Abstract
The estrogen receptors, ERα, ERβ, and GPER, mediate the effects of estrogenic compounds on their target tissues. Estrogen receptors are located in the tissues of the female reproductive tract and breast as one would expect, but also in tissues as diverse as bone, brain, liver, colon, skin, and salivary gland. The purpose of this discussion of the estrogen receptors is to provide a brief overview of the estrogen receptors and estrogen action from perspectives such as the historical, physiological, pharmacological, pathological, structural, and ligand perspectives.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
The literature contains many reviews of estrogen receptors and their ligands [1–9], as well as the effects of estrogens on specific tissue types [4, 10–31]. The reader interested in comprehensive discussion of estrogen receptors is referred to one or more of these excellent reviews. The purpose of this discussion is to provide a brief overview of the estrogen receptors and estrogen action from various perspectives.
2 The Historical Perspective
In evolutionary terms, the estrogen receptor is an ancient protein that is expressed in all vertebrates and a few invertebrates [32]. From a historical perspective, studies to discover the mechanism by which estrogen exerted its effects on target tissues took place in the 1950s and 1960s and culminated in the determination that estrogen bound to a protein in its target cells [33, 34]. Jensen and coworkers [35] describe a fascinating historical account of the early studies that led to the discovery of the estrogen receptor. This first estrogen binding protein is the protein now known as estrogen receptor α (ERα, also known as ER1 or Esr1). Our understanding of the binding of the estrogen-receptor complex to DNA, transcription to RNA, and subsequent protein synthesis evolved over the course of the 1960s and 1970s [33, 36, 37] to the mechanism that we recognize today of ligand-activated transcription factors. This genomic mechanism of action involves transcription and translation of genes so it is characterized by the time that it takes (hours) to develop a response as well as the persistence of the response for the lifespan of the new protein. The second estrogen receptor was identified in 1986 [38] and was named estrogen receptor β (ERβ, also known as ER2 and Esr2). Like ERα, ERβ is a ligand-activated transcription factor, so they share the slow-onset, persistent response genomic mechanism of action.
As early as the 1970s, reports appeared in the literature of actions of estrogen that occurred too rapidly to be mediated by the genomic mechanism of action [39–41]. However, these reports were pushed aside by the, at the time, seemingly more compelling genomic mechanism of action. Nevertheless, reports continued to emerge of rapid, nongenomic actions of estrogen [42] as well as nongenomic actions of other steroid hormones [43]. The ability of estrogen to mediate rapid, nongenomic actions is now widely accepted. An estrogen-responsive G protein coupled receptor called GPR30 or GPER has now been well characterized and is considered by many to be the source of the rapid actions of estrogen [44–52]. However, others have reported that the genomic receptors, ERα and ERβ, can associate with the membrane and mediate rapid actions although the mechanism of association of the genomic ER with the membrane is unclear [53]. Yet others report that mutated forms of ERα are involved in the rapid nongenomic actions of estrogen [54]. The details of rapid actions of estrogen remain controversial; it is quite possible that all of these mechanisms occur, perhaps in different cell types or differentially in physiological versus pathological situations.
3 The Physiological Perspective
We can also examine the estrogen receptor from the perspective of its physiological functions. In the same time frame as the discovery of the first estrogen receptor (late 1950s–1960s), the functional perspective of estrogen was purely as a female reproductive hormone. The reproductive functions of estrogen are the material of textbooks [55, 56]. In the historical context, an estrogenic compound was defined as a substance that could stimulate uterine growth and up-regulate synthesis of the progesterone receptor, both of which serve reproductive functions. As our understanding of the estrogen receptors has evolved, so also has our understanding of the functions of estrogen. In addition to the requirement of estrogen in reproductive function, and therefore, its role in the survival of the species, we now know that estrogen is critical to many other physiological functions. Estrogenreceptors are expressed and estrogenic ligands produce specific effects in the cardiovascular system [10–13], brain [14, 15], bone [16, 17], liver [18, 19], adipose tissue [19–21], colon [22, 23], skin [24, 25, 57], prostate [26, 27], testes [28, 29], epididymis [30, 31], and salivary gland [4]. Thus estrogen receptors serve a truly pleiotropic array of functions.
