Abstract
Heterocyclic compounds are cyclic organic molecules possessing at least one atom other than carbon in the ring structure. They are a widely used class of organic compounds. The heterocyclic scaffolds represent the central framework of many biologically active molecules. The other applications, like agrochemicals, veterinary products, dyes, etc., also make them essential. Due to its high global demand, there is always a need for new and efficient methodology in synthesizing these molecules. The sustainable process with a minimal environmental impact is the need of the present day and biocatalysis supports this. Enzymes are biocatalysts and play a progressively significant role in the synthesis of heterocyclic molecules. This chapter discusses the utility of different enzymes for the synthesis of nitrogen-, oxygen- or both containing heterocyclic molecules.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Heterocyclic compounds are cyclic organic molecules possessing at least one atom other than carbon in the ring structure (Sabir et al. 2015). Their both properties, physical and chemical, are reliant on the presence of heteroatom(s). These compounds find their applications in almost all types of industries, including pharmaceuticals, agrochemicals, dyes, and pigments and others (Joule and Mills 2013; Rudi et al. 2005; Broughton and Watson 2004; Arunkumar 2015; Arora et al. 2012; Kozikowski 1984). Due to its increasing demand, researchers continuously keep an eye on their synthetic routes. They are always trying to develop various efficient and environmentally friendly methodologies (Busto et al. 2011; Feber 2004; Shoda et al. 2016). The conventional methods, like chemical catalysis, electrochemical, microwave-assisted, and using ionic liquids, solid and solution phase synthesis, are some of the well-known methods (Busto et al. 2011; Feber 2004; Shoda et al. 2016). Still, the development of a sustainable synthetic process is widely explored in organic synthesis. Biocatalysis has shown potential towards the synthesis of organic molecules, including heterocyclic molecules in a sustainable and environmentally friendly manner (Milner and Maguire 2012; Mane et al. 2018; Wu et al. 2019; Singh et al. 2006).
Biocatalysis is the use of bio-based catalysts or enzymes in organic synthesis (Dalal et al. 2016; Xie et al. 2013; Li et al. 2008; Xiang et al. 2013, 2014; Xue et al. 2012; Ding et al. 2015). Apart from the other advantages possessed by enzyme-catalyzed reactions, they also take care of the production of single enantiomers instead of racemic mixtures (Singh et al. 2006). The two fundamental properties of catalysts also apply to enzyme-catalyzed reactions: (i) increase in the rate of reaction; and (ii) remain non-consumed after the reaction. The focus of research revolves around the development of stereo-, regio- and chemo-selective reactions (Shoda et al. 2016; Kobayashi et al. 1997, 1996). Different classes of enzymes such as hydrolases, oxidoreductases, transferase, lyase, isomerase and ligase perform different types of reactions (Fig. 1) (Shoda et al. 2016; Singh et al. 2006; Groger and Asano 2012; Webb 1992).
In this chapter, the enzyme-catalyzed synthesis of pyrroles, indoles, phenazines, benzocarbazoles, benzimidazoles, pyrazoles, benzofurans, chromenes, dioxins, lactones and oxazolidinones have been discussed.
2 Enzyme-Mediated Synthesis of Heterocyclic Compounds
Enzyme-mediated synthesis is based on the ability of its active site to allow a particular substrate to enter into it and further get transformed into a suitable product (Yang et al. 2015; Kłossowski et al. 2013). Many name reactions like Morita–Baylis–Hillman reaction (Reetz et al. 2007), Michael addition (Zhang et al. 2017a), aldol addition (Li et al. 2008) and others (Hu et al. 2012; Wang et al. 2017) have been successfully carried out using enzyme-catalyzed method. Some of the important enzyme-mediated reactions have been discussed in this section towards the synthesis of heterocyclic compounds.
2.1 Nitrogen-Containing Heterocycles
Nitrogen heterocycles are mainly found in peptides and alkaloids, and these both are most widely spread in mostly all-natural products. The one, two and more number of nitrogen-containing heterocycles with 5-, 6- and 7-membered aromatic compounds is very important moiety in the field of medicine (Jampilek (2019); Vitaku et al. 2014; Singh and Geetanjali 2011). The synthesis of N-containing heterocyclic molecules has always been explored for sustainable processes (Veer and Singh 2019; Poonam 2019; Chauhan and Geetanjali 2000; Chauhan et al. 2003). There is always demand for the wide-structural diversity of N-heterocycles and hence exploration of synthetic protocols. They are prepared by synthetic reactions as well as via enzymatic methods. The biosynthetic pathways of naturally occurring heterocyclic compounds help in designing their enzymatic synthetic paths (Hemmerling and Hahn 2016; Junghanns et al. 1995).
2.1.1 Pyrroles
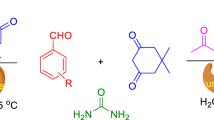
Pyrroles are five-membered ring, N-containing heterocyclic molecule which showed their utility in diverse biological activities, speciality polymeric materials, etc. (Trofimov et al. 2004; Bellina and Rossi 2006). The Paal–Knorr reaction has been used to synthesize the derivatives of N-substituted pyrrole in 60–99% yield catalyzed by α-amylase obtained from hog pancreas. This reaction was standardized using aniline (R=Ph) and 2,5-hexanedione as the starting materials (Fig. 2) (Zheng et al. 2013). The α-amylase from hog pancreas yielded N-phenyl-2,5-dimethylpyrrole in 94% yield; however, from Aspergillus oryzae gave the same product in 65% yield. This suggests that the sources of enzyme also play important role in product formation.
