Abstract
Pretreatment is an important step in lignocellulosic bioethanol production which aims to reduce lignin content and break down lignocellulosic structure thereby increasing the accessibility of enzymes in hydrolysis. Therefore, this research explored the pretreatment process on oil palm empty fruit bunch (EFB) with CO2 impregnation followed by alkali explosion. EFB was impregnated with CO2 at 5 °C for 12 h. After impregnation, EFB was mixed with 2.5 M of NaOH solution (1:5 of S/L ratio) in an alkali explosion reactor. Alkali explosion was conducted at 150 °C, 4 kg/cm2 of pressure with the variation of reaction time for 15, 30, and 45 min. The parameters analyzed in this study include EFB recovery, EFB composition and characteristics, glucose yields, and ethanol yields. EFB composition was analyzed as cellulose, hemicellulose, and lignin while the characteristics of the EFB were examined in functional groups. The results indicated that combined pretreatment using CO2 impregnation followed by 15 min of alkali explosion obtained higher delignification of EFB, glucose, and ethanol yield than only alkali explosion for 30 min. The highest glucose and ethanol yields were 99.33 and 83.80 w/w% of glucose mass in EFB, respectively. These results prove that CO2 can be used as an impregnation agent in lignocellulosic pretreatment and could be combined with alkali explosion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fossil fuel combustion releases emissions that negatively impact the environment and humans [1]. Therefore, many researchers have carried out research to substitute fossil fuels with new and renewable energy (NRE). One of the alternative raw materials for renewable energy that is currently being developed is biomass. Biomass has been the most energy source in the world for heating application [2]. However, modernization of bioenergy production systems is needed so that biomass can be optimally applied as feedstock. Bioethanol is one of the bioenergies that can be produced from biomass, such as starch, sugar, lignocellulosic biomass, and algae [3,4,5]. An alternative non-food material that has the potential to be developed as a source of bioethanol production is oil palm empty fruit bunches (EFB). EFB is the largest waste in the palm oil industry, reaching 22 to 25% of the weight of fresh fruit [6]. EFB has cellulose (20–50%), hemicellulose (23–36%), lignin, and other derivatives (22–51%) [7, 8]. The significant variations of the constituents of EFB due to differences in the sample’s origin such as size, age, growth phase, soil condition, geographic location, and climate leverage related to the oil palm tree, as well as different analytical methods and sample preparation before analysis of EFB [9, 10].
Lignocellulosic bioethanol production mainly has four primary processes: pretreatment, hydrolysis, fermentation, and purification [11]. In the process, the constraint faced is lignin content in lignocellulose, which inhibits the conversion process, and causes a low hydrolysis rate resulting in a low ethanol concentration production [12]. In addition, the structure of lignin molecules is a physical barrier in hydrolysis due to preventing the accessibility of enzymes to cellulose and hemicellulose [13, 14]. Therefore, the pretreatment of lignocellulose is an important step in bioethanol production.
Previous research has been done with various pretreatment methods, such as biological, chemical, mechanical, and thermal processes, as well as a combination of each method to accelerate the hydrolysis of lignocellulose. Alkali explosion is one chemical pretreatment that can provide a high yield of delignification. However, this method requires high temperature and pressure as well as takes a long time to process [15].
To optimize the pretreatment process, several studies reported that the addition of impregnating agents such as H2SO4, SO2, or CO2 could decrease inhibitor production and improve the enzymatic hydrolysis of biomass [16]. Therefore, this study applied CO2 as an impregnating agent before alkali explosion for EFB in bioethanol production. Impregnation refers to the imbuing or saturating process with something [17]. Impregnation of biomass could change the morphology by enhancing cellulose’s permeability and increasing biomass’s surface area [18]. The use of CO2 as impregnating agent is considered to provide benefits such as strong solubility, low toxicity, weak corrosivity, and low occupational risk [19, 20]. Moreover, CO2 is also a by-product of ethanol fermentation; thus, it is enormously available in bioethanol plants [21].
