Abstract
The plasma membrane H+ ATPase is a member of the P-ATPase family transporting H+ from the cytosol to the extracellular space and thus energizing the plasma membrane for the uptake of ions and nutrients. As a housekeeping gene, this protein can be detected in almost every plant cell including the exclusive expression of specific isoforms in pollen grains and tubes where its activity is a prerequisite for successful germination and growth of pollen tubes. This review summarizes the current knowledge on pollen PM H+ ATPases and hypothesizes a central role for pollen-specific isoforms of this protein in tube growth. External as well as cytosolic signals from signal transduction and metabolic pathways are integrated by the PM H+ ATPase and directly translated to tube growth rates, allocating the PM H+ ATPase to an essential node in the signalling network of pollen tubes in their race to the ovule.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollen grains present a highly specialized tissue of angiosperm plants. They can be regarded as two- or three-cellular organisms consisting of the vegetative cell which surrounds a generative cell or two sperm cells. Within the anther tissue these male gametophytes develop from a diploid pollen mother cell (microsporocyte) after meiotic divisions into haploid microspores that further divide by mitosis into the vegetative and the generative cell. In some species the generative cell further divides into two sperm cells in the pollen grain whereas in other species the generation of sperm cells occurs later. In a last step the anther tissue dehydrates and exposes the pollen grains to the environment where they wait for transport to the stigma (Bedinger 1992; Cresti et al. 1992; Tanaka 1993).
The primary task of the pollen grain is to deliver the sperm cells to the egg apparatus that in many cases is not on the same flower, even not on the same plant and sometimes many kilometres away. To reach the "females" the pollen grains are either carried by insects, birds or mammals or are wind-blown over large distances. During their transportation the pollen has to resist harsh environmental conditions and severe damage by their deliverers like mechanical stress, changes in temperatures and humidity. Therefore, they are covered by an additional cell wall, the exine consisting of sporopollenin which resists mechanical and chemical treatments. Furthermore, they are in a dehydrated, quiescent state with water contents sometimes less than 10 % of the fresh weight. This desiccation helps to maintain the pollen viability during transport.
To germinate and to grow a pollen tube as fast as possible after landing on the stigma, the pollen grains generate all mRNAs and proteins that they will need for a fast start during the last phase of pollen development (Mascarenhas 1990; Schrauwen et al. 1990). This was confirmed recently by an increasing number of transcriptomic (e.g., Becker et al. 2003; Bock et al. 2006; Wei et al. 2010) and proteomic studies on developing, mature and tube-growing pollen grains (e.g., Dai et al. 2006; Grobei et al. 2009; Pertl et al. 2009).
A large number of cellular processes are involved in polar growth of the pollen tube and have been studied intensively during the last decades. Initiation of pollen tube germination and the subsequent growth of pollen tubes can be viewed as a temporally and spatially fine-tuned network of interacting cellular processes and pathways like the cytoskeleton dynamics, vesicle trafficking and fusion, Ca2+, pH and phosphoinositide signalling, G-protein signalling and many more (Holdaway-Clarke and Hepler 2003; Cheung and Wu 2008; Cole and Fowler 2006; Michard et al. 2009; Steinhorst and Kudla 2013). It has been postulated that ion fluxes or transport across the plasma membrane may synchronise these individual processes leading to a concerted action for steady tube growth (Feijó et al. 1995, 2001). One of the best-characterized plasma membrane transport proteins in plants is the plasma membrane H+ ATPase (PM H+ ATPase, EC 3.6.3.6) which belongs to the family of P-type ATPases. This article reviews the current knowledge of the physiological role of the PM H+ ATPase in the pollen's life, emphasizes its central role, and discusses some open questions and knowledge gaps that need to be filled for a comprehensive understanding of the H+ pump's role during pollen germination and tube growth.
Activity and function of the plasma membrane H+ ATPase
The plasma membrane H+ ATPase consists of approximately 950 amino acids resulting in a molecular weight of ca. 105 kDa and a pI around 6.5. The protein contains ten transmembrane domains. Its N and C terminus face the cytoplasmic side and together with the small and the large loop more than 70 % of the protein resides in the cytosol and only a small part is exposed to the extracellular side (Fig. 1a). The major role of the PM H+ ATPase is the generation of an electrochemical gradient of H+ across the plasma membrane of plant cells by coupling the energy from ATP hydrolysis to the transport of H+ and thus energizing the plasma membrane for uptake of ions and nutrients. Furthermore, due to the export of H+ from the cytosol to the extracellular space, the PM H+ ATPase is involved in the regulation of cytoplasmic as well as cell wall pH. General and basic principles on the activity and function of the PM H+ ATPase can be found in other review articles in more detail (Serrano 1989; Briskin 1990; Palmgren 1998, 2001; Morsomme and Boutry 2000; Gaxiola et al. 2007). It has to be noted that two other H+ pumps, the V-type H+ ATPase and the H+ pyrophosphatase, which are mainly localized at the tonoplast, also contribute to the cytosolic pH homeostasis and thus may affect pollen tube growth, too.
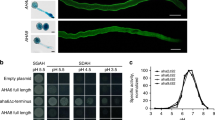
Model of the lily pollen PM H+ ATPase LilHA1. a Membrane topology of LilHA1 (AY029190.2). Transmembrane domains were predicted by HMMTOP (http://www.sacs.ucsf.edu/cgi-bin/hmmtop.py) and the model was drawn with TOPO2 (http://www.sacs.ucsf.edu/TOPO2/). b Three-dimensional model of LiHA1 created by homology modelling (SWISS-Model, http://swissmodel.expasy.org/workspace) using the crystal structure of AHA2 (3b8cA; Pedersen et al. 2007). The complete CTAD of LilHA1 was modelled by I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER). The last part of the CTAD starting as indicated by red bar in a is coloured bright red. Colours in b correspond with marked regions in a. A actuator domain, N nucleotide binding domain, P phosphorylation domain, R regulatory domain (=CTAD)
The activity of the PM H+ ATPase was mainly characterized using plasma membrane vesicles that were isolated by sucrose density gradient centrifugation or aqueous two-phase partitioning (Larsson et al. 1987; Briskin and Hanson 1992; Palmgren 1998). These methods result in a stochastic mixture of inside (cytosolic side)-out and outside-out vesicles. Upon addition of ATP the H+ transport, changes in membrane potential (VM) and ATP hydrolysis can be monitored on inside-out vesicles to characterize the PM H+ ATPase activity (Briskin et al. 1987; Palmgren 1990; Briskin and Reynolds-Niesman 1991). Further purification of the pump protein needs careful solubilisation by detergents to preserve the structure and activity of the protein in reconstituted vesicles. It has to be noted that despite the large number of studies on the biochemical characterization of the PM H+ ATPase activity no specific inhibitor like, for instance ouabain for the Na+,K+ ATPase, has been found. Other H+ ATPases, such as the V-ATPases and the F-ATPases, can be specifically inhibited by bafilomycin A and oligomycin, respectively. Therefore, most studies use ortho-vanadate that blocks the formation of a phosphorylated intermediate in all P-type ATPases as well as in a number of phosphate-metabolizing enzymes, to inhibit the PM H+ ATPase activity. A less well-characterized component, erythrosine B, also inhibits the PM H+ ATPase activity.
