Abstract
With advancing technology in glycobiology research over the past decade, there is now a large and ever-growing number of publications examining the biological and clinical significance of glycosylation in human diseases. In cholangiocarcinoma (CCA), various alterations of global glycosylation, both in core glycosylation of N-glycans and O-Glycans as well as in peripheral fucosylation and sialylation, have been identified. The aberrant glycosylation occurs in a large number of cellular glycoproteins, including secreted, membrane-bound, and nucleocytoplasmic proteins. Several aberrant expressed glycans and glycoconjugates are applicable as tumor markers for diagnosis and prognostic prediction of CCA. Moreover, some specific glycans appear to be associated with short survival of the CCA patients and played important roles in proliferation, migration, invasion, and chemoresistance of CCA cells. Suppression of glycosylation and glycan functions by specific siRNA, lectins, or inhibitors could significantly reduce the metastatic potential of CCA cells and enhance their chemosensitivity. These findings suggest the potential of targeting glycosylation for CCA treatment and will be discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Glycosylation is an enzymatic process that modifies glycans, lipids, or proteins with sugar molecules. The biosynthesis of glycan branches is effectuated through a specific set of glycosylation machinery, e.g., glycosyltransferases and glycosidases, sugar transporters, and activated sugar donors. The resulting glycan microheterogeneity and complexity is intricately involved with many physiological processes, e.g., cell identity, cell-cell cross talk, and cell-environment interactions. The specific terminal glycan modification can confer unique function and properties to oligosaccharides and is often regulated during ontogeny and cellular differentiation [1].
Changes in glycan patterns have been observed as early events in several pathological conditions and used as biomarkers of many diseases, including cancer. Alteration of glycan structure of cell surface glycoproteins was first reported in SV-40-transformed murine fibroblasts [2]. Since then, the significance of glycan patterns in tumor development and progression has been extensively studied [3]. At present, it is well accepted that aberrant glycosylation is a universal feature of cancer cells and plays a crucial role in cancer biology [4]. Aberrant glycans that modify the cell surface or the secreted glycoproteins of cancer cells can be potential cancer biomarkers [5] and therapeutically targeted and manipulated to provide a new avenue to improving cancer treatment [6]. Advancements in high-throughput approaches for glycome analysis have accelerated the glycoproteomics-based discovery of glyco-biomarkers and drawn much attention from researchers to investigate glycomics for medical applications. Lectin-based approaches [7,8,9,10] and mass spectrometry [11, 12] have been applied to determine the aberrant glycosylation in clinical samples from cholangiocarcinoma (CCA) patients.
In this chapter, we present a broad overview of cellular glycosylation processes and the dysregulation of glycosylation in cancer. We then describe the aberrant glycosylation, in core (N- and O-glycosylation) and peripheral glycosylation (fucosylation, sialylation) as well as O-GlcNAcylation and glycosphingolipid synthesis, in the development and progression of CCA. Lastly, we explore the potential of using these glycosylated products to improve diagnosis and treatment of CCA.
Glycans and Glycosylation in Biology
Biochemistry of Glycans
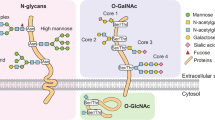
Glycans reported in eukaryotic cells can be classified according to the linkage to proteins or lipids and include glycoproteins, proteoglycans, sulfated glycosaminoglycans, hyaluronan, and glycosphingolipids (GSLs) [13, 14]. A glycoprotein is a glycoconjugate in which a protein carries one or more glycans attached to a polypeptide chain, mostly via N- or O-linkages [15]. An N-linked glycosylation refers to the co- or posttranslational modification of a polypeptide with an oligosaccharide that is covalently linked to the amide nitrogen of an asparagine (Asn) residue and can be classified into three main types: high-mannose, complex, and hybrid types [16]. N-glycosylation is the most common glycosylation observed in a large number of proteins synthesized in humans. O-linked glycosylation refers to the posttranslational linkage of an oligosaccharide to the OH group of a serine (Ser) or threonine (Thr) residue of a polypeptide. The first sugar that attaches to Ser or Thr can be mannose (Man), fucose (Fuc), galactose (Gal), glucose (Glc), N-acetylgalactosamine (GalNAc), N-acetylglucosamine (GlcNAc), or xylose (Xyl). An attachment of GalNAc to Ser/Thr is the most common O-glycosylation of membrane-bound and secreted glycoproteins such as mucin (MUC) glycoproteins; therefore, O-linked GalNAc modifications are generally called mucin-type glycosylation. One of the polypeptide N-acetylgalactosaminyltransferases (GalNAc-Ts) transfers a GalNAc to Ser or Thr of the specific proteins in different cell types and organs [17]. The oligosaccharide is further extended with Gal and GlcNAc, forming eight types of core mucin O-glycan [18, 19]. In addition, the linkage of Ser/Thr with a single molecule of GlcNAc, called O-GlcNAcylation, is another O-glycosylation that has been aggressively studied in the past decade. Several nucleocytoplasmic proteins, especially transcription factors, have been identified as O-GlcNAcylated proteins [20].
Proteoglycans are a polymeric glycoconjugate that contains one or more glycosaminoglycan chains, linear polysaccharides, and uronic acid or galactose, attached to a core protein [21, 22]. Hyaluronan is one of the glycosaminoglycans; it is unique from other classes of glycosaminoglycans in that it is not further modified by sulfation or by epimerization of the glucuronic acid moiety to iduronic acid [22]. A GSL consists of a glycan usually attached via glucose or galactose to the terminal primary hydroxyl group of the lipid moiety ceramide, which is composed of a long chain base (sphingosine) and a fatty acid; therefore, they can be neutral or acidic [21, 22]. A ganglioside is an acidic glycolipid containing one or more residues of sialic acid [23]. All forms of glycosylation are synthesized in the endoplasmic reticulum (ER) and Golgi apparatus, except O-GlcNAcylation.
Glycosylation Process
Unlike DNA, RNA, and protein, glycan structure is not directly encoded from the genome; rather, it is tightly regulated by a cellular process called “glycosylation” [13]. Particular glycan structures are generated and sequentially modified by a variety of glycosyltransferases and glycosidases as well as the presence of sugar donors inside the cells [19]. The sub-compartmentalization of glycan biosynthesis in the Golgi apparatus can also modulate the order of glycosylation [19]. A variety of glycan structures mediate the diversity and complexity of cellular biology. Furthermore, glycan structures and production can reflect small changes in intra- or extracellular environment.
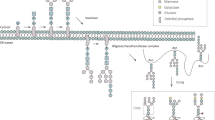
Protein N-linked glycosylation is compartmentalized in the ER. The biosynthesis of N-glycan precursors begins on the outer leaflet of the ER and is completed in the ER lumen, with proficiently glycosylated proteins then being exported to the Golgi apparatus for further elongation and addition of peripheral glycans moieties (Fig. 24.1) [16, 24, 25]. The biosynthesis of the core oligosaccharide (14-sugar glycan) for N-glycan requires several known enzymes. Firstly, the sugar moieties are sequentially added to the dolichol phosphate (Dol-P) on the outer part of the ER membrane by the asparagine-linked glycosylation family of glycosyltransferases [16, 24]. The mature oligosaccharide is then transferred to the acceptor protein in the sequence of asparagine-X-serine/threonine (X can be any amino acid except proline) by the oligosaccharyltransferase (OST) complex [24, 25]. The glucoses of the core oligosaccharide are then removed by glycosidase I–II. Generally, glycoproteins exiting ER to Golgi contain either eight or nine mannose residues of N-glycans. In the cis-Golgi, the oligomannoses are trimmed by α-mannosidases to form Man5, an important intermediate glycoform for synthesizing hybrid and complex N-glycans [16, 19]. In the last step of N-glycan processing, the biosynthesis of hybrid and complex N-glycans is started in the medial Golgi by adding and branching of GlcNAc to mannose residues via the action of N-acetylglucosaminyltransferases (GlcNAc-Ts) [16]. There are several glycosyltransferases, including galactosyltransferases (GalTs), fucosyltransferases (FUTs), and sialyltransferases (STs) involved in the extension of N-glycans to form mature N-glycans which ordinarily occur in the trans Golgi [16, 19].
Different from N-glycosylation, the synthesis of O-linked glycan is initiated by the addition of a monosaccharide to a Ser or Thr residue followed by the stepwise-elongation into an oligosaccharide chain. Biosynthesis of mucin-type O-linked glycosylation is initiated in the Golgi apparatus by the transfer of a GalNAc to a Ser or Thr residue on the peptide chain by one of the UDP-GalNAc enzymes (Fig. 25.1) [18]. The Tn antigen is further elongated to form complex O-glycan by several glycosyltransferases [18, 19].
