Abstract
In the vertebrate tree of life, viviparity or live birth has independently evolved many times, resulting in a rich diversity of reproductive strategies. Viviparity is believed to be a mode of reproduction that evolved from the ancestral condition of oviparity or egg laying, where most of the fetal development occurs outside the body. Today, there is not a simple model of parity transition to explain this species-specific divergence in modes of reproduction. Most evidence points to a gradual series of evolutionary adaptations that account for this phenomenon of reproduction, elegantly displayed by various viviparous squamates that exhibit placentae formed by the appositions of maternal and embryonic tissues, which share significant homology with the tissues that form the placenta in therian mammals. In an era where the genomes of many vertebrate species are becoming available, studies are now exploring the molecular basis of this transition from oviparity to viviparity, and in some rare instances its possible reversibility, such as the Australian three-toed skink (Saiphos equalis). In contrast to the parity diversity in squamates, mammals are viviparous with the notable exception of the egg-laying monotremes. Advancing computational tools coupled with increasing genome availability across species that utilize different reproductive strategies promise to reveal the molecular underpinnings of the ancestral transition of oviparity to viviparity. As a result, the dramatic changes in reproductive physiology and anatomy that accompany these parity changes can be reinterpreted. This chapter will briefly explore the vertebrate modes of reproduction using a phylogenetic framework and where possible highlight the role of potential candidate genes that may help explain the polygenic origins of live birth.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Charles Darwin’s theory of evolution describes a process of natural selection by which allows species to adapt to their environment enhancing reproductive success and survival. In vertebrates, reproductive strategies can be uniform in some lineages and remarkably diverse and flexible in others. Fortunately evolution has left a tantalizing series of clues as to how ancestral physiological, morphological, biochemical, and molecular networks were coopted that led to the species-specific forms of reproductive parity. Firstly, before investigating these hints of nature, simply reproduction can be sexual or asexual, the later a rarity in vertebrates. Decades of literature debate asexual origins, meiotic drivers, and disadvantages to that of sexual reproduction (Fu et al. 2019; Zanders and Unckless 2019) and both modes of reproduction can be associated with viviparity or oviparity. However, the focus of this brief chapter will be the nourishing of a growing fetus by either a viviparous or oviparous system that has left a fascinating legacy of how evolution reshaped the genetic blueprints of life “genomes” toward a preferential mode of parity, oviparity versus viviparity, in particular when altered gene signatures suggest an association with placental development and function.
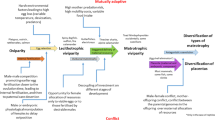
Viviparity, in which eggs are fertilized and embryos develop inside the maternal reproductive tract or body cavity, as opposed to oviparity, in which females deposit fertilized eggs that develop outside of the parent, can differ in even closely related species. The most frequent examples in nature are squamates. A vast literature strongly suggests that viviparous phenotypes, such as sophisticated and diverse placental structures, have evolved convergently from oviparity many times in nature (Blackburn 1999, 2015). Although, evidence in squamates suggests more malleable modes of parity (Blackburn 2015), viviparity is predominant and nearly exclusive in mammals where placental biology has been most studied (Fig. 1). Still, there is no definitive answer as to why viviparity is almost exclusive in certain vertebrate clades, mammals, while it is overwhelmingly oviparity in others, for example, birds, amphibians, and fishes.
A number of theories have been offered to explain the evolution of this phylogenetic distribution of parity. A species range of mobility and territory span, e.g., limited range mammals and the far spanning habitat of birds, is one such contributing factor that has possibly led to separate clades of parity. However, at least some species in nearly all bird families stay in the same place all year, thus avoiding the rigors of migration. The great horned owl is one such example. Moreover, before human habitat disruption occurred, many mammal species migrated and even today some still do in the continent of Africa. A species mobility does allow the placement of the offspring in the best sites for growth, such as the migration of wild ungulates to rich sources of vegetation phenology. But in viviparity, the developing embryos are protected internally and physiologically maintained with mobility and nutritional costs to the mother. Migratory movements of terrestrial mammals are a phenomenon critical to their persistence, wherein some ungulate species prior to domestication, seasonally migrated to enhance lifetime reproductive fitness. Some hypotheses suggest this movement dependence on fluctuations in food supply propels as yet an unknown rapid, non-genomic adaptive mechanism that better fits offspring environment (nutritional), thus giving them a reproductive advantage (Sharpe 2018).