The variation of reaction time during alkali explosion was also explored in this study. According to previous studies, the reaction time for the alkaline explosion of EFB ranged between 15 and 45 min [22, 23], and the optimal delignification process was 30 min [24, 25]. Therefore, optimization of reaction time in combined pretreatment with CO2 impregnation followed by alkali explosion be necessary to explore.
2 Materials and methods
2.1 Materials
This study obtained EFB from oil palm plantation in Sumatra Island, Indonesia. EFB was chopped and milled to obtain in fiber form with the size ± 3 mm. Furthermore, EFB was dried to attain 10% of moisture content. CO2 gas was provided by PT WAP Andalan Indonesia. The enzymes used were Cellic® Ctec2 and Cellic® Htec2 from Novozymes Korea Ltd. Commercial instant dry yeast Saccharomyces cerevisiae was applied in fermentation. All chemicals used were analytical grade.
2.2 Pretreatment process
The pretreatment was performed in a 5-L reactor that was manufactured by Changhae Ethanol Co., Ltd. In the experiments with CO2 as catalyst impregnation, the EFB fiber was placed in a plastic bag, and CO2 was supplied from a gas cylinder at atmospheric pressure. CO2 gas was added as much as 3% w/w based on the moisture content of EFB where the water content in the EFB used in this study was 10% w/w. The weight of CO2 was determined by weighing the bag before and after adding the gas. The sample in a plastic bag was stored at 5 °C for 12 h to complete impregnation [19]. Furthermore, the impregnated EFB was treated in the reactor using 2.5 M NaOH solution with 1:5 of the solid–liquid (S/L) ratio. Alkali explosion was carried out at 150 °C of temperature and 4 kg/cm2 of pressure [15]. The variation of alkali explosion time was 15, 30, and 45 min. Subsequently, the pretreated-EFB are neutralized with water until pH of 6–8 at room temperature and dried for 24 h at 50 °C in the oven. The alkali explosion without CO2 impregnation was also conducted as a blank sample with the process at 150 °C, 4 kg/cm2 for a 30-min process.
2.3 Separate hydrolysis and fermentation (SHF) process
The ethanol was produced by separate hydrolysis and fermentation (SHF) method.
2.3.1 Enzymatic hydrolysis process
The substrate concentration used in the hydrolysis process was 10 w/v% of EFB-treated and, then, was mixed with a citrate buffer and enzymes until 100 mL of total volume. The enzymes used were 30 FPU/g substrate of Cellic®Ctec2. Next, Cellic®Htec2 is added with a volume ratio of 1:5 of Cellic®Ctec2. The hydrolysis process was carried out at a temperature of 50 °C with the agitation of 150 rpm in the shaking incubator for 72 h.
2.3.2 Fermentation Process
After 72 h of enzymatic hydrolysis, 1 w/v% of dried S. cerevisiae was added into hydrolysate for the fermentation process. The process was carried out at 32 °C of temperature, 150 rpm for 72 h.
2.4 Analytical methods
The chemical component of EFB (cellulose, hemicellulose, lignin, and ash) was analyzed using standard biomass analytical procedures from National Renewable Energy Laboratory (NREL) [26]. The structural changes of EFB were evaluated by a Shimadzu FTIR spectrometer. The glucose and ethanol concentration after SHF was determined using high-performance liquid chromatography (HPLC) equipped with HPX-87P (Bio-RAD, CA, USA) column and analyzed with a RID detector. The eluent used as a mobile phase was 5 mM H2SO4 solution at a flow rate of 0.6 mL/min [21].
2.5 Delignification and yield calculation
Percent of delignification, glucose, and ethanol yields were calculated using the equations below:
-
1)
Percentage of delignification [27]
$$\mathrm{\%delignification}=\frac{{\mathrm{lignin\;content\;of\;EFB}}_{i}-{\mathrm{lignin\;content\;of\;EFB}}_{f}(\mathrm{\% w}/\mathrm{w})}{{\mathrm{lignin\;content\;of\;EFB}}_{i}(\mathrm{\% w}/\mathrm{w})}\times\;100\mathrm{\%}$$(1)
where EFBi = EFB before pretreatment and EFBf = EFB after pretreatment.
