Abstract
This chapter reviews recent advancements in the photocatalytic process, along with other similar green technologies such as nanotechnology, nonthermal plasma treatment, ozone-based technologies, etc., with specific emphasis on reducing the environmental impacts of leather production and processing. Leather industries are among the most polluting industries worldwide, and to address the challenges leather industries are facing with respect to environmental pollution much scientific work has been carried out. Photocatalytical processes have been explored for treatment of wastewater from tanneries and leather dyeing and finishing. Green photocatalytic processes exhibit great potential for chromium removal from tanneries’ wastewater, and degradation of dyes and other hazardous chemical compounds usually found in wastewater from leather industries. Nanomaterials and nanomaterial-based photocatalytic processes also provide leather and leather products with diverse types of surface functionalization and antimicrobial finish which is environmentally affable compared to conventional technology. Other similar technologies are nonthermal plasma and ozone technology which is principally based on nonthermal plasma. Nonthermal plasmas-ionized gases at low temperature have a potential for surface modification of leather which can render applications such as sterilization, improved uptake of dyes, chemicals, and natural products, varieties of finish including antimicrobial finish, etc. Being a dry technology the nonthermal plasma processing can significantly reduce environmental impacts compared to wet chemical processing. The ozone-based technologies are also similar in modes of action with that of the photocatalytic process. The ozone-based technologies are explored by contemporary researchers and are reported to have potential applications such as cleaner dehairing, cleaner preservation, treatment of tanneries’ wastewater, hazardous chemicals used in leather manufacturing, dye degradation, etc. The holistic overview provided in this chapter would be certainly useful to researchers working in these areas.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Leather
- Pollution
- Environmental impacts
- Photocatalysis
- Nonthermal plasma
- Ozone
- Waste treatment
- Cr reduction
- Dye degradation
- Wastewater treatment
1 Introduction
Leather production is all about the transformation of proteinaceous skin/hide flayed from dead animals to a stable, economically important commodity with a wide variety of uses. Leather production involves pre-tanning, tanning and post-tanning operations; at each stage huge quantities of chemicals and water are required which give rise to massive loads of waste, both solid and liquid, with substantial environmental impacts [1]. As per a study, each year approximately 6.5 million tons of flayed animal skins and wet salted hides are processed worldwide, with around 4.8 billion square feet of leather production [1]. Other studies report 1.67 × 109 m2 of leather manufactured every year with global sales of US$70 billion [2]. There are around 10,000 tanneries in the world with an estimated US$50 billion turnover; the largest supplier of leather is the European Union which exported 1163.1 thousand tons in the year 2014 [3]. The major production centres of leather are agroeconomic countries such as Argentina, Brazil, Mexico, China, South Korea, India and Pakistan [3]. South Asian leather industries comprise about 5000 tanneries which are small-scale, having a processing capacity of less than 2–3 tons of hides/skins per day, and are scattered all around the region [4]. For an agro-economy, local development can be enhanced by tanneries and leather processing industries but in time this can lead to serious pollution of the environment [3]. As per a statistical estimation, the world capacity of leather production is about 1.5 × 1010 kg hides/skins every year with a per day average discharge of more than 1.5 × 1010 kg of wastewater and an annual generation of 6 × 109 kg of solid waste [5]. India being a country with a huge livestock population, more than 3000 tanneries employing 2.5 million people are present in India, processing 80 million hides and 130 million skin pieces every year. Chrome tanning is commonly perceived in 80% of tanneries in India. India is a leading exporter of leather goods, having an export capacity of of $2.8 billion [6]. Leather production involves several stages/processes as described in Fig. 16.1. These stages/processes can be classified as pre-tanning or beamhouse operations followed by the tanning of pickled hide either by chrome tanning or vegetable tanning, and the associated stages/processes are called tanning yard operations. The leather thus produced is further provided with various finishes as per the product requirements [3].
At each stage of leather production and processing, huge amounts of water, chemicals and energy are required, and waste is produced at each stage which has significant environmental impacts as described in the next section. The challenge to reduce the environmental impacts associated with leather production and processing has been addressed by various technological solutions [7]. In this chapter, we provide an overview for emerging photocatalytic, nonthermal plasma and ozone technology to reduce environmental impacts associated with leather production/processing. As seen in Fig. 16.1, the environmental impacts associated with beamhouse operations can be addressed by ozone technology; for effluent generated from the tanning process photocatalytic and nonthermal plasma-based treatment can provide an effective solution for chromium reduction and removal. All three technologies can be beneficially utilized in the treatment of tannery effluent; the ozonation explored widely for treatment of tannery effluent is described in the following sections.
2 Environmental Impacts Associated with Leather Production and Processing
The environmental impacts associated with leather production and processing are releases of pollutants to water, air and soil. Usage of electricity for rotation of drums/vessels is the main source of air pollution; use of fossil fuel for electricity generation can pollute air with oxide of sulphur, oxides of nitrogen, particulate matter and emission of heavy metals such as vanadium, manganese, nickel, etc. [3]. Air pollution resulting from leather processing includes emission of sulphides, thiols, VOCs and organic solvents, ammonia, powdered dyes, leather dust, etc., while polyaromatic hydrocarbons (PAHs) and halogenated organic compounds, PCDD/furans can be emitted when solid waste from leather production is incinerated for waste to energy combustion [3]. Huge quantities of chemicals and water usage for leather production and processing results in a significant portion converted to by-products and pollution. Nearly 100 different chemicals are used to convert raw hides/skins in to finished leather [8]. As per an estimate, every year 6.5 million tons of flayed animal skins and wet salted hides are processed, using 3.5 million tons of different chemicals for leather processing worldwide [1]. Furthermore, organic matter from flayed hides/skins are a major source of waste and by-products. For a bovine hide, about 20–25% weight of raw bovine hide is transformed into leather, with a further 65% transformed to sole leather, while the remaining material contributes to waste, partially being recovered as by-products [3]. When water is utilized in most leather production and processing operations, generally if 1 ton of raw hide is processed, it results in ~250 kg leather with 15–50 kl of wastewater, 450–730 kg of solid waste and 500 kg of sludge generation [3]. The wastewater produced has an organic load of about 240 kg of chemical oxygen demand (COD), and 100 kg of bio-chemical-oxygen-demand (BOD), 150 kg of suspended solids (SS), 170 kg of sodium chloride (NaCl), 80 kg of sulphates and 5 kg of chromates [3]. Apart from the huge organic load that needs to be treated, tannery wastewater contains chromate, i.e., chromium (VI), oxidized from chromium (III) in tanning, which is of most concern because of its recognized carcinogenic, mutagenic and allergenic potential; for that 3 mg/kg (based on leather weight) is the current legislative limit [3]. With a worldwide emphasis now placed on production of chrome-free wet white leather, still 80–90% of tanneries are producing chrome-tanned wet blue leather, because the alternative treatments cannot produces the same quality leather as with chrome tanning [3]. The wastewater from leather production and processing also produces pollutants of significant concern such as the heavy metals copper, cobalt, barium, antimony, selenium, lead, zinc, mercury, nickel, cadmium compounds and arsenic, along with various toxic organic compound-azo dyes, polychlorinated biphenyl (PCB), formaldehyde resins, pesticide and biocide residues [3].
2.1 Liquid Waste from Leather Industries and Associated Impacts
As per an estimate, 57% of total water is consumed in pre-tanning and tanning processes while 35% of water is consumed for washing [1]. For one ton of raw hide processing 30–35 m3 of wastewater gets produced, with a wide range of wastewater production from 10 to 100 m3 per tons of raw hide depending on the type of hide, the production process and the quality of finished products required [9]. In a study related to the carbon and energy footprint analysis of tannery wastewater treatment it is reported that from 1 kg of raw material 0.3 to 0.4 kg of finished product is obtained with 25 to 45 l of water consumed per kg of raw material, being a water intensity of 0.13 m3/m2 of finished product [10]. Water consumption for leather production is estimated to be more than 5 × 107 m3 per year for the five largest leather producing regions—Brazil, China, India, Italy and Russia, with the highest water footprint of 2.7 × 107 m3 per year for China [10]. The global carbon footprint associated with tannery wastewater treatment is estimated to be 1.49 × 103 tCO2, eq. d−1; the energy intensity for tannery wastewater treatment is estimated to be 3.9 kWh kg−1 bCOD removed which is significantly higher compared to 1.4 kWh kg−1 bCOD for sewage treatment [10].
Preservation of hides by salt preservation can contribute 40% of Total Dissolved Solid (TDS) and 55% of chloride in effluent generated from leather production/processing. Dehairing of skins/hides by the liming process generates 40% and 50% of BOD and COD respectively, and contributes 60–70% to the total pollution load of leather processing [1]. The dehairing stage using sodium sulphide and lime contributes 84% of BOD, 75% of COD and 92% SS in wastewater generated from tannery industries [2, 11]. Conventional dehairing uses sodium sulphide which can affect the efficiency of effluent treatment and cause unfavourable environmental consequences [2]. Overall, wastewater generated from beamhouse operations contains a significantly high concentration of salts, because for the preservation of raw hide during this stage 300–400 kg of salts are required per ton of fresh flayed hide. Furthermore, unabsorbed sulphide and lime from the pelts in the liming process gets discharged in wastewater along with epidermis, broken hair, and non-structural proteins and other material which can increase COD and BOD if wastewater is produced in the soaking and liming stages. Amine additions to wastewater from beamhouse operations such as liming/deliming, and bating along with re-tanning operations can result in anaerobic conditions which are toxic for microorganisms playing an important role in wastewater treatment [3]. In subsequent tanning processes hides/skins after beamhouse operations are subjected to chrome tanning or natural tanning, which is processed with several stages that utilize huge variety of chemicals such as synthetic or natural tanning agent with acids, surfactants, salts, sulphonated oils, etc. Chromium tanning is the most used method; 90% of tanneries worldwide use this method in which chromium sulphate is applied at a concentration level of 8–10% [1].
In the most commonly used chrome tanning process only 60 to 80% of applied chromium salts are taken up by the hides/skins, thus the tannery effluent contains significant amounts of trivalent chromium along with other salts such as sulphide, sodium chloride and high concentration of organics with COD above 3000 mg/l [12]. Chromium is the pollutant of most concern; in order to prevent negative impacts to the environment upon discharge, the chromium concentration should not be more than 10 mg/l in the treated tannery effluent [12]. Cr(III) can be oxidized to Cr(VI) which is highly toxic, and the maximum permissible limit of Cr(VI) is 0.05 mg/l of Cr2O7−2 [12]. Overall, 45–50 m3 wastewater are generated per ton of raw hide processing, and 70% of total BOD, COD and total dissolved solids are resulting from the process [1]. A medium-sized tannery can produce over 300 million m3 wastewater every day, which contain thousands of tons of chemicals along with solid waste [1]. In India, 4000 tons of chrome are salts used every year of which 60–70% are used in tanneries. Effluent generated from chrome tanning contains 1500–3000 mg/l chromium, with an average 30–40 l of effluent generated per kg of hide tanned, and an average 50 l of wastewater generated per kg of hide processed by leather finishing operations. Indian tanneries are discharging 9.42 × 106 l of wastewater every year [6]. In India, the river Ganga and its tributary has significantly affected tanneries situated in the surrounding area, with high concentrations of heavy metals in sediments of this riverine system. In Tamil Nadu state of South India, tanneries have contaminated 55,000 ha of land and 5 million people were affected by low quality drinking water due to salination of rivers, and also by ground water contamination from wastewater discharged from tanneries, which also leads to loss of agriculture productivity [2].
2.2 Solid Waste from Leather Industries and Associated Impacts
Leather production and processing generates a huge amount of solid waste disposal which is problematic for leather industries. As per the statistics, 650 kg of solid waste is produced from processing of 1 ton of wet salted hides [13]. As leather is made from proteinous hides/skins flayed from dead animals, various nonfibrous proteins or fibrous proteins other than collagen such as hair, fleshing wastes, etc., contribute to solid waste along with trimmings from raw hides/skins, chrome shavings and trimmings, buffering dust, strips, cuttings from leather along with chrome sludge, and effluent treatment plant (ETP) sludge generated from primary and secondary treatments [1]. The quantity of trimmings waste depends on the raw material; in general, it accounts for 5% weight by weight for hides and 12 to 15% weight by weight for skins. Every year about 418 × 103 tons of trimming waste are generated globally [13]. The contribution of beamhouse operations in total solid waste generation is 80%, whereas tanning operations contribute by 19% and finishing operations contribute by 1%. As per the scientific literature, 150 kg of finished leather is produced from one ton of raw hides/skins, whereas the remaining 850 kg contributes to solid waste, comprised of 450 kg of collagen waste and 400 kg of fleshing waste [1]. Apart from other solid waste produced from leather production and processing, sludge generated from effluent treatment can significantly impact the environment if not properly handled. Primary treatment of tannery effluent results in settled sludge generation which accounts for 5 to 10% of the total volume of effluent; further sludge quantity increases up to 20% for the case of biological treatment. The sludge has a solid content 3–5% which increases to 25–40% after dewatering [3]. As per an estimation, bovine tanneries in Europe are generating 4 × 105 tons of sludge and the same amount of other solid waste each year [3]. From the different types of solid waste generated from leather production and processing, the chrome shavings are of considerable concern due to the presence of heavy metal chromium, and the disposal of chrome shavings is quite challenging. As per an estimate, every year India is generating 0.2 million tons of chrome shavings whereas worldwide approximately 0.8 million tons of chrome shaving are generated which mostly get disposed of through landfill or incineration [14]. The chrome shavings are fibrous in nature, make up 3–5% of total proteinous waste, contain 30–40% moisture; though chrome shavings contain Cr(III) there is the possibility of its conversion to the 300 times more toxic Cr(VI) [14]. Landfilling of chrome shavings can liberate 40–50% methane gas which can be a significant contribution in global warming, Cr can be leached out from landfill sites and can contaminate ground water, making the soil unfit for cultivation [14]. Solid waste is also generated when leather goods, specially footwear, are manufactured; 15–20% of raw material used for manufacturing of leather goods gets converted to solid waste [11].