Furthermore, the PM H+ ATPase can also be investigated in heterologous expression systems. The choice of the expression system is important because a total reduction of endogenous PM H+ ATPases may be lethal for the organism chosen to express this protein. The PM H+ ATPase is a so-called housekeeping enzyme and hence essential for the survival of the cells. Therefore, the endogenous yeast PMA1 gene was placed under control of a galactose-inducible promoter whereas in the presence of glucose the plant proton pump is expressed (Cid et al. 1987; Villalba et al. 1992; Palmgren and Christensen 1993; Regenberg et al. 1995). Alternatively, the expression of both yeast PM H+ ATPases, PMA1 and PMA2, was inactivated by disrupting their genes. Yeast cells could survive in galactose medium with a special plasmid in which PMA1 and URA3 were placed under the control of the GAL1 promoter whereas the introduced plant proton pump is controlled by the PMA1 promoter. Shifting from galactose to glucose medium induced an exclusive expression of the plant PM H+ ATPase while the PMA1-carrying plasmid was removed simultaneously by selection on URA3 suicidal medium (Morsomme and Boutry 2000; de Kerchove d'Exaerde et al. 1995). These strategies of recombinant expression of the plant-derived H+ ATPase also allow the addition of tags to the recombinant protein which simplifies the identification and the purification for further studies on reconstituted vesicle systems.
In general, a specific enzyme activity of the PM H+ ATPase of 1–2 μmol Pi min−1 mg−1 was measured in purified plasma membrane vesicles with a Km for MgATP ranging between 0.3 and 1.5 mM (Palmgren 1998; Morsomme and Boutry 2000). The pH optimum of the ATPase activity is slightly acidic ranging around pH 6.5–6.8 and the ATP hydrolysis can be stimulated by addition of potassium ions (10–50 mM; Buch-Pedersen et al. 2006). Usually, 1 H+ is transported per hydrolysed ATP but may vary due to specific regulations of the pump activity thus generating a pH gradient of 1.5 to 2 pH units and hyperpolarizing the plasma membrane even more negative than −200 mV (Serrano 1989; Morsomme et al. 1996; Palmgren and Harper 1999; Gaxiola et al. 2007).
Structure and regulation of the plasma membrane H+ ATPase
A C-terminally tagged PM H+ ATPase (AHA2) was expressed in yeast to allow its purification and crystallization. The crystal structure shows three large cytosolic domains N (nucleotide binding), P (phosphorylation domain) and A (actuator domain) in addition to a transmembrane domain with ten helices (Pedersen et al. 2007). Figure 1 shows a homology model of the lily pollen H+ ATPase isoform LilHA1 (access. no. AY029190.2) using the crystal structure of AHA2 as a template. To couple the transmembrane transport of H+ to ATP hydrolysis, the MgATP is bound to the N domain which then moves towards the P domain where a conserved asparagine residue (Asp 329 in AHA2) becomes phosphorylated. Dephosphorylation of the Asp residue involves a large conformation change of domain A simultaneously with conformational changes in the membrane region. The energy released from the hydrolysis of ATP is temporarily stored in a conformational change of the protein exhibiting its H+ binding side either to the cytosol (unphosphorylated) or to the extracellular space (phosphorylated) and thus transports H+ from one side to the other side of the membrane (Buch-Pedersen et al. 2009).
A moderate regulation of the overall activity of PM H+ ATPases in plant cells may occur at the transcriptional level in response to environmental changes (e.g., temperature, salts, sugar; see Lee et al. 2004; Camoni et al. 2006; Janicka-Russak and Klobus 2007). Alternatively, a regulated incorporation of exocytotic vesicles already containing the PM H+ ATPase protein increases the amount of ATPase in the plasma membrane and therefore increases H+ extrusion as has been shown after auxin addition (Hager et al. 1991). However, the most prominent short-term regulation in activity is at the post-translational level due to phosphorylation of the C-terminal autoinhibitory domain (CTAD) that results in the binding of 14-3-3 proteins. In this case the penultimate threonine (Thr) residue at the CTAD is phosphorylated which in turn enables the binding of a 14-3-3 dimer and thus, displacing the C terminus to allow a higher ATP turnover and H+ transport (Fuglsang et al. 1999; Svennelid et al. 1999; Camoni et al. 2000; Maudoux et al. 2000). A crystallographic model showed details of the interaction between the C terminus of the PM H+ ATPase and 14-3-3 proteins and explains how the fungal toxin fusicoccin irreversibly activates the ATPase activity by strengthening the interaction between 14-3-3 s and the PM H+ ATPase C terminus (Korthout and DeBoer 1994; Oecking et al. 1997; Baunsgaard et al. 1998; Fullone et al. 1998). A predicted structure of the CTAD which could not be crystallized, so far, was superimposed onto the LILHA1 model (Fig. 1b). This simple model implies that the C-terminal part (bright red) of the LilHA1-CTAD might reach into the gap between the A, N and P domain thus slowing down the conformational changes necessary for H+ transport. Note that this hypothesis is not confirmed yet by crystallization experiments, and other possible arrangements of the CTAD have been proposed (Axelsen et al. 1999; Palmgren 2001; Pedersen et al. 2007).
Using single particle analysis of electron microscopy images, a wheel-like structure formed by a H+ ATPase hexamer held together by six 14-3-3 proteins was identified that might be the functional unit of the PM H+ ATPase (Ottmann et al. 2007). It has to be noted that other regulatory mechanisms independent from the C terminus or phosphorylation or 14-3-3 protein binding have been reported but still need more investigations to show how these regulatory mechanisms are involved in the physiological function of the PM H+ ATPase (Borch et al. 2002; Morandini et al. 2002; Giacometti et al. 2004; Ekberg et al. 2010; Piette et al. 2011). In addition, phosphorylation of amino acid residues other than the penultimate Thr might inhibit the PM H+ ATPase activity (Lino et al. 1998; Fuglsang et al. 2007; Yang et al. 2010).
If the major regulation of the PM H+ ATPase activity occurs via phosphorylation the question raises which protein kinases and phosphatases are responsible for the phosphorylation and dephosphorylation, respectively? So far, this question is still under investigation and the outcome might result in a very variable picture: each stimulus may activate different signal transduction pathways that finally phosphorylate the PM H+ ATPase at different positions via diverse kinases resulting in a specific response to the respective signal. For instance, in Vicia faba guard cells the blue light activation of the PM H+ ATPase involves phosphorylation by a K252a-independent protein kinase and dephosphorylation by a Mg2+-dependent type 2C phosphatase both co-localizing with the PM H+ ATPase (Hayashi et al. 2010) whereas in Arabidopsis seedlings the phosphorylation by PKS5 inhibits the ATPase activity (Fuglsang et al. 2007). Even in the same organism (Nicotiana sp.), two different ATPase isoforms (PMA2 and PMA4) showed variability in their phosphorylation state (Bobik et al. 2010).
