Abstract
Liver failure is one of the life-threatening illnesses, and it accounts for 3.5% of all deaths worldwide. Liver transplantation is the solution, but due to the long waiting list for an available donor, patients die before they get the chance to get a healthy liver. Shortage supply of donor organs, lifelong need for immunosuppression, and the adult hepatocytes culturing difficulty are the serious limitations for liver transplantation. Therefore, hepatocyte transplantation of stem cells with tissue engineering is the alternative therapeutic approach to liver transplantation. Differentiation of stem cells from many origins into hepatocytes has been reported for therapeutic and research purposes. This book chapter summarizes the differentiation process into hepatocytes from different stem cell types, including mesenchymal, pluripotent, hematopoietic, and umbilical cord stem cells. In addition, it discusses the use of hepatocytes in drug discovery and clinical studies, as well as, in 2D and 3D conformation and liver formation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hepatocytes
- Hepatocytes like stem cells
- Differentiation
- Induced pluripotent stem cells (iPSCs)
- Embryonic stem cells (ESCs)
- Hematopoietic stem cells transplantation
- Umbilical cord
- 2D
- 3D
Introduction
Liver is the body’s biggest organ, which plays an essential role in detoxification, metabolism, protein synthesis, regulation of glucose levels, and control of blood homeostasis. The liver consists of many types of cells. Generally, they are classified as non-parenchymal and parenchymal cells (hepatocytes). The hepatocytes form 70% of the liver mass that is derived from endoderm during embryonic development along with biliary epithelial cells (cholangiocytes). After resection (hepatectomy) or injury, the liver is able to regrow 70% of its mass, by multiplying the hepatocyte cells (Fausto et al., 2006; Michalopoulos, 2007). However, the capability of liver regeneration is insufficient in many diseases, such as advanced cirrhosis and hepatitis that end up in liver failure and thus life-threatening (Alqahtani, 2012). For such problem, liver transplantation is the best solution, but due to the long waiting list for available liver, patients die before they get the chance to get healthy liver of donor. Shortage supply of donor organs, lifelong need for immunosuppression, and the adult hepatocytes culturing difficulty are the serious limitations for liver transplantation (Bodzin & Baker, 2018; Iansante et al., 2018; Ibars et al., 2016; Langer & Vacanti, 2016). Therefore, the use of bio-artificial liver devices or transplantation of isolated hepatocytes is the option to provide a limited liver function (Dan & Yeoh, 2008; Demetriou et al., 2004; Horslen & Fox, 2004; McKenzie et al., 2008). Hepatocyte transplantation of stem cells with tissue engineering is the promising approach of unlimited sources for transplantation (Iansante et al., 2018; Muraca et al., 2002). Stem cells can differentiate into diverse cell progenies, such as hepatocytes, which could be used in liver tissue engineering and hepatocyte transplantation. Hepatocyte primary cell cultures and the use of animal models, such as chicken, mouse, and zebra fish, have identified many of the genes and molecular pathways that regulate the embryonic development of the liver. This scientific data has encouraged scientist to generate practical hepatocytes of stem cells including, embryonic stem cells (ESC), induced pluripotent stem cells (iPSC), hepatic progenitor/stem cells (HPC), and mesenchymal stem cells (MSC) (Ang et al., 2018; Corbett & Duncan, 2019; Ghosheh et al., 2020). Transplantation of these stem cells to generate functional hepatocytes in liver may lead to cure liver diseases. The lack of knowledge of differentiation hindered the differentiation process of stem cells into functional hepatocytes. Commonly, stem cells are differentiated by small molecules or growth factors to induce the cells into becoming hepatocyte-like cells, usually via a stepwise strategy in 2D or 3D (Zhao et al., 2020). Transplantation of human hepatocytes has been used to treat a number of liver disorders such as glycogen storage disease type 1 (Muraca et al., 2002), phenylketonuria (Stéphenne et al., 2012), urea cycle disorders (Meyburg et al., 2009; Mitry et al., 2004; Soltys et al., 2017; Stéphenne et al., 2006), factor VII deficiency (Dhawan et al., 2004), infantile Refsum’s disease (Sokal et al., 2003), acute liver failure (Bilir et al., 2000; Habibullah et al., 1994; Khan et al., 2004; Schneider et al., 2006), and severe infantile oxalosis (Beck et al., 2012).
Isolation and Culture of Hepatocytes
Isolation of hepatocytes was performed firstly in 1957 (Branster & Morton, 1957), followed by advanced techniques to achieve high-quality hepatocytes. Hepatocytes harvesting is based on using two-step collagenase perfusion technique. In general, the first perfusion of liver with buffers containing ethylene glycol tetra acetic acid (EGTA) or ethylene diamine tetraacetic acid (EDTA) is to wash blood out of tissue into waste and to avoid coagulation of leftover blood in the tissue. The second perfusion buffer contains collagenase to dissolve the tissue collagen. Following perfusion with collagenase, the liver is cut into halves and opens the capsule that leads to free the cells. Then a gauze filter is used to separate the cells from the connective tissue and debris. Followed by several washes and the hepatocytes finally separated by centrifugation at 50–100 g and 4 °C (Berry & Friend, 1969; Charni-Natan & Goldstein, 2020; Knobeloch et al., 2012; Lee et al., 2020a, b; Strom et al., 1982; Seglen, 1976). One of the limitations of this method that the ability of each collagenase lot to release hepatocytes must be assessed. As the collagenase, lots vary in their composition, and consequently, its activity will vary among batches. In addition, individual collagenase lots activity may vary between animal models.
Two ways can be followed to access the isolated hepatocyte, one is the trypan blue exclusion, which is considered a poor guide for hepatocyte engraftment and function. Hepatocytes viability has not been shown to correlate with engraftment using this method (Akhter et al., 2007; Matsumura et al., 2019; Mitry et al., 2003). The second way is the adherence of hepatocytes to tissue culture plates after 24 h of seeding that showed a better correlation with engraftment (Holzman et al., 1993). After isolation, the cells will be subjected to direct transplantation, primary cell culture, or storage (cryopreserved) for later use. Cell culture or cryopreserved options has some possible advantages including increasing number of cells of donors for transplantation, tissue matching, and immunological modulation of donor cells if needed. Even though cryopreservation of cells is vital for urgent transplantation needs, this option is damaging the hepatocytes through caspases activation during freezing and thawing steps (Baust et al., 2001; Yagi et al., 2001). Generally, the hepatocytes are placed in liquid nitrogen after resuspended in cryopreservation freezing medium, which contains culture medium, 10% dimethyl sulfoxide, and 10% fetal calf serum (Aoki et al., 2005; Hang et al., 2010; Kusano et al., 2008). Even after thawing this suspension provides valid cell viability and function, it is not reliable for clinical transplantation. Recent methods were introduced to preserve the cells during cryopreservation that includes the modification of freezing medium, such as CryoStor CS10 (Woods et al., 2009) and University of Wisconsin solution, which contains 5% glucose, 10% DMSO, and a cytoprotectant such as the pan-caspase inhibitor benzyloxycarbonyl-Val-Ala-dl-Asp-fluoromethylketone (ZVAD). This freezing medium showed better cell attachment, viability, and function after thawing (Jitraruch et al., 2017). Post-cryopreservation thawing hepatocytes were maintained in culture for 24 h in order to access the cellular function and morphology (Jitraruch et al., 2017).
The best relevant in vitro model to human liver is the primary human hepatocytes culture, which can show drug metabolism profile that is very similar to liver drug metabolism profile (Gómez-Lechón et al., 2003). Thus, it can be used for pharmacological and toxicological experiments, such as drug clearance and hepatotoxicity (Gómez-Lechón et al., 2008; Hewitt et al., 2007; Leist et al., 2017; Soldatow et al., 2013; Vinken & Hengstler, 2018), that will reduce the cost and reduce the number of animal models used for drug discovery and development. Therefore, the researchers tried to establish long-lasting primary human hepatocytes cell culture relevant to in vivo situation using the extracellular matrix (ECM), hormones, growth factors, and cytokines (Clause & Barker, 2013; Michalopoulos et al., 2001; Navarro-Alvarez et al., 2006; Pediaditakis et al., 2001; Tanaka et al., 2006). The updated culture modifications significantly maintained the hepatocytes function and morphology in the culture. The function and morphology of hepatocytes were investigated through, gene expression profiles, levels of cytochrome P450 activity, and functional apical and basal polarity (Jindal et al., 2009; Kidambi et al., 2009).
Hepatocyte Morphological and Functional Characterization
Isolation of pure hepatocytes required functional and morphological evaluation after cryopreservation or primary culture to be suitable for clinical application.
Hepatocytes filters and process blood nutrients, drugs, metabolites and hormones, and synthesis and secret the bile (Treyer & Müsch, 2013). To mediate these functions, the hepatocytes are highly polarized with multiple apical membranes forming bile canaliculi and multiple basolateral membranes facing the sinusoids (Schulze et al., 2019; Slim, 2014; Treyer & Müsch, 2013). Within this particular cell morphology, protein secretion, membrane trafficking, cell signaling, and bile transport are highly organized (McNiven et al., 2009; Schulze et al., 2019; Thi et al., 2020). The examination of cell morphology in cell culture accessed using light microscopy and electron microscopy (SEM and TEM), which presented viable hepatocytes with smooth membrane, precise nuclear membranes, and intact cristae in mitochondria, while the unhealthy or apoptotic hepatocytes showed irregular plasma membranes, condensation of nuclear chromatin, and swelling in mitochondria (Jitraruch et al., 2017; Lillegard et al., 2011; Wu et al., 2014).
Specific characterization of differentiated HLC in comparison with adult hepatocytes is an important indicator of successful functional hepatocyte differentiation. This characterization is required after pharmacological research or transplantation in vivo. Hepatocytes functional activity can be evaluated using many ways, including
-
1.
Gene expression profile of differentiated HLC in compared to mature liver hepatocytes (Gao et al., 2017; Hewitt et al., 2007; Li et al., 1990).
-
2.
Evaluating the enzymatic functions, such as glycogen storage, LDL uptake, and cytochrome P450 (Snykers et al., 2007; Yin et al., 2015; Zheng et al., 2015).
-
3.
Accessing drug metabolism function and other materials such as ammonia and hormones (Duncan et al., 1998; Hewitt et al., 2007; Yoon et al., 2010).
-
4.
Screening for different protein synthesis and/or secretion: albumin, alpha fetoprotein, CK-18, complements, clotting factors, and transporter proteins (Hasan et al., 2017; Raoufil et al., 2015; Wei et al., 2008; Yin et al., 2015; Zhang et al., 2009).
-
5.
Assessment of mitochondrial dehydrogenase activity and cell adherence (Ho et al., 2012; Jitraruch et al., 2017).
-
6.
Measuring the ability of urea production, bile acids clearance, and lipids and lipoproteins secretion (Hasan et al., 2017; Hewitt et al., 2007; Mita et al., 2006; Yoon et al., 2010).
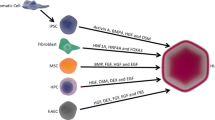
Differentiation of Mesenchymal Stem Cells into Hepatocytes
Functional hepatocytes can be generated by inducing the differentiation of mesenchymal stem cells (MSCs) (Fig. 6.1). MSCs were firstly isolated from bone marrow in 1968 (Friedenstein et al., 1968). There are many resources to isolate MSCs from, including adult tissues (bone marrow, adipose tissue, and peripheral blood) (Chen et al., 2009; Hass et al., 2011; Jung et al., 2013; Kim et al., 2011; Kolanko et al., 2019; Liang & Sun, 2015) and neonatal birth-associated tissues (cord blood, umbilical cord, placenta, chorion, and human amniotic membrane). MSCs are characterized by positive cell surface expression of CD90, CD73, CD44, CD166, and CD105 and negative expression of HLA-DR, CD45, and CD34 (Dominici et al., 2006; Kholodenko et al., 2019; Maleki et al., 2014). MSCs are derived from neonatal birth-associated tissues well known for their differentiation and proliferation capabilities in vitro (Ullah et al., 2015) and in vivo (Danielyan et al., 2014).