For disposal of waste generated from leather production and in processing operations; land filling, anaerobic digestion and thermal incineration are the conventional methods, but environmental impacts and inherent issues are associated with these methods. The presence of chromium in leather production/processing waste can causes severe ground water contamination in the case of ground co-disposal, and chronic air pollution with high concentration of trivalent chromium during thermal incineration [8, 15, 16]. In one study, the method in which tannery wastes were incinerated at 800 ℃ in a thermal incinerator of starved air under various oxygen flow rates to optimize the oxygen flow required to prevent the conversion of Cr from Cr(III) to Cr(VI) oxidation state is reported. The exploratory study of the incineration of waste under the external oxygen supply was conducted under various conditions. Using Portland cement and fine aggregate, the calcined waste has been effectively solidified/stabilized. The unconfined compressive strength of the blocks was within the range of 120–180 kg/cm whereas to determine the degree of leachate and metals, leachability research was conducted on solidified block through the toxicity characterization of leachate procedure (TCLP) which indicate 99.1–99.9% metal fixation and 55–66 mg/l was the dissolved organic concentration in the TCLP leachate [15].
3 Environment Friendly Technology for Leather Processing: Need of Hour
As described in the above sections, the large quantity of water required for production and processing of leather is converted to wastewater; a study of the United Nations Industrial Development Organization (UNIDO) highlights the huge water demand for leather production and processing as an area of environmental concern [17]. For one ton of raw hides/skins, processing results in the generation of 30–40 m3 of wastewater [4]. Fresh water requirements for leather production and processing can be reduced by proper treatment and reuse of tannery wastewater, but reuse of wastewater is not possible without its preliminary analysis, and due to the complexity of the matrix reuse of tannery wastewater is a real challenge. The ultimate quality of leather gets affected with changes in the pH or changes in formulation in cases of reuse and that is why reuse is not possible more than once [17]. Also, environmental impacts associated with discharge of tannery effluents comes under sharp criticism and that is why lots of research work has been carried out to find alternative treatments for leather production/processing wastewater and to improve conventional treatment processes by photocatalytic and other green treatments.
3.1 Photocatalytic Technology
Advanced oxidation by photocatalysis is one of the promising methods for wastewater treatment which is widely used for treatment of wastewater generated from various industrial activities, for degradation of organic pollutants present in wastewater [18]. Photocatalysis is all about the production of receptive oxygen species on acquaintance of a photocatalyst which is illuminated by UV or visible radiation. The produced reactive oxygen species have the capability for degradation and mineralization of a wide variety of synthetic organic contaminants and pollutants [7, 19]. The application of photocatalysis for treatment of wastewater and pollutant degradation may provide many advantages such as negligible generation of secondary pollution, harmless degradation products, with time and reaction requirements being less, etc., with some of the limitations such as band gap dependency and interfacial charge transfer [7]. Currently, extensive research work is under process for the synthesis of catalysts with higher performance and expansion of light response range of photocatalysts using a variety of approaches such as deposition of noble metal, ion modification, coupled semiconductor, optimization of energy band configuration of photocatalysts for specific applications [20]. Heterogenous photocatalysis involving transition metal ions for environmental applications [21], degradation of various organic pollutants [22], photocatalytic dye degradation [23], photocatalytic dye degradation by synergetic effect of adsorption [24], environmental applications of photo-electrocatalytic technologies [25], coupling of photocatalysis and biodegradation, etc., are major research areas. TiO2 and ZNO are the most studied photocatalysts; apart from that layered double hydroxides-based photocatalysts [26], metal-doped TiO2, non-metal doped/co-doped TiO2 and TiO2 nanostructured hybrids [27], MoS2-based photocatalysis [28], metal oxide-cellulose nanocomposites-based photocatalysis [29], nanocomposites based on graphitic carbon nitride (g-C3N4), etc., are explored for environmental applications [30]. The major application of photocatalysis to reduce environmental impacts associated with leather production and processing is treatment of wastewater and reduction and removal of chromium [7, 31]. A reasonable photocatalytic reaction system can be developed for simultaneous treatment of two or more pollutants based on photocatalytic reactions with a redox reaction happening at the same time [31]. This approach can be beneficially utilized for treatment of tannery wastewater as described in the following section [7].
3.2 Nonthermal Plasma
Plasma is the fourth state of matter, it is ionized form of gases, though being a fourth state of matter, it necessarily has high temperature. The types of plasma which are called nonthermal plasmas or nonequilibrium plasmas are plasmas which do not have a high temperature. Nonthermal plasma technology is utilized as a dry, ecofriendly technology for surface modification of textiles and leather without altering the bulk properties. Nonthermal plasmas are of two types: low pressure plasma and atmospheric pressure plasma. Nonthermal plasma can be produced using a variety of gases as well as gas mixtures; it is an ionized gas which consists of charged particles/ions, energetic electrons, unionized gas molecules, reactive species such as hydroxyl radical, atomic oxygen, ozone, superoxide anion, hydrogen peroxide, reactive nitrogen species, gas molecules in ground and excited states and UV and visible photons. Leather being heat labile material, nonthermal plasma can be utilized for surface modification and eco-friendly processing of leather; atmospheric pressure plasmas are particularly suitable for leather processing. With inclusion of nonthermal plasmas the leather manufacturing process can be improved, less consumption of chemicals, water and energy savings can be achieved; thus nonthermal plasma has a huge potential in different areas of leather production and processing. Also, nonthermal plasmas can be utilized for wastewater treatment as described in subsequent section [32].
3.3 Ozone/Ozonation
Ozone is a trioxygen inorganic oxygen molecule with a chemical formula of O3, an allotrope of oxygen with higher electrochemical oxidation potential, more reactivity and less stability compared to diatomic molecular oxygen. The electrochemical oxidation potential of ozone compared to other known oxidizing agents is as follows: fluorine, F2 (3.06 V) > hydroxyl radical (2.80 V) > atomic oxygen O (2.42 V) > ozone (2.08 V) > hydrogen peroxide (1.78 V) > hypochlorite (1.49 V) > Cl2 (1.36 V) > chlorine dioxide (1.27 V) > oxygen gas (1.23 V) [4]. Due to its powerful oxidation potential ozone/ozonation can be extensively utilized for removal of pollutants from industrial effluents. Pollutants present in industrial effluents can be degraded by direct attack of ozone or by free radicals which can a degrade wide variety of organic compounds [33]. There are three techniques for ozone production: (1) ultraviolet techniques in which oxygen exposed to UV radiation can produce ozone in concentrations of 0.0003 g/hour at 1/100 W each; (2) ozone can be produced by perchloric acid electrolysis; and (3) by electrical discharge—a nonthermal plasma-based technique [4].
The advantages of ozone/ozonation for leather production and processing can be summarized as follows: when ozone/ozonation is utilized for treatment of tannery effluent it results in removal of turbidity, colour, bacteria and viruses; also the odour problem in open air stages of effluent treatment plants can be eliminated even with a small capacity (5 g/h) ozone generator [4]. Ozone treatment can result in oxidation of secondary sludge (partial or complete), lysis and partial oxidation of bacterial biomass and other organics to increase availability of food when it recycled as activated sludge, and filamentous bacterial growth and other colloidal structures can be broken down and thus achieve easy dewatering of sludge [4]. For treatment of tannery wastewater when ozone/ozonation is applied with use of high concentration ozone generator in combination with biological treatment, it can decrease treatment time, required discharge standards can be achieved and floor space requirement for ETP can be reduced with properly designed colum/vessel for ozonation [4]. Ozone/ozonation treatment can be easily combined with other advanced oxidation treatments. Residual ozone can be easily destroyed by an UV radiation-based ozone destroyer [34]. Ozonation itself does not generate any sludge, unlike other treatments, and multiple pollution treatment goals can be achieved by a single application of ozonation [33].
4 Chromium Reduction and Removal by Photocatalytic and Nonthermal Plasma Technology
Chromium as basic chromium sulphate is applied for tanning in which processed hide gets converted to wet blue, which is a stable product not degraded further even by microbial action. It is a tanning agent which renders the best quality leather with many additional advantages such as low process cost at high speed, producing a light colour of tanned leather with good stability, all of which make it a most used tanning agent [12]; however, it is a pollutant which of major concern from the environmental point of view [17]. In chrome tanning around 60–80% of the chromium reacts with hides/skins and the rest lost to tannery process effluent [12]. When one ton of hides is converted to 200 kg of leather containing 3 kg of chromium, non-tanned solid waste of 250 kg and chromium-containing tanned solid waste is produced which contains 3 kg of chromium [14], and over the time 50,000 kg of wastewater is produced which contains 5 kg of chromium [8, 35]. In the chrome tanning process 20% of raw materials converts to leather and overall, 60% is chromium lost in solid and liquid waste with many associated environmental impacts such as conversion of Cr(III) to carcinogenic Cr(VI) and contamination of water resources, occupational diseases, potential threats due to skin penetration of Cr(VI) by use of leather products, and entry of chromium into the environment at the end of product life, etc. [35].
Conventionally, chromium is removed from tannery effluent by precipitation using alkalis such as NaOH, Na2CO3 or Ca(OH)2; other methods for chromium removal are liquid–liquid extraction carried out by di(2,4,4trimethylpentyl) phosphonic acid and partially ammoniated di(2-ethylhexyl) phosphoric acid (D2EHPA), ion exchange, four stage extraction/re-extraction with mono(2-ethylhexyl) phosphoric acid (M2EHPA) or (D2EHPA), removal using high temperature and pressure, adsorption of suitable adsorbent such as kaolinite, flotation and removal by oleic acid surfactant and activated charcoal [3, 6]. In the most-used alkali precipitation method, chromium which is used as chromium sulphate for tanning/re-tanning is recovered by multiple washing and filtration. First, alkaline washing is carried out with hydrogen peroxide to oxidize Cr(III) to Cr(VI) to separate it from other metals, and next to that an acidic pH of solution is obtained after alkaline washing by H2SO4 and subsequently FeSO4 and sodium bisulphite added to reduce Cr(VI) to Cr(III). From the reduced chromate solution with addition of NaOH a precipitation of Cr(OH)3 is obtained and recovered by filtration [3]. Currently, many emerging techniques such as electrocoagulation, adsorption, biological treatment, membrane treatment and photocatalysis are explored for chromium removal from wastewater [7]. Significant environmental impacts associated with the most used precipitation and filtration method [12] due to usage of chemicals and usage of solvents in alternative techniques for chromium removal can be addressed by photocatalytic and nonthermal plasma-based treatment for tannery wastewater. Of the above-mentioned treatment the photocatalytic process is widely studied and is a more efficient treatment for chromium removal [36].
4.1 Application of Photocatalytic Process for Reduction and Removal of Chromium
In tanneries effluent chromium is present along with other organic pollutants; this co-existence of chromium with other pollutants creates difficulty in conventional treatment where chromium should be removed prior to any other treatment—otherwise Cr(III) can be converted to toxic Cr(VI). Photocatalysis can be advantageously used for simultaneous removal of chromium and other organic pollutants where toxic Cr(VI) can be reduced to Cr(III) and organic pollutants can be oxidized [7, 31, 37]. Various photocatalysts are studied for reduction and removal of chromium. Modified TiO2 mediated photocatalysis is the most studied approach for the removal of chromium; this includes use of carbon-based advanced materials for TiO2 modification, semiconductor-oxide-modified TiO2, semiconductor sulfide-modified TiO2, noble-metal-modified TiO2, and dye-sensitized TiO2 [38,39,40]. Other photocatalysts which are explored for Cr(VIII) reduction and removal by visible light or solar light are cerium-doped MoS2 nanostructures [41], LiMn2O4/SnO2 catalyst [42], α-Fe2O3 nanocrystals impregnated on g-C3N4-SO3H [43], nonthermal plasma-vulcanized flower-like ZnS/Zn-Al composites for adsorptive photocatalysis [44], graphene nanocomposite photocatalysts [45], RGO/BiOI/ZnO composites [46], phosphorus-doped g-C3N4/SnS nanocomposite [47] CaFe2O4 [48], etc. 3-D hierarchical Ag/ZnO@CF photocatalyst reported for synergistic removal of Cr(VI) and phenol by heterogeneous and homogeneous catalysis [49]. Photocatalysis is reported for Cr(VI) reduction under LED visible light with simultaneous degradation of bisphenol A using S-TiO2/UiO-66-NH2 composite [50], methylene blue using mesoporous BiVO4 photocatalyst using visible light [51], citric acid over TiO2 particles under near UV irradiation [52], humic acid over TiO2 particles under UV irradiation [53], etc. Photocatalytic reduction of Cr(VI) in the presence of polyethylene glycol (PEG), a water soluble non-ionic co-polymer is reported as an eco-friendly approach for removal of chromium from industrial wastewater [54]. Enhancement of Cr(VI) removal efficiency by photocatalysis can be improved via adsorption/photocatalysis synergy using electrospun chitosan/g-C3N4/TiO2 nanofibres [55], or red peanut skin [56]. When Nb2O5 is explored as an alternative catalyst for reduction and removal of Cr(VI) from tannery wastewater, it proved to be 20% more efficient than TiO2 and thus can be considered a promising alternative [57]. Bifunctional MOF/titanate nanotube composites have been studied for both photocatalysis and simultaneous adsorptive removal of formed Cr(III) [58].