The authors have optimized the biocatalyst from eight different enzymes they tried for the reaction. The optimized enzyme, α-amylase from hog pancreas under mild reaction condition such as 50 °C temperature in methanol showed excellent activity using wide variety of primary amines (Zheng et al. 2013). The reactions showed high yields and better efficiency under mild reaction conditions.
2.1.2 Indoles
Spirooxindoles are indole derivatives present in many secondary metabolites and biologically important molecules (Ding et al. 2006; Galliford and Scheidt 2007). These molecules have been synthesized using enzymes as biocatalysts (Chai et al. 2011). The reaction of isatin, malononitrile and ethyl acetoacetate in the presence of lipase from porcine pancreas (PPL) in water–ethanol gave spirooxindole derivatives in 82–95% yield (Fig. 3) (Chai et al. 2011). The reaction condition with respect to reaction time, catalyst, temperature and solvent was optimized by the authors.
The use of others solvents like acetone, dichloromethane, hexane, chloroform, tetrahydrofuran, acetonitrile and dimethylformamide either did not promote the reaction or gave only moderate yield (Chai et al. 2011). Other commercially available hydrolytic enzymes were also evaluated for the reaction but PPL gave better results. The enzymes amano lipase M from Mucor javanicus and Amano lipase A from Aspergillus niger also gave the products in 82 and 84% yield, respectively. The lipase acrylic resin from Candida antarctica gave only trace of spirooxindoles. This was proposed that the specific spatial conformation along with the tertiary structure of PPL gave better yield of spirooxindole derivatives (Chai et al. 2011).
2.1.3 Phenazines
Phenazine is dibenzo annulated pyrazine with the formula (C6H4)2N2. Their derivatives are multifunctional in nature and successfully used in pharmaceuticals (Zhuo et al. 2013; Gamage et al. 2006), energy sectors (Okazaki et al. 2017; Lee et al. 2010), sensors (Pauliukaite et al. 2010), etc. Due to their broad applications, enzymatic synthesis has also been performed on this molecule synthesis (Sousa et al. 2014, 2018). Sousa et al. studied the use of Laccases, a multi-copper oxidase for the synthesis of phenazine and phenoxazinone frameworks from substituted aromatic amines (Fig. 4) (Sousa et al. 2014). This has been observed that the laccase-catalyzed reactions give only water as waste product when reactions are performed in aqueous solvent systems (Witayakran and Ragauskas 2009; Mikolasch and Schauer 2009). The reaction was performed under mild reaction condition using solvent methanol in phosphate buffer (pH 6–7) at temperature of 37 °C under aerobic conditions. The enzymatic oxidation of meta, para-disubstituted amine derivatives afforded phenazines. With the starting reagent, 1,2-diaminobenzene, 2,3-diaminophenazine was formed in 66% yield, whereas with ortho-aminophenol, phenoxazine formed in 83% yield (Sousa et al. 2014). The oxidation of 1-amino-2-naphthol with PPL at pH 7 gave 14H-dibenzo[a,j]phenoxazine-5,6-diol in 59% yield (Fig. 5) (Sousa et al. 2014).
This group further utilized the optimized protocol for a one-step asymmetric phenazines and phenoxazinones synthesis using spore coat protein A (CotA)-laccase enzyme as catalyst from ortho-substituted diamines and ortho-substituted hydroxylamines through aerobic oxidations (Sousa et al. 2018). Some of the other similar molecules synthesized using similar procedure with different reactants are given with their yields in Fig. 6 (Sousa et al. 2014, 2018).
2.1.4 Benzocarbazoles
Carbazole consists of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. This is based on indole structure where a second benzene ring is fused at the 2–3 position of indole. Carbazole derivatives possess pharmaceutical properties (Knölker and Reddy 2002; Pecca and Albonico 1971) and have applications in material sciences (Grazulevicius et al. 2003; Lia and Grimsdale 2010).
Sousa et al. performed the enzymatic synthesis of carbazoles using CotA laccase (Sousa et al. 2015). The oxidation of the meta, para-disubstituted arylamine 2,4-diaminophenyldiamine afforded benzocarbazole derivative in 74% yield and hence developed a clean method to construct in one-step C–C and C–N bonds (Fig. 7) (Sousa et al. 2015). The electrochemical behaviour of the target substrate plays essential role for product formation through an intramolecular oxidative coupling step (Sousa et al. 2015).
2.1.5 Benzimidazoles
Benzimidazole is a bicyclic heteroaromatic system which is composed of a benzene ring fused with an imidazole ring, i.e. five-membered ring with three number of carbon and two nitrogen is attached. It is occurring in nature as part of the vitamin B12 molecule with chemical formula C7H6N2. This type of molecules possesses therapeutic properties including broad-spectrum anthelmintic, fungicidal or antimicrobial action (Salahuddin and Mazumder 2017).
