Abstract
Fish inhabit microbial- and parasite-enriched environments that constrict the adaptive choices and evolutionary pathways of regulatory physiological systems. Teleost immune architecture reflects those selective pressures, and the modulation of immune performance by glucocorticoids and other mediators of perceived stress have sculpted the type, intensity and scope of stress-related immunity in fish. In this chapter, the immune response to stress in teleosts and the role of the principal mediators such as hormones, cytokines and neurotransmitters are reviewed. The interactions between the immune, endocrine and neural regulatory systems and the influence of the local environment in the response are also considered.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
20.1 Introduction
Environmental changes, unpredictable or expected, persistent or serendipitous, biotic or abiotic, mellifluous or obnoxious, constitute the fuel upon which selective processes boost normal or abnormal stress responses (Romero et al. 2009). A majority of these changes may elicit harmless adjustments in the physiological constraints that define the tolerance limits of core temperature, osmolyte levels or acid–base balances. Others, unexpected, rare and intense changes may force stressful, allostatic adjustments, enhancing the activation of neuroendocrinological and immune cascades, in an effort to reverse unsafe levels of endogenous variables. In addition, a minority of those variations, mainly induced by human-related industrial and productive activities, may severely compromise the health of organisms in a species-specific manner, mostly in local scenarios in which the defining parameters of niche minutiae may change abruptly (Halpern et al. 2008). A classic example is the impact in coastal fish of endocrine disruptors, whose concentration may change dramatically due to aquaculture or industrial run-offs, fluvial outflows and seasonal weather episodes, in the reproductive, tiroidal and stress neurohormonal cascades (Gaw et al. 2014; Szwejser et al. 2017). Confronted with such human-induced rapid environmental changes (HIRECs), stressed individuals skew their energetic budgets, dedicated to metabolism, reproduction and growth, in favour of physiological trade-offs, usually weighted by the species-specific scope and versatility of the evolved adaptive behavioural repertories that allow for coping with environmental stressors, but also by the efficiency and unfolding capabilities of the immune response (Balasch and Tort 2019). Accordingly, the stress response becomes a result of interactive processes between nervous, immune and endocrine systems, devoted to overcome the challenge caused by the stressor and to compensate for its effects, a connection already observed some decades ago when several works demonstrated that not only hormones altered immune functions but also that the immune mediators influenced the neuroendocrine system (Chiappelli et al. 1993). As we discuss below, both stress-related and immune physiological systems share common components and pathways, but a precise description of neuroimmunoendocrine crosstalk is still lacking for fish species. In this sense, the molecular, cellular and systemic scaffolding of stress and immune responses may be considered a common mechanism of defences that share the burden of limited metabolic budgets in what can be described as a collaborative network of mutual influences.
Any particular stressor (or combinations of stressors) may generate an evolutionarily conserved adaptive stress response or, alternatively, maladaptive ones if the intensity, persistence and resilience values of the stressor depart from the “classical” selective pressures, such as depredation, parasitism, or intraspecific and interspecific competition for resources and sex partners, in a given ecological niche. Considering fish species, the evolutionary road to immune sense—i.e. the meaningful detection of self- and non-self-antigens—and sensitivity—measured as the minimum defensive response threshold to foreign microorganisms or xenobiotics—is paved with hardwired anticipatory stress responses constructed during the convoluted evolutionary history of survival and extinction in microbial-rich environments.
20.2 The Aquatic Constraints on Fish Immunity: Navigating Stressful Seas
Fish inhabit aquatic environments prone to pathogen spillover and xenobiotic spreading that stress their immune systems, usually less complex, in terms of both diversity of cellular phenotypes and specificity of antigen recognition components, than those of mammals (Flajnik 2018). Ocean microbiota abundances range from estimates of 104 to 106 cells mL−1, or, in the case of virus (dominated by phages), up to 108 viruses mL−1 in open waters and 1031 in the sub-seafloor (Whitman et al. 1998; Middelboe and Brussaard 2017; Cai et al. 2019). In open oceans, the taxonomic and functional composition, distribution and stratification of microbial communities correlate strongly with temperature and dissolved oxygen (Brum et al. 2015; Sunagawa et al. 2015), with salinity, nutrient content and currents seemingly playing minor roles. Microbial communities in aquatic dwellings are characterized by (1) differential local and global clustering of viral and bacterial communities, influenced by host–pathogen interactions, hydrogeographic features or biogeographical regimes (Brum et al. 2015), (2) complex interactions between bacteria and virus (Breitbart 2011; Orsi 2018) that influence biogeochemical cycling, bacterial gene expression (due to horizontal gene transfer) and pathogenicity (extensive to marine invertebrates and vertebrates), (3) high persistence (thousands of years) of viral and bacterial communities in the energy-starved seafloor (Lomstein et al. 2012; Cai et al. 2019) and (4) huge genetic diversity reservoirs, especially in deep-sea sediments crowded by viral phenotypes, which may fuel evolutionary changes (Jousset et al. 2017). Virus and transposable elements have been postulated as the origin of early immune pathways in marine basal vertebrates (Broecker and Moelling 2019), and fish house large numbers of viruses, prone to jump between hosts (Shi et al. 2018; Geoghegan et al. 2018). In fact, the microorganism richness of aquatic realms continues to impact the co-evolution of complex host–pathogen interactions (Zhang et al. 2018), and maybe even influenced the faster rate of genetic evolution in teleost fish compared to mammals (Takezaki 2018). This, in turn, may have facilitated their bewildering morphological and physiological diversity. Fish show extremely diverse lifestyles, having colonized abyssal wastelands (Swann et al. 2020), submerged murky caves (Maldonado et al. 2020), polar waters (Farrell and Franklin 2016), hypoxic sulphidic mangroves (Rossi et al. 2019) and desiccated ponds (Wright and Turko 2016) due to their remarkable phenotypic plasticity. Coupling the expression of fish genomes to changing environmental constraints allows for the expression of plastic phenotypes, but the vast majority of studies of developmental or behavioural plasticity in fish have been focused more in the metabolic adjustments of energy reservoirs, osmoregulation, coupled acid–base respiratory trade-offs and growth in heated, cooled, hypoxic or contaminated dwellings, and less in the effects on immune responses to such environmental insults.
Parasites also contribute to energy and nutrient flows in aquatic ecosystems, modulating host population dynamics and trophic networks, interfering with reproductive seasonality and influencing coevolving morphologies and complex lifecycles involving multiple hosts for retaliating immune strategies of antigen sensing in the host (Marcogliese 2002; Rohde 2002; Rohlenová et al. 2011). The extent of parasite biomass and communal structure is not yet well characterized, but some studies have highlighted that the effect of temperature in virulence and speciation rates in ectoparasites, or endoparasites in ectotherm species, may depend upon differences between freshwater and marine environments (Scharsack et al. 2016; Poulin 2016; da Costa and Val 2020). Temperature may also have a relevant influence in the still unaccounted importance of cryptic parasites, i.e. species genetically distinct but with very similar morphologies (Poulin 2011). Pulse warming events, short-term temperature fluctuations derived from seasonal anomalies and extreme weather related to global warming, has been shown to affect fish immune responses, habitat use and migratory adjustments due to the expansion of parasite ranges, altered life stages and increased virulence in the host–parasite trade-offs (Claar and Wood 2020).
The impact of human-induced rapid environmental changes (HIRECs) on fish immune performance is still in its infancy, and it will require an appropriate conceptual framework that may encompass the multilevel, transgenerational and overlapping influences on immune outcomes of anthropogenic and “natural” (as opposed to human-dominated), changes in aquatic landscapes (Sievers et al. 2018; Donelan et al. 2020). The effects of HIRECs on fish diversity and performance depend largely on habitat distribution, with coastal environments more prone to suffer from human-derived disturbances (Halpern et al. 2008). Human-induced alterations in aquatic ecosystems include, but are not restricted to, climate change, pollution, eutrophication, exploitation, habitat degradation, invasive species and hatchery production, inducing local, ecological and also commercial extinction of the most consumed species (McCauley et al. 2015). Coastal estuarine areas may suffer from acidification due to eutrophication, aquaculture practices, freshwater run-offs and tidal exchanges, besides the continuous increase in atmospheric PCO2 (Duarte et al. 2013). The impairment of connectivity networks between habitats may affect food webs and species abundance and dispersion, altering the host–pathogen cycles (Duarte et al. 2020). Albeit the modelling of the changes in climate patterns is as good as the quality of the data that validate and support the forecasting, it is beyond doubt that, for ectotherms, climate change may influence distribution patterns, species richness, genetic variation along biogeographical clines, colonization and diversification rates and immune responses to pathogen outbursts (Stanley et al. 2018; Clucas et al. 2019; Manel et al. 2020). These and other ecological constrictions limit the scope, performance and diversity of fish immune systems, in what has been increasingly recognized as a complex system of mutual interaction between the needs of immune surveillance and response, and the physical and biotic intricacies of aquatic realms. An ecological immunology approach to analyse the evolutionary road to fish immune constructs is much needed as it may help to prevent and anticipate the changes in aquatic communities due to expanding human activities.
20.3 The Fish Approach to Immune Defence
The pathogenic load, together with the spatial and temporal heterogeneity and resilience to unpredictable changes in aquatic habitats, affects all levels of endogenous responses, from major neurosecretory and hormonal regulatory systems, to plastic behavioural phenotypes and moving population assemblages (Egerton et al. 2018; Flajnik 2018; Broecker and Moelling 2019; Guo et al. 2019). In this sense, the majority of efforts traditionally invested so far in the analysis of immune responsiveness in fish have been dedicated to describe the components of immunity in model or commercial teleost species subjected to pathogenic challenges, sometimes coupled with physiological responses to variable temperature or oxygen levels or common handling procedures in aquaculture practices. Current approaches rely on the analysis of immune genome repertories and the development of transgenic models to elucidate host–pathogen crosstalk in immunocompromised species. However, the influence of past evolutionary events on species-specific immunity is still almost absent in these analyses (Solbakken et al. 2017). Fish are basal roots in the phylogenetic vertebrate branching and have endured a long evolutionary history of diversification and extinction cycles (Near et al. 2012) and several rounds of genome duplications (Glasauer and Neuhauss 2014). They also show extreme differences in morphology and life stories, colonization of extreme, nutrient-scarce habitats (Priede and Froese 2013; Crawford et al. 2020; Maldonado et al. 2020) or adaptive radiations in the aftermath of environmental upside downs and evolutionary transitions (Smithwick and Stubbs 2018; Ribeiro et al. 2018). All these upheavals have coalesced in a panoply of extremely diverse lifestyles, varied reproductive strategies and immune trade-offs that prevent a unified definition of common patterns of defensive responses in fish. In addition, the vast majority of fish retain a hardwired ectothermy that constricts immune function, which is why some species seem to try to become endotherms by means of metabolic and behavioural adjustments (Dickson and Graham 2004).
Broadly speaking, fish immunity encompasses three adaptive strategies evolved to cope with a microbial world, namely the approaches observed in “agnathans” (jawless fish), “chondrichthyes” (cartilaginous fish) and “osteichthyes” (bony, ray-finned fish, Actinopterygii, and lobe-finned fish, Sarcopterygii, including coelacanth and lungfishes). A detailed description of the immune intricacies of jawless and cartilaginous fish is beyond the scope of this review, and the readers are referred to several excellent reviews (Flajnik 2018; Smith et al. 2019). Nevertheless, a very brief sketch of immune features in jawless fish and sharks may be useful to illustrate the high variability of immune components described for marine and freshwater fish. Both cartilaginous and bony fish display the following traits: (1) dedicated organs (thymus, spleen) for the maturation of immune cells (generalist phagocytes, specialized B cells and T cells), (2) production of antimicrobial proteins and mediators of immune responses (cytokines, immunoglobulins with variable domains), (3) the achievement of a reasonable high level of diversity for antigen-recognizing membrane receptors (TCRs) and immunoglobulins due to enzymatic rearrangement of immune genes and (4) the capability of internalization, processing and use of exogenous antigens using products of polymorphic gene sequences (MHCs, see below) as a means to coordinate the mutual activation and regulation of the distinct immune cellular subtypes. The more ancient jawless fish, lampreys and hagfish, lack specialized immune organs, except for the thymoid, an equivalent of thymus. They have B-cell and T-cell-mediated responses, and probably other cellular components of immune responses. They may have cytokines but lack immunoglobulins, and instead of MHC-regulated arrangements of immune receptors, they have evolved exclusive variable lymphocyte receptors (VLRs) as a means to diversify antigen recognition. Genomic analysis uncovers immune genes shared for all fish groups on a regularly basis, and a high degree of convergence is expected regarding common characteristics of cellular phenotypes and molecular mediators between agnathan and gnathostomata fish species. Additionally, at least three rounds of genome duplications have defined the expansion of the fish immune repertoire (Kuraku et al. 2009), two of them before the agnathan/gnathostomata divergence, around 500–800 million years ago (Mya), and the most recent after the split of the teleost lineage, 325–350 Mya, probably helping in their successful adaptive radiation (Glasauer and Neuhauss 2014). A fourth genome duplication event 88–90 Mya has equipped salmonids with several paralogous and duplicate genes still retained in extant species (Christensen and Davidson 2017), and, more recently, around 12 Mya, in carps (Xu et al. 2019).
Teleostei house the vast majority of extant fish (Nelson et al. 2016) and may be considered the most advanced ray-finned fish (Actinopterygii) in terms of alternate functional physiology frameworks, but it is not possible to extrapolate the immune features of teleosts to all fish. In fact, the fuzzy definition of “fishes”, a term used to refer to a multiplicity of morphologies, species and taxonomic phylogenies, is still controversial, even in the phylogenomic era (Hughes et al. 2018), and confuses the description of common fish immune responses. An ever-growing number of fish genomes have been sequenced in non-model species (Buonocore and Gerdol 2016; Wcisel et al. 2017; Hara et al. 2018; Ravi and Venkatesh 2018; Smith et al. 2018), but Actinopterygii have been the object of the vast majority of functional immune studies to date. Therefore, here we will focus on teleost models to discuss the effects of environmental stressors in immune performance.
20.3.1 Teleostean Immunophysiology (I): Common Vertebrate Features
In infected or immunocompromised vertebrates, as it may happen after stress episodes, the return to original homeostatic grounds starts with the innate toolbox of local inflammatory responses. Broadly speaking, this includes a repertoire of several key overlapping processes (Medzhitov 2008), among others: (1) on-site release of PAMPs and DAMPs (pathogen- or tissue-damaged danger-associated molecular patterns, respectively), (2) complement-mediated opsonization cascades (see Chap. 9), (3) degranulation of resident granulocytes, (4) release of antimicrobial proteins (AMPs) and pro-inflammatory/chemotactic cytokines (see Chap. 10), (5) recruitment of circulating phagocytes and cytotoxic natural killer (NK) cells to the site of infection or damage, (6) activation of hepatic acute-phase proteins and (7) antigen (Ag) recognition by dendritic cells, macrophages and other antigen-presenting cells (APCs).
The response to bacterial, viral of fungal PAMPs (see Chap. 2), is orchestrated by an array of germline-encoded soluble, membrane-bound and intracellular pattern recognition receptors (PRRs) (Takeuchi and Akira 2010), such as the highly diverse Toll-like receptors (TLR). PRRs are expressed primarily by immune cells, but also by tissue-specific epithelial/endothelial cells. Interferons (IFN) are the main mediators of antiviral responses (Secombes and Zou 2017). Pathogens can subvert the PRR recognition masking themselves with endogenous antigens, delocalizing or modifying the structure of PRRs to prevent proper bonding to antigens, changing their phosphorylation/ubiquitination features, thus targeting them for degradation, and inhibiting downstream signalling components (Majzoub et al. 2019). Exogenous and endogenous antigens are processed, fragmented in manageable peptides and presented to immune cells through highly polymorphic major histocompatibility complex (MHC) proteins (Kotsias et al. 2019). Widespread exposure to self or altered cytosolic peptides bound with MHC class I molecules in almost all cell surfaces activates cellular crosstalking and polarization of immune phenotypes, fully unrolling the inflammatory process by activation of membrane receptors of CD8+TC (cytotoxic) cells. If the MHC I complex is impaired by viral assault, a “missing self” signal ensues and the infected cell is destroyed by NK cells. Other non-classical MHC I molecules are induced in stressed cells and contribute to the clearance by NK cells upon recognition by their fast-evolving variable receptors, a trademark of these cytotoxic cells (Parham and Moffett 2013). Extracellular antigens, once endocytosed, fragmented and bound to MHC class II molecules constitutively expressed in immune cells (mainly APCs), stimulate CD4+TH (helper) cells that coordinate the nascent immune response. In non-APCs, such as epithelial cells and fibroblasts, interferons (IFN-γ), TLRs, TGF-β and other signalling molecules can stimulate MHC II expression during inflammatory processes, rendering the global network of interactions more complex (Neefjes et al. 2011). Several non-classical MHC molecules also bind small metabolites and participate in the editing of antigen-derived peptides, and activation of T cells and NK cells (D’Souza et al. 2019).
If the containment measures of innate immunity are ineffective, the immune response broadens, and the adaptive, highly specific immune modules, dominated by B- and T-cell subtypes, are brought into play (see Chap 4). Haematopoiesis increases, and lymphocytes with variable surface receptors begin to be mass-produced and selected for improved antigen affinity in specialized lymphoid organs, such as lymph nodes and spleen. To ensure the diversity of antigen-recognizing receptors, at earlier stages of B- and T-cell differentiation, RAG1-RAG2 endonuclease complex helps rearrange the gene segments that encode the different Igs and TCRs, enabling the ulterior clonal selection of high-affinity receptors capable of recognizing a vast array of antigens (Ru et al. 2018).
The vast majority of T cells consist of αβT cells, which participate in the overall adaptive response to antigens, whereas the less abundant, and still less studied, γδT cells have also been implicated in antigen recognition in innate-like immunity (Rampoldi et al. 2020). Immature B cells express IgM/IgD surface receptors, and in mammals, but not in fish (see Chap. 8), if stimulated by antigens and cognate T cells, change the isotype to IgG, IgA and IgE. Once exposed to antigen, B cells also endure several cycles of somatic hypermutation (SHM) of immunoglobulin gene segments, facilitating the cloning and expansion of Ag-specific cellular populations. Once diversified, these cellular populations are confronted by follicular dendritic cells (FDCs), APCs and T follicular helper (TFH) cells in the germinal centres of lymph nodes (absent in fish) and other secondary lymphoid organs. These processes mature the affinity of the differential surface receptors for the antigen in the precursors of Ig-producing B cells. The mutagenic changes in Ig genes elicited by activation-induced deaminase enzyme (AID) rearrange gene segments and, ultimately, favour SHM and immunoglobulin isotype class switch recombination (CSR), which, in turn, refine and enforce the antigen–antibody (Ag-Ab) bond, dramatically improving the overall immune response (Methot and Di Noia 2017). Once matured, B cells produce specific antibodies, whereas CD4+ TH and regulatory (TREG) cells orchestrate the immune crosstalk and reactivity, and CD8+ TC cells neutralize infected cells and collaborate to the overall cytokine signalling. If effective, the adaptive countermeasures end with (1) the controlled and apoptotic-regulated clearance of activated immune cells, (2) the nesting of quiescent populations of long-lived plasma cells (LLPCs), true memory B cells and T cells and probably NK memory cells (Beaulieu 2018) that guarantee rapid responses upon re-exposure to the same antigen and (3) the clean-up of immune debris and tissue healing, mainly by specialized macrophage M2 phenotypes (see Chap. 6) and other tissue-healing cells (Yunna et al. 2020).