-
2)
Glucose yield calculation [21]
-
a)
Glucose yield calculation based on theoretical glucose from cellulose in the substrate
$$\mathrm{\%Ygc}=\frac{\mathrm{ glucose\;in\;hydrolysate }(\mathrm{g})}{\mathrm{theoretical\;glucose\;in\;substrate }(\mathrm{g})}\times\;100\mathrm{\%}$$(2) -
b)
Glucose yield calculation based on the substrate
$$\mathrm{\%Ygs}=\frac{\mathrm{glucose\;in\;hydrolysate\;}(\mathrm{g})}{\mathrm{weight\;of\;substrate\;}(\mathrm{g})}\times 100\mathrm{\%}$$(3)
-
a)
-
3)
Ethanol yield calculation [21]
-
a)
Ethanol yield calculation based on theoretical ethanol from cellulose in the substrate
$$\mathrm{\%Yec}= \frac{\mathrm{ethanol\;in\;broth\;fermentation\;}(\mathrm{g})}{\mathrm{theoretical\;ethanol\;in\;cellulose\;}(\mathrm{g})}\times\;100\mathrm{\%}$$(4) -
b)
Ethanol yield calculation based on the substrate
$$\mathrm{\%Yes}=\frac{\mathrm{ethanol\;in\;broth\;fermentation }(\mathrm{g})}{\mathrm{weight\;of\;substrate }(\mathrm{g})}\times\;100\mathrm{\%}$$(5)
-
a)
3 Results and discussion
3.1 Application of CO2 as an impregnating agent in alkali explosion for EFB
Table 1 shows the recovery percentage of solids generated after pretreatment and washing. The alkali explosion pretreatment with prior CO2 impregnation was about one-third of the initial substrate. The value was almost similar to the percent recovery of the blank sample. The weight losses are considered to be related to the lignin content lost due to the process. Moreover, the decomposition of the polysaccharide compound, silica content removal, and evaporation that occurs in several components (CO, CO2, CH4, and other hydrocarbons) have a major role in sample weight losses [28]. Evaporation could occur during an alkali explosion, which uses high temperatures and pressures. Carbon cellulose chains, hemicellulose, and lignin are degraded due to the high temperature in the pretreatment process [29], such as in an alkali explosion.
The chemical component of EFB before pretreatment as an untreated sample, EFB after alkali explosion as a blank, and EFB after combined pretreatment of CO2 impregnation followed by alkali explosion can be seen in Table 2. The results show that lignin and hemicellulose were degraded after pretreatment. The percent delignification of EFB after combined pretreatment of CO2 impregnation followed by alkali explosion was higher than the blank sample. From the results of combined pretreatment, delignification increased alongside the increasing explosion time. The percentage of delignification of samples A, B, and C was 73.65, 80.03, and 80.54%, respectively. These findings indicate that the percent delignification of sample B at 30 min of explosion time was higher than sample A at 15 min of reaction time but almost similar to sample C at 45 min. Several studies also showed that the optimum reaction time of alkaline pretreatment for biomass was 30 min [25, 30, 31]. According to Monte et al., alkaline pretreatment using NaOH in rice husk could significantly remove silica and hemicellulose as well as partially removed lignin during 30 min of reaction time. After that, the non-cellulosic biomass extraction’s reaction rate weakened and almost stopped after 60 min of reaction time [30].
The percentage of hemicellulose compound in EFB-treated decreased from hemicellulose in EFB-untreated. Pretreatment using an alkali solution such as NaOH, ammonia, or Ca(OH)2 can remove lignin and hemicelluloses [32, 33]. After pretreatment, these components will dissolve in an alkali solution known as black liquor. The ability of NaOH to dissolve lignin is due to the opening of aromatic rings of lignin caused by the use of high temperatures and high pressure and then, the resulting explosive effects can dissolve these components [34]. The explosive effect resulting from the reactor due to rapid temperature drop will help in the delignification process due to an increase in the surface area of the pretreatment sample [35]. Adding CO2 before alkali pretreatment is also believed to increase the pores of lignocellulosic biomass surfaces; thus, the percent delignification of EFB treated using alkali explosion with prior CO2 impregnation was higher than the blank sample. Therefore, decreasing the percent content of lignin and hemicellulose enhanced cellulose percentage in EFB-treated. Cellulose is the main compound that can be converted into glucose using enzymes and subsequently fermented by yeast to produce ethanol.
