Abstract
DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is a novel inducer to promote mitochondrial apoptosis and suppress tumor growth in a variety of cells although its role in cardiovascular diseases remains obscure. This study was designed to examine the role of DNA-PKcs in cardiac ischemia reperfusion (IR) injury and mitochondrial damage. Cardiomyocyte-specific DNA-PKcs knockout (DNA-PKcsCKO) mice were subjected to IR prior to assessment of myocardial function and mitochondrial apoptosis. Our data revealed that IR challenge, hypoxia-reoxygenation (HR) or H2O2-activated DNA-PKcs through post-transcriptional phosphorylation in murine hearts or cardiomyocytes. Mice deficient in DNA-PKcs in cardiomyocytes were protected against cardiomyocyte death, infarct area expansion and cardiac dysfunction. DNA-PKcs ablation countered IR- or HR-induced oxidative stress, mPTP opening, mitochondrial fission, mitophagy failure and Bax-mediated mitochondrial apoptosis, possibly through suppression of Bax inhibitor-1 (BI-1) activity. A direct association between DNA-PKcs and BI-1 was noted where DNA-PKcs had little effect on BI-1 transcription but interacted with BI-1 to promote its degradation. Loss of DNA-PKcs stabilized BI-1, thus offering resistance of mitochondria and cardiomyocytes against IR insult. Moreover, DNA-PKcs ablation-induced beneficial cardioprotection against IR injury was mitigated by concurrent knockout of BI-1. Double deletion of DNA-PKcs and BI-1 failed to exert protection against global IR injury and mitochondrial damage, confirming a permissive role of BI-1 in DNA-PKcs deletion-elicited cardioprotection against IR injury. DNA-PKcs serves as a novel causative factor for mitochondrial damage via suppression of BI-1, en route to the onset and development of cardiac IR injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ample evidence from our laboratory and others has indicated a pivotal role of cardiomyocyte cell death following mitochondrial damage as a cue for cardiac ischemia–reperfusion (IR) injury [14, 55, 57, 58]. The molecular components of mitochondrial apoptosis have been extensively described although pathological regulatory networks responsible for cardiomyocyte mitochondrial dys-synchrony remain essentially elusive [13, 39]. DNA-dependent protein kinase catalytic subunit (DNA-PKcs), a member of the phosphatidylinositol 3-kinase-related family of protein kinases, is mainly turned on in response to stress conditions including aging [45], radiation [19], oxidative stress [35], asthma [11] and type 2 diabetes mellitus [36]. Under physiological conditions, DNA-PKcs primarily binds to Ku80 [38], which functions as a regulatory domain to repair DNA damage via recognition and interaction with broken segment of DNA. To this end, DNA-PKcs functions as an intrinsic repair machinery to promote cell survival [44]. Nonetheless, more recent evidence has documented innovative non-genomic functions of DNA-PKcs. For example, DNA-PKcs deletion attenuates Th2-mediated airway inflammatory response in allergic asthma [35]. Moreover, HIV-mediated CD4+ cell death was reported to be inhibited by DNA-PKcs ablation [9]. At the molecular level, DNA-PKcs may preferentially interact with and phosphorylate p53 at Ser15 to selectively promote p53-dependent apoptosis in response to sustained insult or irreparable cell damages [5, 50]. This notion was consolidated by a recent observation from our laboratory where genetic ablation of DNA-PKcs protected liver against high-fat intake-triggered tissue damage through alleviating fatal mitochondrial fission to preserve adaptive mitophagy [51]. These findings unveiled the possibility that DNA-PKcs may serve as a causative culprit for mitochondrial damage. This notion received further experimental support where DNA-PKcs upregulated Bax and subsequently promoted Bax translocation onto mitochondria, resulting in cytochrome c release and mitochondrial apoptosis in neurodegenerative diseases [33]. Besides, DNA-PKcs was also suppressed by Bcl2 possibly through a negative feedback mechanism [46] and loss of DNA-PKcs preserved mitochondrial integrity in the face of type 2 diabetes mellitus insult [36]. These findings have increasingly prompted a likely role for DNA-PKcs as a master malefactor for mitochondrial dysfunction, especially in association with Bax-related mitochondrial apoptosis.
Bax-mediated mitochondrial apoptosis is initiated through Bax translocation from cytosol onto mitochondrial outer membrane (MOM) whereby insertion of Bax onto MOM makes the mitochondrial membranes more permeable, resulting in executive phase of the apoptotic process (involving caspase-3 and caspase-9 activation) [15]. Bax inhibitor-1 (BI-1), a highly conserved cell death suppressor, was originally identified to suppress Bax-activated mitochondrial death in yeast through interrupting Bax translocation to mitochondria [37]. More findings have later consolidated the important role of BI-1 in the regulation of cell death, involving diverse subcellular compartments such as death receptor regulation, cellular calcium modulation [30], mitochondrial permeability transition pore (mPTP) opening [26], endoplasmic reticulum (ER) stress [32], autophagy [41] and reactive oxygen species (ROS) generation [21]. Although BI-1 is tied with different physiological and pathophysiological processes, including hepatic ischemic injury, diabetes, liver regeneration, and cancer development [1,2,3, 18, 24], limited information is readily available for its role in cardiovascular system. Given that DNA-PKcs is known to promote Bax-related mitochondrial apoptosis, our present study was designed to unveil the potential role of DNA-PKcs in cardiac IR injury and mitochondrial damage with a focus on BI-1. Data from our current study demonstrated that DNA-PKcs was overtly turned on in response to cardiac IR injury, whereas DNA-PKcs activation promoted cardiomyocyte cell death via downregulation of BI-1 and mitochondrial apoptosis. Genetic ablation of DNA-PKcs favored cardiomyocyte survival and alleviated cardiac IR injury, the effect of which was negated in mice with double deletion of DNA-PKcs and BI-1. These data have laid compelling evidence to unveil the paradigm behind DNA-PKcs signaling in cardiac stress such as cardiac IR injury.
Methods
Human study and animal models
The experimental protocols used in this study involving human subjects were conducted in accordance with the Declaration of Helsinki and approved by Ethics Committee of Zhongshan Hospital, Fudan University, Shanghai, China. Failing human hearts were obtained from end-stage heart failure patients admitted to Zhongshan Hospital for heart transplantation. Donor hearts that could not be transplanted for technical reasons were used for controls. The clinical characteristics of patients are shown in Supplemental Table 1. All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, which was published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and were approved by the Zhongshan Hospital, Fudan University and University of Wyoming Institutional Animal Use and Care Committees (Laramie, WY, USA). The generation of DNA-PKcsf/f mice and BI-1 transgenic (BI-1TG) mice was described by our previous studies [51, 56]. The BI-1f/f mice were generated by the Cyagen Biosciences Inc. The exon 2 of BI-1 gene was selected as the conditional knockout region, and deletion of this region resulted in frameshift of this gene. Gene exons were flanked with loxP sites in vivo. Various mouse lines obtained were backcrossed in a similar manner, and wild-type (WT) mice used were littermates of knockout (KO) mice used in each specific experiment. All mice were crossed on a C57BL/6 N background for at least three generations. Cardiomyocyte-specific DNA-PKcs knockout (DNA-PKcsCKO) and BI-1 knockout (BI-1CKO) mice were generated using DNA-PKcsf/fmice or BI-1f/f with α-MHC (alpha myosin heavy chain)-Cre transgenic mice. Subsequently, DNA-PKcsf/f mice were bred to BI-1CKO mice to generate cardiac-specific DNA-PKcs/BI-1 double knockout (DNA-PKcs/BI-1CKO, DNA-PKcsf/f; BI-1f/f; α-MHCCre+) mice. To avoid potential gender-related variation, all experiments were performed using male mice.
IR injury and infarct size measurement
Mice (8 weeks old) were anesthetized with 1% ~ 3% isoflurane through inhalation (Baxter, Deerfield, IL) and then subject to myocardial IR injury (45-min ischemia followed by 0–24-h reperfusion) as our previously described [57, 58] by temporarily occluding the left anterior descending coronary artery (LAD) and then releasing the occlusion. After reperfusion, in order to demarcate the ischemic area at risk (AAR), animals were perfused with 2% Evans blue/saline. Then, hearts were excised and quickly cut into five 1-mm slices and were incubated with 1% 2,3,5-triphenyltetrazolium chloride (TTC) for 10 min at 37 °C to demonstrate infarct size (IS) [29]. The IS, AAR and total LV areas from individual myocardial section were measured using an Image Pro Plus software (Media Cybernetics, Inc., Bethesda, MD, USA). Percentage of IS and AAR of individual myocardial section was multiplied by the weight of the section. IS/AAR was displayed as percentages, and a correlation between area at risk (x-axis: expressed as a percentage of the LV weight) and infarct size (y-axis: expressed as percentage of the LV weight) was analyzed [12].
