Abstract
Sesame (Sesamum indicum L.) is an important but underexploited oilseed crop of tropical and subtropical region having potential to sustain agriculture under changing climatic conditions. Sesame oils have high nutritional and industrial values due to its desirable fatty acid compositions and high amount of antioxidant components, viz., sesamin and sesamolin. Despite this, still sesame is not grown on large acreage due to unavailability of high-yielding cultivars with inbuilt resistance to various biotic and abiotic stresses. Therefore, serious efforts are necessary to develop cultivars having high adaptive potential to the diverse climatic situations along with high yield potential. Classical plant breeding methods impart considerable improvement in sesame, but still a huge gap is left between realized and actual yield potential of sesame. Therefore, efforts should be made toward modern molecular techniques like marker-assisted plant breeding and omics and modern bioinformatics tools to develop climate adaptive, high yield potential along with excellent oil quality cultivars in sesame.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Many developing countries of the world are facing the problem of malnutrition as major part of population is vegetarian and the availability of good-quality nutritional food is limited. Oilseeds are next to cereals in importance which contain superior quality protein, essential fatty acids, vitamins, and minerals. In India, usually nine oilseeds, i.e., soybean, sesame, castor, niger, rapeseed-mustard, groundnut, safflower, sunflower, and linseed, are cultivated. Out of which, sesame (2n = 26) is of great importance and widely known as “Queen of Oilseeds” due to high resistance to rancidity and oxidation of sesame oil (Sarwar et al. 2013), and for the same reason, sesame seeds are also known as the “seeds of immortality ” (Bedigian and Harlan 1986). Tocopherol confers the resistance to oxidative deterioration in sesame oil, bioavailability of which is in turn enhanced by sesamol (Wu 2007).
The oil content is abundant in seeds (32.8–62.7%) (Uzun et al. 2008; Couch et al. 2017). Sesame oil contains significantly high amount of unsaturated essential fatty acids [linoleic acid (37–47%), oleic acid (35–43%)], while the saturated fatty acid content [stearic acid (5–10%), palmitic acid (8–11%), behenic acid, and arachidic acid] is usually low. The seeds also contain 4.3–20.5% carbohydrates, 4.2–6.9% ash, 2.7–6.7% fiber content, and 14.1–29.5% proteins as well as vitamin E, minerals, lignans (sesamolin and sesamin), and tocopherols (Fukuda et al. 1985; Kamal-Eldin et al. 1992; Ashri 1998; Unal and Yalcin 2008; Hassan 2012, Couch et al. 2017). In addition, sesame seeds are also rich in a variety of minerals including potassium (K), magnesium (Mg), phosphorous (P), calcium (Ca), and sodium (Na) (Nzikou et al. 2009; Couch et al. 2017). Sesame oil is often considered as a good protein source because of its balanced amino acid composition, particularly methionine and tryptophan, and is therefore beneficial to patients suffering from kwashiorkor (Pathak et al. 2014a, b). The various chemical compositions of sesame seed are given in Table 15.1.
Sesame is being cultivated from ancient times and its oil was used for spiritual purposes (Weiss 1983). It belongs to division Phanerogams, class Dicotyledonae, subclass Gamopetalae, series Bicarpellatae, order Persoriales, family Pedaliaceae, and genus Sesamum. It is also named as gingelly, beniseed, sim-sim, and til (Shah 2013). It is grown throughout the tropical and subtropical region from 25°N to 25°S. The origin of sesame can be traced back to Africa as several wild relatives of sesame exist in Africa (Sani et al. 2014). India as well as China, Central Asia, Near East, and Abysinia has been recognized as sesame diversity centers in classical studies which is not at all surprising considering the genotypic variability of sesame in India (Zeven and Zhukovsky 1975; Hawkes 1983; Laurentin and Karlovsky 2006).
Sesame is mostly self-pollinated short-day plant, with the probability of 5–68% of cross-pollination (Langham 1944; Ashiri 2007). Generally, its flower opens at morning and withers after 4–6 h of anthesis. While stigma receptivity lasts for 14–24 h after flower opening (Abdel et al. 1976; Yermanos 1980) and varies with genotype (Langham 2007). Anther dehisce just after opening of flower and pollen grains remains viable for 24 h after dehiscence at 24–27 °C (Yermanos 1980). Plants are erect to semi-erect decided by branching types lanceolate to ovate, having pointed leaves with entire or serrate margins and round stem. Flowers are axillary, solitary, short pedicellate and zygomorphic with five fused sepals. Corolla is pendulous, campanulate, and tubular, and one petal is longer than others. Each flower has five (one sterile and four fertile) didynamous and epipetalous stamens with dorsifixed filaments. The gynoecium is multicarpellary with bifid stigma, long style, and a superior ovary. Seeds are very small (4 × 2 mm with 1-mm-thick hilum), pearl shaped, ovate, small, and slightly flattened. Indeterminate growth habit is observed in most of the varieties showing continuous growth of new leaves, regular flowering, and formation of capsules, and as long as the environmental conditions are favorable though, distinct varieties and strains differ considerably in size, growth, form, flower color, seed size, and seed color.
Sesame (Sesamum indicum L.) is a valuable Kharif oilseed crop, mostly grown in light sandy soil as rainfed crop in the arid and semiarid tropics of southern parts of Haryana, Rajasthan, Madhya Pradesh, Gujarat, and Uttar Pradesh. The growth period could be 70–150 days based on the variety and the environmental conditions (Ashri 1998). Sesame seed requires temperature around 20 °C for germination, and more than 23 °C favors good growth and high yields. The crop rarely requires redundant irrigation factually due to its high susceptibility toward the moisture stress. Sesame production is progressively endorsed because of relatively simple cultivation as it can be grown on various kinds of soil, tolerance to high temperatures, less labour-intensives and flexibility to fits in crop rotation schemes (Langham 2007; Dossa et al. 2017a, b). The overall sesame production is below the expectation worldwide mostly due to absence of effective pest control methods, occurrence of biotic and abiotic stress, and notably lack of a pertinent breeding program (Duhoon 2004; Ram et al. 2006). The statistics have shown a decline in production of sesame despite the fact that it is a highly self-sufficient crop (Anthony et al. 2015; FAOSTAT 2015). Declining production and low yield are also of great concern because of its economic importance and high medicinal value. The commercial varieties of sesame are susceptible to biotic and abiotic stress factors including photosensitivity and experience early senescence (Rao et al. 2002). Various studies have elucidated the presence of biotic and abiotic stress-resistant genes in wild species of sesame (Joshi 1961; Weiss 1971; Brar and Ahuja 1979; Kolte 1985).
Late maturing cultivars are proclaimed to have high oil contents than the early maturing counterparts. Amount of oil content also varies according to the location of capsules on the same plant such as the seeds on the main stem from the basal capsules carry more oil than those placed toward the apex and on side branches (Mosjidis and Yermanos 1985). Similarly, brown and white seeded cultivars often have high oil content than black ones, indicating a probable genetic linkage between seed coat color and oil content. Black seed coats are usually thicker than brown- and white-colored coats.
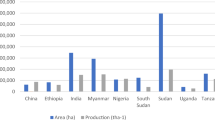
Global sesame production and area coverage were 5,531,948 tons and 9,983,165 hectare, respectively, in 2017 (FAOSTAT 2019). Prime sesame producing countries are Tanzania, India, Nigeria, China, and Ethiopia (FAOSTAT 2019). Worldwide sesame seed consumption in 2018 was reported to be USD 6559.0 million and is approximated to extend up to USD 7244.9 million by 2024, at 1.7% CAGR (compound annual growth rate). India and China are among the top producers of sesame globally, and average yield is highest in China (1223 kg/ha) trailed by Nigeria (729 kg/ha) and Tanzania (720 kg/ha) (FAOSTAT 2020). India was the largest exporter of sesame seeds with annual production recorded 850,000 tons, and total area under cultivation was 1951,000 hectares in 2015–2016. West Bengal was top producer trailed by Madhya Pradesh, Rajasthan, and Uttar Pradesh (Anonymous 2016).
Sesame seeds are used to produce high-quality edible oil also used in fish canning and the production of butter substitutes like margarine (Uzo 1998). Varieties of steroids present in sesame oil increase the insecticide potency of pyrethroids which are used as commercial and household insecticide. Seeds as well as the leaves of sesame are used to prepare food delicacies such as variety of porridges and are also consumed with fried groundnut. Consumption of sesame in the form of porridge is well known from the tribal populations which are eaten by the Fulani tribe mixed with the millet and by Tiv people with the boiled yam (Uzo 1998). Dried sesame leaves after pulverization are used in soups as it is a rich source of minerals and proteins. The seeds are often consumed as a candy and as snacks mixed with sweetener and groundnuts or with partially cooked cowpea. The seeds are used in bread and confectionery industry as well. Sesame flour is more compatible with wheat flour than other oilseeds for producing bread with good loaf volume and crumb texture. The brown and black varieties are fermented to produce local brew beer (Burukutu). Sesame oil is used as a substitute of other solvents in paints, liniments, ointments, and cosmetics (Mbaebie et al. 2010).
Commercially sesame semidrying oil is used as an alternate of olive oil, corn oil, and cotton seed oil for restraining bacterial infection on umbilical cord of infants. Studies have also revealed the anticancer and neuroprotective properties of sesame oil to cure hypoxia or brain damage (Hibasami et al. 2000; Miyahara et al. 2001; Cheng et al. 2006). Antioxidants have been proclaimed to possess health promoting effects like reducing hypertension and cholesterol levels in humans (Noguchi et al. 2001; Sankar et al. 2005). The cake left after oil extraction is rich in phosphorus and calcium and thus used as animal feed. Other uses of sesame seed and oil for medical purposes include the treatment of sores, ulcers, diarrhea, and dysentery. Historically, it is used for customary marriage rites of some tribes in Nigeria. The dried stems of plants are tied together to be made broom, while the ashes of dried shrub are used for the production of black soap. The plant residue is also plowed into the soil to enrich it. The dehulled press cake of sesame is used for the treatment of malnutrition in children because of the presence of globulin as a principal protein in it (Abdullahi 1998).
15.2 Classical Breeding in Sesame
Sesame is often described as the oldest oilseed crop cultivated in ancient times both for its edible seed and mainly for its oil. Sesame oil is esteemed as a best vegetable oil because of its high nutritional quality and stability to oxidative rancidity (Biswas et al. 2018). Despite this, sesame is mainly grown under marginal and submarginal land and also known as poor’s farmer crop. Sesame is an underexploited oilseed crop, yet still it holds tremendous potential for enhanced food value (Manjeet et al. 2020). Sesame is one such crop that justifies imperative and instant consideration of the scientific communal. The sesame plant type is not well adapted to current farming schemes since of its unstipulated growth habit causing varying ripening of capsules, their varying susceptibility to different stresses, and unavailability of non-shattering varieties suited for mechanical harvest (Ashri 1998). The important breeding objectives of sesame are high yield potential along with high oil content; yield stability through tolerance against different abiotic stresses, viz., drought, salinity, heavy metal stress, water logging, and temperature extremities; resistance to biotic stresses like phyllody and charcoal rot, Alternaria blight, leaf curl virus, and several insect pests; and good confectionary quality to meet industrial demands (Ashri 1998; Islam et al. 2016; Sinha et al. 2020). India is a center of origin and diversity for sesame, and several of important germplasm contain economically important traits that are largely underexplored for use in sesame improvement programs (Bisht et al. 1998; Bedigian 2003). However, a systematic screening of these germplasm or their characterizations is still totally lacking. Hence, germplasm evaluation and pre-breeding are still a key approach for sesame improvement. The important breeding objectives in sesame will be discussed in the following heads.
15.2.1 High Seed Yield
In spite of being a great source of very healthy edible oil in terms of presence of huge amounts of polyunsaturated fatty acids and several antioxidants, sesame is cultivated on very small acreage due to availability of poor yielding dehiscent varieties with low harvest index. Lack of improved varieties is the major reasons behind low seed yield in sesame (Pathak et al. 2014a, b). So, productivity enhancement by accumulating desirable alleles into single genetic background is the prime objective for sesame breeders. Sesame seed yield is depending upon its several component traits like number of primary and secondary branches per plant, number of capsules per plant and capsule length, seed weight, and number of seeds per capsule (Teklu et al. 2014; Mustafa et al. 2015; Ramazani 2016; Shakeri et al. 2016;). As yield is a complex trait with low heritability, therefore, indirect selection for yield through its abovementioned attributing traits may enhance productivity in sesame. Another very important yield attributing trait in sesame is harvest index, which directly related with high yield in sesame (Day et al. 2002). However, to improve harvest index in sesame, plant type should be of medium plant height with high density capsule bearing starting from 15 to 20 cm above the ground (Tripathy et al. 2019).
