Abstract
Considerable developments in nuclear medicine imaging technology have occurred in the past decade, with SPECT/CT obtained on hybrid gamma cameras with in-line CT proving to be a powerful diagnostic tool that allows for accurate anatomic localization and characterization of radioactivity distributions. SPECT/CT has substantially improved the interpretation of radioiodine scintigraphy for evaluation of well-differentiated thyroid cancer, using both post-therapeutic and diagnostic 131-I administered activities. SPECT/CT contributes to risk stratification and completion of staging by improved characterization of N and M scores. The information obtained from SPECT/CT impacts patient management and contributes important prognostic information. The new technology of SPECT/CT has significant potential to change the current management protocols and guidelines in thyroid cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Thyroid cancer
- SPECT/CT
- Radioiodine scan
- Pre-ablation scan
- Diagnostic radioiodine scan
- Post-therapy scan
- Radioiodine SPECT/CT
- 131-I SPECT/CT
5.1 Introduction
Thyroid cancer is the most common endocrine malignancy in adults, with 52,890 estimated new cases (12,720 M:F 40,170) and 2180 deaths reported in the United States for 2020 [1]. The incidence of thyroid cancer continues to rise, with a 2.4-fold increase in incidence since 1975, based largely upon detection of small (≤2 cm) tumors, which represents 87% of newly diagnosed cases [2, 3]. Based on histology, thyroid cancers are characterized as 80.2% papillary, 11.4% follicular, 3.1% Hurthle cell (or oxyphil), 3.5% medullary, and 1.7% anaplastic [4]. The 5-year survival rates for well-differentiated thyroid cancer (WDTC), which includes papillary thyroid cancer, follicular, and Hurthle cell histologies, are 99.8% for localized tumors, 97.0% with regional metastases, and 57.3% with distant metastases [5]. In general, stage for stage, the prognosis for papillary thyroid cancer and follicular thyroid cancer is similar; however, certain histologic subtypes of papillary thyroid cancer (such as tall cell variant, columnar cell variant, and diffuse sclerosing variant) and highly invasive follicular thyroid cancer have a worse prognosis [6].
Initial therapy for thyroid cancer includes near-total or total thyroidectomy with or without prophylactic or therapeutic central compartment neck dissection to remove the primary tumor and involved cervical lymph nodes [7]. New ATA guidelines permit withholding surgery or performing only lobectomy for selective patient population as well [7]. Therapeutic lateral neck compartmental dissection is performed for patients with metastatic lateral cervical lymphadenopathy [8,9,10,11]. Cervical nodal metastases are present in 20–50% of patients at initial diagnosis, even with small <1.0 cm intra-thyroidal tumors (microcarcinomas) [12,13,14,15]. The completeness of surgical resection is an important determinant of outcome, because residual metastatic lymph nodes represent the most common site of disease persistence or recurrence [16, 17]. After initial diagnosis, staging and risk stratification are used to individualize treatment decisions, inform on prognosis for an individual patient, decide on the use of postoperative 131-I therapy, and determine the frequency and intensity of follow-up [7].
The most commonly used staging system in thyroid cancer is the TNM staging of the American Joint Committee on Cancer/International Union against Cancer, currently in its eighth edition [7, 18]. Because this staging schema was developed to predict risk for death—not for recurrence—and does not take into account several independent prognostic variables, the American Thyroid Association (ATA) has developed a three-level risk stratification model for thyroid cancer [7]. Radioiodine (131-I) ablation is recommended for distant metastases, a primary tumor that is grossly invasive or larger than 4 cm, and selected patients with 1–4-cm tumors confined to the thyroid, with documented nodal metastases or other high-risk features. The ATA guidelines recommend against 131-I ablation for unifocal or multifocal microcarcinomas without high-risk features [7, 19, 20]. Surveillance of WDTC is usually performed using a combination of radioiodine scintigraphy, neck ultrasound, biochemical thyroglobulin (Tg) levels, and 18-F fluorodeoxyglucose (FDG) PET imaging.
5.2 Radioiodine SPECT/CT
Radionuclide imaging as traditionally performed with planar imaging using 131-I has suboptimal spatial resolution, and image quality is further degraded by septal penetration by energetic 364 keV gamma emissions. The paucity of anatomical information on radioiodine scans makes interpretation challenging; similarly, lesion localization with SPECT is also difficult and not used routinely. Furthermore, diagnostic CT has had a limited role in evaluation of WDTC due to the necessity to avoid iodinated contrast and the frequency of nodal metastases in non-enlarged cervical lymph nodes (<10 mm). Despite these individual limitations, the synergistic combination of functional and anatomical information provided by integrated SPECT/CT has been found to have many advantages over traditional planar imaging in various clinical settings. Optimal co-registration of tomographic volumes of data obtained by gamma-cameras with inline CT, with the patient in the same bed position, allows precise anatomic localization of radioactive foci. Additional benefits include CT-based attenuation correction and morphologic information from unenhanced CT with reduced milliampere seconds (mAs) and kilovoltage (kV) settings.
A growing number of studies confirm that radioiodine 131-I SPECT/CT is a powerful diagnostic tool, overcoming many limitations encountered with planar imaging interpretation [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. Several excellent reviews of the clinical applications of hybrid 131-I/123-I SPECT/CT for imaging thyroid cancer provide context and outline the advantages of SPECT/CT imaging [41, 48,49,50,51,52]. 131-I SPECT/CT has been performed both at the time of first radioablation after total thyroidectomy and during long-term imaging surveillance, using either diagnostic activities between 37 and 450 MBq or post-therapy activities ranging from 1.1 to 8.1+ GBq. Advantages of SPECT/CT applied either selectively or routinely to planar scintigraphy studies have been consistently reported, with precise anatomic localization of foci of radioactivity allowing more accurate assignment of the findings as benign (thyroid remnants or normal physiologic distribution), or metastases in cervical nodes or at distant sites. One of the strengths of 131-I SPECT/CT is to substantially reduce the equivocal findings frequently reported on planar imaging. When unusual radioiodine biodistributions are encountered and a physiologic mimic of disease is suspected, SPECT/CT clarifies the interpretation of planar images, thereby avoiding false-positive diagnoses. SPECT/CT can solve difficult diagnostic interpretations and reveal metastatic lesions to unexpected sites or tissues. Due to CT-based attenuation correction, occasionally SPECT/CT can reveal more foci of pathologic activity as compared to planar studies. An integrated assessment of the size and iodine avidity of metastatic lesions based on the analysis of CT and SPECT components of the hybrid SPECT/CT study provides information about the likelihood of response to 131-I therapy and guides management decisions on alternative therapeutic options such as surgical excision for large metastases or external-beam radiation therapy for non-resectable and non–iodine-avid metastatic deposits.
5.3 Early Use of Radioiodine SPECT/CT
The use of radioiodine SPECT/CT in thyroid cancer was first reported by Even-Sapir et al. in a subgroup of 4 of 27 patients in whom SPECT/CT imaging was performed to evaluate endocrine neoplasms [53]. Subsequently, multiple studies have reported the incremental diagnostic value of SPECT/CT in patients with WDTC. The initial reports described the advantages of SPECT/CT applied to post-therapy 131-I scintigraphy: in a study evaluating co-registration of separately acquired SPECT and CT data with the aid of external fiducial markers, combined SPECT/CT improved diagnostic evaluation compared with SPECT alone in 15 of 17 patients (88%) [42]. Similarly, in a series of 25 patients with post-therapy 131-I scans, SPECT/CT improved diagnostic interpretation compared with planar images in 44% of radioactive foci, resulting in a change in management in 25% of patients [32]. In a study of 71 patients, of whom 54 had post-therapy imaging and 17 had diagnostic 131-I imaging, Tharp et al. described the incremental diagnostic value for SPECT/CT over planar imaging in 57% of patients [36]. One clear advantage of SPECT/CT reported by early investigators and confirmed in subsequent studies is its ability to substantially reduce the number of equivocal foci seen on planar imaging alone. Chen et al. reported that SPECT/CT accurately characterized 85% of foci considered inconclusive on planar imaging, resulting in altered management for 47% patients [25]. Aide et al. analyzed 55 patients studied with post-therapy 131-I scans, finding 29% indeterminate results on planar imaging and only 7% with SPECT/CT. Of the 16 patients with indeterminate planar scans, reclassification with SPECT/CT as positive or negative for disease correlated with the success or failure of radioiodine treatment at follow-up [22]. Our own experience has been primarily with diagnostic pre-ablation 131-I SPECT/CT performed after total thyroidectomy and prior to the first radioablation. In this setting, pre-ablation 131-I scans with SPECT/CT are used to complete postsurgical staging and guide subsequent 131-I therapy. In the neck region, we found incremental value of SPECT/CT over planar imaging in 53/130 (41%) of neck foci and described the typical appearances of thyroglossal duct remnant and thyroid bed remnant, which have long been recognized on planar imaging but are often difficult to distinguish from neck nodal metastases with confidence in the absence of SPECT/CT data [40, 41] (Fig. 5.1).