Differentiation of MSCs was successfully reported to be used for diseases treatment (Afshari et al., 2020; Hu et al., 2013; Lim et al., 2017; Phan et al., 2018; Wu et al., 2020), especially liver disorders. Table 6.1 summarizes some of these examples of approaches in order to treat liver disorders by differentiating MSCs into functional hepatocytes and then liver transplantation.
Differentiation of Pluripotent Stem Cells into Hepatocytes
Human pluripotent stem cells (PSCs, including human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs)) differentiation into HLCs are being widely investigated for generation of functional hepatocyte, because of their potential ability to avoid the immune system, limitless proliferation quantity, and reflect a potential renewable source. Differentiation of PSCs to HLCs is a complex process that involves growth factors or cytokines (Hannan et al., 2013; Tolosa et al., 2015; Wang et al., 2020), small molecules (Asumda et al., 2018; Gao et al., 2020), or microRNAs (Jaafarpour et al., 2020; Jung et al., 2016; Li et al., 2018; Viiri et al., 2019) over different time intervals. All these used materials affect the signaling pathway in the cells to direct it into hepatocyte specifically.
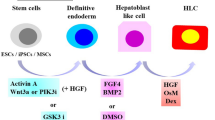
Functional hepatocytes are derived from hiPSCs and hESCs in three stages. First stage, generally involves the usage of Activin A only or along, LY294002, BMP4, and Wnt3a to differentiate hiPSCs and hESCs into definitive endoderm (DE) cells (Cameron et al., 2015; Danoy et al., 2020; Hannan et al., 2013; Hay et al., 2008). These cells keep expressing pluripotent markers along with DE markers that require further differentiation (Carpentier et al., 2016). In the second stage, using DMSO alone or in combination with FGF4, BMP4, and FGF2, resulting in suppression of pluripotency markers and differentiation of the DE cells into hepatoblast (hepatic specification) (Asgari et al., 2013; Cameron et al., 2015; Czysz et al., 2015; Gao et al., 2020; Si-Tayeb et al., 2010). In addition, in this stage other researchers use more materials such as TTNPB, Forskolin, A8301, and C59 to efficiently generate high numbers of hepatocytes (Ang et al., 2018; Loh et al., 2019). The third stage includes the differentiation of hepatoblast into functional hepatocyte using Leibovitz's L-15 or HC-HepatoZYME or HCM mediums supplemented with HGF, oncostatin M (OSM), hydrocortisone (HC), and dexamethasone (Asgari et al., 2013; Cameron et al., 2015; Medine et al., 2011; Si-Tayeb et al., 2010; Tolosa et al., 2015) (Table 6.2; Fig. 6.2). There is a shift of growth factors between the stages depending on the procedure followed by researchers, some examples are summarized in Table 6.1. On the other hand, some researchers used small molecules only to generate mature hepatocytes of hiPSCs and hESCs (Table 6.1). That showed effective differentiation with cost reduction and better reproducibility overgrowth factors (Gao et al., 2020; Siller et al., 2015; Tasnim et al., 2015; Varghese et al., 2019). The differential stages were validated by quantifying the definitive endoderm, hepatoblast, and mature hepatocytes expression markers and measuring the hepatocytes functions (Table 6.2).
The third used application for hepatocyte differentiation is microRNAs, which regulate the gene expression. Overexpression of specific microRNAs such as microRNA-375 (miR-375) and miR-122 (Jaafarpour et al., 2020), microRNA-194 (Jung et al., 2016), miR-192 and miR-372-3p (Li et al., 2018) resulted in changing the cell fate decision to hepatocytes (Table 6.2; Fig. 6.2). This way of differentiation may have a high concern regarding inherent virus genome mutations in the host genome.
Hematopoietic Stem Cells Differentiation into Hepatocyte
HSCs are found in very low numbers in whole bone marrow, umbilical cord, and peripheral blood, and HSCs normally are located in the niche of bone marrow at quiescent mood and will respond to intrinsic or extrinsic signals (Tümpel & Rudolph, 2019) such as growth factors. With cell surface-specific markers in human CD45RA−, CD90±, CD38−, CD34+, Lin−, CD49f+, and RHOl while in mouse the markers as follows Lin−, CD34−, cKit+, Sca-1+, FLK2−, and Slamf1+ (Chotinantakul & Leeanansaksiri, 2012).
HSCs are able to self-renew and differentiation into specialized blood cells (Doulatov et al., 2012) and another type of cells such as liver cells (Sellamuthu et al., 2011), osteochondrocytes (Mehrotra et al., 2010), adipocytes (Sera et al., 2009), endothelial cells (Elkhafif et al., 2011), and pancreatic cells (Minamiguchi et al., 2008). HSCs have been proposed as a replacement for hepatocyte transplantation in liver; as at the first stage of embryogenesis, the liver is considered hematopoietic organ; also, it is an erythrocytes source in the first trimester of pregnancy.
Some approaches were reported as potential cell replacement therapy of hepatocytes, such as inducing bone marrow HSCs differentiation into hepatocytes, which is the cell source for transplant procedures to treat hemophilia patients (Gabr et al., 2014). HSCs derived from umbilical cord, that differentiation in short duration (14 days) into hepatocytes using combination of growth factors (FGF 4 and HGF), this approach indicates that these stem cells have a potential to be used in liver replacement therapy (Sellamuthu et al., 2011). HSCs derived from bone marrow showed functional hepatocyte differentiation and engraftment (Khurana & Mukhopadhyay, 2008). In addition, these HSCs were found effective in curing the liver in the fumarylacetoacetate hydrolase (FAH)-deficient mouse model, the mutant mouse was intravenously injected with different numbers of HSCs, few months later the hepatocyte repopulated, FAH expression was detected, and the activity of hepatocytes was accessed by albumin and β-galactosidase expression (Lagasse et al., 2000). The recovery of animals transplanted with bone marrow-derived HSCs is probably due to HSCs-hepatocyte fusion and reprogramming and not due to their actual differentiation into hepatocytes (Vassilopoulos et al., 2003; Wang et al., 2003). Unfortunately, human liver regeneration by bone marrow HSCs is not clinically relevant at that time (Pilat et al., 2013). Recent reports were showed that human and mouse bone marrow HSCs are successfully incorporated into liver regeneration and transdifferentiating into hepatocytes (Lee et al., 2015). In addition, hematopoietic cell (autologous CD34+) infusion in patients with decompensated cirrhosis of the liver showed significantly improved albumin expression and thus liver function, indicating the importance of HSCs transdifferentiation in liver transplantation (Sharma et al., 2015).
Umbilical Cord Stem Cell Differentiation into Hepatocytes
Umbilical cord (UC) veins, arteries, Wharton’s jelly, perivascular, and lining membrane regions are considered the best source for mesenchymal stem cells (MSC) (Nagamura-Inoue & He, 2014). MSC collection of UC is painless and no tissue damage; in addition, this MSC is fast self-renewable and differentiation cells (Hsieh et al., 2010), promotes tissue repair, modulates immune response (Deuse et al., 2011), and can be used to autologous and allogeneic transplantation. UC-MSC was isolated and differentiated into hepatocytes in vitro using growth factors, small molecules, or microRNAs (Bharti et al., 2018; Campard et al., 2008; Raut & Khanna, 2016; Xue et al., 2016; Zhang et al., 2009). The differentiated hepatocytes were tested for cell activity, protein expression, and gene upregulation, which all showed successful hepatocyte differentiation. UC-MSC was used for the treatment of liver failure in many disease models. For examples, liver fibrosis, cirrhosis, and failure in rats (Chai et al., 2016; Zhang et al., 2017, 2018) and liver failure, chronic injury, ischemia/reperfusion injury in mice (Cui et al., 2017; Feng et al., 2015; Le et al., 2017; Yang et al., 2015; Zheng et al., 2019). In addition, UC-MSC was differentiated into hepatocytes and then transplanted in murine model of CCL4-induced liver injury (Cui et al., 2013; El Baz et al., 2020; Kao et al., 2015), that showed improvement in liver function and restored the liver injury.
UC-MSC is also used in human clinical trials for liver diseases treatments, such as transplantation of UC-MSC for treatment of newly onset type 1 diabetes mellitus (Hu et al., 2013), treatment of severe bronchopulmonary dysplasia in children (Wu et al., 2020), treatment of systemic lupus erythematosus (Liang & Sun, 2015), treatment of decompensated cirrhosis (Zhang et al., 2012), treatment of acute allograft rejection (Shi et al., 2017), and primary biliary cirrhosis (Wang et al., 2013). These clinical trials improved liver function and patients’ life quality. One of the differentiation limitations of MSC derived from umbilical cord is the low number of MSC in the umbilical cord (Han et al., 2013).
Application in Drug Discovery, Preclinical, and Clinical Trials
The new drug discovery needs a lot of testing and validation, hepatocytes fasten this procedure and reduce the use of animal models. Hepatocytes function in drug metabolism (drug elimination of body) involves CYP-dependent oxidation, and CYP enzyme activation or inhibition is necessary for drug–drug interactions prediction (Riley & Grime, 2004). Therefore, hepatocytes were used as tool for testing drug clearance and toxicology, access drug uptake, and drug–drug interaction prediction (Andersson et al., 2012; Bernasconi et al., 2019; Hallifax et al., 2005; Louisse et al., 2020). Biopsy of primary liver cells is considered the best way for in vitro drug screening, as these cells are similar to healthy human cells (Guo et al., 2011). Unfortunately, difficulty of cell supply and difficulty to get cell division for in vitro expansion, as well as their rapid dedifferentiation, which results in losing their function make them unsuitable for research or drug screening (Heslop et al., 2017). For regular supply of hepatocytes for drug screening purposes, researchers successfully differentiated many types of stem cells into functional hepatocytes such as, iPSCs, which are used for screening five drugs after differentiating them into hepatocytes (Choi et al., 2013). In addition, hepatocytes derived from iPSCs were also used to screen for drugs that improve mitochondrial function and reveal that nicotinamide adenine dinucleotide (NAD) is a potential treatment for mtDNA depletion syndrome 3 (Jing et al., 2018).
The liver failure is one of the life-threatening issues, and the liver transplantation is the solution, but many patients die before they get a liver from a donor. Due to that, hepatocyte transplantation was suggested for the replacement of liver transplantation. As a result, many preclinical trials on animal were carried on trying to replace liver cells with functional hepatocytes. These preclinical studies showed functional replacement of hepatocytes that reduce the effect of the liver disease in the animal models (Gilgenkrantz and l’Hortet, 2018; Gramignoli, 2016). These promising results encouraged human clinical trials establishment using hepatocytes like stem cells (HLSCs), which showed no side effect and no need to use immunosuppression, since HLSCs possess immunomodulatory activities (Spada et al., 2020). Some clinical trials examples are listed (Table 6.3).
2D and 3D Hepatocyte Confirmation, Liver Formation
Three-dimensional (3D) and Two-dimensional (2D) cell culture techniques were used to induce hepatocytes differentiation and then characterization by immunocytochemistry, gene expression, electron microscopy, and morphology. Two-dimensional (2D, Monolayer) culture is the most frequent technique used to coax differentiation of isolated stem cells into hepatocytes. Even though 2D provides useful observation of functional tests, low cost, and high efficacy as screening tool for hypertoxicity, there are disadvantages related to differentiated hepatocytes phenotype lasting period, environmental issues, biochemical processes, and loss of tissue-specific architecture (Duval et al., 2017). In order to improve the hepatocyte differentiation, morphology, and liver-specific functions, the researchers start thinking of adding more conditions to the culture that will be similar to in vivo environment (Bachmann et al., 2015). Using extracellular matrix (ECM), stromal cells, soluble factors, and macromolecules showed that the cells differentiate better in these conditions (Clause & Barker, 2013; Yu et al., 2011). In three-dimensional (3D) culture, the researchers try to reach the in vivo condition to give better prediction results and to overcome some disadvantages of 2D, such as maintaining the expansion, differentiation, and function capacity of stem cells for long period. 3D hepatic culture model involves three categories, spheroids, liver on a chip, and bioreactors.
