Abstract
The main objective of this introductory chapter is to provide an overview of the main topics of interest concerning lactose, a carbohydrate uniquely associated with milk of almost all mammals. Some historical milestones of the discovery and research progress in elucidation of the nature and some chemical properties of this disaccharide are briefly mentioned. Topics to follow include lactose biosynthesis and functions in milk; properties and reactions characterizing the behaviour of lactose in various systems and processing conditions; industrial production and uses of isolated lactose and lactose derivatives; and finally biological, technological and nutritional significance of lactose in human foods, including dairy products such as yogurt. Some industrial approaches used to counteract complications that lactose may pose in certain situations such as crystal development in ice cream are touched upon. All these subjects are explored in more depth in the chapters to follow.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1.1 General Introduction and History
Lactose, commonly called milk sugar, is a carbohydrate uniquely associated with milk of almost all mammals, including humans. It is a reducing disaccharide, composed of two monosaccharides glucose and galactose, linked by a β1 → 4 glycosidic bond. Its molecular formula is C12H22O11, its systematic name being β-d-galactopyranosyl-(1 → 4)-d-glucose. As the glucose can exist in two forms (the α-pyranose form or the β-pyranose form), in contrast to galactose (which only has the β-pyranose form), lactose in aqueous solutions can be present in two anomeric forms, α-lactose and β-lactose. The lactose content in human milk is higher than that of all industrially relevant farm animals the milk of which is used in human nutrition, as shown in Table 1.1.
The first documented historical record mentioning lactose as “salt of milk whey” was published in 1633 by Italian physician Fabrizio Bartoletti (often referred to as Bartolettus, 1576–1630). He isolated a “curious whitish salt” by evaporation of water from milk whey, followed by repeated dissolution and coagulation. His method was reprinted in 1688 by the German physician Michael Ettmüller (1644–1683) and mentioned in 1700 by the Venetian pharmacist Lodovico Testi (1640–1707) in his booklet on milk sugar (saccharum lactis). The Swedish German chemist Carl Wilhelm Scheele (1742–1786), remembered mainly for his discovery of oxygen, had wide research interests leading to isolating and characterizing, for the first time, many organic compounds including lactose in 1780. Other notable chemists of the past also contributed to the early knowledge of lactose; thus, Heinrich Vogel (1778–1867) proved in 1812 that glucose will be produced by hydrolyzing lactose, while Louis Pasteur in 1856 isolated galactose as the other lactose component. The exact configuration of the two component sugars was proposed in 1894 by another German chemist, the 1902 Nobel Prize recipient Emil Fischer (1852–1919). The name lactose was coined by the French chemist Jean Baptiste André Dumas (1800–1884).
The interest in lactose as a commercial commodity, as well as a subject of scientific research, increased considerably during the nineteenth century. The foundations of the present knowledge of lactose, especially regarding its chemistry, molecular structure, physical properties, and crystallization behavior were laid during the early twentieth century, including the systematic work of Hudson (1904). This rapidly expanding knowledge has been reviewed numerous times (Whittier 1925; Whitter 1944; Weisberg 1954; Zadow 1984, 1992; Schaafsma 2008; Ganzle et al. 2008; Wong and Hartel 2014). In 2012, the International Dairy Journal published a special issue containing 9 reviews focusing on various aspects of technology, nutrition, and health of lactose and its derivatives (Jelen and Smithers 2012). Most of the major textbooks on Dairy Chemistry, Technology, or Nutrition include a separate chapter on lactose, its properties and/or its nutritive value (e.g., Jenness and Patton 1959; Webb and Johnson 1965; Webb et al. 1974; Renner 1983; Walstra and Jenness 1984; Fox 1985, 1997; Wong et al. 1988; Fox and McSweeney 1998; Walstra 2002; Walstra et al. 1999, 2006; Miller et al. 2007). In the four volumes of the Encyclopedia of Dairy Sciences (Fuquay et al. 2011), there are almost 200 entries related to lactose. The book Lactose and Its Derivatives (Sinelnikov et al. 2007) appeared to be until recently the most comprehensive source of information on the subject, unfortunately available only in Russian. The most recent book, Lactose (Paques and Lindner 2019) presents reviews of the evolutionary role, health effects, and the most current industrial topics related to lactose and some of its commercially interesting derivatives.
The objective of this introductory chapter is to provide a brief summary of the main traditional building blocks of knowledge concerning lactose, leading to better understanding of the next few chapters reviewing selected topics where active lactose-related research is generating new results. Although crystallization has been a main topic of lactose research studied for a long time, the new glass transition data provide additional research angles in this regard. This subject is covered in Chapter 2, while in Chapters 3 and 4 the production and significance of lactose in various dairy products are reviewed in detail. Current research concerning lactose is especially active in areas related to nutrition, including lactose ingestion and malabsorption (Chapter 6), while the subject of oligosaccharides (Chapters 5 and 7) is of great industrial significance presently.
1.2 Lactose Biosynthesis and Functions in Milk
It may be of interest to note that, in contrast to most other sugars being of plant origin, the only significant source of lactose in nature is the mammary gland of lactating mammals. Several million tons of isolated lactose are produced annually as a valuable industrial product just from milk of the few species of mammals used as farm animals for human nutrition purposes; the overall production of lactose by all lactating mammals is obviously many times higher.
The biosynthesis of lactose in the epithelial mammary gland cells has been studied extensively and several exhaustive reviews of the information have been published, mainly towards the end of the last century (Brew and Hill 1975; Jones 1978; Larson 1985, others). These seem to serve as the basis of information found in later texts on lactose, including the previous editions of this series. The latest, rather extensive, review of this topic can be found in the book Lactose (Paques and Lindner 2019) mentioned above. In the following, only the most salient aspects of this somewhat unusual biosynthetic pathway are summarized. It involves two molecules of glucose being absorbed from the blood, one being converted (epimerized) to galactose via the Leloir pathway which is widespread in animal tissues and bacterial cells. This metabolic process also eliminates the galactose toxicity which could exist in its free form. The galactose is phosphorylated and coupled to the second molecule of glucose by a β-1,4-glycosidic linkage, through the action of a unique two-component enzyme, lactose synthase.