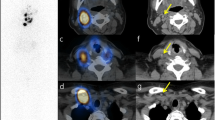
Pre-ablation 131-I SPECT/CT imaging in a 65-year-old-woman with a 1.2 cm papillary thyroid cancer with capsular and vascular invasion, no extra-thyroidal extension, and positive surgical margins; no pathologic evidence for nodal disease (0/3 central nodes submitted); pT1b N0 Mx Stage I. Planar anterior (a) and posterior (b) images depict two foci of neck activity (arrows) corresponding on SPECT/CT (c–e) to soft tissue in bilateral thyroid bed (arrows) compatible with remnant thyroid tissue. However, more inferiorly, the SPECT/CT demonstrates two enlarged, partially calcified lymph nodes in the left lower neck (g, h, arrows), consistent with partially calcified metastatic cervical level IV lymph nodes, restaging: T1b N1b M0, Stage IV A disease. SPECT/CT is able to detect residual non–iodine-avid metastatic disease in patients with elevated thyroglobulin levels out of proportion to the focal activity seen on radioiodine scan. The patient was referred for surgical resection of non-iodine cervical nodal metastases. SPECT/CT can distinguish remnant thyroid tissue from nodal metastases
5.4 Utility of Post-therapy Radioiodine SPECT/CT
The majority of published reports on the utility of SPECT/CT are based on hybrid imaging performed on post-therapy scans following administration of therapeutic 131-I dosages. Maruoka et al. studied 147 patients with post-therapy 131-I SPECT/CT imaging. Compared to planar whole-body scans, SPECT/CT clarified all 25 equivocal interpretations in the neck region as either regional lymph nodes or thyroid remnant. For distant foci, SPECT/CT corrected the interpretation in 21/52 (40%) foci, identifying both metastases and benign physiological mimics, including 20 foci that were equivocal on planar imaging [28].
Kohlfuerst et al. reported on the impact of post-therapy SPECT/CT in a group of 41 patients: in 33 patients with neck lesions, SPECT/CT changed the N status in 12 (36.4%) and led to a treatment change in 8 (24.2%); in 19 patients with lesions distant from the neck, SPECT/CT changed the M status in 4 (21.1%) and led to a treatment change in 2 (10.5%); and for the entire group of 41 patients, SPECT/CT led to a treatment change in 10 (24.4%) [27]. Schmidt et al. published a study on nodal staging in 57 patients who underwent planar imaging and SPECT/CT after the first radioablation and concluded that SPECT/CT determines lymph nodal status more accurately than planar imaging: 6 of 11 lesions previously considered nodal metastases on planar imaging were reclassified as benign, and 11 of 15 lesions considered indeterminate on planar imaging were reclassified as nodal metastases; SPECT/CT provided information that clarified nodal status in 20 of 57 patients (35%), which resulted in a change in risk stratification in 25% [34]. Furthermore, the same group determined that the information obtained with 131-I SPECT/CT performed at the first radioablation can predict the occurrence or persistence of iodine-avid cervical nodal metastases in subsequent follow-up: 94% of nodal metastatic deposits smaller than 0.9 mL were eliminated after radioablation, whereas nodal metastases exceeding this size were less likely to completely resolve with 131-I therapy [33]. Consequently, the size information obtained on CT data from the SPECT/CT study can be used to guide patient selection for excision of larger metastatic deposits. SPECT/CT improved anatomic localization of radioactive foci seen on planar post-therapy scans in 21% of cases, as demonstrated by Wang et al. in a study of 94 patients; in addition, SPECT/CT identified new metastatic foci unsuspected on planar imaging in 7% of patients. Most importantly, the additional information obtained with SPECT/CT resulted in reconsideration of the therapeutic approach in 22 of 94 patients (23%) [37].
The use of post-therapy SPECT/CT at the first radioablation has provided information on the incidence of nodal metastases in patients with T1 tumors (≤2 cm, limited to the thyroid). The most commonly occurring papillary thyroid cancer in the United States is now microcarcinoma (T1a tumors, ≤1.0 cm); 45% of tumors in older patients (age ≥45 years) and 34% of tumors in younger patients (age <45 years) are microcarcinomas [54]. Although the long-term outcome of papillary microcarcinoma is excellent, it frequently spreads to cervical lymph nodes, as documented in 40.9% of cases in a series of 445 patients [14], and may occasionally metastasize to distant sites [55,56,57,58]. In a large, bicentric study of 151 patients, using a combination of nodal staging based on histopathology (pN1) (in 46% patients who underwent surgical neck dissection) and SPECT/CT imaging information (in 54% patients who did not undergo neck dissection), Mustafa et al. reported that nodal metastases occurred in 26% of T1 tumors and in 22% of microcarcinomas [30].
The effect of post-therapy 131-I SPECT/CT on American Thyroid Association (ATA) risk classification was assessed by Grewal et al. in a group of 148 intermediate- and high-risk patients (as initially determined based on clinical and histopathology criteria); 74% of patients underwent a first radioablation after total thyroidectomy, while 26% exhibited a rising trend in thyroglobulin levels and received 131-I therapy for treatment of presumed recurrent or metastatic disease. SPECT/CT changed nodal status in 15% of the postsurgical patients and in 21% of patients suspected of recurrent tumor. Based on the SPECT/CT findings, the ATA risk classification changed due to upstaging in 7 of 109 patients (6.4%). Importantly, review of the CT images of the SPECT/CT study identified non–iodine-avid metastases in 32 of 148 patients (22%) and permitted lesion size determination. Additional imaging studies were avoided in 48% of patients [43]. Ciappucinni et al. demonstrated that post-ablation scintigraphy (planar and SPECT/CT) has prognostic value for predicting the therapeutic outcome of patients with thyroid cancer after total thyroidectomy and first radioablation. In 170 patients followed for a median of 29 months (range, 1.5–4.5 years) after initial 131-I ablative therapy, 32 (19%) patients presented with persistent or recurrent disease in subsequent follow-up: 18 with nodal metastases, 8 with distant metastases, and 6 with both nodal and distant metastases. In all patients free of disease at follow-up evaluations, initial post-ablative SPECT/CT was negative or equivocal for disease [26]. However, SPECT/CT was positive for disease in 78% of patients identified with persistent or recurrent disease at follow-up; post-therapy 131-I whole body scan (WBS) was negative in 22% with persistent or recurrent disease due to the presence of non–iodine-avid metastases, also reported by other groups in 20–30% of patients [43, 59]. The authors conclude that post-ablation scintigraphy (planar WBS and SPECT/CT) has 78% sensitivity and 100% specificity for predicting recurrent or persistent disease and is the sole independent prognostic variable for disease-free survival [26]. In a group of 42 patients studied with dual-phase post-therapy scans (performed at 3 and 7 days after therapeutic 131-I administration), SPECT/CT improved the interpretation of planar images regarding characterization of focal radioiodine accumulations as benign or malignant [44].