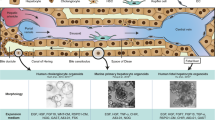
Spheroids are formed in spherical shape in low attachment plates under optimized culture condition to produce uniform spheroid-like structures, such as differentiated HepaRG spheroid culture (Fig. 6.3). The cells in this 3D culture showed polarized and functional hepatocyte for more than 28 days (Ramaiahgari et al., 2017). In addition, extracellular matrix-based hydrogel model was used to generate differentiated hepatocyte spheroids of HepG2 cell line (Ramaiahgari et al., 2014). These spheroids grow to a certain diameter of 118 μm that allows enough oxygen diffusion to the core (Ramaiahgari et al., 2014). The spheroid technique is maintaining the function and metabolism, easy to reproduce 3D form and differentiated hepatocytes showed high sensitivity to toxins, and also it is useful system for analyzing the drug-induced toxic or pathogenesis of diseases effects even though there are disadvantages for this technique such as dedifferentiation, varied physiological response, limited characterization, possible necrosis in the core (Ramaiahgari et al., 2014, 2017; Ryu et al., 2019; Sirenko et al., 2016). However, spheroids showed better differentiation efficiency when compared to 2D (Bratt-Leal et al., 2009).
Liver-on-a-chip device generates 3D organoids in microfluid environment, with preservation of hepatocytes viability, biological activity, and cellular phenotype (Moradi et al., 2020). This kind of culture is permeable to soluble growth factors and oxygen and allows nutrients and waste exchange that will avoid the hypoxia that could happen in spheroids (Ziolkowska et al., 2010) (Fig. 6.3). This system could be replicated in liver environment and offer homogenous distribution, consistent density of the cells in the culture, high cell viability, increased or stable liver enzyme levels, capacity for detoxification, in vivo-like dynamic flow, and chemical gradients even though there are some disadvantages of this system should be noted, such as high cost, limited evaluation tests, lack of scaffold (extracellular matrix), lack of biliary system, and low expression of structural proteins (Bovard et al., 2018; Corrado et al., 2019; Goral & Yuen, 2012; Grix et al., 2018; Lee et al., 2019; Rajan et al., 2020; Utech et al., 2015). Liver on a chip can be used in the application of cellular cytotoxicity, drug metabolism, liver disease model, and drug-induced liver injury.
Bioreactors, it is continuously mixing microfluid environment to form spheroids in a suspension (Lin & Chang, 2008) (Fig. 6.3). Fast stirring speed may damage the spheroids and slow stirring may inhibit spheroid formation as the cells will sink to the bottom (Achilli et al., 2012). This technique enables spheroids formation with the expression of relevant markers and enzymes, maintains cells phenotype and high seeding efficiency. In addition, it increases phases I and II enzyme activity and toxin sensitivity (Baudoin et al., 2011; Freyer et al., 2018; Ryu et al., 2019; Tostões et al., 2012). On the other hand, this technique is high cost and hard to standardize and reproduce, that make it less used (Tostões et al., 2012).
Overall, 3D reflects in vivo culture better than 2D; also, 3D cell culture enables the formation of organoids that result in the expansion of human primary tissues (Broutier et al., 2016; Xu et al., 2018). In addition, 3D is more effective in differentiation process than 2D (Afshari et al., 2020) and provides more realistic physical and biochemical environment than 2D (Chan et al., 2016; Duval et al., 2017). For example, 3D culture is better than 2D culture in lowering transaminase (El Baz et al., 2020), in drug-induced phospholipidosis sensitivity (Lee et al., 2020a; b), in hepatic drug metabolism and hepatoxicity (Corrado et al., 2019), in maintaining functional cell culture for long period (Ramaiahgari et al., 2017).
References
Achilli, T.-M., Meyer, J., & Morgan, J. R. (2012). Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opinion on Biological Therapy, 12, 1347–1360. https://doi.org/10.1517/14712598.2012.707181.
Afshari, A., Shamdani, S., Uzan, G., Naserian, S., & Azarpira, N. (2020). Different approaches for transformation of mesenchymal stem cells into hepatocyte-like cells. Stem Cell Research & Therapy, 11, 54. https://doi.org/10.1186/s13287-020-1555-8.
Akhter, J., Johnson, L. A., Gunasegaram, A., Riordan, S. M., & Morris, D. L. (2007). Hepatocyte transplantation: A review of laboratory techniques and clinical experiences. The Surgeon, 5, 155–164. https://doi.org/10.1016/S1479-666X(07)80043-6.
Alqahtani, S. A. (2012). Update in liver transplantation. Current Opinion in Gastroenterology, 28, 230–238. https://doi.org/10.1097/MOG.0b013e3283527f16.
Andersson, T. B., Kanebratt, K. P., & Kenna, J. G. (2012). The HepaRG cell line: A unique in vitro tool for understanding drug metabolism and toxicology in human. Expert Opinion on Drug Metabolism & Toxicology, 8, 909–920. https://doi.org/10.1517/17425255.2012.685159.
Ang, L. T., Tan, A. K. Y., Autio, M. I., Goh, S. H., Choo, S. H., Lee, K. L., Tan, J., Pan, B., Lee, J. J. H., Lum, J. J., Lim, C. Y. Y., Yeo, I. K. X., Wong, C. J. Y., Liu, M., Oh, J. L. L., Chia, C. P. L., Loh, C. H., Chen, A., Chen, Q., … Lim, B. (2018). A roadmap for human liver differentiation from pluripotent stem cells. Cell Reports, 22, 2190–2205. https://doi.org/10.1016/j.celrep.2018.01.087.
Aoki, T., Koizumi, T., Kobayashi, Y., Yasuda, D., Izumida, Y., Jin, Z., Nishino, N., Shimizu, Y., Kato, H., Murai, N., Niiya, T., Enami, Y., Mitamura, K., Yamamoto, T., & Kusano, M. (2005). A novel method of cryopreservation of rat and human hepatocytes by using encapsulation technique and possible use for cell transplantation. Cell Transplantation, 14, 609–620. https://doi.org/10.3727/000000005783982710.
Asgari, S., Moslem, M., Bagheri-lankarani, K., Pournasr, B., Miryounesi, M., & Baharvand, H. (2013). Differentiation and transplantation of human induced pluripotent stem cell-derived hepatocyte-like cells. Stem Cell Reviews and Reports, 9, 493–504. https://doi.org/10.1007/s12015-011-9330-y.
Asumda, F. Z., Hatzistergos, K. E., Dykxhoorn, D. M., Jakubski, S., Edwards, J., Thomas, E., & Schiff, E. R. (2018). Differentiation of hepatocyte-like cells from human pluripotent stem cells using small molecules. Differentiation, 101, 16–24. https://doi.org/10.1016/j.diff.2018.03.002.
Bachmann, A., Moll, M., Gottwald, E., Nies, C., Zantl, R., Wagner, H., Burkhardt, B., Sánchez, J. J. M., Ladurner, R., Thasler, W., Damm, G., & Nussler, A. K. (2015). 3D cultivation techniques for primary human hepatocytes. Microarrays, 4, 64–83. https://doi.org/10.3390/microarrays4010064.
Banas, A., Teratani, T., Yamamoto, Y., Tokuhara, M., Takeshita, F., Quinn, G., Okochi, H., & Ochiya, T. (2007). Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology, 46, 219–228. https://doi.org/10.1002/hep.21704.
Baudoin, R., Griscom, L., Prot, J. M., Legallais, C., & Leclerc, E. (2011). Behavior of HepG2/C3A cell cultures in a microfluidic bioreactor. Biochemical Engineering Journal, 53, 172–181. https://doi.org/10.1016/j.bej.2010.10.007.
Baust, J. M., Vogel, M. J., Van Buskirk, R., & Baust, J. G. (2001). A molecular basis of cryopreservation failure and its modulation to improve cell survival. Cell Transplantation, 10, 561–571. https://doi.org/10.3727/000000001783986413.
Beck, B. B., Habbig, S., Dittrich, K., Stippel, D., Kaul, I., Koerber, F., Goebel, H., Salido, E. C., Kemper, M., Meyburg, J., & Hoppe, B. (2012). Liver cell transplantation in severe infantile oxalosis—a potential bridging procedure to orthotopic liver transplantation? Nephrology, Dialysis, Transplantation, 27, 2984–2989. https://doi.org/10.1093/ndt/gfr776.
Bernasconi, C., Pelkonen, O., Andersson, T. B., Strickland, J., Wilk-Zasadna, I., Asturiol, D., Cole, T., Liska, R., Worth, A., Müller-Vieira, U., Richert, L., Chesne, C., & Coecke, S. (2019). Validation of in vitro methods for human cytochrome P450 enzyme induction: Outcome of a multi-laboratory study. Toxicology in Vitro, 60, 212–228. https://doi.org/10.1016/j.tiv.2019.05.019.
Berry, M. N., & Friend, D. S. (1969). High-yield preparation of isolated rat liver parenchymal cells. Journal of Cell Biology, 43, 506–520.
Bharti, D., Shivakumar, S. B., Park, J.-K., Ullah, I., Subbarao, R. B., Park, J.-S., Lee, S.-L., Park, B.-W., & Rho, G.-J. (2018). Comparative analysis of human Wharton’s jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell and Tissue Research, 372, 51–65. https://doi.org/10.1007/s00441-017-2699-4.
Bilir, B. M., Guinette, D., Karrer, F., Kumpe, D. A., Krysl, J., Stephens, J., McGavran, L., Ostrowska, A., & Durham, J. (2000). Hepatocyte transplantation in acute liver failure. Liver Transplantation, 6, 32–40. https://doi.org/10.1016/S1527-6465(00)80030-1.
Bodzin, A. S., & Baker, T. B. (2018). Liver transplantation today: where we are now and where we are going. Liver Transplantation, 24, 1470–1475. https://doi.org/10.1002/lt.25320.
Bovard, D., Sandoz, A., Luettich, K., Frentzel, S., Iskandar, A., Marescotti, D., Trivedi, K., Guedj, E., Dutertre, Q., Peitsch, M. C., & Hoeng, J. (2018). A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab on a Chip, 18, 3814–3829. https://doi.org/10.1039/C8LC01029C.
Branster, M. V., & Morton, R. K. (1957). Isolation of intact liver cells. Nature, 180, 1283–1284. https://doi.org/10.1038/1801283a0.
Bratt-Leal, A. M., Carpenedo, R. L., & McDevitt, T. C. (2009). Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnology Progress, 25, 43–51. https://doi.org/10.1002/btpr.139.
Broutier, L., Andersson-Rolf, A., Hindley, C. J., Boj, S. F., Clevers, H., Koo, B.-K., & Huch, M. (2016). Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nature Protocols, 11, 1724–1743. https://doi.org/10.1038/nprot.2016.097.
Cameron, K., Tan, R., Schmidt-Heck, W., Campos, G., Lyall, M. J., Wang, Y., Lucendo-Villarin, B., Szkolnicka, D., Bates, N., Kimber, S. J., Hengstler, J. G., Godoy, P., Forbes, S. J., & Hay, D. C. (2015). Recombinant laminins drive the differentiation and self-organization of hESC-derived hepatocytes. Stem Cell Reports, 5, 1250–1262. https://doi.org/10.1016/j.stemcr.2015.10.016.
Campard, D., Lysy, P. A., Najimi, M., & Sokal, E. M. (2008). Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology, 134, 833–848. https://doi.org/10.1053/j.gastro.2007.12.024.
