Abstract
Human umbilical cord mesenchymal stem cells (hUMSCs) have been shown to have multiple differentiation potentials. However, a key problem is that only a small number of hUMSCs can migrate to damaged tissue after transplantation. According to “The Theory of Kidney Essence” in Traditional Chinese Medicine, some traditional Chinese medicines used for tonifying the kidneys can be applied in promoting the differentiation and migration of stem cells in vivo. Our previous study demonstrated that icariin (ICA) could up-regulate the pluripotent genes of hUMSCs in vitro and induce cell migration in mice in an acute kidney injury model in vivo. The aim of this study was to investigate the effects of ICA-induced hUMSCs in chronic liver injury (CLI) caused by carbon tetrachloride (CCl4). CLI was induced by intraperitoneal injection of CCl4. ICA-treated hUMSCs were transplanted via intra-venous injection. The animals were followed for survival, biochemistry analysis and pathology. The results show that ICA-treated hUMSCs accelerate the recovery of liver function in mice with CLI. In addition, ICA-treated hUMSCs increase the anti-oxidant activities in liver and prevent the progression to hepatic fibrosis. Moreover, ICA induces the migration of hUMSCs to the injured liver tissue. In conclusion, these data demonstrate that ICA-treated hUMSCs exhibit recovery and protective properties in the mice model of CCl4-induced CLI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver injury can be induced either by immune mediated biological factors (e.g., hepatitis virus, bacteria, parasite, etc.), chemical factors (e.g., medicine, industrial poisons, alcohol, etc.) or environmental factors (Holt and Ju 2006). These factors can cause apoptosis and necrosis of hepatic cells (Wang 2014). Chronic hepatic cell necrosis leads to hepatic fibrosis, liver cirrhosis, and even hepatocellular carcinoma. Therefore, the prevention of liver injury is a critical step in protecting the liver against incidence of cirrhosis and liver function failure.
With the development of stem-cell-based replacement therapies, mesenchymal stem cells (MSCs) are now one of the promising options in the treatment of liver disease. MSCs, derived from mesoderm and neural crest, are adult progenitor cells with a multidirectional differentiation potential (Slukvin and Vodyanik 2011; Vodyanik et al. 2010; Takashima et al. 2007). Studies have demonstrated that MSCs can differentiate into hepatocytes because of their mesenchymal differentiation capabilities (Hong et al. 2005; Lee et al. 2004; Snykers et al. 2009). Moreover, MSCs have immunosuppressive properties (Bartholomew et al. 2002; Di Nicola et al. 2002; Glennie et al. 2005; Krampera et al. 2003; Le Blanc and Ringden 2005). Currently, bone marrow is the most common source of MSCs. The adverse reaction that necessitates several weeks of recovery is one of the disadvantages in the collection of bone marrow MSCs. Human umbilical cord can be a vastly available source of mesenchymal stem cells since it is readily derived from medical waste (Taghizadeh et al. 2011). Human umbilical cord mesenchymal stem cells (hUMSCs) are stroma cells isolated from Wharton’s Jelly in the human umbilical cord. It has been demonstrated that hUMSCs have a multiple differentiation potential. hUMSCs can differentiate into various types of cells such as osteoblasts, adipocytes, chondrocytes, islet cells, neurocytes, myocardial cells, hepatocytes, germ cells and so on Wang et al. (2004). In addition, hUMSCs do not express major histocompatibility complex class II antigens and do not activate T-cells in mixed cell cultures (Tse et al. 2003). Moreover, hUMSCs have significant immune modulatory effects such as inhibiting mixed lymphocyte reaction and T cell proliferation after mitogenic stimulation (Le Blanc et al. 2003). Considering the advantages of hUMSCs (e.g., multiple differentiation potential, low rejection reaction, no ethical controversies, vastly available source) given above, hUMSCs seem to be a good source in stem-cell-based replacement therapies. However, a key problem is that only a small number of hUMSCs can migrate to damaged tissue after transplantation. Therefore, accelerating the migration of hUMSCs can improve the efficacy of transplantation.
