Abstract
In this work, self-assembled amphiphilic micelles based on chitosan (CS) and polycaprolactone (PCL) were produced and used as carriers of paclitaxel (PTX) to improve its intestinal pharmacokinetic profile. Chitosan-grafted-polycaprolactone (CS-g-PCL) was synthesized through a carbodiimide reaction by amidation and confirmed by Fourier transform infrared spectroscopy (FTIR), hydrogen nuclear magnetic resonance analysis (1H NMR), and contact angle evaluation. Micelles were produced by solvent evaporation method, and the critical micelle concentration was investigated by conductimetry. The obtained micelles were of 408-nm mean particle size, narrow size distribution (polydispersity index of 0.335) and presented positive surface charge around 30 mV. The morphology of micelles assessed by transmission electron microscopy (TEM) revealed round and smooth surface, in agreement with dynamic light scattering measurements. The association efficiency determined by high-performance liquid chromatography (HPLC) was as high as 82%. The in vitro cytotoxicity of the unloaded and PTX-loaded micelles was tested against Caco-2 and HT29-MTX intestinal epithelial cells, resulting in the absence of cell toxicity for all formulations. Moreover, the permeability of PTX-loaded micelles in Caco-2 monolayer and Caco-2/HT29-MTX co-culture model was determined. Results showed that the permeability of PTX was higher in Caco-2/HT29-MTX co-culture model compared with Caco-2 monolayer due to the mucoadhesive character of micelles, acting as a platform to deliver PTX at the sites of absorption. Therefore, it can be concluded that the PTX-loaded CS-g-PCL micelles, employed for the first time as PTX carriers, may be a potential drug carrier for the intestinal delivery of hydrophobic drugs, particularly anticancer agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oral administration of therapeutic agents is the most common and preferred route of drug delivery for patients due to its ease of administration, which results in greater convenience and, consequently, better patient compliance. However, drugs administered orally are hampered by several factors, such as low solubility in biological fluids, the action of the multidrug efflux transporter P-glycoprotein (P-gp), and great affinity to either intestinal or liver cytochrome P450 enzymes like CYP3A4 [1, 2]. This is the case of paclitaxel (PTX), a drug with limited oral efficiency [3]. PTX is an anticancer drug used in various types of tumors such as colon cancer, metastatic breast cancer, ovarian cancer, among others [4, 5]. Its mechanism of action affects the cell division, leading to hyperstabilization of microtubules that stops mitosis in G2 and M phases, leading to cell death [1, 6]. Being PTX a hydrophobic drug with low solubility in aqueous environment, its commercial intravenous form entails drastic side effects to patients due to the employment of organic excipients to provide suitable solubilization [1, 7]. Even Abraxane®, an albumin nanoparticle-based formulation of PTX, approved by FDA since 2005, is not orally administered due to albumin degradation in gastric conditions [8]. Besides Abraxane®, there are other PTX nanocarriers being tested in clinical trials to be administrated intravenously, such as EndoTAG-I, Paclical, or even Genexol-PM, which is already in the market [9]. Unfortunately, this route of administration is still not ideal due to drug instability after contact with the bloodstream and occurrence of infections and thrombosis [1]. Thus, these biomaterials capable of encapsulating hydrophobic drugs for oral administration, have attracted attention in recent years to overcome these problems.
The use of drug carriers, such as polymeric micelles, is being widely investigated as strategy to improve drug bioavailability due to its capability to encapsulate drugs in a stable way with great efficiency [1, 7, 10, 11]. Polymeric micelles can be spontaneously formed in aqueous solution using amphiphilic polymers, as a result of hydrophobic interactions between the segments of two polymeric sections [12]. These systems have demonstrated better response in the treatment of disease and, specifically micelles with mucoadhesive properties, have the ability to adhere to the intestinal mucosa and release the drug exactly where it should be absorbed [13]. Moreover, the drug can be protected from adverse conditions of the body such as enzymes or biological fluids, preserving their stability and, simultaneously, avoiding most of disadvantages caused by intravenous administration [10]. Furthermore, as nanometric systems, polymeric micelles may not be able to carry a high dose as used in intravenous administration. However, the possibility of releasing the drug at the desired site does not involve the use of high therapeutic doses, making these vehicles promising candidates for effective drug delivery [14].
Chemical modifications of polymers are the most efficient and well-known method to create self-assembled systems. Chitosan (CS), a natural polymer, has already been described, and their properties are primarily based on its biocompatibility, biodegradability, non-toxicity [15, 16], and mucoadhesiveness, which ensures its permanence on the epithelium, improving drug permeation [17, 18]. CS is obtained from chitin by alkaline deacetylation or enzymatic hydrolysis [15]. This polysaccharide is constituted by repeated units of D-glucosamine and N-acetyl-D-glucosamine (GlcNAc) linked by glycosidic linkages β-(1,4) with prevalence of N-acetyl-D-glucosamine units (GlcN) equal or more than ~60% [11, 19]. Besides, it has reactive amino (NH2) and hydroxyl (OH) groups on its backbone and thus can be chemically modified by the addition of hydrophobic moieties of polycaprolactone (PCL). These characteristics are propitious to prepare CS amphiphilic derivatives with self-assembly properties. As such, it is here proposed as the ideal candidate for hydrophilic component of polymeric micelles.
