Abstract
In this chapter, we cover the life history of fish in low-temperature environments, including their overwintering behavior and the physiological mechanisms by which they maintain life in cold environments, based on research to date. There is relatively little research on low-temperature tolerance of fish, compared with research on this phenomenon in mammals and birds, which are also vertebrates, and the mechanisms in fish have not been fully elucidated. First, we cover the life history of fish that overwinter by entering dormancy or hibernation. Next, we describe the mechanism that controls body temperature in fish that survive low-temperature environments. Finally, we introduce the physiological mechanisms for survival in extremely low-temperature environments, particularly antifreeze proteins.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Dormancy
- Hibernation
- Ectothermic fish
- Endothermic fish
- Heat exchange
- Antifreeze glycoprotein (AFGP)
- Antifreeze protein (AFP)
1 Introduction
In fish, which are ectothermic (heterothermic) animals, the temperature of the environment is a major factor controlling phenomena such as growth and breeding because their body temperature is affected by ambient water temperature (Brett 1971; 1979). Fish move in search of a suitable water temperature (Schurmann and Christiansen 1994; Claireaux et al. 1995). Recently, it has been reported that several fishes control their body temperature using a heat generation system, in addition to moving to areas with appropriate water temperature. This group of endothermic fishes includes the bigeye tuna Thunnus obesus and the opah Lampris guttatus (Holland et al. 1992; Wegner et al. 2015) (Fig. 9.1). However, the temperature that they can retain is not high compared with that of homeothermic animals . Furthermore, they lack a heat-radiating mechanism for keeping the body temperature constant, which homeothermic animals possess.
How do fish respond to ambient temperature change? One way is behavioral thermoregulation , in which fish move to an area with suitable water temperature to maintain homeostasis for continuing physiological functions. As the optimum temperature differs for various physiological phases, such as growth and maturation, fish must migrate according to their temperature requirement for each phase in the life cycle.
Migratory fish are able to travel through a wide area, but fishes that have poor swimming ability and inhabit a specific environment are forced to adapt to the ambient temperature , even if the temperature fluctuation is large. These species respond to adverse conditions (i.e., when the water temperature deviates from the appropriate range) by reducing their physiological activity as much as possible. Especially in areas with cold water, fish generally cease physiological activity during the winter season, a condition that is extremely close to the state of hibernation .
Meanwhile, fishes living in environments where the water temperature is low throughout the year, such as the polar zone, have physiological mechanisms for adapting to low water temperatures. Some of these adaptations include the synthesis of an antifreeze protein (AFP) and antifreeze glycoprotein (AFGP ) (Harding et al. 2003; DeVries and Cheng 2005), formation of tubulin that can be synthesized at low temperature (Guderley 2004), and lack of hemoglobin (Hemmingsen 1991).
This chapter will explain adaptations to low water temperatures in fish, focusing on three topics: hibernation, body temperature control, and the mechanism of tolerance to low water temperatures.
2 Dormancy of Fish at Low Temperatures
It is difficult to define hibernation in fish. Hibernation refers to a low metabolic state that animals enter under a low-temperature environment, during which they reduce their basal metabolism and consume less energy. This adaptation to winter is well known in mammals (see Chap. 3). Hibernation is characterized by maintenance of an extremely low metabolic state with unusual physiological conditions such as low breathing, low heart rate , low body temperature , etc. It is necessary to have a mechanism to maintain life even at low temperature. However, the condition is not considered hibernation if the physiological conditions are maintained in an active state like sleep, even if the animals have temporarily stopped active behavior and their metabolic activity is suppressed for a long time. These conditions, which are often observed in fish during the winter season, are considered low-temperature dormancy or winter dormancy (Fig. 9.2). Interestingly, some species of fish, such as the Japanese sandeel, enter dormancy during the summer season, when it is called aestivation (Tomiyama and Yanagibashi 2004). As the sleeping state has been observed in fish not only in winter but in summer, the condition is typically considered dormancy. However, recently fish displaying characteristics that are similar to hibernation have been observed (Campbell et al. 2008).
As described above, fish are classified as heterothermic animals whose body temperature depends on the ambient water temperature, except for some fish species that produce heat by themselves (endothermic fish). Therefore, during periods when the water temperature is low, many fish species move to areas with more suitable temperature where they can maintain normal activities. Other fish species enter a low-temperature dormancy during the winter season. The physiological state of the fish during this low-temperature dormancy, as well as the mechanism causing the dormancy, differs from hibernation in mammals, which are homeothermic animals .
The Pacific sandlance Ammodytes hexapterus (Quinn 1999), the lesser sandeel A. marinus (Winslade 1974), and the black rock cod Notothenia coriiceps, inhabiting the Antarctic (Campbell et al. 2008), are fish that reduce their physiological activity in the winter season. Although it is not described sufficiently in the scientific literature, the phenomenon of low-temperature dormancy is known also in the dojo loach Misgurnus anguillicaudatus and the mudskippers Periophthalmus modestus and Boleophthalmus pectinirostris, which escape by burrowing under sediment in the winter season. However, the strategy for entering a low metabolic state varies by species.
The lesser sandeel, inhabiting the North Sea, burrows into sand during winter when the water temperature is low (Winslade 1974). In the coastal areas of the UK, this burrowing behavior is observed from January, after spawning, until April. Among fish reared in different water temperatures (5 °C, 10 °C, and 15 °C), swimming activity was reduced at 5 °C, although activity levels of fish at 10 °C and 15 °C remained high (Winslade 1974). The burrowing behavior appeared to be a response to decreased water temperature. In addition, the burrowing may be related to fat stores, which are probably at their lowest level after spawning. Burrowing as an overwintering strategy appears to be an effective way to retain energy lost during spawning and also reduces vulnerability to predation. The phenomenon may be regarded as an adaptation to survive a period of unsuitable environment in the fishes’ life cycle.
