Abstract
Pollution of groundwater by seawater intrusion poses a threat to sustainable agriculture in the coastal areas of Korea. Therefore, seawater intrusion monitoring stations were installed in eastern, western, and southern coastal areas and have been operated since 1998. In this study, groundwater chemistry data obtained from the seawater intrusion monitoring stations during the period from 2007 to 2009 were analyzed and evaluated. Groundwater was classified into fresh (<1,500 μS/cm), brackish (1,500–3,000 μS/cm), and saline (>3,000 μS/cm) according to EC levels. Among groundwater samples (n = 233), 56, 7, and 37% were classified as the fresh, brackish, and saline, respectively. The major dissolved components of the brackish and saline groundwaters were enriched compared with those of the fresh groundwater. The enrichment of Na+ and Cl− was especially noticeable due to seawater intrusion. Thus, the brackish and saline groundwaters were classified as Ca–Cl and Na–Cl types, while the fresh groundwater was classified as Na–HCO3 and Ca–HCO3 types. The groundwater included in the Na–Cl types indicated the effects of seawater mixing. Ca2+, Mg2+, Na+, K+, SO4 2−, and Br− showed good correlations with Cl− of over r = 0.624. Of these components, the strong correlations of Mg2+, SO4 2−, and Br− with Cl− (r ≥ 0.823) indicated a distinct mixing between fresh groundwater and seawater. The Ca/Cl and HCO3/Cl ratios of the groundwaters gradually decreased and approached those of seawater. The Mg/Cl, Na/Cl, K/Cl, SO4/Cl, and Br/Cl ratios of the groundwaters gradually decreased, and were similar to or lower than those of seawater, indicating that Mg2+, Na+, K+, SO4 2−, and Br−, as well as Cl− in the saline groundwater can be enriched by seawater mixing, while Ca2+ and HCO3 − are mainly released by weathering processes. The influence of seawater intrusion was evaluated using threshold values of Cl− and Br−, which were estimated as 80.5 and 0.54 mg/L, respectively. According to these criteria, 41–50% of the groundwaters were affected by seawater mixing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater has been used to supply living, agricultural, and industrial water for a long time in many countries. Recently, the use and development of groundwater have increased due to industrialization and urbanization, leading to various problems such as depletion of groundwater, deterioration of water quality, land subsidence, and salinization of groundwater (Zhou et al. 2000; Capaccioni et al. 2005; Hiroshiro et al. 2006; Won et al. 2006). These problems can gradually reduce the amount of available groundwater.
Many studies have been performed to solve these problems (Narayan et al. 2007). Especially, salinization of groundwater becomes an important issue with the groundwater contamination that occurs in many coastal areas (Sivan et al. 2005; Ghabayen et al. 2006), and was mainly caused by evaporite dissolution, fossil seawater, return flow from irrigation water, leakage of wastewater, and seawater intrusion (Bennetts et al. 2006; Ghabayen et al. 2006). Among these salinization processes, salinization of groundwater by seawater intrusion is mainly induced in many coastal areas, where the seawater intrusion occurs frequently due to intensive pumping (Kim et al. 2003b; El Moujabber et al. 2006; Kouzana et al. 2009). So, the efforts optimizing pumping rate have been extensively conducted using aquifer models such as SUTURA model (Gorelick et al. 1984; Ahlfeld and Heidari 1994; Mantoglou et al. 2004; Narayan et al. 2007).
Brackish and saline groundwaters by seawater intrusion are unavailable for living and agricultural water in coastal areas. Therefore, methods such as construction of subsurface barrier and development of recharge well were suggested by some researchers to prevent coastal aquifer from seawater intrusion (Allow 2011). Especially, recharge of water into a coastal aquifer may raise other problems such as inflow of virus and bacteria and quick growth of those (Anders and Chrysikopoulos 2005; Ven Beek et al. 2009). So appropriate set back distance for available wells should be considered when recharge wells are designed (Masciopinto et al. 2008).
