Abstract
Biological fermentation engineering is an important part of biological engineering. Carbohydrates are used to produce various industrial solvents and chemical raw materials by microorganisms, among which energy production through biotransformation is a very important research field. In this chapter, the common organic wastes, including typical rural solid wastes, forestry solid wastes, and naturally-grown aquatic plants such as duckweed, as well as urban waste are discussed as potential feedstock for liquid bioenergy including bioethanol, biobutanol, and bio-olefin. The pretreatment technology and fermentation modes of these wastes as well as the microbial species used are compared and discussed in depth. Duckweed was used as a typical example to evaluate the potential of producing ethanol, butanol, higher alcohols, and biodiesel via fermentation pathways. Moreover, the economic feasibility of producing liquid biofuel through fermentation from different waste feedstocks is evaluated. This chapter provides an important reference and insight for future research on organic waste fermentation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
The oil and natural gas resources suitable for extraction are only enough for 30 years, at most 50 years, and coal reserves are only enough for 300 years (Chen and Qiu 2007). Bioenergy from organic wastes is the only energy product that can replace petroleum fuels on a large scale. Hydro, wind, solar, nuclear, and other new sources of energy apply only to power and heat. Bioenergy products are diverse via fermentation. Energy products include liquid bioethanol and diesel, solid prototype and molding fuels, gaseous biogas, and other energy products. It can replace oil, coal, and natural gas, as well as heat and electricity generation. Bioenergy has diversity in raw materials. Biofuel can use crop straw, forest processing residues, livestock and poultry manure, organic wastewater residue of food processing industry, municipal waste, but also can use low-quality land to grow a variety of energy plants. Bioenergy has a variety of “material” applications, like oil and coal to produce plastic, fiber, and other materials as well as chemical raw materials and other material products, forming a huge biochemical production system. Bioenergy is “recyclable” and “environmentally friendly”. Biofuels are produced in the process of harmless and resource recovery of agricultural and rural organic wastes. All the life materials of biofuel can enter the biological cycle of the earth, and even the carbon dioxide released will be absorbed by plants again and participate in the cycle of the earth, so as to achieve zero emissions. The sustainability of material and the circulability of resources are a modern and advanced production mode. Biofuels can expand agricultural production, promote rural economic development and increase farmers’ income; It can also boost manufacturing, construction, automobile, and other industries. The development of biofuels in China and other countries can also promote agricultural industrialization and the development of small and medium-sized towns and narrow the gap between workers and farmers, which is of great political, economic and social significance.
Biofuel will increase the number of “crude oil” producing countries from 20 to 200. By producing fuel independently, it will suppress the price of imported oil and reduce the cost of imported oil, so that more funds can be used to improve people's lives and fundamentally solve the food crisis. Bioenergy can create jobs and build domestic markets. Brazil's experience shows that for every job in petrochemicals, 152 jobs are created in ethanol. The petrochemical industry invested $220,000 per job, while the fuel industry invested only $11,000. “The renewable energy industry will create 20.4 million jobs by 2030, including 12 million in biofuels,” according to the United Nations Environment Program’s “Green Jobs” report.
However, bioenergy exists in physical form and is the only renewable energy source that can be stored and transported. In addition, it is the most widely distributed, not limited by weather and natural conditions, wherever there is life there is biomass. The carrier source of bioenergy is usually organic waste. Organic waste is a misplaced resource for rural waste such as biomass straw and livestock manure. Forestry solid waste such as biomass waste from forestry. Municipal waste such as domestic waste, kitchen waste, urban sludge, pharmaceutical factory wastewater, etc. is used for fermentation to produce ethanol, butanol, and other renewable energy in different countries. For example, China is a major energy consumer, facing the pressure of traditional fossil energy depletion and carbon emission reduction, the development of renewable energy has become urgent. It has an important role and significance for China to adjust the energy structure and realize the resource utilization of waste.
Despite numerous biomass energy production, collection, and utilization research, many studies reported on the biomass, biomass utilization technology, including biomass production, collection, and processing technology, biomass energy and the environment, the biomass of anaerobic biogas, biomass to ethanol, and diesel oil producing technology, biomass direct combustion technology. However, fermentation methods, modes, and technologies from waste to energy generation include pretreatment, microbial types of energy production including bacteria and fungi, and energy types produced by fermentation including ethanol, butanol, olefin, and other energy sources. Of particular importance is the lack of systematic treatment of wastes (plant species) such as duckweed in the development and application of bioenergy. The purpose of this paper is to introduce systematically the research status of biomass energy fermentation technology, the types of energy, and the microbial species of waste energy fermentation, and the application of duckweed in biomass energy.
7.2 Fermentation Methods, Modes, and Techniques
7.2.1 Solid-State Fermentation
Solid-state fermentation is a microbial fermentation process in which the medium is in a solid state and the fermentation system is carried out in the absence of almost absence of free water present (Xu et al. 2002). Solid-state fermentation substrates are water-insoluble polymers, and the substrate is not only a site for microbial growth and development, but also provides the carbon source, nitrogen source, inorganic salts, water, and other nutrients needed for microbial growth. Compared with other culture methods, solid-state fermentation has the following advantages: (1) the medium is simple and widely available, mostly inexpensive natural substrates such as biomass straw, etc. (2) Low investment and low energy consumption. The technology is simple. (3) The yield of the product is high. (4) Low substrate water content, small bioreactor size, no subsequent wastewater treatment, environmentally friendly, post-treatment processing is relatively simple. (5) The fermentation process generally does not require an aseptic operation. (6) Continuous aeration is not required, and the air is generally not strictly sterile (Huang et al. 2003).
Solid-state fermentation is a multiphase system of gas, liquid, and solid phases as well as microorganisms (Thomas et al. 2013). There is almost no flowing free water in the solid-state fermentation substrate, the content of bound water is about 12–80% (mostly maintained at about 60%), and the oxygen required for microbial growth and development, and metabolism comes mainly from the gas phase of the continuous phase, with relatively low energy consumption. The history of solid-state fermentation can be traced back to thousands of years ago, and with the development of solid-phase fermentation technology, a variety of substances can be used as substrates for fermentation. Biomass feedstocks with more applications at this stage are mainly starchy feedstocks, sugar feedstocks, and lignocellulosic feedstocks. Starchy raw materials such as potatoes and grain grains, sugar raw materials such as sugar cane and sweet sorghum. But the use of these raw materials there is “competition with the people for food, and food for land” problem (Wang et al. 2018). Biomass feedstocks such as biomass straw do not have these problems and are the focus of global research on biological fermentation.