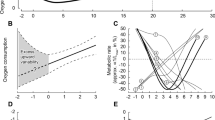
N. coriiceps is a teleost that inhabits the Antarctic (Hubold 1991; Knox 2006) and has antifreeze proteins to prevent the freezing of body fluids, even when the water temperature falls below the freezing point (see Sect. 9.5). Although the annual changes in temperature in the Antarctic marine environment are small and the environment is considered thermally stable, this species also reduced its activity levels during the winter season to save metabolic energy (Campbell et al. 2008). From May to November, when the water temperature decreases rapidly to around −2 °C, the growth rate in N. coriiceps is sharply suppressed and the heart rate (fH) also decreases. The heart rate is positively correlated with oxygen consumption (MO2), and it was found that MO2 also decreases during the period of low water temperature. Campbell et al. (2008) also reported interesting results from behavior tracing of fish, using a static hydrophone array throughout the year.
N. coriiceps had a wide range of activity during the summer season (from December to May). However, from June to August, the low-temperature period, the range of activity was extremely limited. A scuba diver observed that N. coriiceps found at 18 m depth, in water that was −1.8 °C, was not able to move and indicated no response even if the diver was holding the fish. Dormancy in N. coriiceps involves the active suppression of MO2 and fH irrespective of temperature, suggesting that some other cue factor initiates dormancy rather than temperature, such as reduction of light in winter. These changes induce a reduction in the growth rate, as reported in other Antarctic notothenioids (Coggan 1997). Dormancy in N. coriiceps is distinct from the dormancy observed in temperate fish. The degree of physiological suppression in this fish is similar to that of hibernating animals. Therefore, dormancy in N. coriiceps is considered to be hibernation .
The Japanese mudskippers P. modestus and B. pectinirostris enter dormancy during a low-temperature phase. These species have the ability to breathe air and can move on the surface of a muddy tidal flat using their pectoral fin (Pace 2017). They burrow into the mud to nest and spawn in the burrow (Martin and Ishimatsu 2017). Their behavior and spawning depend on water temperature, with an active period from spring to early winter. However, in winter when the temperature drops, the mudskipper escapes into a mud burrow. We observed that P. modestus disappeared from the surface of tidal flats in late November, after which it did not leave the mud burrow. The burrow in the tidal mud remains approximately 3 °C warmer than the surface of the tidal flat, which is exposed to the outside air and experiences low temperatures in the winter (Soyano et al. unpublished data). In Ariake Sound, Japan, the mud temperature remained between 5 and 8 °C at a depth of 30 cm during the coldest season (Takegaki et al. 2006). When we excavated a mud burrow in February, the time of year when the ambient temperature was lowest, the dormant mudskipper was easily captured from the burrow. The fish was a state of dormancy just after capture, although it awakened and moved a short time later when the ambient temperature was lower than that of the mud burrow.
The fH and MO2 in N. coriiceps are reduced during hibernation (Campbell et al. 2008). Unfortunately there are no data about heart rate and body temperature in the mudskipper before and after winter dormancy , but fH and MO2 is expected to decrease during winter dormancy in this species. The mechanism appears to be similar as in hibernation . However, B. pectinirostris often dies during hibernation. In a rearing experiment that explored tolerance to low temperatures, most individuals of this species died within 24 h at 3 °C and within 15 days at 7 °C under continuous low-temperature conditions (Takegaki et al. 2006). This outcome indicates that the temperature tolerance limit of the fish was exceeded.
Another mudskipper species P. koelreuteri and B. boddarti inhabiting Kuwait enters dormancy when the ambient temperature falls below 10 °C in winter, while the fish maintains high activity levels at 14–35 °C. This species also remains in the burrow during cold periods to prevent loss of body temperature (Tytler and Vaughan 1982). The mudskipper also appears to use the burrow to reduce the risk of predation associated with the suppression of behavior accompanying low metabolism.
3 Thermoregulation in Fish
Thermoregulation in ectothermic animals , including most species of fish, depends on the environmental temperature. Ectothermic fish regulate their body temperature by moving to an area with appropriate water temperature to maintain homeostasis and continue normal physiological function, which is known as behavioral thermoregulation. In contrast, fish that generate heat using a physiological thermoregulation mechanism and maintain a body temperature that is higher than that of the ambient water temperature are termed endothermic fish (Holland and Sibert 1994; Nakamura et al. 2015; Wegner et al. 2015). This ability enhances their ability to engage in feeding and swimming behavior and increases their physiological activity. However, fish with this ability constitute fewer than 0.1% of fish species. Moreover, the thermogenic system in fish can warm only a limited part of the body, whereas mammals are completely endothermic animals . Thus, fish with this ability are called regional endotherms (Wegner et al. 2015). They include the swordfish Xiphias gladius (Carey and Robinson 1981), the Atlantic bluefin tuna Thunnus thynnus (Block et al. 2001), the Pacific bluefin tuna T. orientalis (Kitagawa et al. 2006), the bigeye tuna T. obesus (Holland and Sibert 1994), the mako shark Isurus oxyrinchus (Bernal et al. 2001), and other large pelagic predatory fish that dive into deep water to find food. For example, mean body temperature is maintained at 4 °C above the ambient water temperature in the blue shark Prionace glauca, because the rate of warming in the body is higher than the rate of cooling due to the environmental water (Carey and Scharold 1990).
In the bluefin tuna and the skipjack tuna, thermoregulation is carried out by passing oxygenated blood from the gills into the counter-current vascular retia , warm venous blood vessels that go to the heart from the swimming muscles (Carey and Lawson 1973; Stevens et al. 1974, 2000). According to a study that measured the temperature of red muscle, white muscle, and the stomach in the mako shark I. oxyrinchus, body temperature is higher than ambient temperature (Bernal et al. 2001). This study showed that the shark has vascular networks (retia mirabilia) that act as counter-current heat exchangers , allowing metabolic heat retention in certain regions of the body, and the mechanism to regulate heat transfer is similar functionally and morphologically to that in tuna (Bernal et al. 2001) (Fig. 9.3). Such partial elevation of body temperature improves temperature-sensitive physiological processes such as a digestion, metabolism, nervous system function, and locomotion (Graham and Dickson 2001).