Various methods have been used to evaluate groundwater contamination by seawater intrusion. Some specific ions such as Cl−, Na2+, Mg2+, SO4 2−, and Br− in groundwater are enriched by seawater intrusion and can be used as markers of its influence (Sukhija et al. 1996; Capaccioni et al. 2005; de Mondal et al. 2008). In particular, Cl− and Br− of these ions have been used as representative proxies to estimate the influence of seawater on groundwater (Ghabayen et al. 2006). Moreover, ionic ratios such as Cl/HCO3, Ca/Na, Na/Cl, Br/Cl, and Ca/(HCO3 + SO4) ratios of groundwater can be effectively used to evaluate the degree of seawater intrusion (El Moujabber et al. 2006; Ghabayen et al. 2006; de Montety et al. 2008). Recently, threshold values calculated from cumulative frequency curves have often been used in hydrogeochemical research (Russo et al. 2001; Park et al. 2002; Knight et al. 2005; Tutmez 2009). Threshold values are good tools to identify the starting point of chemical impact by seawater intrusion. Park et al. (2005) studied the salinization of groundwater in the western coastal area of Korea. They classified groundwater into four types using threshold values of Cl− and NO3 − estimated from cumulative frequency curves. Lee and Song (2007a) also applied threshold values of Cl− and HNO3 − to evaluate the influence of seawater intrusion and water–rock interaction on groundwater in the western and southern coastal areas of Korea.
In this study, chemical data for the eastern, western, and southern coastal groundwaters during the period from 2007 to 2009 obtained from the seawater intrusion monitoring station installed by the Korea Rural Community Corporation (KRC) were analyzed and evaluated. The study objectives are to determine the chemical compositions of the coastal groundwater and evaluate the influence of seawater intrusion on it.
Materials and methods
Study area
The study area is located along the eastern, western, and southern coastal areas of Korea. According to previous research, groundwater use is concentrated in the western and southern coastal areas compared with the eastern coastal area (MOCT 2002), due to the proximity of many large cities and intensive farming such as rice cropping. Over pumping for irrigation water is closely related with seawater intrusion in coastal areas (El Moujabber et al. 2006; Lee and Song 2007b). In addition, the hydraulic gradient in the western and southern coastal areas is low, because of the predominantly flat lands of these areas (Kim et al. 2006). Owing to these environmental aspects, seawater intrusion was frequently observed in the western and southern coastal areas (Lee and Song 2007a), and research on seawater intrusion has been actively performed in these areas (Park et al. 2002; Kim et al. 2003b, 2006; Song et al. 2007).
Because agricultural activities are intensively practiced in the western and southern coastal areas, much irrigation water is supplied from groundwater. The groundwater usage in Korea increased by ~688 million tons from 2000 to 2008, i.e., an average annual increase of ~76 million ton/year. Agricultural water comprises 42.2 to 46.8% of total groundwater use and has increased by ~460 million tons (~51 million ton/year) during the same period (Fig. 1).
Amount of groundwater used during the period from 2000 and 2008 in Korea. The total groundwater includes domestic, industrial and agricultural water. Data were obtained from National Groundwater Information Management and Service Center (http://www.gims.go.kr)
Seawater intrusion monitoring network
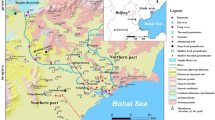
The KRC has installed 92 seawater intrusion monitoring wells in 52 regions and have been managed since 1998 to monitor seawater intrusion (Fig. 2). Each monitoring station had 1–3 wells. Approximately 90% of all seawater intrusion monitoring stations are located in the western and southern coastal areas. All the monitoring wells are within 2 km of the coastal line. The depths of the monitoring wells range from 30 to 200 m (Lee and Song 2007a). The mean groundwater levels of the monitoring wells ranged from −19.7 to 16.2 m above mean sea level (MAF and KRC 2009). Lee et al. (2008) reported that the hydraulic conductivity obtained from aquifer tests ranged from 5.20 × 10−5 to 4.00 × 10−3 cm/sec. A little over a third (37.8%) of the monitoring wells have been actively used for irrigation water (especially, from April to August) and the mean pumping rate of the monitoring wells is 164 m3/day (Lee et al. 2008).