There are many factors that affect the solid-state fermentation process, mainly depending on the type of substrate, the type of microorganism, and the size of the production scale, based on the above conditions, which can be divided into biochemical, physicochemical, and environmental factors, all of which are closely related and cannot be viewed individually. (1) Microbial influence: fungi and bacteria are the microorganisms used more in solid-state fermentation, and relatively speaking inoculation of filamentous fungi is preferable because solid-state fermentation simulates the living environment of filamentous fungi (Soccol et al. 2017). Inoculation of fungal spores is more convenient and flexible, and easy to keep for a longer period of time, but also has the same disadvantages of long-term lag and large spore inoculum. (2) The effect of water and water activity: water is the main medium of solid-state fermentation, and changes in the water content of the substrate have an important impact on the growth and metabolic capacity of microorganisms. The high-water content will reduce the volume of gas in the substrate and the intensity of gas exchange, making it difficult to cool down and ventilate, and increasing the risk of contamination with miscellaneous bacteria. The low water content will inhibit the growth and metabolism of microorganisms and also cause the substrate to swell. The range of water content during solid-state fermentation should be controlled between 30 and 80%, and the amount of water control should be different for inoculating different microorganisms for fermentation. The ability of microorganisms to grow and metabolize on a substrate depends on the water activity (Aw) of that substrate.
For different microbial species, water activity is generally different for bacteria (0.90–0.99), most yeasts (0.80–0.90), and fungi (0.60–0.70). During solid-state fermentation, contamination by trash bacteria can be avoided because of the low water activity requirement of fungi (Li et al. 2011). (3) Substrate and particle size: the general substrate for solid-state fermentation is usually agricultural by-products such as lignocellulose, and these substrates have a macromolecular structure that will wrap the carbon and nitrogen sources, which is not conducive to fermentation, so some pretreatment should be carried out before fermentation. Mainly through physical, chemical, and biological pretreatment means to reduce particle size, release degradable substances and improve the efficiency of fermentation. (4) Temperature and pH: Microorganisms release large amounts of heat during growth and metabolism. A number of microbial life activities such as metabolism, protein synthesis, reproduction, etc. are sensitive to temperature, so this heat should be removed in time to avoid the impact on the growth and metabolism of the bacterium. pH is also one of the key factors affecting the growth and metabolism of microorganisms, and different species of microorganisms have different adaptation ranges for pH. The optimum pH range of fungi is 3.8–6.0, and the optimum pH range of yeast is 4.0–5.0. But the excellent buffering property of some materials in solid-state fermentation helps to reduce the need for pH control. Therefore, in solid-state fermentation, as long as the initial pH is adjusted to the desired value, the fermentation process usually does not need to detect and control pH. (5) Fermentation time: It is very important to control the fermentation time to improve the yield of target products. There is an optimal time phase for microbial fermentation. Too short a time is not enough to obtain the required yield, too long, because the environment has been unfavorable to the growth of bacteria, often resulting in autolysis of bacteria, yield decline. Therefore, the fermentation time should be determined according to the fermentation strain, fermentation process, fermentation products to conduct the corresponding experiments.
7.2.2 Liquid Fermentation
Liquid fermentation is the process of preparing the substrate into a liquid state and then inoculating the strain into the liquid substrate for biological reaction to prepare the product. The uniformity of heat and mass transfer in liquid fermentation is more advantageous than that in solid-state fermentation. Liquid fermentation has the advantages of high density, precise control, and high degree of automation, so industrial mass production is still dominated by liquid fermentation Fig. 7.1.
A large number of studies have been conducted by domestic and foreign scholars on the production of energy substances by liquid fermentation. Wen et al. explored the optimal fermentation conditions for in situ enzymatic saccharifications of rice straw by liquid fermentation using rice straw as substrate and Trichoderma reesei as enzyme-producing microorganism. The results showed that the optimal enzyme-producing fermentation conditions were at fermentation temperature of 30 ℃, initial pH 6.5, fermentation time of 24 h, and rice straw addition of 30 g/L. The specific sugar yield of straw at pH 4.8, enzymatic digestion temperature of 50 ℃, and enzymatic digestion time of 24 h can be as high as 0.350 g/g (Wen et al. 2014). It indicates that liquid enzymatic saccharification is an effective way to achieve resource utilization of biomass straw. Si et al. produced 2,3-butanediol by fermenting corn stover hydrolysate with Klebsiella Oxytoca ZU-03 at an initial pH point of 6.0 and fermentation temperature of 60 ℃ for 64 h. The sugar utilization reached 99.36% and the yield of 2,3-butanediol reached the theoretical 94% of the maximum yield of 0.468 g/g (Si and Xia 2010). Song et al. conducted a study on the production of cellulosic ethanol by fermentation of corn stover saccharate by Pachysolen tannophilus (P-01), and the volume fraction of ethanol was 2.05% under optimal reaction conditions, which was 33.17% higher than that of the control (Song et al. 2008). Sasaki et al. used xylose-assimilating Saccharomyces cerevisiae to ferment rice straw hydrolysate after membrane concentration, and ethanol yield of 5.34–6.44 g/L could be achieved after dilute acid pretreatment (Sasaki et al. 2013).
7.2.3 Simultaneous Saccharification and Fermentation
The general fermentation process of ethanol, butanol, and other biofuels can be divided into four steps: (1) physical, chemical, or biological pretreatment, (2) enzymatic hydrolysis, (3) microbial fermentation, (4) separation and concentration. Due to the different conditions of action of microorganisms and enzymes in the hydrolysis process and fermentation process, the traditional stepwise fermentation hydrolysis process and fermentation process are divided into two vessels of fermentation. The synchronous saccharification fermentation process synchronizes hydrolysis and fermentation process in one fermentation vessel, which occupies less space, shorter fermentation time, and higher yield compared with the traditional fermentation method. It is the most studied fermentation method at present.
