Abstract
In the last few years, a renewal of interest blossom on bacteriophages and other alternative therapies to antibiotics has been evident in the antimicrobial arena. Unfortunately, the dawn of antimicrobial resistance has lead to a ubiquitous decline in the effectiveness of currently available antibiotics. With a dwindling rate of discovery of novel antibiotics, it has become cumbersome to emulate the pace of resistance development. Bacteriophages or phages are the most diverse entities on earth and have a unique ability to act specifically against the infecting bacteria, without affecting the microbiota. They have the potential to be ideal candidates in our search for alternatives to antibiotics for clinical use. However, lack of investment from pharmaceutical companies, unclear regulatory guidelines and ineffective execution of clinical studies has hampered the success of phage therapy. It is time that the scientific community and pharma companies change their perception about phage therapy and conceptualize strategies for diligent development of the therapy by consolidating intellectual property rights and carrying out rigorous clinical trials. In the meantime, compassionate use of phage therapy or a combined approach (cocktail of phages or using with other therapies) can prove beneficial in prolonging the lifetime of the currently available antimicrobial drugs. Thus, this chapter emphasizes on developing a better understanding of bacteriophages (biology, role in infections, benefits and limitations), their transition to clinical use in the form of phage therapy and the challenges faced by this novel treatment along with possible solutions in the journey ahead.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The emergence of resistance amongst antimicrobials has raised grave concerns within the scientific community to further develop antibiotics to fight pathogens. The worldwide rise and spread of new resistant strains of microbes have undermined our capacity to treat common infections, which has resulted in delayed recovery, disability and death (https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance). The development of antimicrobial resistance (AMR) is a continuous process that evolves over time. After a certain period of time, the antibiotic drug loses its effectiveness towards the same pathogenic microbe (such as bacteria, fungi and parasites) it was previously effective. Antimicrobial resistance has led to a lack of availability of efficacious antibiotics and consequently increasing the risk of infectious spreads, severe illnesses and deaths. The year 2019 report released by the United Nations Ad hoc Interagency Coordinating Group (UN IACG) predicts that antimicrobial resistance could cause ten million deaths every year by 2050. Economically, antimicrobial resistance could constrain up to 24 million individuals to extreme poverty by 2030. Currently, at least 700,000 deaths occur every year globally due to drug-resistant diseases. Data show that only in the United States (US), 223,900 cases of antibiotic resistance-driven Clostridioides difficile had been reported in 2017, of which there were 12,800 casualties (https://www.cdc.gov/drugresistance/biggest-threats). Antibiotic resistance also adds to various complications in vulnerable patients going through chemotherapy, surgery, joint replacement and dialysis, and is considered a huge threat to the public health sector of developing countries and throughout the world. Global data from the year 2017 reflect that around ten million cases of tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis, had been reported which included 558,000 cases of rifampicin-resistant patients, of which 82% were multi-drug resistant (MDR). There has always been greater number of TB cases (100 per 100,000 or higher) in underdeveloped and developing countries like sub-Saharan Africa, India, and Southeast Asia due to poor understanding of prescription or treatment, inappropriate choices and insufficient dosing of antibiotics, which attributes to their misuse and further adds to the increase in antibiotic resistance (Park et al. 2019). Extreme use owes to easy accessibility of antimicrobial medications as the same can easily be purchased without the prescription of a qualified health professional. It has been clinically proven that prolonged and unscrupulous use of antimicrobials is probably the main reason behind the development and spread of antimicrobial-resistant infections. Other factors include exceptionally vulnerable immunosuppressed patients (cancer patients, transplant receipt and AIDS patients), delicate elderly patients, or long stay in hospitals (Prestinaci et al. 2015). The inability of antibiotics to treat infectious diseases showcases the uncertainty towards the future of the healthcare system. Hence, there lies a constant need to design and develop new antibiotics that could guard us against widespread microbes causing infections and find alternative strategies to reduce the burden on the current overexploited antibiotic therapy. Several therapies have been developed in the fight against antimicrobial resistance. These include probiotics, prebiotics, synbiotics, bacteriocins, SMAMPs (synthetic mimics of antimicrobial peptides), antimicrobial peptides, IDR (innate defence regulator) peptides, peptidomimetics, bacteriophages, vaccines and immunoglobulins, antibacterial oligonucleotides foldamers, antibacterial nucleic acids and immune stimulation by P4 peptide, etc., each one offering its own benefits and limitations. Amongst the mentioned alternatives, this chapter focuses on bacteriophages and how they can be efficiently used clinically to cure bacterial diseases in the form of phage therapy, covering all the aspects of this alternative treatment.