4 The Pharmacological Perspective
From the perspective of pharmacology, estrogen receptor agonists and estrogen receptor antagonists are both clinically relevant. The clinical uses of estrogen receptor agonists are primarily in the areas of combination hormonal contraceptives and in postmenopausal hormone replacement [58]. In combination hormonal contraceptives, an estrogen is administered with a progestin in the form of a pill, patch, or vaginal ring [59, 60]. These hormonal contraceptives are highly effective in the prevention of pregnancy. Nevertheless, the pleiotropic actions of estrogens incur some adverse effects. For example, the estrogens in contraceptives increase the risk of adverse reactions in the cardiovascular system, especially in women smokers [59, 60]. The primary use of the one pure estrogen receptor antagonist that is available, fulvestrant, is in the treatment of estrogen receptor positive (ER+) breast cancer. Fulvestrant is also sometimes called a selective estrogen receptor downregulator or degrader (SERD) because its binding to receptor leads to proteasomal degradation of the receptor [61]. In addition, an intriguing family of pharmaceutical agents called selective estrogen receptor modulators (SERMs) interacts with the estrogen receptors. The SERMs act as estrogen receptor agonists in some estrogen-sensitive tissues and as antagonists in others [59, 62]. SERMs that are on the market in the USA at this time include tamoxifen, raloxifene, bazedoxifene, and ospemifene. Tamoxifen acts as an estrogen receptor antagonist in the breast and is used in the treatment of ER+ breast cancer. The drawback to tamoxifen is that it acts as an estrogen receptor agonist in the uterus [59]. Raloxifene is an estrogen receptor agonist in bone but an antagonist in breast and uterus; it is used for postmenopausal osteoporosis [59]. Ospemifene is an estrogen receptor agonist on the vaginal epithelium, endometrium, and bone and is antiestrogenic in the breast. It is used for the treatment of dyspareunia that may occur in postmenopausal women [63]. Bazedoxifene is an estrogen receptor agonist in bone, hypothalamus, vagina, and vulva and acts as an antagonist in breast and uterus. Bazedoxifene is approved in the USA to be used in concert with conjugated estrogens for the treatment of vasomotor symptoms associated with the menopause [64]. The underlying concept is that the conjugated estrogens will alleviate the vasomotor symptoms and the bazedoxifene will prevent the adverse effects of estrogen on the breast and endometrium [64].
5 The Pathological Perspective
If we consider the estrogen receptors from the perspective of pathology, it is clear that several diseases are related to the estrogen receptor. Approximately 75 % of breast cancers express estrogen receptors [65, 66], and the presence of estrogen stimulates growth of these tumors. As mentioned above, inhibition of estrogen action in the breast is an important treatment for estrogen-sensitive breast cancer. These treatment strategies include the use of specific estrogen receptor antagonists such as fulvestrant [61], SERMs such as tamoxifen, or prevention of the synthesis of estrogen by use of aromatase inhibitors such as anastrozole, letrozole, and exemestane [59]. Similarly, endometrial cancer is also estrogen sensitive. The administration of estrogen alone for an extended period of time can cause the development of endometrial cancer in postmenopausal women [67].
The development of venous thromboembolism is another pathology associated with estrogen [58]. The risk of venous thromboembolism rises in women who take exogenous estrogens (e.g., contraceptives, postmenopausal hormone replacement therapy), as well as in situations in which endogenous estrogens are high as in women who are pregnant, or in the immediate postpartum period [68].
Autoimmune disease s occur at a higher rate in women than in men [69] and estrogen receptors play a role in the immune system [70]. Several hypotheses that been proposed to explain the discrepancy between men and women. One very intriguing idea is related to the enzyme, activation-induced deaminase (AID), which is involved in class switch recombination during antibody diversification [71]. Estrogen has been shown to activate AID [72], and may affect immune function by this mechanism. Derangement of estrogen regulation of this enzyme may be responsible for at least part of the increased risk of autoimmune disease in women.