Biosynthesis of protein glycosylation in mammalian cells. N-glycosylation is started in the ER by the synthesis of core N-glycan on a dolichol phosphate and then transferred to the peptide and elongated to a more complex oligosaccharide in the Golgi apparatus. Mucin-type O-glycosylation is started and sequentially modified in the Golgi apparatus by several glycosyltransferases to form the complex oligosaccharides
GSL biosynthesis starts with an addition of the first sugar to ceramide (Cer) and then transfer of subsequent sugars by glycosyltransferases. Cer is synthesized at the outer part of the ER and consequently equilibrates to the luminal part and transfers to the Golgi apparatus (Fig. 25.2) [26, 27]. Glucosylceramide (GlcCer) is synthesized at the cytoplasmic face of the ER and cis-Golgi apparatus and then flipped into the Golgi lumen, where it is further elongated by a series of glycosyltransferases. In contrast, galactosylceramide (GalCer) is synthesized inside of the ER lumen and consequentially traffics through the Golgi, where it can be sulfated to form sulfatide [26, 27].
The Significance of Glycans and Glycosylation in Medicine
Glycosylation is one of the important co- and/or posttranslational modifications required for modulating the normal biological function of cells and is necessary to control protein folding, conformation, localization, stability, and activity [14, 28]. Glycans have various structures and biophysical roles, including surface antigens, adhesion molecules, signaling receptors, cell-cell recognition, and cell-matrix interactions [29,30,31]. In addition, glycan elements in the matrix, such as proteoglycans, are important for maintaining tissue structure, porosity, and integrity [31]. Therefore, aberration of glycosylation has been shown to be involved in many human diseases, including cancer [28,29,30].
Several glycan/glycoprotein antigens have been used for detecting and monitoring the growth status of tumors. For CCA, these include carbohydrate antigen 19–9 (CA19–9), i.e., sialyl-Lewis A (sLea), attached to mucin glycoproteins and gangliosides and MUC5AC for screening, surveillance, and prognosis [34,35,36]. Examples in other tumors include CA15–3 (a sialylated O-glycan on MUC1), CA27–29 (recognizes MUC1), and HER2 for breast cancer; CEA for colon cancer; CA125 (i.e., MUC16) and human epididymis protein 4 (HE4) for ovarian cancer; alpha-fetoprotein (AFP) and its fucosylated form (i.e., AFP-L3) for hepatocellular carcinoma (HCC); sialyl-Lewis X (sLex)-related glycans for lung and breast cancers; prostate-specific antigen (PSA) for prostate cancer; and thyroglobulin for thyroid cancer [32, 33]. Although many candidate glycan/glycoprotein markers have been suggested, most of them yield only limited accuracy for cancer screening, diagnosis, prognosis, and/or monitoring. Therefore, the discovery of new biomarkers focused on carbohydrate antigens may improve the quality of diagnostic and prognostic predictions of particular cancers.
From a therapeutic perspective, inhibition of protein glycosylation using the antibiotic tunicamycin (in vitro) can induce cancer cell apoptosis and reduce metastasis of cancer cells via several mechanisms, e.g., ER-stress activation, reduction of stemness ability, inhibition of signaling pathways (TNF-related apoptosis-inducing ligand, MAPK/Erk, EGFR, IGF-1R, and several RTKs), induction of drug sensitivity, etc. [37,38,39,40]. Tunicamycin, however, has not been used in humans due to its severe side effects [41, 42]. Several plant lectins have been shown to inhibit progression of cancer [43]; however, lectins again might cause side effects in human patients such as aggregation of red blood cells. Recently, 5-[(Dimethylamino)sulfonyl]-N-(5-methyl-2-thiazolyl)-2-(1-pyrrolidinyl)-benzamide (NGI-1, ML414), a new inhibitor of protein glycosylation, was established [44]. NGI-1 targets OST subunit and blocks N-linked glycosylation, reducing cancer growth and increasing cancer sensitivity to radiation in vitro and in vivo [44,45,46]. The synergistic effects of NGI-1 with other chemotherapeutics have been reported in glioma and non-small cell lung cancer, especially in drug-resistant cell lines. The low solubility of NGI-1 and its ability to pass through the blood-brain barrier, however, limit the benefit of this compound in cancer treatment [47]. Nevertheless, inhibition of protein glycosylation is still a potential strategy for developing the effective therapy for cancer.
Clinical Relevance of Aberrant Glycosylation and Glycans in Cholangiocarcinoma
Alteration of glycosylation and elevation of cancer-associated glycans and glycoproteins in CCA have been increasingly reported. The aberrantly expressed glycoconjugates play important roles in CCA progression and are potentially useful as markers for detection of the disease. Direct evidence to demonstrate the alteration of glycosylation in CCA has been discovered by glycomics using lectin-based approaches [7,8,9,10] and mass spectrometry [11, 12]. The collective evidence suggests that glycosylation is globally altered in CCA. The alterations can be observed either in N-linked and O-linked glycosylations [12, 48,49,50]. Peripheral glycosylating processes, including fucosylation and sialylation, and synthesis of glycosphingolipids have also been found to be altered in CCA [51,52,53], as described below.
N-Glycans
Alteration in specific N-glycans can serve as a distinct molecular signature for cancer progression. Circulating N-glycoprotein/N-glycoform markers are suggested to be useful for diagnosis, disease monitoring, and assessment of clinical outcomes. Most serum/plasma N-glycoproteins are synthesized by the hepatobiliary system and reflect the status of the liver. In-depth analysis of the glycans in the serum/plasma of CCA patients may facilitate the discovery of novel diagnostic/therapeutic markers of CCA.
The plasma glycoproteome of patients with CCA (n = 60) and control group who were negative for hepatobiliary diseases (n = 95) was determined by Chang et al. using liquid chromatography-tandem mass spectrometry [11]. The analyses revealed four proteins closely related to tumor progression and prognosis of hepatobiliary malignancies. Of these, galectin-3-binding protein, also named MAC-2-binding protein, was found to be highly correlated with tumor stage, tumor grade, recurrence-free survival, and overall survival of CCA patients. Talabnin et al. used positive nanospray ionization-linear ion trap mass spectrometry (NSI-MSn) to determine the serum glycoproteomes and aberrant N-glycans in eight CCA patients compared to four healthy controls [12]. Similar glycan patterns with different relative quantities were obtained. The levels of high-mannose type N-glycan, M6N2, and the complex tri-antennary N-glycan containing a core fucose and terminal tri-sialic acid, NeuAc3H3N3M3N2F, were significantly increased, while levels of M9N2 were decreased, in CCA patients compared to healthy controls. The association of these glycans with clinicopathological features of patients, however, was not observed in this cohort.
For extrahepatic CCA (ECCA), the N-glycome profiling patterns in serum were determined using DNA sequencer-assisted fluorophore-assisted capillary electrophoresis (DSA-FACE) in 106 ECCA patients compared to 60 benign bile tract disease (BBD) and 89 healthy controls [54]. Different N-glycan patterns were observed in CCA vs. BBD and CCA vs. healthy controls, suggesting the N-glycan pattern specific to the disease condition and the potential use of these N-glycans as diagnostic markers. In addition, high levels of branching fucosylated tri-antennary and tetra-antennary N-glycans but not CA19–9 were correlated with positive lymph node metastasis. Using logistic regression coefficients, the authors constructed a mathematical formula for specific N-glycans to separate ECCA patients from normal controls with a higher diagnostic power than CA19–9. The combination of an N-glycan peak and CA19–9 improved the diagnostic accuracy of CA19–9 from 90.8% to 94.4% [54]. Moreover, N-glycan profiles but not CA19–9 levels in pre- and postoperative sera were significantly different. These data suggest circular N-glycan markers as novel and noninvasive markers in the diagnosis and progression monitoring of CCA.
O-Glycans
Alterations of O-glycosylation and an increase in O-glycans can be applied to the diagnosis and prognostic prediction of many types of cancer [5, 55]. In addition, these glycans have also been found to play significant roles in cancer progression and therapeutic resistances [17, 56, 57].
Increases in cancer-associated O-glycans in patient tissues and sera are possibly triggered by the overexpression of carrier glycoproteins. In CCA, elevation of mucin glycoproteins, such as MUC5AC and MUC1, was reported in tissues and sera [34, 36, 58, 59]. MUC5AC was elevated in CCA comparing with normal bile ducts [58, 59] and was demonstrated to be a good candidate for a diagnostic and prognostic marker for CCA. A high level of serum MUC5AC was associated with high tumor stage and short survival of CCA patients [59]. MUC1 and its glycoforms were found to be elevated in CCA [58, 60, 61], and high levels of MUC1 in CCA was associated with vascular invasion and shorter survival of patients [58]. These mucins also play significant roles in CCA progression and metastasis [62, 63].
Not only the elevation of carrier proteins but also the variation of glycan pattern may have clinical significance in CCA. Truncated mucin-type O-glycans such as Tn antigen (GalNAc-Ser/Thr), sialyl-Tn (sTn, Sia-GalNAc-Ser/Thr), Thomsen-Friedenreich antigen (T antigen, Gal-GalNAc-Ser/Thr), and sialyl-T antigen (Sia-Gal-GalNAc-Ser/Thr) are elevated and appear to play important roles in progression and metastasis of cancers; therefore, studies on the potential of using these antigens as targets for immunotherapy have been recently performed [64]. In CCA, Tn-, STn, and T-antigens were found to be elevated in CCA cells [48, 65]; the methods for detection these antigens in patients’ sera are being continuously developed [66, 67].
