Abstract
The 19kd Bcl-2 interacting protein 3 (Bnip3) belongs to a subclass of the Bcl-2 gene family that regulates a wide range of cellular processes including apoptosis, necrosis and autophagy. However, while Bnip3 localizes to the mitochondria in different cell types including cancer cells, its biological function particularly in cardiac myocytes appears to be context specific. This varied response of Bnip3 may be due to differences in Bnip3 abundance, temporal or spatial activation or association with mitochondrial proteins in response to different cell stress conditions. Understanding the biological significance of Bnip3 and the signaling pathways it impinges upon to regulate apoptosis, necrosis and autophagy under disease states is of paramount importance toward developing new therapies to modulate cardiac cell death during cell stress conditions. Herein, we highlight the role of Bnip3 in cardiac cell death under two physiologically important and clinically relevant conditions where Bnip3 is known to be activated, namely ischemic stress and doxorubicin cardiotoxicity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Historically, the adult myocardium has been viewed as a non-proliferative post-mitotic organ with a limited capacity for myocyte regeneration after injury. Hence, the loss of functional cardiac myocytes by programmed apoptosis or necrosis is postulated as a central underlying mechanism for ventricular remodeling and heart failure. Based on morphological criteria alone, apoptosis was initially identified as a programmed death pathway typified by DNA fragmentation and cell shrinkage without loss of membrane integrity [1]. The fact that apoptosis is a regulated event and potentially amenable to therapeutic interventions, my laboratory and others investigated the signaling pathways and molecular factors that govern apoptotic cell death in the heart during disease. Because necrosis was traditionally viewed as an accidental or an unregulated passive response to injury [2], it was largely overlooked or even ignored as a potential form of cell death that could be manipulated by therapeutic or genetic interventions. Consequently, over the past several years a greater appreciation and detailed understanding of the molecular signaling pathways involved in apoptosis and necrosis, coupled with advanced biochemical criteria and cellular markers to discriminate between apoptotic and necrotic cell death, has resulted in an insurgence of recent studies exploring necrosis as a regulated form of cell death during disease.
Apoptosis and Necrosis Cell Death Pathways
Arguably, one of the most intriguing and compelling issues to impact contemporary biology to date is the concept that programmed cell death is a genetically regulated process. Observations made on the basis of distinct morphological criteria alone, more than 30 years ago by Kerr et al. [1], markedly distinguished apoptosis from classical cell death by necrosis. Apoptosis is a highly regulated evolutionary conserved genetic program of cell death essential for normal development and tissue homeostasis [3]. In contrast, necrosis has been traditionally considered as an unregulated passive response to injury [4]. While there is little doubt that necrosis induced by massive cellular trauma is likely an unregulated event, several lines of investigation including new exciting data from our laboratory have challenged this dogma and classical textbook definition that necrotic cell death is merely accidental and unregulated [5,6,7]. This emerging and contemporary view is a paradigm shift in our thinking about how cell fate is regulated. In fact, the concept that necrotic death is regulated has tremendous implications for understanding the pathogenesis of diseases that were previously unexplored as well as developing novel therapies for conditions where necrosis is known to play a significant role.
Bcl-2 Family and Molecular Regulation of Cell Death
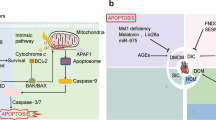
The Bcl-2 gene family is highly conserved group of proteins that are found throughout evolution and associated with a number of cellular processes that include cell-cycle, metabolism and cell fate [8]. The archetypic member of this group of diverse proteins is Bcl-2 (B cell lymphoma 2). Bcl-2 was discovered as a translocation break-point mutation between chromosome (t14:18) in B-cell lymphomas and believed to confer resistance of cancer cells to anti-neoplastic agents [9,10,11]. Since its initial discovery, several other proteins with similar domain structures to Bcl-2 have been identified including the c. elegans ortholog ced 9 [12]. The interesting feature among these proteins is their ability to influence cell fate through regulation of cellular processes involved in cell survival and programmed cell death pathways. Interestingly, these are relate through the presence of distinct structures known as Bcl-2 homology (BH) domains that are conserved among these proteins. Ostensibly, the presence or absence of a given domain confers the ability of these proteins to promote cell death of cell survival which is achieved through the formation of homotypic and heterotypic interactions which influences their cellular distribution and influence on cell fate in response to different physiological/pathophysiological conditions [13]. In this regard, in the most generalized state Bcl-2 and other family members such Bcl-xL or MCl-1 that promote cell survival contain an α-aliphatic N-terminal BH4 domains, BH3, BH2, and BH1 domain, and highly conserved carboxyl terminus domain required for insertion into membranes such as mitochondria, nuclear and endoplasmic reticulum [13]. Other family members such as Bax and Bak which induce cell death retain the N-and C- terminal as well as the conserved BH1, BH2 and BH3 domain but lack the α-aliphatic BH4 domain [13]. Notably, a sub-class of Bcl-2 family proteins known to as BH3 only exist which only contain the N- and -C-terminus and single BH3 like domain. While these proteins, like the other BH3 death proteins (Bax, Bak) can promote cell death in response to pathophysiological conditions, less is known of their ability to influence organelles such as mitochondria and endoplasmic reticulum to promote apoptosis, necrosis or autophagy, Fig. 13.1.
