Abstract
This chapter brings to the reader vast clinical information on severe preeclampsia-eclampsia and obstetric hypertensive crisis (SP-EOHC). Prior to discussing the management of affected women, the author focuses on epidemiological, aetiological and pathophysiological background on SP-EOHC, with evidence-based team approach to critical maternal and perinatal care. Special attention is directed at the management of seizures (using magnesium sulphate) and hypertensive crisis (using nifedipine, hydralazine and labetalol) with emphasis on careful choice of drug medication. Attention is directed at the obstetric management of the mother with emphasis on surveillance aimed at ensuring maternal and perinatal safety by careful intrapartum and postnatal maternal care. The chapter ends with a brief on puerperal and later life obstetric and medical surveillance of mothers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning ObjectivesThis chapter brings to the reader vast clinical information in respect of severe preeclampsia-eclampsia and obstetric hypertensive crisis (SP-EOHC):
-
Epidemiological, aetiological and pathophysiological background on SP-EOHC
-
Focus on evidence-based approach to critical maternal and perinatal care
-
Team approach to patient management
-
Management of seizures and hypertensive crisis with emphasis on careful choice of drug medication
-
Obstetric management with emphasis on surveillance aimed at assuring maternal and perinatal safety
-
Critical intrapartum and postnatal maternal care
-
Puerperal and later-life obstetric and medical surveillance of mothers
1 Introduction
Despite the worldwide decline in maternal mortality rates in the last two decades, high rates still exist, notably in impoverished communities, with over 85% living in sub-Saharan Africa and Southern Asia [1]. At the country level, in 2008, Nigeria (with 50,000 maternal deaths) was second only to India (with 63,000 maternal deaths) out of a global total of 358,000 maternal deaths [2]. And, according to a UNFPA report, India (19% or 56,000) and Nigeria (14% or 40,000) accounted for roughly one-third of the global maternal deaths in 2010 [3].
Latest figures in 2012 show a decreasing global trend with an annual total of 287,000 deaths in 2010 from a total of 543,000 deaths in 1990 [4]. Nigeria does not appear to benefit from this global trend. Nigeria is one of the 10 most unsafe countries for a woman giving birth and is reportedly responsible for 14% of the world’s maternal deaths [5]. Nigeria’s maternal mortality ratio (MMR) is estimated to be 576 deaths per 100,000 live births, with a lifetime risk of 1 in 30 women [5]. Health facility MMRs in different regions in Nigeria covering specific periods of time over the past three decades from 1988 include the following:
Olatunji et al. from Shagamu (1696/100,000 live births); Alabi et al. from Federal Medical Centre, Kogi State (463/100,000 live births); Onakewhor and Gharoro, UBTH, Edo State (454/100,000 live births); Igberase and Ebeigbe, Baptist Medical Centre, Eku, Delta State (2232/100,000 live births); Kullima et al. Federal Medical Centre, Nguru (2849/100,000 live births); and Oladapo et al. OOUTH, Shagamu, (2989/100,000 live births) [6].
Sadly, severe preeclampsia-eclampsia and obstetric hypertensive crisis (SP-EOHC) contributes an increasing prominent quota to these uncontrolled deaths. Preeclampsia and eclampsia are the leading causes of maternal mortality and are responsible for 28% of maternal deaths, followed by haemorrhage (24%) and pregnancy-related infection/sepsis (14.2%) [7]. The proportion of MMR that is attributable to preeclampsia/eclampsia ranges from 12.4% of 97 maternal deaths in South Eastern [8] to 42.2% of 277 maternal deaths in North Western Nigeria [9].
The industrialised countries present a totally different picture. Successive UK triennial Confidential Enquiry into Maternal Deaths (CEMD) reports over the past two decades show a decreasing trend in the contribution of hypertensive crisis to maternal morbidity and mortality [9,10,11]. In the most recent CEMD report (2009–2012) [12], deaths from SP-EOHC are now at the lowest ever recorded rate; the rate decreased significantly between 2006–2008 (19 deaths – 0.83/100,000 maternities) and 2010–2012 (9 deaths – 0.38/100,000 maternities). This follows the introduction of NICE guidelines on hypertension in pregnancy in 2010 [13].
It is pertinent to note that in the 2006–2008 UK triennial report (2011) [14], 9 of the 19 maternal deaths resulting from SP-EOHC occurred among black women, six of whom were black Africans. Black African women seem particularly susceptible to aggressive forms of preeclampsia. In Nigeria, preeclampsia and eclampsia are responsible for about 8000 of the estimated 57,000 maternal deaths that occur annually.
This chapter focuses on evidence-based approach to the critical care management of severe preeclampsia and obstetric hypertensive crisis (SP-EOHC). The aim is to increase the safety and stability of the woman and constantly monitor foetal well-being as an integral part of management protocol.
2 Aetiology of Preeclampsia: Abnormal Placentation
Figure 27.1 [15] presents a graphic representation of events at the placental site with explanation provided on the aetiological sequence of preeclampsia.
In normal placental development, invasive cells of placental origin (trophoblast) invade maternal uterine spiral arteries (a) transforming them from smallcalibre resistance vessels (b) at the myometrial JZ, to high-calibre capacitance vessels, providing adequate perfusion to sustain foetal development (c). (Courtesy Crocker and Heazell [15])
The net result is impaired trophoblast invasion and the spiral arteries remain as small calibre resistance vessels resulting in reduced placental perfusion and ischaemia.
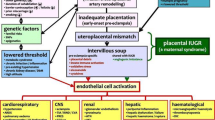
Brosens et al. have painstakingly studied the disorders of deep placentation and provided evidence to implicate this pathology as an important background to major negative obstetric and perinatal outcomes ‘Major Obstetric Syndromes’ in affected women [16] (Table 27.1 below).
