Abstract
Tumor cell dissemination is mediated by a subpopulation of cells referred to as circulating tumor cells (CTCs), which have epithelial to mesenchymal properties and an inherent capacity to intravasate from the primary tumor, extravasate into secondary organs, and survive both in circulation and within the secondary organ. These properties have long been assumed to be cell autonomous; however, it is now clear that CTCs require the aid of secondary cells, such as macrophages. The role of tumor-associated macrophages (TAMs) as direct and indirect players in tumor cell dissemination will be discussed in this chapter. Special emphasis will be placed on the interplay between CTC and macrophages, focusing on the pro-tumorigenic function of TAMs at the level of secreted proteins, how TAMs enhance the invasive nature of tumor cells, the role of TAMs in promoting inflammation and a pro-tumor immune response, and the important role TAMs play in metastasis. Macrophages as therapeutic targets will also be discussed. Finally, the concept that macrophages can physically interact with CTCs or fuse with tumor cells to create giant macrophages or cell fusions that are invisible to the immune system and have enhanced migratory and metastatic capacities will be presented and detailed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Circulating tumor cells
- Macrophages
- Tumor-associated macrophages
- Giant macrophages
- Tumor-leukocyte fusions
11.1 Introduction
The role of macrophages in tumor development and dissemination has been known for several years [1,2,3] and was more recently reviewed by Yang and Zhang in 2017 [4]. Herein, we discuss in depth the role of macrophages and specifically that of tumor-associated macrophages (TAMs) in these various processes, as well as their potential role as clinical biomarkers and therapeutic targets. The following terms describe the different macrophage phenotypes mainly associated with these processes [5].
Inflammatory monocytes are recruited to inflammatory sites and are characterized as follows: CD14+(high), CD16−, CCR2+(high), CSF1R+(high), and LY6G-.
Tumor-associated macrophages (TAMs) are present in the tumor microenvironment and promote tumor development and progression. Human TAMs have the following marker profile: CD11b+, CD14+, CD23+, CD34−, CD45+, CD68+, CD117−, CD133−, CD146−, CD163+ (h), CD204+, CD206+, CCR2+, CSF1R+, CXCR4+, MHC class II+, VEGFR1+, and VEGFR2−. However, different markers are expressed by TAMs with specific tumor-associated functions.
Metastasis-associated macrophages (MAMs) are a subset of inflammatory monocytes that promote tumor dissemination and invasion and the formation of the metastatic niche. Studies in mice have shown that MAMs originate from inflammatory monocytes and have the following marker profile: CD11b+, CD31−, CD45+, CCR2+, CXCR4−, F4/80+, LY6C−, LY6G−, TIE2−, VEGFR1+, and VEGFR2-.
11.2 The Interplay Between Circulating Tumor Cells (CTCs) and Macrophages
The tumor microenvironment (TME) is composed of fibroblasts, immune cells, and vascular endothelial cells. Monocyte-derived macrophages are immune cells that originate from bone marrow-derived blood monocytes. Tumor-associated macrophages (TAMs) are involved in cancer-related inflammation, form part of the tumor microenvironment, and facilitate the dissemination of circulating tumor cell (CTC) and their subsequent seeding in metastatic niches [6, 7]. TAMs are either tissue resident or derived from peripheral sources such as monocytes of bone marrow and spleen [8], although their exact origin and the mechanisms underlying their pro-metastatic function in human tumors is unknown.
Macrophages are extremely plastic and can fluctuate between two states of polarization: “M1” or “M2” state. Classically activated macrophages are known as M1-polarized macrophages, whereas TAMs more closely resemble M2-polarized macrophages, which express higher levels of anti-inflammatory cytokines and angiogenic factors compared to their M1-type counterparts [4]. It is important to note that while TAMs do resemble M2-polarized macrophages, there exist several subpopulations of TAMs that share features of both M1 and M2 macrophages. Thus, the traditional M1 or M2 classification of TAMs may not accurately reflect the differentiated or biological state of these cells, and therefore, researchers have proposed functionally classifying TAMs (e.g., metastasis-promoting macrophage or immunosuppressive macrophage) in lieu of using the traditional M1 and M2 nomenclature [9,10,11,12].