Wang et al. in 2010 developed an efficient synthesis of bioactive compound in an ecologically and economically favourable way (Wang et al. 2010). In their study, they developed the enzyme-mediated synthesis of 2-alkylbenzimidazole. o-Phenylenediamine is used as primary reactant for the synthesis of the desired product (Fig. 8). In this synthesis, o-phenylenedimine reacted with the corresponding ester in the presence of immobilized lipase from Mucor miehei (MML) as a catalyst. This mixture was stirred at 50 °C for 60 h to complete the reaction and achieve the synthesis of benzimidazole derivatives (Wang et al. 2010). The reaction with other hydrolases like lipase acrylic resin from Candida antarctica B, Amano lipase M from Mucor javanicus and lipase from Candida rugosa gave low to poor yields. The spatial conformation of the lipases plays very important role in the product formation (Chai et al. 2011; Wang et al. 2010).
2.1.6 Pyrazoles
Pyrazoles are five-membered heterocyclic molecules possessing two nitrogen atoms in the ring. Pyrazole derivatives are biologically active compounds and also useful for other applications like dyes and luminophores (Mishra and Sasmal 2011; Stellrecht and Chen 2011). These molecules have been synthesized through enzyme-catalyzed reaction. Mane et al. used a whole cell biocatalyst, Saccharomyces cerevisae (Baker’s yeast), with 1,3-dicarbonyl compound and hydrazines at room temperature to give the N-substituted pyrazole derivatives in 70–92% yield through oxidative cyclocondensation reaction (Fig. 9) (Mane et al. 2015). This synthetic method was found to be useful to a series of pyrazole derivatives where fermented Baker’s yeast played role in efficient cyclocondensation 1,3-diketones with hydrazines/hydrazides. The study also suggested that the presence of the enzyme lipase in Baker’s yeast accelerated the reaction leading to the desired pyrazole derivatives (Mane et al. 2015).
Pyranopyrazoles are another important group of heterocyclic compounds. A four-component cyclocondensation reaction of hydrazine hydrate, malononitrile, ethyl acetoacetate and benzaldehyde afforded 6-amino-3-methyl-4-(3-nitrophenyl)-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile using lipase from fungi Aspergillus niger as catalyst (Fig. 10) (Bora et al. 2013). This method of synthesis was successfully utilized for different carbonyl compounds as one of the reactant yielding dihydropyrano[2,3-c]pyrazoles in 75–98% yield (Bora et al. 2013). The cyclic ketones also successfully gave spiro-substituted dihydropyrano[2,3-c]pyrazoles in 70–80% yield. The enzyme showed its utility towards wide range of substrates, reusability and mild reaction condition, i.e. room temperature and ethanol solvent. The study was also done on lipases from different sources such as Pseudomonas cepacia, Amano AK, Penicillium camemberti, Porcine pancreas and Aspergillus niger giving the yield of 65, 72, 75, 91 and 95%, respectively, for the product 6-amino-3-methyl-4-(3-nitrophenyl)-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile.
2.2 Oxygen-Containing Heterocycles
There is no doubt that oxygen-containing heterocycles play important role in industrial, medicinal and nutritional applications due to their diverse biological functions and natural abundance (Venkatachalam and Kumar 2019). Their synthetic methods are always being explored. There are many chemical synthetic methods to produce oxygen-containing heterocycles but due to toxicity and unfriendly approach towards environment and economy, there is demand for green synthesis. Enzyme-mediated synthesis is one of the methodologies to produce oxygen-containing heterocyclic compounds.
2.2.1 Chromenes
4H-chromene derivatives are shown their potential in various pharmaceutical activities (Zhang et al. 2018). They have been synthesized with the help of immobilized mucor miehei lipase (Fig. 11) through multi-component reaction using aldehydes, active methylene compounds and suitable nucleophile in 81–96% yield (Fig. 12) (Zhang et al. 2018). The immobilization of enzymes on magnetite nanoparticles (MNPs) has advantages for low mass transfer resistance, high specific surface area and easy separation from the reaction mixture in the presence of magnetic field (Vaghari et al. 2016; Hola et al. 2015). Zhang et al. used silica-coated MNPs as starting material whose surface was functionalized with 3-aminopropyltriethoxy silane to introduce amino groups. This was further treated with 2,4,6-trichloro-1,3,5-triazine (TCT) to develop support for covalent immobilization of enzyme (Zhang et al. 2018; Abbasi et al. 2016; Ranjbakhsh et al. 2012). Xu et al. also reported lipase-catalyzed synthesis of tetrahydrochromene derivatives using 1,3-dicarbonyl compound, aldehyde and malononitrile (Xu et al. 2011).