Figure 27.2 graphically shows the ‘cascade mechanisms’ of events that finally result in severe preeclampsia and eclampsia and the numerous serious systemic complications. These complications affect the haematological, hepatic, cardiovascular, renal, and central nervous (including the eyes) systems, and the placenta [17].
2.1 Mechanism of Seizures Complicating Preeclampsia
Two hypotheses are propounded regarding the mechanism of seizures complicating preeclampsia [18].
-
1.
The cerebral circulation is in a state of ‘over-autoregulation’ during preeclampsia in response to elevated cerebral perfusion pressure causing transient ischaemia complicated by vasogenic oedema (and reversible clinical course). This pathological process does not progress to ischaemic necrosis that would be complicated by cytotoxic oedema (and irreversible cerebral cortical lesion).
-
2.
This is a form of hypertensive encephalopathy during which a rapid BP rise overcomes the myogenic cerebral arterial (and arteriolar) vasoconstriction, causing the loss of autoregulatory capacity and blood-brain barrier (BBB) disruption with subsequent vasogenic oedema. The unexplained propensity for hyperperfusion and oedema formation in the posterior cerebral cortex is termed the posterior reversible encephalopathy syndrome (PRES) . Suggested possible explanation is the decreased sympathetic innervations of the posterior cerebral arteries.
3 Principles of Management of Severe Preeclampsia and Eclampsia
The WHO Technical Consultation [14, 19] made a total of 23 recommendations on interventions in the management of women with severe preeclampsia and eclampsia. Ten of these interventions have strong supporting evidence, and four strongly lack supporting evidence. These recommended interventions need to be adopted, with local adaptations where necessary, in the protocol for patient management.
4 Team Approach
Success with comprehensive emergency obstetric care (CEmOC), especially with respect to SP-EOHC, is very much tied to team approach that relies on active timely full participation of all team members. Specifically, emphasis is now tilted in the direction of meticulous team approach to maternity care based on the concept of a 24-hour health institutional combat readiness (24H-HICR). The ACOG identifies the continued development of the Obstetric Gynaecologic Hospitalist (Labourist) Model as the potential approach to achieving professional and patient satisfaction while maintaining safe and effective care across delivery settings [20,21,22,23,24]. An expert review by Olson et al. [25] addresses the advantages, challenges, and variety of hospitalist models, focusing on what may be considered an emerging trend and a sustainable model for improved patient care and safety. The RCOG in collaboration with the National Health Service (NHS) has adopted a policy of increased senior medical staff participation in emergency obstetric care with specific injunction on ‘increased consultant presence in delivery suites’. [26,27,28] The team approach to CEmOC constitutes the main focus for the SP-EOHC management protocol.
The key personnel required for sustainable ‘24-HICR’ obstetric team include the following:
-
1.
Obstetric Registrar on call.
-
2.
Anaesthetic Registrar on call.
-
3.
Senior labour ward midwife.
-
4.
Consultant Obstetrician on call.
-
5.
Consultant Anaesthetist/Intensivist [22] on call.
-
6.
Haematology and clinical chemistry laboratory staff need to be aware of case.
-
7.
Hospital administrative officer-on-duty needs to be aware of case.
Criteria for patient inclusion in the management protocol [14]
Any woman with severe proteinuric hypertension where the decision has been made to deliver and with one of the following criteria (1–3, or):
-
1.
Hypertension (>140/90 mm Hg) with proteinuria (>0.3 g/day or >2+) and at least one of the following:
-
(a)
Headache, visual disturbance, epigastric pain.
-
(b)
Clonus (>3 beats).
-
(c)
Platelet count less than 100 × 109/L, ALT (Alanine aminotransferase) >50 iu/l.
-
(d)
Creatinine greater than 100 or creatinine clearance less than 80.
-
(a)
-
2.
Severe hypertension (systolic ≥160 mm Hg or diastolic ≥110 mm Hg) with proteinuria (>0.5 g/day or >2+).
-
3.
Eclampsia.
-
4.
Clinical discretion should be used to include women who present with atypical symptoms.
Identifying life-threatening severe preeclampsia
-
Severe HTN in association with proteinuria.
-
HTN in association with severe proteinuria (≥5 g/24 hours).
-
Clinical evidence of multi-organ involvement:
-
Pulmonary oedema
-
Seizures
-
Oliguria (<500 ml/24 hours)
-
Thrombocytopenia (<105/mm3)
-
Abnormal liver enzymes/epigastric pain/right upper quadrant pain
-
Persistent severe neurological symptoms (altered mental status, headaches, blurred vision or sudden blindness)
-
-
Oedema not a mandatory diagnostic feature but a warning presentation when present.
-
The HELLP syndrome represents a peculiar presentation of severe preeclampsia.
Differential Diagnoses of Eclampsia
It is vitally important that any medical staff summoned to see an obstetric patient presenting with convulsions has the following in mind:
-
1.
Eclampsia
-
2.
Epilepsy
-
3.
Cerebral malaria
-
4.
A. Meningitis
B. Encephalitis
-
5.
Diabetes mellitus
-
Hyperglycaemia
-
Hypoglycaemia
-
-
6.
Drug intoxication
-
7.
SS disease – Cerebral vascular ischaemia
-
8.
Raised intracranial pressure/intracranial vasculopathy:
-
Abscess
-
Tumour
-
Ruptured aneurysm
-
Haemorrhage from other sources
-
Maternal Observations and Investigations
-
1.
Monitoring requires a one-to-one expert nursing care.
-
2.
All maternal observations should be recorded on a specialised pregnancy-induced hypertension chart or an ICU chart.
-
3.
Oxygen saturation should be continuously monitored when possible.
-
4.
Blood pressure recordings should be made every 15–20 minutes.
-
5.
Maternal temperature should be recorded hourly.
