Abstract
Interest in the therapeutic use of bacteriophages (phages) has emerged in recent years, driven mainly by the antimicrobial resistance crisis. This review aimed to summarize some important studies addressing the use of phages as a therapeutic alternative for multiresistant bacterial infections. To this end, a literature search was conducted to address the efficacy and versatility of phage therapy, the advantages and disadvantages of its use, and potential limitations for the application of phage therapy that need to be overcome, especially in Western countries. Thus, this review highlights that phage therapy may be a promising route in the treatment of infections caused by multidrug-resistant pathogens and that a combined approach has the potential to prolong the life of the current available antimicrobials. In addition, standardized clinical trials using monoclonal or polyclonal phages, alone or in combination with antimicrobials, are crucial to determine the real potential of these treatments in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteriophages (phages) are small viruses (20–200 nm) that infect bacteria and are the most abundant biological entities on Earth, about 10 times more than what is estimated for the bacterial population, presenting a high and unknown diversity (Bárdy et al. 2016; Batinovic et al. 2019). The history tells that phages were discovered by Frederick Twort and Félix d'Herelle (Twort 1915; d'Herelle 1917; Kutter et al. 2010) in the early twentieth century, but Dr. José da Costa Cruz, from the Oswaldo Cruz Institute (Rio de Janeiro, Brazil), coordinated the first human phage therapy tests in 1921, during an outbreak of dysentery in Barbacena, Minas Gerais, Brazil (Almeida and Sundberg 2020). The mass testing of phage product from the Oswaldo Cruz Institute during the Paulista revolution of 1924 preceded Felix d’Herelle’s tests in India and the Soviet Union’s tests on military troops (Almeida and Sundberg 2020). It was the beginning of the rational therapeutic use of phages to treat several infectious diseases.
The antibiotics discovery made phage therapy unpopular in the Western and, consequently, the development of phage products for therapeutic purposes was restricted to only a few countries in the former Soviet Union and the Eastern Bloc (Bárdy et al. 2016; Jariah and Hakim 2019). As the problem of antibiotic resistance is becoming increasingly urgent due to the indiscriminate and inappropriate use of these drugs to treat both human and animal infections, to increase growth rate and weight gain in a wide variety of livestock, besides for agricultural use, which was further aggravated by the reduction of new drugs available on the market, phages are progressively being considered as a therapeutic alternative (Kutter et al. 2010; Weber-Dąbrowska et al. 2016; Garvey 2020).
Some authors (Azeredo and Sutherland 2008; Pires et al. 2017) defend that phage therapy is more specific than antibiotic therapy, reducing the destruction of the host’s natural microbiota, it is non-pathogenic for humans, in addition to being self-limiting, since the phages persist only as long as the target bacteria are present. However, given that the bacteriophage vary widely, recent original and review articles presented evidence and also new hypothesis about the roles of bacteriophages in human diseases, including dysbiosis (Divya Ganeshan and Hosseinidoust 2019), cancer (Sanmukh and Felisbino 2017), and Parkinson’s disease (Tetz et al. 2018).
In this review, we discuss the use of bacteriophages as therapeutic alternative to treat multiresistant bacterial infections, also highlighting the potential limitations of phage therapy that need to be overcome.
Threatened by a post-antibiotic era
Bacterial infections remain a challenge to public health with high morbidity and mortality rate worldwide, a situation aggravated by the spread of multidrug-resistant pathogens accompanied by the insufficient production of new drugs (Boucher et al. 2009; Viertel et al. 2014).
The main global concern is imposed by multidrug-resistant bacteria belonging to the “ESKAPE” group. The acronym “ESKAPE” includes six nosocomial pathogens: vancomycin-resistant enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA), Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and extended-spectrum β-lactamase (ESBL)-producing or carbapenem-resistant Enterobacteriales (CRE) responsible for several infections worldwide (Boucher et al. 2009; Magiorakos et al. 2012; Pendleton et al. 2013; WHO 2017).
Indeed, according to many experts, included those from World Health Organization (WHO) and from Center for Disease Control and Prevention (CDC), we are living now in a “post-antibiotic” era (Reardon 2014; CDC 2019). Given this life-threatening scenario, there is a critical need for the development of new therapies and clinical trial designs for the evaluation of unused treatments against the “superbugs”.
Therefore, therapy with bacteriophages, used for the first time almost a century ago, reappeared driven, mainly by the antibiotic crisis, but also due to a better understanding of the biology, genetics, immunology, and pharmacology of these viruses (Gordillo Altamirano and Barr 2019; Rello et al. 2019).
Bacteriophages, a possible weapon
Phages are viruses with DNA or RNA genome, encapsulated in protein capsids and sometimes with complex tail and appendages (Torres-Barceló 2018). As mandatory intracellular parasites, they infect their hosts through a variety of receptors present on the cell surface, such as carbohydrates, lipopolysaccharides, and proteins (Batinovic et al. 2019).