Echocardiogram and electron microscopy
Echocardiography was performed in all mice following reperfusion according to our previous studies [53]. An echocardiogram (14.0 MHz, Sequoia C512; Acuson, Germany) was used to detect both 2-dimensional and M-mode images. Electron microscopy was evaluated. In brief, the whole heart was immediately fixed at 4 °C with 2% glutaraldehyde in a 0.1 mol/L sodium cacodylate buffer and post-fixed for 1 h on ice with 1% osmium tetroxide following IR injury. After completion of slice preparation, samples were stained with lead citrate and uranyl acetate and were observed under a Hitachi H600 Electron Microscope (Hitachi, Japan) [47]. At least 30 cells from a minimum of five randomly selected fields were observed.
Cell culture, HR injury induction and transfection
For in vitro modeling, 45 min of hypoxia followed by 6 h of reoxygenation (HR) was employed to mimic the in vivo IR injury. Primary cardiomyocytes were isolated from mice as described [59]. Adenoviral vector expressing DNA-PKcs was constructed by Shanghai Gene-Pharma Co. (Shanghai, China). Serine at site 2056 of the constitutively inactive form of DNA-PKcs (c.i. DNA-PKcs) was replaced with alanine (unable to be phosphorylated at Ser2056). Transfection was performed as described previously [53].
Cell shortening/relengthening assay and cellular viability detection
Mechanical properties of cardiomyocytes were assessed using a SoftEdge MyoCam system (IonOptix, Milton, MA) per our previous report [17]. Cell shortening and relengthening were assessed using the following indices: resting cell length, peak shortening (PS), time to PS (TPS), time-to-90% relengthening (TR90), and maximal velocity of shortening/relengthening (± dL/dt). Cellular viability was monitored using caspase-3 and caspase-9 activity as well as MTT assay. Caspase-9 and caspase-3 activities were determined using caspase assay kits (Beyotime, China), which detect the production of the chromophore p-nitroanilide after its cleavage from the peptide substrate DEVD-p-nitroanilide and LEHD-p-nitroanilide. MTT assay was conducted in accordance with our previous report [57].
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay and ATP detection
TUNEL assay was used to detect cell death following IR and/or HR injury. For quantification, numbers of TUNEL-positive cells in infarcted areas or cardiomyocytes from at least 5 random fields were counted and data were presented as the ratio between respective experimental and control samples. Cellular ATP levels were measured using a firefly luciferase-based ATP fluorescence assay kit (Beyotime) [7].
Mitochondrial potential (ΔΨm) and mPTP detection
mPTP opening was measured by loading cardiomyocytes with 5 μmol/L calcein-AM (Molecular Probes, Eugene, OR) in the presence of 2–5 mmol/L cobalt chloride, as previously described [49]. Fluorescence signal was normalized to that of control group and was used as an index of mPTP opening rate [52]. ΔΨm was analyzed using JC-1 staining (Beyotime Institute of Biotechnology). Briefly, cells were washed with ice-cold PBS and were then stained with 2.5 g/ml JC-1 for 30 min at 37 °C. After rinsing with a binding buffer, cells were analyzed using fluorescence microscopy. Results were presented as relative aggregate-to-monomer (red/green) fluorescence intensity ratio.
Western blotting
Samples were washed with ice-cold PBS and were lysed with RIPA buffer containing a protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA). Lysates were centrifuged at 14,000 ×g for 15 min at 4 ℃. Protein concentration was quantified with Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). Protein extracts were separated using SDS-PAGE and electrotransferred onto PVDF membranes. Membranes were blocked with 5% fat-free milk in TBS-T buffer for 90 min and incubated overnight at 4 °C with primary antibodies. Primary antibodies for immunoblotting were as follows: Drp1 (1:1000, Abcam, #ab184247), p-Drp1 (1:1000, Cell Signaling Technology, #4494), Mff (1:1000, Cell Signaling Technology, #84580), Mfn1 (1:1000, Abcam, #ab57602), FUNDC1 (1:1000, Abcam, #ab224722), Parkin (1:1000, Cell Signaling Technology, #2132), p-DNA-PKcs (1:1000, Abcam, #ab18192), DNA-PKcs (1:1000, Abcam, #ab70250), BI-1 (1:1000, Abcam, #ab18852), Bax (1:1000, Cell Signaling Technology, #2772), Bad (1:1000, Abcam, #ab32445), Bcl-2 (1:1000, Abcam, #ab59348), caspase-9 (1:1000, Cell Signaling Technology, #9504), pro.caspase-3 (1:1000, Cell Signaling Technology, #9662), cleaved caspase-3 (1:1000, Cell Signaling Technology, #9661), TGF-β (1:1000, Cell Signaling Technology, #3711), IL-6 (1:1000, Cell Signaling Technology, #12912), TNFα (1:1000, Cell Signaling Technology, #11948) and VDAC (1:1000, Cell Signaling Technology, #4866). Representative blots were shown from three independent experiments, and images were taken with an enhanced chemiluminescence (ECL) reagent.
Immunohistochemistry and immunofluorescence
Following reperfusion, mouse heart samples were collected. Tissues were then fixed with formalin and were embedded in paraffin. Sections 4–8 μm in thickness were prepared for immunostaining with the anti-p-DNA-PKcs antibody (1:1000, Abcam, #ab18192). Images were acquired using an Olympus digital camera attached to a light microscope.
For immunofluorescence, samples were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.3% Triton X-100 for 5 min and blocked with 10% goat serum albumin for 1 h at room temperature. Specimens were subsequently incubated with respective primary antibodies overnight at 4 °C, washed with PBS three times and incubated with secondary antibodies for 45 min at room temperature. The primary antibodies for immunofluorescence staining were as follows: BI-1 (1:1000, Abcam, #ab18852), ICAM1 (1:1000, Abcam, #ab119871), Gr1 (1:500, Abcam, #ab25377), troponin T (1:500, Abcam, #ab8295) and mitochondrial antibody (Tom20, 1:500, Abcam, #ab186734). Mitochondrial length was determined, and at least 100 mitochondria from 10 cells were selected [15].
Ubiquitination
Ubiquitination assay was performed as previously described [43]. In brief, cardiomyocytes were transfected with adenovirus and were harvested into buffer A prior to sonication. Whole-cell lysates were incubated with 50 μl equilibrated (50%) Ni–NTA-agarose for 3 h at room temperature. Beads were washed with buffer A twice, buffer A/buffer TI (1:3) twice and buffer TI one time. Precipitated proteins were eluted and were then measured using western blot.
Pulse-chase analysis
Cells were radiolabeled with [35S]-Met (100 mCi) for the indicated durations in normal culture condition. Following rinses with PBS, cells were chased using the complete DMEM medium (with 20% FBS) for the indicated duration [42]. Whole-cell lysates were immunoprecipitated with protein G plus beads coated in the indication antibodies. Precipitated proteins were eluted with SDS-PAGE loading buffer and analyzed through western blots.
qPCR assay
Total RNA was extracted from the cells using TRIzol ® reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and was reverse-transcribed into a total of 1 µl (60 ng/µl) cDNA using a One-Step RT-PCR kit (TransGen Biotech Co., Ltd., Beijing, China), according to our previous study [59]. Quantification of gene expression was performed using an ABI PRISM 7500 Sequence Detection system (Applied Biosystems Life Technologies, Foster City, CA) with SYBR® Green (TransGen Biotech Co., Ltd.). The relative mRNA expression levels were normalized to that of β-actin using the 2−ΔΔCT method. The primer sequences were as follows: MCP1 (forward, 5′-GGATGGATTGCACAGCCATT-3′; reverse, 5′-GCGCCGACTCAGAGGTGT-3′), IL-8 (forward, 5′-AAGAGAGCTCTGTCTGGACC-3′; reverse, 5′-GATATTCTCTTGGCCCTTGG-3′); MMP-9 (forward, 5′-GATGCGTGGAGAGTCGAAAT-3′; reverse, 5′-CACCAAACTGGATGACGATG-3′); BI-1 (forward, 5′-TTTGGAGTGGTAGTAAAAAGGGC-3′; reverse, 5′-TGACATCAGGGACTCAGAGTAG-3′).
Mitochondrial DNA (mtDNA) copy numbers and transcriptional level detection
Relative amounts of mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) content were used to assess the mtDNA copy numbers using PCR. The mtDNA and nuclear amplicons were generated from a complex IV segment and GAPDH segment, respectively.
Co-immunoprecipitation
Co-immunoprecipitation experiments were performed as described previously [52]. Briefly, proteins were cross-linked in 1% paraformaldehyde followed by a PBS rinse buffer containing 100 mmol/L glycine. Cells were then lysed by sonication in PBS with 1% Triton X-100 and were incubated with respective antibodies and protein A/G agarose. Immunoprecipitates were loaded on an SDS-PAGE and were probed with the anti-DNA-PKcs antibody.
Statistical analysis
Data are presented as means ± standard error (SEM). Using GraphPad Prism 5.0 software, results were analyzed using one-way ANOVA among multiple groups followed by the Dunnett T3 post hoc analysis. A p value < 0.05 was considered statistically significant.