In a recent study with 323 patients, Szujo et al. evaluated the role of post-radioiodine therapy SPECT/CT of patients with WDTC in early-risk classification and in prediction of late prognosis. They performed both whole-body planar and neck/chest/abdomen SPECT/CT 3–6 days after the first radioactive iodine treatment. The patients were reevaluated 9–12 months later as well as at the end of follow-up (median 37 months). On the post-radioiodine therapy SPECT/CT, lymph node, lung, and bone involvement were detected in 22% of the patients including 61, 13, and 5 patients, respectively, resulting in early reclassification of 115 cases (36%). No evidence of disease was found in 251 cases at 9–12 months after radioiodine treatment and 269 patients at the end of follow-up. To predict residual disease at the end of follow-up, the sensitivities, specificities, and diagnostic accuracies of the current risk classification systems and SPECT/CT were: ATA: 77%, 47%, and 53%; ETA: 70%, 62%, and 64%; SPECT/CT: 61%, 88%, and 83%, respectively. Based on the study, the accuracy of post-radioiodine SPECT/CT outweighs that of the currently used ATA and ETA risk classification systems in the prediction of long-term outcome of DTC [60].
On a recent prospective study done in Greece, Malamitsi et al. aim to evaluate diagnostic accuracy of post-ablation SPECT/CT as compared to whole-body planar scan in N and M staging. The group also compared SPECT/CT to planar WBS to predict relapse of papillary thyroid cancer by following up the patients for 5 years. On the total number of abnormal uptake, WBS gave 27 false-positive (7.76%) and 23 false-negative (6.61%) results. On cross tabulation of NM stage through WBS and SPECT/CT, the study showed only 24 patients with N0M0 stage and 6 patients with N1M0 stage were classified correctly in the same stage by both methods. Considering N stage, there were 8 cases of upstaging from N0 to N1 and 14 cases of downstaging on SPECT/CT from N1 to N0. Considering M stage, there were 5 cases of upstaging from M0 to M1 and 2 cases of downstaging from M1 to M0. The agreement between the two methods was very low. The accuracy in determining NM stage was low on WBS (51.72%), while that of SPECT/CT 100%, respectively. There was a statistically significant difference in relapse by NM stage when assessed with SPECT/CT (p = 0.033), while with WBS this difference was not significant (p = 0.209) [46] (Table 5.1).
Table 5.1 summarizes the results of studies reporting on the use of post-therapy 131-I scintigraphy with SPECT/CT.
5.5 Utility of Diagnostic and Pre-ablation Radioiodine SPECT/ CT
As emphasized on Martinique Principles, diagnostic radioactive iodine imaging has been shown to contribute to staging and risk stratification, especially by detection of unsuspected lymph node and distant metastases [20]. Surveillance diagnostic 131-I planar imaging is considered to have sensitivity for disease detection ranging between 45% and 75% (depending on the activity dose administered) and a high specificity of 96% and 100% [35, 36, 61,62,63,64]. Another radioiodine isotope, iodine-123 (123-I), may also be used for diagnostic imaging, with the advantages of a lower energy gamma emission and less likelihood for thyroidal tissue stunning. 123-I photon energy of 159 keV is better suited to modern gamma camera equipment; using low-energy, high-resolution collimators permit acquisition of high-quality images. Drawbacks of the use of 123-I are higher costs and a short half-life of 13 h, precluding multiday imaging and increasing the complexity of dosimetry calculations. Barwick et al. demonstrated in a group of 79 consecutive patients studied with surveillance 123-I planar imaging, SPECT, and SPECT/CT that SPECT/CT provided additional diagnostic information in 42% patients and provided further characterization in 70% foci seen on planar images. The authors calculated the diagnostic performance of SPECT/CT compared with planar scans and SPECT alone: planar studies demonstrated a sensitivity of 41%, specificity of 68%, and accuracy of 61%; SPECT studies demonstrated a sensitivity of 45%, specificity of 89%, and accuracy of 78%; SPECT/CT provided significant improvement in specificity, with a calculated sensitivity of 50%, specificity of 100%, and accuracy of 87% (the study group included 11 patients with non–iodine-avid disease demonstrated on anatomic imaging and elevated thyroglobulin levels) [23].
As with post-therapy 131-I scans, the incremental value of SPECT/CT as compared with planar WBS in the diagnostic or pre-ablative setting, results from improved identification and interpretation of focal uptake, correct anatomic localization and characterization of radioactive foci, and precise differentiation between metastatic lesions versus benign uptake in thyroid remnant tissue or physiologic radioiodine distribution in normal organs. Menges et al. reported on the results of 139 scans with SPECT/CT performed in 123 patients: 82 studies were performed for diagnostic follow up evaluation (follow-up diagnostic scans) after radioablation and 57 studies were performed after 131-I therapy (post-therapy scans). The sensitivity of planar and SPECT/CT images were both 62%, with no additional detection of iodine-avid lesions on SPECT/CT compared to planar [29]. However, when non-iodine-avid disease in 27 patients was included in the analysis, SPECT/CT improved sensitivity from 61% to 74%. The main benefit of SPECT/CT was significantly higher specificity than the planar images, 94% versus 74%. We have observed that occasionally SPECT/CT reveals new foci of activity as compared to planar images, particularly when additional anatomic information based on CT dataset increases diagnostic certainty for identification of a new lesion (Fig. 5.2). In a group of 117 patients studied with surveillance planar WBS and SPECT/CT (of whom 108 patients underwent diagnostic 123-I and 9 patients underwent post-therapy 131-I scans), Spanu et al. demonstrated that SPECT/CT has incremental value over planar scanning in 67.8% of patients. SPECT/CT identified more foci of pathologic activity (158 foci on SPECT/CT compared with only 116 foci on planar imaging), changed the treatment approach in 35.6% of patients with disease, and led to avoidance of unnecessary 131-I therapy in 20% of patients without disease [35]. The use of SPECT/CT technology permitted identification of parapharyngeal metastases in 14 of 561 patients (2.5%); in this group, the presence of parapharyngeal metastases was associated with regional or distant metastases [31]. Identification of parapharyngeal lymph node metastases on SPECT/CT is particularly relevant, as these metastatic nodal deposits may remain undetected on routine neck ultrasound (Fig. 5.3).
Pre-ablation 131-I SPECT/CT imaging in a 56-year-old-man with a 7.5 cm minimally invasive follicular thyroid cancer in the left lobe with vascular and capsular invasion, extra-thyroidal extension, and negative surgical margins; with central compartment nodal disease (3+/3 central nodes submitted); pT3 N1a Mx, Stage III disease. Planar posterior chest image (a) depicts faint midline activity in the thorax (arrows) with an appearance suggestive of retained secretions in the esophagus. Correlative axial SPECT (b), axial CT (c), and fused SPECT/CT (d) demonstrate two adjacent foci in the thorax, the first is more intense and localizes to the esophagus compatible with retained secretions (long arrow); however, the second mild focus localizes to a sclerotic focus in the thoracic vertebra compatible with bone metastasis (short arrow). Restaging: T3 N1a M1, Stage IVC disease, leading to administration of 131-I therapy based on dosimetry calculations. Subsequent 131-I scan obtained at 6 months after 230 mCi (8.5 GBq) 131-I therapy documented interval resolution of bone metastasis (not shown)
Pre-ablation 131-I SPECT/CT scan in a 71-year-old-man with multifocal papillary thyroid cancer, the largest lesion measuring 3.0 cm in the left lobe, with extra-thyroidal extension, and negative surgical margins; 8+/21 central and 4+/8 left lateral metastatic lymph nodes were resected; pT3 N1b Mx, Stage IV A disease. Planar scan (a) depicts two foci of neck activity with the superior focus (arrow) corresponding on SPECT/CT (b–d) to a small iodine-avid cervical level IIA lymph node adjacent to the horn of the hyoid bone (arrows). Although pre-ablation I-131 scan did not alter the TNM Stage, the demonstration of an iodine-avid target in the left neck allowed selection of a medium 131-I activity for treatment, instead of low-dose 131-I remnant ablation. The more inferior neck focus was localized to the thyroid bed compatible with remnant thyroid tissue. SPECT/CT allows accurate identification of the epicenter of uptake for precise anatomic lesion localization
In a group of 53 postsurgical patients studied with SPECT/CT at the first radioablation (47 with pre-ablation scans, and 6 with post-therapy scans), Wong et al. demonstrated incremental diagnostic value for SPECT/CT over planar imaging for interpretation of 47.6% of foci, as follows: in 53 of 130 neck activity foci (41%) and 17 of 17 distant foci (100%), SPECT/CT provided clear anatomic lesion localization and lesion size measurement for predicting the likelihood of response to 131-I therapy. Rapid exclusion of physiologic activity or contamination resulted in elimination of equivocal interpretations on planar scans. Reader confidence increased for interpretation of 104 of 147 foci (71%) seen on planar images after the review of SPECT/CT [40]. This study also demonstrated that SPECT/CT imaging using low diagnostic 131-I activity (37 MBq, 1 mCi) is feasible and produces images of excellent diagnostic quality. The same investigators used pre-ablation 131-I scans with SPECT/CT to complement clinical and histopathological data in order to complete staging and risk stratification before radioablation [39]. The patients were staged according to the TNM system using three levels of sequential information: histopathology and chest radiography, planar WBS, and SPECT/CT. The patients were restaged according to the findings on 131-I scintigraphy (planar alone, followed by combined planar and SPECT/CT information). Compared with histopathologic analysis, planar imaging and SPECT/CT changed the postsurgical thyroid cancer stage for 21% of patients. Identification of unsuspected nodal and distant metastases resulted in prescription of appropriately higher therapeutic activities whereas demonstration of residual thyroid tissue (i.e., thyroid remnant) in the absence of high-risk histopathologic features reduced the 131-I ablative dose or eliminated the need for ablation. Information obtained from the diagnostic pre-ablation planar WBS and SPECT/CT scans changed the prescribed radioactivity in 58% patients, as compared with the initially proposed therapy based on histopathologic risk stratification alone.