15.2.2 Early Maturity and Short Plant Stature
Early maturity and short plant stature are the two important agronomic traits in sesame which makes it fit for cultivation for farmers (Uzun and Çagırgan 2006). Early maturity not only helps in reducing crop cultivation cost but also provides enough times for succeeding crop. Short plant stature is very helpful to breed lodging-tolerant sesame and an important component toward mechanical harvesting in sesame (Ashri 1998).
15.2.3 High Oil Content
High oil content is another important breeding objective as oil is chief produce for oilseed crops. Among different oilseed crops, sesame has high oil content (∼55%) which makes it suitable as a key oilseed crop (Yadava et al. 2012). Sesame oil content and its quality are varying with the genotype, color (black to white), and size of the seed. Oil content in sesame gene pool varies from 35% to 63% indicating the presence of sufficient genetic variation for oil content in sesame which is a prerequisite for breeding cultivar with high oil content. Also, genotypes with white seed coat color possess higher oil content than the dark seed coat-colored genotypes. Oil content in sesame is oligogenic to polygenic; therefore, breeders should pay attention on recurrent selection schemes to develop high oil content genotypes (Velasco and Fernández-Martínez 2002; Islam et al. 2016).
15.2.4 Fatty Acid Compositions of Oil
The worth and usefulness of an oilseed crop for both dietary and manufacturing purposes mainly depend upon the fatty acid composition of its oil (Dyer et al. 2008). Varietal development with desirable fatty acid compositions could augment the usefulness of the oil for definite comestible purposes. Sesame geneticists and breeders prefer to select those lines which exhibit high oil content with high polyunsaturated fatty acids like oleic, linoleic, and linoleic acid. Beside this, the presence of several antioxidant compounds like minerals, vitamins, phytosterols, tocopherols, and unique class of lignans such as sesamin and sesamolin adds further nutritional value in sesame. These components mainly help in scavenging of reactive oxygen species and are very helpful for recoverable patients. Hence, breeding for these quality components traits also becomes important for sesame breeders. The high PUFA compositions along with high antioxidant components like tocopherol, sesamin, and sesamolin are desirable for high-quality export value in sesame (Hwang et al. 2005; Gupta 2015). For confectionary purposes, cultivars should have white seed color, bold size, and appealing shape. Beside this, germplasm should be screened for required texture and seed coat thickness and oil flavor using specific descriptors (Tripathy et al. 2019).
15.2.5 Shattering Resistance
Capsule shattering often leads to heavy yield losses in sesame. Most of the sesame varieties are of shattering type, and almost all of the fields are harvested by hand which leads to approximately 60% yields loss (Langham 2007). There is a prerequisite to reorient breeding approach to reduce the high cost of manual harvesting and yield loss due to shattering. Development of new high-yielding cultivars with semi-indehiscent capsules is a possible option to fit mechanized farming.
15.2.6 Abiotic Stress Tolerance
Sesame is usually grown under marginal to submarginal land and faces several types of environmental extremities. However, only limited efforts have been made to develop genotype with high yield potential and improved tolerance to abiotic stresses. Sesame withstands water scarcity to some extent because of its extensive root system but may experience huge yield losses under different environmental stresses such as flooding, salinity, heavy metals stresses, and temperature extremities. This crop is considered moderately salt tolerant and can give gainful output on saline soils. Salinity affects water potential and causes ion imbalance and toxicity in living cells; this altered water status leads to initial growth reduction and reduction in productivity and may lead to death of plant. Stress affects all the major metabolic processes such as germination, seedling growth and survival, accumulation of photosynthetic pigments, photosynthesis, and respiration processes which leads to water scarcity, nutrient imbalance, and oxidative stress in salt affected plant. Abbasdokht et al. (2012) revealed that germination percentage, shoot length, shoot dry weight, root length, and germination rate decreased as the salinity concentration increased in sesame. There was no significant difference between the cultivars up to 0.16 ds.m−1 salinity levels; however, there were significant differences between the cultivars beyond 0.16 ds.m−1, and they also conclude that selection within cultivars for salt tolerance could be possible at germination stage. Bahrami and Razmjoo (2012) concluded that germination and seedling growth were strongly inhibited by 12.05 dSm−1 among the ten cultivars they studied.
Sesame is usually cultivated under rainfed conditions where precipitation is irregular. It is regularly subjected to mild to severe water deficit stress. Vegetative stage is most sensitive to drought stress (Boureima et al. 2011). Drought stress is the main constraint in production potential of the crop in the semiarid regions (Boureima et al. 2012). Drought affects the plant metabolism, growth development, and yield. Different cultivars respond differently to drought stress with some cultivars being highly resistant and others more susceptible (Boureima et al. 2011). Due to extensive rooting system, sesame can overcome drought although it experiences substantial yield losses if drought occurs when it is cultivated on marginal and rainfed areas. Sesame seed yield is more affected by drought than any other morphological characters. Kim et al. (2007) investigated the drought effect on yield, and its component traits in sesame found that water stress significantly decreased sesame yield by decreasing the number of seeds per capsule. Dossa et al. (2019) identified 543 sesame core abiotic stress-responsive genes using meta-analysis of 72 RNA-Seq datasets from drought, water logging, salt, and osmotic stresses using contrasting sesame genotypes. You et al. (2018) performed transcriptomic analysis to study the expression profiling of stress-responsive genes in different tissue and development stage under various abiotic stresses. They found that the genes, namely, SiGolS and SiRS, were significantly regulated by drought, salt, osmotic, and water logging stresses but slightly affected by cold stress. Wang et al. (2018) studied the transcriptomic profiling of SibZIPs gene. Their results indicated that this gene exhibited considerable changes against abiotic stresses, including salt, drought, water logging, osmotic, and cold. Li et al. (2017) studied SiWRKY gene expression patterns and revealed that 33 and 26 SiWRKYs gene expression was strongly responded to water logging and drought stress, respectively. Drought tolerance in sesame is associated with wax depositions, root length, transpiration rate (Sun et al. 2010), and higher activities of few antioxidant enzymes like superoxide dismutase, catalase, polyphenol oxidase, and peroxidase (Fazeli et al. 2007) as well as antioxidant metabolites content, viz., carotenoids (Kadkhodaie et al. 2014), and higher levels of ABA, proline, arginine, lysine, aromatic and branched chain amino acids, GABA, saccharopine, 2-aminoadipate, and allantoin under drought stress (You et al. 2019). Water logging is another important stress, and sesame crop is highly susceptible to flooding, as the crop undergoes immediate senescence and declines within 2–3 days of exposure to waterlogging stress (Anee et al. 2019). High rainfall during monsoon often leads to yield losses in sesame, and there is a need to develop improved cultivars that could survive the waterlogging stress. Anee et al. (2019) observed that lipid peroxidation as well as hydrogen peroxide (H2O2) and methylglyoxal contents increased while leaf relative water content, proline content, and chlorophyll and carotenoid contents decreased under prolonged water logging stress in sesame. Beside this, glutathione and oxidized glutathione contents increased under waterlogging, while the GSH/GSSG ratio and ascorbate content decreased. Ascorbate peroxidase, monodehydroascorbate reductase, glutathione peroxidase, and glyoxalase I activity increased under water logging, while dehydroascorbate reductase, glutathione reductase, and catalase activity showed decreasing trend. Wang et al. (2012) found strong association between cell wall modification and growth pathways, glycolysis, fermentation, mitochondrial electron transport, and nitrogen metabolism with waterlogging tolerance in sesame. These traits should be under consideration when breeding for flooding tolerance in sesame. Salinity often limits sesame cultivation especially in arid and semiarid regions. Sesame cultivars show a considerable variation in the degree of salt tolerance (Bekele et al. 2017). Zhang et al. (2019) compared salt tolerant and sensitive genotype of sesame and revealed that tolerant genotype has higher seed germination percentage, more plant survival rate, as well as better growth rate than susceptible one. Their transcriptome study revealed strongly induced salt-responsive genes in sesame mainly related to amino acid metabolism, carbohydrate metabolism, biosynthesis of secondary metabolites, plant hormone signal transduction, and oxidation-reduction process, while metabolomics investigation revealed amino acid metabolism and sucrose and raffinose family oligosaccharide metabolism impart salt tolerance in sesame. Several antioxidant enzymes, viz., superoxide dismutase, catalase, peroxidase, and ascorbate peroxidase as well as malondialdehyde and proline content, have been found closely related to salt tolerance in sesame (Koca et al. 2007). In addition to this, seed germination percentage, root and shoot length, root to shoot length ratio, and seedling fresh weight are also associated with salinity tolerance particularly at seedling stage (El Harfi et al. 2016). Heavy metal stresses also adversely affect sesame yield considerably. Heavy metal stress tolerance in sesame was associated with accumulation of more dry mass during early growth phase and nitrate reductase activity. Considerable genetic diversity exists in collected sesame germplasm which could exploit to breed cultivar tolerance to abiotic stresses. In addition to this, sesame crop wild relatives have been reported to have agronomically desirable alleles for stress tolerance including both biotic and abiotic stresses.
15.2.7 Biotic Stress Tolerance
Biotic stresses such as pathogens, insects, and weeds adversely affect sesame crop which cause unpredicted losses in productivity and production due to lack of proper management practices including unavailability of resistant varieties (Girmay 2018). Hence, development of resistant cultivars helps in sustainability and in improving yield stability in sesame. The important diseases, their causal organism, and estimated yield losses are presented in Table 15.2. Plants face a combination of different biotic stresses, and to mitigate the effects, they evolved complex signaling pathways. In general, disease resistance in crop plants is two types: whether a hypersensitive or incompatible type is governed by few genes and which is often known as oligogenic or vertical resistance. Second one is partial resistance which is governed by many genes and popularly known as quantitative or horizontal resistance (Singh et al. 2020; Beebe and Corrales 1991; Vale et al. 2001). In sesame, genetics of disease resistance varied as it is was monogenic to oligogenic against phyllody and Alternaria blight, while it was polygenic against charcoal rot (El-Bramawy and Shaban 2007; Eswarappa et al. 2011; Shindhe et al. 2011; Lokesha et al. 2013; Renuka and Lokesha 2013). However, breeding for single disease resistance is often not much effective; hence there is a need to develop cultivars with multiple resistances to the above biotic stresses including both disease and insect pest. The symptoms of various diseases, viz., phyllody, charcoal rot, and leaf curl virus disease, are shown in Figs. 15.1, 15.2, and 15.3.
Symptoms of sesame phyllody in the field. (a) The entire sesame inflorescences are replaced by short twisted leaves closely arranged on top of the stem with very short internodes, but leaves on the lower part of infected plant did not exhibit any visible symptoms. (b) Floral virescence and dark exudates appear on foliage floral parts. (c) Floral virescence
Symptoms of sesame leaf curl virus disease. (a) Severely infected sesame plant showing leaf curling and plant stunting. (b) Close look of sesame twin with severe curling along with thickening of leaves. (c) Underside of an infected sesame leaf showing vein swelling and upward curling along with leaf thickening
15.3 Sesame Classical Breeding Methods
Sesame is known as a predominantly self-pollinated crop. Hence, the selection methods employed for breeding self-pollinated plants are equally effective in sesame. Existence of genetic variation is a precondition for hybridization and accumulation of desirable alleles into single genetic background through selection. Domestication, plant introduction, mass, and pure line selection are the important breeding method applicable for existing genetic variability. Meanwhile, creation of genetic variation through crossing contrasting genotypes followed by pedigree, bulk, and single seed descent selection method is another important breeding scheme for sesame breeders. Recurrent selection and diallel mating selective schemes are the two most effective although time-consuming breeding methods in sesame. However, transferring one or two genes of agronomically important traits from known source to high-yielding, well-adapted genotype is possible through backcrossbreeding schemes. Mutation breeding is another key approach for creating new desirable allele which is totally lacking in germplasm. Mutation breeding is generally employed for quality traits, while backcross method is often used for transferring disease resistance alleles and for transferring genes responsible for male sterility and fertility restoration. Baydar (2005) applied pedigree selection method to improve the ideal type of sesame and revealed that the bicarpels, mono- to tricapsule, with a greater number of branches were considered as ideal plant types in breeding for high-yielding varieties. Ismail et al. (2013) performed pedigree selection in sesame for seed yield per plant and compared yield after two selection cycle. The average yield after two selection cycle of selected families surpassed as compared to their parents. The plant geneticists and breeders are always interested in identifying gene/allele source responsible for desirable agronomic traits and determining the genetic basis of agronomic traits in order to design proper breeding approaches for development of new cultivars. Information about inheritance pattern of any particular trait is indispensable in deciding most appropriate breeding method for the development of elite genotype/variety. The information about nature of gene action for different agronomic traits in sesame is given in Table 15.3, while the gene/allele source for different agronomic traits is given in Table 15.4.