-
6.
A Foley catheter should be in situ and hourly urinary output measured.
-
7.
Routine blood samples should be taken every 12–24 hours, including full blood count, urea and electrolytes, creatinine and liver function tests.
Foetal monitoring
Minimum assessment of foetal well-being should include the following:
-
1.
Growth assessment scans by medical staff.
-
2.
Liquor volume assessment.
-
3.
Continuous external foetal monitoring (CTG).
-
4.
Umbilical cord Doppler if the woman’s condition allows, when possible.
5 Management of Seizures [29]
5.1 Management of Seizures: The +MAGPIE Study
The Eclampsia Trial Collaborative Group (ETCG), co-ordinated by the Perinatal Trials Service in Oxford, conducted a landmark multicentre study on 1687 women with eclampsia from 28 centres in South America, India and Africa comparing Magnesium sulphateFootnote 1 with phenytoin on one hand, and magnesium sulphate with Valium on the other. The ‘MAGPIE Study’Footnote 2 (1995) established that magnesium sulphate is more effective in preventing recurrent seizures following eclampsia than either phenytoin or diazepam [30,31,32,33,34]. Unfortunately, difficulties still exist regarding acceptance and use of MgSO4 in many developing countries, including Nigeria [35].
5.2 Mechanisms of Magnesium Sulphate for Seizure Prevention [18]
The mechanisms by which magnesium sulphate (MgSO4) is effective at preventing eclamptic convulsions are likely multifactorial and have been reviewed in many publications and will only be summarised here. MgSO4 is a calcium antagonist and as such could inhibit vascular smooth muscle contraction. MgSO4 is a potent vasodilator, however, its effects in the cerebral circulation are considerably less effective than systemic vasculature. In addition, the sensitivity to MgSO4 is decreased in cerebral arteries from late-pregnant and post-partum animals, suggesting MgSO4 is not acting as a vasodilator in the cerebral circulation. MgSO4 has been shown to protect the blood-brain barrier (BBB), likely through its calcium antagonistic effects in the cerebral endothelium. When pregnant rats were treated with clinically relevant doses of MgSO4, they had significantly less BBB permeability during acute hypertension. Thus, MgSO4 could prevent recurrent seizures by protecting the BBB. Lastly, MgSO4 is an N-Methyl-D-aspartate (NMDA) receptor antagonist and thus would act as an anticonvulsant if it were in high enough concentration in the brain.
5.3 Management of Seizures
-
1.
General measures [36] valid for all types of seizures: Securing airway, oxygenation circulation:
-
Turn patient to side.
-
Protect patient from injury but do not restrain patient.
-
Maintain airway (insert airway if possible) + give 100% O2 by tight face mask once seizures stop.
-
Suction mouth if necessary.
-
Establish IV access for medication, hydration, blood work.
-
-
2.
All women with eclampsia should be treated with magnesium sulphate as the first-line drug of choice. The intravenous (IV) route is preferable.
-
3.
A loading dose of 4 g magnesium sulphate should be given (IV) over 5–10 minutes. With this bolus dose as an immediate protection, a second (intramuscular) loading dose 10 g (Not favoured by many clinicians) is given as two injections of 5 g in each buttock, followed by a maintenance infusion of 1–2 g/hour IV per hour, continued for at least 24 hours after the last seizure. Recurrent seizures should be treated by a further I.V. bolus of 2 g magnesium sulphate.
-
4.
Use of diazepam on mothers, especially in late pregnancy, is generally discouraged; may be administered in single doses if fits continue, at the discretion of the consultant/neurologist. Prolonged use of diazepam is associated with increased incidence of maternal mortality.
-
5.
Magnesium levels (normal value 0.7–1.0 mmol/litre) should be monitored when repeat fits occur or renal compromise is evident (therapeutic range 2–4 mmol/litre).
-
6.
Deep tendon reflexes should be monitored hourly when magnesium therapy is commenced. If reflexes are absent, or respirations are less than 14 per minute, or SaO2 less than 95%, magnesium therapy should be stopped.
-
7.
Monitor urinary output – should not be less than 25 ml/hour.
-
8.
If the fits continue, it is important to exclude other causes of fits and a CT scan should be considered.
-
9.
Reversal of the depressant effects of MgSO4: Give 10 ml of 10% calcium gluconate (1 g IV) over 10 minutes.
-
10.
Prophylactic H2 antagonists (antacids) should be given until the woman is transferred to normal postnatal care.
6 Use of Magnesium Sulphate: Maternal, Perinatal, Infant and Late Childhood Effects [37,38,39,40,41]
A country-wide multicentre, placebo-controlled, double-blind randomised trial was conducted by Rouse et al. [37] in the USA in 2008, involving 2241 expectant mothers at imminent risk for delivery between 24 and 31 weeks’ gestation. Women assigned to receive MgSO4 were given a 6-g bolus intravenously followed by a constant infusion of 2 g per hour, or matching placebo, for about 3 hours . Maternal effects of MgSO4 treatment, significant but tolerable included flushing, sweating, pain/burning at intravenous site, nausea or vomiting and respiratory depression. Foetal exposure to magnesium sulphate before anticipated preterm delivery did not reduce the combined risk of moderate/severe cerebral palsy or death (stillbirth/infant death) However, the rate of cerebral palsy was reduced among child survivors. Other publications [38,39,40] essentially confirm the foetal neuroprotective effect of MgSO4. A more recent meta-analysis and Cochrane review by Sana Usman et al. [41] from London, UK, on the use of MgSO4 in preterm deliveries provides further evidence in favour of modest neuroprotective effect which is greater the earlier the gestational age at delivery. The authors recommend its use in preterm deliveries less than 30–32 weeks of gestation, guided by local policy.