Until a consensus is established, however, the use of binary M1/M2 classification continues to be widely used [13]. Based on this system, it is believed that macrophage polarization toward a pro-inflammatory, classically activated or “M1” phenotype is mediated by activation of Toll-like receptors upon engagement with bacterial components (e.g., lipopolysaccharides) or via type I helper T (Th1)-secreted cytokines [e.g., interferon (IFN)-γ]. In general, it is assumed that M1 macrophages are involved in Th1 responses to microorganisms, are involved in clearance of dead/apoptotic cells, have enhanced cell killing activity, and are characterized by secretion of a battery of pro-inflammatory cytokines that include IL-12, IL-1β, IL-6, and tumor necrosis factor α (TNFα) and generation of reactive oxygen and nitrogen intermediates [14]. Alternatively and in response to different stimuli or signals, macrophages can polarize toward an alternatively activated “M2” phenotype participating in Th2-type immunity, wound healing, and tissue remodeling [10]. These alternate stimuli can include, but are not limited to IL-4, IL-10, and IL-13 [10]. While M1 macrophages are characterized by secretion of pro-inflammatory cytokines, M2 macrophages are characterized by high expression of scavenging molecules, mannose and galactose receptors, activation of the arginase pathway, production of IL-10, vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), and efficient phagocytic activity [10, 14].
Cells of the myeloid lineage constitute one of the dominant immune cell populations present within tumors. While their initial infiltration into a tumor is dependent on the release of macrophage chemoattractants from tumor cells, such as colony stimulating factor 1 (CSF-1), the chemokines, CCL 2, 3, 4, and 8, vascular endothelial growth factor (VEGF), macrophage inflammatory protein-1 alpha (MIP-1α), and macrophage migration inhibition factor (MIF) [6, 7], once within the tumor, tumor cells promote the differentiation of monocytes (or M1 macrophages) into tumor-conditioned macrophages, also known as TAMs [11]. As mentioned above, while TAMs resemble M2 macrophages and express many of the same cell surface markers as M2 macrophages, to date no single panel of cell surface markers can accurately discriminate TAMs from non-TAMs. In the murine setting, the absence of Gr1 (Ly6G) and the expression of the canonical markers CD11b, F4/80, and CSF-1R in combination with mRNA analysis of additional markers are routinely used to classify macrophage subtypes [9]. In the human setting, antibodies to the glycoprotein CD68, the LPS-co receptor CD14, CD312, CD115, HLA-DR, or FcγRIII (CD16) have been used to identify macrophages, but with mixed and often times contradictory results [15]. Thus, combinations of these markers provide higher specificity and should be used when possible. To more specifically identify M2-like TAMs and subsets, the hemoglobin-scavenger receptor CD163 [16, 17], the macrophage scavenger receptor 1 CD204 [11, 18, 19], the mannose receptor CD206 [20], and more recently the T-cell immunoglobulin and mucin-domain containing protein-3 (Tim-3) [21] have been used with great success.
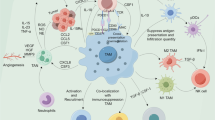
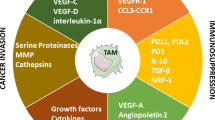
TAMs directly participate in tumor initiation, progression, and metastasis via numerous mechanisms including the following: (1) the secretion of proteolytic molecules such as MMPs to facilitate ECM remodeling [22,23,24,25]; (2) the expression of non-proteolytic proteins like chemokines [26, 27], TGF-β1 [28, 29], ISG15 [30], and hCAP/LL-37 [31, 32] to facilitate tumor cell proliferation, migration, and invasiveness; (3) the expression of angiogenic mediators such as TGF-β, VEGF-A, VEGF-C, platelet-derived growth factor (PDGF), and MMP-9 to sustain the growth of the tumor stroma and promote de novo tumor blood vessel formation [9, 26, 33, 34]; or (4) the expression of immunosuppressive factors including TGF-β, inducible nitric oxide synthase (iNOS), arginase-1, IDO (indoleamine 2,3-dioxygenase), and IL-10 to facilitate T-cell proliferation and activity [35, 36]. While the mechanisms underlying the pro-tumor effects of TAM-secreted factors on bulk tumors has been extensively studied, there is now growing evidence to support that TAMs also enhance tumor cell migration via physical interactions with tumor cells.
11.2.1 Pro-Tumorigenic Function of TAMs
It is well accepted that high TAM content in the tumor microenvironment is associated with a poor prognosis due to their pro-tumor and pro-angiogenesis role [37], and macrophages are found in large numbers at the leading invasion edge of many primary tumors where they degrade the basement membrane to promote tumor progression [1]. Thus, they play an important role in the primary tumor, and at the same time are essential for CTC intravasation. Regarding the latter, previous studies have hypothesized that CTCs intravasate into the circulatory system with TAMs via transendothelial migration [1]. Disseminated tumor cells need to survive in the hostile environment of the blood stream in order to develop metastatic foci at distant sites. Immune cells including macrophages, platelets, and T cells are thought to protect CTCs from the immune system and the environment within the blood vessels [5]. CTCs migrate through the blood stream as single cells or microemboli cell clusters, which consist of cells from the TME such as leukocytes, endothelial cells, or fibroblasts. This hinders the detection and destruction of CTCs by the immune system and also provides a physical protective barrier against damage and destruction in the harsh environment of the blood stream. Thus, the role of TAMs in CTC intravasation may be more complex and dynamic than previously recognized.
