Abstract
The economic expansion of Niger Delta region depends on groundwater resource for various uses. Therefore, there is need for an understanding of the hydrogeological and hydrochemical characteristics as an integral for management of the resource. Hence, this study was aimed at delineating areas of saltwater intrusion in the area. Geological and hydrogeological data were used to delineate two aquifers: alluvial aquifer (upper designated as A and lower designated as B) and a coastal plain aquifer (designated as C). Groundwater in the area was classified as fresh (<1500 μS/cm), brackish (1500–3000 μS/cm), and saline (>3000 μS/cm). Among the groundwater samples (n = 105), 95% from A, B, and C were classified as fresh, while 2 and 3% of the samples from A were classified as brackish and saline, respectively. The main groundwater facies were Na–Cl, Mg–Cl, and Na–HCO3 respectively, for A, B, and C aquifers. The enrichment of Na+ and Cl−, freshwater–seawater mixing ratio, cross plots, and classifications by means of different schemes indicated that seawater intrusion was occurring in the A aquifer. In terms of drinking and irrigation use, the A aquifer water is of poor quality relative to the groundwater from B and C aquifers. The study highlights the potential danger of contaminated groundwater in the coastal areas occupied by low income dwellers. Hence, seawater intrusion should be continuously monitored for sustainable development and management of groundwater in coastal areas.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Niger Delta Region is one of the major petroleum-bearing basins of the world. In this area, groundwater is the major source of water for most drinking, domestic, agricultural, and industrial uses. However, the quantity and quality of groundwater have been affected by natural and human-induced activities associated with rapid development in terms of urbanization and industrialization. In coastal regions like the Niger Delta, salinization is one of the major problems due to over exploitation (Khaska et al., 2013; Anders et al., 2014; Yolcubal et al., 2019). Besides over pumping, other sources and processes which may contribute to salinization of groundwater in coastal regions have been documented in previous studies. Some of these include leakage of saline water trapped in silty and clayey layers (Walter et al., 2017), domestic and industrial effluents, polluted surface water (Fakir et al., 2002; Sanchez-Martos et al., 2002; Carwright et al., 2004; Ghabayen et al., 2006), and discharge from oil and oil-related companies in the Niger Delta Region (Edet, 2005, 2006, 2008, 2009). The Niger Delta Region, which is one of the topmost wetlands in the world, is located in the south most end of Nigeria, sharing a boundary with the Atlantic Ocean in the south. Urban and rural settlements and factories are widespread in the area which host different oil and oil-related industrial facilities mainly flow stations, oil terminals, gas plants, petrochemicals, refineries, liquefied natural gas, fertilizers, etc. These facilities require groundwater for their daily activities, and this is achieved through pumping groundwater.
Several methods have been used to elucidate seawater intrusion into coastal aquifers, for example, parameters such as electrical conductivity (EC), total dissolved solids (TDS), sodium, chloride, and bromide are used as markers of seawater influence (Sukhija et al., 1996; Capaccioni et al., 2005, de Montety et al., 1996). Also ionic ratios (Na/Cl, Cl/HCO3, Ca/Na, Br/Cl, Ca/Cl, Mg/Cl, SO4/Cl, Ca/HCO3 + SO4), seawater mixing ratios, trace elements, and isotopes have been applied to evaluate the degree of saltwater intrusion into freshwater aquifer (Edet & Okereke, 2001; Barbecot et al., 2000; Vengosh et al., 2002; Cartwright et al., 2004; Faye et al., 2005; Walraevens et al., 2005; El Moujabber et al., 2006; Ghabayen et al., 2006; de Montety et al., 2008; Park et al., 2012; Wang & Jiao, 2012; Khaska et al., 2013; Giambastiani et al., 2013; Anders et al., 2014; Abdalla, 2016; Yolcubal et al., 2019). The existence of other processes such as rock weathering, cation exchange, and human activities tends to mask distinguishing salinization process and requires other methods of assessment. Hence, this study applies several tools to determine the degree of groundwater pollution in coastal aquifers due mainly to natural sea water intrusion process and anthropogenic activities.
Most of the available literature on the geology and hydrogeology of the area are localized in scope. Such studies include identification and characterization of the aquifers (Etu-Efeotor and Akpokoje, 1990; Etu-Effeotor, 2000), documentation of aquifer parameters from drilling records (Offodile, 2014), water supply problem (Etu-Efeotor and Odigi, 1983), quality status of groundwater (Etu-Effeotor, 1981; Edet, 1993; Ofoma et al., 2005; Udom & Amah, 2006; Udom et al., 1998, 1999; Esu & Amah, 1999; Onwuka & Omonona, 2017), and seawater intrusion (Amadi et al., 1989; Amadi & Amadi, 1990; Edet & Okereke, 2001, Edet, 2008, 2017, 2018; Edet & Worden, 2009; Edet et al., 2003, 2012). This study is therefore a conglomeration of historical and recent data to unravel and further contribute to the current situation of groundwater for sustainable development of the Niger Delta Basin. The objectives of the present study are (i) to further delineate and characterize the different aquifers in area, (ii) to determine the major ion chemistry of groundwater and delineate areas of saltwater intrusion using different approaches, and (iii) to assess the groundwater suitability for drinking and agricultural use.
2 Study Area Description
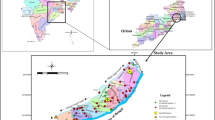
The study area is located in southern parts of Nigeria and is roughly bounded by latitudes 4o 20′–5° 20′ North and longitudes 5° 30′–8° 00′ East (Fig. 1). The area experiences tropical climate with distinct wet and dry seasons. The basin is characterized by high temperatures and rainfall. The temperature range is 21–43°, and the average annual rainfall is about 2500 mm. The average annual rainfall exceeds 3500 mm along the coast and decreases to about 2000 mm inland (Akpokoje, 1987).
The geomorphology and geology of the Niger Delta have been described in details by various authors (Allen, 1965a, b; Akpokoje, 1987; Reyment, 1965; Short & Stauble, 1967; Weber, 1971; Murat, 1972; Kogbe, 1976; Petters, 1982; Whiteman, 1982; Wright, 1989). The formation of the present Niger Delta started during Early Paleocene, and it resulted from the buildup of fine grained sediments eroded and transported by river Niger and its tributaries. The subsurface geology consists of three lithostratigraphic units: Akata, Agbada, and Benin Formations. These formations are overlain by Quaternary deposits. The Benin Formation which underlies the study area is composed of sand of various varieties with intercalations of clay and silts (Allen, 1965a; b). The Quaternary deposits generally consist of alternating sequences of gravel, sand, silt, and clay (Etu-Efeotor & Akpokoje, 1990). Hydrogeology of the Niger Delta Basin has been studied by several workers. Etu-Effeotor and Akpokoje (1990) gave a detailed stratigraphic analysis of the various geomorphological and geologic units in order to delineate local and regional aquifers in the basin, while Etu-Effeotor (2000) studied the hydraulic properties of the subsurface materials in different wells and identified four aquiferous horizons. Recent work in the area is mainly localized on dynamics of static water level (Ngah & Nwankwoala, 2013), estimation of porosity and hydraulic conductivity (Okiongbo & Soronnadi-Ononiwu, 2015; George et al., 2017), groundwater potential and aquifer protective capacity (Rasaq, 2017), and groundwater quality assessment (Ejiro et al., 2015; Nwankwoala & Ngah, 2014).
3 Materials, Methods, and Data Handling
3.1 Geological and Hydrogeological Data Acquisition
Geological data were collected from existing records and those acquired during the duration of this study. The data were geological and hydrogeological information such as lithology, depth of wells and boreholes, water levels, and aquifer characteristics. Field surveys were conducted at various times to study the surficial geology, measure groundwater levels, and collect groundwater samples for chemical analyses. Groundwater level monitoring was undertaken at some locations within the study area across different seasons and tidal changes using existing hand-dug wells.
Lithological logs were used to draw hydrogeological cross sections and estimate the storage capacity of the aquifers as area × depth × porosity. The porosity of the aquifers was assumed to be 23% (George et al., 2017).
Recharge (R) to the aquifers was estimated from rainfall data by means of chloride mass balance (CMB) method (Wood & Sandford, 1995) as:
where Clp and Clgw are chloride concentrations in precipitation and groundwater, respectively.