Even today, despite the advent of molecular biology, there is no single method for classifying bacteriophages. Properties used for phage’s taxonomy include: the nature of nucleic acid, the morphology of the viral particle (virion), physical–chemical properties, and genomic data (Ackermann 2012; Wittebole et al. 2014; Lefkowitz et al. 2018). Electron microscopy has been widely used to identify morphotype, complementing the results from molecular/genomic data analysis (Ackermann 2012; Lefkowitz et al. 2018). Currently, the international organism responsible for viral taxonomy is the International Committee on Taxonomy of Viruses-ICTV (www.ictvonline.org/) (Ackermann 2012; Lefkowitz et al. 2018).
More than 96% of the population of bacteriophages belong to the order Caudovirales, which includes caudate viruses, with double-stranded DNA (Lopes et al. 2014). The virions have an icosahedral capsid, containing the DNA and a tail, which is responsible for the connection to the host and the transport/injection of genetic material into the bacterial cell (Casjens 2005; Ackermann 2007; Wittebole et al. 2014). The order Caudovirales is composed of three families: Myoviridae, with the largest capsid (≈150 nm) and a contractile tail; Siphoviridae, with a relatively small capsid (≈50–60 nm) and with a long, flexible, and non-contractile tail; and Podoviridae, characterized by a small capsid (≈50–60 nm) and a short tail (Drulis-Kawa et al. 2012). Since only phenotypic observation does not allow an accurate distinction of phages, molecular tools are essential for the proper classification (Lopes et al. 2014). The other 4% of the phages exhibit greater morphological diversity, presenting themselves as cubic, filamentous, and pleomorphic viruses (Ackermann 2012).
The oldest battle of planet: phage–bacteria interaction
Bacteriophages can have different biological cycles and thus are classified as lytic or virulent and lysogenic or temperate (Jamal et al. 2019).
During a cycle of lytic infection, the phage binds to the receptor(s) on the surface of bacteria, delivers its genetic material, undergoes replication in the cytosol, and after the formation of new viral particles, escapes from the cytoplasm through the lysis of the bacterial cell (Kortright et al. 2019).
Some bacteriophages produce proteins called amurins which cause bacterial lysis through inhibition of cell wall synthesis. However, most of them utilize the holin–lysin system to kill the host cell (Woźnica et al. 2015; Cisek et al. 2017). Bacterial lysis is initiated when holin accumulates in the cytoplasmic membrane forming small holes that allow other proteins, the endolysins, escape from the cytoplasm to the periplasm and degrade the peptidoglycan. In addition, some phages are also able to synthesize pinholins, another type of holin protein characterized by forming heptameric channels in the membrane, which leads to its depolarization. The change in the electric potential of the bacterial membrane can lead to the activation of SAR-type endolysins, which were in their inactive state in the periplasm, resulting in the enzymatic degradation of the peptidoglycan. In this way, both types of holins control endolysin-mediated peptidoglycan degradation but do so by different mechanisms (Cahill and Young 2019). For Gram-negative hosts, the rupture of the outer membrane is also necessary. The lysis of this layer is mediated by spanin complex, which is usually formed by two components: an outer membrane lipoprotein (o-spanin), and an integral inner membrane protein (i-spanin). The activation of the spanin complex results in the fusion of the inner and outer membranes, resulting in the destruction of the outer membrane, promoting bacterial lysis (Woźnica et al. 2015).
The obligatory lytic phage seems to be the best candidate for the development of phage therapy; however, lysogenic phages prevail in nature (Jamal et al. 2019; Kortright et al. 2019). In lysogeny, phages do not kill the bacterial cell, but incorporates its DNA into the host’s genome (prophages) being able to synthesize a repressor peptide that prevents the synthesis of enzymes necessary for DNA replication and protein synthesis. The prophage is inherited by daughter bacterial cells during binary fission (Tsao et al. 2018). Usually, prophages carry genes that benefit the hosts increasing their virulence and survival; therefore, also expanding the fitness of the bacteriophage (Domelier et al. 2009; Tsao et al. 2018). Just as the prophage is important for bacterial control, it also plays a role in the destruction of cells when they are subjected to some stressful situation, such as UV irradiation. In this situation, the phage repressor peptide ceases to act, and the DNA of the bacteriophage is then duplicated, leading to protein synthesis and formation of new phage particles inside the host. Finally, the bacterial cell is lysed causing the release of virions in environment (Ebrahimizadeh and Rajabibazl, 2014; Howard-Varona et al. 2017).