In addition to the described regulation of the PM H+ ATPase by phosphorylation and interaction with 14-3-3 s, a novel interacting protein, PPI1 (proton pump interactor 1) has been discovered (Morandini et al. 2002; Muniz Garcia et al. 2011). PPI1 also stimulates the ATPase activity by binding to the CTAD but to a different domain than 14-3-3 s (Viotti et al. 2005). So far, the role of PPIs in pollen was not investigated but might become important because high expression levels of the PPI1-mRNA (AT4G27500) can be observed when analyzing micro array data (www.genvestigator.com).
Activity, localization and regulation of the PM H+ ATPase in pollen
Pollen grains of many species can be easily cultured in synthetic culture media that contain sugars as an osmoticum, millimolar amounts of K+, Ca2+, boric acid and an acid pH. For instance, several germination media were reported for Lilium longiflorum, Nicotiana tabacum or Arabidopsis thaliana (Tupy et al. 1977; Dickinson 1978; Daher et al. 2009; Pertl-Obermeyer et al. 2013). During in vitro cultivation, an acidification of the germination medium could be observed due to H+ extrusion from the pollen grains (Southworth 1983; Rodriguez-Rosales et al. 1989; Tupy and Rihova 1984). Using in vivo (Rodriguez-Rosales et al. 1989; Certal et al. 2008; Pertl et al. 2010) and biochemical studies on pollen membrane fractions (Obermeyer et al. 1996; Pertl et al. 2001, 2005) it was demonstrated that the PM H+ ATPase is responsible for the medium acidification. Additionally, all antagonists of the PM H+ ATPase also inhibited pollen germination and tube growth, whereas stimulators of the PM H+ ATPase activity were also able to boost the germination frequency as well as the tube growth rates as summarized in Fig. 2 (see also Fricker et al. 1997; Certal et al. 2008). The inhibitors vanadate and erythrosine B can totally block lily pollen germination. A stimulation of germination by 20 % was achieved with fusicoccin, which promotes the binding of 14-3-3 proteins to the PM H+ ATPase (Pertl et al. 2001), whereas AICAR (5-aminoimidazole-4-carboxamide ribonucleoside monophosphate) which blocks the binding of 14-3-3 proteins to the PM H+ ATPase (Paul et al. 2005), prevents germination of pollen grains. Additionally, less well-known environmental parameters, such as the concentration of boric acid in the germination medium or even weak electrical AC fields, can increase the germination frequency of lily pollen and it was demonstrated that both parameters act on the PM H+ ATPase activity (Obermeyer et al. 1996; Plätzer et al. 1997). All studies showed a direct correlation between the PM H+ ATPase activity and the pollen germination as well as tube growth and thus demonstrate an active PM H+ ATPase as a prerequisite for successful pollen germination and tube growth.
Effect of inhibitors and promoters of the PM H+ ATPase activity on lily pollen germination. Pollen grains of Lilium longiflorum were cultivated in germination medium (10 % w/v sucrose, 1 mM KCl, 0.1 mM CaCl2, 1.6 mM H3BO3, pH 5.6) in the presence of the respective chemicals. For control experiments (0 μM), the appropriate solvent was added at the highest concentration (e.g., ethanol for fusicoccin). AICAR 5-aminoimidazole-4-carboxamide ribonucleoside monophosphate
Furthermore, since the first studies on extracellular currents in lily pollen by Manfred Weisenseel and Lionel Jaffe it is well-known that pollen grains and especially tubes drive steady electric currents in the range of hundred picoamperes through themselves (Weisenseel et al. 1975). More recent investigations (reviewed by Feijó et al. 2001; Holdaway-Clarke and Hepler 2003; Michard et al. 2008) gave a detailed image of these currents: calcium ions enter the tip of a growing pollen tube generating a tip-localized gradient of the free cytosolic Ca2+ concentration ([Ca2+]cyt) that acts like a built-in navigation system (Obermeyer and Weisenseel 1991; Rathore et al. 1991; Pierson et al. 1994). Potassium ions (Messerli et al. 1999) and anions (Cl–) are flowing into the tube and in addition, chloride ions show a large efflux at the tube tip (Zonia et al. 2002). The currents carried by H+ can be distinguished as a large efflux at the pollen grain, small influxes along the tube and a large influx at the very tip followed by a remarkable efflux ca. 20 μm behind the tip (Feijó et al. 1999; Certal et al. 2008). This spatial difference in H+ fluxes along the tube's plasma membrane creates variations in the cytosolic pH. It is now generally accepted that growing pollen tubes show a slightly acidic pH in the tip cytosol and an alkaline band approximately at the beginning of the clear cap (Turian 1981; Feijó et al. 1999; Michard et al. 2008), although two studies were unable to monitor the pH gradients probably due to the high buffer capacity of the applied intracellular pH indicator (Parton et al. 1997; Fricker et al. 1997). Recently, it was shown that the H+ effluxes at the pollen grain as well as in the region of the alkaline band are mainly caused by a vanadate-inhibited PM H+ ATPase (Certal et al. 2008 and supplements therein) although the pH of the alkaline band is slightly different from the pH optimum of the PM H+ ATPase. To solve this mismatch in pH dependence, a detailed biochemical characterization of the transport properties of pollen-specific PM H+ ATPase isoforms is necessary.
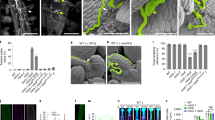
Generally, the observed H+ efflux pattern correlates quite well with the localization of the PM H+ ATPase. Immunogold localization in lily pollen and localization of a NtAHA::GFP fusion protein in tobacco pollen showed an accumulation of the H+ ATPase in the plasma membrane of pollen grains where it is responsible for the H+ efflux (Obermeyer et al. 1992; Certal et al. 2008). No fluorescence-labelled PM H+ ATPases were detected in the PM of the tip region of the clear cap thus allowing a net influx of H+. Additionally, immunogold staining of the PM H+ ATPase was also observed in the ER and the Golgi apparatus thus demonstrating the ATPases' way to the plasma membrane through the secretory pathway. The distribution of the PM H+ ATPase in pollen ER and Golgi membranes was recently confirmed by a proteomic study used to identify membrane proteins from organelles of lily pollen (Pertl et al. 2009). The GFP::ATPase fusion protein could also be detected in the V-shaped cone of vesicles in the tip region of growing tubes (Certal et al. 2008) whereas no tip-localized vesicles were stained during immunogold labelling (Obermeyer et al. 1992). It still needs to be clarified where the PM H+ ATPase is incorporated into the plasma membrane. Is it delivered by the tip-targeted secretory vesicles to the tip zone or by another secretory pathway?