Carpentier, A., Nimgaonkar, I., Chu, V., Xia, Y., Hu, Z., & Liang, T. J. (2016). Hepatic differentiation of human pluripotent stem cells in miniaturized format suitable for high-throughput screen. Stem Cell Research, 16, 640–650. https://doi.org/10.1016/j.scr.2016.03.009.
Chai, N.-L., Zhang, X.-B., Chen, S.-W., Fan, K.-X., & Linghu, E.-Q. (2016). Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World Journal of Gastroenterology, 22, 6036–6048. https://doi.org/10.3748/wjg.v22.i26.6036.
Chan, H. F., Zhang, Y., & Leong, K. W. (2016). Efficient one-step production of microencapsulated hepatocyte spheroids with enhanced functions. Small (weinheim an Der Bergstrasse, Germany), 12, 2720–2730. https://doi.org/10.1002/smll.201502932.
Charni-Natan, M., & Goldstein, I. (2020). Protocol for primary mouse hepatocyte isolation. STAR Protocols, 1, 100086. https://doi.org/10.1016/j.xpro.2020.100086.
Chen, M.-Y., Lie, P.-C., Li, Z.-L., & Wei, X. (2009). Endothelial differentiation of Wharton’s jelly–derived mesenchymal stem cells in comparison with bone marrow–derived mesenchymal stem cells. Experimental Hematology, 37, 629–640. https://doi.org/10.1016/j.exphem.2009.02.003.
Choi, S. M., Kim, Y., Shim, J. S., Park, J. T., Wang, R.-H., Leach, S. D., Liu, J. O., Deng, C.-X., Ye, Z., & Jang, Y.-Y. (2013). Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology, 57, 2458–2468. https://doi.org/10.1002/hep.26237.
Chotinantakul, K., & Leeanansaksiri, W. (2012). Hematopoietic stem cell development, niches, and signaling pathways. Bone Marrow Research, 2012. https://doi.org/10.1155/2012/270425.
Clause, K. C., & Barker, T. H. (2013). Extracellular matrix signaling in morphogenesis and repair. Current Opinion in Biotechnology, 24, 830–833. https://doi.org/10.1016/j.copbio.2013.04.011.
Corbett, J. L., & Duncan, S. A. (2019). iPSC-derived hepatocytes as a platform for disease modeling and drug discovery. Frontiers in Medicine, 6, 265. https://doi.org/10.3389/fmed.2019.00265.
Corrado, B., Gregorio, V. D., Imparato, G., Attanasio, C., Urciuolo, F., & Netti, P. A. (2019). A three-dimensional microfluidized liver system to assess hepatic drug metabolism and hepatotoxicity. Biotechnology and Bioengineering, 116, 1152–1163. https://doi.org/10.1002/bit.26902.
Cui, H., Liu, Z., Wang, L., Bian, Y., Li, W., Zhou, H., Chu, X., & Zhao, Q. (2017). Icariin-treated human umbilical cord mesenchymal stem cells decrease chronic liver injury in mice. Cytotechnology, 69, 19–29. https://doi.org/10.1007/s10616-016-0034-7.
Cui, L., Shi, Y., Zhou, X., Wang, X., Wang, J., Lan, Y., Wang, M., Zheng, L., Li, H., Wu, Q., Zhang, J., Fan, D., & Han, Y. (2013). A set of microRNAs mediate direct conversion of human umbilical cord lining-derived mesenchymal stem cells into hepatocytes. Cell Death & Disease, 4, e918–e918. https://doi.org/10.1038/cddis.2013.429.
Czysz, K., Minger, S., & Thomas, N. (2015). DMSO efficiently down regulates pluripotency genes in human embryonic stem cells during definitive endoderm derivation and increases the proficiency of hepatic differentiation. PLoS ONE, 10, e0117689. https://doi.org/10.1371/journal.pone.0117689.
Dan, Y. Y., & Yeoh, G. C. (2008). Liver stem cells: a scientific and clinical perspective. Journal of Gastroenterology and Hepatology, 23, 687–698. https://doi.org/10.1111/j.1440-1746.2008.05383.x.
Danielyan, L., Beer-Hammer, S., Stolzing, A., Schäfer, R., Siegel, G., Fabian, C., Kahle, P., Biedermann, T., Lourhmati, A., Buadze, M., Novakovic, A., Proksch, B., Gleiter, C. H., Frey, W. H., & Schwab, M. (2014). Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplantation, 23, 123–139. https://doi.org/10.3727/096368914X684970.
Danoy, M., Tauran, Y., Poulain, S., Arakawa, H., Mori, D., Araya, K., Kato, S., Kido, T., Kusuhara, H., Kato, Y., Miyajima, A., Plessy, C., Sakai, Y., & Leclerc, E. (2020). Analysis of hiPSCs differentiation toward hepatocyte-like cells upon extended exposition to oncostatin. Differentiation, 114, 36–48. https://doi.org/10.1016/j.diff.2020.05.006.
Demetriou, A. A., Brown, R. S., Busuttil, R. W., Fair, J., McGuire, B. M., Rosenthal, P., Am Esch, J. S., Lerut, J., Nyberg, S. L., Salizzoni, M., Fagan, E. A., de Hemptinne, B., Broelsch, C. E., Muraca, M., Salmeron, J. M., Rabkin, J. M., Metselaar, H. J., Pratt, D., De La Mata, M., … Solomon, B. A. (2004). Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Annals of Surgery, 239, 660–670. https://doi.org/10.1097/01.sla.0000124298.74199.e5.
Deuse, T., Stubbendorff, M., Tang-Quan, K., Phillips, N., Kay, M. A., Eiermann, T., Phan, T. T., Volk, H.-D., Reichenspurner, H., Robbins, R. C., & Schrepfer, S. (2011). Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplantation, 20, 655–667. https://doi.org/10.3727/096368910X536473.
Dhawan, A., Mitry, R. R., Hughes, R. D., Lehec, S., Terry, C., Bansal, S., Arya, R., Wade, J. J., Verma, A., Heaton, N. D., Rela, M., & Mieli-Vergani, G. (2004). Hepatocyte transplantation for inherited factor VII deficiency. Transplantation, 78, 1812–1814. https://doi.org/10.1097/01.TP.0000146386.77076.47.
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D. S., Deans, R. J., Keating, A., Prockop, D. J., & Horwitz, E. M. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy, 8, 315–317. https://doi.org/10.1080/14653240600855905.
Doulatov, S., Notta, F., Laurenti, E., & Dick, J. E. (2012). Hematopoiesis: a human perspective. Cell Stem Cell, 10, 120–136. https://doi.org/10.1016/j.stem.2012.01.006.
Duncan, S. A., Navas, M. A., Dufort, D., Rossant, J., & Stoffel, M. (1998). Regulation of a transcription factor network required for differentiation and metabolism. Science, 281, 692–695. https://doi.org/10.1126/science.281.5377.692.
Duval, K., Grover, H., Han, L.-H., Mou, Y., Pegoraro, A. F., Fredberg, J., & Chen, Z. (2017). Modeling physiological events in 2D vs. 3D cell culture. Physiology, 32, 266–277. https://doi.org/10.1152/physiol.00036.2016.
El Baz, H., Demerdash, Z., Kamel, M., Hammam, O., Abdelhady, D. S., Mahmoud, S., Hassan, S., Mahmoud, F., Atta, S., Riad, N. M., & Gaafar, T. (2020). Induction of hepatic regeneration in an experimental model using hepatocyte-differentiated mesenchymal stem cells. Cellular Reprogramming, 22, 134–146. https://doi.org/10.1089/cell.2019.0076.
Elkhafif, N., Baz, H. E., Hammam, O., Hassan, S., Salah, F., Mansour, W., Mansy, S., Yehia, H., Zaki, A., & Magdy, R. (2011). CD133+ human umbilical cord blood stem cells enhance angiogenesis in experimental chronic hepatic fibrosis. APMIS, 119, 66–75. https://doi.org/10.1111/j.1600-0463.2010.02693.x.
Fausto, N., Campbell, J. S., & Riehle, K. J. (2006). Liver regeneration. Hepatology, 43, S45–S53. https://doi.org/10.1002/hep.20969.
Feng, T., Zhang, J., Zeng, G., Zhou, R., Tang, X., Cui, C., Li, Y., Wang, H., Li, T., Zhu, W., & Yu, Z. (2015). Therapeutic potential of umbilical cord mesenchymal stem cells in mice with acute hepatic failure. International Journal of Artificial Organs, 38, 271–276. https://doi.org/10.5301/ijao.5000390.
Freyer, N., Greuel, S., Knöspel, F., Gerstmann, F., Storch, L., Damm, G., Seehofer, D., Foster Harris, J., Iyer, R., Schubert, F., & Zeilinger, K. (2018). Microscale 3D liver bioreactor for in vitro hepatotoxicity testing under perfusion conditions. Bioengineering, 5, 24. https://doi.org/10.3390/bioengineering5010024.
Friedenstein, A. J., Petrakova, K. V., Kurolesova, A. I., & Frolova, G. P. (1968). Heterotopic transplants of bone marrow. Transplantation, 6, 230–247.
Gabr, H., Zayed, R., ElBeshlawy, A., Hegazy, L., Fawzy, R., Samir, M., & Mosa, H. (2014). Differentiation of bone marrow hematopoietic stem cells into FVIII-producing hepatocytes: Approach to hemophilia treatment. Comparative Clinical Pathology, 23, 193–198. https://doi.org/10.1007/s00580-012-1595-2.
Gai, H., Nguyen, D. M., Joon Moon, Y., Aguila, J. R., Fink, L. M., Ward, D. C., & Ma, Y. (2010). Generation of murine hepatic lineage cells from induced pluripotent stem cells. Differentiation, 79, 171–181. https://doi.org/10.1016/j.diff.2010.01.002.
Gao, X., Li, R., Cahan, P., Zhao, Y., Yourick, J. J., & Sprando, R. L. (2020). Hepatocyte-like cells derived from human induced pluripotent stem cells using small molecules: Implications of a transcriptomic study. Stem Cell Research & Therapy, 11, 393. https://doi.org/10.1186/s13287-020-01914-1.
Gao, Y., Zhang, X., Zhang, L., Cen, J., Ni, X., Liao, X., Yang, C., Li, Y., Chen, X., Zhang, Z., Shu, Y., Cheng, X., Hay, D. C., Lai, D., Pan, G., Wei, G., & Hui, L. (2017). Distinct gene expression and epigenetic signatures in hepatocyte-like cells produced by different strategies from the same donor. Stem Cell Reports, 9, 1813–1824. https://doi.org/10.1016/j.stemcr.2017.10.019.
Ghosheh, N., Küppers-Munther, B., Asplund, A., Andersson, C. X., Björquist, P., Andersson, T. B., Carén, H., Simonsson, S., Sartipy, P., & Synnergren, J. (2020). Human pluripotent stem cell-derived hepatocytes show higher transcriptional correlation with adult liver tissue than with fetal liver tissue. ACS Omega, 5, 4816–4827. https://doi.org/10.1021/acsomega.9b03514.
Gilgenkrantz, H., de l’Hortet, A. C. (2018). Understanding liver regeneration: From mechanisms to regenerative medicine. The American Journal of Pathology, 188, 1316–1327. https://doi.org/10.1016/j.ajpath.2018.03.008.
Gómez-Lechón, M. J., Castell, J. V., & Donato, M. T. (2008). An update on metabolism studies using human hepatocytes in primary culture. Expert Opinion on Drug Metabolism & Toxicology, 4, 837–854. https://doi.org/10.1517/17425255.4.7.837.
Gómez-Lechón, M., Donato, M., & Jover, R. (2003). Human hepatocytes as a tool for studying toxicity and drug metabolism. Current Drug Metabolism, 4, 292–312. https://doi.org/10.2174/1389200033489424.
Goral, V. N., & Yuen, P. K. (2012). Microfluidic platforms for hepatocyte cell culture: New technologies and applications. Annals of Biomedical Engineering, 40, 1244–1254. https://doi.org/10.1007/s10439-011-0453-8.