2.2.2 Dioxins
Dioxin is 6-membered heterocyclic, non-aromatic organic molecule which consists of four carbon atoms and two oxygen atoms. The molecular formula is C4H4O2. It is also refereed to 1,4-dioxin or p-dioxin. There is an isomeric form which is 1,2-dioxin (o-dioxin) and is very unstable due to its peroxide nature. Agarwal et al. in 2014 proposed an enzymatic synthesis of polybrominated dioxins by using monooxygenase halogenase CPY450 Bmp7 as catalyst in the reaction using bromocatechol as substrates (Fig. 13) (Agarwal and Moore 2014). Both bromocatechol and the electrophilic quinone, i.e. 3,5-dibromo-1,2-dibenzoquinone undergo coupling reaction in the presence of CYP450 Bmp7 enzyme as catalyst to produce dibenzo-p-dioxins. In this case, the benzoquinone provided both 1,4-dioxin oxygen atoms (Fig. 13) (Agarwal and Moore 2014). This reaction method is indicative of synthetic hetero-Diels–Alder coupling between orthoquinones and enamines leading to the formation of 1,4-benzodioxin frameworks. The whole reaction has very mild reaction conditions. The synthesis of desired dioxins can be achieved at room temperature. Excess bromine was quenched by the addition of sodium thiosulphate, and the reaction was extracted twice with the ethyl acetate. This methodology was simple, quite easy to handle and environment-friendly (Agarwal and Moore 2014).
2.2.3 Lactones
Lactones are cyclic esters and have potential applications in the field of synthetic intermediates, pharmaceutical molecules and polymers (Fischer and Pietruszka 2010). A monoclonal antibody (Fig. 14) was utilized as biocatalyst for the synthesis of -lactone (Kitazume et al. 1996). This antibody behaved as enzyme-like catalyst (abzyme) leading to the formation of carbon–carbon bond through the generation of carbanion and the internal nucleophilic attack on the carbonyl carbon to give -lactone (Fig. 15) (Kitazume et al. 1996). Drozdz et al. gave a chemoenzymatic method for the synthesis of lactone using catalyst acyltransferase from Mycobacterium smegmatis in high yield of 84–99% through Baeyer–Villiger (BV) oxidation method (Drożdż et al. 2016). The enzyme retained its activity even in harsh reaction condition like oxidation with 60% aq. H2O2 at 45 °C. The practical potential of this method was established by the use of different ketones as starting material to give their corresponding lactones (Drożdż et al. 2016).
2.2.4 Benzofuran
A five-membered ring possessing an oxygen atom fused with a benzene ring is known as benzofuran. Benzofuran derivatives have wide range of applications mainly in the field of pharmaceutical industries. They exhibited selective cytotoxicity against tumourigenic cell lines (Hayakawa et al. 2004), antiviral and antitumor and activities (Kim et al. 2006), and pharmaceutical agents (Murata et al. 2003; Murota et al. 1990). Kidwai et al. gave an enzymatic synthesis of this molecule (Kidwai et al. 2013). They studied the enzymatic oxidation of catechols/hydroquinones in aqueous solution using laccase as a catalyst and pyrazolin-5-ones as co-substrate (Fig. 16). Here, the enzyme laccase performs one-electron oxidation on catechol to quinone which undergoes 1,4-addition reaction with co-substrate to develop furan ring leading to benzofuro[2,3-c]pyrazolin-5-ones derivatives (Fig. 16) (Kidwai et al. 2013). The optimized synthetic process has been successfully extended towards the synthesis of a new series of benzofuropyrazole derivatives through the coupling of 3-methyl-1-phenyl-pyrazolin-5-one/3-methyl-pyrazolin-5-one and catechols/hydroquinones (Kidwai et al. 2013).
2.3 Nitrogen- and Oxygen-Containing Heterocycles
Heterocycles possessing nitrogen and oxygen in single ring or in a molecule are well-known moiety in medicinal chemistry. Their utility as immunomodulator, antifungal, psychotropical, antibacterial, neuro-related drugs, etc., has been established (Bhattacharya et al. 1991; Kakeya et al. 1998; Danielmeier and Steckhan 1995; Mishra et al. 2019). This section discusses the enzymatic synthesis of those heterocyclic molecules which have nitrogen and oxygen as heteroatoms.
2.3.1 Oxazolidinones
Oxazolidinones are nitrogen- and oxygen-containing five-membered heterocyclic molecules possessing varied applications (Kakeya et al. 1998; Danielmeier and Steckhan 1995). Yadav et al. studied the synthesis of 3-ethyl-1,3-oxazolidin-2-one using Novozyme 435 as catalyst from 2-aminoalcohol and dimethyl carbonate in 61–89% yield (Fig. 17) (Yadav and Pawar 2014). Among the eight immobilized lipases studied, the Candida antarctica lipase B (Novozyme 435) was considered as the choice of the catalyst for the reaction. The authors optimized the effect of various parameters like catalyst loading, temperature, agitation speed, solvent and mole ratio for the reaction (Yadav and Pawar 2014).
The lipase-catalyzed reaction was also utilized for the synthesis of enantioenriched oxazolidinone derivatives with excellent enantiopurities (Zhang et al. 2015). The reaction of 2-(methylamino)-1-phenylethanol and disubstituted carbonate as substrates yielded corresponding oxazolidinone in 46% yield with an absolute (S)-configuration as the major enantiomer (ee 92%) (Fig. 18) (Zhang et al. 2015). Different lipases such as from Burkholderia (Pseudomonas) cepacia, Pseudomonas fluorescens and Candida antarctica were studied. The immobilized P. cepacia gave better result and faster substrate transformation in chosen solvent tert-butyl methyl ether (Zhang et al. 2015). Various enzyme-mediated synthesized heterocyclic compounds have been represented in Table 1.
