Abstract
The spinal accessory nerve (SAN) is a vital structure encountered in many surgeries of the head and neck. The anatomy of the SAN is complex, and identification intraoperatively is key in preventing injury. Compromise of the SAN leads to an array of findings known as postoperative shoulder syndrome, which has a significant impact on patients’ quality of life and contributes to a significant amount of malpractice litigations. Surgeries where the SAN is most at risk include neck dissection, surgery of the posterior triangle such as lymph node biopsies, and lateral skull base surgery. While the frequency of postoperative shoulder syndrome has been mitigated by the trend toward SAN-sparing surgeries in head and neck cancer, it remains a worrisome complication. Intraoperative nerve monitoring is widely used in various head and neck surgeries and has more recently been applied to the SAN. Literature to support the use is variable and relatively limited.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Spinal accessory
- Nerve monitoring
- Cranial nerve eleven
- Head and neck surgery
- Postoperative shoulder syndrome
Introduction
The spinal accessory nerve (SAN) is a vital structure encountered in many surgeries of the head and neck. The anatomy is complex, and identification intraoperatively is key to preventing injury and subsequent postoperative shoulder syndrome. Intraoperative nerve monitoring is widely used in various head and neck surgeries; however, it has more recently been applied to the SAN, although literature to support the use is variable and relatively limited. This chapter will detail the anatomy of the SAN, review the presentation and prevalence of postoperative shoulder syndrome, summarize surgeries that put the SAN at risk, and finally review the literature on SAN monitoring in head and neck surgery.
Anatomy
The SAN or cranial nerve 11 (CN XI) has both a spinal and cranial root joined together only briefly as they course through the jugular foramen. The cranial root originates in the dorsolateral surface of the medulla oblongata and eventually joins with the superior ganglion of the vagus nerve [1]. The spinal root, which provides somatic motor function to the sternocleidomastoid (SCM) and trapezius muscles, arises from the cervical spinal nerves of vertebral levels C1 to C5 within the accessory nucleus of the dorsolateral part of the ventral horn. The nerves course between the dorsal and ventral spinal roots to form a trunk that ascends to enter the posterior cranial fossa through the foramen magnum, exiting through the jugular foramen [2, 3]. Though the spinal portion arises from cervical rootlets, the fibers join briefly with the cranial fibers in the jugular foramen, prior to exiting the cranial cavity. The nerve then courses in proximity to the internal jugular vein (IJV) . At the level of the superior border of the posterior belly of the digastric, the SAN most commonly courses lateral to the IJV, but can less commonly course medially and rarely directly through the IJV [4]. The nerve continues its complex course through the neck traversing anterior to the transverse process of the atlas and descending medial to the styloid process and stylohyoid and digastric muscles. It subsequently enters the deep surface of the SCM, where it may anastomose with fibers of C2–C5 and rarely with C1 [5]. The contribution of these cervical fibers to motor function is not fully understood [6]. This segment of the SAN also serves as an important landmark for subdividing level II of the neck, marking the boundary between levels IIa and IIb [7]. The proximal IJV is used as a landmark to identify the proximal SAN in the anterior triangle and Erb’s point in the posterior triangle. If an imaginary line is drawn from this point to the thyroid notch, the SAN will enter the posterior triangle within 2 cm above this level and exit within 2 cm below it [8]. The SAN begins its course through the posterior triangle of the neck as it emerges from the posterior border of the SCM , approximately 7–9 cm above the clavicle, passing about 1–2 cm superiorly to Erb’s point [1]. Within the posterior triangle of the neck, the nerve crosses superficial to the levator scapulae and enters the trapezius muscle approximately 5 cm above the clavicle [2, 3].
Postoperative Shoulder Syndrome
Any surgery that injures the SAN can result in postoperative shoulder syndrome, which is caused by trapezius muscle denervation. When first described in the 1950s, the findings of postoperative shoulder syndrome were viewed as minor and acceptable side effects following radical neck dissection (RND) [9]. Most neck dissections now are function sparing, which include preservation of the SCM and SAN making postoperative shoulder syndrome less common [10]. Patients with postoperative shoulder syndrome present with pain, weakness, and deformity of the shoulder girdle. They can have destabilization of the scapula with progressive flaring, drooping, lateral and anterior rotation, as well as decreased ability to abduct the shoulder above 90 degrees. Secondary glenohumeral stiffness from scapulohumeral girdle muscle weakness and postoperative immobility can also contribute to shoulder disability [11]. For those with SAN preservation, improvement in symptoms from postoperative shoulder syndrome can be seen 6 months to a year postoperatively as the nerve fibers recover and regenerate [12].