Regulation of pollen PM H+ ATPase is suggested to follow the same general scheme as in other tissue. Several isoforms of regulatory 14-3-3 proteins have been identified in lily pollen (Pertl et al. 2011) and a fusicoccin-induced accumulation of 14-3-3 s with the membrane fraction was observed (Pertl et al. 2001). Although the direct interaction between these two pollen proteins still needs to be investigated in detail, a regulatory role for 14-3-3 proteins in osmoregulation was shown in lily pollen (Pertl et al. 2010). A hyper-osmotic treatment causes a hyperpolarization of the plasma membrane and an increased acidification of the germination medium due to an increased activity of the PM H+ ATPase which, in turn, was caused by recruitment of more 14-3-3 proteins to the plasma membrane probably binding to the PM H+ ATPase. So far, all PM H+ ATPases known to be expressed in pollen contain all sequence motifs necessary for phosphorylation and subsequent binding of 14-3-3 proteins.
Expression of PM H+ ATPase in pollen
Because of its important role in energizing the plasma membrane, the H+ ATPase is probably expressed in every single cell of a plant with the exception of the generative cells of pollen grains (Gehwolf et al. 2002) where an active PM H+ ATPase would acidify the cytosol of the vegetative cell. The PM H+ ATPase belongs to the P3 sub-family of the P-type ATPase family (Pedersen et al. 2012) and consists of 12 isoforms in Arabidopsis thaliana, nine isoforms in Nicotiana plumbaginifolia, ten isoforms in rice, and ten isoforms in maize with still increasing information on isoforms as the number of sequenced organisms will increase in the future. A phylogenetic tree has been generated using the amino acid sequences of isoforms from Arabidopsis, Medicago, tobacco, tomato, potato, wheat, barley, rice, maize and lily (Fig. 3). It can be noticed that the PM H+ ATPase isoforms cluster in five subfamilies as it has been described earlier (Arango et al. 2003). However, a closer look reveals that isoforms mainly expressed in pollen can be found in subfamily IV. The PM H+ ATPase isoforms LilHA1 and LilHA2 have been identified from lily pollen (Gehwolf et al. 2002; Pertl et al. 2009), and NtHA1 was isolated from tobacco pollen (Certal et al. 2008). Furthermore, expression of isoform NpPMA5 (Nicotiana plumbaginifolia) was observed in pollen, too (Lefebvre et al. 2005). Analyzing the expression of the Arabidopsis thaliana isoforms AHA1 to AHA10 at the transcription level using micro array data stored at Genevestigator (www.genevestigator.com) also shows a fascinating picture on the tissue-specific expression of H+ ATPase isoforms (Fig. 4). In the majority of tissues AHA1 and AHA2 are the dominating isoforms, whereas in pollen their transcription level decreases dramatically. In pollen the isoform AHA8 shows by far the highest transcription level followed by AHA7, AHA6 and AHA9. This extreme difference between the transcription levels in pollen compared to sporophytic tissues might correspond to specific physiological functions of the PM H+ ATPase in pollen. This means that AHA8 is regulated by a special promoter tailored to the specific expression in pollen and that the AHA8 protein is specifically adapted to the life conditions of pollen grains and their special tasks. Especially the different hydration states of pollen grains may confront membrane proteins with a problem: it has been postulated that membrane lipids reorganize from the hexagonal (HII) phase to the lamellar (bilayer) phase (Simon 1974; Heslop-Harrison 1979) during the uptake of water, thus forcing membrane proteins to refold into the regenerating bilayer membrane again without losing their activity. One may therefore assume that specific isoforms of membrane proteins are expressed in pollen when compared with other water-containing tissues that face less dramatic changes in the hydration state. We favour this idea as all of the known pollen-specific PM H+ ATPase isoforms cluster in subfamily IV meaning that they have common features in their amino acid sequence that are lacking in other ATPase isoforms. Therefore, in addition to slightly variations in their protein structure, one might expect differences in the biochemical properties of the enzyme suiting its activity to the specific needs of pollen grains. These specific properties may help it to stay intact during the phase transitions of the lipid bilayer during de- and rehydration of the mature pollen grain or to allow a H+ export in the region of the alkaline band of the pollen tube that is actually out of the pH optima known from biochemically characterized isoforms like AHA2, NpPMA2 or NpPMA4. However, this hypothesis has to be proven by future experiments. Additionally, we hypothesize that all PM H+ ATPase isoforms from other organisms, which cluster in subfamily IV, are also mainly expressed in pollen.
Phylogenetic tree of PM H+ ATPase isoforms from several species. CLUSTAL X2.1 was used to align the PM H+ ATPase amino acid sequences and to construct the phylogenetic tree. The GeneBank accession numbers of the analyzed PM H+ ATPases are as follows: Arabidopsis thaliana: AHA1, NP_179486; AHA2,NP_194748; AHA3, NP_200545; AHA4, NP_190378; AHA5, NP_180028; AHA6, NP_178762; AHA7, NP_191592; AHA8, NP_189850; AHA9 NP_178181; AHA10, NP_173169; AHA11, NP_201073; Nicotiana tabacum: NtHA1, AY383599; Nicotiana plumbaginifolia: NpPMA1, Q08435; NpPMA2, Q42932; NpPMA3, Q08436; NpPMA4, Q03194; NpPMA5, AAV49160.1 (truncated); NpPMA6, AAD46186; NpPMA8, AAD46187; NpPMA9, AAD46188; Lilium longiflorum: LilHA1, AY029190.2; LilHA2, EF397610; Oryza sativa: OsHA1, D10207; OsHA2, D31843; OsHA3, AJ440001; OsHA4, AJ440002; OsHA5, AJ440216; OsHA6, AJ440217; OsHA7, AJ440218; OsHA8, AJ440219; OsHA9, AJ440220; OsHA10, AJ440221; Zea mays: ZmHA1, NP_001105360; ZmHA2, NP_001105470; Triticum aestivum: TaHA1, AAV71150; Solanum lycopersicum: SlHA1, NP_001234775; SlHA2, AAD55399; SlHA4, AAB17186; Solanum tuberosum: PHA1, CAA54046; PHA2, CAA54045; Hordeum vulgare: HvHA1, AY136627; Medicago truncatula: MtrHA1, CAB85494; MtrHA2, XM_003603484; MtrHA3, XM_003610033; MtrHA4, XM_003610032; MtrHA5, XM_003594906; MtrHA6, CAB85495. The annotation of the subfamilies follows Arango et al. (2003). Subfamily IV that was highlighted as pollen-specific expression is proposed for these isoforms