Gordon, M. Y., Levičar, N., Pai, M., Bachellier, P., Dimarakis, I., Al-Allaf, F., M’Hamdi, H., Thalji, T., Welsh, J. P., Marley, S. B., Davies, J., Dazzi, F., Marelli-Berg, F., Tait, P., Playford, R., Jiao, L., Jensen, S., Nicholls, J. P., Ayav, A., … Habib, N. A. (2006). Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells, 24, 1822–1830. https://doi.org/10.1634/stemcells.2005-0629.
Gramignoli, R. (2016). Therapeutic use of human amnion-derived products: Cell-based therapy for liver disease. Current Pathobiology Reports, 4, 157–167. https://doi.org/10.1007/s40139-016-0112-8.
Grix, T., Ruppelt, A., Thomas, A., Amler, A.-K., Noichl, B. P., Lauster, R., & Kloke, L. (2018). Bioprinting perfusion-enabled liver equivalents for advanced organ-on-a-chip applications. Genes, 9, 176. https://doi.org/10.3390/genes9040176.
Guo, L., Dial, S., Shi, L., Branham, W., Liu, J., Fang, J.-L., Green, B., Deng, H., Kaput, J., & Ning, B. (2011). Similarities and Differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metabolism and Disposition, 39, 528–538. https://doi.org/10.1124/dmd.110.035873.
Habibullah, C. M., Syed, I. H., Qamar, A., & Taher-Uz, Z. (1994). Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation, 58, 951–952.
Hallifax, D., Rawden, H. C., Hakooz, N., & Houston, J. B. (2005). Prediction of metabolic clearance using cryopreserved human hepatocytes: Kinetic characteristics for five benzodiazepines. Drug Metabolism and Disposition, 33, 1852–1858. https://doi.org/10.1124/dmd.105.005389.
Han, Y.-F., Tao, R., Sun, T.-J., Chai, J.-K., Xu, G., & Liu, J. (2013). Optimization of human umbilical cord mesenchymal stem cell isolation and culture methods. Cytotechnology, 65, 819–827. https://doi.org/10.1007/s10616-012-9528-0.
Hang, H., Shi, X., Gu, G. X., Wu, Y., Gu, J., & Ding, Y. (2010). In vitro analysis of cryopreserved alginate–poly-l-lysine–alginate-microencapsulated human hepatocytes. Liver International, 30, 611–622. https://doi.org/10.1111/j.1478-3231.2009.02197.x.
Hannan, N. R., Segeritz, C.-P., Touboul, T., & Vallier, L. (2013). Production of hepatocyte like cells from human pluripotent stem cells. Nature Protocols, 8, 430–437.
Hasan, M. H., Botros, K. G., El-Shahat, M. A., Abdallah, H. A., & Sobh, M. A. (2017). In vitro differentiation of human umbilical cord blood mesenchymal stem cells into functioning hepatocytes. Alexandria Journal of Medicine, 53, 167–173. https://doi.org/10.1016/j.ajme.2016.05.002.
Hass, R., Kasper, C., Böhm, S., & Jacobs, R. (2011). Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling, 9, 12. https://doi.org/10.1186/1478-811X-9-12.
Hay, D. C., Fletcher, J., Payne, C., Terrace, J. D., Gallagher, R. C. J., Snoeys, J., Black, J. R., Wojtacha, D., Samuel, K., Hannoun, Z., Pryde, A., Filippi, C., Currie, I. S., Forbes, S. J., Ross, J. A., Newsome, P. N., & Iredale, J. P. (2008). Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci U S A, 105, 12301–12306. https://doi.org/10.1073/pnas.0806522105.
Heslop, J. A., Rowe, C., Walsh, J., Sison-Young, R., Jenkins, R., Kamalian, L., Kia, R., Hay, D., Jones, R. P., Malik, H. Z., Fenwick, S., Chadwick, A. E., Mills, J., Kitteringham, N. R., Goldring, C. E. P., & Park, B. K. (2017). Mechanistic evaluation of primary human hepatocyte culture using global proteomic analysis reveals a selective dedifferentiation profile. Archives of Toxicology, 91, 439–452. https://doi.org/10.1007/s00204-016-1694-y.
Hewitt, N. J., Lechón, M. J. G., Houston, J. B., Hallifax, D., Brown, H. S., Maurel, P., Kenna, J. G., Gustavsson, L., Lohmann, C., Skonberg, C., Guillouzo, A., Tuschl, G., Li, A. P., LeCluyse, E., Groothuis, G. M. M., & Hengstler, J. G. (2007). Primary hepatocytes: Current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metabolism Reviews, 39, 159–234. https://doi.org/10.1080/03602530601093489.
Ho, C.-M., Dhawan, A., Hughes, R. D., Lehec, S. C., Puppi, J., Philippeos, C., Lee, P.-H., & Mitry, R. R. (2012). Use of indocyanine green for functional assessment of human hepatocytes for transplantation. Asian Journal of Surgery, 35, 9–15. https://doi.org/10.1016/j.asjsur.2012.04.017.
Holzman, M. D., Rozga, J. A., Neuzil, D. F., Griffin, D. O., Moscioni, A. D., & Demetriou, A. A. (1993). Selective intraportal hepatocyte transplantation in analbuminemic and Gunn rats [WWW Document]. Transplantation. https://doi.org/10.1097/00007890-199306000-00002.
Horslen, S. P., & Fox, I. J. (2004). Hepatocyte transplantation. Transplantation, 77, 1481–1486. https://doi.org/10.1097/01.TP.0000113809.53415.C2.
Hsieh, J.-Y., Fu, Y.-S., Chang, S.-J., Tsuang, Y.-H., & Wang, H.-W. (2010). Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton’s Jelly of umbilical cord. Stem Cells and Development, 19, 1895–1910. https://doi.org/10.1089/scd.2009.0485.
Hu, J., Yu, X., Wang, Z., Wang, F., Wang, L., Gao, H., Chen, Y., Zhao, W., Jia, Z., Yan, S., & Wang, Y. (2013). Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocrine Journal, 60, 347–357. https://doi.org/10.1507/endocrj.EJ12-0343.
Iansante, V., Mitry, R. R., Filippi, C., Fitzpatrick, E., & Dhawan, A. (2018). Human hepatocyte transplantation for liver disease: Current status and future perspectives. Pediatric Research, 83, 232–240. https://doi.org/10.1038/pr.2017.284.
Ibars, E. P., Cortes, M., Tolosa, L., Gómez-Lechón, M. J., López, S., Castell, J. V., & Mir, J. (2016). Hepatocyte transplantation program: Lessons learned and future strategies. World Journal of Gastroenterology, 22, 874–886. https://doi.org/10.3748/wjg.v22.i2.874.
Jaafarpour, Z., Soleimani, M., Hosseinkhani, S., Geramizadeh, B., Yaghmaei, P., Mobarra, N., & Karimi, M. H. (2020). Overexpression of microRNA-375 and microRNA-122 promotes the differentiation of human induced pluripotent stem cells into hepatocyte-like cells. Biologicals, 63, 24–32. https://doi.org/10.1016/j.biologicals.2019.12.005.
Jindal, R., Nahmias, Y., Tilles, A. W., Berthiaume, F., & Yarmush, M. L. (2009). Amino acid-mediated heterotypic interaction governs performance of a hepatic tissue model. The FASEB Journal, 23, 2288–2298. https://doi.org/10.1096/fj.08-114934.
Jing, R., Corbett, J. L., Cai, J., Beeson, G. C., Beeson, C. C., Chan, S. S., Dimmock, D. P., Lazcares, L., Geurts, A. M., Lemasters, J. J., & Duncan, S. A. (2018). A screen using iPSC-derived hepatocytes reveals NAD+ as a potential treatment for mtDNA depletion syndrome. Cell Reports, 25, 1469-1484.e5. https://doi.org/10.1016/j.celrep.2018.10.036.
Jitraruch, S., Dhawan, A., Hughes, R. D., Filippi, C., Lehec, S. C., Glover, L., & Mitry, R. R. (2017). Cryopreservation of hepatocyte microbeads for clinical transplantation. Cell Transplantation, 26, 1341–1354. https://doi.org/10.1177/0963689717720050.
Jung, J., Choi, J. H., Lee, Y., Park, J.-W., Oh, I.-H., Hwang, S.-G., Kim, K.-S., & Kim, G. J. (2013). Human placenta-derived mesenchymal stem cells promote hepatic regeneration in CCl4-injured rat liver model via increased autophagic mechanism. Stem Cells, 31, 1584–1596. https://doi.org/10.1002/stem.1396.
Jung, K. H., McCarthy, R. L., Zhou, C., Uprety, N., Barton, M. C., & Beretta, L. (2016). MicroRNA regulates hepatocytic differentiation of progenitor cells by targeting YAP1. Stem Cells, 34, 1284–1296. https://doi.org/10.1002/stem.2283.
Kao, S.-Y., Shyu, J.-F., Wang, H.-S., Hsiao, C.-Y., Su, C.-H., Chen, T.-H., Weng, Z.-C., & Tsai, P.-J. (2015). Transplantation of hepatocytelike cells derived from umbilical cord stromal mesenchymal stem cells to treat acute liver failure rat. Journal of Biomedical Sciences, 4. https://doi.org/10.4172/2254-609X.100002.
Khan, A. A., Habeeb, A., Parveen, N., Naseem, B., Babu, R. P., Capoor, A. K., & Habibullah, C. M.88 (2004). Peritoneal transplantation of human fetal hepatocytes for the treatment of acute fatty liver of pregnancy: a case report [WWW Document]. Tropical Gastroenterology: Official Journal of the Digestive Diseases Foundation. URL http://pubmed.ncbi.nlm.nih.gov/15682663/?dopt=Abstract. Accessed August 12, 2020.
Kholodenko, I. V., Kurbatov, L. K., Kholodenko, R. V., Manukyan, G. V., & Yarygin, K. N. (2019). Mesenchymal stem cells in the adult human liver: Hype or hope? Cells, 8, 1127. https://doi.org/10.3390/cells8101127.
Khurana, S., & Mukhopadhyay, A. (2008). In vitro transdifferentiation of adult hematopoietic stem cells: An alternative source of engraftable hepatocytes. Journal of Hepatology, 49, 998–1007. https://doi.org/10.1016/j.jhep.2008.05.019.
Kidambi, S., Yarmush, R. S., Novik, E., Chao, P., Yarmush, M. L., & Nahmias, Y. (2009). Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proceedings of the National Academy of Sciences of the United States of America, 106, 15714. https://doi.org/10.1073/pnas.0906820106.
Kim, M. J., Shin, K. S., Jeon, J. H., Lee, D. R., Shim, S. H., Kim, J. K., Cha, D.-H., Yoon, T. K., & Kim, G. J. (2011). Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: A comparative analysis of their potential as placenta-derived stem cells. Cell and Tissue Research, 346, 53–64. https://doi.org/10.1007/s00441-011-1249-8.
Knobeloch, D., Ehnert, S., Schyschka, L., Büchler, P., Schoenberg, M., Kleeff, J., Thasler, W. E., Nussler, N. C., Godoy, P., Hengstler, J., Nussler, A. K. (2012). Human hepatocytes: Isolation, culture, and quality procedures. In Human cell culture protocols, methods in molecular biology (pp. 99–120). Humana Press. https://doi.org/10.1007/978-1-61779-367-7_8.
Kolanko, E., Kopaczka, K., Koryciak-Komarska, H., Czech, E., Szmytkowska, P., Gramignoli, R., & Czekaj, P. (2019). Increased immunomodulatory capacity of human amniotic cells after activation by pro-inflammatory chemokines. European Journal of Pharmacology, 859, 172545. https://doi.org/10.1016/j.ejphar.2019.172545.