After immune challenge or stress, the complex intricacies of coordinated networking between all the participants at each stage of immune responses result in high time-dependent malleability of cellular phenotypic changes as defensive actions progress. All these cellular immune phenotypes, innate or adaptive, either recirculating or anchored to specific tissues, come in several forms, developmental stages and polarized functions that usually coexist spatially and functionally and can contribute to blur the distinction between innate reactivity and adaptive reactivity (Van Kaer et al. 2019). Differentiated tissue-resident macrophages, for example, are a heterogeneous composite of embryonic, steady state and adult monocyte-derived active cells that adds up to the complexity of local, tissue-specific inflammatory responses (Ginhoux and Guilliams 2016). On the adaptive side, naïve TH cells adopt several phenotypes, TH1, TH2, TH17 or TREG, depending on the timely production of specific cytokines during inflammation. This differential cellular polarization may bias TH responses towards general cytotoxicity against cancer cells or intracellular pathogens (TH1 cells), immunosuppressive processes (TH2 cells), intestinal microbiota homeostasis (TH17/TREG), regulation of immune responses against self-antigens (TREG) and protection against extracellular bacteria and fungi (TH17), although there could be some functional overlap between the different TH phenotypes (Yamaguchi et al. 2015). Both innate myeloid and lymphoid cells may display a short-term memory, antigen-independent, that enhances reactivity to repeated exposure to the same antigen. Once active, monocytes, macrophages and NK cells undergo epigenetic reprogramming that change their chromatin profiles, enhance glycolytic metabolism and increase cellular responsiveness after being immunologically “trained” (Netea et al. 2016). Overall, this suggests that it is not possible to divide the vertebrate immune responses between rapid, unspecific, early-responding innate components, and long-lasting latecomers, highly specific ones, capable of establishing a memory of recent infections. As has been recognized in the last decades, many participants of adaptive immunity in vertebrates educate, and are triggered by, the concerted action of innate immune components (Iwasaki and Medzhitov 2015) in what is now considered a continuum of progressive defensive responses, and, more importantly, a source of immune plasticity when confronted with environmental insults.
20.3.2 Teleostean Immunophysiology (II): Particularities and Drawbacks
Teleosts differ from mammalian counterparts in the organography, components, onset, duration, memory and functional effectiveness of immune responses (Salinas 2015; Geven and Klaren 2017; Secombes and Zou 2017; Flajnik 2018; Reverter et al. 2018).
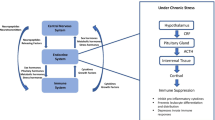
The main regulatory hub and primary lymphoid tissue of both stress and immune responses in fish is the head kidney (Fig. 20.1), where mixing of neuroendocrine and haematopoietic/immune cell populations occurs, resulting in a constant molecular crosstalk, and can be considered a functional analogue of mammalian bone marrow and the adrenal gland (Geven and Klaren 2017). Fish tend to diminish the barriers between capsulated and structured organs devoted to a single function and, instead, possess multifunctional integrative organs (see Chap. 1). In this sense, in teleosts, the head kidney coordinates the neuroimmunoendocrine crosstalk derived from the innervation of lymphoid organs (Balasch and Tort 2019). Head kidney cells produce and secrete the two most relevant stress hormones after stressful stimuli: adrenaline by chromaffin cells and cortisol by interrenal cells (Fig. 20.1). Moreover, the co-localization of these two cell types enables paracrine interactions between these cells, thus enabling cross-influences (Rotllant et al. 2006). Cortisol and catecholamines are important inducers of cytokines from macrophages, as these cells have both α-adrenergic and β-adrenergic receptors (Maciuszek et al. 2019) and glucocorticoid receptors. Moreover, together with cytokines, glucocorticoids evoke a strong synergistic enhancement for most acute-phase proteins (APPs) (Bayne and Gerwick 2001). The presence of adrenergic and hormonal receptors in immune cells modulates cytokine secretion that, in turn, docks in immune receptors in neuroendocrine cells, closing the homeostatic circle (see Verburg-van Kemenade et al. 2017 for a thorough review).
Stress axis and immune dedicated tissues and associated organs in teleosts. Head kidney and spleen are considered the main lymphoid organs, facilitating cell trafficking and harbouring melanomacrophage centres (MMCs), involved in antigen trapping and processing. Mucosa-associated lymphoid tissues in gills, intestine and skin endure parasite attachment and ever-growing microbial communities that modify and educate host immunity. Liver produces acute-phase response antimicrobial peptides and innate complement factors. The influence of immune responses reaches the overall metabolic trade-offs and affect and is affected by the activation of several main regulatory axes, among them the catecholamine-producing sympatho-chromaffin (SC) axis, and the cortisol-producing hypothalamic–pituitary–interrenal (HPI) axis. Under stressful environmental changes, metabolic trade-offs influenced by temperature changes, parasite load and other environmental stressors may impair growth and overall allostatic adjustments
The thymus acts also as a primary lymphoid organ dedicated to the development of T cells and, as described for mammals, regresses with age, but only in some species (Bowden et al. 2005). The spleen and the lymphoid infiltrates in mucosal tissues are considered secondary lymphoid organs in an immune system that not only harbours less immune cell phenotypes and molecular effectors than mammals (Flajnik 2018) but also lacks mammalian-like highly structured intestinal lymphoid nodules, using melanomacrophage centres as surrogates of lymph nodes (Stosik et al. 2019).
Upon detection by dendritic cells, macrophages, granulocytes and IgT/Z+ B cells in mucosal tissues (see Chap. 12), clearance of undesired antigens or malfunctioning cells in fish requires the coordinated participation of the head kidney’s immune axis (Fig. 20.1), catecholamine-producing sympatho-chromaffin (SC) axis and cortisol-producing hypothalamic–pituitary–interrenal (HPI) axis (Khansari et al. 2018; Balasch and Tort 2019). Inconclusive immune outcomes require additional recruiting of cellular and molecular components of the overall defensive responses, including the hypothalamic–pituitary–thyroid (HPT) axis (Geven and Klaren 2017) or hypothalamic–pituitary–gonadal (HPG) axis and growth hormone (GH)/insulin-like growth factor (IGF) axis, especially in migratory species such as salmonids (Ueda 2019).
Overall, cellular and humoral responses in teleosts seem to follow the same pattern as in mammals (Rebl and Goldammer 2018; Smith et al. 2019). However, several mammalian-like and fish-specific TLRs have been described, being highly variable between species (Palti 2011). An illustrative example of the alternative, non-mammalian ways of pathogen sensing in fish is TLR4, an essential PRR for the recognition of bacterial lipopolysaccharide (LPS). Many teleost fish lack a functional TLR4 framework and have greater tolerance to LPS doses than mice or humans in endotoxaemic models (Swain et al. 2008). Alternative sensing pathways to LPS in transcriptomic models of Schizothorax prenanti, a cultured fish species in China, challenged with Aeromonas hydrophila and Escherichia coli LPS include expression of regulatory cytokines (IL-1β, IL-10 and IL-8) and PRR (TLR5, TLR25, PTX3 and C1q) transcripts that have been suggested to mediate the LPS responses in this teleost species (Li et al. 2020). However, the higher tolerance to LPS in teleosts remains intriguing, and, again, the microbial-rich characteristics of dwelling in an aquatic environment seem to be involved (Lieschke and Currie 2007).
All T-cell subsets (TH, TREG and TC) seem to be present in teleosts (see Chaps. 3 and 4), but the morphological and functional description of cellular immune phenotypes in fish is still far for being complete, especially for innate lymphoid cells. Thymic homing from haematopoietic tissues and RAG-mediated selection for non-self-reactive T-cell phenotypes are believed to be similar in teleosts and mammals, but little is known about the commitment of αβ and γδ T-cell categories in teleosts (Bajoghli et al. 2019). B cells develop and mature in the head kidney and migrate to secondary lymphoid organs such as the spleen and posterior kidney, where the antigen-mediated stimulation takes place (Saunders et al. 2010). Once matured, the final plasma cell phenotype recirculates to the head kidney (Ye et al. 2011). Unlike mammals, teleosts produce only three types of immunoglobulins (see Chap. 7), IgM, the main circulating Ig, IgD and IgT, the latter more abundant in mucosal tissues, similar to IgA in mammals, and involved in the mutual regulation of complex host–microbe interaction (Zhang et al. 2010). IgD is still poorly studied, but the presence of IgD+IgM− precursor B cells in mucosal surfaces suggests that, as in mammals, IgD participates in pattern recognition (Gutzeit et al. 2018; Perdiguero et al. 2019). Notwithstanding the lack of germinal centres and FDCs in fish, affinity maturation seems to be present in teleosts. Melanomacrophage centres (MMCs), clusters of melanomacrophages (MMs) enriched in melanin, lipofuscin and hemosiderin that are present in lymphoid tissues, are postulated to be the analogues of germinal centres in fish. These are sites where antigen trapping and presentation to activated IgM+B cells may enhance Ag-Ab bonding, coordinated by MMs acting as functional analogues of FDCs (Stosik et al. 2019). However, the main function of MMCs is to accumulate and destroy metabolites and discarded cells. Indeed, the highly interspecific variability and temperature sensitivity of the activation-induced deaminase (AID) enzymatic activity suggest that the maturation of affinity may not reach the levels described in mammals, where up to 1000-fold higher affinity for the antigen is seen in plasma cells relative to that of the original mature naïve B cell, compared to a 100-fold increase described for ectotherms (Magor 2015). Affinity maturation has been described recently for long-lived plasma cells (LLPCs), primarily in the head kidney but also in the spleen of channel catfish (Ictalurus punctatus). Upon immunization, LLPCs appear in the head kidney 4 weeks post-immunization, after activation in the spleen and other peripheral lymphoid tissues, and secrete high-affinity antibodies, with serum titres that reach a peak 6 weeks post-immunization (Wu et al. 2019). It has been suggested that secreted teleost IgM tetramers undergo variable disulphide polymerization that may also contribute to affinity maturation of antigen recognition (Ye et al. 2010). Class switch recombination (CSR) in activated B cells, a change in the constant region of the Ig heavy chain that leaves the variable region untouched but expands the immune interaction and recognition capabilities by producing different Ig isotypes, has not been described in fish (Patel et al. 2018). Overall, antigen recognition and Ig-mediated responses in teleosts lack the efficacy and diversity observed in endotherm vertebrates.
It is relevant to note that erythrocytes are nucleated cells in fish, and hence can perform gene transcription and within their transcriptome are immune-related genes. For example, RNA-Seq analysis after poly (I:C) treatment shows diverse cohorts of mRNA transcripts related to immune function in erythrocytes. Moreover, erythrocytes can express a type 1 IFN response (Morera et al. 2011) and in vitro can endocytose TNF-α and VHSV G-protein fragments (Puente-Marin et al. 2019). Therefore, erythrocytes play a role in the immune response of fish. It is not yet clear whether this is a primary or a subsidiary or complementary role, particularly under stress situations, but it should be noted that there is a huge quantity of red blood cells compared to leucocytes, so even a small individual role will have an impact in the overall immune response (Morera and MacKenzie 2011; Anderson et al. 2018).
Surprisingly, some species, including Gadiformes, anglerfish and pipefish, have lost the MHC II. The evolution and maintenance of MHC diversity in teleosts (see Chap. 11), and therefore the competence to recognize self- and non-self-antigens effectively, depend on the delicate interplay between the unavoidable requirements of physiological trade-offs in specific niches. This is guaranteed by the ecological relevance of parasite load and their intrusive abilities when confronted with mucosal barriers, and the great plasticity of immune responses in fish. The loss of MHC II pathway components in pipefishes (Syngnathus), for example, has been attributed to their sex role reversed parental care strategy. Syngnathus feature male pregnancy, housing fertilized eggs in inverted tail brood pouches, feeding the developing embryos through placenta-like organs that allow for both the transmission of oxygen and also immune components. Gene expression studies of Vibrio spp. and Tenacibaculum maritimum-challenged pipefish suggested differential parental immune priming of F1 and even F2 offspring, with parent males influencing strongly on offspring innate immunity and complement system components (Beemelmanns and Roth 2016; Beemelmanns and Roth 2017). Interestingly, sex-specific methylation patterns of immune genes were also observed, which are influenced by sex in other fish (Caballero-Huertas et al. 2020) and may be related to predictable, and stable parasitic load pressures over time. As described for Gadiformes (see below), in addition to the loss of MHC II, and CD4 components, diversification of MHC I pathway genes was also found in pipefish. However, MHC I transcripts and pro-inflammatory- and adaptive-related immune genes were found to be downregulated (Roth et al. 2020). This indicates, as the authors suggest, a convergent strategy to minimize the perils of embryo rejection as described for pregnant mammals, in which TREG-mediated suppression or downregulation of MHC I and MHC II genes in the placental barrier ensures no immune reactivity to the antigens of the other parent (Prabhu Das et al. 2015).
At early stages, the development of primary and secondary lymphoid organs, together with the production of associated B/T cellular phenotypes, depends on environmental variables (such as temperature and food abundance) and varies greatly on a species-specific basis. Small eggs correlate with shorter yolk sac stages and fast hatching, but spleen, thymus and the haematopoietic/immune cellular subtypes produced in the head kidney appear several days after hatching, during the fast growth period characteristic of teleost larvae (Falk-Petersen 2005; Zapata et al. 2006). During the yolk stage, maternal transference of immunoglobulins, components of the complement system and other antimicrobial proteins (AMPs) help the developing embryo to keep pathogens at bay (Swain and Nayak 2009; Roth et al. 2018). This transgenerational immune priming is a strategy shared also by several invertebrate taxa (Tetreau et al. 2019). In addition, as suggested by transcriptomic analyses of immune gene expression across several stages post-hatching in the common sole (Solea solea), PPR sensing is also enhanced at hatch and first feeding, whereas increased abundance of MHC and TCRs transcripts correlates with the maturation of lymphoid organs during the metamorphosis (Ferraresso et al. 2016). The same study failed to detect RAG1 or CD4+ and CD8+ markers, suggesting that at the end of metamorphosis T-cell differentiation is still incomplete in this species. In fact, the maturation of immune organs constricts the efficacy of temperature-dependent antiviral and pro-inflammatory responses during larval stages, as demonstrated for zebrafish (Danio rerio) and European eel (Anguilla anguilla). This would impair the immune response to pathogens (Dios et al. 2010; Miest et al. 2019), even in anadromous and catadromous species undergoing the metamorphosis-like rearrangements prior to migration (McMenamin and Parichy 2013; Yada et al. 2018). Juvenile Atlantic salmon (Salmo salar) enduring the osmoregulatory and metabolic strengthening from the smoltification process prior to the downstream migration to open sea usually are affected by immunosuppression (Johansson et al. 2016). Hence, thermal stress may modify the timing of migratory behaviour, biasing metabolic, growth and immune trade-offs, ultimately changing the structure and composition of local age classes in the population (Cline et al. 2019). The reliance on innate/unspecific immunity during the first stages of development renders fish larvae more prone to high mortalities, not only during the yolk phase, when high mortalities are expected, but also during the juvenile and early adult stages, that are more sensitive to stressful changes in environmental variables due to the incomplete maturation of adaptive defence mechanisms.
Overall, ecological factors limit the development and the functional performance of mature immune systems in fish, determining the extent to which individuals may cope with complex environmental stressors. In aquatic dwellings, immunity serves as a high-demanding metabolic sieve to screen for opportunist and virulent pathogens that crave for mucosal epithelia to attach in a seasonal, cyclic way, influenced by rises in temperature in a system dominated by ectotherms.
20.4 The Scalability of Stress Responses in Fish: From Stressed Cells to Systemic Immunomodulation
The processing of relevant information by the components of the immune system requires metabolically expensive parallel and distributed complex computation (Cohen and Efroni 2019) to solve the related problems of identifying cellular and humoral friends and foes, inflammation management, tissue repair, cancer control and, especially, symbiont housing, leaving sticky notes of the immune progression in the form of specialized receptors in neuroendocrine cells. In this computational sense, the overall performance of immune activation resembles those of the neuroendocrine machinery in terms of complexity and scope, and can be considered as an emergent feature based upon a plethora of underlying local cellular and molecular interactions and cross-recognitions between modular (i.e. classical innate and adaptive) components (Sotiropoulos and Tsihrintzis 2017). All vertebrate immune systems can be modelled as problem-solving algorithms dedicated to live, learn and remember the stressful changes in the pathogenic and xenobiotic environment (Fig. 20.2).
Nested loops of neuroimmune control in fish. A series of nested loops participate in the mutual regulation of neural and immune responses (Irwin and Cole 2011). In fish, environmental stressors activate the hypothalamic–pituitary–interrenal axis (HP), the sympatho-chromaffin (SC) axis, and the haematopoietic/immune tissue in the head kidney (HK). The crosstalk between chromaffin, interrenal and haematopoietic cell populations in the HK guarantees a constant neuroimmunoendocrine local regulatory feedback that helps in the activation of immune modules. The transcriptional outcome of the inflammatory processes depends on the intensity of the environmental insults, and the species-specific determinants of the allostatic processes in the organism (extrinsic control of the immune response), as well as the local recognition of soluble or membrane-bound evolutionary conserved pathogen-associated molecular patterns (PAMPs) by means of pathogen recognition receptors (PRRs) in host cells (intrinsic control of immune local inflammation). Once started, inflammation may progress to systemic immune responses, recruiting cells and tissues to mount an adaptive immune response that, in turn, may alter the behavioural phenotype of stressed organisms. Several subloops (not shown in the figure), such as the crosstalk between innate and adaptive immune components, or the delicate equilibrium between host symbionts and mucosal immune surfaces of gills, gut, skin and nasopharyngeal spaces contribute to the fine regulation of neuroimmunoendocrine circuitry in fish
Fish may endure resilient human-induced environmental changes and thrive throughout sustained global pathogenic blooms, marine heating or acidification in part because of in-built plastic neural and behavioural adaptive mechanisms (Ebbesson and Braithwaite 2012; Maruska et al. 2019). However, these capabilities tend to be highly species- and niche-specific, and strongly constricted by each particular evolutionary history of physiological regulatory systems (Lee 2006; Solbakken et al. 2017). In this sense, higher lifestyle diversity and niche occupation may be beneficial in altered ecosystems, but the particular idiosyncrasies of each species or individuals constitute a major drawback for establishing a common unified framework to describe, evaluate and palliate the effects of compromised immune performances in the context of neuroendocrine responses (Balasch and Tort 2019; Taborsky et al. 2021).
