Abstract
Key message
SiDWF1 encodes a gibberellin receptor GID1B-like protein controlling the internode length and plant height in sesame.
Abstract
Sesame is a high-height crop. Here we systematically analyzed the morphological and genetic characters of the sesame dwarf mutant dw607 (dwf1 type). The plant height and the internode length of dw607 significantly declined, while the thousand seed weight (TSW) significantly increased (P < 0.01). The cell size of stem parenchyma and pith tissue reduced, and vascular bundle cells and parenchyma tissue arranged much tighter in the dwarf mutant. Based on the cross-population association mapping of a RIL population of the cross ‘dw607 (dwf1) × 15N41 (wt type),’ the target interval linked to the short internode length was located on C9.scaffold2 of SiChr.4 in sesame. We further screened the 58 variants using the genomic variant data of 824 germplasm and BSA DNA pools and determined the target gene Sidwf1. The SNP mutation of C1057 to T1057 resulted in the amino acid change of P150 (proline) to S150 (serine) in SiDWF1. SiDWF1 gene allele is 1,638 bp and encodes a gibberellin receptor GID1B-like protein. Transcription profile assay reflected that Sidwf1 is highly expressed in leaf, stem, bud, and capsule tissues. The dynamic variation in endogenous GA3 content in dw607 and the wild type was also monitored in this study. The results revealed the molecular genetic mechanism of the internode length and plant height trait in sesame for the first time. The findings supply the theoretical and material basis for developing the marker-assisted selection (MAS) breeding in sesame.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sesame (Sesamum indicum L., 2n = 26) is an important and specific oilseed crop with high nutrition and oil quality (Zhang et al. 2019). Sesame seeds contain abundant nutritional substances including oil (50–55%), proteins (18–20%), carbohydrate (13–25%), digestible fiber (high to 9.8%), and antioxidants (high to 1.1%) (Jimoh and Aroyehun 2011; Zhang et al. 2012, 2019; Makinde and Akinoso 2013; Prakash and Naik 2014; Sene et al. 2017). At present, sesame is cultivated mainly in the developing countries for its labor-consuming production process. Sesame is a high-height crop with long stem or branches. Plant height is positively correlated with seed yield. However, sesame varieties with higher height and longer capsule stems are more susceptible to lodging damage by strong wind or hurricanes and bring about more difficulties for mechanized harvest (Langham et al. 2002; Langham 2008; Zhang et al. 2019). Thus, reducing plant height as well as increasing or stabilizing the final seed yield is one of the crucial breeding objectives in sesame.
Plant height is one of the most important agronomic traits for crops (Li et al. 2018a). Dwarfing and semidwarfing mutations can affect the plant height and contribute to high lodging resistance and yield, as the maturity and harvest index of dwarf and semidwarf mutants are always stimulated (Ashikari et al. 1999). As dwarf wheat lines have been applied since the 1960s, the world wheat production significantly increases. Till now, hundreds of dwarf and semidwarf mutants with short internode length have been found for many crops. Dozens of genes regulating the dwarf and semidwarf phenotypes have been cloned from wheat, maize, rice, soybean, and other plants (Peng et al. 1999; Sasaki et al. 2002; Feng et al. 2015; Thomas 2017; Wang et al. 2017b; Li et al. 2018a, b). Moreover, a great deal of studies revealed that the dwarf or semidwarf phenotype in most mutants was associated with the content variation in endogenous hormones, such as gibberellins (GAs), indole-3-acetic acid (IAA), auxin, brassinosteroid (BR), and Jasmonate (JA) (Rebetzke et al. 2011; Feng et al. 2015; Kurotani et al. 2015; Avila et al. 2016; Hirano et al. 2017; Wang et al. 2017b). In wheat, the reduced height 1 (Rht-1) dwarfing mutation was regulated by either of Rht-B1b and Rht-D1b (also named ‘green evolution genes’) (Peng et al. 1999; Thomas 2017). The results proved that Rht-B1b and Rht-D1b encode the short N-terminal GID1-GA binding peptide fragments which are required for binding the GID1-GA receptor complex and promoting GA-responsive growth. Nucleotide substitution and premature stop codon formation of Rht-B1b and Rht-D1b resulted in the truncated products and the dwarfism in wheat. Similarly, a gene sd-1 identified from a semidwarf rice mutant Dee-Geo-Woo-Gen-type sd-1 was proved to encode a gibberellin 20 oxidase and took part in the gibberellin biosynthesis pathway (Monna et al. 2002). A 383-bp deletion from exon 1 to exon 2 resulted in a frameshift and the formation of a termination codon and finally affected the gene expression (Monna et al. 2002).
For sesame, previous studies indicate that plant height trait is a quantitative trait (Sharmila et al. 2007; Satish 2013). Dozens of QTLs and SNP markers linked with plant height were identified in sesame (Wei et al. 2015; Wang et al. 2016). However, to our knowledge, no genes controlling the plant height trait have been cloned in sesame till now. Recently, a dwarf mutant dw607 (dwf1 type) has been created using ethyl methanesulfonate (EMS) mutagenesis by Chinese sesame scientists (Wang et al. 2017a). Compared with the wild type (Yuzhi 11), the plant height of dw607 declined by more than 40%, and the internode length reduced about 50%. More importantly, the yield of dwarf varieties derived from dw607 significantly increased under rich fertilizers and water culture conditions (data not shown). Thus, creation of dwarf mutant dw607 supplies a valuable material for exploring the genetic mechanism of plant height trait and dwarf variety breeding in sesame.
Here, the objectives of this study are: (1) to systematically investigate the morphological characteristics of the sesame dwarf mutant dw607; (2) to identify the first sesame dwarf gene Sidwf1 using a RIL population derived from mutant dw607 and wild type with normal internode length and plant height traits using the high efficient mapping association and genome-wide variants screening method; and (3) to exploit the mutagenesis character and expression profiles of SiDWF1 gene alleles in sesame. The findings supply the theoretical basis for developing the molecular marker-assisted selection (MAS) breeding in sesame.
Materials and methods
Plant materials and populations
A dwarf mutant dw607 with the short internode length was induced from a subline (90-1) of Yuzhi 11 using EMS mutagenesis (Zhang et al. 2019). The mutant dw607 and the wild type (var. Yuzhi 11) as control were cultured at the Yuanyang Experimental Station (113°97′E and 35°05′N) for phenotype trait investigation and histological and physiological analysis during 2013–2016. Two cross-combinations of dw607 (dwf1, P1) × Yuzhi 11 (wt, P2) and dw607 (dwf1, P1) × 15N41 (wt, P2) and the six generation populations (i.e., two parents, F1, BC1, BC2, and F2) of each combination were cultured for target trait segregation analysis in 2012 and 2013 (Table 2).