The prevalence of shoulder dysfunction varies by the type of surgery. Following posterior triangle lymph node biopsy, the prevalence is between 3% and 8% [8] [13]. The prevalence of findings following neck dissection is variable and is highest after RND when the SAN is sacrificed. The presence of shoulder droop following RND ranges from 44% to 100%, modified radical neck dissection with SAN preservation (MRND) 0% to 30%, selective neck dissection (SND) levels II–V 56%, and SND levels I–III 13%. Reduction in shoulder active abduction range of motion following unilateral RND ranges between 92% and 94%, bilateral RND 100%, and MRND 23%. The prevalence of reduced neck range of motion following RND is as high as 45% and 13% following MRND [14].
Postoperative shoulder syndrome may have a significant impact on patient’s quality of life (QOL) and is a significant source of malpractice litigations [15]. The impact on QOL after neck dissection has been evaluated using a variety of validated questionnaires – SF-36 [16], SF-12 [17], or HNQOL [18] . Validated patient-reported outcomes have been reported in the literature that specifically evaluate shoulder function such as the Shoulder and Pain Disability Index (SPADI) and Constant Shoulder Score [19]. While the impact on quality of life is multifactorial, in general, those with the highest decline in QOL tend to be those patients who had SAN resection [12].
Surgeries that Risk the SAN
Compromise of the SAN is a known complication of many head and neck surgeries, in particular those closer in proximity to the posterior triangle and lateral skull base.
Neck Dissection
Neck dissection , also referred to as cervical lymphadenectomy, is a commonly performed procedure for head and neck cancer and widely known to risk the SAN. SAN injury can occur even when the nerve is macroscopically intact. Injuries can be on a spectrum of neural dysfunction, from neuropraxia and axonotmesis to neurotmesis [20]. The observed morbidity and disability of postoperative shoulder syndrome motivated a trend from RND toward MRND. Demonstration of similar survival and regional control with MRND over RND further supported this transition [21, 22]. SND is also becoming more accepted for appropriate patients [23]. As alluded to above QOL seems to be less affected in those patients who undergo SND involving levels I–III, although care should be taken to protect the proximal segment of the SAN as it courses along the IJV toward the SCM in level II of the neck. Neck dissections involving level V tend to be associated with higher risk to the distal portion of the SAN as it emerges from the posterior border of the SCM and courses through the posterior triangle.
Surgery in the Posterior Triangle
Surgery in the area of the posterior triangle, such as lymph node biopsy, puts the SAN at risk and makes up a majority of malpractice claims related to SAN iatrogenic injury [15]. SAN injury is estimated to occur after 3–8% of posterior triangle lymph node biopsies [8, 13]. As the nerve courses through the posterior triangle, it is surrounded by fibrofatty tissue and associated with a chain of five to ten lymph nodes; however, the nerve may also course superficially to these nodes [8].
Lateral Skull Base Surgery
The SAN is at particular risk in lateral skull base surgery that involves the jugular foramen. Here, the SAN exits the skull base with cranial nerves IX and X, and all these nerves are at risk. The jugular foramen is divided into three compartments, CN IX exits through the anterior compartment and CN X and XI through the middle compartment [24]. Care must be taken to preserve the SAN in this complex space. The most common tumors of this area are glomus jugulare, schwannomas, and meningiomas [25].
SAN Monitoring
SAN injury has a significant impact on patients, and despite the trend toward nerve-sparing surgeries, SAN injury still remains a worrisome complication. The SAN function can be preserved in a variety of ways intraoperatively. Visible and palpable muscle response of the SCM and trapezius can signal proximity of the nerve without the use of electrodes. Given the size of these muscles, this response is most often noticeable. Electromyography (EMG) measures action potentials of a muscle via electrodes placed into the muscle of interest. The SAN can be monitored by placement of electrodes into the trapezius muscle. EMG can be further divided into evoked, passive, and continuous monitoring, the former two methods can be utilized for SAN monitoring. Evoked nerve monitoring involves the surgeon stimulating the nerve to create a measured response. In contrast, passive nerve monitoring relies on analysis of various discharge patterns that occur throughout the operation and does not involve active stimulation of the nerve. Continuous monitoring involves continuously stimulating the nerve of interest for the entire procedure while measuring response; this method is available only for select nerves, not including the SAN [26].
EMG SAN monitoring is set up in a similar manner to that of facial nerve monitoring, with electrodes placed into the trapezius muscle [27]. Typically, bursts and trains of motor unit potential activity during surgery are continuously monitored in addition to deliberate electrical stimulation of the nerve while recording compound muscle action potential of the innervated muscle [20]. SAN monitoring can aid in identification of the nerve and alert the surgeon of proximity even prior to identification. The surgeon can stimulate the nerve directly at the end of the case to assess function, with the goal of preventing postoperative shoulder syndrome [27].