Opponents’ evasion and defense mechanisms
Bacteria and bacteriophages are locked in a constant battle. Bacteria can become resistant to phages by developing subpopulations with stable and resistant phenotypes to protect themselves against the invasion of foreign DNA. The resistance can arise due to mutations of DNA or be acquired by the exchange of genetic material with other bacteria, for example, through the acquisition of plasmids (Wittebole et al. 2014; Hill et al. 2018). The mechanisms of resistance include mutation or loss of membrane receptors, degradation of phage genetic material by restriction–modification (R–M) systems through restriction endonucleases (REase) which cut invading foreign DNA at specific recognition sites, and activation of the clustered regularly interspaced short palindromic repeats (CRISPR) CRISPR-associated proteins (Cas) system, a primitive immune system specialized in destruction of bacteriophages (Wittebole et al. 2014; Hill et al. 2018).
Similarly, several strategies are used by phages to by-pass bacterial antiviral systems such as adaptation to new receptors; bacterial restriction enzymes inhibition (e.g., hydrolysis/degradation of cofactors of the R–M systems); anti-CRISPR (Acr) proteins that interfere with the host CRISPR–Cas system after multiple failed bacteriophage infections and the delivery of a sufficient dose of these proteins to the recipient bacterial cell (Samson et al. 2013; Hwang and Maxwell 2019; Laanto et al. 2020; Secor and Dandekar 2020).
Advantages and limitations of phage therapy
The renewed interest in phage therapy is related to many factors, among them, the capacity for self-programming and exponential multiplication of phages in the scenario of an infection; the potent lytic activity for its bacterial targets; the selective toxicity given by the inability to infect eukaryotic cells; the limited number of bacterial hosts, which allows them to act without affecting the microbiome widely; the ability to destroy biofilm and the possibility of synergistic use with antibiotics (Pelfrene et al. 2016; Malik et al. 2017; Aslam and Schooley 2019). Another benefit of phage therapy is versatility since, due to phage genetic diversity, abundance and ubiquity, there are practically unlimited sources of phages.
Phages can be used alone, as a cocktail to broaden the spectra of activity, or in combination with other antimicrobials to improve their efficacy (Pires et al. 2017; Melo et al., 2020). According to Romero-Calle and colleagues (2019) the use of phage cocktails, each one targeting different receptors and from diverse genetic clades will enhance the ability to mitigate the host protection mechanisms. Additionally, genetic engineering may also provide ways to broaden the diversity and targeting efficiency of phages for the avoidance of phage-resistance.
In the early days of studies about phage therapy, bacteriophages were isolated from stool samples from patients with diarrhea and used successfully in the treatment of bacterial dysentery (d'Herelle 1917; Weber-Dąbrowska et al. 2016; Almeida and Sundberg 2020). Nowadays, in humans, phages have been used successfully to treat a wide variety of infections, both local and systemic (Kutter et al. 2010; Weber-Dąbrowska et al. 2016; Dąbrowska 2019; Gordillo Altamirano and Barr 2019; Kortright et al. 2019).
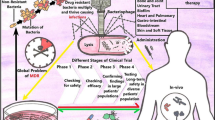
Many clinical studies have been carried out to evaluate the safety and efficacy of phage therapy. In Table 1, we provided an overview of recently concluded, or currently running clinical studies with bacteriophages registered at https://clinicaltrials.gov/.
The first phase I randomized clinical trial, carried out in the United States, was published by Rhoads et al. (2009). The safety of a cocktail of phage directed against Escherichia coli, S. aureus and P. aeruginosa was evaluated in 42 patients with chronic venous leg ulcers with no side effects attributed to the cocktail. Still in 2009, Wright and colleagues published another randomized, double-blind phase I/II clinical trial, in which they tested the action of a phage cocktail in the treatment of chronic otitis by antibiotic-resistant P. aeruginosa in 24 patients (Wright et al. 2009). The researchers evaluated inflammation signs, patient symptoms and quantified bacterial and viral loads. The cocktail improved all variables evaluated compared to placebo after 42 days of follow-up.
Case studies with bacteriophages involving patients with bacterial infections that could no longer be successfully treated by regular antibiotic therapy have already demonstrated the effectiveness of phage therapy in treating patients with eye infections (Fadlallah et al. 2015), diabetic foot ulcers (Fish et al. 2016), pancreatitis (Schooley et al. 2017), and urinary tract infection (Ujmajuridze et al. 2018). Fadlallah et al. (2015) reported a case study of a 65-year-old patient who suffered from a secondary eye infection with VRSA (vancomycin-resistant Staphylococcus aureus), treated with eye drops containing a well-characterized lytic phage, SATA-8505 (ATCC PTA-9476), against MRSA (meticillin-resistant Staphylococcus aureus). After 6 months of treatment, the patient had negative cultures for VRSA. This case study demonstrated that bacteriophage eye drops can be used as an alternative treatment for infectious keratitis caused by multidrug-resistant (MDR) pathogens. Fish et al. (2016) reported a case series involving six patients with diabetic foot ulcers who were treated with a commercial preparation of anti-staphylococcal phage (Sb-1). The reported average healing/use time was seven weeks, and amputations were avoided. Schooley et al. (2017) described the case of a 68-year-old diabetic patient with necrotizing pancreatitis complicated by an infection caused by MDR A. baumannii. The patient responded to therapy with a cocktail of nine lytic phages, administered intravenously and percutaneously, with evident clinical improvement. Ujmajuridze et al. (2018) used Pyophage® to treat nine patients suffering from urinary tract infections. The first phase of this study involved cycles of adaptation of this commercial preparation to increase its sensitivity in relation to uropathogen. In the second phase, six of the nine patients responded to treatment with bacteriophage showing a significant reduction in the concentration of pathogens in urine.