The role of estrogen and the estrogen receptors in several other pathologies, such as cardiovascular disease and dementia, remains controversial. The rate of most cardiovascular diseases is lower in premenopausal women than in men, but rapidly rises after the menopause [73]. This disease pattern suggests that estrogen is protective to the cardiovascular system. However, studies such as the Women’s Health Initiative (WHI) showed that replacement of estrogen in postmenopausal women could trigger cardiovascular events (heart attack and stroke) [74]. Further analysis of data from the WHI study showed that the women who experienced cardiovascular events were more likely to be many years past the menopause. These data suggested that estrogen was more likely to trigger cardiovascular events in women who had been many years without the hormone and in whom cardiovascular disease may have become well-established although still silent. Thus the concept has developed that estrogen may be protective to a healthy cardiovascular system [11] but deleterious to a cardiovascular system in which disease such as atherosclerosis has developed [73, 75]. More recent data suggest that administration of estrogen soon after the menopause prevents the development of cardiovascular disease and does not precipitate CV events [12, 58, 76, 77]. A similar controversy exists regarding the effect of estrogen on dementia in postmenopausal women [78].
6 The Structural Perspective
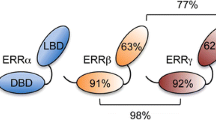
Another perspective from which to examine the estrogen receptors is that of structure. ERα and ERβ share the same general structure, that is, a ligand-binding domain, DNA-binding domain, and two activation function (AF) domains [79]. The two receptors share a high degree of amino acid sequence homology except in the N-terminal domain (the AF-2 domain). Proteins called coactivators (e.g., NCOA1, NCOA2, NCOA3, CREBBP, PPARBP, P68, and SRA) and corepressors (e.g., NCOR1, NRIP) can interact with ligand-bound ERα or ERβ and influence the ability of the receptor to activate or inhibit expression of a gene [80–82]. Phosphorylation of the estrogen receptor can affect its activity [83]. Ligand-bound ERα and ERβ bind to the same DNA sequence, the estrogen response element (ERE), whose sequence is defined as GGTCAnnnTGACC. For many estrogen-responsive genes, the ERE may be a significant distance upstream of the start site [84–86]. The promoters of many estrogen-responsive genes may contain only a half-site sequence of the ERE rather than the complete ERE, and ligand-bound ERα or ERβ can form protein-protein complexes with other transcription factors which then bind to their own response elements in the promoters of regulated genes [87]. Transcription factors with which ERα and ERβ can interact include Sp-1 [87, 88], Ap-1 [89, 90], and NF-κB [91]. Although they utilize the same ERE and interact with the same coregulators, ERα and ERβ exhibit differential tissue distribution and different biological effects [3–5]. Thus there remain complexities to be deciphered.
In contrast, the structure of GPER is dramatically different from that of ERα and ERβ, as one would expect of the membrane protein, and of course its mechanism of action is dramatically different as well. GPER has been reported to activate several signal transduction pathways which culminate in the phosphorylation of substrate proteins, some of which are transcription factors [46]. One of the transcription factors reported to be downstream of GPER is ERα [92], suggesting an interaction between nongenomic and genomic estrogen receptors.
7 The Ligand Perspective
Another perspective from which to examine the estrogen receptors is that of their ligands. Estrogen is a generic term that typically refers to the primary endogenous estrogenic compounds 17β-estradiol, estrone, and estriol (from most to least potent). Metabolites of these estrogens have been shown to regulate ERα and ERβ [93–95], as have other endogenous substances such as 27-hydroxycholesterol [96, 97]. Exogenous substances that can activate the estrogen receptors include phytoestrogens such as genistein, daidzein, and equol [98–101] as well as environmental substances [102–104].