Although a precise definition of stress is difficult due to the number of aspects associated with this concept, there is a general agreement in considering stress as an altered situation of the organism from its normal physiological scope as a result of a real or symbolic challenge. The stressor is defined as the challenging agent causing such a response. Stress is a universal phenomenon, and thus, any living organism may experience situations that cause these reactive or compensatory physiological responses, the so-called, stress response. It should be remembered that, since all organisms are subjected to challenges, all organisms experience stress responses and it is therefore observed that some of the basic components of the stress response have been maintained in all living organisms, and consequently, different taxa show similar molecular components and pathway mechanisms. In most cases, one can identify a specific number of molecules mediating the stress response that are common among animals. For instance, a group of these molecules, namely corticosteroids, adrenaline or cytokines, do exert the same networking function in the stress response in all groups of vertebrates (Hau et al. 2016; Miles et al. 2019). Thus, although the stress concept was defined for mammals by Hans Selye in the last century, it is currently applied to all types of animals, plants and even to different levels of organizations (cell stress, organ stress and whole organism).
Stressed cells share common activation pathways to limit molecular damage and preserve macromolecular structure (and, hence functionality) by maintaining a robust proteostasis network of chaperones, components of the proteasome complex and other mediators that help to synthesize, fold, traffic and degrade proteins, guaranteeing a low level of misfolded or aggregated molecules in the proteome (Sala et al. 2017). Among pre-transcriptional responses, chromatin remodelling involves control over transcription, as there are a number of phenomena that will modify the translation of messenger RNA, such as alternative splicing, or gene silencing by miRNA. Regarding the post-translational response, the ubiquitination system uses small proteins that covalently modify other proteins in cells with consequences in transcription, proliferation, DNA repair, protein degradation and nuclear localization (Mazzucotelli et al. 2008). Under stress conditions, altered or wounded cells, ubiquitins upregulate the NKG2D (natural killer group 2, member D)-activating receptor in mammals, through high levels of MIC ligands (MHC class I polypeptide-related) and activating NK cells and T cells that, ultimately, eliminate altered cells (Stern-Ginossar et al. 2008). The evolution of metazoan bauplans requires the coordinated participation of several cell lineages across different tissue and systemic regulatory networks, which in turn rely on paracrine crosstalk to transfer the cellular distress between nervous, endocrine and immune systems. Unsurprisingly, some components of the proteostasis network, and also several intracellular pathways that allow for their activation, are shared between stress and immune main activation axis in vertebrates (Miles et al. 2019). At the cellular level, the response of the organisms to any type of tissue damage and infection will involve immediate-specific reactions in order to repair the damage, promote wound healing, protect against invading organisms and contribute to host defence mechanisms, in particular the innate immune response. These rapid reactions involve a number of specific proteins, the acute-phase proteins involved in the so-called acute-phase response, APR, mainly produced in the liver that may enhance or depress inflammatory responses (Black 2003). Some of these molecules involved in cell stress responses are well conserved through evolution and have been used as molecular indicators of any kind of stress including immune stress. Therefore, such proteins would be involved in the regulation of both cell immunity, cell damage or altered metabolism. Among the specific proteins associated with immunity and stress is NF-kβ, a highly conserved family of related protein complexes in metazoans (Ghosh et al. 1998) that, once activated by bacterial or viral antigens, activates or represses hundreds of genes in stress-related immune responses (Zhang et al. 2017), including inflammation, cell migration, cell repair and metabolic activation. Map kinases (MAPKs) are signal transduction mediators that are central for the correct function of many aspects of cell physiology, including cell defence against pathogens. MAPKs also activate SAPKS or JNK after alterations in cell osmolality, stress stimuli, inflammatory signals, variations in the level of reactive oxygen and other signs of cell stress associated with defence and immunity (Kyriakis and Avruch 2012). Another kinase, AMPK, is activated when there is a low level of intracellular ATP, cell growth arrest and biosynthetic reduction. Its activation induces glucose and fatty acid uptake and oxidation to recover cell energetics in a subset of lymphocytes, T regulatory cells, that, together with M2 macrophages, the alternative anti-inflammatory phenotype of classically activated M1 macrophages (Locati et al. 2020), regulate the termination of inflammation (Michalek et al. 2011).
Among stress proteins, one group that has been widely associated with stress is the heat shock protein group (HSP), a superfamily of proteins that have been well conserved through evolution, thus present in bacteria, fungi, plants and animals (Feder and Hofmann 1999; Timperio et al. 2008). Although they were discovered after heat shock treatments, they are induced by a huge variety of environmental stressors. HSP and other stress-related proteins work as chaperones, responsible for maintaining the integrity of the molecules in the cell that are transcribed in response to stressful stimuli and they are conserved in all domains of life, but they can also act as DAMPs, damage-associated molecular patterns that initiate inflammatory processes (Schaefer 2014). In mammals, several HSP families have been described to activate immune responses, participate in the pro-inflammatory and apoptotic responses to viral infection, usually enhancing the immune reactivity, but also helping to replicate or assembly the viral particles during dysregulation episodes in HSP function (Wan et al. 2020). The production of hepatic C-reactive protein (CRP), an AP protein, stimulated by an inflammatory cytokine, interleukin 6 (IL-6), has also been associated, in addition to immune responses, with fatty acid metabolism in muscle, feeding, regulation of adipose tissue and overall metabolic maintenance (Del Giudice and Gangestad 2018), processes that may interlock in part during stressful outcomes and suggest a multifactorial role of immune mediators in stress-related metabolic trade-offs. For example, in mammals, chronic stress enhances several inflammatory cytokines, including IL-6 and CRP, probably by means of desensitization of immune cells to the effects of glucocorticoid inhibition (Cohen et al. 2012).
20.4.1 The Central Role of Glucocorticoids
As chemical messengers secreted to blood mainly from the pituitary and head kidney, fish hormones are principal mediators of the systemic stress response but also influence the immune system. Their effects normally take some time because hormones have to reach the receptors in the target tissues that subsequently regulate processes like gene expression, morphological changes or protein synthesis. Nonetheless, it has also been shown that some hormones can be produced locally, which enables them to act acutely through a paracrine process, and therefore have no delay due to transit time by the bloodstream (Calisi and Saldanha 2015). Among the many hormones activated after stress, glucocorticoids or corticosteroids are crucial for modulating the response of the immune system, in addition to also modulating many other functional processes, including metabolism, development or behaviour. Thus, following stress challenges, glucocorticoids will affect multiple physiological, behavioural (Kelly and Vitousek 2017) and life-history (Crespi et al. 2013) elements as components of the homeostatic response. Hence, circulating glucocorticoids represent a plastic phenotype coming from a single genotype that allows individuals to release different concentrations of these circulating messengers depending on the varying environmental conditions and individual perception (Romero and Gormally 2019). One of the sites where endogenous glucocorticoids exert powerful effects is on inflammatory and immune processes. Nevertheless, as pointed out by evolutionary and comparative biologists, in terms of the overall understanding of the glucocorticoid effects, it becomes paramount to quantify the individual variation of these mediators, as this would help to evaluate the current variability in immunity, behaviour, physiology and overall response performance (Guindre-Parker 2020). As a consequence, the recognition of such variability has shown that analysis of cortisol alone is insufficient when used as a single bioindicator to measure the overall stress response and its consequences (Romero and Gormally 2019; Telemeco and Gangloff 2020).
Cortisol modulation entirely depends on the presence of glucocorticoid receptors in the cells. The pleiotropic effects of glucocorticoids will then largely depend on the extension of binding to the different glucocorticoid receptors found in fish tissues (Landys et al. 2006). Thus, it is relevant to point out several features that are important regarding cortisol receptors in fish. First, fish present two glucocorticoid receptors (GR1 and GR2) and one mineralocorticoid receptor (MR) (Faught and Vijayan 2018). Second, alternative splicing variants of glucocorticoid receptors have been found under stress conditions (Romero et al. 2020). Third, receptors are ubiquitously expressed in all tissues including immune tissues. Therefore, the receptor-mediated response is complex in fish and for other vertebrate species.
Altogether, thanks to their ubiquity and complexity, cortisol receptors bring a high plasticity in terms of regulation of physiological and immune responses after stress. For example, high-affinity and low-capacity mineralocorticoid receptors are sensitive to relatively low concentrations of circulating cortisol that will be helpful in regulating metabolism and energetics, whereas high cortisol concentrations in the blood will initiate acute alarm responses including immune signalling (Landys et al. 2006). Receptor type, receptor density, time course and transcriptional effects of circulating glucocorticoids in the blood will involve differential effects across tissues and environmental contexts, thus modulating the action of cortisol and therefore the influence on the immune response (Breuner et al. 2013; Guindre-Parker 2020). Two mechanisms of action have been described after activation of glucocorticoid receptors. First is the genomic signalling, which takes hours to days to express the corresponding effects (Nicolaides et al. 2010). Second, the non-genomic signalling, a rapid mechanism taking only seconds to minutes, that activates plasma membrane proteins using second messengers such as cytoplasmic free calcium (Panettieri et al. 2019; Johnstone et al. 2019; Faught and Vijayan 2019). These signals will modulate the transcription of key genes of the pathways concerned with the generation of immune responses.
Once glucocorticoid receptor signalling is activated, some pathways of innate immunity are enhanced, but most pathways of adaptive immunity are suppressed (Cain and Cidlowski 2017). Low concentrations of endogenous corticosteroids upregulate PRRs, thus sensitizing the innate immune system through cytokine receptors and complement factors, thus allowing the induction of acute responses to danger signals (Cain and Cidlowski 2017). High concentrations of glucocorticoids, by contrast, suppress signals that are mediated by PRRs and cytokine receptors, thereby preventing excessive and/or prolonged immune responses. The inhibition of the transcription of immune response genes is mediated by three mechanisms. First, suppression of the glucocorticoid receptor to gene promoter sequences, second, induction of glucocorticoid receptor-mediated transcription of anti-inflammatory genes (such as NF-κβ-IA) and, third, non-genomic antagonism of pro-inflammatory transcription factors (such as NF-κβ) or via protein–protein interaction (Verburg-Van Kemenade et al. 2009). It should also be noted that the reverse signalling pathway has been observed, whereby GR expression is affected by immune stimulation. Thus, injection of LPS increases expression of GR in the spleen of gilthead sea bream (Acerete et al. 2007), and in common carp, GR1 expression in peritoneal leucocytes increased after 1 and 2 days of zymosan treatment (Stolte et al. 2009).
In addition to the modulation of inflammation, not only the effects of glucocorticoids on the immune system are pleiotropic and varied, but also the effects depend on the specific cell or tissue. They mediate antiviral responses including signals that are induced by affected host cells, such as necrosis or apoptosis to generate an enhanced immune gene transcription response. In addition, environmental information reaching the brain allows initiation of immune response programmes via hormones and neurotransmitters in order to mitigate autotoxic risks associated with excessive signalling through enhanced cytokine activation (Verburg-van Kemenade et al. 2011). Thus, it has been shown that glucocorticoid treatments at low concentrations or concentrations that do not involve a significant systemic challenge can enhance inflammatory responses. At higher concentrations, glucocorticoids result in suppressive effects through cytokine signalling, for instance, inhibiting the production of B cells and T cells (Cain and Cidlowski 2017).
The suppressive effects of glucocorticoids have also been shown under in vitro experiments: cortisol in vitro has been shown to reduce phagocytosis in common carp, tilapia and silver sea bream head kidney leucocytes (Law et al. 2001). It also inhibits respiratory burst activity, phagocytosis and chemotaxis dose-dependently in a goldfish macrophage cell line (Wang and Belosevic 1995). Cortisol also decreases respiratory burst activity in sea bass (Vizzini et al. 2007) and sea bream leucocytes (Esteban et al. 2004). Glucocorticoids upregulate IL-10, IL-4 and TGF-β production but downregulate IL-12, TNF-α, and IFN-γ (Verburg-van Kemenade et al. 2011). In vitro cortisol treatment of head kidney leucocytes significantly depresses phagocytosis, chemotaxis and respiratory burst activity in carp, tilapia and silver sea bream (Wang and Belosevic 1995; Verburg-Van Kemenade et al. 2009). Decreased respiratory burst activity has also been observed in sea bream head kidney leucocytes (Esteban et al. 2004). Additionally, cortisol inhibits the LPS-induced expression of serum amyloid protein (Fast et al. 2002; Stolte et al. 2006), inhibits the proliferation of monocytes/macrophages (Pagniello 2002) and induces apoptosis in silver sea bream and Atlantic salmon macrophages isolated from stressed fish, which also show decreased survival when exposed to Aeromonas salmonicida (Fast et al. 2008).
Recently, it has been shown that glucocorticoids also appear to be involved in the polarization of macrophages in fish (Maciuszek et al. 2019), which would explain the contribution of cortisol in the regulation of the macrophage after stress. After pathogen challenge, LPS treatment or, after stress, macrophages polarize inducing an increase in specific cytokines such as TNF-α, which will regulate the resolution of inflammation. Thus, stimulated monocytes produce 11β-HSD1 that converts inert internal cortisol to active internal cortisol, which plays a key role in regulating polarization by promoting the transition from M1- to M2-type macrophages (Thieringer et al. 2001; Maciuszek et al. 2019). Macrophages initiate the immune reaction for pathogen eradication as polarized M1 macrophages under the influence of IFN-γ and/or LPS, but for the modulation of this reaction and for tissue regeneration/wound healing, the polarized M2 macrophages are needed, which appear upon IL-4 or cortisol stimulation.
Although less studied, immune modulation has also been found in the adaptive arm of the immune system after stress. Corticosteroids regulate adaptive immunity by inhibiting lymphocyte activation and promoting lymphocyte apoptosis. In carp, in vitro stimulation with cortisol reduced IgM secretion by head kidney cells, spleen and blood lymphocytes (Saha et al. 2004). In vivo, thermal stress treatments induced decreased antibody responses after immunization (Verburg-van kemenade et al. 1999), inhibition of proliferation and induction of lymphocyte apoptosis (Saha et al. 2004).
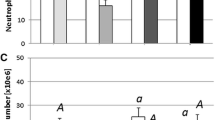
Nevertheless, not all effects are suppressive. Interestingly, for instance, neutrophilic granulocytes remain protected and they do not show decreased numbers after acute stress or glucocorticoid treatment: while a significant lymphopenia is observed after stress treatments, a significant increase in promyelocytes and myelocytes as well as metamyelocytes and mature polymorphonuclear neutrophilic granulocytes takes place (Wojtaszek et al. 2002; Engelsma 2003). Therefore, a dual-cell response has been observed depending on the leucocyte type. As neutrophilic granulocytes are of great importance to the first line of defence, in particular for the phagocytic response, it would be dangerous to leave the whole immune system without a fast active response. Therefore, granulocytes would be beneficial in situations when pathogens may attack an animal subjected to acute stress or injury, having the other responses in a depressed status.
20.4.2 The Neuro-Immune Circuitry Under Stress
In fish under inflammatory episodes, interaction between the neuroendocrine and immune system is always taking place and glucocorticoids are one of the regulators of such inflammatory responses through programming of macrophages. As a consequence, a regulatory balance between pro-inflammatory and anti-inflammatory actions mediated by glucocorticoids is customary. Inflammatory reactions have to be carefully controlled, as high concentration of pro-inflammatory cytokines or the prolonged induction of these cytokines, reactive oxygen species or nitric oxide may be detrimental for host tissues. Glucocorticoids can modulate an excessive inflammatory reaction. Conditions that are associated with acute or chronic stress may either suppress or potentiate control pathways leading to disease progression through modulation of systemic or local pro/anti-inflammatory cytokines and mediators and, consequently, the Th1/Th2 (T helper cells) balance (Verburg-Van Kemenade et al. 2009). Thus, during the activation of the systemic stress response, the immune and inflammatory mechanisms are modulated towards induction of a Th2 shift. In this way, the system protects the organism from excessive activation of Th1 pro-inflammatory cytokines (Calcagni and Elenkov 2006) that could end up with a cytokine storm. Corticosteroids are also able to inhibit inflammation by decreasing signal transduction downstream of PRRs, cytokine receptors and high-affinity IgE receptors (Fce receptors).
In addition to the global modulatory effect of glucocorticoids, a second pathway mediated by the sympathetic nervous system also contributes to the modulation of pro-inflammatory and anti-pathogen programmes of the immune response (Fig. 20.1). Therefore, the regulation of the immune response becomes more complex since activation or deactivation of immune response genes is conditioned by both external influences driven by neural sensors and brain pathways, and internal factors such as cell damage or pathogenic alterations. Furthermore, it should be remembered that the interaction is reciprocal; i.e. regulation of neural activity is modulated by immune response activity. Other important factors such as biological rhythms, previous experience or time course are also processed primarily through the brain and neural circuits, thus influencing the immune response. As the regulation of immune defence in complex organisms such as vertebrates including fish is exerted through leucocytes, such cells will integrate the influences coming from intrinsic immune-related inputs associated with the interaction with microorganism invasion, and the extrinsic inputs derived from the coordinating role of the brain. Hence, in both mammals and fish, catecholamines directly affect immune responses.
Activation of the sympathetic nervous system has been found to alter the production, release and circulation of leucocytes, through upregulation of myelopoiesis, activation of haematopoietic stem cells, NK cells and release of neutrophils and monocytes from the spleen. One example of this crosstalk is the brain-mediated suppression of the sickness and depressed status or the immobilizing behaviours following inflammation, as a way to facilitate other urgent responses such as fight-or-flight behaviour in order to avoid predation or aggression. So far, only a limited number of studies concerning the effects of catecholamines on the expression of inflammatory mediators have been performed compared to studies on glucocorticoids. In carp, secretion of adrenaline reduced the percentage of monocytes/macrophages at the site of inflammation after induced peritonitis while maintaining the total number of leucocytes, which may be related to elevated apoptotic activity and a reduction in mature monocyte/macrophage populations in the peritoneum (Kepka et al. 2012). Stress-related hormones, and particularly adrenaline, modulated the expression of pro-inflammatory and anti-inflammatory cytokines in cultured sea bream leucocytes (Castillo et al. 2008). Whereas cortisol reduced the expression of the assessed cytokines, the effects of adrenaline were not general, less evident and time-dependent. Noradrenaline via stimulation of β-adrenergic receptors modulated gene expression of leucocytes, associated with the signalling cascade of second messengers such as adenylyl cyclase, cyclic AMP and protein kinases. After β-adrenergic signalling, the transcription of IL-4 and IL-5 (Th2 cytokine genes) is stimulated in lymphocytes, whereas the expression of IFN-γ and IL-1β (T1 genes) is suppressed. Thus, biogenic amines not only modulate the adaptive immune response but also can affect innate response programmes, such as suppression of antiviral type I IFN-mediated responses and upregulation of pro-inflammatory cytokine genes, such as IL-1β, IL-6 and TNF (Verburg-Van Kemenade et al. 2009). In addition, it should be noted that catecholamines may act as glucocorticoid secretagogues in the head kidney interrenal tissue. As demonstrated in sea bass (Rotllant et al. 2006), an intracellular adenylate kinase pathway after β-adrenoreceptor activation leads to increased cortisol production. Thus, additional regulation of immune function by adrenaline through cortisol increase can be induced.