The 113 F6 individuals of the RIL population derived from dw607 and 15N41 and the two parents were cultured at Sanya Experimental Station (109°50′E and 18°25′N) for genome re-sequencing and target gene location in 2013. Other 100 individuals with dwf1 and wt genotype, respectively, were chosen from the F2-3 population of dw607 (dwf1, P1) × Yuzhi 11 (wt, P2) and applied to construct two DNA BSA (bulked segregant analysis) pools for Illumina sequencing. A total of 600 F2-3 lines of the combination population of dw607 (dwf1, P1) × Yuzhi 11 (wt, P2) were collected at Yuanyang Experimental Station for candidate SNP validation in 2014. Meanwhile, 500 germplasm accessions (partial list in Supplementary Fig. 2) with normal internode length trait were randomly chosen from the sesame germplasm reservoir of Henan Sesame Research Center, Henan Academy of Agricultural Sciences (HRSC, HAAS) (Zhengzhou, China), and cultured at Sanya Experimental Station for phenotype observation and leaf collection. All the above germplasm and populations were available from HSRC, HAAS.
Young leaf tissues of all the above materials were harvested, immersed in liquid nitrogen, and frozen at − 80 °C for genomic DNA extraction. For internode length trait assay, the average internode length of mature plantlet was calculated according to the capsule stem length and the internode number.
Histological analysis of mutant dw607 and the wild type
In order to explore the stem tissue structure of dwf1 and wt type, the 6- and 16-day-old seedlings of dw607 and Yuzhi 11, respectively, were cultured in growth chamber. The fresh stem segments of each sample were collected and immediately fixed in formalin–glacial acetic acid–alcohol (FAA) solution containing 3.8% formalin, glacial acetic acid, and 70% alcohol (V:V:V = 1:1:18) for 24 h. During fixing, the air was extracted through a pump.
The paraffin sections were prepared according to the method of Zhang et al. (2018). The samples were dehydrated with a series of ethanol and stained with 1% safranin. After embedded in paraffin, all the cross sections (10 μm) were cut and stained with 0.1% fast green. The specimens were observed, and the images were photographed under the Leica DM6000 microscope (Leica, Germany) equipped a cooled CCD500 camera (SPOT, Diagnostic Instruments, USA). Images were optimized in Adobe Photoshop 7.0 (Adobe, USA).
Evaluation of endogenous GA3 amount in dw607 and the wild type
ELISA assay was applied to evaluate the contents of gibberellic acid (GA3), indole-3-acetic acid (IAA), abscisic acid (ABA), and brassinosteroid (BR) in sesame samples. Samples of root tip, leaf, and shoot tip of dw607 and Yuzhi 11 were collected in every 5 days after 18 DAS (days after sowing) and immersed in liquid nitrogen. ELISA test kits for plant hormone essay were developed and supplied by Crop Chemistry Control Research Center, China Agricultural University. Endogenous GA3, IAA, ABA, and BR in samples were individually extracted according to the procedures of the ELISA test kit manufacture instruction. Three biological replicates were set for each treatment. Each sample was measured with triple replicates.
Genomic DNA extraction, library construction, and Illumina sequencing
Genomic DNA of each sample was extracted using DNeasy Plant Mini Kits (QIAGEN, Hilden, Germany) and fragmented by sonication. Paired-end (PE) libraries were constructed according to Illumina sequencing preparation guidelines. Illumina HiSeq2500 platform (Illumina, San Diego, USA) was applied for genome re-sequencing according to the procedures of Zhang et al. (2016).
Sequencing data analysis and candidate SNP detection
All obtained raw reads of the genome re-sequencing population were filtered using Trimmomatic 0.33 (Bolger et al. 2014). Data alignment to the reference sesame genome was carried out using BWA 0.7.15 (Li and Durbin 2009). Putative SNPs and InDels of 115 individuals were screened using Genome Analysis Tool Kit (GATK3.7) packages (Poplin et al. 2017). All the variants from all the 115 sequencing samples were filtered by the criteria of minimal variant count ≥ 100 and minimum frequency of 0.1. The fine sesame genome (var. Yuzhi 11) (version 2.0) was applied as the reference genome in this study (Zhang et al. 2016). The chromosome position of target gene was determined according to the integration of genome assembly data (version 2.0) and the chromosome annotation information (Zhang et al. 2016; Zhao et al. 2018).
Association analysis and candidate gene location
TASSELE 5.0 and GLM (general linear model) model were used to perform association analysis of all the variants with the phenotype data of the internode length trait for the RIL population. Target gene locus was determined according to the specific interval with the lowest P value. Home-made scripts were applied to screen candidate variants according to the procedures of Zhang et al. (2018). Two groups of sesame variant database, i.e., genomic variant data of 824 sesame accessions (wt) (target region data shown in Supplementary Table 3) and SNP/InDel database of dwf1 and wt population BSA pools (Supplementary Table 5), were applied to screen candidate variants.
PCR-based SNP markers were designed according to the candidate SNP locus using Primer Premier 5.0 program (https://www.premierbiosoft.com/prierdesign/index.html). PCR was carried out on a PTC-225 machine (MJ Research, Waltham, MA) according to the description of Wei et al. (2014). All the PCR products were electrophoresed in 8% non-denaturing polyacrylamide gels for SNP validation (Liang et al. 2008).
Cloning and annotation of Sidwf1 gene
To obtain the full gDNA and cDNA sequences of Sidwf1 alleles in sesame, the primer pairs were designed using Primer Premier 5.0 (Supplementary Table 7). DNA amplification was performed on a PTC-225 machine (MJ Research, USA) (Zhang et al. 2018). Sanger sequencing with three replications was applied. BLASTP and BLAST2GO were carried out to explore the non-redundant (NR) protein and KEGG (Kyoto Encyclopedia of Genes and Genomes) annotations (https://www.genome.jp/kegg/pathway.html) of the target gene.
RNA extraction and expression profile assay of Sidwf1 gene
Total RNA was extracted from tissues using TriZOL Reagent (Ambion, Life Technologies, USA). The primer pairs of Sidwf1 gene alleles for quantitative real-time PCR (qRT-PCR) analyses were designed using Primer Premier 5.0 program (https://www.premierbiosoft.com/prierdesign/index.html). Real-time PCR was performed on a Mastercyclerrealplex (Eppendorf, Germany) according to the standard method of Zhang et al. (2018). Endogenous reference gene Siβ-tubulin was applied for qPCR assay (Wei et al. 2013). Transcript amount of SiDWF1 gene alleles was normalized against the β-tubulin gene and compared using △△Ct method according to the method of Wei et al. (2013).
