Abstract
The rapid evolving knowledge of glioblastoma molecular biomarkers and their association to prognosis and treatment calls for clinicians to keep abreast of the latest literature on the recommendations that have an impact on clinical practice. The presence or absence of IDH mutations and MGMT methylation continue to be essential molecular markers that indicate prognosis and response to treatment. New emerging data on the presence of other alterations such as TERT promoter mutation, EGFR amplification, and/or the combination of gain of entire chromosome 7 and loss of entire chromosome 10 (+7/−10) in the case of IDH-wildtype astrocytomas and the presence of CDKN2A/B in IDH-mutant astrocytomas have become significant as these mutations are associated with more aggressive tumor behavior. Other mutations such as EGFRvIII expression, FGFR-TACC gene fusions, PTEN deletion, PDGFRA and BRAF V 600E have elucidated important pathways for targeted therapies and aid in prognosis assessment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Glioblastoma
- IDH
- MGMT
- TERTp
- EGFR
- CDKN2A/B
- FGFR-TACC
- PTEN deletion
- PDGFRA
- BRAFV 600E
- Targeted therapies
- Molecular biomarkers
- Tumor behavior
Introduction
Neuro-oncology clinical practice has evolved at a rapid pace over the last decade, especially in the most recent years. The World Health Organization (WHO) 2016 classification of central nervous system (CNS) tumors became a pivotal point in the diagnosis and management of brain tumors. The incorporation of molecular biomarkers in addition to histology has importantly impacted the clinical management of gliomas, leading to more accurate diagnosis, better prognostication of tumor behavior, and overall survival (OS) and, at the same time, it has opened new horizons in term of therapeutic approaches [1,2,3].
The Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) was announced in December 2016 as a response to the accelerated expansion of advances on novel molecular markers and their clinical implication in the management of CNS tumors, providing regular and timely updates in between WHO CNS tumor classification editions and proposing future changes to future CNS tumor classifications [4, 5]. In April 2020, cIMPACT-NOW published their 6th update and their recommendations will be further discussed [2].
In this chapter, we will review the molecular markers that are relevant in clinical practice for glioblastoma (GBM) along with emerging novel biomarkers with a potential diagnostic or therapeutic role (Table 2.1).
Isocitrate Dehydrogenase (IDH)
Isocitrate dehydrogenases (IDH1, IDH2, IDH3) are metabolic enzymes that participate in the Krebs cycle by catalyzing the oxidative carboxylation of isocitrate to α-ketoglutarate and carbon dioxide, resulting in the production of nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) or nicotinamide adenine dinucleotide hydrogen in the case of IDH3 [6,7,8,9,10,11].
Mutations in the IDH1 and IDH2 genes result in a single amino acid substitution and are considered to be mutually exclusive [8, 11]. The most common mutation of IDH1 in glioma is found at the arginine codon 132 (R132) with the most frequent substitution is of arginine by histidine (R132H), which occurs in more than 90%. On the IDH2 gene, the most common mutation is at codon 172 (R172) and (R140) which is analogous for IDH1 [8, 9]. Mutations in IDH1 and IDH2 have been identified in other malignancies such as chondrosarcoma, intrahepatic cholangiocarcinoma, acute myeloid leukemia and myelodysplastic syndromes [10, 12, 13].
Mutations in IDH result in the production of the oncometabolite R(−)-2-hydroxyglutarate (2HG). 2HG competitively inhibits α-ketoglutarate-dependent enzymes affecting histone and DNA demethylation, and adaption to hypoxia, leading to abnormalities of epigenetic regulation, genetic instability , T cell differentiation, and tumor immunity. 2HG also impairs cellular differentiation in a variety of cell lineages promoting oncogenic transformation in association with other cancer genes [6, 9, 10, 14, 15].
IDH1 mutations are present in up to 7% of GBM and in over 70% of grade II and grade III gliomas. Mutations have been also identified in the IDH2 gene in approximately 4–8% of gliomas [13, 15, 16]. IDH mutation has been recognized as an early event in gliomagenesis. It has become a fundamental element for diagnosis, treatment decision and prognostication of tumor behavior.