In this work, an amphiphilic CS derivative, chitosan-grafted-polycaprolactone (CS-g-PCL), was synthesized by a carbodiimide reaction, and its physical-chemical properties were studied, resulting in a copolymer with self-assembled properties. Polymeric micelles were produced by evaporation method, and its ability to encapsulate PTX was investigated. The in vitro potential cytotoxicity of the micellar system was assessed in colorectal cancer cells as well as its permeability across Caco-2 monoculture model and in Caco-2/HT29-MTX co-culture model, regarding the application as PTX intestinal delivery system.
Experimental
Chemical materials
Chitosan (CS, molecular weight (M w) ≈ 87 kDa and degree of deacetylation (DD) ≈ 82%) was purchased from Sigma-Aldrich (St. Louis, MO, USA), and polycaprolactone (PCL) was acquired from PolySciTech (45–55 kDa, West Lafayette, IN, USA). The carboxylic end groups of the PCL were activated by N,N′-dicyclohexylcarbodiimide (DCC, Alfa Aesar, Haverhill, MA, USA) and N-hydroxysuccinimide (NHS, Sigma-Aldrich, St. Louis, MO, USA) for direct conjugation with CS by amidation reaction. Dichloromethane (DCM) and dimethylformamide (DMF) were purchased from Merck Millipore (Billerica, MA, USA) and were used to solubilize PCL. A 10 kDa cutoff dialysis membrane (Thermo Scientific, Waltham, MA, USA) was used to purify the activated PCL. Paclitaxel (PTX) was a gift from Indena SpA (Viale Ortles, Milano, Italy).
Cell line and culture
Two colorectal cancer cell lines were used: C2BBe1 clone of Caco-2 obtained from American Type Culture Collection (ATCC, USA) and HT29-MTX cell line, kindly provided by Dr. T. Lesuffleur (INSERMU178, Villejuif, France). Both cell lines were cultured with Dulbecco’s modified Eagle medium (DMEM, Lonza, Verviers, Belgium) supplemented with 10% (v/v) fetal bovine serum (FBS), 1% (v/v) penicillin (100 U/mL), streptomycin (100 μg/mL), and 1% (v/v) non-essential amino acids (NEAA). All these supplements were purchased from Merck Millipore (Billerica, MA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Triton X-100 1% from Spi-Chem (West Chester, PA, USA), and culture flasks, 96-well tissue culture plates, and 12-well plates Transwell® were purchased from Corning Inc., (Steuben County, NY, USA). The cells were maintained in an incubator (ESCO CelCulture® CO2 Incubator, Singapore) at 37 °C temperature and 5% CO2 in a water-saturated atmosphere.
Synthesis of chitosan-grafted-polycaprolactone
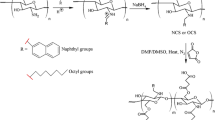
The synthesis of CS-g-PCL was performed using a similar protocol described before [20]. Briefly, CS (2%, w/v) was solubilized in acetic acid (2%, v/v) with moderate stirring following pH adjustment to 7–8 with 3 M sodium hydroxide (NaOH, Sigma, St. Louis, MO, USA). PCL (1 g) was dissolved in 50 mL of dimethylformamide/dichloromethane (DMF/DCM, 1:1, v/v, Sigma, St. Louis, MO, USA), and after complete dissolution, 2.5 mmol of DCC and 2.5 mmol NHS were added to the solution. The activation reaction of the carboxyl group of the PCL (which will react with the amine groups of CS through NHS using DCC as a catalyst) was carried out for 12 h at room temperature (RT). The insoluble by-product of this reaction, dicyclohexylurea, was removed by centrifugation for 20 min at 15 °C and 4300 rpm (Thermo Scientific Heraeus Megafuge 1.0R, Waltham, MA, USA). The obtained solution was further filtered under vacuum. To remove the excess of DCC/NHS, the activated PCL solution was dialyzed against 10-fold volume of DMF/DCM using 10 kDa cutoff dialysis membrane (Thermo Scientific, Waltham, MA, USA) for 2 days with two changes of volumes. Lastly, 12.5 mL of activated PCL solution was added into 50 mL of CS solution, and the final solution was allowed stirring for 24 h at 50 °C.
Characterization of chitosan-grafted-polycaprolactone
The characterization of CS-g-PCL amphiphilic polymer was performed by attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR), proton nuclear magnetic resonance (1H NMR), contact angle, and critical micelle concentration (CMC).
ATR-FTIR spectra of each sample were generated by ABB MB3000 FTIR spectrometer from ASEA Brown Boveri (ABB Zurich, Switzerland) equipped with a MIRacle single reflection attenuated total reflectance (ATR) accessory from PIKE Technologies (Madison, WI, USA). All spectra were collected with 256 scans and a 4 cm−1 resolution in the region of 4000–600 cm−1.
1H NMR spectra were acquired at 80 °C using a Bruker AVANCE III 400 spectrometer. These analyses were conducted on HCl/D2O 1% (v/v) for CS and CS-g-PCL and CDCl3 for PCL.