Sidwf1 homolog detection and phylogenetic analysis
Sidwf1 homolog(s) was screened from sesame reference genome (var. Yuzhi 11) using BLASTP. Alignment of amino acid sequences of SiDWF1 and Sidwf1 with the homologs of other crops was carried out using DNAMAN (https://www.lynnon.com/pc/framepc.html). All the 20 GID1B homologs of Erythranthe guttata, Ricinus communis, Vitis vinifera, Nicotiana tabacum, Solanum lycopersicum, Arabidopsis thaliana, Glycine max, Prunus persica, Gossypium hirsutum, Brassica napus, Triticum aestivum, Zea mays, and Oryza sativa were downloaded from NCBI dataset. MEGA 5.2 was applied to perform sequence comparison and phylogenetic tree construction of SiDWF1 and homologs [49] (Tamura et al. 2011).
Results
Phenotypic comparison of the dwarf mutant dw607 and the wild type
In order to explore the dwarf mutagenesis mechanism in sesame, we firstly investigated and compared the 9 key agronomic traits (i.e., plant height, first capsule position, tip length, capsule node number, average internode length, capsule length, capsule width, thousand seed weight, and oil content) of the dwarf mutant dw607 and Yuzhi 11 (wild type) under standard cultivation conditions in 2012–2014 (Table 1; Fig. 1). For dw607, the phenotypic characters of root, leaf, stem, capsule, seed, and other organs differed from the wild type (Fig. 1). The stem of dw607 during germination (Fig. 1c) to flowering stages (Fig. 1b) grew shorter than that of the wild type. Compared with Yuzhi 11 (wt) during mature stage, the plant height of dw607 (dwf1) significantly declined from 176.00 to 118.25 cm, and the internode length reduced from 6.82 to 4.31 cm (P < 0.01). Meanwhile, the thousand seed weight (TSW) also significantly increased (P < 0.01). Correlation analysis indicted that both internode length and thousand seed weight were significantly affected by plant height trait (Table 1). Correspondingly, we evaluated the difference of plant height between dw607 and Yuzhi 11 during the cycle life (Supplementary Fig. 1) (Supplementary Table 1). Results showed that the plant height of dw607 plantlets increased slowly at seedling stage (1–28 days after sowing, DAS). In early flowering and flowering stages (34–63 DAS), the plant height gap between dw607 and Yuzhi 11 was getting more evident (Supplementary Fig. 1). As to the growth rhythm, the initiation of flowering period in dw607 delayed about 5 days, and the whole life period prolonged 7 days (Supplementary Table 1).
Phenotypic comparison of dwarf mutant dw607 with short internode and the wild type Yuzhi 11. a Plant morphology of dw607 at late flowering stage; b plant height and capsule stem length comparison of Yuzhi 11 (left) and dw607 (right) at mature stage; c seedling comparison of dw607 (left) and Yuzhi 11 (right) for 3 days after germination; d internode length comparison of Yuzhi 11 (left) and dw607 (right); e seed size comparison of the wild type (upper two lows) and dw607 (lower two rows)
Subsequently, we performed the cytological character analysis of dw607 and Yuzhi 11 (Fig. 2). Transverse and vertical sections of stems of 6-day-old and 16-day-old seedlings were observed and compared. For 6-day-old dwarf 607 and Yuzhi 11 seedlings, the cell length and width of vertical hypocotyl section were about 80.9 μm and 40.0 μm and 148.1 μm (*) (P < 0.05) and 38.84 μm, respectively. Interestingly, for 16-day-old dw607 and Yuzhi 11 seedlings, the cell length and width of vertical parenchyma tissue section were about 33.4 μm and 37.7 μm and 102.4 μm (*) and 67.4 μm (*), respectively. The difference in cell length and width of parenchyma tissue (Pc) and pith (Pi) between dwarf mutant and the wild type was more evident (Fig. 2a–h). Thus, the vascular bundle cells (VC) and Pc tissue of dw607 arranged much tighter, and the xylem tissue became thicker than the wild type (Fig. 2g, h).
Cross section of stem organ of dw607 and Yuzhi 11. a and b Transverse stem section of 6-day-old seedlings of dw607 and Yuzhi 11, respectively; c and d transverse stem section of 16-day-old seedlings of dw607 and Yuzhi 11, respectively; e and f vertical stem section of 6-day-old seedlings of dw607 and Yuzhi 11, respectively; g and h vertical stem section of 16-day-old seedlings of dw607 and Yuzhi 11, respectively. Ep epidermis; Pc parenchyma tissue; Pi pith; phloem; VC vascular bundle cell. Red bar = 100 μm (color figure online)
Inheritance analysis of dwarf mutation with short internode length trait
Sesame is a crop with indefinite inflorescence. Plant height, as well as the stem length trait, is affected by the development of inflorescence meristem and flowering period in sesame. For dw607, ‘plant height’ and ‘internode length’ traits are significantly associated with the dwarf phenotype (Table 1). Then, we chose the internode length trait to explore the inheritance of dwarfing mutation. We firstly performed the cross-combination of ‘dw607 (dwf1) and 15N41 (wt type)’ and ‘dw607 (dwf1) and Yuzhi 11 (wt type)’ for target trait inheritance analysis (Table 2). For the F1 and F2 population derived from the cross ‘dw607 (dwf1) × Yuzhi 11 (wt type),’ the average internode length of dw607 (P1) and Yuzhi 11 (P2) was 4.0 cm and 6.1 cm, respectively (Fig. 3). The average internode length of F1 individuals was high (6.0 cm) and similar to that of normal genotype. Meanwhile, the internode length of F2 individuals continuously varied from 2.5 to 10.0 cm with the peak number of 6.5–7.0 cm. In order to accurately determine the phenotype segregation ratio of each F2 population, the individuals with internode length of ≤ 5.0 cm (close to the value of P1 (dwf1)) were referred to ‘dwf1 type,’ while the others with the internode length of more than 5.0 cm (close to the value of P2 (wt)) were ‘wild type.’
In the two combinations between dwf1 and normal genotype, the backcrosses (BC2) developed from F1 and wt type displayed the normal internode length (Table 2). The segregation ratio of dwf1 backcrosses (BC1) and F2 populations between the short internode length (dwf1) and the normal phenotype (wt) fitted the expected ratios of 1 (dwf1): 1 (wt) ratio and 3 (wt): 1 (dwf1), respectively. Further, Chi-squared tests (P > 0.05) confirmed that the segregation of the short internode length trait in sesame fitted the Mendelian inheritance mode. The dwarf mutation with short internode length trait is controlled by a recessive gene allele in sesame. Here the dwarf mutation locus in mutant dw607 and the normal allele in Yuzhi 11 were annotated as Sidwf1 and SiDWF1, respectively.