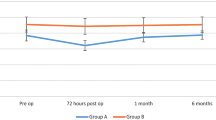
Currently, there is no standard of care when it comes to intraoperative SAN monitoring in head and neck surgery, leaving the decision at the discretion of the surgeon. As SAN monitoring is a relatively newer innovation, literature on the topic is limited [20] [28]. Data supporting SAN monitoring to prevent postoperative shoulder syndrome is variable. Intraoperative SAN monitoring during MRND has been shown to improve postoperative EMG scores; however, clinical scores related to postoperative shoulder syndrome were similar, whether nerve monitoring was used or not [29]. Lee and colleagues also studied 25 consecutive patients undergoing selective neck dissection with spinal accessory nerve monitoring and had no patient with postoperative shoulder syndrome sequelae other than mild pain; however, there was no comparative control group [28].
If the decision is made to use SAN monitoring, certain parameters can aid in predicting which patients may suffer from shoulder function decline postoperatively. These include threshold increment of greater than 0.25–0.5 mA [20, 30] and amplitude decrement of greater than 72% during surgery [30]. These findings may not always correlate with clinical outcomes and seem to have more specificity than sensitivity in predicting shoulder dysfunction [20, 31]. While these parameters are not completely predictive, the information can help in counseling patients in the postoperative period and may help in setting expectations for recovery. We were not able to identify any literature documenting the frequency of SAN monitoring use during neck dissection among surgeons.
Conclusion/Summary
The spinal accessory nerve (SAN) is a vital structure encountered in many surgeries of the head and neck. The anatomy of the SAN is complex, and identification intraoperatively is key in preventing injury. Postoperative shoulder syndrome resulting from SAN compromise has a significant impact on patient’s QOL. Intraoperative SAN monitoring can be used to identify the SAN, as well as assess nerve integrity during and at the end of surgery. Literature to support the use of SAN monitoring is variable and relatively limited, and decision for use is based on surgeon preference and discretion.
References
Overland J, Hodge JC, Breik O, Krishnan S. Surgical anatomy of the spinal accessory nerve: review of the literature and case report of a rare anatomical variant. J Laryngol Otol. 2016;130(10):969.
Wilkinson JL. Neuroanatomy for medical students [Internet]. Butterworth-Heinemann; 1992. Available from: https://books.google.com/books?id=wbp8yQEACAAJ.
Rea P. Chapter 11 – Spinal accessory nerve. In: Rea PBT-CA of the CN, editor. San Diego: Academic Press; 2014. p. 117–25. Available from: http://www.sciencedirect.com/science/article/pii/B9780128008980000117.
Hinsley ML, Hartig GK. Anatomic relationship between the spinal accessory nerve and internal jugular vein in the upper neck. Otolaryngol Neck Surg. 2010;143(2):239–41.
Standring S. Gray’s anatomy E-book: the anatomical basis of clinical practice. Elsevier Health Sciences; 2016.
Weisberger EC. The efferent supply of the trapezius muscle: a neuroanatomic basis for the preservation of shoulder function during neck dissection. Laryngoscope. 1987;97(4):435–45.
Robbins KT, Clayman G, Levine PA, Medina J, Sessions R, Shaha A, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology–Head and Neck Surgery. Arch Otolaryngol Neck Surg [Internet]. 2002;128(7):751–8. Available from: https://doi.org/10.1001/archotol.128.7.751.
Nason RW, Abdulrauf BM, Stranc MF. The anatomy of the accessory nerve and cervical lymph node biopsy. Am J Surg. 2000;180(3):241–3.
Ewing MR, Martin H. Disability following “radical neck dissection”. An assessment based on the postoperative evaluation of 100 patients. Cancer. 1952;5(5):873–83.
Goldstein DP, Ringash J, Bissada E, Jaquet Y, Irish J, Chepeha D, et al. Scoping review of the literature on shoulder impairments and disability after neck dissection. Head Neck. 2014;36(2):299–308.
Johnson JT, Rosen CA. Bailey’s head & neck surgery otolaryngology. Lippincott; 2014.
Terrell JE, Welsh DE, Bradford CR, Chepeha DB, Esclamado RM, Hogikyan ND, et al. Pain, quality of life, and spinal accessory nerve status after neck dissection. Laryngoscope [Internet]. 2000;110(4):620–6. Available from: https://doi.org/10.1097/00005537-200004000-00016.