Mitochondrion Regulated Cell Death Pathways
In addition to energy production, the mitochondrion has been identified as major signaling platform for apoptosis and necrosis, respectively, Fig. 13.2. The mitochondrial events that discriminate apoptosis versus necrosis signaling events remain poorly understood. However, a growing body of evidence supports a paradigm in which permeability changes to the outer mitochondrial membrane (OMM) or inner mitochondrial membrane (IMM) distinguish apoptosis and necrosis respectively [14]. Hence, in response to cell stress such as genotoxic DNA damage, hypoxia, reactive oxygen species or nutrient deprivation many of the Bcl-2 proteins particularly Bax and Bak engage the intrinsic mitochondrial death pathway and activate apoptosis [15]. The activation and oligomerization of Bax and Bak proteins, results in permeabilization of the mitochondrial outer membrane (MOMP) through the formation of ion conductance Bax/Bak channels [15]. MOMP results in the dissipation of mitochondrial membrane potential (ΔΨm) on the IMM where electron transport and respiration occurs and release of apoptogens from mitochondria including cytochrome c, through an undefined mechanism that associates with the mitochondrial protein SMAC, caspase 9 and dATP form the apoptosome in the cytosol [16]. Once formed the apoptosomes triggers the activation of death effector caspases 3, 6, and 8 which through the proteolytic cleavage of intracellular substrates leads to the demise of the cell through apoptosis [16], Fig. 13.3. Notably, the loss of ΔΨm during apoptosis is a relatively late event compared to necrosis which is a relatively early event [17]. Conversely, early permeability changes resulting from the formation of a permeability transition pore on the IMM (mPTP) is considered an integral feature of necrotic cell death [17]. mPTP causes structural and functional derangements to the mitochondrion that include impaired respiration, loss of ΔΨm, ATP synthesis, redistribution of ions, osmotic imbalance, mitochondrial matrix swelling, and increased Ca2+ that eventually leads to OMM rupture [17]. Linkage of the intrinsic death pathway to the extrinsic death receptor pathway is achieved through the processing and activation of initiation caspases such as 8 and 10 result in the proteolytic cleavage of Bid to truncated Bid (tBid) which upon translocation to the mitochondria triggers MOMP and apoptosis [16]. Further involvement of death receptors to necroptosis involves the intracellular adapter kinases Receptor Interacting Protein 1 Kinase, (RIP1K RIP3) and mix linage kinase (MLKL) which presumably disrupt mitochondrial metabolism leading to necrotic cell death through a poorly understood mechanism involving RIP3 [18]. Importantly, many of the processes that lead to MOMP or IMM defects leading to apoptosis or necrosis are not well understood but can be suppressed by over-expression of Bcl-2 or B-cell lymphoma—extra larger (Bcl-XL) proteins. Together, these observations highlight the importance of Bcl-2 family proteins in regulating apoptotic and necrotic cell death, respectively. Given the overlapping nature of some Bcl-2 proteins to promote cell survival or cell death, it remains to be elucidated how the cell discriminates between apoptosis and necrosis pathways in response cell stress. Recent data suggests that selective post-translational modifications such as phosphorylation may alter the putative function and cell fate and context specific manner. For example, the site specific phosphorylation of Beclin-1 can switch its ability to interact with Bcl-2 and promote cell death through displacement of Bax from Bcl-2 or promote autophagy and cell survival [19].