According to “The Theory of Kidney Essence” in Traditional Chinese Medicine, some traditional Chinese medicines which have the properties of tonifying the kidney and strengthening yang can increase the proliferation and differentiation of stem cells (Zhang et al. 2004). Studies have shown that serum containing Carapax et Plastrum Testudinis astragalin can promote the proliferation and differentiation of mesenchymal stem cells in vitro (Zeng et al. 2007; Zhou et al. 2005). Zuo-Gui-Wan, one of the most popular prescriptions for kidney tonifying can up-regulate the pluripotent gene (Oct-4) expression in embryonal stem cells and, therefore, inhibit the apoptosis and promote the proliferation of stem cells. You-Gui-Wan can increase cell proliferation gene (Notch) and inhibit the P16 gene expression in stem cells (Hu et al. 2007; An et al. 2007).
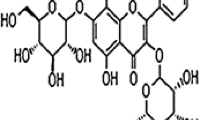
Icariin (ICA), with a molecular weight of 676.65, is extracted from Epimedii, its formula is C33H40O15. Our previous study demonstrated that ICA, at a concentration of 100 μmol/L, could up-regulate the pluripotent Oct-4 gene expression of hUMSCs after being treated for 1 week in vitro and ICA-treated hUMSCs exert significant cell migration to kidney tissue in mice in an acute kidney injury model in vivo (Chu and Wang 2016). It has also been demonstrated that ICA can enhance the proliferation of rat bone mesenchymal stem cells via stimulating the ERK and p38MAPK signaling pathway (Qin et al. 2015). In addition, research has shown that activating the PI3 K/Akt signaling pathway is one of the mechanisms for ICA to enhance the osteogenic differentiation of rat bone stromal cells (Zhai et al. 2014). Moreover, icariin could delay the cirrhotic effect of hepatocytes induced by CCL4 in vitro (Qian et al. 2011). The aim of this study is to investigate the protective effects of icariin (ICA) induced hUMSCs on chronic liver injury in mice and to evaluate the possible mechanism of anti-hepatic injury. Normal hUMSCs were used as the positive control. Liver function in serum and pathological staining in liver were measured to evaluate the hepatic protective effect of ICA-treated hUMSCs. Moreover, anti-oxidant activities and gene expression related to fibrosis in liver were assessed in an effort to elucidate the possible mechanisms by which ICA-treated hUMSCs exert their protective hepatic activities. In addition, the cells were fluorescence-labeled in order to observe the difference in migration of ICA-treated hUMSCs to the liver in comparison to normal hUMSCs. Terminal-deoxynucleoitidyl transferase mediated nick end labeling (TUNEL) was used to study cell apoptosis in liver tissue in each group.
Material and method
Animals and reagents
Kunming mice (Beijing HFK Bioscience Company, Beijing, China); icariin standard substance (C33H40O15, molecular weight: 676.6617 kD, National Institute for Control of Pharmaceutical and Biology Product, Beijing, China); DMEM/F12, Fetal Bovine Serum (FBS) and PBS solution (all from Hyclone, Logan, UT, USA); Penicillin and Streptomycin (PS) and l-glutamine (LG, Solarbio, Beijing, China); trypsin-EDTA solution (Sigma, St. Louis, MO, USA); cross-linkable membrane dye (DiR), carbon tetrachloride (CCl4), cereal third transaminase (ALT), aspartate aminotransferase (AST), albumin (ALB), total protein (TP), superoxide dismutase (SOD), malonaldehyde (MDA), glutathione (GSH), terminal-deoxynucleoitidyl transferase mediated nick end labeling experiment (TUNEL) test kit (all from Nanjing Jiancheng Biological Engineering Institute, Nanjing, China); total RNA extraction kit, first-stand cDNA reverse transcription kit, polymerase chain reaction kit and primers (TianGen Biotechnology Co., Ltd, Beijing, China).
Method
Cell culture
Human umbilical cord was obtained from a healthy donor (upon obtaining informed patient consent). It was immediately transferred to the laboratory. Under class III laminar-flow hood the cord was exposed to Wharton’s Jelly tissue and cut into 1 mm3 fragments. They were then immersed in 15 mL DMEM/F12 supplemented with 1% penicillin/streptomycin, 1% L-glutamine and 10% FBS in T75 tissue culture flasks. The tissue culture flasks were placed in an incubator with saturated humidity at 37 °C containing 5% CO2. Fibroblast-like cells started to migrate out of the tissue pieces after 7 days. They proliferated and were sub-cultured up to passage 4 (P4) in which homogenous cell populations were obtained.