Results
DNA-PKcs is activated by cardiac IR stress and promotes myocardial damage
To evaluate changes of cardiac DNA-PKcs in response to IR injury, pan and phosphorylated DNA-PKcs were examined. Compared to the sham group, DNA-PKcs phosphorylation at Ser2056 was dramatically elevated following reperfusion peaking at 6 h following reperfusion (Fig. 1a, b). Prolonged reperfusion beyond 6 h failed to provoke further rises in DNA-PKcs phosphorylation. Thus, 6 h was used as the designated reperfusion time for the remaining of our study. Similarly, the level of p-DNA-PKcs was also increased in failing human hearts (Supplemental Fig. 1a–c). In vitro, hypoxia-reoxygenation (HR)-induced DNA-PKcs phosphorylation at 6-h reoxygenation was inhibited by the antioxidant NAC (N-acetylcysteine) and the NADPH oxidase protein inhibitor DPI (diphenyleneiodonium chloride) (Fig. 1c, d), in line with the earlier notion for a role of oxidative stress in DNA-PKcs activation (phosphorylation) [35].
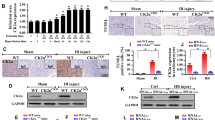
DNA-PKcsCKO mouse hearts are protected against global IR injury. a, b Ventricular homogenates were prepared from DNA-PKcsf/f mice and subjected to western blots at different times point. Forty-five minutes ischemia and 6-h reperfusion significantly elevated the expression of p-DNA-PKcs (n = 6 mice per group). c, d. Cardiomyocyte was isolated from DNA-PKcsf/f mice, and then, hypoxia-reoxygenation injury was used to induce reperfusion injury. Western blots analysis for DNA-PKcs phosphorylation. NAC and DPI were used to attenuate oxidative stress. (n = 6 mice per group). e DNA-PKcsf/f mice and DNA-PKcsCKO mice were subjected to global IR injury (45-min ischemia and 6-h reperfusion). Then, the expression of p-DNA-PKcs was determined in the tissue isolated from reperfused hearts. f Area at risk (AAR) and infarct size (IS) were determined using Evans blue and TTC staining, respectively. The percentages of IS and AAR of each myocardial slice were multiplied by the weight of the section. IS/AAR was presented as percentages. A correlation between area at risk (x-axis: expressed as a percentage of the LV weight) and infarct size (y-axis: expressed as percentage of the LV weight) was analyzed. In all groups, infarct size was positively and linearly related to area at size. g After reperfusion injury, ventricular was collected and the cardiomyocyte death was determined via TUNEL staining. h–i Cardiomyocytes were infected with adenovirus-loaded β-gal (Ad-β-gal) or DNA-PKcs (Ad-DNA-PKcs) at the indicated multiplicity of infection (MOI). Then, cardiomyocytes were treated with HR injury and the expression of p-DNA-PKcs was determined. j–k Cellular death as assessed by TUNEL staining after infection with Ad-β-gal or Ad-DNA-PKcs in the presence of HR injury. Scale bar: 100 μm. Experiments were repeated three times with similar results. Data are shown as the means ± SEM, n = 6 mice or 3 independent cell isolations per group. *p < 0.05. ANOVA between multiple groups followed by Dunnett T3 post hoc
To further discern the role of DNA-PKcs in IR injury, cardiomyocyte-specific DNA-PKcs knockout (DNA-PKcsCKO) mice were generated and were subjected to IR challenge. Western blots (Fig. 1e) and immunohistochemistry assay (Supplemental Fig. 1d, e) revealed that IR-induced DNA-PKcs phosphorylation was abolished in DNA-PKcsCKO mice, validating the murine model. More importantly, IR injury-induced myocardial infarction (Fig. 1f) and TUNEL apoptosis (Fig. 1g) were significantly alleviated by DNA-PKcs ablation.
The beneficial effect of DNA-PKcs deletion against IR injury was further consolidated in cardiomyocytes isolated from DNA-PKcsf/f and DNA-PKcsCKO mice. In vitro caspase-3 activity (Supplemental Fig. 1f) and MTT assay (Supplemental Fig. 1g) displayed that DNA-PKcs deficiency mitigated HR-triggered cardiomyocyte death while generating little effect on cardiomyocyte viability and apoptotic index itself. Next, we employed an adenoviral vector (Ad-DNA-PKcs) to assess DNA-PKcs signaling in mouse cardiomyocytes. Ad-DNA-PKcs transfection exhibited no effect on DNA-PKcs phosphorylation probably due to the physiological redox setting (Fig. 1h, i). However, in the face of HR challenge, Ad-DNA-PKcs transfection promoted DNA-PKcs phosphorylation in a dose-dependent manner compared with Ad-β-gal transfection (Fig. 1h, i). These data favored that DNA-PKcs may be particularly controlled by reperfusion injury. Moreover, overexpression of DNA-PKcs in HR-treated cardiomyocyte led to a robust cellular death in a dose-dependent manner, as evidenced by a significant rise in cell death (as assessed by TUNEL staining) (Fig. 1j, k) and a loss in cell membrane integrity (as assessed by LDH release) (Supplemental Fig. 1h). Taken together, these findings depicted a role for IR-mediated oxidative status in DNA-PKcs activation, resulting in oxidative cardiomyocyte death.
DNA-PKcs deletion sustains cardiac function and attenuates myocardial inflammation caused by IR injury
In Fig. 2a–c, little difference was observed between DNA-PKcsf/f and DNA-PKcsCKO mice in the absence of IR challenge although DNA-PKcs deletion greatly alleviated IR-compromised cardiac function. Consistently, electron microscopic examination revealed that IR injury evoked mitochondrial swelling (white arrowheads), cardiomyocyte dissolution (yellow arrowheads) and Z line disappearance (red arrowheads). However, DNA-PKcs knockout preserved cardiac structural organization with IR challenge (Fig. 2d). Besides, DNA-PKcs deletion overtly decreased levels of cardiac injury markers such as LDH, troponin T and CK-MB (Supplemental Fig. 2a–c). In agreement with in vivo findings, DNA-PKcs deletion reversed cardiomyocyte contractile dysfunction in IR injury (Supplemental Fig. 2d–i).
Reperfusion-mediated cardiac dysfunction and cardiac inflammation response are alleviated in DNA-PKcsCKO mouse. a–c Cardiac contractile function [ejection fraction (EF) and fractional shortening (FS)] and diastolic function [left ventricular diastolic dimension (LVDd)] were assessed by echocardiography in DNA-PKcsf/f and DNA-PKcCKO mice. d Electron microscope was used to observe the ultra-structure of myocardium in DNA-PKcsf/f and DNA-PKcCKO mice after reperfusion. Scale bar: 2 μm. e, f After reperfusion injury, ventricular was collected and the expression of ICAM-1 was determined via immunohistochemistry. Scale bar: 250 μm. g, h Gr-1 inflammation cells accumulation was observed via immunofluorescence. Troponin T was used to stain myocardium. Scale bar: 125 μm. i–l After reperfusion injury, ventricular was collected and the expression of inflammation factors were measured via western blots. Experiments were repeated three times with similar results. Data are shown as the means ± SEM, n = 6 mice or 3 independent cell isolations per group. *p < 0.05. ANOVA between multiple groups followed by Dunnett T3 post hoc
Our data further revealed profound myocardial inflammation in response to IR challenge. In Fig. 2e, f, IR injury overtly elevated the adhesion molecule ICAM-1, the effect of which was alleviated by DNA-PKcs depletion. In addition, decreased adhesion molecule in DNA-PKcsCKO hearts led to reduced recruitment of Gr1+ neutrophil in myocardium, suggesting an inductive role for DNA-PKcs in myocardial reperfusion inflammation response (Fig. 2g, h). Along the same line, DNA-PKcs depletion suppressed reperfusion-mediated rises in pro-inflammatory factors, as evidenced by data from western blotting (Fig. 2i–l) and qPCR (Supplemental Fig. 2j–l). These findings suggested that disruption of DNA-PKcs confers protection against global IR injury-mediated cardiac malfunction.
DNA-PKcs deficiency prevents cardiomyocytes against mitochondria-related cell death
Mitochondrial apoptosis is a critical event leading to execution of cell death in IR injury. Given the pathogenic role of DNA-PKcs in mitochondrial damage noted in obesity and aging [8, 28, 36], we went on to examine whether IR-mediated cardiomyocyte mitochondrial apoptosis is attributable to DNA-PKcs activation. Our data revealed that HR injury promoted mPTP opening (Fig. 3a) and caspase-9/3 activation while these responses were suppressed by DNA-PKcs deletion (Fig. 3b–h).