Lactose serves two important functions in milk. It is a ready source of energy for the neonate, providing about 30% of the caloric value of bovine milk, and it influences osmotic pressure of milk, which is isotonic with blood and hence is essentially constant. The lactose contributes about half of the total milk osmotic pressure, with the diffusable ions (especially K+, Na+, and Cl−) being the other contributors. There is a strong inverse relationship between the concentration (mM) of lactose and the osmolality (mM) of milk (Holt and Jenness 1984; Holt 1985).
An interesting question has been posed frequently as to whether there is a readily explainable evolutionary reason for lactose to be the carbohydrate of milk, considering the complex pathway of formation. It was proposed that, since a given weight of lactose exerts only half the osmotic pressure that the same weight of a monosaccharide would, a given osmotic effect provides twice as much energy. Since the osmotic pressure is fixed in milk due to the osmotic pressure of blood being constant, the inclusion of a disaccharide seems advantageous on this account, but this does not explain a reason why specifically galactose is formed by the rather convoluted pathway and included in the disaccharide molecule. A recent review on galactose metabolism (Coelho et al. 2015) offers a detailed explanation, as it indicates the biological importance of galactose, not only for the neonatal development but generally as a crucial structural element in macromolecules. A recent factsheet issued by the International Dairy Federation (IDF 2017) has brought further attention to the positive role of galactose in various aspects of human health and nutrition.
The principles of lactose synthesis in the mammary gland are the same for all mammals. However, the concentration of lactose in milk of various species of mammals fluctuates significantly, even though in average values, the lactose content in milk of most mammals used as milking animals worldwide is similar, 4.3–5.0%, w/w (Table 1.1). The average lactose content in milk of bovine breeds most often used for industrial milk production varies only slightly, while the variations among individual animals of a given breed can be much larger. The synthesis of lactose draws water osmotically into the Golgi vesicles from the blood and hence affects the total milk yield; milk production would increase with increased lactose synthesis. At the same time, this also explains the relatively constant lactose content (% w/w) of milk, and the variations in lactose levels between cows, which is much smaller than the variation in the other two macronutrients, fat and protein. It is also important to realize that lactose synthesis is only one of the numerous processes of the total milk synthesis, all occurring in the mammary gland. The synthesis of other milk components, particularly the triglycerides and milk proteins, also proceeds at the same time, based on the availability of glucose from blood. One of the milk proteins being secreted by the mammary epithelial cells is α-lactalbumin, one of the main whey proteins, which is a part of the lactose synthase system. The other component of this complex is a galactosyltransferase enzyme; the lactose synthase complex catalyzes the final formation of lactose from glucose and galactose. All these reactions proceed simultaneously and depend on blood glucose availability and thus on the overall metabolic status of the lactating female, with the lactose synthesis playing several key roles in the overall milk synthesis in the mammary gland.
Thus, the key roles of lactose in the overall milk production can be summarized as follows. Lactose determines the volume of milk produced; its significant contribution to the milk osmotic pressure is a crucial factor in milk yield; and the production of oligosaccharides is inextricably linked to lactose synthesis. In addition, the lactose synthesis process, drawing on the available glucose, exerts significant effects on the production of other major milk components, the synthesis of which is also based on the supply of glucose; this includes the secretion of α-lactalbumin, indispensable for lactose production, illustrating the interrelationships of all these reactions, with lactose being the center-piece element.
1.3 Properties and Reactions
The properties of lactose are generally similar to those of other sugars but differ in some technologically important respects. Lactose is a reducing sugar, i.e., it has a free, or potentially free, carbonyl group (an aldehyde group in the case of lactose). Like other reducing sugars, lactose exists partially as an open-chain form with an aldehyde group which can form a hemi-acetal and, thus, a ring structure. The formation of a hemi-acetal creates a new chiral center (asymmetric carbon) which may exist as two isomers (enantiomorphs), α or β. In an aqueous solution, the equilibrium between the α and β forms will be established by the mutarotation process, i.e., by alternatively opening and forming the ring structure. The α and β anomers of lactose have very different properties, the most important of which are specific rotation, [a]20D (+91.1° and + 33.2° for α- and β-lactose, respectively; Walstra et al. 2006) and solubility (74 and 480 g/1000 g H2O, for α- and β-lactose, respectively, at 20 °C; Sienkiewicz and Kirst 2006). These significantly differing properties will lead to establishment of the equilibrium ratio of β- to α-lactose in aqueous solutions, which at 20 °C is 1.68 (62.7/37.3), the corresponding equilibrium [a]20D being about +55.3° and the final lactose solubility at this equilibrium about 192 g/1000 g H2O. The α-lactose solubility (and thus also the final solubility) increase steadily with temperature, being 96.5 and 248 g/1000 g H2O at 30 °C and 233.5 and 584 g/1000 g H2O at 60 °C (Schuck 2011). Mutarotation is a first-order reaction, the rate of which increases sharply with increasing temperature. The mutarotatory equilibrium is established almost instantaneously at 75 °C, while it takes several hours at ambient temperatures, as first estimated by Bell (1930) from the seminal data of Hudson (1904).
The aqueous solubility of lactose, especially of the α-enantiomorph, is low at ambient temperature compared to other sugars, but increases rapidly with increasing temperatures.
The solubility of α-lactose is significantly more temperature-dependent than that of the β anomer. At temperatures >93.5 °C, α-lactose becomes more soluble that β-lactose. Hence, it is the β form of lactose which crystallizes above this temperature, while the usual commercial form of lactose (the α-lactose) is being crystallized at <93.5 °C, such conditions being much more easily attainable industrially. For special purposes, the β form of lactose may be manufactured by crystallization well above the cross-over temperature. The α-lactose crystallizes as a monohydrate (C12H22O11·H2O), while β-lactose forms anhydrous crystals; thus, the yield of α-lactose is ~5% higher than that of β-lactose. Some details concerning solubility, crystallization, mutarotation, and hygroscopicity of lactose are found in Chapters 2, 3, and 4, or in specialized texts, e.g., that of Schuck (2011). These interrelationships need to be well understood as they play a significant role in the production of isolated lactose, or in various industrial problems, especially concerning concentration and drying of products containing substantial amounts of lactose, as discussed later.