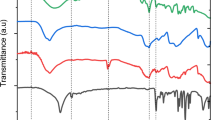
Figure 1 shows The FTIR spectra from EFB before and after pretreatment. The wavenumber of EFB-treated (blank, A, B, C samples) at around 3279 – 3552 cm−1 appeared clearer than EFB-untreated. The wavenumber indicated hydrogen-bonded (O–H) stretching absorption. O–H stretching region at a peak of 3000–3600 cm−1 of EFB spectra was more assigned to the O–H stretching region from cellulose [36]. From the results, the clear peak of O–H stretching from the cellulose of EFB treated indicated that the cellulose content in EFB treated was higher as compared to EFB-untreated. The CH stretching mode at around 2923 cm−1 was shifted to a lower wavenumber at around 2854 cm−1 after pretreatment. Then, the wavenumbers between 1425 and 1427 cm−1, 1155 and 1159 cm−1, and around 895 cm−1 were found in the EFB after pretreatment. The wavenumber around 1429 cm−1 and 1157 cm−1 indicates CH2 bending and C–O–C asymmetric stretching known as cellulose-related bands [37]. Their appearance wavenumbers in EFB after pretreatment indicate an increase in cellulose amount. Moreover, the C–H–O stretching of the β-(1–4)-glycosidic linkage bands at around 895 cm−1 appear stable enough in EFB-treated [36]. The changes in intensity were also found in the band at wavenumber 1026–1038 cm−1. The band at wavenumber around 1032 cm−1 was indicated to the C–O stretch in cellulose and hemicellulose [36]. The intensity of this band was increased after pretreatment.
3.2 Separate hydrolysis and fermentation (SHF) of EFB
Figure 2 shows the changes in glucose production during the hydrolysis of EFB. It was observed that glucose production of EFB after combined pretreatment using CO2 impregnation followed by alkali explosion was higher than EFB after alkali explosion (blank sample). The tendency of glucose production in the A, B, and C samples was similar. After 72 h of hydrolysis, 0.81–0.85 g glucose/g substrate was obtained from the A, B, and C samples, while a blank sample provided 0.68 g glucose/g substrate. Moreover, the glucose production of sample A with 15 min of reaction alkali explosion process was higher than glucose of blank sample with 30 min of alkali explosion process. Therefore, the utilization of CO2 as an impregnating agent could reduce the time of alkali explosion. CO2 impregnation allows the biomass’s pores to expand, improving the result of alkali explosion and enhancing enzyme accessibility during the hydrolysis step [21, 38]. An accomplishment rate of the hydrolysis process is influenced by the reduction of lignin levels in biomass, the disruption of component structure of lignocellulose, and the breakdown of the crystallinity of cellulose [39].
According to Table 3, hydrolysis of EFB after combined pretreatment using CO2 impregnation followed by alkali explosion achieved more than 98% of glucose yield (cellulose basis) and 80% of glucose yield (substrate basis). These results were higher than the blank sample, obtaining 97.94% of glucose yield (cellulose basis) and 68.00% of glucose (substrate basis). Therefore, it indicated the significance of utilization of CO2 as an impregnation agent before alkali explosion to enhance the hydrolysis result.
Table 4 shows ethanol production and ethanol yield from the fermentation of EFB. After 72 h of fermentation, the ethanol concentration of blank, A, B, and C samples was 27.29, 32.80, 35.30, and 37.60 g/L broth fermentation, respectively. The ethanol yield of EFB after combined pretreatment using CO2 impregnation followed by alkali explosion was higher than EFB after alkali explosion (blank sample). Ethanol yield (cellulose basis) of A, B, and C samples was between 76.84 and 83.80 w/w% and a range of 32.80 – 37.60 w/w% (substrate basis), while ethanol yield of the blank sample was 76.22 w/w% (cellulose basis) and 27.29 w/w% (substrate basis). The results indicated that ethanol yield increased in line with increasing explosion time in combined pretreatment. More interesting, after combined pretreatment (A) at 15-min reaction time, the sample obtained higher ethanol production than the sample after only alkali explosion (blank). Adding CO2 impregnation before alkali pretreatment could reduce reaction time in alkali explosions that use high temperatures and pressure. Thus, energy and cost saving for the pretreatment process could be achieved.