Mitochondrial function is sustained in DNA-PKcCKO mice. a mPTP opening rate was measured via analyzing the arbitrary mPTP opening time. b–h Cardiomyoyctes were infected with Ad-β-gal or Ad-DNA-PKcs. Then, western blots were used to evaluate the parameters related to mitochondrial apoptosis, especially mitochondrial Bax translocation. VDAC is the loading control for mitochondrial Bax. Experiments were repeated three times with similar results. Data are shown as the means ± SEM, n = 6 mice or 3 independent cell isolations per group. *p < 0.05. ANOVA between multiple groups followed by Dunnett T3 post hoc
Given the apparent positive tie between DNA-PKcs and Bax, we tested whether DNA-PKcs deletion could counter mitochondrial apoptosis through scavenging mitochondrial Bax (mito-Bax). In vitro findings revealed that IR injury upregulated, whereas DNA-PKcs deficiency downregulated the levels of mito-Bax (Fig. 3b–h). In addition, DNA-PKcs deletion was also closely associated with augmented mitochondrial anti-apoptotic factors such as Bcl2. However, the pro-apoptotic element for mitochondrial death, such as Bad, was greatly reduced in response to DNA-PKcs deficiency (Fig. 3b–h). Reminiscent results were obtained in vivo (Supplemental Fig. 3a–f). Taken together, these data indicate that DNA-PKcs deletion retards Bax-related mitochondrial apoptosis triggered by IR injury.
To establish a relationship between partial activation of DNA-PKcs and mitochondrial dysfunction as well as cardiomyocyte damage, changes in mitochondria function and cardiomyocyte viability were measured at 30 min post-reperfusion. As shown in Supplemental Fig. 3g, caspase-3 activity in myocardium was significantly elevated at 30 min of reperfusion and remained elevated at 6 h post-reperfusion. Similar changes in mitochondrial function and cardiomyocyte viability were also observed in vitro through mitochondrial potential assay (Supplemental Fig. 3h, i) and MTT assay (Supplemental Fig. 3j), respectively. These data revealed that IR-mediated early myocardial damage and mitochondrial injury could be attenuated by DNA-PKcs deletion.
BI-1 is stabilized in DNA-PKcsCKO myocardium
BI-1 is a pro-survival molecule which interrogates Bax activation. Therefore, these characteristics of BI-1 might contribute to the protective properties of DNA-PKcs deletion against IR injury-induced mitochondrial damage. In failing human hearts (Supplemental Fig. 1a–c), expression of BI-1 was markedly downregulated. Similarly, IR injury caused a significant decrease in BI-1 expression in DNA-PKcsf/f mice, and this effect also occurred at an early stage of reperfusion (30 min after reperfusion) (Supplemental Fig. 4a, b). However, DNA-PKcs deletion prevented BI-1 downregulation at both early and late stages of reperfusion (Supplemental Fig. 4a, b). These data were confirmed in HR-challenged cardiomyocytes in vitro (Fig. 4a, b), revealing an inverse correlation between BI-1 and DNA-PKcs in IR injury.
DNA-PKcs deficiency stabilized BI-1 in cardiomyocytes. a, b BI-1 expression was determined in cardiomyocytes infected with Ad-β-gal or Ad-DNA-PKcs via western blots in the presence of HR injury. c The expression of BI-1 was determined in cardiomyocyte in the presence and/or absence of MG132. d, e The half-life of BI-1 protein is determined in cardiomyocytes through a pulse-chase assay. The expression of BI-1 was determined via western blots. f DNA-PKcs directly prompts BI-1 ubiquitination in a dose-dependent manner. In vitro, ubiquitination assays were performed with the indicated HA-ubiquitin and Ad-DNA-PKcs. g, h Endogenous BI-1 and DNA-PKcs proteins communication. Co-immunoprecipitation (IP) assays were performed in the presence of HR injury. i–k DNA-PKcs mutant, a constitutively inactive form of DNA-PKcs (c.i. DNA-PKcs) with serine 2056 replaced with alanine (cannot be phosphorylated), was transfected into DNA-PKcs-deleted cardiomyocytes. Then, western blots were used to obverse the expression of p-DNA-PKcs and BI-1. l, m The half-life of BI-1 protein is determined in cardiomyocytes infected with c.i. DNA-PKcs through a pulse-chase assay. The expression of BI-1 was determined via western blots. Experiments were repeated three times. Data are shown as the means ± SEM, n = 6 mice or 3 independent cell isolations per group. *p < 0.05. ANOVA between multiple groups followed by Dunnett T3 post hoc
To further consolidate the unique effect of DNA-PKcs on BI-1 expression, we overexpressed DNA-PKcs in cardiomyocytes from WT mice through Ad-DNA-PKcs transfection. Under normoxic condition, Ad-DNA-PKcs displayed little effect on DNA-PKcs phosphorylation (Fig. 1h, i) and BI-1 expression (Fig. 4a, b). However, in the face of HR challenge, Ad-DNA-PKcs transfection dose-dependently provoked DNA-PKcs phosphorylation (Fig. 1h, i) and consequently reduced BI-1 expression compared with Ad-β-gal transfection (Fig. 4a, b). In contrast, deletion of DNA-PKcs reversed IR-induced loss in BI-1 levels (Fig. 4a, b), reconfirming the essential role of DNA-PKcs as an upstream negative regulator for BI-1. Unexpectedly, BI-1 mRNA levels did not differ between DNA-PKcs-deleted or DNA-PKcs-overexpressed cardiomyocytes in the absence or presence of HR challenge (Supplemental Fig. 4c), suggesting that DNA-PKcs regulates BI-1 at the post-transcriptional level. Interestingly, Western analysis showed that Ad-DNA-PKcs transfection-induced loss of BI-1 protein levels was negated by MG132, a proteasomal inhibitor (Fig. 4c). Notably, the pulse-chase analysis demonstrated that Ad-DNA-PKcs transfection increased, whereas DNA-PKcs deficiency decreased, the degradation rate of endogenous BI-1 protein in cardiomyocytes with HR challenge (Fig. 4d, e). Consistent with these findings, the poly-ubiquitination of BI-1 was significantly decreased in DNA-PKcs-depleted cells and was dose-dependently upregulated along with Ad-DNA-PKcs transfection (Fig. 4f). Constitutive interaction between endogenous DNA-PKcs and BI-1 was verified using co-immunoprecipitation (Fig. 4g, h), suggesting that DNA-PKcs negatively impacts BI-1 stabilization through promoting proteasomal degradation, independent of transcription regulation.
To elucidate whether DNA-PKcs phosphorylation was obligatory for BI-1 degradation, the DNA-PKcs mutant, a constitutively inactive form of DNA-PKcs (c.i. DNA-PKcs) with Ser2056 being replaced with alanine (unable to be phosphorylated at Ser2056), was transfected into cardiomyocytes. With c.i.DNA-PKcs transfection, there was no change in the transcription of BI-1 (Supplemental Fig. 4d). However, HR-mediated DNA-PKcs phosphorylation was disrupted (Fig. 4i–k), whereas the half-life of BI-1 was prolonged (Fig. 4l, m), in comparison with the Ad-β-gal transfection. These data highlighted that DNA-PKcs needs to be phosphorylated prior to its binding with BI-1 to initiate BI-1 degradation.
As observed above, DNA-PKcs was activated by oxidative stress (Fig. 1c, d) which might induce covalent and conformational changes of BI-1 for its elimination. To understand the role of oxidative stress in DNA-PKcs-induced BI-1 downregulation, NAC was used in HR-challenged cardiomyocytes. Following HR challenge, both of BI-1 stabilization (Supplemental Fig. 4e,f) and expression (Supplemental Fig. 4g, h) were reduced, and these alterations were greatly obliterated by NAC supplementation. Conversely, in cardiomyocytes with DNA-PKcs deletion, HR-mediated BI-1 downregulation was inhibited, and thus, NAC exhibited little notable effect of BI-1 expression (Supplemental Fig. 4g, h), suggesting that oxidative stress participates in ischemia or hypoxia-triggered BI-1 downregulation, a process dependent on DNA-PKcs activation.
Genetic inhibition of BI-1 abolishes the protection observed in DNA-PKcsCKO mice
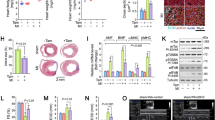
Considering that DNA-PKcs deletion was associated with an increase in BI-1 stabilization and expression, in junction with the inhibitory property of BI-1 on Bax-mediated mitochondrial apoptosis, we questioned whether cardioprotection afforded by DNA-PKcs deletion against IR injury is attributed to BI-1 upregulation. To test our hypothesis, cardiomyocyte-specific DNA-PKcs/BI-1 double-deficient mice were generated. Loss of protein levels of both DNA-PKcs and BI-1 proteins was confirmed using western blot analysis (Fig. 5a–c). Compared with the sham group, IR injury imposed more pronounced cardiac damage in BI-1CKO mice (Supplemental Fig. 5a–g), in contrary to reduced infarcted area (Fig. 5d–f), less TUNEL+ cells (Fig. 5g, h), attenuated cardiac damage (Fig. 5i–k) and improved cardiac function (Fig. 5l, m) in DNA-PKcsCKO mice. Interestingly, genetic ablation of BI-1 in DNA-PKcsCKO mice nullified the protective effects of DNA-PKcs ablation with expanded infarct zone, exacerbated cardiac function and cell death. Ultrastructural image revealed extensive mitochondrial vacuolization and myocyte dissolution in hearts from control DNA-PKcs/BI-1f/f mice subjected to IR injury (Fig. 5n). However, DNA-PKcs knockout-preserved myocardial histology was negated by BI-1 depletion (Fig. 5n).