Contact angle of CS and CS-g-PCL was measured on an optical contact angle measuring device (OCA15, Dataphysics Instruments Co. Ltd., Filderstadt, Germany) to determine the hydrophilicity/hydrophobicity of the samples. For this test, 200 mg of each sample was subjected to vacuum during 2 min followed by 8 t of pressure for 30 s, to form a tablet. For the drop, 4 μL of distilled water was released from a needle (0.50 mm) falling on the tablet. Contact angles were measured using Laplace-Young method with brightness of 96 and contrast of 511 at 15 s after the fall of the drop, focused by a high-performance CCD camera.
The CMC of CS-g-PCL was determined by conductimetric titration (Consort C863, Consort bvba, Turnhout, Belgium) carried out at 25 °C [21]. The procedure consists on the conductivity measurement of various copolymer concentrations in 0.1 M acetic acid ranged between 1 and 1× 10−6 mg/mL. The CMC was obtained from the inflection point of the curve plotted by the specific conductivity against the logarithm of the concentration of the copolymer.
Preparation of paclitaxel-loaded chitosan-grafted-polycaprolactone micelles
Paclitaxel-loaded chitosan-grafted-polycaprolactone (PTX-loaded CS-g-PCL) micelles were produced by the well-established solvent evaporation method [22]. Briefly, 5 mg of copolymer was dissolved in 5 mL of acetic acid (0.1 M) and DMF (1:1, v/v). Subsequently, different volumes of PTX (1 mg/mL, dissolved in 70% ethanol) were added dropwise to the previous solution to form a theoretical loading of 0.5–5% (w/w). The mixture was allowed under stirring for 4 h for ethanol evaporation. Posteriorly, the solution was sonicated for 5 min using a probe-type sonicator (Vibra-Cell, Sonics Material Inc., Danbury, USA) in iced bath. All the solutions were centrifuged through Amicon® Ultra-15 100 kDa at 5000 rpm during 10 min to remove the no-encapsulated PTX. After filtration, PTX-loaded CS-g-PCL polymeric micelles were collected.
Characterization of paclitaxel-loaded chitosan-grafted-polycaprolactone micelles
In order to characterize micelle formulations, the mean particle size, polydispersity index (PdI), and surface charge were determined using dynamic light scattering (DLS) and electrophoretic light scattering (ELS) (ZetaSizer Nano ZS, Malvern, UK), respectively.
The morphological features were observed by transmission electron microscope (TEM). Samples were prepared by placing 10 μL of micellar solution on 300-mesh nickel grids and examined under a JEOL JEM 1400 (JEOL Ltd., Tokyo, Japan). Images were digitally recorded using a Gatan SC 1100 ORIUS CCD camera (Warrendale, PA, USA).
The association efficiency (AE) and drug loading (DL) of PTX were assessed by high-performance liquid chromatography (HPLC) using a Merck Hitachi LaChrom® system (Merck Millipore, NJ, USA) equipped with a D-7000 Interface, a L-7455 Diode Array Detector, a L-7200 Autosampler, and a L-7100 Pump. The PTX samples were injected (20 μL) on a LiChrospher® 100 RP-18 (125 × 4 mm, 5 μm, Merck Millipore, NJ, USA) with a LiChrospher® 100 RP-18 guard column (4 × 4 mm, 5 μm, Merck Millipore, NJ, USA) and mobile phase consisting of methanol and water (65:35, v/v) at a flow rate of 1.0 mL/min. PTX elution was monitored at 227 nm. The AE and DL were determined indirectly by quantifying the PTX in the supernatant after centrifugation of micelles, using the following formulas, respectively:
The standard solutions used for the calibration curve were prepared considering the mobile phase of the method and the solution in which the micelles were prepared. Thus, standard solutions were prepared on the day of the experiment in methanol/acetic acid 0.1 M (80:20, v/v). The retention time for PTX was 7.9 min.
Cytotoxicity studies
Potential cytotoxicity CS-g-PCL and PTX-loaded CS-g-PCL micelles was tested against Caco-2 (C2BBe1 clone, passage 51–74) and HT29-MTX (passage 41–63) colorectal cells, using MTT reagent. Caco-2 and HT29-MTX cells grew separately in culture flasks in a complete medium, as described before, where the culture medium was replaced every other day. After detachment, cells were seeded into 96-well plates at density of 2 × 104 cells/well for Caco-2 and 1 × 104 cells/well for HT29-MTX cell lines and were incubated at 37 °C in 5% CO2 atmosphere for 24 h. Subsequently, the medium was removed, and cells were washed twice with phosphate buffered saline (PBS). Cells were incubated with complete medium with different concentrations (0.01–100 μg/mL) of the mentioned samples. A negative control (1% Triton X-100) and positive control (DMEM with cell incubation) were also included. After 4 and 24 h of incubation, the medium was discarded and cells were washed twice with PBS. The MTT solution at final concentration of 0.5 mg/mL was added and incubated for 4 h in the dark. Finally, the MTT solution was removed, and 200 μL of DMSO was added to dissolve the MTT formazan crystals formed. The plates were placed in orbital shaker for 15 min in the dark at RT, and after that, the absorbance was measured using a multimode plate reader (Biotek Synergy 2, Winooski, VT, USA) at 570 and 630 nm. The results were analyzed according to the following equation:
Permeability studies
For the permeability experiment, 1 × 105 cells/cm2 were seeded in 12-Transwell® cell culture inserts (pore diameter 3.0 μm) for Caco-2 monoculture and Caco-2/HT29-MTX co-culture model in a proportion of 90:10. The cells were allowed to grow and differentiate for 21 days with medium replacement every other day, as previously reported [23, 24]. First, the medium was removed from both sides of the Transwell®, and the cells were washed twice with pre-warmed Hank’s buffered salt solution (HBSS, Gibco, Grand Island, NY, USA). Afterwards, the Transwell® was replaced by fresh warm HBSS and allowed to equilibrate for 30 min at 37 °C in an orbital shaker at 100 rpm before starting the permeability experiment. Similarly, permeability studies were run during 1 h, and a volume of 0.5 mL of PTX-loaded CS-g-PCL micelles and free PTX at concentration of 50 μg/mL, prepared in HBSS and DMSO/HBSS (1%, v/v), respectively, were added to the apical side of the Transwell®. Still, a volume of 1.5 mL of HBSS was added to the basolateral side. At different time points (15, 30, 45, and 60 min), 200 μL of each sample was collected from the basolateral side of the Transwell® and the same volume of fresh warm HBSS was added to replace the removed volume. All experiments were performed in triplicate, and the PTX was quantified by HPLC, as described above.