Liu considered synchronous saccharification fermentation as a promising process for biotransformation of lignin biomass and studied the effect of synchronous saccharification fermentation of corn stover for ethanol production after steam blast pretreatment, with ethanol yield and final ethanol concentration of 77.2% and 59.8 g/L, respectively, under optimal reaction conditions (Liu et al. 2016). Du et al. investigated high temperature brewer's yeast using corn stover as a raw material for synchronous saccharification. The optimal conditions for ethanol production were obtained as 7.4% inoculum, 34.2 ℃ temperature, 5.0 initial pH, and 49.36 U/g enzyme concentration, and the ethanol yield was 59.88% at 150.12 h of fermentation under optimal fermentation conditions (Du et al. 2016). The effect of conditions on ethanol production by simultaneous saccharification fermentation of wheat straw was investigated and found that the concentration of ethanol reached a maximum of 38.32 g/L after 120 h of fermentation at 38 ℃, 16.0% solids content, 35 FPU/g cellulase dosing, and 8 g/L yeast concentration, with a yield of 71.71% of the theoretical differential rate (Zhang et al. 2012). Saha et al. used wheat straw as raw material and The maximum ethanol yield was 36.0 g/L and 0.43 g/L at pH 6.0 and fermentation temperature 35 ℃ for 83 h (Saha et al. 2015). Lin et al. investigated the simultaneous saccharification fermentation of wheat straw at an initial pH of 4.6, enzyme addition of 30 FPU/g and temperature of 37.5 ℃, and the maximum ethanol. The maximum ethanol yield could reach 70.76% at an initial pH of 4.6, enzyme addition of 30 FPU/g and temperature of 37.5 ℃ (Zhang et al. 2013). Synchronous saccharification fermentation can be applied not only to the production of alcohols from biomass straw but also to the production of biofuels from other wastes. Zhang et al. conducted research work on the production of ethanol from synchronous saccharification fermentation of kitchen waste. The results showed that the optimal reaction conditions were glycosylase addition concentration of 100 U/g, protease addition of 150 U/g, cellulase of 100 U/g, pH 5.3, and the concentration of ethanol could reach 54.6 g/L after 100 h of fermentation time (Zhang et al. 2015). Yan et al. investigated the induction of cellulase by cow manure and the possibility of converting cow manure feedstock into bioethanol by simultaneous saccharification and fermentation of ethanol yield of 25.65 g/L (Yan et al. 2018).
7.2.4 Pretreatment Techniques
Pretreatment technologies can be classified according to their nature as physical, chemical, combined physical and chemical methods, and biological methods. Lignocellulosic raw materials such as biomass straw, forestry waste, municipal waste, and other wastes such as livestock manure, municipal sludge, and pharmaceutical plant wastewater can be used to ferment biomass for fuel production due to their high organic matter content.
China is a largely agricultural country that produces a large amount of biomass straw every year, and the national production of biomass straw was 816 million t in 2016, of which corn straw was the largest, accounting for 36.88% of the total (Zhang et al. 2018). The use of biomass straw to produce biofuels such as ethanol and butanol has attracted increasing interest from researchers due to its wide source and high economic efficiency. The lignocellulosic feedstock has a complex structure, with hemicellulose and lignin intertwined and covering the wood surface to form a dense structure, making it impossible for cellulase to act directly with the cellulose. The component content of the main biomass straws is shown in Table 7.1 (Wang 2015). Direct fermentation yield is low, so pretreatment is performed to destroy the dense structure formed by cellulose, lignin, and hemicellulose. For lignocellulosic materials, the main physical pretreatment techniques are mechanical crushing, ultrasonic pretreatment, microwave method, high energy radiation, and liquid hydrothermal method. Chemical pretreatment technology mainly includes acid hydrolysis method, alkali treatment method, organic solvent method, wet oxidation method, etc. Physicochemical methods mainly include steam blasting method, ammonia fiber blasting method, etc. The biological method mainly uses certain microorganisms for pretreatment.
Chen et al. investigated the effect of irradiation pretreatment of rice straw on the production of ethanol by enzymatic saccharification and fermentation, and the results showed that there was an increase in cellulose conversion and ethanol conversion after irradiation pretreatment compared to the control group (Chen et al. 2015). Sun et al. conducted a study on hydrothermal pretreatment of corn straw and found that 56.08% hemicellulose was leached after hydrothermal pretreatment, which showed that hydrothermal pretreatment destroyed the dense structure of lignocellulosic (Sun et al. 2019). Lian et al. determined the optimal reaction conditions of enzymatic digestion time of 9 h, fermentation time of 7 d, and fermentation temperature of 34 ℃ by pretreating corn stover with hydrogen peroxide to produce fuel ethanol using semi-synchronous saccharification fermentation. Under the optimal conditions, ethanol conversion reached 76.54% and ethanol concentration reached 23.64 g/L (Lian et al. 2018). Kim et al. explored the effect on ethanol production using nitric acid pretreatment of rice straw. The results showed that 0.65% nitric acid at 158.8 ℃ and 5.86 min reaction time enzymatic digestion was best with a digestibility rate of 83.0% and ethanol yield increased from 10.92 to 14.50 g/L (Kim et al. 2014). Amiri et al. conducted a study on the production of ethanol, butanol, and acetone by pretreatment of rice straw with organic solvents and found that pretreatment at 180 ℃ gave a glucose yield of 46.2%, after fermentation, 22.5 g of ethanol, 22.1 g of propanol, and 80.3 g of butanol could be obtained (Amiri et al. 2014). Kuamr et al. used green solvent (GS) pretreatment of rice straw as raw material to produce cellulosic ethanol, and the results showed that the saccharification efficiency after green solvent pretreatment was as high as 87.1%, and the maximum yield of reducing sugar was 226.7 g/L. After 36 h of fermentation, 36.7 g/L of ethanol could be obtained yield, with a conversion rate of 90.1% (Kumar et al. 2016). Arora considered biological pretreatment as a green pretreatment method and it used white-rot fungus, Trametes hirsuta pretreatment of rice straw and compared it with steam blast pretreatment to investigate the effect on the production of bioethanol. It was found that the lignin removal rate of the biological pretreatment was higher than that of the steam blasting pretreatment, and the ethanol yields after biological pretreatment and steam blasting pretreatment were 0.86 g/L and 1.13 g/L, respectively, which showed that the ethanol yield of the steam blasting pretreatment was better than that of the biological pretreatment (Arora et al. 2016). It is evident that lignocellulosic feedstocks, after pretreatment, are able to increase the ethanol yield and production rate.