Location of Sidwf1 gene using association mapping and genome variants screening
To locate the target gene locus related to the short internode length and dwarf trait, we firstly performed the cross-population association mapping using a RIL population of the cross ‘dw607 (dwf1) × 15N41 (wt type)’ with 831 F6 individuals. The two parents (dw607 and 15N41) and the 113 F6 individuals of the RIL population were re-sequenced using Illumina sequencing platform (Table 3). In total, 748.13 Gb of raw data of the 115 samples was obtained. The average genome coverage was 18.3-fold per sample. All mapped reads were aligned to the sesame reference genome (var. Yuzhi 11, PRJNA315784) for SNP calling. A total of 780,199 unique SNPs were found in the two parents.
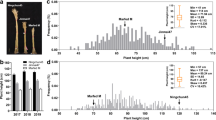
Subsequently, all the 780,199 unique SNPs were applied for variants screening in the 113 individuals of the RIL population. After filtered, 496,486 SNP/InDel variants were detected for Joint calling. According to the reference sesame genome (Yuzhi 11) and the nomination of sesame chromosome set (Zhang et al. 2018; Zhao et al. 2018), a total of 423,138 SNP/InDel variants were plotted on the 13 chromosomes (Fig. 4). Association mapping results showed that the variant site (C9_7086504) with the lowest of P value (2.59E−15) located on Contig C9.scaffold2 of SiChr.4. To screen the target variants from the target contig, we chose the up- and downstream 200-Kb flanking sequences of C9_7086504 as the target interval linked to the short internode length trait (dwf1).
Cross-population association mapping of Sidwf1 gene locus in sesame. Manhattan plot of SNP/InDel association mapping of the short internode trait is performed using a RIL population. The peak of − log 10 (P) is located on SiChr. 4. After screened using the genome variants data, 12 variants (green dots) are retained as the candidate markers associated with the short internode length trait in sesame. Blue line indicates the P value of E−8 (color figure online)
The interval C9 between C9_6861138 and C9_7285379 markers contained 58 variants with the P value variation of 9.08E−05 to 2.59E−15 (Supplementary Table 2). Then, we filtered these detected 58 SNP/InDel variants using the regional genome variants data of 824 sesame accessions (wild type). For the 824 accessions, 8,985 SNPs/InDels existed in the region of C9_6861138 and C9_7285379 (Supplementary Table 3). Genomic variants screening results showed that 12 variant loci (green dots in Fig. 4) were retained in the target interval for the population (Supplementary Table 4). Furthermore, we screened the above plotted variant sites using the genome data of two BSA pools of dwarf type (dwf1) and wild type (wt) of the F2−3 population (uploaded to NCBI dataset) (Supplementary Table 5). As a result, 5 SNP/InDel sites, i.e., C9_6958525, C9_6986819, C9_6989486, C9_7080799, and C9_7225874, were retained in the target interval (Supplementary Table 6).
Interestingly, differing from the other 4 variants distributed in intergenic regions, a SNP C9_6989486 is located in gene coding region (C9.scaffold2.572) and caused the missense mutation from C to T in C9.scaffold2.572. To confirm this SNP site in dwf1 genotype, we designed the primer pair of SiSNPdwf1 (Supplementary Table 7) and screened the test population including 600 F2-3 individuals of test population and 500 sesame germplasm accessions with normal internode length (Supplementary Fig. 2). PCR screening results proved that the SiSNPdwf1 alleles in gene C9.scaffold2.572 entirely accorded with the phenotype segregation in the test population. Then, C9.scaffold2.572 gene was regarded as the target gene controlling the short internode length and dwarf trait in dw607 and was named Sidwf1.
Structure analysis of SiDWF1 gene and homolog in sesame
With the aid of the reference genome information of var. Yuzhi 11, we designed the primer pairs and amplified the entire cDNA and DNA sequences of SiDWF1 (C9.scaffold2.572) allele (Supplementary Table 8). Sanger sequencing and gene alignment results proved that the full sequence length of SiDWF1 gene (NCBI Accession No. KY649623) in Yuzhi 11 was 1,638 bp and comprised of 2 exons (Fig. 5a). Correspondingly, SiDWF1 encodes 343 amino acids. For dw607, the SNP mutation of C/T occurred in Exon 2 of Sidwf1 gene (Fig. 5a). The nucleotide C at 1,057-bp position was mutated into T, and the amino acid P150 (proline) in the conserved sequence changed into S150 (serine) (Fig. 6). Non-redundant (NR) protein annotation results revealed that SiDWF1 gene was identified to encode a gibberellin receptor GID1B-like protein (Supplementary Table 5).
Protein sequence comparison of SiDWF1 and homologs in sesame and other plant species. Eight ortholog proteins homologous to SiDWF1 (ARD08849.1) and SiDWF2 (XP_011079771.1) are AtGID1a (NP 187163.1), AtGID1b (OAP02516.1), and AtGID1c (NP 198084.1) in Arabidopsis thaliana, PpeGID1c (ALS35481.1) in Prunus persica, TaGID1-A1 (CBW30246.1), TaGID1-B1 (CBW30247.1), and TaGID1-D1 (CBW30245.1) in Triticum aestivum, OsGID1 (Q6L545.1) in Oryza sativa. Identical residues are shaded in black; conserved residues are shaded in gray; and residues with low identity are shaded in light gray. The black dot indicates amino acid gap. Asterisk indicates the mutation site of SiDWF1 to Sidwf1 protein in highly conserved region. Conserved bases in red frames are HGG and GXSXG motifs, which exist in hormone-sensitive lipase (HSL) family,
BlastP analysis indicated that there was a homolog gene, i.e., evm.model.C4.1012 (the same annotated as a gibberellin receptor GID1B-like protein) with SiDWF1 in sesame (Fig. 5b). The homolog evm.model.C4.1012 was 1,574 bp with the gene resemblance of 86.6% to SiDWF1. Herein, this homologous gene evm.model.C4.1012 was named SiDWF2. Amino acid sequence comparison indicated that 33 amino acids of SiDWF1 differed from those of SiDWF2 (Fig. 5b). Moreover, we performed the interspecies comparative analyses of 22 SiDWF1 homologs in 14 crops (partial results in Fig. 6). The results showed that SiDWF1 had the high resemblance to the GID1B homologs from the other 13 species, as the resemblance rate varied from 60.2% (TaGID1-D1 and ZmGID1) to 88.0% (EgGID1b) (Fig. 7). Compared with the 20 GID1B homologs in 13 plant species with normal internode length phenotype, Sidwf1 protein carried the specific amino acid mutation of Pro/Ser in the conserved sequence. Of the 22 homologs, EgGID1b (XP 012832770.1) in Erythranthe guttata displayed the closest relationship with SiDWF1.