BI-1 ablation abolishes the protection exerted by DNA-PKcs deletion on reperfused hearts. a–c DNA-PKcs/BI-1f/f, DNA-PKcsCKO, BI-1CKO and DNA-PKcs/BI-1CKO mice were generated. Ventricular homogenates from all groups were subjected to western blots analysis to determine levels of DNA-PKcs and BI-1 protein. D–f All groups were subjected to IR injury (45-min ischemia and 6-h reperfusion). Area at risk (AAR) and infarct size (IS) were determined using Evans blue and TTC staining, respectively. The percentages of IS and AAR of each myocardial slice were multiplied by the weight of the section. IS/AAR was presented as percentages. A correlation between area at risk (x-axis: expressed as a percentage of the LV weight) and infarct size (y-axis: expressed as percentage of the LV weight) was analyzed. In all groups, infarct size was positively and linearly related to area at size. g, h Representative images from TUNEL staining of heart sections after IR injury were shown. Sections were counterstained with DAPI (blue) and troponin-T (red) to identify nuclei and cardiomyocytes, respectively (merged). Scale bar: 175 μm. i–k After reperfusion injury, blood was collected, and then, the concentration of cardiac damage markers was determined via ELISA in all groups. l, m Parameters of ventricular function were determined by echocardiography. n Electron microscope was used to observe the ultra-structure of myocardium in all groups after reperfusion. Scale bar: 2 μm. o Ventricular homogenates from all groups were collected, and then, western blots were used to evaluate the parameters related to mitochondrial apoptosis, especially mitochondrial Bax translocation. VDAC is the loading control for mitochondrial Bax. Experiments were repeated three times. Data are shown as the means ± SEM, n = 6 mice or 3 independent cell isolations per group. *p < 0.05. ANOVA between multiple groups followed by Dunnett T3 post hoc
Similar findings were noted in cardiomyocytes isolated from control DNA-PKcs/BI-1f/f mice, DNA-PKcsCKO, BI-1CKO and DNA-PKcs/BI-1CKO mice. Cell viability and survival rate, evaluated using TUNEL staining (Supplemental Fig. 5h, i) and caspase-3 activity assay (Supplemental Fig. 5j), were drastically decreased in response to HR insult, the effect of which was reverted to normal levels with DNA-PKcs deficiency. Likewise, BI-1 deletion nullified the protective effect of DNA-PKcs knockout on cardiomyocyte viability and survival index. We went on to evaluate Bax-related mitochondrial apoptosis. Our data revealed that HR-mediated mitochondrial Bax translocation was greatly attenuated by DNA-PKcs deletion in a BI-1-dependent manner both in vivo (Fig. 5o) and in vitro (Supplemental Fig. 5k–o). Taken together, these observations depicted that the restored BI-1 activity accounts for DNA-PKcs deletion-offered beneficial effects against mitochondrial damage and cardiac IR injury.
BI-1 maintains mitochondrial structure and function in IR injury setting
In addition to Bax-related mitochondrial apoptosis, BI-1 maintains mitochondrial function and structure via multiple mechanisms [22, 31, 41]. In light of the cardinal role of mitochondria in cardiac IR injury, we went on to examine the role of DNA-PKcs/BI-1 signaling cascade in mitochondrial homeostasis. Mitochondrial morphology was evaluated using immunofluorescence assay. Reperfusion stimulus forced mitochondria to divide into fragments (Fig. 6a, b), accompanied with an increase in the expression of mitochondrial fission-related proteins such as p-Drp1 and Mff (Fig. 6c–h). Whereas mitochondrial fusion-dependent factor such as Mfn1 and mitophagy parameters such as FUNDC1 and Parkin were both markedly reduced in response to reperfusion challenge (Fig. 6c–h). Interestingly, DNA-PKcs deficiency preserved mitochondrial network structure, repressed fission-related factors, reversed fusion-associated elements and upregulated mitophagy. However, BI-1 deficiency negated the protective effects of DNA-PKcs deletion on mitochondrial dynamic homeostasis.
Mitochondrial dynamics are maintained by DNA-PKcs deficiency via BI-1 in reperfused hearts. a, b Cardiomyocytes were isolated from DNA-PKcs/BI-1f/f mice, DNA-PKcsCKO, BI-1CKO and DNA-PKcs/BI1CKO mice. Mitochondria immunofluorescence assay. The average length of mitochondria was determined in each cardiomyocyte. n = 10 cells per group and experiments were repeated three times. Scale bar: 10 μm. c–h Ventricular homogenates from all groups were collected, and then, western blots were used to evaluate the parameters related to mitochondrial dynamics. Phosphorylated Drp1 and Mff are the factors associated with mitochondrial fission. Mfn1 is the mitochondrial fusion-related proteins. FUNDC1 and Parkin are the mediators involving receptor-dependent and receptor-independent mitophagy. Experiments were repeated three times. Data are shown as the means ± SEM, n = 6 mice or 3 independent cell isolations per group. *p < 0.05. ANOVA between multiple groups followed by Dunnett T3 post hoc
Based on our previous evidence [57, 58], the functional consequence of aberrant mitochondrial dynamics is the imbalance distribution of mitochondrial genome into daughter mitochondria. In Supplemental Fig. 6a–c, HR injury impaired mtDNA copy and transcripts, and these effects of which were inhibited by DNA-PKcs in a BI-1-dependent manner. The electron transport chain complexes’ (ETC) activity is mainly controlled by mtDNA. However, HR reduced the activity of ETC (Supplemental Fig. 6d–f), which was reversed via DNA-PKcs deletion in a BI-1-dependent manner.
BI-1 overexpression mediates cardioprotection under reperfusion insults
To further consolidate the beneficial role of BI-1 in reperfused hearts, BI-1TG mice were used. Compared to the sham group, BI-1 levels were downregulated by reperfusion (Fig. 7a, b), associated with increased histological injury including expanded infarct zone (Fig. 7c–e) and impaired cardiac function (Fig. 7f–h). However, BI-1TG mice displayed a significant drop in infarct area size and improved myocardial function following IR injury. Moreover, reperfusion-mediated cardiomyocyte death (Fig. 7i) and mitochondrial Bax-related apoptosis (Fig. 7j–l) were greatly attenuated in BI-1TG mice. These data indicated that reintroduction of BI-1 protein benefits hearts and maintains mitochondrial integrity in the face of cardiac reperfusion injury.
BI-1 transgenic mice (BI-1TG) are protected against myocardial reperfusion injury. a, b Ventricular homogenates were prepared from wild-type mice (WT) and BI-1TG mice subjected to IR injury (45-min ischemia and 6-h reperfusion). Ventricular homogenates from all groups were subjected to western blots analysis to determine levels of BI-1 protein. c–e All groups were subjected to IR injury. Area at risk (AAR) and infarct size (IS) were determined using Evans blue and TTC staining, respectively. The percentages of IS and AAR of each myocardial slice were multiplied by the weight of the section. IS/AAR was presented as percentages. A correlation between area at risk (x-axis: expressed as a percentage of the LV weight) and infarct size (y-axis: expressed as percentage of the LV weight) was analyzed. In all groups, infarct size was positively and linearly related to area at size. f–h Parameters of ventricular function were determined by echocardiography. i Ventricular homogenates were prepared from wild-type cells (WT) and BI-1TG mice subjected to IR injury. Then, caspase-3 activity was measured via ELISA assay. j–l Ventricular homogenates from all groups were collected, and then, western blots were used to evaluate the parameters related to mitochondrial apoptosis, especially mitochondrial Bax translocation. VDAC is the loading control for mitochondrial Bax. Experiments were repeated three times with similar results. Data are shown as the means ± SEM, n = 6 mice or 3 independent cell isolations per group. *p < 0.05. ANOVA between multiple groups followed by Dunnett T3 post hoc
Discussion
The salient finding from our present study revealed a unique role for DNA-PKcs and its downstream mediator BI-1 in the onset and development of cardiac IR injury possibly through preservation of mitochondrial homeostasis. Our results revealed that (1) DNA-PKcs was significantly upregulated in reperfused hearts; (2) higher DNA-PKcs expression promoted scar expansion, myocardial dysfunction and cardiomyocyte death in IR injury; (3) mechanistic examination revealed that DNA-PKcs activation imposed overt damage to mitochondrial structure/function and rendered cardiomyocytes to mitochondrial apoptosis via promoting BI-1 degradation; (4) loss of DNA-PKcs reversed BI-1 levels and, consequently, suppressed pathological mitochondrial fission, promoted mitophagy, disrupted Bax-related mitochondrial apoptosis, limited infarction zone formation and maintained myocardial function under IR injury; (5) genetic ablation of BI-1 abolished DNA-PKcs deletion-offered protection both in vivo and in vitro; and (6) reintroduction of BI-1 benefited hearts against reperfusion through sustaining mitochondrial homeostasis. Our finding revealed a novel pathological regulatory role for DNA-PKcs activation, BI-1 inhibition and mitochondrial damage in the face of IR injury.