2 Antimicrobial Resistance (AMR): The Crisis
Antimicrobial resistance is a natural phenomenon that occurs when microbes are constantly exposed to antibiotic drugs. Whenever an antibiotic is used against disease-causing microbes, the most susceptible microbes are either inhibited or killed, while some are resilient and have a natural resistance to the attack, as expressed in the species. Significant factors contributing to AMR include the unscrupulous use of antimicrobials, patient incompetence to follow the prescribed therapy properly, and unavailability of new drugs within a particular class of antimicrobials that could replace the ineffective ones. Broadly, resistance can be of two types: intrinsic resistance and acquired resistance. In the case of intrinsic resistance, microbes do not possess the target for the antibiotic, while in the case of acquired resistance, bacteria device a mechanism that allows them to escape the action of the antibacterial drug molecule. Acquired resistance arises through the acquisition of host genetic material or mutations. These two forms of resistance are the most prominent ones and impart greater chances for the survival and multiplication of pathogen (Annunziato 2019; Prestinaci et al. 2015). Antibiotics have consistently been considered the most significant discovery over the last 70 years. Antibiotics are agents used to prevent and treat microbial infections in humans. Between 1950 and 1970, the use of antibiotics formally began with the so-called “golden age” of antimicrobial drugs. Unfortunately, the beginning of the antibiotics era tragically corresponds to the rise of the phenomenon of antimicrobial resistance (Fig. 15.1). Since the discovery of penicillin, the first antibiotic, Alexander Flemming had expressed concerns and cautioned the scientific community for the higher demand of antibiotics which later could lead to their abuse. This has been widely acknowledged now that, while the utilization of antimicrobials has prompted the control and even elimination of infectious disease; their abuse and/or overuse has led to the development of resistant strains. After a couple of years of the golden age of antimicrobials, an alarming sign of developing resistance has been observed (Annunziato 2019).
Diagram showing major milestones in the journey of antibiotics and evolution of antimicrobial resistance (Annunziato 2019)
3 Phage Biology Basics
Bacteriophages or simply phages are viruses that infect bacteria instead of humans, small in size (20–200 nm), diverse and the most abundant biological entities estimated to be around 1031, amounting to ten folds the bacterial population. These are composed of protein or proteolipidic polyhedral capsid containing nucleic acid (mainly DNA and RNA) fragments and complex appendages. The capsid is generally joined to a tail made up of a helical protein structure necessary for the adsorption of the virion (viral particle) to the bacterial cell (Brives and Pourraz 2020). Characterized as natural intracellular bacterial parasites, phages lack the ability to reproduce independently and completely depend on the host bacterial cell for its survival. Phages bind to specific receptors on the bacterium’s surface and inject the genetic material into the host cell. After entry into the host, phages may follow a lysogenic or temperate and lytic or virulent path. In the first case, these may amalgamate their genetic material into the host’s genome and reproduce to form daughter cells. In the latter case, phages take control over the bacterial replication machinery and produce the next generation of phages followed by consequent lysis of the host cell (Lin et al. 2017). Figure 15.2 demonstrates the lytic and lysogenic life cycle of a bacteriophage. As illustrated, phage attaches to the host cell and rapidly kills the infected cell at the end of the growth cycle. However, in the lysogenic phase, the phage introduces its genetic material into the host genome or stays undetected as plasmids inside their host cells by embedding into the bacterial chromosome (the prophage state) (Inal 2003).
Diagram showing schematic representation of lytic and lysogenic replication cycles of bacteriophage (Brives and Pourraz 2020)
Despite the advances in molecular biology techniques, it still is a cumbersome task to classify the vast varieties of phages. The International Committee on Taxonomy of Viruses (ICTV), an international organization, emphasizes the classification of phages based upon taxonomical properties such as the nature of genetic material, morphology of the virion, physico-chemical characteristics and genome sequencing data. The largest order of bacteriophages, i.e. Caudovirales, constitutes more than 96% population of all the bacteriophages, includes caudate viruses having double-stranded DNA and virions having an icosahedral capsid with the DNA and a tail (enabling connection between host and phage to transport its genetic material into the bacterial cell). The order Caudovirales further constitute three families: Myoviridae (capsid size ~150 nm and a contractile tail); Siphoviridae (capsid size ~50–60 nm, with a long, flexible and non-contractile tail) and Podoviridae (capsid size ~50–60 nm and a short tail). The remaining 4% of the phage population demonstrate greater morphological diversity (representing as filamentous, cubic or pleomorphic viruses) and hence are studied on an individual basis (Royer et al. 2021).
4 Discovery and Early History of Bacteriophages
Earliest reports for bacteriophages date back to 1896 by Ernst Hankin who reported the antibacterial activity of waters from the rivers Ganges and Yamuna in India against Vibrio cholera infection (Hankin 1896). Two years later, a Russian bacteriologist also reported similar phenomenon while working with Bacillus subtilis bacteria (Samsygina and Boni 1984). Later it was in the early twentieth century that phages were concurrently discovered by British microbiologist Frederick Twort (1915) and French-Canadian microbiologist Felix d’Herelle (1917). It was Frederick Twort who first hypothesized the possibility of a virus to be exhibiting activity against bacteria. However, Twort did not pursue phage research further but it was d’Herelle who methodically explored the nature of phages and investigated their capacity to work as therapeutic agents (Summers 2016). While working at the Pasteur Institute in Paris, d’Herelle utilized the newly discovered phage therapy during World War I, being in-charge of the bacteriological examination of the cases. During this period, he investigated extreme and atypical cases of bacillary dysentery amongst French soldiers stationed at Maisons-Laffitte commune on the north-western suburbs of Paris. While working on the faecal samples, d’Herelle found out that the not so familiar bacterial culture tend to lyse and clear in some time. When small portions of the cleared cultures were transferred to other samples of bacterial cultures, these would clear them as well. The mysterious antimicrobial activity is retained even after the removal of bacteria from the culture solutions. Following these observations, he went on to coin the term “Bacteriophage”with reference to assumed microbes that bring about the lysis and the lysis phenomenon itself (Ackermann 1917). d’Herelle expanded his research to United States, France, and Soviet Georgia by setting up phage therapy centres at several places (Carlton 1999; Myelnikov 2018).