Ti/TiO2 photo anode with sodium sulphate was used to check the feasibility of UV irradiation-based photobleaching of leather dye acid red 151, anionic surfactant, and photo-electrocatalytic reduction of Cr(VI). With use of pH 2 and 0.1 mol l−1 sodium sulphate, 100% dye decolourization can be obtained with reduction of 98–100% of Cr(VI) and abatement of 95% of the original total organic carbon. The findings of this study indicate that photo-electrocatalytic oxidation can be considered as an exceptional alternative for treatment of tannery wastewater containing dyes, surfactants and toxic hexavalent chromium; at lower concentration of pollutants complete removal can be obtained [59]. In a similar study, parthenium weed activated carbon loaded with zinc oxide nanoparticles (ZnO-NPs-PWAC) was used for the simultaneous removal of methylene blue and Cr(VI), which was further studied with real tannery wastewater. In this study, detailed characterization was carried out to test photocatalytic activity of ZnO-NPs and ZnONPs-PWAC. ZnONPs alone provided more than 93% efficiency for decolourization of malachite green, congo red, and methylene blue under sunlight irradiation. PWAC provides more than 99% removal of Methylene Blue in 130 min where as ZnO-NPs-PWAC provides the same removal efficiency within 60 min. Similarly, PWAC provides more than 99% removal of Cr(VI) in 160 min, whereas ZnO-NPs-PWAC provides the same removal efficiency within 90 min. Combined reactions of photocatalysis and adsorption provides enhancement in removal efficiency; ZnO-NPs-PWAC also provided more than 92% removal efficiency for realistic tannery wastewater [60]. Other than TiO2 and ZNO, silver chromate nanocrystals provided a fresh and feasible opportunity that can dispose of wastewater containing chromium and at the same time, producing a new visible-light catalyst that can degrade the organic pollutant in the wastewater. Ag2CrO4 nanocrystals were prepared by ultrasonic synthesis, template and hydrothermal. A comparative study of the product was investigated. Best results were reported with the product synthesized by the ultrasonic method (dye degradation within 8 min) than with the other two methods reported (dye degradation occurred in 42 min) [61]. Synthesis of spherical TiO2 catalytic materials with hollow structure reported for photo-electrocatalytic reduction of Cr(VI); the result of the study indicates that a photocatalytical removal rate of Cr(VI) is 0.0126/min whereas the removal rate by photo-electrocatalysis is 0.0362/min which is three time faster than the former one. The spherical TiO2 based photo-electrocatalysis studied for the actual tannery wastewater samples collected from three different tanning procedures, and excellent activity was also obtained with realistic tannery wastewater. This indicates the great potential of photocatalytic-based technology for Cr(VI) reduction and removal from tannery effluents [62]. Hydroxylated α-Fe2O3 was utilized for synergetic photocatalytic reduction of Cr(VI) and degradation of leather preservative 4-Chlorophenol under visible light. For one hour of visible light irradiation, Cr(VI) reduction was obtained in a range of 24.8% to 70.2%, while degradation of 4-Chlorophenol increased from 13.5 to 47.8%. The photocatalyst can be reused again and again; good degradation is obtained even after nine cycles of degradation [63].
4.2 Applications of Nonthermal Plasma for Chromium Reduction and Removal
Applications of nonthermal plasma are also reported for chromium reduction and removal. In a study, simultaneous Cr(VI) reduction and As(III) oxidation in aqueous solutions reported using a glow discharge plasma; experiments were carried out to study effects of input energy, pH value and concentrations for redox transformation of Cr(VI) and As(III). In the glow discharge treatment synergetic effect observed between Cr(VI) and As(III), the presence of Cr(VI) can significantly enhance As(III) oxidation. Increase in voltage inputs from 530 to 600 V can increase conversion of Cr(VI) from 96 to 100% and As (III) from 53 to 77%. In acidic pH reduction of Cr(VI) (96% reduction) proceeds rapidly compare to As(III); the optimum pH for As(III) is 7. The conversion is due to H2O2 generated in glow discharge which reduce Cr(VI) and hydroxyl radicals which can oxidize As(III). This has opened up a new possibility for treatment of chromium and arsenic containing wastewater [64]. In a similar study, removal of Cr(VI) and methylene blue was simultaneously carried out by atmospheric pressure argon plasma jet. In an acidic medium, the highest amount of hydrogen peroxide is formed which reduces Cr(VI) to Cr(III), and simultaneous removal of methylene blue and Cr(VI) is more beneficial than individual pollutants [65]. The same atmospheric pressure argon plasma jet was studied for simultaneous removal of As(III) and Cr(VI), where both the pollutants complement redox natura and simultaneous removal is beneficial [66]. For Cr(VI) removal CoFe2O4/multiwalled carbon nanotubes (MWNTs)/sponge electrodes were prepared which enhances the performance of DBD. Cr(VI) and phenolic pollutants such as phenol, hydroquinone, nitrobenzene and p-nitrophenol were simultaneously removed using these plasma discharge systems. In this study optimum concentration of CoFe2O4 nanowires estimated 0.5 g/l and with that 98.52% degradation of phenol achieved [67]. In a similar study, simultaneous oxidation of phenol and reduction of Cr(VI) is studied with contact glow discharge electrolysis [68]. Simultaneous removal of Cr(VI) and an azo dye acid orange 7 has been studied; due to synergistic effects both pollutants improve degradation of each other. Under acidic condition 94% reduction of Cr(VI) is achieved; furthermore, with an increase in the input power from 80 to 120 V, Cr(VI) removal increased from 54 to 88% and removal of dye acid orange increased from 62 to 89% [69]. Atmospheric pressure argon glow discharge plasma at gas solution interface was explored for reduction of Cr(VI); here in the experiments a small quantity of ethanol was added as a hydroxyl radical scavenger. Further in the study, the same experiments were carried out using air glow discharge; 89% of Cr(VI) got removed from 25 ml 80 mg/l K2Cr2O7 solution in presence of 2% v/v ethanol after a 15-min treatment with the air discharge [70]. In a similar study Cr(VI) reduction in aqueous solution was achieved in presence of ethanol as hydroxyl radical scavenger by micro-plasma [71]. In a study, Cr(VI) ion imprinted polypropylene (PP) fibres were fabricated by plasma mediated grafting which is effective and selective for Cr(VI) adsorption. The surfaces of PP fibres were activated by nonthermal RF plasma (argon and air) followed by gaseous phase acrylic acid grafting which is further amidated with triethylenetetramine and subjected to Cr(VI) template imprinting. It further explored for Cr(VI) removal in which highest adsorption capacity 167 mg/g obtained at pH 3. Adsorbed Cr(VI) eluate rapidly and effectively by 0.2% NaOH solution and adsorption efficiency maintained more than 80% even after ten regenerations [72].
5 Photocatalytic Process for Treatment of the Leather Industry’s Wastewater and Other Applications
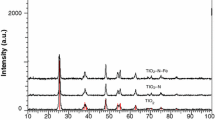
Various studies report application of TiO2, and other nanoparticles used persuasively for photocatalytic dye degradation and the removal of organics and heavy metals from tannery wastewater. In a study, ITO (indium tin oxide coated glass) supported TiO2 nanoparticles were utilized for photo-oxidation of dye released by leather industries. In the study, complete degradation and mineralization of dye into inorganic product is reported, while decolourization ratios of more than 90% were reported for the time of 480 min [73]. In another study, TiO2 nanoparticles were prepared on alumina and glass beads; after that photocatalytic activity was studied for photo-oxidation of acid brown 14 leather dye in aqueous solution illuminated with solar light. Characterization techniques like HPLC and UV–Vis helps to keep track on changes in the concentration of acid brown 14 after photocatalytic degradation. The effectual results were reported for TiO2 supported on alumina beads in an acidic condition [74]. Degradation of dermacid red (CAS: 6406-56-0), dermacid black RVE (CAS: 99576-15-5) and dermacid brown (CAS: 8011-86-7) leather dyes was carried out by photocatalysis with TiO2 and a comparative study carried out for degradation of these dyes using ultrasound irradiation, photocatalysis with TiO2, Fenton/photo Fenton and a combination of those techniques. For photocatalysis and photo Fenton UV illumination was used, whereas for ultrasound irradiation at low (20 kHz) and high frequencies (860 kHz) was utilized. Due to the different natures and structures of the azo dyes, each dye has a different optimization parameter of photocatalytic degradation by different combinations of advanced oxidation process; combination of these advanced oxidation processes has synergetic effects for dye decolourization. Maximum degradation of the chromophore group was reported in the first two hours of experiment [75]. Other than TiO2, silver chromate nanocrystals provided a fresh and feasible opportunity that can dispose of wastewater containing chromium and at the same time, produce a new visible-light catalyst that can degrade the organic pollutant in the wastewater. Ag2CrO4 nanocrystals were prepared by ultrasonic synthesis, template and hydrothermal methods and utilized for photocatalytic degradation of dye rhodamine B. In a comparative study of Ag2CrO4 nanocrystals prepared by the different methods, best results were obtained with the Ag2CrO4 nanocrystals synthesized by the ultrasonic method (dye degradation within 8 min) than with the other two methods reported (dye degradation occurred in 42 min) [61]. Apart from dye degradation, photocatalysis is explored for degradation of organic pollutants for wastewater. In a study, ZnO photocatalyst was also used to treat tannery wastewater, with 1gm/litre ZNO catalyst in effluent diluted by 1:200 proportion, which is irradiated by mercury vapour lamp irradiation (1850 μW cm−2, Topcon UVR-2) for four hours at pH of 8.0. The treatment resulted in a reduction in physiochemical parameters—chemical oxygen demand (COD) from 15,023 to 350 mg/l, biochemical oxygen demand (BOD) from 4374 to 10 mg/l, total solids from 28,500 to 188 mg/l, total organic carbon from 4865 to 4.93 mg/l and turbidity from 331 to 1.15 NTU. The treatment also resulted in decrease in toxicity as tested by the lethality assay of microcrustacean Artemia salina L, with LC50 increasing from 14.90 to 56.82% [76]. In another study, ZnO-ZnFe2O4 composite photocatalyst supported by activated carbon studied for reduction in biochemical oxygen demand (BOD5) of tannery wastewater under visible light irradiation. It is reported in the study that by adsorption only 9% of BOD5 was removed while with the photocatalytic treatment 90% reduction in BOD5 was obtained in a two-hour treatment [77]. The removal of residential tributyltin (TBT) from tannery wastewater was carried out by effective electro-field-assisted-photocatalytic technique using hierarchical TiO2 microspheres. The rate removal 0.0052 min−1 was obtained with photocatalysis while 0.0488 min−1 was the rate of reaction by electro-field-assisted-photocatalytic removal of TBT which is nine times faster compared to photocatalysis [78]. In a study, immobilization of TiO2 was carried out on macro-defect free cements to form photoactive polymer-cement composites which utilized for tannin degradation. Using MDF containing embedded TiO2 nanoparticles 98% photodegradation of tannins obtained with six-hour irradiation with 254 nm [79]. Polyvinyl thiol (PVASH) assisted Ag NPs are reported for surface enhanced raman spectroscopy (SERS) detection and photocatalytic degradation of tannery waste landfill leachate with degradation rate of 0.0025 min−1 and 0.0039 min−1 for visible and UV light irradiation respectively [80]. Heterostructured BiVO4-ZnO mixed oxide catalysts were utilized for sunlight-driven photocatalytic degradation of post-tanning wastewater. The photocatalyst has an optical band gap of 3.02 eV, which is suitable for sunlight-driven degradation; higher photocatalytic activity is observed for mixed oxide catalyst; 64% reduction in COD is achieved with this mixed oxide catalyst under optimal experimental conditions in the presence of sunlight for a six-hour treatment duration [81]. Various refractory organic compounds such as nonadec-1-ene, 2-phenylethanol, 2,4-di-tert-butylphenol and other organic compounds present in tannery effluent were identified and 2-phenylethanol photocatalytically degraded using standard Degussa P-25 TiO2 (100 mg) under UV light. 2-phenylethanol was transformed into 2-tert-butyl-4,6-dimethylphenol, 2,6-di-tert-butyl-4-methylphenol by photocatalytic degradation and after prolong exposure of 30 h 100% degradation of 2-phenylethanol was achieved [82]. From leather skin waste carbonaceous N-containing materials are derived which are found to have photocatalytic activity due to crystalline species of Cr2O3and TiO2 having degradation potential for contaminates such as phenol [83]. From collagen biowaste bi-functional iron embedded carbon nanostructures have been prepared which have photocatalytic activity and applications for lithium ion batteries [84].