The integrity of the cell monolayers was checked before and after the permeability experiment by measuring the transepithelial electric resistance (TEER) using an EVOM Epithelial Voltohmmeter Instrument with chopstick electrodes (World Precision Instruments, Sarasota, FL, USA). The permeability results were expressed in percentage of permeability and apparent permeability (P app). P app was calculated using the following equation:
where C 0 is the initial concentration in the apical side of the Transwell® (μg/mL), A is the surface area of the insert (cm2), ∆t is the time during which experiment occurred (seconds), and ∆Q is the amount of compound detected in the basolateral side (μg).
Statistical analysis
Experiments were performed in triplicate for proper statistical analysis and are represented as mean ± standard deviation (SD). One-way ANOVA with post hoc Tukey’s test with a p value ≤0.05 was used to achieve significant differences between DLS, ELS, and HPLC data. A two-way ANOVA with Bonferroni multiple/post hoc group comparisons was used to analyze the cytotoxicity data. GraphPadPrism software Inc., USA, was used, and the level of significance was set at probabilities of *p < 0.05, **p < 0.01, and ***p < 0.001.
Results and discussion
Synthesis and characterization of chitosan-grafted-polycaprolactone
A carbodiimide reaction by amidation was selected to reach CS-g-PCL copolymer, as the initial approach to obtain an amphiphilic material. As described before, from modified PCL with a terminal carboxyl group, it was possible to graft CS backbone using amine coupling agent DCC/NHS at an alkaline pH.
The reaction between PCL and CS led to the formation of secondary amide bonds (O=C–NH) at 650 cm−1 (N–H wagging), at 1570 cm−1 (N–H bending), at 1650 cm−1 (−C=O stretching), and at 3360 cm−1 (N–H stretching) (Fig. 1a) [25, 26]. PCL-NHS reacts with primary amines of CS only in alkaline conditions and, consequently, leads to the formation of secondary amide bonds [27]. On the other hand, it was also expected the appearance of the characteristic peaks of CS and PCL. The band at 890–1100 cm−1 was assigned to ether groups of the saccharine structures of CS (C–O–C stretching) (Fig. 1c) [10]. Lastly, several characteristic bands for PCL (−CO–O–C–) were located at 1725 cm−1 (−C=O stretching) and the broad band at 2800–3000 cm−1 (−CH2 stretching) (Fig. 1b) [28]. The grafted copolymer presented characteristic bands of both CS and PCL and, more importantly, was shown the appearance of the secondary amide bands, a result of the reaction of the two polymers, which implied the success of the reaction.
The 1H NMR spectra of PCL, CS, and CS-g-PCL are shown in Fig. 2. The characteristic peaks were observed in the CS spectrum at 2.06 ppm due to CH3 of acetamido groups of GlcNAc units, 3.6–4.0 ppm, related to the hydrogens H3–H6 of GlcN unit and H2 of GlcNAc unit and 3.2 ppm as a result of H2 of GlcN units. The 1H NMR spectrum of CS-g-PCL (Fig. 2a) showed characteristic peaks of PCL at 1.4, 1.6–1.7, 2.3, and 4.1 ppm, which correspond to the γ, β and δ, α, and ε signals of the graft segment in CS-g-PCL copolymer, respectively. Also, the characteristic peaks of CS at 3.6–4.0 and 2.06 ppm are referents to 3, 4, 5, 6, 6′, and 7 of GlcNAc and GlcN units of CS, respectively. The chemical structure of CS-g-PCL was clearly confirmed by the presence of the characteristic peaks of CS and PCL in the 1H NMR spectrum [10, 11, 29]. So, it could be assumed that CS-g-PCL was successfully prepared through carbodiimide reaction by amidation.
To further demonstrate the hydrophilic/hydrophobic behavior of CS-g-PCL copolymer, the contact angle of developed material was assessed. The contact angle is proportional to the hydrophobic surface that contacts the droplet of water. Accordingly, as depicted in Fig. 3, the contact angle of CS was lower than CS-g-PCL and, therefore, a more prominent hydrophilic feature can be assigned. The difference between both contact angles was, still, minor, which may indicate a low degree of substitution of CS-g-PCL; nonetheless, it does not cease to be indicative of the presence of the PCL hydrophobic groups grafted on CS backbone during synthesis.