In fish, adrenergic agonists decreased phagocytosis of fish macrophages (Roy and Rai 2008). Furthermore, adrenaline and isoproterenol (adrenaline receptor agonist) reduced the production of reactive oxygen species (ROS) in rainbow trout (Oncorhynchus mykiss) pronephric phagocytes (Flory and Bayne 1991), while noradrenaline promoted the respiratory burst in O. mykiss and Channa punctata leucocytes. Both ligands generated this effect via adrenergic receptors. In vitro administration of adrenaline reduced the synthesis of ROS and nitric oxide, while enhancing arginase activity in carp phagocytes. Furthermore, in vitro adrenaline inhibited the expression of pro-inflammatory cytokines, chemokines and their receptors. It was therefore hypothesized that adrenaline will downregulate phagocyte skewing towards innate polarization (Kepka et al. 2012). Innervation of lymphoid tissue has been found in the spleen of Coho salmon (Oncorhynchus kisutch), where nerve fibres are associated with the vasculature and the MMCs. Moreover, immune cells express receptors for neurotransmitters, including adrenergic receptors. So far, adrenergic receptors have been sequenced in zebrafish, goldfish, trout and catfish leukocytes (Kepka et al. 2012).
Regarding brain structures, the blood–brain barrier is similar in fish compared to mammals, showing both molecular and functional similarities, as observed in studies on D. rerio (Jeong et al. 2008). So, the fish brain would be accessible or sensitive to cytokines or other mediator molecules induced by cytokines. However, although the cytokine receptor IL-1RI mRNA was found in the preoptic area of carp brain (Metz et al. 2006) it is not clear whether IL-1β or other important cytokines from outside the brain interact at the brain level, since there are large and hydrophilic molecules that are unlikely to pass through the blood–brain barrier by passive diffusion. Prostaglandins could be the mediators, as they are smaller, lipophilic and neuroactive (Maier 2003).
Evidence suggests that signalling immune mechanisms to the brain in fish may also present high similarities to mammals. It has been proposed that the vagus nerve could serve as a cytokine-to-brain communication route (Maier 2003). Nonetheless, as stressors can induce the increase in peripheral cytokines by the activation of systems such as the noradrenergic pathway, these in turn may lead to increased brain IL-1β via one of the immune to brain routes. Again, few studies have investigated the signalling routes from peripheral blood molecules to the brain, and so, the role of cytokines in the central regulation of the stress reaction and HPI and sympatho-chromaffin (SC) axes activation in fish is not well defined. Cytokine modulation has been shown by adrenaline in fish (Khansari et al. 2017a, b), which could induce the activation of signalling pathways to the brain. Within the brain, pro-inflammatory cytokines reduce the activity of neurotransmitters such as noradrenaline, dopamine and serotonin, which will significantly determine the modulation of behavioural patterns (Weber et al. 2015). Moreover, cytokines activate several other signalling pathways into the brain to integrate both peripheral pro-inflammatory and antigenic signals with physiological and behavioural responses, such as fever, aggression or social interaction. All these mechanisms will work together under stress episodes to integrate the physiological and behavioural response with the necessary protective reaction of immune cells.
Finally, other types of indirect regulation can occur in fish, for instance associated with the connection between the gut and the brain. It has been shown both in mammals and in fish that information by chemical signals is taking place between both organs. Different strains of Gram-negative bacteria are particularly responsive to catecholamines (Butt and Volkoff 2019). Many other hormones are concerned regarding stress episodes and endocrine influences. Therefore, functions like reproduction or growth will be also affected since the neuro-immuno-endocrine interaction is effectively triggered. A clear case study is the Sockeye salmon (Oncorhynchus nerka) in which degeneration of a number of glands and organs has been observed associated with very high levels of steroids and the loss of immunocompetence, particularly innate responses, during the migration period (Dolan et al. 2016). Cortisol and other reproductive steroids divert energy from several biological processes including immune functions to mobilize energy that ensures the fish are able to spawn. Sex steroids have also been found to modulate cortisol production in the interrenal of salmonids (McQuillan et al. 2003). Other hormones are released to the circulation after stress, although they do not show the ample consequences of corticosteroids and catecholamines (Yada and Tort 2016). For instance, the teleost CRF hormone family is tightly associated with immune responses against microbial pathogens. In goldfish (Carassius auratus) and tilapia (Oreochromis mossambicus), brain CRF expression is increased following immune stimulation (Volkoff and Peter 2004; Pepels and Balm 2004). Urocortin, a neuropeptide belonging to the CRF family of hypothalamic hormones, induces changes in many peripheral tissues such as heart, kidney, skin, muscles, spleen and immune cells including macrophages, fibroblasts, lymphocytes and mastocytes in which its receptors CRF1R and CRF2R are highly expressed (Choy et al. 2020). Urocortin has also been shown to help in the regulation of inflammation, interacting with IL-6 and cytokine production, promoting macrophage phagocytosis and bacterial killing, and inducing antimicrobial activity against Gram-positive and Gram-negative bacteria in mammals (Campos-Salinas et al. 2013), thus reducing bacterial burden. In addition, peripheral secretions of urocortin are involved in modulating the peripheral immune response by acting on specific receptors in different populations of immune cells to produce a wide range of effects (Dermitzaki et al. 2018).
20.4.3 Metabolic Trade-Off in Stress-Related Immune Responses
A relevant component of the stress response in animals including fish is the energetic component. Several authors have previously described such an energetic approach by defining the concept of allostatic load, i.e. the fact that alterations in the homeostasis of the animal will produce a cost in energetic terms that will have to be paid back in order to maintain the metabolic and physiological equilibrium, either in the short term or in the longer term (Romero et al. 2009). Thus, any surplus in energy expenditure devoted to overcome the stress situation will generate compensations associated with disposal of energetic resources, otherwise used for other purposes. This compensation may affect the efficacy of some functions, in particular immune induction and cell proliferation, which are highly energy-demanding: in mammals, under hypoxic (1–4% oxygen) conditions, T cells cease to proliferate, cytokines are not produced, and even granulocytes that depend on anaerobic glycolysis may be impaired (Ohta 2018). Resting immune cells, such as circulating monocytes, memory T cells, plasma B cells and their naive phenotypes, tend to rely on minimal metabolism fuelled by oxidative phosphorylation, low-level glycolysis and fatty acid oxidation, but once activated during inflammation, switch to metabolic reprogramming that translates in enhanced glucose uptake, aerobic glycolysis and synthesis of fatty acids (Gaber et al. 2017). This sustains proliferation, clonal expansion, chemokine and cytokine release, and regulation of lymphoid lineages during stress-derived pro-inflammatory processes, controlled by the activation of the hypoxia-inducible factor 1α (HIF-1α) and mTOR pathways, whereas AMP activation results in anti-inflammatory onsets that inhibit mTOR signalling and reduce cell responses (O’Neill and Hardie 2013). Overall, mounting a sustained inflammatory response increases the basal metabolic rate (BMR) by an estimate of 30–50% in homoeothermic vertebrates, with half of the energetic requirements dedicated to produce the hepatic acute-phase response (Lochmiller and Deerenberg 2000; Straub et al. 2010). In fish, vaccinated rainbow trout may endure a 20% increase in BMR over the course of 1 month (Ackerman et al. 2000; Skinner et al. 2010), whereas E. coli lipopolysaccharide (LPS) elicited a transient (48 h) increase in BMR in mosquitofish (Gambusia holbrooki) to overcompensate for the inflammatory response (Bonneaud et al. 2016). Interestingly, a recent study about the impact in juvenile O. mykiss of several stressors failed to show a strong correlation between metabolic and immune trade-offs (Wernicke von Siebenthal et al. 2018). The authors assessed the effects on resource allocation in trout enduring limited food availability (thus mimicking the seasonally scarcity of resources in the original habitat), parasitic infection by Tetracapsuloides bryosalmonae, a myxozoan parasite that causes proliferative kidney disease (PKD) in salmonids (Sudhagar et al. 2019), and exposure to ethinyloestradiol (EE2), a common endocrine disruptor (Aris et al. 2014). The compensatory responses favoured the production of immune mediators in infected fish, but also the growth of immune organs in the resource-depleted experimental group, albeit with little metabolic trade-offs between EE2-induced vitellogenesis, infection and growth. These and other results indicate the importance of metabolic plasticity in acute vs chronic inflammation, but also the complex intricacies, still unresolved, of the species-specific combined effects of the type and dosage of the stressor in fish, even if the ecological niche or population specificities are considered.
20.5 Immune Futures: A Glimpse of the Complexities of Environmental Influences in Stress-Related Immune Responses
Searching for a conceptualization of the unavoidable scalability of stress-related immune responses and mechanisms across cells, tissues, organs, individuals and ecological assemblages, several authors have proposed theoretical approaches to the whole concept of biological stress, such as the control theory model (Del Giudice et al. 2018) based on the regulatory mechanisms to recover physiological balance, or the damage fitness model (Wada and Heidinger 2019; Breuner and Berk 2019), based on the mechanisms to avoid or recover from damage and maintain the overall health (Romero 2004). However, the mismatch between the multiple dimensions of the physiological response and the individual tools and indicators used to quantify and evaluate such responses makes it difficult to obtain fine-grain resolution of such multilevel theoretical approaches required for analysing the complexity of real-world scenarios (Telemeco and Gangloff 2020). Moreover, even if the effects of stress-related molecular mediators on the susceptibility to diseases may be similar in metazoans (Costantini et al. 2011), the secretory patterns of the same molecules that are the trademark of stress overtaking organism homeostasis in vertebrates, such as corticosteroids, are highly variable between sexes, populations, species, life-history strategies, lifecycles, social status and biotopes (Breuner et al. 2013; Hau et al. 2016), impairing the formulation of a cohesive conceptualization of the coupling of stress responses to environmental changes. Defining the factors that constrict the immune features and capabilities of fish enduring unexpected and persistent stressors in ever-changing environments demands a multilevel approach based on the evolutionary history of each species (Fig. 20.2). As described elsewhere, such stressotopes, adaptive scenarios with variable but measurable selective pressures over multiscale immune responses, niche-specific and capable of generating allostatic loads (Balasch and Tort 2019), may enable, together with conceptualizations of stress and immune responses based upon evolutionary roadmaps (Becker et al. 2020; Taborsky et al. 2021), a precise framework to analyse probable outcomes of immune response in stressful environments.
It is expected that, confronted with human-induced abrupt changes, fish may rely on developmental plasticity mechanisms to cope with stressful environments (Petitjean et al. 2019), but to what extent altered phenotypes may influence survival and unexpected changes in environmental variables is still unknown. An example may help to outline the difficulties of analysing a common stressor in the context of the fish immune response. Fish (with the exception of a few pelagic species with high metabolic basal rates) are ectothermic and their immune response diminishes in cold waters (Tong and Li 2020). Therefore, temperature changes are the main driver behind the evolution of immune repertories in fish, with pathogenic as an undesired direct effect of rising temperatures that strongly modulates host–pathogen interactions.
Any attempt to describe the effects of temperature on teleost immunity ends, as often happens with interspecies studies in fish, with a hard to summarize list of enhancements and impairments of temperature-regulated ups and downs of immune functionality. This is due to different experimental approaches, variable effects of optimal temperature-dependent virulence factors in pathogenic bacteria, several differences in the thermopreferendum of hosts and combined stressors (such as low oxygen levels, xenobiotics, emergent contaminants or hormone-mimicking pharmaceuticals) and the fact that temperature effects on teleost defensive responses are strongly seasonal, highly variable between species, populations with different life histories and even between individuals and sexes (Butler et al. 2013; Guijarro et al. 2015; Abram et al. 2017; Stewart et al. 2018; Marchand et al. 2019; Petitjean et al. 2020). Although no specific rules seem to apply to what is clearly a multifactorial sum of effects, recent surveys suggest that low temperatures may impair adaptive immune responses (especially T-cell-mediated immunity) and potentiate innate, unspecific defensive mechanisms, especially when several stressors are combined (Wentworth et al. 2018; Defo et al. 2019; Ignatz et al. 2020; Sun et al. 2020). In this sense, it has been suggested that infected or immunocompromised fish tend to actively search for hot spots to increase body temperature (Gräns et al. 2012; Boltaña et al. 2013). Inducing such behavioural fever (Covert and Reynolds 1977) contributes to enhance immune dynamics, mimicking the immune performance, both innate and adaptive, showed by temperate teleost species in spring (Buchtíková et al. 2011; Brown et al. 2016). Seasonal oscillations in temperature affect immunocompetence directly, by enhancing the circannual gene expression of immune-related genes (Gracey et al. 2004; Boltaña et al. 2013; Stewart et al. 2018; Abolfathi et al. 2020), but also indirectly, by interaction with the programmed onset of the reproductive hormonal axis, and seasonal cycles of pathogen abundance (Baekelandt et al. 2020). Long-term acclimation to non-optimal temperature regimes may improve an otherwise sluggish immune response at low temperatures, and accelerate phenotypic plasticity of immune-related gene expression responses, even in high-temperature scenarios (Scott and Johnston 2012; Abram et al. 2017; Tong and Li 2020).
To further complicate matters, the seasonality of immune function is multifactorial (Fig. 20.2), and the effects of temperature can be overridden by exogenous and endogenous variables, usually related to the onset of the reproductive hypothalamic–pituitary–gonadal (HPG) axis during the mating season, which affects immunocompetence. For example, male three-spine sticklebacks (Gasterosteus aculeatus) parasitized by Schistocephalus solidus and exposed to 17β-oestradiol (E2) suffered greater parasite load than females (Macnab et al. 2016; Ling et al. 2020), indicating sex-specific (and also host-specific) effects on the interaction between the HPG and immune axis. Similarly, the effects of altered temperature regimes, combined with acute gill net entanglement and air exposure, two common stressors in aquaculture practices, suppressed female maturation and enhanced immune responses, increasing the parasite burden in a sex-dependent manner (Teffer et al. 2019). As mentioned before, shared receptors between immune and endocrine cells (Fig. 20.1), together with the intimate housing of the HPI and HPG axis in the teleost head kidney, facilitate neuroimmunoendocrine crosstalk, and estrogens have been deemed the culprit of immune modulations in fish (Szwejser et al. 2017; Cabas et al. 2018; Chaves-Pozo et al. 2018). The seasonal surges of E2 in teleost mature breeding females may affect immune responses, decreasing IgM levels and impairing cytokine networks (Cuesta et al. 2007; Seemann et al. 2016), altering the expression of Foxp3, a transcription factor for the development of TREG cells (Wei et al. 2013). However, these and other immunosuppressive effects are markedly species-specific and still not well understood.
In addition, the link between temperature changes and pathogen distribution and virulence is expected to alter the immune plasticity of fishes (Claar and Wood 2020). In an ocean of microbes, temperature-driven pathogen outbreaks may act as an adaptive cue to boost metabolism, substitute behavioural fever for genuine endothermic fever, and abandon ectothermic metabolism in favour of a more efficient, memory-based, adaptive immunity, less dependent on low temperatures. In ectotherms, it has been argued that an immunity with increasingly cell consuming efficient affinity maturation of Ig in well-structured lymphoid organs cannot be realized due to an unaffordable metabolic cost by most ectotherms with slow metabolism (Lee 2006; Sandmeier and Tracy 2014). Besides, investing in increasingly efficient pathogen detectors may set the basis for an arm’s race that selects for strategies of immune evasion and rapid changes in virulence from pathogens (Hedrick 2004; Guo et al. 2019), which would throw both host and pathogen to unending Red Queen evolutionary dynamics (Liow et al. 2011) of continuous adaptations simply to remain unharmed. These processes may have precluded an evolutionary urge for the development of fully functional endothermy in fish. Clearly, the origins of endothermy are far more complex and more species-specific than to be reduced only to immune solutions to compromised or stressful habitats (Nespolo et al. 2017; van de Pol et al. 2017), but the very few cases of functional endothermy found to date in fish (Soyano and Mushirobira 2018) seem to suggest that fish have adopted an immune strategy of resistance to pathogens, which appears somewhat defective compared with the mammalian-type hyper-specialized systems (such as immune receptor affinity maturation, multiple niche- and organ-specific cellular phenotypes, Ig isotype diversification and switching, and long-lasting memory). Between tolerance to pathogens (Råberg and Stjernman 2012), as showed by some aquatic invertebrates, or resistance, teleosts seem to have opted by fitting ectothermic metabolism to already highly plastic immune capabilities, suggesting that, in teleosts, the evolvability of the immune system has contributed to their evolutionary success, high diversity of lifestyles and widespread adaptive radiations (Solbakken et al. 2017).