3.2 Groundwater Sampling and Analysis
A total of 105 groundwater samples were collected between 1990 and 2018 from monitoring wells, hand-dug wells, and boreholes for physicochemical parameters’ determinations and assessment. The locations of the sampling points are presented as Table 1. Temperature, pH, electrical conductivity (EC), total dissolved solids (TDS), and dissolved oxygen (DO) were measured in the field using standard equipment. Groundwater samples were collected in polyethylene bottles, which were soaked in HNO3 for 24 hr and rinsed several times prior to use. At the sampling sites, the bottles were rinsed with the water to be sampled prior to filling the bottles with the samples. The samples were filtered through 0.45 μm membrane filters into sterilized polyethylene bottles. Samples collected for major ions analysis were preserved by acidifying with HNO3 to achieve a pH of ≤2. The samples were kept at 4 °C in laboratory prior to analyses.
The samples were analyzed at various times for major cations (Na, K, Ca, Mg) and anions (Cl, SO4, NO3) using ion chromatograph (Dionex Dx-120) at the Institute of Geosciences, University of Tuebingen (Germany) and Thermo Scientific X Series 2 high-resolution ICP-MS, Ion Chromatography Metrohm 872 Extension Module, and Ion Chromatography Metrohm 881 Extension Module at the Department of Hydrogeology, TU Bergakademie, Freiberg (Germany). Bicarbonate was estimated by titration with H2SO4 standard solution using methyl orange as indicator at the Institute of Oceanography, University of Calabar (Nigeria).
3.3 Seawater Contamination Assessment
The Niger Delta Basin is in direct contact with the Atlantic Ocean; hence, it was necessary to access the level of sea water intrusion into the freshwater system. The sea water fraction in groundwater was estimated using chloride concentration since chloride has been considered as a conservative tracer, not affected by ion exchange (Custodio, 1987) and calculated as follows (Appelo & Postma, 1999):
where Cclsample is Cl− concentration in sample, Cclfresh is Cl− concentration in freshwater, and Clclsea Cl− concentration in seawater.
The fsea is used to calculate the theoretical concentration of each cation resulting from conservative mixing of seawater and freshwater using the following formula:
where Ci mix is conservative mixing, Ci sea is concentration of cation in seawater, and Ci fresh is concentration of cations in freshwater. For each cation, the difference between the concentration of conservative mixing (Ci mix) and measured concentration of the cation simply represents the change in concentration (delta, ∆), resulting from any chemical reaction occurring with mixing (Fidelibus, 2003; Zghibi et al., 2013):
When ∆Ci is positive, groundwater is enriched for that particular ion i, whereas a negative value indicates depletion compared to theoretical mixing (Appelo & Postma, 1999; Anderson et al., 2005; Slama, 2010).
3.4 Geochemical Classification of Groundwater
3.4.1 Chloride and Bicarbonate
Revelle (1941) and Krishnakumar et al. (2014) used the ratio of Cl−/HCO3− to classify the effect of seawater on fresh groundwater system. The classification is as follows: <0.5 (not affected by sea water); 0.5–6.6 (slightly/moderately affected by sea water), and >6.6 (strongly affected by sea water).
3.4.2 Chloride-Nitrate Classification
The groundwater samples were classified into four groups using concentrations of chloride and nitrate which, respectively, reflect the influences of sea water and human activities (Cardona et al., 2004). This was done by obtaining threshold values of Cl− and NO3− from the inflection points of cumulative frequency distribution plots (Sinclair, 1974, 1976).
3.4.3 Stuyfzand Classification
Groundwater classification by Stuyfzand (1986, 1993) method involves four levels: primary, type, subtype, and class (Table 2). The primary type is based on chloride content; type is determined on the basis of hardness, while classification into subtypes is based on dominant cations and anions (facies). Lastly, the class is determined on the basis of Na+ + K+ + Mg2+ (meq/l) corrected for sea water contribution according to the formula:
The parameter is tested against √0.5Cl− as a margin of error in order to arrive at a meaningful positive (marine cation surplus, positive cation code), negative (marine cation deficit; negative cation exchange code), or equilibrium value (cation exchange code Ø). Mtoni et al. (2013), further added that, the classification allows for the recognition of cation exchange.
4 Results and Discussion
4.1 Aquifer Framework, Characteristics and Recharge
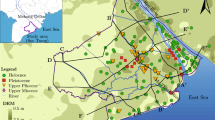
Groundwater occurs in the Niger Delta Basin in two main aquifers, an upper unconfined shallow aquifer, designated as alluvial aquifer, and a lower unconfined to semi-confined deep aquifer, designated as coastal plain aquifer. The alluvial aquifer dominates the coastal area, while the coastal plain characterizes the inland area. Upper parts of the alluvial aquifer are harnessed through hand-dug wells, (designated as A) and the lower parts through shallow boreholes (designated as B), while the coastal aquifer is harnessed through deep boreholes (designated as C).
The alluvial aquifer is at a depth of generally <30 m. It is composed of clayey sand to sand with varying proportions of silt and gravel. The aquifer is found within the alluvial deposits, especially within the coastal areas and ranges in thickness from < 0 to 30 m (Fig. 2a). Water levels vary between 0.75 and 15.0 m with an average value of 4.28 m. The aquifer is limited in lateral extent due to clay/shale intercalations. The hydraulic conductivities of shallow aquifer estimated from grain size distribution curve using Hazen’s method (Fetters, 2001; Al-Shaibani, 2008) and pumping test data, ranged between 0.001 and 68 m/day with an average value of 11.02 m/day. The average transmissivity value was 259.7 m2/day with range of 204.5–315 m2/day. Specific yield values varied from 108 to 432 m3/hr/m (average 214.8 m3/hr/m). The estimated volume of storage for the alluvial aquifer is about 29 M m3 based on data on Table 3. Abstraction of water from this aquifer is through hand-dug wells and shallow boreholes.
The coastal plain aquifer is the main aquifer within the Niger Delta Basin. The aquifer serves as the main source of water for the entire area, and abstraction is through shallow and deep boreholes. The aquifer occurs at depth >30 m with thickness reaching >150 m (Fig. 2b). The aquifer is characterized by sand, gravelly sand, and gravel with intercalations of clay/shale and silt. Static water level varies between 2.9 and 21.0 m with a mean 8.4 m below the ground surface. For this aquifer, the specific yield is in the range 10.5–1404 m3/hr/m with average value of 216.8 m3/hr/m (Offodile, 2014). Transmissivities of the aquifer varied between 154.5 and 6500 m2/day with average of 1227.0 m2/day, and well discharge ranged between 1392 and 7200 m3/day with an average of 3345 m3/day. Hydraulic conductivities of the coastal plain aquifer ranged between 1.4 and 75.0 m/day averaging 16.2 m/day. The estimated volume of storage for the coastal aquifer is about 1304 M m3 using data in Table 3.
The groundwater level contour map shown on Fig. 3 summarizes the distribution of piezometric head in the aquifer system within the Niger Delta Basin. The general groundwater flow is north–south with variations in northeast–southwest and northwest–southeast directions under hydraulic gradient of 0.00007–0.00104. Detailed groundwater level measurements within the shallow aquifer at six locations (8A, 31A, 42A, 49A, 63C, and 69C, Table 1) indicate fall in static water level with respect to the ground level (SWLwrtgl) by about 4.50 m on the average in dry season and corresponding increase in wet season by an average of 3.50 m. Change (ΔSWL) in water level between wet (May–October) and dry (November–April) seasons varied between 0.40 and 2.70 m (average 1.00). Also, local monitoring at three locations (4A, 6A, and 7A, Table 1) indicated SWLwrtgl at low tide varied from 0.25 to 2.00 m with an average of 0.91 m, while at high tide, SWLwrtgl varied from 0.14 to 1.75 m with an average of 0.74 m. The change in water levels between low and high tides ranged between 0.11 and 0.25 m with an average of 0.18 m. This is due to shallow water level (<1.0 m) and porous alluvial materials. These variations in water levels during different seasons and tidal changes are the main form of recharge mechanism in the basin.
Regional groundwater flow map of the Niger Delta (Updated and modified from Edet, 2008)
Chloride mass balance (Eq. 1) was used to estimate the recharge in the area. The input data for this estimation are given in Table 3. In the area, chloride concentration in groundwater increases toward the coast with average values varying from 272.9 mg/l in A (coastal area) through 66.9 mg/l in B (between the coastal and inland area) to 21.9 for C (inland area). From CMB, the recharge estimate for the area varied from 6 mm through 15–25 mm representing 0.2, 0.6, and 1.25% of precipitation, respectively, for areas A, B, and C.