In a larger cohort of 320 patients studied with pre-ablation 131-I scans, the impact of diagnostic pre-ablation 131-I planar and SPECT/CT scintigraphy on N and M scores, and TNM stage was assessed in younger age <45 years, n = 138 (43%) and older age ≥45 years, n = 182 (57%) patients [65]. Subgroup analysis regarding the detection of regional and distant metastases for small tumors was also performed in 49 patients (15%) with T1a (size ≤1.0 cm) and 67 patients (21%) with T1b (size >1.0 and ≤2.0 cm).The study demonstrated that in younger patients pre-ablation 131-I scans with SPECT/CT detected distant metastases in 5/138 patients (4%), and nodal metastases in 61/138 patients (44%), including unsuspected nodal metastases in 24 of 63 (38%) patients initially assigned pN0 or pNx. In older patients, distant metastases were detected in 18/182 patients (10%), and nodal metastases in 51/182 patients (28%), including unsuspected nodal metastases in 26 of 108 (24%) patients initially assigned pN0 or pNx. Pre-ablation scans with SPECT/CT detected distant metastases in 2/49 (4%) T1a and 3/67 (4.5%) T1b patients.
For the entire group of 320 patients, pre-ablation 131-I scans with SPECT/CT detected regional metastases in 35%, and distant metastases in 8% of patients. Information acquired with pre-ablation scans changed staging in 4% of younger, and 25% of older patients. In summary, pre-ablation planar and SPECT/CT imaging contributed to staging in thyroid cancer and facilitated the selective use of 131-I therapy for targeting regional and distant metastases identified with radioiodine scintigraphy.
Diagnostic pre-ablation 131-I scans are unlikely to underestimate the extent of metastatic disease, as demonstrated by the high concordance rate (94%) between pre-ablation and post-therapy scans. In only 2% of patients did post-therapy scans reveal metastatic lesions which upstaged disease status as compared to diagnostic 131-I scan. When SPECT/CT technology is applied to pre-ablation WBS scans, the information obtained completes postsurgical staging before management decisions regarding 131-I therapy are made. Performing diagnostic radioiodine scans with planar and SPECT/CT imaging provides the opportunity to identify patients with unsuspected regional and distant metastases, defines the target of 131-I therapy, and permits adjustment of prescribed therapeutic radioactivity and dosimetry calculations, when high-dose 131-I therapy is considered [39, 66]. Avoidance of unnecessary 131-I therapy is equally important for patients in whom residual and/or metastatic disease has been excluded. SPECT/CT improves characterization of focal central neck activity as benign thyroid remnant when pathology review demonstrates no evidence of extra-thyroidal tumor extension and negative surgical excision margins, justifying omission of radioiodine administration in such patients [49] (Table 5.2).
Table 5.2 summarizes the results of studies reporting on the use of diagnostic radioiodine scintigraphy with SPECT/CT.
5.6 SPECT/CT Evaluation of Unusual Radioactive Distributions
Unusual patterns of radioiodine biodistribution which mimic metastatic disease are well recognized and may raise a diagnostic dilemma for interpretation of planar radioiodine scintigraphy [67, 68]. Physiological radioiodine activity is seen in salivary glands, mucosa, breast, thymus, stomach, bowel, kidneys, and bladder. Salivary and urinary contamination should also be considered when unexpected focal activity distribution occurs. SPECT/CT is an excellent diagnostic tool for rapid evaluation of suspected physiologic mimics and can accurately localize radioiodine distribution to salivary glands, dental fillings, nasolacrimal secretions, retrosternal goiter, esophageal or airway secretions, hiatal hernias, bowel diverticula, breast, skin contamination, and benign uptake related to radioiodine retention in cysts, bronchiectasis, esophageal diverticulum, thymus, spermatocele, functional ovarian cyst, benign struma ovarii, teratoma, or menstruating uterus [38, 40, 69,70,71,72,73,74,75,76,77,78]. Several authors have demonstrated the utility of SPECT/CT for the evaluation of cryptic findings on radioiodine scintigraphy [24] including a wide range of physiological mimics of disease [79]. In a pictorial review of radioiodine scintigraphy for thyroid cancer evaluation, we have demonstrated the utility of SPECT/CT imaging for problem-solving in difficult interpretation cases, including salient examples of focal radioiodine uptake in the neck, thorax, and body, where benign and pathological etiologies are virtually indistinguishable on planar imaging, yet easily characterized with SPECT/CT [80].
Several reports outline the usefulness of SPECT/CT for revealing unusual metastatic lesions in the liver, kidney, central nervous system, erector spinae muscle, and rectus abdominis muscle [81,82,83,84,85]. SPECT/CT is useful for evaluation of distant metastatic disease [25, 27, 35, 37, 40, 86, 87]. SPECT/CT can confirm osseous and pulmonary sites of metastases, providing additional anatomical diagnostic information to guide management decisions. In the thorax, SPECT/CT can precisely localize malignancy to bone (ribs or spine), lung, or mediastinal lymph nodes (Fig. 5.4).