The presence of IDH1/2 mutation in gliomas has been associated with younger age and better prognosis [11, 17]. The mean age at diagnosis for IDH-mutant GBM is 40 years and median overall survival (mOS) 27–31 months. For IDH-wildtype GBM the mean age at diagnosis is 64 years and mOS 15–18 months [17,18,19].
Recent research advances suggest that IDH-wildtype and IDH-mutant GBM are two separate diseases, with a completely different age of presentation, molecular profile, and overall survival. This has been translated to the clinical research setting where most clinical trial studies designed for newly diagnosed or recurrent GBM are focused on IDH-wildtype GBM and exclude IDH-mutant tumors. In the recent years, IDH mutation has been explored as a potential therapeutic target in glioma [20,21,22], with clinical trials designed for IDH-mutant solid tumors including dedicated arms for IDH-mutant gliomas [23, 24].
The third update of cIMPACT-NOW recognized WHO grade II diffuse astrocytic glioma, IDH-wildtype, with molecular features of GBM as an equivalent to a WHO grade IV tumor. This new concept applies to lower grade astrocytic tumors by histology, that contain the presence of TERT promoter mutation, EGFR gene amplification, and/or the combination of gain of entire chromosome 7 and loss of entire chromosome 10 (+7/−10); given that their behavior is similar than classic IDH-wildtype GBM [25, 26]. This was further reviewed in the sixth update from cIMPACT-NOW. On an effort to simplify nomenclature, and clinical trial eligibility, it was proposed that IDH-wildtype diffuse astrocytic gliomas can be classified as GBM, IDH-wildtype WHO grade 4 (now suggesting the use of Arabic numerals) in the presence of one or more of the aforementioned mutations. For IDH-mutant astrocytomas with microvascular proliferation or necrosis or CDKN2/B homozygous deletion, or any combination of any of these features will be designated astrocytoma, IDH-mutant, WHO grade 4 [2].
These guidelines from C-IMPACT-NOW are giving the clinician timely updates based on recent validated findings for more accurate diagnosis and prognostication that may change clinical management in daily practice, allowing the physician to provide a more tailored treatment recommendation and the possibility to offer a clinical trial that better suit the molecular profile for each patient’s tumor.
O6-Methylguanine-DNA Methyl- Transferase (MGMT)
Despite the fact it was not incorporated to the 2016 WHO classification of CNS tumors, MGMT promoter methylation status is one of the most relevant biomarkers used in the management of GBM as its presence predicts benefit from alkylating chemotherapy in patients with glioblastoma [27, 28]. The MGMT gene is located on chromosome 10 (10q26). It encodes the repair protein MGMT that reverses the damage created by alkylating agents by repairing damaged guanine nucleotides by transferring the methyl at O6 site of guanine to its cysteine residues. Epigenetic modification of the cytosine-phosphate-guanine (CpG) island at specific CpG sites within the MGMT promoter silences the gene, causing defective repair of DNA alkylation, promoting gene mutation and cell death [29, 30].
MGMT promoter methylation has been associated with better OS in glioma [28, 31,32,33]. MGMT promoter methylation status has been defined according to the percentage or level of methylation detected. Different testing methods have been studied, however, there is no agreement on the best test modality. Among the testing with the most reliability are methylation specific PCR (qMSP) and pyrosequencing [32, 34, 35]. Thresholds on the level of methylation have been studied in GBM: Unmethylated ≤9%, indeterminate or “gray zone” 10–29% and methylated >30%. Methylation levels above 30% have been correlated with better PFS and OS than below 30% (25.2 vs 15.2 months) [32]. Additional studies demonstrated that patients in the indeterminate methylation status also benefit of radiation and temozolomide therapy reflecting an OS of 10–17 months for truly unmethylated, 15.4–20 months for indeterminate and 19.7–34.1 months in methylated patients [35, 36]. It is important to underline that these studies did not correlate consistently with IDH status of the tumor samples studied.