We at CCS Haryana Agricultural University, Hisar, India, have been maintaining about 550 germplasm lines, and these were evaluated for various agronomic traits during Kharif, 2014. The range of variation for different agronomic traits revealed substantial and potential variability in different genotypes. Among these germplasm lines, four lines, viz., TC 180, TC 26, TC 104, and TC 174, were promising for seed yield per plant, while the genotypes, viz., TC 302, TC 354, TC 301, TC 183, F 45, and TC 353, were promising for early flowering and maturity. For the development of short stature cultivars, the genotypes, viz., TC 302, TC 24, TC 325, TC 327, TC 167, TC 342, TC 27, and TC 45, were having desirable short plant height. These genotypes might be useful for development of high-yielding variety in sesame. Two high-yielding sesame varieties, viz., HT 1 and HT 2 (HT 9713), were developed at CCS HAU for farmer’s cultivation. (Fig. 15.4). The variety HT 1 possesses medium to long height and dark green-colored leaves, resistant to phyllody and leaf curl virus with an average yield of 2.4 q/acre. The variety HT 2 is a short stature, early maturing, high-yielding variety developed from pedigree selection of population developed from the cross HT 1 × HT 15, and it possessed both high yield and disease resistance as it showed resistant reaction to all the major diseases (phyllody and leaf curl virus) as well as to leaf roller/capsule borer.
Sesamum varieties developed by CCS, HAU, Hisar. A. Variety HT I possessing resistance to phyllody and leaf curl virus with an average seed yield of 2.4 q/acre. B. HT 2, a dwarf, early maturing and high-yielding variety. C. White-colored bold seeds of variety HT1. D. White-colored bold seeds of variety HT2
15.3.1 Heterosis Breeding in Sesame
In spite of many efforts through classical breeding methods , viz., mass, pedigree, backcross and recurrent selection, still there have not been accomplished a foremost yield revolution in sesame productivity. Therefore, heterosis breeding could have potential to break yield plateau in sesame by exploiting the advantage of heterosis or hybrid vigor phenomenon (Mothilal and Ganesan 2005; Monpara and Pawar 2016). Heterosis is the term used for superiority of F1 hybrids as compared to their parents. Heterosis was already exploited in many important oilseed’s crops including rapeseed-mustard, groundnut, and soybean to some extent. Recently, several workers reported the existence of noteworthy heterosis in certain cross combinations of sesame (Murty 1975; Sasikumar and Sardana 1990; Jiarong 1991; Quoada and Layrisse 1995; Uzun et al. 2004; Sumathi and Muralidharan 2008; Banerjee and Kole 2010; Jadhav and Mohrir 2013; Parimala et al. 2013; Saravanan and Nadarajan 2002; Lal Jatothu et al. 2013; Vavdiya et al. 2013; Hassan and Sedeck 2015;). Hence, there is urgent need to exploit this heterosis to capitalize on seed yield and oil content in sesame. Effective male sterility-fertility restoration system might provide opportunity to develop efficient system for commercial hybrid seed production in sesame. Several workers reported genetic male sterility in sesame like corolla recessive genic male sterile mutant (Langham 1947), greenish anther color at pollen dehiscence-associated male sterility (Osman and Yermanos 1982), short anther’s filaments and cold night temperature-based recessive genetic male sterility (Brar 1982), and mutagen-induced monogenic recessive genetic male sterility (Rangaswamy and Rathinam 1982). Cytoplasmic male sterility was also identified in sesame wild relative Sesamum malabaricum (Prabakaran et al. 1995; Bhuyan and Sarma, 2003; Prabakaran 1998). This CMS system was used to develop 36 cross combinations with high heterotic effects (77–540%) for many seed and oil yield (Tripathy et al. 2019). Several experimental F1 cross combinations have been developed in India which exhibited heterotic effect of 31.0–44.3% in seed yield and 13–48% in oil yield over commercial pure line variety TKG 22 (Gangaiah 2008).
We at CCS HAU evaluates several lines in different cross-combinations for exploiting heterosis in sesame through cytoplasmic genetic male sterility system (CMS) and also to develop resistant lines against insect-pests and diseases. Also, the possibility for exploitation of inter-specific hybridization was explored at CCS HAU by exploiting Sesamum malabaricum. The genotypes, viz., IC 043144–1 and JJK/MIS10–67 of S. malabaricum, were crossed with HT 1, HT 2, HT 9316, HT 9907, HT 9913, TKG 22, MT 11–8-2, LT 210, HTC 1, and KMR 60. The 49 newly developed F1 hybrids were evaluated for seed yield and its component traits. Out of these cross combinations, eight showed higher seed yield over local check HT 2. Highest seed yield per plant was observed in hybrid HT 20 × HT 2 (7.3 g), followed by HT 45 × RT 125 (6.7 g), CST 2001–9 × HT 2000 (6.5 g), OC 201 × HT 9316 (6.0 g), OC 251 × HT 2000 (6.0 g), T 78 × HT 2 (4.5 g), KMR 41 × RT 125 (4.4 g), RH 54 × HT 9316 (4.0 g), and local check HT 2 (3.6 g) during Kharif, 2015. This indicates the potential of F1 hybrids for breaking yield plateau in sesame. For charcoal rot resistance breeding at CCS HAU, 24 germplasm lines, viz., NIC 7837, NIC 7875, HT 1, NIC 17274, HT 2, NIC 17849, HT 15, SI 2174–1, SI 3296, IS 92–2, and HT 9913, were found moderately resistant.
15.4 Molecular Breeding in Sesame
Crop breeding together with improved agronomic practices resulted in the Green Revolution in the 1960s with spectacular yield gains, particularly for staple crops like wheat and paddy in developing countries (Lenaerts et al. 2019). However, crop yield augmentation has been slowing down more in recent times. Changes in climatic patterns, arable land, and water accessibility now endow with further challenges for ensuring yield steadiness across varied environment. Changing climatic conditions affect farming and foodstuff formation in multifaceted ways. It influences food production straightforwardly by altering in agroclimatic environment and on another way by distressing development and allocation of income and thus demands for agricultural outcomes (Shetty et al. 2013). Advanced biological techniques in plant breeding like genomics, proteomics, bioinformatics tools, molecular breeding, and plant tissue culture and genetic engineering have already led to significant impacts on several important crops including rice, wheat, rapeseed-mustard, soybean, maize, potato, sorghum, and pearl millet (Varshney et al. 2005). Sesame is an underexploited crop of tropical and subtropical region of the world. As sesame is the crop of developing countries, major efforts for sesame improvement were made only through classical plant breeding methods (Gupta 2015). However, under changing climatic conditions and evergreen increasing human populations, efforts should be directed toward the use of recent biotechnological techniques to boost up the sesame production and productivity. The molecular breeding work in sesame began very late with only one genetic map published and no information on QTL mapping before 2013 (Dossa et al. 2017a, b). However, over the last decade, some noteworthy advancement has been made in sesame breeding programs to use advanced molecular biology techniques including plant tissue culture techniques; highly informative molecular marker techniques like SNPs; high density linkage and genetic maps; omics studies including genomics, proteomics, transcriptomics, and metabolomics; and advanced bioinformatics tools (Tripathy et al. 2019). In addition, the draft of sesame genome triggered functional analyses of candidate genes related to important agronomic traits (Wei et al. 2017a, b; Zhang et al. 2013a, b). With these invaluable efforts, sesame has some important genomic resources and platforms for improvement, and presently, sesame has shifted from an “orphan crop” to a “resource-rich crop.” Among different advanced techniques, molecular marker technologies have considerably accelerated the classical sesame breeding programs in enhancing the genetic gain and minimizing the breeding cycles in many crop species (Dossa et al. 2017a, b). Molecular marker technologies in sesame are witnessing significant progress, and it is clear that sesame is no longer far behind large crops in this field. Various kinds of molecular markers have been developed and used to sesame genotyping including randomly amplified polymorphic DNA (RAPD), amplified fragment length polymorphism (AFLP), microsatellites or simple sequence repeat (SSR), and inter-simple sequence repeats (ISSR) employed mainly for genetic diversity analysis at DNA level. The next class of markers concerned mostly of expressed sequence tags-SSR (EST-SSR), cDNA-SSR, genome sequence-SSR (gSSR), and chloroplast SSR (cpSSR) which were mainly employed for association mapping, germplasm characterizations, and molecular breeding in sesame (Dar et al. 2017; Dixit et al. 2005; Wei et al. 2014a, b; Kizil et al. 2020; Cui et al. 2017; Li-Bin et al. 2008;Wei et al. 2011; Zhang et al. 2013a, b; Kumar and Sharma 2011). Recently, with the discovery of next-generation sequencing technology (NGS) , another class of molecular markers emerged. SNPs are more useful as genetic markers than many other simple markers because they are the most abundant and stable form of hereditary difference in most genomes (Uncu et al. 2016; Wei et al. 2014a, b). Therefore, high-throughput methods available for SNP detection and genotyping have been used in sesame research including restriction site-associated DNA sequencing (RAD-seq), specific length amplified fragment sequencing (SLAF-seq), RNA-Seq, whole-genome sequencing (WGS), genotyping by sequencing (GBS), and insertion/deletions (Indels) (Uncu et al. 2016). Using these marker techniques, several important genes and QTLs were mapped and validated in sesame till now (Table 15.5). Different types of molecular markers have been developed and used successfully for genetics and breeding activities in Sesamum indicum. The following sections provide brief information related to different types of molecular markers used in sesame based on their detection method.
15.4.1 RFLP (Restriction Fragment Length Polymorphism)
RFLP was the first molecular marker and the barely marker system based on hybridization. Polymorphism occurs among individuals of same species as a result of insertion/deletion (InDels), translocation, duplications, inversions, and point mutations. RFLP begins with the isolation of pure genomic DNA, after which isolated DNA is treated with restriction enzymes resulting in a large number of fragments varying in length. Agarose or polyacrylamide gel electrophoresis (PAGE) is used to study the polymorphism among genomic DNA (Kundan et al. 2014).
15.4.2 RAPD (Randomly Amplified Polymorphic DNA)
Williams et al. and Welsh and McClelland independently developed RAPD technique (Welsh and Mcclelland 1990; Williams et al. 1990). Simple, short (ten nucleotides), and random primers were used for PCR amplification of genomic DNA. When two hybridization sites are similar and in the opposite direction, PCR amplification takes place. Sharma et al. studied the characterization and analysis of genetic variance in Indian sesame (Sesamum indicum L.) genotypes. To find out the extent of genetic diversity between 60 sesame varieties in diverse geographical regions of India, 20 phenotypic (qualitative and quantitative) traits and 200 RAPD markers were used. In accessing the diversity, 14 RAPD markers were found to be useful. Among the population, high level of genetic variability (HT = 0.1991) and within population less variability (HS = 0.0749) were observed. Among the sesame population, mean coefficient of gene differentiation (GST = 0.6238) was 62.38% and 37.62% within the population. The above information suggests that the Indian sesame lines are genetically different, which should be used to improve the sesame crop (Sharma et al. 2014). Dar et al. reported the assessment of genetic variance in sesame using 22 RAPD, and 18 SSR primers were used for the study of 47 diverse sesame accessions cultivated in different agroclimatic regions of India. One hundred ninety-one polymorphic bands were observed with RAPD primers while SSR gives 64 bands. Maximum PIC was reported with SSRs (0.194) compared to RAPD (0.186). In describing genetic variation between the varieties studied, RAPD primer RPI-B11 and SSR primer S16 were the most informative (Dar et al. 2017).
15.4.3 AFLP (Amplified Fragment Length Polymorphism)
The combination RFLP and RAPD markers results in the development of AFLP markers, in which digestion of genomic DNA is followed by PCR amplification. AFLP is a cost-effective technique, in which there’s no need of former sequence information. In AFLP, two restriction enzymes (a frequent cutter and a rare cutter) are used. After restriction digestion, oligonucleotide fragments were used for PCR amplification (Vos et al. 1995). Laurentin and Karlovsky studied the genetic variance in a sesame germplasm set using AFLP. Great genetic variability was studied within the 32 sesame associations from the Venezuelan Germplasm Collection which represents genotypes from 5 diversity centers (India, Africa, China-Korea-Japan, Central Asia, and Western Asia). Out of the 457 AFLP markers recorded, 93% were polymorphic. The Jaccard similarity coefficient ranged from 0.38 to 0.85 between pairs of accessions. According to geographical origin, five groups of genetic diversity study discovered that only 20% of the total diversity was due to diversity among groups that used Nei’s coefficient for population differentiation. Similarly, only 5% of the total diversity is accredited to differences between groups through analysis of molecular variance (AMOVA). This study showed that 32 sesame associations were genetically highly variable and did not show a link between geographical origin and AFLP patterns. This suggests that there was a large gene flow among diversity centers (Laurentin & Karlovsky 2006).