Model of Bnip3 mediated cardiac cell death. Transcriptional activation of Bnip3 during hypoxia or in response to doxorubicin leads to the mitochondrial integration of Bnip3 where it disrupts interaction between UCP3 and COXI on the mitochondrial inner membrane (IMM). This leads to impaired mitochondrial respiration, loss of mitochondrial membrane potential ΔΨm, ATP synthesis, and cell death
Intersection of Bnip3 with Apoptosis, Necrosis and Autophagy. Left Panel: Bnip3 activates the intrinsic mitochondrial death pathway. Bnip3 triggers mitochondrial perturbations leading to cytochrome release, caspase activation and apoptosis, Middle Panel: Bnip3 promotes mitophagy and clearance of damaged mitochondria. Mitochondrial perturbations induced by Bnip3 serves as a docking site for the recruitment of LC3II to depolarized mitochondria along with other autophagy related proteins which target damaged mitochondria for removal by mitochondrial autophagy, Right Panel: Bnip3 promotes necrotic cell death of cardiac myocytes. The activation and integration of Bnip3 into mitochondrial membranes disrupts calcium regulation resulting in mitochondrial calcium loading possibly through the voltage dependent ion channel VDAC or mitochondrial calcium uniporter (MCU) leading to mPTP opening and necrotic cell death
Hypoxia-Inducible Expression of Bnip3
The adenovirus Bcl-2 19Kd interacting protein 3, (Bnip3) is a member of BH3 domain like members of the Bcl-2 gene family. Bnip3 was initially identified as cellular protein that formed protein–protein interactions with the 19kD anti-death gene of adenovirus in cells infected with adenovirus [20]. Indeed, to circumvent premature lysis of infected cells, adenovirus and several other human viruses produce anti-death genes that suppress cell death of the infected host cell to ensure sufficient viral progeny has replicated during lytic infection. Since these initial observations, several laboratories including our own, identified Bnip3 as critical regulator of cell death in a number of different cell types including cardiac myocytes [6]. To this end, we identified Bnip3 as a Bcl-2 interacting protein in cardiac myocytes. Notably, the 19kD anti-death gene of adenovirus is the viral homologue to Bcl-2 [21]. Our excitement surrounding the identification of Bnip3 is predicated on the fact that in contrast to other Bcl-2 family members known to promote cell death by apoptosis, Bnip3 is the only member of this family that is highly expressed in the heart and specifically cardiac myocytes under hypoxic conditions [6]. Indeed, in contrast to other death promoting proteins such as Bax, or Bak which are known to promote MOMP and cell death in response to a variety of cell stress signaling, Bnip3 was found to be the only protein activated in cardiac myocytes during hypoxia. The unique feature and hypoxia-inducible nature of Bnip3 identifies it a potentially important cellular target that could be manipulated therapeutically to prevent cell death and cardiac dysfunction during ischemic or hypoxic stress. During the course of our previous studies we established that Bnip3 resides under basal conditions loosely associate with the mitochondrial outer member, in contrast to Bax or Bak which reside in the cytoplasm and translocate to the mitochondrial outer membrane in response to apoptosis signaling [6, 13]. The hypoxia induced activation of Bnip3 involves not only induction of its promoter activity resulting in increased Bnip3 cellular content but also in its integration to mitochondrial inner membranes [6]. We have demonstrated that the integration of Bnip3 into the mitochondrial IMM is contingent upon its carboxy-terminal transmembrane domain [6]. Earlier studies from our group and others revealed that the BH3 like domain and the N-terminal domains of Bnip3 are dispensable for cell killing, again in contrast to Bax and Bak which require their respective BH3 domains for inducing apoptosis, however, the carboxyl terminal transmembrane (TM) was identified to be crucial for triggering mitochondrial and cell death of cardiac myocytes during hypoxic stress [6]. These findings are substantiated by studies in which engineered mutations or complete deletion of the TM domain completely abrogated mitochondrial dysfunction and cell death of cardiac myocytes. Further genetic ablation of Bnip3 through transgenesis resulted in mice with greater functional recovery and smaller infarct sizes following myocardial infarction than corresponding wild type animals [7]. Collectively, our data identify Bnip3 as an inducible death factor and critical regulator of mitochondrial dysfunction and cell death of cardiac myocytes during ischemic injury, Figs. 13.2 and 13.3.