Kharkov Mechnikov Institute was established in Kharkov, Soviet Ukraine in the year 1886 to carry out bacteriological studies investigated the Donbass region affected by frequent outbreaks of typhoid, scarlet fever and dysentery between years 1929 and 1935. Several therapies such as scarlet fever serum, typhoid vaccine and bacteriophage against dysentery were tried. The scientists and technicians were successful in making phage preparations against Shiga strain of dysentery bacillus (Shigella dysenteriae). Later Mel’nyk also isolated bacteriophages from areas near those water sources and used phage preparations against Shiga sub-strains (cultured from the faecal matter of patients). Similar to d’Herelle’s findings, the phages were found to lyse the infective microbes, as depicted by clear cultures. The consequent clear cultures were first mixed and then filtered through the bacterial filter to trap bacteria and debris; permitting the passage of phage for further use. This area-specific use of phages to fight localized strains helped deducing the specificity of bacteriophages (Myelnikov 2018). However, with the advent of antibiotics, a decline in further development and use of phage therapy was observed. Initially, two audits on phage therapy were published in the Journal of the American Medical Association (JAMA) in 1934 and 1941, explaining challenges in the utilization of phages and issues concerning their efficacy. During the same period, several derivatives of sulphonamides or sulfa drugs (potent antibacterial compounds) were introduced in Germany and there was enormous production and utilization of antibiotics in the United States in the 1940s. Hence, these sulfa drugs were considered one of the noteworthy accomplishments in the history of therapeutic agents also contributing to the sharp decline of phage therapy in the western world (Brives and Pourraz 2020). However, to overcome the earnest problem of antibiotic resistance arising due to multi-drug-resistant bacteria, interest in phage therapy has revived.
In the year 2017, WHO (World Health Organization) released a list of some disease-causing microbes consisting of antibiotic-resistant bacteria that pose the greatest risk to global well-being. Some susceptible microbes included carbapenem-resistant Acinetobacter, Candida auris, Clostridioides difficile, carbapenem-resistant Enterobacteriaceae, drug-resistant Neisseria gonorrhoeae, drug-resistant Campylobacter, drug-resistant Candida vancomycin-resistant Enterococci (VRE), erythromycin-resistant Group A Streptococcus and clindamycin-resistant group B Streptococcus. Therefore, the need to develop phage therapy for the treatment of common bacterial infections like sepsis, some forms of diarrhoea and sexually transmitted infections increases substantially as the world is running out of effective antibiotics (https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis). A brief timeline of events occurring in the development of bacteriophages and phage therapy has been summarized in Fig. 15.3.
Chronology of major events in the history of research on phages and advances in the phage therapy (Gordillo Altamirano and Barr 2019)
5 Mechanism of Action of Bacteriophages
Based upon replication cycle, bacteriophages can proceed through lytic and lysogenic mechanisms (Fig. 15.2). Bacterial lysis occurs when the infected cells tend to release the lytic phage progeny. Scientific investigations on lytic mechanism of phages provide insights into the phage therapy. Some bacteriophages utilize amurins (single proteins) which inhibit the synthesis of bacterial peptidoglycan whereas others use the holin-lysin systems. In the year 1992, a model was hypothesized which stated that lysis by phage involved two proteins: holins and endolysins. The model was principally based on molecular and genetic studies carried out on phage lambda. During the morphogenesis of the infectious cycle, the holin encoded by lambda S gets accumulated in the membrane, forming large pores in the cytoplasmic membrane and assisting the endolysins to escape and attack the peptidoglycan and consequently affecting the physical integrity of cell wall of bacteria (Moghadam et al. 2020; Young 2014). Holins are an extremely different class of small phage-encoded membrane proteins. There are three classes of holins based on the differences in amino acids sequence. Class I incorporates proteins that have more than 95 amino acids residues and are represented by Escherichia coli phage λ S105 protein and Staphylococcus aureus bacteriophage p68 hol15 protein. Class II holins have 65–95 amino acid residues and are characterized by Clostridium perfringens bacteriophage Ф3626 hol3626 protein and Lambdoid phage 21 S protein. Class III holins are represented by phage ФCP26F holin (Dewey et al. 2010). Genetic and biochemical methodologies on S105 suggest that it is the most extensively studied holin. It is a 105 amino acid residue polypeptide with three transmembrane domains (TMDs) encoded by the S gene of phage. S105 produces lethal lesions (~340 nm in diameter) in the bacterial lipid bilayer.
Endolysins, also known as phage lysins, are enzymatic proteins acting through hydrolysing peptidoglycan and subsequent cell wall degradation. They possess muralytic action on the bacterial cell wall mediated through amidase, endopeptidase or glycosidase action leading to bacterial cell destruction. At the end of their phage replication cycle, penetration into the peptidoglycan layer leads to osmotic lysis which eventually causes bacterial cell death and promotes virion progeny release (Cisek et al. 2017). The structural differences in the cell wall of gram-positive and gram-negative bacteria reflect upon the differences between their enzyme targets (Schmelcher et al. 2012). The impact of endolysins on bacterial cell walls is scheduled by certain holin genes which are specified in the dual start model. In this model, the holin gene is an open reading frame that encodes two proteins, holins and antiholins. The proportion of holin to antiholin (holin antagonist) decides the time of release of endolysins. For instance, Class I holin gene, the S gene of bacteriophage lambda not only encodes the effector holin, S105 but also an inhibitor, S107 with a Met1-Lys2-Met3 extension at the terminus (Shi et al. 2012).