Lignocellulosic material is considered an effective feedstock for bioethanol and butanol production, but other wastes also have some potential for biofuel production. The production of biofuels from other biomass wastes and the pretreatment methods have been studied by domestic and foreign scholars. Liu et al. used campus kitchen waste as raw material and fermented it with brewer's yeast after pretreatment using Rhizobium and Bacillus subtilis for ethanol production. It was found that the maximum yield of ethanol could reach 6.67% under optimal fermentation conditions with 22.79% substrate water content, 15% brewer's yeast inoculum, 30 ℃, and 5 d fermentation time (Liu et al. 2013). Woldesenbet et al. used different concentrations of dilute sulfuric acid (0.2, 0.4, 0.6, 0.8, and 1.0 mol/L) to pretreat livestock manure to investigate livestock manure production The potential of bioethanol was investigated and it was shown that pretreatment of chicken manure with 0.8 mol/L released more reducing sugars with a maximum ethanol yield of 50 g/L (Woldesenbet et al. 2013). Ji conducted a study on the preparation of fuel ethanol from MSW and the ethanol yield per gram of dry kitchen waste was 0.255 mL/g (Ji 2014).
7.3 Types of Energy from Fermentation
In recent years, with the rapid growth of the global economy, the world’s energy consumption has increased significantly. In the face of the increasing depletion of traditional fossil energy sources such as coal, oil, and natural gas, the increasingly serious environmental pollution, and the greenhouse effect caused by the massive emission of greenhouse gases, the energy issue has become one of the most important problems plaguing countries around the world. The development of new renewable energy sources such as ethanol, butanol, olefins, etc. has received increasing attention from all over the world Fig. 7.2.
7.3.1 Ethanol
Ethanol with molecular formula C2H6O, commonly known as alcohol, is a flammable, volatile, colorless, and transparent liquid at room temperature and pressure. Fuel ethanol generally refers to anhydrous ethanol with a volume fraction of 99.5% or more, which is a good octane blending component and gasoline oxygenating agent. Ethanol gasoline can effectively reduce the emission of PM2.5 and CO in automobile exhaust and can supplement fossil fuel resources, which has important research significance and value for reducing the foreign dependence on petroleum resources, reducing greenhouse gas emissions, and environmental pollution.
Since the 1970s, biofuel ethanol has been extensively researched around the world as a vehicle fuel, and ethanol is considered one of the most important renewable fuels of the future. After decades of development fuel ethanol is now globally recognized as the most mature alternative fuel to gasoline. Several countries around the world have spared no effort to develop bioethanol and use ethanol as an additive or fuel alternative to petroleum, and have developed a series of supportive policies. The United States and Brazil have the largest fuel ethanol applications in the world, accounting for more than 10% and 50% of their gasoline fuel consumption, respectively. China's current fuel ethanol usage accounts for more than 2.1% of gasoline usage, with huge room for development. The development of renewable bio-liquid fuels has a very important role in reducing the dependence on fossil fuels, reducing greenhouse gas emissions, and activating the rural economy in China (Guo et al. 2016). The current first generation fuel ethanol, which uses food as the main raw material, has been gradually abandoned due to high cost and endangering land and food security. Second-generation fuel ethanol technologies based on non-food feedstocks such as waste, straw, and algae are being actively developed (Li et al. 2013).
Lignocellulose is the most abundant renewable resource on earth, and the production of bioethanol from lignocellulosic feedstocks has good prospects for development. The development of bioenergy from lignocellulosic feedstock by modern biotechnological means has become an important part of the energy development strategy of major countries in the world. Khaleghian et al. studied the production of bioethanol from rice straw by simultaneous saccharification and fermentation of the straw after pretreatment with sodium carbonate, and the results showed that the enzymatic hydrolysis yield was up to 100% and the ethanol yield was increased by more than 40% (Khaleghian et al. 2015). Phitsuwan et al. conducted a study on the production of ethanol from rice straw, which showed that rice straw is a promising biomass feedstock for ethanol production (Phitsuwan et al. 2016). Suriyachai et al. optimized the process of ethanol production from rice straw using Simultaneous saccharification and co-fermentation (SSCF) fermentation, and the optimized ethanol yield could reach a maximum of 15.2 g/L and ethanol concentration up to 28.6 g/L (Suriyachai et al. 2013). Wu et al. conducted a study on ethanol production from biomass straw treated with Trichoderma reesei Aq-5b and Trichoderma viride NSW-XM and found that pretreatment greatly improved the ethanol production efficiency and shortened the fermentation cycle, and the ethanol yield was as high as 2.17 g/(L h) after pretreatment (Wu et al. 2016).
Although the cellulose ethanol production technology is maturing and entering the industrial demonstration process, there is still room for improvement in pretreatment, enzyme preparation and enzymatic process, pentose/hexose co-fermentation strains and process, and equipment and equipment.
7.3.2 Butanol
Butanol is a colorless and transparent liquid with the molecular formula C4H9OH, has a special odor, is slightly soluble in water, and is miscible in any ratio with various organic solvents such as ethanol and ether. Although bioethanol is generally favored as a gasoline blending component, the development of its application is somewhat limited due to its low energy density, high vapor pressure, and corrosive transport pipelines. The main properties of ethanol and butanol are shown in Table 7.2 (Liu et al. 2008). Compared with bioethanol, biobutanol has the advantages of high energy density and fuel calorific value, miscibility with gasoline in any ratio, and low corrosiveness for pipeline transportation (Gao et al. 2018). After bioethanol, biobutanol has become a hot spot for research and development of a new generation of renewable energy.