6 Nonthermal Plasma and Ozone for Treatment of the Leather Industry’s Wastewater
Both nonthermal plasma and ozone are advanced oxidation approaches, and as discussed in previous sections the most common and most efficient method for ozone generation is based on nonthermal plasma—specifically dialectic barrier discharge or silent discharge. The utilization of ozone for wastewater treatment is purely a chemical treatment owed to the higher oxidation potential of ozone [85], whereas the nonthermal plasma treatment of wastewater is based on advanced oxidation as well as physical treatment; either by nonthermal plasma discharge generated at the gas–liquid interface or within liquid [86]. The wastewater treatment and pollutant degradation by nonthermal plasma is associated with generation of powerful active species such as hydroxyl radical (⋅OH), super oxide, ozone and hydrogen peroxide, as well as pollution degradation by dissociation high energetic electrons, ultrasonic waves and UV radiation [86].
6.1 Nonthermal Plasma Treatment for Degradation of Leather Preservatives and Other Applications
Nonthermal plasma generated by dielectric barrier discharge (ceramic plate electrodes coupled with pulsed generator Minipuls 6, GBS Elektronic, Rossendrof, Germany) using different gas combinations such as argon, argon:oxygen (80:20) and air. These have been utilized for degradation of 2,4-dichlorophenol (2,4-DCP) (biocide used for leather preservation) [87] in aqueous solution; also the plasma treatment explored in combination of advanced oxidation such as the Fenton process. Plasma discharge created using Ar gas results in complete degradation of 2,4-DCP within 15 min; however, when 20% oxygen is added to Ar degradation of 2,4-DCP is delayed but mineralization improved due to accelerated oxidation of intermediate products by reactive species [86]. The addition of 10 mg/l ferrous ions to an aqueous solution of 2,4-DCP induced the Fenton process; better degradation and mineralization was achieved due to the fact that it resulted in the combination of two advanced oxidation processes—nonthermal plasma and the Fenton process [86]. Degradation of leather fungicide p-nitrophenol was carried out using microwave atmospheric plasma in aqueous solution; 100 mg/l of p-nitrophenol got removed completely with a 12-min treatment, and TOC removal of 57.6% was obtained. The pH of a solution has no significant impact on degradation; hydroxyl radical generated by plasma discharge playing a key role in degradation of p-nitrophenol [88]. Atmospheric pressure air DBD was reported for the sterilization of wastewater samples collected from leather processing plants. Aliquot of the wastewater sample was treated with air DBD for 75.5, 81.94, and 85.34 mA with a discharge current and exposure time of 30, 60 and 90 s. The plasma exposure at 75.5A can greatly change dominant bacterial groups at 30 and 60 s exposure, while 80% reduction in bacterial population is observed at 90 s exposure. The same trend is observed for plasma treatment at 81.94 A; the plasma treatment at 85.34 mA can eliminate all bacterial groups at 60 or 90 s exposure. This sterilization effect of the air DBD is conferred to active species such as NO, N2+, UV radiation; air DBD can be environmentally affable and a promising tool for wastewater treatment to eliminate microorganisms from treated wastewater without use of chlorine [89].
6.2 Ozone/Ozonation to Increase Biodegradability of Tannery Wastewater
Though ozone can be produced in three ways, nonthermal plasma-based silent discharge is the prevalent, most effective and economic method for ozone generation, which is greatly explored for treatment of wastewater [4, 90]. The ozone technique can be applicable for treatment of wastewater generated from beamhouse operations, and for treatment of tannery wastewater only after removal of chromium by precipitation using pre-alkalization [12]. Ozone has an electrochemical oxidation potential of 2.08 V, higher compared to molecular oxygen (1.23 V) from which it is produced, and thus it can be a preferred advanced oxidation method to improve performance of biological treatment of tannery wastewater [4, 36]. With bench scale experiments, ozone produced by silent discharge was explored as a tertiary treatment for tannery effluent collected from a CETP and ETP. Atmospheric air at a flow rate of 1.2 l/m was passed through silent discharge and ozone generated at a concentration of 0.5 g/m3 purged through 1-l effluent sample for three hours using a diffuser having pore size of 160 µm. For the CETP effluent sample the ozone treatment resulted in 85% reduction in COD, the colour changed from dark brown to colourless and there was complete removal of odour. With the same method for ETP effluent sample, ozone treatment resulted in 81% reduction in COD, 40% reduction in BOD and more than 95% reduction in sulfides, with a colour change from dark green to colourless and complete elimination of odour [4]. In a field level experiment at tannery ETP a small ozone generator having capacity of 0.5 g/h, 1.5% w/w, 4 l/min utilized for injection of ozone with H2O2 in the ratio of H2O2/O3 of 1:2 in a aeration tank (45 m3) for 24 h. This resulted in a 25% reduction in BOD value and 20% reduction in COD value due to the production of perozone or hydroxyl radical having 60% more oxidation efficiency [4]. Strategic application of ozone as pre-treatment can increase the biodegradability of tannery effluent by anaerobic treatment due to simplification of complex and high molecular weight compounds. Pre-ozonation of tannery effluent can increase assimilable organic carbon in effluent by faction of 1:3 from 36 µg/l for raw effluent to 109 µg/l after ozonation; deoxygenation coefficient for BOD removal increased from 0.1/day to 0.25/day. Thus, treatment efficiency can be improved, treatment time can be reduced and with fewer problems of solid handling [4].
In another study, sequencing batch biofilter granular reactor (SBBGR) coupled with ozonation is explored for the treatment of tannery wastewater, where integration of ozonation is reported to improve efficiency of the biological treatment. In the study, wastewater from SBBGR flowed through the ozone reactor (0.25m3) at a rate of 2 m3/hour and recirculated again for final biological treatment. Due to high oxidation potential, ozone can degrade recalcitrant material present in tannery wastewater and thus a high removal rate is obtained for COD, BOD, TSS, TKN; colour and surfactant can be obtained with very less sludge production (0.1 kg dry sludge/m3 of effluent treated). Without ozonation 91 ± 1% removal for COD, 99 ± 1% removal for BOD, 87 ± 2% removal for dissolved organic carbon (DOC), 88 ± 4% removal for TSS, 84 ± 4% removal for TKN, 93 ± 4% removal for surfactants and 24 ± 3% removal for colour is obtained by SBBGR. Whereas when ozonation integrated with the SBBGR 97.5 ± 0.5% removal for COD, 99 ± 1% removal for BOD, 93 ± 1% removal for dissolved organic carbon (DOC), 96 ± 1% removal for TSS, 91 ± 2% removal for TKN, 98 ± 5% removal for surfactant, 96 ± 2% removal for colour obtained, while 20 ± 2 mg/l ozone was consumed. The treatment cost after integration of ozonation with SBBGR is estimated at about 1€ per m3 of wastewater which is four time lesser than the estimated cost without ozonation (about 4–5€ per m3 of wastewater), whereas sludge generation (about 3 kg dry sludge/m3 produced when the plant operated without ozonation) is 30 time less [34]. Ozonation has been applied for simultaneous removal of COD and colour from tannery effluent which resulted in enhanced biodegradability of tannery effluent and colour present in the removed material due to oxidative cleavage of chromophore groups of dyes and colour imparting substances. Effluent containing direct brown diazo dye and realistic effluent from wet tanning processes was treated with diffusion of ozone from an ozone generator at various process variables. For real tannery wastewater at optimum process condition 92% COD removal efficiency obtained, and BOD/COD ratio increase from 0.18 for without treatment to 0.49 after 30-min ozone treatment. For the both effluents, efficiency of ozonation depends upon the initial concentration of pollutants and ozone dosage, and pollution degradation follows pseudo first-order kinetics [33]. Ozonation combined with coagulation flocculation (CF-OZ) is studied to improve treatment of tannery wastewater and compared with coagulation flocculation and adsorption (CF-ADS). With CF-ADS 50.04% removal TOC, 53.13% removal of COD, 17.05% removal of Na+, 61.13%, colour removal achieved and compared to that CF-OZ resulted in 46.50% removal TOC, 56.25%, removal of COD, 11.10% removal of Na+, 85.34% colour removal. Further, ozonation decreases the COD/TOC ratio from 2.79 for untreated effluent to 2.66 for ozonated effluent and the BOD/COD ratio increased from 0.24 for untreated to 0.60 for CF-OZ and 0.47 for CF-ADS. Thus, both treatments facilitate further biological treatment of tannery effluent, ozonation being more beneficial compared to the other one [91]. Ozonation pre-treatment of two separated streams of wastewater generated from tanneries, i.e., beamhouse operation wastewater and tanning yard effluent were explored. The results of this study indicate that for both streams, ozonation prior to biological treatment breaks down refractory compounds. In the case of tanning yard effluent, pre-ozonation before aerobic treatment resulted in 95% COD removal and 91% DOC removal. For both the effluents, above-mentioned effect is found in the optimal range of ozone dose of 1 to 3 g per gram of DOC; further ozone dose is wasted in the destruction of already degradable compounds [92].
Advanced oxidation treatments, ozone and the Fenton process, were explored for treatment of tannery effluents at a central effluent treatment plant (CETP). The tannery effluents received at CETP has a BOD/COD ratio about 0.1 to 0.25 and even after physicochemical treatment the effluent has low biodegradability. The above-mentioned advanced oxidation treatments provided to effluent from primary settling tank of the CETP improved the biodegradability of effluents in subsequent activated sludge processes. Thirty to fifty percent COD removal was achieved by Fenton treatment and 15–20% COD removal obtained by ozonation at the primary stage; and after secondary treatment 60–70% COD removal obtained by ozonation and 50 to 60% COD obtained by the Fenton treatment. Ozonation was further explored for treatment of tannery effluents at CETP; coagulation and extended aeration followed ozonation considered as the best treatment which can provide 80–90% reduction in COD [93]. Ozonation as post-treatment is also found beneficial for treatment of tannery wastewater. In a study on the effect of ozonation, before, within and after biological treatment, it was found that application of ozone after biological treatment is most promising where the highest COD removal can be achieved by a low feeding time of five minute at a flow rate of 42.8 mg/l. Conversion of nonbiodegradables to readily biodegradable is possible due to ozonation and thus an improvement in biological degradation [94]. Catalytical ozonation using Mn-Cu/Al2O3 catalyst also explored for tertiary treatment of real tannery effluent which results in higher COD removal efficiency compared to ozonation alone. In catalytical ozonation COD removal increases with the quantity of catalyst from 0.5 to 3.0 g/l and ozone volume from 0.2 to 0.4 g/h. The highest 88.6% COD removal was obtained at 7pH, catalyst load of 2.0 g/l and ozone flow rate of 0.3 g/hour for treatment time of 60 min [95].
Ozone/ozonation as a chemical treatment/advanced oxidation treatment can be utilized for treatment of wastewater generated from chromium tanning after the removal chromium by pre-alkalization. The study was performed as a bench scale utilizing an ozone generator (COW-025, Afraz Mohit Sahand Ltd.) with a capability of 25 g/h ozone generation when the generator was fed with pure oxygen. The ozone generator connected with the ozone contactor (3-l cylinder), with a total reaction volume of 5 l including volume of the circulation loop. Ozonation as a chemical treatment/advanced oxidation treatment able to remove COD from 30 to 70% by providing treatment for 120 min with an ozone flowrate of 1 to 8 g/hour. From statistical analysis, an ozone dose of more than 15 g/h for 120 min predicated for complete COD removal. Colour removal by ozonation increased from 40 to 64% at a flowrate of 1 g/hour when pH decreased from 9 to 3; 85% colour removal obtained for ozonation at a flow rate of 8 g/hour at any pH. At optimum conditions with ozonation 88% COD removal and 93% colour removal was obtained, however when high concentration of ozone such as 8 g/hour is utilized, Cr(III) in wastewater can be converted to toxic Cr(VI) and therefore chromium removal by pre-alkalization is a necessity when ozone is directly applied for treatment of tannery wastewater [12]. Ozone/ozonation is reported to enhance biological treatment of wastewater generated from tanning processes utilizing plant-based polyphenols as natural tanning agents. Natural tanning is environmentally friendly compared to chrome tanning; however, poor biodegradability of tannins cause problems in biological treatment such as absorption of light and heat by tannins, reduction of efficiency of biological treatment due to persistent nature tannins remaining in effluent even after conventional treatment of tannery wastewater. In a study, a bubble column bench scale reactor attached with a ozone generator (Model No L6G, Faraday Instruments, India) was utilized for treatment of wastewater generated from natural tanning of leather. The ozone generator used in the study can produce ozone at 2 g/h by using oxygen as feed gas and produced ozone diffused through the wastewater by ceramic diffuser and treatment carried out for 30 min. Ozone due to its superior oxidation potential can degrade tannins, the BOD5/COD ratio of untreated wastewater is 0.196 due to poor biodegradability of tannins which increased to 0.298 after the ozonation. Ozone pre-treatment resulted in a 20% reduction in COD and 49% removal of total phenols; improvement in subsequent biological treatment resulted in 60 mg/l BOD5 and 350 mg/l COD in the treated effluent [96]. Ozonation has been explored for degradation of syntans used in leather tanning. Syntans are synthetic organic materials used for tanning, and like chrome tanning and natural tanning, syntans are not completely absorbed by hide and unreacted syntans are lost into effluent. Commercially available syntan which is aromatic sulfonic acid condensation product is degraded by a bench scale reactor connected with an ozone generator (Faraday Model No. L6G, India) which can produce 1–6 g/h ozone using oxygen as feed gas. Experiments were conducted using 100–700 ppm and ozone dose of 1 g/h. Tannin removal efficiency was found to be 98%, and 91% COD removal for syntan concentration of 100 ppm was obtained whereas 72% tannin removal and 48% COD reduction obtained for 700 ppm concentration [97]. Phenol sulfonic acid-syntan (PSAS) degraded by ozonation using a bench scale glass reactor of 3 l capacity connected with a lab-scale ozone generator (Faraday Instruments, Model No. L6G, India) which can generate 1–3 g/hour ozone using pure oxygen as feed gas. The optimum conditions for syntan degradation are found to be 7 pH, 5.2 × 10–3 mmol/l ozone concentration, 500 mg/l concentration of substrate. The ozonation treatment improved biodegradability of the syntan which was reflected as an increase in the BOD/COD ratio from 0.03 for untreated to 0.42; the degradation follows pseudo first order reactions, with increase in ozone dose maximum of 84.2% COD removal and 58% (dissolved organic carbon) DOC removal achieved at 7 pH [98].