Heat generation and exchange system in fish. There are two type of body warming in fish. (a) Partially body warming. The bluefin tuna and swordfish are known as this type, which utilize the red muscle as heat generator. (b) Whole body warming. The opah is known as this type, which has the characteristic structure in gill and pectoral musculature for heat generation and exchange
Interestingly, fish can use temperature control to enhance the function of the eyes and brain. Swordfish have a particularly high ability to use their heating function to increase the temperature of the brain and eyes (Fritsches et al. 2005). This mechanism is fundamentally different from that found in the tuna. The eyes of ectothermic fishes are the same temperature as the surrounding environmental water, so it is expected that the vision potential in the eye will be diminished when the eye temperature decreases due to entering a low-temperature zone. Endothermic open-ocean predators have the ability to warm the retinal area to maintain visual function. The retinal warming in the eye prevents a decline in visual resolution due to the drop in water temperature caused by locomotion to a deep-sea area, thereby helping the predator to capture prey.
As described above, the bluefin tuna and mako shark merely enhance their vision and movement potential temporarily by partially increasing the body temperature . However, the opah L. guttatus is able to warm its whole body by introducing a special heat exchange system (Wegner et al. 2015) (Fig. 9.3). This ability is related to the vascular structure around the heart from the gills. The opah produces heat by flapping its winglike pectoral fins. The warmed blood is sent from the afferent filament artery to the gill via the heart. However, the deoxygenated blood warmed by the flapping of pectoral fins loses heat when the blood undergoes gas exchange at the surface before entering the efferent branchial arteries. The afferent and efferent arteries filament are closely coupled and stacked in an alternating pattern within the gill arch. This structure is very important to rewarm the oxygenated blood after it has cooled in the surfaces of the gill filament. The warmed blood is delivered to the whole body. By employing such a heat exchanging system, the opah can keep its body temperature several degrees higher than the external water temperature. Although this mechanism is different from the low-temperature tolerance of fish inhabiting polar regions , it is considered an important physiological mechanism for adapting to low water temperatures that are experienced on a daily basis.
4 Use of Antifreeze Protein to Adapt to Low-Temperature Environment
In the Arctic and Antarctic regions , the water temperature can drop below zero due to supercooling. Fishes in these regions use antifreeze mechanisms to adapt to the extreme temperatures. The plasma freezing point of the bald notothen Trematomus borchgrevinki inhabiting the Antarctic Ocean is −2.75 °C, whereas in the black perch Embiotoca jacksoni, which is distributed in the temperate zone, it is −0.7 °C. Therefore, body fluid in some fishes does not freeze even if the water temperature drops below zero (DeVries 1982). The mechanism for this phenomenon is antifreeze protein. Glycoproteins that enable a lower plasma freezing point have been isolated from the plasma of fish belonging to the Notothenioidei suborder inhabiting the Antarctic Ocean, and several proteins that can reduce the plasma freezing point are found in other species of fish (Harding et al. 2003). These proteins, called antifreeze glycoproteins (AFGPs) and antifreeze proteins (AFPs), inhibit the growth of ice crystals in plasma by covering the water-accessible surface of ice, resulting in a lower freezing point for plasma and enabling polar fish to survive in seawater below the freezing point.
4.1 Characteristics of Antifreeze Proteins
Antifreeze proteins found in fish have been classified in a single class of AFGP and four classes of AFPs (types I–IV). AFGP, a glycoprotein with a molecular weight of 2.6–33 kDa, consists of a number of repeating units of alanine-alanine-threonine and has a side chain of threonine modified with disaccharide, which is involved in binding to ice crystals (Table 9.1). A total of eight AFGPs with different molecular weights were purified from a single fish species and were classified roughly into two types, high molecular (AFGP1–5) and low molecular (AFGP6–8) types (Harding et al. 2003). These proteins have been isolated in Notothenioidei and Gadidae living in cold water (DeVries 1982; Burcham et al. 1984).
Type I AFP is a monomeric protein with an α-helical folded structure and a molecular weight of 3.3–4.4 kDa. The protein consists of a large amount of alanine, threonine, and aspartic acid (Duman and DeVries 1976). AFP was isolated from the winter flounder Pseudopleuronectes americanus, shorthorn sculpin Myoxocephalus scorpius, and other species, and multiple molecules were purified (Duman and DeVries 1976; Hew et al. 1980). Type 1 AFP is classified into two types, the liver type and the skin type (Gong et al. 1996; Low et al. 1998). Whereas the liver type is a secreted protein, the skin type has no signal peptide and is considered to function intracellularly. Hyperactive AFP, which has an activity level 10–100-fold higher than that of the conventional type I AFP, was isolated from the plasma of winter flounder (Marshall et al. 2005). The molecular weight of this novel AFP was 16,683 Da, and 60% or more of its amino acid composition was alanine, as in the conventional type. The novel AFP is a long rod-like structure with dimeric α-helix.
Type II AFPs are globular proteins with a molecular weight of 11–24 kDa. They have a folding structure consisting of two helices and nine β-strands in two β-sheets, with five disulfide bonds (Gronwald et al. 1998). These proteins are divided into two types, calcium (Ca2+)-dependent and calcium-independent (Ewart et al. 1992). The Ca2+-dependent type II AFP was isolated from the smelt Osmerus mordax (Ewart et al. 1992) and the Atlantic herring Clupea harengus (Liu et al. 2007). This type of protein was purified from the Japanese smelt Hypomesus nipponensis. Its function in the body appears to be enabling biological activity to continue in the absence of Ca2+ (Yamashita et al. 2003). The Ca2+-independent type has been isolated from the sea raven Hemitripterus americanus (Slaughter et al. 1981), the longsnout poacher Brachyosis rostratus (Nishimiya et al. 2008), and others. This type of protein has no Ca2+ binding sites in the sea raven (Ewart et al. 1992).
Type III AFPs are globular proteins with a molecular weight of 6–7 kDa and a folding structure that includes an α-helix, three 310-helices, and two β-strands (Choi et al. 2015). These proteins do not contain abundant alanine nor high levels of half-cysteine residues in the primary structure (Hew et al. 1984), and there is an ice-binding site in the C-terminal part (Sönnichsen et al. 1996). The globular protein purified from the Antarctic eelpout Lycodichthys dearborni and the ocean pout Macrozoarces americanus also belongs to type III AFP, and multiple AFP molecules have been purified from individual fish species (Hew et al. 1984; Ko et al. 2003). These molecules can be divided into the QAE Sephadex binding groups and SP Sephadex (or CM Sephadex) binding groups due to the difference in the binding property with ion exchange carriers (Li et al. 1985).