The phenomena related to growth kinetics and morphology of especially α-lactose monohydrate crystals have been studied extensively in the past, and various crystal forms have been published. The tomahawk appearance of the fully developed crystal has been accepted since its first appearance in the literature (Hunziker and Nissen 1927) and reprinted innumerable times, especially as redrawn in the crystallographic canon by Kreveld and Michaels (1965) even though such a complex form is rarely if ever encountered in real situations. Rather, the crystals can assume many other shapes, prisms, needles, pyramids, flat triangles, “half-moons,” etc.; pictures of all these and similar forms can be found in the literature. In the constant supersaturation conditions attainable especially in laboratory studies using the single crystal methodology (Bhargava and Jelen 1996; Jelen, 1972), the α-lactose monohydrate crystal grows in a quasi-pyramid-like form, with the only active face of the pyramid being the bottom (Fig. 1.1). However, the constant supersaturation conditions are rarely encountered in industrial conditions, where the crystal shape will be affected by the varying conditions during the crystallization process, including the supersaturation, temperature, cooling rate, and especially the presence of impurities in the lactose solution (Jelen and Coulter 1973; Bhargava and Jelen 1996, others). Understanding the mechanism of crystalline growth and its kinetics is of particular importance for production of isolated lactose by the usual industrial crystallization process, as well as in various situations where crystallization constitutes one of the technological steps for ensuring optimal product quality, particularly concerning dried lactose-rich powders or concentrated products with high lactose content.
Single crystals of α-lactose monohydrate produced by growing them individually in aqueous lactose solutions of constant supersaturation and used in single crystal experiments (Jelen 1972; weight of the crystals approx. 0.25 g, length about 1.5 cm)
Several other physical properties of lactose can be found in specialized treatises; these include density, melting point, heat of combustion, specific heat, etc. and are of lesser importance in understanding the subjects covered in subsequent chapters.
Compared to other sugars, lactose has a low level of sweetness; at least 3 times more lactose is needed to achieve equal sweetness in comparisons to sucrose standards of 1% or 5% aqueous solutions (Wong et al., 1998). However, even with the limited sweetness, adding small amounts of isolated lactose to compensate for the lower total solids when ultrafiltration permeates were used for protein down-standardization seemed to have a noticeable sweetening effect with as little as 0.75% added (Jelen and Michel 1999). β-lactose has been shown to be sweeter than α-lactose, but after reaching the mutarotatory equilibrium the sweetness difference became insignificant. The difference in sweetness of the two anomers is too small to be of practical significance in food applications like coating sugar in baking (Wong et al., 1998). In general, lactose has limited value as a sweetening agent in foods but isolated lactose, UF whey permeate (with the lactose content over 80% dry matter), or even dried whey (about 73% lactose) are useful in applications where excessive sweetness is undesirable, e.g., bulking agent, protein standardization of milk, etc.
Like all reducing sugars, lactose can participate in the non-enzymatic browning reaction referred to as the Maillard reaction (MR), resulting in the production of (off-) flavor compounds and brown polymers and causing the color change, sedimentation, and other potential problems. Milk, containing both a reducing sugar and proteins (the two compound species participating in one of the several forms of the MR), offers suitable conditions for the reaction to proceed, most noticeably during the application of heat in production of concentrated milk products such as evaporated or sweetened condensed milk. The rate of the reaction is dependent on the process temperature and the concentration of the principal reactants. However, at the very high temperatures, such as used in UHT processing of milk, the reaction is initiated even though the reactant concentrations are much lower than in the milk concentrates. The reaction between the lactose and the available milk protein α- and ε- amino groups proceeds not only during the heating process but, once initiated, continues also during the storage, even at or below ambient temperatures. This could lead to formation of melanoidins, known to be formed at the late stages of the very complex series of intermediate MR steps. Formation of melanoidins leads to increase in molecular size and loss of solubility in storage of, e.g., spray-dried whey. The same general MR-type pathways, involving polymerization of casein and whey proteins, were used in the past to explain the age gelation phenomenon, but later studies suggested that the interaction between lactose and the ε-NH2 lysine residues (the reaction sometimes referred to as lactosylation), occurring at the initial stages of the MR, may be of importance for sedimentation in highly heated liquid milk. This was recently confirmed by Malmgren et al. (2017) in storage experiments with directly heated UHT milk at accelerated storage temperature of 40 °C where no age gelation (but heavy sedimentation) occurred.
Several other reactions involving lactose take place during heat treatment of milk or other lactose-containing dairy products. One such reaction is the isomerization of lactose into lactulose, caused by conversion of the glucose moiety into fructose. The reaction of lactulose with the free ε- amino group of lysine produces an Amadori compound called lactulosyl-lysine (galactosefructoselysine), found in milk during the early stages of the MR. Quantification of lactulose has been proposed in the past to estimate the intensity of the heat treatment used in dairy processing. A more recent development in this regard involves an indirect determination of lactulosyl-lysine after its acid hydrolysis into pyridosine and furosine and measuring the furosine value by high performance liquid chromatography (HPLC). A study by Rattray, Gallmann, and Jelen Rattray et al. (1997) showed that the lactulosyl-lysine production upon heating of milk is affected by the lactose and protein concentrations, as well as the heating process and the temperatures used for storage of the final product.
Direct determination of lactose in various materials including foods may be accomplished by several chemical reactions including the redox titration using alkaline CuSO4 (Fehling’s solution) or chloramine-T, as the principal standard method for the quantitative determination of lactose. The phenol-sulfuric acid method of Dubois et al. (1956) is simple but can only be used for determination of lactose in systems where no other reducing sugars are present, e.g., in cheese, milk, whey, or experimental pure lactose solutions. Nowadays, in large laboratories, lactose is usually determined by HPLC or by infra-red spectrophotometry. It may also be determined by polarimetry, enzymatically (using an enzyme assay kit), or by cryoscopy. The enzymatic methods are based on the hydrolysis reaction, which converts lactose to its monosaccharide constituents, glucose and galactose.