DNA-PKcs is a recognized signaling molecule sensing DNA damage to initiate DNA double-strand repair [38]. Later evidence has suggested that DNA-PKcs may modulate cell fate beyond DNA repair. In particular, DNA-PKcs was primarily activated in response to pathological stress including radiation [19], aging [8], diabetes [36], cardiomyopathy [48] and heart failure [4]. Although DNA-PKcs is believed to be essential for preservation of genomic stability by repairing the apurinic/apyrimidinic sites, excessive DNA-PKcs activation may be malignant. Moderate DNA-PKcs activation preferentially cooperates and helps Ku80 to recognize and interact the broken segments of DNA [40], although hyperactivation of DNA-PKcs unfortunately phosphorylates p53 to selectively initiate p53-dependent apoptosis in hepatocarcinnoma [50]. This notion received further support from earlier studies whereby DNA-PKcs inhibition promotes human endothelial cell proliferation [34], alleviates doxorubicin-induced cardiac toxicity [23] and counters obesity-induced cardiomyocyte apoptosis, fatty liver disease progression [54] and mitochondrial maladaptation in metabolism during aging [36]. These findings have uncovered that DNA-PKcs hyperactivation directly promotes cell damage although basal levels of DNA-PKcs are expected to function as a cell survival cue to maintain DNA homeostasis. The distinct effects of DNA-PKcs on cell fate ranging from survival to death may be dependent upon the levels of oxidative stress, and the nature of upstream activators for DNA-PKcs. In fact, physiological levels of reactive oxygen species function as signal molecular to mediate necessary biological responses, whereas excessive oxidative stress such as superoxide anion produced during IR injury is fatal to cardiomyocyte survival. This observation is in line with a previous study that oxidative stress-mediated DNA-PKcs activation was negated by antioxidants, resulting in attenuated airway inflammation [35]. Interestingly, an early report illuminated that DNA damage may be more pronounced in coronary artery disease (CAD) and an inverse correlation may exist between DNA damage and total antioxidant capacity in patients with confirmed coronary artery diseases [10]. In contrary, a recent report revealed that ROS overproduction seems to be evoked by DNA-PKcs inactivation and DNA-PKcs deficiency augments H2O2-mediated death in a colon cancer cell line [27]. Thus, potential interaction between DNA-PKcs and oxidative stress may display tissue and cell specificity. Moderate activation of DNA-PKcs seems to be protective, whereas the excessive DNA-PKcs hyperactivation may be the consequence of uncontrolled oxidative injury. This hypothesis deserves some further scrutiny to determine the “fatal threshold” of DNA-PKcs activation.
Findings from our present study demonstrated that BI-1 upregulation is involved in the protective effect of DNA-PKcs deletion on cardiac IR injury. DNA-PKcs has been proven to be associated with Bax-related mitochondrial apoptosis via promoting Bax release from the Ku70-Bax complex [33]. Moreover, DNA-PKcs was also suppressed by Bcl2 [46] and loss of DNA-PKcs sustains mitochondrial function and protects against type 2 diabetes mellitus [36]. These findings are in favor of the role of DNA-PKcs as an initiator of cell death via triggering Bax-related mitochondrial apoptosis in various diseases, and also our current experimental setting. In addition, we noted that BI-1 degradation accounts for the DNA-PKcs-mediated Bax-dependent mitochondrial apoptosis. In response to cardiac IR injury, BI-1 transcription remains relatively unchanged, whereas its protein expression was downregulated due to DNA-PKcs activation. Mechanistically, DNA-PKcs was able to directly interact with and promoted BI-1 degradation in a proteasome-dependent manner, indicating that DNA-PKcs post-transcriptionally manages BI-1 activity. Loss of DNA-PKcs restored BI-1 activity and protected hearts against lethal consequence of IR injury, whereas DNA-PKcs and BI-1 double-deficient mice failed to display protection against cardiac IR injury, providing a piece of compelling evidence for a role of BI-1 downstream of DNA-PKcs. Notably, BI-1 is believed to protect liver, kidney and brain against reperfusion injury through retarding ER stress [6]. Consistent with these findings, our study offered the first piece of evidence for an endogenous protective role of BI-1 in cardiac reperfusion injury through gatekeeping mitochondrial homeostasis. Moreover, our data have enriched the upstream regulatory system of BI-1 stabilization. Although the transcriptional regulatory mechanism for BI-1 expression has not been well recognized, its protein degradation was fine-tuned by DNA-PKcs especially at the stage of reperfusion. These findings denoted DNA-PKcs/BI-1 signaling pathways as a potential therapeutic target in the management of acute cardiac stress.
One intriguing finding from our study suggests that the DNA-PKcs/BI-1 signaling cascade participates in the regulation of mitochondrial dynamics including mitochondrial fission and mitophagy, in addition to its well-established impact on mitochondrial apoptosis. In our hands, IR-induced excessive mitochondrial fission and defective mitophagy were mitigated by DNA-PKcs deletion in a BI-1-dependent manner. Our earlier studies have denoted the deleterious impact of mitochondrial fission and the benefit of mitophagy on mitochondrial damage and cardiac IR injury [52, 57, 58]. Here in this study, we depicted that fission and mitophagy may share a common upstream trigger, using the DNA-PKcs/BI-1 axis as the intricate and unique regulatory module to modulate mitochondrial apoptosis, mitochondrial fission and mitophagy. These findings should help to enrich the concept for mitochondrial homeostasis in cardiac IR injury. At the molecular levels, several mechanisms may be considered for the BI-1-related mitochondrial fission arrest following IR injury, including ROS scavenging and calcium buffering [16, 25], commonly known as the initial signals for mitochondrial fission [20]. Future study is warranted to elucidate DNA-PKCs/BI-1-modified mitophagy activity.
Several experimental limitations exist for our present study. First, DNA-PKcs was turned on at a relatively early stage of reperfusion, with its expression peaking after 6-h reperfusion. Although DNA-PKcs activation may be one of the initial molecular signals for BI-1 downregulation, mitochondrial dysfunction and cardiac functional damage, it is still premature to draw a conclusion that DNA-PKcs activation, BI-1 downregulation and mitochondrial damage all occur within a same time window. The apparent match in timing among DNA-PKcs activation, BI1 downregulation and cardiomyocyte damage deserves further scrutiny. Moreover, myocardial IR injury is a rather complex and rapidly progressing process. Our data just focused on the role of DNA-PKcs activation in BI-1 stabilization and cardiomyocyte mitochondrial damage. More studies are needed to further explore the roles of time-dependent and/or dose-dependent DNA-PKcs activation in BI-1 downregulation and IR-induced myocardial damage.
Taken together, data from our study suggested that DNA-PKcs is activated in response to IR injury, leading to cardiomyocytes mitochondrial apoptosis through BI-1 inhibition. These findings helped to delineate for the first time the pivotal role of DNA-PKcs/BI-1 cascade in the pathogenesis of mitochondrial disharmony and myocardial death, and the therapeutic potential of targeting DNA-PKcs-BI-1 in the management of myocardial reperfusion injury.