Type IV AFP has a molecular weight of 12 kDa and has been isolated from the longhorn sculpin Myoxocephalus octodecimspinosis (Deng et al. 1997; Deng and Laursen 1998). It has a high number of α-helices and four-helix bundle structures and contains a large amount of glutamine. This protein causes ice crystals to grow as hexagonal trapezohedra, unlike other AFPs.
4.2 Acquisition and Molecular Evolution of AFP
AFGP is only found in the Antarctic notothenioid and northern gadid fishes (DeVries 1982; Burcham et al. 1984). Although AFGP of the Antarctic notothenioid is derived from pancreatic trypsinogen, the origin of AFGP in the northern gadid is different from that of the Antarctic notothenioid due to difference in genomic sequence and partial structure, suggesting that AFGP developed in these fishes as a result of convergent evolution (Chen et al. 1997a, b).
Type II AFP is considered to have evolved from pre-existing calcium-dependent C-type lectins because the protein is highly homologous with the sugar chain recognition region of the calcium-dependent lectin (Ewart et al. 1992). In addition, it is possible that type II AFP in certain fish species was acquired by lateral gene transfer (Graham et al. 2012; Sorhannus 2012). For example, although the Atlantic herring and the smelt are systematically separated, the primary structure of AFP is very similar between both species, compared with other orthologous genes.
The sequence of Type III AFP is homologous to the C-terminal region of mammalian sialic acid synthase, suggesting that the synthase is the ancestral protein of Type III AFP (Baardsnes and Davies 2001). Type III AFP is a multicopy gene that is present with approximately 150 copies, many of which are closely linked but irregularly spaced (Hew et al. 1988) in the Newfoundland ocean pout populations. In more southerly population of ocean pout in the New Brunswick, the AFP level is lower, and there are only about one-quarter as many AFP copies. As the gene dosage and the AFP levels show a strong correlation, it appears that low-temperature tolerance was acquired by the multiplicity of genes. The ancestral protein of type I AFP is not well understood. However, it is known that type I AFP shows a multiplicity of genes, similar to type III AFP (Scott et al. 1985; Hew et al. 1988). Thus, species with type I may have acquired low-temperature tolerance by amplification of the AFP gene.
Type IV AFP may have originated in apolipoprotein E3, as this AFP has a similar structure to the LDLR-binding region of apolipoprotein E3 (Deng et al. 1997).
4.3 Synthesis and Regulation Mechanism of AFP
AFGP was once thought to be synthesized in the liver and distributed within the circulatory system to prevent the blood from freezing. However, recent research has revealed that the major site of AFGP synthesis in the Antarctic notothenioids is the exocrine pancreas, not the liver (Cheng et al. 2006). AFGP of Arctic cod, acquired as a result of convergent evolution, is also synthesized in the pancreas. The pancreatic AFGP enters the intestinal lumen via the pancreatic duct to prevent ingested ice from nucleating the intestinal fluid. The source of AFGP in plasma is the reabsorbed pancreas-derived AFGP in intestinal fluid. Seasonal changes in plasma AFGP levels have been reported in the saffron cod Eleginus gracilis which is in the Arctic cod family and inhabits the northern part of the range, although there are no seasonal changes in AFGP levels in notothenioids inhabiting Antarctic waters, where the annual water temperature hardly changes (Burcham et al. 1984).
Moreover, levels of plasma proteins, including AFGP, were high in the winter, which is thought to increase plasma osmolality, in the saffron cod (Ogawa et al. 1997). The protein levels and osmolality decreased after intraperitoneal injection of salmon prolactin (PRL), suggesting that PRL may act on the kidneys and remove the AFGP from plasma by increasing glomerular filtration. When the glomeruli in the kidneys of saffron cod were observed throughout the year, the glomeruli in fish collected in winter showed atrophy in comparison with the functional glomeruli in fish collected in summer (Kitagawa et al. 1990). These results indicate that the reduction of glomerular function in association with a decline in AFGP drainage function depends on seasonal changes in glomeruli morphology. Interestingly, the kidneys of Antarctic notothenioids are aglomerular or functionally aglomerular (DeVries 1982; Eastman and DeVries 1986), and the glomerular reduction or aglomerularism are considered to be important mechanisms for conservation of small molecular weight AFGP compounds, vital to living in the polar zone.
As described above, two different forms of type I AFP, the liver type and the skin type, have been isolated from the winter flounder (Gong et al. 1996). Although the liver type is mainly expressed in the liver, the skin type is strongly expressed in the liver and exterior tissues, such as the skin, scales, fin, and gills. Type I AFP in the blood seasonally changes in this species, as does AFGP in the saffron cod (Hew and Fletcher 1979). However, AFP levels in plasma dropped when winter flounder were exposed to pituitary extract, including growth hormone (GH) fraction, even though AFGP of the saffron cod was reduced by PRL treatment in winter (Idler et al. 1989). Moreover, the hypophysectomized winter flounder retained high levels of AFP in plasma (Hew and Fletcher 1979), suggesting that AFP synthesis was suppressed by substances in the pituitary, including GH. As there was no seasonal change in the structure of the kidney in the winter flounder (Boyd and DeVries 1983), it is speculated that the transcription of AFP is promoted due to the reduced secretion of GH in the winter; in consequence, tolerance to low temperatures increases.
Although AFP II, III, and IV are mainly expressed in the liver, their expression is also seen in the pancreas (Cheng et al. 2006). However, there is little information about the mechanism that regulates these AFP types, unlike AFGP and type I AFP. Moreover, it appears that type IV AFP lacks the ability to prevent the blood from freezing on its own because its levels are too low in the blood (Gauthier et al. 2008). As type IV AFP has been detected only in fish that also have type I AFP, it is possible that type IV AFP has other physiological functions besides its role in low-temperature tolerance.
5 Biological Factors Related to Low-Temperature Tolerance and Cold Shock
In the previous section , we described the antifreeze proteins and antifreeze glycoproteins that some species utilize to survive extremely low temperatures. A related challenge is surviving a sudden drop in water temperature (cold shock), which induces various physiological changes in fish, including effects on growth, ion regulation, and immune function (Donaldson et al. 2008). Biological factors related to low-temperature tolerance have been reported in association with this response.