Kusano, T., Aoki, T., Yasuda, D., Matsumoto, S., Jin, Z., Nishino, N., Hayashi, K., Odaira, M., Yamada, K., Koizumi, T., Izumida, Y., Mitamura, K., Enami, Y., Niiya, T., Murai, N., Kato, H., Shimizu, Y., Kou, K., Furukawa, Y., … Kusano, M. (2008). Microencapsule technique protects hepatocytes from cryoinjury. Hepatology Research, 38, 593–600. https://doi.org/10.1111/j.1872-034X.2007.00311.x.
Lagasse, E., Connors, H., Al-Dhalimy, M., Reitsma, M., Dohse, M., Osborne, L., Wang, X., Finegold, M., Weissman, I. L., & Grompe, M. (2000). Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nature Medicine, 6, 1229–1234. https://doi.org/10.1038/81326.
Langer, R., & Vacanti, J. (2016). Advances in tissue engineering. Journal of Pediatric Surgery, 51, 8–12. https://doi.org/10.1016/j.jpedsurg.2015.10.022.
Lázaro-Diéguez, F., & Müsch, A. (2014). The special case of hepatocytes. Bioarchitecture, 4, 47–52. https://doi.org/10.4161/bioa.29012.
Le, T. V., Nguyen, N. H., Do, H. Q., Le, H. M., & Truong, N. H. (2017). Transplantation of umbilical cord blood-derived mesenchymal stem cells to treat liver cirrhosis in mice: a comparison of tail and portal vein injection. Progress Stem Cell, 4, 201–216. https://doi.org/10.15419/psc.v4i2.365.
Lee, H., Chae, S., Kim, J. Y., Han, W., Kim, J., Choi, Y., & Cho, D.-W. (2019). Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Biofabrication, 11, 025001. https://doi.org/10.1088/1758-5090/aaf9fa.
Lee, J.-Y., Han, H.-J., Lee, S.-J., Cho, E.-H., Lee, H.-B., Seok, J.-H., Lim, H. S., & Son, W.-C. (2020). Use of 3D Human Liver Organoids To Predict Drug-Induced Phospholipidosis. International Journal of Molecular Sciences, 21, 2982. https://doi.org/10.3390/ijms21082982.
Lee, S. M. L., Bertinetti-Lapatki, C., Schiergens, T. S., Jauch, K.-W., Roth, A. B., & Thasler, W. E. (2020). Concurrent isolation of hepatic stem cells and hepatocytes from the human liver. Vitro Cellular Developmental Biology-Animal, 56, 253–260. https://doi.org/10.1007/s11626-020-00433-w.
Lee, S.-G., Moon, S.-H., Kim, H.-J., Lee, J. Y., Park, S.-J., Chung, H.-M., Ha, T. Y., Song, G.-W., Jung, D.-H., Park, H., Kwon, T.-W., & Cho, Y.-P. (2015). Bone marrow-derived progenitor cells in de novo liver regeneration in liver transplant. Liver Transplantation, 21, 1186–1194. https://doi.org/10.1002/lt.24099.
Leist, M., Ghallab, A., Graepel, R., Marchan, R., Hassan, R., Bennekou, S. H., Limonciel, A., Vinken, M., Schildknecht, S., Waldmann, T., Danen, E., van Ravenzwaay, B., Kamp, H., Gardner, I., Godoy, P., Bois, F. Y., Braeuning, A., Reif, R., Oesch, F., … Hengstler, J. G. (2017). Adverse outcome pathways: Opportunities, limitations and open questions. Archives of Toxicology, 91, 3477–3505. https://doi.org/10.1007/s00204-017-2045-3.
Li, L., Miu, K.-K., Gu, S., Cheung, H.-H., & Chan, W.-Y. (2018). Comparison of multi-lineage differentiation of hiPSCs reveals novel miRNAs that regulate lineage specification. Scientific Reports, 8, 1–15. https://doi.org/10.1038/s41598-018-27719-0.
Li, Y. C., Wang, D. P., & Chiang, J. Y. (1990). Regulation of cholesterol 7 alpha-hydroxylase in the liver. Cloning, sequencing, and regulation of cholesterol 7 alpha-hydroxylase mRNA. Journal of Biological Chemistry, 265, 12012–12019.
Liang, J., & Sun, L. (2015). Mesenchymal stem cells transplantation for systemic lupus erythematosus. International Journal of Rheumatic Diseases, 18, 164–171. https://doi.org/10.1111/1756-185X.12531.
Lillegard, J. B., Fisher, J. E., Nedredal, G., Luebke-Wheeler, J., Bao, J., Wang, W., Amoit, B., & Nyberg, S. L. (2011). Normal atmospheric oxygen tension and the use of antioxidants improve hepatocyte spheroid viability and function. Journal of Cellular Physiology, 226, 2987–2996. https://doi.org/10.1002/jcp.22651.
Lim, R., Hodge, A., Moore, G., Wallace, E. M., & Sievert, W. (2017). A pilot study evaluating the safety of intravenously administered human amnion epithelial cells for the treatment of hepatic fibrosis. Frontiers in Pharmacology, 8, 549. https://doi.org/10.3389/fphar.2017.00549.
Lin, R.-Z., & Chang, H.-Y. (2008). Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnology Journal, 3, 1172–1184. https://doi.org/10.1002/biot.200700228.
Loh, K. M., Palaria, A., & Ang, L. T. (2019). Efficient differentiation of human pluripotent stem cells into liver cells. JoVE (Journal of Visualized Experiments) e58975. https://doi.org/10.3791/58975.
Louisse, J., Alewijn, M., Peijnenburg, A. A. C. M., Cnubben, N. H. P., Heringa, M. B., Coecke, S., & Punt, A. (2020) Towards harmonization of test methods for in vitro hepatic clearance studies. Toxicology in Vitro, 63, 104722. https://doi.org/10.1016/j.tiv.2019.104722.
Maleki, M., Ghanbarvand, F., Reza Behvarz, M., Ejtemaei, M., & Ghadirkhomi, E. (2014). Comparison of mesenchymal stem cell markers in multiple human adult stem cells. International Journal of Stem Cells, 7, 118–126. https://doi.org/10.15283/ijsc.2014.7.2.118.
Matsumura, M., Imura, T., Inagaki, A., Ogasawara, H., Fukuoka, K., Fathi, I., Miyagi, S., Ohashi, K., Unno, M., Kamei, T., Satomi, S., & Goto, M. (2019). A simple and useful predictive assay for evaluating the quality of isolated hepatocytes for hepatocyte transplantation. Science and Reports, 9, 1. https://doi.org/10.1038/s41598-019-42720-x.
McKenzie, T. J., Lillegard, J. B., & Nyberg, S. L. (2008). Artificial and bioartificial liver support. Seminars in Liver Disease, 28, 210–217. https://doi.org/10.1055/s-2008-1073120.
McNiven, M. A., Wolkoff, A. W., & Hubbard, A. (2009). A stimulus needed for the study of membrane traffic in hepatocytes. Hepatology, 50, 345–348. https://doi.org/10.1002/hep.23004.
Medine, C. N., Lucendo-Villarin, B., Zhou, W., West, C. C., & Hay, D. C. (2011). Robust generation of hepatocyte-like cells from human embryonic stem cell populations. Journal of Visualized Experiments, e2969. https://doi.org/10.3791/2969.
Mehrotra, M., Rosol, M., Ogawa, M., & LaRue, A. C. (2010). Amelioration of a mouse model of osteogenesis imperfecta with hematopoietic stem cell transplantation: Micro-computed tomography studies. Experimental Hematology, 38, 593–602. https://doi.org/10.1016/j.exphem.2010.04.008.
Meyburg, J., Das, A. M., Hoerster, F., Lindner, M., Kriegbaum, H., Engelmann, G., Schmidt, J., Ott, M., Pettenazzo, A., Luecke, T., Bertram, H., Hoffmann, G. F., & Burlina, A. (2009). One liver for four children: First clinical series of liver cell transplantation for severe neonatal urea cycle defects. Transplantation, 87, 636–641. https://doi.org/10.1097/TP.0b013e318199936a.
Michalopoulos, G. K. (2007). Liver regeneration. Journal of Cellular Physiology, 213, 286–300. https://doi.org/10.1002/jcp.21172.
Michalopoulos, G. K., Bowen, W. C., Mulè, K., & Stolz, D. B. (2001). Histological organization in hepatocyte organoid cultures. American Journal of Pathology, 159, 1877–1887.
Minamiguchi, H., Ishikawa, F., Fleming, P. A., Yang, S., Drake, C. J., Wingard, J. R., & Ogawa, M. (2008). Transplanted human cord blood cells generate amylase-producing pancreatic acinar cells in engrafted mice. Pancreas, 36, e30. https://doi.org/10.1097/MPA.0b013e3181584656.
Mita, S., Suzuki, H., Akita, H., Hayashi, H., Onuki, R., Hofmann, A. F., & Sugiyama, Y. (2006). Inhibition of bile acid transport across Na+/taurocholate cotransporting polypeptide (SLC10A1) and bile salt export pump (ABCB 11)-coexpressing LLC-PK1 cells by cholestasis-inducing drugs. Drug Metabolism and Disposition, 34, 1575–1581. https://doi.org/10.1124/dmd.105.008748.
Mitry, R. R., Dhawan, A., Hughes, R. D., Bansal, S., Lehec, S., Terry, C., Heaton, N. D., Karani, J. B., Mieli-Vergani, G., & Rela, M. (2004). one liver, three recipients: Segment IV from split-liver procedures as a source of hepatocytes for cell transplantation. Transplantation, 77, 1614–1616. https://doi.org/10.1097/01.TP.0000122224.98318.19.
Mitry, R. R., Hughes, R. D., Aw, M. M., Terry, C., Mieli-Vergani, G., Girlanda, R., Muiesan, P., Rela, M., Heaton, N. D., & Dhawan, A. (2003). Human hepatocyte isolation and relationship of cell viability to early graft function. Cell Transplantation, 12, 69–74. https://doi.org/10.3727/000000003783985197.
Moradi, E., Jalili-Firoozinezhad, S., & Solati-Hashjin, M. (2020). Microfluidic organ-on-a-chip models of human liver tissue. Acta Biomaterialia, 116, 67–83. https://doi.org/10.1016/j.actbio.2020.08.041.
Muraca, M., Gerunda, G., Neri, D., Vilei, M. T., Granato, A., Feltracco, P., Giron, G., & Burlina, A. B. (2002). Hepatocyte transplantation as a treatment for glycogen storage disease type IA. Journal of Hepatology, 36, 41. https://doi.org/10.1016/S0168-8278(02)80123-4.
Nagamura-Inoue, T., & He, H. (2014). Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells, 6, 195–202. https://doi.org/10.4252/wjsc.v6.i2.195.
Navarro-Alvarez, N., Soto-Gutierrez, A., Rivas-Carrillo, J. D., Chen, Y., Yamamoto, T., Yuasa, T., Misawa, H., Takei, J., Tanaka, N., & Kobayashi, N. (2006). Self-assembling peptide nanofiber as a novel culture system for isolated porcine hepatocytes. Cell Transplantation, 15, 921–927. https://doi.org/10.3727/000000006783981387.
Nazarov, I., Lee, J. W., Soupene, E., Etemad, S., Knapik, D., Green, W., Bashkirova, E., Fang, X., Matthay, M. A., Kuypers, F. A., & Serikov, V. B. (2012). Multipotent stromal stem cells from human placenta demonstrate high therapeutic potential. Stem Cells Translational Medicine, 1, 359–372. https://doi.org/10.5966/sctm.2011-0021.
Pediaditakis, P., Lopez-Talavera, J. C., Petersen, B., Monga, S. P. S., & Michalopoulos, G. K. (2001). The processing and utilization of hepatocyte growth factor/scatter factor following partial hepatectomy in the rat. Hepatology, 34, 688–693. https://doi.org/10.1053/jhep.2001.27811.
Phan, T. G., Ma, H., Lim, R., Sobey, C. G., & Wallace, E. M. (2018). Phase 1 trial of amnion cell therapy for ischemic stroke. Frontiers in Neurology, 9, 198. https://doi.org/10.3389/fneur.2018.00198.