8 Summary
Research on the estrogen receptors has been ongoing for over five decades; this brief overview can only provide a meager glimpse of the broad range of knowledge that is available regarding these receptors. In spite of the significant quantity of information that we have about the estrogen receptors, substantial questions remain. To name just a few: if ERα and ERβ use the same ERE and interact with the same coregulators, and are expressed in many of the same tissues, what is the mechanism by which they exert different biological effects? To what extent do ERα, ERβ, and GPER interact and do those interactions differ among estrogen-sensitive tissues? Can we delineate specific functions for each of the three receptors? Knockout animals for ERα, ERβ, and GPER have answered some of the biological questions regarding individual functions of these receptors, but not all of the models agree [4, 105, 106]. In spite of the 50+ years of investigation, the field of estrogen receptor research remains strong and vibrant, with notable questions remaining to carry the field forward into the future.
References
Katzenellenbogen BS, Montano MM, Ediger TR et al (2000) Estrogen receptors: selective ligands, partners, and distinctive pharmacology. Recent Prog Horm Res 55:163–193
Katzenellenbogen BS, Choi I, Delage-Mourroux R et al (2000) Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol 74:279–285
Hall JM, Couse JF, Korach KS (2001) The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276:36869–36872
Imamov O, Shim GJ, Warner M, Gustafsson JA (2005) Estrogen receptor beta in health and disease. Biol Reprod 73:866–871
Dahlman-Wright K, Cavailles V, Fuqua SA et al (2006) International union of pharmacology. LXIV. Estrogen receptors. Pharmacol Rev 58:773–781
Planey SL, Kumar R, Arnott JA (2014) Estrogen receptors (ERα versus ERβ): friends or foes in human biology? J Recept Signal Transduct Res 34:1–5
Vrtačnik P, Ostanek B, Mencej-Bedrač S, Marc J (2014) The many faces of estrogen signaling. Biochem Med (Zagreb) 24:329–342
Helsen C, Claessens F (2014) Looking at nuclear receptors from a new angle. Mol Cell Endocrinol 382:97–106
Bondesson M, Hao R, Lin CY, Williams C, Gustafsson JA (2015) Estrogen receptor signaling during vertebrate development. Biochim Biophys Acta 1849:142–151
Karas RH, Patterson BL, Mendelsohn ME (1994) Human vascular smooth muscle cells contain functional estrogen receptor. Circulation 89:1943–1950
Miller VM, Duckles SP (2008) Vascular actions of estrogens: functional implications. Pharmacol Rev 60:210–241
Clarkson TB, Mehaffey MH (2009) Coronary heart disease of females: lessons learned from nonhuman primates. Am J Primatol 71(9):785–793
Xing D, Nozell S, Chen YF, Hage F, Oparil S (2009) Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol 29:289–295
Tetel MJ, Pfaff DW (2010) Contributions of estrogen receptor-α and estrogen receptor-β to the regulation of behavior. Biochim Biophys Acta 1800:1084–1089
McEwen BS, Davis PG, Parsons B, Pfaff DW (1979) The brain as a target for steroid hormone action. Annu Rev Neurosci 2:65–112
Manolagas SC, O’Brien CA, Almeida M (2013) The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 9:699–712
Laurent M, Antonio L, Sinnesael M, Dubois V, Gielen E, Classens F, Vanderschueren D (2014) Androgens and estrogens in skeletal sexual dimorphism. Asian J Androl 16:213–222
Shi L, Feng Y, Lin H, Ma R, Cai X (2014) Role of estrogen in hepatocellular carcinoma: is inflammation the key? J Transl Med 12:93
Jia G, Aroor AR, Sowers JR (2014) Estrogen and mitochondria function in cardiorenal metabolic syndrome. Prog Mol Biol Transl Sci 127:229–249
Barros RPA, Gustafsson JA (2011) Estrogen receptors and the metabolic network. Cell Metab 14:289–299
Kim JH, Cho HT, Kim YJ (2014) The role of estrogen in adipose tissue metabolism: insights into glucose homeostasis regulation. Endocr J 61:1055–1067
Caiazza F, Ryan EJ, Doherty G, Winter DC, Sheahan K (2015) Estrogen receptors and their implications in colorectal carcinogenesis. Front Oncol 5:19