3 Summary and Outlook
Heterocyclic compounds are industrially essential molecules. Their history started in the eighteenth century, and since then, they developed themselves as both natural products and synthetic molecules. There is always a demand for the wide-structural diversity of heterocycles and hence exploration of synthetic protocols. Due to the environmental issues related to chemical synthesis, the researchers started looking for alternatives to chemical synthesis in accordance with green and sustainable chemistry. Out of the several modified methods, biocatalysis or using enzymes for synthesis showed potential. The enzymatic heterocyclic synthesis has contributed significantly to the structural diversity of heterocyclic compounds. This also allowed their applicability in various fields. No doubt, the chemical synthesis has also provided structural diversity, but for many asymmetric syntheses has better been performed using enzymatic methods.
This chapter discussed the enzymatic synthesis of nitrogen- and oxygen-containing or possessing both the atoms heterocyclic molecules. The synthesis of pyrroles, indoles, phenazines, benzocarbazoles, benzimidazoles, pyrazoles, benzofurans, chromenes, dioxins, lactones and oxazolidinones has been discussed. This has been observed that the enzyme-catalyzed reactions are more suitable than chemical reactions due to their high selectivity and mild reaction conditions. With the development of genetic engineering and enzyme engineering, the cost of enzymes continues to decrease. Enzyme activity and stability continue to increase. The superiority of enzymatic clean production is definitely promoting breakthrough development in this field. Enzyme-catalyzed biotransformation technology represents the future development of this synthetic industry.
References
Abbasi M, Amiri R, Bordbar AK, Ranjbakhsh E, Khosropour AR (2016) Improvement of the stability and activity of immobilized glucose oxidase on modified iron oxide magnetic nanoparticles. Appl Surf Sci 364:752–757
Agarwal V, Moore BS (2014) Enzymatic synthesis of polybrominated dioxins from the marine environment. ACS Chem Biol 9:1980–1984
Arora P, Arora V, Lamba HS, Wadhwa D (2012) Importance of heterocyclic chemistry: a review. Int J Pharma Sci Res 3:2947–2954
Arunkumar SS (2015) A review on synthetic hetrocyclic compounds in agricultural and other applications. Int J PharmTech Res 8:170–179
Bellina F, Rossi R (2006) Synthesis and biological activity of pyrrole, pyrroline and pyrrolidine derivatives with two aryl groups on adjacent positions. Tetrahedron 62:7213–7256
Bhattacharya SK, Clow A, Przyborowska A, Halket J, Glover V, Sandler M (1991) Effect of aromatic amino acids, pentylenetetrazole and yohimbine on isatin and tribulin activity in rat brain. Neurosci Lett 132:44–46
Bora PP, Bihani M, Bez G (2013) Multicomponent synthesis of dihydropyrano[2,3-c]pyrazoles catalyzed by lipase from Aspergillus niger. J Mol Catal B: Enzym 92:24–33
Broughton HB, Watson IA (2004) Selection of heterocycles for drug design. J Mol Graph Model 23:51–58
Bruyneel F, Payen O, Rescigno A, Tinant B, Marchand-Brynaert J (2009) Laccase-mediated synthesis of novel substituted phenoxazine chromophores featuring tuneable water solubility. Chem Eur J 15:8283–8295
Busto E, Fernandez VG, Gotor V (2011) Hydrolases in the stereoselective synthesis of N-heterocyclic amines and amino acid derivatives. Chem Rev 111:3998–4035
Chai SJ, Lai YF, Xu JC, Zheng H, Zhu Q, Zhanga PF (2011) One-pot synthesis of spirooxindole derivatives catalyzed by lipase in the presence of water. Adv Synth Catal 353:371–375
Chauhan SMS, Geetanjali, Singh R (2000) A mild and efficient synthesis of 10-substituted isoalloxazines in the presence of solid acids. Ind J Heterocycl Chem 10:157–158
Chauhan SMS, Singh R, Geetanjali (2003) Microwave assisted synthesis of 10-substituted isoalloxazines in the presence of solid acids. Synth Commun 33:1179–1184
Dalal KS, Wagh YB, Trivedi DR, Dalal DS, Chaudhari BL (2016) Bovine Serum Albumin catalyzed one pot, three-component synthesis of dihydropyrano[2,3-c]pyrazole derivatives in aqueous ethanol. RSC Adv 6:14868–14879
Danielmeier K, Steckhan E (1995) Efficient pathways to (R)- and (S)-5-hydroxymethyl-2-oxazolidinone and some derivatives. Tetrahedron Asymmetry 6:1181–1190