Pre-ablation 131-I scan in a 19-year-old-woman with unifocal papillary thyroid cancer (2 cm tumor in the left lobe with focal lymphovascular invasion and extrathyroidal extension) associated with lymph nodal metastases in the left neck at presentation: 3+/17 lymph nodes resected in the surgical specimen of total thyroidectomy; T3 N1b Mx, Stage I disease. Planar anterior (a) and posterior (b) images demonstrate the presence of pulmonary metastatic disease. T1b N1b M1, Stage II disease. Correlative coronal, axial CT (c and e), and fused SPECT/CT (d and f) images reveal multifocal chest activity corresponding to multiple bilateral pulmonary nodules, the largest of which measures 5 mm. Identification of unsuspected pulmonary metastases on pre-ablation scan permitted administration of high-dose 131-I therapy based on dosimetry calculations
5.7 Changes to Clinical Management
Most of the changes in the new eighth edition of the American Joint Committee on Cancer/International Union against Cancer will be to downstage a significant number of patients into lower stages and aim to reflect their low risk of dying from thyroid cancer more accurately. It is important to remember that the risk of death from thyroid cancer does not match the risk of recurrence in many patients. Therefore, more individualized and accurate assessments of the risk of recurrence and dying from the disease should have a substantial impact on both initial therapeutic decision-making (e.g., extent of thyroid surgery, need for radioactive iodine treatment, and/or need for TSH suppressive therapy) and on follow-up management strategies [18]. In conjunction with pre-ablation or post-therapy scans, SPECT/CT has been used for completion of thyroid cancer staging, impacting clinical management, and providing important prognostic information. It is a powerful diagnostic tool which allows precise localization of radioactivity and improved characterization of benign and malignant radioactivity distributions compared to planar imaging. Routine implementation of SPECT/CT imaging with radioiodine scintigraphy may lead to reassessment of current management protocols in thyroid cancer. The use of radioiodine SPECT/CT has been reported to change clinical management in a significant numbers of patients, both when used routinely on all consecutive patients and when used on selected patients with inconclusive planar images. Potential changes in management include decisions such as administration or withholding radioiodine treatment, referral for surgical reintervention and guiding the extent of surgery, patient selection for external-beam radiation therapy, and the use of alternative imaging strategies such as 18F-FDG PET/CT when non–iodine-avid metastatic disease is suspected. Depending on the clinical context, the timing of the radioiodine scan (pre-ablation or post-therapy), and the therapeutic protocols at each institution, a change in management has been reported in 11–58% patients in various studies [24, 26, 28, 33, 36, 37, 40, 51].
Radioiodine SPECT/CT also provides prognostic information regarding success of radioiodine treatment as determined by clinical follow-up and surveillance diagnostic 131-I whole-body imaging. Schmidt et al. using short-term follow-up reported that almost all patients with negative post-therapy SPECT/CT (60/61 patients) had a negative 5-month diagnostic 131-I scan, and even the majority with positive post-therapy SPECT-CT (17/20 patients) still had negative diagnostic scan [33]. The authors identified a neck nodal volume of <0.9 mL on SPECT/CT to be highly likely to respond to 131-I therapy and commented that surgical reintervention for resection of lymph nodal metastases of this size would be excessive. The positivity or negativity for disease as determined by post-therapy SPECT/CT correlated more closely to success or failure of radioiodine treatment than the planar imaging findings [22]. Identification of unsuspected distant metastases in 4% of younger patients and 10% of older patients was associated with an upstage in disease status and led to prescription of therapeutic 131-I activity based on dosimetric calculations. In patients for whom 131-I therapy is considered based on histopathologic prognostic factors, pre-ablation 131-I scintigraphy can detect metastases in normal-size cervical lymph nodes (which may not appear suspicious on postoperative neck ultrasonography), can identify pulmonary micro-metastases (which are too small to be detected on routine chest X-ray and may remain undetected on computed tomography), and can diagnose bone metastases at an early stage before cortical disruption is identified on bone X-rays. The information obtained with diagnostic radioiodine studies has the potential to impact management decisions, e.g., whether to proceed or omit therapeutic 131-I administration, refer for surgical debulking prior to 131-I therapy, or perform additional imaging studies when non–iodine-avid disease is demonstrated by negative radioiodine scans but elevated thyroglobulin levels. The benefits of obtaining this information at an early postoperative time point in the disease process are not negligible, because 131-I therapy is most effective for smaller metastatic deposits, and early identification of regional and distant metastases is important for successful therapy [33, 88] (Fig. 5.5).
Diagnostic 131-I SPECT/CT imaging in a 56-year-old-woman with 1.2 cm papillary thyroid cancer without capsular invasion, no extra-thyroidal extension, and negative surgical margins; no pathologic evidence for nodal disease (0/3 central nodes submitted); pT1b N0 M0, Stage I disease. Planar scan (a) depicts focal neck activity characterized on SPECT/CT as remnant thyroid tissue. There is a faint focus of uptake in the right lower thorax on planar anterior whole-body image (arrow) Correlative axial SPECT (b), CT (c), and fused SPECT/CT (d) reveal focal activity in the liver corresponding to a 0.8 cm hypodense lesion (arrows) also confirmed on liver US and MRI, consistent with hepatic metastasis. Restaging: T1b N0 M1, Stage IVC disease. Identification of unsuspected hepatic metastasis on pre-ablation scan permitted administration of high-dose 131-I therapy instead of low-dose 131-I remnant ablation. Subsequent 131-I scan obtained at 6 months after 200 mCi (7.4 GBq) 131-I therapy confirmed interval resolution of liver metastasis (not shown)
To determine the effectiveness of a management strategy based on radiotheragnostics principles, Avram et al. analyzed their routine evaluation with postoperative diagnostic 131-I SPECT/CT scans for completion of staging and risk stratification and individualized 131-I therapy guided by diagnostic scan findings in addition to surgical pathology and postoperative stimulated thyroglobulin (Tg) levels on 320 patients. The study showed that the patient-individualized therapeutic strategy is associated with good clinical outcomes with complete response after a single postoperative 131-I treatment in 88% of patients presenting with histopathology risk factors and regional metastases and in 42% patients with distant metastatic disease [89].
5.8 Comparison to Other Imaging Modalities
Qiu et al. studied patients with suspected bone metastases and compared the test performance of 99mTc-methylene diphosphonate (MDP) bone scan, 131-I SPECT/CT, and FDG PET. They found that 131-I SPECT/CT sensitivity 93% and specificity 97% were higher than FDG PET sensitivity 86% and specificity 94%, and both were higher than bone scintigraphy sensitivity 79% and specificity 84%. FDG PET positivity was associated with poor prognostic features, survival rate of 69% compared to 93% in FDG PET negative patients [90].
Oh et al. studied the performance of WBS, SPECT/CT, and FDG PET. The 131-I SPECT/CT sensitivity of 65% and specificity of 95% were similar to FDG PET sensitivity of 61% and specificity of 98%, and both were higher than planar whole-body scan sensitivity 65% and specificity 55%. Subgroup analysis found that 131-I-SPECT/CT sensitivity was better in patients with a single 131-I treatment, whereas FDG PET had higher sensitivity patients with multiple 131-I challenges [91]. On a recent study performed with Bulzacka and Macarawicz, they evaluated the utility of neck US for detection of thyroid remnants and compared the diagnostic capability of identification of lymph node metastases in neck with post-therapy 131-I SPEC/CT and neck ultrasound in patients after thyroidectomy for differentiated thyroid cancer They found 341 positive foci in 150 patients (97.4%) by SPECT/CT and 213 corresponding positive finding in 118 patients (76.6%) by US. Ultrasound detected 30–46% of iodine uptake foci in superior lateral regions, 49% in pyramidal lobe/thyroglossal duct area (both p < 0.05), 74–77% in inferior lateral regions, and 22% in isthmus (both p > 0.05). They concluded that US is less sensitive than post-therapy 131-I SPECT/CT scans for remnant detection in patients after thyroidectomy, especially for remnants located above the lower margin of thyroid cartilage [92].
5.9 Utility of Radioiodine SPECT/CT for Lesional Dosimetry
Future directions for the use of hybrid radioiodine imaging involve the use of SPECT/CT to perform lesion-specific dosimetry. Lesion radioiodine uptake and retention can be quantified on SPECT, and tumor volume can be measured on the CT component of the SPECT/CT study, permitting calculation of radiation absorbed dose to tumor. Follow-up with SPECT/CT can be used to determine therapeutic responses and assess tumor shrinkage. An example of this approach has been reported in a patient with a large skull metastasis causing infringement on the brain [93]. Other authors have demonstrated the feasibility of patient-specific three-dimensional dosimetry using multiple SPECT/CT images, in which the patient’s own anatomy and spatial distribution of radioactivity over time are factored into the calculation of radiation absorbed dose to tumor (lesion dosimetry) or to an organ of interest (organ dosimetry) [94]. The CT images of SPECT/CT studies are used to provide the density and composition of each voxel for use in a Monte Carlo calculation and to define organs or regions of interest for computing spatially averaged doses. The longitudinal series of SPECT images is used to perform time integration of activity in each voxel and to obtain the cumulated activity per voxel [94]. The goal of patient-specific voxel-based absorbed dose calculations is to improve prediction of the biologic effects of radionuclide therapy.