Phage therapy has also been used in combination with other antimicrobials for greater effectiveness in the treatment of infections (Markoishvili et al. 2002; Trelińska et al. 2010; Khawaldeh et al. 2011; Schooley et al. 2017; Aslam et al. 2018; Chan et al. 2018), as well as against bacterial biofilms (Jo et al. 2016; Chaudhry et al. 2017; Pires et al. 2017). As an example, Chan et al. (2018) reported the case of a 76-year-old patient who underwent aortic arch replacement surgery with a Dacron graft and was later diagnosed with infection by MDR P. aeruginosa. As the patient was at high risk for surgical graft replacement and treatment with intravenous ceftazidime and superficial debridement of the chest wall was unsatisfactory, a single dose of OMKO1 phage and ceftazidime was used simultaneously. The treatment was well tolerated and although the patient had other complications, the infection by P. aeruginosa receded without recurrence, despite the interruption of antibiotics. Finally, Pires et al. (2017) in a review discussed some of the most important studies about the effectiveness of phages against bacterial biofilm. The author concluded that phage therapy is an attractive option for preventing and controlling biofilm-related infections, despite the difficulty of effectively controlling a population with only one phage. Furthermore, the authors proposed the use of combined therapies, including phages cocktails and antimicrobials, to overcome biofilm barriers. However, the combined use of phages with antibiotics, it is important to note that is difficult to distinguish between the effect of phages and antibiotics alone, or a potential synergistic effect, because in most clinical cases, patients are receiving phages in association with antibiotic therapy.
Despite the advantages of phages over antibiotics, they also have some disadvantages, which result from the fact that they are relatively large and highly specific for bacterial strains. Among the disadvantages are: (1) the total size of the viral particle, which varies from about 20 to 200 nm, with some larger phages being quickly recognized by the reticuloendothelial system, resulting in loss of effectiveness; (2) the phage components are immunogenic and could trigger immune responses after entering the bloodstream; (3) high specificity can be a disadvantage, since only one set of strains is targeted by the phages; (4) phages are able to transfer (by transduction) genetic material between host bacteria, which drives the evolution of the bacterial population; (5) the possibility of transferring genes that encode virulence and/or resistance to antibiotics; (6) phage therapy combined with antibiotics increases the interactions between phages and bacteria, expanding gene exchange by transduction, leading to the adaptation of the microbiota and, possibly, of MDR bacteria (Górski et al. 2012; Modi et al. 2013; Kim et al. 2019; Melo et al. 2020).
Bacteriophages are naturally present within the human body, most of them as prophages in our bacterial microbiome. Under different conditions, prophages can be induced to the lytic cycle leading to disturbance of the balance of microbiota and, consequently, dysbiosis. It is important to consider the potential interaction between lytic phages, used for therapy, and the commensal phages (or prophages) in the microbiota, specifically the possibility of induction of prophages, directly or indirectly, as a result of lytic phage action (Divya Ganeshan and Hosseinidoust 2019).
Although phage-based clinical products are commercially available in some Eastern European countries (Stafal®, Sextaphage®, PhagoBioDerm™ and Pyophage® [Mulani et al. 2019]), in Western Europe and USA, no phage products for clinical applications have yet reached the commercial stage (Pelfrene et al. 2019). The phage therapy still not have the approval for human administration from the Food and Drug Administration (FDA) or the European Medicines Agency (EMA), but it is allowed for compassionate use in some countries (McCallin et al. 2019; Sybesma et al. 2018).
There is a lack of well-curated public phage libraries available for therapeutic use. Research labs in academia, industry, and army research centers have small to mid-sized collections that are mostly not shared with the broader scientific community (Divya Ganeshan and Hosseinidoust 2019). The Phage Directory (https://phage.directory) is an initiative that aims to organize such sharing, allowing the access, the use, and build upon the world’s phage knowledge (Gibson et al. 2019).
Finally, other issues have been pointed out as limitations to the application of the bacteriophage therapy in the Western World in the twenty-first century: quality and quantity of previously conducted study designs; bacteriophage-cocktail production, composition, and application methods in the context of the current legal framework; unfamiliarity among health professionals and the general public about the potential use of bacteriophage therapy, and limitations in intellectual property protection for bacteriophage therapeutic applications (Sybesma et al. 2018).