Another interesting feature of Bnip3 that readily distinguishes it from the other Bcl-2 family members which predominately trigger apoptosis, is its ability to promote necrotic cell death by a mechanism that impinges on the IMM [5]. As stated above, the mitochondrion plays a critical role in oxidative metabolism and cellular respiration for ATP synthesis which is crucial for maintaining cellular homeostasis and day to day cardiac contractile function [22]. This is achieved through the stepwise univalent electron transport from the entry of NADH and FADH generated by cellular metabolism from glucose and fatty acid oxidation, which enter the respiratory chain complexes I to IV which produce an electrochemical proton gradient across the IMM. The flow of protons across the IMM through the mitochondrial F0/F1ATPase is responsible for ATP generation 23. Disruption of the electron transport chain proteins through oxidation or carbonylation of the mitochondrial complex proteins on the IMM triggers calcium mediated mPTP opening and necrotic cell death [23]. Importantly, our work has demonstrated that the mitochondrial integration of Bnip3 in response to cell stress disrupts the IMM resulting in cell death with features of necrosis [5], Fig. 13.3.
Another mode by which Bnip3 can provoke mPTP opening, involves Cyclophilin D (CypD), the known mPTP regulator. CypD is a peptidyl prolyl cis–trans-isomerase and normally resides in the mitochondrial matrix. Under mitochondrial stress conditions CypD translocates to the intermembrane space (IMS) of the mitochondria. How CypD translocates from matrix to intermembrane space under stress conditions is largely unknown, but post translational modifications of CypD, such as phosphorylation and acetylation, have been proposed as an underlying mechanisms. Calcium overload through the mitochondrial calcium uniporter (MCU) has also been suggested to activate CypD, resulting in its recruitment to IMS [24,25,26,27]. Increased interaction between CypD and F1F0 ATPase has been implicated in mitochondrial perturbations. We recently found that Bnip3 protein interacts with CypD in doxorubicin treated cardiomyocytes, Fig. 13.3. The significance of interaction between Bnip3 and CypD is not clear at the moment, but the fact that Bnip3 induced mitochondrial defects and cell death could be supressed by genetic knock down of CypD or by use of chemical inhibitor Cyclosporin A (CSA), suggest that CypD might be the effector downstream from Bnip3. Taken together our studies unravel a new mechanism of Bnip3 mediated mitochondrial defects which are obligatorily linked to CypD [28].
Bnip3 and Autophagy
Autophagy is a cellular quality control process essential for maintaining tissue homeostasis and organ function [29]. Defects in autophagy have been linked to a variety of diseases entities including Danon Disease as well as heart failure [30]. Generally speaking, autophagy plays a key role in the removal of damaged organelles, proteins and pathogens such as viral particles and bacteria from the cell through an elaborate lysosome system [29, 31]. Autophagy can be classified as macro- micro and chaperone mediated autophagy. In most cases, macro-autophagy referred to as within is critical for the selective removal of oxidized proteins and lipids as well as damaged organelles such as mitochondria, Golgi and Endoplasmic reticulum [31]. In this regard, autophagy or mitophagy of damaged or irreparable mitochondria plays as an essential cellular quality control mechanism for ensuring a healthy pool of mitochondrial for oxidative metabolism, respiration and ATP production [32, 33]. The inability to effectively clear damaged mitochondria through defective quality control mechanisms leads to the accumulation of mitochondria producing reactive oxygen species which would be toxic to cells [32, 34]. Hence, clearing mitochondria is of paramount importance in maintaining cell viability and tissue quality control. In this regard, a less well defined feature of Bnip3 and related protein Bnip3L, is to serve as a docking site for the autophagy regulator proteins such as microtubule associated light chain 3 II (LC3II) and p62 for the autophagic clearance of damaged mitochondria through mitophagy [35, 36]. Indeed, the N-terminus of Bnip3 and Bnip3L contain LC3II-Interacting Regions which allow these proteins to interact with LC3II [35], Fig. 13.3.
In this way, Bnip3 may contribute to mitochondrial quality control mechanisms through association with LC3II or other autophagy related proteins (Atgs) to ensure the adequate removal of ROS producing mitochondria during stress conditions such as nutrient deprivation or hypoxia. However, the role of Bnip3 as a major participant in mitochondrial quality control is not well defined since germ-line deletion of Bnip3 does not appear to influence mitochondrial turnover under basal or stress conditions, suggesting that Bnip3 is either dispensable for mitochondrial autophagy or other proteins such as Bnip3L may functionally substitute for the absence of Bnip3 in controlling mitochondrial turn-over. Further, the relationship between Bnip3 and necrotic cell death in relation to mitophagy activation or inhibition requires further examination since the interplay between these two cellular processes remains poorly understood.