The enzymatic hydrolysis reaction is catalyzed by the enzyme β-galactosidase (EC3.2.1.23), obtained from a multitude of microbial sources. The exact chemical structures of these enzymes can differ widely, as can the optimum reaction conditions (especially pH); the unifying property for the whole β-galactosidase family is the catalytic effectiveness in the lactose hydrolysis reaction. The reaction can be accomplished by adding the free soluble enzyme preparation to the milk or other solution containing lactose, by using the immobilized enzyme column, or in an enzyme reactor. Bacteria fermenting lactose have the capability of producing lactase, and they can be utilized as a crude source of the enzyme as well (Vasiljevic and Jelen 2003). Enzymatic lactose hydrolysis can be used in production of lactose-free dairy foods, and as one method to increase the sweetness of certain products (e.g., yogurts), as the glucose–galactose mixture resulting from the hydrolysis reaction is at least 3× sweeter than the original lactose solution. Significant research and marketing emphasis has been expended in Australia and elsewhere on developments of sweetening hydrolyzed lactose syrups in 1980s, and numerous reviews on the lactose hydrolysis technologies appeared at that time. However, the present economic relationships make this method of using lactose for sweetening purposes doubtful.
Application of severe heat (about 120 °C or more) and very low pH (about 1.5) will also hydrolyze lactose. However, at these drastic conditions, other reactions will also take place and thus the acid hydrolysis of lactose is of academic interest only. Interestingly, lactose is much more resistant to acid hydrolysis in comparison to other disaccharides, e.g., sucrose.
1.4 Production and Uses of Lactose and Lactose Derivatives
In comparison to other macronutrients of milk (proteins, fat), lactose has been often called the least valuable milk component. It is also contained in milk in amounts significantly greater than any of the other components except water, and, as it is discarded in whey in the manufacture of cheese and industrial casein, it presents a major waste problem for the dairy industry. The search for industrially and economically viable uses of lactose has been an ongoing subject of scientific and industrial research for a very long time.
Much basic research elucidating the lactose properties and reactions described above has been aimed at defining conditions suitable for production of isolated lactose and various lactose-rich or lactose-based products. Earlier, these were made almost invariably from whey but nowadays the source of choice is often the protein-free permeate resulting from whey or milk processing by membrane technology. The newest contributions to the scientific underpinning of some of these processes are explored in Chapters 2, 3, and 4.
About 95% of the world’s whey stream originates from cheese production (Brewster, 2020). This is the single most important source of isolated lactose and other lactose-rich ingredients. The principal products from unmodified whey are various dry whey powders containing about 71-73 % lactose. The UF permeates resulting from fractionation of whey into whey protein concentrates or isolates – or by concentration and fractionation of milk – contain over 80% lactose in dry matter and are being used as the source material from which lactose is being produced by crystallization, as well as for other applications in liquid, concentrated or dried form. The various permeates have become an important ingredient and its use by various sectors of the food industry is rapidly increasing, at an average compound annual growth rate of 17% for the bakery industry, by far the most important user of the permeates presently (Brewster, 2020). Other segments of the food industry where permeates are making significant inroads are confectionery, dairy, and producers of hot drinks and snacks. The main types of dried products originating from whey are listed in Table 1.2, and most of these contain lactose as their main component.
The traditional crystallization technology and the adjunct downstream processing steps are essentially similar to those used for sucrose or other sugars, but the principal crystallization step is usually accomplished in batch crystallizers upon controlled cooling. The use of evaporative continuous crystallizers used routinely in sucrose crystallization is much less common, due to the mineral impurities found in whey (or milk) permeates or whey itself, necessitating additional steps such as demineralization (Wong and Hartel, 2014). Also, as most of the growth occurs on only one (the bottom) face of the lactose crystal pyramid (Dincer et al., 2009; Bhargava and Jelen, 1996), the rate of lactose crystal growth is much slower than that of a sucrose crystal, which grows on all faces of its hexagonal prism structure. Thus, with higher levels of supersaturation, often reached during evaporative crystallization, spontaneous secondary nucleation (“false grain”) would predominate, rather than the growth of the crystals needed for ease of the downstream operations, especially centrifugal separation and washing.
Owing to its relatively low sweetness and low solubility, the applications of lactose are different from those of sucrose or glucose. One of the principal applications of isolated lactose is as ingredient in the production of “humanized” infant formulae based on bovine milk, which has a significantly lower lactose content than human milk. The lactose used may be a crystalline product or demineralized whey (for physiological reasons, it is necessary to reduce the concentration of inorganic salts in bovine whey).
Lactose, either in its isolated form or as the main component of dried whey or UF permeate, is used in a number of special applications in the food industry, e.g., as a free-flowing or agglomerating agent, to accentuate/enhance the flavor of some foods, to improve some desired functionality, and as a bulking agent in many processed foods including products of the dairy industry such as ice cream. As a reducing sugar with limited sweetness, it is widely used in the bakery and confectionery industries for production of the golden crust of many baked goods, a desirable effect of the otherwise detrimental Maillard reaction.
One of the important traditional non-food uses of the crystalline lactose is by the pharmaceutical industry for pill formation, due to its ease of molding, tablet compression, and low hygroscopicity. Several companies produce isolated lactose especially formulated for tableting efficiency. This special subject is reviewed in detail in one of the chapters of the Paques and Lindner’s (2019) book Lactose. However, in general, the global market for both isolated lactose and the dried whey is rather static and new approaches to utilization of the ever-increasing supply of lactose worldwide continue to be actively sought. Some other novel uses of lactose will be reviewed in later chapters.
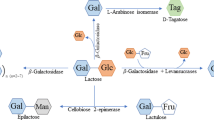
Similar to other sugars, the lactose molecule has a number of functional groups with reactivities that can be used to convert lactose to several food-grade derivatives using either chemical or enzymatic pathways. The following groups are the primary targets in the derivatization processes: (a) the glycosidic linkage between glucose and galactose; (b) the free hydroxyl groups; (c) the reducing group of glucose; and (d) the carbon–carbon bonds. There are several commercially viable lactose derivatives being produced industrially. Reviews of the main lactose derivatives of interest have been published in the recent past, and these can be consulted for more specific information. In addition to the glucose-galactose syrups mentioned above, the following is a summary of the main derivatives of interest.