Final considerations
Considering the limited arsenal of antibiotics available, as well as an insufficient production of new drugs, and despite all its limitations, bacteriophages are a natural, safe, and effective strategy for the treatment of bacterial infections, with the additional advantage of preventing and controlling multi-resistant organisms. A combined approach, in which two or more therapies are used together with the aim of overcoming the individual limitations of each, has the potential to extend the useful life of the antimicrobials currently available. For this, standardized clinical trials using phage, monoclonal or polyclonal, in monotherapy or in combination therapy with antimicrobials, will allow to ascertain the real potential of these treatments in clinical practice, but must be carefully designed to be safe, inclusive and to generate data for comparison with previous studies.
Basis of the foregoing and the real threat to human health caused by MDR microorganisms, efforts in research and development of new treatments are being prioritized. Although some knowledge gaps need to be filled before the use of phage therapy is standardized, the field is advancing rapidly. Initiatives to share information and strains are already being developed in different research institutions around the world. Also, there is no single effective approach to the clinical use of phage therapy, and its diversity and versatility are among its greatest advantages. Finally, although the use of phage therapy seems challenging, its realization will bring social, commercial, economic and, above all, clinical benefits.
Therefore, we propose a consolidation of public bacteriophage libraries initiatives for phage therapy as the Phage Directory (https://phage.directory) available for compassionate treatment of antibiotic-resistant in protocols in humans. Enough cases can be used to gather adequate information and increase the availability of phages as a therapeutic alternative.
Finally, the consolidation of public phage libraries, with the sharing of strains, in addition to contribute to the implementation of randomized double blind placebo control studies, would allow the compassionate use of phages which, in the future, producing enough data to attest the safety and efficacy of phage therapy.
References
Abedon ST, Thomas-Abedon C, Thomas A, Mazure H (2011) Bacteriophage prehistory: is or is not Hankin, 1896, a phage reference? Bacteriophage 1(3):174–178. https://doi.org/10.4161/bact.1.3.16591
Ackermann HW (2007) Salmonella phages examined in the electron microscope. Methods Mol Biol 394:213–234. https://doi.org/10.1007/978-1-59745-512-1_11
Ackermann HW (2012) Bacteriophage electron microscopy, vol 82. Academic Press, Cambridge, MA, USA, pp 1–32
Almeida GMF, Sundberg LR (2020) The forgotten tale of Brazilian phage therapy. Lancet Inf Dis 20(5):e90–e101. https://doi.org/10.1016/s1473-3099(20)30060-8
Aslam S, Schooley RT (2019) What’s old is new again—bacteriophage therapy in the 21st century. Antimicrob Agents Chemother 64(1):e01987-10. https://doi.org/10.1128/AAC.01987-19
Aslam S, Yung G, Dan J, Reed S, LeFebvre M, Logan C et al (2018) Bacteriophage treatment in a lung transplant recipient. J Heart Lung Transplant 37(4):S155–S156. https://doi.org/10.1016/j.healun.2018.01.376
Azeredo J, Sutherland IW (2008) The use of phages for the removal of infectious biofilms. Curr Pharm Biotechnol 9(4):261–266. https://doi.org/10.2174/138920108785161604
Bárdy P, Pantůček R, Benešík M, Doškař J (2016) Genetically modified bacteriophages in applied microbiology. J Appl Microbiol 121(3):618–633. https://doi.org/10.1111/jam.13207
Batinovic S, Wassef F, Knowler SA, Rice DTF, Stanton CR, Rose J et al (2019) Bacteriophages in natural and artificial environments. Pathogens 8(3):100. https://doi.org/10.3390/pathogens8030100
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB et al (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48(1):1–12. https://doi.org/10.1086/595011
Cahill J, Young R (2019) Phage lysis: multiple genes for multiple barriers. Adv Virus Res 103:33–70. https://doi.org/10.1016/bs.aivir.2018.09.003
Casjens SR (2005) Comparative genomics and evolution of the tailed-bacteriophages. Curr Opin Microbiol 8(4):451–458. https://doi.org/10.1016/j.mib.2005.06.014