Barzi A, Lenz AM, Labonte MJ, Lenz HJ (2013) Molecular pathways: estrogen pathway in colorectal cancer. Clin Cancer Res 19:5842–5848
Thornton MJ (2013) Estrogens and aging skin. Dermatoendocrinology 5:264–270
Stevenson S, Thornton J (2007) Effect of estrogens on skin aging and the potential role of SERMs. Clin Interv Aging 2:283–297
Yeh CR, Da J, Song W, Fazili A, Yeh S (2014) Estrogen receptors in prostate development and cancer. Am J Clin Exp Urol 2:161–168
Nelson AW, Tilley WD, Neal DE, Carroll JS (2014) Estrogen receptor beta in prostate cancer: friend or foe? Endocr Relat Cancer 21:T219–T234
Chimento A, Sirianni R, Casaburi I, Pezzi V (2014) GPER signaling in spermatogenesis and testicular tumors. Front Endocrinol (Lausanne) 5:30
Royer C, Lucas TF, Porto CS (2012) 17Beta-estradiol signaling and regulation of proliferation and apoptosis of rat Sertoli cells. Biol Reprod 86:108
Hess RA, Fernandes SA, Gomes GR, Oliveira CA, Lazari MF, Porto CS (2011) Estrogen and its receptors in efferent ductules and epididymis. J Androl 32:600–613
Shayu D, Hardy MP, Rao AJ (2007) Delineating the role of estrogen in regulating epididymal gene expression. Soc Reprod Fertil Suppl 63:31–43
Eick GN, Thornton JW (2011) Evolution of steroid receptors from an estrogen-sensitive ancestral receptor. Mol Cell Endocrinol 334:31–38
Toft D, Gorski J (1966) A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci U S A 55:1574–1581
Toft D, Shyamala G, Gorski J (1967) A receptor molecule for estrogens: studies using a cell-free system. Proc Natl Acad Sci U S A 57:1740–1743
Jensen EV, Jacobson HI, Walf AA, Frye CA (2010) Estrogen action: a historic perspective on the implications of considering alternative approaches. Physiol Behav 99:151–162
Mueller GC, Gorski J, Aizawa Y (1961) The role of protein synthesis in early estrogen action. Proc Natl Acad Sci U S A 47:164–169
De Sombre ER, Puca GA, Jensen EV (1969) Purification of an estrophilic protein from calf uterus. Proc Natl Acad Sci U S A 64:148–154
Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA (1996) Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A 93:5925–5930
Pietras RJ, Szego CM (1975) Endometrial cell calcium and oestrogen action. Nature 253:357–359
Pietras RJ, Szego CM (1977) Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature 265:69–72
Pietras RJ, Szego CM (1980) Partial purification and characterization of oestrogen receptors in subfractions of hepatocyte plasma membranes. Biochem J 191:743–760
Pietras RJ, Arboleda J, Reese DM et al (1995) HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene 10:2435–2446
Revelli A, Massobrio M, Tesarik J (1998) Nongenomic actions of steroid hormones in reproductive tissues. Endocr Rev 19:3–17
Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630
Filardo EJ, Graeber CT, Quinn JA et al (2006) Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res 12:6359–6366
Mizukami Y (2010) In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen: from discovery to functions in vivo. Endocr J 57:101–107
Kolkova Z, Casslén V, Henic E, Ahmadi S, Ehinger A, Jirstrom K, Casslén B (2012) The G protein-coupled estrogen receptor 1 (GPER/GPR30) does not predict survival in patients with ovarian cancer. J Ovarian Res 5:9
Filardo EJ, Thomas P (2012) Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 153:2953–2962
Soltysik K, Czekaj P (2013) Membrane estrogen receptors—is it an alternative way of estrogen action? J Physiol Pharmacol 64:129–142
Han G, White RE (2014) G-protein-coupled estrogen receptor as a new therapeutic target for treating coronary artery disease. World J Cardiol 6:367–375
Barton M, Prossnitz ER (2015) Emerging roles of GPER in diabetes and atherosclerosis. Trends Endocrinol Metab 26:185–192
Méndez-Luna D, Martinez-Archundia M, Maroun RC et al (2015) Deciphering the GPER/GPR30-agonist and antagonists interactions using molecular modeling studies, molecular dynamics, and docking simulations. J Biomol Struct Dyn 14:1–12