Induction
hUMSCs were sub-cultured in 15 mL DMEM/F12 supplemented with 1% penicillin/streptomycin, 1% L-glutamine and 10% FBS in T75 tissue culture flasks. One week before hUMSCs were administered, the expansion medium was changed to ICA containing medium (supplemented with 100 μmol/L ICA, 10% FBS, 1% PS and 1% LG) and cultured for 1 week with the medium being changed every 3 days. As a control, normal hUMSCs were cultured in DMEM/F12 supplemented with 1% penicillin/streptomycin, 1% L-glutamine and 10% that was changed every 3 days.
Cell labeling
Before the hUMSCs were administered, they were labelled with the cross-linkable membrane dye, DiR. 2 × 106. Cells were incubated in 1 mL of the DiR cell-labeling solution at 37 °C for 30 min. The unbound dye was removed by washing with PBS, and the labeled cells were re-suspended in 0.9% saline solution.
Animals and treatments
Mice were housed at room temperature (23 ± 1 °C) with a 12 h light and 12 h dark cycle (lights on from 6:00 am to 6:00 pm). Food and water were available ad libitum. The experiments were carried out according to the institutional regulations and national criteria for animal experimentation. The Institution Animal Ethics Committee reviewed the entire animal protocol prior to conducting the experiments. Mice were randomly assigned to the following four groups: Group A: Control group (n = 10); Group B: CCl4 model group (n = 10); Group C: normal hUMSCs group (n = 6); Group D: ICA-treated hUMSCs group (n = 6).
After a three-day acclimatization period, chronic liver injury was induced as described previously (Yoshiji and Sahashi 2002). In brief, Groups B, C and D received an intraperitoneal injection of 20% CCl4 solution diluted in olive oil (2 mL/kg body weight), twice weekly for 4 weeks. Group A received intraperitoneal injection of olive oil only (2 mL/kg body weight). After 4 weeks CCl4 was withdrawn. Thereafter, in Groups A and B normal saline was injected into the tail vein; in Group C hUMSCs (2 × 106) were diluted in normal saline and injected into the tail vein. In Group D ICA-treated hUMSCs (2 × 106) were diluted in normal saline injected into the tail vein.
On the 14th day after administration of the cells the mice were anesthetized with ether and blood was obtained from the retrobulbar plexus for serum biochemistry analysis. Blood samples were centrifuged at 3000 rpm for 15 min and serum was collected and kept at −80 °C for analysis as described below.
The animals were then decapitated and their livers removed. The livers were dissected into two equal parts. For each liver, one half was fixed in 10% formalin for histological analysis; the others were stored at −80 °C for the biochemistry analysis below.
Serum and tissue analysis
Serum ALT, AST, ALB, TP and liver SOD, MDA, GSH were measured on a Plate Reader using experimental reagent kits.
RNA isolation and real-time reverse transcription quantitative polymerase chain reaction (RT-PCR)
Total RNA was isolated from mice liver tissues using the RNA extraction kit and first strand cDNA was synthesized from 1ug of total RNA according to the manufacturer’s instructions. Quantitative RT-PCR (qRT-PCR) was used to detect the expression of β-actin, TGF-a, TGF-β1, COL-I and COL-III. All samples were run in triplicate and detected by BIORAd iQ5 (Biorad, Hercules, CA, USA). β-actin was used as a loading control. The sequences of all primers are listed in Table 1.
Histological analysis
Liver tissue sections were dissected and fixed in 10% formalin, then embedded in paraffin, sectioned to 5 μm thickness, stained with hematoxylin and eosin (H&E). The extent of CC14-induced liver injury was evaluated by assessing morphological changes in liver sections.
Frozen section and TUNEL staining
The liver tissue sections were dissected, frozen and sectioned to 5 μm thickness. The distribution of hUMSCs was observed under a fluorescence microscope. TUNEL experiment was conducted using experimental reagent kit and the section observed under a fluorescence microscope.
Statistics
Quantitative data were expressed as Mean ± SD and compared using ANOVA K independent samples test when the variances were heterogeneous. Results were considered to be statistically significant when P < 0.05.
Results
Isolation and characterization of hUMSCs
One week after the primary work, adherent cells with fibroblastic morphology could be observed (Fig. 1a). At approximately 3 weeks, cells reached 80% confluence and passage could be undertaken (Fig. 1b).