In each monitoring well, water level, electrical conductivity (EC), and water temperature had been measured hourly using a multi-probe equipped with a data logger in each monitoring well (Lee et al. 2008; MAF and KRC 2009). The chemical compositions of groundwater had been analyzed for Ca2+, Mg2+, Na+, K+, Cl−, SO4 2−, HCO3 −, and Br− every year. According to data collected from 2005 to 2009, seawater had intruded inland in 26% of the monitoring wells (n = 24), and its regression occurred in 16% of them (n = 15) (MAF and KRC 2009). According to monitoring data in 2009, influence of seawater on groundwater increased at 15 monitoring wells compared with results monitored in 2008, and 67% (n = 10) and 27% (n = 4) of these monitoring wells are distributed in southern and western coastal areas, respectively (MAF and KRC 2009). These results were closely concerned with agricultural activities in these areas and causing lack of agricultural water. Thus, the development of alternative groundwater resources is demanded in the coastal area where seawater intrusion occurred and government is making a long-term plan to supply agricultural water based on monitoring data of seawater intrusion (MAF and KRC 2009).
Results and discussion
General hydrochemistry
Groundwater affected by seawater can be classified into fresh (<1,500 μS/cm), brackish (1,500–3,000 μS/cm), and saline (>3,000 μS/cm) according to the EC levels (Rai 2004; Mondal et al. 2010). Based on these classification standards, the fresh, brackish, and saline groundwaters formed 56, 7, and 37% of the groundwater in the study area, respectively (Fig. 3), indicating that 44% of the groundwaters are affected by seawater mixing. The influence of seawater mixing on groundwater chemistry is demonstrated in detail below. The EC levels did not show any correlation with distance from the coast in Fig. 3, because the study area includes both alluvial and bedrock coastal aquifers. According to previous studies, the correlations between EC levels and distance from the coast are affected by the characteristics of coastal aquifer in Korea. EC levels and distance from the coast show good negative correlation in alluvial coastal aquifers (Kim et al. 2003a), but they do not show any correlation in bedrock coastal aquifers (Kim et al. 2006). In this study, the study area could not be divided into alluvial and bedrock coastal aquifers, because detailed information for the aquifer profile was not obtained.
Distribution of EC levels of the groundwaters with distance from the coast. The fresh, brackish, and saline have range of <1,500, 1,500–3,000, and >3,000 μS/cm, respectively (Rai 2004)
Table 1 summarizes the EC levels and concentrations of major dissolved components of groundwater. The EC levels and major dissolved components in the brackish and saline groundwaters showed wide ranges and large standard deviations compared with those in the fresh groundwater, and this difference was attributed to the influence of seawater mixing (Mondal et al. 2010). Figure 4 shows the concentration ranges of dissolved components of the fresh, brackish, and saline groundwaters. The concentrations of all dissolved components of the brackish and saline groundwaters were significantly enriched compared with those of the fresh groundwater, especially the enrichments of Na+ and Cl−. In Table 1, the concentrations of Na+ and Cl− in the saline groundwater increased by ~69 and 84 times, respectively, compared with those in the fresh groundwater, while those of Mg2+, K+, and SO4 2− increased by ~28, 24, and 30 times, respectively. In particular, the maximum concentrations of Cl− and Mg2+ in saline groundwater (Cl− = 18,793 mg/L and Mg2+ = 1,249 mg/L) are very similar to those in seawater (Cl− = 18,211 mg/L and Mg2+ = 1,248 mg/L). However, the concentrations of Ca2+ and HCO3 − increased ~14 and 2.5 times, respectively. Substantial amounts of HCO3 − and Ca2+ indicate contribution by water–rock interaction (Hem 1985; Vengosh and Rosenthal 1994; Mondal et al. 2010).