Specifically, the effects of magnesium sulphate on intrapartum foetal CTG and perinatal outcome have been scrutinised in a study by Duffy et al. [42] In a 4-year retrospective cohort study (from Washington University, St Louis, MO, USA) of 5387 consecutive term deliveries, of whom 248 (4.6%) had prenatal MgSO4 for severe preeclampsia/eclampsia, women exposed were compared to women not exposed to magnesium. The results show that maternal exposure to magnesium is associated with lower fetal heart rate (FHR) baseline within the accepted normal range, decreased variability, and fewer prolonged decelerations without evidence of adverse effect on neonatal outcome. These findings suggest that magnesium minimally blunts foetal response towards tachycardia, marked variability and prolonged decelerations, thus implying an overall minimal masking effect of magnesium on signs of foetal distress, not severe enough to influence the overall perinatal outcome.
7 Management of Hypertension
Women with acute severe late pregnancy and intrapartum hypertension have significantly higher risk of severe maternal morbidity than women without severe hypertension [43]. Severe hypertension is defined as greater than 160/110 mm Hg or mean arterial pressure (MAP) greater than 125 mm Hg. Significantly, while diastolic BP is a useful index of severity of preeclampsia, elevated systolic BP (≥160) carries the serious risk of intracranial (intracerebral) haemorrhage [10, 12]. Cerebrovascular accident accounts for 15–20% of deaths from eclampsia. The risk of haemorrhagic stroke correlates directly with the degree of elevation in systolic blood pressure and is less related to, but not independent of, the diastolic blood pressure [17].
Aggressive antihypertensive therapy is recommended at sustained systolic and diastolic blood pressures of 160 and 110 mm Hg, respectively. The commonly used drugs for obstetric hypertensive crisis are hydralazine, immediate release oral nifedipine and labetalol. Protocols that include additional requirements in order to provide urgent IV hypertension therapy lead to unnecessary and dangerous delays (that can be fatal) in reducing time to treatment for severe hypertension for pregnant and post-partum patients [29].
It is pertinent to consider the danger attendant in induction of general anaesthesia in the midst of uncontrolled hypertension. Induction of anaesthesia and endotracheal intubation carry the grave risk of dangerously increasing an already high blood pressure to a catastrophic level that imperils the brain (cerebral haemorrhage), the heart (myocardial ischaemia) and the lungs (pulmonary oedema), all important causes of maternal death. Blood pressure must be brought down to safe levels before induction of general anaesthesia [29, 44, 45].
8 Hydralazine
Hydralazine was until recently considered the first-line drug of choice for management of severe hypertension titrated against blood pressure, except in the presence of tachycardia (greater than 120 bpm). The agreed dose is 5 mg IV repeated every 20 minutes, to a maximum cumulative dose of 20 mg (Fig. 27.3).
8.1 Obstetric Side Effects of Hydralazine
Hydralazine is still considered useful and effective for treatment of acute hypertensive crisis by advocates but many obstetricians now shy away from its adoption as a first-line drug of choice. The adverse effects are considered grave and include severe maternal ‘overshoot’ hypotension and considerably reduced placental blood flow that may be disproportional to administered doses, with resulting adverse effects on foetal heart rates – maternal and foetal tachycardia. The attendant increased caesarean section rates may occasionally be secondary to the foetal distress caused by these side effects. Other recorded side effects include increased incidence of placental abruption and maternal oliguria [45,46,47]. These side effects are considered sufficiently serious to warrant discontinuation of hydralazine use as a first-line drug in increasing number of obstetric units [45].
9 Adalat (Nifedipine)
Nifedipine notably in the form of immediate release oral nifedipine has now found a favourable place as a useful first-line antihypertensive medication. Oral nifedipine and intravenous labetalol regimes were found to be similarly effective, or nifedipine more effective in the control of acute hypertensive crisis [48, 49]. The ACOG has now added nifedipine as a first-line treatment option in the management of obstetric hypertensive crisis [29].
When sustained severe obstetric hypertension is established, the common regimen of oral nifedipine administered is 10 mg, repeated every 20 minutes with patient’s vital signs under surveillance. Usually, a cumulative maximum dose of 50 mg is required to bring the blood pressure to safe level – 140–150/90–100. Maintenance dose of nifedipine, 10 mg 8-hourly or long-acting nifedipine (Adalat-Retard), 20 mg 12-hourly, is administered. Other regimens include additional use of hydralazine or labetalol in combination with nifedipine [29].
Caution needs to be exercised in its use, notably when MgSO4 is in use because of the synergistic Ca++ – blocking effect, which can lead to profound hypotension and shock [29]. Sublingual nifedipine must be avoided at all cost because of possible irreversible severe hypotension with consequent severe maternal and perinatal morbidity and mortality.
10 Labetalol (Fig. 27.4)
10.1 Labetalol: Clinical Pharmacology
Labetalol combines both selective, competitive α1 – adrenergic blocking and non-selective, competitive β – adrenergic blocking activity in a single substance. In man, the ratio of α- to β-blockade are about 1:3 and 1:7 following oral and IV administration respectively. Labetalol produces dose-related falls in blood pressure without reflex tachycardia and without significant reduction in heart rate, through a mixture of its α- and β-blocking effects. Haemodynamic effects are variable with small non-significant changes in cardiac output.
Doses of labetalol that control hypertension do not affect renal function in mild-to-severe hypertensive patients with normal renal function.
Due to the α1-receptor blocking activity of labetalol, BP is lowered more in the standing than in the supine position, and symptoms of postural hypotension can occur. This should be considered during IV administration and positioning of patients. Such patients should not be allowed to move to an erect position unmonitored until their ability to do so is established.