5.10 Disadvantages and Limitations of SPECT/CT
Disadvantages of SPECT/CT include additional imaging time and possible patient discomfort and claustrophobia from lying in a confined position for approximately 20 min in the tightly enclosed space of the SPECT/CT gantry, and additional radiation exposure from the CT component of the study (1–4 mSv with each acquisition) [95]. SPECT/CT has an axial field of view limited to 40 cm in the current imaging systems; therefore, evaluation of both neck and distant radioactive foci may require two separate SPECT/CT acquisitions [95]. Analysis of benefit and potential risk should be performed on an individual basis in the young female and particularly in the pediatric population [96]. Kim studied 13 children with 131-I/123-I diagnostic SPECT/CT reporting that the SPECT/CT helped clarify equivocal foci in the neck, helping to inform decisions including not to proceed to 131-I treatment [97].
Recognizing the limitations of SPECT/CT is important: the spatial resolution of SPECT is limited by the partial-volume effect in small lesions; although metastases in normal-sized neck lymph nodes are frequently diagnosed, micro-metastatic lesions cannot be detected with SPECT/CT. Similarly, SPECT/CT is insensitive for the detection of residual locally invasive thyroid cancer after surgery, unless there is gross residual tumor volume or anatomic findings of invasion. Therefore, SPECT/CT radioiodine studies must always be interpreted in the context of the surgical pathology report, which clarifies the presence or absence of tumor invasion into local structures and completion of surgical resection. These elements are of critical importance when one is interpreting the significance of paratracheal central neck activity as benign thyroid remnant versus residual disease. When pathologic review of the surgical resection specimen of total thyroidectomy demonstrates no evidence of tumor extrathyroidal extension and surgical excision margins are negative, focal paratracheal central neck activity can be characterized as a benign thyroid remnant. Non–iodine-avid disease, which occurs in 20–30% of differentiated thyroid cancer [59, 98], may remain undetected on SPECT/CT and lead to false-negative interpretations (Fig. 5.1). The possibility of non–iodine-avid disease needs to be considered in the context of Hurthle cell thyroid cancer, papillary thyroid cancer with unfavorable histology (e.g., tall cell, columnar, or cribriform variants), and poorly differentiated thyroid cancer (such as trabecular, insular, or solid variants). Therefore, the review of histopathology and biochemical data—thyroglobulin, thyrotropin, free thyroxine—remains essential for accurate interpretation of the findings on radioiodine planar and SPECT/CT scintigraphy.
5.11 Conclusion
Considerable progress in the interpretation of radioiodine scintigraphy in thyroid cancer has been achieved by the introduction of SPECT/CT. SPECT/CT is a powerful diagnostic tool that allows accurate anatomic localization and characterization of radioiodine foci and has substantially improved the interpretation of classic radioiodine scintigraphy. SPECT/CT reduces the number of equivocal radioiodine foci encountered in the neck and body and allows more precise characterization of the etiology (benign vs. malignant) of focal radioiodine uptake seen on WBS, contributing to completion of staging in thyroid cancer by improved characterization of N and M scores. Accurate staging and risk stratification are important for thyroid cancer management as they determine the prognosis for survival and risk of recurrence and guide therapeutic decisions and intensity of surveillance. The additional information obtained with SPECT/CT impacts management in a significant number of patients. The new technology of SPECT/CT has changed the field and may lead to reassessment of current management protocols and guidelines in thyroid cancer.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–7.
Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. 2015;25(10):1127–36.
Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see comments]. Cancer. 1998;83(12):2638–48.
Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62(2):118–28.
Volante M, Landolfi S, Chiusa L, Palestini N, Motta M, Codegone A, et al. Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns: a clinicopathologic study of 183 patients. Cancer. 2004;100(5):950–7.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
Gemsenjager E, Perren A, Seifert B, Schuler G, Schweizer I, Heitz PU. Lymph node surgery in papillary thyroid carcinoma. J Am Coll Surg. 2003;197(2):182–90.
Goropoulos A, Karamoshos K, Christodoulou A, Ntitsias T, Paulou K, Samaras A, et al. Value of the cervical compartments in the surgical treatment of papillary thyroid carcinoma. World J Surg. 2004;28(12):1275–81.
Kupferman ME, Patterson M, Mandel SJ, LiVolsi V, Weber RS. Patterns of lateral neck metastasis in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130(7):857–60.
Asimakopoulos P, Nixon IJ, Shaha AR. Differentiated and medullary thyroid cancer: surgical management of cervical lymph nodes. Clin Oncol (R Coll Radiol). 2017;29(5):283–9.
Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-prognostic significance of lymph node metastasis and multifocality. Cancer. 2003;98(1):31–40.
Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996;5(1):43–63.
Mercante G, Frasoldati A, Pedroni C, Formisano D, Renna L, Piana S, et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid. 2009;19(7):707–16.
Scheumann GF, Gimm O, Wegener G, Hundeshagen H, Dralle H. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg. 1994;18(4):559–67; discussion 67–8.
Shah MD, Hall FT, Eski SJ, Witterick IJ, Walfish PG, Freeman JL. Clinical course of thyroid carcinoma after neck dissection. Laryngoscope. 2003;113(12):2102–7.
Wang TS, Dubner S, Sznyter LA, Heller KS. Incidence of metastatic well-differentiated thyroid cancer in cervical lymph nodes. Arch Otolaryngol Head Neck Surg. 2004;130(1):110–3.
Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (eighth edition): what changed and why? Thyroid. 2017;27(6):751–6.
Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2018;68(1):55–63.
Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. Controversies, consensus, and collaboration in the use of (131)I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid. 2019;29(4):461–70.
Lee SW. SPECT/CT in the treatment of differentiated thyroid cancer. Nucl Med Mol Imaging. 2017;51(4):297–303.
Aide N, Heutte N, Rame JP, Rousseau E, Loiseau C, Henry-Amar M, et al. Clinical relevance of single-photon emission computed tomography/computed tomography of the neck and thorax in postablation (131)I scintigraphy for thyroid cancer. J Clin Endocrinol Metab. 2009;94(6):2075–84.
Barwick T, Murray I, Megadmi H, Drake WM, Plowman PN, Akker SA, et al. Single photon emission computed tomography (SPECT)/computed tomography using Iodine-123 in patients with differentiated thyroid cancer: additional value over whole body planar imaging and SPECT. Eur J Endocrinol. 2010;162(6):1131–9.
Blum M, Tiu S, Chu M, Goel S, Friedman K. I-131 SPECT/CT elucidates cryptic findings on planar whole-body scans and can reduce needless therapy with I-131 in post-thyroidectomy thyroid cancer patients. Thyroid. 2011;21(11):1235–47.
Chen L, Luo Q, Shen Y, Yu Y, Yuan Z, Lu H, et al. Incremental value of 131I SPECT/CT in the management of patients with differentiated thyroid carcinoma. J Nucl Med. 2008;49(12):1952–7.
Ciappuccini R, Heutte N, Trzepla G, Rame JP, Vaur D, Aide N, et al. Postablation (131)I scintigraphy with neck and thorax SPECT-CT and stimulated serum thyroglobulin level predict the outcome of patients with differentiated thyroid cancer. Eur J Endocrinol. 2011;164(6):961–9.
Kohlfuerst S, Igerc I, Lobnig M, Gallowitsch HJ, Gomez-Segovia I, Matschnig S, et al. Posttherapeutic (131)I SPECT-CT offers high diagnostic accuracy when the findings on conventional planar imaging are inconclusive and allows a tailored patient treatment regimen. Eur J Nucl Med Mol Imaging. 2009;36(6):886–93.
Maruoka Y, Abe K, Baba S, Isoda T, Sawamoto H, Tanabe Y, et al. Incremental diagnostic value of SPECT/CT with 131I scintigraphy after radioiodine therapy in patients with well-differentiated thyroid carcinoma. Radiology. 2012;265(3):902–9.