Reliable and consistent assessment of MGMT methylation status at the first clinic visit is of utmost importance in the evaluation of patients with GBM due to its role in patient counseling and clinical trials eligibility. MGMT status has become relevant in the design of clinical trials for newly diagnosed GBM with some trials excluding patients with MGMT promoter hypermethylated tumors, other trials include it as a parameter for randomization.
A special population in which the value of MGMT has been particularly important for consideration of treatment decision, is the elderly. Multiple trials have demonstrated that concomitant treatment with temozolomide and hypofractionated radiotherapy increased OS regardless of the MGMT promoter methylation status [37,38,39,40,41]. However, for older patients who are not candidates for a combined-modality approach because of poor functional status or significant comorbidity, MGMT promoter methylation has a particularly important role. Emerging data support the use of temozolomide chemotherapy as an alternative to radiation therapy, in those patients with MGMT methylated tumors. Radiation therapy alone is an effective alternative for patients with MGMT unmethylated tumors.
Pseudoprogression is defined as a new or expanding area(s) of contrast enhancement that occur early after the end of radiation therapy, within the first 6 months (typically between 3 and 4 months), in the absence of true tumor growth, and that tends to stabilize or resolve without a change in therapy. In GBM, MGMT promoter methylation was associated with a 3.5-fold greater risk of developing pseudoprogression in up to 30% of patients treated with chemoradiation with concomitant temozolomide and has been linked to a better outcome. Pseudoprogression can also occur in unmethylated tumors, but to a lesser frequency. The response assessment in neuro-oncology (RANO) criteria recommended that patients should be excluded from clinical trials for recurrent disease within the first 12 weeks after radiation therapy, unless progression is clearly outside the radiation field or there is histologic documentation of progression [42,43,44,45,46]. In the clinical setting, magnetic resonance perfusion and spectroscopy and 18-FDG brain PET may aid in the differentiation between pseudoprogression versus progressive disease although histopathological analysis continues to be the gold standard.
Epidermal Growth Factor Receptor (EGFR)
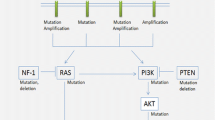
Epidermal growth factor receptor (EGFR) is a member of the ErbB family of receptor tyrosine kinase (RTK). Its structure includes an N-terminal extracellular domain, a transmembrane domain, an extracellular kinase domain, and a cytoplasmic C-terminal tail containing several phosphorylation sites that serve as signal transduction modules. Binding of one of several ligands to the extracellular ligand-binding domain induces receptor homo-dimerization or hetero-dimerization and results in kinase activation. In normal cells, this leads to DNA synthesis, cell proliferation, migration, and adhesion. EGFR mutations lead to production of mitogenic RTKs that inhibit the activity of p53 [47, 48].
EGFR is one of the first oncogenes identified in GBM. EGFR gene amplification is presented in about 40% of GBM [47, 49,50,51]. EGFRvIII mutation is found in 20% of GBM and it is particularly interesting as it is constitutively active and a potential neoantigen. The presence of EGFR amplification supports a GBM diagnosis and differentiates from other gliomas [47, 48, 50, 52,53,54]. EGFRvIII mutation alone is not predictive of outcome, however, the downstream altered molecular pathways associated as a result of its deletion may have a clinical impact [50, 52, 55]. EGFR has become one of the hallmark alterations that, if present in IDH-wildtype anaplastic astrocytoma, it supports the diagnosis of WHO grade IV astrocytoma [25].
EGFR has been extensively studied as therapeutic target. However, the results of multiple clinical trials evaluating EGFR tyrosine kinase inhibitors (TKI) and peptide treatment/vaccine, such as rindopepimut, in recurrent and/or newly diagnosed GBM patients have been disappointing [56, 57]. Clinical trials with newer generation EGFR TKI, EGFRvIII CAR-T cells alone and in combination with PD-1 inhibitors are currently ongoing and may elucidate the precise role of EGFR as a therapeutic target in GBM [50].