Bnip3 and Doxorubicin Cardiomyopathy
Doxorubicin (DOX) is a widely used chemotherapeutic used to treat cancers in both children and adults [5]. It has been proven to play a major role in the effective treatment of a number of cancers, including leukemia, breast cancer and non-Hodgkin’s lymphoma [37]. Though its clinical efficacy is well documented, patients treated with DOX are observed to have a higher risk of aberrant arrhythmias, myocardial infarction and cardiomyopathy [38]. Furthermore, over a third of patients receiving a dosage greater than 601 mg/m2 of body surface area suffered congestive heart failure [38]. In attempt to maximize the clinical reliability of DOX, the cardiotoxic effects of the chemotherapeutic should be minimized, while keeping its antitumor properties intact. The mechanisms that underlie DOX toxicity in cardiac myocytes remain poorly understood. There are reports demonstrating that DOX acts upon topoisomerase II activity to disrupt DNA synthesis and inhibit cell proliferation in cancer cells [39], while other reports demonstrate increased oxidative stress and reactive oxygen species (ROS) form iron overload [40]. Indeed, a recent report the Ardehali laboratory demonstrated that DOX treatment resulted in mitochondrial iron overloaded from defects in the ABC cassette iron transporter protein [41]. Restoration or normalization of iron homeostasis suppressed mitochondrial damage induced by DOX.
At the cellular level, close inspection by electron microscopy revealed severe ultrastructural defects that include disruption of the sarcomeres, mitochondrial swelling with rarefaction of mitochondrial cristae [5]. Interestingly, the cellular and mitochondrial defects induced by DOX were reminiscent of the cellular injury induced by Bnip3, raising the intriguing possibility that Bnip3 may underlie the cardiotoxic effects of DOX. In fact, because the heart is abundantly rich in mitochondria, predisposes it to potential damaging effects of drugs such as DOX. To explore this possibility as a step toward understanding the mechanisms that underlie DOX cardiotoxicity, we tested whether Bnip3 is involved in the mitochondrial defects and cardiac dysfunction in cardiac myocytes treated with DOX. For these studies we treated mice with saline or DOX 5 mg/kg for 4 days for a cumulative DOX dose of 20 mg [5]. One week following treatment, mice were assessed for cardiac function, parallel studies were conducted in isolated primary cultured cardiac myocytes to assess cell viability, mitochondrial respiration, mitochondrial membrane potential ΔΨm and permeability transition pore opening mPTP following DOX (5ug/ml) treatment [5]. Interestingly, western blot analysis revealed a marked increase in Bnip3 expression in cardiac myocytes in vivo and in vitro which corresponded with a reduction in cell viability [5]. The increased cell death in vitro, was concordant with ultrastructural injury and impaired contractile function in vivo [5].
Further, mitochondria isolated from mice hearts following DOX treatment exhibited diminished oxygen consumption rates and respiratory reserve capacity, consistent with impaired mitochondrial respiration and ATP production [5]. Impaired respiration also coincided with a loss of mitochondrial membrane potential, mitochondrial calcium, and mPTP opening. A dramatic increase in LDH and cTnT release was observed in mice treated with DOX, a finding consistent with increased mitochondrial damage impaired contractile function [5]. These findings substantiate that defects to the mitochondrial IMM, trigger mPTP opening and necrotic cell death of cardiac myocytes. Given that Bnip3 was highly expressed and integrated into mitochondrial membranes, we were curious if Bnip3 was responsible for the cytotoxic effects induced by DOX in vitro and in vivo. Therefore, to test this possibility, we tested whether genetic knock-down or ablation of Bnip3 would influence the mitochondrial injury and cardiac dysfunction induced by DOX. To this end, we observed that in contrast to wild type hearts treated with DOX which displayed impaired mitochondrial morphology and contractile function, Bnip3−/− mice treated with DOX were relatively resistant to DOX treatment and were indistinguishable from vehicle treated saline controls with respect to cell morphology and cardiac contractility. Further, knock-down of Bnip3 or mutations of Bnip3 defective