A number of preferential and detrimental attributes are associated with oviparity as well. Multiple offspring offset the higher mortality accompanying the external exposure of eggs to predators and their exposure to changing environment (weather, temperature). Higher offspring numbers also serves in increasing germline recombination events, one key process of a species genetic adaptation to the changing environment. Consequently, some genomic regions of reproductive mode relevance are likely subject to intense natural selection or alternatively have evolved sequence structures, such as CG dinucleotide expansions, that allow more fluid gene regulatory modifications. One can summarize these observed “reproductive strategies,” as having advantages as well as disadvantages that affect their species-specific evolution (Table 1).
Genome-wide studies have sought sequence structures, mostly genes and their pathways, that display shared features, across species that evolved viviparity or oviparity. As organisms naturally experiment with these new sequence variants, new species emerge and continue to evolve. In the 1850s, when Darwin described this engine of natural selection, the underlying molecular mechanisms were unknown. But over the past century, advances in genetics and molecular biology have outlined a modern, neo-Darwinian theory of how evolution works: DNA sequences randomly mutate, and organisms with the specific sequences best adapted to the environment will multiply and prevail or disappear. In an era of many vertebrate genomes being available, each with unique reproductive strategies, newly developed computational methods, such as the use of pangenome variant graphs (Hickey et al. 2020), may uncover the complex structural changes that occurred along the oviparity to viviparity trajectory. Not unexpectedly gene expression changes play a role in likely differentiating parity modes (Foster et al. 2020; Gao et al. 2019). Some have been closely associated with viviparity prominently genomic imprinting in mammals (Kaneko-Ishino and Ishino 2019). When within species, occurrence of both oviparous and viviparous reproductive strategies naturally exist, such as the lizard species of Saiphos equalis (Smith and Shine 1997), and the resolution of genetic change is even more pronounced. Even seasonal shifts along a oviparity-viviparity continuum in some lizard populations suggest a novel advantage to viviparity under these unique conditions (Shine et al. 2018).
Viviparity has its independent origins across a broad span of the vertebrate phylogenetic tree, including bony and cartilaginous fishes, amphibians, mammals, and squamate reptiles (Fig. 1). Hundreds of studies have documented the anatomical and physiological features of viviparity and oviparity and debated their origins. Is oviparity ancestral, what are the molecular mechanisms responsible for the evolution of viviparity, and are these molecular mechanisms the same for separate origins of viviparity? Thus far, very few studies have asked the question what are the fundamental molecular networks that species have used to evolve to a viviparity state, and possibly reverse itself within certain clades (Gao et al. 2019). New comparative studies at species, genus, and family levels are needed if we are to find the essential genetic components related to the extraordinary reproductive strategy of viviparity. Such transitions of oviparous to viviparous modes of reproduction require many adaptations including reduction in eggshell construction, delayed oviposition, altered maternal immune system responses, not to mention the placenta development of intricate blood supply for fetal nutrition and gas exchange. These questions are best considered using examples of specific transitions among closely-related squamates, fishes, amphibians, and mammals. Each of these life-bearing strategies have been studied from the standpoints of morphology, physiology, endocrinology, ecology, and evolution. In this brief exploration of viviparous origins prior to the terrestrial life of vertebrates, we appropriately look back over 400 million years ago and start with the fishes.
2 Fishes
Among the estimated 32,672 species of fishes (Froese and Pauly 2018), viviparity is a rarity, with approximately 510 classified as viviparous (Parenti and Grier 2004). In addition, some species display an intermediate ovoviviparous mode of reproduction where nutrient supply is deposited before fertilization (lecithotropy) (Macfarlane and Bowers 1995) which allows contrasts to matrotrophic species which have extensive maternal nourishment. In fishes, viviparity is clearly a specialized phenotype, again likely an oviparity derived mode of reproduction. In some viviparous fishes, novel embryo provisioning processes have been observed when compared to the nutrient partitioning in eutherian mammals by blood plasma passing to the fetus via the placenta and umbilical cord (Bell and Ehrhardt 2002). The most studied is the process of vitellogenesis, a provisioning of vitellogenin (vtg) proteins during embryonic development. These yolk protein nutrients are lost in eutherian mammals. Some viviparous fish species have maintained the vtg proteins that provision the yolk protein in oviparous species, and a large amount of yolk nutrient is contained in their eggs (Sawaguchi et al. 2005; Vega-Lopez et al. 2007). The bony fishes of the teleost family Goodeidae are viviparous, but unlike most all other viviparous species have not lost the vtg proteins. In one member of Goodeidae, Xenotoca eiseni, the vtg proteins are suggested to be a contributing matrotrophic factor supplied to the intraovarian embryo, a nourishing mechanism lost in a majority of viviparous vertebrates. For all other viviparous fishes, the vtg genes have been lost through a coevolution mechanism with casein genes (Brawand et al. 2008).