Many countries around the world are vigorously developing biobutanol technology in the face of the energy and resource revolution. The U.S. biobutanol fuel project has been put into operation in 2009. The UK has already accounted for about 10% of the market share of biofuels in 2015. China's research on biobutanol has also made substantial progress. Wang conducted a study on the production of biobutanol from corn stover, using 2% NaOH, 1.5% H2SO4, steam blasting, and steam blasting in combination with 2% NaOH and 1.5% H2SO4 to pretreat corn stover to investigate the effect of enzymatic hydrolysis and the effect of butanol yield. The results showed that 2% NaOH pretreatment was the most effective, with a high lignin removal rate of 81.7% and enzymatic hydrolysis rate of 70.5% for corn stover. After ABE fermentation, the butanol concentration could reach 212.0–232.0 g/L (Wang 2016). Tian conducted a study on the production of butanol by hydrolysis and fermentation of corn stover and studied the intention of citric acid-sodium citrate instead of traditional acetic acid-sodium acetate as a buffer for enzymatic hydrolysis of corn stover pretreatment, and the experiment proved that the concentration of summary reducing sugar in the hydrolysate was 14% higher, and through UV mutagenesis, a mutant strain CM20 was used to ferment the hydrolysate with a butanol yield of 10.8 g/L (Tian 2015). Boonsombuti et al. studied the effects of ionic liquids, acids, and bases on the production of butanol after pretreatment of rice straw, respectively, and the results showed that the alkali treatment was more effective and that isobutanol appeared in the NaOH pretreated fermentation broth, indicating that isobutanol can be produced under suitable conditions (Boonsombuti et al. 2020). Moradi et al. similarly studied the effect of NaOH and concentrated phosphoric acid pretreatment of rice straw for butanol production and the results showed that 163.5 and 44.2 g of butanol per kg of substrate was hydrolyzed after alkali pretreatment with glucose. After concentrated phosphoric acid pretreatment, 192.3 g of glucose, as well as 44.2 g of butanol per kg of substrate, was hydrolyzed (Moradi et al. 2013).
Lignocellulosic feedstock is a hot spot for research on the production of biobutanol, and other wastes such as kitchen waste have received some attention and research due to their high organic matter content as a potentially cheap and high-quality biomass resource. Zhang et al. conducted a study on the direct use of strain an amylolytic Clostridium sp. strain BOH3 for the production of butanol from kitchen waste without pretreatment. The strain BOH3 was found to have the gene encoding amylase and could produce 14.1 g/L of butanol from food waste (Zhang et al. 2020). The butanol production process is affected by various factors such as pH. Shi et al. conducted a study on the fermentation of kitchen waste by Clostridium beijerinckii NCIMB 8052 for the production of biobutanol and showed that the butanol yield was only 5.96 g/L without the addition of any nutrients, and the addition of pH buffer increased the butanol yield and Wang et al. investigated the feasibility of direct butanol fermentation from kitchen waste without saccharification and achieved a butanol yield of 12.1 g/L and a maximum production rate of 0.725 g/(L h) at a solid-to-liquid ratio of 1:1 (Wang et al. 2019).
7.4 Plants
The source and cost of biomass feedstock are one of the major constraints on the development of bioenergy, so many scientists are focusing on lignocellulosic feedstock, which is cheaper and more widely available. Han et al. determined the fiber composition and the potential for ethanol production in reed, kelp, and shore moss 3. Ethanol fermentation experiments were conducted on reed with high cellulose content and controlled with straw, and the ethanol content was 0.43–0.47% higher than that of straw at 0.29–0.31% under the same conditions of fermentation for 20 h (Han et al. 2019).
It is evident that reed, kelp, and shorea feedstocks have great potential for ethanol production. Li explored the feasibility of kelp for biofuel production using enzymatic saccharification technology and fermentation of ethanol and butanol, and the results showed that ethanol and butanol of 0.58% (V/V) by volume and 0.34% (V/V) by volume, respectively, could be obtained after fermentation (Li 2013). Zhou et al. investigated the feasibility of ethanol production by fructose-based fermentation of the energy plant inulin as the sole carbon source and showed that bioethanol production from inulin could be well converted using Saccharomyces cerevisiae strain BY47442, with no significant difference compared to ethanol production using glucose or fructose as substrates (Zhou et al. 2008). Yang likewise conducted a study on the production of ethanol by simultaneous saccharification fermentation of inulin and showed that ethanol fermentation using Saccharomyces cerevisiae strain Y05 resulted in ethanol concentrations up to 71.18 g/L (Yang 2010). Sornvoraweat et al. conducted a study on the production of bioethanol using water hyacinth as a raw material for isolated hydrolytic fermentation (SHF) and obtained an ethanol yield of 0.25 g/g at a concentration of 3.39 g/L under optimal fermentation conditions (Sornvoraweat et al. 2010).
7.4.1 Biodiversity of Floating Duckweed
Duckweed, a floating aquatic plant, consists of about 40 species in five genera, namely, Spirodela, Landoltia, Lemna, Wolffiella, Wolffia, among which Duckweed is the smallest flowering plant in the world. Duckweed is widely distributed in various freshwater environments, such as lakes, ponds, and rice paddies. The duckweed plant has a simple structure, which is composed of phylloids and pseudoroots (Spirodeka, Landoltia, Lemna) or only phylloids (Wolffiella, Wolffia). It is easy to culture and fast to grow asexually (the biomass doubles about 24 h) (Yang et al. 2021). Studies have shown that the starch content in duckweed can be controlled by controlling the growth conditions, such as pH of culture medium and phosphate concentration (Cui and Cheng 2015). Due to the high biomass accumulation, short reproduction cycle, and rich organic matter of duckweed, duckweed is a high-quality raw material for the development of biomass energy such as ethanol, butanol, and biogas Fig. 7.3.
7.4.2 Floating Ethanol
With the increasing scarcity of fossil energy and the increasing ecological load, countries around the world are successively taking alternative energy sources as an important energy policy to achieve sustainable development. Lignocellulosic fermentation for ethanol production is considered as one of the most mature processes for biofuel production, but the dense structure of lignin and hemicellulose composition of lignocellulosic feedstock and pretreatment technology become one of the main factors limiting its development, leading to high costs.