Menges M, Uder M, Kuwert T, Schmidt D. 131I SPECT/CT in the follow-up of patients with differentiated thyroid carcinoma. Clin Nucl Med. 2012;37(6):555–60.
Mustafa M, Kuwert T, Weber K, Knesewitsch P, Negele T, Haug A, et al. Regional lymph node involvement in T1 papillary thyroid carcinoma: a bicentric prospective SPECT/CT study. Eur J Nucl Med Mol Imaging. 2010;37(8):1462–6.
Qiu ZL, Xu YH, Song HJ, Luo QY. Localization and identification of parapharyngeal metastases from differentiated thyroid carcinoma by 131I-SPECT/CT. Head Neck. 2011;33(2):171–7.
Ruf J, Lehmkuhl L, Bertram H, Sandrock D, Amthauer H, Humplik B, et al. Impact of SPECT and integrated low-dose CT after radioiodine therapy on the management of patients with thyroid carcinoma. Nucl Med Commun. 2004;25(12):1177–82.
Schmidt D, Linke R, Uder M, Kuwert T. Five months’ follow-up of patients with and without iodine-positive lymph node metastases of thyroid carcinoma as disclosed by (131)I-SPECT/CT at the first radioablation. Eur J Nucl Med Mol Imaging. 2010;37(4):699–705.
Schmidt D, Szikszai A, Linke R, Bautz W, Kuwert T. Impact of 131I SPECT/spiral CT on nodal staging of differentiated thyroid carcinoma at the first radioablation. J Nucl Med. 2009;50(1):18–23.
Spanu A, Solinas ME, Chessa F, Sanna D, Nuvoli S, Madeddu G. 131I SPECT/CT in the follow-up of differentiated thyroid carcinoma: incremental value versus planar imaging. J Nucl Med. 2009;50(2):184–90.
Tharp K, Israel O, Hausmann J, Bettman L, Martin WH, Daitzchman M, et al. Impact of 131I-SPECT/CT images obtained with an integrated system in the follow-up of patients with thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2004;31(10):1435–42.
Wang H, Fu HL, Li JN, Zou RJ, Gu ZH, Wu JC. The role of single-photon emission computed tomography/computed tomography for precise localization of metastases in patients with differentiated thyroid cancer. Clin Imaging. 2009;33(1):49–54.
Wong KK, Avram AM. Posttherapy I-131 thymic uptake demonstrated with SPECT/CT in a young girl with papillary thyroid carcinoma. Thyroid. 2008;18(8):919–20.
Wong KK, Sisson JC, Koral KF, Frey KA, Avram AM. Staging of differentiated thyroid carcinoma using diagnostic 131I SPECT/CT. AJR Am J Roentgenol. 2010;195(3):730–6.
Wong KK, Zarzhevsky N, Cahill JM, Frey KA, Avram AM. Incremental value of diagnostic 131I SPECT/CT fusion imaging in the evaluation of differentiated thyroid carcinoma. AJR Am J Roentgenol. 2008;191(6):1785–94.
Wong KK, Zarzhevsky N, Cahill JM, Frey KA, Avram AM. Hybrid SPECT-CT and PET-CT imaging of differentiated thyroid carcinoma. Br J Radiol. 2009;82(982):860–76.
Yamamoto Y, Nishiyama Y, Monden T, Matsumura Y, Satoh K, Ohkawa M. Clinical usefulness of fusion of 131I SPECT and CT images in patients with differentiated thyroid carcinoma. J Nucl Med. 2003;44(12):1905–10.
Grewal RK, Tuttle RM, Fox J, Borkar S, Chou JF, Gonen M, et al. The effect of posttherapy 131I SPECT/CT on risk classification and management of patients with differentiated thyroid cancer. J Nucl Med. 2010;51(9):1361–7.
Wakabayashi H, Nakajima K, Fukuoka M, Inaki A, Nakamura A, Kayano D, et al. Double-phase (131)I whole body scan and (131)I SPECT-CT images in patients with differentiated thyroid cancer: their effectiveness for accurate identification. Ann Nucl Med. 2011;25(9):609–15.
Spanu A, Nuvoli S, Gelo I, Mele L, Piras B, Madeddu G. Role of diagnostic (131)I SPECT/CT in long-term follow-up of patients with papillary thyroid microcarcinoma. J Nucl Med. 2018;59(10):1510–5.
Malamitsi JV, Koutsikos JT, Giourgouli SI, Zachaki SF, Pipikos TA, Vlachou FJ, et al. I-131 postablation SPECT/CT predicts relapse of papillary thyroid carcinoma more accurately than whole body scan. In Vivo. 2019;33(6):2255–63.
Lee CH, Jung JH, Son SH, Hong CM, Jeong JH, Jeong SY, et al. Risk factors for radioactive iodine-avid metastatic lymph nodes on post I-131 ablation SPECT/CT in low- or intermediate-risk groups of papillary thyroid cancer. PLoS One. 2018;13(8):e0202644.
Avram AM. Role of SPECT/CT, versus traditional practices, in individualizing treatment of thyroid carcinoma: reply. J Nucl Med. 2012;53(11):1819.
Avram AM. Radioiodine scintigraphy with SPECT/CT: an important diagnostic tool for thyroid cancer staging and risk stratification. J Nucl Med. 2012;53(5):754–64.
Barwick TD, Dhawan RT, Lewington V. Role of SPECT/CT in differentiated thyroid cancer. Nucl Med Commun. 2012;33(8):787–98.
Toubert ME, Vija L, Vercellino L, Banayan S, Faugeron I, Berenger N, et al. Additional diagnostic value of hybrid SPECT-CT systems imaging in patients with differentiated thyroid cancer. Am J Clin Oncol. 2014;37(3):305–13.
Xue YL, Qiu ZL, Song HJ, Luo QY. Value of (131)I SPECT/CT for the evaluation of differentiated thyroid cancer: a systematic review of the literature. Eur J Nucl Med Mol Imaging. 2013;40(5):768–78.
Even-Sapir E, Keidar Z, Sachs J, Engel A, Bettman L, Gaitini D, et al. The new technology of combined transmission and emission tomography in evaluation of endocrine neoplasms. J Nucl Med. 2001;42(7):998–1004.
Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 2011;21(3):231–6.
Hay ID, Grant CS, van Heerden JA, Goellner JR, Ebersold JR, Bergstralh EJ. Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery. 1992;112(6):1139–46; discussion 46–7.
Hay ID, Hutchinson ME, Gonzalez-Losada T, McIver B, Reinalda ME, Grant CS, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144(6):980–7; discussion 7–8.
Ross DS, Litofsky D, Ain KB, Bigos T, Brierley JD, Cooper DS, et al. Recurrence after treatment of micropapillary thyroid cancer. Thyroid. 2009;19(10):1043–8.
Strate SM, Lee EL, Childers JH. Occult papillary carcinoma of the thyroid with distant metastases. Cancer. 1984;54(6):1093–100.
Mian C, Barollo S, Pennelli G, Pavan N, Rugge M, Pelizzo MR, et al. Molecular characteristics in papillary thyroid cancers (PTCs) with no 131I uptake. Clin Endocrinol. 2008;68(1):108–16.
Szujo S, Sira L, Bajnok L, Bodis B, Gyory F, Nemes O, et al. The impact of post-radioiodine therapy SPECT/CT on early risk stratification in differentiated thyroid cancer; a bi-institutional study. Oncotarget. 2017;8(45):79825–34.
Delbeke D, Schoder H, Martin WH, Wahl RL. Hybrid imaging (SPECT/CT and PET/CT): improving therapeutic decisions. Semin Nucl Med. 2009;39(5):308–40.
Filesi M, Signore A, Ventroni G, Melacrinis FF, Ronga G. Role of initial iodine-131 whole-body scan and serum thyroglobulin in differentiated thyroid carcinoma metastases. J Nucl Med. 1998;39(9):1542–6.