15.4.4 SSR or Microsatellites (Simple Sequence Repeats)
SSRs are short tandem repeats of one to six nucleotides having simple sequence length polymorphism, which are present profusely in the genome of different taxa. Microsatellites are distributed throughout the whole genome, viz., nuclear and mitochondrial as well as chloroplast genes. They are also present in the protein coding genes and expressed sequence tags (ESTs). Due to the presence of different numbers of repeats in microsatellite regions, high polymorphism is easily detected by PCR (Kalia et al. 2011). Zhang et al. studied the development and validation of genic-SSR markers in sesame by RNA-seq. In this study, 75 bp and 100 bp paired RNA seq were used to sequence 24 cDNA libraries, and 42,566 uni-transcripts were collected from more than 260 million filtered readings. The total length of uni-transcript was 47.99 Mb, and 7324 SSRs (SSRs ≥15 bp) and 4440 SSRs (SSRs ≥18 bp) were acknowledged. On a usual, there was one genic-SSR per 6.55 kb (SSRs≥15 bp) or 10.81 kb (SSRs≥18 bp). A total of 2164 genic-SSR markers have been developed in sesame using transcriptomic sequencing. Two hundred seventy-six of 300 validated primer pairs successfully yielded PCR amplicons in 24 cultivated sesame accessions (Zhang et al. 2012). Park et al. reported the genetic diversity, phylogenetic conditions, and population structure of 227 connections of sesame seed collections collected from 15 countries in 4 different continents. Among sesame accessions, a total of 158 alleles were detected, with an average of 11.3 alleles per locus. The average polymorphism content value was 0.568. It indicates a high genetic variance at 14 loci both among and within the population. UPGMA and the unweighted pair group method formed four robust clusters among the 277 core collection accessions of sesame. Similar patterns were obtained using country-based dendrograms and model-based analysis, as certain geographically distant connections were grouped in the same cluster (Park et al. 2014). Surapaneni et al. (2014) studied the genetic characterization of 68 Indian sesame cultivars and 3 related wild species using 102 SSR markers. By constructing the genomic libraries, 62 novel sesame-specific microsatellites were isolated from the study. The content of polymorphic information in the markers of the markers ranged from 0.43 to 0.88 with an average of 0.66. All connections were grouped into two large clusters with a genetic similarity between 0.40 and 0.91 by UPGMA cluster analysis. A high percentage of variation (87.1%) was observed within the population by AMOVA analysis. An overall Fs of 0.11 among the populations indicated low population differentiation. The study reveals that the development of SSR markers will be constructive for genetic analysis, linkage mapping, and selection of parents in future breeding programs. Uncu et al. (2015) used a pyro-sequencing approach for the development of genomic SSR markers. They approached successfully in identifying 19,816 nonredundant SSRs, 5727 of which were identified in a coting assembly that covers 19.29% of the sesame genome. Molecular genetic diversity and population structure in a collection of world affiliations were analyzed using a subset of the newly identified SSR markers. The results of two analyses almost overlapped and suggested a correlation between genetic similarity and geographical closeness. Iqbal et al. (2018) reported on the calculation of the genetic diversity of sesame genotypes using morphological traits and SSR gene markers. To access the molecular genetic diversity at the molecular level of 70 genotypes from ecogeographic regions of the world, 235 gene markers were developed by mining expression sequence tag data from the NCBI database. The PIC content ranged between 0.36 and 0.82 with an average of 0.61. Neighbor-joining (NJ) analysis discovered that the five main groups and grouping were independent of geographic origin. Stavridou et al. (2021) studied the characterization of genetic diversity present in a varied sesame landrace set using seven expressed sequence tag-simple sequence repeat (EST-SSR) markers coupled with a high-resolution melting (HRM) analysis. The PIC value of 0.82 indicates that the selected markers were highly polymorphic . The sesame genotypes were classified into four major clades based on the principal coordinate analysis and dendrogram reconstruction of molecular data.
15.4.5 ISSR (Inter-simple Sequence Repeat)
Zietkiewicz et al. (1994) developed the ISSR marker technique. ISSR is based on the amplification of DNA segments situated in between two identical but oppositely oriented microsatellite repeat regions, at a distance which allows amplification. Parsaeian et al. (2011) conducted a research to study the genetic variations between 18 genotypes of sesame taken from diverse agroclimatic parts of Iran along with 6 exotic genotypes from the Asian countries by means of combined agro-morphological and ISSR marker traits. Total 13 ISSR primers were chosen for molecular analysis revealed 170 bands, of which 130 (76.47%) were polymorphic. On the basis of ISSR profiles , the generated dendrogram divided the genotypes into seven groups. A nonsignificant co-phenetic correlation was observed in the Mantel test by studying genetic variation in sesame using agro-morphological traits and ISSR markers.
15.4.6 SNP (Single Nucleotide Polymorphism)
Single base pair changes present in the sequence of an individual’s genome are known as SNPs. SNPs are results of transition or transversion, and in plants, SNP frequency ranges between 1 SNP in every 100–300 bp. On the basis of different molecular mechanism, diverse types of SNP genotyping assays have been developed, and among them, allele-specific hybridization, invasive cleavage, primer extension, and oligonucleotide ligation are most important (Sobrino and Carracedo 2005). Several recent high-throughput genotyping methods such as chip-based NGS, GBS, and NGS and allele-specific PCR make SNPs the most attractive markers for genotyping (Agarwal et al. 2008). Uncu et al. (2016) reported an identification and mapping of high-throughput SNPs in the sesame genome with genotyping by sequencing (GBS) analysis. SNPs preferred through a high stringency filtering protocol (770 SNPs) for better map precision were used in concurrence with SSR markers (50 SSRs) in linkage analysis. This results in 13 linkage groups spanning a total genetic distance of 914 cM with 432 markers (420 SNP, 12 SSR). Wei et al. studied the three kinds of markers (SNPs, InDels, and SSRs) used for DNA fingerprinting of 151 sesame cultivars released in China. The 140 polymorphic markers used (47 SNPs, 47 InDels, and 46 SSRs) bare a narrow range of genetic variations. Of the 151 cultivars, 3 cultivars (AH03, AH04, and AH05 from Anhui Province) were considered synonymous cultivars due to their high coefficients of genetic similarity (> 98%). To distinguish all sesame cultivars overall, 15 SNPs, 14 InDels, and 9 SSRs were sufficient (Wei et al. 2017a, b).
15.5 Plant Tissue Culture in Sesame
Sesame is prevalently self-pollinated; however, the hybrids by conventional crosses with wild types were difficult to produce because of the sexual incompatibility (Tiwari et al. 2011; Kulkarni et al. 2017). Protoplast fusion and somatic hybridization using tissue culture techniques are effective strategies to overcome sexual incompatibility. Interspecific hybrids have been successfully developed between cultivated variety of sesame and its wild relatives S. occidentale and S. radiatum through ovule and ovary culture by Dasharath et al. (2007). Rajeswari et al. (2010) have standardized an efficient protocol to produce hybrids of a cross between Sesamum indicum and S. alatum using ovule culture. The developed hybrids were resistant against phyllody disease. In vitro culture has been largely investigated in sesame which may result in somaclonal variations. Somaclonal variations are induced during callus induction and callus proliferation (Hoffman et al. 1982). Repeated and prolonged subculturing of calli enhances the frequency of gross chromosomal aberrations and gene mutations (Sanal and Mathur 2008). Regenerants resulting from such cultures are liable to carry heritable variations for seedling vigor, growth, capsule dehiscence, placental thickness, seed dormancy, seed size, yield, oil content, and oil quality (Bairu et al. 2006; Ram et al. 1990). Sesame is exceedingly recalcitrant for in vitro regeneration.
Attempts have been made for direct as well as indirect regeneration and callus induction of sesame in tissue cultures using various explants such as cotyledons and/or hypocotyl (Younghee 2001; Baskaran and Jayabalan 2006; Were et al. 2006a, b; Chakraborti and Ghosh 2009; Yadav et al. 2010; Al-Shafeay et al. 2011; Shashidhara et al. 2011; Rao and Honnale 2011; Honnale and Rao 2013; Pusadkar et al. 2015), shoot tips (Lee et al. 1985; George et al. 1987; Baskaran and Jayabalan 2006), de-embryonated cotyledon (Seo et al. 2007; Lokesha et al. 2012; Malaghan et al. 2013; Chowdhury et al. 2014; Pusadkar et al. 2016), embryo (Saravanan and Nadarajan 2005), and nodes (Gangopadhyay et al. 1998). Cotyledon or hypocotyl has proven significantly successful as an explant for plant regeneration by somatic embryogenesis (Younghee 2001; Baskaran and Jayabalan 2006; Yadav et al. 2010; Honnale and Rao 2013). All these studies achieved varying degree of success in terms of callus growth and regeneration. Callus induction and shoot regeneration frequency were significantly enhanced by supplementing cytokinins and auxins with nutrient media (Baskaran and Jayabalan 2006, Wadeyar and Lokesha 2011; Honnale and Rao 2013; Zamik et al. 2017; Gayatri and Basu 2020). Auxins and cytokines alone as well were found capable of promoting regeneration in cultures (Baskaran and Jayabalan 2006; Yadav et al. 2010). Genotypes, explant type, type of growth regulators, concentration of growth regulators, age of the explants, and the developmental stage of explants were revealed as crucial factors that governed in vitro shoot regeneration and somatic embryogenesis (Mary and Jayabalan 1997; Venkatachalam et al. 1999; Seo et al. 2007; Malaghan et al. 2013; Zimik and Arumugam 2017). However, some cotyledonary explants were also found to undergo necrosis after supplementing with cytokinins (Baskaran and Jayabalan 2006; Were et al. 2006a, b). Auxin and cytokinin treatment has been found to induce rooting in cotyledon culture of sesame (Were et al. 2006a, b; Seo et al. 2007; Zimik et al. 2017). ABA and AgNO3 were also reported to enhance shoot regeneration if supplemented with plant growth regulators (Seo et al. 2007, W deyar and Lokesha 2011). AgNO3 inhibits ethylene which is produced during in vitro culture (Chi and Pua 1989) and responsible for recalcitrant behavior of tissues in culture (Chi and Pua 1989; Shashidhara 2005). AgNO3 have also been reported to enhance conversion of somatic embryos to plants (Honnale and Rao 2013; Xu et al. 1997). Abscisic acid promotes seed maturation and inhibits seed germination (Zeevaart and Creelman 1988). The impact of ABA is broadly studied in somatic embryogenesis. ABA treatment prevents the precocious germination of embryos (somatic) and generation of secondary embryo (Choi and Jeong 2002; García-Martín et al. 2005).
15.6 Concluding Remarks
Due to increasing health awareness, people are more concerned about nutrition quality of food. Sesame is a very promising crop of future prospects due to its high-quality oil for nutritional and industrial purposes. Sesame is an underutilized and poor farmer’s crop and is fit for sustainable agricultural development. Improvement of the sesame crop can be achieved by various methods such as conventional and molecular breeding methods to obtain agronomically elite lines. Breeding efforts so far made in the country have not resulted in any substantial and significant breakthroughs in terms of productivity and yield stability. Data so far available from yield trials carried out at CCS HAU clearly show that none of the high-yielding varieties are available till date with incorporating multiple stress tolerance. The future of sesame as a commercial crop totally now totally depends upon developing high-yielding strains with inbuilt resistance to various biotic and abiotic stresses to overcoming the present yield barriers. Unluckily, the on hand germplasm materials have not yet been fully and thoroughly exploited in sesame crop improvement program. Recent years have witnessed a continuously increasing number of functional genes discovered for key agronomic traits in sesame, thanks to the availability of omics tools. In this regard, the future strategies in the sesame breeding are the functional validation of these gene resources through genetic engineering approaches and marker-assisted breeding.