11.2.2 TAMs Enhance the Invasive Nature of Tumor Cells
Clinical and experimental evidence both in vivo and in vitro show that macrophages play an important role in tumor progression and dissemination and are therefore, potential targets for therapy. The relationship between poor disease prognosis and the presence of TAMs has been shown in tumor types such as breast, lung, and pancreas [38,39,40].
Macrophages are associated with chronic inflammation, and Balkwill et al. showed in 2005 that treatment with anti-inflammatory agents reduced cancer risk [41]. NF-κB appears to be important in the inflammatory response. In fact, it has been shown in vivo that NF-κB activation leads to the upregulation of anti-apoptotic genes, such as BCL-XL, BFL1, and GADD45β and therefore prevents apoptosis of tumorigenic cells [42]. Inhibition of NF-κB could be a potential therapeutic strategy to target macrophages, as this would not only restore apoptosis of malignant cells but also inhibit the expression of growth and survival factors in macrophages.
Furthermore, Lin et al. in 2001 showed that a homozygous null breast cancer mouse model for the colony-stimulating factor-1 (CSF-1) gene had reduced tumor progression with almost no metastasis. Whereas, overexpression of CSF-1 accelerated tumor progression and metastasis [43]. Furthermore, blocking the expression of CSF-1 in a human xenograft mouse model reduced tumor growth and metastatic capacity [44], thus supporting the notion of macrophages as enhancers of tumor progression. Tumors cells appear to “educate” macrophages in order to promote tumor invasion and intravasation into the blood vessels and the circulation to form secondary metastatic lesions [5]. In 2010, Wu et al. showed using co-cultures of macrophages or macrophage-conditioned medium with tumor cells an enhanced invasive phenotype, which appears to be dependent on NF-κB and SNAIL [45]. Thus, the tumor-macrophage interaction is fundamental for tumor invasion and dissemination. Grivennikov et al. showed in 2012 that TAMs secrete inflammatory cytokines such as IL-23 and IL-17 that promote cancer progression [46]. IL-23 was mainly produced by tumor-associated myeloid cells in response to tumor-elicited inflammation by microbial products in colon tumors. A recent study by Krug et al. in pancreatic neuroendocrine tumors (PNET) showed that TAMs play a critical role in the malignant phenotype of PNET. The number of infiltrating TAMs correlated with tumor invasiveness and metastatic potential. Specifically, in vivo and in vitro experiments of myeloid cell inhibition with liposomal clodronate showed a reduced malignant transformation of insulinomas with an associated reduction in angiogenesis and the number of infiltrating TAMs [47]. Similarly, Michl and colleagues showed in a genetic model of pancreatic cancer that clodronate-mediated depletion of macrophages markedly reduced metastasis formation and was associated with reduced CD4+CD25+ T cell levels and impaired angiogenesis. Interestingly, tumor incidence was only slightly reduced, suggesting that TAMs likely are more important in dissemination rather than tumorigenesis, at least for pancreatic cancer [48].
TAMs also produce proteases, such as Cathepsin B, matrix metallopeptidase (MMP) 2, MMP7, and MMP9 that destroy the components of the extracellular matrix (ECM), and therefore facilitate the invasion and migration of tumor cells [4]. In fact, Finkernagel et al. recently demonstrated in ovarian tumors that the transcriptional profile of TAMs was similar to that of resident macrophages. This included functions such as bacteria phagocytosis and antigen presentation. However, there was a subset of genes that were specifically upregulated in TAMs that were associated with the re-organization of the extracellular matrix [49].
TAMs are also involved in angiogenesis and promote the formation of intra-tumoral blood vessels that provide essential nutrition to the growing tumor. They also secrete pro-angiogenesis factors including colony stimulating factor-1 (CSF-1), various chemokines such as IL-8 and IL-1β, CCL2, CCL3, CCL4, CCL5, and CCL8, as well as macrophage migration inhibition factor (MIF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), tumor-necrosis factor-α (TNF-α), and thymidine phosphorylase [6]. Specifically, macrophage infiltration of the tumor site was significantly reduced in a CSF-1-null mouse model of breast cancer, with a consequent impaired development of the vasculature of the tumor and reduced vessel density due to VEGF depletion in the surrounding stroma [33]. Furthermore, human breast cancer spheroids had an increased angiogenic response and more pronounced vascularization when implanted into nude mice if they were infiltrated with macrophages. This was likely due to the release of VEGF by the spheroid cultures [6].
11.2.3 TAMs, Inflammation, and the Immune Response
The role of inflammation in cancer development is clear, and TAMs connect inflammation and cancer. The recruitment of macrophages to the primary tumor is crucial to establish and maintain an inflammatory TME. Epithelial-mesenchymal transition (EMT) increases the motility and invasiveness of tumor cells and is a key mechanism in the metastatic process. The transcription factor, Snail, induces EMT via the repression of the cell adhesion protein E-cadherin and is a crucial factor for inflammation-initiated invasion and metastasis. The inflammatory cytokine, TNFα, stabilizes Snail via the activation of the NF-κB pathway [45].