Ding K, Lu Y, Coleska ZN, Wang G, Qiu S, Shangary S, Gao W, Qin D, Stuckey J, Krajewsky K, Roller PP, Wang S (2006) Structure-based design of spiro-oxindoles as potent, specific small-molecule inhibitors of the MDM2−p53 interaction. J Med Chem 49:3432–3435
Ding Y, Ni X, Gu M, Li S, Huang H, Hu Y (2015) Knoevenagel condensation of aromatic aldehydes with active methylene compounds catalyzed by lipoprotein lipase. Catal Commun 64:101–104
Drożdż A, Hanefeld U, Szymańska K, Jarzębski A, Chrobok A (2016) A robust chemo-enzymatic lactone synthesis using acyltransferase from Mycobacterium smegmatis. Catal Commun 81:37–40
Farhat W, Biundo A, Stamm A, Malmström E, Syrén PO (2020) Lactone monomers obtained by enzyme catalysis and their use in reversible thermoresponsive networks. J Appl Polym 137:48949
Feber K (2004) Biotransformations in organic chemistry, 5th edn. Springer, Berlin, Heidelberg, p 454
Fischer T, Pietruszka J (2010) Key building blocks via enzyme-mediated synthesis. In: Piel J (eds) Natural products via enzymatic reactions. Topics in current chemistry, vol 297. Springer, Berlin
Galliford CV, Scheidt KA (2007) Natural pyrrolidinylspirooxindoles as templates for the development of medicinal agents. Angew Chem 119:8902–8912
Gamage SA, Rewcastle GW, Baguley BC, Charlton PA, Denny WA (2006) Phenazine-1-carboxamides: structure–cytotoxicity relationships for 9-substituents and changes in the H-bonding pattern of the cationic side chain. Bioorg Med Chem 14:1160–1168
Grazulevicius JV, Strohriegl P, Pielichowski J, Pielichowski K (2003) Carbazole-containing polymers: synthesis, properties and applications. Prog Polym Sci 28:1297–1353
Groger H, Asano Y (2012) Principle of enzyme catalysis. In: Drauz K, Groger H, May O (eds) Enzyme catalysis in organic synthesis, 3rd edn. Wiley-VCH Verlag GmbH & Co KGaA, pp 1–42
Hayakawa I, Shioya R, Agatsuma T, Furukawa H, Naruto S, Sugano Y (2004) 4-Hydroxy-3-methyl-6-phenylbenzofuran-2-carboxylic acid ethyl ester derivatives as potent anti-tumor agents. Bioorg Med Chem Lett 14:455–458
Hemmerling F, Hahn F (2016) Biosynthesis of oxygen and nitrogen-containing heterocycles in polyketides. Beil J Org Chem 12:1512–1550
Hola K, Markova Z, Zoppellaro G, Tucek J, Zboril R (2015) Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol Adv 33:1162–1176
Hu W, Guan Z, Deng X, He YH (2012) Enzyme Catalytic promiscuity: the papain-catalyzed knoevenagel reaction. Biochimie 94:656–661
Jampilek J (2019) Heterocycles in medicinal chemistry. Molecules 24:3839
Joule JA, Mills K (2013) Heterocyclic chemistry at a glance: applications and occurrences of heterocycles in everyday life, 2nd edn. Wiley, New York, pp 180–194
Junghanns KT, Kneusel RE, Baumert A, Maier W, Gröger D, Matern U (1995) Molecular cloning and heterologous expression of acridone synthase from elicited Ruta graveolens L. cell suspension cultures. Plant Mol Biol 27:681–692
Kakeya H, Morishita M, Kobinata K, Osono M, Ishizuka M, Osada H (1998) Isolation and biological activity of a novel cytokine modulator, cytoxazone. J Antibiot 51:1126–1128
Kidwai M, Jain A, Sharma A, Kuhad RC (2013) Laccase—a natural source for the synthesis of benzofuro[2,3-c]pyrazolin-5-ones. Catal Sci Technol 3:230–234
Kim S, Salim AA, Swanson SM, Kinghorn AD (2006) Potential of cyclopenta[b]benzofurans from Aglaia species in cancer chemotherapy. Anti-Cancer Agents Med Chem 6:319–345
Kitazume T, Tsukamoto T, Murata K, Yoshimura K (1996) Preparation of heterocyclic compounds via carbon-carbon bond formation catalyzed by an antibody. J Mol Catal B: Enzym 2:27–31
Kłossowski S, Wiraszka B, Berłożecki S, Ostaszewski R (2013) Model studies on the first enzyme-catalyzed Ugi reaction. Org Lett 15:566–569
Knölker HJ, Reddy KR (2002) Isolation and synthesis of biologically active carbazole alkaloids. Chem Rev 102:4303–4428
Kobayashi S, Okamoto E, Wen X, Shoda S (1996) Chemical synthesis of native-type cellulose and its analogues via enzymatic polymerization. J Macromol Sci Part A: Pure Appl Chem 33:1375–1384
Kobayashi S, Shoda S, Wen X, Okamoto E, Kiyosada T (1997) Choroselective enzymatic polymerization for synthesis of natural polysaccharides. J Macromol Sci Part a: Pure Appl Chem 34:2135–2142
Kozikowski A (1984) Comprehensive heterocyclic chemistry. In: Katritzky AR, Rees CW (eds), vol 1. Pergamon Press Ltd., Oxford, p 567
Lee DC, Cao B, Jang K, Forster PM (2010) Self-assembly of halogen substituted phenazines. J Mater Chem 20:867–873