As a comparison with other studies, Table 5 shows several studies that have been reported regarding EFB pretreatment and pretreatment technology using CO2. Pretreatment of EFB using steam explosion and combined CO2-added steam explosion [21] obtained lower percent delignification and glucose yield as well as ethanol yield than results from this study. Alkali explosion [31] or superheated steam explosion followed by alkaline autoclaving pretreatment [40] of EFB also provided slightly lower percent delignification and glucose yield as compared to results in this study. Moreover, the application of CO2 in pretreatment was also used for other biomasses such as sugarcane bagasse and leaves, rice straw, corn cob, and corn stalk [19, 41,42,43,44]. From their studies, CO2 was considered to apply in pretreatment as a catalyst or impregnation agent.
4 Conclusion
Pretreatment of EFB using CO2 impregnation followed by alkali explosion could provide higher percent delignification, glucose, and ethanol yield as compared to pretreatment alkali explosion. EFB from combined pretreatment with CO2 impregnation followed by 15 min of alkali explosion obtained 73.65% of delignification. This result was higher than the delignification of EFB from 30 min of alkali explosion (blank sample) of 68.46%. Furthermore, in the combined pretreatment, a longer time in alkali explosion leads to an increase in the percent of delignification, yield of glucose, and ethanol. The highest result was 80.54 w/w% of delignification, 99.33 w/w% of glucose yield, and 83.80 w/w% of ethanol yield from EFB after combined pretreatment with 45 min of alkali explosion. Therefore, adding CO2 as an impregnation agent in alkali pretreatment is believed to enhance the delignification, glucose, and ethanol yield. Further studies are needed to optimize the NaOH concentration and temperature of CO2 impregnation on combined pretreatment to reduce chemicals and energy uses in the process.
Data availability
Not applicable.
References
Perera F (2018) Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: solutions exist. Int J Environ Res Public Health 15(1), 16:1–17. https://doi.org/10.3390/ijerph15010016
Perea-Moreno MA, Samerón-Manzano E, Perea-Moreno AJ (2019) Biomass as renewable energy: worldwide research trends. Sustain 11(3), 863:2–19. https://doi.org/10.3390/su11030863
Bušić A, Marđetko N, Kundas S, Morzak G, Belskaya H, Šantek MI, Komes D, Novak S, Šantek B (2018) Bioethanol production from renewable raw materials and its separation and purification: a review. Food Technol. Biotechnol. 56:289–311. https://doi.org/10.17113/ftb.56.03.18.5546
Bibi R, Ahmad Z, Imran M, Hussain S, Ditta A, Mahmood S, Khalid A (2017) Algal bioethanol production technology: a trend towards sustainable development. Renew Sustain Energy Rev 71:976–985. https://doi.org/10.1016/j.rser.2016.12.126
Lamichhane G, Acharya A, Poudel DK, Aryal B, Gyawali N, Niraula P, Phuyal SR, Budhathoki P, Bk G, Parajuli N (2021) Recent advances in bioethanol production from Lignocellulosic biomass. Int J Green Energy 18:731–744. https://doi.org/10.1080/15435075.2021.1880910
Ling JH, Lim YT, Leong WK, Sia HT (2021) Utilization of oil palm empty fruit bunch in cement bricks. J Adv Civ Environ Eng 4(1):1–10. https://doi.org/10.30659/jacee.4.1.1-10
Sukiran MA, Abnisa F, Wan Daud WMA, Abu Bakar N, Loh SK (2017) A review of torrefaction of oil palm solid wastes for biofuel production. Energy Convers Manag 149:101–120. https://doi.org/10.1016/j.enconman.2017.07.011