Due to the hydrophobic and hydrophilic natures of PCL and CS, respectively, CS-g-PCL can spontaneously form micelles in aqueous environment. To determine the CMC of CS-g-PCL, the conductimetric method was employed due to easy, low-cost, and reproducible results. A substantial increase of specific conductivity was observed, which represents the beginning of the formation of micelles. The CMC value was set to 9.55 × 10−4 mg/mL, obtained by the intersection between straight lines of the graph of specific conductivity against the logarithmic concentration of the copolymer (Fig. 4). Vehicles such as polymeric micelles are known to have the advantage of presenting lower CMC than low M w surfactants [30]. Moreover, micelles with low CMC are generally more stable, promoting drug stability in body fluids as well as in blood circulation [31, 32]. Thus, these micelles subjected to a large dilution, for example, when administered orally, will continue to be stable, maintaining their morphology and drug retention. In fact, CS-g-PCL form micelles with approximately 10 μg/mL concentration. This value is one of the most important characteristic of this system since it can be diluted avoiding micelle disassociation. Similarly, this low CMC value is common in other polymeric micelles based on CS [10, 32, 33].
Characterization of paclitaxel-loaded chitosan-grafted-polycaprolactone micelles
PTX was encapsulated into CS-g-PCL micelles by solvent evaporation method, and their physical-chemical properties such as size, PdI, zeta potential, morphology, percent drug association efficiency, and drug loading were evaluated. Several theoretical PTX loadings were tested in order to elect the formulation with better properties to be used in in vitro studies.
The size distribution of micelles was determined by DLS and TEM, as depicted in Table 1 and Fig. 5, respectively. For all formulations of theoretical PTX loading ranging from 0 to 2.5%, micelles presented similar properties of mean particle size around 420 nm and positively charged. However, the formulation with 5% of PTX showed large size, which, correlated with lower AE value, may indicate PTX saturation and precipitation of the drug. Visual observation of the micelles revealed residual particles on the bottom of the tub, corroborating this hypothesis. Similar other studies regarding hydrophobic modifications on CS backbone have been performed, and they are in agreement with in this work [32, 34]. Regarding the PdI, the results showed high values in almost all formulations. However, these high values can be explained by the fact that micelles were mostly constituted of a natural polymer, CS, where the size of the chains cannot be controlled and, therefore, the size of the micelles can be inconstant.
All the micelles showed positive zeta potential, which evidence that micelles were properly constituted with positively charged CS in their shell. Consistent with previous published data [35, 36], the presence of PTX compared with free-drug nanocarriers had no significant effect on zeta potential.
TEM microphotographs (Fig. 5) assessed the micellar morphology of unloaded and 2.5% PTX-loaded formulations. Results suggested good dispersity without aggregations and spherical shape before and after PTX encapsulation. A variability in micelle size can justify the high PdI value, previously measured by DLS. Also, it was possible to observe that most of the micelles found present mean particle size smaller than 400 nm, compared to DLS results. Additionally, it occurred probably due to the sample treatment prior TEM analysis, as their size can decrease during drying preparation due to polymer contraction [37].
The quantification of encapsulated PTX in micelles was performed by HPLC, as described above. As depicted in Table 1, for a range of 0–5% theoretical loading, the AE was relatively similar, around 80%. However, for 5% of PTX, the AE decreased slightly, probably due to saturation of the micellar system, as mentioned before.
Thus, the 2.5% loading formulation was selected for further experiments, considering their smaller size allied to the interactions with hydrophobic segments. Also, it has been thought that the increase of PTX interaction with hydrophobic core of micelles may result in higher stability [38]. These interactions may improve the drug entrapment and, consequently, lead to a more controlled release of the drug [39]. Regarding in vivo studies performed in mice, Taxol® and Genexol-PM were administered intravenously at concentration of 2.5 and 21.5 mg/kg, respectively [40]. Although this system has a low drug concentration, the increased bioavailability and the possibility of drug release directly into the colon cancer cells will lead to a reduction in the required therapeutic dose, without compromising its efficacy.
In vitro cytotoxicity of paclitaxel-loaded chitosan-grafted-polycaprolactone micelles
In order to evaluate the safety and biocompatibility of PTX-loaded CS-g-PCL micelles regarding intestinal delivery, in vitro experiments using Caco-2 and HT29-MTX intestinal epithelial cells were performed. Samples of micelles and controls were incubated with cells for 4 and 24 h, and cell viability was assessed by MTT assay.