5.1 The Cell Division Cycle Protein 48 (CDC48)
The cell division cycle protein 48 (CDC48) , which is involved in cold tolerance, has been identified, and the gene that codes for it has been cloned (Yamashita et al. 1996; Imamura et al. 2003). The protein is a polypeptide consisting of 806 amino acid residues that promote cell division. CDC48 belongs to the AAA (ATPases associated with diverse cellular activities) ATPase family and is considered an essential factor in cell division and cell cycle progression, as well as playing an important part in cell homeostasis (Moir et al. 1982; Dantuma and Hoppe 2012; Meyer et al. 2012). Its primary function is in the endoplasmic reticulum-associated protein degradation, in which it has a key role in promoting quality control in the degradation (Latterich et al. 1995; Hoppe et al. 2000; Ye et al. 2001; Wolf and Stolz 2012; Gallagher et al. 2014).
The CDC48 gene was isolated from zebrafish, and the effect of temperature on its expression level was investigated using a zebrafish embryo-derived cultured cell line (Imamura et al. 2002, 2003). CDC48 mRNA and protein levels increased as the temperature declined. Interestingly, cell proliferation was enhanced in the cells that overexpressed CDC48, which were transfected with cDNA constructs for CDC48, under low-temperature condition (Imamura et al. 2003). In addition, expression of this gene increased during the embryonic stage, particularly in the nervous system (Imamura et al. 2012). These findings indicate that the role of CDC48 is degradation of ubiquitinated proteins via activation of ubiquitin-proteasome system function to promote neural development (Imamura et al. 2012). Cold-inducible CDC48 appears to be an important protein with an essential role in controlling cell proliferation and repressing apoptosis in low-temperature conditions in fish.
5.2 Hormonal Regulation of Physiological Phenomena in Low-Temperature Conditions
Hormones induce and regulate physiological phenomena in organisms. The synthesis and release of hormones in fish is influenced by ambient water temperature (Fig. 9.4). The brain, the central organ of the nervous system, is also a central part of the endocrine system , and information about the external environment, including temperature, is concentrated in the brain of vertebrates (Crawshaw et al. 1985; Boulant 2000). The hypothalamus is one of the most important parts of the brain for transmitting endocrine information converted from external information. Information about water temperature is also processed in the hypothalamus and is transmitted to the whole body through the endocrine system, centered in the pituitary gland, which is called the hypothalamus-pituitary axis. Hormones synthesized in the pituitary gland include growth hormone (GH), thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), gonadotropic hormone (GTH), prolactin (PRL), melanophore-stimulating hormone (MSH), and somatolactin (SL) (Takei et al. 2016). The secretion and action of these hormones are strongly affected by temperature. In addition, other hormones secreted by stimulation of the pituitary hormone in various organs, including thyroid hormone (TH), sex steroid, and glucocorticoid, are also affected by temperature, directly or indirectly. Although the effect of temperature on the secretion or action of hormones has been investigated in fish, much of the research was conducted in the context of growth, migration, and reproduction in species that are useful for aquaculture and fisheries (Wootton and Smith 2015).
Many studies that examined the relationship between low temperature and hormones have addressed the annual changes in hormone levels related to environmental water temperature and the effect of low temperature on hormone synthesis by temperature manipulation. Unfortunately, there is limited information about the role of hormones in low-temperature tolerance and physiological phenomena at low temperature. TH is known to be a regulator of thermal acclimation in fish (Little et al. 2013). TH has a modulatory function of the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) , a protein associated with muscle and heart function in cold water (Little and Seebacher 2013, 2014). In addition, the heart rate and SERCA activity of fish in which hypothyroidism was induced by propylthiouracil and iopanoic acid were reduced by cold acclimation , while these levels in normal fish acclimated to cold water were high (Little and Seebacher 2013). Moreover, TH treatment in hypothyroid fish restored heart rate and SERCA activity, suggesting that TH plays an important role in maintenance of heart function during cold acclimation.
GH synthesis in fish is modulated by water temperature and is higher during the warmer seasons of the year (Deane and Woo 2009). GH is involved with the process of temperature acclimatization. One of its actions is to control AFP synthesis (Idler et al. 1989). GH synthesis is suppressed during the winter, when AFP levels are high (Fletcher et al. 1989). Moreover, when pituitary extracts including GH were injected in flounder in the wintertime, AFP levels decreased, indicating that GH is one of the regulatory factors of AFP synthesis (Idler et al. 1989).
Cold shock is a stressor that affects various physiological phenomena (Donaldson et al. 2008). The primary response to cold shock is the release of corticosteroids and catecholamines via a neuroendocrine response of the central nervous system (Barton 2002). In tilapia (Oreochromis aureus) exposed to cold water, levels of cortisol and catecholamines (epinephrine and norepinephrine) were examined. As a result of acute cold shock, plasma epinephrine, norepinephrine, and cortisol increased with the decreasing water temperature (Chen et al. 2002). These results indicate that cold shock promoted hormone secretion by the hypothalamic-pituitary-adrenal cortical axis. These hormones cause the physiological changes necessary to maintain homeostasis as a secondary response (Barton 2002). However, no further evidence has been obtained indicating the role of cortisol in the low-temperature tolerance of fish.
To understand the mechanism of low-temperature tolerance of fish, it is important to study the role of hormones, including their response to low temperature, because hormones are key factors that mediate the response of organs and cells to environmental conditions.
6 Perspectives
As climate change wreaks changes in oceans, rivers, and lakes, it is important to elucidate the mechanism of low-temperature tolerance in fish in order to understand the biological effects of environmental fluctuation and to take necessary measures to conserve aquatic animals. However, there is too little information on the biological responses of fish to low temperatures, which is fundamental for understanding the physiological mechanism of low-temperature tolerance. In addition to gathering these data, it is important to clarify the mechanisms of low-temperature tolerance, through research on the expression of genes and proteins affected by temperature fluctuation, the functional analysis of these genes and proteins, etc.
Information about the ecological and physiological responses of fish to low temperatures in polar regions is increasing. However, research on this issue should not only target fish inhabiting polar regions. Research should also be conducted on other fish species that show special responses to low temperatures.