Overall, even a common stressor of fish, namely temperature changes, requires an evolutionary background to delineate the foreground characteristics of stress-related immune performance. The consequences of the extreme diversity of fish lifecycles on stress-related immune function are overwhelmingly complex, but immensely rewarding for testing evolutionary models of immune system development coupled with ecological parameters. To date, few multiscale functional studies have addressed the intricacies of host–pathogen interaction in teleosts beyond the snapshot-like analysis of transcriptomes, proteomic surveys and metabolomic assessments of biological pathways. Genomic data are incredibly useful to systematize fish immune diversity and have rapidly becoming the by-default approach to evaluate environmental insults in fish physiology. However, integrated multiscale efforts are needed to discriminate between evolutionary changes in phenotypic plasticity in order to clarify the impact of combined stressors in host-pathogen immune negotiations in changing ecological contexts. Life story trade-offs often rest upon tortuous evolutionary paths across multiple interactions between overlapping species, competitive scenarios and unexpected environmental changes, sometimes ill-suited to human-induced extensive remodelling of contemporary environments. Combined analysis of environmental stressors in species-specific preferred niches may help to elucidate direct or indirect immune-related effects of environmental variables, which, to date, seem to affect immune responses in an indirect and elusive way. Ocean acidification, for example, seems to affect mainly acid–base homeostasis, branchial osmoregulation and overall metabolic scope, resulting in behavioural alterations, again in a species-specific manner (Esbaugh 2018). However, some results are controversial (Clark et al. 2020), and immune function under rising CO2 partial pressures is poorly studied. Transcriptional analysis of acidification effects in gill tissues of barramundi (Lates calcarifer) suggests that acidified seawater modulates the expression of transcripts related to antigen processing and presentation, mucosal Ig and NK-mediated cytotoxicity (Ma et al. 2020). Similarly, Senegalese Sole (Solea senegalensis) enduring severe aquatic hypercapnia showed a short-term (up to 24 h) increase in plasma total bactericidal and antiprotease activities in mucosal lymphoid branchial tissues (Machado et al. 2020). However, chronic exposure (4 weeks) enhanced the expression of a limited repertoire of cytokine (IL-1β, IL-10) and cyclooxygenase 2 (COX2) transcripts related to inflammatory responses. Even if these results indicate an activated immune response in branchial tissues, the systemic effects of acidification are far from being resolved, and may be restricted mainly to local inflammation in gill mucosae or a result of impaired metabolic trade-offs that prime other physiological systems. In any case, as demonstrated by the evolutionary intricacies of amphibious fishes (Wright and Turko 2016), phenotypic and developmental plasticity must be included in the short- to long-term response to rapid environmental changes in aquatic CO2 levels in fish. In this sense, resolving the fish immune tree of life will necessarily require a compilation of branching ecological influences on host lifetime, together with hologenomic approaches and niche-specific preferences.
20.6 Conclusion
Vertebrate immune systems suffer from acute horror vacui. Confronted with heavily microorganism-loaded environments, and constantly changing ornamental antigens anchored to pathogen surfaces, aquatic animals respond with functional redundancy of defensive components, multifunctional organs, constant production of unrequested naive lymphocytes and iterative cellular selection processes that completely fill the physiological tapestry of organisms. From an ecological perspective, fish immune phenotypes change over evolutionary time resembling developmentally and evolutionarily labile dose–response frameworks (Hedrick 2004; Grimholt 2018; Ravi and Venkatesh 2018). They are nurtured by the constant confrontation with stressors that question self-, non-self- and altered-self-antigenic components, but also by abrupt changes in environmental shelters, for instance, human-induced disturbances in preferred niches, or reproductive and metabolic adjustments to harsh habitats in the heart of a microbial-enriched environment (McCauley et al. 2015; Kelly and Salinas 2017; Ling et al. 2020). Fish possess the unspecific immune response to foreign molecules characteristic of invertebrates, reinforced by the canonical adaptive, specific and energetically expensive inflammatory reactivity of vertebrates (Palti 2011; Flajnik 2018; Patel et al. 2018; Rebl and Goldammer 2018). However, as discussed, a handful of exclusive characteristics and drawbacks differentiate fish immunity from that found in endothermic (i.e. birds and mammals) vertebrates. Several factors account for these differences, the most preeminent being the (1) physical, microbiological and human-influenced characteristics of aquatic dwellings, (2) long-vanished palaeoclimatic events (Solbakken et al. 2017) and (3) the high species specificity of immune responses, partly ameliorated by phenotypic and developmental plasticity to environmental changes (Farrell and Franklin 2016).
Overall, the immune reactivity of fish to environmental insults is influenced by the type and intensity of exogenous stressors (alone or combined), may be modulated by the phenotypic plasticity of behavioural responses, depends on sex-, population- species-specific and niche-related differences, and suffers the lags in the maturation of adaptive immunocompetence in larvae and adults. Even though stress-related neuroendocrine interactions have been and continue to be thoroughly analysed in teleosts, and several studies addressed neuroimmunoendocrine influences in stressed fish (Verburg-van Kemenade et al. 2017; Das et al. 2018; Shepherd et al. 2018; Hou et al. 2019), the impact and crosstalk of immune altered states in the activation of stress-related neurohormonal frameworks are still not fully characterized in fish. This is partly due to the model-centric methodological approach that favours some laboratory-friendly, novel translational research-related or simply commercial teleost species, at the expense of less known but more abundant and diversified fish (Schartl 2014). In an attempt to describe a more global picture of allostatic load effects in stressed fish, the focus now is on multibiomarker approaches that may render accurate descriptions of fish immunoreactivy in realistic multifactorial scenarios, where several combined parameters (temperature, pathogen load, multiplicity of stressors, behavioural phenotypes, etc.) can be analysed simultaneously in an integrated framework (Khansari et al. 2018; Silva et al. 2018; Vargas et al. 2018; Dallarés et al. 2020). Unfortunately, we are still far from the development of an analytical framework, such as stressotopes (Balasch and Tort 2019) that may allow for the comprehensive multilevel description of evolved adaptive interactions between an individual’s physiology and their preferred niche. However, a promising step may be the hologenomic approach (Limborg et al. 2018), in which the diverse methodologies for the expression analysis of fish host genomes, together with commensal and pathogenic mucosal microbiota, coalesce to integrate molecular and functional data in the light of evolutionary choices under environmental pressures.
Abbreviations
- Ag:
-
antigen
- AID:
-
activation-induced deaminase enzyme
- AMP:
-
antimicrobial protein
- APC:
-
antigen-presenting cells
- APP:
-
acute-phase protein
- APR:
-
acute-phase response
- BMR:
-
basal metabolic rate
- CSR:
-
class switch recombination
- DAMPs:
-
danger/damage-associated molecular patterns
- E2:
-
17β-oestradiol
- EE2:
-
ethinyloestradiol
- FDC:
-
follicular dendritic cell
- GR:
-
glucocorticoid receptor
- HIRECs:
-
human-induced rapid environmental changes
- HPG:
-
hypothalamic–pituitary–gonadal axis
- HPI:
-
hypothalamic–pituitary–interrenal axis
- HPT:
-
hypothalamic–pituitary–thyroid axis
- IFN:
-
interferon
- IGF:
-
growth hormone (GH)/insulin-like growth factor axis
- LLPC:
-
long-lived plasma cell
- LPS:
-
bacterial lipopolysaccharide
- MHC:
-
major histocompatibility complex
- MMC:
-
melanomacrophage centre
- MR:
-
mineralocorticoid receptor
- NK:
-
natural killer cells
- PAMP:
-
pathogen-associated molecular pattern
- PKD:
-
proliferative kidney disease
- PRR:
-
pattern recognition receptor
- RAG:
-
recombination-activating gene
- ROS:
-
reactive oxygen species
- SC:
-
sympatho-chromaffin axis
- SHM:
-
somatic hypermutation
- TCR:
-
T-cell receptor
- TLR:
-
Toll-like receptor
- VLRs:
-
variable lymphocyte receptors
References
Abolfathi M, Akbarzadeh A, Hajimoradloo A, Joshaghani HR (2020) Seasonal changes of hydrolytic enzyme activities in the skin mucus of rainbow trout, Oncorhynchus mykiss at different body sizes. Dev Comp Immunol 103:103499. https://doi.org/10.1016/j.dci.2019.103499
Abram QH, Dixon B, Katzenback BA (2017) Impacts of low temperature on the teleost immune system. Biology (Basel) 6(4). https://doi.org/10.3390/biology6040039
Acerete L, Balasch JC, Castellana B, Redruello B, Roher N, Canario AV, Planas JV, MacKenzie S, Tort L (2007) Cloning of the glucocorticoid receptor (GR) in gilthead seabream (Sparus aurata): Differential expression of GR and immune genes in gilthead seabream after an immune challenge. Comp Biochem Physiol B: Biochem Mol Biol 148(1):32–43. https://doi.org/10.1016/j.cbpb.2007.04.015
Ackerman PA, Iwama GK, Thornton JC (2000) Physiological and immunological effects of adjuvanted aeromonas salmonicida vaccines on juvenile rainbow trout. J Aquat Anim Health 12(2):157–164. https://doi.org/10.1577/1548-8667(200006)012<0157:PAIEOA>2.0.CO;2
Anderson HL, Brodsky IE, Mangalmurti NS (2018) The evolving erythrocyte: red blood cells as modulators of innate immunity. J Immunol 201(5):1343–1351. https://doi.org/10.4049/jimmunol.1800565
Aris AZ, Shamsuddin AS, Praveena SM (2014) Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: a review. Environ Int 69:104–119. https://doi.org/10.1016/j.envint.2014.04.011
Baekelandt S, Milla S, Cornet V, Flamion E, Ledoré Y, Redivo B, Antipine S, Mandiki SNM, Houndji A, El Kertaoui N, Kestemont P (2020) Seasonal simulated photoperiods influence melatonin release and immune markers of pike perch Sander lucioperca. Sci Rep 10:2650. https://doi.org/10.1038/s41598-020-59568-1
Bajoghli B, Dick AM, Claasen A, Doll L, Aghaallaei N (2019) Zebrafish and Medaka: two teleost models of T-cell and thymic development. Int J Mol Sci 20(17):4179. https://doi.org/10.3390/ijms20174179
Balasch JC, Tort L (2019) Netting the stress responses in fish. Front Endocrinol 10:62. https://doi.org/10.3389/fendo.2019.00062
Bayne CJ, Gerwick L (2001) The acute phase response and innate immunity of fish. Dev Comp Immunol 25(8–9):725–743. https://doi.org/10.1016/S0145-305X(01)00033-7
Beaulieu AM (2018) Memory responses by natural killer cells. J Leukoc Biol 104(6):1087–1096. https://doi.org/10.1002/JLB.1RI0917-366R
Becker DJ, Albery GF, Kessler MK, Lunn TJ, Falvo CA, Czirják GÁ, Martin LB, Plowright RK (2020) Macroimmunology: the drivers and consequences of spatial patterns in wildlife immune defence. J Anim Ecol 89(4):972–995. https://doi.org/10.1111/1365-2656.13166
Beemelmanns A, Roth O (2016) Biparental immune priming in the pipefish Syngnathus typhle. Zoology 119(4):262–272. https://doi.org/10.1016/j.zool.2016.06.002
Beemelmanns A, Roth O (2017) Grandparental immune priming in the pipefish Syngnathus typhle. BMC Evol Biol 17(1):44. https://doi.org/10.1186/s12862-017-0885-3
Black PH (2003) The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun 17(5):350–364. https://doi.org/10.1016/S0889-1591(03)00048-5
Boltaña S, Rey S, Roher N, Vargas R, Huerta M, Huntingford FA, Goetz FW, Moore J, Garcia-Valtanen P, Estepa A, MacKenzie S (2013) Behavioural fever is a synergic signal amplifying the innate immune response. Proc R Soc B Biol Sci 280(1766):20131381. https://doi.org/10.1098/rspb.2013.1381
Bonneaud C, Wilson RS, Seebacher F (2016) Immune-challenged fish up-regulate their metabolic scope to support locomotion. PLoS One 11(11):e0166028. https://doi.org/10.1371/journal.pone.0166028
Bowden TJ, Cook P, Rombout JHWM (2005) Development and function of the thymus in teleosts. Fish Shellfish Immunol 19(5):413–427. https://doi.org/10.1016/j.fsi.2005.02.003
Breitbart M (2011) Marine viruses: truth or dare. Annu Rev Mar Sci 4(1):425–448. https://doi.org/10.1146/annurev-marine-120709-142805
Breuner C, Berk S (2019) Using the van Noordwijk and de Jong resource framework to evaluate glucocorticoid-fitness hypotheses. Integr Comp Biol 59:243–250. https://doi.org/10.1093/icb/icz088
Breuner CW, Delehanty B, Boonstra R (2013) Evaluating stress in natural populations of vertebrates: total CORT is not good enough. Funct Ecol 27(1):24–36. https://doi.org/10.1111/1365-2435.12016
Broecker F, Moelling K (2019) Evolution of immune systems from viruses and transposable elements. Front Microbiol 10:51. https://doi.org/10.3389/fmicb.2019.00051
Brown M, Hablützel P, Friberg IM, Thomason AG, Stewart A, Pachebat JA, Jackson JA (2016) Seasonal immunoregulation in a naturally-occurring vertebrate. BMC Genomics 17:369. https://doi.org/10.1186/s12864-016-2701-7
Brum JR, Ignacio-Espinoza JC, Roux S, Doulcier G, Acinas SG, Alberti A, Chaffron S, Cruaud C, de Vargas C, Gasol JM, Gorsky G, Gregory AC, Guidi L, Hingamp P, Iudicone D, Not F, Ogata H, Pesant S, Poulos BT, Schwenck SM, Speich S, Dimier C, Kandels-Lewis S, Picheral M, Searson S, Coordinators TO, Bork P, Bowler C, Sunagawa S, Wincker P, Karsenti E, Sullivan MB (2015) Patterns and ecological drivers of ocean viral communities. Science 348(6237):1261498. https://doi.org/10.1126/science.1261498
Buchtíková S, Šimková A, Rohlenová K, Flajšhans M, Lojek A, Lilius E-M, Hyršl P (2011) The seasonal changes in innate immunity of the common carp (Cyprinus carpio). Aquaculture 318(1):169–175. https://doi.org/10.1016/j.aquaculture.2011.05.013
Buonocore F, Gerdol M (2016) Alternative adaptive immunity strategies: coelacanth, cod and shark immunity. Mol Immunol 69:157–169. https://doi.org/10.1016/j.molimm.2015.09.003
Butler MW, Stahlschmidt ZR, Ardia DR, Davies S, Davis J, Guillette LJ, Johnson N, McCormick SD, McGraw KJ, DeNardo DF, Associate Editor: Williams TD, Editor: Bronstein JL (2013) Thermal sensitivity of immune function: evidence against a generalist-specialist trade-off among endothermic and ectothermic vertebrates. Am Nat 181(6):761–774. doi:https://doi.org/10.1086/670191.
Butt RL, Volkoff H (2019) Gut microbiota and energy homeostasis in fish. Front Endocrinol 10:6–8. https://doi.org/10.3389/fendo.2019.00009
Caballero-Huertas M, Moraleda-Prados J, Joly S, Ribas L (2020) Immune genes, IL1β and Casp9, show sexual dimorphic methylation patterns in zebrafish gonads. Fish Shellfish Immunol 97:648–655. https://doi.org/10.1016/j.fsi.2019.12.013
Cabas I, Chaves-Pozo E, Mulero V, García-Ayala A (2018) Role of estrogens in fish immunity with special emphasis on GPER1. Dev Comp Immunol 89:102–110. https://doi.org/10.1016/j.dci.2018.08.001
Cai L, Jørgensen BB, Suttle CA, He M, Cragg BA, Jiao N, Zhang R (2019) Active and diverse viruses persist in the deep sub-seafloor sediments over thousands of years. ISME J 13(7):1857–1864. https://doi.org/10.1038/s41396-019-0397-9
Cain DW, Cidlowski JA (2017) Immune regulation by glucocorticoids. Nat Rev Immunol 17(4):233–247. https://doi.org/10.1038/nri.2017.1
Calcagni E, Elenkov I (2006) Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci 1069:62–76. https://doi.org/10.1196/annals.1351.006
Calisi RM, Saldanha CJ (2015) Neurohormones, brain, and behavior: a comparative approach to understanding rapid neuroendocrine action. Integr Comp Biol 55(2):264–267. https://doi.org/10.1093/icb/icv007
Campos-Salinas J, Caro M, Cavazzuti A, Forte-Lago I, Beverley SM, O’Valle F, Gonzalez-Rey E (2013) Protective role of the neuropeptide urocortin II against experimental sepsis and leishmaniasis by direct killing of pathogens. J Immunol 191(12):6040–6051. https://doi.org/10.4049/jimmunol.1301921
Castillo J, Castellana B, Acerete L, Planas JV, Goetz FW, Mackenzie S, Tort L (2008) Stress-induced regulation of steroidogenic acute regulatory protein expression in head kidney of Gilthead seabream (Sparus aurata). J Endocrinol 196(2):313–322. https://doi.org/10.1677/JOE-07-0440
Chaves-Pozo E, García-Ayala A, Cabas I (2018) Effects of sex steroids on fish leukocytes. Biology (Basel) 7(1):9. https://doi.org/10.3390/biology7010009
Chiappelli F, Franceschi C, Ottaviani E, Farne M, Faisal M (1993) Phylogeny of the neuroendocrine-immune system: Fish and shellfish as model systems for social interaction stress research in humans. Annu Rev Fish Dis 3(written):327–346. doi:https://doi.org/10.1016/0959-8030(93)90042-A.