As can be seen in Fig. 6a, c, after 4 h of incubation, no toxicity was found for PTX-loaded micelles in a range of 0.01 to 100 μg/mL (in respect to PTX concentration), similarly to empty micelles, demonstrating the innocuous behavior of the copolymer particle formation. In the case of free PTX, some toxicity was found, being more critical for the highest concentration in both cell lines. This incubation time is usually tested considering the residence time of drugs in the intestinal epithelium [41, 42]. After 24 h of incubation (Fig. 6b, d), micelles demonstrated a safety profile even for the highest concentration, where cell viability levels were settled at 76 and 88% for HT29-MTX and Caco-2 cell lines, respectively. According to ISO standards, the obtained values of cell viability set aside the hypothesis that these samples have a cytotoxic effect [43]. The same safety profile of unloaded CS-g-PCL micelles was also reported by other authors [11]. Regarding the free PTX, it was evidenced a decrease in cell viability for all concentrations, presenting cell viability values close to 10 and 18% at the highest concentration (100 μg/mL) for HT29-MTX and Caco-2 cells, respectively, corroborating its known toxic effect against intestinal cells [35, 44]. Also, it is believed that such low cell viability levels are not due to a direct antitumor effect, since the incubation time was less than the doubling time of these cell lines [45, 46]. Therefore, this cytotoxicity obtained is not expected to be due to the pharmacological effect of the drug, but due to other non-specific mechanisms. Thus, it was clear that PTX CS-g-PCL micelles provide a better safety profile of PTX compared with PTX in solution.
Cell viability of unloaded CS-g-PCL micelles, PTX-loaded micelles, and free PTX against Caco-2 (a, b) and HT29-MTX (c, d) cell lines, respectively, at concentrations between 0.01–100 μg/mL after incubation for 4 h (a, c) and for 24 h (b, d). Values were reported as mean ± SD (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001 denotes a significant difference
In vitro permeability of paclitaxel-loaded chitosan-grafted-polycaprolactone micelles
Lastly, the intestinal permeability of PTX was investigated in order to understand to what extent this system could enhance the bioavailability of PTX across the intestinal barrier. PTX-loaded CS-g-PCL micelles were tested on Caco-2 monoculture model and Caco-2/HT29-MTX co-culture model at physiological proportions (90/10) [23, 24]. This Caco-2/HT29-MTX co-culture intestinal model was used to evaluate the effect of mucus in the permeation of PTX in the intestinal epithelium, regarding the mucoadhesive effect of chitosan.
As mentioned previously, cells were seeded for both models and allowed to grow for 21 days. The TEER values were monitored before and after the experiment in order to assess the integrity of the monolayer. The registered values were not less than 200 Ω cm2 (data not shown), which means that the integrity of the cell monolayers was achieved.
As depicted in Fig. 7, the permeability of PTX encapsulated into micelles started somewhat later in Caco-2 monolayer compared to Caco-2/HT29-MTX co-culture model. It is thought that this occurred due to the stronger interaction between micelles and mucus produced by HT29-MTX cells, providing a higher local concentration of PTX at the sites of absorption. The mucoadhesive character of CS present in these micelles may promote adherence to the mucosal layer of the model, favoring the immediate permeation of PTX. Moreover, it has already been described by other authors that, indeed, CS has an ability to open tight junctions (TJs) [47]. In addition, in co-culture model, the tightness of TJ is known to be not so high as Caco-2 cells, but more close to the human condition.
In vitro cumulative permeability profiles of PTX-loaded CS-g-PCL micelles across Caco-2 monoculture model (circles) and across Caco-2/HT29-MTX co-culture model (squares). All experiments were conducted from the apical to basolateral compartment in HBSS at 37 °C. Error bars represent mean ± SD (n = 3)
After 60 min of experiment, the overall amount of PTX reaching the basolateral side was around 5% when tested in Caco-2 model, while for co-culture model, it increased to 7%.
Regarding the PTX-loaded CS-g-PCL micelles in Caco-2 monolayer, the P app obtained was 7.5 ± 7.0 × 10−6 cm/s. This result was 2.4-fold higher than for free PTX (Fig. 8). In addition, in a work developed by Mo et al., with micelles based on CS, the P app obtained was 5-fold improved in comparison with free PTX [1]. The authors related this increase to the mucoadhesive character of CS as well as the positive charge of the micelles which promotes the opening of TJs [48], which is in agreement with the work developed. However, in the present work, lower PTX concentration was used, which may justify the different results.
Apparent permeability coefficients of free PTX and PTX-loaded micelles across Caco-2 monoculture model (white columns) and across Caco-2/HT29-MTX co-culture model (filled columns). All experiments were conducted from the apical to basolateral compartment in HBSS at 37 °C. Error bars represent mean ± SD (n = 3)
For Caco-2/HT29-MTX co-culture model, P app of PTX from CS-g-PCL micelles was slightly higher compared to the Caco-2 model (8.8 ± 7.8 × 10−6 cm/s). These P app were approximately 2-fold compared to the free drug, which was a good improvement of PTX bioavailability in the model that more closely represents the intestinal barrier due to the mucus production and the formation of TJs less tight as Caco-2 monolayer [24]. In general, PTX-loaded CS-g-PCL micelles improved PTX permeation through intestinal models, mainly in the co-culture model, where a higher and more controlled delivery was achieved, enhancing 2-fold PTX permeation.
Conclusion
In this work, CS-g-PCL was synthetized by a carbodiimide reaction with the ability to form micelles by self-assembly. The chemical synthesis was successfully confirmed based on FTIR, 1H NMR, and contact angle technique. The chosen micellar system presented an average size of 408 ± 40 nm and spherical shape. The positively charged micelles presented high PTX association efficiency, convenient to be used as drug delivery system. The in vitro assays demonstrated PTX-loaded CS-g-PCL micelles as safe carrier of PTX and a promising system to improve their bioavailability. The cytotoxicity studies of micelles showed safety levels for 4 and 24 h of incubation for both colorectal cancer cells unlike the free drug, which showed evident toxicity patterns. Moreover, micelles were able to improve the intestinal permeability of PTX in two intestine cell-based models, more evident in presence of mucus, highlighting the mucoadhesiveness properties of the polymeric micelles. Therefore, CS-g-PCL micelles may be a potential candidate as vehicle of PTX intestinal delivery.