Aquatic plants grow without taking up land, grow faster compared to terrestrial plants, and have a high content of starch as well as cellulose components that can be converted into fermentable sugars, making them potential feedstocks for raw fuel ethanol (Xue et al. 2013). Aquatic puffballs are fast-growing plants, rich in starch and cellulose, and have become a research hotspot for the fermentation of bioethanol production due to their excellent biochemical properties. Xu et al. concluded that floating duckweed has a starch content of 31–45.8% (dry weight) and after fermentation, can convert up to 94.7% of starch to ethanol, which is much higher than the ethanol yield of most other potential crops (Xu et al. 2012). Lee et al. conducted a comparative study of ethanol production from floating duckweed and corn starch and showed that floating duckweed plants have a lower ethanol yield than corn starch, but a higher ethanol conversion rate (Lee et al. 2016). Xu et al. conducted a study on the conversion of high amylose floating duckweed to bioethanol and found that after enzymatic fermentation by yeast, the ethanol conversion was 94.7% of the theoretical yield, which was higher than about 50% of the ethanol produced with corn as substrate (Xu et al. 2011). Calicioglu and Brennan conducted a study on continuous fermentation of floating duckweed for ethanol and methane production and found that the combined bioethanol-biomethane fermentation process obtained 70.4% more bioenergy from floating duckweed compared to fermentation alone (Calicioglu and Brennan 2018).
As floating duckweed contains lignocellulose, pretreatment is required to destroy the structure of lignocellulose, increase the accessibility of enzymes, and improve the yield and efficiency of ethanol. Zhao et al. pretreated floating duckweed by steam blasting and later produced ethanol using simultaneous saccharification fermentation technique, and found that the ethanol yield was 70% higher than that of the unpretreated control (Zhao et al. 2015). Fontinelle Souto conducted a hydrothermal pretreatment followed by simultaneous saccharification fermentation after hydrothermal pretreatment and found that ethanol yield reached 88.81% of the theoretical yield under optimal conditions pretreatment conditions (200 ℃, 10 min) (Fontinelle Souto et al. 2019). Chen et al. investigated the effect of pectinase pretreatment of floating duckweed on bioethanol yield and found that the maximum glucose yield after pretreatment was 218.64 ± 3.10 mg/g and ethanol concentration was 30.8 ± 0.8 g/L with a yield of 2.20 g/(L h) (Chen et al. 2012).
7.4.3 Duckweed Butanol
Butanol is a clean and efficient new energy source. The production of butanol by biological fermentation is a research hotspot in recent years. At present, the key factor restricting the development of biobutanol industry is its economy, so it is very important to choose raw materials with wide sources and good economy easily. Duckweed is a non-food aquatic plant with high organic matter content and its source has been widely concerned.
Long carried out a study on high-starch duckweed butanol fermentation and found that the duckweed fermentation with an initial sugar concentration of 60 g/L could yield 11.65 g/L butanol, which was 99.57% of corn butanol fermentation yield and 98.9% of cassava butanol fermentation yield (Long, 2012). It can be seen that duckweed is an effective substitute for food crops to produce butanol by fermentation of aquatic plants.
7.4.4 Duckweed Advanced Alcohol
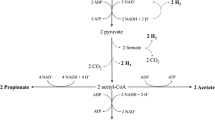
Facing the energy crisis, it is necessary to develop new alternative energy sources. Ethanol is considered an ideal biofuel to replace petroleum, but bioethanol has some shortcomings such as low calorific value and certain corrosivity. Ethanol renewable alcohol obtained by biological fermentation can be used as an effective substitute for ethanol. Su et al. conducted a study on the production of biofuel advanced alcohols by duckweed fermentation Fig. 7.4 and found that 12.03 g/L butanol could be produced by C. acetobutylicum CPCC 8012. In addition, 16.24 mg/L butanol, 4.68 mg/L isoamyl alcohol, and 198.85 mg/L amyl alcohol can be produced by fermentation of Escherichia coli bioengineered strains (Su et al. 2014).
SC: Saccharomyces cerevisiae AH109; EC: Escherichia coli; CA: Clostridium acetobutylicum CICC 8012. PFM: fermentation substrate (duckweed) for acid hydrolysate was filtered with a 0.22-micron membrane. NFM: the fermentation substrate without filtration. EP: Pretreatment with enzymatic hydrolysis. AP: Pretreatment with acid hydrolysis. AHD: acid hydrolysate of duckweed as the fermentation substrate, meaning the products resulting from pretreatment of duckweed with acid hydrolysis. EHD: enzymatic hydrolysate as the fermentation substrate, meaning the products resulting from pretreatment of duckweed with enzymatic hydrolysis.
7.5 Conclusions
There are various technical methods for fermentation to produce energy, and simultaneous saccharification fermentation technology is the most applied fermentation technology, and the screening of efficient strains of homosaccharide fermentation and the optimization of the process is the focus of future research. The fermentation of biomass straw to produce biomass energy is an effective way to alleviate the depletion of fossil energy and realize the resource utilization of waste, but because biomass straw is rich in lignin and cellulose, pretreatment is required for fermentation. Therefore, research on new efficient pretreatment means and development of new fermentation processes are hot spots for future research.
With its rich biodiversity and fast growth rate rich in starch and cellulose, duckweed is a good potential feedstock for biofuel production. The application of biotechnology and a deeper understanding of the microscopic fermentation process will bring a technological revolution in the production of biofuels from floating duckweed. In addition, the development of an efficient and clean production process of floating pondweed as well as pretreatment technology is a hot spot for future research on floating pondweed produced fuels.