Metastatic tumor cells are immunogenic and should be recognized and neutralized by immune cells such CD8+ T cells natural killer (NK) cells. However, these metastatic tumor cells develop several strategies to overcome detection and destruction by the immune system, such as the recruitment of immunosuppressive cells [5]. TAMs are involved in immune suppression in the TME via the inhibition of the cytotoxic T lymphocyte (CTL) response via IL-10 and the induction of the expression PD-L1 in monocytes [4]. The anti-inflammatory cytokines produced by M2 TAMs reprogram the immunosuppressive microenvironment to promote tumor progression.
11.2.4 TAMs Play an Important Role in Metastasis
TAMs are important players in the development of a premalignant niche for the establishment of metastatic lesions [4] and also play a crucial role in the regulation of EMT, which enhances the metastatic capabilities of tumor cells [4]. TAM-derived TNF-α, VEGF, and TGF-β induce macrophages to produce S100A8 (aka SMA1) and serum amyloid A3, which recruit macrophages and tumor cells to the metastatic site [4]. Metastasis-associated macrophages (MAMs) are characterized by the expression of the markers CD11b, VEGF receptor 1 (VEGFR1), CXC-chemokine receptor 3 (CXCR3), and CC-chemokine receptor 2 (CCR2) and do not express GR1, angiopoietin 1 receptor (TIE2), and CD11c [5].
In pancreatic cancer, IFNβ produced by primary human PDAC cells can induce TAMs to secrete IFN-stimulated gene 15 (ISG15), a protein with both anti-viral and pro-tumorigenic properties. TAM-derived ISG15 can then stimulate pancreatic cancer stem cell (CSC) self-renewal and tumor-initiating properties, for example increased EMT [30]. Sainz et al. demonstrated that PDAC CSCs secrete the TGF-β superfamily members Nodal/Activin A and TGF-β1, which promote macrophage polarization to an M2 phenotype. As a consequence, polarized TAMs secrete a number of pro-tumoral proteins, including the antimicrobial peptide hCAP-18/LL-37. This antibacterial peptide binds to its receptors, including formyl peptide receptor 2 (FPR2) and P2X purinoceptor 7 receptor (P2X7R) and enhances the metastatic potential of pancreatic tumor cells. Specifically, the authors show that tumor cells pre-treated with LL-37 have significantly higher metastatic capacity than those treated with a scrambled peptide control [32].
11.3 Macrophages as a Therapeutic Target
The targeting of TAMs represents a novel strategy in cancer treatment and may be achieved in various ways such as blocking the recruitment of macrophages to tumors and re-educating the TME to a more anti-tumor phenotype and macrophage cytoreductive strategies. In mouse models, the CCL2-blocking agent (carlumab, CNTO88) was shown to inhibit tumor growth in a phase 2 study in metastatic castration-resistant prostate cancer in 2013 [50].
Furthermore, Sanford et al. showed in 2013 in a pancreatic cancer mouse model that the CCR2 antagonist (PF-04136309) blocks the mobilization of CCR2+ monocytes, which leads to a depletion of TAMs, reducing the metastatic potential [51]. Inflammatory macrophages were increased in the blood compared to the bone marrow in pancreatic cancer patients, which was a predictor of poorer survival in patients that had undergone a surgical resection. Pancreatic tumors with high CCL2 expression and low CD8 T-cell infiltrate have significantly decreased survival rates as tumor cells secrete CCL2, which recruits immunosuppressive CCR2+ macrophages to the primary tumor [51]. In a recent dose-finding study by Nywening et al., researchers were able to translate these findings directly to patients, by showing that the addition of an inhibitor of monocyte recruitment, specifically a small molecule CCR2 inhibitor PF-04136309 to FOLFIRINOX resulted in tumor shrinkage in 48% of patients with pancreatic cancer [13], double the historical response rate of FOLFIRINOX alone.
Re-education of TAMs to an M1-like phenotype using bioconjugated manganese dioxide nanoparticles or Pseudomonas aeruginosa mannose-sensitive hemagglutinin have been shown to enhance chemotherapy [39, 52]. In this way, antitumor macrophages scavenge and destroy phagocytosed tumor cells [53]. The CSF1/CSF1 receptor (CSF1R) is critical for monocyte progenitor generation and TAM polarization and is therefore a potential cytoreductive treatment target [4]. Furthermore, macrophage-specific surface markers are potential therapeutic targets, such as the mannose receptor CD206, the scavenger receptor A, and CD52 [4]. Several phase I clinical trials have been performed with antibodies against CSF1, which leads to a reduction in the number of TAMs (ClinicalTrials.gov identifier: NCT01316822, NCT01444404 and NCT01004861). However, there are currently no phase II or III clinical trials that specifically target macrophages or TAMs [5]. TAMs appear to modulate the cytotoxic effects of chemotherapy in animal models via various mechanisms. The M2 subtype of TAMs has been shown to be involved in revascularization of the tumor after chemotherapy, resulting in tumor relapse that is partly regulated by VEGF-A. In fact, the number of M2 TAMs around the blood vessels reduced after pharmacological inhibition of CXCR4, which subsequently diminished tumor revascularization and regrowth [54].