Liver function tested in serum after transplantation
Hepatic protective activities of ICA-treated hUMSCs were assessed by hepatic histology and by measuring levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin (ALB) and total protein (TP) of serum, which are frequently-used parameters of evaluating liver function. Administration of CC14 for 4 weeks caused significant elevation of serum ALT, and AST activity in mice compared with mice treated with olive oil (P < 0.01; Table 2). On the 14 days after administration of cells, the increase in ALT and AST activity remained significantly higher in Group B than in Group A (P < 0.01). Serum AST and ALT activity was significantly lower in group D than in Group B (P < 0.05; Table 3) suggesting that ICA-treated hUMSCs accelerate the recovery of liver function in mice with chronic liver injury.
Anti-oxidant activities tested in liver after transplantation
Oxidative damage appeared in all stages of chronic liver injury. Anti-oxidant activities of ICA-treated hUMSCs were accessed through investigating the levels of superoxide dismutase (SOD), malondialdehyde (MDA) and glutathione (GSH) in liver. Activity of SOD and level of GSH were significantly lower in Group B compared to Group A (P < 0.05; Fig. 2a, c). Group D demonstrated an increase in SOD activity and GSH level compared to Group B, the difference was statistically significant (P < 0.05; P < 0.01, respectively, Fig. 2a, c). MDA level was increased in Group B compared with Group A (P < 0.01; Fig. 2b). MDA levels were decreased in Group D compared to Group B (P<0.05; Fig. 2b). In conclusion, ICA treated hUMSCs showed an increase in anti-oxidant activity in mice with chronic liver injury.
Anti-oxidant activities tests in liver after hUMSCs transplantation. a SOD activity in liver after chronic CCl4 injection and subsequent hUMSCs and ICA-treated hUMSCs administration. b MDA level in liver after chronic CCl4 injection and subsequent hUMSCs and ICA-treated hUMSCs administration. c GSH level in liver after chronic CCl4 injection and subsequent hUMSCs and ICA-treated hUMSCs administration. Group A Control group (n = 6); Group B CCl4 model group (n = 10); Group C hUMSCs administration group (n = 6); Group D ICA-treated hUMSCs administration group (n = 6). ## P < 0.01 compared with the control group, *P < 0.05 compared with the CCl4 model group, **P < 0.01 compared with the CCl4 model group. Groups B, C and D were treated with CCl4 to set up the chronic liver injury model, Group C was transplanted hUMSCs and Group D was transplanted ICA treated hUMSCs
Gene expression related to hepatic injury after administration of cells
Long periods of chronic liver injury could progress to liver fibrosis and liver cirrhosis. COL-I, COL-III and TGF-β1 genes contribute to the progression of liver fibrosis and TGF-a gene is related to promoting the regeneration of hepatic cells. The gene expression of COL-1, COL-III, TGF-β1 and TGF-α were investigated to study the mechanism of ICA-treated hUMSCs exert on the anti-hepatic injury effect. COL-I, COL-III and TGF-β1 and mRNA expressions were increased in Group B compared to Group A (P < 0.01; Fig. 3a, b, d). COL-I and COL-III expressions were down-regulated in Group D compared to Group B (P < 0.01; P < 0.05, respectively, Fig. 3a, b). TGF-β1 mRNA expression was increased in Groups C and D compared to Group B (P < 0.01; Fig. 3d). Group B demonstrated up-regulation of TGF-a mRNA expression compared to Group A (P < 0.05; Fig. 3c), the TGF-a mRNA expression was increased in Group D compared to Group B (P < 0.01; Fig. 3c). The results show that ICA-treated hUMSCs transplantation could improve hepatic injury through down-regulating the COL-I, COL-III and TGF-β1gene expression as well as up-regulating the TGF-a gene expression in liver.
Liver injury genes expression tests in liver after hUMSCs administration. a COL-I mRNA expression in liver after chronic CCl4 injection and subsequent hUMSCs and ICA-treated hUMSCs administration. b COL-III mRNA expression in liver after chronic CCl4 injection and subsequent hUMSCs and ICA-treated hUMSCs administration. c TGF-a mRNA expression in liver after chronic CCl4 injection and subsequent hUMSCs and ICA-treated hUMSCs administration. d TGF-β1 mRNA expression in liver after chronic CCl4 injection and subsequent hUMSCs and ICA treated hUMSCs administration. Group A Control group; Group B CCl4 model group; Group C hUMSCs administration group; Group D ICA-treated hUMSCs administration group. ## P < 0.01 compared with the control group, *P < 0.05 compared with the CCl4 model group, **P < 0.01 compared with the CCl4 model group. Groups B, C and D were treated with CCl4 to set up the chronic liver injury model, Group C was transplanted hUMSCs and Group D was transplanted ICA treated hUMSCs
Histological findings
CCl4 treatment induced a significant amount of liver injury and extensive changes in liver morphology, including the infiltration of inflammatory cells and obvious cellular swelling of the liver, as observed with H&E staining. Liver injury was attenuated in Groups C and D after 4 weeks. Most importantly, pathological changes in Group D were less than those in Group C, reflected as less cellular swelling and infiltration of inflammatory cells as well as orderly arrangement of hepatic cord cells, suggesting that the inflammation, mitochondrial damage and liver parenchyma injury were improved after the hUMSCs administration, especially after the ICA-treated hUMSCs transplantation (Fig. 4).