References
Mo R, Jin X, Li N, Ju C, Sun M, Zhang C, et al. The mechanism of enhancement on oral absorption of paclitaxel by N-octyl-O-sulfate chitosan micelles. Biomaterials. 2011;32(20):4609–20.
Malingré MM, Beijnen JH, Schellens JH. Oral delivery of taxanes. Invest New Drug. 2001;19(2):155–62.
Sparreboom A, Van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, et al. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci. 1997;94(5):2031–5.
Rowinsky E, Wright M, Monsarrat B, Lesser G, Donehower RC. Taxol:Pharmacology, metabolism and clinical implications. Cancer Surv. 1993;17:283–304.
Rowinsky EK, Onetto N, Canetta RM, Arbuck SG. Taxol: the first of the taxanes, an important new class of antitumor agents. Semin Oncol. 1992;19(6):646–62.
Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235(1):179–92.
Huo M, Zhang Y, Zhou J, Zou A, Yu D, Wu Y, et al. Synthesis and characterization of low-toxic amphiphilic chitosan derivatives and their application as micelle carrier for antitumor drug. Int J Pharm. 2010;394(1):162–73.
Landry F, Bazile D, Spenlehauer G, Veillard M, Kreuter J. Degradation of poly (D, L-lactic acid) nanoparticles coated with albumin in model digestive fluids (USP XXII). Biomaterials. 1996;17(7):715–23.
Pillai G. Nanomedicines for cancer therapy: an update of FDA approved and those under various stages of development. SOJ Pharm Pharm Sci. 2014;1(2):13.
Duan K, Zhang X, Tang X, Yu J, Liu S, Wang D, et al. Fabrication of cationic nanomicelle from chitosan-graft-polycaprolactone as the carrier of 7-ethyl-10-hydroxy-camptothecin. Colloids Surf B Biointerfaces. 2010;76(2):475–82.
Gu C, Le V, Lang M, Liu J. Preparation of polysaccharide derivates chitosan-graft-poly (ɛ-caprolactone) amphiphilic copolymer micelles for 5-fluorouracil drug delivery. Colloids Surf B Biointerfaces. 2014;116:745–50.
Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24(1):1–16.
Ahmad Z, Shah A, Siddiq M, Kraatz H-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014;4(33):17028–38.
Joshi N, Saha R, Shanmugam T, Balakrishnan B, More P, Banerjee R. Carboxymethyl-chitosan-tethered lipid vesicles: hybrid nanoblanket for oral delivery of paclitaxel. Biomacromolecules. 2013;14(7):2272–82.
Dash M, Chiellini F, Ottenbrite R, Chiellini E. Chitosan—a versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci. 2011;36(8):981–1014.
Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliver Rev. 2010;62(1):3–11.
Sosnik A, das Neves J, Sarmento B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: a review. Prog Polym Sci. 2014;39(12):2030–75.
Hsu L-W, Lee P-L, Chen C-T, Mi F-L, Juang J-H, Hwang S-M, et al. Elucidating the signaling mechanism of an epithelial tight-junction opening induced by chitosan. Biomaterials. 2012;33(26):6254–63.
Silva DS, Delezuk JA, La Porta FA, Longo E, Campana-Filho SP. Comparison of experimental and theoretical data on the structural and electronic characterization of chitin and chitosan. Curr Phys Chem. 2015;5(3):206–13.
Wiens M, Elkhooly TA, Schröder H-C, Mohamed TH, Müller WE. Characterization and osteogenic activity of a silicatein/biosilica-coated chitosan-graft-polycaprolactone. Acta Biomater. 2014;10(10):4456–64.
Khan AM, Shah SS. Determination of critical micelle concentration (CMC) of sodium dodecyl sulfate (SDS) and the effect of low concentration of pyrene on its CMC using ORIGIN software. J Chem Soc Pak. 2008;30(2):186.
Rao JP, Geckeler KE. Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci. 2011;36(7):887–913.
Antunes F, Andrade F, Araújo F, Ferreira D, Sarmento B. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur J Pharm Biopharm. 2013;83(3):427–35.
Araújo F, Sarmento B. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int J Pharm. 2013;458(1):128–34.
Van de Velde K, Kiekens P. Structure analysis and degree of substitution of chitin, chitosan and dibutyrylchitin by FT-IR spectroscopy and solid state 13 C NMR. Carbohydr Polym. 2004;58(4):409–16.
Le Tien C, Lacroix M, Ispas-Szabo P, Mateescu M-A. N-acylated chitosan: hydrophobic matrices for controlled drug release. J Control Release. 2003;93(1):1–13.
Brinkley M. A brief survey of methods for preparing protein conjugates with dyes, haptens and crosslinking reagents. Bioconjug Chem. 1992;3(1):2–13.
