Abstract
Nanoparticles (NPs) have been variously used in the areas of agriculture, biomedicine, nanotechnology, human health, and biological applications. The potential human and animal exposures to NPs are increasing as the range of consumer products containing engineered NPs increases over time. Thus, there has been a surge in research focusing on the interactions and toxicity of NPs with living organisms. The toxicity of metallic nanoparticles such as Ag has been extensively studied in bacterial cell studies. However, one of the most widely used nanomaterials includes metal oxide NPs. Metal oxide NPs are generally utilized for their semiconductor properties. NPs such as TiO2, ZnO, and CuO have been used in different applications including photocatalysis, energy storage, and biomedical applications. This chapter focuses on the interactions of TiO2, ZnO, and CuO NPs and their adverse effects on live organisms and cells. Special emphasis is given in this chapter to discuss recent results reported on the interactions of algal cells: Diatoms/plankton species, bivalve species, fish species. Some studies on the exposure of human cell lines and mammalian models are also included. The common effects of metal oxide nanoparticles exhibited across the species of organisms include, predominately, oxidative stress and decreased cellular viability. To gain a comprehensive picture of NP toxicity, the identification of surfactant toxicity is important, as many of the stabilizing agents used in NP synthesis are highly toxic. Finally, the stability of the NPs is important to the discussion of NP toxicity. Stability of NPs and NP toxicity can be seen as the toxicological differences in the phases of TiO2. Rutile is the most thermodynamically stable form of TiO2 and is less toxic than the anatase form of TiO2. As well, CuO and ZnO NPs are sparingly soluble in aqueous solution, which can generate Cu2+ and Zn2+ ions in solution. Both Cu2+ and Zn2+ ions are toxic and complicate the interpretations of CuO and ZnO NP toxicity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction and Background
Nanostructured materials have been widely used in different applications including energy storage, catalysis, agriculture systems, and biomedicine. Nanomaterials (NMs) are an anomalous class of compounds that are known for their high toxicity when exposed to living organisms. The investigation of the behavior of toxic nanomaterials for human exposure (e.g., nanoparticles, nanoflakes, and nanorods) has attracted much attention in recent years due to the fact that there is no quantitative evaluation to address its effects on human health and the environment. The anomalous behavior of nanomaterials such as nanoparticles (NP) is originated by the fact that these materials have strained surfaces, and generally have an oxidized layer of atoms at the surface (Zhang et al. 2014; Strasser et al. 2010; Casaletto et al. 2006). NMs have both natural and anthropogenic sources (Griffin et al. 2018; Rogers 2016; Jeevanandam et al. 2018; Currie and Silica in Plants 2007; Hough et al. 2008). NPs are commonly found in the environment from natural sources, for example, nanoparticles such as SiO2, and Fe3O4 are commonly found in soils and plants (Griffin et al. 2018; Rogers 2016; Jeevanandam et al. 2018; Currie and Silica in Plants 2007; Hough et al. 2008). However, concerns of toxicity can raise awareness when using nanoparticles of anthropogenic origins in consumer products (Vance et al. 2015; Fröhlich and Roblegg 2012; Calzolai et al. 2012; Tulve et al. 2015). For example, currently there are more than 1800 consumer products based on NPs used in the market (Vance et al. 2015). Some of the applications of these products are intended for direct use in human health such as skin protection or treatment; for example, new cosmetics are being produced compounds based on NPs such as zinc oxide (ZnO) nanoparticles used in sunscreens (Vance et al. 2015; Fröhlich and Roblegg 2012; Calzolai et al. 2012; Tulve et al. 2015). Health drinks, antibacterial, antimicrobial products are also being produced from noncompounds containing metallic NPs such as silver and copper (Vance et al. 2015; Fröhlich and Roblegg 2012; Calzolai et al. 2012; Tulve et al. 2015). A further example of the invasive application of NPs in consumer products is the generation of clothing impregnated with metallic NPs in the fabric in order to reduce the odor-producing bacteria (Tulve et al. 2015). However, the actual human and environmental health effects of NPs are not well understood. The literature on the toxicity of NPs is lacking and in some cases contradictory in nature. As time progresses, the risk of exposure to anthropogenic synthesized nanoparticles has been constantly increasing and may have unknown possible health effects. The toxic effect of these nanomaterials with respect to the effects on human health has not been systematically investigated.