Phylogeny analyses of SiGID1B protein in sesame and other plants. Data above the branches indicate the support with 1000 bootstrap replications. The cluster includes SiDWF1 and SiDWF2 in Sesamum indicum and 20 homologs in 13 plants, i.e., EgGID1b (XP 012832770.1) in Erythranthe guttata, RcGID1b (XP 002524767.1) in Ricinus communis, VvGID1b (XP 002271700.1) in Vitis vinifera, NtGID1b-like (XP 009604447.1) in Nicotiana tabacum, SlGID1b-like (NP 001234767.2) in Solanum lycopersicum, AtGID1a (NP 187163.1), AtGID1b (OAP02516.1), and AtGID1c (NP 198084.1) in Arabidopsis thaliana, GmSlGID1b-like (XP 003518940.1) in Glycine max, PpeGID1b (XP 007200347.1) and PpeGID1c (ALS35481.1) in Prunus persica, GhGID1b (ABQ96123.1) in Gossypium hirsutum, BnGID1a (XP 013750692.1), BnGID1b (NP 001302770.1), and BnGID1c (XP 013643894.1) in Brassica napus, TaGID1-A1 (CBW30246.1), TaGID1-B1 (CBW30247.1), and TaGID1-D1 (CBW30245.1) in Triticum aestivum, ZmGID1 (NP 001309908.1) in Zea mays, and OsGID1 (Q6L545.1) in Oryza sativa
Expression profiles of SiDWF1 alleles and dynamic GA3 variation in sesame
To reveal the expression profiles of SiDWF1 alleles in dwf1 and wild type, we monitored the transcription level of SiDWF1 and Sidwf1 in root, leaf, stem, bud, and capsule tissues of Yuzhi 11 and Dw607, respectively, using real-time quantitative PCR (RT-qPCR) (Fig. 8). The results displayed that SiDWF1 and Sidwf1 genes highly expressed in most tissues (i.e., root, stem, leaf, shoot, bud, and 0-d capsule) except for 5-d-old capsule pericarp and seed. The highest relative expression level (1/2△△CT) of dw607 and Yuzhi 11 occurred in bud tissue with the peak value of 2.6 and 2.1, respectively. Compared with SiDWF1, Sidwf1 expressed significantly higher in leaves and buds of mutant dw607.
Expression profiles of SiDWF1 gene alleles in Yuzhi 11 and dw607, respectively. The tissues of root, stem, leaf, bud, 0-d capsule (0-d c), 5-d capsule pericarp (5-d cp), and 5-d seed (5-d s) are collected from plantlets of Yuzhi 11 and Dw607 at flowering stage. Gene expression of each tissue is measured with triple biological replications
As GID1B proteins take part in the GA biosynthesis pathway and promote the GA-responsive growth, we also monitored the content variation in endogenous hormones of GA3, IAA, ABA (abscisic acid), and BR in Yuzhi 11 and mutant dw607, respectively (Supplementary Fig. 3). The results displayed that the amount of endogenous GA3 per gram tissue varied from 4–22 μg in dw607 during the life cycle. From seedling stage to late flowering stage, GA3 content in shoot, root, and leaf tissues fluctuated. Meanwhile, a highlighted tendency presented in leaf organ (Supplementary Fig. 3a-c). Compared with the wild type, the level of endogenous GA3 in dw607 leaf varied with time lag. In shoot and root tip, the GA3 accumulation variation between dw607 and Yuzhi 11 presented with the same tendency.
In addition, the accumulations of IAA, ABA, and BR in dw607 and Yuzhi 11, respectively, were compared. For IAA, the variation tendency in dw607 and Yuzhi 11 was similar. The peak amount of IAA always occurred in shoot tip and leaf at flowering stage (Supplementary Fig. 3d-f). The IAA content in root tip maintained lower than those of leaf and shoot tip. Meanwhile, comparison results indicated that the ABA level and variation tendency in dw607 were similar to those of Yuzhi 11, even though the ABA content in some samples of Yuzhi 11 changed violently (Supplementary Fig. 3g-i). During flowering stage, the peak amount of ABA in shoot tips of Yuzhi 11 and dw607 was 140.3 μg g−1 and 149.8 μg g−1, respectively. As for BR content, the evaluation results showed that the peak content of BR in shoot, root, and leaf tissues of dw607 delayed, while the variation tendency between dw607 and Yuzhi 11 was similar (Supplementary Fig. 3j-l).
Discussion
Plant height affects plant architecture, lodging resistance, yield, and even harvest style for crops. For sesame, to reduce the plant height and concurrently increase the seed yield and the adaption for harvest mechanization is one of the key breeding objectives. In 2009, the dwarf sesame mutant dw607 with short internode length and high seed weight traits was created by EMS mutagenesis (Wang et al. 2017a). For the dwarf mutant, the plant height and internode length decline about 50%. The yield per plantlet and thousand seed weight increase, even though the capsule node number and capsule number per plant were not affected. Notably, the first dwarf sesame variety Yuzhi Dw607 (China variety authorization no. CNA013391E) was bred from the mutant dw607 and applied for sesame production in China since 2015. The highest yield level of var. Yuzhi Dw607 touched more than 3300 kg per ha in Xinjiang production region of China in 2017 (data not shown) and displays the high yield potential. Similar to the first determinate variety Yuzhi DS899, the dwarf variety Yuzhi Dw607 represents the outstanding varieties in recent sesame breeding history (Zhang et al. 2019).
In this study, the morphological and genetic analysis of dw607 was systematically performed. The target gene, Sidwf1 controlling the plant height and internode length traits, was cloned, based on the combination strategy of cross-population association mapping and genomic variants screening. The results proved that the above gene cloning method is highly efficient and convenient for quality trait-related genetics research in sesame (Zhang et al. 2018). For dw607, the development characters of stem and plant architecture are affected by the mutation of SiDWF1 gene alleles, which encodes a GID1B-like protein with HGG and GXSXG motifs (Fig. 6). Till now, GID1 receptor genes have been characterized in Arabidopsis thaliana, ferns, cotton, maize, barley, oilseed rape, wheat, and other plants (Griffiths et al. 2006; Hirano et al. 2007; Aleman et al. 2008; Chandler et al. 2008; Suzuki et al. 2009; Zeng et al. 2011; Li et al. 2013). Different from other homologs in other crops, Sidwf1 gene contained the only missense change of SNP C1057, which was mutagenized to T1057 and resulted in the amino acid change of P150 (proline) to S150 (serine) (Fig. 5). In plants, GID1B-like proteins are conservative. Compared to the homologs of monocotyledonous crops, the resemblance rate of SiDWF1 is still high to 60% (Fig. 7).