Labetalol is now considered by increasing number of physicians as the first-line drug of choice for management of acute hypertensive crisis. Labetalol is considered to be at least as effective, more predictable, more acceptable, and produces no change in heart rate compared to hydralazine [45, 50]. Indeed, some studies suggest possible advantages and no apparent disadvantages for the foetus during its use in pregnancy [51]. The protocol of combined use of magnesium sulphate and Labetalol (Labet) adopted in Benin City, Nigeria was found to be beneficial in the plan to reduce mortality complicating severe preeclampsia, eclampsia and severe hypertensive crisis in pregnancy [52].
Treatment is commenced with parenteral labetalol (Trandate, Labet, Normodyne), 20 mg bolus, followed at 10–20 minute intervals by 40 mg, 80 mg, 80 mg and 80 mg boluses till the desired BP (140–150/85–95 mm Hg) is achieved. The maximum cumulative dose that can be given is 300 mg. This is followed up with oral labetalol, through nasogastric tube if necessary, commencing with a dose of 200 mg 8-hourly. The usual maintenance dosage of labetalol is between 200 and 400 mg twice daily (the maximum daily dose of 2400 mg is hardly applicable to obstetric patients).
11 Intravenous Use of Labetalol for Hypertensive Crisis
Two methods recommended:
-
1.
Repeated IV injections:
Initial dose of 20 mg by slow injection for 2 minutes; repeat injection of 40 mg after 10–20 minutes; and then repeated doses of 80 mg given at 10–20-minute intervals till the desired BP (130–150/85–95) is achieved. The maximum cumulative dose of labetalol that can be administered is 300 mg. The maximum effect on BP usually occurs within 5 minutes of administration. Continue antihypertensive treatment with oral Trandate (usually 200 mg 8-hourly).
-
2.
Slow continuous infusion:
Effective IV dose is usually in the range of 50–200 mg given at infusion rate of 2 mg minute. A total of 300 mg may be required. Monitor BP during infusion, and continuous infusion till satisfactory response is obtained. After discontinuation, oral labetalol is initiated.
Method: The contents of 2 vialsFootnote 3 (40 ml) are added to 160 ml of compatible IV fluids. The resultant 200 ml of solution will contain 200 mg labetalol (1 mg/1 ml). The diluted solution should be administered (using an Ivac or a Cardiff infusion pump at a rate of 2 ml/min) to deliver 2 mg/minute.
11.1 Side Effects
-
1.
Postural hypotension and scalp tingling.
-
2.
Bradycardia (Severe bradycardia controlled with atropine 1–2 mg intravenously).
-
3.
Nausea, vomiting, headache and epigastric pain.
-
4.
Foetal bradycardia and distress (caused by severe maternal bradycardia).
11.2 Precautions [29, 50]
-
1.
Patient should be kept in the supine position during IV administration.
-
2.
Labetalol injection should not normally be given to patients with digitalis resistant heart failure or atrio ventricular block.
-
3.
Caution with asthmatic patients or individuals prone to bronchospasm. Resulting bronchospasm is controlled by using selective – acting bronchodilator, for example, salbutamol, or atropine 1 mg IV.
-
4.
Caution with diabetics, as it may prevent appearance of features (e.g. tachycardia) of acute hypoglycaemia. Beta-blockade also reduces release of insulin in response to hyperglycaemia. Therefore, necessity for adjustment of diabetes treatment arises.
-
5.
Anaesthesia – With labetalol therapy, patient requires IV atropine prior to induction of GA; the effect of halothane on BP may be enhanced by labetalol.
-
6.
Caution with women with history of impaired hepatic function. Severe hepatocellular injury is a recorded very rare complication.
-
7.
Paradoxical hypertensive responses have been recorded with treatment of pheochromocytoma. Chung et al. reported intraoperative dramatic elevation of blood pressure during resection of a pheochromocytoma [53] This was caused by elevated systemic vascular resistance index (SVRI) and decreased cardiac index (CI), secondary to β-adrenergic blockade with labetalol. A suggestion is made for prompt replacement of labetalol with β-adrenergic blockers or other vasodilators when such a condition arises.
12 Treatment of Resistant Hypertension [29]
On the rare occasion when IV labetalol, hydralazine, or immediate release oral nifedipine fails to relieve severe hypertension, urgent consultation with an anaesthesiologist, intensivist, or maternal-foetal medicine specialist to discuss second-line intervention is recommended. Second-line alternatives include nicardipine or esmolol by infusion pump and sodium nitroprusside. Sodium nitroprusside should be reserved for extreme cases, and used for the shortest time, because of potential for maternal, foetal, and neonatal cyanide and thiocyanate toxicity, and increased maternal intracranial pressure and worsening of cerebral oedema.
13 Fluid Balance Management
The following guidelines need to be strictly adhered to in order to maintain safe fluid and electrolyte balance.
-
1.
Fluid intake should be restricted to 85 ml/hour because of the risk of renal and respiratory sequelae; the risk of grave respiratory complications, notably pulmonary oedema, is greater than renal dysfunction or failure [54].
-
2.
Urinary output should be measured hourly.
-
3.
500 ml human albumin solution (HAS) should be considered:
-
prior to antihypertensive therapy
-
prior to caesarean section
-
if oliguria is evident (defined as urinary output less than 100 ml in a consecutive 4-hour period)
-
prior to administration of regional anaesthesia.
-
-
4.
If HAS has previously been administered, the insertion of a central venous pressure (CVP) line should be considered. If further complications occur, especially oliguria, after the administration of HAS, a line is recommended. Although the success rate of the subclavian-vein route is greater than the antecubital-fossa route, clinicians should use the route with which they are more familiar.
-
5.
If the central venous pressure value is greater than 10 mm Hg, 20 mg furosemide should be considered.
-
6.
If the central venous pressure value is less than 10 mm Hg, HAS 500 ml should be considered.
-
7.
A further dose of 20–40 mg furosemide should be considered if there is persistent oliguria.