In Figure 5, despite the wide range of concentrations of the dissolved components, Ca2+, K+, HCO3 −, and Br− show good lognormal distributions but those of K+ and Br− have a tail at the high concentration range. This indicated that Ca2+ and HCO3 − are mainly affected by simple processes, and some of the samples showing high concentrations of K+ and Br− can be considered as being affected by anomalous pollution such as seawater mixing. However, Mg2+, Na+, Cl−, and SO4 2− do not show lognormal distributions, which suggests that these components may be supplied by complex processes such as water–rock interaction, seawater mixing and anthropogenic contamination (Barbecot et al. 2000; Alcalá and Custodio 2008; de Montety et al. 2008).
Most of the fresh groundwater was plotted on an area of Na–HCO3 and Ca–HCO3 types, and most of the brackish and saline groundwaters on an area of Ca–Cl and Na–Cl types (Fig. 6). It clearly shows the evolution of groundwater due to seawater encroachment. The water types of coastal groundwater may be gradually transformed from Na–HCO3 and/or Ca–HCO3 types into Ca–Cl and/or Na–Cl types by cation exchange reaction and seawater mixing processes (Appelo and Postma 1994; Capaccioni et al. 2005; Mondal et al. 2008).
Enrichment of dissolved components
When seawater encroachment occurred, enrichments of Na+ and Cl− were generally observed (Mondal et al. 2010), indicating that such enrichments can play an important role as indicators of seawater intrusion. However, whereas Cl− is suitable as a proxy of seawater intrusion, Na+ is not. As well as seawater mixing, Na+ can also be enriched by water–rock interaction processes (silicate mineral weathering processes) and anthropogenic contamination, while Cl− is dominantly supplied from seawater only. Therefore, Cl− has been used as proxy to indicate seawater encroachment. Figure 7 shows the correlation between Ca2+, Mg2+, Na+, K+, SO4 2−, HCO3 −, and Br− with Cl−. Most of the components besides HCO3 − (r = 0.413) showed a good positive correlation with Cl− of over r = 0.624. Of these components, Mg2+, SO4 2−, and Br−, with strong correlation with Cl− of over r = 0.823, showed the distinct mixing trends between the fresh groundwater and seawater. The correlation coefficients of the components were calculated using SPSS computer software. Some of the Ca2+, Na+ and HCO3 − results were higher than would be expected with only seawater mixing, suggesting the action of additional supply sources of Ca2+, Na+, and HCO3 − besides seawater mixing. These sources are related with the water–rock interaction (Vengosh and Rosenthal 1994; Oliva et al. 2004). Especially, the concentrations of HCO3 − hardly increase due to seawater mixing, because those of HCO3 − in groundwater (mean value of groundwater = 173 mg/L, n = 233) were not greatly different compared with those in seawater (mean value of seawater around Korea = 135 mg/L).
Ionic ratios
Ionic ratios of groundwater have been used to evaluate the influence of seawater intrusion on coastal groundwater (Sukhija et al. 1996; Vengosh et al. 1999; El Moujabber et al. 2006; Kouzana et al. 2009). Figure 8 shows the ionic ratios of the fresh, brackish, and saline groundwaters. All ionic ratios of the fresh groundwater were higher than those of the brackish and saline groundwaters because of the enrichment of Cl− due to seawater encroachment. Some of the Mg/Cl, Na/Cl, K/Cl, and SO4/Cl ratios of groundwaters were much lower than expected by simple seawater mixing. This was owing to additional processes supplying Cl− besides seawater intrusion (Alcalá and Custodio 2008). Generally, Mg2+, Na+, K+, and SO4 2− are hardly removed from an aqueous system because chemical species, including these components are unsaturated in aqueous environment. However, because the amount of Cl− supplied from additional processes was much lower than expected by simple seawater mixing, the concentrations of Cl− released from contaminants can be ignored in the evaluation of seawater intrusion. The mean Ca/Cl ratio of the fresh, brackish, and saline groundwaters was 0.967, 0.147, and 0.154, respectively, and of HCO3/Cl ratio was 3.867, 0.587, and 0.274, respectively. Most of the Ca/Cl and HCO3/Cl ratios of groundwaters were much higher than those of seawater (Ca/Cl = 0.021 and HCO3/Cl = 0.007) but gradually approach those of seawater according to increasing EC levels (seawater intrusion). The mean of Mg/Cl, Na/Cl, K/Cl, SO4/Cl, and Br/Cl ratios of the fresh groundwater were 0.312, 0.837, 0.108, 0.408, and 0.010, respectively, and were higher than those of seawater. However, the mean of Mg/Cl, Na/Cl, K/Cl, SO4/Cl, and Br/Cl ratios of the brackish and saline groundwaters were greatly decreased compared with those of the fresh groundwater, and those of the saline groundwater were similar to those of seawater. This indicated that the chemical compositions of coastal groundwater can be changed by seawater encroachment, and that seawater mixing can supply a large amount of Mg2+, Na+, K+, Cl−, SO4 2−, and Br− to coastal groundwater (Sukhija et al. 1996; Capaccioni et al. 2005; Mondal et al. 2008).