The GID1 receptor gene was initially identified in rice (OsGID1), which is involved in gibberellin biosynthesis pathway (Ueguchi-Tanaka et al. 2005). In a plant species, GID1B or GID1B-like proteins always have one or more homologs which reveal the sequence conservation and the complexity of gibberellin biosynthesis pathway and regulation. In wheat, both of the homologous DELLA genes Rht-B1 and Rht-D1 cause dwarfing (Peng et al. 1999). For sesame, two ortholog genes (i.e., SiDWF1 and SiDWF2) were proved to encode GID1B-like proteins (Fig. 6). A SNP mutation (C1057T) of SiDWF1 caused the short internode length in dw607. Thus, we inferred that SiDWF2 might take different functions from SiDWF1. In addition, compared with the wild type, the development rhythm of plant architecture in dw607 delayed. Mutagenesis of the conserved domain of SiDWF2 might also cause the architecture change in sesame.
Till now, hundreds of dwarf mutants have been found in many species. Most of these mutations are involved in the biosynthesis and signal transduction of the GA3, BR, or other plant hormones (Chen et al. 2017; Yan et al. 2017). In this study, the sesame dwarf mutant dw607 also exhibited the change of stem development process. The internode length and stem length were significantly reduced (Table 1). Similar to emf2b in rice (Zhong et al. 2018) and m34 in maize (Li et al. 2018a), cell expansion of stem tissue in dw607 was seriously inhibited (Fig. 2). Moreover, evaluation results of GA3, IAA, ABA, and BR content reflected that the accumulation pattern of the four endogenous hormones in dw607 and the wild type was similar (Supplementary Fig. 3). The variation in GA3 content in root, leaf, and shoot tissues of dwarfing mutant presented the delayed rhythm, consistent with the growth and development styles of dw607 (Supplementary Fig. 3; Table 1). SiDWF1 belongs to gibberellin-insensitive protein. Theoretically, the dwarfing mutation of dwf1 type cannot be entirely restored even with the artificial regulation at specific stage (Ashikari et al. 1999). Further GA3 complement experiment proved that the dwarfing trait of dw607 could not be recovered under the artificial supplement of GA3 (data not shown). Thus, the dwarfing type of dw607 is similar to that of the gibberellin-insensitive rice mutant dwarf 1 (d1) (Ashikari et al. 1999).
In soybean, Gazara et al. (2018) found a GID1b homolog (Gmax.GID1b3) expressed highly in roots and nodules, as well as in flowers. Similarly, we detected that the transcripts of Sidwf1 gene were highly expressed in root, leaf, stem, buds, and 0-d capsule tissues (Fig. 8). Moreover, the main tissues and stages with high expression of SiDWF1 alleles also accumulated more GA3, which would reflect the inner relationship of the dwarfing gene and the main biological process regulation. The findings supply the foundation for elucidating the regulation of the molecular mechanism on the short internode length and dwarf trait in sesame. The developed SNP marker for Sidwf1 also improves the development of molecular breeding in sesame.
Conclusion
The short internode length and dwarfing trait in mutant dw607 is controlled by a recessive gene allele (Sidwf1). Cell size of stem parenchyma and pith tissues in dw607 significantly reduce. SiDWF1 is a gibberellin receptor GID1B-like protein. The SNP mutation of C1057 to T1057 resulted in an amino acid change and affected the gene function of SiDWF1. The findings revealed the molecular genetic mechanism of the short internode length and dwarf trait in sesame for the first time.
Availability of data and materials
The cDNA sequence of SiDWF1 gene was available in NCBI with Accession No. KY649623. The BSA data of the dwf1 and the wild type were available in NCBI under bioproject PRJNA555174 with SRA Accession No. SRR9733676-SRR9733681.
Abbreviations
- ABA:
-
Abscisic acid
- BR:
-
Brassinosteroid
- BSA:
-
Bulked segregant analysis
- DAS:
-
Days after sowing
- ELISA:
-
Enzyme-linked immunosorbent assay
- EMS:
-
Ethyl methanesulfonate
- Ep:
-
Epidermis
- FAA:
-
Formalin–glacial acetic acid–alcohol
- GAs:
-
Gibberellins
- GID1:
-
Gibberellin-insensitive dwarf 1
- GLM:
-
General linear model
- GWAS:
-
Genome-wide association studies
- IAA:
-
Indole-3-acetic acid
- InDel:
-
Insertion–deletion
- LG:
-
Linkage group
- JA:
-
Jasmonate
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- MAS:
-
Marker-assisted selection
- NGS:
-
Next-generation sequencing technology
- NR:
-
Non-redundant
- PC:
-
Parenchyma cell
- PE:
-
Paired end
- Pi:
-
Pith
- qRT-PCR:
-
Quantitative real-time PCR
- QTL:
-
Quantitative trait loci
- RIL:
-
Recombinant inbred line
- SNP:
-
Single-nucleotide polymorphism
- TSW:
-
Thousand seed weight
- VC:
-
Vascular bundle cells
References
Aleman L, Kitamura J, Abdel-mageed H, Lee J, Sun Y, Nakajima M, Ueguchi-Tanaka M, Matsuoka M, Allen RD (2008) Functional analysis of cotton orthologs of GA signal transduction factors GID1 and SLR1. Plant Mol Biol 68:1–16
Ashikari M, Wu J, Yano M, Sasaki T, Yoshimura A (1999) Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the alpha-subunit of GTP-binding protein. P Natl Acad Sci USA 96:10284–10289
Avila LM, Cerrudo D, Swanton C, Lukens L (2016) Brevis plant1, a putative inositol polyphosphate 5-phosphatase, is required for internode elongation in maize. J Exp Bot 67(5):1577–1588
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120
Chandler PM, Harding CA, Ashton AR, Mulcair MD, Dixon NE, Mander LN (2008) Characterization of gibberellin receptor mutants of barley (Hordeum vulgare L.). Mol Plant 1:285–294
Chen J, Zhao H, Zheng X, Liang K, Guo Y, Sun X (2017) Recent amplification of Osr4 LTR-retrotransposon caused rice D1 gene mutation and dwarf phenotype. Plant Divers 39(2):73–79
Feng Y, Yin Y, Fei S (2015) Down-regulation of BdBRI1, a putative brassinosteroid receptor gene produces a dwarf phenotype with enhanced drought tolerance in Brachypodium distachyon. Plant Sci 234:163–173
Gazara RK, Moharana KC, Bellieny-Rabelo D, Venancio TM (2018) Expansion and diversification of the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1 (GID1) in land plants. Plant Mol Biol 97(4–5):435–449
Griffiths J, Murase K, Rieu I, Zentella R, Zhang Z, Powers SJ, Gong F, Phillips AL, Hedden P, Sun T, Thomas SG (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18:3399–3414
Hirano K, Nakajima M, Asano K, Nishiyama T, Sakakibara H, Kojima M, Katoh E, Xiang H, Tanahashi T, Hasebe M, Banks JA, Ashikari M, Kitano H, Ueguchi-Tanaka M, Matsuoka M (2007) The GID1-mediated gibberellin perception mechanism is conserved in the Lycophyte Selaginella moellendorffii but not in the Bryophyte Physcomitrella patens. Plant Cell 19:3058–3079
Hirano K, Yoshida H, Aya K, Kawamura M, Hayashi M, Hobo T, Sato-lzawa K, Kitano H, Ueguchi-Tanaka M, Matsuoka M (2017) SMALL ORGAN SIZE 1 and SMALL ORGAN SIZE 2/DWARF AND LOW-TILLERING form a complex to integrate auxin and brassinosteroid signaling in rice. Mol plant 10(4):590–604
Jimoh WA, Aroyehun HT (2011) Evaluation of cooked and mechanically defatted sesame (Sesamum indicum) seed meal as a replacer for soybean meal in the diet of African catfish (Clarias gariepinus). Turkish J Fish Aquat Sci 11(2):185–190
Kurotani K, Hattori T, Takeda S (2015) Overexpression of a CYP94 family gene CYP94C2b increases internode length and plant height in rice. Plant Signal Behav 10(7):e1046667
Langham DR (2008) Growth and development of sesame. American Sesame Grower Association, San Antonio, p 44
Langham DR, Wiemers T, Janick J, Whipkey A (2002) Progress in mechanizing sesame in the US through breeding. In: Janick J (ed) Trends in new crops and new uses, ASHS Press, Alexandria, pp:157–173.