Phosphatase and Tensin Homolog (PTEN) Deletion
PTEN plays a major role regulating multiple biological functions at the level of the membrane and nucleus. It regulates genomic stability, cellular proliferation, migration and survival, tumor microenvironment among other functions. It has been implicated in multiple malignancies including gliomas. The loss of PTEN expression has been associated with glioma cells proliferation. This alteration is present in approximately 40% of primary GBM, and its relevance in OS has been under debate. However, it is considered an additional biomarker in the diagnosis of GBM [58,59,60]. Recent clinical trials have focused on the PI3K/Akt pathway, with targeted therapies such as buparlisib, sonolisib, pilaralisib, dactolisib, alone or in combination with mTOR inhibitors, are ongoing or have demonstrated no clinical benefit [61, 62].
BRAF V600E Mutation
The BRAF protein is an intermediary in the RAS-RAF pathway. After a ligand-mediated receptor tyrosine kinase is triggered by extracellular growth factors, it activates RAS, which initiates BRAF-mediated activation of MEK and ERK, causing transcription of factors for cell proliferation. The BRAF V600E mutation results in constitutive activation of the MEK-ERK pathway and uncontrolled cell division [63, 64]. BRAF mutations are drivers of oncogenesis in approximately 6% of human malignancies including melanoma, thyroid, colorectal and non-small cell lung cancer [65]. BRAF V600E mutations have been identified in a variety of primary brain tumors such as pleomorphic xanthoastrocytoma (up to 60%) [66, 67] and 47–58% ganglioglioma [68, 69], but they are uncommon in GBM (1–2%), except for the epithelioid variant in which is present in about 56% [65, 70, 71]. Epithelioid GBM is a rare and aggressive variant that is more common in children and young adults. It carries a dismal prognosis of about 6 months OS and frequently has leptomeningeal dissemination [72,73,74].
Even though BRAF mutations are rare in GBM, it is important to consider testing for it, especially in the younger population, as the use of BRAF and MEK inhibitors have shown a dramatic response on imaging and prolonged PFS [65, 74].
FGFR-TACC Gene Fusions
Fibroblast growth factor receptor-transforming acidic coiled-coil (FGFR-TACC)gene fusions are present in 3% of GBM. In astrocytes, fusions between FGFR3 and TACC3 genes can lead to malignant transformation and GBM progression due to the activation of mitogenic, antiapoptotic and migratory functions. Preliminary data of a Phase 2 trial with infigratinib showed PFS6 of 16% with a mOS of 6.7 months, demonstrating a partial response or stable disease in approximately one-third of patients with recurrent GBM and other glioma subtypes [75]. Futibatinib, another FGFR inhibitor, has shown to be well tolerated, however, efficacy results have not been published yet [76].
Platelet Derived Growth Factor Receptor Alpha (PDGFRA)
PDGFRA amplification is found in approximately 15–20% of adult GBM, especially in cerebellar variant. PDGFRA amplification increases with grade and is associated with a less favorable prognosis in WHO grade II and III IDH1-mutant astrocytoma comparable to WHO grade IV [49, 52]. In GBM harboring H3F3A-K27M mutation, positive PDGFRA expression was linked to even worse prognosis [77]. PDGFRA has been studied as a potential target in the treatment for GBM using dasatinib and other multikinase inhibitors alone or in combination with bevacizumab with no significant results [78, 79].
CDKN2A/B Homozygous Deletion
Cyclin Dependent Kinase Inhibitor 2A (CDKN2A) encodes Ink4a and Arf proteins, which play an important role in activating Rb and p53, respectively. CDKN2B encodes the tumor suppressor p15INK4b. Ink4a and p15INK4b inhibit CDK4 and CDK6 and maintain the growth-suppressive function of the Rb gene. When dysregulated, uncontrolled cell growth occurs [52, 80].
The prevalence of homozygous deletion of CDKN2A/B has been reported in 22–35% of all gliomas (16–47% IDH-mutant GBM and up to 58% of IDH-wildtype GBM) [54]. The presence of CDKN2A homozygous deletion in LGG and IDH-mutant GBM was associated with lower PFS and OS when compared to CDKN2A intact tumors [81]. The fifth and sixth updates of C-IMPACT-NOW have incorporated CDKN2A/B homozygous deletion as a marker for malignant behavior IDH-mutant WHO grade 2 and 3 astrocytomas, upgrading them to WHO grade 4 astrocytoma [2, 82].