Cumulative frequency curves and threshold values
Quantitative evaluation of the influence of seawater intrusion on groundwater is very important in coastal areas of many areas, because groundwater usually supplies drinking or irrigation water. Therefore, many studies on the determination and evaluation of seawater intrusion in coastal areas have been performed. In Korea, some studies using regional threshold values calculated from cumulative frequency curves were performed to indicate seawater encroachment and to evaluate the influence of seawater mixing (Park et al. 2002; Park et al. 2005; Lee and Song 2007a, b; Kim et al. 2009). These studies were mainly performed in the western coastal area, and some were performed in the southern coastal area. The present study included the eastern coastal area as well as the western and southern coastal areas (Table 2).
The cumulative frequency curves for major dissolved components can distinguish ‘anomalous’ values from ‘background’ values (Russo et al. 2001; Park et al. 2002; Knight et al. 2005; Tutmez 2009). The threshold values in cumulative frequency curves indicate a starting point of chemical impact caused by the inflow of a water body showing high concentrations of some components. Of potential water bodies, seawater can be preferentially considered in coastal areas. Figure 9 shows threshold values of major components estimated from the cumulative frequency curves. The threshold values of all components lay between the mean of the concentration of each component in the fresh groundwater and that in the brackish groundwater. To confirm the confidence of these values, the present study results are compared with those of previous studies (Table 2). The present study results differed slightly from those of Lee and Song (2007a) and Kim et al. (2009), but widely from those of Park et al. (2005) and Lee and Song (2007b). These results may be attributed to differences within the study area. The eastern and southern coastal areas were not included in their studies. The regional threshold values of the western coastal area may be higher than those of the eastern and southern coastal areas, because of the influence of old seawater in the reclaimed areas predominantly located on the west coast (Kim et al. 2003a). Approximately 2% of the total land surface of Korea has been reclaimed from the sea or tidal flats in the western coastal area (Koo et al. 1998). Threshold values of HCO3 −, Cl−, and Br− of major components have been frequently used to evaluate the influence of water–rock interaction and seawater influence. Threshold values of HCO3 −, Cl−, and Br were estimated as 119, 80.5, and 0.54 mg/L, respectively. In this study, to confirm the possibility of threshold value of Br− as a proxy of seawater intrusion, the results obtained from the threshold values of Cl− and HCO3 − were compared with those of Br− and HCO3 − (Fig. 10). According to threshold values of Cl− and HCO3 −, 7.9% of the groundwaters were dominantly influenced by water–rock interaction, while 24.7% was significantly affected by seawater, and 24.7% was influenced by both (Fig. 10a). In addition, based on the threshold values of Br− and HCO3 −, 10.5, 22.0, and 19.4% of the groundwaters were affected by water–rock interaction, seawater encroachment, and both water–rock interaction and seawater mixing, respectively (Fig. 10b). The values estimated by the threshold values of Br− and HCO3 − were higher than those of Cl− and HCO3 −, but the difference was not significant. Using the threshold values of Cl− and Br− to consider only seawater mixing besides water–rock interaction, 41.4% of the groundwaters were affected by seawater mixing (Fig. 11). The results obtained from the threshold values of Cl− and Br− were slightly lower than those shown in Fig. 10.