CDC (2019) Centers for Disease Control and Prevention. CDC’s antibiotic resistance threats in the United States. 2019 AR threats report. https://www.cdc.gov/drugresistance/Biggest-Threats.html. Accessed 11 October 2020
Chan BK, Turner PE, Kim S, Mojibian HR, Elefteriades JA, Narayan D (2018) Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health 2018(1):60–66. https://doi.org/10.1093/emph/eoy005
Chaudhry WN, Concepción-Acevedo J, Park T, Andleeb S, Bull JJ, Levin BR (2017) Synergy and order effects of antibiotics and phages in killing Pseudomonas aeruginosa biofilms. PLoS ONE 12(1):e0168615. https://doi.org/10.1371/journal.pone.0168615
Cisek AA, Dąbrowska I, Gregorczyk KP, Wyżewski Z (2017) Phage therapy in bacterial infections treatment: one hundred years after the discovery of bacteriophages. Curr Microbiol 74(2):277–283. https://doi.org/10.1007/s00284-016-1166-x
Dąbrowska K (2019) Phage therapy: what factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med Res Rev 39(5):2000–2025. https://doi.org/10.1002/med.21572
Divya Ganeshan S, Hosseinidoust Z (2019) Phage therapy with a focus on the human microbiota. Antibiotics 8(3):131. https://doi.org/10.3390/antibiotics8030131
d’Herelle F (1917) Sur un microbe invisible antagoniste des bacilles dysentériques. CR Acad Sci Paris 165:373–375
Domelier AS, van der Mee-Marquet N, Sizaret PY, Héry-Arnaud G, Lartigue MF, Mereghetti L, Quentin R (2009) Molecular characterization and lytic activities of Streptococcus agalactiae bacteriophages and determination of lysogenic-strain features. J Bacteriol 191(15):4776–4785. https://doi.org/10.1128/JB.00426-09
Drulis-Kawa Z, Majkowska-Skrobek G, Maciejewska B, Delattre AS, Lavigne R (2012) Learning from bacteriophages—advantages and limitations of phage and phage-encoded protein applications. Curr Protein Pept Sci 13(8):699–722. https://doi.org/10.2174/138920312804871193
Ebrahimizadeh W, Rajabibazl M (2014) Bacteriophage vehicles for phage display: biology, mechanism, and application. Curr Microbiol 69(2):109–120. https://doi.org/10.1007/s00284-014-0557-0
Fadlallah A, Chelala E, Legeais JM (2015) Corneal infection therapy with topical bacteriophage administration. Open Ophthalmol J 9:167–168. https://doi.org/10.2174/1874364101509010167
Fish R, Kutter E, Wheat G, Blasdel B, Kutateladze M, Kuhl S (2016) Bacteriophage treatment of intransigent diabetic toe ulcers: a case series. J Wound Care 25:27–33. https://doi.org/10.12968/jowc.2016.25.Sup7.S27
Garvey M (2020) Bacteriophages and the one health approach to combat multidrug resistance: is this the way? Antibiotics 9(7):414. https://doi.org/10.3390/antibiotics9070414
Gibson SB, Green SI, Liu CG, Salazar KC, Clark JR, Terwilliger AL et al (2019) Constructing and characterizing bacteriophage libraries for phage therapy of human infections. Front Microbiol 10:2537. https://doi.org/10.3389/fmicb.2019.02537
Gordillo Altamirano FL, Barr JJ (2019) Phage therapy in the postantibiotic era. Clin Microbiol Rev 32(2):e00066-18. https://doi.org/10.1128/CMR.00066-18
Górski A, Międzybrodzki R, Borysowski J, Dąbrowska K, Wierzbicki P, Ohams M et al (2012) Phage as a modulator of immune responses: practical implications for phage therapy. Adv Virus Res 83:41–47. https://doi.org/10.1016/B978-0-12-394438-2.00002-5
Hill C, Mills S, Ross RP (2018) Phages & antibiotic resistance: are the most abundant entities on earth ready for a comeback? Future Microbiol 13:711–726. https://doi.org/10.2217/fmb-2017-0261
Howard-Varona C, Hargreaves KR, Abedon ST, Sullivan MB (2017) Lysogeny in nature: mechanisms, impact and ecology of temperate phages. ISME J 11(7):1511–1520. https://doi.org/10.1038/ismej.2017.16
Hwang S, Maxwell KL (2019) Meet the anti-CRISPRs: widespread protein inhibitors of CRISPR-Cas systems. CRISPR J 2(1):23–30. https://doi.org/10.1089/crispr.2018.0052
Jamal M, Bukhari SMAUS, Andleeb S, Ali M, Raza S, Nawaz MA et al (2019) Bacteriophages: an overview of the control strategies against multiple bacterial infections in different fields. J Basic Microbiol 59(2):123–133. https://doi.org/10.1002/jobm.201800412
Jariah ROA, Hakim MS (2019) Interaction of phages, bacteria, and the human immune system: evolutionary changes in phage therapy. Rev Med Virol 29(5):e2055. https://doi.org/10.1002/rmv.2055
Jault P, Leclerc T, Jennes S, Pirnay JP, Que YA, Resch G et al (2019) Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect Dis. 19(1):35–45. https://doi.org/10.1016/S1473-3099(18)30482-1