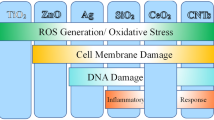
Based on the interaction studies of a large number of species, the main toxicity effects or routs of toxicity can be summarized as in Fig. 5.1.
Most of the investigations reported in this area discuss the toxicity effects of NPs on living organisms, which have been focused for the most part on bacterial studies. However, studies have been reported on the toxicity of NPs in eukaryotic and complex organisms such as algae and multicellular aquatic organisms, but a clear mechanism and the mode of nanomaterial actions are lacking. The generation of ROS species has been shown to occur in natural waters using photoactive nanoparticles; the generation of ROS has been shown to occur in algal cells (Li et al. 2003). The generation of ROS species produced from photoactive NPs can be exemplified in Table 5.1. The generation of radical species including ROS is the process that occurs in natural water systems in the presence of sunlight. Interestingly, the reactive species generated from these chemical reactions may be amplified in the presence of NPs. Radical species are also formed within cells of living organisms and there are mechanisms in place to reduce the damage from the generation of the ROS and other radial species. However, an overproduction of radical species in cells can overwhelm the defense mechanisms causing cellular damage and death.
The enhancement of the generation of radical species using photoactive NPs has been well documented in the literature (Parke and Sapota 1996; Nosaka and Generation 2017; Sharma et al. 2019). The application of NPs in photocatalytic destruction of organic chemicals has also been achieved through applying the ability of NPs to generate ROS species or through the electron transfers from the NPs (Deng et al. 2015; Le et al. 2015; Haldorai et al. 2014; Kumar et al. 2014).
In the studies on bacterial species, results show a high correlation between nanoparticles and their differential toxicity to different bacterial strains (Prabhu and Poulose 2012; Hwang et al. 2008; Durán et al. 2010; Fabrega et al. 2009; Greulich et al. 2012; Durán et al. 2016). Perhaps, the best example of NP toxicity in the literature is Ag-NP toxicity on E. coli as well as other common bacteria (Prabhu and Poulose 2012; Hwang et al. 2008; Durán et al. 2010; Fabrega et al. 2009; Greulich et al. 2012; Durán et al. 2016). These studies have shown that Ag in the NP form is typically more toxic than the dissolved Ag+ ion. Bacterial studies have focused on the bactericidal effects of metallic nanoparticles such as silver NPs. Silver nanoparticles have shown to be toxic to both gram-negative and gram-positive bacteria. It is interesting that nanoparticles affect both the gram-positive and -negative bacteria, and NPs have been shown to transport across the cell walls of bacteria. Figs. 5.2 and 5.3 show E. coli and Bacillus subtilis after treatment of sodium citrate (control group), citrate-stabilized Ag NPs, and silver ions, respectively (the TEM size bars represent 500 nm). As can be seen in the NP-treated bacteria, the cells look very different from both the AgNO3-treated and citrate-treated bacteria. The cell walls/cell membranes of the Ag NP-treated cells are difficult to see and are ill-defined.
The oxidative and reductive dissolution of NPs is well discussed in the literature (Schnippering et al. 2008; Misra et al. 2012; Wang et al. 2013; Ho et al. 2010). The dissolution of NPs in solution is especially well documented in metal oxide NPs, where the average size of NPs has been shown to change with time due to the reaction with dissolved species (Schnippering et al. 2008; Misra et al. 2012; Wang et al. 2013; Ho et al. 2010). The redox-based dissolution of NPs is due to the transfer of electrons from the surface of the NP to the dissolve species, which can be either organic or ionic in nature. Note that dissolution of metal oxides in solution has been well documented for different naturally occurring minerals such as hematite and magnetite as well as Iron (oxy)hydroxides (Sulzberger and Laubscher 1995; Borghi et al. 1991; Postma 1993). For example, the dissolution of NPs in solution has been shown to be enhanced in the presence of light generating ionic species, which may be more toxic to particular organisms in the water column (Shibata et al. 2004; Dasari et al. 2013).