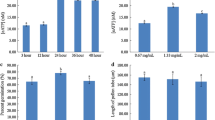
Expression levels of Arabidopsis PM H+ ATPase isoforms. a Transcript levels of AHA1 to AHA10 of different Arabidopsis tissues were calculated from micro-array experiments using Genevestigator (www.genevestigator.com) and presented as log2 values. b Linear expression values of all 11 PM H+ ATPase isoforms in Arabidopsis pollen. Mean value ± SD
Integration of signal transduction and metabolic pathways by the PM H+ ATPase
According to our current knowledge on biological systems, pollen germination and tube growth may be viewed as a complex but robust network in which cellular processes, signal transduction and metabolic pathways interact with each other thus forming a network that is well regulated in space and time. Whatever parameter is experimentally changed, either an entire cellular process (e.g., vesicle transport [Geitmann et al. 1996], actin polymerisation [Cai et al. 1997; see also Cheung and Wu 2008 for a recent review]) or a single enzyme activity (protein phosphatase 2A [Obermeyer et al. 1998] or Rho-GTPases [Kost 2008]) or even a small ion (tip-localized Ca2+ concentration; Malhó and Trewavas 1996), the system responds more or less in the same way: complete or temporary arrest of tube growth depending on the strength of the stimulus. A network may be seen as a large number of nodes linked by edges. A node with a large number of links to other nodes may become a central process in the network. As presented above the PM H+ ATPase may be such an important node. Modulation of the ATPase activity by independent and different parameters, leads immediately to a change in the germination frequency and in tube growth speed. By transition from a low activity state to a high activity state the pollen tube grows faster or slower if changing to a low activity state (summarized in Fig. 5). However, different independent signals and/or signal transduction pathways may have opposite effects on the PM H+ ATPase activity and thus the PM H+ ATPase has to be able to integrate signals from different sources. Priority signals might exist which can overrule other parameters and due to the length of the tube one might also assume that the PM H+ ATPase might be differentially regulated at different parts of the tube. For instance, a local increase in external pH (e.g., from pH 5.6 of the usual germination medium to pH 7.0) reduces the proton motif force at the plasma membrane locally and may result in a local activation of the H+ pump. In general, signal transduction pathways that modulate the phosphorylation state of proteins also change the PM H+ ATPase activity. So far, several phosphorylation sites have been identified in PM H+ ATPases (Rudashevskaya et al. 2012) and protein kinases and phosphatases that are involved in the phosphorylation of the H+ pump (Lino et al. 1998; Camoni et al. 1998; Fuglsang et al. 2006, 2007; Hayashi et al. 2010) were identified, but none could be found that specifically phosphorylates the penultimate threonine residue responsible for 14-3-3 binding. It is probable that metabolic pathways that affect the cytosolic ATP/ADP ratio (Obermeyer et al. 2013) may temporally lead to a change in the phosphorylation state of the PM H+ ATPase, and apart from that, low ATP concentrations will cause a decrease in H+ transport. Different external signals (hormones, light, salt stress) are well known to influence the H+ pump activity. Recently, changes in the water potential influenced the pollen PM H+ ATPase activity (Pertl et al. 2010) resulting in a 14-3-3-dependent (phosphorylation?) activation of the H+ pump and its contribution to osmosensing and osmoregulation. In this context, it has to be emphasized that regulation of the PM H+ ATPase may involve interaction with other plasma membrane proteins, e.g., receptor kinases, as has been shown for the brassinosteroid receptor BRI1 that interacted with AHA1 (Caesar et al. 2011). This research area on ATPase interacting proteins is just emerging, and no data have been obtained so far for pollen PM H+ ATPases and their regulation.
However, it is important for the pollen grain and particularly, for the tube to respond to changes in environmental parameters as fast as possible to be able to maintain its optimal growth speed for reaching the ovules in time. Only pollen tubes which negotiate all obstacles on their way to the egg cells can contribute to fertilization and influence the next generation of plants. Based on the studies on the pollen PM H+ ATPase reviewed here, one may conclude that by modulating the ATPase activity, the pollen grain and tube is able to respond fast to small environmental perturbation on its way to the ovule. However, vast disturbances will definitely inhibit tube growth, but most small changes that may happen in the style tissue (e.g., pH differences, osmolarity, oxygen supply) may be compensated by speeding up or slowing down the tube growth speed via modulation of the PM H+ ATPase activity. Further research on the still unanswered questions on the pollen specificity of certain PM ATPase isoforms, their biochemical and structural properties, the trafficking of PM H+ ATPases to the pollen plasma membrane and putative regulatory interaction partners may reveal new features of this protein corresponding to the specific demands of a highly specialized cell, the pollen.
References
Arango M, Gevaudant F, Oufattole M, Boutry M (2003) The plasma mebrane proton pump ATPase: the significance of gene subfamilies. Planta 216:355–365
Axelsen KB, Venema K, Jahn T, Baunsgaard L, Palmgren MG (1999) Molecular dissection of the C-terminal regulatory domain of the plant plasma membrane H+ ATPase AHA2: mapping of residues that when altered give rise to an activated enzyme. Biochemistry 38(22):7277–7234
Baunsgaard L, Fuglsang AT, Jahn T, Korthout HAAJ, De Boer AH, Palmgren MG (1998) The 14-3-3 proteins associate with the plant plasma membrane H+ ATPase to generate a fusicoccin binding complex and a fusicoccin responsive system. Plant J 13:661–671
Becker JD, Boavida LC, Carneiro J, Haury M, Feijo J (2003) Transcriptional profiling of Arabidopsis tissues reveals the unique characterictics of the pollen transcriptome. Plant Physiol 133:713–725
Bedinger P (1992) The remarkable biology of pollen. Plant Cell 4:879–887