Jo A, Ding T, Ahn J (2016) Synergistic antimicrobial activity of bacteriophages and antibiotics against Staphylococcus aureus. Food Sci Biotechnol 25(3):935–940. https://doi.org/10.1007/s10068-016-0153-0
Khawaldeh A, Morales S, Dillon B, Alavidze Z, Ginn AN, Thomas L et al (2011) Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection. J Med Microbiol 60(Pt 11):1697–1700. https://doi.org/10.1099/jmm.0.029744-0
Kim BO, Kim ES, Yoo YJ, Bae HW, Chung IY, Cho YH (2019) Phage-derived antibacterials: harnessing the simplicity, plasticity, and diversity of phages. Viruses 11(3):268. https://doi.org/10.3390/v11030268
Kortright KE, Chan BK, Koff JL, Turner PE et al (2019) Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25(2):219–232. https://doi.org/10.1016/j.chom.2019.01.014
Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S et al (2010) Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol 11(1):69–86. https://doi.org/10.4161/bact.1.2.15845
Laanto E, Mäkelä K, Hoikkala V, Ravantti JJ, Sundberg LR (2020) Adapting a phage to combat phage resistance. Antibiotics 9(6):291. https://doi.org/10.3390/antibiotics9060291
Lefkowitz EJ, Dempsey DM, Hendrickson RC, Orton RJ, Siddell SG, Smith DB (2018) Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res 46(D1):D708–D717. https://doi.org/10.1093/nar/gkx932
Leitner L, Sybesma W, Chanishvili N, Goderdzishvili M, Chkhotua A, Ujmajuridze A et al (2017) Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomized, placebo-controlled, double-blind clinical trial. BMC Urol. 17(1):90. https://doi.org/10.1186/s12894-017-0283-6
Leitner L, Ujmajuridze A, Chanishvili N, Goderdzishvili M, Chkonia I, Rigvava S et al (2020) Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomised, placebo-controlled, double-blind clinical trial. Lancet Infect Dis. 16:S1473. https://doi.org/10.1016/S1473-3099(20)30330-3
Lopes A, Tavares P, Petit MA, Guérois R, Zinn-Justin S (2014) Automated classification of tailed bacteriophages according to their neck organization. BMC Genomics 15(1):1027. https://doi.org/10.1186/1471-2164-15-1027
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3):268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x
Malik DJ, Sokolov IJ, Vinner GK, Mancuso F, Cinquerrui S, Vladisavljevic GT et al (2017) Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv Colloid Interface Sci 249:100–133. https://doi.org/10.1016/j.cis.2017.05.014
Markoishvili K, Tsitlanadze G, Katsarava R, Morris JG Jr, Sulakvelidze A (2002) A novel sustained-release matrix based on biodegradable poly(ester amide)s and impregnated with bacteriophages and an antibiotic shows promise in management of infected venous stasis ulcers and other poorly healing wounds. Int J Dermatol 41(7):453–458. https://doi.org/10.1046/j.1365-4362.2002.01451.x
McCallin S, Sacher JC, Zheng J, Chan BK (2019) Current state of compassionate phage therapy. Viruses 11(4):343. https://doi.org/10.3390/v11040343
Melo LD, Oliveira H, Pires DP, Dabrowska K, Azeredo J (2020) Phage therapy efficacy: a review of the last 10 years of preclinical studies. Crit Rev Microbiol 46(1):78–99. https://doi.org/10.1080/1040841X.2020.1729695
Międzybrodzki R, Borysowski J, Weber-Dąbrowska B, Fortuna W, Letkiewicz S, Szufnarowski K et al (2012) Clinical aspects of phage therapy. Adv Virus Res. 83:73–121. https://doi.org/10.1016/B978-0-12-394438-2.00003-7
Modi SR, Lee HH, Spina CS, Collins JJ (2013) Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature 499(7457):219–222. https://doi.org/10.1038/nature12212
Mulani MS, Kamble EE, Kumkar SN, Tawre MS, Pardesi KR (2019) Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol 10:539. https://doi.org/10.3389/fmicb.2019.00539
Pelfrene E, Willebrand E, Cavaleiro Sanches A, Sebris Z, Cavaleri M (2016) Bacteriophage therapy: a regulatory perspective. J Antimicrob Chemother 71(8):2071–2074. https://doi.org/10.1093/jac/dkw083
Pelfrene E, Sebris Z, Cavaleri M (2019) Comment on Fauconnier, A. Phage therapy regulation: from night to dawn. Viruses 11(9):771. https://doi.org/10.3390/v11090771
Pendleton JN, Gorman SP, Gilmore BF (2013) Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 11(3):297–308. https://doi.org/10.1586/eri.13.12
Phage Directory. Available online https://phage.directory/. Accessed 21 October 2020.