for integrating into the IMM, were sufficient to suppress DOX-induced mitochondrial injury. In fact, loss or inactivation of Bnip3 in the presence of DOX, normalized mitochondrial respiration, and mitochondrial ΔΨm. Further, mitochondrial calcium overload which is critical for mPTP was suppressed in cells deficient for Bnip3 [5]. This suggests that Bnip3 may trigger intracellular calcium release from the endoplasmic reticulum to promote mPTP and necrotic cell death. Importantly, DOX induced mPTP opening and necrotic cell death in vitro and in vivo was abrogated following Bnip3 inactivation. Taken together, these data strongly suggest that Bnip3 is a critical effector of DOX induced mitochondrial injury and cell death of cardiac myocytes. To address the mode by which Bnip3 perturbs mitochondrial respiration on the IMM, we reasoned that Bnip3 may disrupt one or more of the electron chain transport complexes which would impair respiration and trigger mPTP. In this regards, we identified that intra-mitochondrial complexes between uncoupling protein 3 (UCP3) and cytochrome c oxidase subunit I (COXI) of complex IV were disrupted in cardiac myocytes following DOX treatment. Notably, genetic ablation of Bnip3 prevented DOX induced disruption of UCP3-COXI and impaired respiration in vivo and in vitro. These findings fully support a model in which the activation of Bnip3 in cardiac myocytes treated with DOX promotes mitochondrial perturbations resulting in mPTP opening and necrotic cell death [5], Fig. 13.2.
Concluding Comments
The Bcl-2 protein Bnip3 is a highly conserved evolutionary protein that is vastly transcriptionally induced in cardiac myocytes subjected to hypoxia. Bnip3 is also induced in cardiac myocytes treated with DOX. The activation and integration of Bnip3 into mitochondrial IMM promotes perturbations resulting in loss of mitochondrial ΔΨm, impaired respiration, and mPTP. The ability of Bnip3 to provoke mitochondrial mPTP on the IMM is an underlying feature of necrotic cell death. The ability of Bnip3 to serve as a mitochondrial quality control mechanism by promoting mitophagy through the adapter protein for LC3II is another feature of Bnip3 that requires further investigation. How Bnip3 intersects with key cellular process that underlie apoptosis, necrosis and autophagy remains to be elucidated, Fig. 13.3.
References
Kerr JFR, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Kung G, Konstantinidis K, Kitsis RN (2011) Programmed necrosis, not apoptosis, in the heart. Circ Res 108:1017–36
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219
Dhingra R, Margulets V, Chowdhury SR, Thliveris J, Jassal D, Fernyhough P, Dorn GW, Kirshenbaum LA (2014) Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proc Natl Acad Sci 111:E5537–E5544
Regula KM, Ens K, Kirshenbaum LA (2002) Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res 91:226–31
Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, Li H, Kirshenbaum LA, Hahn HS, Robbins J, Jones WK, Dorn GW (2007) Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest 117:2825–2833.
Marie Hardwick J, Soane L (2013) Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol 5
Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM (1984) Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 226:1097–1099
Tsujimoto Y, Cossman J, Jaffe E, Croce CM (1985) Involvement of the bcl-2 gene in human follicular lymphoma. Science 228:1440–1443
Nunez G, Seto M, Seremetis S, Ferrero D, Grignani F, Korsmeyer SJ, Dalla-Favera R (1989) Growth- and tumor-promoting effects of deregulated BCL2 in human B-lymphoblastoid cells. Proc Natl Acad Sci U S A 86:4589–4593
Hengartner MO, Horvitz HRC (1994) Elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 76:665–676
Kvansakul M, Hinds MG (2013) Structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis 4:e909
Sesso A, Belizário JE, Marques MM, Higuchi ML, Schumacher RI, Colquhoun A, Ito E, Kawakami J (2012) Mitochondrial swelling and incipient outer membrane rupture in preapoptotic and apoptotic cells. Anat Rec 295:1647–1659
Fulda S, Gorman AM, Hori O, Samali A (2010) Cellular stress responses: cell survival and cell death. Int J Cell Biol 214074
Xiong S, Mu T, Wang G, Jiang X (2014) Mitochondria-mediated apoptosis in mammals. Protein Cell 5:737–749
Hurst S, Hoek J, Sheu SS (2017) Mitochondrial Ca2+ and regulation of the permeability transition pore. J Bioenerg Biomembr 49:27–47