References
Bailly-Maitre B, Bard-Chapeau E, Luciano F, Droin N, Bruey JM, Faustin B, Kress C, Zapata JM, Reed JC (2007) Mice lacking bi-1 gene show accelerated liver regeneration. Cancer Res 67:1442–1450. https://doi.org/10.1158/0008-5472.CAN-06-0850
Bailly-Maitre B, Belgardt BF, Jordan SD, Coornaert B, von Freyend MJ, Kleinridders A, Mauer J, Cuddy M, Kress CL, Willmes D, Essig M, Hampel B, Protzer U, Reed JC, Bruning JC (2010) Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance. J Biol Chem 285:6198–6207. https://doi.org/10.1074/jbc.M109.056648
Bailly-Maitre B, Fondevila C, Kaldas F, Droin N, Luciano F, Ricci JE, Croxton R, Krajewska M, Zapata JM, Kupiec-Weglinski JW, Farmer D, Reed JC (2006) Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci USA 103:2809–2814. https://doi.org/10.1073/pnas.0506854103
Bartunek J, Vanderheyden M, Knaapen MW, Tack W, Kockx MM, Goethals M (2002) Deoxyribonucleic acid damage/repair proteins are elevated in the failing human myocardium due to idiopathic dilated cardiomyopathy. J Am Coll Cardiol 40:1097–1103 (discussion 1104–1095)
Cao J, Lin G, Gong Y, Pan P, Ma Y, Huang P, Ying M, Hou T, He Q, Yang B (2016) DNA-PKcs, a novel functional target of acriflavine, mediates acriflavine's p53-dependent synergistic anti-tumor efficiency with melphalan. Cancer Lett 383:115–124. https://doi.org/10.1016/j.canlet.2016.09.029
Chae HJ, Kim HR, Xu C, Bailly-Maitre B, Krajewska M, Krajewski S, Banares S, Cui J, Digicaylioglu M, Ke N, Kitada S, Monosov E, Thomas M, Kress CL, Babendure JR, Tsien RY, Lipton SA, Reed JC (2004) BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell 15:355–366. https://doi.org/10.1016/j.molcel.2004.06.038
Chen W, Zou P, Zhao Z, Chen X, Fan X, Vinothkumar R, Cui R, Wu F, Zhang Q, Liang G, Ji J (2016) Synergistic antitumor activity of rapamycin and EF24 via increasing ROS for the treatment of gastric cancer. Redox Biol 10:78–89. https://doi.org/10.1016/j.redox.2016.09.006
Chung JH (2018) The role of DNA-PK in aging and energy metabolism. FEBS J 285:1959–1972. https://doi.org/10.1111/febs.14410
Cooper A, Garcia M, Petrovas C, Yamamoto T, Koup RA, Nabel GJ (2013) HIV-1 causes CD4 cell death through DNA-dependent protein kinase during viral integration. Nature 498:376–379. https://doi.org/10.1038/nature12274
Demirbag R, Yilmaz R, Kocyigit A (2005) Relationship between DNA damage, total antioxidant capacity and coronary artery disease. Mutat Res 570:197–203. https://doi.org/10.1016/j.mrfmmm.2004.11.003
Ghonim MA, Pyakurel K, Ju J, Rodriguez PC, Lammi MR, Davis C, Abughazleh MQ, Mansy MS, Naura AS, Boulares AH (2015) DNA-dependent protein kinase inhibition blocks asthma in mice and modulates human endothelial and CD4(+) T-cell function without causing severe combined immunodeficiency. J Allergy Clin Immunol 135:425–440. https://doi.org/10.1016/j.jaci.2014.09.005
Hausenloy DJ, Barrabes JA, Botker HE, Davidson SM, Di Lisa F, Downey J, Engstrom T, Ferdinandy P, Carbrera-Fuentes HA, Heusch G, Ibanez B, Iliodromitis EK, Inserte J, Jennings R, Kalia N, Kharbanda R, Lecour S, Marber M, Miura T, Ovize M, Perez-Pinzon MA, Piper HM, Przyklenk K, Schmidt MR, Redington A, Ruiz-Meana M, Vilahur G, Vinten-Johansen J, Yellon DM, Garcia-Dorado D (2016) Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol 111:70. https://doi.org/10.1007/s00395-016-0588-8
Heusch G (2018) 25 years of remote ischemic conditioning: from laboratory curiosity to clinical outcome. Basic Res Cardiol 113:15. https://doi.org/10.1007/s00395-018-0673-2
Heusch G (2019) Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol 114:45. https://doi.org/10.1007/s00395-019-0756-8
Hockings C, Alsop AE, Fennell SC, Lee EF, Fairlie WD, Dewson G, Kluck RM (2018) Mcl-1 and Bcl-xL sequestration of Bak confers differential resistance to BH3-only proteins. Cell Death Differ 25:719–732. https://doi.org/10.1038/s41418-017-0010-6
Hossain MK, Saha SK, Abdal Dayem A, Kim JH, Kim K, Yang GM, Choi HY, Cho SG (2018) Bax inhibitor-1 acts as an anti-influenza factor by inhibiting ROS mediated cell death and augmenting heme-oxygenase 1 expression in influenza virus infected cells. Int J Mol Sci. https://doi.org/10.3390/ijms19030712
Hu N, Han X, Lane EK, Gao F, Zhang Y, Ren J (2013) Cardiac-specific overexpression of metallothionein rescues against cigarette smoking exposure-induced myocardial contractile and mitochondrial damage. PLoS ONE 8:e57151. https://doi.org/10.1371/journal.pone.0057151
Hunsberger JG, Machado-Vieira R, Austin DR, Zarate C, Chuang DM, Chen G, Reed JC, Manji HK (2011) Bax inhibitor 1, a modulator of calcium homeostasis, confers affective resilience. Brain Res 1403:19–27. https://doi.org/10.1016/j.brainres.2011.05.067
Ihara M, Ashizawa K, Shichijo K, Kudo T (2019) Expression of the DNA-dependent protein kinase catalytic subunit is associated with the radiosensitivity of human thyroid cancer cell lines. J Radiat Res 60:171–177. https://doi.org/10.1093/jrr/rry097
Jhun BS, Ou J, Adaniya SM, Cypress MW, Yoon Y (2018) Adrenergic regulation of Drp1-driven mitochondrial fission in cardiac physio-pathology. Antioxid (Basel Switz). https://doi.org/10.3390/antiox7120195
Kim HR, Lee GH, Cho EY, Chae SW, Ahn T, Chae HJ (2009) Bax inhibitor 1 regulates ER-stress-induced ROS accumulation through the regulation of cytochrome P450 2E1. J Cell Sci 122:1126–1133. https://doi.org/10.1242/jcs.038430
Kim JH, Lee ER, Jeon K, Choi HY, Lim H, Kim SJ, Chae HJ, Park SH, Kim S, Seo YR, Kim JH, Cho SG (2012) Role of BI-1 (TEGT)-mediated ERK1/2 activation in mitochondria-mediated apoptosis and splenomegaly in BI-1 transgenic mice. Biochim Biophys Acta 1823:876–888. https://doi.org/10.1016/j.bbamcr.2012.01.016
L’Ecuyer TJ, Aggarwal S, Zhang JP, Van der Heide RS (2012) Effect of hypothermia on doxorubicin-induced cardiac myoblast signaling and cell death. Cardiovasc Pathol 21:96–104. https://doi.org/10.1016/j.carpath.2011.02.001
Lee GH, Ahn T, Kim DS, Park SJ, Lee YC, Yoo WH, Jung SJ, Yang JS, Kim S, Muhlrad A, Seo YR, Chae SW, Kim HR, Chae HJ (2010) Bax inhibitor 1 increases cell adhesion through actin polymerization: involvement of calcium and actin binding. Mol Cell Biol 30:1800–1813. https://doi.org/10.1128/MCB.01357-09
Lee GH, Hwang JD, Choi JY, Park HJ, Cho JY, Kim KW, Chae HJ, Kim HR (2011) An acidic pH environment increases cell death and pro-inflammatory cytokine release in osteoblasts: the involvement of BAX inhibitor-1. Int J Biochem Cell Biol 43:1305–1317. https://doi.org/10.1016/j.biocel.2011.05.004
Lee GH, Lee HY, Li B, Kim HR, Chae HJ (2014) Bax inhibitor-1-mediated inhibition of mitochondrial Ca2+ intake regulates mitochondrial permeability transition pore opening and cell death. Sci Rep 4:5194. https://doi.org/10.1038/srep05194
Li M, Lin YF, Palchik GA, Matsunaga S, Wang D, Chen BP (2014) The catalytic subunit of DNA-dependent protein kinase is required for cellular resistance to oxidative stress independent of DNA double-strand break repair. Free Radical Biol Med 76:278–285. https://doi.org/10.1016/j.freeradbiomed.2014.08.019
Li Y, Goronzy JJ, Weyand CM (2018) DNA damage, metabolism and aging in pro-inflammatory T cells: rheumatoid arthritis as a model system. Exp Gerontol 105:118–127. https://doi.org/10.1016/j.exger.2017.10.027
Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G (2018) Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314:H812–H838. https://doi.org/10.1152/ajpheart.00335.2017
Lisak D, Schacht T, Gawlitza A, Albrecht P, Aktas O, Koop B, Gliem M, Hofstetter HH, Zanger K, Bultynck G, Parys JB, De Smedt H, Kindler T, Adams-Quack P, Hahn M, Waisman A, Reed JC, Hovelmeyer N, Methner A (2016) BAX inhibitor-1 is a Ca(2+) channel critically important for immune cell function and survival. Cell Death Differ 23:358–368. https://doi.org/10.1038/cdd.2015.115
Lisak DA, Schacht T, Enders V, Habicht J, Kiviluoto S, Schneider J, Henke N, Bultynck G, Methner A (2015) The transmembrane Bax inhibitor motif (TMBIM) containing protein family: Tissue expression, intracellular localization and effects on the ER CA(2)(+)-filling state. Biochim Biophys Acta 1853:2104–2114. https://doi.org/10.1016/j.bbamcr.2015.03.002
Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, Glavic A, Kress C, Lin JH, Walter P, Reed JC, Glimcher LH, Hetz C (2009) BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell 33:679–691. https://doi.org/10.1016/j.molcel.2009.02.017
Liu J, Naegele JR, Lin SL (2009) The DNA-PK catalytic subunit regulates Bax-mediated excitotoxic cell death by Ku70 phosphorylation. Brain Res 1296:164–175. https://doi.org/10.1016/j.brainres.2009.07.101
Mannell H, Hammitzsch A, Mettler R, Pohl U, Krotz F (2010) Suppression of DNA-PKcs enhances FGF-2 dependent human endothelial cell proliferation via negative regulation of Akt. Cell Signal 22:88–96. https://doi.org/10.1016/j.cellsig.2009.09.015
Mishra A, Brown AL, Yao X, Yang S, Park SJ, Liu C, Dagur PK, McCoy JP, Keeran KJ, Nugent GZ, Jeffries KR, Qu X, Yu ZX, Levine SJ, Chung JH (2015) Dendritic cells induce Th2-mediated airway inflammatory responses to house dust mite via DNA-dependent protein kinase. Nat Commun 6:6224. https://doi.org/10.1038/ncomms7224
Park SJ, Gavrilova O, Brown AL, Soto JE, Bremner S, Kim J, Xu X, Yang S, Um JH, Koch LG, Britton SL, Lieber RL, Philp A, Baar K, Kohama SG, Abel ED, Kim MK, Chung JH (2017) DNA-PK Promotes the mitochondrial, metabolic, and physical decline that occurs during aging. Cell Metab 25(1135–1146):e1137. https://doi.org/10.1016/j.cmet.2017.04.008
Poreba M, Groborz K, Navarro M, Snipas SJ, Drag M, Salvesen GS (2019) Caspase selective reagents for diagnosing apoptotic mechanisms. Cell Death Differ 26:229–244. https://doi.org/10.1038/s41418-018-0110-y
Radhakrishnan SK, Lees-Miller SP (2017) DNA requirements for interaction of the C-terminal region of Ku80 with the DNA-dependent protein kinase catalytic subunit (DNA-PKcs). DNA Repair (Amst) 57:17–28. https://doi.org/10.1016/j.dnarep.2017.06.001
Ren J, Zhang Y (2017) Editorial: new therapetic approaches in the management of ischemia reperfusion injury and cardiometabolic diseases: opportunities and challenges. Curr Drug Targets 18:1687–1688. https://doi.org/10.2174/138945011815171019092703
Reynolds P, Anderson JA, Harper JV, Hill MA, Botchway SW, Parker AW, O'Neill P (2012) The dynamics of Ku70/80 and DNA-PKcs at DSBs induced by ionizing radiation is dependent on the complexity of damage. Nucleic Acids Res 40:10821–10831. https://doi.org/10.1093/nar/gks879
Sano R, Hou YC, Hedvat M, Correa RG, Shu CW, Krajewska M, Diaz PW, Tamble CM, Quarato G, Gottlieb RA, Yamaguchi M, Nizet V, Dahl R, Thomas DD, Tait SW, Green DR, Fisher PB, Matsuzawa S, Reed JC (2012) Endoplasmic reticulum protein BI-1 regulates Ca(2)(+)-mediated bioenergetics to promote autophagy. Genes Dev 26:1041–1054. https://doi.org/10.1101/gad.184325.111
Santamaria PG, Floristan A, Fontanals-Cirera B, Vazquez-Naharro A, Santos V, Morales S, Yuste L, Peinado H, Garcia-Gomez A, Portillo F, Hernando E, Cano A (2018) Lysyl oxidase-like 3 is required for melanoma cell survival by maintaining genomic stability. Cell Death Differ 25:935–950. https://doi.org/10.1038/s41418-017-0030-2
Shang Y, He J, Wang Y, Feng Q, Zhang Y, Guo J, Li J, Li S, Wang Y, Yan G, Ren F, Shi Y, Xu J, Zeps N, Zhai Y, He D, Chang Z (2017) CHIP/Stub1 regulates the Warburg effect by promoting degradation of PKM2 in ovarian carcinoma. Oncogene 36:4191–4200. https://doi.org/10.1038/onc.2017.31
Song Z, Xie Y, Guo Z, Han Y, Guan H, Liu X, Ma T, Zhou PK (2019) Genome-wide identification of DNA-PKcs-associated RNAs by RIP-Seq. Signal Transduct Target Ther 4:22. https://doi.org/10.1038/s41392-019-0057-6
Tian X, Seluanov A, Gorbunova V (2017) Beyond making ends meet: DNA-PK, metabolism, and aging. Cell Metab 25:991–992. https://doi.org/10.1016/j.cmet.2017.04.022
Wang Q, Gao F, May WS, Zhang Y, Flagg T, Deng X (2008) Bcl2 negatively regulates DNA double-strand-break repair through a nonhomologous end-joining pathway. Mol Cell 29:488–498. https://doi.org/10.1016/j.molcel.2007.12.029
Winiarska K, Dzik JM, Labudda M, Focht D, Sierakowski B, Owczarek A, Komorowski L, Bielecki W (2016) Melatonin nephroprotective action in Zucker diabetic fatty rats involves its inhibitory effect on NADPH oxidase. J Pineal Res 60:109–117. https://doi.org/10.1111/jpi.12296
Yoshida M, Shiojima I, Ikeda H, Komuro I (2009) Chronic doxorubicin cardiotoxicity is mediated by oxidative DNA damage-ATM-p53-apoptosis pathway and attenuated by pitavastatin through the inhibition of Rac1 activity. J Mol Cell Cardiol 47:698–705. https://doi.org/10.1016/j.yjmcc.2009.07.024
Yurkova N, Shaw J, Blackie K, Weidman D, Jayas R, Flynn B, Kirshenbaum LA (2008) The cell cycle factor E2F–1 activates Bnip3 and the intrinsic death pathway in ventricular myocytes. Circ Res 102:472–479. https://doi.org/10.1161/CIRCRESAHA.107.164731
Zhao BX, Chen HZ, Du XD, Luo J, He JP, Wang RH, Wang Y, Wu R, Hou RR, Hong M, Wu Q (2011) Orphan receptor TR3 enhances p53 transactivation and represses DNA double-strand break repair in hepatoma cells under ionizing radiation. Mol Endocrinol 25:1337–1350. https://doi.org/10.1210/me.2011-0081
Zhou H, Du W, Li Y, Shi C, Hu N, Ma S, Wang W, Ren J (2018) Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J Pineal Res. https://doi.org/10.1111/jpi.12450
Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu P, Ma Q, Tian F, Chen Y (2017) Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc. https://doi.org/10.1161/JAHA.116.005328
Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S, Zhang Y, Han T, Ren J, Cao F, Chen Y (2017) Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitophagy pathways. J Pineal Res. https://doi.org/10.1111/jpi.12438
Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J, Hu S, Chen Y, Zhang Y (2018) Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. J Pineal Res 65:e12503. https://doi.org/10.1111/jpi.12503
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ, Chen Y (2018) Protective role of melatonin in cardiac ischemia-reperfusion injury: From pathogenesis to targeted therapy. J Pineal Res. https://doi.org/10.1111/jpi.12471
Zhou H, Shi C, Hu S, Zhu H, Ren J, Chen Y (2018) BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis 21:599–615. https://doi.org/10.1007/s10456-018-9611-z
Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu S, Ren J, Chen Y (2018) NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res Cardiol 113:23. https://doi.org/10.1007/s00395-018-0682-1
Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y (2018) Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ 25:1080–1093. https://doi.org/10.1038/s41418-018-0086-7
Zhou H, Yang J, Xin T, Zhang T, Hu S, Zhou S, Chen G, Chen Y (2015) Exendin-4 enhances the migration of adipose-derived stem cells to neonatal rat ventricular cardiomyocyte-derived conditioned medium via the phosphoinositide 3-kinase/Akt-stromal cell-derived factor-1α/CXC chemokine receptor 4 pathway. Mol Med Rep 11:4063–4072. https://doi.org/10.3892/mmr.2015.3243
Acknowledgements
This work was supported in part by National Key R&D Program of China (2017YFA0506000), China Postdoctoral Science Foundation (2019TQ0128) and the NSFC (81900252, 81770261, 81870249, 81900254 and 91749128).
Author information
Authors and Affiliations
Contributions
HZ, PJZ and JW were involved in conception and design, performance of experiments, data analysis and interpretation and manuscript writing; ST and PZ were involved in the development of methodology; HZ and PJZ were involved in the data acquisition; JR and YZ were involved data analysis and interpretation; and HZ, JR and YZ involved in study supervision and final approval of manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, H., Toan, S., Zhu, P. et al. DNA-PKcs promotes cardiac ischemia reperfusion injury through mitigating BI-1-governed mitochondrial homeostasis. Basic Res Cardiol 115, 11 (2020). https://doi.org/10.1007/s00395-019-0773-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-019-0773-7