There are many lines of evidence pointing to the elevation of O-GalNAc modification or mucin-type glycosylation in CCA [8, 48, 61, 68]. Matsuda et al. analyzed the glycan profiles in CCA and normal bile ducts using lectin microarray and showed that a GalNAc-binding lectin, Wisteria floribunda agglutinin (WFA), provided the highest power in differentiating CCA from normal bile duct epithelia [61]. In addition, lectin histochemistry studies revealed that Gal/GalNAc-binding lectins, Sophora japonica agglutinin (SJA) and Vicia villosa lectins (VVL), provided strong reactivity with hyperplastic/dysplastic bile ducts and CCA compared with normal bile ducts and hepatocytes (Fig. 25.3) [8, 48, 68]. SJA-binding N-acetylgalactosamine-associated glycan (SNAG) appeared to be applicable as a diagnostic and prognostic marker for CCA; it was highly detected in sera from CCA patients compared to non-CCA controls and associated with short survival of CCA patients [68]. In addition, VVL-binding GalNAc glycan (VBG) has been found to play important roles in CCA metastasis in vitro [48]. Synthesis of VBG and its metastatic potential were recently shown to be related with the activity of polypeptide GalNAc-transferase 5 (GalNAcT5) in CCA cell lines [48]. Suppression of GalNAcT5 expression significantly reduced the migration and invasion abilities of CCA cell lines, while the overexpression of GalNAcT5 reversed these features. The molecular basis underlying this event involved AKT/ERK signaling pathways. In addition, an immunohistochemistry-based study showed that diffusely positive staining of GalNAcT3 in cancer cells was associated with lymph node metastasis of CCA, suggesting the usefulness of preoperative GalNAcT3 investigation in clinical management [69].
Expression of CCA-associated glycans in patient tissues. Lectin immunohistochemistry staining was used to detect VVL-binding glycan (VBG), SJA-binding GalNAc-associated glycan (SNAG), terminal fucose glycan (TFG), carbohydrate antigen (CA)-S27, CA-S121, and MAL-II-binding glycan (MAL-SG) in CCA tissues. Normal bile ducts (NBD) were for all CCA-associated glycans, except CA-S27. Hyperplastic/dysplastic (HP/DP) bile ducts were positive for all the glycans except MAL-SG, while CCA was positive for all examined glycans. Bar = 50 μm
Experiments in animal models are useful to better understand the association of glycan modification and CCA tumor biology. Using a hamster model of liver fluke-associated CCA [70, 71], O-GalNAc modifications, VBG and SNAG, were detected in hyperplastic/dysplastic bile ducts of hamster liver tissues as early as 1 month after liver fluke infection and CCA induction [48, 68]. No signal of VBG and SNAG was detected in normal bile ducts and hepatocytes. This finding suggested the association of VBG and SNAG in the development of carcinogenesis.
Collectively, these observations point to the importance of O-GalNAc modified glycans in CCA development and metastasis, suggesting the possibility of using the enzymes involved in O-GalNAc modification, e.g., GalNAcT5, as a target for CCA treatment in the future.
O-GlcNAcylation
O-GlcNAcylation is a dynamic posttranslational modification by adding a GlcNAc moiety on Ser or Thr residues of proteins via O-β-glycosidic linkage without any elongation. The process is regulated by two enzymes; O-linked β-N-acetylglucosaminyltransferase (OGT) and β-N-acetylglucosaminidase (OGA). OGT transfers GlcNAc from uridine diphospho-N-acetylglucosamine (UDP-GlcNAc) to -OH group of Ser or Thr, whereas OGA catalyzes the reversed reaction. Unlike the general N-linked or O-linked glycosylation, O-GlcNAcylation is a reversible process [20]. The rapid modification of proteins by O-GlcNAcylation can dynamically modulate protein function, stability, and activity. Several cellular processes including transcription regulation, translation control, inhibition of proteasomal degradation, stress response, and modulation of signal transduction can be regulated by O-GlcNAcylation [72, 73]. The dynamic interplay between O-GlcNAcylation and other posttranslational modifications, e.g., phosphorylation, has also been reported [20, 72, 74]. The balance between O-GlcNAcylation and phosphorylation of proteins is required for normal cell growth and development; hence, the alteration of these modifications may lead to the pathobiological processes and then disease [72, 75].
O-GlcNAcylation in CCA has been intensively studied in recent years. An increase in O-GlcNAcylated proteins (OGPs) in correlation with high OGT and low OGA levels was demonstrated in tumor tissues of CCA patients [50]. High expression of OGT (similar to high levels of SNAG, as discussed above [68]) was found to be associated with poor prognosis and shorter survival of CCA patients, suggesting the involvement of O-GlcNAcylation in CCA development and progression. The roles of O-GlcNAcylation on metastasis were studied in CCA cell lines; without any effect on cell proliferation, the migration and invasion abilities of CCA cell lines were dramatically reduced when O-GlcNAcylation was suppressed using specific siRNA against OGT. In contrast, enhancing O-GlcNAcylation by siOGA significantly increased migration and invasion abilities of CCA cell lines [76]. This effect was shown to be via O-GlcNAcylation of a transcription factor, NF-κB. Nuclear translocation of NF-κB was regulated by O-GlcNAc modification, which in turn induced expression of matrix metalloprotease enzymes [76].
Besides NF-κB, the glycoproteomics has identified several novel CCA-associated OGPs [77]. Among these, heterogeneous nuclear ribonucleoprotein-K (hnRNP-K) was abundantly detected in highly metastatic CCA cell lines [77]. O-GlcNAcylation was found to be an important modification to mediate nuclear translocation of hnRNP-K, which subsequently activated expression of several downstream genes, including cyclin D1, X-linked inhibitor of apoptosis protein 1 (XIAP1), epithelial to mesenchymal transition (EMT) markers, matrix metalloproteinase 2 (MMP2), and MMP7. Suppression of hnRNP-K negatively affected proliferation, migration, and invasion of CCA cell lines. In addition, immunohistochemistry of tumor tissues from CCA patients revealed that the nuclear localization of hnRNP-K could predict metastatic status and poor patient survival [77].
A recent study in CCA cell lines revealed that O-GlcNAcylation could indirectly mediate the N-glycan pattern of the membrane-bound glycoproteins via α1-mannosidase 1A (MAN1A1), an enzyme that reduces the high-mannose type N-glycan [49]. Suppression of O-GlcNAcylation using si-OGT could reduce the level of high-mannose type N-glycan and consequently repressed metastatic ability of CCA cells. Decreased O-GlcNAcylation was concomitant with the repression of PI3K/Akt and MAPK/Erk signaling pathways, which enhanced the stability of forkhead box O3 (FOXO3), the transcriptional factor regulating MAN1A1 expression [49]. Masking the high-mannose type N-glycan on the CCA cell surface using Pisum sativum agglutinin (PSA), a mannose specific lectin, reduced the metastatic ability of CCA cells. The correlation between O-GlcNAcylation, high-mannose type N-glycan, and CCA metastasis was also demonstrated in tumor tissues from CCA patients [49].
Aside from the enzymes OGT and OGA, the concentration of nutrient-sensing UDP-GlcNAc can also modulate intracellular O-GlcNAcylation. UDP-GlcNAc is synthesized via the hexosamine biosynthesis pathway in which glutamine-fructose amidotransferase (GFAT) is a rate-limiting enzyme. Recent studies have indicated an association between diabetes mellitus and poor prognosis of CCA patients. In vitro experiments have demonstrated that high-glucose media can promote the aggressiveness of CCA cells via mediating O-GlcNAcylation [78, 79]. Cultured cells in high-glucose conditions could enhance the expression of OGT and GFAT, resulting in an increase of O-GlcNAcylation and metastatic abilities of CCA cell lines. Vimentin was found to be highly stabilized under high-glucose conditions. The GFAT inhibitor, 6-Diazo-5-oxo-L-norleucine (DON), significantly suppressed O-GlcNAcylation, migration, and vimentin stability of CCA cells [79]. The association between O-GlcNAcylation and the expression level of GFAT in human CCA tissues were also confirmed using immunohistochemistry [79].
These findings strongly suggest that a high level of O-GlcNAcylation supports progressive phenotypes of CCA cells in several ways (Fig. 25.4). CCA-associated OGPs may be of clinical use, either as a prognostic marker or a potential target for CCA treatment. Before use in a clinical setting, however, further preclinical and clinical studies are needed to confirm the true indicators and utility of these CCA-associated OGPs for prognosis and treatment of CCA.