However, there is a problem with determining the concentration of NPs in solution and making it comparable to the dissolved ion concentration. To further explore the differences, one must look at the traditional understanding of colloids in solution. A colloid has been traditionally defined as a particle ranging in size from 0.1 to 1000 nm, which is suspended in a medium, such as a solid in liquid (Gregory 2005). Interestingly, colloids have charged surfaces depending on their composition (e.g., structure and morphology) and can either be positive or negative (McDonogh et al. 1984; Zhong et al. 2004; Fafarman et al. 2011). Alternatively, using surfactants, NPs can have zero charge and very low dispersibility in water. The toxicity of chemical compounds can be linked to a few different factors; however, toxicity can be linked to dose, availability, and duration of exposure (Zhong et al. 2004; Fafarman et al. 2011).
2 Effect of Surfactant on Toxicity
Surfactants can play a vital role in the toxicity of nanoparticle and dispersion within the environment and subsequently within organisms. The earlier works on the synthesis of nanoparticles have reported the use of reducing agents such as citric acid, ascorbic acid, and sodium borohydride (Wuithschick et al. 2015; Kimling et al. 2006; Song et al. 2009). These reducing agents have been used to generate poly-dispersed mixtures of nanoparticles, and in some cases the reducing agents become surface coating agents. The geometry of nanoparticles can also affect their dispersion in a solution. Fig. 5.4 exhibits formation of geometrically regular nanoparticles using citrate. However, these geometries extend past the metallic NPs into the metal oxide as well as metal sulfide NPs (Grzelczak et al. 2008; Lim et al. 2007; Kitchens et al. 2005; Scarabelli et al. 2013; Rodriguez et al. 1996; Luis & Liz-Marzán 2002; Personick et al. 2011).
However, more recently the shift in nanoscience has been for the addition of surfactants to a solution to control the geometry of the resulting nanoparticle (Grzelczak et al. 2008; Lim et al. 2007; Kitchens et al. 2005; Scarabelli et al. 2013; Rodriguez et al. 1996; Luis & Liz-Marzán 2002; Personick et al. 2011). The desire to control the shape of nanoparticles can be related to the desired function of the nanoparticles. Nevertheless, the use of surfactants has one major drawback to their use, which is the coating of the nanoparticle surface with a chemical that may inhibit the way the particle interacts with the environment. Examples of a surface coated Au-NP with cetyltrimethylammonium bromide (CTAB) surfactant and citrate are shown in Fig. 5.5. The CTAB-extracted nanoparticles show a definite layer around the edge and the citrate-stabilized nanoparticle show a halo effect around the edge of the particle.
The development of surfactant-stabilized NPs has raised the following interesting question: What causes the toxicity of nanoparticles? In addition, the development of “biological methods” of NP synthesis for better biocompatibility of NPs raises even more questions that need to be answered about NP toxicity, the distribution of NPs in organisms, and the toxicity of the surfactant used for nanoparticle stabilization. As surfactants coat NPs, their ability to cross the cellular membrane increases, which may change the toxicity of the NP, or may cause carcinogenic effects. In addition, NP toxicity may be related to the stress of the organism and inability for the immune system to respond to pathogens.
The various nanomaterials currently synthesized or developed for human health-related applications include metal oxide nanoparticles, metallic nanoparticles, organic nanoparticles, nanofibers, and carbon nanoparticles. Moreover, there are many questions about NP toxicity and the validity of the toxicity, some particles show toxic effects in one organism but do not show the same in another organism. Current literature shows that the toxicity of NPs has been linked to the oxidation ability of the NPs, the ability to generate radical species such as the ROS and reactive nitrogen species (RNS) (Parke and Sapota 1996; Kimura et al. 2005; Hurst and Lymar 1997). Another important question about NP toxicity is the toxicity due to the NP itself or to dissolved ions in solution, or the generation of secondary species. NP toxicity has also been linked in some cases to the ability to attach to the cell walls in some organisms (Chwalibog et al. 2010; Hajipour et al. 2012). The focus of this book chapter is to highlight the toxicity of metal and metal oxide nanoparticles in different biological systems. The discussion of studies that have focused primarily on the accumulation or purely on the uptake of NPs by organisms is beyond the scope of this work.