The Poecilid fishes, guppies and mollies, offer an altogether different dichotomy, of placental evolution a clade of entirely live-bearing species. As a result, a theory of genomic conflict has been deliberated, that is the viviparity-driven conflict hypothesis where the variation in placentation among Poecilia is proposed to effect rates of male sexual selection and drive greater speciation (Furness et al. 2019). Pollux et al. (2014), empirically tested if the evolution of the placenta was driving sexual selection in live-bearing fish and concluded that there are no male sexual traits that effect the evolution of the placenta (Pollux et al. 2014); instead a more complex interaction between female reproductive mode, male sexual selection, and the rate of speciation is a better working model (Furness et al. 2019). With high-quality genome assemblies now available for members of the viviparous Poecilid genus, advancing computational methods can delineate gene expansion and contraction more accurately as well as lineage-specific changes among putative regulatory sequences to differentiate this evolved state of viviparity.
More recently, genomic and transcriptomic analysis revealed remarkable analogies in the evolution of live-bearing rockfish (Sebastes schlegelii) (He et al. 2019). In these fish, sperm storage precedes a fertilization lengthy development of the embryo in the ovary and the ovarian wall has a presumed role similar to the mammalian uterus. Remarkably during implantation and development in the ovary, an upregulation of a number of genes involved in placentation, cell adhesion, invasion, and blood vessel function as well as oxygen and nutrient transfer occurs. This revealed that zona pellucida proteins function to retain sperm while metalloproteinases, such as high choriolysin enzyme (hce1-like) that is also described as hatchling enzyme, may play a role in releasing the embryo from the chorion at pre-hatching. In this first genome-wide study of an unusual viviparous teleost, sperm storage and development of the fry in the ovary suggests a selective advantage over egg-laying due to unpredictable and seasonal changes in nutrient supply. Only continued research of other rare viviparous fishes will further improve our comprehension of live-birth rationale.
3 Amphibians
A viviparous mode of reproduction has evolved independently in all three orders of amphibians—salamanders, frogs, and caecilians. Of the approximately 8000 amphibian species, a vast majority of them are egg layers. The few instances of viviparity are mostly confined to several species of caecilians, although a few frogs and the fire salamander enjoy this distinction. The study of the first “live-bearing” Asian amphibian, Gegeneophis seshachari, emphasizes the need to probe deeper into their phylogenetic reproductive strategies since it is the only known caecilian genus to have both oviparous and viviparous species (Gower et al. 2008). Anurans feature an extraordinary variety of reproductive modes and parental care for offspring, for example, gastric brooding (Haddad and Prado 2005). As in other vertebrates, the modes of reproduction are developing eggs retained in the oviducts, oviparity, and viviparity that include oviductal retention of developing embryos with the young born as tadpoles (Iskandar et al. 2014), metamorphosed juveniles, or froglets (Wells 2008). Then again out of the ordinary reproductive approaches in amphibians are observed in intraoviductal cannibalism and retention of externally fertilized ova in the skin of the mother’s back. Other stark parity twists are also observed in amphibians. The diversity of amphibian oviduct structure and function is a furtherance of this reproduction epitome (Wake and Dickie 1998). Oviduct morphology is regionally differentiated to secrete varying products that encompass the egg that across all three orders offer species-specific avenues of investigation into ancestral transitions of oviparity to varying forms of viviparity. Our understanding of the developmental and functional bases for fetal adaptations in amphibians is undeveloped (Wake 2015) as very few studies have attempted to identify genes and pathways involved in parity transition in amphibia. Jantra et al. (2007) analyzed immune gene expression patterns in amphibia with different parity modes and found differential expression possibly linked with viviparity as one example.