Molecular mechanisms of O-GlcNAcylation promote the progression of CCA cells. In high-glucose conditions, CCA cells might increase glucose uptake and the hexosamine biosynthesis pathway (HBP) via upregulating glutamine-fructose amidotransferase (GFAT), resulting in an increase of UDP-GlcNAc. Together with increased UDP-GlcNAc, OGT is increased while OGA is decreased, leading to the elevation of OGP in CCA cells. Increasing O-GlcNAcylation in CCA cells promotes CCA metastasis via many mechanisms, including (i) induction of nuclear translocation of NF-κB and hnRNP-K, (ii) activation of Akt and Erk signaling pathways, (iii) modulation of vimentin and FOXO3 stability, and (iv) induction of high-mannose type N-glycan at the cell surface
Fucosylation
Fucosylation is a glycosylation step catalyzed by 1 of 13 fucosyltransferases (FUTs) that use GDP-fucose as a donor substrate [81]. FUT adds a fucose to oligosaccharides with through various linkages, providing products with various glycan structures (Fig. 25.5). FUTs can be classified into four subfamilies based on the glycosidic linkage formed. The first group, FUT1 and FUT2, transfers a fucose residue to the terminal galactose to form α1,2-linkage, yielding H blood group antigen and related structures. The second group, α1,3/4-FUTs, FUT3, FUT4, FUT5, FUT6, FUT7, and FUT9, is involved in the synthesis of Lewis blood group antigens. The third group is comprised of FUT8, an α1,6-FUT which directly adds a fucose to the innermost GlcNAc of the N-linked oligosaccharides on glycoproteins to produce core fucosylation. Finally, protein O-fucosyltransferase 1 (POFUT1) and POFUT2 transfer a fucose residue via an α-linkage to Ser or Thr to produce O-fucosylation.
Fucose is added to an oligosaccharide chain in the final step in the late cisternae of the Golgi apparatus to increase the complexity of glycan structures. This specific glycan modification can confer unique function and properties to oligosaccharides and is often regulated during ontogeny and cellular differentiation [1]. Abnormal fucosylation has been observed in various disease states including cancer. Monitoring fucosylation changes across the spectrum of carcinogenesis can be useful for early cancer detection and management [57]. Exploring fucosylation in CCA development and progression, therefore, may offer an opportunity for early diagnosis and targeted treatment.
Blood Group Antigens
Fucosylation is involved in the biosynthesis of blood group-related antigens, such as A, B, H, Lea, Leb, Lex, Ley, sLea, and sLex. Several fucosylated products are potential biomarkers for CCA. Immunohistochemistry of these blood group-related antigens has been reported in 75 cases of CCA tissues (31 peripheral type and 44 hilar type CCA) [80]. Expression of A, B, and H were detected in the large bile ducts, whereas Lea, Leb, and Ley were variably observed in small and large bile ducts of nonneoplastic tissues. In CCA, expression of the blood group A, Lea, Leb, Ley, and sLea antigens were differentially expressed according to the histological type of cancer, suggesting that the distribution of blood group-related antigens may relate to the differentiation of CCA.
Terminal α1, 2-Fucose Glycans
Clinical relevance of terminal α1,2-fucose glycan (TFG) in CCA was reported by Indramanee et al. (2019) [82]. Lectin histochemistry of human CCA tissues using Ulex europaeus agglutinin-I (UEA-I) that recognizes TFG was performed in 79 paraffin-embedded tumors from CCA patients [8]. Neither hepatocytes nor normal bile duct epithelia expressed TFG; in contrast, 47% of CCA specimens showed high expression of TFG (Fig. 25.3), which was correlated with shorter patient survival, suggesting aberrant terminal fucosylation in CCA and a possible prognostic indicator. The involvement of TFG in carcinogenesis and progression of CCA has been demonstrated in the liver fluke-associated CCA hamster model. UEA-I lectin histochemistry of hamster liver sections demonstrated that TFG was absent in normal bile duct epithelia but elevated in hyperproliferative bile ducts and gradually increased during CCA development. TFG was expressed in CCA but was negative in all HCC tissues tested, suggesting TFG as a potential biomarker for differentiating CCA from HCC.
Significance of TFG on the efficiency of EGF-EGFR binding and/or activation has also been examined [82]. Suppression of TFG expression using siFUT1 or neutralizing the surface TFG with UEA-I in CCA cell lines effectively inhibited migration, invasion, and adhesion abilities in vitro. The observation was concurrent with the reduction of Akt/Erk signaling and EMT. The effect was further shown to be driven by the decreasing of EGF-EGFR activation that consequently reduced the Akt/Erk cascades.
Carbohydrate Antigen-S27
A novel carbohydrate antigen, CA-S27 , recognized by the S27 monoclonal antibody (mAb) [83, 84], was proven to be a Lea-associated glycan using glycoconjugate microarray [84]. The clinical relevance of CA-S27 was reported by Silsirivanit et al. (2013) [84]. Immunohistochemistry of 45 human CCA tissues revealed a high reactivity of CA-S27 in almost all CCA tissues but not hepatocytes (Fig. 25.3). Additionally, a quantitative determination of serum CA-S27 by sandwich ELISA was developed using the CA-S27 monoclonal antibody and soybean agglutinin. Using this method, serum CA-S27 of CCA patients (n = 96) was found to be significantly higher than those of the control groups (patients with gastrointestinal cancers, HCC, benign hepatobiliary diseases, and healthy subjects [n = 190]) and distinguished CCA patients from controls with 87% sensitivity and 59% specificity. Serum CA-S27 was secreted from CCA tissues, and serum CA-S27 level declined dramatically after tumor removal. Moreover, a high serum CA-S27 level was associated with shorter survival of CCA patients. MUC5AC mucin, a secretory mucin-related to poor prognosis in CCA [36], was shown to be the major glycoprotein possessed by CA-S27 in serum [84].
The significance of CA-S27 in promoting CCA progression was demonstrated in CCA cell lines [84]. FUT3, a key enzyme for Le synthesis was highly expressed in CCA cells with high CA-S27expression. Silencing of FUT3 expression by siFUT3 or neutralizing surface CA-S27 by CA-S27 mAb effectively decreased invasion, migration, adhesion, and proliferation abilities of CCA cells.
Collectively, these data suggest important roles and significance of CA-S27 in CCA. In particular, serum CA-S27 might be a serum marker for diagnosis and progression of CCA, a prognostic factor for clinical outcomes of CCA, and a potential therapeutic target for metastatic CCA.
Carbohydrate Antigen-S121
Carbohydrate antigen-S121 (CA-S121 or CCA-CA) is an unidentified sugar structure recognized by a monoclonal antibody S121 [83]. The glycan epitope was found on MUC5AC mucin and strongly detected in hyperplastic/dysplastic and neoplastic bile duct epithelia but not in normal bile ducts or hepatocytes (Fig. 25.3). Serum CA-S121 assessment by lectin sandwich ELISA was able to distinguish CCA patients from several controls, e.g., healthy individuals, Opisthorchis viverrini-infected individuals, patients with benign hepatobiliary diseases, and patients with various gastrointestinal cancers or HCC with 87.63% sensitivity and 89.58% specificity. CCA patients with high serum CA-S121 had a shorter survival than those with low serum CA-S121. Moreover, the combination of serum CA-S121 with serum alkaline phosphatase resulted in sensitivity, specificity, positive predictive value, and negative predictive value all >95% [85]. Using the combination of these two markers may be useful for screening people who are risk of CCA.
sLea or CA19–9
sLea or CA19–9 has been used clinically since 1997 for diagnosis and surveillance of patients with gastrointestinal cancers, especially CCA, pancreatic adenocarcinoma, and gallbladder adenocarcinoma [86, 87]. Although CA19–9 is not a specific biomarker for CCA, it is the most frequent and best studied marker for identifying CCA in clinical practice. Recently, use of CA19–9 has also been recommended as part of CCA surveillance in primary sclerosing cholangitis (PSC), as discussed in greater detail elsewhere in this book (Chap. 20, Ali et al.) [88,89,90,91,92].
The biosynthesis of CA19–9 is based on the enzymatic activity of FUT3 irrespective of FUT2 activity [93, 94]. In contrast, inactivity of FUT2 increases levels of serum CA19–9 [95]. Based on these observations, Wannhoff A et al. (2013) suggested to use a new optimal cutoff value for CA19–9 based on individual FUT2/3 genotype [96]. The approach could improve the power of CA19–9 in differentiating PSC from CCA with 90% sensitivity and a 43% reduction of false-positive results. Serum CA19–9 and FUT genotyping is clinically beneficial and may enhance the early detection of CCA in clinical practice.
The clinical relevance of sLea in CCA has been demonstrated in various studies. For example, Juntavee et al. (2005) found that sLea was highly expressed in tissue of the mass-forming type of CCA and correlated well with vascular invasion and unfavorable patient outcomes [51]. The significance of sLea in vascular invasion was signified by the fact that CCA cells that possessed high sLea expression adhered and transmigrated to IL-1β-activated endothelial cells of the human umbilical vein more than CCA cells without sLea expression. Moreover, these abilities were significantly diminished in the presence of neutralizing antibodies specific to either sLea or E-selectin.