3 Methodologies of Study
Perhaps one of the most important and yet least uniform parts of nanotoxicity studies is the methodology used in the synthesis (e.g., predicting and measuring toxicity). There are two basic ways to observe NP toxicity in biological systems: (1) using chemical techniques of analysis or (2) biological/cellular techniques. Each set of techniques has advantages and disadvantages when used to observe NP toxicity. Testing the toxicity of NPs can be broken down into in vitro and/or in vivo testing. The results of toxicity testing performed in vivo and in vitro do not necessarily align with each other. However, this may be due to the fact that there is no direct effect (rather, in direct effect) of NP toxicity on the organismal development or behavior. In fact, some of the earlier studies on NP-plant interactions have shown positive effects of NPs on plant growth (Gardea-Torresdey et al. 2002; Rico et al. 2014; Arora et al. 2012; Kumar et al. 2013). For example, the treatment of Medicago sp. with potassium tetrachloroaurate salt resulted in the generation of gold NPs inside the live plants (Gardea-Torresdey et al. 2002). However, the treated plants showed enhanced growth when compared to the control plants. Similarly, this effect was shown in wheat and Arabidopsis thaliana (Rico et al. 2014; Arora et al. 2012; Kumar et al. 2013). Alternatively, the generation of reactive oxygen species (ROS) and other radical species have been shown to be important in investigating the toxicity of some NP species. Perhaps the dissolved ions, stabilizing agents, and secondary generated ROS are more toxic than the NPs are to the organism (Lee et al. 2009; Bozich et al. 2014; Yasun et al. 2015).
4 Chemical and Physical Methods to Observe NPs in Biological Systems
Taking a broad overview of the characterization methods used for NPs, one can summarize them as follows: dynamic light scattering (DLS), UV-Vis, X-ray diffraction (XRD), transmission electron microscopy (TEM), atomic force microscopy (AFM), selected area electron diffraction (SAED), atomic emission spectroscopy (AES), electron energy loss spectroscopy (EELS), and X-ray photoemission spectroscopy (XPS). These techniques are utilized for the characterization of the NPs themselves, at the same time also used for the evaluation of changes in NPs after uptake. In addition, ICP-MS, ICP-AES, fluorescence spectroscopy, flow cytometry, and Neutron activation analysis (NAA) have been used to determine NPs uptake as well (Jansa and Huo 2012; Pauksch et al. 2014; Govindaraju et al. 2008; Sun et al. 2005; Raffi et al. 2008; Akhtar et al. 2012; Asharani et al. 2008; Tetard et al. 2008; Kerkmann et al. 2004; Saif et al. 2008; Absar et al. 2005; Hu et al. 2007; Abdel-Mohsen et al. 2013; Heike et al. 2014; Merrifield et al. 2018).
As technology advances, the ability to evaluate the effects of NPs on organisms also gets refined. In recent years, there have been tremendous instrumental advances specifically in the area of Inductively Coupled Plasma Mass Spectroscopy (ICP-MS) for NP analysis (Heike et al. 2014; Merrifield et al. 2018; Love et al. 2012; Marquis et al. 2009). The development of the Single Particle (SP) ICP-MS has given investigators the ability to characterize true NP solution concentrations. Furthermore, as SP-ICP-MS is available, it can be utilized to detect the difference between NPs and dissolved ions (Heike et al. 2014; Merrifield et al. 2018). In addition, the improvement in TEM techniques has also allowed for better imaging of NPs within cells (Love et al. 2012; Marquis et al. 2009). As well as TEM technology has advanced the ability to image and determine the phase of NPs in cells. The attachment of Selected SAED to TEM may allow, in the future, capturing the diffraction analysis of NPs in individual cells.
Fluorescence spectroscopy has also been utilized for the quantitative and qualitative determination of NPs in cells (Love et al. 2012; Marquis et al. 2009). The downside of the fluorescence microscopy method is the limitation of the quantification of the NPs that depends on the fluorescence properties of the NPs. The limitation can further exasperate by adding a fluorescence molecule to the particle, which may alter the uptake and NP behavior. To overcome this problem, spinning disk confocal microscopy was developed and has been used to monitor the movement of quantum dots within cells (Love et al. 2012; Marquis et al. 2009).
One of the most important issue to address NP toxicity is the reproducibility of the data reported on the same material by different research groups. The reproducibility problems sometimes can be related to the intrinsic properties of the NPs. For example, the attachment of a surfactant to a NP surface will change the global behavior of the NP such as the point of zero charge (PZC), and the interaction with the cellular walls and lipid membrane in cells (Love et al. 2012; Marquis et al. 2009). In addition, the surfactants can also change the agglomeration of the nanoparticles in solution, which can directly affect the transport of the NPs. Surfactants are commonly used as a stabilizer during the synthesis of the NPs to generate specific size range and geometrical shape.