Bobik K, Duby G, Nizet Y, Vandermeeren C, Stiernet P, Kanczewsky J, Boutry M (2010) Two widely expressed plasma membrane H+ ATPase isoforms of Nicotiana tabacum are differentially regulated by phosphorylation of their penultimate threonine. Plant J 62:291–301
Bock KW, Honys D, Ward JM, Padmanaban S, Nawrocki EP, Hirschi KD, Twell D, Sze H (2006) Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiol 140:1151–1168
Borch J, Bych K, Roepstorff P, Palmgren MG, Fuglsang AT (2002) Phosphorylation-independent interaction between 14-3-3 protein and the plant plasma membrane H+ ATPase. Biochem Soc Trans 30:411–415
Briskin DP (1990) The plasma membrane H+ ATPase of higher plant cells: biochemistry and transport function. Biochim Biophys Acta 1019:95–109
Briskin DP, Hanson JB (1992) How does the plasma membrane H+ ATPase pump protons? J Exp Bot 248:269–289
Briskin DP, Reynolds-Niesman I (1991) Determination of H+/ATP stoichiometry for the plasma membrane H+ ATPase from red beet (Beta vulgaris L.) storage tissue. Plant Physiol 95:242–250
Briskin DP, Leonard R, Hodges TK (1987) Isolation of the plasma membrane: membrane markers and general principles. Meth Enzymol 148:542–558
Buch-Pedersen MJ, Rudashevskaya EL, Berner TS, Venema K, Palmgren MG (2006) Potassium as an intrinsic uncoupler of the plasma membrane H+-ATPase. J Biol Chem 281:38285–38292
Buch-Pedersen MJ, Pedersen BP, Veierskov B, Nissen P, Palmgren MG (2009) Protons and how they are transported by proton pumps. Pflugers Arch 457:573–579
Caesar K, Elgass K, Chen Z, Hiuppenberger P, Witthöft J, Schleifenbaum F, Blatt MR, Oecking C, Harter K (2011) A fast brassinolide-regulated response pathway in the plasma membrane of Arabidopsis thaliana. Plant J 66:528–540
Cai G, Moscatelli A, Cresti M (1997) Cytoskeletal organization and pollen tube growth. Trends Plant Sci 2:86–91
Camoni L, Fullone MR, Marra M, Aducci P (1998) The plasma membrane H+ ATPase from maize roots is phosphorylated in the C-terminal domain by a calcium-dependent protein kinase. Physiol Plant 104:549–555
Camoni L, Iori V, Marra M, Aducci P (2000) Phosphorylation-dependent interaction between plant plasma membrane H+-ATPase and 14-3-3 proteins. J Biol Chem 275(14):9919–9923
Camoni L, Marra M, Garufi A, Visconti S, Aducci P (2006) The maize plasma membrane H+ ATPase is regulated by a sugar-induced transduction pathway. Plant Cell Physiol 47:743–747
Certal AC, Almeida RB, Carvalho LM, Wong E, Moreno N, Michard E, Carneiro J, Rodriguez-Leon J, Wu H-M, Cheung AY, Feijo J (2008) Exclusion of a proton ATPase from the apical membrane is associated with cell polarity and tip growth in Nicotiana tabacum pollen tubes. Plant Cell 20:614–634
Cheung AY, Wu H-M (2008) Structural and signaling networks for the polar cell growth machinery in pollen tubes. Ann Rev Plant Biol 59:547–572
Cid A, Perona R, Serrano R (1987) Replacement of the promoter of the yeast plasma membrane ATPase gene by a galactose-dependent promoter and its physiological consequences. Curr Genet 12:105–110
Cole RA, Fowler JE (2006) Polarized growth: maintaining focus on the tip. Curr Opin Plant Biol 9:579–588
Cresti M, Blackmore S, van Went JL (1992) Atlas of sexual reproduction in flowering plants. Springer Verlag, Berlin
Daher FB, Chebli Y, Geitmann A (2009) Optimization of conditions for germination of cold-stored Arabidopsis thaliana pollen. Plant Cell Rep 28:347–357
Dai S, Li L, Chen T, Chong K, Xue Y, Wang T (2006) Proteomic analysis of Oriza sativa pollen reveal novel proteins associated with pollen germination and tube growth. Proteomics 6:2504–2529
de Kerchove d'Exaerde A, Supply P, Dufour JP, Bogarts P, Thines D, Goffeau A, Boutry M (1995) Functional complementation of a null mutation of the yeast Saccharomyces cerevisae plasma membrane H+ ATPase by a plant H+ ATPase gene. J Biol Chem 270:23828–23837
Dickinson DB (1978) Influence of borate and penta-erythritol concentrations on germination and tube growth of Lilium longiflorum pollen. J Am Soc Hortic Sci 103:413–416
Ekberg K, Palmgren MG, Veierskov B, Buch-Pedersen MJ (2010) A novel mechanism of the P-type ATPase autoinhibition involving both termini of the protein. J Biol Chem 285:7344–7350
Feijó JA, Malhó R, Obermeyer G (1995) Ion dynamics and its possible role during in vitro pollen germination and tube growth. Protoplasma 187:155–167
Feijó JA, Sainhas J, Hackett G, Kunkel JG, Hepler PK (1999) Growing pollen tubes possess a constitutive alkaline band in the clear zone and a growth-dependent acidic tip. J Cell Biol 144:483–496
Feijó JA, Sainhas J, Holdaway-Clarke T, Cordeiro S, Kunkel JG, Hepler PK (2001) Cellular oscillations and the regulation of growth: the pollen tube paradigm. BioEssays 23(1):86–94
Fricker MD, White NS, Obermeyer G (1997) pH gradients are not associated with tip growth in pollen tubes of Lilium longiflorum. J Cell Sci 110:1729–1740
Fuglsang AT, Visconti S, Drumm K, Jahn T, Stensballe A, Mattei M, Jensen ON, Aducci P, Palmgren MG (1999) Binding of 14-3-3 protein to the plasma membrane H+ ATPase AHA2 involves the three C-terminal residues Tyr (946)–Thr–Val and requires phosphorylation of the THR (947). J Biol Chem 274:36774–36780
Fuglsang AT, Tulinius G, Cui N, Palmgren MG (2006) Protein phosphatase 2A-scaffolding subunit A interacts with plasma membrane H+-ATPase C-terminus in the same region as 14-3-3 protein. Physiol Plant 128:334–340
Fuglsang AT, Guo Y, Cuin TA, Qiu Q, Song C, Kristiansen KA, Bych K, Schulz A, Shabala S, Schumaker KS, Palmgren MG, Zhu J-K (2007) Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+ ATPase by preventing interaction with 14-3-3 protein. Plant Cell 19:1617–1634
Fullone MR, Visconti S, Marra M, Fogliano V, Aducci P (1998) Fusicoccin effect on the in vitro interaction between plant 14-3-3 proteins and plasma membrane H+-ATPase. J Biol Chem 273(13):7698–7702
Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581:2204–2214
Gehwolf R, Griessner M, Pertl H, Obermeyer G (2002) First patch, then catch: measuring the activity and the mRNA transcripts of a proton pump in individual Lilium pollen protoplasts. FEBS Lett 512:152–156
Geitmann A, Wojciechowicz K, Cresti M (1996) Inhibition of intracellular pectin transport in pollen tubes by monensin, brefeldin A and cytochalasin D. Bot Acta 109:373–381