Fucosylated Fetuin-A and Kininogen
The aberrant N-linked glycans observed in serum can be used as a diagnostic or prognostic marker for specific cancers, including CCA. Betesh et al. used a glycomic approach to analyze and compare N-linked glycans in sera from CCA patients and controls. Increased levels of serum alpha-1,6 linked core and alpha 1,3 linked outer arm fucosylation in CCA patients were noted [97]. Furthermore, the fucosylated proteome of sera from CCA patients identified numerous fucosylated glycoproteins, e.g., alpha-2-macroglobulin, kininogen, hemopexin, fetuin-A, and ceruloplasmin. The relative proportion of fucosylation of these proteins was further determined using lectin fluorophore-linked immunosorbent assay (lectin-FLISA). The technique detects the amount of fucosylation present on an equal number of captured molecules independently of the total amount of protein tested. Of these, fucosylated fetuin-A and kininogen were significantly elevated in sera from CCA patients compared with those from PSC. Fucosylated fetuin-A could differentiate PSC from CCA with 62% sensitivity and 90% specificity, while fucosylated kininogen could differentiate CCA from the control group with similar diagnostic performance. In addition, these markers, either used alone or in combination, provide better detection of CCA than CA-19-9, indicating the potential of these two glycoproteins as diagnostic markers for CCA [97].
Alpha-L-Fucosidase
Alpha-L-fucosidase (AFU), a lysosomal enzyme, hydrolyzes the cleavage of fucose 𝛼-1,2, 𝛼-1,3, 𝛼-1,4, and 𝛼-1,6 linkages in the glycosylation chains to maintain homeostasis of fucose metabolism. It has been used as a tumor marker for various cancers, e.g., HCC and colorectal cancer [98, 99]. A high level of serum AFU has been shown to be associated with poor outcomes in HCC [100], though the reversed outcome was observed in breast cancer [101]. For CCA, AFU activity in serum was determined in 148 intrahepatic CCA cases by an automated analyzer. Based on ROC analysis and a cutoff of AFU <20.85 U/L, it was found that AFU level was an independent prognostic factor in patients with intrahepatic CCA [102]; patients with a high serum AFU level exhibited better outcomes. Treating CCA cells with AFU diminished the invasion capacity of CCA cells by suppression of MMP-2 and MMP-9 expression. Hence, serum AFU has been proposed to be a prognostic indicator for CCA.
Sialylation
Sialylation , the addition of sialic acid to subterminal sugar residues on oligosaccharides, is an important peripheral glycosylation process for maturation of glycoproteins and glycolipids (Fig. 25.6). The patterns of sialylation in cells are regulated by levels of nucleotide sugar donor, CMP-sialic acid, and the expressions of sialyltransferases (STs) and sialidases. STs are a family of enzymes responsible for transferring a sialic acid from a nucleotide sugar donor (CMP-sialic acid) to a glycoconjugate acceptor. Desialylation, the process of removing terminal sialic acid from glycoconjugates, is driven by sialidases or neuraminidases (NEUs). Altered expression of STs and/or NEUs, resulting in increases in uncommon sialylated glycans, has been reported in many cancer types [103]. These sialylated glycans were found to promote tumor progression and therapeutic resistance in several cancers.
Sialylation in human cells. (a) The addition of sialic acid to glycoprotein is catalyzed by sialytransferases (STs) and the removal reaction by sialidases. (b) The linkage of sialic acids to subterminal sugar residues is performed by four groups of STs: (1) ST3GalTs for Sia-𝛼2,3-Gal linkage; (2) ST6GalT for Sia-𝛼2,6-Gal linkage; (3) ST6GalNAcT for Sia-𝛼2,6-GalNAc linkage, and (4) ST8Sia for Sia-𝛼2,8-Sia linkage
Accumulating data over the past few decades have demonstrated alterations of sialylation in CCA. Serum sialic acid was found to be increased in CCA patients and capable of differentiating CCA patients from those with benign biliary diseases and healthy controls [104]. The elevation of serum sialic acid in CCA patients may be due to increases in core glycans/glycoproteins such as MU5AC and sLea [34]. Moreover, sialic acid residues on sLea or sLex (section Fucosylation) are important for the binding of these molecules with E-selectin during extravasation [105, 106].
Lectin histochemistry of CCA tissues using Maackia amurensis lectin-II (MAL-II) and Sambucus nigra agglutinin (SNA) has revealed that alpha-2,3-sialylated glycan (MAL-II-binding glycan, MAL-SG) and alpha-2,6-sialylated glycan (SNA-binding glycan, SNA-SG) were overexpressed in CCA tissues compared to normal bile ducts (Fig. 25.3) [52]. A high level of MAL-SG in CCA tissues was associated with shorter survival of CCA patients, suggesting the potential of MAL-SG as a prognostic indicator for CCA. In addition, in vitro drug sensitivity assays have shown that suppression of sialylation by a sialyltransferase inhibitor significantly enhanced the sensitivity of CCA cell lines to 5-fluorouracil (5-FU), a common chemotherapeutic drug used for CCA treatment [52]. In addition to 5-FU, the involvement of sialylation in drug resistance to cisplatin and paclitaxel has also been reported (in other cancers) [107, 108]; a similar effect may be expected for CCA, in which cisplatin and paclitaxel are also drugs of choice.
Glycosphingolipids
GSLs are an important membrane component which play important roles in forming functional membranous microdomains. Synthesis of GSLs is separated into two phases: (1) CER synthesis and (2) glycosylation (Fig. 25.2). Hydrophobic interactions between the ceramide part of GSLs and other membrane components, such as cholesterol, proteins, and sphingomyelin, are important for determining the functions of microdomains [109,110,111,112,113]. The heterogeneity of GSLs is attributable to either glycan or ceramide compositions [114, 115]. The glycan part of a GSL can be monosaccharide, such as glucose and galactose, or oligosaccharide such as lactose, forming glucosyl-ceramide (GlcCer), galactosylceramide (GalCer), and lactosylceramide (LacCer), respectively. The ceramide part of a GSL can be composed of either hydroxylated or non-hydroxylated forms of fatty acids with C16 to C24. Aberrant expression of GSLs has been reported in many cancers, including breast, endometrial, and lung, and may be due to dysregulated expression of ceramide synthases (CERSs), GTs, and fatty acid-2-hydroxylases (FA2H) [116,117,118,119,120,121,122]. Indeed, altered expression of these enzymes has been associated with tumor growth and metastasis [116, 117, 120, 121, 123].
There is limited information regarding GSL expression in CCA. A recent study using LC-MS/MS analysis revealed that GSLs were elevated in CCA tissues compared with the adjacent normal liver [53]. High level of hydroxylated fatty-containing GSL was associated with shorter survival of CCA patients, suggesting the role of fatty acid hydroxylation in tumor progression of CCA [53]. These findings suggest increased activity of GSL-associated enzymes, e.g., ceramide synthase and fatty acid hydroxylase, in CCA as well as potential prognostic implications. Further study, however, is needed in this regard.
Conclusions and Perspectives
Glycoconjugates are one of the major components in cells that modulate key biological and physiological processes to maintain cellular homeostasis. Aberrant glycosylation of cell surface molecules alters cellular functions which can contribute to human diseases, including cancer. With the advanced technology in glycobiology research, several glycan structures and functions related to diseases have been revealed. The association of abnormal glycosylation patterns and aggressive phenotypes, e.g., tumor growth and metastasis, have been reported in CCA. Moreover, several CCA-associated glycans have been validated and are applicable for diagnosis and prognostic prediction. Directly targeting the synthesis of these glycans for cancer treatment, however, remains to be validated and is an area of ongoing investigation. A number of glycosylation inhibitors have been developed and studied for their antitumor activities, many of which appear to effectively suppress tumor growth and metastasis and enhance chemosensitivity of cancer cells. A combination of glycosylation inhibitors with other therapeutic agents or therapy may be a promising strategy to improve the treatment of CCA.
Further research into the molecular basis of glycosylation in CCA is expected to enhance understanding of cell-cell interactions, extracellular communications, and cancer immunology and which may reveal new targets for CCA treatment. Furthermore, the integration of large data analysis of glycomics/glycoproteomics and several other “-omics,” e.g., genomics, transcriptomics, proteomics as well as metabolomics, in CCA cell lines/tissues from patients will provide an avenue for greater impact on developing novel approaches for the screening, diagnosis, prognosis, and targeted treatment for this highly lethal malignancy.