6.3 Ozone/Ozonation for Dye Degradation
Ozone treatment, when applied to tannery wastewater treatment, can be advantageously utilized for degradation of dyes, preservatives/biocides applied during leather production and processing which would be otherwise difficult to degrade in conventional treatment due to its stable chemical structure and toxicity [85]. The advantages of ozonation for dye decolourization is no sludge generation, less cost and effective degradation. Degradation of sandopel brown BRR dye in aqueous solution carried out by ozone generated using ambient air by ozone generator (Model No-SA001, India) which can produce 3 g ozone/ hour. The dye decolourization by ozonation was carried out in a 5-l cylindrical glass reactor filled with various dye concentrations from 30 to 360 mg/l, pH 4 to 11 and through that ozone bubbled at a concentration of 1.6 mg/l for different times. Decolourization was faster at in alkaline pH; at pH 11 95% dye decolourization was achieved in 20 min whereas at pH of 4 the same results were obtained at 35 min. A maximum 97% dye decolourization obtained, decolourization decreased with an increase in initial dye concentration; for dye concentration of 30 mg/l, 97% decolourization was achieved in 30 min; where it took 170 min for dye concentration of 360 mg/l, dye decolourization by ozone follows first order kinetics [99]. In another study, ozonation and ozone along with hydrogen peroxide was explored for degradation of four leather dyes—direct black 168, acid black 241, acid brown 83 and acid brown 191 using a 1-l contact reactor connected to an ozone generator (Marca Iberozono, model 80). For dye degradation the contact reactor filled with 40 mg/l dye solution and ozonation was carried out at saturation for 60 min; the effect of pH was explored by adjusting pH 2 to 10. A similar experiment was performed for ozone + H2O2 treatment but adding 6 to 60 mg H2O2 (30% p/v) per 1 l of the dye solutions. Contact with ozone for a prolonged exposure time of 100 min resulted in complete decolourization of all the dyes; however, in the case of ozone treatment coupled with H2O2 greater colour reduction obtained at a shorter period of time in 4 to 5 min but afterwards no further improvement was observed [100]. Leather dye acid black 52 was degraded by ozonation using a packed bed reactor with different process variables such as pH, dye concentration, and contact time. One hundred per cent colour removal and 61% COD reduction was achieved at optimum conditions of 1.96 pH, 1159 mg/l dye concentration and 10.6 min contact time and 4.8 pH, 1159 mg/l dye concentration and 17 min contact time respectively [101]. In other similar studies three azo leather dyes, CI acid black 1 (AB1), CI acid yellow 19 (AY19) and CI acid orange 7 (AO7) degraded using a batch reactor of 500 ml volume connected with an ozone generator (model 1000 Nano Paak Sayyal, Iran). When experimental variables such as ozone dose, dye concentration, pH, temperature, etc., were studied; greater than 75% colour removal obtained under optimum conditions within the first five minutes of removal treatment, initial dye concentration and pH of dye solution are the most influential factors for dye degradation using ozonation [102]. Acid dye navitan bordeaux MB (C.I. acid violet 90), 100 ppm synthetic effluent sample was degraded by ozonation using a bubble column reactor and an experiment studied the influence of ozone dose, dye concentration and pH on the decolourization rate. The results of this study indicate that at a high ozone dose 90% removal can be achieved in 1–2 min, whereas at a low ozone dose of 90% removal can be achieved in 4–5 min, complete colour removal can be achieved in 5 min and 30 mg/l ozone concentration is required for 90% decolourization when pure oxygen gas is used for ozone generation. 50 mg/l ozone concentration required for 90% decolourization and complete decolourization achieved in 7 min when ozone is generated using ambient air [103]. In a study, ozonation was explored for decolourization of three leather dyes CI direct blue 1, CI green G and CI fast red B base and compared with electrocoagulation. The experimental set up was composed of a reactor (1100 l) connected with an ozone generator (Carbar—03A2-7 W). Molecular ozone is selective in dye degradation; in certain dye structures attached by ozone, dye degradation was carried out using a volume of 200 l of 50 mg/l dye solution. After 210 min 50% degradation was observed for CI direct blue 1 and 55% for CI fast red B base, and 61–89% for CI green G respectively [104]. Leather dye acid black 210 in aqueous solution degraded by ozonation and UV/ozonation; influence of pH, dye concentration, effect of UV radiation were studied, decolourization and mineralization studied by UV and TOC analysis. At optimum conditions 100% decolourization and 55% mineralization was achieved within a short time of 15 min [105].
6.4 Ozone/Ozonation for Degradation of Leather Preservatives
Chloro-substituted phenolic compounds are widely used as biocides/preservatives in leather production/processing [87], which can cause water pollution problems due to acute toxicity and poor biodegradability and the persistent nature of compounds [85]. These compounds are reported to have carcinogenic and mutagenic properties and are identified as priority pollutants by USEPA [85]. Ozonation is reported to be useful in degradation of 2,4-dichlorophenol (2,4-DCP), a commonly used biocide for preservation during leather production/processing. A laboratory scale study has been carried out using an ozone generator with different flowrates in the range of 100 to 400 mg ozone/hour with/without CuO/ZnO catalyst and ultrasound with 36 kHz frequency and output power of 150 W. The influence of different operation parameters such as temperature, catalysis loading rate, concentration of 2,4-dichlorophenol, etc., were studied to optimized degradation of 2,4-dichlorophenol. With ultrasound only, a maximum degradation of 28.85% was achieved, whereas combined treatment with ultrasound and ozonation gave maximum degradation of 95.66% when ZnO was used as the catalyst, and 97.03% when CuO was used as the catalyst for a 120-min treatment time. The degradation products for these combined ozonation processes are found to be nontoxic to microorganisms [85]. Another study reports the application of ozonation as pretreatment to improve the biodegradation of 2,4-dichlorophenol in wastewater along with other biodegradable organic matter. Ozonation provided to 2-l samples of various synthetic wastewaters containing 2,4-dichlorophenol using a ozone generator (oxygen feed) (WEDECO GSO30) having 6 g/h ozone flowrate, and treatment provided for 2, 5 and 10 min, the pH of wastewater samples adjusted to 9 and ozonated wastewater was further subjected to biological treatment. Ozone pretreatment results in a decreased concentration of 2,4-DCP and an increase in chloride concentration, which indicates conversion of 2,4-DCP to de-halogenated intermediates, the degradation of 2,4-DCP by ozonation follows pseudo first order kinetics, ozonation decrease toxicity of 2,4-DCP and further biodegradation is required for shorter time for degradation in case of the ozone pretreatment [106]. The further effect of ozonation as pretreatment for biodegradation of 2,4-DCP containing wastewater was explored using a pilot scale bioreactor where pre-ozonation results in improved sludge settleability and less sludge production; whereas in the case of non-ozonated 2,4-DCP containing wastewater sludge settleability was hampered [107]. In a study, ozonation is compared with photocatalysis (TiO2 photocatalyst) based advanced oxidation process and nonthermal plasma-dielectric barrier discharge (DBD) in different gases using a planar falling film reactor for degradation of 2,4-DCP (100 mg/l, experimental volume 0.5 l). The outcome of the study indicates that ozone (ozone generator-Fischer, model OZ 502/10, Germany, 130 ± 5 mg/l ozone, feed gas-oxygen) is highly effective in degradation of 2,4-DCP; however, complete mineralization is not achieved as found by TOC analysis. When ozonation is coupled with photocatalysis, complete mineralization of 2,4-DCP was achieved after 60 min of treatment [86]. Ozonation as pretreatment can improve the biodegradability of 2,4-DCP; when 100 ppm solution of 2,4-DCP is treated with ozone dose of 0.12 g/l, the BOD5/COD ratio increased from zero without treatment to 0.25 (BOD21/COD = 0.48). With pre-ozonation, 80% of TOC removal can be achieved when the solution gets treated biologically by mixing with sewage and 70% TOC removal can be achieved when 100% of the pretreated solution was further treated biologically with short hydraulic retention time between 12 to 48 h [108].
Leather preservative chlorophene was degraded by ozone, UV and UV/ozone-based advanced oxidation and studied with experimental variables such as pH, concentration of the pollutants, ozone dose and intensity of UV light. Of these, O3 and UV/ O3 can effectively degrade chlorophene. Maximum degradation can be achieved at pH of 8 and degradation increases with increase in ozone dose and intensity of UV. UV can enhance degradation of chlorophene, the pseudo-first-order rate constant found lowest, 9.8 × 10−4 min−1 for UV and 2.4 × 10−2 and 6.4 × 10−2 min−1 for ozonation and UV/Ozone treatment. The presence of another organic matter retarded degradation by 38% [109]. Biocide o-phenylphenol, a commonly used fungicide to prevent fungal attack of untanned and tanned hide and an endocrine disrupting compound degraded by ozonation and influence of pH tested. Degradation of o-phenylphenol by ozonation increases with the increase in pH, thus ozonation is an effective and quick method degradation of o-phenylphenol [110]. Leather antifungal agent 2-Mercaptobenzothiazole (MBT) was degraded by ozonation in pure water and tannery effluent in a fast and efficient manner. 2-Mercaptobenzothiazole (MBT) and its degradation products have a high affinity towards ozone, thus ozone treatment results in oxidation and partial mineralization; benzothiazole is an ozonation product which can form in concentrations of 60 mole per cent of the original concentration [111]. There are a few studies which deal with the toxicity of tannery effluent treated by ozonation. Ozonation of tannery effluent results into oxidation and partial mineralization of pollutants, and the destruction and improvement in biodegradability which can be measured by COD, BOD, TOC and DOC. The degradation of by-products can be formed which can be studied by separation and analytical techniques and toxicity of degradation products studied by biotoxicity testing. In a study, the toxicity of tannery wastewater before after ozonation was evaluated using Daphnia magna Straus and Vibrio fischeri bioassays. The study indicates a minor increase in the toxicity of effluent after ozonation for bacteria by V. fischeri bioassays, while no toxicity changed after ozonation against Daphnia magna Straus [112]. In other similar studies a slight decrease in the toxicity of tannery effluent was observed after ozonation and other treatments based on advanced oxidation [113]. A study viewed the generation of endocrine disruptive substances after ozone treatment of tannery wastewater which are qualitatively identified as nonylphenol ethoxylate (NPEO) degradation products: short chain NPEOs, nonylphenol carboxylates (NPECs) and nonylphenol (NP) by LC–MS. The study reports no increase in V. fischeri or D. magna toxicity but an increase in estrogenic potential after ozone treatment by enzyme-linked receptor assay (ELRA) [114].
7 Other Applications
7.1 Nonthermal Plasma Surface Modification of Leather for Ecofriendly Processing and Providing Functional Finish
Atmospheric pressure DBD plasma of air and ammonia gas was studied to provide surface functionalization and improvement in dyeing with natural dyes [115, 116]. Atmospheric pressure air DBD was utilized for the sterilization of goat hide, and thus the application of nonthermal plasma can be explored for leather preservation which can reduce the quantity of synthetic chemical-based antimicrobial agents [117]. Further treatment with air DBD results into better uptake of natural products; an antimicrobial finish was provided to hide/leather by treating it with bark extract of Cassia renigera and Cassia fistula [117]. Atmospheric pressure argon plasma was reported for inactivation of Syndrome coronavirus 2 (SARS-CoV-2) virus in leather and various other surfaces, thus atmospheric pressure plasma opens up new possibilities for control of COVID-19 as an efficient surface sterilization technique [118]. Antifungal properties that can be imparted to natural leather by surface modification with low temperature plasma treatment which was carried out with tetrafluoromethane (CF4), hexafluoroethane (C2F6) and perfluoropropane (C3F8) [119].