4.2 Groundwater Chemistry and Quality
Detailed and average values of the various physicochemical constituents for all and the different aquifers are presented in Table 4 and Online Resource 1.
4.2.1 General Physicochemical Composition of Niger Delta Basin
The electrical conductivity (EC) of the groundwater ranged from 16.28 to 21,583 µS/cm. Ten percent of all the groundwater samples exceeds the WHO (2004, 2006) maximum limit for drinking water for in respect of EC, suggesting influence of sea water. According to electrical conductivity (EC) levels (Rai, 2004; Mondal et al., 2010), groundwater was classified as fresh (<1500 μS/cm), brackish (1500–3000 μS/cm), and saline (>3000 μS/cm). Among the groundwater samples (n = 105), 95% from A, B, and C were classified as fresh, while 2 and 3% of the samples from A were classified as brackish and saline, respectively. The water is acidic to alkaline in nature, with pH values ranging from 3.72 to 8.5. Sixty-five percent of all the groundwater samples were not within the WHO (1993,2004) range of 6.5–8.5 for drinking water. Dissolved oxygen (DO) concentration varied from 1.19 to 10.7 mg/l. Low DO value (<5.0 mg/l) for 54% of the groundwater samples is attributed to high organic matter. Na+ is the dominant cation. It ranged from 0.06 mg/l in the coastal plain aquifer (sample 67C) to 1280.80 mg/l in the upper alluvial aquifer (sample 17A). This represents an average of 52% of all the cations. Six out of 105 samples representing about 6% of all the samples are higher than the WHO (1993) maximum permissible of 200 mg/l for Na+ in drinking water. Ca2+ ranged between 0.01 (82C & 83C) and 520.3 mg/l (25A), while Mg2+ varied from 0.01 (86C) to 230.7 (17A) mg/l. These average values represents 21.8 and 20.5% of the total cations for Ca2+ and Mg2+, respectively, in the groundwater samples. However, 4 and 5% of Ca2+ and Mg2+ exceed the WHO (1993) maximum limit for drinking water. K+ concentrations represent on the average 5.7% of the total cations in groundwater samples. The concentration of K+ ranged from 0.01 (55B) to 159.6 (25A) mg/l. Concentration of Cl− varied from 0.32 (sample 80C) to 2670 (sample 17A) mg/l with 11% of the groundwater samples exceeding the WHO maximum limit of 250 mg/l. Bicarbonate concentrations ranged from 0.2 (67C) to 787.2 (25A) mg/l. 2% of the groundwater samples exceeds the WHO limit (2004) of 600 mg/l. SO42− concentrations ranged from 0 (79C, 80C, 81C, 82C, 83C, 84C, 85C, 86C, 87C, 88C, 89C, 91C, 92C, 96C, 97C, 98C, and 99C) to 968.9 (24A) mg/l. Two samples of groundwater analyzed had SO42− higher than WHO (2004) maximum drinking water limit of 250 mg/l. The concentration of NO3− varied between 0 (80C) and 59.60 (73C) mg/l. The mean concentrations of Na and Cl (Table 4) increased by ~80 and 1000 times relative to those of freshwater (rainwater), while those of K, Ca, Mg, and SO4 increased by ~70, 20, 175, and 55 times, respectively, suggesting influence of seawater. However, the values of all the physicochemical parameters are below that of typical sea water (Table 4).
4.2.2 Physicochemical Composition of Different Aquifer Systems
Upper Alluvial Aquifer Harnessed Through Hand-Dug Wells (A)
EC of the upper alluvial aquifer ranged from 100 to 21,538 µS/cm. Groundwater on the average is acidic with 48% of all the samples not within the WHO limit for drinking and domestic purposes. Dissolved oxygen (DO) concentration varied from 1.30 to 8.79 mg/l. Low DO values (<5.0 mg/l) was recorded for 48% of the samples. Na+ is the dominant cation, ranging from 0.56 to 1280.8 mg/l. This represents an average of 48% of all the cations. Ca2+ ranged between 0.02 and 520.30 mg/l, while Mg2+ varied from 0.47 to 230.7 mg/l. These values represent, respectively, 24.6 and 20.3% of Ca2+ and Mg2+ relative to the total cations. K+ represents on the average 5.7% of total cations in groundwater samples of the basin. Concentration of K+ varied from 0.40 to 159.60 mg/l. Concentration of Cl− varied from 3.54 to 2670 mg/l. Bicarbonate concentration ranged from 1.0 to 787.20. SO42− concentration varied between 0.22 and 968.90 mg/l. Concentration of NO3− varied from 0.08 to 51.62 mg/l. All the parameters showed varying exceedances above WHO standard limits for drinking. Statistical evaluation by means of Pearson´s correlation (Table 5) of the groundwater analyses show that EC had significant positive correlation at p < 0.05 with Na+, K+, Ca2+, Mg2+, Cl−, HCO3−, and SO42−. All the parameters (Na+, K+, Ca2+, Mg2+, Cl−, HCO3−, and SO42−) showed significant positive correlation with each other, except with NO3−. Besides, evaluation of the concentration of the major ions indicate Na-Ca–Cl-HCO3 water type suggesting seawater intrusion as the major process controlling water chemistry, while ion exchange and silicate weathering as the minor processes.
Lower Alluvial Aquifer Harnessed Through Shallow Boreholes (B)
Groundwater samples from lower alluvial aquifer showed that the pH varied from 4.08 to 6.40 indicating acid groundwater. EC varied from 21 to 80 µS/cm. These values of EC are within acceptable limit for drinking and domestic purposes. DO concentration varied from 4.00 to 8.40 mg/l. Among the cations, Na+ varied from 2.20 to 21.80 mg/l; K+ from 0.01 to 6.70 mg/l; Ca2+ from 1.10 to 46.10 mg/l and Mg2+ from 0.10 to 60.20 mg/l. Among the anions, Cl− varied between 10.20 and 307.40 mg/l; HCO3− varied from 0.50 to 18.90 mg/l; SO42− from 1.24 to 10.70 mg/l, and NO3− from 0.09 to 0.82 mg/l. These values are within the limits of WHO for drinking and domestic purposes, except for DO and K+ in some samples. In lower alluvial aquifer as presented in Table 5, EC is positively correlated with NO3−, while Na + is positively correlated with K+, Ca2+, and Cl−. Also K+ positively correlated with Ca2+ and Cl−. Cl− correlates positively with Ca2+ and Mg2+, while SO42− positively correlates with Mg2+ and Cl−. This pattern of relationship is attributable to cation exchange due mainly to significant positive correlation between Na+ and Ca2+ and Ca2+ and Cl−. The main groundwater chemical facies are Mg-Na-Cl. Significant positive correlation between EC with NO3− is attributable to anthropogenic pollution.
Coastal Plain Aquifer Harnessed Through Deep Boreholes (C)
Groundwater pH values from coastal plain aquifer ranged from 3.72 to 7.16. EC values ranged from 16.28 to 457.80 µS/cm. Based on EC values, all the samples analyzed are classified as fresh (EC < 1500 µS/cm) according to Rai (2004) and Mondal et al. (2010). DO concentration varied from 1.19 to 10.7 mg/l. Concentration of Na+ varied from 0.06 to 64.60 mg/l, while K+ varied from 0.02 to 30.30 mg/l. Ca2+ and Mg2+ in the analyzed groundwater samples from coastal plain aquifer ranged from 0.01 to 67.80 mg/l and 0.01 to 28.30 mg/l, respectively. Cl− concentration ranged from 0.32 to 116 mg/l. The concentration of NO3− in groundwater ranged from 0.00 to 59.60 mg/l. The values of SO42− in the groundwater samples ranged from 0.00 to 47.47 mg/l, while HCO3− concentration ranged from 0.20 to 117.50 mg/l. These values within the various limits are specified by WHO for drinking and domestic purposes, except for pH, DO, and NO3− in some samples. (Table 4). In the coastal plain aquifer, EC correlated positively with Na+ and NO3−, while Na+ correlated positively with K+, Mg2+, Cl−, and HCO3−. In addition, Cl− correlated positively with K+, Ca2+, and Mg2+, while HCO3− is also positively correlated with K+, Ca2+, Mg2+, and Cl−. It is observed that Na+, K+, Ca2+, Mg2+, Cl−, and HCO3− were poorly correlated with SO42− and NO3− due to contribution from anthropogenic pollution. However, significant correlation between Na+ and HCO3− suggests contribution from silicate weathering. Na-Ca-HCO3 constitutes the dominant hydrochemical facies.