Abbreviations
- 5-FU:
-
5-fluorouracil
- DON:
-
6-Diazo-5-oxo-L-norleucine
- MAN1A1:
-
α1-mannosidase 1A
- AFP:
-
Alpha-fetoprotein
- AFU:
-
Alpha-L-fucosidase
- Asn:
-
Asparagine
- BB:
-
Benign bile tract disease
- CA19–9:
-
Carbohydrate antigen 19–9
- CA-S27:
-
Carbohydrate antigen-S27
- Cer:
-
Ceramide
- CERS:
-
Ceramide synthase
- CCA:
-
Cholangiocarcinoma
- Dol-P:
-
Dolichol phosphate
- EMT:
-
Epithelial to mesenchymal transition
- ECCA:
-
Extrahepatic CCA
- FA2H:
-
Fatty acid-2-hydroxylase
- FOXO3:
-
Forkhead box O3
- Fuc:
-
Fucose
- FUT:
-
Fucosyltransferase
- Gal:
-
Galactose
- GalCer:
-
Galactosylceramide
- GalT:
-
Galactosyltransferase
- GalNAcT5:
-
GalNAc-transferase 5
- Glc:
-
Glucose
- GlcCer:
-
Glucosylceramide
- GFAT:
-
Glutamine-fructose amidotransferase
- GSL:
-
Glycosphingolipid
- HC:
-
Healthy controls
- hnRNP-K:
-
Heterogeneous nuclear ribonucleoprotein-K
- HE4:
-
Human epididymis protein 4
- LacCer:
-
Lactosylceramide
- MAL:
-
Maackia amurensis lectin
- MAL-SG:
-
MAL-II-binding glycan
- Man:
-
Mannose
- MMP:
-
Matrix metalloproteinase
- mAb:
-
Monoclonal antibody
- MUC:
-
Mucin
- GalNAc:
-
N-acetylgalactosamine
- GlcNAc:
-
N-acetylglucosamine
- GlcNAc-T:
-
N-acetylglucosaminyltransferase
- NEU:
-
Neuraminidase
- POFUT1:
-
O-fucosyltransferase 1
- OGP:
-
O-GlcNAcylated protein
- OGT:
-
O-linked β-N-acetylglucosaminyltransferase
- OST:
-
Oligosaccharyltransferase
- PSA:
-
Pisum sativum agglutinin
- PSC:
-
Primary sclerosing cholangitis
- PSA:
-
Prostate-specific antigen
- SNA:
-
Sambucus nigra agglutinin
- Ser:
-
Serine
- sLea:
-
Sialyl-Lewis A
- sLex:
-
Sialyl-Lewis X
- sTn:
-
Sialyl-Tn
- ST:
-
Sialyltransferase
- SNAG:
-
SJA-binding N-acetylgalactosamine-associated glycan
- SNA-SG:
-
SNA-binding glycan
- SJA:
-
Sophora japonica agglutinin
- SBA:
-
Soybean agglutinin
- TFG:
-
Terminal α1,2-fucose glycan
- Thr:
-
Threonine
- GalNAc-T:
-
UDP-GalNAc-polypeptide GalNAc-transferase
- UEA-I:
-
Ulex europaeus agglutinin-I
- UDP-GlcNAc:
-
Uridine diphospho-N-acetylglucosamine
- VVL:
-
Vicia villosa lectin
- VBG:
-
VVL-binding GalNAc glycan
- WFA:
-
Wisteria floribunda agglutinin
- XIAP:
-
X-linked inhibitor of apoptosis protein
- Xyl:
-
Xylose
- OGA:
-
β-N-acetylglucosaminidase
References
Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13(7):41R–53R.
Meezan E, Wu HC, Black PH, Robbins PW. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by sephadex chromatography. Biochemistry. 1969;8(6):2518–24.
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–55.
Munkley J, Elliott DJ. Hallmarks of glycosylation in cancer. Oncotarget. 2016;7(23):35478–89.
Kailemia MJ, Park D, Lebrilla CB. Glycans and glycoproteins as specific biomarkers for cancer. Anal Bioanal Chem. 2017;409(2):395–410.
Munkley J, Mills IG, Elliott DJ. The role of glycans in the development and progression of prostate cancer. Nat Rev Urol. 2016;13(6):324–33.
Hirabayashi J, Yamada M, Kuno A, Tateno H. Lectin microarrays: concept, principle and applications. Chem Soc Rev. 2013;42(10):4443–58.
Indramanee S, Silsirivanit A, Pairojkul C, Wongkham C, Wongkham S. Aberrant glycosylation in cholangiocarcinoma demonstrated by lectin-histochemistry. Asian Pac J Cancer Prev. 2012;13 Suppl:119–24.
Kuno A, Matsuda A, Unno S, Tan B, Hirabayashi J, Narimatsu H. Differential glycan analysis of an endogenous glycoprotein: toward clinical implementation--from sample pretreatment to data standardization. Methods Mol Biol. 2014;1200:265–85.
Matsuda A, Kuno A, Matsuzaki H, Kawamoto T, Shikanai T, Nakanuma Y, et al. Glycoproteomics-based cancer marker discovery adopting dual enrichment with Wisteria floribunda agglutinin for high specific glyco-diagnosis of cholangiocarcinoma. J Proteome. 2013;85:1–11.
Chang TT, Cheng JH, Tsai HW, Young KC, Hsieh SY, Ho CH. Plasma proteome plus site-specific N-glycoprofiling for hepatobiliary carcinomas. J Pathol Clin Res. 2019;5(3):199–212.
Talabnin K, Talabnin C, Ishihara M, Azadi P. Increased expression of the high-mannose M6N2 and NeuAc3H3N3M3N2F tri-antennary N-glycans in cholangiocarcinoma. Oncol Lett. 2018;15(1):1030–6.
Varki A, Kornfeld S. Historical background and overview. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, et al., editors. Essentials of glycobiology. New York: Cold Spring Harbor; 2015. p. 1–18.
Varki A. Biological roles of glycans. Glycobiology. 2017;27(1):3–49.
Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12(4):43R–56R.
Stanley P, Taniguchi N, Aebi M. N-Glycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, et al., editors. Essentials of glycobiology. New York: Cold Spring Harbor; 2015. p. 99–111.
Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22(6):736–56.
Brockhausen I, Stanley P. O-GalNAc glycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, et al., editors. Essentials of glycobiology. New York: Cold Spring Harbor; 2015. p. 113–23.
Stanley P. Golgi glycosylation. Cold Spring Harb Perspect Biol. 2011;3(4)
Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446(7139):1017–22.
Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15(12):771–85.
Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339(1):237–46.
Yu RK, Tsai YT, Ariga T, Yanagisawa M. Structures, biosynthesis, and functions of gangliosides–an overview. J Oleo Sci. 2011;60(10):537–44.
Aebi M. N-linked protein glycosylation in the ER. Biochim Biophys Acta. 2013;1833(11):2430–7.
Kelleher DJ, Gilmore R. An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology. 2006;16(4):47R–62R.
D'Angelo G, Capasso S, Sticco L, Russo D. Glycosphingolipids: synthesis and functions. FEBS J. 2013;280(24):6338–53.
Schnaar RL, Kinoshita T. Glycosphingolipids. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, et al., editors. Essentials of glycobiology. New York: Cold Spring Harbor; 2015. p. 125–35.
Li X, Wang X, Tan Z, Chen S, Guan F. Role of glycans in cancer cells undergoing epithelial-mesenchymal transition. Front Oncol. 2016;6:33.
Ghazarian H, Idoni B, Oppenheimer SB. A glycobiology review: carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem. 2011;113(3):236–47.
Freeze HH, Kinoshita T, Varki A. Glycans in acquired human diseases. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, et al., editors. Essentials of glycobiology. New York: Cold Spring Harbor; 2015. p. 583–95.
Varki A, Gagneux P. Biological functions of glycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, et al., editors. Essentials of glycobiology. New York: Cold Spring Harbor; 2015. p. 77–88.
Kirwan A, Utratna M, O'Dwyer ME, Joshi L, Kilcoyne M. Glycosylation-based serum biomarkers for cancer diagnostics and prognostics. Biomed Res Int. 2015;2015:490531.
Stowell SR, Ju T, Cummings RD. Protein glycosylation in cancer. Annu Rev Pathol. 2015;10:473–510.
Wongkham S, Sheehan JK, Boonla C, Patrakitkomjorn S, Howard M, Kirkham S, et al. Serum MUC5AC mucin as a potential marker for cholangiocarcinoma. Cancer Lett. 2003;195(1):93–9.
Xuan J, Li J, Zhou Z, Zhou R, Xu H, Wen W. The diagnostic performance of serum MUC5AC for cholangiocarcinoma: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95(24):e3513.
Boonla C, Wongkham S, Sheehan JK, Wongkham C, Bhudhisawasdi V, Tepsiri N, et al. Prognostic value of serum MUC5AC mucin in patients with cholangiocarcinoma. Cancer. 2003;98(7):1438–43.
Barkeer S, Chugh S, Karmakar S, Kaushik G, Rauth S, Rachagani S, et al. Novel role of O-glycosyltransferases GALNT3 and B3GNT3 in the self-renewal of pancreatic cancer stem cells. BMC Cancer. 2018;18(1):1157.
Wu J, Chen S, Liu H, Zhang Z, Ni Z, Chen J, et al. Tunicamycin specifically aggravates ER stress and overcomes chemoresistance in multidrug-resistant gastric cancer cells by inhibiting N-glycosylation. J Exp Clin Cancer Res. 2018;37(1):272.
Abhari BA, McCarthy N, Le Berre M, Kilcoyne M, Joshi L, Agostinis P, et al. Smac mimetic suppresses tunicamycin-induced apoptosis via resolution of ER stress. Cell Death Dis. 2019;10(3):155.
Hou H, Ge C, Sun H, Li H, Li J, Tian H. Tunicamycin inhibits cell proliferation and migration in hepatocellular carcinoma through suppression of CD44s and the ERK1/2 pathway. Cancer Sci. 2018;109(4):1088–100.
Foufelle F, Fromenty B. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol Res Perspect. 2016;4(1):e00211.