4 Reptiles
Squamata reptiles include snakes and lizards, representing over 10,700 extant species (www.reptile-database.org) with vastly differing modes of reproduction. The exclusive use of oviparity in some reptile branches exclude any insight about molecular origins of viviparity. In birds as descendants of the dinosaur, oviparity is the only reproductive strategy but with remarkable post egg-laying oddities, such as brood parasitism behavior in some lineages (Feeney and Riehl 2019). Interestingly, a live-bearing species of Archosauromorpha reptile that is today represented by birds and crocodilians suggests an estimated transition to live birth occurred some 50 million years earlier in this clade (Liu et al. 2017). Still, within squamate reptiles, oviparity has transitioned to viviparity over 100 times (Blackburn 2015) that is astounding against a total of ~140 origins across all vertebrates (Blackburn 2000). This makes squamates a prime lineage to investigate the evolution of viviparity from oviparity that is generally thought to originate with lengthened periods of egg retention. This derived nature of viviparity is considered irreversible but theoretical models of its evolution, particularly among squamates, are not always steadfast (Pyron and Burbrink 2015). The reasoning for so-called “switchbacks” among squamates contribute to this debate (Blackburn 2015). Simulations of phylogenetic origins for viviparity are complicated to broadly interpret due to at least ten species that practice reproductive bimodality and imply parity mode is a malleable trait (Pyron and Burbrink 2014). However, there are unifying trait features such as eggshells that significantly share species-wide structure and their oviduct glands that synthesize egg fibers, thus providing strong predictive evidence that oviparity is ancestral. Even so, current squamate genomic studies are just now endeavoring to grasp the beginnings of viviparity.
Some closely related lizards within the genus Phrynocephalus are oviparous and viviparous. With an estimated divergence time of 13 million years for this monophylogentic clade of viviparous, lizard species compared to their oviparous relatives’ gene expression differences in the maternal oviduct throughout development of the eggs and embryos were explored (Gao et al. 2019). As a result, numerous stage-specific gene regulatory differences were found in both species. A stasis in viviparous gene expression through birth was suggested to encourage prolonged uterine gene expression as opposed to rapid shifts seen in oviparous species. Only 3 of 148 oviparous differentially expressed genes that were shared in the viviparous lizard showing these genes are downregulated in viviparous lizards. Importantly, changes in the underlying gene sequences, a possible result of natural selection, were not found thus suggesting gene expression is the major factor associated with parity differences in these lizard species. Very few candidate genes have been proposed to be associated with placenta development and stability, some include Hβ58 (Paulesu et al. 2001), various proteases (Cornetti 2018), estrogen receptor (Gao et al. 2019), and a variety of its effected genes (Eyster 2016) and the cadherins role in attachment maintenance (Wu et al. 2011). Of these candidates, estrogen’s role in modulating viviparity is the most widely studied (Huet-Hudson et al. 1990; Fujimoto et al. 2005). A recent transcriptome analysis between viviparous and oviparous populations of the lizard Saiphos equalis provided a unique opportunity to study the transition between parity modes. The long egg retention populations showed vastly different transcriptome profiles compared to oviparous lizards supporting the idea that long egg retention is an intermediate towards viviparity (Foster et al. 2020). Candidate squamate genes associated with viviparous origins in these few studies offer a unique avenue in understanding how evolution reworked gene networks toward one parity type or the other among amniotes (Fig. 2). As in mammals, the viviparous squamate develops placental membranes that interact with adjacent uterine tissues responsible for adhesion to the uterus, respiratory gas exchange, water transport, and nutrient transport justifying additional comparative investigations (Fig. 2).