References
Amiri H, Karimi K, Zilouei H (2014) Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Biores Technol 152:450–456
Arora A, Priya S, Sharma P, Sharma S, Nain L (2016) Evaluating biological pretreatment as a feasible methodology for ethanol production from paddy straw. Biocatal Agric Biotechnol 8:66–72
Boonsombuti A, Trisinsub O, Luengnaruemitchai A (2020) Comparative study of three chemical pretreatments and their effects on the structural changes of rice straw and butanol production. Waste Biomass Valorization 11:2771–2781
Calicioglu O, Brennan RA (2018) Sequential ethanol fermentation and anaerobic digestion increases bioenergy yields from duckweed. Biores Technol 257:344–348
Chen H, Qiu W (2007) The crucial problems and recent advance on producing fuel alcohol by fermentation of straw. Prog Chem Z2:1116–1121 (in Chinese)
Chen L, Su X, Xiong X, Chen J, Wu X, Hu Q, Zhang Y, Wang K (2015) Enzymatic saccharification and fermentation of irradiated rice straw for ethanol production. Acta Nucifera Sin 29(04):704–709 (in Chinese)
Chen Q, Jin Y, Zhang G, Fang Y, Xiao Y, Zhao H (2012) Improving production of bioethanol from duckweed (Landoltia punctata) by pectinase pretreatment. Energies 5:3019–3032
Cui W, Cheng JJ (2015) Growing duckweed for biofuel production: a review. Plant Biol 17:16–23
Du J, Bu W, Hu X, Wang Y (2016) Experimental study on ethanol production by simultaneous saccharization of corn straw. Acta Energiae Solaris Sin 37(09):2251–2256 (in Chinese)
Fontinelle Souto LR, da Silva IF, Ninow JL, Collins SRA, Elliston A, Waldron KW (2019) Effect of hydrothermal pre-treatment on duckweed (Landoltia punctata) biomass for simultaneous saccharification and fermentation process. Biomass Bioenergy 127
Gao Y, Guo X, Yang Y, Zhang M, Li W, Lu D (2018) Research progress of biobutanol fermentation. Biotechnol Bull 34(08):27–34 (in Chinese)
Guo X, Luo H, Deng L (2016) Progress of global fuel ethanol industry. Contem Chem Ind 45(09):2244–2248 (in Chinese)
Han W, Ming K, Ma Y, Lv J, Zhang B (2019) Study on the possibility of several coastal plants as biomass materials of producing ethanol. Anhui Agric Sci 47(05):77–79 (in Chinese)
Huang D, Wu Q, Lu J, Guan G (2003) Current research progress of solid state fermentation technology and equipment. Food Ferment Ind 06:87–91(in Chinese)
Ji L (2014) Study on fuel ethanol production from municiple solid wastes. Beijing Univ Chem Technol (in Chinese)
Khaleghian H, Karimi K, Behzad T (2015) Ethanol production from rice straw by sodium carbonate pretreatment and Mucor hiemalis fermentation. Ind Crops Prod 76:1079–1085
Kim I, Lee B, Park JY, Choi S-A, Han J-I (2014) Effect of nitric acid on pretreatment and fermentation for enhancing ethanol production of rice straw. Carbohyd Polym 99:563–567
Kumar AK, Parikh BS, Shah E, Liu LZ, Cotta MA (2016) Cellulosic ethanol production from green solvent-pretreated rice straw. Biocatal Agric Biotechnol 7:14–23
Lee CJ, Yangcheng H, Cheng JJ, Jane JL (2016) Starch characterization and ethanol production of duckweed and corn kernel (vol 67, pp 348, 2015). Starch-Starke 68:581–581
Li F (2013) The explored research of fuel ethanol and butanol production from Laminaria japonica fermentation. Guangxi Univ Sci Technol (in Chinese)
Li L, Yang X, Xue Y (2011) Research progress of modern solid state fermentation technology, equipment and application. J Henan Univ Technol (Nat Sci Edn) 32(01):89–94 (in Chinese)
Li Z, Li D, Huang G, Wei H (2013) Insights on current development of fuel ethanol. Prog Chem Indus 32(07):1457–1467 (in Chinese)
Lian Z, LV Z, Liu B, Liu X, Li J, Tian B, Zhuang Q, Liu T (2018) Study on alkaline pretreatment and semi synchronous saccharification and fermentation of high concentration corn straw to produce ethanol. Food Ferment Indus 44(10):124–129 (in Chinese)
Liu A, Xu S, Cai X, Peng P, Lu C (2013) Fuel ethanol production from food waste by being mixed microbical fermentation. Chin J Environ Eng 7(02):727–731 (in Chinese)
Liu Y, Liu H, Zhang J, Cheng K, Chen Z (2008) Research progress in new biofuel butanol. Mod Chem Indus 06:28–31 + 33 (in Chinese)
Liu Z, Chen H (2016) Simultaneous saccharification and co-fermentation for improving the xylose utilization of team exploded corn stover at high solid loading. Bioresour Technol 201:15–26
Liu ZH, Qin L, Zhu JQ et al (2016) Simultaneous saccharification and fermentation of steam-exploded corn stover at high glucan loading and high temperature. Biotechnol Biofuels 7
Long F (2012) Study on butanol conversion technology of Canna edulis and duckweed. Univ Chin Acad Sci (in Chinese)
Moradi F, Amiri H, Soleimanian-Zad S, Ehsani MR, Karimi K (2013) Improvement of acetone, butanol and ethanol production from rice straw by acid and alkaline pretreatments. Fuel 112:8–13
Phitsuwan P, Permsriburasuk C, Waeonukul R, Pason P, Tachaapaikoon C, Ratanakhanokchai K (2016) Evaluation of fuel ethanol production from aqueous ammonia-treated rice straw via simultaneous saccharification and fermentation. Biomass Bioenerg 93:150–157
Saha BC, Nichols NN, Qureshi N, Kennedy GJ, Iten LB, Cotta MA (2015) Pilot scale conversion of wheat straw to ethanol via simultaneous saccharification and fermentation. Biores Technol 175:17–22
Sasaki K, Sasaki D, Sakihama Y, Teramura H, Yamada R, Hasunuma T, Ogino C, Kondo A (2013) Ethanol fermentation by xylose-assimilating Saccharomyces cerevisiae using sugars in a rice straw liquid hydrolysate concentrated by nanofiltration. Biores Technol 147:84–88