More recently, in vitro experiments with sorafenib, an oral multikinase, was shown to inhibit polarized macrophage-induced epithelial mesenchymal transition (EMT) in hepatocellular carcinoma cell lines [55]. Specifically, secretion of the EMT stimulation factor, hepatocyte growth factor (HGF), was decreased in macrophages after sorafenib treatment. Consequently, HGF-Met signaling activation by polarized macrophage-conditioned medium was reduced. These effects were not observed in normal hepatocytes. Furthermore, pre-treatment of polarized macrophages with sorafenib reduced the migration of hepatocellular carcinoma cells.
In humans, histological examination of hepatocellular carcinoma tumors treated with sorafenib showed a reduced number of tumor-infiltrating CD68+ macrophages and a reduced expression of the EMT markers, fibronectin and vimentin. Furthermore, the plasma levels of the EMT stimulation factor, hepatocyte growth factor (HGF), were significantly reduced after 24 weeks of therapy with sorafenib in patients with hepatocellular carcinoma, thus, suggesting that sorafenib inhibits HGF secretion.
11.4 TAMs as a Biomarker in Oncology
A high number of infiltrating TAMs in the primary tumor are associated with an aggressive behavior and poor prognosis [4]. The cell surface markers CD163, CD14, CD204, and CD206 may be used to identify TAMs, although they are not tumor-site specific [4, 56]. Serum CD163 levels may also be used as a prognostic marker in some tumor types [4]. Cell-surface vimentin–positive macrophage-like circulating tumor cells were identified in blood from patients with gastrointestinal stromal tumors (GISTs). These cells express the macrophage markers CD68 and CD14, tumor cell markers DOG-1, C-kit and are negative for CD45 [57].
11.4.1 Circulating Tumor Microemboli (CTM)
Circulating tumor clusters or microemboli (CTMs) have been reported in various tumor types including lung, breast, colon, prostate, and pancreas. CTMs have been identified via a variety of approaches including cell microscopy, immunocapture, and microfluidic chips [58,59,60,61]. As with the detection of CTCs, higher numbers of CTMs per ml of blood correlates with a poorer progression-free and overall survival. CTMs are thought to provide a survival advantage for CTCs in the harsh environment of the bloodstream and protect them from anoikis [62]. CTMs are thought to be cell clusters that have collectively shed from the primary tumor and consist of cells with both epithelial and mesenchymal phenotypes [63].
11.4.2 CTC-Macrophage Fusions
Cell fusion occurs when two or more cells become merged via the plasma membranes, and the progeny are known as hybrids. The tumor-leucocyte cell fusion theory of metastatic potential was proposed many years ago, whereby a tumor cell fuses with a migratory blood cell in order to promote tumor cell dissemination around the body [64,65,66]. Many tumor cell types have fusogenic properties and this was proposed as a mechanism to promote their malignant potential, resistance to drugs, and apoptosis [67]. In fact, malignant plasma cells in multiple myeloma (MM) are highly fusogenic and form multinucleated osteoclasts that express CSC markers with a high metastatic potential [68]. This concept of leukocyte-tumor cell fusion as a driver of cancer progression has been recently reviewed by Sutton et al. [69].
Giant circulating cancer-associated macrophage-like cells (CAMLs) were identified in 2013 [70] and are thought to be exclusively found in cancer patients. These cells range from 25 to 300 μm in size, with enlarged nuclei and express the pro-angiogenic markers CD14 and CD11c as well as CD45, cytokeratin, and epithelial markers CK 8, 18, and 19, and EpCAM [70] (Fig. 11.1). CAMLs are disseminated TAMs with the ability to seed, proliferate, and neovascularize the metastatic niche and are also involved in the phagocytosis of neoplastic cells within the primary tumor. In fact, higher CAML counts were found after chemotherapy treatment compared to untreated or hormone-based therapy. CAMLs or tumor cell-macrophage hybrids have been found in various tumor types such as breast, prostate, esophageal, colorectal, and pancreas, and the majority (over 83%) of patients with early and advanced stage disease are positive for CAMLs [69]. However, healthy controls and patients with benign disease were negative [70]. Here below we summarize the most relevant studies of tumor cell-macrophage fusions in various tumor types (Figs. 11.2 and 11.3).