-
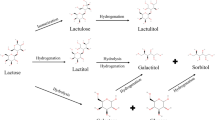
Lactulose. Probably the most commercially successful derivative of lactose, produced by the epimerization of the glucose moiety of lactose to fructose under mildly alkaline conditions. Lactulose has many applications including use as a bifidogenic factor in infant formulas and health foods, and as a mild laxative. It is listed in the US Pharmacopoeia, European Pharmacopoeia, and Japanese Pharmacopoeia. A major portion of the Seki and Saito’s (2012) review of the main lactose derivatives is devoted to details of the production, properties, and applications of this probiotic.
-
Lactosucrose, a trisaccharide comprising galactose, glucose, and fructose, is a potential prebiotic oligosaccharide produced by enzymatic polymerization with sucrose. Its importance in maintaining human gastrointestinal homeostasis has been reported.
-
Lactobionic acid is a saccharic acid comprising galactose and gluconic acid; it is a sweet-tasting acid, which is a rare property that can be exploited in processed foods. Lactobionic acid has application as a bifidus factor and as calcium chelator in dietary supplements. The most interesting non-food use is for preservation of transplant organs and as a humectant in skincare products.
-
Lactitol. The carbonyl group of lactose can be reduced to lactitol (the alcohol of lactose). Since the aldehyde group of the Glu moiety is reduced to the OH group, it does not participate in a Maillard reaction and its heat stability is high. Its application can be as a sweetener as its taste is similar to that of sucrose. As a special use of lactitol, its effectiveness in protection of water logged archeological relics has been mentioned.
-
Tagatose, the keto analogue of galactose, is usually included in the group of lactose derivatives, even though it is not produced from lactose directly but from the galactose obtained from lactose. Tagatose is nearly as sweet as sucrose, has a good quality sweet taste, and enhances flavor of other sweeteners. It is absorbed poorly from the small intestine and thus is considered as a low calorie sweetener.
-
Galactooligosaccharides (GOS) are a special group of lactose derivatives, produced as a result of transferase activity of the β-galactosidase (lactase) enzyme, used normally for its hydrolytic activity in splitting lactose to its monosaccharide constituents as discussed above. Under certain conditions (mainly at high lactose concentrations) the transgalactosylation activity will predominate and oligosaccharides, usually containing 2 to 9 monosaccharides, will be produced. The GOS are composed of galactose, with glucose or galactose at the reducing end. In its transgalactosylation function, the lactase enzyme is catalyzing the addition of galactose units to the lactose molecule. The relative rates of the hydrolysis vs. transgalactosylation reactions depend on the enzyme source and other variables. Detailed GOS reaction pathways have been described in a number of publications, e.g., Chan and Ganzle, Chen and Gänzle 2017. Over 30 different di-, tri-, and tetrasaccharides with defined structures were identified as products of enzymatic transgalactosylation. The main reason for the currently keen interest in the GOS is their similarity with the native human milk oligosaccharides (HMO) present in relatively large quantities in human milk. The GOS used in infant formula to mimic the functions of HMO oligosaccharides, have other interesting physico-chemical and probiotic properties and may be useful also as food ingredients. Chapter 5 provides an up-to-date review of these two related but substantially different subjects.
In addition to the above described lactose derivatives obtained by chemical or biochemical reactions, lactose can serve as a substrate fermentable by some bacteria or yeasts. Using the classical microbial fermentation technology, various products can be obtained if the economies of the applicable processes are favorable. The value of lactose and some byproducts of the modern whey processing (especially the protein-free UF permeates containing relatively large amounts of what can be termed “crude lactose”) has been fluctuating rather wildly in the recent times and may signal revitalization of some of the fermentation processes that are presently economically non-competitive.
Lactic acid bacteria, capable of using lactose as the main fermentation substrate, must possess the ability of producing intracellular β-galactosidase to hydrolyze the lactose first, before turning it into the various metabolic byproducts. Some lactose fermenting bacteria are capable of combining the two monosaccharide molecules produced by the hydrolysis into long carbohydrate chains referred to as exopolysaccharides. This is being exploited by the contemporary dairy industry for improving texture of some products such as yogurt in using these types of bacteria as starter cultures. What has been in the past considered as one of the major defects of yogurts, the so-called ropiness, has become a desirable trait. Similarly, other lactic acid bacteria are capable of producing specific flavor compounds. As an example, diacetyl, a desirable flavor compound in buttermilk, sour cream, or cultured butter, is produced by Leuconostoc spp. co-starter bacteria for these products. This flavor compound can also be produced by fermenting lactose separately, isolating the diacetyl, and adding to the sweet butter to improve its sensory impact without the need to ferment the cream first.
The production of ethanol from lactose by fermentation using Kluyveromyces lactis or K. fragilis has been at a commercial level for at least 40 years. If the ethanol is used in potable products, this process is economically viable but whey-derived ethanol may not be classified as potable in some countries. The continued interest in new bioenergy sources could open new opportunities for lactose-derived industrial ethanol but such applications may not be cost-competitive and will depend strongly on local taxation policy. The oxidation of ethanol by Acetobacter aceti to acetic acid for vinegar or other applications is technically feasible but in most cases presently not cost-effective.
The in situ fermentation of lactose by lactic acid bacteria to lactic acid is widespread in the production of fermented dairy products. The same pathway can be used in large scale lactose fermentation to lactic acid for food or industrial applications (including the biodegradable plastic, polylactic acid), but once again, its cost-competitiveness with other fermentation substrates or with the chemical synthesis is problematic.
1.5 Biological, Technological, and Nutritional Significance of Lactose
Contrary to being considered the least important of the main milk components, the lactose plays several very significant roles in the whole agri-food chain. In the primary milk production, it determines the milk yield and influences other reactions in the mammary gland. The significance of lactose for the newborn is both as a source of energy and a source of the galactose important for the cerebral and neurological development of the infant, as well as a principal building block for the equally nutritionally important galactooligosaccharides. The relative ease of hydrolysis in the digestive system of all neonates is an added benefit of lactose in the early life nutrition.