References
Abbasali M, Gholipouri A, Tobeh A, KhoshkholghSima NA, Ghalebi S (2017) Identification of drought tolerant genotypes in the Sesame (Sesamum indicum L.) Collection of National Plant Gene Bank of Iran. Irani J Field Crop Sci 48(1):275–289
Abbasdokht H, Ashrafi E, Taheri S (2012) Effects of different salt levels on germination and seedling growth of sesame (Sesamum indicum L.) cultivars. Tech J Eng Appl Sci 2(10):309–313
Abdel AIM, Serry M, El-Ahmar BA (1976) Some factors affecting self and artificial pollination insesame (Sesamum indicum L). Agric Res Rev 54:155–159
Abdelraouf RE, Anter AS (2020) Response of new sesame lines (Sesamum indicum L.) to deficit irrigation under clay soils conditions. Plant Arch 20(2):2369–2377
Abdullahi A (1998) The potentials of beniseed as a source of raw materials in Nigeria. In: Misari SM, Idown AA, Busari LD (eds) Beniseed in Nigeria: opportunities for research, production and marketing. RMRDC, Abuja, pp 120–122
Adam EY, Abdallgader O, Yousif AA (2020) A note on sesame gall midge SphondyliaSesamiFelt.(Diptera; Cecidomyidae) in the Blue Nile State, Sudan. Int J Environ Agric Res (IJOEAR) 6(9):23–26
Abd Elaziz GB, Ghareeb ZE (2018) Gene Action and Combining Ability for Seed Yield and its Components in Eight Sesame Genotyps Diallel Crosses. J. Plant Prod. 9:695–702
Agarwal M, Shrivastava N, Padh H (2008) Advances in molecular marker techniques and their applications in plant sciences. In Plant Cell Rep (Vol. 27, 4, pp. 617–631). Springer. https://doi.org/10.1007/s00299-008-0507-z
Akhtar KP, Sarwar G, Sarwar N, Elahi MT (2013) Field evaluation of sesame germplasm against sesame phyllody disease. Pak J Bot 45:1085–1090
Al-Shafeay AF, Ibrahim AS, Nesiem MR, Tawfik MS (2011) Establishment of regeneration and transformation system in Egyptian sesame (Sesamum indicum L.) cv Sohag1. GM Crops 2:182–192
Aladji-Abatchoua MMI, Noubissie-Tchiangam JB, Njintang-Yanou N (2014) Genetic analysis of seed yield component in sesame (Sesamum indicum L.) at Mora (Cameroon). Sch. Acad. J. Biosci. 2:318–325
Anee TI, Nahar K, Rahman A, Mahmud JA, Bhuiyan TF, Alam MU, Fujita M, Hasanuzzaman M (2019) Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants 8(7):196
Anter AS, El-Sayed AB (2020) Screening new sesame (Sesamum indicumL.) lines at two levels of Na Cl incorporated with algae for salt tolerance. Plant Arch 20(2):2271–2276
Anthony O, Ogunshakin R, Vaghela S, Patel B (2015) Towards sustainable intensification of sesame-based cropping systems diversification in northwestern India. J Food Secur 3(1):1–5
Anyanga WO, Rubaihayo P, Gibson P, Okori P (2016) Combining ability and gene action in sesame (Sesamum indicum L) elite genotypes by diallel mating design. J. Plant Breed. Crop Sci. 8:250–256
Asadi H, Baradaran R, Seghatoleslami MJ, Mousavi SG (2020) Evaluation of drought tolerance in some sesame (Sesamum indicum L.) genotypes based on stress tolerance indices. Iran J Field Crop Res 18(4):413–433
Asekova S, Oh E, Kulkarni KP, Siddique MI, Lee MH, Kim JI, Lee JD, Kim M, Oh KW, Ha TJ, Kim SU (2021) An integrated approach of QTL mapping and genome-wide association analysis identifies candidate genes for phytophthora blight resistance in sesame (Sesamum indicum L.). Front. plant sci 12:1–15
Ashiri A (2007) Sesame (Sesamum indicum L.). In: Singh RJ (ed) Genetic resources, chromosome engineering and crop improvement, oilseed crops. CRC Press, Boca Raton, pp 231–289
Ashri A (1998) Sesame breeding. Plant Breed Rev 16:179–228
Bahrami H, Razmjoo J (2012) Effect of salinity stress (NaCl) on germination and early seedling growth of ten sesame cultivars (Sesamum indicum L.). Int J Agric Sci 2(6):529–537
Bairu MW, Fennell CW, Van Staden J (2006) The effect of plant growth regulators on somaclonal variation in Cavendish banana (Musa AAA cv. Zelig). Sci Hortic 108:347–351
Banerjee P, Kole P (2010) Heterosis, inbreeding depression and their relationship with genetic divergence in sesame (Sesamum indicum L.). Acta Agron Hung 58(3):313–321
Baskaran P, Jayabalan N (2006) In vitro mass propagation and diverse callus orientation on Sesamum indicum L.-an important oil plant. J Agric Technol 2:259–269
Baydar H (2005) Breeding for the improvement of the ideal plant type of sesame. Plant Breeding 124:263-267
Baydar H, Turgut I, Turgut K (1999) Variation of certain characters and line selection for yield, oil, oleic and linoleic acids in the Turkish sesame (Sesamum indicum L.) populations. Turk J Agric For 23(4):431–442
Bedawy I, Moharm M (2019) Reaction and Performance of Some Sesame Genotypes for Resistance to Macrophomina phaseolina, the Incitant of Charcoal Rot Disease. Alexandria Sci. Exchange J. 40:12–18
Bedigian D (2003) Evolution of sesame revisited: domestication, diversity and prospects. Genet Resour Crop Evol 50(7):779–787
Bedigian D, Harlan JR (1986) Evidence for cultivation of sesame in the ancient world. Econ Bot 40(2):137–154
Beebe SE, Corrales MP (1991) Breeding for disease resistance. In: Common beans: research for crop improvement, pp 561–617
Bekele A, Besufekad Y, Adugna S, Yinur D (2017) Screening of selected accessions of Ethiopian sesame (Sesame indicum L.) for salt tolerance. Biocatal Agric Biotechnol 9:82–94
Belay Y (2018) Screening of fusarium wilt, bacterial blight and phyllody diseases resistant sesame genotypes in sesame growing areas of northern Ethiopia. J Agric Ecol Res Int:1–12
Bhuyan J, Sarma MK (2003) Identification of heterotic crosses involving cytoplasmic-genic male sterile lines in sesame (Sesamum indicum L.). Sesame and Safflower Newsletter 18:7–11
Bisht IS, Mahajan RK, Loknathan TR, Agrawal RC (1998) Diversity in Indian sesame collection and stratification of germplasm accessions in different diversity groups. Genet Resour Crop Evol 45(4):325–335
Biswas S, Natta S, Ray DP, Mondal P, Saha U (2018) Til (Sesamum indicum L.)-an underexploited but promising oilseed with multifarious applications: a review. Int J Bioresour Sci 5(2):127–139
Boureima S, Eyletters M, Diouf M, Diop T, Van Damme P (2011) Sensitivity of seed germination and seedling radicle growth to drought stress in sesame Sesamum indicum L. Res J Environ Sci 5(6):557
Boureima S, Oukarroum A, Diouf M, Cisse N, Van Damme P (2012) Screening for drought tolerance in mutant germplasm of sesame (Sesamum indicum) probing by chlorophyll a fluorescence. Environ Exp Bot 81:37–43
Boureima S, Diouf S, Amoukou M, Van Damme P (2016) Screening for sources of tolerance to drought in sesame induced mutants: assessment of indirect selection criteria for seed yield. Int J Pure Appl Biosci 4(1):45–60
Brar GS (1982) Male sterility in sesame. Indian J Genet 42:23–27
Brar GS, Ahuja L (1979) Sesame: its culture, genetics, breeding and biochemistry. Annu Rev Plant Sci 1:245–313
Chakraborti P, Ghosh A (2009) Variation in callus induction and root shoot bud formation depend on seed coat of sesame genotypes. Res J Bot 5:14–19
Cheng FC, Jinn TR, Hou RCW, Tzen JTC (2006) Neuroprotective effects of sesamin and sesamolin on gerbil brain in cerebral ischemia. Int J Biomed Sci 2:284–288
Chi CL, Pua EC (1989) Ethylene inhibitor enhanced de novo shoot regeneration from cotyledon of Brassica campestris spp chinensis (Chinese cabbage) in vitro. Plant Sci 64:243–250
Choi YE, Jeong JH (2002) Dormancy induction of somatic embryos of Siberian ginseng by high sucrose concentrations enhances the conservation of hydrated artificial seeds and dehydration resistance. Plant Cell Rep 20:1112–1116
Chowdhury S, Basu A, Kundu S (2014) A new high-frequency Agrobacterium-mediated transformation technique for Sesamum indicum L. using de-embryonated cotyledon as explant. Protoplasma 251:1175–1190
Chowdhury S, Basu A, Kundu S (2017) Overexpression of a new osmotin-like protein gene (SindOLP) confers tolerance against biotic and abiotic stresses in sesame. Front Plant Sci 8:1–16
Couch A, Gloaguen RM, Langham DR, Hochmuth GJ, Bennett JM, Rowland DL (2017) Non-dehiscent sesame (Sesamum indicumL.): its unique production potential and expansion into thesoutheastern USA. J Crop Improv 72
Cui C, Mei H, Liu Y, Zhang H, Zheng Y (2017) Genetic diversity, population structure, and linkage disequilibrium of an association-mapping panel revealed by genome-wide SNP markers in sesame. Front Plant Sci 8:1189
Dar AA, Mudigunda S, Mittal PK, Arumugam N (2017) Comparative assessment of genetic diversity in Sesamum indicum L. using RAPD and SSR markers. 3 Biotech 7(1):1–12. https://doi.org/10.1007/s13205-016-0578-4
Dasgupta P, Lal JP, Kumar H (2018) Role of epistatic gene action in inheritance of quantitative traits in sesame (Sesamum indicum L.). J. Pharm. Innov. 7:592–595
Dasharath K, Sridevi O, Salimath PM, Ramesh T (2007) Production of interspecific hybrids in sesame through embryo rescue. Ind J Crop Sci 2:193–196
Day JS, Langham DR, Wongyai W (2002) Potential selection criteria for the development of high-yielding determinate sesame varieties. Sesame Safflower Newsl 17:29–35
Deepthi P, Shukla CS, Verma KP, Reddy E (2014a) Identification of charcoal rot resistant lines of Sesamum indicum and chemical management of Macrophomina phaseolina. Med Plants-Int J Phytomed Relat Ind 6(1):36–42
Deepthi P, Shukla CS, Verma KP, Reddy SS (2014b) Yield loss assessment and influence of temperature and relative humidity on charcoal rot development in sesame (Sesamum indicumL.). Bioscan 9:193–195
Dixit A, Jin MH, Chung JW, Yu JW, Chung HK, Ma KH, Park YJ, Cho EG (2005) Development of polymorphic microsatellite markers in sesame (Sesamum indicum L.). Mol Ecol Notes 5(4):736–738
Dossa K, Diouf D, Cisse N (2016) Genome-wide investigation of Hsf genes in sesame reveals their segmentalduplication expansion and their active role in drought stress response. Front Plant Sci 7:1522
Dossa K, Konteye M, Niang M, Doumbia Y, Cisse N (2017a) Enhancing sesame production in West Africa’s Sahel: a comprehensive insight into the cultivation of this untapped crop in Senegal and Mali. Agric Food Secur 6(1):1–15
Dossa K, Diouf D, Wang L, Wei X, Zhang Y, Niang M, Fonceka D, Yu J, Mmadi MA, Yehouessi LW, Liao B (2017b) The emerging oilseed crop Sesamum indicum enters the “Omics” era. Front Plant Sci 8:1154
Dossa K, Mmadi MA, Zhou R, Zhang T, Su R, Zhang Y, Wang L, You J, Zhang X (2019) Depicting the core transcriptome modulating multiple abiotic stresses responses in sesame (Sesamum indicum L.). Int J Mol Sci 20(16):3930
Dossou SSK, Wang L, Wei X, Zhang Y, Li D, Yu J, Zhang X (2020) Transcriptome analysis of black and white sesame seed reveals candidate genes associated with black seed development in sesame (Sesamum Indicum)
Duhoon SS (2004) Exploitation of heterosis for raising productivity in sesame in new directions for a diverse planet. In: Proceedings of the 4th international crop science congress, Brisbane, Australia, vol 26
Dyer JM, Stymne S, Green AG, Carlsson AS (2008) High-value oils from plants. Plant J 54(4):640–655
El Harfi M, Hanine H, Rizki H, Latrache H, Nabloussi A (2016) Effect of drought and salt stresses on germination and early seedling growth of different color-seeds of sesame (Sesamum indicum). Int J Agric Biol 18(6)
El-Bramawy MAS, Shaban WI (2007) Nature of gene action for yield, yield components and major diseases resistance in sesame (Sesamum indicum L.). Res J Agric Biol Sci 3(6):821–826