Conclusions
The hydrochemistry of groundwaters in the eastern, western, and southern coastal areas of Korea was examined using data collected from KRC during 2007–2009. Many methods have been used in attempts to examine such hydrochemistry. In this study, the enrichments of major dissolved components, ionic ratios, and threshold values were used to confirm seawater intrusion and to evaluate its influence. Groundwaters were classified into fresh (56%), brackish (7%), and saline (37%) according to their EC levels. All dissolved components in the brackish and saline groundwaters were enriched compared with those in the fresh groundwater, and these trends were significant in Na+ and Cl−. Thus, the brackish and saline groundwaters were classified as Ca–Cl and Na–Cl types, while the fresh groundwater was classified as Na–HCO3 and Ca–HCO3 types.
Ca2+, Mg2+, Na+, K+, SO4 2−, and Br− showed good correlations with Cl− of over r = 0.624. Of these components, Mg2+, SO4 2−, and Br had the strongest correlations with Cl− of over r = 0.823, which demonstrated the mixing trend between fresh groundwater and seawater. The Ca/Cl and HCO3/Cl ratios of the groundwaters gradually decreased due to seawater encroachment but most of the Ca/Cl and HCO3/Cl ratios of the groundwaters are higher than those of seawater. The Mg/Cl, Na/Cl, K/Cl, SO4/Cl and Br/Cl ratios of the fresh groundwater were higher than those of seawater. However, those of the brackish and saline groundwaters were similar to or lower than those of seawater. These results indicated that the main source of Ca2+ and HCO3 − were different from those of Mg2+, Na+, K+, SO4 2−, and Br−. The former pair dominantly resulted from water–rock interaction, while the latter group was mainly supplied from seawater mixing processes. Therefore, the Mg/Cl, Na/Cl, K/Cl, SO4/Cl and Br/Cl ratios can be applied as useful indicators to evaluate seawater intrusion.
In this study, the influence of seawater intrusion on groundwater was quantitatively assessed using the threshold values of major dissolved components. The threshold values of HCO3 −, Cl−, and Br− were suitable as proxies to evaluate the degree of water–rock interaction and seawater mixing, because their sources are relatively simple compared with those of the others. The threshold values of HCO3 −, Cl−, and Br− were 119, 80.5, and 0.54 mg/L, respectively, indicating that approximately 41–50% of the groundwaters were influenced by seawater mixing.
From these study results, the occurrence of seawater intrusion was confirmed in many coastal areas. This highlights the potential danger of reduced groundwater resources in many coastal areas. Any decrease of groundwater resources would be very detrimental, due to the growing dependence on groundwater in coastal areas. Therefore, seawater intrusion should be continuously monitored and these monitoring data can be helpful for making a strategy securing sustainable groundwater resources.
References
Ahlfeld DP, Heidari M (1994) Applications of optimal hydraulic control to ground-water systems. J Water Resour Plan Manag 120:350–365
Alcalá FJ, Custodio E (2008) Using the Cl/Br ratio as a tracer to identify the origin of salinity in aquifers in Spain and Portugal. J Hydrol 359:189–207
Allow KA (2011) The use of injection wells and a subsurface barrier in the prevention of seawater intrusion: a modelling approach. Arab J Geosci. doi:10.1007/s12517-011-0304-9
Anders R, Chrysikopoulos CV (2005) Virus fate and transport during artificial recharge with recycled water. Water Resour Res 41:W10415. doi:10.1029/2004WR003419
Appelo CAJ, Postma D (1994) Geochemistry. Groundwater and pollution, Balkema, Rotterdam