The ability of the digestive system of young mammals to hydrolyze lactose is an evolutionary trait necessitated by the natural selection of the lactose to be the principal carbohydrate for the neonate. Lactose provides about 40% of the energy needs of the young, but for its digestion it must be first converted to the two monosaccharides. Thus, the secretion of intestinal lactase by cells in the brush border of the small intestine is essential for neonatal development. The intestinal lactase secretion decreases with progressive weaning until it stops entirely, as commonly observed with almost all adult mammals which do not use milk as food after weaning. In the case of humans, when milk became a component of regular daily diet (about 9–10,000 years ago, in the Neolithic period) this had an evolutionary impact, whereby the β-galactosidase secretion sometimes does not cease but continues into the adulthood. As a result, two phenotypes of adults emerged; those that can digest lactose due to the continued ability to produce the intestinal β-galactosidase, and those that cannot. It has been estimated that about 35% of total world population are of the former type; however, the geographical distribution of the lactase persistence (LP) condition varies widely, with over 90% of northern Europeans being considered LP, in contrast to as little as 11% LP in Southern Europe and about 1% among native Americans. The lactase non-persistence (LNP) condition leads to one of the several complications that lactose causes for the dairy and food industry, referred to as lactose malabsorption or, in lay language, lactose intolerance. This subject is explored fully in Chap. 6.
The dairy industry has at its disposal several technological avenues to offer consumers, experiencing the LNP condition, dairy products that avoid the problem. These were listed and discussed by Harju, Kallioinen, and Tossavainen Harju et al. (2012) and include, apart from the practically lactose-free cheese, many other regular dairy products in which the lactose is either hydrolyzed or has been removed. The hydrolysis route, using exogenous, industrially produced β-galactosidase preparations, has the disadvantage of leading to products with increased sweetness. As mentioned above, the glucose–galactose mixture thus obtained is several times sweeter than the original lactose solution. Especially in the case of liquid milk products (pasteurized or UHT milk), the sweetness is not readily acceptable to regular consumers. However, the process is rather simple and does not require special equipment. A whole family of hydrolyzed lactose products in which the sweetness is not a problem (or may even be advantageous) has been developed (Jelen and Tossavainen 2003). The alternative route, removing lactose from the liquid milk altogether, using chromatographic columns or by membrane filtration, may lead to products indistinguishable from the regular liquid milk, if enough residual lactose is left in the milk and hydrolyzed to produce the same sweetness as lactose does in the original milk. The disadvantage is the higher cost and the need for additional specialized equipment; the added benefit, with the contemporary concern regarding obesity, is a substantially lower caloric content than in the regular milk.
The amounts of lactose in the fermented dairy foods like yogurt, sour cream, or cultured buttermilk are reduced by the bacterial fermentation by about 30–40%, but not entirely eliminated. Still, there is some evidence that the residual lactose in these products may be tolerated better by the LNP consumers than in the liquid milk where the pH is much higher. Some of the possible explanations of this still controversial subject are related to the presence of the live lactic bacteria, possibly combined with the higher viscosity of these products, resulting in longer oro-cecal transit time during which the hydrolysis can be sufficiently advanced in the upper gastrointestinal tract. When the unhydrolyzed lactose enters the lower intestine, it results in extra water being drawn into the large intestine, causing diarrhea; the lactose is then metabolized by intestinal bacteria with the production of gas (carbon dioxide, methane, and hydrogen) causing cramps and flatulence.
A different category of complications that lactose may cause in some industrially processed dairy foods and ingredients is related to its physical properties, in particular low solubility, crystallization behavior, and hygroscopicity. In products such as sweetened condensed milk, Dulce de Leche, or the Norwegian brown cheese Mysost, the concentration of the dry matter containing lactose has been significantly increased by partial removal of water. As a result, the lactose-in-water concentration greatly exceeds the maximum solubility limit, causing crystallization. If not counteracted by the appropriate processing techniques developed over many years, the appearance of the α-lactose monohydrate crystals could be detected and, if these are large enough, would make the product unacceptable. This is also true for ice cream, where much of the water, converted to pure ice crystals, is no longer available as a solvent and the lactose concentration in the unfrozen portion of the ice cream mix again exceeds the lactose solubility limit. If not properly managed by the mix formulation, the appearance of lactose crystals large enough to be detected in the mouth may cause a serious sensory defect termed sandiness. This is much more serious than appearance of large ice crystals caused sometimes by temperature fluctuation in the ice cream storage. Large ice crystals, even though also detectable in the mouth, will melt quickly upon the consumption of the ice cream, while the poorly soluble lactose crystals will persist and will cause a very unpleasant sensation in the throat while swallowing the ice cream.
In production of dry products such as skim milk, whey, or permeate powders, the fast drying process does not allow the slow growing crystals to develop. Any lactose which has not been pre-crystallized before the drying process will be present in the powder in the amorphous “glass” form, which is very hygroscopic. Powders made without the pre-crystallization step need to be packaged using water-vapor-impermeable packaging; otherwise, α-lactose crystals will form gradually by interacting with water vapor from the surrounding air. Firstly, liquid bridges between the powder particles will be formed. This will reduce the glass transition temperature, leading in turn to sticky surfaces of the particles, and finally formation of α-lactose crystals whereby the water binding the particles together enters the monohydrate crystal structure. The resulting interlocking mass of clumps causes the package to cake irreversibly.
To produce a non-caking, non-hygroscopic powder, the lactose must be pre-crystallized before the drying step. This is accomplished by holding the concentrate for several hours under the conditions suitable for production of small crystals, not to interfere with the spray-drying technology (see Chap. 3).
Crystalline lactose in the α-hydrate form has very low hygroscopicity and can be used, e.g., in icing sugar blends. The free moisture in isolated crystalline α-lactose or dried permeate powders, needs to be rigorously controlled below 3 g/kg powder. The free moisture is not bound by the crystalline material and may cause excessive mold development. The problem is less acute in dried whey powders with pre-crystallised lactose where the presence of whey protein with its strong water binding properties will counteract the free water effect. The free moisture naturally does not include the water of crystallization contained in the α-lactose crystals (approx. 45 g/kg lactose); this has no effect on the aw in the package.