Choy KW, Tsai AP-Y, Lin PB-C, Wu M-Y, Lee C, Alias A, Pang C-Y, Liew H-K (2020) The role of urocortins in intracerebral hemorrhage. Biomol Ther 10(1):96. https://doi.org/10.3390/biom10010096
Christensen KA, Davidson WS (2017) Autopolyploidy genome duplication preserves other ancient genome duplications in Atlantic salmon (Salmo salar). PLoS One 12(2):e0173053. https://doi.org/10.1371/journal.pone.0173053
Claar DC, Wood CL (2020) Pulse heat stress and parasitism in a warming world. Trends Ecol Evol 35(8):704–715. https://doi.org/10.1016/j.tree.2020.04.002
Clark TD, Raby GD, Roche DG, Binning SA, Speers-Roesch B, Jutfelt F, Sundin J (2020) Ocean acidification does not impair the behaviour of coral reef fishes. Nature 577(7790):370–375. https://doi.org/10.1038/s41586-019-1903-y
Cline TJ, Ohlberger J, Schindler DE (2019) Effects of warming climate and competition in the ocean for life-histories of Pacific salmon. Nat Ecol Evol 3(6):935–942. https://doi.org/10.1038/s41559-019-0901-7
Clucas GV, Lou RN, Therkildsen NO, Kovach AI (2019) Novel signals of adaptive genetic variation in northwestern Atlantic cod revealed by whole-genome sequencing. Evol Appl 12(10):1971–1987. https://doi.org/10.1111/eva.12861
Cohen IR, Efroni S (2019) The immune system computes the state of the body: crowd wisdom, machine learning, and immune cell reference repertoires help manage inflammation. Front Immunol 10. https://doi.org/10.3389/fimmu.2019.00010
Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB (2012) Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. PNAS 109(16):5995–5999. https://doi.org/10.1073/pnas.1118355109
Costantini D, Marasco V, Møller AP (2011) A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B 181(4):447–456. https://doi.org/10.1007/s00360-011-0566-2
Covert JB, Reynolds WW (1977) Survival value of fever in fish. Nature 267(5606):43–45. https://doi.org/10.1038/267043a0
Crawford DL, Schulte PM, Whitehead A, Oleksiak MF (2020) Evolutionary physiology and genomics in the highly adaptable killifish (Fundulus heteroclitus). Compr Physiol 10(2):637–671. https://doi.org/10.1002/cphy.c190004
Crespi EJ, Williams TD, Jessop TS, Delehanty B (2013) Life history and the ecology of stress: How do glucocorticoid hormones influence life-history variation in animals? Funct Ecol 27(1):93–106. https://doi.org/10.1111/1365-2435.12009
Cuesta A, Vargas-Chacoff L, García-López A, Arjona FJ, Martínez-Rodríguez G, Meseguer J, Mancera JM, Esteban MA (2007) Effect of sex-steroid hormones, testosterone and estradiol, on humoral immune parameters of gilthead seabream. Fish Shellfish Immunol 23(3):693–700. https://doi.org/10.1016/j.fsi.2007.01.015
D’Souza MP, Adams E, Altman JD, Birnbaum ME, Boggiano C, Casorati G, Chien Y, Conley A, Eckle SBG, Früh K, Gondré-Lewis T, Hassan N, Huang H, Jayashankar L, Kasmar AG, Kunwar N, Lavelle J, Lewinsohn DM, Moody B, Picker L, Ramachandra L, Shastri N, Parham P, McMichael AJ, Yewdell JW (2019) Casting a wider net: Immunosurveillance by nonclassical MHC molecules. PLoS Pathog 15(2):e1007567. https://doi.org/10.1371/journal.ppat.1007567
da Costa JC, Val AL (2020) Extreme climate scenario and parasitism affect the Amazonian fish Colossoma macropomum. Sci Total Environ 726:138628. https://doi.org/10.1016/j.scitotenv.2020.138628
Dallarés S, Dourado P, Sanahuja I, Solovyev M, Gisbert E, Montemurro N, Torreblanca A, Blázquez M, Solé M (2020) Multibiomarker approach to fipronil exposure in the fish Dicentrarchus labrax under two temperature regimes. Aquat Toxicol 219:105378. https://doi.org/10.1016/j.aquatox.2019.105378
Das C, Thraya M, Vijayan MM (2018) Nongenomic cortisol signaling in fish. Gen Comp Endocrinol 265:121–127. https://doi.org/10.1016/j.ygcen.2018.04.019
Defo MA, Gendron AD, Head J, Pilote M, Turcotte P, Marcogliese DJ, Houde M (2019) Cumulative effects of cadmium and natural stressors (temperature and parasite infection) on molecular and biochemical responses of juvenile rainbow trout. Aquat Toxicol 217:105347. https://doi.org/10.1016/j.aquatox.2019.105347
Del Giudice M, Gangestad SW (2018) Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun 70:61–75. https://doi.org/10.1016/j.bbi.2018.02.013
Del Giudice M, Buck CL, Chaby L, Gormally B, Taff C, Thawley C, Vitousek M, Wada H (2018) What is stress? A systems perspective. Integr Comp Biol 58:1019–1032. https://doi.org/10.1093/icb/icy114
Dermitzaki E, Venihaki M, Tsatsanis C, Gravanis A, Avgoustinaki PD, Liapakis G, Margioris AN (2018) The multi-faceted profile of corticotropin-releasing factor (CRF) family of neuropeptides and of their receptors on the paracrine/local regulation of the inflammatory response. Curr Mol Pharmacol 11(1):39–50. https://doi.org/10.2174/1874467210666170109164430
Dickson KA, Graham JB (2004) Evolution and consequences of endothermy in fishes. Physiol Biochem Zool 77(6):998–1018. https://doi.org/10.1086/423743
Dios S, Romero A, Chamorro R, Figueras A, Novoa B (2010) Effect of the temperature during antiviral immune response ontogeny in teleosts. Fish Shellfish Immunol 29(6):1019–1027. https://doi.org/10.1016/j.fsi.2010.08.006
Dolan BP, Fisher KM, Colvin ME, Benda SE, Peterson JT, Kent ML, Schreck CB (2016) Innate and adaptive immune responses in migrating spring-run adult chinook salmon, Oncorhynchus tshawytscha. Fish Shellfish Immunol 48:136–144. https://doi.org/10.1016/j.fsi.2015.11.015
Donelan SC, Hellmann JK, Bell AM, Luttbeg B, Orrock JL, Sheriff MJ, Sih A (2020) transgenerational plasticity in human-altered environments. Trends Ecol Evol (Amst) 35(2):115–124. https://doi.org/10.1016/j.tree.2019.09.003
Duarte CM, Hendriks IE, Moore TS, Olsen YS, Steckbauer A, Ramajo L, Carstensen J, Trotter JA, McCulloch M (2013) Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuar Coasts 36(2):221–236. https://doi.org/10.1007/s12237-013-9594-3
Duarte CM, Agusti S, Barbier E, Britten GL, Castilla JC, Gattuso J-P, Fulweiler RW, Hughes TP, Knowlton N, Lovelock CE, Lotze HK, Predragovic M, Poloczanska E, Roberts C, Worm B (2020) Rebuilding marine life. Nature 580(7801):39–51. https://doi.org/10.1038/s41586-020-2146-7
Ebbesson LOE, Braithwaite VA (2012) Environmental effects on fish neural plasticity and cognition. J Fish Biol 81(7):2151–2174. https://doi.org/10.1111/j.1095-8649.2012.03486.x
Egerton S, Culloty S, Whooley J, Stanton C, Ross RP (2018) The gut microbiota of marine fish. Front Microbiol 9:873. https://doi.org/10.3389/fmicb.2018.00873
Engelsma M (2003) Multiple acute temperature stress affects leucocyte populations and antibody responses in common carp, Cyprinus carpio L. Fish Shellfish Immunol 15(5):397–410. https://doi.org/10.1016/S1050-4648(03)00006-8
Esbaugh AJ (2018) Physiological implications of ocean acidification for marine fish: emerging patterns and new insights. J Comp Physiol B Biochem Syst Environ Physiol 188(1):1–13. https://doi.org/10.1007/s00360-017-1105-6
Esteban MA, Rodríguez A, Ayala AG, Meseguer J (2004) Effects of high doses of cortisol on innate cellular immune response of seabream (Sparus aurata L.). Gen Comp Endocrinol 137(1):89–98. https://doi.org/10.1016/j.ygcen.2004.02.006
Falk-Petersen IB (2005) Comparative organ differentiation during early life stages of marine fish. Fish Shellfish Immunol 19(5):397–412. https://doi.org/10.1016/j.fsi.2005.03.006
Farrell AP, Franklin CE (2016) Recognizing thermal plasticity in fish. Science 351(6269):132–133. https://doi.org/10.1126/science.351.6269.132-b
Fast MD, Sims DE, Burka JF, Mustafa A, Ross NW (2002) Skin morphology and humoral non-specific defence parameters of mucus and plasma in rainbow trout, coho and Atlantic salmon. Comp Biochem Physiol Part A Mol Integr Physiol 132(3):645–657. https://doi.org/10.1016/S1095-6433(02)00109-5
Fast MD, Hosoya S, Johnson SC, Afonso LOB (2008) Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short- and long-term stress. Fish Shellfish Immunol 24(2):194–204. https://doi.org/10.1016/j.fsi.2007.10.009
Faught E, Vijayan MM (2018) The mineralocorticoid receptor is essential for stress axis regulation in zebrafish larvae. Sci Rep 8(1):1–11. https://doi.org/10.1038/s41598-018-36681-w
Faught E, Vijayan M (2019) Glucocorticoid and mineralocorticoid receptor activation modulates postnatal growth. J Endocrinol 244. https://doi.org/10.1530/JOE-19-0358
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282. https://doi.org/10.1146/annurev.physiol.61.1.243
Ferraresso S, Bonaldo A, Parma L, Buonocore F, Scapigliati G, Gatta PP, Bargelloni L (2016) Ontogenetic onset of immune-relevant genes in the common sole (Solea solea). Fish Shellfish Immunol 57:278–292. https://doi.org/10.1016/j.fsi.2016.08.044
Flajnik MF (2018) A cold-blooded view of adaptive immunity. Nat Rev Immunol 18(7):438–453. https://doi.org/10.1038/s41577-018-0003-9
Flory CM, Bayne CJ (1991) The influence of adrenergic and cholinergic agents on the chemiluminescent and mitogenic responses of leukocytes from the rainbow trout, Oncorhynchus mykiss. Dev Comp Immunol 15:135–142
Gaber T, Strehl C, Buttgereit F (2017) Metabolic regulation of inflammation. Nat Rev Rheumatol 13(5):267–279. https://doi.org/10.1038/nrrheum.2017.37
Gaw S, Thomas KV, Hutchinson TH (2014) Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Philos Trans R Soc Lond Ser B Biol Sci 369(1656). https://doi.org/10.1098/rstb.2013.0572
Geoghegan JL, Di Giallonardo F, Cousins K, Shi M, Williamson JE, Holmes EC (2018) Hidden diversity and evolution of viruses in market fish. Virus Evol 4(2):vey 031. https://doi.org/10.1093/ve/vey031
Geven EJW, Klaren PHM (2017) The teleost head kidney: integrating thyroid and immune signalling. Dev Comp Immunol 66:73–83. https://doi.org/10.1016/j.dci.2016.06.025
Ghosh S, May MJ, Kopp EB (1998) NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16:225–260. https://doi.org/10.1146/annurev.immunol.16.1.225
Ginhoux F, Guilliams M (2016) Tissue-resident macrophage ontogeny and homeostasis. Immunity 44(3):439–449. https://doi.org/10.1016/j.immuni.2016.02.024
Glasauer SMK, Neuhauss SCF (2014) Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol Gen Genomics 289(6):1045–1060. https://doi.org/10.1007/s00438-014-0889-2
Gracey AY, Fraser EJ, Li W, Fang Y, Taylor RR, Rogers J, Brass A, Cossins AR (2004) Coping with cold: an integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate. PNAS 101(48):16970–16975. https://doi.org/10.1073/pnas.0403627101
Gräns A, Rosengren M, Niklasson L, Axelsson M (2012) Behavioural fever boosts the inflammatory response in rainbow trout Oncorhynchus mykiss. J Fish Biol 81(3):1111–1117. https://doi.org/10.1111/j.1095-8649.2012.03333.x
Grimholt U (2018) Whole genome duplications have provided teleosts with many roads to peptide loaded MHC class I molecules. BMC Evol Biol 18(1):25. https://doi.org/10.1186/s12862-018-1138-9
Guijarro JA, Cascales D, García-Torrico AI, García-Domínguez M, Méndez J (2015) Temperature-dependent expression of virulence genes in fish-pathogenic bacteria. Front Microbiol 6:700. https://doi.org/10.3389/fmicb.2015.00700
Guindre-Parker S (2020) Individual variation in glucocorticoid plasticity: considerations and future directions. Integr Comp Biol 60(1):79–88. https://doi.org/10.1093/icb/icaa003
Guo CJ, He J, He JG (2019) The immune evasion strategies of fish viruses. Fish Shellfish Immunol 86:772–784. https://doi.org/10.1016/j.fsi.2018.12.013
Gutzeit C, Chen K, Cerutti A (2018) The enigmatic function of IgD: some answers at last. Eur J Immunol 48(7):1101–1113. https://doi.org/10.1002/eji.201646547
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319(5865):948–952. https://doi.org/10.1126/science.1149345
Hara Y, Yamaguchi K, Onimaru K, Kadota M, Koyanagi M, Keeley SD, Tatsumi K, Tanaka K, Motone F, Kageyama Y, Nozu R, Adachi N, Nishimura O, Nakagawa R, Tanegashima C, Kiyatake I, Matsumoto R, Murakumo K, Nishida K, Terakita A, Kuratani S, Sato K, Hyodo S, Kuraku S (2018) Shark genomes provide insights into elasmobranch evolution and the origin of vertebrates. Nat Ecol Evol 2(11):1761–1771. https://doi.org/10.1038/s41559-018-0673-5
Hau M, Casagrande S, Ouyang JQ, Baugh AT (2016) Chapter two- glucocorticoid-mediated phenotypes in vertebrates: multilevel variation and evolution. In: Naguib M, Mitani JC, Simmons LW, Barrett L, Healy S, Zuk M (eds) Advances in the study of behavior. Academic, pp. 41–115.
Hedrick SM (2004) The acquired immune system: a vantage from beneath. Immunity 21(5):607–615. https://doi.org/10.1016/j.immuni.2004.08.020
Hou Z-S, Wen H-S, Li J-F, He F, Li Y, Qi X (2019) Effects of long-term crowding stress on neuro-endocrine-immune network of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 95:180–189. https://doi.org/10.1016/j.fsi.2019.10.011
Hughes LC, Ortí G, Huang Y, Sun Y, Baldwin CC, Thompson AW, Arcila D, Betancur-R R, Li C, Becker L, Bellora N, Zhao X, Li X, Wang M, Fang C, Xie B, Zhou Z, Huang H, Chen S, Venkatesh B, Shi Q (2018) Comprehensive phylogeny of ray-finned fishes (Actinopterygii) based on transcriptomic and genomic data. Proc Natl Acad Sci U S A 115(24):6249–6254. https://doi.org/10.1073/pnas.1719358115
Ignatz EH, Braden LM, Benfey TJ, Caballero-Solares A, Hori TS, Runighan CD, Fast MD, Westcott JD, Rise ML (2020) Impact of rearing temperature on the innate antiviral immune response of growth hormone transgenic female triploid Atlantic salmon (Salmo salar). Fish Shellfish Immunol 97:656–668. https://doi.org/10.1016/j.fsi.2019.12.081
Irwin MR, Cole SW (2011) Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol 11(9):625–632. https://doi.org/10.1038/nri3042
Iwasaki A, Medzhitov R (2015) Control of adaptive immunity by the innate immune system. Nat Immunol 16(4):343–353. https://doi.org/10.1038/ni.3123
Jeong J-Y, Kwon H-B, Ahn J-C, Kang D, Kwon S-H, Park JA, Kim K-W (2008) Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res Bull 75(5):619–628. https://doi.org/10.1016/j.brainresbull.2007.10.043
Johansson L-H, Timmerhaus G, Afanasyev S, Jørgensen SM, Krasnov A (2016) Smoltification and seawater transfer of Atlantic salmon (Salmo salar L.) is associated with systemic repression of the immune transcriptome. Fish Shellfish Immunol 58:33–41. https://doi.org/10.1016/j.fsi.2016.09.026
Johnstone WM, Honeycutt JL, Deck CA, Borski RJ (2019) Chapter Two - Nongenomic glucocorticoid effects and their mechanisms of action in vertebrates. In: Galluzzi L (ed) International review of cell and molecular biology. Academic, pp 51–96
Jousset A, Bienhold C, Chatzinotas A, Gallien L, Gobet A, Kurm V, Küsel K, Rillig MC, Rivett DW, Salles JF, van der Heijden MGA, Youssef NH, Zhang X, Wei Z, Hol WHG (2017) Where less may be more: how the rare biosphere pulls ecosystems strings. ISME J 11(4):853–862. https://doi.org/10.1038/ismej.2016.174
Kelly C, Salinas I (2017) Under pressure: interactions between commensal microbiota and the teleost immune system. Front Immunol 8:559. https://doi.org/10.3389/fimmu.2017.00559
Kelly AM, Vitousek MN (2017) Dynamic modulation of sociality and aggression: an examination of plasticity within endocrine and neuroendocrine systems. Philos Trans R Soc B Biol Sci 372(1727). https://doi.org/10.1098/rstb.2016.0243
Kepka M, Verburg-van Kemenade BML, Chadzinska M (2012) Neuroendocrine modulation of the inflammatory response in common carp: adrenaline regulates leukocyte profile and activity. Gen Comp Endocrinol 188(1):102–109. https://doi.org/10.1016/j.ygcen.2012.11.014
Khansari AR, Parra D, Reyes-López FE, Tort L (2017a) Cytokine modulation by stress hormones and antagonist specific hormonal inhibition in rainbow trout (Oncorhynchus mykiss) and gilthead sea bream (Sparus aurata) head kidney primary cell culture. Gen Comp Endocrinol. https://doi.org/10.1016/j.ygcen.2017.06.005
Khansari AR, Parra D, Reyes-López FE, Tort L (2017b) Modulatory in vitro effect of stress hormones on the cytokine response of rainbow trout and gilthead sea bream head kidney stimulated with Vibrio anguillarum bacterin. Fish Shellfish Immunol 70:736–749. https://doi.org/10.1016/j.fsi.2017.09.009
Khansari AR, Balasch JC, Vallejos-Vidal E, Parra D, Reyes-López FE, Tort L (2018) Comparative immune- and stress-related transcript response induced by air exposure and vibrio anguillarum bacterin in rainbow trout (Oncorhynchus mykiss) and gilthead seabream (Sparus aurata) mucosal surfaces. Front Immunol 9:856. https://doi.org/10.3389/fimmu.2018.00856
Kotsias F, Cebrian I, Alloatti A (2019) Antigen processing and presentation. Int Rev Cell Mol Biol 348:69–121. https://doi.org/10.1016/bs.ircmb.2019.07.005
Kuraku S, Meyer A, Kuratani S (2009) Timing of genome duplications relative to the origin of the vertebrates: did cyclostomes diverge before or after? Mol Biol Evol 26(1):47–59. https://doi.org/10.1093/molbev/msn222
Kyriakis JM, Avruch J (2012) Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev 92(2):689–737. https://doi.org/10.1152/physrev.00028.2011
Landys MM, Ramenofsky M, Wingfield JC (2006) Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol 148(2):132–149. https://doi.org/10.1016/j.ygcen.2006.02.013
Law WY, Chen WH, Song YL, Dufour S, Chang CF (2001) Differential in vitro suppressive effects of steroids on leukocyte phagocytosis in two teleosts, tilapia and common carp. Gen Comp Endocrinol 121(2):163–172. https://doi.org/10.1006/gcen.2000.7593
Lee KA (2006) Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol 46(6):1000–1015. https://doi.org/10.1093/icb/icl049
Li Y, Xia P, Wu J, Huang A, Bu G, Meng F, Kong F, Cao X, Han X, Yu G, Pan X, Yang S, Zeng X, Du X (2020) The potential sensing molecules and signal cascades for protecting teleost fishes against lipopolysaccharide. Fish Shellfish Immunol 97:235–247. https://doi.org/10.1016/j.fsi.2019.12.050
Lieschke GJ, Currie PD (2007) Animal models of human disease: zebrafish swim into view. Nat Rev Genet 8(5):353–367. https://doi.org/10.1038/nrg2091
Limborg MT, Alberdi A, Kodama M, Roggenbuck M, Kristiansen K, Gilbert MTP (2018) Applied hologenomics: feasibility and potential in aquaculture. Trends Biotechnol 36(3):252–264. https://doi.org/10.1016/j.tibtech.2017.12.006
Ling F, Steinel N, Weber J, Ma L, Smith C, Correa D, Zhu B, Bolnick D, Wang G (2020) The gut microbiota response to helminth infection depends on host sex and genotype. ISME J:1–13. doi:https://doi.org/10.1038/s41396-020-0589-3.