A recent study in breast cancer cells by Zhang et al. showed that tumor-macrophage hybrid cells had enhanced tumorigenic and metastatic capacities such as increased proliferation, colony formation, migration, and invasion capacity with resistance to apoptosis. These effects appeared to be induced by EMT and Wnt/β-catenin signaling, with an associated downregulation of E-cadherin and an increased expression of N-cadherin, vimentin, Snail, as well as MMP-2, MMP-9, and S100A4 [71]. Another study showed that MCF-7 breast tumor cells and macrophage hybrids occurred by spontaneous fusions at a rate of around 2%. These fusions showed phenotypic and genetic traits from both maternal cells such as CD163 and CD45 expression and short tandem repeat (STR) genetic markers [72]. Another recent study described the isolation, cultivation, and characterization of macrophage-tumor cell fusions (MTFs) from the blood of pancreatic ductal adenocarcinoma (PDAC) patients. The MTFs consisted of M2-polarized macrophages, and the cells were generally aneuploid with characteristics associated with epithelial, macrophage, and stem cells and also expressed markers associated with tumor progression and metastasis. Furthermore, when transplanted orthotopically into a murine pancreas, MTFs grew as well-differentiated cell colonies in many different organs, without forming an established tumor. Thus, suggesting that these structures disseminate from the primary tumor and form a metastatic niche [73]. Furthermore, a study in melanoma showed that 2 circulating tumor cell (CTC) populations were detectable, one cytokeratin positive only and a second that was also positive for CD45 and the monocyte differentiation marker CD14, thus, suggesting the presence of leukocyte/macrophage-tumor cell fusion hybrids in these patients [74]. In fact, these macrophage-CTC fusions enter into the blood stream and generate metastatic lesions due to their ability to secrete cytokines to prepare the metastatic niche and colonize the secondary organ [75]. A recent study by Lindström et al. in breast cancer showed that cell fusions of MCF-7 cells with macrophages resulted in an increased radio resistance and enhanced DNA-repair capacity after exposure to Gy γ-radiation [76]. Another study in breast cancer, regarding the tumor-initiating and metastatic capacities of M2 macrophages and MCF-7 or MDA-MB-231 cell line hybrids in NOD/SCID mice showed that the hybrids had a more aggressive phenotype, including increased migration, invasion, and tumorigenicity. However, their proliferative ability was reduced and the hybrid phenotype was CD44+CD242/low with overexpression of EMT associated genes, indicative of stem-like properties [77]. Although, a study in a murine model of spontaneous neu+ breast cancer demonstrated that even though macrophages are most commonly fused with tumor cells, they were present at low levels in the primary tumor and undetectable in metastasis [78]. These studies suggest that TAMs may promote the metastatic potential of breast cancer cells via cell fusion, and the hybrids may gain a BCSC phenotype, compared with the parental lines (Fig. 11.4).
Several studies have addressed the specific mechanism by which macrophages promote the metastatic potential of tumor cells via cell-cell fusion. In fact, acute myeloid leukemia (AML) cells spontaneously fused with macrophages, dendritic and endothelial cells in a murine in vivo model. The hybrid cells gave rise to leukemia with 100% penetrance when implanted into mice, and data suggest that tumor cell-macrophage fusion may be a mechanism of gene transfer to promote tumor dissemination [79]. Furthermore, a study by Powell et al. in 2011 suggested that the cellular properties of macrophages, such as migration and immune evasion, are transferred to tumor cells via cell fusion as a mechanism of the metastatic conversion of cancer cells [80].
These cell fusions could provide new diagnostic, prognostic, and treatment response biomarkers in oncology as their presence seems to correlate with many clinical criteria. However, this presents a challenge due to their low prevalence in blood and the difficulty to isolate these cells in sufficient quantities in order to perform profiling studies. Likewise, the concept and existence of giant macrophages or CTC-macrophage fusions is still under scrutiny, and the true biological relevance of these cells has yet to be definitely determined. Without a doubt, the challenges associated with identifying and isolating these cells are many and include the scarcity of these cells, and the methodologies available to isolate them. Regarding the latter, many systems that have been developed to isolate CTCs include an immune cell elimination step. Thus, giant macrophages or CTC-macrophage fusions would be eliminated based on the expression of immune cell markers. Such systems include the CellSearch® platform (Menarini Silicon Biosystems, Inc. 2019). Thus, for the detection of these cell hybrids, other methodologies based on cell size would likely prove more beneficial. For example, the OncoQuick® system represents a simple-to-use, rapid, and efficient system for the enrichment of CTCs. OncoQuick® tubes consists of 50 ml polypropylene tube with a porous barrier which is inserted above a specially developed separation medium, which allows for density gradient centrifugation of cells from up to 30 ml of anticoagulated whole blood. Disseminated CTCs are enriched in the interphase, and following centrifugation, cells can be validated via various techniques, including immunofluorescence, RTqPCR, WB, or in vitro cell culture. This technique was used by Clawson GA et al. to identify and culture macrophage-tumor cell fusions from blood of patients with pancreatic ductal adenocarcinoma [81]. Indeed, other systems exists, but more research is needed to determine the best method for isolating these cells. Until then, we can only speculate that these cells play an important role in tumor cell dissemination.