Extraembryonic membranes of viviparous reptiles and mammals. Both develop membranes that form a placenta during pregnancy to serve the nutritional needs of the developing embryo and fetus. Many ancestral genes, a subset listed here, have been putatively repurposed to facilitate viviparous transitions across vertebrate phylogenies. (Figure modified with permission from shmoop.com)
5 Mammals
In mammals, the only lineage that evolved or retained oviparity is the monotremes (platypus and echidna). Other mammals feature life birth at different stages, and if eggs and their prolonged retention are an ancient precursor to the mammalian placenta, the marsupials represent an ideal intermediate group with a more simplified intrauterine development for the study of evolutionary paths to eutherian placental structures (Frankenberg and Renfree 2018). Confusion can arise though in the characterization of mammalian placental biology. Eutherian mammals sometimes mistakenly classified as placental mammals are to the exclusion of marsupials and monotremes while in fact all three major groups of mammals feature placentae. Guernsey et al. (2017) describe the marsupial placenta that similarly mediates early embryonic development but despite a shared mechanism of nourishing the infant. Even in the egg-laying monotreme, the embryo is supported by a simple placenta during early development. Both the monotreme hatchling and marsupial newborn rely on a far more complex repertoire of milk proteins for further development. In many viviparous species, the mother provides nutrients to the embryo during gestation, a pattern known as “matrotrophy.” Yet, when observing the life history of mammalian viviparity, at the base of mammalian phylogeny one enigma remains. Only five species of the 5416 extant mammals are not viviparous, the oviparous monotremes of echidna (four extant species) and platypus. The discovery of egg-laying in platypus and echidna was a sensation among European Zoologists and was remarkably reported on the same day in 1884 by Haake in echidna and Caldwell in platypus. The various attributes of the monotremes and how their oviparous mode of reproduction, as well as many other unexpected phenotypes, conflict with existing theories of species evolution remains under study. The platypus genome was a harbinger of evidence about what the ancient mammal-like reptile genomes may have lost in the burst of eutherian species. The ancestral descent of known physiological systems that were counterintuitive, such as the ability to lay eggs (Warren et al. 2008), was revealed in a collection of studies. The remnants of genes that are associated with venom (Whittington et al. 2009), lactation, egg-laying, testes descent, sensory gene evolution, gastric function (Ordonez et al. 2008), immune gene family expansions (Whittington et al. 2008), jumping genes (retrotransposed elements), and many others were characterized (Warren et al. 2008). Despite the variety of mammalian placental types and differences among viviparous squamates, all are derived from the trophoectodermal layer of cells in the developing embryo and unique gene adaptations offer partial explanations (Fig. 2). In a search for genes that drive the trophoectoderm differentiation, a precursor to the functioning placenta, the oviparous platypus has offered some transcription factors that putatively control trophoectoderm destiny, specifically POU domain transcription factors (Niwa et al. 2008; Frankenberg et al. 2010). More comparative studies will be needed to firmly associate this unique monotreme regulatory sequence as a key precursor in directing cellular differentiation toward placental structures that establish uterine nourishment of embryos. Other derived eutherian genic examples of placental support also exist. For instance, the proper cis-regulatory gene regulation of prolactin in endometrial cells by HoxA-11 transcription factors is necessary for proper placentation. These maternal stemmed cis-regulatory elements that control prolactin endometrial expression are exclusive to eutherian mammals (Lynch et al. 2008). Some studies suggest that inflammation has been repurposed as a way for the mother and fetus to communicate. Only eutherians display a dramatic downregulation of inflammation after implantation, thus averting premature parturition (Chavan et al. 2017). Gene duplication events after the divergence of eutherians for the Tripartite-motif family-like genes, in particularly the unique eutherian existence of TRIML2, suggests a specific role in the control of placental induced inflammation (Zhang et al. 2020). Also, the parent of origin-dependent monoallelic gene expression or genomic imprinting, which is seen as an important driver of placentation and viviparity on mammals, is absent in monotremes (Keverne 2014). The candidate gene examples noted here and others validate the vibrant use of the marsupial and monotreme genomes to further define the origins of mammalian placental complexity.
6 Conclusion
The evolutionary transition from oviparity to viviparity is now better understood and can occur in intermediate steps via internal incubation of the eggs or more or less extensive nourishment through placental tissues. Vertebrates offer a variety of evolutionary solutions to balancing fecundity, number of offspring, and environmental uncertainty with the challenges of internal development of the fetus (Amoroso 1952). While many gene signaling pathways involved in adhesion, invasion, immunosuppression, and nutrient and gas exchange have been associated with viviparity, different genes among them have been recruited to support parity. Together, a compendium of data supports the evolution of viviparity allowed for the gradual loss of yolk-dependent nourishment of the developing fetus. Illustrations of oviparous coupled genes and their partnering molecular networks that collectively have evolved toward a role in sustaining the developing fetus via the placenta offer exciting expansions in parity knowledge. On the other hand, the immense complexity observed when comparing oviparous and viviparous gene systems intimates uncertainty in this broad statement. Continuing studies that offer compelling counter arguments of parity beginnings will hopefully systemize the gene networks potentially utilized to achieve the appropriate level of reproductive success. Certainly, with many high-quality genomes now available for various oviparous and viviparous species, scientists are empowered to test many gene centric theories on the molecular plan deployed to confer one mode of reproduction over another. Perhaps E.C. Amoroso summarized it simplistically and best when asked to define the placental features of mammals that distinguish them from lower vertebrates when he noted one key word—“protection” and then followed “This protection conferred by viviparity reaches its climax in mammals.”