Average concentrations of all the parameters were higher concentration in groundwater from A relative to groundwater from B and C aquifers. However, the average values of DO, Ca2+, Mg2+, and Cl− were higher in B relative to C. Considering analytical results for the entire study area, the standard deviation (SD) is high for EC, TDS Na+, K+,Ca2+, Mg2+,Cl−, NO3− SO42−, and HCO3− suggesting dispersion of ionic concentration from average value (Table 4). SD was low for pH and DO suggesting their limited variability (Vasu et al., 2017).
4.2.3 Seawater Contamination
The ratio of Na+/Cl− for analyzed groundwater samples ranged from 0.01 to 15.64 for the entire groundwater with an average of 1.73 ± 2.28. The average values for A, B, and C were 0.95 ± 0.68, 0.44 ± 0.38, and 2.73 ± 3.01, respectively. The deviation of these values relative to that of seawater (0.86) is shown in Fig. 4a, b. Figure 4a, b includes freshwater and seawater dilution line, which shows simple mixing of both freshwater and seawater end members (Mtoni et al., 2013). A deviation from the dilution line is related to ion exchange, suggesting an excess or depletion of Na+ relative to Cl− (Walraevens & Van Camp, 2005). Excess Na+ in groundwater plotting above mixing line (1:1 line) in Fig. 4b indicates silicate weathering or freshening. On the other hand, depletion of Na+ in groundwater indicated by plotting below the mixing line (Fig. 4b) suggests seawater intrusion. In addition, concentrations of EC and Cl− are good indicators of sea water contamination (El Moujabber et al., 2006). Contamination of seawater is represented by elevated concentration of EC > 1500 µS/cm and Cl− (>250 mg/l). The concentrations of EC and Cl− in the basin varied from 16 to 21, 538 µS/cm and from 0.32 to 2670 mg/l, respectively. Values of EC (1992.5–21,538 µS/cm) at 1A, 5A, 17A, 24A, and 25A (upper alluvial aquifer) and Cl− (307.4–2324.0 mg/l) at 1A, 2A, 3A, 5A, 17A, 24A, 25A, 27A, 35A, 38A (upper alluvial aquifer), and 53B (lower alluvial aquifer) (Appendix 1) further support intrusion of seawater.
a Plot of Na+/Cl− versus Cl− for upper alluvial (A), lower alluvial (B) and coastal plain (C) aquifers, b Plot of Na+ versus Cl− for upper alluvial (A), lower alluvial (B) and coastal plain (C) aquifers, c Plot of K+ versus Cl− for upper alluvial (A), lower alluvial (B) and coastal plain (C) aquifers, d Plot of Ca2+ versus Cl− for upper alluvial (A), lower alluvial (B) and coastal plain (C) aquifers, e Plot of Mg2+ versus Cl− for upper alluvial (A), lower alluvial (B) and coastal plain (C) aquifers
To quantify the mixing ratios of seawater and freshwater in the basin, the chloride mass balance (Eqs. 2–4) was applied. The computed values of fraction of seawater in freshwater (% F) are presented in Table 6. The delta values varied from 0.0002 to 13.8% for the entire basin. The average values for the different aquifers were 1.41 ± 2.83, 0.34 ± 0.51, and 0.11 ± 0.15 for A, B, and C, respectively. The F values > 1% (range 1.13–13.8%) at 1A, 2A, 3A, 5A, 7A, 8A, 17A, 24A, 25A, 26A, 27A, 35A, and 38A in upper alluvial aquifer and 53B in lower alluvial aquifer also support evidence of seawater contamination. Figure 4b–e demonstrate that there is an enrichment of Cl− with respect to cations (Na+, K+, Ca2+, and Mg2+) with distinctive patterns that are lower than the theoretical mixing line. This is confirmed by the computed ionic delta and presented as Table 6 and Fig. 5. Most of the groundwater samples display enrichment of Na+ compared to pure mixing model, ranging between -3.35 and 3.13 with an average value 1.83 ± 1.36, except in 1A, 2A, 3A, 5A, 8A, 17A, 24A, and 25A (A aquifer) which have negative values. The depletion of Na+ is accompanied by enrichment of K + , Ca2+, and Mg2+. Enrichment of Ca2+ and Mg2+ suggest ion exchange process (Pulido-Leboeuf, 2004; Trabelsi et al., 2012).
The clayey sand layers in the aquifer could be the required ion exchanger. This phenomenon may be responsible for low Na+/Cl− ratios observed in some locations. The plot of Cl−/HCO3− vs Cl− (Fig. 6) indicates Cl−/HCO3− ratios ranging between 0.05 and 695.13 with an average value of 16.88 ± 70.48 for all the groundwater samples. The different aquifers displayed ratios in the following range: 0.12 to 695.13 (average 26.27 ± 99.25) for upper alluvial aquifer; 1.29 to 120.63 (average 40.42 ± 48.15) for lower alluvial aquifer, and 0.05 to 101.28 (average 3.36 ± 101.38) for coastal plain aquifer.
4.2.4 Classification of Groundwater
Chloride and Bicarbonate
On the basis of Cl−/HCO3− classification scheme, 47.6% of the groundwater samples were not affected by sea water intrusion, 6.7% are slightly/moderately affected by sea water intrusion, while 45.7% are strongly affected by sea water intrusion. The unaffected samples were obtained from coastal plain aquifer. Twenty-five and 75% of the samples from A aquifer were not affected and strongly affected by sea water intrusion, respectively, while 54, 6, and 40% of groundwater samples from B aquifer were not affected, slightly/moderately affected, and strongly affected by sea water intrusion. For the groundwater samples from C aquifer, 73.7% were not affected; 21.1% were slightly/moderately affected, while 5.3% are strongly affected by sea water intrusion (Table 7). Above show that samples from the A aquifer were strongly affected by sea water relative to the B and C aquifers.
Chloride and Nitrate Classification
Analytical results indicated that about 20 and 14% of the groundwater samples from A and B aquifers exceeded the WHO (1993) limit for chloride and 2% of same set of groundwater samples show nitrate concentrations greater than WHO (1993). The threshold values following method of Sinclair (1974, 1976) were 35 and 2 mg/l for Cl− and NO3−, respectively. The classification of groundwater of the study area is presented in Fig. 7. Group 1 account for 20, 37.5, and 29.55%, respectively, for A, B, and C aquifers and are relatively low in chloride and nitrate (Cl− < 35 mg/l and NO3− < 2 mg/l). Sixty percent, 62.5, and 13.64% of the groundwater, respectively, for A, B, and C aquifers were classified as group 3, which is enriched in chloride and poor in nitrate (Cl− > 35 mg/l and NO3− < 2 mg/l). Group 2 with low chloride and high nitrate (Cl− < 35 mg/l and NO3− > 2 mg/l) contained 12% from A aquifer and 56.82% from C aquifer, while Group 4 with high chloride and high nitrate content (Cl− > 35 mg/l and NO3− > 2 mg/l) has 8% of samples from A aquifer. Majority of the groundwater samples in Groups 3 are from A and B aquifers suggesting influence of seawater contamination, while the majority of samples in Group 2 are from C aquifer suggesting input from human activities (Table 7). The arithmetic plot for Cl− and NO3− shows two pathways (Fig. 8). One pathway shows an increasing trend for chloride values along the y-axis. This pathway reflects seawater contamination. The second pathway is an increase in nitrate values along the x-axis and suggests human activities. This is in line with the work of Kim et al. (2004), Min et al. (2003), and Stumm and Morgen (1996). From above, samples from upper and lower alluvial aquifers are affected by high concentration of chloride, suggesting influence of sea water, while samples from coastal plain aquifer are affected by high nitrate due to human activities.
Stuyfzand Classification
Majority of the groundwater samples are grouped as Fresh, F (Table 8) accounting for 72, 88.5, and 100% for A, B, and C aquifers, respectively. The second most important group is Brackish (B) accounting for 14 and 12.5%, respectively, for A and B aquifers. The third group is fresh-brackish (Fb) and accounts for 8% of groundwater in A aquifer. Brackish-salt (Bs) is the least account for 6% of samples from the A aquifer.