Mohammad IN, Ongkudon CM, Misson M (2020) Physicochemical properties and lignin degradation of thermal-pretreated oil palm empty fruit bunch. Energies 13(22):5966. 1–12. https://doi.org/10.3390/en13225966
Elgharbawy AA, Alam MZ, Moniruzzaman M, Kabbashi NA, Jamal P (2018) Chemical and structural changes of pretreated empty fruit bunch (EFB) in ionic liquid-cellulase compatible system for fermentability to bioethanol. 3 Biotech 8:236. https://doi.org/10.1007/s13205-018-1253-8
Windiastuti E, Suprihatin, Bindar Y, Hasanudin U (2022) Identification of potential application of oil palm empty fruit bunches (EFB): a review. IOP Conf Ser Earth Environ Sci 1063:012024–012024-11. https://doi.org/10.1088/1755-1315/1063/1/012024
Dey P, Pal P, Kevin JD, Das DB (2020) Lignocellulosic bioethanol production: prospects of emerging membrane technologies to improve the process—a critical review. Rev Chem Eng 36:333–367. https://doi.org/10.1515/revce-2018-0014
Robak K, Balcerek M (2018) Review of second-generation bioethanol production from residual biomass. Food Technol Biotechnol 56(2):174–187. https://doi.org/10.17113/ftb.56.02.18.5428
Ladeira Ázar RIS, Bordignon-Junior SE, Laufer C, Specht J, Ferrier D, Kim D (2020) Effect of lignin content on cellulolytic saccharification of liquid hot water pretreated sugarcane bagasse. Molecules 25(3), 623:1–12. https://doi.org/10.3390/molecules25030623
Djajadi DT, Jensen MM, Oliveira M, Jensen A, Thygesen LG, Pinelo M, Glasius M, Jørgensen H, Meyer AS (2018) Lignin from hydrothermally pretreated grass biomass retards enzymatic cellulose degradation by acting as a physical barrier rather than by inducing nonproductive adsorption of enzymes. Biotechnol Biofuels 11:1–13. https://doi.org/10.1186/s13068-018-1085-0
Muryanto TE, Abimanyu H, Cahyono A, Cahyono ET, Sudiyani Y (2015) Alkaline delignification of oil palm empty fruit bunch using black liquor from pretreatment. Procedia Chemistry 16:99–105. https://doi.org/10.1016/j.proche.2015.12.032
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11. https://doi.org/10.1016/S0960-8524(01)00212-7
Weidner E (2018) Impregnation via supercritical CO2–What we know and what we need to know. J Supercrit Fluids 134:220–227. https://doi.org/10.1016/j.supflu.2017.12.024
Morais ARC, Da Costa Lopes AM, Bogel-Łukasik R (2015) Carbon dioxide in biomass processing: contributions to the green biorefinery concept. Chem Rev 115:3–27. https://doi.org/10.1021/cr500330z
Ferreira-Leitão V, Perrone CC, Rodrigues J, Franke APMH, MacRelli S, Zacchi G (2010) An approach to the utilization of CO2 as impregnation agent in steam pretreatment of sugar cane bagasse and leaves for ethanol production. Biotechnol Biofuels 3:1–8. https://doi.org/10.1186/1754-6834-3-7
Rezakazemi M, Darabi M, Soroush E, Mesbah M (2019) CO2 absorption enhancement by water-based nanofluids of CNT and SiO2 using hollow-fiber membrane contactor. Sep Purif Technol 210:920–926. https://doi.org/10.1016/j.seppur.2018.09.005
Triwahyuni E, Miftah AK, Muryanto Maryana R, Sudiyani Y (2021) Effect of CO2-added steam explosion on oil palm empty fruit bunch for bioethanol production. Cellul Chem Technol 55:839–847. https://doi.org/10.35812/CelluloseChemTechnol.2021.55.71
Han M, Kim Y, Kim SW, Choi GW (2011) High efficiency bioethanol production from OPEFB using pilot pretreatment reactor. J Chem Technol Biotechnol 86:1527–1534. https://doi.org/10.1002/jctb.2668
Duangwang S, Sangwichien C (2013) Optimizing alkali pretreatment of oil palm empty fruit bunch for ethanol production by application of response surface methodology. Adv Mater Res 622:117–121. https://doi.org/10.4028/www.scientific.net/AMR.622-623.117