Lind P, Kohlfurst S. Respective roles of thyroglobulin, radioiodine imaging, and positron emission tomography in the assessment of thyroid cancer. Semin Nucl Med. 2006;36(3):194–205.
van Sorge-van Boxtel RA, van Eck-Smit BL, Goslings BM. Comparison of serum thyroglobulin, 131I and 201Tl scintigraphy in the postoperative follow-up of differentiated thyroid cancer. Nucl Med Commun. 1993;14(5):365–72.
Avram AM, Fig LM, Frey KA, Gross MD, Wong KK. Preablation 131-I scans with SPECT/CT in postoperative thyroid cancer patients: what is the impact on staging? J Clin Endocrinol Metab. 2013;98(3):1163–71.
Van Nostrand D, Aiken M, Atkins F, Moreau S, Garcia C, Acio E, et al. The utility of radioiodine scans prior to iodine 131 ablation in patients with well-differentiated thyroid cancer. Thyroid. 2009;19(8):849–55.
Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627–35.
Shapiro B, Rufini V, Jarwan A, Geatti O, Kearfott KJ, Fig LM, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115–32.
Dumcke CW, Madsen JL. Usefulness of SPECT/CT in the diagnosis of intrathoracic goiter versus metastases from cancer of the breast. Clin Nucl Med. 2007;32(2):156–9.
Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688–9.
Rachinsky I, Driedger A. Iodine-131 uptake in a menstruating uterus: value of SPECT/CT in distinguishing benign and metastatic iodine-positive lesions. Thyroid. 2007;17(9):901–2.
Jammah AA, Driedger A, Rachinsky I. Incidental finding of ovarian teratoma on post-therapy scan for papillary thyroid cancer and impact of SPECT/CT imaging. Arq Bras Endocrinol Metabol. 2011;55(7):490–3.
Mebarki M, Menemani A, Medjahedi A, Boualou F, Slama A, Ouguirti S, et al. Radioiodine accumulation in a giant ovarian cystadenofibroma detected incidentally by 131-I whole body scans. Case Rep Radiol. 2012;2012:295617.
Sakahara H, Yamashita S, Suzuki K, Imai M, Kosugi T. Visualization of nasolacrimal drainage system after radioiodine therapy in patients with thyroid cancer. Ann Nucl Med. 2007;21(9):525–7.
Savas H, Wong KK, Saglik B, Hubers D, Ackermann RJ, Avram AM. SPECT/CT characterization of oral activity on radioiodine scintigraphy. J Clin Endocrinol Metab. 2013;98(11):4410–6.
Albano D, Motta F, Baronchelli C, Lucchini S, Bertagna F. 131I whole-body scan incidental uptake due to spermatocele. Clin Nucl Med. 2017;42(11):901–4.
Jang HY, Kim BH, Kim WJ, Jeon YK, Kim SS, Kim YK, et al. False-positive radioiodine uptake in a functional ovarian cyst in a patient treated with total thyroidectomy for papillary cancer. Intern Med. 2013;52(20):2321–3.
Rizzo A, Zagaria L, Perotti G, Annunziata S, Salvatori M. Accumulation of 131I in esophageal diverticulum diagnosed by SPECT/CT. Clin Nucl Med. 2018;43(11):e410–e1.
Oh JR, Ahn BC. False-positive uptake on radioiodine whole-body scintigraphy: physiologic and pathologic variants unrelated to thyroid cancer. Am J Nucl Med Mol Imaging. 2012;2(3):362–85.
Glazer DI, Brown RK, Wong KK, Savas H, Gross MD, Avram AM. SPECT/CT evaluation of unusual physiologic radioiodine biodistributions: pearls and pitfalls in image interpretation. Radiographics. 2013;33(2):397–418.
Agriantonis DJ, Hall L, Wilson MA. Utility of SPECT/CT as an adjunct to planar whole body I-131 imaging: liver metastasis from papillary thyroid cancer. Clin Nucl Med. 2009;34(4):247–8.
Aide N, Lehembre E, Gervais R, Bardet S. Unusual intratracheal metastasis of differentiated thyroid cancer accurately depicted by SPECT/CT acquisition after radioiodine ablation. Thyroid. 2007;17(12):1305–6.
Qiu ZL, Luo QY. Erector spinae metastases from differentiated thyroid cancer identified by I-131 SPECT/CT. Clin Nucl Med. 2009;34(3):137–40.
von Falck C, Beer G, Gratz KF, Galanski M. Renal metastases from follicular thyroid cancer on SPECT/CT. Clin Nucl Med. 2007;32(9):751–2.
Zhao LX, Li L, Li FL, Zhao Z. Rectus abdominis muscle metastasis from papillary thyroid cancer identified by I-131 SPECT/CT. Clin Nucl Med. 2010;35(5):360–1.
Barwick T, Murray I, Megadmi H, Drake W, Plowman PN, Akker S, et al. SPECT/CT using Iodine-123 in patients with differentiated thyroid cancer—additional value over whole body planar imaging and SPECT. Eur J Endocrinol. 2010;162(6):1131–9.
Wong KK, Sisson JC, Koral KF, Frey KA, Avram AM. Staging of differentiated thyroid carcinoma using diagnostic 131-I SPECT-CT. AJR Am J Roentgenol. 2010;195(3):730–6.
Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–9.
Avram AM, Esfandiari NH, Wong KK. Preablation 131-I scans with SPECT/CT contribute to thyroid cancer risk stratification and 131-I therapy planning. J Clin Endocrinol Metab. 2015;100(5):1895–902.
Qiu ZL, Xue YL, Song HJ, Luo QY. Comparison of the diagnostic and prognostic values of 99mTc-MDP-planar bone scintigraphy, 131I-SPECT/CT and 18F-FDG-PET/CT for the detection of bone metastases from differentiated thyroid cancer. Nucl Med Commun. 2012;33(12):1232–42.
Oh JR, Byun BH, Hong SP, Chong A, Kim J, Yoo SW, et al. Comparison of (1)(3)(1)I whole-body imaging, (1)(3)(1)I SPECT/CT, and (1)(8)F-FDG PET/CT in the detection of metastatic thyroid cancer. Eur J Nucl Med Mol Imaging. 2011;38(8):1459–68.
Bulzacka I, Makarewicz J. Postablative 131I SPECT/CT is much more sensitive than cervical ultrasonography for the detection of thyroid remnants in patients after total thyroidectomy for differentiated thyroid cancer. Clin Nucl Med. 2020;45(12):948–53.
Sisson JC, Dewaraja YK, Wizauer EJ, Giordano TJ, Avram AM. Thyroid carcinoma metastasis to skull with infringement of brain: treatment with radioiodine. Thyroid. 2009;19(3):297–303.
Song H, He B, Prideaux A, Du Y, Frey E, Kasecamp W, et al. Lung dosimetry for radioiodine treatment planning in the case of diffuse lung metastases. J Nucl Med. 2006;47(12):1985–94.
Buck AK, Nekolla SG, Ziegler SI, Drzezga A. SPECT/CT. J Nucl Med. 2008;49(8):1305–19.
Gelfand MJ, Lemen LC. PET/CT and SPECT/CT dosimetry in children: the challenge to the pediatric imager. Semin Nucl Med. 2007;37(5):391–8.
Kim HY, Gelfand MJ, Sharp SE. SPECT/CT imaging in children with papillary thyroid carcinoma. Pediatr Radiol. 2011;41(8):1008–12.
Min JJ, Chung JK, Lee YJ, Jeong JM, Lee DS, Jang JJ, et al. Relationship between expression of the sodium/iodide symporter and 131I uptake in recurrent lesions of differentiated thyroid carcinoma. Eur J Nucl Med. 2001;28(5):639–45.
Acknowledgments
None.
Disclosure Statement: The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Avram, A.M., Savas, H. (2022). SPECT/CT for Thyroid Cancer Imaging. In: Ahmadzadehfar, H., Biersack, HJ., Herrmann, K. (eds) Clinical Applications of SPECT-CT. Springer, Cham. https://doi.org/10.1007/978-3-030-65850-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-65850-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65849-6
Online ISBN: 978-3-030-65850-2
eBook Packages: MedicineMedicine (R0)