Total hardness varies from very soft (*) with value of 0.02 mmol/l for sample 86C from C aquifer to extremely hard (7) with value of 646.7 mmol/l for groundwater sample 25A from A aquifer. Most of the groundwater samples are hard (11.4%) to very hard (15.2%) in C aquifer and extremely hard (4, 5, 6) constituting 17.1, 17.1, and 15.2%, respectively, in coastal plain aquifer. On the basis of dominant ions, most of the groundwater is classified as Na-Cl representing 44, 25, and 23% in A, B, and C aquifers. Mg-Cl is the major groundwater type in B aquifer, while Na-HCO3 constitutes the main groundwater type in C aquifer. Most of the groundwater samples analyzed from the area have positive cation exchange code (+) for C aquifer suggesting freshening or recharge water, while negative cation exchange code (−) dominates groundwater from A aquifer, suggesting salt water contamination. Again this supports the fact that the upper alluvial aquifer is affected by sea water relative to the lower alluvial and coastal plain aquifers.
4.2.5 Impact of Human Activities
Human activities such as irrigation including application of fertilizers and disposal of waste have been linked to variations of electrical conductivity, total dissolved solids, cations, and nitrate in groundwater (Jalali, 2009; Marghade et al., 2012). This is supported by the significant correlation (r = > 0.50, p < 0.05) between TDS with Na+ (r = 0.80), Cl− (r = 0.76), and SO42− (r = 0.72). However, correlation between NO3− with Cl− (r = 0.17); TDS with (NO3− + Cl−)/Na+ (r = 0.0022) and TDS with (NO3− + Cl−)/HCO3− (r = 0.21) does not suggest human activities, rather more of halite dissolution, sea water intrusion on one hand and mineral dissolution on the other (Choi et al., 2005).
4.2.6 Analysis of Historical and Recent Data
An analysis of the historical and recent data was for the coastal aquifer since data were only available for this aquifer. Comparative assessment shows that the impact of seawater contamination cannot be deciphered between the two hydrochemical data sets as given in Table 9. From Table 9, the average seawater indicators (EC, TDS, Na+, and Cl−) for historical period of study were 143.18 μS/cm, 130.59 mg/l, 16.77 mg/l, and 22.21 mg/l, respectively. For the recent study period, the values for the same indicators were 129.86 μS/cm, 75.45 mg/l, 14.11 mg/l, and 17.70 mg/l, respectively. This is attributable to limited number of data for the recent time (n = 17) relative to historical time (30).
4.2.7 Assessment of Groundwater Quality for Drinking and Irrigation Use
Groundwater in the study area is assessed for drinking and irrigation purposes. Drinking water used for drinking is compared with WHO (2006) standard, while for irrigation use, assessment is based on electrical conductivity (EC), sodium adsorption ratio (SAR), and percent sodium (%Na).
Drinking Purpose
Groundwater samples from upper alluvial aquifer show elevated concentrations above WHO limits for drinking in all parameters ranging between 2% for nitrate and 59% for pH (Table 4). Groundwater samples show concentrations higher than WHO limits in dissolved oxygen (DO), potassium, and chloride representing 50, 25, and 13% of all the samples from lower alluvial samples (B), while 77, 62, and 2% of groundwater samples from coastal plain aquifer exceeded WHO limits in pH, DO, and nitrate (Table 4). The groundwater samples with elevated concentrations are not suitable for drinking purpose. Seawater contamination represented by high EC, TDS, Na+, and Cl− and improper waste disposal represented by low DO and high NO3− maybe responsible for groundwater contamination.
In order to provide an overall water quality assessment, the water quality index (WQI) approach was applied to the analyzed data. Water quality approached has been used by several workers to assess the quality of water. Such studies include but not limited to Banoeng-Yakubo et al. (2009), Bhutiani et al. (2016), Cude (2001), Edet et al. (2013), Ewaid and Abed (2017), Kumar et al. (2015), Li et al. (2010); Oni (2016), and Saeedi et al. (2009). The procedures of using WQI to assess water quality are well documented in the above studies and will not be introduced in this write up. The classification of water for overall assessment is as follows: WQI < 25 (Excellent quality), 25 < WQI < 50 (Good quality), 50 < WQI < 100 (Medium quality) 100 < WQI < 150 (Poor quality), and WQI > 150 (Very poor quality), and assessment is given in Table 9.
As given in Table 9, of all the samples, 58, 88, and 98% from upper alluvial, lower alluvial, and coastal plain aquifers, respectively, are of excellent quality, while 22, 13, and 2% are of good quality. In the samples from upper alluvial aquifer, 8, 4, and 8%, respectively, are of medium, poor, and very poor quality.
Irrigation Purpose
The United States Department of Agriculture (USDA, 1954) was used to classify the groundwater for irrigation use with respect to electrical conductivity (EC), sodium adsorption ratio (SAR), and percent sodium (%Na). SAR and %Na are computed as Eqs. 6and7 with units in meq/l:
The calculated SAR ranges from 0.02 to 17.51, 0.14 to 1.47, and 0.01 to 8.48 for upper alluvial, lower alluvial, and coastal plain aquifers, respectively. Almost all the samples are in the excellent (SAR < 10) and good (SAR 10–18) irrigation water classes (Table 10). The USDA (1954) also classified irrigation water on the basis of EC as presented in Table 10. Most of the groundwater samples in lower alluvial and coastal plain aquifers are in the low salinity class (EC < 250 µS/cm), while most samples in upper alluvial aquifer (46%) and 26% of samples in coastal plain aquifer are in the medium salinity class (EC 250–750 µS/cm). Thirty-six percent, 10%, and 8% of the samples from upper alluvial aquifer are in low, high, and very high salinity classes, respectively. Percent sodium (%Na) was also used to evaluate sodium hazard and computed as given in Table 9. According to Khodpanah et al. (2009), water with %Na > 60% may result in sodium accumulations that will result in breakdown of physical properties of soil. The values of %Na range from 0.80 to 82.92%, 6.41 to 60.97%, and 0.77 to 95.53%, respectively, for upper alluvial, lower alluvial, and coastal plain aquifers. It is observed that 34% (upper alluvial aquifer), 13% (lower alluvial aquifer), and 60% (coastal plain aquifer) have very high %Na, suggesting very poor quality for irrigation.
In addition, the USSL (1954) scheme was applied in this study to further evaluate the quality of groundwater for irrigation use. The scheme classifies water on the basis of EC and SAR. EC is classified into four salinity zones (C1 [<250 µS/cm], C2 [250–750 µS/cm], C3 [750–2250 µS/cm], and C4 [>2550 µS/cm]) and SAR into four hazard zones (S1 [<10], S2 [10–18], S3 [18–26], and S4 [>26]). Classification of groundwater is presented in Table 9. The data show that 36, 100, and 77% of groundwater from upper alluvial, lower alluvial, and coastal plain aquifers, respectively, are characterized as C1-S1 (low salinity-low sodium hazard), which is very suitable for irrigation use, while 46 and 23% of samples from upper alluvial and coastal plain aquifers are in the class C2-S1 (medium salinity-low sodium hazard), classed as suitable. 10, 6, and 2%, respectively, of groundwater samples from A are classed as C3-S1 (medium salinity-low sodium hazard), C4-S1 (very high salinity-low sodium hazard), and C4-S2 (very high salinity-low sodium hazard).
5 Conclusions
Historical and recent geological, hydrogeological, and hydrochemical data were used to study the aquifers of the Niger Delta Region of Nigeria. Lithologic logs and aquifer characteristics including water levels, well and boreholes depths, hydraulic conductivity, and transmissivity were used to delineate two aquifers: alluvial aquifer (upper and lower) and coastal plain aquifers in the Niger Delta Region (Nigeria). Many methods have been used to attempt to examine the processes controlling groundwater chemistry. The methods applied include enrichment of Na+, K+, Ca2+, Mg2+, Cl−, HCO3−, and SO42−, and seawater mixing ratio, cross plots, and different classification schemes were used to confirm seawater intrusion, anthropogenic pollution, and processes controlling groundwater chemistry. The groundwater is acidic, while most of the groundwater samples (95%) from A, B, and C are fresh, while 2 and 3% of the samples from A are brackish and saline, respectively. The main groundwater facies are Na-Cl, Mg-Cl, and Na-HCO3, respectively, from A, B, and C.