5 Biochemical Assays for the Determination of NPs
Warheit et al. have developed a base set of toxicity tests using ultrafine TiO2 NPs (Warheit et al. 2007). The crystal size, chemical composition, and surface reactivity of the NPs were determined using different experimental techniques such as hazard tests: pulmonary assay, skin irritation, skin sensitization, oral toxicity, and eye irritation. Additional genotoxicity and aquatic screening experiments were also performed on the NPs to study their NPs behavior in organisms. However, when strictly discussing methodologies from the biochemical/ molecular biological point of view, one delves into cellular assays, which have been developed to assess the vitality of cells. Cellular assays include, but are not limited to, the assays of the following: metabolic activity, hemolysis, apoptosis and necrosis, exocytosis, cell proliferation, oxidative stress, immunogenicity, gene expression, and DNA damage (Love et al. 2012; Marquis et al. 2009).
Proliferation can be determined using assays through assessing different cell properties and/or processes including metabolic shifts, DNA synthesis, and the ability to form colonies. Metabolic processes and properties are assessed using either MMT, XXT, or WST-1 assays; membrane integrity can be measured using dye uptake such as Alamar blue or Thymidine incorporation. Necrosis can be determined using LDH to determine cellular material leakage or uptake of dyes such as trypan blue, neutral red, and propidium iodide. Apoptosis can be assayed through the uptake and transport of Annexin-V and DNA laddering among other techniques. The determination of the mechanisms or mode of action of NPs is generally done using DNA damage assays such as the studying of the fragmentation, and breakage of DNA (Love et al. 2012; Marquis et al. 2009). This can be assessed through Comet, CSE, and TUNEL (which can be utilized to study cellular apoptosis) techniques (Love et al. 2012; Marquis et al. 2009). The other major mechanism of NP toxicity, oxidative stress is determined through the determination of ROS, lipid peroxidation, lipid hydroperoxides, depletion of antioxidants, SOD activity, and the expression of SOD . These cellular properties are usually determined using the interaction with dyes, or fluorescence probes, or the formation of stable radical species (Love et al. 2012; Marquis et al. 2009).
For in vivo studies of whole organisms, more techniques become available to investigators, which allow for the determination of the effects of “long-term” exposure to NPs. The in vivo studies allow for the determination of EC50 or LD50 for treatment of animal with NPs. Furthermore, in vivo studies allow for the determination of the movement of NPs in an organism between organisms and allow for the determination of the toxicity of a given NP and the route of exposure through the three common routs of exposure inhalation, dermal contact, or through ingestion. To determine the effects of NPs on living organisms, researchers have utilized studies such as histopathology/histology to observe morphology changes in the organs of the test animal. Hematology and serum chemistry were used to investigate at the composition of blood and serum as well as the changes occurring after treatment with NPs. The distribution of the nanoparticles in the body is another aspect that has been studied, which can be achieved using X-ray or MRI imaging as well as the extraction of the organs and determination of the effects of the NPs. All the techniques utilized previously allow for a better or clearer picture of the distribution and transport of NPS in living organisms. These studies also provide valuable information of the effect of biological systems on the toxicity of NPs.
6 Metal Oxide NP Toxicity: Case Studies
6.1 TiO2 NP Toxicity
The toxicity of TiO2 NPs has been investigated extensively in many different organisms including algae, plants human cells, and fish as outlined in the following sections. The interest in TiO2 NP toxicity has been developed through its use and the application in consumer products such as sunscreens (Vance et al. 2015). TiO2 is found in three different crystal structures, anatase, brookite, and rutile, of which, rutile is the most thermodynamically stable form, followed by anatase, and then brookite. In sunscreens, there are commonly two forms of TiO2 present in the anatase and the rutile forms and are added for their UV absorption properties. Due to the low solubility of TiO2, the toxicity of the NPs appears to be a function of the NPs and not to solubilized ions in solution. The commonly observed toxic effects of TiO2 NPs on the different organisms are summarized below in Table 5.2.