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinfomatics 25(14):1754–1760
Li A, Yang W, Li S, Liu D, Guo X, Sun J, Zhang A (2013) Molecular characterization of three GIBBERELLIN-INSENSITIVE DWARF1 homologous genes in hexaploid wheat. Plant Physiol 170:432–443
Li J, Ali SA, Xiao G, Chen F, Yuan L, Gu R (2018a) Phenotypic characterization and genetic map of the dwarf mutant m34 in maize. J Integr Agr 17:60345–60347
Li Z, Guo Y, Ou L, Hong H, Wang J, Liu Z, Guo B, Zhang L, Qiu L (2018b) Identification of the dwarf gene GmDW1 in soybean (Glycine max L.) by combining mapping-by-sequencing and linkage analysis. Theor Appl Genet 131:1001–1016
Liang H, Wang C, Li Z, Luo XZ, Zou G (2008) Improvement of the silver-stained technique of polyacrylamide gel electrophoresis. Hereditas 30(10):1379–1382
Makinde FM, Akinoso R (2013) Nutrient composition and effect of processing treatments on antinutritional factors of Nigerian sesame (Sesamum indicum Linn) cultivars. Int Food Res J 20(5):2293–2300
Monna L, Kitazawa N, Yoshino R, Suzuki J, Masuda H, Maehara Y, Tanji M, Sato M, Nasu S, Minobe Y (2002) Positional cloning of rice semidwarfing gene, sd-1: rice “green revolution gene” encodes a mutant enzyme involved in gibberellin synthesis. DNA Res 9(1):11–17
Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP (1999) ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400(6741):256–261
Poplin R, Ruano-Rubio V, DePristo MA, Fennell TJ, Carneiro MO, Van der Auwera GA, Kling DE, Gauthier LD, Levy-Moonshine A, Roazen D, Shakir K, Thibault J, Chandran S, Whelan C, Lek M, Gabriel S, Daly MJ, Neale B, MacArthur DG, Banks E (2017) Scaling accurate genetic variant discovery to tens of thousands of samples. BioRxiv (https://doi.org/10.1101/201178).
Prakash K, Naik SN (2014) Bioactive constituents as a potential agent in sesame for functional and nutritional application. J Bioresour Eng Technol 1:48–66
Rebetzke GJ, Ellis MH, Bonnett DG, Condon AG, Falk D, Richiards RA (2011) The Rht13 dwarfing gene reduces peduncle length and plant height to increase grain number and yield of wheat. Field Crops Res 124(3):323–331
Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, Kitano H, Matsuoka M (2002) Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416(6882):701–702
Satish RG (2013) Genetic analysis of sesame (Sesamum indicum L.) for traits related to moisture stress tolerance with reference to root traits. Doctoral dissertation, University of Agricultural Sciences, Bengaluru
Sene B, Sarr F, Sow MS, Diouf D, Niang M, Traore D (2017) Physico-chemical composition of the sesame variety (Sesamum indicum L.) 32–15 and characterization of its derived products (seeds, oil and oilcake) in Senegal. Food Sci Qual Manag 65:5–10
Sharmila V, Ganeshi SK, Gunasekaran M (2007) Generation mean analysis for quantitative traits in sesame (sesamum indicum l.) crosses. Genet Mol Biol 30(1):80–84
Suzuki H, Park S, Okubo K, Kitamura J, Ueguchi-Tanaka M, Iuchi S, Katoh E, Kobayashi M, Yamaguchi I, Matsuoka M, Asami T, Nakajima M (2009) Differential expression and affinities of Arabidopsis gibberellin receptors can explain variation in phenotypes of multiple knock-out mutants. Plant J 60:48–55
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Thomas SG (2017) Novel Rht-1 dwarfing genes: tools for wheat breeding and dissecting the function of DELLA proteins. J Exp Bot 68(3):354–358
Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YI, Kitano H, Yamaguchi I, Matsuoka M (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437:693–698
Wang L, Xia Q, Zhang Y, Zhu X, Zhu X, Li D, Ni X, Gao Y, Xiang H, Wei X, Yu J, Quan Z, Zhang X (2016) Updated sesame genome assembly and fine mapping of plant height and seed coat color QTLs using a new high-density genetic map. BMC Genomics 17(1):31
Wang H, Zhang H, Ma Q, Wei L, Ju M, Li C, Duan Y, Miao H (2017a) Optimization of EMS mutagenesis condition and screening of mutants in sesame. J Henan Agri Sci 46(1):36–41
Wang N, Xing Y, Lou Q, Feng P, Liu S, Zhu M, Yin W, Fang S, Lin Y, Zhang T, Sang X, He G (2017b) Dwarf and short grain 1, encoding a putative U-box protein regulates cell division and elongation in rice. J Plant Physiol 209:84–94
Wei L, Miao H, Zhao R, Han X, Zhang T, Zhang H (2013) Identification and testing of reference genes for Sesame gene expression analysis by quantitative real-time PCR. Planta 237(3):873–889
Wei L, Miao H, Li C, Duan Y, Niu J, Zhang T, Zhao Q, Zhang H (2014) Development of SNP and InDel markers via de novo transcriptome assembly in Sesamum indicum L. Mol Breed 34(4):2205–2217
Wei X, Liu K, Zhang Y, Feng Q, Wang L, Zhao Y, Li D, Zhao Q, Zhu X, Zhu X, Li W, Fan D, Gao Y, Lu Y, Zhang X, Tang X, Zhou C, Zhu C, Liu L, Zhong R, Tian Q, Wen Z, Weng Q, Han B, Huang X, Zhang X (2015) Genetic discovery for oil production and quality in sesame. Nat Commun 6:8609
Yan J, Liao X, He R, Zhong M, Feng P, Li X, Tang D, Liu X, Zhao X (2017) Ectopic expression of GA 2-oxidase 6 from rapeseed (Brassica napus L.) causes dwarfism, late flowering and enhanced chlorophyll accumulation in Arabidopsis thaliana. Plant Physiol Bioch 111:10–19