Cl− showed significant correlation with Na+, K+, Ca2+, Mg2+, Cl−, HCO3−, and SO42− in upper and lower alluvial aquifer and Cl− with Na+, K+, Ca2+, Mg2+, Cl−, and HCO3− in coastal plain aquifer which demonstrated the mixing of seawater and freshwater. However, correlation of EC and NO3− in lower alluvial and coastal plain aquifer indicate anthropogenic pollution. The cross plots of Na+/Cl−, K+/Cl−, Ca2+/Cl−, and Mg2+/Cl− with Cl−/HCO3− and Cl−/NO3− and classification indicated seawater intrusion was occurring in the upper alluvial aquifer and decreased inland through lower alluvial aquifer to the coastal plain aquifer. In terms of drinking and irrigation use, the upper alluvial aquifer water is of poor quality relative to the groundwater from lower alluvial and coastal plain aquifers. The study has shown that the upper alluvial aquifer had relatively higher salinity and is due to sea water intrusion. This study highlights the potential danger of contaminated groundwater in the coastal areas the home of low income dwellers. Hence, seawater intrusion should be continuously monitored for sustainable management of coastal groundwater affected by seawater.
References
Abdalla, F. (2016). Ionic ratios as tracers to assess seawater intrusion and to identify salinity sources in Jazan coastal aquifer, Saudi Arabia. Arabian Journal of Geosciences, 9, 40. https://doi.org/10.1007/s12517-015-2065-3.
Akpokoje, E. G. (1987). The Engineering geological characteristics and classification of major superficial soils of Niger Delta. Engineering Geology, 23, 193–211.
Al-Shaibani, A. M. (2008). Hydrogeology and hydrochemistry of a shallow alluvial aquifer, western Saudi Arabia. Hydrogeology Journal, 16, 155–165.
Allen, J. R. L. (1965). Late Quatenary Niger Delta and adjacent areas: Sedimentary environment and lithofacies. American Association of Petroleum Geologists, 49, 549–600.
Allen, J. R. L. (1965b). Aspects of the geology of Nigeria (133p.). Nigeria: University of Ibadan Press.
Amadi, P. A., Ofoegbu, C. O., & Morrison, T. (1989). Hydrochemical assessment of groundwater quality in parts of the Niger Delta, Nigeria. Environmental Geology and Water Sciences, 14(3), 195–202.
Amadi, U. M. P., & Amadi, P. A. (1990). Saltwater migration in coastal aquifers of southern Nigeria. Journal of Mining and Geology, 26(1), 35–44.
Anders, R., Mendez, G. O., Futa, K., & Danskin, W. R. (2014). A geochemical approach to determine sources and movement of saline groundwater in a coastal aquifer. Groundwater, 52(5), 756–768.
Andersen, M. S., Nyvang, V., Jakobsen, R., & Postma, D. (2005). Geochemical processes and solute transport/freshwater interface of a sandy aquifer. Geochimica Et Cosmochimica Acta, 69, 3979–3994.
Appelo, C. A. J., & Postma, D. (1999). Geochemistry, groundwater and pollution (p. 536). Rotterdam: AA Balkema.
Banoeng-Yakubo, B., Yidana, S. M., Emmanuel, N., Akabzaa, T., & Asiedu, D. (2009). Analysis of groundwater quality using water quality index and conventional graphical methods: The Volta region. Ghana Environmental Earth Sciences, 59, 867–879.
Barbecot, F., Marlin, C., Gilbert, E., & Dever, L. (2000). Hydrochemical and isotopic characterization of the Bathonian and Bajocian coastal aquifer of the Caen area (northern France). Applied Geochemistry, 15, 791–805.
Bhutiani, R., Khanna, D. R., & Kulkarni, D. B. (2016). Assessment of Ganga river ecosystem at haridwar, Uttarakhand, India with reference to water quality indices. Applied Water Science, 6, 107–113.
Capaccioni, B., Didero, M., Paletta, C., & Didero, L. (2005). Saline intrusion and refreshening in a multilayer coastal aquifer in the Catanian Plain (Sicily, Southern Italy): Dynamics of degradation processes according to hydrochemical characteristics of groundwaters. Journal of Hydrology, 307, 1–16.
Cardona, A., Rivera, J. J. C., Alvarez, R. H., & Castro, G. E. (2004). Salinization in coastal aquifers of arid zone: An example from Santo Domingo, Baja California Sur, Mexico. Environmental Geology, 45, 350–366.
Carwright, I., Weaver, T. R., Fulton, S., Nichol, C., Reid, M., & Cheng, X. (2004). Hydrogeochemical and isotropic constrains on the origins of dryland salinity, Murray basin, Victoria, Australia. Applied Geochemistry, 19, 1233–1254.
Chadha, D. K. (1999). A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeology Journal, 7, 431–439.
Choi, B. Y., Yun, S. T., Yu, S. Y., Lee, P. K., Park, S. S., & Chae, G. T. (2005). Hydrochemistry of urban groundwater in Seoul, South Korea: Effect of landuse and pollution recharge. Environmental Geology, 48, 979–990.
Cude, C. (2001). Oregon water quality index: A tool for evaluating water quality management effectiveness. Journal of the American Water Resources Association, 37, 125–137.
Custodio, E. (1987). Hydrogeochemistry and tracers. In Custodio, E. (Ed.), Groundwater problems in coastal areas studies and reports in hydrology (Vol 45, pp. 213–269. UNESCO, Paris
de Montety, V., Radakovitch, O., Vallet-Coulomb, C., Blavoux, B., Hermitte, D., & Valles, V. (2008). Origin of groundwater salinity and hydrogeochemical processes in a confined coastal aquifer. Case of the Rhône delta (southern France). Applied Geochemistry, 23, 2337–2349.
El Moujabber, M., BouSamra, B., Darwish, T., & Atallah, T. (2006). Comparison of different indicators for groundwater contamination by seawater intrusion on the Lebanese Coast. Water Resources Management, 20, 161–180.
Edet, A. E. (1993). Groundwater quality assessment in parts of eastern Niger delta, Nigeria. Environmental Geology., 22, 41–46.
Edet, A. E., & Okereke, C. S. (2001). A regional study of saltwater intrusion in southeastern Nigeria based on analysis of geoelectrical and hydrochemical data. Environmental Geology, 40(10), 1278–1289.
Edet, A. (2005–2006). Groundwater vulnerability assessment using soil and hydrogeological data in the petroliferous Niger Delta, southern Nigeria. Journal of Environmental Systems, 32(2), 109–123
Edet, A. (2008). Hydrogeochemical characteristics of groundwater in the in parts of the Niger Delta. In Proceedings 1st Postgraduate Researchers Conference on Meeting Environmental Challenges in the Coastal Regions of Nigeria. 29–30 September 2008. University of Abertay Dundee, United Kingdom.
Edet, A. E. (2009). Characterization of groundwater contamination using factor analysis in the Niger Delta (Nigeria). Journal of Hydrologic Engineering, 14(11), 1255–1261.
Edet, A (2017) Hydrogeology and groundwater evaluation of a shallow coastal aquifer, southern Aiwa Ibom State (Nigeria). Applied Water Science, 7(5), 2397–2412. https://doi.org/10.1007/s13201-016-0432-1.
Aniekan, E. (2018). Seasonal and spatio-temporal patterns, evolution and quality of groundwater in Cross River State, Nigeria: Implications for groundwater management. Sustainable Water Resources Management. https://doi.org/10.1007/s40899-018-0236-6.
Edet, A., & Worden, R. (2009). Monitoring of Physical parameters and evaluation of the chemical composition of river and groundwater in Calabar (Southeastern Nigeria). Environmental Monitoring Assessment, 157, 243–258.
Edet, A. E., Worden, R. H., Mohammed, E. A., & Preston, M. R. (2012). Hydrogeochemical processes in a populated shallow Coastal Plain Sand Aquifer southeastern Nigeria. Environmental Earth Sciences, 65, 1933–1953.
Edet, A. E., Merkel, B. J., & Offiong, O. E. (2003). Trace element hydrochemical assessment of the Calabar Coastal Plain Sands, southeastern Nigeria using statistical methods. Environmental Geology, 44, 137–149.
Edet, A., Ukpong, A., & Nganje, T. (2013). Hydrochemical studies of Cross River Basin (southeastern Nigeria) river systems using cross plots, statistics and water quality index. Environmental Earth Sciences, 70, 3043–3056.
Ejiro, A. K., Anthony, A. I.. & Oghenero, O.-A. (2015). Characterization of groundwater quality from surface geoelectrics: The case of the Sombreiro – Warri Deltaic Plain Aquifer, Western Niger Delta, Nigeria. Journal of Environment and Earth Science, 5(8), 5–12.