7 TiO2 Toxicity in the Treatment of Algae
Algae, particularly microalgae, are simple organisms that are abundantly found in lakes, rivers, or on lands. The toxicity of nanoparticles in these organisms can be studied where the whole organism can be exposed to nanoparticles in the growth medium. When considering the toxicity of nanoparticles to algal species, it is a whole organism treatment being studied, whose treatment with TiO2 NPs appear to induce oxidative stress in the organisms and subsequently affect cell viability. Multiple studies on the toxicity of TiO2 NPs have been performed and are summarized in the following examples extracted from the literature.
Chen et al. investigated the toxic effects of TiO2 NP on unicellular green alga Chlamydomonas reinhardtii (Chen et al. 2012). The authors investigated TiO2 NPs with an average size range of 21 nm at varying concentrations from 0.1 mg/L to 100 mg/L. The exposure of the algae to the TiO2 showed inhibition of photosynthetic efficiency and cellular growth. However, the amount of chlorophyll (a) in the algae was not different than in the control samples but the carotenoids and chlorophyll (b) were observed to increase with increasing concentration. The authors also showed that as the concentration of TiO2 NPs was increased, the cells were damaged while the chloroplasts were degraded, and other organelles were reduced. It was noted that the TiO2 NPs were found inside the cells, coating the cell walls, and coating the cellular membranes. Along the same lines, Aruoja et al. investigated the toxicity of TiO2 nanoparticles on a microalgae Pseudokirchneriella subcapitata (Aruoja et al. 2009). The authors showed that bulk TiO2 had an EC50 of 35.9 mg Ti/l whereas the TiO2 NP had an EC50 equal to 5.83 mg Ti/L (Aruoja et al. 2009). The data showed almost a sixfold increase in the EC50 for the NP compared with the bulk crystal phase. The authors also showed that the TiO2 NPs formed characteristic aggregates that entrapped the algal cells. It was suggested that the toxicity of the TiO2 NPs occurred through either the generation of reactive oxygen species (ROS), the inactivation of the cells through a combination of TiO2 and visible light, or through the destruction of the cellular surface. However, the toxicity of the TiO2 NPs was caused by the generation of the ROS-mediated toxicity through the hydroxyl radical species (Aruoja et al. 2009). Lee and An studied TiO2 NP toxicity on green algae using irradiation from UV-A and UV-B light sources, visible light, as well as without irradiation (Lee and An 2013). The authors investigated the toxic effects of 21 nm TiO2 NPs on the green alga Pseudokirchneriella subcapitata , which is found in fresh water. It was found that as the concentration of NPs was increased, the algal growth rate was observed to decrease. These effects were not observed in the pre-irradiation conditions and attributed to the effects of the photo catalytic ability of the TiO2 NPs. Under visible light, the TiO2 nanoparticles showed EC50 of 2.53 under visible light; however, under UV-A light, the EC50 increased slightly to 3.00 and was 2.95 using UV-B irradiation (Lee and An 2013). The authors tied the toxic effects of the TiO2 nanoparticles to the formation of the superoxide ion by the TiO2 which caused observed effects in the algae. Sadiq et al. have investigated the toxic effects of TiO2 anatase NPs on Scenedesmus sp. and Chlorella sp. microalgal species (Sadiq et al. 2011). Inhibition of the growth for both microalgal species was observed. The alga species were treated with concentrations of 3–196 mg/L. The observed EC50 at 72 h were 16.12 for Chlorella sp. and 21.2 mg/L for Scenedesum sp. Through FTIR analysis of the samples, the authors observed the attachment of the nanoparticles to the cellular membrane during the study. Photocatalytic TiO2 Degausa P-25 (a mixture of anatase and rutile) mixtures have also been studied for their toxic effects on the marine algae Pseudokirchneriella subcapitata (Sadiq et al. 2011). The authors investigated dose response experiments, where lipid peroxidation, chlorophyll A concentration, as well as direct cell counts were measured for toxicity. The study showed an EC50 of 6.5 particles per cell, and it was discovered that the critical particle size ranged from 4 to 30 nm for detrimental effects to be observed. Further analysis of the samples by the authors using SEM showed agglomeration of the NPs on the surface of the cells in layers. Metzler et al. also indicated the NPs showing lipid peroxidation in conjunction with the surface coverage may have been the cause of the negative effects of the NPs (Metzler et al. 2011). Fu et al. investigated the effects of TiO2 nanoparticle on