Valtonen EJ, Lilius HG. Late sequelae of iatrogenic spinal accessory nerve injury. Acta Chir Scand. 1974;140(6):453–5.
Gane EM, Michaleff ZA, Cottrell MA, McPhail SM, Hatton AL, Panizza BJ, et al. Prevalence, incidence, and risk factors for shoulder and neck dysfunction after neck dissection: a systematic review. Eur J Surg Oncol [Internet]. 2017;43(7):1199–218. Available from: http://www.sciencedirect.com/science/article/pii/S0748798316309660.
Morris LGT, Ziff DJS, DeLacure MD. Malpractice litigation after surgical injury of the spinal accessory nerve: an evidence-based analysis. Arch Otolaryngol Neck Surg [Internet]. 2008;134(1):102–7. Available from: https://doi.org/10.1001/archotol.134.1.102.
Ware Jr JE. SF-36 health survey. 1999;
Ware JE, Keller SD, Kosinski M. SF-12: how to score the SF-12 physical and mental health summary scales. Health Institute, New England Medical Center; 1995.
Terrell JE, Nanavati KA, Esclamado RM, Bishop JK, Bradford CR, Wolf GT. Head and neck cancer—specific quality of life: instrument validation. Arch Otolaryngol Neck Surg. 1997;123(10):1125–32.
Constant CR, Murley AG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–4.
McGarvey AC, Hoffman GR, Osmotherly PG, Chiarelli PE. Intra-operative monitoring of the spinal accessory nerve: a systematic review. J Laryngol Otol. 2014;128(9):746.
Chu W, Strawitz JG. Results in suprahyoid, modified radical, and standard radical neck dissections for metastatic squamous cell carcinoma: recurrence and survival. Am J Surg [Internet]. 1978;136(4):512–5. Available from: http://www.sciencedirect.com/science/article/pii/0002961078902726.
Jesse RH, Ballantyne AJ, Larson D. Radical or modified neck dissection: a therapeutic dilemma. Am J Surg [Internet]. 1978;136(4):516–9. Available from: http://www.sciencedirect.com/science/article/pii/0002961078902738.
Muzaffar K. Therapeutic selective neck dissection: a 25-year review. Laryngoscope [Internet]. 2003;113(9):1460–5. Available from: https://doi.org/10.1097/00005537-200309000-00005.
Lloyd S. Accessory nerve: anatomy and surgical identification. J Laryngol Otol [Internet]. 2007;121(12):1118–25. Available from: http://libproxy.temple.edu/login?url=https://search.proquest.com/docview/274820196?accountid=14270.
Griessenauer CJ, McGrew B, Matusz P, De Caro R, Loukas M, Tubbs RS. Surgical approaches to the Jugular Foramen: a comprehensive review. J Neurol Surg B Skull Base [Internet]. 2015/11/16. 2016;77(3):260–4. Available from: https://pubmed.ncbi.nlm.nih.gov/27175322.
Stankovic P, Wittlinger J, Georgiew R, Dominas N, Hoch S, Wilhelm T. Continuous intraoperative neuromonitoring (cIONM) in head and neck surgery—a review. HNO. 2020:1–7.
Midwinter K, Willatt D. Accessory nerve monitoring and stimulation during neck surgery. J Laryngol Otol. 2002;116(4):272–4.
Lee C-H, Huang N-C, Chen H-C, Chen M-K. Minimizing shoulder syndrome with intra-operative spinal accessory nerve monitoring for neck dissection. Acta Otorhinolaryngol Ital. 2013;33(2):93.
Lanišnik B, Žitnik L, Levart P, Žargi M, Rodi Z. The impact on post-operative shoulder function of intraoperative nerve monitoring of cranial nerve XI during modified radical neck dissection. Eur Arch Oto Rhino Laryngol. 2016;273(12):4445–51.
Birinci Y, Genc A, Ecevit MC, Erdag TK, Guneri EA, Oztura I, et al. Spinal accessory nerve monitoring and clinical outcome results of nerve-sparing neck dissections. Otolaryngol Neck Surg. 2014;151(2):253–9.
Witt RL, Rejto L. Spinal accessory nerve monitoring in selective and modified neck dissection. Laryngoscope. 2007;117(5):776–80.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Molin, N., Liu, J.C. (2022). Spinal Accessory Nerve Monitoring in Head and Neck Surgery. In: Scharpf, J., Randolph, G.W. (eds) Intraoperative Cranial Nerve Monitoring in Otolaryngology-Head and Neck Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-84916-0_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-84916-0_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84915-3
Online ISBN: 978-3-030-84916-0
eBook Packages: MedicineMedicine (R0)