Giacometti S, Camoni L, Albumi C, Visconti S, De Michelis MI, Aducci P (2004) Tyrosine phosphorylation inhibits the interaction of 14-3-3 proteins with the plant plasma mebrane H+ ATPase. Plant Biol 6:422–431
Grobei MA, Qeli E, Brunner E, Rehrauer H, Zhang R, Roschitzki B, Basler K, Ahrens CH, Grossniklaus U (2009) Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Res 19:1786–1800
Hager A, Debus G, Edel H-G, Stransky H, Serrano R (1991) Auxin induces exocytosis and the rapid synthesis of a high-turnover pool of plasma-membrane H+ ATPase. Planta 185: 5327–5537
Hayashi Y, Nakamura S, Takemiya A, Takahashi Y, Shimazaki K-I, Kinoshita T (2010) Biochemical characterization of in vitro phosphorylation and dephosphorylation of the plasma membrane H+ ATPase. Plant Cell Physiol 51:1186–1196
Heslop-Harrison J (1979) An interpretation of the hydrodynamics of pollen. Am J Bot 66:737–743
Holdaway-Clarke T, Hepler PK (2003) Control of pollen tube growth: role of ion gradients and fluxes. New Phytol 159:539–563
Janicka-Russak M, Klobus G (2007) Modification of plasma membrane and vacuolar H+ ATPases in response to NaCl and ABA. Plant Physiol 164:295–302
Korthout HAAJ, DeBoer AH (1994) A fusicoccin binding protein belongs to the family of 14-3-3 brain protein homologs. Plant Cell 6:1681–1692
Kost B (2008) Spatial control of Rho (rRac.Rop) signaling in tip-growing plant cells. Trends Cell Biol 18:119–127
Larsson C, Widell S, Kjellbom P (1987) Preparation of high-purity plasma membranes. Meth Enzymol 148:350–382
Lee SH, Singh AP, Chung GC, Ahn SJ, Noh EK, Steudle E (2004) Exposure of roots of cucumber (Cucumis sativus) to low temperature severely reduces root pressure, hydraulic conductivity and active transport of nutrients. Physiol Plant 120:413–420
Lefebvre B, Arango M, Oufattole M, Crouzet J, Purnelle B, Boutry M (2005) Identification of a Nicotiana plumbaginifolia plasma membrane H+ ATPase gene expressed in the pollen tube. Plant Mol Biol 58:775–787
Lino B, Baizabel-Aguirre VM, Gonzalez de la Vara LE (1998) The plasma membrane H+ ATPase from beet root is inhibited by a calcium-dependent phosphorylation. Planta 204:352–359
Malhó R, Trewavas AJ (1996) Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell 8:1935–1949
Mascarenhas JP (1990) Gene activity during pollen development. Annu Rev Plant Physiol Plant Mol Biol 41:317–338
Maudoux O, Batoko H, Oecking C, Gevaert K, Vandekerckhove J, Boutry M, Morsomme P (2000) A plant plasma membrane H+-ATPase expressed in yeast is activated by phosphorylation at its penultimate residue and binding of 14-3-3 regulatory proteins in the absence of fusicoccin. J Biol Chem 275(23):17762–17770
Messerli M, Danuser G, Robinson KR (1999) Pulsatile influxes of H+, K+ and Ca2+ lag growth pulses of Lilium longiflorum pollen tubes. J Cell Sci 112:1497–1509
Michard E, Dias P, Feijo JA (2008) Tobacco pollen tubes as cellular models for ion dynamics: improved spatial and temporal resolution of extracellular flux and free cytosolic concentration of calcium and protons using pHluorin and YC3.1 CaMeleon. Sex Plant Reprod 21:169–181
Michard E, Alves F, Feijo JA (2009) The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. Int J Dev Biol 53:1609–1622
Morandini P, Valera M, Albumi C, Bonza MC, Giacometti S, Ravera G, Murgia I, Soave C, De Michelis MI (2002) A novel interaction partner of the C-terminus of Arabidopsis thaliana plasma membrane H+ ATPase (AHA1 isoform): site and mechanism of action on H+ ATPase activity differ from those of 14-3-3 proteins. Plant J 31:487–497
Morsomme P, Boutry M (2000) The plant plasma membrane H+ ATPase: structure, function and regulation. Biochim Biophys Acta 1465:1–16
Morsomme P, Kerchove d'Exaerde A, de Meester S, Thines D, Goffeau A, Boutry M (1996) Single point mutations in various domains of a plant plasma membrane H+ ATPase expressed in Saccharomyces cerevisiae increase H+ pumping and permit yeast growth at low pH. EMBO J 15:5513–5526
Muniz Garcia MN, Pais SM, Tellez-Inon MT, Capiati DA (2011) Characterization of StPPI1, a proton pump interactor from Solanum tuberosum L. that is up-regualted during tube formation and by abiotic stress. Planta 233:661–674
Obermeyer G, Weisenseel MH (1991) Calcium channel blocker and calmodulin antagonists affect the gradient of free calcium ions in lily pollen tubes. Eur J Cell Biol 56:319–327
Obermeyer G, Lützelschwab M, Heumann H-G, Weisenseel MH (1992) Immunolocalisation of H+ ATPases in the plasma membrane of pollen grains and pollen tubes of Lilium longiflorum. Protoplasma 171:55–63
Obermeyer G, Kriechbaumer R, Strasser D, Maschessnig A, Bentrup F-W (1996) Boric acid stimulates the plasma membrane H+ ATPase of ungerminated lily pollen grains. Physiol Plant 98:281–290
Obermeyer G, Klaushofer H, Nagl M, Höftberger M, Bentrup F-W (1998) In-vitro germination and growth of lily pollen tubes is affected by protein phosphatase inhibitors. Planta 207:303–312
Obermeyer G, Fragner L, Lang V, Weckwerth W (2013) Dynamic adaption of metabolic pathways during germination and growth of lily pollen tubes after inhibition of the lectron transport chain. Plant Physiol 162:1822–1833
Oecking C, Piotroski M, Hagemeier J, Hagemann K (1997) Topology and target interaction of the fusicoccin-binding 14-3-3 homologs of Commenlina communis. Plant J 12:441–453
Ottmann C, Marco S, Jaspert N, Marcon C, Schauer N, Weyand M, Vandermeeren C, Duby G, Boutry M, Wittinghofer A, Rigaud J-L, Oecking C (2007) Structure of a 14-3-3 coordinated hexamer of the plant plasma membrane H+ ATPase by combining X-ray crystallography and electron cryomicroscopy. Mol Cell 25:427–440
Palmgren MG (1990) An H+ ATPase assay: proton pumping and ATPase activity determined simultaneously in the same sample. Plant Physiol 94:882–886
Palmgren MG (1998) Proton gradients and plant growth: role of the plasma membrane H+ ATPase. Adv Bot Res 28:2–70
Palmgren MG (2001) Plant plasma membrane H+ ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52:817–845
Palmgren MG, Christensen G (1993) Complementation in situ of the yeast plasma membrane H+ ATPase gene pma1 by a H+ ATPase gene from a heterologous species. FEBS Lett 317:216–222
Palmgren MG, Harper JF (1999) Pumping with plant P-type ATPases. J Exp Bot 50:883–893
Parton RM, Fischer S, Malhó R, Papasouliotis O, Jelitto TC, Leonard T, Read ND (1997) Pronounced cytoplasmic pH gradients are not required for tip growth in plant and fungal cells. J Cell Sci 110:1187–1198