Cells migration and the apoptosis of cells in the liver
Our previous study demonstrated that ICA induced hUMSC migration to the kidney in acute renal injury. To verify the previous discovery, the hUMSCs labeled with DIR were observed in liver tissue through fluorescence microscope. In addition, the cell apoptosis in the liver was directly observed via TUNEL staining. The DIR-labelled hUMSCs were detected in liver sections in Groups C and D. More DiR-labelled hUMSCs were observed in Group D compared with Group C under the fluorescence microscope (Fig. 5a, b). TUNEL experiment demonstrated that there were more cells undergoing apoptosis in Group B than in Group C and D and the apoptosis of cells in Group D were less than in Group C. No apoptosis was observed in Group A (Fig. 6a–d). In agreement with the previous results, the current results show that ICA could induce hUMSCs migration to the damaged tissue. Moreover, ICA-treated hUMSCs transplantation attenuated the hepatic cell apoptosis in the chronic liver injury model.
Discussion
CCl4-induced liver injury, which shows many similarities with human liver injury, is a common model for studying the mechanisms of liver injury and the therapeutic effects of drugs (Fort et al. 1998; Tamayo 2007). In this study, CCl4-induced chronic liver injury was established to examine the effects of ICA-treated hUMSCs administration in the chronic liver injury model. AST, ALT, ALB and TP, which are conventional indicators of chronic liver injury, were measured to investigate the hepatic protective effect of ICA-treated hUMSCs. This study revealed changes in the levels of AST and ALT in the CCl4 model group at the end of the fourth week, indicating considerable hepatocellular injury. After ICA-treated hUMSCs were administered (2 × 106) for 14 days, increasing levels of ALT and AST in serum were observed.
Moreover, ICA-treated hUMSCs activity in chronic liver injury may relate to an increase in the SOD activity and GSH level as well as decrease of the MDA level in the liver. It has been demonstrated that oxidative damage is closely related to the CCl4-induced liver injury model. In the liver, CCl4 inhibited metabolism and activated hepatic cytochrome P450s to produce trichloromethyl radicals CCl3 and OOCCl3. These free radicals contribute to lipid peroxidation that disrupts calcium homeostasis, gradually leading to the necrosis of hepatocytes and cell membrane injury surrounding the central vein. Finally, the necrosis of hepatocytes and hypo-immunity result in liver injury (Morrison et al. 1965; Xu and Qu 2008; Bockhold et al. 2005; Fausto 2006). SOD and GSH could participate in eliminating the generation of free radical (O2 −) in order to relieve the damage of trichloromethyl radicals in liver. SOD can react with free radical (O2 −) to produce H2O2 and GSH can convert H2O2 to H2O (Wang et al. 2011; Sun et al. 2008). MDA is the end product in the lipid peroxidation, so measuring the concentration of MDA in the liver can indicate the extent of damage of hepatic cells indirectly (Li et al. 2006).
In addition, regulating the transforming growth factors and hepatic injury cytokines may be one of the genetic mechanisms of ICA treated hUMSCs in preventing chronic liver injury. TGF-α can accelerate the synthesis of DNA in cells, therefore promoting the regeneration and restoration of hepatic cells (Waseem and Lane 1990; Chen et al. 2003). It was demonstrated in this study that ICA-treated hUMSCs can up-regulate the gene expression of TGF-α in the liver. Transforming growth factor-beta (TGF-β) can be secreted from hepatic stellate cells (HSCs), Kupffer cells and hepatic cells. TGF-β is considered the most important cytokine in the development of hepatic injury and hepatic fibrosis. TGF-β1, one of the isoforms in the TGF-β family, can stimulate the HSCs producing extracellular matrix such as COL-I and COL-III which can promote the necrosis of hepatic cells (Kong et al. 2006; Bissell et al. 2001). This study has shown that ICA-treated hUMSCs can down-regulate the TGF-β1, COL-I COL-III gene expressions in the liver and prevent chronic liver injury.