El Moujabber, M., Bou Samra, B., Darwish, T., & Atallah, T. (2006). Comparison of different indicators for groundwater contamination by seawater intrusion on the Lebanese coast. Water Resources Management, 20, 161–180.
Esu, E. O., & Amah, E. A. (1999). Physico-chemical and bacteriological quality of natural waters in parts of Akwa Ibom and Cross river States, Nigeria. Global Journal of Pure and Applied Sciences, 5(4), 525–552.
Etu-Effeotor, J. O. (1981). Preliminary hydrogeochemical investigations of subsurface waters in parts of the Niger Delta. Journal of Geology and Mining, 18(1), 103–107.
Etu-Effeotor, J. O., & Odigi, M. I. (1983). Water Supply problems in the eastern Niger Delta. Journal Mining and Geology, 20(1 & 2), 183–193.
Etu-Effeotor, J. O., & Akpokoje, E. G. (1990). Aquifer systems of the Niger Delta. Journal of Mining and Geology, 26(2), 279–284.
Etu-Effeotor, J. O. (2000). Hydraulic characteristics of the aquifers within the Oligocene-Recent Coastal Plains Sands part of southern Nigeria. Global Journal of Pure and Applied Sciences, 6(1), 107–115.
Ewaid, S. H., & Abed, S. A. (2017). Water quality index for Al-Gharraf River, southern Iraq. Egypt Journal of Aquatic Research, 43(2), 117–122.
Fakir, Y., Mernissi, M. E., Kreuser, T., & Berjami, B. (2002). Natural tracer approach to characterize groundwater in coastal Sahel of Qualidia (Morocco). Environmental Geology, 43, 197–202.
Faye, S. P., Maloszewski, W., Stichler, P., Trimborn, S. C., & Gaye, C. B. (2005). Groundwater salinization in the Saloum (Senegal) delta aquifer: Minor elements and isotropic indicators. Science of the Total Environment, 343, 243–259.
Fetters, C. W. (2001). Applied hydrogeology (4th ed., p. 598). Eaglewood Cliffs, NJ, USA: Prentice Hall.
Fidelibus, M. D. (2003). Environmental tracing in coastal aquifers: Old problems and new solutions. In Coastal Aquifer Intrusion Technology: Mediterranean Countries (Vol II, pp. 79–111). Publ. IGME, Madrid.
George, N. J., Akpan, A. E., & Akpan, F. S. (2017). Assessment of spatial distribution of porosity and aquifer geohydraulic parameters in parts of the Tertiary – Quaternary hydrogeoresource of south-eastern Nigeria. NRIAG Journal of Astronomy and Geophysics, 6(2), 422–433. https://doi.org/10.1016/j.nrjag.2017.09.001.
Ghabayen, S. M. S., McKee, M., & Kemblowski, M. (2006). Ionic and isotopic ratios for identification of salinity sources and missing data in the Gaza aquifer. Journal of Hydrology, 318(1–4), 360–373.
Giambastiani, B. M. S., Colombani, N., & Mastrocicco, M. (2013). Characterization of the lowland coastal aquifer of Comacchio (Ferrara, Italy): Hydrology, hydrochemistry and evolution of the system. Journal of Hydrology, 50, 53–44.
Jalali, M. (2009). Geochemistry characterization of groundwater in an agricultural area of Razan, Hamadan, Iran. Environmental Geology, 56, 1479–1488.
Khaska, M., La Salle, C.L., Lancelot, J., Aster Team, Mohammad, A., Verdoux, P., Noret, A., & Simler, R. (2013). Origin of groundwater salinity (current seawater vs saline deep water) in coastal karst aquifer based on Sr and Cl isotopes. Case study of the La Clape massif (southern France). Applied Geochemistry, 37, 212–227.
Khodpanah, L., Sulaiman, W. N. A., & Khodpanah, N. (2009). Groundwater quality assessment for different purposes in Eshtehard District, Tehran, Iran. European Journal of Scientific Research, 36(4), 543–553.
Kim, K., Rajmohan, N., Kim, H. J., Hwang, G. S., Cho, M. J. (2004). Assessment of groundwater chemistry in coastal region (Kunsan, Korea) having a complex contaminant sources: A stoichiometric approach. Environmental Geology, 46, 763–774. https://doi.org/10.1007/s00254-004-1109-x
Kogbe, C. A. (1976). Geology of Nigeria. Lagos: Elizabeth Publishing Co.
Krishnakumar, P., Lakshumanan, C., Kishore, V. P., Sundararajan, M., Santhiya, G., & Chidambaram, S. (2014). Assessment of groundwater quality in and around Vedaraniyam, South India. Environmental Earth Sciences, 71, 2211–2225.
Kumar, S. K., Logeshkumaran, A., Magesh, N. S., Godson, P. S., & Chandrasekar, N. (2015). Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, Part of Chennai City, Tamil Nadu, India. Applied Water Science, 5, 335–343.
Li, P., Qian, H., & Wu, J. (2010). Groundwater quality assessment based on improved water quality index in Pengyang County, Ningxia, Northwest China. European Journal of Advanced Chemistry Research, 7(S1), S209–S216. https://doi.org/10.1155/2010/451304.
Marghade, D., Malpe, D. B., & Zade, A. B. (2012). Major ion chemistry of shallow groundwater of a fast growing city of Central India. Environmental Monitoring and Assessment, 184, 2405–2418. https://doi.org/10.1007/s10661-011-2126-3.
Min, J. H., Yun, S. T., Kim, K., Kim, H. S., & Kim, D. J. (2003). Geologic controls on the chemical behavior of nitrate in Riverside alluvial aquifers, Korea. Hydrological Processes, 17, 1197–1211.
Mondal, N. C., Singh, V. P., Singh, V. S., & Saxena, V. K. (2010). Determining the interaction between groundwater and saline water through groundwater major ion chemistry. Journal of Hydrology, 388, 100–111.
Mtoni, Y., Mjemah, I. C., Bakundukize, C., Camp, M. V., Martens, K., & Walraevens, K. (2013). Saltwater intrusion and nitrate pollution in the coastal aquifer of Dar es Salaam, Tanzania. Environment and Earth Science, 70, 1091–1111.
Murat, R. C. (1972). Stratigraphy and paleogeography of the Cretaceous and Lower Tertiary in southern Nigeria. In T. F. G. Dessauvagie & A. J. Whiteman (Eds.), African Geology (pp. 251–266). Geology Department: University of Ibadan.
Ngah, S. A., & Nwankwoala, H. O. (2013). Assessment of Static Water Level Dynamics in parts of the Eastern Niger Delta. The International Journal of Engineering and Science, 2(11), 136–141.
Nwankwoala, H. O., & Ngah, S. A. (2014). Groundwater resources of Niger Delta: Quality implications and management considerations. International Journal of Water Resources and Environmental Engineering, 6(5), 155–163.
Offodile, M. E. (2014). Hydrogeology: Groundwater supply and development in Nigeria (3rd ed., 636pp).
Ofoma, A. E., Omologbe, D. A., & Aigberua, P. (2005). Physico-chemical quality of groundwater in parts of Port-Harcourt city eastern Niger Delta, Nigeria. Water Resources, 16, 18–24.
Okiongbo, K. S., & Soronnadi-Ononiwu, G. C. (2015). Estimation of porosity and hydraulic conductivity of shallow quaternary alluvial aquifer in Yenogoa, southern Nigeria using electrical measurments. IFE Journal of Science, 17(2), 493–504.
Oni, O., & Fasakin, O. (2016). The use of water quality index method to determine the potability of surface water and groundwater in the vicinity of a municipal solid waste dumpsite in Nigeria. American Journal of Engineering Research, 5(10), 96–101.
Onwuka, O.S., & Omonona, O.V. (2017). Hydrogeochemical characteristics of coastal aquifers from Port Harcourt, southern Nigeria. Environmental Earth Sciences, 76(17), 609. https://doi.org/10.1007/s12665-017-6933-x.
Park, Y., Lee, J.-Y., Kim, J.-H., & Song, S.-H. (2012). National scale evaluation of groundwater chemistry in Korea coastal aquifers: Evidences of seawater intrusion. Environment and Earth Science, 66, 707–718.
Petters, S. W. (1982). Central West African Cretaceous-Tertiary Benthic Foraminifera and Stratigraphy. Paloeontographica, A., 179, 1–104.