Chang JY, Korolev VV. Specific toxicity of tunicamycin in induction of programmed cell death of sympathetic neurons. Exp Neurol. 1996;137(2):201–11.
Yau T, Dan X, Ng CC, Ng TB. Lectins with potential for anti-cancer therapy. Molecules. 2015;20(3):3791–810.
Lopez-Sambrooks C, Shrimal S, Khodier C, Flaherty DP, Rinis N, Charest JC, et al. Oligosaccharyltransferase inhibition induces senescence in RTK-driven tumor cells. Nat Chem Biol. 2016;12(12):1023–30.
Baro M, Lopez Sambrooks C, Quijano A, Saltzman WM, Contessa J. Oligosaccharyltransferase inhibition reduces receptor tyrosine kinase activation and enhances glioma radiosensitivity. Clin Cancer Res. 2019;25(2):784–95.
Lopez Sambrooks C, Baro M, Quijano A, Narayan A, Cui W, Greninger P, et al. Oligosaccharyltransferase inhibition overcomes therapeutic resistance to EGFR tyrosine kinase inhibitors. Cancer Res. 2018;78(17):5094–106.
Wahl DR, Lawrence TS. No sugar added: a new strategy to inhibit glioblastoma receptor tyrosine kinases. Clin Cancer Res. 2019;25(2):455–6.
Detarya M, Sawanyawisuth K, Aphivatanasiri C, Chuangchaiya S, Saranaruk P, Sukprasert L, et al. The O-GalNAcylating enzyme GALNT5 mediates carcinogenesis and progression of cholangiocarcinoma via activation of AKT/ERK signaling. Glycobiology. 2020;30(5):312–24.
Phoomak C, Silsirivanit A, Park D, Sawanyawisuth K, Vaeteewoottacharn K, Wongkham C, et al. O-GlcNAcylation mediates metastasis of cholangiocarcinoma through FOXO3 and MAN1A1. Oncogene. 2018;37(42):5648–65.
Phoomak C, Silsirivanit A, Wongkham C, Sripa B, Puapairoj A, Wongkham S. Overexpression of O-GlcNAc-transferase associates with aggressiveness of mass-forming cholangiocarcinoma. Asian Pac J Cancer Prev. 2012;13 Suppl:101–5.
Juntavee A, Sripa B, Pugkhem A, Khuntikeo N, Wongkham S. Expression of sialyl Lewis(a) relates to poor prognosis in cholangiocarcinoma. World J Gastroenterol. 2005;11(2):249–54.
Wattanavises S, Silsirivanit A, Sawanyawisuth K, Cha'on U, Waraasawapati S, Saentaweesuk W, et al. Increase of MAL-II binding alpha2,3-sialylated glycan is associated with 5-FU resistance and short survival of cholangiocarcinoma patients. Medicina (Kaunas). 2019;55(12)
Silsirivanit A, Phoomak C, Teeravirote K, Wattanavises S, Seubwai W, Saengboonmee C, et al. Overexpression of HexCer and LacCer containing 2-hydroxylated fatty acids in cholangiocarcinoma and the association of the increase of LacCer (d18:1-h23:0) with shorter survival of the patients. Glycoconj J. 2019;36(2):103–11.
Wang M, Fang M, Zhu J, Feng H, Warner E, Yi C, et al. Serum N-glycans outperform CA19-9 in diagnosis of extrahepatic cholangiocarcinoma. Electrophoresis. 2017;38(21):2749–56.
Silsirivanit A. Glycosylation markers in cancer. Adv Clin Chem. 2019;89:189–213.
Tarp MA, Clausen H. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta. 2008;1780(3):546–63.
Vajaria BN, Patel PS. Glycosylation: a hallmark of cancer? Glycoconj J. 2017;34(2):147–56.
Boonla C, Sripa B, Thuwajit P, Cha-On U, Puapairoj A, Miwa M, et al. MUC1 and MUC5AC mucin expression in liver fluke-associated intrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11(32):4939–46.
Bamrungphon W, Prempracha N, Bunchu N, Rangdaeng S, Sandhu T, Srisukho S, et al. A new mucin antibody/enzyme-linked lectin-sandwich assay of serum MUC5AC mucin for the diagnosis of cholangiocarcinoma. Cancer Lett. 2007;247(2):301–8.
Xu H, Inagaki Y, Tang W, Guo Q, Wang F, Seyama Y, et al. Elevation of serum KL-6 mucin levels in patients with cholangiocarcinoma. Hepato-Gastroenterology. 2008;55(88):2000–4.
Matsuda A, Kuno A, Kawamoto T, Matsuzaki H, Irimura T, Ikehara Y, et al. Wisteria floribunda agglutinin-positive mucin 1 is a sensitive biliary marker for human cholangiocarcinoma. Hepatology. 2010;52(1):174–82.
Danese E, Ruzzenente A, Montagnana M, Lievens PM. Current and future roles of mucins in cholangiocarcinoma-recent evidences for a possible interplay with bile acids. Ann Transl Med. 2018;6(17):333.
Kasprzak A, Adamek A. Mucins: the old, the new and the promising factors in hepatobiliary carcinogenesis. Int J Mol Sci. 2019;20(6)
Fu C, Zhao H, Wang Y, Cai H, Xiao Y, Zeng Y, et al. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. HLA. 2016;88(6):275–86.
Sasaki M, Yamato T, Nakanuma Y. Expression of sialyl-Tn, Tn and T antigens in primary liver cancer. Pathol Int. 1999;49(4):325–31.
Rangel MGH, Silva MLS. Detection of the cancer-associated T antigen using an Arachis hypogaea agglutinin biosensor. Biosens Bioelectron. 2019;141:111401.
Tanaka-Okamoto M, Hanzawa K, Mukai M, Takahashi H, Ohue M, Miyamoto Y. Correlation of serum sialyl Tn antigen values determined by immunoassay and SRM based method. Anal Biochem. 2018;544:42–8.
Saentaweesuk W, Silsirivanit A, Vaeteewoottacharn K, Sawanyawisuth K, Pairojkul C, Cha'on U, et al. Clinical significance of GalNAcylated glycans in cholangiocarcinoma: values for diagnosis and prognosis. Clin Chim Acta. 2018;477:66–71.
Inoue T, Eguchi T, Oda Y, Nishiyama K, Fujii K, Izumi H, et al. Expression of GalNAc-T3 and its relationships with clinicopathological factors in 61 extrahepatic bile duct carcinomas analyzed using stepwise sections - special reference to its association with lymph node metastases. Mod Pathol. 2007;20(2):267–76.
Thamavit W, Bhamarapravati N, Sahaphong S, Vajrasthira S, Angsubhakorn S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978;38(12):4634–9.
Thamavit W, Kongkanuntn R, Tiwawech D, Moore MA. Level of Opisthorchis infestation and carcinogen dose-dependence of cholangiocarcinoma induction in Syrian golden hamsters. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;54(1):52–8.
Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–58.
Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18(7):452–65.
Hu P, Shimoji S, Hart GW. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett. 2010;584(12):2526–38.
Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29(19):2831–42.
Phoomak C, Vaeteewoottacharn K, Sawanyawisuth K, Seubwai W, Wongkham C, Silsirivanit A, et al. Mechanistic insights of O-GlcNAcylation that promote progression of cholangiocarcinoma cells via nuclear translocation of NF-kappaB. Sci Rep. 2016;6:27853.
Phoomak C, Park D, Silsirivanit A, Sawanyawisuth K, Vaeteewoottacharn K, Detarya M, et al. O-GlcNAc-induced nuclear translocation of hnRNP-K is associated with progression and metastasis of cholangiocarcinoma. Mol Oncol. 2019;13(2):338–57.
Saengboonmee C, Seubwai W, Pairojkul C, Wongkham S. High glucose enhances progression of cholangiocarcinoma cells via STAT3 activation. Sci Rep. 2016;6:18995.
Phoomak C, Vaeteewoottacharn K, Silsirivanit A, Saengboonmee C, Seubwai W, Sawanyawisuth K, et al. High glucose levels boost the aggressiveness of highly metastatic cholangiocarcinoma cells via O-GlcNAcylation. Sci Rep. 2017;7:43842.
Minato H, Nakanuma Y, Terada T. Expression of blood group-related antigens in cholangiocarcinoma in relation to non-neoplastic bile ducts. Histopathology. 1996;28(5):411–9.
Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16(12):158R–84R.
Indramanee S, Sawanyawisuth K, Silsirivanit A, Dana P, Phoomak C, Kariya R, et al. Terminal fucose mediates progression of human cholangiocarcinoma through EGF/EGFR activation and the Akt/Erk signaling pathway. Sci Rep. 2019;9(1):17266.
Silsirivanit A, Araki N, Wongkham C, Pairojkul C, Narimatsu Y, Kuwahara K, et al. A novel serum carbohydrate marker on mucin 5AC: values for diagnostic and prognostic indicators for cholangiocarcinoma. Cancer. 2011;117(15):3393–403.
Silsirivanit A, Araki N, Wongkham C, Vaeteewoottacharn K, Pairojkul C, Kuwahara K, et al. CA-S27: a novel Lewis a associated carbohydrate epitope is diagnostic and prognostic for cholangiocarcinoma. Cancer Sci. 2013;104(10):1278–84.