14 Obstetric Management
In the obstetric management of the patients, the choice lies between expectant and interventionist options. Expectant management [55, 56] includes in-patient care with steroids (with gestations remote from term), magnesium sulphate (as required), antihypertensive drugs and close maternal and foetal monitoring to identify indications for delivery. Also to be considered is in-utero transfer to centres with facility for intensive neonatal care for preterm babies. Figure 27.5 provides a clinical algorithm for the proposed management of severe preeclampsia remote from term. Table 27.2 provides a summary of indications for interventionist option in management [56, 57].
Proposal for management of severe preeclampsia remote from term. (Courtesy Sibai and Norwitz [55])
15 Eclampsia: Mode of Delivery
Caesarean section should no longer be considered a better option for women with eclampsia; indeed, abdominal surgery can place both mother and foetus at increased risk of adverse events, notably, if the mother is unstable and general endotracheal anaesthesia is used. Immediate delivery does not necessarily imply caesarean section; a properly managed and successful vaginal delivery is less haemodynamically stressful to the mother while providing additional advantages to term or late preterm neonate [57, 58]. A number of studies, including a randomised controlled pilot study by Seal et al. [59] provide evidence that a policy of early caesarean section delivery with ≥34 weeks’ gestation, is not associated with better maternal or perinatal outcomes.
The decision to proceed with delivery through abdominal or vaginal route should be individualised based on such factors as parity, gestational age, Bishop’s score, foetal status and maternal desire for vaginal birth. Prolonged inductions/labour must be avoided [60].
16 Post-partum SP-OHC [61]
The following need to be considered in the immediate post-partum surveillance of the woman with SP-OHC during the puerperium, notably in the first week:
-
1.
New-onset HTN-preeclampsia
-
2.
Persistent gestational HT N – preeclampsia
-
3.
Late-onset eclampsia
-
4.
HELLP syndrome
-
5.
Pre-existing/undiagnosed HTN – renal, adrenal, thyroid diseases
-
6.
Cerebral vasoconstriction syndrome
-
7.
Cerebral venous thrombosis/stroke
-
8.
TTP/haemolytic uraemic syndrome
Increased awareness of differential diagnosis constitutes a cornerstone in the management of the patient. Team approach with a stepwise multidisciplinary approach makes for timely accurate diagnosis and successful management. Ideally, post-partum surveillance, including monitoring of the woman’s blood pressure, requires an interval postnatal visit before the traditional 6 weeks postnatal visit. Thereafter women with persistent residual medical (cardiovascular, renal, neurological) challenges should be referred for appropriate medical consultation and follow-up.
17 SP-EOHC: Later-Life Medical Diseases
SP-EOHC may well represent a ‘milestone’ or an ‘eye-opener’ event that reflects a woman’s lifetime syndrome of medical disability that will, in later life, culminate in serious medical conditions such as chronic hypertension [62, 63], diabetes mellitus [64], kidney [65] and cardiovascular diseases and death [66]. A spectacular systematic review and meta-analysis by Bellamy et al. [67] on long-term consequences of preeclampsia revealed that the relative risks (RRs) for hypertension were 3.70 after 14 years follow-up, for ischaemic heart disease 2.16 after 12 years, for stroke 1.81 after 10 years, and for venous thromboembolism 1.87 after 5 years. Overall mortality after preeclampsia was increased 1.5-fold after 14 years. Long-term follow-up studies also reveal that babies born preterm or growth-restricted are at increased risk of developing hypertension, coronary artery disease, and diabetes mellitus, and for growth-restricted female neonates, preeclampsia in later life [68, 69].
18 Summary and Recommendation
This chapter has explored the severe morbidity and mortality attending SP-EOHC and the important criteria that determine the successful outcome of obstetric and perinatal care of patients. Team approach, attention to timely emergency care of mothers, and use of appropriate anticonvulsant and antihypertensive medication are crucial to successful outcome in obstetric and perinatal management. Careful postnatal and puerperal care and later-life meticulous multidisciplinary surveillance, in alliance with internists, is an essential follow-up component of patients’ care.
Notes
- 1.
(MgSO4·7H2O = Epsom Salt).
- 2.
MAGPIE = MAGnesium for PreventIon of Eclampsia.
- 3.
Two vials (20 ml/vial) of Trandate and Normodyne brands and four vials (10 ml/vial) of Labet brand.
References
Trends in Maternal Mortality 1990–2015. Estimates developed by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: WHO; 2015. p. 1–100.
Trends in Maternal Mortality 1990–2008. Estimates developed by WHO, UNICEF, UNFPA, and The World Bank. Geneva: WHO; 2010. Assessed 7 Sept 2010. www.who.int/en/reproductivehealth.
Trends in Maternal Mortality: 1990 to 2010. WHO, UNICEF, UNFPA, and The World Bank estimates. Geneva: WHO; 2012.
Department of Health. Why mothers die, 1997–1999: the confidential enquiries into maternal deaths in the UK. London: RCOG; 2001.
NPC & ICF International. Nigeria demographic and health survey 2013; 2014.
Kirk K, Chattopadhyay I. A systematic review of the treatment and management of preeclampsia and eclampsia in Nigeria. Washington, DC: Population Council, USAID; 2015. p. 13.
Oladapo O, Adetoro O, Ekele B, Chama C, Etuk S, Aboyej A, Gulmezoglu A. When getting there is not enough: a nationwide cross-sectional study of 998 maternal deaths and 1451 near-misses in public tertiary hospitals in a low-income country. Br J Obstet Gynaecol. 2015. https://doi.org/10.1111/1471-0528.13450.
Nwagha UI, Nwachukwu D, Dim C, Ibekwe PCP, Onyebuchi A. Maternal mortality trend in South East Nigeria: less than a decade to the millennium development goals. J Womens Health. 2002;19(2):323–7.