To evaluate the potential mechanisms by which ICA-treated hUMSCs ameliorated chronic liver injury, we first determined whether the cells survived and migrated from the peripheral circulation and entered the liver of mice in which they were administered. The cells were labeled with the cross-linkable membrane dye in order to observe the difference in migration between the normal hUMSCs administration group and ICA-treated hUMSCs administration group. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), which is a method for detecting DNA fragmentation by labeling the terminal end of nucleic acids, was used to investigate the difference in cell apoptosis between the normal hUMSCs administration group and ICA-treated hUMSCs administration group. A clear positive observation in this study was that the fluorescent signal in the ICA-treated hUMSCs administration group was stronger compared to the normal hUMSCs administration group in the liver, indicating that ICA induction can promote the migration of hUMSCs to the liver. TUNEL experiment also demonstrated that cell apoptosis was less in the ICA-treated hUMSCs group compared to the normal hUMSCs group and the CCL4 model group.
In conclusion, ICA-treated hUMSCs administration possesses potent abilities to alleviate chronic liver injury, suggesting that ICA-treated hUMSCs exert this effect by enhancing the anti-oxidant abilities, preventing the development of hepatic fibrosis and promoting regeneration of hepatic cells. Compared to hUMSCs, ICA administration resulted in an increase in migration and reduced the apoptosis of hUMSCs in the liver. In addition, accumulating studies have demonstrated that mesenchymal stem cells could secret cytokines after migrating to the target organ (Togel 2005). Further study will be carried out to explore the levels of cytokines, chemotactic factors and growth factors of hUMSCs after being treated with ICA in order to illustrate the possible cellular functions of ICA on enhancing the migration of hUMSCs.
References
An HM, Hu B, Shi YF, Xu LW, Shi XF, Chen FM, Gu Y, Pan LX, Wang Z (2007) Effect of nourishing kidney-yin of embryonal stem cells. Chin J Inf Tradit Chin Med 14:24–26
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R (2002) Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30:42–48
Bissell DM, Roulot D, George J (2001) Transforming growth factor beta and the liver. Hepatology 34:859–867
Bockhold K, Riely CA, Jeffreys C (2005) Overcoming obstacles to immunization in patients with chronic liver disease. Am J Med 118:40–45
Chen P, Li K, Dong JH, Han BL (2003) Changes of TGF-α, HGF, PCNA and IGFBP-1 s mRNA after partial hepatectomy in rat liver. World Chin J Digestol 11:434–443
Chu XQ, Wang L, Li W, Duya P, Bian Y-H (2016) Effect of Chinese materia medica with tonifying kidney function on transplantation of multipotency mesenchymal stem cells from human umbilical cord in mice model of acute kidney injury. Chin Herb Med 8:173–181
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843
Fausto Nelson (2006) Involvement of the innate immune system in liver regeneration and injury. J Hepatol 45:347–349
Fort J, Pilette C, Veal N, Oberti F, Gallois Y, Douay O, Rosenbaum J, Calès PJ (1998) Effects of long-term administration of interferon alpha in two models of liver fibrosis in rats. Hepatol 29:263–270
Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F (2005) Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105:2821–2827
Holt MP, Ju C (2006) Mechanisms of drug-induced liver injury. AAPS J 8:48–54
Hong SH, Gang EJ, Jeong JA, Ahn C, Hwang SH, Yang IH, Park HK, Han H, Kim H (2005) In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun 330:1153–1161
Hu B, An HM, Shi YF, Xu LW, Shi XF, Chen FM, Gu Y, Pan LX, Wang Z (2007) Effect of nourishing kidney-yin on functions of key genes of embryonal stem cells. Chin J Basic Med Tradit Chin Med 13:365–367
Kong L, Zhang YG, Wang RQ, Yao SK (2006) The effects of transforming growth factor beta1/Smads signaling pathway changes on the pathogenesis of liver fibrosis. Chin J Hepatol 14:842–844
Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F (2003) Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101:3722–3729
Le Blanc K, Ringden O (2005) Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 11:321–334
Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O (2003) Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 57:11–20
Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK (2004) In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 40:1275–1284
Li WP, Li XB, Xiao SH, Huang XW, Deng CL, Chen F, Tang KH (2006) The effects of LingQiJuanGan liquor on oxygen free radicals and NO in rats with chronic liver damage. Li Shi Zhen Med Meteria Medica Res 5:756–757
Morrison GR, Brock FE, Karl IE, Biophys RE (1965) Quantitative analysis of regenerating and degenerating areas within the lobule of the carbon tetrachloride- injured liver. Arch Biochem 111:448–460
Ohta Y, Sahashi D (2002) l-tryptophan administration promotes the reversion of pre-established chronic liver injury in rats treated with carbon tetrachloride. J Nutr Biochem 13:550–559
Qian HH, Liu P, Li J, Chen L, Cao L, Zhang RX (2011) Icaritin delays CCl4-induced hepatic cirrhosis in rats by protecting hepatocytes from oxidative injury. Acad J Second Mil Medical Univ 31:625–629
Qin SY, Zhou W, Liu SY, Chen PX, Wu HJ (2015) Icariin stimulates the proliferation of rat bone mesenchymal stem cells via ERK and p38 MAPK signaling. Int J Clin Exp Med 8:7125–7133
Slukvin II, Vodyanik M (2011) Endothelial origin of mesenchymal stem cells. Cell Cycle 10:1370–1373
Snykers S, De Kock J, Rogiers V, Vanhaecke T (2009) In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells 27:577–605
Sun SZ, Lu J, Guan ST, Zhao J, Zhang HM (2008) Effect of coriolus versicolor polysaccharides on the content of antioxidase, Free Radicals and NO in Liver Injury. Li Shi Zhen Med Meteria Medica Res 6:1439–1440
Taghizadeh RR, Cetrulo KJ, Cetrulo CL (2011) Wharton’s Jelly stem cells: future clinical applications. Placenta 32:311–315
Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa S (2007) Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 129:1377–1388
Tamayo RP (2007) Is cirrhosis of the liver experimentally produced by CCl4 an adequate model of human cirrhosis? Hepatology 3:112–120
Togel F (2005) Amelioration of acute renal failure by stem cell therapy—paracrine secretion versus transdifferentiation into resident cells: administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol E-pub February 15, 2005. J Am Soc Nephrol 16:1153–1163
Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC (2003) Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 75:389–397
Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, Slukvin II (2010) A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 7:718–729
Wang KW (2014) Molecular mechanisms of liver injury: apoptosis or necrosis. Exp Toxicol Pathol 66:351–356
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC (2004) Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 22:1330–1337
Wang JM, Cui Y, Wang ZT, Ji LL, Cui YX, Liu H, Wang CH (2011) Research Progress of Superoxide Dismutase Involved in Liver Injury. Chin J Exp Tradit Med Formulae 17:265–268
Waseem NH, Lane DP (1990) Monoclonal antibody analysis of the proliferating cell nuclear antigen(PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci 96:121–129
Xu X, Qu CQ (2008) Mechanisms of drug-induced liver injury. Med Recapitulate 14:747–749
Zeng YR, Fan YG, Liu H, Zhang DX, Xia XZ, Fan HJ (2007) Effect of serum containing kidney-tonifying and blood-activating chinese herbal medicine on the proliferation of rat bone marrow mesenchymal stem cells in vitro. Tradit Chin Drugs Res Clin Pharmacol 18:93–96
Zhai YK, Guo XY, Ge BF, Zhen P, Ma XN, Zhou J, Ma HP, Xian CJ, Chen KM (2014) Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3 K/AKT/eNOS/NO/cGMP PKG. Bone 66:189–198
Zhang J, Xu ZW, Du SH (2004) Essence and stem cell. Chin Arch Tradit Chin Med 22:1198
Zhou JH, Chen DF, Li H, Du SH, Li YW, Deng RD, Zhang SX (2005) Effects of serum containing Carapax et Plastrum Testudinis on in- vitro proliferation of rat mesenchymal stem cells. J Guangzhou Univ Chin Med 22:35–38
Acknowledgements
This work is supported by Tianjin Research Program of Application Foundation and Advanced Technology(15JCYBJC26100) and Program for Changjiang Schilars and Innovative Research Team in University (PCSIRT): IRT_14R41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cui, H., Liu, Z., Wang, L. et al. Icariin-treated human umbilical cord mesenchymal stem cells decrease chronic liver injury in mice. Cytotechnology 69, 19–29 (2017). https://doi.org/10.1007/s10616-016-0034-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-016-0034-7