These recent changes are quite impactful to daily clinical practice, as the presence of this mutation in astrocytic tumors dramatically changes the prognosis. CDKN2A/B deletion should be obtained routinely in the pathological analysis of IDH-mutant astrocytoma of any grade [83]. This marker could become a landmark parameter for clinical trial inclusion criteria in the near future [82, 84,85,86].
Telomerase Reverse Transcriptase Promoter Mutation (TERTp)
Telomerase reverse transcriptase (TERT) is a rate-limiting catalytic subunit of telomerase, an RNA-dependent DNA polymerase that lengthens telomeric DNA to maintain shorter telomeres in human cells function to prevent uncontrolled cellular proliferation. TERT promoter mutations result in the upregulation of TERT transcription, have been identified in over 50 different types of cancer, including several CNS neoplasms [87, 88]. Somatic hot spot mutations in TERTp occur in IDH-wildtype GBM and in 1p/19q co-deleted IDH-mutant oligodendroglioma. ATRX mutations are found to be mutually exclusive with TERTp mutations in adult GBM [89,90,91]. TERTp mutation has been linked to worse prognosis if found on IDH-wildtype astrocytoma WHO grade II or III as their clinical course resembles to the one of a WHO grade IV GBM [25, 90, 92]. Although TERT promoter mutation has not become a major pharmacological target for cancer therapy yet, it has significant role in glioma diagnosis and prognosis.
Conclusion
Advances in tumor molecular profiling technologies have allowed molecular characterization of GBM as never before. The addition of molecular biomarkers and histology to the 2016 WHO Classification of CNS Tumors has deeply impacted the clinical management of gliomas providing not only a more accurate diagnosis and prognostication, but also the opportunity to develop innovative clinical trials tailored to the genetic and epigenetic alterations of each tumor. The inclusion of next generation sequence-based assays and other molecular methods in the evaluation of newly diagnosed and recurrent GBM is becoming essential, as it may dramatically impact the diagnosis and management of our patients.
As more discoveries rapidly arise, and the pathogenesis of GBM continues to be better understood, it is likely that more markers will become part of additional classifications of this complex and heterogeneous tumor.
References
van den Bent MJ, et al. A clinical perspective on the 2016 WHO brain tumor classification and routine molecular diagnostics. Neuro-Oncology. 2017;19(5):614–24.
Louis DN, et al. cIMPACT-NOW update 6: new entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020;30(4):844–56.
Louis DN, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20.
Louis DN, et al. Announcing cIMPACT-NOW: the consortium to inform molecular and practical approaches to CNS tumor taxonomy. Acta Neuropathol. 2017;133(1):1–3.
Louis DN, et al. cIMPACT-NOW (the consortium to inform molecular and practical approaches to CNS tumor taxonomy): a new initiative in advancing nervous system tumor classification. Brain Pathol. 2017;27(6):851–2.
Zhang C, et al. IDH1/2 mutations target a key hallmark of cancer by deregulating cellular metabolism in glioma. Neuro-Oncology. 2013;15(9):1114–26.
Parsons DW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–12.
Romanidou O, Kotoula V, Fountzilas G. Bridging cancer biology with the clinic: comprehending and exploiting IDH gene mutations in gliomas. Cancer Genomics Proteomics. 2018;15(5):421–36.
Miyata S, et al. Comprehensive metabolomic analysis of IDH1R132H clinical glioma samples reveals suppression of β-oxidation due to carnitine deficiency. Sci Rep. 2019;9(1):9787.
Clark O, Yen K, Mellinghoff IK. Molecular pathways: isocitrate dehydrogenase mutations in cancer. Clin Cancer Res. 2016;22(8):1837–42.
Hartmann C, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–74.
Dang L, Yen K, Attar EC. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol. 2016;27(4):599–608.
Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19(4):764.
Waitkus MS, Diplas BH, Yan H. Isocitrate dehydrogenase mutations in gliomas. Neuro-Oncology. 2016;18(1):16–26.