6 Benefits and Limitations of Phage Therapy
Phage therapy has numerous advantages that make it an attractive option in contrast to antibiotics. Bacteriophages have high specificity for bacterium, unlike antibiotics, which are more active against a wide spectrum of microorganisms and probably cause secondary infections, e.g. with yeast, dysbiosis and other side effects (Romero-Calle et al. 2019). In addition, phages are dominant in nature and producing new phages takes fewer time rather than production of antibiotics. Thus, the development of phage therapy is inexpensive as compared to the production of antibiotics. One of the reasons why antibiotics are rendered ineffective is their metabolism and excretion by the body without reaching the site of infection. However, phages on administration replicate at the site of infection where the host organisms are located and then spread throughout the body. A comparative between phage therapy with conventional antibiotics has been summed up in Table 15.1 (El-Shibiny and El-Sahhar 2017; Romero-Calle et al. 2019).
Generally, phages target the surface receptors of the bacterial cell through virulence factors which later kill the bacteria by rupturing cell wall and cell membrane and eventually leading to the death of bacteria. Also, it is difficult for bacteria to develop resistance against phages but there are a few reports citing incidents of resistance within bacterial species attributed mainly to alteration and attenuation of phage virulence (El-Shibiny and El-Sahhar 2017). On comparison of phage therapy to traditional antibiotic treatments for use in the cure of bacterial infections, phage therapy offers extraordinary advantages over them. However, the phage therapy like other therapies is not devoid of its limitations. Despite being the largest group of organic entities on earth and offering numerous advantages, application of phage therapy is accompanied by various limitations as outlined in Table 15.2 (Moghadam et al. 2020).
7 Phage Therapy against Bacterial Infections in Humans
Since their discovery in early 1900s, bacteriophages have been widely used clinically to treat numerous bacterial infections accompanied by cases of conflicting outcomes in phage trials in the 1930s. Issues concerning the safety and efficacy of phage therapy were also raised in this period because of lack and inappropriate characterization, problems in production and purification of phage preparation (Gordillo Altamirano and Barr 2019). To overcome these obstacles, several clinical trials have been carried out and a few have been completed in recent years (Furfaro et al. 2018). Studies have shown that humans are constantly exposed to bacteriophages everyday and they help in their well-being. However, a number of factors still need to be explored while performing the clinical trials (Parracho et al. 2012). Bacteriophages have been utilized to treat bacterial diseases occurring in diverse body locales with different arrangements. Eastern Europe has a long history of using bacteriophages against bacterial infections (Summers 1999). Various mixed phage cocktails are sold in Russian drug stores as registered items. The bacteriophage therapy has been best examined for topical application on bacterial infections occurring in human skin (Sulakvelidze et al. 2001). The first human phage therapy has been accounted for the treatment of skin infections caused by Staphylococcus aureus. During the 1980s, phage preparations at the Eliava Institute, Georgia were given to human volunteers with no reported adverse effects. They were mostly effective on immunocompromised patients, new born children and in cases of pelvic inflammatory illnesses. However, it has also been observed that phage preparations have high efficacy in early phases of diseases (Abedon et al. 2011). It is a known fact that burn surfaces quickly get colonized by microorganisms which are fit for producing biofilms and are regularly impervious to multiple antibiotics. Phage treatment might have been utilized to treat burns and prevent sepsis during and after world war II in Eastern Europe (Morozova et al. 2018).
The largest clinical trial on phage therapy carried out in Europe was the Phago Burn trial, in the year 2013. This clinical trial fulfilled the criteria for both Good Manufacturing Practices (GMP) and Good Clinical Practices (GCP). In this randomized but controlled phase I/II clinical trial, 27 patients experiencing burn wounds infections were enrolled from hospitals situated in France and Belgium. Patients were randomly treated with phage therapy with a cocktail of 12 lytic phages and a standard preparation (1% sulfadiazine silver emulsion cream) to compare efficacy and tolerability of both therapies in patients infected by Pseudomonas aeruginosa. Both the formulations were applied topically for 7 days with a 14-day check. In general, bacterial load in burn wounds was less in treated groups but the process was slower than in the control group. No adverse effects were observed in the phage treated group. The restricted adequacy of the phage cocktail was accounted for an essential drop of phage titre. This resulted in lower concentration of phages in the patients than the ones assessed first. Also, in these participants, phage treatment failed as bacteria were found to be resistant to low doses of phage (Jault et al. 2019).
A combination of eight bacteriophages targeted against P. aeruginosa, S. aureus and Escherichia coli were applied on venous leg ulcers in around 39 patients and no side effects were observed relating to the treatment (Rhoads et al. 2009). Another randomized clinical trial was conducted on a group of 24 patients with a persistent otitis externa condition using a topical phage preparation against P. aeruginosa and the patients were successfully treated without any adverse effects. Besides this, a combination of P. aeruginosa and S. aureus phages was developed for application on the skin of infected patients (Wright et al. 2009). In Eastern Europe, oral phage delivery has also been utilized against intestinal diseases nowadays. Nonetheless, before studying the efficacy of phage therapy on numerous people in clinical trials, it is fundamental to first evaluate their safety in humans to guarantee that they do not cause adverse reactions when given orally (Sulakvelidze et al. 2001). While most clinical trials have failed to provide promising evidence of the efficacy of the phage therapy, some of the few case analyses show phage therapy as a successful method to treat life-threatening infections (Pires et al. 2020). For example, a novel method has been used to prepare personalized therapeutic bacteriophages cocktails to protect patients from life-threatening multidrug resistance caused by Acinetobacter baumanni infection. This case further consolidated the utilization of phage therapy in treating patients experiencing MDR bacterial infections with limited therapeutic options (Schooley et al. 2017). It is important that the western world should acknowledge the phage therapy and ramp up the clinical trials for their commercial use. There are numerous observational studies that have been performed but have encountered restrictions such as sample sizes and inadequacy in control groups. Every clinical case requires robust clinical trial data to be submitted to the regulators for the improvement of existing clinical guidelines (Payne and Jansen 2003).