Dhuriya YK, Necroptosis SD (2018) A regulated inflammatory mode of cell death. J Neuroinflammation 15:199
Marquez RT, Xu L (2012) Bcl-2: Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am J Cancer Res 2:214–21
Chen G, Ray R, Dubik D, Shi L, Cizeau J, Bleackley RC, Saxena S, Gietz RD, Greenberg AH (1997) The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. J Exp Med 186:1975–1983
White E (1996) Life, death, and the pursuit of apoptosis. Genes Dev 10:1–15
Wang K, Xu Y, Sun Q, Long J, Liu J, Ding J (2018) Mitochondria regulate cardiac contraction through ATP-dependent and independent mechanisms. Free Radic Res 52:1256–1265
Osellame LD, Blacker TS, Duchen MR (2012) Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab 26:711–723
Bauer TM, Murphy E (2020) Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ Res 126(2):280–293
Dhingra R, Lieberman B, Kirshenbaum LA. Cyclophilin D (2018) phosphorylation is critical for mitochondrial calcium uniporter regulated permeability transition pore sensitivity. Cardiovasc Res 115(2):261–263
Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, York AJ, Zhang J, Bers DM, Molkentin JD (2015) The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep 12:15–22
Lambert JP, Luongo TS, Tomar D, Jadiya P, Gao E, Zhang X, Lucchese AM, Kolmetzky DW, Shah NS, Elrod JW (2019) MCUB regulates the molecular composition of the mitochondrial calcium uniporter channel to limit mitochondrial calcium overload during stress. Circulation 140:1720–1733
Dhingra R, Guberman M, Rabinovich-Nikitin I, Gerstein J, Margulets V, Gang H, Madden N, Thliveris J, Kirshenbaum LA (2019) Impaired NF-κB signalling underlies cyclophilin D-mediated mitochondrial permeability transition pore opening in doxorubicin cardiomyopathy. Cardiovasc Res 116(6):1161–1174
Mughal W, Dhingra R, Kirshenbaum LA (2012) Striking a balance: autophagy, apoptosis, and necrosis in a normal and failing heart. Curr Hypertens Rep 14:540–547
Kirshenbaum LA (2012) Regulation of autophagy in the heart in health and disease. J Cardiovasc Pharmacol 60:109
Parzych KR, Klionsky DJ (2014) An overview of autophagy: morphology, mechanism, and regulation. Antioxidants Redox Signal. 20:460–473
Saito T, Sadoshima J (2015) Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circ Res 116:1477– 1490
Shirakabe A, Fritzky L, Saito T, Zhai P, Miyamoto S, Gustafsson ÅB, Kitsis RN, Sadoshima J (2016) Evaluating mitochondrial autophagy in the mouse heart. J Mol Cell Cardiol 92:134–139
Dorn GW, Kitsis RN (2015) The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble. Circ Res 116:167–182
Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson ÅB (2012) Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem 287:19094–19104
Ma X, Godar RJ, Liu H, Diwan A (2012) Enhancing lysosome biogenesis attenuates BNIP3-induced cardiomyocyte death. Autophagy 8:297–309
Young RC, Ozols RF, Myers CE (1981) The anthracycline antineoplastic drugs. N Engl J Med 305:139–153
Singal PK, Iliskovic N (1998) Doxorubicin-induced cardiomyopathy. N Engl J Med 339:900–905
Nitiss JL (2009) Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer 9:338–350
Gammella E, Recalcati S, Cairo G (2016) Dual role of ROS as signal and stress agents: iron tips the balance in favor of toxic effects. Oxid Med Cell Longev 8629024
Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, Mutharasan RK, Jairaj Naik T, Ardehali H (2014) Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 124:617–630
Acknowledgements
This work was supported by a Foundation grant to L.A.K from the Canadian Institute for Health Research (CIHR) L.A.K. holds a Canada Research Chair in Molecular Cardiology. IRN holds a post-doctoral fellowship from the CIHR.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Disclousures
None.
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rabinovich-Nikitin, I., Gerstein, J., Dhingra, R., Guberman, M., Kirshenbaum, L.A. (2022). Regulation of Cell Death Signaling Pathways in Cardiac Myocytes by Mitochondrial Bnip3. In: Kirshenbaum, L.A. (eds) Biochemistry of Apoptosis and Autophagy. Advances in Biochemistry in Health and Disease, vol 18. Springer, Cham. https://doi.org/10.1007/978-3-030-78799-8_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-78799-8_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-78798-1
Online ISBN: 978-3-030-78799-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)