References
Amoroso EC (1952) Placentation. In: Parkes AS (ed) Marshall’s physiology of reproduction, vol 2, 3rd edn. Longmans, London, pp 127–311
Bell AW, Ehrhardt RA (2002) Regulation of placental nutrient transport and implications for fetal growth. Nutr Res Rev 15(2):211–230
Blackburn DG (1999) Viviparity and oviparity: evolution and reproductive strategies. In: Encyclopedia of reproduction. Academic Press, New York, pp 994–1003
Blackburn DG (2000) Classification of the reproductive patterns of amniotes. Herpetol Monogr 14:371–377
Blackburn DG (2015) Evolution of viviparity in squamate reptiles: reversibility reconsidered. J Exp Zool B Mol Dev Evol 324(6):473–486
Brawand D, Wahli W, Kaessmann H (2008) Loss of egg yolk genes in mammals and the origin of lactation and placentation. PLoS Biol 6(3):e63
Chavan AR, Griffith OW, Wagner GP (2017) The inflammation paradox in the evolution of mammalian pregnancy: turning a foe into a friend. Curr Opin Genet Dev 47:24–32
Cornetti LEA (2018) Candidate genes involved in the evolution of viviparity: a RAD sequencing experiment in the lizard Zootoca vivipara (Squamata: Lacertidae). Zool J Linnean Soc 183:196–207
Eyster KM (2016) The estrogen receptors: an overview from different perspectives. Methods Mol Biol 1366:1–10
Feeney WE, Riehl C (2019) Monogamy without parental care? Social and genetic mating systems of avian brood parasites. Philos Trans R Soc Lond Ser B Biol Sci 374(1769):20180201
Foster CSP, Thompson MB, Van Dyke JU, Brandley MC, Whittington CM (2020) Emergence of an evolutionary innovation: gene expression differences associated with the transition between oviparity and viviparity. Mol Ecol 29(7):1315–1327
Frankenberg S, Renfree MB (2018) Conceptus coats of marsupials and monotremes. Curr Top Dev Biol 130:357–377
Frankenberg S, Pask A, Renfree MB (2010) The evolution of class V POU domain transcription factors in vertebrates and their characterisation in a marsupial. Dev Biol 337(1):162–170
Froese R, Pauly D (2018) Fish base. World Wide Web Electronic Publication
Fu C, Coelho MA, David-Palma M, Priest SJ, Heitman J (2019) Genetic and genomic evolution of sexual reproduction: echoes from LECA to the fungal kingdom. Curr Opin Genet Dev 58–59:70–75
Fujimoto J, Nakagawa Y, Toyoki H, Sakaguchi H, Sato E, Tamaya T (2005) Estrogen-related receptor expression in placenta throughout gestation. J Steroid Biochem Mol Biol 94(1–3):67–69
Furness AI, Pollux BJA, Meredith RW, Springer MS, Reznick DN (2019) How conflict shapes evolution in poeciliid fishes. Nat Commun 10(1):3335
Gao W, Sun YB, Zhou WW, Xiong ZJ, Chen L, Li H, Fu TT, Xu K, Xu W, Ma L, Chen YJ, Xiang XY, Zhou L, Zeng T, Zhang S, Jin JQ, Chen HM, Zhang G, Hillis DM, Ji X, Zhang YP, Che J (2019) Genomic and transcriptomic investigations of the evolutionary transition from oviparity to viviparity. Proc Natl Acad Sci U S A 116(9):3646–3655
Gower DJ, Giri V, Dharne MS, Shouche YS (2008) Frequency of independent origins of viviparity among caecilians (Gymnophiona): evidence from the first ‘live-bearing’ Asian amphibian. J Evol Biol 21(5):1220–1226
Guernsey MW, Chuong EB, Cornelis G, Renfree MB, Baker JC (2017) Molecular conservation of marsupial and eutherian placentation and lactation. Elife 6
Haddad CFB, Prado CPA (2005) Reproductive modes in frogs and their unexpected diversity in the Atlantic forest of Brazil. Bioscience 55:207–217