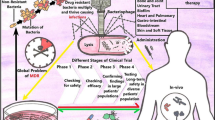
8 Phage Therapy: Problems and Alternative Solutions
As an antibacterial therapeutic, phage therapy has not been successful in completely replacing antibiotics as alternative. In order to establish phages as therapeutics, lot more investigations need to be done to uncover every aspect of bacteriophages. Due to reluctance of using phages, they have not been tested for their effects systemically in animal models and hence, they have a rather simplified pharmacokinetic profile. Merrils and coworkers in 1973 first reported infusion of high titre of lambda phage into non-immune germ-free mice. They concluded that phages were quickly cleared by the spleen, other filtering organs and liver (Geier et al. 1973). It has been observed that selected phage strains can remain in circulation for over a period of many days and continue to be accessible for interaction with possible systemic bacterial infectious agents by genetic selection method (Vitiello et al. 2005). This capacity of phages to stay in the circulation for a longer period and clear the toxins and antibiotic resistance genes may be helpful in the administration of phages. This property can further help design and develop phages which are effective with improved activity for specific bacterial strain (Adhya et al. 2014). Another limitation of phages is their moderately narrow spectrum of activity. Apparently, it was thought that the fundamental restricting factor for their clinical use is the number of phages accessible to doctors, which appears to be irrational after recognition of phages to be the most abundant organic entity on earth. The uniqueness of phages for bacterial strains infers the need to have a wide variety of phages with strong lytic action for fighting pathogenic bacteria in the given patient.
After the First International Congress on Microbes and Viruses in 2010 at the Pasteur Institute in Paris, an article on bacteriophages was published in 2011 in which the authors proposed two approaches known as ‘prêt-à porter’ and ‘sur-mesure’. In the ‘prêt-à-porter’ approach, fixed cocktails of several phages (around 10) are brought together to ensure that at least one the phage will be effective on the bacterial strain carried by the patient. This approach helps standardization of phage cocktail for commercial use whereas in the ‘sur-mesure’ approach, the patients will be administered only few phages that are specifically active on the infecting strain (Brives and Pourraz 2020). Phage preparations are often contaminated by bacterial debris, especially, macromolecules derived from the host bacteria and culture media with significant amount of endotoxins (Raetz et al. 2007). Injection of even modest quantities can result in cell injury, toxic shock and can be fatal to patients. However, various endotoxin removal methodologies and commercial kits have been developed which help in purification of phage preparations (Merabishvili et al. 2009).
Other key problem associated with phages is the inability to establish scientific proof of phage efficacy. Several articles in defence of phage therapy have been published in Europe in the recent couple of years, stating that the deficiency of clinical preliminaries exhibits the efficacy of phage due to their specificity whereas in infectious diseases, the antibiotics developed in the course of recent years are themselves quite specific, which has not kept them from demonstrating their adequacy (Morrison and Ulevitch 1978).
9 Future Challenges and Role of Bacteriophages
Unlike other antimicrobials such as antibiotics, phages show greater diversity in mechanism of action and can be much safer in many instances (Chan et al. 2013). The challenges for phage therapy include: how to put forward the positive attributes of phage therapy in presence of existing regulatory practices and how phages would find a way into the current economic models that support the distribution and utilization of antibacterial agents (Fig. 15.4) (Międzybrodzki et al. 2012). Phage therapy appears to flourish especially in regions where the regulatory authorities are moderate or with lesser restrictions (for example, in Poland and some countries of undivided Soviet Union). Scientists and researchers believe that phage therapy shall show expansion in years to come as promising candidates for use as antibacterial agents to overcome infections in western medicine system. Thus, it is anticipated that in the following 5–10 years, phage therapy would ascertain its way into clinical practice to cure life-threatening, ongoing chronic bacterial infections that cannot be treated using available antibacterial drugs (Sommer and Dantas 2011). To scale up the production of phages, there are significant issues that have not been satisfactorily addressed. These include removal of endotoxins and pyrogens which are delivered from the ruptured cells during phage lysis after administration of phage preparation. However, it is quite possible to optimize such outcomes. Recently, an endotoxin removal kit has been introduced which purifies the phage preparation before use in clinical trials (Merabishvili et al. 2009). Literature reports show that preliminary clinical studies have been attempted on cocktail of phages and have cleared phase I, showing no adverse reactions related to the utilization of phage cocktails, thus paving way to phase II clinical trials (e.g. APS Biocontrol Limited, Dundee, United Kingdom). Clearance of phage therapy in phase I and II encourages positive model for subsequent investigation in phase III (Rhoads et al. 2009). Another major barrier in bacteriophage delivery is the removal of phages by the immune system. Kim et al. studied the immune response by human body after phage administration by conjugating phage with polyethylene glycol. Results revealed that there was a decrease in the levels of helper T cells and an increase in the blood circulation time in contrast to unmodified phages (Kim et al. 2008).