Zeng X, Zhu L, Chen Y, Qi L, Pu Y, Wen J, Yi B, Shen J, Ma C, Tu J, Fu T (2011) Identification, fine mapping and characterisation of a dwarf mutant (bnaC.dwf) in Brassica napus. Theor Appl Genet 122:421–428
Zhang H, Wang X, Wang H, Wei S (2012) Sesame production technology. Henan People Press, Zhengzhou, p p203
Zhang H, Miao H, Wang L, Qu L, Liu H, Wang Q, Yue M (2013) Genome sequencing of the important oilseed crop Sesamum indicum L. Genome Biol 14(1):401
Zhang H, Miao H, Li C, Wei L, Duan Y, Ma Q, Kong J, Xu F, Chang S (2016) Ultra-dense SNP genetic map construction and identification of SiDt gene controlling the determinate growth habit in Sesamum indicum L. Sci Rep 6:31556
Zhang H, Miao H, Wei L, Li C, Duan Y, Xu F, Qu W, Zhao R, Ju M, Chang S (2018) Identification of a SiCL1 gene controlling leaf curling and capsule indehiscence in sesame via cross-population association mapping and genomic variants screening. BMC Plant Biol 18(1):296
Zhang H, Miao H, Ju M (2019) Potential for adaption to climate change through genomic breeding in sesame. In: Kole C (ed) Genomic designing of climate-smart oilseed crops. Springer press, USA, pp 371–440
Zhao R, Miao H, Song W, Chen C, Zhang H (2018) Identification of sesame (Sesamum indicum L.) chromosomes using the BAC-FISH system. Plant Biol 20(1):85–92
Zhong J, Peng Z, Peng Q, Cai Q, Peng W, Chen M, Yao J (2018) Regulation of plant height in rice by the Polycomb group genes OsEMF2b, OsFIE2 and OsCLF. Plant Sci 267:157–167
Acknowledgements
This work was supported by the Key Laboratory of Specific Oilseed Crops Genomics of Henan Province. This work was financially supported by the earmarked fund for China Agriculture Research System (CARS-14), the Plan for Scientific Innovation Talent of Henan Province (184200510002), the Key Project of Science and Technology in Henan Province (151100111200), the Henan Province Specific Professor Position Program (SPPP2016), the Distinguished Professor Program of Institutions of Higher Learning in Henan Province (DPPIHL2017), the Innovation Scientists and Technicians Troop Construction Projects of Henan Province (ISTTCPHP2016), the Henan Natural Science Foundation (162300410159), and the International Cooperation and Exchanges Project of Henan Province (182102410040).
Author information
Authors and Affiliations
Contributions
ZH conceived the technical route and guided the manuscript for publishing. MH guided the experiments, performed the data analysis, and drafted the manuscript. LC and DY conducted the main data analysis and experiments. WL and JM performed the genetic experiments and participated in result validation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Matthew N Nelson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
122_2019_3441_MOESM1_ESM.xlsx
Supplementary Table 1 Growth profile comparison of dw607 and Yuzhi11. * Data are collected at Yuanyang experimental station in 2013; Supplementary Table 2 Variant information of the candidate interval controlling the short internode length trait in sesame; Supplementary Table 3 Variants information of 824 sesame germplasm accessions in target region; Supplementary Table 4 Information of candidate variants screened using genome resequencing data of 824 sesame germplasm accessions; Supplementary Table 5 Genome re-sequencing information of the BSA pools for internode length trait. a: The genome coverage is calculated based on the sesame genome size of 354 Mb estimated by K-mer (Zhang et al. 2013); Supplementary Table 6 Information of the five candidate variants for short internode length trait in sesame; Supplementary Table 7 Primer pair information of the SNP marker design of Sidwf1 gene alleles; Supplementary Table 8 Primer pair information of the cDNA and DNA sequences of SiDWF1 Supplementary file1 (XLSX 21888 kb)
122_2019_3441_MOESM2_ESM.pdf
Supplementary Figure 1 Comparison of plant height of dw607 and Yuzhi 11 during cycle life. Data are collected at Yuanyang experimental station in 2013; Supplementary Figure 2 Amplification validation of the SiSNPdwf1 marker using the test population and germplasm accessions. M: DNA marker; Lane 1–10: F2-3 individuals with short internode length trait (dwf1 type); Lane 11–20: F2-3 individuals with normal internode length trait (wt); Lane 21–40: 20 sesame accessions with normal internode length trait (wt); Supplementary Figure 3 Dynamic content variation of GA3, IAA, ABA, BR and ZR in shoot tip, root tip, and leaf of dw607 and Yuzhi11. a-c: Dynamic variation of GA3 content in shoot tip, root tip, and leaf of dw607 and Yuzhi11, respectively; d-f: Dynamic variation of IAA content in shoot tip, root tip, and leaf of dw607 and Yuzhi11, respectively; g-i: indicate the dynamic variation of ABA content in shoot tip, root tip, and leaf of dw607 and Yuzhi11, respectively; j-l: Dynamic variation of BR content in shoot tip, root tip, and leaf of dw607 and Yuzhi11, respectively Supplementary file2 (PDF 532 kb)
Rights and permissions
About this article
Cite this article
Miao, H., Li, C., Duan, Y. et al. Identification of a Sidwf1 gene controlling short internode length trait in the sesame dwarf mutant dw607. Theor Appl Genet 133, 73–86 (2020). https://doi.org/10.1007/s00122-019-03441-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-019-03441-x