Hydrophobic coating can be carried out on leather by plasma polymerization using different HMDSO/ toluene mixture, by the introduced silicon atoms on the natural leather surface [120]. Whereas superhydrophilicity and deformation resistance can be provided to natural leather by low pressure O2/H2O plasma treatment. This improvement in hydrophilicity is due to the incorporation of large numbers of hydrophilic groups such as hydroxyl, carboxyl, amines, etc. This groups increases reaction sites for binding of metal ions or dye molecules, etc., thus improvements in metal pickup, tanning or any other subsequent processing can be achieved [121]. In a study, the surface modification of pickled goat hides was carried out using low pressure oxygen plasma which increase in roughness due to the etching effect and the incorporation of oxygen containing functional groups and improvement in hydrophilicity. This results in improved uptake of chromium in chrome tanning and thus less chromium loss in wastewater. A 10-min treatment with low pressure oxygen plasma results in maximum chrome exhaustion, and surface chrome content increased from 1.09 to 1.31% [122]. Similar results were obtained by atmospheric pressure argon corona discharge for surface modification of crust sheep leather by different time durations from 3 to 9 s, which improved re-tanning and decreased the pollution load as studied by characterization of wastewater [123].
Low pressure plasma treatment with He/O2 mixture as plasma forming gas was studied to control surface wettability, improve dyeability, and wet rubbing property, whereas a waterproof property to natural leather can be imparted by CF4 plasma [124]. Atmospheric pressure diffuse ambient air plasma was utilized for surface modification of dyed natural leather to improve wettability and adhesion of glue [125]. Surface modification of chrome tanned leather by atmospheric argon plasma improves surface wettability and improvement in dyeability of chrome tanned leather with acid and metal complex dyes [126]. Upholstery leather was provided flame retardant treatment using borax by atmospheric pressure argon plasma treatment; the plasma treatment results in the modification of surface and activation of surface by incorporating functional groups which results in better uptake of borax to impart the flame retardant property [127]. Dielectric barrier discharge was utilized to impart water repellency and flame retardant properties to leather and other materials [128]. Microwave excitation low pressure oxygen plasma is reported to enhance the adhesion of polyurethane coated leather and polyurethane foam [129]. Chrome shavings can be effectively disposed of with plasma pyro-gasification, and heavy metals from chrome shavings can be recovered by chemical pretreatment to precipitate out heavy metals from chrome shavings [130].
7.2 Ozone/Ozonation for Eco-friendly Preservation of Leather and Other Applications
Ozonation has been utilized as an eco-friendly alternative to conventional salts preservation [131] which requires 50 to 60% of common salt NaCl per unit weight of rawhide, which generates wastewater with salts concentration not less than 60,000 to 65,000 ppm. For the study, fresh goat skin was treated with ozone at a concentration of 2 g/h for 0.5 to 2 h in a specially designed reactor. The samples treated with ozone are kept in the open atmosphere and sealed bags at room temperature; ozone treatment for 30 min also can eliminate microorganisms present on raw skin, and samples could be preserved for more than two weeks. Thus, with ozonation flayed skins can be preserved for short periods, and the use of a high concentration of salts in conventional salt curing/preservation can be eliminated [132]. In a similar study, DBD-based ozone generation was utilized for preservation of goat skin; the generator was fed with oxygen gas to generate ozone at a concentration of 2 g/h and experimental goat skin was exposed to ozone for two hours. In this study, ozonation is also able to preserve the skin similarly with conventional salt curing [133]. Ozone can be utilized as ecofriendly biocides alternative for toxic and environmentally harmful chemical biocides which are added to beamhouse operations to prevent the microbial decomposition of hide. With 15-min ozonation per hour bacterial growth can be prevented, whereas 5-to-10-min ozonation is sufficient to keep the bacterial population under control where no damage to hide or skins is caused by bacteria [134]. Ozonation was also explored for sulphide-free dehairing of hides and skins; by using ozone complete dehairing can be achieved with significantly less environmental impacts compared to convention dehairing using sulphide [135]. With ozone-based dehairing, leather with similar characteristics obtained to that of conventional lime and sodium sulphide-based dehairing. The further benefits of ozone-based dehairing are more chromium absorption in tanning, more hair recovery and COD reduction in beamhouse effluents; plus reuse of effluent is possible [136].
Ozonation has been explored as an ecofriendly bleaching treatment for the decolourization of leather products. Bleaching is a necessary step for the removal of colour impurities; also for chrome tanned leather which is called wet-blue due to the blue colour imparted by chromium; conventional bleaching is a wet chemical process involving chemicals and thus environmental impacts associated with the process. Decolourization of chrome tanned leather and sheepskin dyed with black dye was carried out with ozonation using a laboratory-scale ozonator (Lundell Aquametrics, Inc.) having a 180 mg/h ozone generation capacity connected to the applicator. Ozone was produced using oxygen gas as feed, and with the ozone flowrate set to 3 l/min and treatment carried out from 3 to 30 min. An ozonation time of 30 min was found to be the optimum for decolourization of leather products [137]. Ozonation has been explored for chamois leather, which is one of the most popular and economically important leather articles, conventionally made from lamb or goat skin by the oil tanning technique. With ozonation of oil treated skins, leather products with similar characteristics to conventional chamois leather can be produced; ozonation can reduce the oxidation time to 60 min compared to an oxidation period of 10–12 days required in conventional methods [138].
8 Conclusion
The chapter provides an overview of photocatalysis, nonthermal plasma and ozonation-based technology for a reduction in the environmental impacts associated with leather production and processing. In this chapter, the leather production process was described, and the environmental impacts associated with leather production and processing were displayed. All three technologies are advanced oxidation processes with different mode of action. Photocatalysis and its variations as well as nonthermal plasma are considered as physicochemical treatments, whereas ozonation is considered as a purely chemical treatment, though the ozone generator is chiefly based on the nonthermal plasma silent discharge as air or oxygen. All three techniques were explored extensively for treatment and control of pollution from leather production and processing. As set forth in this chapter, photocatalysis, nonthermal plasma and ozonation all exhibit great potential for the control and treatment of pollution generated from leather industries. Thus, this chapter will certainly be useful to researchers and stockholders working in this field.
References
Kanagaraj J, Senthilvelan T, Panda RC, Kavitha S (2015) Eco-friendly waste management strategies for greener environment towards sustainable development in leather industry: a comprehensive review. J Clean Prod 89:1–17. https://doi.org/10.1016/j.jclepro.2014.11.013
Dixit S, Yadav A, Dwivedi PD, Das M (2015) Toxic hazards of leather industry and technologies to combat threat: a review. J Clean Prod 87:39–49. https://doi.org/10.1016/j.jclepro.2014.10.017
Tasca AL, Puccini M (2019) Leather tanning: life cycle assessment of retanning, fatliquoring and dyeing. J Clean Prod 226:720–729. https://doi.org/10.1016/j.jclepro.2019.03.335
Balakrishnan PA, Arunagiri A, Rao PG (2002) Ozone generation by silent electric discharge and its application in tertiary treatment of tannery effluent. J Electrostat 56:77–86. https://doi.org/10.1016/S0304-3886(02)00031-1
Hu J, Xiao Z, Zhou R et al (2011) Ecological utilization of leather tannery waste with circular economy model. J Clean Prod 19:221–228. https://doi.org/10.1016/j.jclepro.2010.09.018
Garg SK, Tripathi M, Srinath T (2012) Strategies for chromium bioremediation of tannery effluent. Rev Environ Contam Toxicol 217:75–140. https://doi.org/10.1007/978-1-4614-2329-4_2
GracePavithra K, Jaikumar V, Kumar PS, SundarRajan P (2019) A review on cleaner strategies for chromium industrial wastewater: present research and future perspective. J Clean Prod 228:580–593. https://doi.org/10.1016/j.jclepro.2019.04.117
Jiang H, Liu J, Han W (2016) The status and developments of leather solid waste treatment: a mini-review. Waste Manag Res 34:399–408. https://doi.org/10.1177/0734242X16633772
Lofrano G, Meriç S, Zengin GE, Orhon D (2013) Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: a review. Sci Total Environ 461–462:265–281. https://doi.org/10.1016/j.scitotenv.2013.05.004
Giaccherini F, Munz G, Dockhorn T et al (2017) Carbon and energy footprint analysis of tannery wastewater treatment: a Global overview. Water Res Ind 17:43–52. https://doi.org/10.1016/j.wri.2017.03.001
Sivaram NM, Barik D (2019) Chapter 5—Toxic waste from leather industries. In: Barik D (ed) Energy from toxic organic waste for heat and power generation. Woodhead Publishing, pp 55–67
Houshyar Z, Khoshfetrat AB, Fatehifar E (2012) Influence of ozonation process on characteristics of pre-alkalized tannery effluents. Chem Eng J 191:59–65. https://doi.org/10.1016/j.cej.2012.02.053
Sathish M, Madhan B, Raghava Rao J (2019) Leather solid waste: an eco-benign raw material for leather chemical preparation—a circular economy example. Waste Manag 87:357–367. https://doi.org/10.1016/j.wasman.2019.02.026
Pati A, Chaudhary R, Subramani S (2014) A review on management of chrome-tanned leather shavings: a holistic paradigm to combat the environmental issues. Environ Sci Pollut Res Int 21:11266–11282. https://doi.org/10.1007/s11356-014-3055-9
Sekaran G, Swarnalatha S, Dandaiah S (2007) Solid waste management in leather sector. J Des Manuf Technol 1:47–52. https://doi.org/10.18000/ijodam.70008
Kanagaraj J, kandukalpatti chinnaraj V, Babu N, Sayeed S (2006) Solid wastes generation in the leather industry and its utilization for cleaner environment. J Sci Ind Res 65:541–548. https://doi.org/10.1002/chin.200649273
de Aquim PM, Hansen É, Gutterres M (2019) Water reuse: an alternative to minimize the environmental impact on the leather industry. J Environ Manag 230:456–463. https://doi.org/10.1016/j.jenvman.2018.09.077
Rueda-Marquez JJ, Levchuk I, Fernández Ibañez P, Sillanpää M (2020) A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J Clean Prod 258:120694. https://doi.org/10.1016/j.jclepro.2020.120694
Gnanaprakasam A, Sivakumar VM, Thirumarimurugan M (2015) Influencing parameters in the photocatalytic degradation of organic effluent via nanometal oxide catalyst: a review. Ind J Mater Sci 2015:1–16. https://doi.org/10.1155/2015/601827
Jiang L, Wang Y, Feng C (2012) Application of photocatalytic technology in environmental safety. Proc Eng 45:993–997. https://doi.org/10.1016/j.proeng.2012.08.271
Litter MI (1999) Heterogeneous photocatalysis: transition metal ions in photocatalytic systems. Appl Catal B 23:89–114. https://doi.org/10.1016/S0926-3373(99)00069-7
Parul Kaur K, Badru R et al (2020) Photodegradation of organic pollutants using heterojunctions: a review. J Environ Chem Eng 8:103666. https://doi.org/10.1016/j.jece.2020.103666
Lau GE, Che Abdullah CA, Wan Ahmad WAN et al (2020) Eco-friendly photocatalysts for degradation of dyes. Catalysts 10:1129. https://doi.org/10.3390/catal10101129
Natarajan S, Bajaj HC, Tayade RJ (2018) Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J Environ Sci 65:201–222. https://doi.org/10.1016/j.jes.2017.03.011
Daghrir R, Drogui P, Robert D (2012) Photoelectrocatalytic technologies for environmental applications. J Photochem Photobiol A 238:41–52. https://doi.org/10.1016/j.jphotochem.2012.04.009