Currently, phage therapy has been investigated only on fast dividing bacterial species and not on slow dividing bacterial species, where it needs to be developed and explored. Phage therapy can provide a promising solution for extremely slow dividing Mycobacterium africanum and Mycobacterium leprae (Dąbrowska 2019; Van Belleghem et al. 2019). Many challenges lie ahead for the therapeutic application of phages such as elimination of endotoxins in the final product, delivery system for the host, inadequacy in identification of quick and immediate therapy, activity potential of phages against specific host bacteria, public awareness and the absence of regulatory guidelines (Manohar et al. 2019). A huge amount of effort is required from government, organizations and academic researchers to translate phage therapy from clinical trials to markets.
10 Emerging Approaches in Phage Therapy
Phage therapy has been an area of interest that offers endless advantages over other antimicrobial therapies already threatened by antibiotic-resistant pathogens. Combining antibiotic and phage therapy, or the use of different phages as cocktails might be the most reassuring strategies for the treatment of bacterial infections (Manohar et al. 2019). For example, effectiveness of bacteriophages alone or in combination of amoxicillin, for eradication of biofilm formed by Klebsiella pneumoniae B5055 and planktonic cells has been assessed (Bedi et al. 2009). It has also been observed that individual treatments had restricted success rates. However, repeated phage treatments have shown to increase the biovolume of biofilms, as reported in P. aeruginosa PA01 (Henriksen et al. 2019). In contrast, the combination of phage and antibiotics leads to biofilm eradication (Díaz-Pascual et al. 2019). Consequently, when an antibiotic is administered in combination with specific bacteriophage, significant destruction of biofilm has been observed. Hence, the phages could be used effectively in conjugation to antibiotic therapy (Bedi et al. 2009). Mechanistically, antibiotics have substantial effects on biofilm structures and enhance the intrusion of biofilm by bacteriophages. An additional benefit of this combination therapy would be its ability to control emergence of resistant mutants that, in any case, progress effectively after utilizing antibiotic and phage therapies separately. It has also been observed that while using antibiotics and phages simultaneously, synergism does not occur for all the phage-antibiotic combinations. It has been reported that higher concentration of antibiotics to phages in combination therapy can also antagonize the proliferation of bacteriophages (Jansen et al. 2018).
Increase in the antimicrobial activity of phages has also been reported when administered along with enzymes. The use of enzyme depolymerase alongside phages has lead to prevention and dispersion of Staphylococcus biofilms (Gutiérrez et al. 2015). Other agents that can be effective in combination therapy with phages include hydrogen peroxide, honey, probiotics, xylitol, chlorine and cobalt (II) sulphate (Pires et al. 2020).
Advancement in synthetic biology and engineering methods has enabled improvement in phages by producing novel genomes. Engineered phages have enhanced antimicrobial activity along with the limitations of phage therapies (narrow host range and host immunity) (Kim et al. 2019). Engineered phages based upon lytic mechanism of phages have been developed. One or more temperate phages are genetically engineered with a lytic phage for use as therapeutic. The most common approach comprises of turning the phage into lytic one by genetic engineering. An illustration of this approach is the utilization of a cocktail made from one natural lytic phage and two engineered temperate phages to effectively treat a 15-year-old patient with cystic fibrosis with a disseminated Mycobacterium abscessus infection (Dorscht et al. 2009).
A well-known approach to overcome the problem of narrow range of activity is to use a combination of phages with different spectra in a single cocktail. However, the use of cocktails requires greater optimization to improve their performance. In addition, mixing up a diverse group of phages can lead to more challenges for approval and manufacturing. Subsequently, it would be ideal to create engineered phages with broad-spectrum and enhanced antimicrobial activity instead of simply combining multiple phages. For example, use of a recombinant phage (T3/7) produced by combination of a T3-based hybrid phage whose tail fibre gene I7 is a recombinant between those of T3 and T7. The hybrid phage T3/7 has been found to show better adsorption, effectiveness and a wider host range than both T3 and T7 phages used independently. To enhance the phage antimicrobial activity against biofilms, some enzymes such as lactonase and dispersin B have also been engineered into phage T7 (Lin et al. 2012). Researchers have proposed the use of a restriction enzyme on a non-specific target of a host genome that can be fundamentally extended to ‘CRISPR antibacterial’ technique which focuses on a specific gene vital for bacterial growth and survival and even more significantly, antibacterial species with their accessible phages (Kim et al. 2019). Hence, engineering methodologies can potentially improve phages’ antimicrobial properties, and such phages should be considered therapeutic choices. Additionally, phages that are engineered have simpler patentability and hence are of more commercial interest.
11 Conclusions
Undoubtedly, bacteriophages offer vast diversity and have huge potential for development as antimicrobial therapy in a ‘post-antibiotic era’ world which is short of medications essential for our fight against surging microbial infections. To ensure maximum clinical efficacy of phage therapy, fast-track research needs to be carried out on pathogenic bacteria affecting patients, followed by isolation, identification and screening against individual phage strains or available approved phage cocktails. So far, antimicrobial resistance continues to be one of the preeminent threats to global health, which requires significant intervention at different strata of the society. Considering the limited armamentarium of antibiotics available and an insufficiency in production of newer ones, and despite all its limitations, phages are a nature’s gift to humanity which is safe and effective strategy for combating bacterial infections, with the added advantage of controlling and preventing multidrug-resistant organisms.
To overcome individual limitations of a therapy, a combination therapy approach, wherein two or more therapies can be concurrently used, holds potential in extending the effectiveness of the currently available antimicrobials. The prospects of phage preparations to be used in combination with antibiotics, probiotics and vaccines against chronic infections and resistant pathogenic bacteria can help reduce human illnesses significantly. Measures can be taken in this direction to encourage pharmaceutical giants to invest in development of phage therapy and promote international collaborations amongst academia, doctors and pharma sector, resulting in ensuring advancements in phage therapy. Hence, comprehensive research is the requisite to achieve approval of the regulatory agencies for their inclusion to our repertoire of treatments as well as commercial use.