Pires DP, Melo L, Vilas Boas D, Sillankorva S, Azeredo J (2017) Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr Opin Microbiol 39:48–56. https://doi.org/10.1016/j.mib.2017.09.004
Reardon S (2014) WHO warns against ‘post-Antibiotic’ era. Nature 2014:15135. https://doi.org/10.1038/nature.2014.15135
Rello J, Parisella FR, Perez A (2019) Alternatives to antibiotics in an era of difficult-to-treat resistance: new insights. Expert Rev Clin Pharmacol 12(7):635–642. https://doi.org/10.1080/17512433.2019.1619454
Rhoads DD, Wolcott RD, Kuskowski MA, Wolcott BM, Ward LS, Sulakvelidze A (2009) Bacteriophage therapy of venous leg ulcers in humans results of a phase I safety trial. J Wound Care 18(6):237–243. https://doi.org/10.12968/jowc.2009.18.6.42801
Romero-Calle D, Guimarães Benevides R, Góes-Neto A, Billington C (2019) Bacteriophages as alternatives to antibiotics in clinical care. Antibiotics 8(3):138. https://doi.org/10.3390/antibiotics8030138
Samson JE, Magadán AH, Sabri M, Moineau S (2013) Revenge of the phages: defeating bacterial defences. Nat Rev Microbiol 11(10):675–687. https://doi.org/10.1038/nrmicro3096
Sanmukh SG, Felisbino SL (2017) Bacteriophages in cancer biology and therapies. Clin Oncol 2:1295. https://clinicaltrials.gov/
Sarker SA, Sultana S, Reuteler G, Moine D, Descombes P, Charton F et al (2016) Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine. 4:124–137. https://doi.org/10.1016/j.ebiom.2015.12.023
Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L et al (2017) Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 61(10):e00954-17. https://doi.org/10.1128/AAC.00954-17
Secor PR, Dandekar AA (2020) More than simple parasites: the sociobiology of bacteriophages and their bacterial hosts. mBio 11:e00041-20. https://doi.org/10.1128/mBio.00041-20
Sybesma W, Rohde C, Bardy P, Pirnay JP, Cooper I, Caplin J et al (2018) Silk route to the acceptance and re-implementation of bacteriophage therapy—part II. Antibiotics 7(2):35. https://doi.org/10.3390/antibiotics7020035
Tetz G, Brown SM, Hao Y, Tetz V (2018) Parkinson’s disease and bacteriophages as its overlooked contributors. Sci Rep 8:10812. https://doi.org/10.1038/s41598-018-29173-4
Torres-Barceló C (2018) The disparate effects of bacteriophages on antibiotic-resistant bacteria. Emerg Microbes Infect 7(1):168. https://doi.org/10.1038/s41426-018-0169-z
Trelińska J, Stolarska M, Zalewska-Szewczyk B, Kuzański W, Młynarski W (2010) The use of bacteriophage therapy in Pseudomonas aeruginosa infection in patients with acute leukemias - two cases report. Onkol Polska 1:50–53
Tsao Y, Taylor VL, Kala S, Bondy-Denomy J, Khan AN, Bona D et al (2018) Phage morons play an important role in Pseudomonas aeruginosa phenotypes. J Bacteriol 200:e00189-e218. https://doi.org/10.1128/JB.00189-18
Twort FW (1915) Investigation on the nature of the ultramicroscopic viruses. Lancet 186:1241–1243
Ujmajuridze A, Chanishvili N, Goderdzishvili M, Leitner L, Mehnert U, Chkhotua A et al (2018) Adapted bacteriophages for treating urinary tract infections. Front Microbiol 9:1832. https://doi.org/10.3389/fmicb.2018.01832
Viertel TM, Ritter K, Horz HP (2014) Viruses versus bacteria-novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J Antimicrob Chemother 9:2326–2336. https://doi.org/10.1093/jac/dku173
Weber-Dąbrowska B, Jończyk-Matysiak E, Żaczek M, Łobocka M, Łusiak-Szelachowska M, Górski A (2016) Bacteriophage procurement for therapeutic purposes. Front Microbiol 7:1177. https://doi.org/10.3389/fmicb.2016.01177
Wittebole X, De Roock S, Opal SM (2014) A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 5(1):226–235. https://doi.org/10.4161/viru.25991
World Health Organization (WHO) (2017) Global priority list of antibiotic-resistant bacteria to guide research, discovery and development of new antibiotics. https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/. Accessed 25 February 2020
Woźnica WM, Bigos J, Łobocka MB (2015) Liza komórek bakteryjnych w procesie uwalniania bakteriofagów – kanoniczne i nowo poznane mechanizmy [Lysis of bacterial cells in the process of bacteriophage release–canonical and newly discovered mechanisms]. Postepy Hig Med Dosw (Online) 23(69):114–126
Wright A, Hawkins CH, Anggård EE, Harper DR (2009) A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 34(4):349–357. https://doi.org/10.1111/j.1749-4486.2009.01973.x
Funding
CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for a student scholarship.
Author information
Authors and Affiliations
Contributions
Literature search and data analysis (Sabrina Royer, Aléxia Pinheiro Morais and Deivid William da Fonseca Batistão). Drafted and/or critically revised the work (Sabrina Royer and Deivid William da Fonseca Batistão).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Royer, S., Morais, A.P. & da Fonseca Batistão, D.W. Phage therapy as strategy to face post-antibiotic era: a guide to beginners and experts. Arch Microbiol 203, 1271–1279 (2021). https://doi.org/10.1007/s00203-020-02167-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-02167-5