Yet another possible difficulty that lactose poses for processed dairy powders is its propensity for Maillard reaction. The MR proceeds not only during elevated heat processing but also, at a much slower rate, in milk and whey powders, especially during storage under adverse conditions of temperature and high in-package humidity (see Chap. 3). The rate of MR is the fastest at approx. aw = 0.6, so the proper packaging and/or proper lactose pre-crystallization before the drying step is doubly important. The MR in these powders especially if stored for extended periods of time can lead to significant discoloration and loss of nutritive value.
Similar effects of the MR in terms of development of browning are noticeable in the concentrated fluid or semi-solid dairy products mentioned above – sweetened condensed milk, the South American specialty Dulce-de-Leche (“milk honey”), and the Norwegian specialty whey cheeses known as Gjetost, Gudbrandsdalsost or simply mysost (whey cheese) or brunost (brown cheese). The brown colour development is inevitable, especially in the latter product, as the final water removal step to reach the approx. 85 % dry matter typical of the sliceable product, or about 70% in the case of mysost spread, is carried in an atmospheric kettle. This high temperature evaporation is necessary to keep the highly viscous mass flowable during the important rapid cooling step, resulting in forced nucleation producing lactose crystals small enough to avoid the problem of sandiness. In some products being offered as speciality ingredients, such as caramelized sweetened condensed milk, the MR effects are advantageous.
The monosaccharides, glucose and galactose, are much more reactive than lactose; thus, powders containing hydrolyzed lactose are even more susceptible to MR. The hydrolysis of lactose by β-galactosidase markedly increases the heat stability of milk and concentrated milk, especially around the pH of minimum solubility (Tan and Fox 1996). The mechanism of stabilization has not been elucidated fully but is probably due to the carbonyls formed in the Maillard reaction; unfortunately, such lactose-hydrolyzed milk products are very susceptible to intense browning.
The nutritional significance of lactose and its most important derivatives, the oligosaccharides, has been long recognized for the human infant. The acceptance of its positive role in human nutrition in general is emerging much more slowly, mostly as a pushback against its negative image due primarily to the lactose intolerance. Some of the positive nutritional claims made previously, especially as enhancer of calcium absorption, are still unsettled. The available data, although showing a positive tendency, have not been accepted by the European Food Safety Authority as sufficient to support the health claim that lactose improves calcium absorption (EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) 2011).
There are other possible positive effects of lactose that are only now being slowly recognized, including its immunomodulatory functions and the various important effects of its monosaccharide component galactose. The IDF has been stepping up its advocacy of lactose as an important nutrient by the publication of a “position paper” on lactose (IDF 2020).
1.6 Conclusion
Systematic knowledge about lactose has been developing for more than 200 years, but is still incomplete. While the earlier research concentrated mainly on the physical properties and classical lactose chemistry, present investigations are focused predominantly on the biochemical reactions and the role that lactose plays in the nutrition of the young and old. As one of the unique, naturally occurring disaccharides, lactose has become a valuable commodity for both the food and non-food uses. However, due to the ever-increasing production of cheese worldwide, the availability of lactose keeps growing faster than the opportunities of its use, both as a pure carbohydrate and as a principal component of whey, the by-product of cheese production. The industrial processes used in the lactose production and utilization contribute to the generation of new knowledge through synergy between basic and applied research. Some of the new discoveries coming from both the basic and applied research streams are described in the following chapters of this volume.
References
Bell, R. W. (1930). Some methods of preparing quickly soluble lactose. Industrial and Engineering Chemistry, 22(1), 51–54.
Bhargava, A., & Jelen, P. (1996). Lactose solubility and crystal growth as affected by mineral impurities. Journal of Food Science, 61(1), 180–184.
Brew, K., & Hill, R. L. (1975). Lactose biosynthesis. Reviews of Physiology, Biochemistry and Pharmacology, 72, 105.
Brewster, E. (2020). Dairy-derived proteins expand the playing field. Food Technology, 74(11), 54–63.
Chen, X. Y., & Gänzle, M. G. (2017). Lactose and lactose-derived oligosaccharides: More than prebiotics? A review. International Dairy Journal, 67, 61–72.
Coelho, A. I., Berry, G. T., & Rubio-Gozalbo, M. E. (2015). Galactose metabolism and health. Current Opinion in Clinical Nutrition and Metabolic Care, 18(4), 422–427.
Dubois, M., Gilles, K. A., Hamilton, J. K., Robers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). (2011). Scientific opinion on the substantiation of health claims related to lactose and increase in calcium absorption leading to an increase in calcium retention (ID 668) pursuant to article 13(1) of regulation (EC) no 1924/2006. EFSA Journal, 9, 1–13.
Fox, P. F. (1985). Developments in dairy chemistry. In Lactose and minor constituents (Vol. 3). London: Elsevier Applied Science.
Fox, P. F. (1997). Advanced dairy chemistry. In Lactose, water, salts and vitamins (Vol. 3, 2nd ed.). London: Chapman & Hall.
Fox, P. F., & McSweeney, P. L. H. (1998). Dairy chemistry and biochemistry. London: Chapman & Hall.
Fuquay, J. W., Fox, P. F., & McSweeney, P. L. J. (2011). Encyclopedia of dairy sciences. Oxford: Elsevier Academic Press.
Ganzle, M. G., Hasse, G., & Jelen, P. (2008). Lactose-crystallization, hydrolysis and value-added products. International Dairy Journal, 18, 685–694.
Harju, M., Kallioinen, H., & Tossavainen, O. (2012). Lactose hydrolysis and other conversions in dairy products: Technological aspects. A review. International Dairy Journal, 22(2), 104–109.
Holt, C. (1985). The milk salts: Their secretion, concentrations and physical chemistry. In P. F. Fox (Ed.), Developments in dairy chemistry, Vol. 3. Lactose and minor constituents (pp. 143–181). London: Elsevier Applied Science Publishers.
Holt, C., & Jenness, R. (1984). Interrelationships of constituents and partition of salts in milk samples from eight species. Comparative Biochemistry and Physiology, 77A, 275–282.
Hudson, C. S. (1904). The hydration of milk-sugar in solution. Journal of the American Chemical Society, 26(9), 1065–1082.
Hunziker, O. F., & Nissen, B. H. (1927). Lactose solubility and lactose crystal formation: II. Lactose crystal formation. Journal of Dairy Science, 10(2), 139–154.