Paul A-L, Sehnke P, Ferl RJ (2005) Isoform-specific subcellular localisation among 14-3-3 proteins in Arabidopsis seems to be driven by client interaction. Mol Biol Cell 16:1735–1743
Pedersen BP, Buch-Pedersen MJ, Morth JP, Palmgren MG, Nissen P (2007) Crystal structure of the plasma membrane proton pump. Nature 450:1111–1117
Pedersen CNS, Axelsen KB, Harper JF, Palmgren MG (2012) Evolution of plant P-type ATPases. Front Plant Sci 3. DOI: 10.3389/fpls.2012.00031
Pertl H, Himly M, Gehwolf R, Kriechbaumer R, Strasser D, Michalke W, Richter K, Ferreira F, Obermeyer G (2001) Molecular and physiological characterisation of a 14-3-3 protein from lily pollen grains regulating the activity of the plasma membrane H+ ATPase during pollen grain germination and tube growth. Planta 213:132–141
Pertl H, Gehwolf R, Obermeyer G (2005) The distribution of membrane-bound 14-3-3 proteins in organelle-enriched fractions of germinating lily pollen. Plant Biol 7:140–147
Pertl H, Schulze WX, Obermeyer G (2009) The pollen organelle membrane proteome reveals highly spatial–temporal dynamics during germination and tube growth of lily pollen. J Proteome Res 8:5142–5152
Pertl H, Poeckl M, Blaschke C, Obermeyer G (2010) Osmoregulation in Lilium pollen grains occurs via modulation of the plasma membrane H+ ATPase activity by 14-3-3 proteins. Plant Physiol 154:1921–1928
Pertl H, Rittmann S, Schulze WX, Obermeyer G (2011) Identification of lily pollen 14-3-3 isoforms and their subcellular and time-dependent expression profile. Biol Chem 392:249–262
Pertl-Obermeyer H, Obermeyer G (2013) Pollen cultivation and preparation for proteomic studies. In: Jorrin-Novo JV (ed) Plant proteomics: methods and protocols, vol 1072. Methods in Molecular Biology, Springer Verlag. doi:10.1007/978-1-62703-631-3_30
Pierson ES, Miller DD, Callaham DA, Shipley AM, Rivers BA, Cresti M, Hepler PK (1994) Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell 6:1815–1828
Piette A-S, Derua R, Waelkens E, Boutry M, Duby G (2011) A phosphorylation in the C-terminal auto-inhibitory domain of the plant plasma membrane H+ ATPase activates the enzyme with no requiremnet for regugulatory 14-3-3 proteins. J Biol Chem 286:18474–18482
Plätzer K, Obermeyer G, Bentrup F-W (1997) AC fields of low frequency and amplitude stimulate pollen tube growth possible via stimulation of the plasma membrane H+ ATPase. Bioelectrochem Bioenerg 44:95–102
Rathore KS, Cork RJ, Robinson KR (1991) A cytoplasmic gradient of Ca2+ is correlated with the growth of lily pollen tubes. Dev Biol 148:612–619
Regenberg B, Villalba JM, Lanfermeijer FC, Palmgren MG (1995) C-terminal deletion analysis of plant plasma membrane H+ ATPase: Yeast as a model system for solute transport across the plant plasma membrane. Plant Cell 7:1655–1666
Rodriguez-Rosales MP, Roldán M, Belver A, Donaire JP (1989) Correlation between in vitro germination capacity and proton extrusion in olive pollen. Plant Physiol Biochem 27:723–728
Rudashevskaya EL, Ye J, Jensen ON, Fuglsang AT, Palmgren MG (2012) Phosphosite mapping of P-type plasma membrane H+ ATPase in homologous and heterologous environments. J Biol Chem 287:4904–4913
Schrauwen JAM, de Groot PFM, Van Herpen MMA, van der Lee T, Reynen WH, Weterings K, Wullems GJ (1990) Stage-related expression of mRNAs during pollen development in lily and tobacco. Planta 182:298–304
Serrano R (1989) Structure and function of plasma membrane ATPase. Ann Rev Plant Physiol Plant Mol 40:61–94
Simon EW (1974) Phospholipids and plant membrane permeability. New Phytol 73:377–420
Southworth D (1983) pH changes during pollen germination in Lilium longiflorum. In: Mulcahy DL, Ottaviano E (eds) Pollen: biology and implications for plant breeding. Elsevier, New York, pp 61–65
Steinhorst L, Kudla J (2013) Calcium - a central regulator of pollen germination and tube growth. Biochim Biophys Acta 1833:1573–1581
Svennelid F, Olsson A, Piotroski M, Rosenquist M, Ottman C, Larsson C, Oecking C, Sommarin M (1999) Phosphorylation of Thr-948 at the C-terminus of the plasma membrane H+ ATPase creates a binding site for the regulatory 14-3-3 protein. Plant Cell 11:2379–2391
Tanaka I (1993) Development of male gametes in flowering plants. J Plant Res 106:55–63
Tupy J, Rihova L (1984) Changes and growth effect of pH in pollen tube culture. J Plant Physiol 115:1–10
Tupy J, Hrabetova E, Balatkova V (1977) A simple rapid method of determining pollen tube growth in mass culture. Plant Sci Lett 9:285–290
Turian G (1981) Decreasing pH-gradient toward the apex of germinating pollen tubes. Bot Helv 91:161–167
Villalba JM, Palmgren MG, Berberian GE, Ferguson C, Serrano R (1992) Functional expression of plant plasma membrane H+ ATPase in yeast endoplasmic reticulum. J Biol Chem 267:12341–12349
Viotti C, Luoni L, Morandini P, De Michaelis MI (2005) Characterization of the interaction between the plasma membrane H+ ATPase of Arabidopsis thaliana and a novel interactor (PPI1). FEBS J 272:5864–5871
Wei LQ, Xu WY, Deng ZY, Su Z, Xue Y, Wang T (2010) Genome-scale analysis and comparison of gene expression profiles in developing and germinated pollen in Oryza sativa. BMC Genomics 11:338
Weisenseel MH, Nuccitelli R, Jaffe LA (1975) Large electrical currents traverse growing pollen tubes. J Cell Biol 66:556–567
Yang Y, Qin Y, Xie C, Zhao F, Zhao J, Liu D, Chen S, Fuglsang AT, Palmgren MG, Schumaker KS, Deng XW, Guo Y (2010) The Arabidopsis chaperone J3 regulates the plasma membrane H+ ATPase through interaction with the PKS5 kinase. Plant Cell 22:1313–1332
Zonia L, Cordeiro S, Tupy J, Feijó JA (2002) Oscillatory chloride efflux at the pollen tube apex has a role in growth and cell volume regulation and is targeted by inositol 3,4,5,6-tetrakisphosphate. Plant Cell 14:2233–2249
Acknowledgements
Experimental work on pollen PM H+ ATPases was partially financed by a grant of the Austrian Science Fund (FWF, P21298).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Friedrich W. Bentrup
V. Lang and H. Pertl-Obermeyer contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lang, V., Pertl-Obermeyer, H., Safiarian, M.J. et al. Pump up the volume - a central role for the plasma membrane H+ pump in pollen germination and tube growth. Protoplasma 251, 477–488 (2014). https://doi.org/10.1007/s00709-013-0555-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-013-0555-2