In conclusion, the looming crisis of antimicrobial resistance has left us with no other choice but to device multidimensional strategies to deal with widespread bacterial infections. The phage therapy offers enormous possibilities and can be instrumental in redefining modern biology by assisting in understanding the biological processes at the molecular level. Therefore, it is time to deliberate the benefits of phage therapy in its entirety with its targeted compassionate use on patients left with no other alternative treatment.
References
Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM (2011) Phage treatment of human infections. Bacteriophage 1(2):66–85
Ackermann H-W (1917) On an invisible microbe antagonistic to dysentery bacilli. C R Acad Sci 165:373–375
Adhya S, Merril CR, Biswas B (2014) Therapeutic and prophylactic applications of bacteriophage components in modern medicine. Cold Spring Harb Perspect Med 4(1):a012518
Annunziato G (2019) Strategies to overcome antimicrobial resistance (AMR) making use of non-essential target inhibitors: a review. Int J Mol Sci 20(23):5844
Bedi MS, Verma V, Chhibber S (2009) Amoxicillin and specific bacteriophage can be used together for eradication of biofilm of Klebsiella pneumoniae B5055. World J Microbiol Biotechnol 25(7):1145–1151
Brives C, Pourraz J (2020) Phage therapy as a potential solution in the fight against AMR: obstacles and possible futures. Palgrave Commun 6(1):1–11
Carlton RM (1999) Phage therapy: past history and future prospects. Arch Immunol Ther Exp 47:267–274
Chan BK, Abedon ST, Loc-Carrillo C (2013) Phage cocktails and the future of phage therapy. Future Microbiol 8(6):769–783
Cisek AA, Dąbrowska I, Gregorczyk KP, Wyżewski Z (2017) Phage therapy in bacterial infections treatment: one hundred years after the discovery of bacteriophages. Curr Microbiol 74(2):277–283
Dąbrowska K (2019) Phage therapy: what factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med Res Rev 39(5):2000–2025
Dewey JS, Savva CG, White RL, Vitha S, Holzenburg A, Young R (2010) Micron-scale holes terminate the phage infection cycle. Proc Natl Acad Sci 107(5):2219–2223
Díaz-Pascual F, Hartmann R, Lempp M, Vidakovic L, Song B, Jeckel H, Thormann KM, Yildiz FH, Dunkel J, Link H (2019) Breakdown of Vibrio cholerae biofilm architecture induced by antibiotics disrupts community barrier function. Nat Microbiol 4(12):2136–2145
Dorscht J, Klumpp J, Bielmann R, Schmelcher M, Born Y, Zimmer M, Calendar R, Loessner MJ (2009) Comparative genome analysis of listeria bacteriophages reveals extensive mosaicism, programmed translational frameshifting, and a novel prophage insertion site. J Bacteriol 191(23):7206–7215
El-Shibiny A, El-Sahhar S (2017) Bacteriophages: the possible solution to treat infections caused by pathogenic bacteria. Can J Microbiol 63(11):865–879
Furfaro LL, Payne MS, Chang BJ (2018) Bacteriophage therapy: clinical trials and regulatory hurdles. Front Cell Infect Microbiol 8:376
Geier MR, Trigg ME, Merril CR (1973) Fate of bacteriophage lambda in non-immune germ-free mice. Nature 246(5430):221–223
Gordillo Altamirano FL, Barr JJ (2019) Phage therapy in the postantibiotic era. Clin Microbiol Rev 32(2):e00066–e00018
Gutiérrez D, Briers Y, Rodríguez-Rubio L, Martínez B, Rodríguez A, Lavigne R, García P (2015) Role of the pre-neck appendage protein (Dpo7) from phage vB_SepiS-phiIPLA7 as an anti-biofilm agent in staphylococcal species. Front Microbiol 6:1315
Hankin E (1896) An OUTBREAK of CHOLERA in an OFFICERS’ MESS. Br Med J 2(1878):1817
Henriksen K, Rørbo N, Rybtke ML, Martinet MG, Tolker-Nielsen T, Høiby N, Middelboe M, Ciofu O (2019) P. aeruginosa flow-cell biofilms are enhanced by repeated phage treatments but can be eradicated by phage–ciprofloxacin combination: —monitoring the phage–P. aeruginosa biofilms interactions. Pathog Dis 77(2):ftz011
Inal JM (2003) Phage therapy: a reappraisal of bacteriophages as antibiotics. Arch Immunol Ther Exp 51(4):237–244
Jansen M, Wahida A, Latz S, Krüttgen A, Häfner H, Buhl EM, Ritter K, Horz H-P (2018) Enhanced antibacterial effect of the novel T4-like bacteriophage KARL-1 in combination with antibiotics against multi-drug resistant Acinetobacter baumannii. Sci Rep 8(1):1–12
Jault P, Leclerc T, Jennes S, Pirnay JP, Que Y-A, Resch G, Rousseau AF, Ravat F, Carsin H, Le Floch R (2019) Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect Dis 19(1):35–45
Kim KP, Cha JD, Jang EH, Klumpp J, Hagens S, Hardt WD, Lee KY, Loessner MJ (2008) PEGylation of bacteriophages increases blood circulation time and reduces T-helper type 1 immune response. Microb Biotechnol 1(3):247–257
Kim B-O, Kim ES, Yoo Y-J, Bae H-W, Chung I-Y, Cho Y-H (2019) Phage-derived antibacterials: harnessing the simplicity, plasticity, and diversity of phages. Viruses 11(3):268