Sudiyani Y, Triwahyuni E, Muryanto Burhani D, Waluyo J, Sulaswaty A, Abimanyu H (2016) Alkaline pretreatment of sweet sorghum bagasse for bioethanol production. Int J Renew Energy Dev 5:113–118. https://doi.org/10.14710/ijred.5.2.113-118
Muryanto Sudiyani Y, Abimanyu H (2016) Optimization of NaOH alkali pretreatment of oil palm empty fruit bunch for bioethanol. Indones J Appl Chem 18:27–35. https://doi.org/10.14203/jkti.v18i01.37
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass determination of structural carbohydrates and lignin in biomass. Technical Report. NREL/TP-510–42618:1–15. https://www.nrel.gov/docs/gen/fy13/42618.pdf
Hazwan M, Bowra S, Cox P (2020) Purity and structural composition of lignin isolated from Miscanthus x giganteus by sub-critical water extraction with associated modifiers. J Agric Food Eng 1:1–12. https://doi.org/10.37865/jafe.2020.0010
Bahrin EK, Ibrahim MF, Abdul Razak MN, Abd-Aziz S, Hassan MA, Baharuddin AS, Sulaiman A, Shirai Y, Nishida H (2012) Physicochemical property changes and enzymatic hydrolysis enhancement of oil palm empty fruit bunches treated with superheated steam. BioResources 7:1784–1801. https://doi.org/10.15376/biores.7.2.1784-1801
Baruah J, Nath BK, Sharma R, Kumar S, Deka RC, Baruah DC, Kalita E (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res 6:1–19. https://doi.org/10.3389/fenrg.2018.00141
Monte LS, Escócio VA, de Sousa AMF, Furtado CRG, Leite MCAM, Visconte LLY, Pacheco EBAV (2018) Study of time reaction on alkaline pretreatment applied to rice husk on biomass component extraction. Biomass Conv Bioref 8:189–197. https://doi.org/10.1007/s13399-017-0271-9
Sudiyani Y, Triwahyuni E, Muryanto, Marno S, Putri N (2020) Bioethanol production from alkali steam explosion of oil palm of empty fruit bunch fiber. IOP Conf Ser Mater Sci Eng 854:012030–1-012030-7. https://doi.org/10.1088/1757-899X/854/1/012030
Tan J, Li Y, Tan X, Wu H, Li H, Yang S (2021) Advances in pretreatment of straw biomass for sugar production. Front Chem 9:1–28. https://doi.org/10.3389/fchem.2021.696030
Dewi RK, Zuhroh ST, Zulaikha S (2018) Delignification of chandlenut shell waste with alkali pretreatment method as an alternative fuel feedstock. Int J Mech Eng Technol 9(10):271–278. https://iaeme.com/MasterAdmin/Journal_uploads/IJMET/VOLUME_9_ISSUE_10/IJMET_09_10_027.pdf. Accessed 10 Oct 2022
Rezania S, Oryani B, Cho J, Talaiekhozani A, Sabbagh F, Hashemi B, Rupani PF, Mohammadi AA (2020) Different pretreatment technologies of lignocellulosic biomass for bioethanol production: an overview. Energy. 199:117457. https://doi.org/10.1016/j.energy.2020.117457
Waluyo J, Anggraini RIF, Kurniawan, HH, Sudiyani Y (2018) Physical chemical characterization of alkali pretreatment for oil palm empty fruit bunch. AIP Conf Proc 2024:020072-1-020072-11. https://doi.org/10.1063/1.5064358
Isroi IMM, Millati R, Syamsiah S, Cahyanto MN, Niklasson C, Taherzadeh MJ (2012) Structural changes of oil palm empty fruit bunch (OPEFB) after fungal and phosphoric acid pretreatment. Molecules 17:14995–15012. https://doi.org/10.3390/molecules171214995
Putri AMH, Triwahyuni E, Sudiyani Y (2016) Statistical analysis of NaOH pretreatment effects on sweet sorghum bagasse characteristics. AIP Conf Proceeding 1803:020008-1-020008–7. https://doi.org/10.1063/1.4973135
Cha YL, Yang J, Park Y, An GH, Ahn JW, Moon YH, Yoon YM, Yu GD, Choi IH (2015) Continuous alkaline pretreatment of Miscanthus sachhariflorus using a bench-scale single screw reactor. Bioresour Technol 181:338–344. https://doi.org/10.1016/j.biortech.2015.01.079