He Y, Chang Y, Bao L, Yu M, Li R, Niu J, Fan G, Song W, Seim I, Qin Y, Li X, Liu J, Kong X, Peng M, Sun M, Wang M, Qu J, Wang X, Liu X, Wu X, Zhao X, Wang X, Zhang Y, Guo J, Liu Y, Liu K, Wang Y, Zhang H, Liu L, Wang M, Yu H, Wang X, Cheng J, Wang Z, Xu X, Wang J, Yang H, Lee SM, Liu X, Zhang Q, Qi J (2019) A chromosome-level genome of black rockfish, Sebastes schlegelii, provides insights into the evolution of live birth. Mol Ecol Resour 19(5):1309–1321
Hickey G, Heller D, Monlong J, Sibbesen JA, Siren J, Eizenga J, Dawson ET, Garrison E, Novak AM, Paten B (2020) Genotyping structural variants in pangenome graphs using the vg toolkit. Genome Biol 21(1):35
Huet-Hudson YM, Chakraborty C, De SK, Suzuki Y, Andrews GK, Dey SK (1990) Estrogen regulates the synthesis of epidermal growth factor in mouse uterine epithelial cells. Mol Endocrinol 4(3):510–523
Iskandar DT, Evans BJ, McGuire JA (2014) A novel reproductive mode in frogs: a new species of fanged frog with internal fertilization and birth of tadpoles. PLoS One 9(12):e115884
Jantra S, Bigliardi E, Brizzi R, Ietta F, Bechi N, Paulesu L (2007) Interleukin 1 in oviductal tissues of viviparous, oviparous, and ovuliparous species of amphibians. Biol Reprod 76(6):1009–1015
Kaneko-Ishino T, Ishino F (2019) Evolution of viviparity in mammals: what genomic imprinting tells us about mammalian placental evolution. Reprod Fertil Dev 31(7):1219–1227
Keverne EB (2014) Mammalian viviparity: a complex niche in the evolution of genomic imprinting. Heredity (Edinb) 113(2):138–144
Liu J, Organ CL, Benton MJ, Brandley MC, Aitchison JC (2017) Live birth in an archosauromorph reptile. Nat Commun 8:14445
Lynch VJ, Tanzer A, Wang Y, Leung FC, Gellersen B, Emera D, Wagner GP (2008) Adaptive changes in the transcription factor HoxA-11 are essential for the evolution of pregnancy in mammals. Proc Natl Acad Sci U S A 105(39):14928–14933
Macfarlane R, Bowers M (1995) Matrotrophic viviparity in the yellowtail rockfish Sebastes flavidus. J Exp Biol 198:1197–1206
Niwa H, Sekita Y, Tsend-Ayush E, Grutzner F (2008) Platypus Pou5f1 reveals the first steps in the evolution of trophectoderm differentiation and pluripotency in mammals. Evol Dev 10(6):671–682
Ordonez GR, Hillier LW, Warren WC, Grutzner F, Lopez-Otin C, Puente XS (2008) Loss of genes implicated in gastric function during platypus evolution. Genome Biol 9(5):R81
Parenti LR, Grier HJ (2004) Evolution and phylogeny of gonad morphology in bony fishes. Integr Comp Biol 44(5):333–348
Paulesu L, Cateni C, Romagnoli R, Chellini F, Angelini F, Guarino FM, Rider V, Imakawa K, Bigliardi E (2001) Evidence of H beta 58, a gene involved in mammalian placental development, in the three-toed skink, Chalcides chalcides (Squamata: Scincidae), a viviparous placentotrophic reptile. Placenta 22(8–9):735–741
Pollux BJ, Meredith RW, Springer MS, Garland T, Reznick DN (2014) The evolution of the placenta drives a shift in sexual selection in livebearing fish. Nature 513(7517):233–236
Pyron RA, Burbrink FT (2014) Early origin of viviparity and multiple reversions to oviparity in squamate reptiles. Ecol Lett 17(1):13–21
Pyron RA, Burbrink FT (2015) Contrasting models of parity-mode evolution in squamate reptiles. J Exp Zool B Mol Dev Evol 324(6):467–472
Sawaguchi S, Koya Y, Yoshizaki N, Ohkubo N, Andoh T, Hiramatsu N, Sullivan CV, Hara A, Matsubara T (2005) Multiple vitellogenins (Vgs) in mosquitofish (Gambusia affinis): identification and characterization of three functional Vg genes and their circulating and yolk protein products. Biol Reprod 72(4):1045–1060
Sharpe RM (2018) Programmed for sex: nutrition-reproduction relationships from an inter-generational perspective. Reproduction 155(3):S1–S16