Nwobodo E, Ahmed Y. Maternal mortality associated with eclampsia in Sokoto, Nigeria. Orient J Med. 2011;23:1–4.
The confidential enquiry into maternal and child health (CEMACH). Why mothers die, 2000–2002. Report on confidential enquiries into maternal deaths in the United Kingdom. London: RCOG; 2004.
The confidential enquiry into maternal health (CEMACH): saving mothers’ lives. Reviewing maternal deaths to make motherhood safer, 2003–2005. CEMD in the UK. London: RCOG; 2007.
Knight M, Kenyon S, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk JJ, editors. Saving lives, improving mothers’ care: lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009–2012. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014. p. 1–97.
NICE – National Institute for Health and Care Excellence. CG107 hypertension in pregnancy. 2010. From http://www.nice.org.uk/guidance/CG107.
The confidential enquiry into maternal and child health (CMACE). Saving mothers’ lives. Reviewing maternal deaths to make motherhood safer: 2006–2008. 8th report of the CEMD in the UK. BJOG, An Int J Obstet Gynaecol. 2011;118(Suppl. 1):1–205.
Crocker I, Heazell A. Chapter 2: The pathophysiology of hypertension. In: Heazell A, Norwitz ER, Kenny LC, Baker P, editors. Hypertension in pregnancy. 1st ed. Cambridge: Cambridge University Press; 2010. p. 19–33.
Brosens I, Pijnenborg R, Vercruysse L, Romero R. “The great obstetrical syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201.
Norwitz ER, Chaur-Gong H, Repke JT. Acute complications of preeclampsia. Clin Obstet Gynecol. 2002;45(2):308–29.
Cipolla MJ, Kraig RP. Seizures in women with preeclampsia: mechanisms and management. Fetal Matern Med Rev. 2011;22(2):91–108.
World Health Organization. WHO Recommendations for prevention and treatment of preeclampsia and eclampsia. Geneva: WHO Press; 2011.
McCue B. What is an obstetrics/gynecology hospitalist? Obstet Gynecol Clin N Am. 2015;14(3):457–61.
Stevens TA, Swaim LS, Clark SL. The role of obstetrics/gynecology hospitalists in reducing maternal mortality. Obstet Gynecol Clin N Am. 2015;42(3):463–75.
American College of Obstetricians and Gynecologists. Committee opinion no. 459: the obstetric-gynecologic hospitalist. Obstet Gynecol. 2010;116:237–9.
Garite TJ, Levine L, Olson R. Business and organizational models of obstetric and gynecologic hospitalist groups. Obstet Gynecol Clin N Am. 2015;42(3):533–40.
American College of Obstetricians and Gynecologists (ACOG) Committee Opinion Number 657. The Obstetric and Gynecologic Hospitalist. ACOG Committee on patient safety, quality improvement and obstetric practice. Obstet Gynecol. 2016;127(2):419.
Olson R, Garite TJ, Fishman A, Andress IF. Obstetrician/gynecologist hospitalists: can we improve safety and outcomes for patients and hospitals and improve lifestyle for physicians? Am J Obstet Gynecol. 2012;207(2):81–6.
Edmonds S, Allenby K. Experiences of a 24-hour resident consultant service. TOG: Obstet Gynaecol. 2008;10:107–11.
Royal College of Obstetricians and Gynaecologists (RCOG). The future workforce in obstetrics and gynaecology in England and Wales: overview summary. London: RCOG; 2009.
Royal College of Obstetricians and Gynaecologists (RCOG). Labour ward solutions. RCOG Good Practice No. 10, January 2010.
American College of Obstetricians and Gynecologists (ACOG) Committee Opinion Number 692. Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. J Obstet Gynaecol. 2017;129:769–70.
Eclampsia Trial Collaborative Group. Which anticonvulsant for women with Eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet. 1995;345:1455–63.
Atallah. The Eclampsia Trial, a model of international collaborative study with worldwide benefits. Sao Paulo Med J. 1995;113(4):927–8.
Duley L, Henderson-Smart DJ, Chou D. Magnesium versus Phenytoin for Eclampsia. Cochrane Database Syst Rev. 2010;(10):CD000128.
Duley L, Henderson-Smart DJ, Walker GJ, Chou D. Magnesium sulphate versus diazepam for eclampsia. Cochrane Database Syst Rev. 2010;12:CD000127.
Fawcett WJ, Haxby EJ, Male DA. Magnesium: physiology and pharmacology (Review Article). Br J Anaesth. 1999;83:302–20. (pp 304–307).
Ekele BA. Use of magnesium sulphate to manage preeclampsia and eclampsia in Nigeria: overcoming the odds. Ann Afr Med. 2009;8(2):73–5.
Anthony J. Chapter 78: Critical care of the obstetric patient. In: James DK, Steer PJ, Weiner CP, Gonik B, Crowther CA, Robson SC, editors. High risk pregnancy, management options. 4th ed. St Louis: Elsevier Saunders; 2011. p. 1347–60.
Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulphate for the prevention of cerebral palsy. N Engl J Med. 2008;359(9):895–905.
Stanley FJ, Crowther C. Antenatal magnesium sulfate for neuroprotection before preterm birth? N Engl J Med. 2008;359(9):962–4.
Doyle LW, Crowther CA, Middleton P, Marret S. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2007;3:CD000025.
Royal College of Obstetricians and Gynaecologists (RCOG). The management of severe preeclampsia/eclampsia. Guideline No. 10(A). London: RCOG; 2006.
Usman S, Foo L, Tay J, Bennett PR, Lee C. Use of magnesium sulfate in preterm deliveries for neuroprotection of the neonate. RCOG – Obstet Gynaecol. 2017;19(1):21–8.