Baig KS (2020) Interaction of enzymes with lignocellulosic materials: causes, mechanism and influencing factors. Bioresour Bioprocess 7(21):1–19. https://doi.org/10.1186/s40643-020-00310-0
Thamsee T, Choojit S, Cheirsilp B, Yamseangsung R, Ruengpeerakul T, Sangwichien C (2019) Combination of superheated steam explosion and alkaline autoclaving pretreatment for improvement of enzymatic digestibility of the oil palm tree residues as alternative sugar sources. Waste Biomass Valorization 10:3009–3023. https://doi.org/10.1007/s12649-018-0292-z
Cha YL, Yang J, Ahn JW, Moon YH, Yoon YM, Yu GD, An GH, Choi IH (2014) The optimized CO2-added ammonia explosion pretreatment for bioethanol production from rice straw. Bioprocess Biosyst Eng 37:1907–1915. https://doi.org/10.1007/s00449-014-1165-x
Gao M, Xu F, Li S, Ji X, Chen S, Zhang D (2010) Effect of SC-CO2 pretreatment in increasing rice straw biomass conversion. Biosyst Eng 106:470–475. https://doi.org/10.1016/j.biosystemseng.2010.05.011
de CarvalhoSilvello MA, Martínez J, Goldbeck R (2020) Application of supercritical CO2 treatment enhances enzymatic hydrolysis of sugarcane bagasse. Bioenergy Res 13:786–796. https://doi.org/10.1007/s12155-020-10130-x
Yin J, Hao L, Yu W, Wang E, Zhao M, Xu Q, Liu Y (2014) Enzymatic hydrolysis enhancement of corn lignocellulose by supercritical CO 2 combined with ultrasound pretreatment. Chinese J Catal 35:763–769. https://doi.org/10.1016/S1872-2067(14)60040-1
Funding
This work was supported by Research Center for Chemistry, National Research and Innovation Agency (BRIN).
Author information
Authors and Affiliations
Contributions
ET is the main contributor who conceptualized the study and methodology, collected and analyzed the data, and wrote the original paper. Meanwhile, AKM conducted pretreatment experiments and data collection. MM and RM performed hydrolysis and fermentation processes and analyzed data. YS supervised the study and also the review and editing of the paper.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human or animal participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Statement of novelty
Indonesia as one of the largest oil palm producers in the world not only produces oil palm but also generates huge amounts of lignocellulosic biomass such as empty fruit bunch (EFB). This biomass can be converted into energy, for example, bioethanol. Pretreatment is an important stage in lignocellulosic bioethanol production. This study inquires about the application of CO2 as an impregnating agent in the alkali explosion of EFB for bioethanol production. This research reports for the first time that adding CO2 as an impregnating agent before alkali explosion could enhance the percentage of delignification, hydrolysis, and fermentation yield in bioethanol production from EFB. This report was assessed in terms of the percentage of EFB solid recovery after pretreatment, the percentage of the chemical composition of EFB, the characteristics of EFB, the yield of glucose, and ethanol yield after separate hydrolysis and fermentation (SHF).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Triwahyuni, E., Miftah, A.K., Muryanto, M. et al. Conversion of oil palm empty fruit bunch into bioethanol through pretreatment with CO2 as impregnating agent in alkali explosion. Biomass Conv. Bioref. 14, 19131–19138 (2024). https://doi.org/10.1007/s13399-023-04102-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04102-2