Jalbert LE, et al. Metabolic profiling of IDH mutation and malignant progression in infiltrating glioma. Sci Rep. 2017;7:44792.
Ostrom QT, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro-Oncology. 2019;21(Suppl_5):v1–v100.
Yan H, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–73.
Molinaro AM, et al. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019;15(7):405–17.
Christians A, et al. The prognostic role of IDH mutations in homogeneously treated patients with anaplastic astrocytomas and glioblastomas. Acta Neuropathol Commun. 2019;7(1):156.
Tejera D, et al. Ivosidenib, an IDH1 inhibitor, in a patient with recurrent, IDH1-mutant glioblastoma: a case report from a phase I study. CNS Oncol. 2020;9:Cns62.
Mellinghoff, I. K., et al. Ivosidenib in Isocitrate Dehydrogenase 1-Mutated Advanced Glioma. J Clin Oncol 2020;38(29):3398–406.
De La Fuente MI, et al. A phase Ib/II study of olutasidenib in patients with relapsed/refractory IDH1 mutant gliomas: safety and efficacy as single agent and in combination with azacitidine. J Clin Oncol. 2020;38(15_Suppl):2505.
Galanis E, et al. Integrating genomics into neuro-oncology clinical trials and practice. Am Soc Clin Oncol Educ Book. 2018;38:148–57.
Popovici-Muller J, et al. Discovery of AG-120 (Ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med Chem Lett. 2018;9(4):300–5.
Brat DJ, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–10.
Tesileanu CMS, et al. Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the cIMPACT-NOW criteria. Neuro-Oncology. 2019;22(4):515–23.
Stupp R, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66.
Binabaj MM, et al. The prognostic value of MGMT promoter methylation in glioblastoma: a meta-analysis of clinical trials. J Cell Physiol. 2018;233(1):378–86.
Yu W, et al. O(6)-methylguanine-DNA methyltransferase (MGMT): challenges and new opportunities in glioma chemotherapy. Front Oncol. 2020;9:1547.
Mansouri A, et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: refining the approach based on emerging evidence and current challenges. Neuro-Oncology. 2019;21(2):167–78.
Dahlrot RH, et al. Posttreatment effect of MGMT methylation level on glioblastoma survival. J Neuropathol Exp Neurol. 2019;78(7):633–40.
Brigliadori G, et al. Defining the cutoff value of MGMT gene promoter methylation and its predictive capacity in glioblastoma. J Neuro-Oncol. 2016;128(2):333–9.
Hegi ME, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003.
Estival A, et al. Pyrosequencing versus methylation-specific PCR for assessment of MGMT methylation in tumor and blood samples of glioblastoma patients. Sci Rep. 2019;9(1):11125.
Hsu C-Y, et al. Prognosis of glioblastoma with faint MGMT methylation-specific PCR product. J Neuro-Oncol. 2015;122(1):179–88.
Pinson H, et al. Weak MGMT gene promoter methylation confers a clinically significant survival benefit in patients with newly diagnosed glioblastoma: a retrospective cohort study. J Neuro-Oncol. 2020;146(1):55–62.
Malmström A, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–26.
Kalra B, Kannan S, Gupta T. Optimal adjuvant therapy in elderly glioblastoma: results from a systematic review and network meta-analysis. J Neuro-Oncol. 2020;146(2):311–20.
Wee CW, et al. Chemoradiation in elderly patients with glioblastoma from the multi-institutional GBM-molRPA cohort: is short-course radiotherapy enough or is it a matter of selection? J Neuro-Oncol. 2020;148(1):57–65.
Hanna C, et al. Treatment of newly diagnosed glioblastoma in the elderly: a network meta-analysis. Cochrane Database Syst Rev. 2020;3(3):Cd013261.
Perry JR, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–37.
Zhou M, et al. The value of MGMT promote methylation and IDH-1 mutation on diagnosis of pseudoprogression in patients with high-grade glioma: a meta-analysis. Medicine. 2019;98(50):e18194.
Brandes AA, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–7.