References
Condeelis J, Pollard JW. Minireview macrophages: obligate partners for tumor cell migration. Invasion Metastasis. 2006:263–6. https://doi.org/10.1016/j.cell.2006.01.007.
Noy R, Pollard JW. Tumor-associated macrophages : from mechanisms to therapy. Immunity. 2015;41:49–61.
Shih J-Y, Yuan A, Chen JJ-W, Yang P-C. Tumor-associated macrophage: its role in cancer invasion and metastasis. J Cancer Mol. 2006;2:101–6.
Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10:1–12. https://doi.org/10.1186/s13045-017-0430-2.
Kitamura T, Qian B, Pollard JW, Avenue MP. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15:73–86.
Huang S, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134–42.
Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related. Inflammation. 2009;86:1065–73.
Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2015;41:21–35.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51.
Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69.
Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Investig. 2012;122:787–95.
Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216.
Heusinkveld M, et al. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol. 2011;187:1157–65.
Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83.
Laoui D, et al. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol. 2011;55:861–7.
Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55.
Yan W, et al. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut. 2015;64:1593–604.
Gocheva V, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55.
Nagakawa Y, Aoki T, Kasuya K, Tsuchida A, Koyanagi Y. Histologic features of venous invasion, expression of vascular endothelial growth factor and matrix metalloproteinase-2 and matrix metalloproteinase-9, and the relation with liver metastasis in pancreatic cancer. Pancreas. 2002;24:169–78.
Sousa S, et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015;17:101.
Wang R, et al. Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer. 2011;74:188–96.
Bohrer LR, Schwertfeger KL. Macrophages promote fibroblast growth factor receptor-driven tumor cell migration and invasion in a CXCR2-dependent manner. Mol Cancer Res. 2012;10:1294–305.
Fang W, et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis. 2014;35:1780–7.
Ye X, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol. 2012;189:444–53.
Singh R, Shankar BS, Sainis KB. TGF-β1-ROS-ATM-CREB signaling axis in macrophage mediated migration of human breast cancer MCF7 cells. Cell Signal. 2014;26:1604–15.
Sainz B, Martín B, Tatari M, Heeschen C, Guerra S. ISG15 is a critical microenvironmental factor for pancreatic cancer stem cells. Cancer Res. 2014;74:7309–20.
Li D, et al. Tumor-produced versican V1 enhances hCAP18/LL-37 expression in macrophages through activation of TLR2 and vitamin D3 signaling to promote ovarian cancer progression in vitro. PLoS One. 2013;8:e56616.
Sainz B, et al. Microenvironmental hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by activating its cancer stem cell compartment. Gut. 2015;64:1921–35.
Lin EY, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–46.
Murdoch C, Muthana M, Coffelt SB, C L. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44.
Galdiero MR, et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–10.
Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;8:254–65.
Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia. 2002;7:147–62.
Yang L, et al. CD163 + tumor-associated macrophage is a prognostic biomarker and is associated with therapeutic effect on malignant pleural effusion of lung cancer patients. Oncotarget. 2015;6:10592–603.
D’Errico G, et al. Tumor-associated macrophage-secreted 14-3-3ζ signals via AXL to promote pancreatic cancer chemoresistance. Oncogene. 2019;38:5469–85.
Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7.
Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59.
Lin BEY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40.
Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8.
Wu Y, et al. Stabilization of snail by NF-κB is required for inflammation- induced cell migration and invasion. Cancer Cell. 2010;15:416–28.
Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Österreicher CH, Hung KE, Datz C. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–8.
Krug S, et al. Therapeutic targeting of tumor-associated macrophages in pancreatic neuroendocrine tumors. Int J Cancer. 2018;143:1806–16.
Griesmann H, et al. Pharmacological macrophage inhibition decreases metastasis formation in a genetic model of pancreatic cancer. Gut. 2017;66:1278–85.
Finkernagel F, et al. The transcriptional signature of human ovarian carcinoma macrophages is associated with extracellular matrix reorganization. Oncotarget. 2016;7:75339–52.
Pienta KJ, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Investig New Drugs. 2013;31:760–8.
Sanford DE, et al. In pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404–15.
Song M, Liu T, Shi C, Zhang X, Chen X. Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia. ACS Nano. 2016;10:633–47.
Liu X, Kwon H, Li Z, Fu Y. Is CD47 an innate immune checkpoint for tumor evasion ? J Hematol Oncol. 2017:1–7. https://doi.org/10.1186/s13045-016-0381-z.