Zhang G, Zhang X, Meng Y et al (2020) Layered double hydroxides-based photocatalysts and visible-light driven photodegradation of organic pollutants: a review. Chem Eng J 392:123684. https://doi.org/10.1016/j.cej.2019.123684
Basavarajappa PS, Patil SB, Ganganagappa N et al (2020) Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int J Hydrogen Energy 45:7764–7778. https://doi.org/10.1016/j.ijhydene.2019.07.241
Li Z, Meng X, Zhang Z (2018) Recent development on MoS2-based photocatalysis: a review. J Photochem Photobiol C 35:39–55. https://doi.org/10.1016/j.jphotochemrev.2017.12.002
Oyewo OA, Elemike EE, Onwudiwe DC, Onyango MS (2020) Metal oxide-cellulose nanocomposites for the removal of toxic metals and dyes from wastewater. Int J Biol Macromol 164:2477–2496. https://doi.org/10.1016/j.ijbiomac.2020.08.074
Ismael M (2020) A review on graphitic carbon nitride (g-C3N4) based nanocomposites: synthesis, categories, and their application in photocatalysis. J Alloy Compd 846:156446. https://doi.org/10.1016/j.jallcom.2020.156446
Ajiboye TO, Oyewo OA, Onwudiwe DC (2021) Simultaneous removal of organics and heavy metals from industrial wastewater: a review. Chemosphere 262:128379. https://doi.org/10.1016/j.chemosphere.2020.128379
Tudoran C, Roşu M-C, Coroş M (2020) A concise overview on plasma treatment for application on textile and leather materials. Plasma Process Polym 17:2000046. https://doi.org/10.1002/ppap.202000046
Preethi V, Parama Kalyani KS, Iyappan K et al (2009) Ozonation of tannery effluent for removal of cod and color. J Hazard Mater 166:150–154. https://doi.org/10.1016/j.jhazmat.2008.11.035
Di Iaconi C, Ramadori R, Lopez A (2009) The effect of ozone on tannery wastewater biological treatment at demonstrative scale. Biores Technol 100:6121–6124. https://doi.org/10.1016/j.biortech.2009.06.022
Kolomaznik K, Adamek M, Andel I, Uhlirova M (2008) Leather waste—potential threat to human health, and a new technology of its treatment. J Hazard Mater 160:514–520. https://doi.org/10.1016/j.jhazmat.2008.03.070
Rameshraja D, Suresh S (2011) Treatment of tannery wastewater by various oxidation and combined processes. Int J Environ Res 5:349–360. https://doi.org/10.22059/ijer.2011.320
Litter MI (2009) Treatment of chromium, mercury, lead, uranium, and arsenic in water by heterogeneous photocatalysis. In: de Lasa HI, Serrano Rosales B (eds) Advances in chemical engineering. Academic Press, pp 37–67
Athanasekou C, Romanos GEm, Papageorgiou SK et al (2017) Photocatalytic degradation of hexavalent chromium emerging contaminant via advanced titanium dioxide nanostructures. Chem Eng J 318:171–180. https://doi.org/10.1016/j.cej.2016.06.033
Acharya R, Naik B, Parida K (2018) Cr(VI) remediation from aqueous environment through modified-TiO2-mediated photocatalytic reduction. Beilstein J Nanotechnol 9:1448–1470. https://doi.org/10.3762/bjnano.9.137
Litter MI (2017) Last advances on TiO2-photocatalytic removal of chromium, uranium and arsenic. Curr Opin Green Sustain Chem 6:150–158. https://doi.org/10.1016/j.cogsc.2017.04.002
Wang H, Wen F, Li X et al (2016) Cerium-doped MoS2 nanostructures: efficient visible photocatalysis for Cr(VI) removal. Sep Purif Technol 170:190–198. https://doi.org/10.1016/j.seppur.2016.06.049
Douafer S, Lahmar H, Benamira M et al (2019) Chromate reduction on the novel hetero-system LiMn2O4/SnO2 catalyst under solar light irradiation. Surf Interfaces 17:100372. https://doi.org/10.1016/j.surfin.2019.100372
Balu S, Chen Y-L, Juang R-C et al (2020) Morphology-controlled synthesis of α–Fe2O3 nanocrystals impregnated on g-C3N4–SO3H with ultrafast charge separation for photoreduction of Cr (VI) under visible light. Environ Pollut 267:115491. https://doi.org/10.1016/j.envpol.2020.115491
Zheng X, Liu D, Wen J, Lv S (2020) Nonthermal plasma-vulcanized flower-like ZnS/Zn-Al composites from Zn-Al layered double hydroxides for the adsorption-photo-reduction of Cr(VI). Sep Purif Technol 117934. https://doi.org/10.1016/j.seppur.2020.117934
Dakhinaray T, Dash BP, Sahoo PK et al (2020) Role of graphene nanocomposite photocatalysts in photo-reduction of Cr (VI) for wastewater treatment. Mater Today Proc. https://doi.org/10.1016/j.matpr.2020.09.335
Yang L, Xu C, Wan F et al (2019) Synthesis of RGO/BiOI/ZnO composites with efficient photocatalytic reduction of aqueous Cr(VI) under visible-light irradiation. Mater Res Bull 112:154–158. https://doi.org/10.1016/j.materresbull.2018.12.019
Sun H, Park S-J (2020) Phosphorus-doped g-C3N4/SnS nanocomposite for efficient photocatalytic reduction of aqueous Cr(VI) under visible light. Appl Surf Sci 531:147325. https://doi.org/10.1016/j.apsusc.2020.147325
Kenfoud H, Nasrallah N, Baaloudj O et al (2020) Photocatalytic reduction of Cr(VI) onto the spinel CaFe2O4 nanoparticles. Optik 223:165610. https://doi.org/10.1016/j.ijleo.2020.165610
Liang H, Li T, Zhang J et al (2020) 3-D hierarchical Ag/ZnO@CF for synergistically removing phenol and Cr(VI): heterogeneous vs. homogeneous photocatalysis. J Colloid Interface Sci 558:85–94. https://doi.org/10.1016/j.jcis.2019.09.105
Li Y-X, Wang X, Wang C-C et al (2020) S-TiO2/UiO-66-NH2 composite for boosted photocatalytic Cr(VI) reduction and bisphenol A degradation under LED visible light. J Hazard Mater 399:123085. https://doi.org/10.1016/j.jhazmat.2020.123085
Xie G, Wang H, Zhou Y et al (2020) Simultaneous remediation of methylene blue and Cr(VI) by mesoporous BiVO4 photocatalyst under visible-light illumination. J Taiwan Inst Chem Eng 112:357–365. https://doi.org/10.1016/j.jtice.2020.05.014
Meichtry JM, Brusa M, Mailhot G et al (2007) Heterogeneous photocatalysis of Cr(VI) in the presence of citric acid over TiO2 particles: relevance of Cr(V)–citrate complexes. Appl Catal B 71:101–107. https://doi.org/10.1016/j.apcatb.2006.09.002
Yang J-K, Lee S-M (2006) Removal of Cr(VI) and humic acid by using TiO2 photocatalysis. Chemosphere 63:1677–1684. https://doi.org/10.1016/j.chemosphere.2005.10.005
Liu J, Huang K, Xie K et al (2016) An ecological new approach for treating Cr(VI)-containing industrial wastewater: photochemical reduction. Water Res 93:187–194. https://doi.org/10.1016/j.watres.2016.02.025
Li Q-H, Dong M, Li R et al (2021) Enhancement of Cr(VI) removal efficiency via adsorption/photocatalysis synergy using electrospun chitosan/g-C3N4/TiO2 nanofibers. Carbohyd Polym 253:117200. https://doi.org/10.1016/j.carbpol.2020.117200
Kebir M, Chabani M, Nasrallah N et al (2011) Coupling adsorption with photocatalysis process for the Cr(VI) removal. Desalination 270:166–173. https://doi.org/10.1016/j.desal.2010.11.041
Josué TG, Almeida LNB, Lopes MF et al (2020) Cr (VI) reduction by photocatalyic process: Nb2O5 an alternative catalyst. J Environ Manag 268:110711. https://doi.org/10.1016/j.jenvman.2020.110711
Wang X, Liu W, Fu H et al (2019) Simultaneous Cr(VI) reduction and Cr(III) removal of bifunctional MOF/titanate nanotube composites. Environ Pollut 249:502–511. https://doi.org/10.1016/j.envpol.2019.03.096
Paschoal FMM, Anderson MA, Zanoni MVB (2009) Simultaneous removal of chromium and leather dye from simulated tannery effluent by photoelectrochemistry. J Hazard Mater 166:531–537. https://doi.org/10.1016/j.jhazmat.2008.11.058
Kamaraj M, Srinivasan NR, Assefa G et al (2020) Facile development of sunlit ZnO nanoparticles-activated carbon hybrid from pernicious weed as an operative nano-adsorbent for removal of methylene blue and chromium from aqueous solution: Extended application in tannery industrial wastewater. Environ Technol Innovation 17:100540. https://doi.org/10.1016/j.eti.2019.100540
Shen J, Lu Y, Liu J-K, Yang X-H (2016) Photocatalytic activity of silver chromate materials by various synthesis methods. J Exp Nanosci 11:650–659. https://doi.org/10.1080/17458080.2015.1110624
Zhao Y, Chang W, Huang Z et al (2017) Enhanced removal of toxic Cr(VI) in tannery wastewater by photoelectrocatalysis with synthetic TiO2 hollow spheres. Appl Surf Sci 405:102–110. https://doi.org/10.1016/j.apsusc.2017.01.306
Wang J-C, Ren J, Yao H-C et al (2016) Synergistic photocatalysis of Cr(VI) reduction and 4-Chlorophenol degradation over hydroxylated α-Fe2O3 under visible light irradiation. J Hazard Mater 311:11–19. https://doi.org/10.1016/j.jhazmat.2016.02.055
Jiang B, Guo J, Wang Z et al (2015) A green approach towards simultaneous remediations of chromium(VI) and arsenic(III) in aqueous solution. Chem Eng J 262:1144–1151. https://doi.org/10.1016/j.cej.2014.10.064
Chandana L, Lakshminarayana B, Subrahmanyam Ch (2015) Influence of hydrogen peroxide on the simultaneous removal of Cr(VI) and methylene blue from aqueous medium under atmospheric pressure plasma jet. J Environ Chem Eng 3:2760–2767. https://doi.org/10.1016/j.jece.2015.09.030
Chandana L, Subrahmanyam Ch (2017) Non-thermal discharge plasma promoted redox transformation of arsenic(III) and chromium(VI) in an aqueous medium. Chem Eng J 329:211–219. https://doi.org/10.1016/j.cej.2017.05.114
Long Y, Nie J, Yuan C et al (2021) Preparation of CoFe2O4/MWNTs/sponge electrode to enhance dielectric barrier plasma discharge for degradation of phenylic pollutants and Cr(VI) reduction. Appl Catal B 283:119604. https://doi.org/10.1016/j.apcatb.2020.119604
Liu Y (2009) Simultaneous oxidation of phenol and reduction of Cr(VI) induced by contact glow discharge electrolysis. J Hazard Mater 168:992–996. https://doi.org/10.1016/j.jhazmat.2009.02.116
Zhang C, Sun Y, Yu Z et al (2018) Simultaneous removal of Cr(VI) and acid orange 7 from water solution by dielectric barrier discharge plasma. Chemosphere 191:527–536. https://doi.org/10.1016/j.chemosphere.2017.10.087
Ke Z, Huang Q, Zhang H, Yu Z (2011) Reduction and removal of aqueous Cr(VI) by glow discharge plasma at the gas-solution interface. Environ Sci Technol 45:7841–7847. https://doi.org/10.1021/es201680m
Du C, Yan J (2017) Reduction and removal of Cr(VI) from aqueous solution by microplasma. In: Du C, Yan J (eds) Plasma remediation technology for environmental protection. Springer, Singapore, pp 41–59
Luo Z, Xu J, Zhu D et al (2019) Ion-imprinted polypropylene fibers fabricated by the plasma-mediated grafting strategy for efficient and selective adsorption of Cr(VI). Polymers 11:1508. https://doi.org/10.3390/polym11091508
Macedo LC, Zaia DAM, Moore GJ, de Santana H (2007) Degradation of leather dye on TiO2: a study of applied experimental parameters on photoelectrocatalysis. J Photochem Photobiol A 185:86–93. https://doi.org/10.1016/j.jphotochem.2006.05.016
Sakthivel S, Shankar MV, Palanichamy M et al (2002) Photocatalytic decomposition of leather dye: comparative study of TiO2 supported on alumina and glass beads. J Photochem Photobiol A 148:153–159. https://doi.org/10.1016/S1010-6030(02)00085-0
Maroudas A, Pandis PK, Chatzopoulou A et al (2021) Synergetic decolorization of azo dyes using ultrasounds, photocatalysis and photo-fenton reaction. Ultrason Sonochem 71:105367. https://doi.org/10.1016/j.ultsonch.2020.105367
Hasegawa MC, de Daniel JFS, Takashima K et al (2014) COD removal and toxicity decrease from tannery wastewater by zinc oxide-assisted photocatalysis: a case study. Environ Technol 35:1589–1595. https://doi.org/10.1080/09593330.2013.874499
Mohammed A (2013) BOD5 removal from tannery wastewater over ZnO-ZnFe2O4 composite photocatalyst supported on activated carbon. J Chem Eng Mater Sci 4:80–86. https://doi.org/10.5897/JCEMS2013.0165
Zhao Y, Huang Z, Chang W et al (2017) Microwave-assisted solvothermal synthesis of hierarchical TiO2 microspheres for efficient electro-field-assisted-photocatalytic removal of tributyltin in tannery wastewater. Chemosphere 179:75–83. https://doi.org/10.1016/j.chemosphere.2017.03.084
Baltes L, Patachia S, Tierean M et al (2018) Photoactive polymer-cement composites for tannins removal from wastewaters. J Environ Chem Eng 6:4357–4367. https://doi.org/10.1016/j.jece.2018.06.039
Aarthi A, Umadevi M, Parimaladevi R, Sathe GV (2019) Polyvinyl thiol assisted Ag NPs as an efficient SERS analyzer and visible light photocatalyst for tannery waste landfill leachate. Vacuum 161:125–129. https://doi.org/10.1016/j.vacuum.2018.12.022
Kumar ETD (2017) Solar light driven degradation of post tanning water at heterostructured BiVO4-ZnO mixed oxide catalyst interface. Surf Interfaces 7
Natarajan TS, Natarajan K, Bajaj HC, Tayade RJ (2013) Study on identification of leather industry wastewater constituents and its photocatalytic treatment. Int J Environ Sci Technol 10:855–864. https://doi.org/10.1007/s13762-013-0200-9