Eswarappa V, Kumar KTV, Rani HV (2011) Inheritance study and identification of RAPD marker linked to alternaria blight resistance in sesame (Sesamum indicum L.). Curr Biotica 4(4):453–460
FAOSTAT (2015) Food and agricultural organization of the united nations. Statistical Database
Faostat (2019) FAOSTAT Data Acessed in 2019
Farooq S, Mohyo-Ud-Din A, Naz S, Siddique M, Khan SN (2019) Screening of sesame (Sesamum indicum l.) germplasms for resistance against charcoal rot disease caused by Macrophomina phaseolina (Tassi) Goid. Int J Biol Biotechnol 16(2):407–410
Fazeli F, Ghorbanli M, Niknam V (2007) Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol Plant 51(1):98–103
Food and Agriculturre Organization Statistical Databases (FAOSTAT). Available online: http://faostat.fao.org/. Accessed on 5 Feb 2020
Fukuda Y, Osawa T, Namiki M (1985) Studies on antioxidative substances in sesame seed. Agric Biol Chem 49(2):301–306
Gaikwad KB, Lal JP, Kumar H (2009) Genetic architecture of yield and yield attributing characters in sesame (Sesamum indicum L.). Crop Improv 36:31–36
Gaikwad KB, Lal JP, Kumar H (2010) Gene effects for yield and its components in sesame (Sesamum indicum L.). Environ & Ecol 28(2):873–878
Ganem Junior EDJ, Segnana LG, Kitajima EW, Bedendo IP (2019) Sesame phyllody associated with a 16SrI-B phytoplasma, a ‘Candidatus Phytoplasma asteris’-related strain, in Paraguay. Sci Agric 76(1):47–50
Gangaiah B (2008) Agronomy–kharif crops. World 7(3.28):441
Gangopadhyay G, Poddar R, Gupta S (1998) Micropropagation of sesame (Sesamum indicum L.) by in vitro multiple shoot production from nodal explants. Phytomorphology 48:83–90
García-Martín G, Manzanera JA, González-Benito ME (2005) Effect of exogenous ABA on embryo maturation and quantification of endogenous levels of ABA and IAA in Quercus suber somatic embryos. Plant Cell Tissue Organ Cult 80:171–177
Garmaroodi HS, Mansouri S (2014) Primary evaluation of sesame germplasm for resistance to charcoal rot disease in laboratory condition. Seed Plant Improv J 30(3)
Gayatri T, Basu A (2020) Development of reproducible regeneration and transformation system for Sesamum indicum. Plant Cell Tissue Organ Cult 143(2):441–456
George L, Bapat VA, Rao PS (1987) In vitro multiplication of sesame (Sesamum indicum) through tissue culture. Ann Bot 60(1):17–21
Girmay AB (2018) Sesame production, challenges and opportunities in Ethiopia. Agric Res Technol Open Access J 15(5):555972
Gupta SK (ed.) (2015) Breeding oilseed crops for sustainable production: opportunities and constraints. Academic
Hassan MAM (2012) Studies on Egyptian sesame seeds (Sesamum indicum L.) and its products 1–Physicochemical analysis and phenolic acids of roasted Egyptian sesame seeds. World J Dairy Food Sci 7(2):195–201
Hassan MS, Sedeck FS (2015) Combining ability and heterosis estimates in sesame. World Appl Sci J 33(5):690–698
Hawkes J (1983) The diversity of crop plants. Harvard Unirversity Press, Cambridge
He Q, Lee TR, Yu J, Oo WH, Yoon MY, Min MH, Chu SH, Kim KW, Lee YS, Park YJ (2020) Genotypic variation in fatty acids in whole grain sesame (fatty acids in whole grain sesame). J Crop Sci Biotechnol 23(1):9–20
Hibasami H, Fujikawa T, Takeda H, Nishibe S, Satoh T, Fujisawa T et al (2000) Induction of apoptosis by Acanthopanax senticosus HARMS and its component, sesamin in human stomach cancer KATO III cells. Oncol Rep 7:1213–1216
Hoffman F, Thomas E, Wenzel G (1982) Anther culture as a breeding tool in a rape, II. Progeny analysis of androgenetic lines and induced mutants from haploid cultures. Theor Appl Genet 61:225–232
Honnale H, Rao S (2013) Direct somatic embryogenesis in Sesamum indicum (L.) Cv-E8 from cotyledon and hypocotyl explants. Int J Appl Biol Pharm Technol 4:120–127
Hwang LS, Lee MH, Su NW (2005) Sesame oil. In: Bailey’s industrial oil and fat products, pp 1–39
Iqbal A, Bhattacharyya U, Akhtar R, Dasgupta T (2018) Genetic diversity computation in sesame genotypes using morphological traits and genic. SSR Markers 78(3):348–356. https://doi.org/10.31742/IJGPB.78.3.12
Islam F, Gill RA, Ali B, Farooq MA, Xu L, Najeeb U, Zhou W (2016) Sesame. In: Gupta SK (ed) Breeding oilseed crops for sustainable production. Academic, San Diego, pp 135–147
Ismail AA, Abo-Elwafa A, Sedeck FS, Abd-Elsaber A (2013) Pedigree selection for yield and its components in sesame (Sesamum indicum L.) 1-response to selection for yield, correlation and path coefficients analyses
Jadhav RS, Mohrir MN (2013) Heterosis studies for quantitative traits in sesame (Sesamum indicumL.). Electron J Plant Breed 4(1):1056–1060
Jiarong TLWW (1991) Studies on heterosis, combining ability and reciprocal effects in sesame [J]. Acta Agric Boreal Sin 4
Joshi AB (1961) Sesamum: A monograph. Indian Central Oilseed Committee, Hyderabad
Kadkhodaie A, Razmjoo J, Zahedi M, Pessarakli M (2014) Selecting sesame genotypes for drought tolerance based on some physiochemical traits. Agron J 106(1):111–118
Kalia RK, Rai MK, Kalia S, Singh R, Dhawan AK (2011) Microsatellite markers: an overview of the recent progress in plants. Euphytica 177(3):309–334). Kluwer Academic Publishers. https://doi.org/10.1007/s10681-010-0286-9
Kamal-Eldin A, Appelqvist LA, Yousif G, Iskander GM (1992) Seed lipids of Sesamum indicum and related wild species in Sudan. The sterols. J Sci Food Agric 59(3):327–334
Khammari M, Ghanbari A, Rostami H (2013) Evaluation indicator of drought stress in different cultivars of sesame. Int J Manag Sci Bus Res 2(9):28
Khuimphukhieo I, Khaengkhan P, Sarepoua E (2020) Inheritance, heritability and association of agronomic traits and sesamin and sesamolin contents in sesame (Sesamum indicum L.). Agric Nat Resour 54(4):439–446
Kim KS, Park SH, Jenks MA (2007) Changes in leaf cuticular waxes of sesame (Sesamum indicum L.) plants exposed to water deficit. J Plant Physiol 164(9):1134–1143
Kizil S, Basak M, Guden B, Tosun HS, Uzun B, Yol E (2020) Genome-wide discovery of InDel markers in sesame (Sesamum indicum L.) using ddRADSeq. Plants 9(10):1262
Koca H, Bor M, Özdemir F, Türkan İ (2007) The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ Exp Bot 60(3):344–351
Kolte SJ (1985) Disease of annual edible oil seed crops. Vol II: Rape seed-mustard and sesame diseases. CRC Press, Boca Raton
Kotcha A (2012) Inheritance of shatter resistance capsules in sesame. Genomics and Genetics 5:149–158
Komivi D, Mareme N, Achille EA, Ndiaga C, Diaga D (2016) Whole genome homology-based identificationof candidate genes for drought tolerance in sesame (Sesamum indicum L.). African J. Biotechnology 15:1464–1475
Kulkarni VV, Ranganatha CN, Shankergoud I (2017) Interspecific crossing barriers in sesame (Sesamum indicum L.). Int J Curr Microbiol App Sci 6:4894–4900
Kumar V, Sharma SN (2011) Comparative potential of phenotypic, ISSR and SSR markers for characterization of sesame (Sesamum indicum L.) varieties from India. J Crop Sci Biotechnol 14(3):163–171
Kumari V, Singh A, Chaudhary HK, Kumar A, Prasad R, Jambhulkar S, Sanju S (2019) Identification of Phytophthora blight resistant mutants through induced mutagenesis in sesame (Sesamum indicum L.). Indian Phytopathol 72(1):71–77
Kundan MK, Vinita T, Yachana J, Madhumati B (2014) Potential and application of molecular markers techniques for plant genome analysis. Int J Pure App Biosci 2(1):169–188. www.ijpab.comwww.ijpab.com
Lal Jatothu J, Dangi KS, Kumar SS (2013) Evaluation of sesame crosses for heterosis of yield and yield attributing traits. J Trop Agric 51(1):84–91
Langham DG (1944) Natural and controlled pollination in sesame. J Hered 35:254–256
Langham DR (2007) Phenology of sesame. Issues in New Crops and New Uses, Janick & Whipkey, eds., ASHS Press, Alexandria, VA, pp.144-182
Laurentin HE, Karlovsky P (2006) Genetic relationship and diversity in a sesame (Sesamum indicum L.) germplasm collection using amplified fragment length polymorphism (AFLP). BMC Genet 7:10
Lee JI, Park YH, Park YS, Im BG (1985) Propagation of sesame (Sesamum indicum L.) through shoot tip culture. Korean J Breed
Lenaerts B, Collard BC, Demont M (2019) Improving global food security through accelerated plant breeding. Plant Sci 287:110207
Li C, Miao H, Wei L, Zhang T, Han X, Zhang H (2014) Association mapping of seed oil and protein contentin Sesamum indicum L. Using SSR markers. PLoS One 9:e105757
Li D, Liu P, Yu J, Wang L, Dossa K, Zhang Y, Zhou R, Wei X, Zhang X (2017) Genome-wide analysis of WRKY gene family in the sesame genome and identification of the WRKY genes involved in responses to abiotic stresses. BMC Plant Biol 17(1):152
Li-Bin WEI, Zhang HY, Zheng YZ, Wang-Zhen GUO, Zhang TZ (2008) Developing EST-derived microsatellites in sesame (Sesamum indicum L.). Acta Agron Sin 34(12):2077–2084
Liu H, Yang M, Wu K, Zhou X, Zhao Y (2013) Development, inheritance and breeding potential of a recessive genic male sterile line D248A in sesame (Sesamum indicum L.). Springer Plus 2(1):1–7
Lokesha R, Naik MK (2011) Selection of somaclones resistant to Alternaria blight in sesame (Sesamum Indicum L.) through in VitroCellLineMethod. Indian J Fundam Appl Life Sci 1(4):338–346
Lokesha R, Rahaminsab J, Ranganatha ARG, Dharmaraj PS (2012) Whole plant regeneration via adventitious shoot formation from de-embryonated cotyledon explants of sesame (Sesamum indicum L.). World J Sci Technol 2:47–51
Lokesha R, Banakar CN, Goudappagoudar R (2013) Inheritance of phyllody resistance in sesame (Sesamum indicum L.). BIOINFOLET Q J Life Sci 10(1b):177–179
Mahadevaprasad TN, Karuna K, Jayashree MK, Reddy KM (2017) Field evaluation of sesame lines against phyllody. Electron J Plant Breed 8(3):945–949
Malaghan SV, Lokesha R, Savitha R, Ranganatha ARG (2013) Adventitious shoot regeneration in sesame (Sesamum indicum L.) (Pedaliaceae) via deembryonatedcotyledonary explants. Res J Biol 1:31–35
Manjeet, Avtar R, Kumar A, Sheoran RK, Verma PK (2020) Screening of sesame (Sesamum indicum L.) genotypes for major diseases. Electron J Plant Breed 11(04):1227–1232
Mary RJ, Jayabalan N (1997) Influence of growth regulators on somatic embryogenesis in sesame. Plant Cell Tissue Organ Cult 49(1):67–70
Mbaebie B, Omosun G, Uti A, Oyedemi S (2010) Chemical composition of Sesamum indicum L.(Sesame) grown in southeastern Nigeria and the physicochemical properties of the seed oil. Seed Sci Biotech 4:69–72
Mei H, Liu Y, Du Z, Wu K, Cui C, Jiang X (2017) High-density genetic map construction and gene mapping of basal branching habit and flowers per leaf axil in sesame plant materials and trait investigation. Front Plant Sci 8:636
Miao H, Li C, Duan Y, Wei L, Ju M, Zhang H (2020) Identification of a Sidwf1 gene controlling short internode length trait in the sesame dwarf mutant dw607. Theor Appl Genet 133(1):73–86
Miyahara Y, Hibasami H, Katsuzaki H, Imai K, Komiya T (2001) Sesamolin from sesame seed inhibits proliferation by inducing apoptosis inhuman lymphoid leukemia Molt 4B cells. Int J Mol Med 7:369–371. https://doi.org/10.3892/ijmm.7.4.369
Monpara BA, Pawar AK (2016) Evaluation of hybrids for heterosis breeding in sesame (Sesamum indicum L.). Electron J Plant Breed 7(4):1183–1187
Mosjidis J, Yermanos DM (1985) Plant position effect on seed weight, oil content and oil compositon in sesame (SesamumindicumL.). Euphytica 34:193–200
Mothilal A, Ganesan KN (2005) Heterosis studies in sesame (Sesamum indicum L.). Agric Sci Dig 25(1):74–76