Hughes R, et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Microenviron Immunol. 2015;75:3479–92.
Deng YR, Liu WB, Lian ZX, Li X, Hou X. Sorafenib inhibits macrophage-mediated epithelial-mesenchymal transition in hepatocellular carcinoma. Oncotarget. 2016;7:38292–305.
Wang F, et al. CD163+CD14+ macrophages, a potential immune biomarker for malignant pleural effusion. Cancer Immunol Immunother. 2015;64:965–76.
Li H, Meng QH, Noh H, Somaiah N, Torres KE. Cell-surface vimentin – positive macrophage-like circulating tumor cells as a novel biomarker of metastatic gastrointestinal stromal tumors. Oncoimmunology. 2018;7:e1420450.
Chang M, et al. Clinical significance of circulating tumor microemboli as a prognostic marker in patients with pancreatic ductal adenocarcinoma. Clin Chem. 2016;513:505–13.
Aceto N, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2015;158:1110–22.
Cohen SJ, et al. Relationship of circulating tumor cells to tumor response, progression-free survival , and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2019;26:3213–21.
Cho EH, et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys Biol. 2013;9:1–13.
Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445.
Hou J, et al. Short communication circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011;178:989–96.
Lazova R, Chakraborty A, Pawelek JM. Leukocyte-cancer cell fusion: initiator of the Warburg effect in malignancy? Adv Exp Med Biol. 2011;714:151–72.
Dittmar T, Zänker KS. Introduction. Adv Exp Med Biol. 2011;950:1–3.
Dittmar T, et al. Recurrence cancer stem cells – made by cell fusion? Med Hypotheses. 2009;73:542–7.
Duelli D, Lazebnik Y. Cell fusion: A hidden enemy ? Cancer Cell. 2003;3:445–8.
Silvestris F, Ciavarella S, Strippoli S, Dammacco F. Cell fusion and hyperactive osteoclastogenesis in multiple myeloma. Adv Exp Med Biol. 2011;714:113–28.
Sutton TL, Walker BS, Wong MH. Circulating hybrid cells join the fray of circulating cellular biomarkers. Cell Mol Gastroenterol Hepatol. 2019;8:595–607.
Adams DL, et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc Natl Acad Sci U S A. 2014;111:3514–9. https://doi.org/10.1073/pnas.1320198111.
Zhang L-N, Huang Y-H, Zhao L. Fusion of macrophages promotes breast cancer cell proliferation, migration and invasion through activating epithelial-mesenchymal transition and Wnt/β-catenin signaling pathway. Arch Biochem Biophys. 2019;676:108137.
Shabo I, et al. Macrophage traits in cancer cells are induced by macrophage-cancer cell fusion and cannot be explained by cellular interaction. BMC Cancer. 2015;15:1–11.
Clawson GA, et al. ‘Stealth dissemination’ of macrophage-tumor cell fusions cultured from blood of patients with pancreatic ductal adenocarcinoma. PLoS One. 2017;12(9):e0184451.
Clawson GA, et al. Circulating tumor cells in melanoma patients. PLoS One. 2017;28:12(9):e0184451.
Clawson GA, et al. Macrophage-tumor cell fusions from peripheral blood of melanoma patients. PLoS One. 2015;10:e0134320.
Lindström A, Midtbö K, Arnesson L-G, Garvin S, Shabo I. Fusion between M2-macrophages and cancer cells results in a subpopulation of radioresistant cells with enhanced DNA-repair capacity. Oncotarget. 2017;8:51370–86.
Ding J, Jin W, Chen C, Shao Z, Wu J. Tumor associated macrophage × cancer cell hybrids may acquire cancer stem cell properties in breast cancer. PLoS One. 2012;7:e41942.
Lizier M, et al. Fusion between cancer cells and macrophages occurs in a murine model of spontaneous neu+ breast cancer without increasing its metastatic potential. Oncotarget. 2016;7:60793–806.
Martin-Padura I, et al. Spontaneous cell fusion of acute leukemia cells and macrophages observed in cells with leukemic potential. Neoplasia. 2012;14:1057–66.
Powell AE, et al. Fusion between intestinal epithelial cells and macrophages in a cancer context results in nuclear reprogramming. Cancer Res. 2011;71:1497–505.
Clawson GA, et al. “Stealth dissemination” of macrophage-tumor cell fusions cultured from blood of patients with pancreatic ductal adenocarcinoma. PLoS One. 2017;12:e0184451.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Earl, J., Sainz, B. (2021). Giant Macrophages: Characteristics and Clinical Relevance. In: Chinen, L.T.D. (eds) Atlas of Liquid Biopsy. Springer, Cham. https://doi.org/10.1007/978-3-030-69879-9_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-69879-9_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69878-2
Online ISBN: 978-3-030-69879-9
eBook Packages: MedicineMedicine (R0)