Abbreviations
- ACTH:
-
Adrenocorticotropic hormone
- AFGP:
-
Antifreeze glycoprotein
- AFP:
-
Antifreeze protein
- CDC48:
-
Cell division cycle protein 48
- GH:
-
Growth hormone
- GTH:
-
Gonadotropic hormone
- LDLR:
-
Low density lipoprotein receptor
- MO2 :
-
Muscle oxygen consumption
- MSH:
-
Melanophore-stimulating hormone
- PRL:
-
Prolactin
- SERCA:
-
Sarco-endoplasmic reticulum Ca2+ ATPase
- SL:
-
Somatolactin
- TH:
-
Thyroid hormone
- TSH:
-
Thyroid-stimulating hormone
References
Baardsnes J, Davies PL (2001) Sialic acid synthase: the origin of fish type III antifreeze protein? Trends Biochem Sci 26:468–469
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integ Comp Biol 42:517–525
Bernal D, Sepulveda C, Graham JB (2001) Water-tunnel studies of heat balance in swimming mako sharks. J Exp Biol 204:4043–4054
Block BA, Dewar H, Blackwell SB, Williams TD, Prince ED, Farwell CJ, Boustany A, Teo SL, Seitz A, Walli A, Fudge D (2001) Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science 293:1310–1314
Boulant JA (2000) Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis 31:S157–S161
Boyd RB, DeVries AL (1983) The seasonal distribution of anionic binding sites in the basement membrane of the kidney glomerulus of the winter flounder Pseudopleuronectes americanus. Cell Tissue Res 234:271–277
Brett JR (1971) Energetic responses of salmon to temperature-study of some thermal relations in physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerka). Am Zool 11:99–113
Brett JR (1979) Environmental factors and growth. In: Hore WS, Randall DJ, Brett JR (eds) Fish physiology. Academic, New Yolk, pp 599–675
Burcham TS, Osuga DT, Chino H, Feeney RE (1984) Analysis of antifreeze glycoproteins in fish serum. Anal Biochem 139:197–120
Campbell HA, Fraser KPP, Bishop CM, Peck LS, Egginton S (2008) Hibernation in an Antarctic fish: on ice for winter. PLoS One 3:e1743
Carey FG, Lawson KD (1973) Temperature regulation in free-swimming bluefin tuna. Comp Biochem Physiol 44A:375–392
Carey FG, Robinson BH (1981) Daily patterns in the activities of swordfish, Xiphias gladius, observed by acoustic telemetry. Fish Bull US 79:277–292
Carey FG, Scharold JV (1990) Movements of blue sharks (Prionace glauca) in depth and course. Mar Biol 106:329–342
Chen L, DeVries AL, Cheng CH (1997a) Evolution of antifreeze glycoprotein gene from a trypsinogen gene in Antarctic notothenioid fish. Proc Natl Acad Sci U S A 94:3811–3816
Chen L, DeVries AL, Cheng CH (1997b) Convergent evolution of antifreeze glycoproteins in Antarctic notothenioid fish and Arctic cod. Proc Natl Acad Sci U S A 94:3817–3822
Chen WH, Sun LT, Tsai CL, Song YL, Chang CF (2002) Cold-stress induced the modulation of catecholamines, cortisol, immunoglobulin M, and leukocyte phagocytosis in tilapia. Gen Comp Endocrinol 126:90–100
Cheng CH, Cziko PA, Evans CW (2006) Nonhepatic origin of notothenioid antifreeze reveals pancreatic synthesis as common mechanism in polar fish freezing avoidance. Proc Natl Acad Sci U S A 103:10491–10496
Choi YG, Park CJ, Kim HE, Seo YJ, Lee AR, Choi SR, Lee SS, Lee JH (2015) Comparison of backbone dynamics of the type III antifreeze protein and antifreeze-like domain of human sialic acid synthase. J Biomol NMR 61:137–150
Claireaux G, Webber DM, Kerr SR, Boutilier RG (1995) Physiology and behaviour of free-swimming Atlantic cod (Gadus morhua) facing fluctuating temperature conditions. J Exp Biol 198:49–60
Coggan R (1997) Seasonal and annual growth rates in the Antarctic fish Notothenia coriiceps R. J Exp Mar Biol Ecol 213:215–229
Crawshaw L, Grahn D, Wollmuth L, Spimson L (1985) Central nervous regulation of body temperature in vertebrates: comparative aspects. Pharmacol of body temperature in vertebrates: comparative aspect. Pharmacol Ther 30:19–30
Dantuma NP, Hoppe T (2012) Growing sphere of influence: Cdc48/p97 orchestrates ubiquitin-dependent extraction from chromatin. Trends Cell Biol 22:483–491
Deane EE, Woo NYS (2009) Modulation of fish growth hormone levels by salinity, temperature, pollutants and aquaculture related stress: a review. Rev Fish Biol Fish 19:97–120
Deng G, Laursen RA (1998) Isolation and characterization of an antifreeze protein from the longhorn sculpin, Myoxocephalus octodecimspinosis. Biochim Biophys Acta 1388:305–314
Deng G, Andrews DW, Laursen RA (1997) Amino acid sequence of a new type of antifreeze protein, from the longhorn sculpin Myoxocephalus octodecimspinosis. FEBS Lett 402:17–20
DeVries AL (1982) Biological antifreeze agents in coldwater fishes. Comp Biochem Physiol A 73:627–640
DeVries AL, Cheng CHC (2005) Antifreeze proteins and organismal freezing avoidance in polar fishes. In: Farrell AP, Steffenson JF (eds) The physiology of polar fishes. Fish physiology series, vol 22. Academic, San Diego, pp 155–201
Donaldson MR, Cooke SJ, Patterson DA, Macdonald JS (2008) Review paper, cold shock and fish. J Fish Biol 73:1491–1530
Duman JG, DeVries AL (1976) Isolation, characterization, and physical properties of protein antifreezes from the winter flounder, Pseudopleuronectes americanus. Comp Biochem Physiol B 54:375–380
Eastman JT, DeVries AL (1986) Renal glomerular evolution in Antarctic notothenioid fishes. J Fish Biol 29:649–662
Ewart KV, Rubinsky B, Fletcher GL (1992) Structural and functional similarity between fish antifreeze proteins and calcium-dependent lectins. Biochem Biophys Res Commun 185:335–340
