid. J Agric Food Chem 46(5):2001–2006
Varghese KS, Pandey MC, Radhakrishna K et al (2014) Technology, applications and modelling of ohmic heating: a review. J Food Sci Technol-Mysore 51(10):2304–2317
Vicente AA, Castro I, Teixeira JA (2005) Ohmic heating for food processing: new technologies and quality issues. In: Sun DW (ed) Thermal processing in food processing. CRC Press, Boca Raton, FL, pp 426–468
Vicente AA, Castro I, Teixeira JA (2006) Innovations in thermal food processes. In: Sun DW (ed) Thermal food processing: new technologies and quality issues. CRC Press, Taylor & Francis Group, Boca Raton, FL, USA, pp 424–468
Vikram VB, Ramesh MN, Prapulla SG (2005) Thermal degradation kinetics of nutrients in orange juice heated by electromagnetic and conventional methods. J Food Eng 69(1):31–40
Vorobiev E, Lebovka N (2010) Enhanced extraction from solid foods and biosuspensions by pulsed electrical energy. Food Eng Rev 2(2):95–108
Wang CS, Chu C (2003) Study of vacuum evaporation by using ohmic heating. IFT Annual Meeting, Chicago, IL, USA
Wang WC, Sastry SK (1993) Salt diffusion into vegetable tissue as a pretreatment for ohmic heating: electrical conductivity profiles and vacuum infusion studies. J Food Eng 20(4):299–309
Wang WC, Sastry SK (1997a) Changes in electrical conductivity of selected vegetables during multiple thermal treatments. J Food Process Eng 20(6):499–516
Wang WC, Sastry SK (1997b) Starch gelatinization in ohmic heating. J Food Eng 34(3):225–242
Wang WC, Sastry SK (2002) Effects of moderate electrothermal treatments on juice yield from cellular tissue. Innov Food Sci Emerg Technol 3:371–377
Wang CS, Kuo SZ, Kuo-Huang LL et al (2001) Effect of tissue infrastructure on electric conductance of vegetable stems. J Food Sci 66(2):284–288
Wattanayon W, Udompijitkul P, Kamonpatana P (2021) Ohmic heating of a solid-liquid food mixture in an electrically conductive package. J Food Eng 289:110180
Yildiz H, Bozkurt H, Icier F (2009) Ohmic and conventional heating of pomegranate juice: effects on rheology, color, and total phenolics. Food Sci Technol Int 15(5):503–512
Yildiz H, Icier F, Baysal T (2010) Changes in beta-carotene, chlorophyll and color of spinach puree during ohmic heating. J Food Process Eng 33(4):763–779
Yoon SW, Lee CYJ, Kim KM et al (2002) Leakage of cellular materials from Saccharomyces cerevisiae by ohmic heating. J Microbiol Biotechnol 12(2):183–188
Acknowledgments
This book chapter was written within the framework of MEFPROC (Improving Sustainability in Food Processing using Moderate Electric Fields (MEF) for Process Intensification and Smart Processing). This transnational project is part of the ERA-Net SUSFOOD2 with funding provided by national/ regional sources of the Federal Ministry of Food and Agriculture and co-funding by the European Union’s Horizon 2020 research and innovation programme.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Baier, A.K., Rauh, C. (2022). Influence of Ohmic Heating on Food Bioactives. In: Jafari, S.M., Capanoglu, E. (eds) Retention of Bioactives in Food Processing. Food Bioactive Ingredients. Springer, Cham. https://doi.org/10.1007/978-3-030-96885-4_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-96885-4_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-96884-7
Online ISBN: 978-3-030-96885-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)