Pilat, N., Unger, L., & Berlakovich, G. A. (2013). Implication for Bone marrow derived stem cells in hepatocyte regeneration after orthotopic liver transplantation [WWW Document]. International Journal of Hepatology. https://doi.org/10.1155/2013/310612.
Rajan, S. A. P., Aleman, J., Wan, M., Pourhabibi Zarandi, N., Nzou, G., Murphy, S., Bishop, C. E., Sadri-Ardekani, H., Shupe, T., Atala, A., Hall, A. R., & Skardal, A. (2020). Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform. Acta Biomaterialia, 106, 124–135. https://doi.org/10.1016/j.actbio.2020.02.015.
Ramaiahgari, S. C., den Braver, M. W., Herpers, B., Terpstra, V., Commandeur, J. N. M., van de Water, B., & Price, L. S. (2014). A 3D in vitro model of differentiated HepG2 cell spheroids with improved liver-like properties for repeated dose high-throughput toxicity studies. Archives of Toxicology, 88, 1083–1095. https://doi.org/10.1007/s00204-014-1215-9.
Ramaiahgari, S. C., Waidyanatha, S., Dixon, D., DeVito, M. J., Paules, R. S., & Ferguson, S. S. (2017). From the cover: three-dimensional (3D) HepaRG spheroid model with physiologically relevant xenobiotic metabolism competence and hepatocyte functionality for liver toxicity screening. Toxicological Sciences, 159, 124–136. https://doi.org/10.1093/toxsci/kfx122.
Raoufil, A., Aminil, A., Azadbakht, M., Farhadifar, F., Rahram Nikhn Frrfin Fthi, N. F. (2015). Production of hepatocyte-like cells from human umbilical vein mesenchymal stem cells. Italian Journal of Anatomy and Embryology = Archivio italiano di anatomia ed embriologia, 120, 150–161.
Raut, A., & Khanna, A. (2016). Enhanced expression of hepatocyte-specific microRNAs in valproic acid mediated hepatic trans-differentiation of human umbilical cord derived mesenchymal stem cells. Experimental Cell Research, 343, 237–247. https://doi.org/10.1016/j.yexcr.2016.03.015.
Resca, E., Zavatti, M., Maraldi, T., Bertoni, L., Beretti, F., Guida, M., La Sala, G. B., Guillot, P. V., David, A. L., Sebire, N. J., De Pol, A., & De Coppi, P. (2015). Enrichment in c-Kit improved differentiation potential of amniotic membrane progenitor/stem cells. Placenta, 36, 18–26. https://doi.org/10.1016/j.placenta.2014.11.002.
Riley, R. J., & Grime, K. (2004). Metabolic screening in vitro: Metabolic stability, CYP inhibition and induction. Drug Discovery Today: Technologies, 1, 365–372. https://doi.org/10.1016/j.ddtec.2004.10.008.
Roelandt, P., Pauwelyn, K. A., Sancho-Bru, P., Subramanian, K., Bose, B., Ordovas, L., Vanuytsel, K., Geraerts, M., Firpo, M., De Vos, R., Fevery, J., Nevens, F., Hu, W.-S., & Verfaillie, C. M. (2010). Human embryonic and rat adult stem cells with primitive endoderm-like phenotype can be fated to definitive endoderm, and finally hepatocyte-like cells. PLoS ONE, 5, e12101. https://doi.org/10.1371/journal.pone.0012101.
Ryu, N.-E., Lee, S.-H., & Park, H. (2019). Spheroid culture system methods and applications for mesenchymal stem cells. Cells, 8, 1620. https://doi.org/10.3390/cells8121620.
Salama, H., Zekri, A.-R., Zern, M., Bahnassy, A., Loutfy, S., Shalaby, S., Vigen, C., Burke, W., Mostafa, M., Medhat, E., Alfi, O., & Huttinger, E. (2010). Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplantation, 19, 1475–1486. https://doi.org/10.3727/096368910X514314.
Schneider, A., Attaran, M., Meier, P. N., Strassburg, C., Manns, M. P., Ott, M., Barthold, M., Arseniev, L., Becker, T., & Panning, B. (2006). Hepatocyte transplantation in an acute liver failure due to mushroom poisoning. Transplantation, 82, 1115–1116. https://doi.org/10.1097/01.tp.0000232451.93703.ab.
Schulze, R. J., Schott, M. B., Casey, C. A., Tuma, P. L., & McNiven, M. A. (2019). The cell biology of the hepatocyte: A membrane trafficking machine. Journal of Cell Biology, 218, 2096–2112. https://doi.org/10.1083/jcb.201903090.
Seglen, P. O. (1976). Preparation of isolated rat liver cells. In Prescott, D. M. (Ed.), Methods in cell biology (pp. 29–83). Academic Press. https://doi.org/10.1016/S0091-679X(08)61797-5.
Sellamuthu, S., Manikandan, R., Thiagarajan, R., Babu, G., Dinesh, D., Prabhu, D., & Arulvasu, C. (2011). In vitro trans-differentiation of human umbilical cord derived hematopoietic stem cells into hepatocyte like cells using combination of growth factors for cell based therapy. Cytotechnology, 63, 259–268. https://doi.org/10.1007/s10616-011-9337-x.
Sera, Y., LaRue, A. C., Moussa, O., Mehrotra, M., Duncan, J. D., Williams, C. R., Nishimoto, E., Schulte, B. A., Watson, P. M., Watson, D. K., & Ogawa, M. (2009). Hematopoietic stem cell origin of adipocytes. Experimental Hematology, 37, 1108-1120.e4. https://doi.org/10.1016/j.exphem.2009.06.008.
Sharma, M., Rao, P. N., Sasikala, M., Kuncharam, M. R., Reddy, C., Gokak, V., Raju, B., Singh, J. R., Nag, P., & Reddy, D. N. (2015). Autologous mobilized peripheral blood CD34+ cell infusion in non-viral decompensated liver cirrhosis. World Journal of Gastroenterology, 21, 7264–7271. https://doi.org/10.3748/wjg.v21.i23.7264.
Shi, M., Liu, Z., Wang, Y., Xu, R., Sun, Y., Zhang, M., Yu, X., Wang, H., Meng, L., Su, H., Jin, L., & Wang, F. (2017). A pilot study of mesenchymal stem cell therapy for acute liver allograft rejection. Stem Cells Translational Medicine, 6, 2053–2061. https://doi.org/10.1002/sctm.17-0134.
Siller, R., Greenhough, S., Naumovska, E., & Sullivan, G. J. (2015). Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Reports, 4, 939–952. https://doi.org/10.1016/j.stemcr.2015.04.001.
Sirenko, O., Hancock, M. K., Hesley, J., Hong, D., Cohen, A., Gentry, J., Carlson, C. B., & Mann, D. A. (2016). Phenotypic characterization of toxic compound effects on liver spheroids derived from iPSC using confocal imaging and three-dimensional image analysis. ASSAY and Drug Development Technologies, 14, 381–394. https://doi.org/10.1089/adt.2016.729.
Si-Tayeb, K., Noto, F. K., Nagaoka, M., Li, J., Battle, M. A., Duris, C., North, P. E., Dalton, S., & Duncan, S. A. (2010). Highly efficient generation of human hepatocyte–like cells from induced pluripotent stem cells. Hepatology, 51, 297–305. https://doi.org/10.1002/hep.23354.
Snykers, S., Vanhaecke, T., De Becker, A., Papeleu, P., Vinken, M., Van Riet, I., & Rogiers, V. (2007). Chromatin remodeling agent trichostatin A: A key-factor in the hepatic differentiation of human mesenchymal stem cells derived of adult bone marrow. BMC Developmental Biology, 7, 24. https://doi.org/10.1186/1471-213X-7-24.
Sokal, E. M., Smets, F., Bourgois, A., Van Maldergem, L., Buts, J.-P., Reding, R., Bernard Otte, J., Evrard, V., Latinne, D., Vincent, M. F., Moser, A., & Soriano, H. E. (2003). Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: Technique, safety, and metabolic follow-up1. Transplantation, 76, 735–738. https://doi.org/10.1097/01.TP.0000077420.81365.53.
Soldatow, V. Y., LeCluyse, E. L., Griffith, L. G., & Rusyn, I. (2013). In vitro models for liver toxicity testing. Toxicol Res (camb), 2, 23–39. https://doi.org/10.1039/C2TX20051A.
Soltys, K. A., Setoyama, K., Tafaleng, E. N., Soto Gutiérrez, A., Fong, J., Fukumitsu, K., Nishikawa, T., Nagaya, M., Sada, R., Haberman, K., Gramignoli, R., Dorko, K., Tahan, V., Dreyzin, A., Baskin, K., Crowley, J. J., Quader, M. A., Deutsch, M., Ashokkumar, C., … Fox, I. J. (2017). Host conditioning and rejection monitoring in hepatocyte transplantation in humans. Journal of Hepatology, 66, 987–1000. https://doi.org/10.1016/j.jhep.2016.12.017.
Spada, M., Porta, F., Righi, D., Gazzera, C., Tandoi, F., Ferrero, I., Fagioli, F., Sanchez, M. B. H., Calvo, P. L., Biamino, E., Bruno, S., Gunetti, M., Contursi, C., Lauritano, C., Conio, A., Amoroso, A., Salizzoni, M., Silengo, L., Camussi, G., & Romagnoli, R. (2020). Intrahepatic administration of human liver stem cells in infants with inherited neonatal-onset hyperammonemia: A phase I study. Stem Cell Reviews and Reports, 16, 186–197. https://doi.org/10.1007/s12015-019-09925-z.
Stéphenne, X., Debray, F. G., Smets, F., Jazouli, N., Sana, G., Tondreau, T., Menten, R., Goffette, P., Boemer, F., Schoos, R., Gersting, S. W., Najimi, M., Muntau, A. C., Goyens, P., & Sokal, E. M. (2012). Hepatocyte transplantation using the domino concept in a child with tetrabiopterin nonresponsive phenylketonuria. Cell Transplantation, 21, 2765–2770. https://doi.org/10.3727/096368912X653255.
Stéphenne, X., Najimi, M., Sibille, C., Nassogne, M., Smets, F., & Sokal, E. M. (2006). Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology, 130, 1317–1323. https://doi.org/10.1053/j.gastro.2006.01.008.
Strom, S. C., Jirtle, R. L., Jones, R. S., Novicki, D. L., Rosenberg, M. R., Novotny, A., Irons, G., McLain, J. R., & Michalopoulos, G. (1982). Isolation, culture, and transplantation of human hepatocytes. Journal of the National Cancer Institute, 68(5), 771–778.
Tanaka, K., Soto-Gutierrez, A., Navarro-Alvarez, N., Rivas-Carrillo, J. D., Jun, H.-S., & Kobayashi, N. (2006). Functional hepatocyte culture and its application to cell therapies. Cell Transplantation, 15, 855–864. https://doi.org/10.3727/000000006783981332.
Tasnim, F., Phan, D., Toh, Y.-C., & Yu, H. (2015). Cost-effective differentiation of hepatocyte-like cells from human pluripotent stem cells using small molecules. Biomaterials, 70, 115–125. https://doi.org/10.1016/j.biomaterials.2015.08.002.
Thi, V. L. D., Wu, X., Belote, R. L., Andreo, U., Takacs, C. N., Fernandez, J. P., Vale-Silva, L. A., Prallet, S., Decker, C. C., Fu, R. M., Qu, B., Uryu, K., Molina, H., Saeed, M., Steinmann, E., Urban, S., Singaraja, R. R., Schneider, W. M., Simon, S. M., & Rice, C. M. (2020). Stem cell-derived polarized hepatocytes. Nature Communications, 11, 1–13. https://doi.org/10.1038/s41467-020-15337-2.