Fletcher GL, Idler DR, Vaisius A, Hew CL (1989) Hormonal regulation of antifreeze protein gene expression in winter flounder. Fish Physiol Biochem 7:387–393
Fritsches KA, Brill RW, Warrant EJ (2005) Warm eyes provide superior vision in swordfishes. Curr Biol 15:55–58
Gallagher PS, Candadai SVC, Gardner RG (2014) The requirement for Cdc48/p97 in nuclear protein quality control degradation depends on the substrate and correlates with substrate insolubility. J Cell Sci 127:1980–1991
Gauthier SY, Scotter AJ, Lin FH, Baardsnes J, Fletcher GL, Davies PL (2008) A re-evaluation of the role of type IV antifreeze protein. Cryobiology 57:292–296
Gong Z, Ewart KV, Hu Z, Fletcher GL, Hew CL (1996) Skin antifreeze protein genes of the winter flounder, Pleuronectes americanus, encode distinct and active polypeptides without the secretory signal and prosequences. J Biol Chem 271:4106–4112
Graham JB, Dickson KA (2001) Anatomical and physiological specializations for endothermy. In: Block BA, Dtevens ED (eds) Tuna: physiology, ecology, and evolution. Academic, San Diego, pp 121–168
Graham LA, Li J, Davidson WS, Davies PL (2012) Smelt was the likely beneficiary of an antifreeze gene laterally transferred between fishes. BMC Evol Biol 12:190
Gronwald W, Loewen MC, Lix B, Daugulis AJ, Sonnichsen FD, Davies PL, Sykes BD (1998) The solution structure of type II antifreeze protein reveals a new member of the lectin family. Biochemistry 37:4712–4721
Guderley H (2004) Metabolic responses to low temperature in fish muscle. Biol Rev 79:409–427
Harding MM, Anderberg P, Haymet AD (2003) ‘Antifreeze’ glycoproteins from polar fish. Eur J Biochem 270:1381–1392
Hemmingsen EA (1991) Respiratory and cardiovascular adaptations in hemoglobin-free fish: resolved and unresolved problems. In: di Prisco G, Maresca B, Tota B (eds) Biology of Antarctic fish. Springer-Verlag, Berlin, pp 191–203
Hew CL, Fletcher GL (1979) The role of pituitary in regulating antifreeze protein synthesis in the winter flounder. FEBS Lett 99:337–339
Hew CL, Fletcher GL, Ananthanarayanan VS (1980) Antifreeze proteins from the shorthorn sculpin, Myoxocephalus scorpius: isolation and characterization. Can J Biochem 58:377–383
Hew CL, Slaughter D, Joshi SB, Fletcher GL, Ananthanarayanan VS (1984) Antifreeze polypeptides from the Newfoundland ocean pout, Macrozoarces americanus: presence of multiple and compositionally diverse components. J Comp Physiol B 155:81–88
Hew CL, Wang NC, Joshi S, Fletcher GL, Scott GK, Hayes PH, Buettner B, Davies PL (1988) Multiple genes provide the basis for antifreeze protein diversity and dosage in the ocean pout, Macrozoarces americanus. J Biol Chem 263:12049–12055
Holland KN, Sibert JR (1994) Physiological thermoregulation in bigeye tuna, Thunnus obesus. Environ Biol Fish 40:319–327
Holland KN, Brill RW, Chang RK, Sibert JR, Fournier DA (1992) Physiological and behavioural thermoregulation in bigeye tuna (Thunnus obesus). Nature 358:410–412
Hoppe T, Matuschewski K, Rape M, Schlenker S, Ulrich HD, Jentsch S (2000) Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell 102:577–586
Hubold G (1991) Ecology of notothenioid fishes in the Weddell Sea. In: di Prisco G, Maresca B, Tota B (eds) Biology of Antarctic fish. Springer-Verlag, Berlin Heidelberg, pp 3–22
Idler DR, Fletcher GL, Belkhode S, King MJ, Hwang SJ (1989) Regulation of antifreeze protein production in winter flounder: a unique function of growth hormone. Gen Comp Endocrinol 74:327–334
Imamura S, Ojima N, Yamashita M (2002) Molecular cloning and cold-inducible gene expression of the cell division cycle gene CDC48 in zebrafish cells. Fish Sci 68:1291–1292
Imamura S, Ojima N, Yamashita M (2003) Cold-inducible expression of the cell division cycle gene CDC48 and its promotion of cell proliferation during cold acclimation in zebrafish. FEBS Lett 549:14–20
Imamura S, Yabu T, Yamashita M (2012) Protective role of cell division cycle 48 (CDC48) protein against neurodegeneration via ubiquitin-proteasome system dysfunction during zebrafish development. J Biol Chiem 287:23047–23056
Kitagawa Y, Ogawa M, Fukuchi M (1990) On the kidney of the saffron cod, Eleginus gracilis and its cold adaptation. Proc NIPR Symp Polar Biol 3:71–75
Kitagawa T, Kimura S, Nakata H, Yamada H (2006) Thermal adaptation of Pacific bluefin tuna Thunnus orientalis to temperate waters. Fish Sci 72:149–156
Knox GA (2006) Biology of the Southern Ocean, 2nd edn. CRC Press, London
Ko TP, Robinson H, Gao YG, Cheng CH, DeVries AL, Wang AH (2003) The refined crystal structure of an eel pout type III antifreeze protein RD1 at 0.62-A resolution reveals structural microheterogeneity of protein and solvation. Biophys J 84:1228–1237
Latterich M, Frohlich KU, Schekman R (1995) Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell 22:885–893
Li XM, Trinh KY, Hew CL, Buettner B, Baenziger J, Davies P (1985) Structure of an antifreeze polypeptide and its precursor from the ocean pout, Macrozoarces americanus. J Biol Chem 260:12904–12909
Little AG, Seebacher F (2013) Thyroid hormone regulates muscle function during cold acclimation in zebrafish (Danio rerio). J Exp Biol 216:3514–3521
Little AG, Seebacher F (2014) Thyroid hormone regulates cardiac performance during cold acclimation in zebrafish (Danio rerio). J Exp Biol 217:718–725
Little AG, Kunisue T, Kannan K, Seebacher F (2013) Thyroid hormone actions are temperature-specific and regulate thermal acclimation in zebrafish (Danio rerio). BMC Biol 11:26