Shine R, Wapstra E, Olsson M (2018) Seasonal shifts along the oviparity-viviparity continuum in a cold-climate lizard population. J Evol Biol 31(1):4–13
Smith SA, Shine R (1997) Intraspecific variation in reproductive mode within the scincid lizard Saiphos equalis. Aust J Zool 45:435–445
Vega-Lopez A, Ortiz-Ordonez E, Uria-Galicia E, Mendoza-Santana EL, Hernandez-Cornejo R, Atondo-Mexia R, Garcia-Gasca A, Garcia-Latorre E, Dominguez-Lopez ML (2007) The role of vitellogenin during gestation of Girardinichthys viviparus and Ameca splendens; two goodeid fish with matrotrophic viviparity. Comp Biochem Physiol A Mol Integr Physiol 147(3):731–742
Wake MH (2015) Fetal adaptations for viviparity in amphibians. J Morphol 276(8):941–960
Wake MH, Dickie R (1998) Oviduct structure and function and reproductive modes in amphibians. J Exp Zool 282(4–5):477–506
Warren WC, Hillier LW, Marshall Graves JA, Birney E, Ponting CP, Grutzner F, Belov K, Miller W, Clarke L, Chinwalla AT, Yang SP, Heger A, Locke DP, Miethke P, Waters PD, Veyrunes F, Fulton L, Fulton B, Graves T, Wallis J, Puente XS, Lopez-Otin C, Ordonez GR, Eichler EE, Chen L, Cheng Z, Deakin JE, Alsop A, Thompson K, Kirby P, Papenfuss AT, Wakefield MJ, Olender T, Lancet D, Huttley GA, Smit AF, Pask A, Temple-Smith P, Batzer MA, Walker JA, Konkel MK, Harris RS, Whittington CM, Wong ES, Gemmell NJ, Buschiazzo E, Vargas Jentzsch IM, Merkel A, Schmitz J, Zemann A, Churakov G, Kriegs JO, Brosius J, Murchison EP, Sachidanandam R, Smith C, Hannon GJ, Tsend-Ayush E, McMillan D, Attenborough R, Rens W, Ferguson-Smith M, Lefevre CM, Sharp JA, Nicholas KR, Ray DA, Kube M, Reinhardt R, Pringle TH, Taylor J, Jones RC, Nixon B, Dacheux JL, Niwa H, Sekita Y, Huang X, Stark A, Kheradpour P, Kellis M, Flicek P, Chen Y, Webber C, Hardison R, Nelson J, Hallsworth-Pepin K, Delehaunty K, Markovic C, Minx P, Feng Y, Kremitzki C, Mitreva M, Glasscock J, Wylie T, Wohldmann P, Thiru P, Nhan MN, Pohl CS, Smith SM, Hou S, Nefedov M, de Jong PJ, Renfree MB, Mardis ER, Wilson RK (2008) Genome analysis of the platypus reveals unique signatures of evolution. Nature 453(7192):175–183
Wells KD (2008) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Whittington CM, Papenfuss AT, Bansal P, Torres AM, Wong ES, Deakin JE, Graves T, Alsop A, Schatzkamer K, Kremitzki C, Ponting CP, Temple-Smith P, Warren WC, Kuchel PW, Belov K (2008) Defensins and the convergent evolution of platypus and reptile venom genes. Genome Res 18(6):986–994
Whittington CM, Koh JM, Warren WC, Papenfuss AT, Torres AM, Kuchel PW, Belov K (2009) Understanding and utilising mammalian venom via a platypus venom transcriptome. J Proteome 72(2):155–164
Wu Q, Thompson MB, Murphy CR (2011) Changing distribution of cadherins during gestation in the uterine epithelium of lizards. J Exp Zool B Mol Dev Evol 316(6):440–450
Zanders SE, Unckless RL (2019) Fertility costs of meiotic drivers. Curr Biol 29(11):R512–R520
Zhang X, Pavlicev M, Jones HN, Muglia LJ (2020) Eutherian-specific gene TRIML2 attenuates inflammation in the evolution of placentation. Mol Biol Evol 37(2):507–523
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Warren, W.C., Grutzner, F. (2021). The Evolution of Viviparity in Vertebrates. In: Geisert, R.D., Spencer, T. (eds) Placentation in Mammals. Advances in Anatomy, Embryology and Cell Biology, vol 234. Springer, Cham. https://doi.org/10.1007/978-3-030-77360-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-77360-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-77359-5
Online ISBN: 978-3-030-77360-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)