Levin ER (2009) Plasma membrane estrogen receptors. Trends Endocrinol Metab 10:477–482
Kang L, Zhang X, Xie Y et al (2010) Involvement of estrogen receptor variant ER-α36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol 24(4):709
Bashay VE, Carr B (2011) The normal menstrual cycle and the control of ovulation. In: De Groot LJ, Beck-Peccoz P, Chrousos G et al (eds) Endotext [Internet]. MDText.com, Inc., South Dartmouth, MA
Bulun SE (2011) Physiology and pathology of the female reproductive axis. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM (eds) Williams Textbook of Endocrinology, 12th edn. Saunders, Philadelphia, PA, pp 581–660
Jackson RL, Greiwe JS, Schwen RJ (2011) Ageing skin: oestrogen receptor β agonists offer an approach to change the outcome. Exp Dermatol 20:879–882
Bassuk SS, Manson JE (2015) Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol 25:193–200
Levin ER, Hammes SR (2011) Estrogens and progestins. In: Brunton L, Chagner B, Knollman B (eds) Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th edn. McGraw Hill Medical, New York, pp 1163–1194
Sonalkar S, Schreiber CA, Barnhart KT (2014) Contraception. In: De Groot LJ, Beck-Peccoz P, Chrousos G et al (eds) Endotext [Internet]. MDText.com, Inc., South Dartmouth, MA
Ciruelos E, Pascual T, Vozmediano ML et al (2014) The therapeutic role of fulvestrant in the management of patients with hormone receptor-positive breast cancer. Breast 23:201–208
Riggs L, Hartmann LC (2003) Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med 348:618–629
Grant MD, Marbella A, Wang AT et al (2015) Menopausal symptoms: comparative effectiveness of therapies [Internet]. AHRQ Comparative Effectiveness Rev Mar. Report No.: 15-EHC005-EF
Goldberg T, Fidler B (2015) Conjugated estrogens/bazedoxifene (Duavee). A novel agent for the treatment of moderate-to-severe vasomotor symptoms associated with menopause and the prevention of postmenopausal osteoporosis. P T 40:178–182
Pritchard KI, Gelmon KA, Rayson D et al (2013) Endocrine therapy for postmenopausal women with hormone receptor-positive HER2-negative advanced breast cancer after progression or recurrence on nonsteroidal aromatase inhibitor therapy: a Canadian consensus statement. Curr Oncol 20:48–61
Blok EJ, Derks MGM, van der Hoeven JJM, van de Velde CJH, Kroep JR (2015) Extended adjuvant endocrine therapy in hormone-receptor positive early breast cancer: current and future evidence. Cancer Treat Rev 41:271–276
Gambrel RD Jr, Bagnell CA, Greenblatt RB (1983) Role of estrogens and progesterone in the etiology and prevention of endometrial cancer: review. Am J Obstet Gynecol 146:696–707
Marshall AL (2014) Diagnosis, treatment, and prevention of venous thromboembolism in pregnancy. Postgrad Med 126:25–34
Whitacre CC (2001) Sex differences in autoimmune disease. Nat Immunol 2(9):777–780
Cunningham M, Gilkeson G (2011) Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol 40:66–73
Muramatsu M, Kinoshita K, Fagarasan S et al (2000) Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102:553–563
Pauklin S, Sernandez IV, Bachmann G et al (2009) Estrogen directly activates AID transcription and function. J Exp Med 206:99–111
Clarkson TB (2007) Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause 14:373–384
Rossouw J, Anderson G, Prentice R et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333
Dubey RK, Imthurn B, Barton M, Jackson EK (2005) Vascular consequences of menopause and hormone therapy: importance of timing of treatment and type of estrogen. Cardiovasc Res 66:295–306
Clarkson TB, Melendez GC, Appt SE (2013) Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause 20(3):342–353
Mirkin S, Archer DF, Pickar JH, Komm BS (2015) Recent advances help understand and improve the safety of menopausal therapies. Menopause 22:351–360
Rocca WA, Grossardt BR, Shuster LT (2014) Oophorectomy, estrogen, and dementia: a 2014 update. Mol Cell Endocrinol 389:7–12
Aranda A, Pascual A (2001) Nuclear hormone receptors and gene expression. Physiol Rev 81:1269–1304
Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M (2000) Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852
DiRenzo J, Shang Y, Phelan M et al (2000) BRG-1 is recruited to estrogen-responsive promoters and cooperates with factors involved in histone acetylation. Mol Cell Biol 20:7541–7549
Stashi E, York B, O’Malley BW (2014) Steroid receptor coactivators: servants and masters for control of systems metabolism. Trends Endocrinol Metab 25:337–347
Lannigan DA (2003) Estrogen receptor phosphorylation. Steroids 68:1–9
Carroll JS, Brown M (2006) Estrogen receptor target gene: an evolving concept. Mol Endocrinol 20:1707–1714
Carroll JS, Meyer CA, Song J et al (2006) Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297
Bourdeau V, Deschênes J, Métivier R et al (2004) Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol 18:1411–1427
Safe S, Kim K (2008) Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol 41:263–275
Safe S (2001) Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm 62:231–252
Paech K, Webb P, Kuiper GG et al (1997) Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 277:1508–1510
Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL (2001) Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem 276:13615–13621
Biswas DK, Singh S, Shi Q, Pardee AB, Iglehart JD (2005) Crossroads of estrogen receptor and NF-kappaB signaling. Sci STKE 2005(288):pe27
Clark S, Rainville J, Zhao X, Katzenellenbogen BS, Pfaff D, Vasudevan N (2014) Estrogen receptor-mediated transcription involves the activation of multiple kinase pathways in neuroblastoma cells. J Steroid Biochem Mol Biol 139:45–53
Dubey RK, Jackson EK (2009) Potential vascular actions of 2-methoxyestradiol. Trends Endocrinol Metab 20:374–379
Dubey RK, Tofovic SP, Jackson EK (2004) Cardiovascular pharmacology of estradiol metabolites. J Pharmacol Exp Ther 308:403–409
Ruan X, Seeger H, Wallwiener D, Huober J, Mueck AO (2015) The ratio of the estradiol metabolites 2-hydroxyestrone (2-OHE1) and 16α-hydroxyestrone (16-OHE1) may predict breast cancer risk in postmenopausal but not in premenopausal women: two case-control studies. Arch Gynecol Obstet 291:1141–1146
Umetani M, Shaul PW (2011) 27-Hydroxycholesterol: the first identified endogenous SERM. Trends Endocrinol Metab 22:130–135
Nelson ER, Wardell SE, McDonnell DP (2013) The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: implications for the treatment and prevention of osteoporosis. Bone 53:42–50
Setchell KDR (1998) Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 68(Suppl):1333S–1346S
Brzezinski A, Debi A (1999) Phytoestrogens: the “natural” selective estrogen receptor modulators? Eur J Obstet Gynecol Reprod Biol 85:47–51
Muthyala RS, Ju YH, Sheng S et al (2004) Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem 12:1559–1567
Jackson RL, Greiwe JS, Schwen RJ (2011) Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist. Nutr Rev 69:432–448
Aris AZ, Shamsuddin AS, Praveena SM (2014) Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: a review. Environ Int 69:104–119
Lu Z, Gan J (2014) Analysis, toxicity, occurrence and biodegradation of nonylphenol isomers: a review. Environ Int 73:334–345
Wall EH, Hewitt SC, Case LK, Lin CY, Korach KS, Teuscher C (2014) The role of genetics in estrogen responses: a critical piece of an intricate puzzle. FASEB J 28:5042–5054
Walker VR, Korach KS (2004) Estrogen receptor knockout mice as a model for endocrine research. ILAR J 45:455–461
Prossnitz ER, Barton M (2009) Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat 89:89–97
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Eyster, K.M. (2016). The Estrogen Receptors: An Overview from Different Perspectives. In: Eyster, K.M. (eds) Estrogen Receptors. Methods in Molecular Biology, vol 1366. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3127-9_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3127-9_1
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3126-2
Online ISBN: 978-1-4939-3127-9
eBook Packages: Springer Protocols