Murty DS (1975) Heterosis, combining ability and reciprocal effects for agronomic and chemical characters in sesame. Theor Appl Genet 45(7):294–299
Mustafa HSB, Ali Q, Anwar M, Aftab M, Mahmood T (2015) Selection criteria for improvement in sesame (Sesamum indicum L.). J Exp Agric Int 9:1–13
Noguchi T, Ikeda K, Sasaki Y, Yamamoto J, Yamori Y (2001) Effects of vitamin E and sesamin on hypertension and cerebral thrombogenesis in stroke-prone spontaneously hypertensive rats. Hypertens Res 24:735–742
Nzikou JM, Matos L, Kalou GB et al (2009) Chemical composition on the seeds and oil of sesame (Sesamum indicum L.) grown in Congo-Brazzaville. Adv J Food Sci Technol 1(1):6–11
Osman HE, Yermanos DM (1982) Genetic male sterility in sesame: reproductive characteristics and possible use in hybrid seed production. Crop Sci 22(3):492–498
Pandey SK, Das A, Dasgupta T (2013) Genetics of seed coat color in sesame (Sesamum indicum L.). Afr. J. Biotechnol 12:6061–6067
Pandey S, Jaglan RS, Yadav S (2018) Biology of leaf webber and capsule borer, Antigastracatalaunalis (Duponchel) on sesame. J Entomol Zool Stud 6:1731–1734
Parimala K, Devi IS, Bharathi V, Raghu B, Srikrishnalatha K, Reddy AV (2013) Heterosis for yield and its component traits in sesame (Sesamum indicum L.). Electron J Plant Breed, v4 n3: 1246-1250
Park JH, Suresh S, Cho GT, Choi NG, Baek HJ, Lee CW, Chung JW (2014) Assessment of molecular genetic diversity and population structure of sesame (Sesamum indicum L.) core collection accessions using simple sequence repeat markers. Plant Genet Resour Charact Util 12(1):112–119. https://doi.org/10.1017/S1479262113000373
Parsaeian M, Mirlohi A, Saeidi G (2011) Study of genetic variation in sesame (Sesamum indicum L.) using agro-morphological traits and ISSR markers. Russ J Genet 47(3):314–321. https://doi.org/10.1134/S1022795411030136
Pathak N, Rai AK, Kumari R, Thapa A, Bhat KV (2014a) Sesame crop: an underexploited oilseed holds tremendous potential for enhanced food value. Agric Sci 5:519
Pathak N, Rai AK, Kumari R, Bhat KV (2014b) Value addition in sesame: a perspective on bioactive components for enhancing utility and profitability. Pharmacogn Rev 8(16):147
Pawar DV, Suryawanshi AP, Kadam VA (2019) Occurrence and distribution of sesame Alternaria leaf blight disease in nine agro climatic zones of Maharashtra state, India. Int J Curr Microbiol App Sci 8(10):2326–2343
Prabakaran AJ (1998) Cytoplasmic male sterility in sesame from the species cross. Sesame and Safflower Newsletter P.1
Prabakaran AJ, Rangasamy SS, Ramalingam RS (1995) Identification of cytoplasm-induced male sterility in sesame through wide hybridization. Curr Sci:1044–1047
Pusadkar PP, Kokiladevi E, Aishwarya V, Gnanam R, Sudhakar D, Balasubramanian P (2015) Efficacy of growth hormone for callus induction in sesame (Sesamum indicum L.). J Cell Tissue Res 15:5067–5071
Pusadkar P, Kokiladevi E, Shilpa B (2016) Efficacy of plant growth hormones for shoot induction and regeneration in sesame (Sesamum indicumL.). Res J Biotechnol 11:27–30
Quoada P, Layrisse A (1995) Heterosis and combining ability in hybrids among 12 commercial varieties of sesame (Sesamum indicum L.). Plant Breed 114(3):239–242
Raikwar RS (2018) Combining ability studies for assessment of yield and its components in sesame (Sesamum indicum L.). Electron J. Plant Breed 9:777–781
Rajeswari S, Thiruvengadam V, Ramaswamy NM (2010) Production of interspecifi c hybrids between Sesamum alatum Thonn and Sesamum indicum L. through ovule culture and screening for phyllody disease resistance. S Afr J Bot 76:252–258
Ram R, Catlin D, Romero J, Cowley C (1990) Sesame: new approaches for crop improvement. In: Janick J, Simon JE (eds) Advances in new crops. Timber Press, Portland, pp 225–228
Ram SG, Sundaravelpandian K, Kumar M, Vinod KK, Bapu JK, Raveendran TS (2006) Pollen–pistil interaction in the inter-specific crosses of Sesamum sp. Euphytica 152:379–385
Ramazani SHR (2016) Surveying the relations among traits affecting seed yield in sesame (Sesamum indicum L.). J Crop Sci Biotechnol 19(4):303–309
Rangaswamy M, Rathinam M (1982) Mutagen induced male sterile lines in sesame. Indian J Genet 42:142–143
Rao S, Honnale HN (2011) Callus induction and organogenesis in Sesamum indicum L. Cv. E 8. Curr Trends Biotechnol Pharm 5:1462–1468
Rao KR, Kishor PBK, Vaidyanath K (2002) Bio-technology of sesame-an oil seed crop. Plant Cell Bio-technol Mol Biol 3:101–110
Rao PVR, Anuradha G, Prada MJ, Shankar VG, Reddy KR, Reddy NE, Siddiq EA (2012) Inheritance of powdery mildew tolerance in sesame. Arch Phytopathol Plant Protect 45(4):404–412
Rao PVR, Prasuna K, Anuradha G, Srividya A, Vemireddy LP, Shankar VG, Sridhar S, Jayaprada M (2014) Molecular mapping of important agro-botanic traits in sesame. Electron J. Plant Breed 5:475-488
Rajput SD, Harer PN, Kute NS (2017) Combining ability analysis for yield and its component traits in sesame (Sesamum indicum L). Electron J. Plant Breed 8:1307–1309
Renganathan P (2020) Chapter-6 sesame root rot-occurrence, economic losses, symptomology and pathogenic variability. Curr Res Innov Plant Pathol:113
Renuka G, Lokesha R (2013) Inheritance of Alternaria blight resistance in sesame. Int J Plant Sci (Muzaffarnagar) 8(1):110–112
Sadeghiy Garmaroudi H, Mansouri S, Nazari A (2003) Evaluation the resistance of sesame improved cultivars and lines against charcoal rot diseases caused by Macrophominaphaseolina
Saha RR, Ahmed F, Mokarroma N, Rohman MM, Golder PC (2016) Physiological and biochemical changes in waterlog tolerant sesame genotypes. SAARC J Agric 14(2):31–45
Sanal PK, Mathur VL (2008) Chromosomal instability in callus culture of Pisum sativum. Plant Cell Tissue Org Cult 78(3):267–271
Sani I, Okpalaoka CC, Bello F, Warra AA, Abdulhamid A (2014) Flavonoid content and antioxidant potential of white and brownsesame seed oils. Eur J Biomed Pharm 1(3):56–63
Sankar D, Sambandam G, Ramakrishna RM, Pugalendi KV (2005) Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin Chim Acta 355:97–104
Saravanan S, Nadarajan N (2002) Studies on heterosis in sesame (Sesamum indicum L.). Indian J Genet 62:271–272
Saravanan S, Nadarajan N (2005) Effect of media supplements on in vitro response of sesame (Sesamum indicum L.) genotypes research. Res J Agric Biol Sci 1(1):98–100
Sarwar G, Akhtar KP, Haq MA, Jamil FF, Alam SS (2006) Prevalence of phyllody and sesame leaf curl disease in sesame and their impact on seed yield. Pak J Phytopathol (Pak)
Sarwar MF, Sarwar MH, Sarwar M, Qadri NA, Moghal S (2013) The role of oilseeds nutrition in human health: a critical review. J Cereal Oilseed 4(8):97–100
Sasikumar B, Sardana S (1990) Heterosis for yield and yield components in sesame. Indian J Genet 50(1):87–88
Seo HY, Kim YJ, Park TI, Kim HS, Yun HS (2007) High-frequency plant regeneration via adventitious shoot formation from deembryonated cotyledon explants of Sesamum indicum L. In Vitro Cell Dev Biol Plant 43:209–214
Shabana R, Abd El-Mohsen AA, Khalifa MMA, Saber AA (2014) Quantification of resistance of F6 sesame elite lines against Charcoal-rot and Fusarium wilt diseases. AdvAgric Biol 1:144–150
Shah NC (2013) Sesamum indicum (sesame or til): Seeds & oils – an historical and scientificevaluation from indian prospective. Indian J Hist Sci 48(2):151–174
Shakeri E, Modarres-Sanavy SAM, Amini Dehaghi M, Tabatabaei SA, Moradi-Ghahderijani M (2016) Improvement of yield, yield components and oil quality in sesame (Sesamum indicum L.) by N-fixing bacteria fertilizers and urea. Arch Agron Soil Sci 62(4):547–560
Sharma SN, Kumar V, Sharma C, Shukla N, Gupta A, Tripathi A, Paroha S (2014) Characterization and analysis of genetic diversity in Indian sesame (Sesamum indicum L.) genotypes. Indian J Genet Plant Breed 74(3):344–352. https://doi.org/10.5958/0975-6906.2014.00852.9
Sharmila V, Ganesh SK, Gunasekaran M (2007) Generation mean analysis for quantitative traits in sesame (Sesamum indicum L.) crosses. Genet. Mol. Biol. 30:80–84
Shashidhara N (2005) Shoot regeneration via rapid method of callusing in sesame (Sesamum indicum L.). M.Sc. thesis, Univ. Agric. Sci. Dharwad
Shashidhara N, Ravikumar H, Ashoka N, Santosh DT, Pawar P, Lokesha R, Janagoudar BS (2011) Callus induction and subculturing in sesame (Sesamum indicum L.): a basic strategy. Int J Agric Environ Biotechnol 4:153–156
Shetty PK, Ayyappan S, Swaminathan MS (2013) Climate change and sustainable food security (NIAS Books and Special Publications No. SP4-2013). NIAS; ICAR
Sheng C, Song S, Zhou R, Li D, Gao Y, Cui X, Tang X, Zhang Y, Tu J, Zhang X, Wang L (2021) QTL-seq and transcriptome analysis disclose major QTL and candidate genes controlling leaf size in Sesame (Sesamum indicum L.). Front. Plant Sci. 12:1-13
Shindhe GG, Lokesha R, Naik MK, Ranganath AGR (2011) Inheritance study on phyllody resistance in sesame (Sesamum indicum L.). Plant Arch. 11:775–776
Singh M, Avtar R, Pal A, Punia R, Singh VK, Bishnoi M, Singh A, Choudhary RR, Mandhania S (2020) Genotype-Specific Antioxidant Responses and Assessment of Resistance Against Sclerotinia sclerotiorum Causing Sclerotinia Rot in Indian Mustard. Pathogens 9:892
Singh PK, Akram M, Vajpeyi M, Srivastava RL, Kumar K, Naresh R (2007) Screening and development of resistant sesame varieties against phytoplasma. Bull Insectol 60(2):303
Sinha S, Kumar A, Sohane RK, Kumar R (2020) Breeding of oilseeds: a challenge for self-sufficiency. In: Proceedings-cum-Abstract Book
Sobrino B, Carracedo A (2005) SNP typing in forensic genetics: a review. Methods Mol Biol (Clifton, NJ) 297:107–126. https://doi.org/10.1385/1-59259-867-6:107
Solanki ZS, Gupta D (2003) Inheritance studies for seed yield in sesame. Sesame Safflower Newsl 18:25–28
Song Q, Joshi M, Wang S, Johnson CD, Joshi V (2020) Comparative analysis of root transcriptome profiles of sesame (Sesamum indicum L.) in response to osmotic stress. J Plant Growth Regul:1–15
Stavridou E, Lagiotis G, Kalaitzidou P, Grigoriadis I, Bosmali I, Tsaliki E, Tsiotsiou S, Kalivas A, Ganopoulos I, Madesis P (2021) Characterization of the genetic diversity present in a diverse sesame landrace collection based on phenotypic traits and est-ssr markers coupled with an hrm analysis. Plants 10(4). https://doi.org/10.3390/plants10040656
Sumathi P, Muralidharan V (2008) Study of gene action and heterosis in monostem/shybranching genotypes in sesame (Sesamum indicum L.). Indian J Genet Plant Breed 68(3):269
Sumathi P, Muralidharan V (2009) Genetic analysis of the hybrids, involving monostem/shy branching genotypes in sesame (Sesamum indicum L.). Electron J. Plant Breed. 1:65–69
Sun J, Rao Y, Yan T, Yan X, Zhou H (2010) Effects of drought stress on sesame growth and yield characteristics and comprehensive evaluation of drought tolerance. Chin J Oil Crop Sci 32(4):525–533
Surapaneni M, Yepuri V, Vemireddy LR, Ghanta A, Siddiq EA (2014) Development and characterization of microsatellite markers in Indian sesame (Sesamum indicumL.). Mol Breed 34(3):1185–1200. https://doi.org/10.1007/s11032-014-0109-0
Teboul N, Gadri Y, Berkovich Z, Reifen R, Peleg Z (2020) Genetic architecture underpinning yield components and seed mineral–nutrients in sesame. Gene 11(10):1221
Teklu DH, Kebede SA, Gebremichael DE (2014) Assessment of genetic variability, genetic advance, correlation and path analysis for morphological traits in sesame genotypes. Asian J Agric Res 8(4):181–194