Barbecot F, Marlin C, Gibert E, Dever L (2000) Hydrochemical and isotopic characterization of the Bathonian and Bajocian coastal aquifer of the Caen area (northern France). Appl Geochem 15:791–805
Bennetts DA, Webb JA, Stone DJM, Hill DM (2006) Understanding the salinisation processes for groundwater in an area of south-eastern Australia, using hydrochemical and isotopic evidence. J Hydrol 323:178–192
Capaccioni B, Didero M, Paletta C, Didero L (2005) Saline intrusion and refreshening in a multilayer coastal aquifer in the Catania Plain (Sicily, Sthohern Italy): dynamics of degradation processes according to the hydrochemical characteristics of groundwaters. J Hydrol 307:1–16
de Montety V, Radakovitch O, Vallet-Coulomb C, Blavoux B, Hermitte D, Valles V (2008) Origin of groundwater salinity and hydrogeochemical processes in a confined coastal aquifer: Case of the Rhône delta (Southern France). Appl Geochem 23:2337–2349
El Moujabber M, BouSamra B, Darwish T, Atallah T (2006) Comparison of different indicators for groundwater contamination by seawater intrusion on the Lebanese Coast. Water Resour Manag 20:161–180
Ghabayen SMS, McKee M, Kemblowski M (2006) Ionic and isotopic ratios for identification of salinity sources and missing data in the Gaza aquifer. J Hydrol 318:360–373
Gorelick SM, Voss CI, Gill PE, Murray W, Saunders MA, Wright MH (1984) Aquifer reclamation design: the use of contaminant transport simulation combined with non-linear programming. Water Resour Res 20:415–427
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, 3rd edn. USGS, Aleandria
Hiroshiro Y, Jinno K, Berndtsson R (2006) Hydrogeochemical properties of a salinity-affected coastal aquifer in western Japan. Hydrol Process 20:1425–1435
Kim JH, Yum BW, Kim RH, Koh DC, Cheong TJ, Lee J, Chang HW (2003a) Application of cluster analysis for the hydrogeochemical factor of saline groundwater in Kimje, Korea. Geosci J 7:313–322
Kim Y, Lee KS, Koh DC, Lee DH, Lee SG, Park WB, Koh GW, Woo NC (2003b) Hydrogeochemical and isotopic evidence of groundwater salinization in a coastal aquifer: a case study in Jeju volcanic island, Korea. J Hydrol 270:282–294
Kim RH, Kim JH, Ryu JS, Chang HW (2006) Salinization properties of a shallow groundwater in a coastal reclaimed area, Yeonggwang, Korea. Environ Geol 49:1180–1194
Kim HJ, Hamm SY, Kim NH, Cheong JY, Lee JH, Jang S (2009) Characteristics of groundwater caused by seawater intrusion and agricultural activity in Sacheon and Hadong areas, Republic of Korea. Econ Environ Geol 42:575–589 (in Korean with English abstract)
Knight JH, Gilfedder M, Walker GR (2005) Impact of irrigation and dryland development on groundwater discharge to rivers-a unit response approach to cumulative impacts analysis. J Hydrol 303:79–91
Koo JW, Choi JK, Son JW (1998) Soil properties of reclaimed tidal lands and tidelands of western sea coast in Korea. J Korean Soc Soil Sci Fert 31:120–127 (in Korean with English abstract)
Kouzana L, Mammou AB, Felfoul MS (2009) Seawater intrusion and associated processes: case of the Korba aquifer (Cap-Bon, Tunisia). C R Geosci 341:21–35
Lee JY, Song SH (2007a) Evaluation of groundwater quality in coastal areas: implications for sustainable agriculture. Environ Geol 52:1231–1242
Lee JY, Song SH (2007b) Groundwater chemistry and ionic ratios in a western coastal aquifer of Buan, Korea: implication for seawater intrusion. Geosci J 11:259–270
Lee JY, Yi MJ, Song SH, Lee GS (2008) Evaluation of seawater intrusion on the groundwater data obtained from the monitoring network in Korea. Water Int 33:127–146
MAF (Ministry of Agriculture, Forestry), KRC (Korea Rural Community Corporation) (2009) An annual report on investigation of seawater intrusion. MAF and KRC, Uiwang, Korea
Mantoglou A, Papantoniou M, Giannoulopoulos P (2004) Management of coastal aquifers based on non-linear optimization and evolutionary algorithms. J Hydrol 297:209–228