International Dairy Federation. (2017). Reasons why galactose is good for you. Factsheet 002/2017. Brussels, Belgium.
International Dairy Federation. (2020). Lactose, an important nutrient: Advocating a revised policy approach for dairy and its intrinsic sugar. Brussels, Belgium.
Jelen, P. (1972). An investigation of certain factors determining the applicability of high temperatures in industrial crystallization of lactose from whey. Ph.D. thesis, University of Minnesota.
Jelen, P., & Coulter, S. T. (1973). Effect of certain salts and other whey substances on the growth of lactose crystals. Journal of Food Science, 38(7), 1186–1189.
Jelen, P., & Michel, C. (1999). Sensory impact of lactose in protein-standardized milk. Milchwissenschaft, 54(8), 438–441.
Jelen, P., & Smithers, G. (2012). Nutrition and health aspects of lactose and its derivatives. International Dairy Journal, 22(2), 87–158.
Jelen, P., & Tossavainen, O. (2003). Low lactose and lactose-free milk and dairy products—Prospects, technologies and applications. Australian Journal of Dairy Technology, 58, 161–165.
Jenness, R., & Patton, S. (1959). Principles of dairy chemistry. Wiley.
Jones, E. A. (1978). Lactose biosynthesis. In B. L. Larson & V. R. Smith (Eds.), Lactation—A comprehensive treatise (Vol. IV, pp. 371–385). New York: Academic Press.
Larson, B. L. (Ed.). (1985). Lactation (p. 276). Ames, Iowa: Iowa State University Press.
Malmgren, B., Ardö, Y., Langton, M., Altskär, A., Bremere, M. G. E. G., Dejmek, P., & Paulsson, M. (2017). Changes in proteins, physical stability and structure in directly heated UHT milk during storage at different temperatures. International Dairy Journal, 71, 60–75.
Miller, G. D., Jarvis, J. K., & McBean, L. D. (2007). Handbook of dairy foods and nutrition (3rd ed.). Boca Raton, FL: CRC Press.
Paques, M., & Lindner, C. (2019). Lactose: Evolutionary role, health effects, and applications (p. 310). Oxford: Academic Press
Rattray, W., Gallmann, P., & Jelen, P. (1997). Influence of protein standardization and UHT heating on the furosine value and freezing point of milk. Le Lait, 77, 297–305.
Renner, E. (1983). Milk and dairy products in human nutrition. Munchen: Volkswirtschaftlicher Verlag.
Schaafsma, G. (2008). Lactose and lactose as bioactive ingredients in human nutrition. International Dairy Journal, 18(5), 458–465.
Schuck, P. (2011). Lactose: Crystallization. In J. W. Fuquay, P. F. Fox, & P. L. J. McSweeney (Eds.), Encyclopedia of dairy sciences. Oxford: Elsevier Academic Press.
Seki, N., & Saito, H. (2012). Lactose as a source for lactulose and other functional lactose derivatives. A review. International Dairy Journal, 22(2), 110–115.
Sienkiewicz, T., & Kirst, E. (2006). Analytik von Milch und Milcherzeugnissen. Hamburg: B. Behr’s Verlag GmbH & Co.
Sinelnikov, B. M., Khramtsov, A. G., Evdokimov, I. A., Ryabtseva, S. A., & Serov, A. V. (2007). Lactose and its derivatives (p. 768). Saint Petersburg: Izdatelstvo Professija
Tan, R. H., & Fox, P. F. (1996). Effect of enzymatic hydrolysis of lactose on the heat stability of milk or concentrated milk. Netherlands Milk and Dairy Journal, 50, 267–277.
Van Kreveld, A., & Michaels, A. S. (1965). Measurement of crystal growth of α-lactose. Journal of Dairy Science, 48, 259–268.
Vasiljevic, T., & Jelen, P. (2003). Oligosaccharide production and proteolysis during lactose hydrolysis using crude cellular extracts from lactic acid bacteria. Le Lait, 83, 453–467.
Walstra, P. (2002). Physical chemistry of foods. New York: Marcel Dekker.
Walstra, P., & Jenness, R. (1984). Dairy chemistry and physics. New York: Wiley.
Walstra, P., Geurts, T. J., Noomen, A., Jellema, A., & van Boekel, M. A. J. S. (1999). Dairy technology: Principles of milk properties and processes. New York: Marcel Dekker Inc.
Walstra, P., Wouters, J. T., & Geurts, T. J. (2006). Dairy science and technology. Boca Raton, FL: CRC Press.
Webb, B. H., & Johnson, A. H. (1965). Fundamentals of dairy chemistry. Westport, CT: The AVI Publishing Company, Inc.
Webb, B. H., Johnston, A. H., & Alford, J. A. (1974). Fundamentals of dairy chemistry (2nd ed.). Westport, CT: The AVI Publishing Company, Inc.
Weisberg, S. M. (1954). Recent progress on the manufacture and use of lactose: A review. Journal of Dairy Science, 37, 1106–1115.
Whitter, E. O. (1944). Lactose and its utilization: A review. Journal of Dairy Science, 27, 505–537.
Whittier, E. O. (1925). Lactose: A review. Chemical Reviews, 2, 85–125.
Wong, S. Y., & Hartel, R. W. (2014). Crystallization in lactose refining. Journal of Food Science, 79(3), R257–R272.
Wong, N. P., Jenness, R., Kenny, M., & Marth, E. H. (1988). Fundamentals of dairy chemistry (3rd ed.). Westport, CT: The AVI Publishing Company, Inc.
Zadow, J. G. (1984). Lactose: Properties and uses. Journal of Dairy Science, 67, 2654–2679.
Zadow, J. G. (1992). Lactose and whey processing. London: Elsevier Applied Science.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jelen, P. (2022). Lactose: Occurrence, Properties, Reactions, and Significance. In: McSweeney, P.L.H., O'Mahony, J.A., Kelly, A.L. (eds) Advanced Dairy Chemistry. Springer, Cham. https://doi.org/10.1007/978-3-030-92585-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-92585-7_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92584-0
Online ISBN: 978-3-030-92585-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)