Liow LH, Van Valen L, Stenseth NC (2011) Red Queen: from populations to taxa and communities. Trends Ecol Evol (Amst) 26(7):349–358. https://doi.org/10.1016/j.tree.2011.03.016
Locati M, Curtale G, Mantovani A (2020) Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol 15:123–147. https://doi.org/10.1146/annurev-pathmechdis-012418-012718
Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88(1):87–98. https://doi.org/10.1034/j.1600-0706.2000.880110.x
Lomstein BA, Langerhuus AT, D’Hondt S, Jørgensen BB, Spivack AJ (2012) Endospore abundance, microbial growth and necromass turnover in deep sub-seafloor sediment. Nature 484(7392):101–104. https://doi.org/10.1038/nature10905
Ma Z, Zheng X, Fu Z, Lin S, Yu G, Qin JG (2020) Transcriptional analysis reveals physiological response to acute acidification stress of barramundi Lates calcarifer (Bloch) in coastal areas. Fish Physiol Biochem 46(5):1729–1741. https://doi.org/10.1007/s10695-020-00824-6
Machado M, Arenas F, Svendsen JC, Azeredo R, Pfeifer LJ, Wilson JM, Costas B (2020) Effects of water acidification on senegalese sole solea senegalensis health status and metabolic rate: implications for immune responses and energy use. Front Physiol 11:26. https://doi.org/10.3389/fphys.2020.00026
Maciuszek M, Rydz L, Świtakowska I, Verburg-van Kemenade BML, Chadzińska M (2019) Effects of stress and cortisol on the polarization of carp macrophages. Fish Shellfish Immunol 94:27–37. https://doi.org/10.1016/j.fsi.2019.08.064
Macnab V, Katsiadaki I, Tilley CA, Barber I (2016) Oestrogenic pollutants promote the growth of a parasite in male sticklebacks. Aquat Toxicol 174:92–100. https://doi.org/10.1016/j.aquatox.2016.02.010
Magor BG (2015) Antibody affinity maturation in fishes—our current understanding. Biology (Basel) 4(3):512–524. https://doi.org/10.3390/biology4030512
Maier SF (2003) Bi-directional immune-brain communication: implications for understanding stress, pain, and cognition. Brain Behav Immun 17(2):69–85. https://doi.org/10.1016/S0889-1591(03)00032-1
Majzoub K, Wrensch F, Baumert TF (2019) The innate antiviral response in animals: an evolutionary perspective from flagellates to humans. Viruses 11(8). https://doi.org/10.3390/v11080758
Maldonado E, Rangel-Huerta E, Rodriguez-Salazar E, Pereida-Jaramillo E, Martínez-Torres A (2020) Subterranean life: behavior, metabolic, and some other adaptations of Astyanax cavefish. J Exp Zool B Mol Dev Evol. https://doi.org/10.1002/jez.b.22948
Manel S, Guerin P-E, Mouillot D, Blanchet S, Velez L, Albouy C, Pellissier L (2020) Global determinants of freshwater and marine fish genetic diversity. Nat Commun 11(1):1–9. https://doi.org/10.1038/s41467-020-14409-7
Marchand A, Tebby C, Beaudouin R, Hani YMI, Porcher J-M, Turies C, Bado-Nilles A (2019) Modelling the effect of season, sex, and body size on the three-spined stickleback, Gasterosteus aculeatus, cellular innate immunomarkers: a proposition of laboratory reference ranges. Sci Total Environ 648:337–349. https://doi.org/10.1016/j.scitotenv.2018.07.381
Marcogliese DJ (2002) Food webs and the transmission of parasites to marine fish. Parasitology 124(Suppl):S83–S99. https://doi.org/10.1017/s003118200200149x
Maruska K, Soares MC, Lima-Maximino M, Henrique de Siqueira-Silva D, Maximino C (2019) Social plasticity in the fish brain: neuroscientific and ethological aspects. Brain Res 1711:156–172. https://doi.org/10.1016/j.brainres.2019.01.026
Mazzucotelli E, Mastrangelo AM, Crosatti C, Guerra D, Stanca AM, Cattivelli L (2008) Abiotic stress response in plants: when post-transcriptional and post-translational regulations control transcription. Plant Sci 174(4):420–431. https://doi.org/10.1016/j.plantsci.2008.02.005
McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR (2015) Marine defaunation: animal loss in the global ocean. Science 347(6219). https://doi.org/10.1126/science.1255641
McMenamin SK, Parichy DM (2013) Metamorphosis in teleosts. Curr Top Dev Biol 103:127–165. https://doi.org/10.1016/B978-0-12-385979-2.00005-8
McQuillan HJ, Lokman PM, Young G (2003) Effects of sex steroids, sex, and sexual maturity on cortisol production: an in vitro comparison of chinook salmon and rainbow trout interrenals. Gen Comp Endocrinol 133(1):154–163. https://doi.org/10.1016/S0016-6480(03)00163-1
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454(7203):428–435. https://doi.org/10.1038/nature07201
Methot SP, Di Noia JM (2017) Molecular mechanisms of somatic hypermutation and class switch recombination. Adv Immunol 133:37–87. https://doi.org/10.1016/bs.ai.2016.11.002
Metz J, Huising M, Leon K, Verburg-van Kemenade BML, Flik G (2006) Central and peripheral interleukin-1β and interleukin-1 receptor I expression and their role in the acute stress response of common carp, Cyprinus carpio L. J Endocrinol 191:25–35. https://doi.org/10.1677/joe.1.06640
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186(6):3299–3303. https://doi.org/10.4049/jimmunol.1003613
Middelboe M, Brussaard CPD (2017) Marine viruses: key players in marine ecosystems. Viruses 9(10). https://doi.org/10.3390/v9100302
Miest JJ, Politis SN, Adamek M, Tomkiewicz J, Butts IAE (2019) Molecular ontogeny of larval immunity in European eel at increasing temperatures. Fish Shellfish Immunol 87:105–119. https://doi.org/10.1016/j.fsi.2018.12.048
Miles J, Scherz-Shouval R, van Oosten-Hawle P (2019) Expanding the organismal proteostasis network: linking systemic stress signaling with the innate immune response. Trends Biochem Sci 44(11):927–942. https://doi.org/10.1016/j.tibs.2019.06.009
Morera D, MacKenzie SA (2011) Is there a direct role for erythrocytes in the immune response? Vet Res 42(1):89. https://doi.org/10.1186/1297-9716-42-89
Morera D, Roher N, Ribas L, Balasch JC, Doñate C, Callol A, Boltaña S, Roberts S, Goetz G, Goetz FW, MacKenzie SA (2011) Rna-seq reveals an integrated immune response in nucleated erythrocytes. PLoS One 6(10). https://doi.org/10.1371/journal.pone.0026998
Near TJ, Eytan RI, Dornburg A, Kuhn KL, Moore JA, Davis MP, Wainwright PC, Friedman M, Smith WL (2012) Resolution of ray-finned fish phylogeny and timing of diversification. Proc Natl Acad Sci U S A 109(34):13698–13703. https://doi.org/10.1073/pnas.1206625109
Neefjes J, Jongsma MLM, Paul P, Bakke O (2011) Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol 11(12):823–836. https://doi.org/10.1038/nri3084
Nelson J, Grande T, Wilson M (2016) Fishes of the world, 5th edn. Wiley
Nespolo RF, Solano-Iguaran JJ, Bozinovic F (2017) Phylogenetic analysis supports the aerobic-capacity model for the evolution of endothermy. Am Nat 189(1):13–27. https://doi.org/10.1086/689598
Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, O’Neill LAJ, Xavier RJ (2016) Trained immunity: a program of innate immune memory in health and disease. Science 352(6284). https://doi.org/10.1126/science.aaf1098
Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E (2010) The human glucocorticoid receptor: molecular basis of biologic function. Steroids 75(1):1–12. https://doi.org/10.1016/j.steroids.2009.09.002
O’Neill LAJ, Hardie DG (2013) Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493(7432):346–355. https://doi.org/10.1038/nature11862
Ohta A (2018) Oxygen-dependent regulation of immune checkpoint mechanisms. Int Immunol 30(8):335–343. https://doi.org/10.1093/intimm/dxy038
Orsi WD (2018) Ecology and evolution of seafloor and subseafloor microbial communities. Nat Rev Microbiol 16(11):671–683. https://doi.org/10.1038/s41579-018-0046-8
Pagniello K (2002) Effect of corticosteroids on viability and proliferation of the rainbow trout monocyte/macrophage cell line, RTS11. Fish Shellfish Immunol 13(3):199–214. https://doi.org/10.1006/fsim.2001.0395
Palti Y (2011) Toll-like receptors in bony fish: from genomics to function. Dev Comp Immunol 35(12):1263–1272. https://doi.org/10.1016/j.dci.2011.03.006
Panettieri RA, Schaafsma D, Amrani Y, Koziol-White C, Ostrom R, Tliba O (2019) Non-genomic effects of glucocorticoids: an updated view. Trends Pharmacol Sci 40(1):38–49. https://doi.org/10.1016/j.tips.2018.11.002
Parham P, Moffett A (2013) How did variable NK-cell receptors and MHC class I ligands influence immunity, reproduction and human evolution? Nat Rev Immunol 13(2):133–144. https://doi.org/10.1038/nri3370
Patel B, Banerjee R, Samanta M, Das S (2018) Diversity of immunoglobulin (Ig) isotypes and the role of activation-induced cytidine deaminase (AID) in fish. Mol Biotechnol 60(6):435–453. https://doi.org/10.1007/s12033-018-0081-8
Pepels PPLM, Balm PHM (2004) Ontogeny of corticotropin-releasing factor and of hypothalamic–pituitary–interrenal axis responsiveness to stress in tilapia (Oreochromis mossambicus; Teleostei). Gen Comp Endocrinol 139(3):251–265. https://doi.org/10.1016/j.ygcen.2004.09.013
Perdiguero P, Martín-Martín A, Benedicenti O, Díaz-Rosales P, Morel E, Muñoz-Atienza E, García-Flores M, Simón R, Soleto I, Cerutti A, Tafalla C (2019) Teleost IgD+IgM- B cells mount clonally expanded and mildly mutated intestinal IgD responses in the absence of lymphoid follicles. 29(13):Cell Rep, 4223–C4235.e5. https://doi.org/10.1016/j.celrep.2019.11.101
Petitjean Q, Jean S, Gandar A, Côte J, Laffaille P, Jacquin L (2019) Stress responses in fish: from molecular to evolutionary processes. Sci Total Environ 684:371–380. https://doi.org/10.1016/j.scitotenv.2019.05.357
Petitjean Q, Jean S, Côte J, Lamarins A, Lefranc M, Santos R, Perrault A, Laffaille P, Jacquin L (2020) Combined effects of temperature increase and immune challenge in two wild gudgeon populations. Fish Physiol Biochem 46(1):157–176. https://doi.org/10.1007/s10695-019-00706-6
Poulin R (2011) Uneven distribution of cryptic diversity among higher taxa of parasitic worms. Biol Lett 7(2):241–244. https://doi.org/10.1098/rsbl.2010.0640
Poulin R (2016) Greater diversification of freshwater than marine parasites of fish. Int J Parasitol 46(4):275–279. https://doi.org/10.1016/j.ijpara.2015.12.002
Prabhu Das M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, McCune JM, Mor G, Schulz L, Soares M, Spencer T, Strominger J, Way SS, Yoshinaga K (2015) Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol 16(4):328–334. https://doi.org/10.1038/ni.3131
Priede IG, Froese R (2013) Colonization of the deep sea by fishes. J Fish Biol 83(6):1528–1550. https://doi.org/10.1111/jfb.12265
Puente-Marin S, Thwaite R, Mercado L, Coll J, Roher N, Del Mar O-VM (2019) Fish red blood cells modulate immune genes in response to bacterial inclusion bodies made of TNFα and a g-VHSV fragment. Front Immunol 10:1–11. https://doi.org/10.3389/fimmu.2019.01055
Råberg L, Stjernman M (2012) The evolutionary ecology of infectious disease virulence. In: Demas GE, Nelson RJ (eds) Ecoimmunology. Oxford University Press, Oxford, pp 548–578
Rampoldi F, Ullrich L, Prinz I (2020) Revisiting the interaction of γδ T-cells and B-cells. Cells 9(3). https://doi.org/10.3390/cells9030743
Ravi V, Venkatesh B (2018) The divergent genomes of teleosts. Annu Rev Anim Biosci 6:47–68. https://doi.org/10.1146/annurev-animal-030117-014821
Rebl A, Goldammer T (2018) Under control: the innate immunity of fish from the inhibitors’ perspective. Fish Shellfish Immunol 77:328–349. https://doi.org/10.1016/j.fsi.2018.04.016
Reverter M, Tapissier-Bontemps N, Lecchini D, Banaigs B, Sasal P (2018) Biological and ecological roles of external fish mucus: a review. Aust Fish 3. https://doi.org/10.3390/fishes3040041
Ribeiro E, Davis AM, Rivero-Vega RA, Ortí G, Betancur RR (2018) Post-cretaceous bursts of evolution along the benthic-pelagic axis in marine fishes. Proc Biol Sci 285(1893):20182010. https://doi.org/10.1098/rspb.2018.2010
Rohde K (2002) Ecology and biogeography of marine parasites. Adv Mar Biol 43:1–86. https://doi.org/10.1016/s0065-2881(02)43002-7
Rohlenová K, Morand S, Hyršl P, Tolarová S, Flajšhans M, Šimková A (2011) Are fish immune systems really affected by parasites? An immunoecological study of common carp (Cyprinus carpio). Parasit Vectors 4:120. https://doi.org/10.1186/1756-3305-4-120
Romero LM (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol (Personal edition) 19(5):249–255. https://doi.org/10.1016/j.tree.2004.03.008
Romero LM, Gormally BMG (2019) How truly conserved is the “well-conserved” vertebrate stress response? Integr Comp Biol 59(2):273–281. https://doi.org/10.1093/icb/icz011
Romero LM, Dickens MJ, Cyr NE (2009) The reactive scope model – a new model integrating homeostasis, allostasis, and stress. Horm Behav 55(3):375–389. https://doi.org/10.1016/j.yhbeh.2008.12.009
Romero A, Vega M, Santibáñez N, Spies J, Pérez T, Enríquez R, Kausel G, Oliver C, Oyarzún R, Tort L, Vargas-Chacoff L (2020) Salmo salar glucocorticoid receptors analyses of alternative splicing variants under stress conditions. Gen Comp Endocrinol 293(March). https://doi.org/10.1016/j.ygcen.2020.113466
Rossi GS, Tunnah L, Martin KE, Turko AJ, Taylor DS, Currie S, Wright PA (2019) Mangrove fishes rely on emersion behavior and physiological tolerance to persist in sulfidic environments. Physiol Biochem Zool 92(3):316–325. https://doi.org/10.1086/703117
Roth O, Beemelmanns A, Barribeau SM, Sadd BM (2018) Recent advances in vertebrate and invertebrate transgenerational immunity in the light of ecology and evolution. Heredity (Edinb) 121(3):225–238. https://doi.org/10.1038/s41437-018-0101-2
Roth O, Solbakken MH, Tørresen OK, Bayer T, Matschiner M, Baalsrud HT, Hoff SNK, Brieuc MSO, Haase D, Hanel R, Reusch TBH, Jentoft S (2020) Evolution of male pregnancy associated with remodeling of canonical vertebrate immunity in seahorses and pipefishes. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1916251117
Rotllant J, Ruane NM, Dinis MT, Canario AVM, Power DM (2006) Intra-adrenal interactions in fish: catecholamine stimulated cortisol release in sea bass (Dicentrarchus labrax L.). Comp Biochem Physiol A Mol Integr Physiol 143(3):375–381. https://doi.org/10.1016/j.cbpa.2005.12.027
Roy B, Rai U (2008) Role of adrenoceptor-coupled second messenger system in sympatho-adrenomedullary modulation of splenic macrophage functions in live fish Channa punctatus. Gen Comp Endocrinol 155(2):298–306. https://doi.org/10.1016/j.ygcen.2007.05.008
Ru H, Zhang P, Wu H (2018) Structural gymnastics of RAG-mediated DNA cleavage in V(D)J recombination. Curr Opin Struct Biol 53:178–186. https://doi.org/10.1016/j.sbi.2018.11.001
Saha NR, Usami T, Suzuki Y (2004) In vitro effects of steroid hormones on IgM-secreting cells and IgM secretion in common carp (Cyprinus carpio). Fish Shellfish Immunol 17(2):149–158. https://doi.org/10.1016/j.fsi.2004.01.001
Sala AJ, Bott LC, Morimoto RI (2017) Shaping proteostasis at the cellular, tissue, and organismal level. J Cell Biol 216(5):1231–1241. https://doi.org/10.1083/jcb.201612111
Salinas I (2015) The mucosal immune system of teleost fish. Biology (Basel) 4(3):525–539. https://doi.org/10.3390/biology4030525
Sandmeier FC, Tracy RC (2014) The metabolic pace-of-life model: incorporating ectothermic organisms into the theory of vertebrate ecoimmunology. Integr Comp Biol 54(3):387–395. https://doi.org/10.1093/icb/icu021
Saunders HL, Oko AL, Scott AN, Fan CW, Magor BG (2010) The cellular context of AID expressing cells in fish lymphoid tissues. Dev Comp Immunol 34(6):669–676. https://doi.org/10.1016/j.dci.2010.01.013
Schaefer L (2014) Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem 289(51):35237–35245. https://doi.org/10.1074/jbc.R114.619304
Scharsack JP, Franke F, Erin NI, Kuske A, Büscher J, Stolz H, Samonte IE, Kurtz J, Kalbe M (2016) Effects of environmental variation on host-parasite interaction in three-spined sticklebacks (Gasterosteus aculeatus). Zoology (Jena) 119(4):375–383. https://doi.org/10.1016/j.zool.2016.05.008
Schartl M (2014) Beyond the zebrafish: diverse fish species for modeling human disease. Dis Model Mech 7(2):181–192. https://doi.org/10.1242/dmm.012245
Scott GR, Johnston IA (2012) Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. PNAS 109(35):14247–14252. https://doi.org/10.1073/pnas.1205012109
Secombes CJ, Zou J (2017) Evolution of interferons and interferon receptors. Front Immunol 8:209. https://doi.org/10.3389/fimmu.2017.00209