Masciopinto C, Mantia RL, Chrysikopoulos CV (2008) Fate and transport of pathogens in a fractured aquifer in the Salento area, Italy. Water Resour Res 44:W01404. doi:10.1029/2006WR005643
MOCT (Ministry of Construction, Transportation of Korea) (2002) Basic plan for groundwater management. MOCT, Gwacheon, Korea
Mondal NC, Singh VS, Saxena VK, Prasad RK (2008) Improvement of groundwater quality due to fresh water ingress in Potharlanka Island, Krishna delta, India. Environ Geol 55:595–603
Mondal NC, Singh VP, Singh VS, Saxena VK (2010) Determining the interaction between groundwater and saline water through groundwater major ions chemistry. J Hydrol 388:100–111
Narayan KA, Schleeberger C, Bristow KL (2007) Modelling seawater intrusion in the Burdekin delta irrigation area, north Queensland, Australia. Agric Water Manag 89:217–228
Oliva P, Dupré B, Martin F, Viers J (2004) The role of trace minerals in chemical weathering in a high elevation granitic watershed (Estibére, France): chemical and mineralogical evidence. Geochim Cosmochim Acta 68:2223–2243
Park SC, Yun ST, Chae GT, Lee SK (2002) Hydrogeochemistry of shallow groundwaters in western coastal area of Korea: a study on seawater mixing in coastal aquifers. J KoSSGE 7:63–77
Park SC, Yun ST, Chae GT, Yoo IS, Shin KS, Heo CH, Lee SK (2005) Regional hydrochemical study on salinization of coastal aquifers, western coastal area of South Korea. J Hydrol 313:182–194
Rai NS (2004) Role of mathematical modeling in groundwater resources management. Sri Vinayaka Enterprises, Hyderabad
Russo D, Zaidel J, Laufer A (2001) Numerical analysis of flow and transport in a combined heterogeneous vadose zone-groundwater system. Adv Water Resour 24:49–62
Sivan O, Yechieli Y, Herut B, Lazar B (2005) Geochemical evolution and timescale of seawater intrusion into the coastal aquifer of Israel. Geochim Cosmochim Acta 69:579–592
Song SH, Lee JY, Park N (2007) Use of vertical electrical soundings to delineate seawater intrusion in a coastal area of Byunsan, Korea. Environ Geol 52:1207–1219
Sukhija BS, Varma VN, Nagabhushanam P, Reddy DV (1996) Differentiation of paleomarine and modern seawater intruded salinities in coastal groundwaters (of Karaikal and Tanjavur, India) based on inorganic chemistry, organic biomarker fingerprints and radiocarbon dating. J Hydrol 174:173–201
Tutmez B (2009) Assessing uncertainty of nitrate variability in groundwater. Ecol Inf 4:42–47
Ven Beek CGEM, Breedveld RJM, Juhasz-Holterman M, Oosterhof A, Stuyfzand PJ (2009) Cause and prevention of well bore clogging by particles. Hydrogeol 17:1877–1886
Vengosh A, Rosenthal E (1994) Saline groundwater in Israel: its bearing on the water crisis in the country. J Hydrol 156:389–430
Vengosh A, Spivack AJ, Artzi Y, Ayalon A (1999) Geochemical and boron, strontium, and oxygen isotopic constraints on the origin of the salinity in groundwater from the mediterranean coast of Israel. Water Resour Res 35:1877–1894
Won JH, Lee JY, Kim JW, Koh GW (2006) Groundwater occurrence in Jeju Island, Korea. Hydrogeol J 14:532–547
Zhou X, Chen M, Ju X, Wang J (2000) Numerical simulation of sea water intrusion near Beihai, China. Environ Geol 40:223–233
Acknowledgments
The chemical data used in this study were collected as “investigation of seawater intrusion” project by MAF and KRC. Comments of two anonymous reviewers greatly improved the initial manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, Y., Lee, JY., Kim, JH. et al. National scale evaluation of groundwater chemistry in Korea coastal aquifers: evidences of seawater intrusion. Environ Earth Sci 66, 707–718 (2012). https://doi.org/10.1007/s12665-011-1278-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1278-3