Tolosa, L., Caron, J., Hannoun, Z., Antoni, M., López, S., Burks, D., Castell, J. V., Weber, A., Gomez-Lechon, M.-J., & Dubart-Kupperschmitt, A. (2015). Transplantation of hESC-derived hepatocytes protects mice from liver injury. Stem Cell Research & Therapy, 6, 1–17. https://doi.org/10.1186/s13287-015-0227-6.
Tostões, R. M., Leite, S. B., Serra, M., Jensen, J., Björquist, P., Carrondo, M. J. T., Brito, C., & Alves, P. M. (2012). Human liver cell spheroids in extended perfusion bioreactor culture for repeated-dose drug testing. Hepatology, 55, 1227–1236. https://doi.org/10.1002/hep.24760.
Treyer, A., & Müsch, A. (2013). Hepatocyte Polarity. Comprehensive Physiology, 3, 243–287. https://doi.org/10.1002/cphy.c120009.
Tümpel, S., & Rudolph, K. L. (2019). Quiescence: Good and bad of stem cell aging. Trends in Cell Biology, 29, 672–685. https://doi.org/10.1016/j.tcb.2019.05.002.
Ullah, I., Subbarao, R. B., & Rho, G. J. (2015). Human mesenchymal stem cells—current trends and future prospective. Bioscience Reports, 35, e00191. https://doi.org/10.1042/BSR20150025.
Utech, S., Prodanovic, R., Mao, A. S., Ostafe, R., Mooney, D. J., & Weitz, D. A. (2015). Microfluidic generation of monodisperse, structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture. Advanced Healthcare Materials, 4, 1628–1633. https://doi.org/10.1002/adhm.201500021.
Varghese, D. S., Alawathugoda, T. T., & Ansari, S. A. (2019) Fine tuning of hepatocyte differentiation from human embryonic stem cells: Growth factor vs. small molecule-based approaches. Stem Cells International, 2019. https://doi.org/10.1155/2019/5968236.
Vassilopoulos, G., Wang, P.-R., & Russell, D. W. (2003). Transplanted bone marrow regenerates liver by cell fusion. Nature, 422, 901–904. https://doi.org/10.1038/nature01539.
Viiri, L. E., Rantapero, T., Kiamehr, M., Alexanova, A., Oittinen, M., Viiri, K., Niskanen, H., Nykter, M., Kaikkonen, M. U., & Aalto-Setälä, K. (2019). Extensive reprogramming of the nascent transcriptome during iPSC to hepatocyte differentiation. Scientific Reports, 9, 1–12. https://doi.org/10.1038/s41598-019-39215-0.
Vinken, M., & Hengstler, J. G. (2018). Characterization of hepatocyte-based in vitro systems for reliable toxicity testing. Archives of Toxicology, 92, 2981–2986. https://doi.org/10.1007/s00204-018-2297-6.
Wang, L., Li, J., Liu, H., Li, Y., Fu, J., Sun, Y., Xu, R., Lin, H., Wang, S., Lv, S., Chen, L., Zou, Z., Li, B., Shi, M., Zhang, Z., & Wang, F.-S. (2013). A pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. Journal of Gastroenterology and Hepatology, 28, 85–92. https://doi.org/10.1111/jgh.12029.
Wang, Q., Sun, D., Liang, Z., Wang, J., Zhong, X., Lyu, Y., Cao, J., Lin, Z., Du, Y., Miao, Z., Lu, S., Li, C., Xu, J., Shi, Y., & Deng, H. (2020). Generation of human hepatocytes from extended pluripotent stem cells. Cell Research, 30, 810–813. https://doi.org/10.1038/s41422-020-0293-x.
Wang, X., Willenbring, H., Akkari, Y., Torimaru, Y., Foster, M., Al-Dhalimy, M., Lagasse, E., Finegold, M., Olson, S., & Grompe, M. (2003). Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature, 422, 897–901. https://doi.org/10.1038/nature01531.
Wei, X., Wang, C., Liu, Q., Li, J., Li, D., Zhao, F., Lian, J., Xie, Y., Wang, P., Bai, X., & Jia, Z. (2008). In vitro hepatic differentiation of mesenchymal stem cells from human fetal bone marrow. Journal of International Medical Research, 36, 721–727. https://doi.org/10.1177/147323000803600414.
Woods, E. J., Perry, B. C., Hockema, J. J., Larson, L., Zhou, D., & Goebel, W. S. (2009). Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology, 59, 150–157. https://doi.org/10.1016/j.cryobiol.2009.06.005.
Wu, X., Xia, Y., Zhou, O., Song, Y., Zhang, X., Tian, D., Li, Q., Shu, C., Liu, E., Yuan, X., He, L., Liu, C., Li, J., Liang, X., Yang, K., Fu, Z., Zou, L., Bao, L., & Dai, J. (2020). Allogeneic human umbilical cord-derived mesenchymal stem cells for severe bronchopulmonary dysplasia in children: Study protocol for a randomized controlled trial (MSC-BPD trial). Trials, 21, 125. https://doi.org/10.1186/s13063-019-3935-x.
Wu, Y.-H., Hu, S.-Q., Liu, J., Cao, H.-C., Xu, W., Li, Y.-J., & Li, L.-J. (2014). Nature and mechanisms of hepatocyte apoptosis induced by D-galactosamine/lipopolysaccharide challenge in mice. International Journal of Molecular Medicine, 33, 1498–1506. https://doi.org/10.3892/ijmm.2014.1730.
Xu, H., Jiao, Y., Qin, S., Zhao, W., Chu, Q., & Wu, K. (2018). Organoid technology in disease modelling, drug development, personalized treatment and regeneration medicine. Experimental Hematology & Oncology, 7, 1–12. https://doi.org/10.1186/s40164-018-0122-9.
Xu, W., He, H., Pan, S., Chen, Y., Zhang, M., Zhu, S., Gao, Z., Peng, L., & Li, J. (2019). Combination treatments of plasma exchange and umbilical cord-derived mesenchymal stem cell transplantation for patients with hepatitis B virus-related acute-on-chronic liver failure: A clinical trial in China [WWW Document]. Stem Cells International. https://doi.org/10.1155/2019/4130757.
Xue, G., Han, X., Ma, X., Wu, H., Qin, Y., Liu, J., Hu, Y., Hong, Y., & Hou, Y. (2016). Effect of microenvironment on differentiation of human umbilical cord mesenchymal stem cells into hepatocytes in vitro and in vivo. BioMed Research International, 2016, 8916534. https://doi.org/10.1155/2016/8916534.
Yagi, T., Hardin, J. A., Valenzuela, Y. M., Miyoshi, H., Gores, G. J., & Nyberg, S. L. (2001). Caspase inhibition reduces apoptotic death of cryopreserved porcine hepatocytes. Hepatology, 33, 1432–1440. https://doi.org/10.1053/jhep.2001.24560.
Yang, J.-F., Cao, H.-C., Pan, Q.-L., Yu, J., Li, J., & Li, L.-J. (2015). Mesenchymal stem cells from the human umbilical cord ameliorate fulminant hepatic failure and increase survival in mice. Hepatobiliary & Pancreatic Diseases International, 14, 186–193. https://doi.org/10.1016/S1499-3872(15)60354-X.
Yin, L., Zhu, Y., Yang, J., Ni, Y., Zhou, Z., Chen, Y., & Wen, L. (2015). Adipose tissue-derived mesenchymal stem cells differentiated into hepatocyte-like cells in vivo and in vitro. Molecular Medicine Reports, 11, 1722–1732. https://doi.org/10.3892/mmr.2014.2935.
Yoon, H.-H., Jung, B.-Y., Seo, Y.-K., Song, K.-Y., & Park, J.-K. (2010). In vitro hepatic differentiation of umbilical cord-derived mesenchymal stem cell. Process Biochemistry, 45, 1857–1864. https://doi.org/10.1016/j.procbio.2010.06.009.
Yu, Y.-D., Kim, K.-H., Lee, S.-G., Choi, S.-Y., Kim, Y.-C., Byun, K.-S., Cha, I.-H., Park, K.-Y., Cho, C.-H., & Choi, D.-H. (2011). Hepatic differentiation from human embryonic stem cells using stromal cells. Journal of Surgical Research, 170, e253–e261. https://doi.org/10.1016/j.jss.2011.06.032.
Yu, Y.-B., Song, Y., Chen, Y., Zhang, F., & Qi, F.-Z. (2018). Differentiation of umbilical cord mesenchymal stem cells into hepatocytes in comparison with bone marrow mesenchymal stem cells. Molecular Medicine Reports, 18, 2009–2016. https://doi.org/10.3892/mmr.2018.9181.
Zhang, G.-Z., Sun, H.-C., Zheng, L.-B., Guo, J.-B., & Zhang, X.-L. (2017). In vivo hepatic differentiation potential of human umbilical cord-derived mesenchymal stem cells: Therapeutic effect on liver fibrosis/cirrhosis. World Journal of Gastroenterology, 23, 8152–8168. https://doi.org/10.3748/wjg.v23.i46.8152.
Zhang, Y., Li, Y., Li, W., Cai, J., Yue, M., Jiang, L., Xu, R., Zhang, L., Li, J., & Zhu, C. (2018). Therapeutic effect of human umbilical cord mesenchymal stem cells at various passages on acute liver failure in rats. Stem Cells International, 2018, 7159465. https://doi.org/10.1155/2018/7159465.
Zhang, Y.-N., Lie, P.-C., & Wei, X. (2009). Differentiation of mesenchymal stromal cells derived from umbilical cord Wharton’s jelly into hepatocyte-like cells. Cytotherapy, 11, 548–558. https://doi.org/10.1080/14653240903051533.
Zhang, Z., Lin, H., Shi, M., Xu, R., Fu, J., Lv, J., Chen, L., Lv, S., Li, Y., Yu, S., Geng, H., Jin, L., Lau, G. K. K., & Wang, F.-S. (2012). Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. Journal of Gastroenterology and Hepatology, 27, 112–120. https://doi.org/10.1111/j.1440-1746.2011.07024.x.
Zhao, X., Zhu, Y., Laslett, A. L., & Chan, H. F. (2020). Hepatic differentiation of stem cells in 2D and 3D biomaterial systems. Bioengineering, 7, 47. https://doi.org/10.3390/bioengineering7020047.
Zheng, G., Liu, Y., Jing, Q., & Zhang, L. (2015). Differentiation of human umbilical cord-derived mesenchymal stem cells into hepatocytes in vitro. Bio-Medical Materials and Engineering, 25, 145–157. https://doi.org/10.3233/BME-141249.
Zheng, J., Li, H., He, L., Huang, Y., Cai, J., Chen, L., Zhou, C., Fu, H., Lu, T., Zhang, Y., Yao, J., & Yang, Y. (2019). Preconditioning of umbilical cord-derived mesenchymal stem cells by rapamycin increases cell migration and ameliorates liver ischaemia/reperfusion injury in mice via the CXCR4/CXCL12 axis. Cell Proliferation, 52, e12546. https://doi.org/10.1111/cpr.12546.
Ziolkowska, K., Jedrych, E., Kwapiszewski, R., Lopacinska, J. M., Skolimowski, M., & Chudy, M. (2010). PDMS/glass microfluidic cell culture system for cytotoxicity tests and cells passage. Sensor Actuator B-CHEM, Sensors and Actuators B, Sensors and Actuators B: Chemical : International Journal Devoted to Research and Development of Physical and Chemical Transducers, Sensors and Actuators b: Chemicalinterna, 145, 533–542. https://doi.org/10.1016/j.snb.2009.11.010.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alomari, M. (2021). Differentiation of Stem Cells into Hepatocyte Lineage: In Vitro Cell Culture, In Vivo Transplantation in Animal Models. In: Khan, F.A. (eds) Advances in Application of Stem Cells: From Bench to Clinics. Stem Cell Biology and Regenerative Medicine, vol 69. Humana, Cham. https://doi.org/10.1007/978-3-030-78101-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-78101-9_6
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-78100-2
Online ISBN: 978-3-030-78101-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)