Pattanapairoj S, Silsirivanit A, Muisuk K, Seubwai W, Cha'on U, Vaeteewoottacharn K, et al. Improve discrimination power of serum markers for diagnosis of cholangiocarcinoma using data mining-based approach. Clin Biochem. 2015;48(10–11):668–73.
Safi F, Schlosser W, Kolb G, Beger HG. Diagnostic value of CA 19-9 in patients with pancreatic cancer and nonspecific gastrointestinal symptoms. J Gastrointest Surg. 1997;1(2):106–12.
Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692(2–3):103–19.
Ali AH, et al. Surveillance for hepatobiliary cancers in patients with primary sclerosing cholangitis. Hepatology. 2018;67(6):2338–51.
Charatcharoenwitthaya P, et al. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48(4):1106–17.
Fung BM, Lindor KD, Tabibian JH. Cancer risk in primary sclerosing cholangitis: epidemiology, prevention, and surveillance strategies. World J Gastroenterol. 2019;25(6):659–71.
Lindor KD, et al. ACG clinical guideline: primary sclerosing cholangitis. Am J Gastroenterol. 2015;110(5):646–59. quiz 660
Tabibian JH, Ali AH, Lindor KD. Primary Sclerosing cholangitis, part 2: cancer risk, prevention, and surveillance. Gastroenterol Hepatol (N Y). 2018;14(7):427–32.
Mollicone R, Reguigne I, Kelly RJ, Fletcher A, Watt J, Chatfield S, et al. Molecular basis for Lewis alpha(1,3/1,4)-fucosyltransferase gene deficiency (FUT3) found in Lewis-negative Indonesian pedigrees. J Biol Chem. 1994;269(33):20987–94.
Nishihara S, Narimatsu H, Iwasaki H, Yazawa S, Akamatsu S, Ando T, et al. Molecular genetic analysis of the human Lewis histo-blood group system. J Biol Chem. 1994;269(46):29271–8.
Narimatsu H, Iwasaki H, Nakayama F, Ikehara Y, Kudo T, Nishihara S, et al. Lewis and secretor gene dosages affect CA19-9 and DU-PAN-2 serum levels in normal individuals and colorectal cancer patients. Cancer Res. 1998;58(3):512–8.
Wannhoff A, Hov JR, Folseraas T, Rupp C, Friedrich K, Anmarkrud JA, et al. FUT2 and FUT3 genotype determines CA19-9 cut-off values for detection of cholangiocarcinoma in patients with primary sclerosing cholangitis. J Hepatol. 2013;59(6):1278–84.
Betesh L, Comunale MA, Wang M, Liang H, Hafner J, Karabudak A, et al. Identification of fucosylated Fetuin-A as a potential biomarker for cholangiocarcinoma. Proteomics Clin Appl. 2017;11(9–10)
Delacadena M, Fernandez J, Decarlos A, Martinezzorzano V, Gilmartin E, Rodriguezberrocal F. Low levels of alpha-L-fucosidase activity in colorectal cancer are due to decreased amounts of the enzymatic protein and are related with Dukes' stage. Int J Oncol. 1996;9(4):747–54.
Deugnier Y, David V, Brissot P, Mabo P, Delamaire D, Messner M, et al. Serum alpha-L-fucosidase: a new marker for the diagnosis of primary hepatic carcinoma? Hepatology. 1984;4(5):889–92.
Wang K, Guo W, Li N, Shi J, Zhang C, Lau WY, et al. Alpha-1-fucosidase as a prognostic indicator for hepatocellular carcinoma following hepatectomy: a large-scale, long-term study. Br J Cancer. 2014;110(7):1811–9.
Yuan K, Listinsky CM, Singh RK, Listinsky JJ, Siegal GP. Cell surface associated alpha-L-fucose moieties modulate human breast cancer neoplastic progression. Pathol Oncol Res. 2008;14(2):145–56.
Shuang Z, Mao Y, Lin G, Wang J, Huang X, Chen J, et al. Alpha-L-fucosidase serves as a prognostic Indicator for intrahepatic cholangiocarcinoma and inhibits its invasion capacity. Biomed Res Int. 2018;2018:8182575.
Munkley J, Scott E. Targeting aberrant sialylation to treat cancer. Medicines (Basel). 2019;6(4)
Wongkham S, Boonla C, Kongkham S, Wongkham C, Bhudhisawasdi V, Sripa B. Serum total sialic acid in cholangiocarcinoma patients: an ROC curve analysis. Clin Biochem. 2001;34(7):537–41.
Ben-David T, Sagi-Assif O, Meshel T, Lifshitz V, Yron I, Witz IP. The involvement of the sLe-a selectin ligand in the extravasation of human colorectal carcinoma cells. Immunol Lett. 2008;116(2):218–24.
Trinchera M, Aronica A, Dall'Olio F. Selectin ligands sialyl-lewis a and sialyl-lewis x in gastrointestinal cancers. Biology (Basel). 2017;6(1)
Rillahan CD, Antonopoulos A, Lefort CT, Sonon R, Azadi P, Ley K, et al. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat Chem Biol. 2012;8(7):661–8.
Schultz MJ, Swindall AF, Wright JW, Sztul ES, Landen CN, Bellis SL. ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J Ovarian Res. 2013;6(1):25.
Chiricozzi E, Ciampa MG, Brasile G, Compostella F, Prinetti A, Nakayama H, et al. Direct interaction, instrumental for signaling processes, between LacCer and Lyn in the lipid rafts of neutrophil-like cells. J Lipid Res. 2015;56(1):129–41.
Diaz-Rohrer BB, Levental KR, Simons K, Levental I. Membrane raft association is a determinant of plasma membrane localization. Proc Natl Acad Sci U S A. 2014;111(23):8500–5.
Iwabuchi K, Prinetti A, Sonnino S, Mauri L, Kobayashi T, Ishii K, et al. Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconj J. 2008;25(4):357–74.
Ohno Y, Suto S, Yamanaka M, Mizutani Y, Mitsutake S, Igarashi Y, et al. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci U S A. 2010;107(43):18439–44.
Prinetti A, Loberto N, Chigorno V, Sonnino S. Glycosphingolipid behaviour in complex membranes. Biochim Biophys Acta. 2009;1788(1):184–93.
Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–64.
Hakomori SI, Handa K. GM3 and cancer. Glycoconj J. 2015;32(1–2):1–8.
Alderson NL, Hama H. Fatty acid 2-hydroxylase regulates cAMP-induced cell cycle exit in D6P2T schwannoma cells. J Lipid Res. 2009;50(6):1203–8.
Chen J, Li X, Ma D, Liu T, Tian P, Wu C. Ceramide synthase-4 orchestrates the cell proliferation and tumor growth of liver cancer in vitro and in vivo through the nuclear factor-kappaB signaling pathway. Oncol Lett. 2017;14(2):1477–83.
Patt LM, Grimes WJ. Cell surface glycolipid and glycoprotein glycosyltransferases of normal and transformed cells. J Biol Chem. 1974;249(13):4157–65.
Schiffmann S, Sandner J, Birod K, Wobst I, Angioni C, Ruckhaberle E, et al. Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis. 2009;30(5):745–52.
Suzuki M, Cao K, Kato S, Komizu Y, Mizutani N, Tanaka K, et al. Targeting ceramide synthase 6-dependent metastasis-prone phenotype in lung cancer cells. J Clin Invest. 2016;126(1):254–65.
Wang S, Beeghly-Fadiel A, Cai Q, Cai H, Guo X, Shi L, et al. Gene expression in triple-negative breast cancer in relation to survival. Breast Cancer Res Treat. 2018;171(1):199–207.
Wegner M, Neddermann D, Piorunska-Stolzmann M, Jagodzinski PP. Role of epigenetic mechanisms in the development of chronic complications of diabetes. Diabetes Res Clin Pract. 2014;105(2):164–75.
Nagano M, Takahara K, Fujimoto M, Tsutsumi N, Uchimiya H, Kawai-Yamada M. Arabidopsis sphingolipid fatty acid 2-hydroxylases (AtFAH1 and AtFAH2) are functionally differentiated in fatty acid 2-hydroxylation and stress responses. Plant Physiol. 2012;159(3):1138–48.
Acknowledgments
We are grateful for the support from the Integration and Innovation Research grant (I62-01-02); the Center for Translational Medicine, Faculty of Medicine; and Mekong Health Science Research Institute (12/2561), Khon Kaen University, Thailand.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Silsirivanit, A., Phoomak, C., Wongkham, S. (2021). Glycosylation in Cholangiocarcinoma Development and Metastasis: Diagnostic and Therapeutic Considerations. In: Tabibian, J.H. (eds) Diagnosis and Management of Cholangiocarcinoma. Springer, Cham. https://doi.org/10.1007/978-3-030-70936-5_25
Download citation
DOI: https://doi.org/10.1007/978-3-030-70936-5_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-70935-8
Online ISBN: 978-3-030-70936-5
eBook Packages: MedicineMedicine (R0)