Lia J, Grimsdale AC (2010) Carbazole-based polymers for organic photovoltaic devices. Chem Soc Rev 39:2399–2410
Li C, Feng XW, Wang N, Zhou YL, Yu XQ (2008) Biocatalytic promiscuity: the first lipase-catalysed asymmetric aldol reaction. Green Chem 10:616–618
Mane A, Salokhe P, More P, Salunkhe R (2015) An efficient practical chemo enzymatic protocol for the synthesis of pyrazoles in aqueous medium at ambient temperature. J Mol Catal B: Enzym 121:75–81
Mane AH, Patil AD, Kamat SR, Salunkhe RS (2018) Biocatalyst mediated synthesis of tryptanthrins performed under ultrasonication. Chem Select 3:6454–6458
Mihovilovic MD (2006) Enzyme mediated Baeyer-Villiger oxidations. Curr Org Chem 10:1265–1287
Mikolasch A, Schauer F (2009) Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol 82:605–624
Milner SE, Maguire AR (2012) Recent trends in whole cell and isolated enzymes in enantioselective synthesis. ARKIVOC 1:321–382
Mishra N, Sasmal D (2011) Development of selective and reversible pyrazoline based MAO-B inhibitors: virtual screening, synthesis and biological evaluation. Bioorg Med Chem Lett 21:1969–1973
Mishra D, Fatima A, Singh R, Munjal NS, Mehta V, Malairaman U (2019) Design, synthesis and evaluation of coumarin-phenylthiazole conjugates as cholinesterase inhibitors. Chem Biol Lett 6:23–30
Murata K, Kumagai H, Kawashima T, Tamitsu K, Irie M, Nakajima H, Suzu S, Shibuya M, Kamihira S, Nosaka T, Asano S, Kitamura T (2003) Selective cytotoxic mechanism of GTP-14564, a novel tyrosine kinase inhibitor in leukemia cells expressing a constitutively active Fms-like tyrosine kinase 3 (FLT3). J Biol Chem 278:32892–32898
Murota S, Morita I, Suda N (1990) The control of vascular endothelial cell injury. Ann N Y Acad Sci 598:182–187
Okazaki M, Takeda Y, Data P, Pander P, Higginbotham H, Monkman AP, Minakata S (2017) Thermally activated delayed fluorescent phenothiazine–dibenzo[a, j]phenazine–phenothiazine triads exhibiting tricolor-changing mechanochromic luminescence. Chem Sci 8:2677–2686
Pauliukaite R, Ghica ME, Barsan MM, Brett CMA (2010) Phenazines and polyphenazines in electrochemical sensors and biosensors. Anal Lett 43:1588–1608
Pecca JG, Albonico SM (1971) Synthetic trypanocides. 2. Substituted 5,6-dihydro[c]benzocarbazoles. J Med Chem 14:448–449
Poonam, Singh R (2019) Facile one-pot synthesis of highly functionalized pyrazoles using alumina-silica-supported MnO2 as recyclable catalyst in water. Res Chem Intermed 45:4531–4542
Ranjbakhsh E, Bordbar AK, Abbasi M, Khosropour AR, Shams E (2012) Enhancement of stability and catalytic activity of immobilized lipase on silica-coated modified magnetite nanoparticles. Chem Eng J 179:272–276
Reetz MT, Mondière R, Carballeira JD (2007) Enzyme promiscuity: first protein-catalyzed Morita-Baylis-Hillman reaction. Tetrahedron Lett 48:1679–1681
Rudi A, Erez, Y, Benayahu Y, Kashman Y (2005) Omriolide A and B; two new rearranged spongian diterpenes from the marine sponge Dictyodendrilla aff. Retiara. Tetrahedron Lett 46:8613–8616
Sabir S, Alhazza MI, Ibrahim AA (2015) A review on heterocyclic moiety and their applications. Catal Sustain Energy 2:99–115
Salahuddin SM, Mazumder A (2017) Benzimidazoles: a biologically active compounds. Arab J Chem 10:S157–S173
Shoda S, Uyama H, Kadokawa J, Kimura S, Kobayashi S (2016) Enzymes as green catalyst for precision macromolecular synthesis. Chem Rev 116:2307–2413
Singh R, Geetanjali, Singh V (2011) Exploring alkaloids as inhibitors of selected enzyme. Asian J Chem 23:483–490
Singh R, Sharma R, Tewary N, Geetanjali, Rawat DS (2006) Nitrilase and its application as a green catalyst. Chem Biodiv 3:1279–1287
Sousa AC, Oliveira MC, Martins LO, Robalo MP (2014) Towards the rational biosynthesis of substituted phenazines and phenoxazinones by laccases. Green Chem 16:4127–4136
Sousa AC, Fátima M, Piedade MM, Martins LO, Robalo MP (2015) An enzymatic route to a benzocarbazole framework using bacterial CotA laccase. Green Chem 17:1429–1433
Sousa AC, Oliveira MC, Martins LO, Robalo MP (2018) A sustainable synthesis of asymmetric phenazines and phenoxazinones mediated by CotA-Laccase. Adv Synth Catal 360:575–583
Stellrecht CM, Chen LS (2011) Transcription inhibition as a therapeutic target for cancer. Cancers 3:4170–4190
Trofimov BA, Sobenina LN, Demenev AP, Mikhaleva AI (2004) C-vinylpyrroles as pyrrole building blocks. Chem Rev 104:2481–2506