Duffy RC, Odibo AO, Roehl KA, Macones GA. Effect of magnesium sulfate on fetal heart rate patterns in the second stage of labor. Obstet Gynecol. 2012;119(6):1129–36.
Kilpatrick SJ, Abreo A, Greene N, Melsop K, Peterson N, Shields LE, Main EK. Severe maternal morbidity in a large cohort of women with acute severe intrapartum hypertension. Am J Obstet Gynecol. 2016;215(1):91.e1–7.
Unit of Perinatal and Paediatric Epidemiology, University of Liverpool. Regional audit of the management of severe pre eclampsia and eclampsia. Mersey perinatal annual report. Liverpool: University of Liverpool; 1996.
Dekker G. Chapter 35: Hypertension. In: James DK, Steer PJ, Weiner CP, Gonik B, et al., editors. High risk pregnancy, management options. 4th ed. St Louis: Elsevier Saunders; 2011. p. 599–626.
American College of Obstetricians and Gynecologists – Practice Bulletin # 125. Chronic hypertension in pregnancy. Obstet Gynecol. 2012;119(2):396–407.
Magee LA, Cham C, Waterman EJ, et al. Hydralazine for the management of hypertension in pregnancy: meta analysis. Br Med J. 2003;327:955–60.
Raheem IA, Saaid R, Omar SZ, Tan PC. Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: a randomized trial. Br J Obstet Gynaecol. 2012;119(1):78–85.
Shekhar S, Sharma C, Thakur S, Verma S. Oral nifedipine or intravenous labetalol for hypertensive emergency in pregnancy: a randomized controlled trial. Obstet Gynecol. 2013;122(5):1057–63.
Walker JJ, Greer I, Calder AA. Treatment of acute pregnancy-related hypertension: labetalol and hydralazine compared. Postgrad Med J. 1983;59(Suppl 3):168–70.
Pickles CJ, Symonds EM, Broughton PF. The fetal outcome in a randomized double-blind control trial of labetalol versus placebo in pregnancy-induced hypertension. Br J Obstet Gynaecol. 1989;96(1):38–43.
Unuigbe JA, Ebomwonyi IO, Sule Z, Agbon-Ojeme GE, Uwaifo JO, Omorogbe SO. Management of severe pre-eclampsia, eclampsia, and severe obstetric hypertensive crisis using a combination protocol of Magnesium Sulphate and Labetalol (Labet). Presented at the 9th RCOG International Conference in Athens, Greece, September 27–29, 2011.
Chung PCH, Li AH, Lin CC, Yang MW. Elevated vascular resistance after labetalol during resection of a pheocromocytoma. Can J Anesth. 2002;49(2):148–50.
Tuffnell DJ, Jankowicz D, Lindlow SW, et al. Outcome of severe preeclampsia/eclampsia in Yorkshire, 1999–2003. Br J Obstet Gynecol. 2005;112:875–80.
Sibai BM, Norwitz ER. Chapter 9: Management of severe preeclampsia. In: Heazell H, Norwitz ER, Kenny LC, et al., editors. Hypertension in pregnancy. 1st ed. Cambridge: Cambridge University Press; 2010. p. 129–40.
Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment and delivery indications. Am J Obstet Gynecol. 2007;196:514.el−9.
Repke JT, Norwitz ER. Management of eclampsia. In: Heazell A, Norwitz ER, Kenny LC, Baker PN, editors. Hypertension in pregnancy. 1st ed. Cambridge: Cambridge University Press; 2010. p. 141–58.
Churchill D, Duley L. Interventionist versus expectant care for preeclampsia before term. Cochrane Database Syst Rev. 2002;(3):CD003106.
Seal SL, Gosh D, Kamilya G, et al. Does route of delivery affect maternal and perinatal outcome in women with eclampsia? A randomized controlled pilot study. Am J Obstet Gynecol. 2012;206(6):484.e1–7.
Nassar AH, Adra AAM, Chakhtoura N. Severe preeclampsia remote from term: labor induction or elective caesarean delivery? Am J Obstet Gynecol. 1998;179:1210–3.
Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206(6):470–5.
Chelsey LC, Annitto JE, Cosgrove RA. The remote prognosis of eclamptic women. Am J Obstet Gynecol. 1976;124:446–59.
Sibai BM, Sarinoglu C, Mercer BM. Eclampsia V11. Pregnancy after eclampsia and long-term prognosis. Am J Obstet Gynecol. 1992;166:1757–61.
Sibai BM, el Nazer A, Gonzalez-Ruiz A. Severe preeclampsia-eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986;155:1011–6.
Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800–8009.
Funai EF, Friedlander Y, Paltiel O, et al. Long-term mortality after preeclampsia. Epidemiology. 2005;16:206–15.
Bellamy L, Casas JP, Hingorani AD, Williams DJ. Preeclampsia and the risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Br Med J. 2007;335:974–85.
Barker DJ. The developmental origins of well-being. Philos Trans R Soc Lond B Biol Sci. 2004;359:1359–66.
Innes KE, Marshall JA, Byers TE, Calonge N. A woman’s own birth weight and gestational age predict her later risk of developing preeclampsia, a precursor of chronic disease. Epidemiology. 1999;10:153–60.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Unuigbe, J.A. (2021). Critical Care Management of Severe Preeclampsia-Eclampsia and Obstetric Hypertensive Crisis. In: Okonofua, F., Balogun, J.A., Odunsi, K., Chilaka, V.N. (eds) Contemporary Obstetrics and Gynecology for Developing Countries . Springer, Cham. https://doi.org/10.1007/978-3-030-75385-6_27
Download citation
DOI: https://doi.org/10.1007/978-3-030-75385-6_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-75384-9
Online ISBN: 978-3-030-75385-6
eBook Packages: MedicineMedicine (R0)