Wen PY, et al. Response assessment in neuro-oncology clinical trials. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(21):2439–49.
Thust SC, van den Bent MJ, Smits M. Pseudoprogression of brain tumors. J Magn Reson Imaging. 2018;48(3):571–89.
Chukwueke UN, Wen PY. Use of the response assessment in neuro-oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 2019;8(1):CNS28.
Maire CL, Ligon KL. Molecular pathologic diagnosis of epidermal growth factor receptor. Neuro-Oncology. 2014;16 Suppl 8(Suppl 8):viii1–6.
Cimino PJ, et al. A wide spectrum of EGFR mutations in glioblastoma is detected by a single clinical oncology targeted next-generation sequencing panel. Exp Mol Pathol. 2015;98(3):568–73.
Brennan CW, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–77.
An Z, et al. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018;37(12):1561–75.
Mellinghoff IK, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–24.
Aldape K, et al. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829–48.
Neftel C, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178(4):835–849.e21.
Kessler T, et al. Molecular profiling-based decision for targeted therapies in IDH wild-type glioblastoma. Neurooncol Adv. 2020;2(1):vdz060.
Brito C, et al. Clinical insights gained by refining the 2016 WHO classification of diffuse gliomas with: EGFR amplification, TERT mutations, PTEN deletion and MGMT methylation. BMC Cancer. 2019;19(1):968.
Alexandru O, et al. Receptor tyrosine kinase targeting in glioblastoma: performance, limitations and future approaches. Contemp Oncol (Pozn). 2020;24(1):55–66.
Weller M, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–85.
Milella M, et al. PTEN: multiple functions in human malignant tumors. Front Oncol. 2015;5:24.
Han F, et al. PTEN gene mutations correlate to poor prognosis in glioma patients: a meta-analysis. Onco Targets Ther. 2016;9:3485–92.
Yang J-M, et al. Characterization of PTEN mutations in brain cancer reveals that pten mono-ubiquitination promotes protein stability and nuclear localization. Oncogene. 2017;36(26):3673–85.
Rosenthal M, et al. Buparlisib plus carboplatin or lomustine in patients with recurrent glioblastoma: a phase Ib/II, open-label, multicentre, randomised study. ESMO Open. 2020;5(4):e000672.
Wen PY, et al. Phase I, open-label, multicentre study of buparlisib in combination with temozolomide or with concomitant radiation therapy and temozolomide in patients with newly diagnosed glioblastoma. ESMO Open. 2020;5(4):e000673.
Maraka S, Janku F. BRAF alterations in primary brain tumors. Discov Med. 2018;26(141):51–60.
Bond CE, Whitehall VLJ. How the BRAF V600E mutation defines a distinct subgroup of colorectal cancer: molecular and clinical implications. Gastroenterol Res Pract. 2018;2018:9250757.
Kushnirsky M, et al. Prolonged complete response with combined dabrafenib and trametinib after BRAF inhibitor failure in BRAF-mutant glioblastoma. JCO Precis Oncol. 2020;4:44–50.
Ida CM, et al. Pleomorphic xanthoastrocytoma: natural history and long-term follow-up. Brain Pathol. 2015;25(5):575–86.
Dias-Santagata D, et al. BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PLoS One. 2011;6(3):e17948.
Koelsche C, et al. Mutant BRAF V600E protein in ganglioglioma is predominantly expressed by neuronal tumor cells. Acta Neuropathol. 2013;125(6):891–900.
Phadnis S, et al. Rare-20. BRAF mutations in pediatric gangliogliomas and the clinical significance an MD Anderson Cancer Center experience. Neuro-Oncology. 2018;20(Suppl_6):vi240.
Korshunov A, et al. Epithelioid glioblastomas stratify into established diagnostic subsets upon integrated molecular analysis. Brain Pathol. 2018;28(5):656–62.
Behling F, et al. Frequency of BRAF V600E mutations in 969 central nervous system neoplasms. Diagn Pathol. 2016;11(1):55.
Zeng Y, et al. Clinicopathological, immunohistochemical and molecular genetic study on epithelioid glioblastoma: a series of fifteen cases with literature review. Onco Targets Ther. 2020;13:3943–52.