Thiyagu K, Kandasamy G, Manivannan N, Muralidharan V, Manoranjitham SK (2007) Identification of resistant genotypes to root rot disease (Macrophominaphaseolina) of sesame (Sesamum indicum L.). Agric Sci Dig 27(1):34–37
Tiwari S, Kumar S, Gontia I (2011) Biotechnological approaches for sesame (Sesamum indicum L.) and niger (GuizotiaabyssinicaLf Cass.). Asia-Pac J Mol Biol Biotechnol 19:2–9
Tripathy SK, Mishra DR, Mohapatra PM, Pradhan KC, Mishra D, Mohanty SK, Dash S, Raj KR, Swain D, Mohanty MR, Panda S (2016a) Genetic analysis of seed yield in sesame (Sesamum indicum L.). Int J Agric Sci 6(9):1128–1132
Tripathy K, Mishra R, Mishra D, Mohanty K, Dash S, Swain D, Mohapatra M, Pradhan C, Panda S, Reshmi R, Mohanty R (2016b) Inheritance pattern of oil content in sesame (Sesamum indicum L.). Plant Gene Trait 7
Tripathy SK, Kar J, Sahu D (2019) Advances in sesame (Sesamum indicum L.) breeding. In: Advances in plant breeding strategies: industrial and food crops. Springer, Cham, pp 577–635
Ubor W, Gibson P, Anyanga W, Rubaihayo P (2015) Inheritance of resistance to sesame gall midge in Uganda. Afr Crop Sci J 23(4):355–363
Unal MK, Yalcin H (2008) Proximate composition of Turkish sesame seeds and characterization of their oils. Grasas Aceites 59(1):23–26
Uncu AO, Gultekin V, Allmer J, Frary A, Doganlar S (2015) Genomic simple sequence repeat markers reveal patterns of genetic relatedness and diversity in sesame. Plant Genome 8(2). https://doi.org/10.3835/plantgenome2014.11.0087
Uncu AO, Frary A, Karlovsky P, Doganlar S (2016) High-throughput single nucleotide polymorphism (SNP) identification and mapping in the sesame (Sesamum indicum L.) genome with genotyping by sequencing (GBS) analysis. Mol Breed 36(12):1–12. https://doi.org/10.1007/s11032-016-0604-6
Uzo JO (1998) Beniseed: a neglected oil wealth of Nigeria. In: Misari SM, Idowu AA, Busari LD (eds) Beniseed in Nigeria: opportunities for research, production and marketing, pp 1–17
Uzun B, Çagırgan MI (2006) Comparison of determinate and indeterminate lines of sesame for agronomic traits. Field Crop Res 96(1):13–18
Uzun B, OnurÖzbaş M, Çanci H, İlhanÇağirgan M (2004) Heterosis for agronomic traits in sesame hybrids of cultivars× closed capsule mutants. Acta Agric Scand B Soil Plant Sci 54(2):108–112
Uzun B, Arslan C, Furat S (2008) Variation in fatty acid composition, oil content and oil yield in a germplasm collection of sesame (Sesamum indicum L.). J Am Oil Chem Soc 85:1135–1142
Vale FXR, Parlevliet JE, Zambolim L (2001) Concepts in plant disease resistance. Fitopatol Bras 26(3):577–589
Varshney RK, Graner A, Sorrells ME (2005) Genomics-assisted breeding for crop improvement. Trends Plant Sci 10(12):621–630
Vavdiya PA, Dobariya KL, Babariya CA, Sapovadiya MV (2013) Heterosis for seed yield and its components in sesame (Sesamum indicum L.). Electron J Plant Breed 4(3):1246–1250
Velasco L, Fernández-Martínez JM (2002) Breeding oilseed crops for improved oil quality. J Crop Prod 5(1–2):309–344
Venkatachalam P, Geeta N, Rao KS, Jayabalan N, Saravanababu S (1999) BAP regulated direct shoot organogenesis from cultured seedling explants of Ground nut (Arachis hypogaea L.). Indian J. Exp Biol 37:807–812
Vos P, Hogers R, Bleeker M, Reijans M, Van De Lee T, Hornes M, Friters A, Pot J, Paleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23(21):4407–4414. https://doi.org/10.1093/nar/23.21.4407
Wadeyar BS, Lokesha R (2011) Studies on high frequency shoot regeneration in sesame (Sesamum indicum L.). Plant Tissue Cult Biotechnol 21(1):45–52
Wang LH, Zhang YX, Li DH, Zhang XY, Lv HX, Wei WL, Sun J, Zhang XR (2011) Waterlogging effect and evaluation method on waterlogging tolerance of germinated sesame [J]. Chin J Oil Crop Sci 6
Wang L, Zhang Y, Qi X, Li D, Wei W, Zhang X (2012) Global gene expression responses to waterlogging in roots of sesame (Sesamum indicum L.). Acta Physiol Plant 34(6):2241–2249
Wang L, Xia Q, Zhang Y, Zhu XX, Zhu XX, Li D, Ni X, Gao Y, Xiang H, Wei X et al (2016) Updatedsesame genome assembly and fine mapping of plant height and seed coat color QTLs using a new high-densitygenetic map. BMC Genomics 2016(17):1–13
Wang L, Zhang Y, Zhu X, Zhu X, Li D, Zhang X, Gao Y, Xiao G, Wei X (2017) Development of an SSR-based genetic map in sesame and identification of quantitative trait loci associated with charcoal rot resistance. Sci Rep 7(1):1–8
Wang Y, Zhang Y, Zhou R, Dossa K, Yu J, Li D, Liu A, Mmadi MA, Zhang X, You J (2018) Identification and characterization of the bZIP transcription factor family and its expression in response to abiotic stresses in sesame. PLoS One 13(7):e0200850
Wei W, Qi X, Wang L, Zhang Y, Hua W, Li D, Lv H, Zhang X (2011) Characterization of the sesame (Sesamum indicum L.) global transcriptome using Illumina paired-end sequencing and development of EST-SSR markers. BMC Genomics 12(1):1–13
Wei L, Miao H, Li C, Duan Y, Niu J, Zhang T, Zhao Q, Zhang H (2014a) Development of SNP and InDel markers via de novo transcriptome assembly in Sesamum indicum L. Mol Breed 34(4):2205–2217
Wei X, Wang L, Zhang Y, Qi X, Wang X, Ding X, Zhang J, Zhang X (2014b) Development of simple sequence repeat (SSR) markers of sesame (Sesamum indicum) from a genome survey. Molecules 19(4):5150–5162
Wei X, Liu K, Zhang Y, Feng Q, Wang L, Zhao Y, Li D, Zhao Q, Zhu XXX, Zhu XXX et al (2015) Genetic discovery for oil production and quality in sesame. Nat Commun 6:1–10
Wei X, Zhu X, Yu J, Wang L, Zhang Y, Li D, Zhou R, Zhang X (2016) Identification of sesame genomic variations from genome comparison of landrace and variety. Front Plant Sci 7:1–12
Wei X, Gong H, Yu J, Liu P, Wang L, Zhang Y, Zhang X (2017a) SesameFG: an integrated database for the functional genomics of sesame. Sci Rep 7(1):1–10
Wei L, Miao H, Xu F, Kong J, Zhang H (2017b) Chinese sesame cultivars, DNA fingerprinting, and two-dimensional barcodes using single nucleotide polymorphisms, insertions or deletions, and simple sequence repeat markers. Crop Sci 57(4):1941–1947. https://doi.org/10.2135/cropsci2016.10.0888
Weiss EA (1971) Castor, sesame and safflower. Leonard Hill, London, pp 311–525
Weiss EA (1983) Oilseed crops. Longman Inc, New York, p 660
Welsh J, Mcclelland M (1990) Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res 18(24):7213–7218. https://doi.org/10.1093/nar/18.24.7213
Were BA, Onkware AO, Gudu S, Welander M, Carlsson AS (2006a) Seed oil content and fatty acid composition in East African sesame (Sesamum indicum L.) accessions evaluated over 3 years. Field Crop Res 97(2-3):254–260
Were BA, Gudu S, Onkware AO, Carlsson AS, Welander M (2006b) In vitro regeneration of sesame (Sesamum indicum L.) from seedling cotyledon and hypocotyl explants. Plant Cell Tissue Organ Cult 85:235–239
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18(22):6531–6535. https://doi.org/10.1093/nar/18.22.6531
Wu WH (2007) The contents of lignans in commercial sesame oils of Taiwan and their changes during heating. Food Chem 104:341–344
Wu K, Liu H, Yang M, Tao Y, Ma H, Wu W, Zuo Y, Zhao Y (2014) High-density genetic map construction andQTLs analysis of grain yield-related traits in sesame (Sesamum indicum L.) based on RAD-Seq techonology. BMC Plant Biol 14:1–14
Xu ZQ, Jia JF, Hu ZD (1997) Somatic embryogenesis in Sesamum indicum L. cv. Nigrum. J. Plant Physiol 150:755–758
Yadav M, Sainger DCM, Jaiwal PK (2010) Agrobacterium tumefaciens- mediated genetic transformation of sesame (Sesamum indicum L.). Plant Cell Tissue Organ Cult 103(3):377–386. https://doi.org/10.1007/s11240-010-9791-8
Yadava DK, Vasudev S, Singh N, Mohapatra T, Prabhu KV (2012) Breeding major oil crops: present status and future research needs. Technol Innov Major World Oil Crop 1:17–51
Yermanos DM (1980) Sesame. In: Fehr WR, Hadley HH (eds) Hybridization of crop plants. ASA, Madison, pp 549–563
Yol E (2017) Inheritance of long and dense capsule characteristics in sesame. Turk J Field Crop 22(1):8–13
Yol E, Uzun B (2011) Inheritance of number of capsules per leaf axil and hairiness on stem, leaf and capsule of sesame (Sesamum indicum L.). Aust J Crop Sci 5(1):78–81
You J, Wang Y, Zhang Y, Dossa K, Li D, Zhou R, Wang L, Zhang X (2018) Genome-wide identification and expression analyses of genes involved in raffinose accumulation in sesame. Sci Rep 8(1):4331
You J, Zhang Y, Liu A, Li D, Wang X, Dossa K, Zhou R, Yu J, Zhang Y, Wang L, Zhang X (2019) Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol 19(1):1–16
Younghee K (2001) Effects of BA, NAA, 2, 4-D and AgNO3 treatments on callus induction and shoot regeneration from hypocotyl and cotyledon of sesame (Sesamum indicum L.). J Korean Soc Hortic Sci 42:70–74
Zaker M, Sadeghi Garmaroodi H, Rezaii S (2020) Resistance evaluation of sesame cultivars and lines to charcoal rot disease in greenhouse condition. Iran J Plant Prot Sci 50(2):213–220
Zeevaart JAD, Creelman RAM (1988) Etabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol 39:439–473
Zeven A, Zhukovsky P (1975) Dictionary of cultivated plants and their centres of diversity. PUDOC, Wageningen
Zhang H, Miao H, Li C, Wei L, Duan Y, Ma Q, Kong J, Xu F, Chang S (2016) Ultra-dense SNP genetic map construction and identification of SiDt gene controlling the determinate growth habit in Sesamum indicum L. Sci. rep. 6:1–13
Zhang H, Wei L, Miao H, Zhang T, Wang C (2012) Development and validation of genic-SSR markers in sesame by RNA-seq. BMC Genomics 13(1). https://doi.org/10.1186/1471-2164-13-316
Zhang Y, Wang L, Xin H, Li D, Ma C, Ding X, Hong W, Zhang X (2013a) Construction of a high-density genetic map for sesame based on large scale marker development by specific length amplified fragment (SLAF) sequencing. BMC Plant Biol 13(1):1–12
Zhang H, Li C, Miao H, Xiong S (2013b) Insights from the complete chloroplast genome into the evolution of Sesamum indicum L. PLoS One 8(11):e80508
Zhang Y, Li D, Zhou R, Wang X, Dossa K, Wang L, Zhang Y, Yu J, Gong H, Zhang X, You J (2019) Transcriptome and metabolome analyses of two contrasting sesame genotypes reveal the crucial biological pathways involved in rapid adaptive response to salt stress. BMC Plant Biol 19(1):1–14
Zhao Y, Yang M, Wu K, Liu H, Wu J, Liu K (2013) Characterization and genetic mapping of a novel recessivegenic male sterile gene in sesame (Sesamum indicum L.). Mol Breed 32:901–908
Zhou R, Dossa K, Li D, Yu J, You J, Wei X, Zhang X (2018) Genome-wide association studies of 39 seed yield-related traits in sesame (Sesamum indicum L.). Int. J. Mole. Sci. 19:2794
Zietkiewicz E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 20(2):176–183. https://doi.org/10.1006/geno.1994.1151
Zimik M, Arumugam N (2017) Induction of shoot regeneration in cotyledon explants of the oilseed crop Sesamum indicum L. J Genet Eng Biotechnol 15(2):303–308
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Singh, M., Chahar, S., Avtar, R., Singh, A., Kumar, N. (2022). Advances in Classical and Molecular Breeding in Sesame (Sesamum indicum L.). In: Gosal, S.S., Wani, S.H. (eds) Accelerated Plant Breeding, Volume 4. Springer, Cham. https://doi.org/10.1007/978-3-030-81107-5_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-81107-5_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-81106-8
Online ISBN: 978-3-030-81107-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)