Vaghari H, Jafarizadeh-Malmiri H, Mohammadlou M, Berenjian A, Anarjan N, Jafari N, Nasiri S (2016) Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol Lett 38:223–233
Veer B, Singh R (2019) Facile synthesis of 2-arylimidazo[1,2-a]pyridines catalyzed by DBU in aqueous ethanol. P Roy Soc A-Math Phy 475:1–12
Venkatachalam H, Kumar NVA (2019) Heterocycles: synthesis and biological activities: the oxygen-containing fused heterocyclic compounds. Nandeshwarappa BP, Sadashiv SO (eds) IntechOpen
Vitaku E, Smith D T, Njardarson J T (2014) Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J Med Chem 57:10257–10274
Wang L, Li C, Wang N, Li K, Chen X, Yu XQ (2010) Enzyme-mediated domino synthesis of 2-alkylbenzimidazoles in solvent-free system: a green route to heterocyclic compound. J Mol Catal B: Enzym 67:16–20
Wang Z, Chen X, Wang C, Zhang L, Li FX, Zhang WA, Chen P, Wang L (2017) A mild and efficient dakin reaction mediated by lipase. Green Chem Lett Rev 10:269–273
Webb E C (1992) Enzyme nomenclature 1992, recommendation of the nomenclature committee of the international union of biochemistry and molecular biology on the nomenclature and classification of enzymes. International Union of biochemistry and molecular biology, 6th edn. Academic Press, San Diego, p 863
Witayakran S, Ragauskas AJ (2009) Synthetic applications of Laccase in green chemistry. Adv Synth Catal 351:1187–1209
Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y, Qin L, Wei H (2019) Nanomaterials with enzyme-like characteristics (nanozymes): next generation artificial enzymes (II). Chem Soc Rev 48:1004–1076
Xiang Z, Liu Z, Chen X, Wu Q, Lin X (2013) Biocatalysts for cascade reaction: porcine pancreas lipase (PPL)-catalyzed synthesis of bis(indolyl)alkanes. Amino Acids 45:937–945
Xiang Z, Liang Y, Chen X, Wu Q, Lin X (2014) D-aminoacylase-initiated cascade aldol condentation/robinson annulation for the synthesis of substituted cyclohex-2-enones from simple aldehydes and acetones. Amino Acids 46:1929–1937
Xie ZB, Wang N, Jiang GF, Yu XQ (2013) Biocatalytic asymmetric aldol reaction in buffer solution. Tetrahedron Lett 54:945–948
Xue Y, Li L, He Y, Guan Z (2012) Protease-catalsed direct asymmetric mannich reaction in organic solvent. Sci Rep 2:761
Xu JC, Li WM, Zheng H, Lai YF, Zhang PF (2011) One-pot synthesis of tetrahydrochromene derivatives catalyzed by lipase. Tetrahedron 67:9582–9587
Yadav GD, Pawar SV (2014) Novelty of immobilized enzymatic synthesis of 3-ethyl-1,3-oxazolidin-2-one using 2-aminoalcohol and dimethylcarbonate: mechanism and kinetic modeling of consecutive reactions. J Mol Catal B: Enzym 109:62–69
Yang FJ, Wang HR, Jiang LY, Yue H, Zhang H, Wang Z, Wang L (2015) A green and one-pot synthesis of Benzo[g]chromene Derivatives through a multi-component reaction catalyzed by lipase. RSC Adv 5:5213–5216
Zhang Y, Ren Y, Ramström O (2015) Synthesis of chiral oxazolidinone derivatives through lipase-catalyzed kinetic resolution. J Mol Catal B: Enzym 122:29–34
Zhang MJ, Li R, He YH, Guan Z (2017a) Pepsin-catalyzed vinylogous Michael addition of deconjugated butenolides and maleimides in water. Catal Commun 98:85–89
Zhang WA, Zhao ZY, Wang Z, Guo C, Wang CY, Zhao R, Wang L (2017b) Lipase-catalyzed synthesis of indolyl 4H-chromenes via a multicomponent reaction in ionic liquid. Catalysts 7:185
Zhang W, Chen P, Zhao Z, Wang L, Wang S, Tang Y, Wang B, Wang Z, Zhuang H (2018) Synthesis of functionalized 4H-chromenes catalyzed by lipase immobilized on magnetic nanoparticles. Green Chem Lett Rev 11:246–253
Zheng H, Shi Q, Du K, Mei Y, Zhang P (2013) A novel enzyme-catalyzed synthesis of N-substituted pyrrole derivatives. Mol Divers 17:245–250
Zhuo ST, Li CY, Hu MH, Chen SB, Yao PF, Huang SL, Ou TM, Tan JH, An LK, Li D, Gu LQ, Huang ZS (2013) Synthesis and biological evaluation of benzo[a]phenazine derivatives as a dual inhibitor of topoisomerase I and II. Org Biomol Chem 11:3989–4005
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rathore, D., Geetanjali, Singh, R. (2021). Enzyme-Mediated Synthesis of Heterocyclic Compounds. In: Inamuddin, Boddula, R., Ahamed, M.I., Khan, A. (eds) Advances in Green Synthesis. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-030-67884-5_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-67884-5_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67883-8
Online ISBN: 978-3-030-67884-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)