Kanemaru Y, et al. Dramatic response of BRAF V600E-mutant epithelioid glioblastoma to combination therapy with BRAF and MEK inhibitor: establishment and xenograft of a cell line to predict clinical efficacy. Acta Neuropathol Commun. 2019;7(1):119.
Burger MC, et al. Dabrafenib in patients with recurrent, BRAF V600E mutated malignant glioma and leptomeningeal disease. Oncol Rep. 2017;38(6):3291–6.
Lassman A, et al. OS10.6 Infigratinib (BGJ398) in patients with recurrent gliomas with fibroblast growth factor receptor (FGFR) alterations: a multicenter phase II study. Neuro-Oncology. 2019;21:iii21–2.
Meric-Bernstam F, et al. Abstract CT238: TAS-120 in patients with advanced solid tumors bearing FGF/FGFR aberrations: a phase I study. Cancer Res. 2019;79(13 Supplement):CT238.
Zhang R-Q, et al. Biomarker-based prognostic stratification of young adult glioblastoma. Oncotarget. 2016;7(4):5030–41.
Lassman AB, et al. Phase 2 trial of dasatinib in target-selected patients with recurrent glioblastoma (RTOG 0627). Neuro-Oncology. 2015;17(7):992–8.
Galanis E, et al. A phase 1 and randomized, placebo-controlled phase 2 trial of bevacizumab plus dasatinib in patients with recurrent glioblastoma: Alliance/North Central Cancer Treatment Group N0872. Cancer. 2019;125(21):3790–800.
Jiao Y, Feng Y, Wang X. Regulation of tumor suppressor gene CDKN2A and encoded p16-INK4a protein by covalent modifications. Biochem Mosc. 2018;83(11):1289–98.
Lu VM, et al. The prognostic significance of CDKN2A homozygous deletion in IDH-mutant lower-grade glioma and glioblastoma: a systematic review of the contemporary literature. J Neuro-Oncol. 2020;148(2):221–9.
Brat DJ, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139(3):603–8.
Yang RR, et al. IDH mutant lower grade (WHO Grades II/III) astrocytomas can be stratified for risk by CDKN2A, CDK4 and PDGFRA copy number alterations. Brain Pathol. 2020;30(3):541–53.
Appay R, et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro-Oncology. 2019;21:1519–28.
Mirchia K, et al. Total copy number variation as a prognostic factor in adult astrocytoma subtypes. Acta Neuropathol Commun. 2019;7(1):92.
Reis GF, et al. CDKN2A loss is associated with shortened overall survival in lower-grade (World Health Organization Grades II–III) astrocytomas. J Neuropathol Exp Neurol. 2015;74(5):442–52.
Patel B, et al. TERT, a promoter of CNS malignancies. Neurooncol Adv. 2020;2(1):vdaa025.
Yuan X, Larsson C, Xu D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: old actors and new players. Oncogene. 2019;38(34):6172–83.
Killela PJ, et al. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014;5(6):1515–25.
Bollam SR, Berens ME, Dhruv HD. When the ends are really the beginnings: targeting telomerase for treatment of GBM. Curr Neurol Neurosci Rep. 2018;18(4):15.
Lee Y, et al. The frequency and prognostic effect of TERT promoter mutation in diffuse gliomas. Acta Neuropathol Commun. 2017;5(1):62.
Reifenberger G, et al. Advances in the molecular genetics of gliomas — implications for classification and therapy. Nat Rev Clin Oncol. 2017;14(7):434–52.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
del Pilar Guillermo Prieto, M., de La Fuente, M.I. (2021). The Role of Molecular Genetics of Glioblastoma in the Clinical Setting. In: Otero, J.J., Becker, A.P. (eds) Precision Molecular Pathology of Glioblastoma. Molecular Pathology Library. Springer, Cham. https://doi.org/10.1007/978-3-030-69170-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-69170-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69169-1
Online ISBN: 978-3-030-69170-7
eBook Packages: MedicineMedicine (R0)