Si Y, Xia L (2010) 2, 3-butanediol was produced by fermentation of corn straw hydrolysate. Food Ferment Indus 36(02):26–29 (in Chinese)
Soccol CR, da Costa ESF, Letti LAJ, Karp SG, Woiciechowski AL, de Vandenberghe LPS (2017) Recent developments and innovations in solid state fermentation. Biotechnol Res Innov 1(1):52–71
Song A, Liu Y, Dong Y, Du F (2008) Optimization of fiber fuel system for corn straw saccharification liquid fermentation. Food Ferment Ind 34(12):65–68 (in Chinese)
Sornvoraweat B, Kongkiattikajorn J (2010) Separated hydrolysis and fermentation of water hyacinth leaves for ethanol production. Asia-Pacific J Sci Technol 15(9):794–802
Sornvoraweat B, Kongkiattikajorn J, Kasetsart U (2010) Development of separate hydrolysis and fermentation of water hyacinth for ethanol production, 48th Kasetsart University Annual Conference, Thailand, pp 268–275
Su H, Zhao Y, Jiang J, Lu Q, Li Q, Luo Y, Zhao H, Wang M (2014) Use of duckweed (Landoltia punctata) as a fermentation substrate for the production of higher alcohols as biofuels. Energy Fuels 28:3206–3216
Sun M, Wang G, Han X, Zhang C (2019) Effect of hydrothermal pretreatment on characteristics of corn straw hydrolysate. Acta Solar Energy Sin 40(06):1656–1663 (in Chinese)
Suriyachai N, Weerasaia K, Laosiripojana N, Champreda V, Unrean P (2013) Optimized simultaneous saccharification and co-fermentation of rice straw for ethanol production by Saccharomyces cerevisiae and Scheffersomyces stipitis co-culture using design of experiments. Biores Technol 142:171–178
Thomas L, Larroche C, Pandey A (2013) Current developments in solid-state fermentation. Biochem Eng J 146–161
Tian L (2015) Study on butanol fermentation using corn straw hydrolysate as substrate. Qingdao University (in Chinese)
Wang L (2015) Techno-economical ananlysis of cellulosic ethanol production process by different pretreatments. Dalian University of technology (in Chinese)
Wang L, Zhao Q, Chen H (2018) Research progress in solid state fermentation of bio-energy. Biotechnology 03:41–44 (in Chinese)
Wang S (2016) Biobutanol production from corn stover and product separation. Dalian Univ Technol (in Chinese)
Wang Y, Gao M, Bodjui Olivier A, Wang F, Wang Q (2019) Bio-Butanol production from direct fermenation of kitchen wastes. Environ Eng 37(04):137–142 (in Chinese)
Wen X, Bai G, Li T, Ma Y, Zou W (2014) Optimization of in situ enzymatic hydrolysis for saccharification of rice straw by liquid fermentation. Food Ferment Ind 47(04):166–172+181 (in Chinese)
Woldesenbet AG, Gizachew S, Bhagwan SC (2013) Bio-ethanol production from poultry manure at Bonga Poultry Farm in Ethiopia. Afr J Environ Sci Technol 7(6):435–440
Wu X, Zhang J, Xu E, Liu Y, Cheng Y, Addy M, Zhou W, Griffith R, Chen P, Ruan R (2016) Microbial hydrolysis and fermentation of rice straw for ethanol production. Fuel 180:679–686
Xu F, Chen H, Li Z (2002) New developments in engineering aspects of solid-state fermentation. Prog Biotechnol 01:44–48 (in Chinese)
Xu J, Cui W, Cheng JJ, Stomp A-M (2011) Production of high-starch duckweed and its conversion to bioethanol. Biosys Eng 110:67–72
Xu J, Zhao H, Stomp A-M, Cheng J (2012) The production of duckweed as a source of biofuels. Biofuels 3(5):589–601
Xue H, Dong Z, Fang Y, Jin Y, Zhao H (2013) Producing fuel ethanol from energy hygrophyte duckweed. Renew Energy 31(07):55–59 (in Chinese)
Yan Q, Liu X, Wang Y, Li H, Li Z, Zhou L, Qu Y, Li Z, Bao X Cow manure as a lignocellulosic substrate for fungal cellulase expression and bioethanol production. Amb Express
Yang J, Zhao X, Li G, Hu S, Chen Y, Sun Z, Hou H (2021) Research and application in duckweeds: a review. Sci Bull 66(09):1026–1045 (in Chinese)
Yang L (2010) (in Chinese) Study on simultaneous saccharification and fermentation of Jerusalem artichoke to produce fuel ethanol. Hebei Univ Sci Technol
Zhang C, Li T, Su G, HE J (2020) Enhanced direct fermentation from food waste to butanol and hydrogen by an amylolytic Clostridium. Renew Energy 153:522–529
Zhang W, Li W, Zhao J, Lin Y, Peng Z, Wang X (2012) Fuel ethanol was prepared by simultaneous saccharification and fermentation of wheat straw. Food Ferment Ind 38(12):50–54 (in Chinese)
Zhang W, Lin Y, Zhang Q, Wang X, Wu D, Kong H (2013) Optimisation of simultaneous saccharification and fermentation of wheat straw for ethanol production. Fuel 112:331–337
Zhang Y, Chen X, Gu Y, Zhou X (2015) A physicochemical method for increasing methane production from rice straw: extrusion combined with alkali pretreatment. Appl Energy 160:39–48
Zhang Y, Guo D, Wang K, Wu X, Qi H, Deng M, Chen L (2018) Fermentation for ethanol production with γ ray irradiation-pretreated corn straw. Brewed China 37(09):58–61 (in Chinese)
Zhao X, Moates GK, Elliston A, Wilson DR, Coleman MJ, Waldron KW (2015) Simultaneous saccharification and fermentation of steam exploded duckweed: improvement of the ethanol yield by increasing yeast titre. Biores Technol 194:263–269
Zhou Z, Cao H, Zhu Y, Li S, Bai X, Zhao X, Du Y (2008) The primary study of ethanol production by fermenting of jerusalem artichoke instead of corn. J Northwest Agric 04:297–301 + 305 (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lin, J., Zhang, Y., Song, T., Su, H. (2022). Waste Fermentation for Energy Recovery. In: Abomohra, A.EF., Wang, Q., Huang, J. (eds) Waste-to-Energy. Springer, Cham. https://doi.org/10.1007/978-3-030-91570-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-91570-4_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91569-8
Online ISBN: 978-3-030-91570-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)