Liu Y, Li Z, Lin Q, Kosinski J, Seetharaman J, Bujnicki JM, Sivaraman J (2007) Structure and evolutionary origin of Ca2+-dependent herring type II antifreeze protein. PLoS One 2:e548
Low WK, Miao M, Ewart KV, Yang DS, Fletcher GL, Hew CL (1998) Skin-type antifreeze protein from the shorthorn sculpin, Myoxocephalus scorpius. Expression and characterization of a Mr 9, 700 recombinant protein. J Biol Chem 273:23098–23103
Marshall CB, Chakrabartty A, Davies PL (2005) Hyperactive antifreeze protein from winter flounder is a very long rod-like dimer of alpha-helices. J Biol Chem 280:17920–17929
Martin KLM, Ishimatus A (2017) Review of reproductive strategies. In: Jaafar Z, Murdy EO (eds) Fishes out of water, biology and ecology of mudskippers. CBC Press, New York, pp 209–236
Meyer H, Bug M, Bremer S (2012) Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol 14:117–123
Moir D, Stewart SE, Osmond BC, Botstein D (1982) Cold-sensitive cell-division-cycle mutants of yeast: isolation, properties, and pseudoreversion studies. Genetics 100:547–563
Nakamura I, Goto Y, Sato K (2015) Ocean sunfish rewarm at the surface after deep excursions to forage for siphonophores. J Anim Ecol 84:590–603
Nishimiya Y, Kondo H, Takamichi M, Sugimoto H, Suzuki M, Miura A, Tsuda S (2008) Crystal structure and mutational analysis of Ca2+-independent type II antifreeze protein from longsnout poacher, Brachyopsis rostratus. J Mol Biol 382:734–746
Ogawa M, Sugai T, Murata J, Watanuki T (1997) Effects of salmon prolactin and growth hormone on plasma osmolality, Na+ concentration and protein content in the saffron cod. Fish Physiol Biochem 17:289–293
Pace C (2017) Aquatic and terrestrial locomotion. In: Jaafar Z, Murdy EO (eds) Fishes out of water, biology and ecology of mudskippers. CBC Press, New York, pp 195–208
Quinn T (1999) Habitat characteristics of an intertidal aggregation of Pacific sandlance (Ammodytes hexapterus) at a North Puget Sound Beach in Washington. Northwest Sci 73:44–49
Schurmann H, Christiansen JS (1994) Behavioral thermoregulation and swimming activity of two Arctic teleosts (subfamily gadinae) – the Polar cod (Boreogadus saida) and the navaga (Eleginus navaga). J Therm Biol 19:207–212
Scott GK, Hew CL, Davies PL (1985) Antifreeze protein genes are tandemly linked and clustered in the genome of the winter flounder. Proc Natl Acad Sci U S A 82:2613–2617
Slaughter D, Fletcher GL, Ananthanarayanan VS, Hew CL (1981) Antifreeze proteins from the sea raven, Hemitripterus americanus. Further evidence for diversity among fish polypeptide antifreezes. J Biol Chem 256:2022–2026
Sönnichsen FD, DeLuca C, Davies PL, Sykes BD (1996) Refined solution structure of type III antifreeze protein: hydrophobic groups may be involved in the energetics of the protein-ice interaction. Structure 4:1325–1337
Sorhannus U (2012) Evolution of type II antifreeze protein genes in teleost fish: a complex scenario involving lateral gene transfers and episodic directional selection. Evol Bioinforma 8:535–544
Stevens ED, Lam HM, Kendall J (1974) Vascular anatomy of the counter-current heat exchange of skipjack tuna. J Exp Biol 61:145–153
Stevens ED, Kanwisher JW, Carey FG (2000) Muscle temperature in free-swimming giant Atlantic bluefin tuna (Thunnus thynnus L.). J Therm Biol 25:419–423
Takegaki T, Fujii T, Ishimatsu A (2006) Overwintering habitat and low-temperature tolerance of the young mudskipper Boleophthalmus pectinirostris. Nippon Suisan Gakkaishi 72:880–885
Takei Y, Ando H, Tsutui K (2016) Handbook of hormones. Academic, Oxford
Tomiyama M, Yanagibashi S (2004) Effect of temperature, age class, and growth on induction of aestivation in Japanese sandeel (Ammodytes personatus) in Ise Bay, central Japan. Fish Oceanogr 13:81–90
Tytler P, Vaughan T (1982) Thermal ecology of the mudskippers, Periophthalmus koelreuteri (Pallas) and Boleophthalmus boddarti (Pallas) of Kuwait Bay. J Fish Biol 23:327–337
Wegner NC, Snodgrass OE, Dewar H, Hyde JR (2015) Whole-body endothermy in a mesopelagic fish, the opah, Lampris guttatus. Science 348:786–789
Winslade P (1974) Behavioural studies on the lesser sandeel Ammodytes marinus (Raitt) III. The effect of temperature on activity and the environmental control of the annual cycle of activity. J Fish Biol 6:587–599
Wolf DH, Stolz A (2012) The Cdc48 machine in endoplasmic reticulum associated protein degradation. Biochem Biophys Acta 1823:117–124
Wootton RJ, Smith C (2015) Reproductive biology of teleost fish. Wiley Blackwell, West Sussex
Yamashita M, Ojima N, Sakamoto T (1996) Induction of proteins in response to cold acclimation of rainbow trout cells. FEBS Lett 382:261–264
Yamashita Y, Miura R, Takemoto Y (2003) Type II antifreeze protein from a mid-latitude freshwater fish, Japanese smelt (Hypomesus nipponensis). Biosci Biotechnol Biochem 67:461–466
Ye Y, Meyer HH, Rapoport TA (2001) The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into cytosol. Nature 414:652–656
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Soyano, K., Mushirobira, Y. (2018). The Mechanism of Low-Temperature Tolerance in Fish. In: Iwaya-Inoue, M., Sakurai, M., Uemura, M. (eds) Survival Strategies in Extreme Cold and Desiccation. Advances in Experimental Medicine and Biology, vol 1081. Springer, Singapore. https://doi.org/10.1007/978-981-13-1244-1_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-1244-1_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1243-4
Online ISBN: 978-981-13-1244-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)