Lin T-Y, Lo Y-H, Tseng P-W, Chang S-F, Lin Y-T, Chen T-S (2012) A T3 and T7 recombinant phage acquires efficient adsorption and a broader host range. PLoS One 7(2):e30954
Lin DM, Koskella B, Lin HC (2017) Phage therapy: an alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther 8(3):162
Manohar P, Tamhankar AJ, Leptihn S, Ramesh N (2019) Pharmacological and immunological aspects of phage therapy. Infect Microbes Dis 1(2):34–42
Merabishvili M, Pirnay J-P, Verbeken G, Chanishvili N, Tediashvili M, Lashkhi N, Glonti T, Krylov V, Mast J, Van Parys L (2009) Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One 4(3):e4944
Międzybrodzki R, Borysowski J, Weber-Dąbrowska B, Fortuna W, Letkiewicz S, Szufnarowski K, Pawełczyk Z, Rogóż P, Kłak M, Wojtasik E (2012) Clinical aspects of phage therapy. Adv Virus Res 83:73–121
Moghadam MT, Amirmozafari N, Shariati A, Hallajzadeh M, Mirkalantari S, Khoshbayan A, Jazi FM (2020) How phages overcome the challenges of drug resistant bacteria in clinical infections. Infect Drug Resist 13:45
Morozova VV, Kozlova YN, Ganichev DA, Tikunova NV (2018) Bacteriophage treatment of infected diabetic foot ulcers. In: Bacteriophage therapy. Springer, Berlin, pp 151–158
Morrison D, Ulevitch R (1978) The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol 93(2):526
Myelnikov D (2018) An alternative cure: the adoption and survival of bacteriophage therapy in the USSR, 1922–1955. J Hist Med Allied Sci 73(4):385–411
Park M, Satta G, Kon OM (2019) An update on multidrug-resistant tuberculosis. Clin Med 19(2):135
Parracho HM, Burrowes BH, Enright MC, McConville ML, Harper DR (2012) The role of regulated clinical trials in the development of bacteriophage therapeutics. J Mol Genet Med 6:279
Payne RJ, Jansen VA (2003) Pharmacokinetic principles of bacteriophage therapy. Clin Pharmacokinet 42(4):315–325
Pires DP, Costa AR, Pinto G, Meneses L, Azeredo J (2020) Current challenges and future opportunities of phage therapy. FEMS Microbiol Rev 44(6):684–700
Prestinaci F, Pezzotti P, Pantosti A (2015) Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health 109(7):309–318
Raetz CR, Reynolds CM, Trent MS, Bishop RE (2007) Lipid a modification systems in gram-negative bacteria. Annu Rev Biochem 76:295–329
Rhoads D, Wolcott R, Kuskowski M, Wolcott B, Ward L, Sulakvelidze A (2009) Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J Wound Care 18(6):237–243
Romero-Calle D, Guimarães Benevides R, Góes-Neto A, Billington C (2019) Bacteriophages as alternatives to antibiotics in clinical care. Antibiotics 8(3):138
Royer S, Morais AP, da Fonseca Batistão DW (2021) Phage therapy as strategy to face post-antibiotic era: a guide to beginners and experts. Arch Microbiol 203:1271–1279
Samsygina G, Boni E (1984) Bacteriophages and phage therapy in pediatric practice. Pediatriia (4):67–70
Schmelcher M, Donovan DM, Loessner MJ (2012) Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7(10):1147–1171
Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S (2017) Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 61(10):e00954–e00917
Shi Y, Yan Y, Ji W, Du B, Meng X, Wang H, Sun J (2012) Characterization and determination of holin protein of Streptococcus suis bacteriophage SMP in heterologous host. Virol J 9(1):1–11
Sommer MO, Dantas G (2011) Antibiotics and the resistant microbiome. Curr Opin Microbiol 14(5):556–563
Sulakvelidze A, Alavidze Z, Morris JG Jr (2001) Bacteriophage therapy. Antimicrob Agents Chemother 45(3):649–659
Summers WC (1999) Felix dHerelle and the origins of molecular biology. Yale University Press, Yale
Summers WC (2016) Félix Hubert d’Herelle (1873–1949): history of a scientific mind. Bacteriophage 6(4):e1270090
Van Belleghem JD, Dąbrowska K, Vaneechoutte M, Barr JJ, Bollyky PL (2019) Interactions between bacteriophage, bacteria, and the mammalian immune system. Viruses 11(1):10
Vitiello CL, Merril CR, Adhya S (2005) An amino acid substitution in a capsid protein enhances phage survival in mouse circulatory system more than a 1000-fold. Virus Res 114(1–2):101–103
Wright A, Hawkins C, Änggård E, Harper D (2009) A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 34(4):349–357
Young R (2014) Phage lysis: three steps, three choices, one outcome. J Microbiol 52(3):243–258
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kaur, R., Sethi, N. (2022). Phage Therapy as an Alternative Treatment in the Fight Against AMR: Real-World Problems and Possible Futures. In: Akhtar, N., Singh, K.S., Prerna, Goyal, D. (eds) Emerging Modalities in Mitigation of Antimicrobial Resistance. Springer, Cham. https://doi.org/10.1007/978-3-030-84126-3_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-84126-3_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84125-6
Online ISBN: 978-3-030-84126-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)