Colmenares JC, Lisowski P, Bermudez JM et al (2014) Unprecedented photocatalytic activity of carbonized leather skin residues containing chromium oxide phases. Appl Catal B 150–151:432–437. https://doi.org/10.1016/j.apcatb.2013.12.038
Telay Mekonnen B, Meiyazhagan A, Ragothaman M et al (2019) Bi-functional iron embedded carbon nanostructures from collagen waste for photocatalysis and Li-ion battery applications: a waste to wealth approach. J Clean Prod 210:190–199. https://doi.org/10.1016/j.jclepro.2018.10.319
Barik AJ, Gogate PR (2017) Degradation of 2,4-dichlorophenol using combined approach based on ultrasound, ozone and catalyst. Ultrason Sonochem 36:517–526. https://doi.org/10.1016/j.ultsonch.2016.08.017
Hama Aziz KH, Miessner H, Mueller S et al (2018) Comparative study on 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenol removal from aqueous solutions via ozonation, photocatalysis and non-thermal plasma using a planar falling film reactor. J Hazard Mater 343:107–115. https://doi.org/10.1016/j.jhazmat.2017.09.025
Favaro G, De Leo D, Pastore P et al (2008) Quantitative determination of chlorophenols in leather by pressurized liquid extraction and liquid chromatography with diode-array detection. J Chromatogr A 1177:36–42. https://doi.org/10.1016/j.chroma.2007.10.106
Zhao C, Xue L, Zhou Y et al (2020) A microwave atmospheric plasma strategy for fast and efficient degradation of aqueous p-nitrophenol. J Hazard Mater 124473. https://doi.org/10.1016/j.jhazmat.2020.124473
Ws E-S, Sa O, Aa M (2015) Deterioration to extinction of wastewater bacteria by non-thermal atmospheric pressure air plasma as assessed by 16S rDNA-DGGE fingerprinting. Front Microbiol 6:1098. https://doi.org/10.3389/fmicb.2015.01098
Rice RG (1996) Applications of ozone for industrial wastewater treatment—a review. Ozone: Sci Eng 18:477–515. https://doi.org/10.1080/01919512.1997.10382859
Mella B, Barcellos BS de C, Costa DE da S, Gutterres M (2018) Treatment of leather dyeing wastewater with associated process of coagulation-flocculation/adsorption/ozonation. Ozone Sci Eng 40:133–140. https://doi.org/10.1080/01919512.2017.1346464
Jochimsen JC, Schenk H, Jekel MR, Hegemann W (1997) Combined oxidative and biological treatment for separated streams of tannery wastewater. Water Sci Technol 36:209–216. https://doi.org/10.1016/S0273-1223(97)00389-2
Sivagami K, Sakthivel KP, Nambi IM (2018) Advanced oxidation processes for the treatment of tannery wastewater. J Environ Chem Eng 6:3656–3663. https://doi.org/10.1016/j.jece.2017.06.004
M.D SD, Genceli EA, Babuna FG, Orhon D (2004) Ozonation of nonbiodegradable organics in tannery wastewater. J Environ Sci Health Part A 39:1705–1715. https://doi.org/10.1081/ESE-120037871
Huang G, Pan F, Fan G, Liu G (2016) Application of heterogeneous catalytic ozonation as a tertiary treatment of effluent of biologically treated tannery wastewater. J Environ Sci Health Part A 51:626–633. https://doi.org/10.1080/10934529.2016.1159863
Kalyanaraman C, Kameswari KSB, Rao JR (2015) Studies on enhancing the biodegradation of tannins by ozonation and Fenton’s oxidation process. J Ind Eng Chem 25:329–337. https://doi.org/10.1016/j.jiec.2014.11.012
Rema T, Parivallal B, Ramanujam RA (2010) Studies on degradation of syntan used in leather tanning process using ozone. Int J Environ Sci Dev 1:264–267. https://doi.org/10.7763/IJESD.2010.V1.51
Thankappan R, Srinivasan SV, Suthanthararajan R, Sillanpää M (2018) Studies on removal of phenol sulfonic acid-syntan in aqueous medium using ozonation. Environ Technol 39:2434–2446. https://doi.org/10.1080/09593330.2017.1355936
Srinivasan SV, Rema T, Chitra K et al (2009) Decolourisation of leather dye by ozonation. Desalination 235:88–92. https://doi.org/10.1016/j.desal.2007.07.032
Prats D, Var P, Yagiie C (2003) Colour elimination through oxidation technologies in leather finishing industry waste waters. 65:10
Vedaraman N, Shamshath Begum S, Srinivasan SV (2013) Response surface methodology for decolourisation of leather dye using ozonation in a packed bed reactor. Clean Techn Environ Policy 15:607–616. https://doi.org/10.1007/s10098-012-0544-8
Kasiri MB, Modirshahla N, Mansouri H (2013) Decolorization of organic dye solution by ozonation; optimization with response surface methodology. Int J Ind Chem 4:3. https://doi.org/10.1186/2228-5547-4-3
Sekar ASS (2008) Removal of colour from tannery dye wastewater using ozone. Nat Environ Pollut Technol 7:4
Lambert JA, Vega MM, Isarain-Chávez E, Peralta-Hernández JM (2013) Ozone and electrocoagulation processes for treatment of dye in leather industry wastewater: a comparative study. Int J Emerg Technol Adv Eng 3:1–9
de Carvalho CB, de Franco ME, Souza FS, Féris LA (2018) Degradation of acid black 210 by advanced oxidative processes: O3 and O3/UV. Ozone Sci Eng 40:372–376. https://doi.org/10.1080/01919512.2018.1435258
Van Aken P, Van den Broeck R, Degrève J, Dewil R (2015) The effect of ozonation on the toxicity and biodegradability of 2,4-dichlorophenol-containing wastewater. Chem Eng J 280:728–736. https://doi.org/10.1016/j.cej.2015.06.019
Van Aken P, Van den Broeck R, Degrève J, Dewil R (2017) A pilot-scale coupling of ozonation and biodegradation of 2,4-dichlorophenol-containing wastewater: the effect of biomass acclimation towards chlorophenol and intermediate ozonation products. J Clean Prod 161:1432–1441. https://doi.org/10.1016/j.jclepro.2017.05.124
Contreras S, Rodrı́guez M, Momani FA et al (2003) Contribution of the ozonation pre-treatment to the biodegradation of aqueous solutions of 2,4-dichlorophenol. Water Res 37:3164–3171. https://doi.org/10.1016/S0043-1354(03)00167-2
He Z, Zhang A, Li Y et al (2011) Chlorophene degradation by combined ultraviolet irradiation and ozonation. J Environ Sci Health Part A 46:1–8. https://doi.org/10.1080/10934529.2011.526065
Olak-Kucharczyk M, Miller JS, Ledakowicz S (2012) Ozonation kinetics of o-phenylphenol in aqueous solutions. Ozone Sci Eng 34:300–305. https://doi.org/10.1080/01919512.2012.694339
Fiehn O, Wegener G, Jochimsen J, Jekel M (1998) Analysis of the ozonation of 2-mercaptobenzothiazole in water and tannery wastewater using sum parameters, liquid- and gas chromatography and capillary electrophoresis. Water Res 32:1075–1084. https://doi.org/10.1016/S0043-1354(97)00332-1
Schrank SG, Gebhardt W, José HJ et al (2017) Ozone treatment of tannery wastewater monitored by conventional and substance specific wastewater analyses. Ozone Sci Eng 39:159–187. https://doi.org/10.1080/01919512.2016.1273090
Schrank SG, José HJ, Moreira RFPM, Schröder HF (2004) Comparison of different advanced oxidation process to reduce toxicity and mineralisation of tannery wastewater. Water Sci Technol 50:329–334
Schrank SG, Bieling U, José HJ et al (2009) Generation of endocrine disruptor compounds during ozone treatment of tannery wastewater confirmed by biological effect analysis and substance specific analysis. Water Sci Technol 59:31–38. https://doi.org/10.2166/wst.2009.762
Dave H, Ledwani L, Nema SK (2016) Surface modification by atmospheric pressure air plasma treatment to improve dyeing with natural dyes: an environment friendly approach for leather processing. Plasma Chem Plasma Process 36:599–613. https://doi.org/10.1007/s11090-015-9687-9
Dave H, Ledwani L, Nema SK (2017) Improvement in natural dyeing with the aid of atmospheric pressure plasma treatment: a green solution for leather processing. Curr Environ Eng 4:140–149. https://doi.org/10.2174/2212717804666161208125652
Vajpayee M, Singh M, Dave H et al (2020) Antimicrobial finishing of hide/leather by atmospheric pressure plasma and extracts of Cassia renigera and Cassia fistula bark. Rend Fis Acc Lincei 31:1105–1116. https://doi.org/10.1007/s12210-020-00954-2
Chen Z(陈支通), Garcia G, Arumugaswami V, Wirz RE (2020) Cold atmospheric plasma for SARS-CoV-2 inactivation. Phys Fluids 32:111702. https://doi.org/10.1063/5.0031332
Ando H, Kataoka S, Kuwata M et al (1998) Low temperature fluorocarbon gas plasma treatment and fungal resistance on leather. J Japan Soc Colour Mater 71:91–99. https://doi.org/10.4011/shikizai1937.71.91
Kayaoğlu BK, Öztürk E (2013) Imparting hydrophobicity to natural leather through plasma polymerization for easy care effect. Fibers Polym 14:1706–1713. https://doi.org/10.1007/s12221-013-1706-y
You X, Gou L, Tong X (2016) Improvement in surface hydrophilicity and resistance to deformation of natural leather through O2/H2O low-temperature plasma treatment. Appl Surf Sci 360:398–402. https://doi.org/10.1016/j.apsusc.2015.11.030
Jiang Y, Li J, Liu F et al (2019) The effects of surface modification using O2 low temperature plasma on chrome tanning properties of natural leather. J Ind Text 49:534–547. https://doi.org/10.1177/1528083718804205
Açıkel S, Çelik C, Kaygusuz M, Aslan A (2020) Effect of argon atmospheric pressure plasma (APP) treatment on the properties of sheep leathers and wastewater oft he retanning processes. J Soc Leather Technol Chem 104:120–129
Choi JH, Lee ES, Baik HK et al (2003) Surface modification of natural leather using low-pressure parallel plate plasma. Surf Coat Technol 171:257–263. https://doi.org/10.1016/S0257-8972(03)00282-2
Štěpánová V, Kelar J, Slavíček P et al (2017) Surface modification of natural leather using diffuse ambient air plasma. Int J Adhes Adhes 77:198–203. https://doi.org/10.1016/j.ijadhadh.2017.05.004
Acikel S, Aslan A, Oksuz L, Aktan T (2013) Effects of atmospheric pressure plasma treatments on various properties of leathers. J Am Leather Chem Ass 108:266–276
Açıkel S meriç (2019) Investigation of flame retardancy of Borax in upholstery leather modified with atmospheric pressure plasma. Eur J Sci Technol 17:844–851. https://doi.org/10.31590/ejosat.641656
Gaidau C, Surdu L, Niculescu M et al (2017) Research on cold plasma treatment of leather and fur based materials as ecological alternative. Industria textilă 68. https://doi.org/10.35530/IT.068.05.1365
Seul SD, Lim JM, Ha SH, Kim YH (2005) Adhesion enhancement of polyurethane coated leather and polyurethane foam with plasma treatment. Korean J Chem Eng 22:745–749. https://doi.org/10.1007/BF02705793
Pietrelli L, Ferro S, Reverberi AP, Vocciante M (2020) Removal and recovery of heavy metals from tannery sludge subjected to plasma pyro-gasification process. J Clean Prod 273:123166. https://doi.org/10.1016/j.jclepro.2020.123166
Wu J, Zhao L, Liu X et al (2017) Recent progress in cleaner preservation of hides and skins. J Clean Prod 148:158–173. https://doi.org/10.1016/j.jclepro.2017.01.113
Sivakumar V, Balakrishnan PA, Muralidharan C, Swaminathan G (2010) Use of ozone as a disinfectant for raw animal skins—application as short-term preservation in leather making. Ozone Sci Eng 32:449–455. https://doi.org/10.1080/01919512.2010.515524
Vaduganathan L (2017) The ozone treatment for elimination of toxic waste—an alternate for preservation of goat skins and enhancement of bleaching property of starch. 1 13:6005–6010. https://doi.org/10.24297/jac.v13i11.5883
MehmetM Mutlu, Cadircl BH, Ozgunay H et al (2009) Ozone as a biocide in soaking. J Soc Leather Technol Chem 93:18–20
Sundar VJ, Vedaraman N, Balakrishnan PA et al (2006) Sulphide free unhairing–studies on ozone based depilation. J Am Leather Chem Assoc 101:231–234
Balakrishnan PA, Vedaraman N, Rangasamy T, Muralidharan C (2006) An eco-benign approach for unhairing goat skin using ozone. Ozone Sci Eng 28:341–346. https://doi.org/10.1080/01919510600900274
Onem E, Yorgancioglu A, Namirti G et al (2017) Ozonation as an environmentally friendly method to decolorize the leather products. Ozone Sci Eng 39:454–461. https://doi.org/10.1080/01919512.2017.1322487
Sundar VJ, Muralidharan C, Sadulla S (2007) Research note: ozonation—an emerging inevitable option for chamois making. Ozone Sci Eng 29:405–409. https://doi.org/10.1080/01919510701573434
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dave, H., Vajpayee, M., Ledwani, L. (2022). Photocatalytic and Other Similar Green Technologies for Reducing Environmental Impacts of Leather Industries. In: Garg, S., Chandra, A. (eds) Green Photocatalytic Semiconductors. Green Chemistry and Sustainable Technology. Springer, Cham. https://doi.org/10.1007/978-3-030-77371-7_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-77371-7_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-77370-0
Online ISBN: 978-3-030-77371-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)