Elzein T, Nasser-Eddine M, Delaite C, Bistac S, Dumas P. FTIR study of polycaprolactone chain organization at interfaces. J Colloid Interface Sci. 2004;273(2):381–7.
Yu H, Wang W, Chen X, Deng C, Jing X. Synthesis and characterization of the biodegradable polycaprolactone-graft-chitosan amphiphilic copolymers. Biopolymers. 2006;83(3):233–42.
Lee K, Kwon I, Kim Y-H, Jo W, Jeong S. Preparation of chitosan self-aggregates as a gene delivery system. J Control Release. 1998;51(2):213–20.
Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine. 2010;5(3):485–505.
Wang X-H, Tian Q, Wang W, Zhang C-N, Wang P, Yuan Z. In vitro evaluation of polymeric micelles based on hydrophobically-modified sulfated chitosan as a carrier of doxorubicin. J Mater Sci Mater Med. 2012;23(7):1663–74.
Hu F-Q, Ren G-F, Yuan H, Du Y-Z, Zeng S. Shell cross-linked stearic acid grafted chitosan oligosaccharide self-aggregated micelles for controlled release of paclitaxel. Colloids Surf B Biointerfaces. 2006;50(2):97–103.
Zhu H, Liu F, Guo J, Xue J, Qian Z, Gu Y. Folate-modified chitosan micelles with enhanced tumor targeting evaluated by near infrared imaging system. Carbohydr Polym. 2011;86(3):1118–29.
Danhier F, Lecouturier N, Vroman B, Jérôme C, Marchand-Brynaert J, Feron O, et al. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: in vitro and in vivo evaluation. J Control Release. 2009;133(1):11–7.
Garinot M, Fiévez V, Pourcelle V, Stoffelbach F, des Rieux A, Plapied L, et al. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J Control Release. 2007;120(3):195–204.
Soppimath KS, Tan DW, Yang YY. pH-triggered thermally responsive polymer core–shell nanoparticles for drug delivery. Adv Mater. 2005;17(3):318–23.
Muley P, Kumar S, El Kourati F, Kesharwani SS, Tummala H. Hydrophobically modified inulin as an amphiphilic carbohydrate polymer for micellar delivery of paclitaxel for intravenous route. Int J Pharm. 2016;500(1):32–41.
Duong HHP, Yung L-YL. Synergistic co-delivery of doxorubicin and paclitaxel using multi-functional micelles for cancer treatment. Int J Pharm. 2013;454(1):486–95.
Werner ME, Cummings ND, Sethi M, Wang EC, Sukumar R, Moore DT, et al. Preclinical evaluation of Genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;86(3):463–8.
Araújo F, Shrestha N, Shahbazi M-A, Fonte P, Mäkilä EM, Salonen JJ, et al. The impact of nanoparticles on the mucosal translocation and transport of GLP-1 across the intestinal epithelium. Biomaterials. 2014;35(33):9199–207.
Sgorla D, Almeida A, Azevedo C, Bunhak ÉJ, Sarmento B, Cavalcanti OA. Development and characterization of crosslinked hyaluronic acid polymeric films for use in coating processes. Int J Pharm. 2016;511(1):380–9.
ISO10993-05. Biological evaluation of medical devices—part 5: tests for in vitro cytotoxicity. Geneva: International Organization for Standardization; 2009.
Zhang L, He Y, Ma G, Song C, Sun H. Paclitaxel-loaded polymeric micelles based on poly (ɛ-caprolactone)-poly (ethylene glycol)-poly (ɛ-caprolactone) triblock copolymers: in vitro and in vivo evaluation. Nanomedicine: NBM. 2012;8(6):925–34.
Grès M-C, Julian B, Bourrié M, Meunier V, Roques C, Berger M, et al. Correlation between oral drug absorption in humans, and apparent drug permeability in TC-7 cells, a human epithelial intestinal cell line: comparison with the parental Caco-2 cell line. Pharm Res. 1998;15(5):726–33.
Lesuffleur T, Barbat A, Dussaulx E, Zweibaum A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990;50(19):6334–43.
Yeh T-H, Hsu L-W, Tseng MT, Lee P-L, Sonjae K, Ho Y-C, et al. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials. 2011;32(26):6164–73.
Lin Y-H, Mi F-L, Chen C-T, Chang W-C, Peng S-F, Liang H-F, et al. Preparation and characterization of nanoparticles shelled with chitosan for oral insulin delivery. Biomacromolecules. 2007;8(1):146–52.
Acknowledgements
This work was financed by Fundo Europeu de Desenvolvimento Regional (FEDER) funds through the COMPETE 2020—Operacional Programme for Competitiveness and Internationalization (POCI), Portugal 2020 (NORTE-01-0145-FEDER-000012), and by Portuguese funds through Fundação para a Ciência e a Tecnologia (FCT)/Ministério da Ciência, Tecnologia e Inovação in the framework of the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274). This research was also partially supported by CESPU/IINFACTS under the project NanoGum-CESPU-2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Almeida, A., Silva, D., Gonçalves, V. et al. Synthesis and characterization of chitosan-grafted-polycaprolactone micelles for modulate intestinal paclitaxel delivery. Drug Deliv. and Transl. Res. 8, 387–397 (2018). https://doi.org/10.1007/s13346-017-0357-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0357-8