Pulido-Leboeuf, P. (2004). Seawater intrusion and associated processes in a small coastal complex aquifer (Castell de Ferro, Spain). Applied Geochemistry, 19, 1517–1527.
Rai, N. S. (2004). Role of mathematical modelling in groundwater resources management. Hyderabad: Sri Vinayaka Enterprises.
Rasaq, B. (2017). Investigation of Groundwater Potential and Aquifer Protective Capacity of Part of Effurun, Delta State, Nigeria. International Journal Geology and Mining, 3(3), 141–150.
Reyment, R. A. (1965). Aspects of the geology of Nigeria (p. 145p). Nigeria: University of Ibadan Press.
Revelle, R. (1941). Criteria for recognition of sea water in groundwater. Transactions. American Geophysical Union, 22(593), 597.
Saeedi, M., Abessi, O., Sharifi, F., & Meraji. (2009). Development of groundwater index. Environmental Monitoring and Assessment. https://doi.org/10.1007/s/10661-009-0837-5
Sanchez-Martos, F., Pulido-Bosch, A., Molina-Sachez, L., & Vallejos-Izquierdo, A. (2002). Identification of the origin of salinization in groundwater using minor ions (Lower Andarax, Southeast Spain). Science of the Total Environment, 297, 43–58.
Short, K. C., & Stauble, A. J. (1976). Outline of the geology of the Niger Delta. Bulletin—American Association of Petroleum Geologists, 54, 761–779.
Sinclair, A. J. (1974). Selection of thresholds in geochemical data using probability graphs. Journal of Geochemical Exploration, 3, 129–149.
Sinclair, A. J. (1976). Application of probability graphs in mineral exploration (p. 95). Association of Exploration Geochemists, Rexdale.
Slama, F. (2010). Field experimentation and modelling of salt transfer in Korba coastal plain: Impact of the seawater intrusion and irrigation practices. Ph.D. Thesis. University of Neuchatel, Centre of Hydrogeology, p. 112.
Stumm, W., & Morgen, J. J. (1996). Aquatic chemistry. New York: Wiley.
Stuyfzand, P. J. (1986). A new hydrogeological classification of water types: principles and application to the coastal dunes aquifer system of the Netherlands. In: Proceedings 9th Salt Water Intrusion Meeting (SWIM): Delft, The Netherlands, pp 641–656.
Stuyfzand, P. J. (1993). Hydrochemistry and hydrology of the coastal dune area of the Western Netherlands. PhD dissertation. Free University (VU), Amsterdam. 90-74741-01-0:366
Sukhija, B. S., Varma, V. N., Nagabhushanam, P., & Reddy, D. V. (1996) Differentiation of paleomarine and modern seawater intruded salinities in coastal groundwaters (of Karaikal and Tanjavur, India).
Trabelsi, R., Abid, K., Zouari, K., & Yahyaoui, H. (2012). Groundwater salinization processes in shallow coastal aquifer of Djeffara plain of Medenine, Southern Tunisia. Environmental Earth Sciences, 66(2), 641–653.
Udom, G. J., Esu, E. O., & Ekwere, S. J. (1998). Quality status of groundwater in Calabar Municipality, southeastern Nigeria. Global Journal of Pure and Applied Sciences, 4(2), 163–169.
Udom, G. J., Etu-Efeotor, J. O., & Esu, E. O. (1999). Hydrochemical evaluation of groundwater in parts of Port Harcourt and Tai-Eleme Local Government Areas, Rivers State. Global Journal of Pure and Applied Sciences, 5(5), 545–552.
Udom, G. J., & Amah, E. A. (2006). Quality status of groundwater in Yenegoa and its environs, Bayelsa State, Nigeria. Journal of Scientific and Industrial Studies, 4(1), 45–51.
USDA. (1954). Agriculture handbook No 60. Washington DC, USA: United States Department of Agriculture, US Government Print Office.
United States Salinity Laboratory (USSL). (1954). Diagnosis and improvement of saline and alkali soils. In Agriculture handbook No 60 (pp. 69–81). US Department of Agriculture (USDA), Washington.
Vasu, D., Singh, S. K., Tiwary, P., Sahu, N., Ray, S. K., Butte, P., & Duraisami, V. P. (2017). Influence of geochemical processes on hydrochemistry and irrigation suitability of groundwater in parts of semi-arid Deccan Plateau, India. Applied Water Science. https://doi.org/10.1007/s13201-017-0528-2.
Vengosh, A., Gill, J., Davisson, M. L., & Hudson, G. B. (2002). A multi-isotope (B, Sr, O, H and C) and age dating (3H, 3He and 14C) study of groundwater from Salinas Valley, California: Hydrochemistry, dynamics and contamination processes. Water Resources Research, 38(1), 1008. https://doi.org/10.1029/2001WR000517.
Walraevens, K., Van Camp, M. (2005). Advances in understanding natural groundwater quality controls in coastal aquifers (pp 451–460). 18 Saltwater Intrusion Meeting (SWIM). Cartagena 2004, Spain.
Walter, J., Chesnaux, R., Cloutier, V., & Gaboury, D. (2017). The influence of water/rock-water/clay interactions and mixing in sanilization processes in groundwater. Journal of Hydrology, 13, 168–188.
Wang, Y., & Jiao, J. J. (2012). Origin of groundwater salinity and hydrogeochemical processes in the confined quaternary aquifer of Pearl River Delta, China. Journal of Hydrology, 325(1–4), 35–55.
Weber, K. J. (1971). Sedimentological aspects of oil fields in Niger delta. Geologie En Mijnbouw, 50, 559–576.
Whiteman, A. J. (1982). Nigeria: Its petroleum geology resources and potential (Vols. 1 and 2, 394p). London: Graham and Trotman.
Winograd, I. J., & Farlekas, G. M. (1974). Problem in 14C dating of waters from aquifers of deltaic origin. Isotope hydrology (pp. 69–93). Vienna: IAEA.
Wood, W. W., & Sandford, W. E. (1995). Chemical and isotopic methods for quantifying groundwater recharge in a regional, semi-arid environment. Groundwater, 33, 458–468.
World Health Organization (WHO). (1993). Guidelines for drinking water quality. In Revision of the 181 Guidelines Final Task Group Meeting, 21–25 Sept 1992, WHO Geneva, Switzerland.
World Health Organization (WHO). (2004). Guidelines for drinking water quality vole 1 recommendations (3rd ed.). Switzerland: WHO Geneva.
World Health Organization (WHO). 2006. Guidelines for drinking water quality vol 1 recommendations (3rd ed.) Geneva, Switzerland: WHO.
WHO (2008) The guideline for drinking-water quality 3rd edition incorporating the first and second addenda Vol 1 recommendations. Geneva: World Health Organization.
Wright, J. B. (1989). Review of the origin and evolution of the Benue Trough in Nigeria. In: C. A. Kogbe (Ed.), Geology of Nigeria (pp. 359–376). Rock View (Nigeria) Limited.
Yolcubal, I., Gündüz, Ö. C. A., & Kurtuluş, N. (2019). Origin of salinization and pollution sources and geochemical processes in urban coastal aquifer (Kocaeli NW Turkey). Environmental Earth Sciences, 78, 181. https://doi.org/10.1007/s12665-019-8181-8.
Zghibi, A., Tarhouni, J., & Zouhri, L. (2013). Assessment of seawater intrusion and nitrate contamination on the groundwater quality in the Korba coastal plain of Cap_Bon (North-east of Tunisia). Journal of African Earth Sciences, 87, 1–12.
Acknowledgements
Literature for this work was possible as a result of Fellowship from Alexander von Humboldt Foundation, Germany. Also to my hosts Prof Dr BJ Merkel and Prof Dr Peter Grathwhöl in Germany at various times for facilitating the sample analyses and for their hospitality.
Compliance with Ethical Standards
Funding: Part of this study was funded through a fellowship by Alexander von Humboldt, Germany (3.4-NRI/101071296 STP) at TU Bergakademie, Freiberg, and Institute of Geosciences, University of Tübingen, Germany.
Conflict of Interest
The author declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Edet, A. (2021). Aquifer Characteristics and Evidence of Saltwater Intrusion in Coastal Groundwater of Niger Delta (Nigeria) Based on Historical and Recent Data. In: Al-Maktoumi, A., et al. Water Resources in Arid Lands: Management and Sustainability. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-030-67028-3_29
Download citation
DOI: https://doi.org/10.1007/978-3-030-67028-3_29
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67027-6
Online ISBN: 978-3-030-67028-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)