Seemann F, Knigge T, Duflot A, Marie S, Olivier S, Minier C, Monsinjon T (2016) Sensitive periods for 17β-estradiol exposure during immune system development in sea bass head kidney. J Appl Toxicol 36(6):815–826. https://doi.org/10.1002/jat.3215
Shepherd BS, Spear AR, Philip AM, Leaman DW, Stepien CA, Sepulveda-Villet OJ, Palmquist DE, Vijayan MM (2018) Effects of cortisol and lipopolysaccharide on expression of select growth-, stress- and immune-related genes in rainbow trout liver. Fish Shellfish Immunol 74:410–418. https://doi.org/10.1016/j.fsi.2018.01.003
Shi M, Lin X-D, Chen X, Tian J-H, Chen L-J, Li K, Wang W, Eden J-S, Shen J-J, Liu L, Holmes EC, Zhang Y-Z (2018) The evolutionary history of vertebrate RNA viruses. Nature 556(7700):197–202. https://doi.org/10.1038/s41586-018-0012-7
Sievers M, Hale R, Parris KM, Swearer SE (2018) Impacts of human-induced environmental change in wetlands on aquatic animals. Biol Rev Camb Philos Soc 93(1):529–554. https://doi.org/10.1111/brv.12358
Silva AT, Midwood JD, Aarestrup K, Pottinger TG, Madsen SS, Cooke SJ (2018) The influence of sex, parasitism, and ontogeny on the physiological response of European Eels (Anguilla anguilla) to an abiotic stressor. Physiol Biochem Zool 91(4):976–986. https://doi.org/10.1086/698689
Skinner LA, Schulte PM, Balfry SK, McKinley RS, LaPatra SE (2010) The association between metabolic rate, immune parameters, and growth performance of rainbow trout, Oncorhynchus mykiss (Walbaum), following the injection of a DNA vaccine alone and concurrently with a polyvalent, oil-adjuvanted vaccine. Fish Shellfish Immunol 28(2):387–393. https://doi.org/10.1016/j.fsi.2009.11.026
Smith JJ, Timoshevskaya N, Ye C, Holt C, Keinath MC, Parker HJ, Cook ME, Hess JE, Narum SR, Lamanna F, Kaessmann H, Timoshevskiy VA, Waterbury CKM, Saraceno C, Wiedemann LM, Robb SMC, Baker C, Eichler EE, Hockman D, Sauka-Spengler T, Yandell M, Krumlauf R, Elgar G, Amemiya CT (2018) The sea lamprey germline genome provides insights into programmed genome rearrangement and vertebrate evolution. Nat Genet 50(2):270–277. https://doi.org/10.1038/s41588-017-0036-1
Smith NC, Rise ML, Christian SL (2019) A comparison of the innate and adaptive immune systems in cartilaginous fish, ray-finned fish, and lobe-finned fish. Front Immunol 10:2292. https://doi.org/10.3389/fimmu.2019.02292
Smithwick FM, Stubbs TL (2018) Phanerozoic survivors: actinopterygian evolution through the Permo-Triassic and Triassic-Jurassic mass extinction events. Evolution 72(2):348–362. https://doi.org/10.1111/evo.13421
Solbakken MH, Voje KL, Jakobsen KS, Jentoft S (2017) Linking species habitat and past palaeoclimatic events to evolution of the teleost innate immune system. Proc R Soc B Biol Sci 284(1853):20162810. https://doi.org/10.1098/rspb.2016.2810
Sotiropoulos D, Tsihrintzis G (2017) Artificial immune systems. Springer, Cham, pp 159–235
Soyano K, Mushirobira Y (2018) The mechanism of low-temperature tolerance in fish. Adv Exp Med Biol 1081:149–164. https://doi.org/10.1007/978-981-13-1244-1_9
Stanley RRE, DiBacco C, Lowen B, Beiko RG, Jeffery NW, Wyngaarden MV, Bentzen P, Brickman D, Benestan L, Bernatchez L, Johnson C, Snelgrove PVR, Wang Z, Wringe BF, Bradbury IR (2018) A climate-associated multispecies cryptic cline in the northwest Atlantic. Sci Adv 4(3):eaaq 0929. https://doi.org/10.1126/sciadv.aaq0929
Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N, Mandelboim M, Mandelboim O (2008) Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol 9(9):1065–1073. https://doi.org/10.1038/ni.1642
Stewart A, Hablützel PI, Watson HV, Brown M, Friberg IM, Cable J, Jackson JA (2018) Physical cues controlling seasonal immune allocation in a natural piscine model. Front Immunol 9:582. https://doi.org/10.3389/fimmu.2018.00582
Stolte EH, Van Kemenade BMLV, Savelkoul HFJ, Flik G (2006) Evolution of glucocorticoid receptors with different glucocorticoid sensitivity. J Endocrinol 190:17–28. https://doi.org/10.1677/joe.1.06703
Stolte EH, Chadzinska M, Przybylska D, Flik G, Savelkoul HFJ, Verburg-van Kemenade BML (2009) The immune response differentially regulates Hsp70 and glucocorticoid receptor expression in vitro and in vivo in common carp (Cyprinus carpio L.). Fish Shellfish Immunol 27(1):9–16. https://doi.org/10.1016/j.fsi.2008.11.003
Stosik MP, Tokarz-Deptuła B, Deptuła W (2019) Melanomacrophages and melanomacrophage centres in Osteichthyes. Cent Eur J Immunol 44(2):201–205. https://doi.org/10.5114/ceji.2019.87072
Straub RH, Cutolo M, Buttgereit F, Pongratz G (2010) Energy regulation and neuroendocrine–immune control in chronic inflammatory diseases. J Intern Med 267(6):543–560. https://doi.org/10.1111/j.1365-2796.2010.02218.x
Sudhagar A, Kumar G, El-Matbouli M (2019) The malacosporean myxozoan parasite tetracapsuloides bryosalmonae: a threat to wild salmonids. Pathogens 9(1). https://doi.org/10.3390/pathogens9010016
Sun J-L, Zhao L-L, Liao L, Tang X-H, Cui C, Liu Q, He K, Ma J-D, Jin L, Yan T, Zhou J, Yang S (2020) Interactive effect of thermal and hypoxia on largemouth bass (Micropterus salmoides) gill and liver: Aggravation of oxidative stress, inhibition of immunity and promotion of cell apoptosis. Fish Shellfish Immunol 98:923–936. https://doi.org/10.1016/j.fsi.2019.11.056
Sunagawa S, Coelho LP, Chaffron S, Kultima JR, Labadie K, Salazar G, Djahanschiri B, Zeller G, Mende DR, Alberti A, Cornejo-Castillo FM, Costea PI, Cruaud C, d’Ovidio F, Engelen S, Ferrera I, Gasol JM, Guidi L, Hildebrand F, Kokoszka F, Lepoivre C, Lima-Mendez G, Poulain J, Poulos BT, Royo-Llonch M, Sarmento H, Vieira-Silva S, Dimier C, Picheral M, Searson S, Kandels-Lewis S, Coordinators TO, Bowler C, de Vargas C, Gorsky G, Grimsley N, Hingamp P, Iudicone D, Jaillon O, Not F, Ogata H, Pesant S, Speich S, Stemmann L, Sullivan MB, Weissenbach J, Wincker P, Karsenti E, Raes J, Acinas SG, Bork P (2015) Structure and function of the global ocean microbiome. Science 348(6237). https://doi.org/10.1126/science.1261359
Swain P, Nayak SK (2009) Role of maternally derived immunity in fish. Fish Shellfish Immunol 27(2):89–99. https://doi.org/10.1016/j.fsi.2009.04.008
Swain P, Nayak SK, Nanda PK, Dash S (2008) Biological effects of bacterial lipopolysaccharide (endotoxin) in fish: a review. Fish Shellfish Immunol 25(3):191–201. https://doi.org/10.1016/j.fsi.2008.04.009
Swann JB, Holland SJ, Petersen M, Pietsch TW, Boehm T (2020) The immunogenetics of sexual parasitism. Science 369(6511):1608–1615. https://doi.org/10.1126/science.aaz9445
Szwejser E, Verburg-van Kemenade BML, Maciuszek M, Chadzinska M (2017) Estrogen-dependent seasonal adaptations in the immune response of fish. Horm Behav 88:15–24. https://doi.org/10.1016/j.yhbeh.2016.10.007
Taborsky B, English S, Fawcett TW, Kuijper B, Leimar O, McNamara JM, Ruuskanen S, Sandi C (2021) Towards an evolutionary theory of stress responses. Trends Ecol Evol 36(1):39–48. https://doi.org/10.1016/j.tree.2020.09.003
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140(6):805–820. https://doi.org/10.1016/j.cell.2010.01.022
Takezaki N (2018) Global rate variation in bony vertebrates. Genome Biol Evol 10(7):1803–1815. https://doi.org/10.1093/gbe/evy125
Teffer AK, Hinch S, Miller K, Jeffries K, Patterson D, Cooke S, Farrell A, Kaukinen KH, Li S, Juanes F (2019) Cumulative effects of thermal and fisheries stressors reveal sex-specific effects on infection development and early mortality of adult coho salmon (Oncorhynchus kisutch). Physiol Biochem Zool 92(5):505–529. https://doi.org/10.1086/705125
Telemeco RS, Gangloff EJ (2020) Analyzing stress as a multivariate phenotype. Integr Comp Biol 60(1):70–78. https://doi.org/10.1093/icb/icaa005
Tetreau G, Dhinaut J, Gourbal B, Moret Y (2019) Trans-generational immune priming in invertebrates: current knowledge and future prospects. Front Immunol 10. https://doi.org/10.3389/fimmu.2019.01938
Thieringer R, Grand CBL, Carbin L, Cai T-Q, Wong B, Wright SD, Hermanowski-Vosatka A (2001) 11β-hydroxysteroid dehydrogenase type 1 is induced in human monocytes upon differentiation to macrophages. J Immunol 167(1):30–35. https://doi.org/10.4049/jimmunol.167.1.30
Timperio AM, Egidi MG, Zolla L (2008) Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP). J Proteome 71(4):391–411. https://doi.org/10.1016/j.jprot.2008.07.005
Tong C, Li M (2020) Transcriptomic signature of rapidly evolving immune genes in a highland fish. Fish Shellfish Immunol 97:587–592. https://doi.org/10.1016/j.fsi.2019.12.082
Ueda H (2019) Sensory mechanisms of natal stream imprinting and homing in Oncorhynchus spp. J Fish Biol 95(1):293–303. https://doi.org/10.1111/jfb.13775
van de Pol I, Flik G, Gorissen M (2017) Comparative physiology of energy metabolism: fishing for endocrine signals in the early vertebrate pool. Front Endocrinol (Lausanne) 8:36. https://doi.org/10.3389/fendo.2017.00036
Van Kaer L, Postoak JL, Wang C, Yang G, Wu L (2019) Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cell Mol Immunol 16(6):531–539. https://doi.org/10.1038/s41423-019-0221-5
Vargas R, Balasch JC, Brandts I, Reyes-López F, Tort L, Teles M (2018) Variations in the immune and metabolic response of proactive and reactive Sparus aurata under stimulation with Vibrio anguillarum vaccine. Sci Rep 8(1):17352. https://doi.org/10.1038/s41598-018-35863-w
Verburg-van Kemenade BML, Nowak B, Engelsma MY, Weyts FAA (1999) Differential effects of cortisol on apoptosis and proliferation of carp B-lymphocytes from head kidney, spleen and blood. Fish Shellfish Immunol 9(5):405–415. https://doi.org/10.1006/fsim.1998.0197
Verburg-Van Kemenade BML, Stolte EH, Metz JR, Chadzinska M (2009) Chapter 7 Neuroendocrine–immune interactions in teleost fish. In: Fish physiology. Academic, pp 313–364
Verburg-van Kemenade BML, Ribeiro CMS, Chadzinska M (2011) Neuroendocrine-immune interaction in fish: differential regulation of phagocyte activity by neuroendocrine factors. Gen Comp Endocrinol 172(1):31–38. https://doi.org/10.1016/j.ygcen.2011.01.004
Verburg-van Kemenade BML, Cohen N, Chadzinska M (2017) Neuroendocrine-immune interaction: Evolutionarily conserved mechanisms that maintain allostasis in an ever-changing environment. Dev Comp Immunol 66:2–23. https://doi.org/10.1016/j.dci.2016.05.015
Vizzini A, Vazzana M, Cammarata M, Parrinello N (2007) Peritoneal cavity phagocytes from the teleost sea bass express a glucocorticoid receptor (cloned and sequenced) involved in genomic modulation of the in vitro chemiluminescence response to zymosan. Gen Comp Endocrinol 150:114–123. https://doi.org/10.1016/j.ygcen.2006.07.016
Volkoff H, Peter RE (2004) Effects of lipopolysaccharide treatment on feeding of goldfish: role of appetite-regulating peptides. Brain Res 998(2):139–147. https://doi.org/10.1016/j.brainres.2003.11.011
Wada H, Heidinger B (2019) Damage-fitness model: evaluation and synthesis. Integr Comp Biol 59. https://doi.org/10.1093/icb/icz060
Wan Q, Song D, Li H, He M (2020) Stress proteins: the biological functions in virus infection, present and challenges for target-based antiviral drug development. Signal Transduct Target Ther 5. https://doi.org/10.1038/s41392-020-00233-4
Wang R, Belosevic M (1995) The in vitro effects of estradiol and cortisol on the function of a long-term goldfish macrophage cell line. Dev Comp Immunol 19(4):327–336. https://doi.org/10.1016/0145-305X(95)00018-O
Wcisel DJ, Ota T, Litman GW, Yoder JA (2017) Spotted gar and the evolution of innate immune receptors. J Exp Zool B Mol Dev Evol 328(7):666–684. https://doi.org/10.1002/jez.b.22738
Weber RA, Pérez Maceira JJ, Aldegunde MJ, Peleteiro JB, García Martín LO, Aldegunde M (2015) Effects of acute handling stress on cerebral monoaminergic neurotransmitters in juvenile Senegalese sole Solea senegalensis. J Fish Biol 87(5):1165–1175. https://doi.org/10.1111/jfb.12774
Wei J, Yu L, Sun L, Zhang X, Li M, Qi W, Zhou L, Wang D (2013) Molecular cloning and expression analysis of Foxp 3 from Nile tilapia. Vet Immunol Immunopathol 155(1–2):48–56. https://doi.org/10.1016/j.vetimm.2013.06.004
Wentworth SA, Thede K, Aravindabose V, Monroe I, Thompson AW, Molyneaux N, Owen CL, Burns JR, Gonzalez-Vicente A, Garvin JL, Packer RK (2018) Transcriptomic analysis of changes in gene expression of immune proteins of gill tissue in response to low environmental temperature in fathead minnows (Pimephales promelas). Comp Biochem Physiol Part D Genomics Proteomics 25:109–117. https://doi.org/10.1016/j.cbd.2017.11.004
Wernicke von Siebenthal E, Rehberger K, Bailey C, Ros A, Herzog EL, Segner H (2018) Trade-Offs underwater: physiological plasticity of rainbow trout (Oncorhynchus mykiss) confronted by multiple stressors. Aust Fish 3(4):49. https://doi.org/10.3390/fishes3040049
Whitman WB, Coleman DC, Wiebe WJ (1998) Prokaryotes: the unseen majority. PNAS 95(12):6578–6583. https://doi.org/10.1073/pnas.95.12.6578
Wojtaszek J, Dziewulska-Szwajkowska D, Lozińska-Gabska M, Adamowicz A, Dzugaj A (2002) Hematological effects of high dose of cortisol on the carp (Cyprinus carpio L.): cortisol effect on the carp blood. Gen Comp Endocrinol 125(2):176–183. https://doi.org/10.1006/gcen.2001.7725
Wright PA, Turko AJ (2016) Amphibious fishes: evolution and phenotypic plasticity. J Exp Biol 219(15):2245–2259. https://doi.org/10.1242/jeb.126649
Wu L, Fu S, Yin X, Guo Z, Wang A, Ye J (2019) Long-lived plasma cells secrete high-affinity antibodies responding to a T-dependent immunization in a teleost. Fish Front Immunol:10. https://doi.org/10.3389/fimmu.2019.02324
Xu P, Xu J, Liu G, Chen L, Zhou Z, Peng W, Jiang Y, Zhao Z, Jia Z, Sun Y, Wu Y, Chen B, Pu F, Feng J, Luo J, Chai J, Zhang H, Wang H, Dong C, Jiang W, Sun X (2019) The allotetraploid origin and asymmetrical genome evolution of the common carp Cyprinus carpio. Nat Commun 10(1):4625. https://doi.org/10.1038/s41467-019-12644-1
Yada T, Tort L (2016) 10 - Stress and Disease resistance: immune system and immunoendocrine interactions. In: Schreck CB, Tort L, Farrell AP, Brauner CJ (eds) Fish physiology. Academic, pp 365–403
Yada T, Mekuchi M, Ojima N (2018) Molecular biology and functional genomics of immune-endocrine interactions in the Japanese eel, Anguilla japonica. Gen Comp Endocrinol 257:272–279. https://doi.org/10.1016/j.ygcen.2017.11.001
Yamaguchi T, Takizawa F, Fischer U, Dijkstra JM (2015) Along the axis between type 1 and type 2 immunity; principles conserved in evolution from fish to mammals. Biology (Basel) 4(4):814–859. https://doi.org/10.3390/biology4040814
Ye J, Bromage ES, Kaattari SL (2010) The strength of B cell interaction with antigen determines the degree of IgM polymerization. J Immunol 184(2):844–850. https://doi.org/10.4049/jimmunol.0902364
Ye J, Kaattari I, Kaattari S (2011) Plasmablasts and plasma cells: reconsidering teleost immune system organization. Dev Comp Immunol 35(12):1273–1281. https://doi.org/10.1016/j.dci.2011.03.005
Yunna C, Mengru H, Lei W, Weidong C (2020) Macrophage M1/M2 polarization. Eur J Pharmacol 877:173090. https://doi.org/10.1016/j.ejphar.2020.173090
Zapata A, Diez B, Cejalvo T, Gutiérrez-de Frías C, Cortés A (2006) Ontogeny of the immune system of fish. Fish Shellfish Immunol 20(2):126–136. https://doi.org/10.1016/j.fsi.2004.09.005
Zhang Y-A, Salinas I, Li J, Parra D, Bjork S, Xu Z, LaPatra SE, Bartholomew J, Sunyer JO (2010) IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat Immunol 11(9):827–835. https://doi.org/10.1038/ni.1913
Zhang Q, Lenardo MJ, Baltimore D (2017) 30 years of NF-κB: a blossoming of relevance to human pathobiology. Cell 168(1–2):37–57. https://doi.org/10.1016/j.cell.2016.12.012
Zhang Y-Z, Wu W-C, Shi M, Holmes EC (2018) The diversity, evolution and origins of vertebrate RNA viruses. Curr Opin Virol 31:9–16. https://doi.org/10.1016/j.coviro.2018.07.017
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tort, L., Balasch, J.C. (2022). Stress and Immunity in Fish. In: Buchmann, K., Secombes, C.J. (eds) Principles of Fish Immunology . Springer, Cham. https://doi.org/10.1007/978-3-030-85420-1_20
Download citation
DOI: https://doi.org/10.1007/978-3-030-85420-1_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-85419-5
Online ISBN: 978-3-030-85420-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)