ROS production and inhibition of growth in freshwater algae (Fu et al. 2015). The authors used Pseudokirchneriella subcapitata (new name, Raphidocelis subcapitata) as the algal species and exposed these algae to TiO2 NPs with UV irradiation for 3 h with and without a UV filter. The effects of TiO2 NPs on algae pre-exposed to UV light were also investigated. The data showed that exposure to TiO2 NPs with and without UV filters decreased algal growth. It was also determined that the EC50 were 8.7 and 6.3 mg/L for the algae exposed to UV with and without filters, respectively (Fu et al. 2015). Finally, it was concluded from the study that the exposure to the TiO2 NPs the ROS species were not directly involved in the sublethal effects in the algae. Moreover, Li et al. studied the effects of TiO2 on alga1 species and the production of ROS species (Li et al. 2015). In that work, two algal species Karenia brevis and Skeletonema costatum were used where the algal species were exposed to TiO2 NPs with diameters in the range of 5–10 nm in the anatase phase. The authors observed that the TiO2 NPs were transported inside the cells. The growth of both algal species was inhibited with an EC50 of 10.69 and 7.37 for Karenia brevis and Skeletonema costatum after 72 h treatment, respectively. The effects on the algal species were attributed to the oxidative stress caused by ROS generation inside the algal cells. Inhibition of electron transport showed the ROS generation site was the chloroplast for the K. brevis (Li et al. 2015). Clement et al. investigated the toxicity of anatase and rutile TiO2 NPs on caldocerans, algae, and rotifers (Clément et al. 2013). The authors exposed the organisms to TiO2 nanoparticles with crystallite sizes of 15, 32, and 25 nm as determined using XRD. The authors also exposed the organisms to microparticles of anatase and rutile. The treatments consisted of exposing the organisms to 100 mg/L of a specific material for up to 72 hrs (Clément et al. 2013). Chlorella vulgaris at a TiO2 concentration of 100 mg/L 25 nm anatase and 1 um rutile with the average of 5.7% and 4.53% were observed, respectively. With D. magna, the authors performed tests at 48 and 72 h after exposure. Acute toxicity was observed and was directly related to the increase in particle concentrations (Clément et al. 2013). As the exposure time was extended to 72 h, it was determined that the EC50 was 1.30 mg/L, which was observed for the 15 nm anatase NPs. EC50 were greater when concentrations of 100 mg/L of the other nanoparticles were tested. More importantly, the anatase form was more toxic than the rutile form of the particles (Clément et al. 2013).
8 TiO2 Toxicity in the Treatment of Diatoms
Similar to other algae, plankton and diatoms show toxicity to TiO2 exposures. As diatoms are single-celled algae, they are also treated as a whole organism in the environmental toxicity assays. As in other forms of organisms, TiO2 NPs induce oxidative stress in diatoms affecting their survival and growth. Clement et al. extended his nanotoxicity study to include Phaeodactylum tricornutum (Clément et al. 2013). All forms of TiO2 used for exposure caused toxicity in this diatom, except the nanoparticles and microparticles. From the EC50 values, it was indicated that the 15 nm nanoparticles were more toxic than the other nanoparticles. In fact, the nanoparticles were toxic to the diatoms as is K2Cr2O7, a well-known carcinogen. The authors also investigated Brachionus plicatilis , a rotifer species, and demonstrated that the micro-particles were much less toxic than K2Cr2O7 while the 15 nm Anatase NPs were the most toxic (Clément et al. 2013). Plankton Daphnia magna were studied by Dabrunz et al. for the toxic effects of TiO2 NPs (Dabrunz et al. 2011). The authors studied the plankton stressed with TiO2 NPs with an approximate diameter of ~100 nm. It was found that concentrations of 3.8 and 0.73 ppm lead to toxic effects in the plankton (Dabrunz et al. 2011). It was also reported that increasing the particle size to ~200 nm, the toxicity was greatly reduced. A mechanism of action for the toxicity of the nanoparticles was proposed, which required the development of a surface coating of the TiO2 NPs over the outside of the plankton, which lead to a molting disruption. Additionally, the treatment of the plankton with 2 ppm of TiO2 NPs lead to the development of the surface coating; however after 36 h, the coating was not present in the treated samples (Dabrunz et al. 2011). However, after 96 h of treatment with surface coating, the mortality rate of the plankton was found to be 90%. In a similar study by Zhu et al., the toxicity and bioaccumulation of TiO2 NPs in Daphnia magna was investigated (Zhu et al. 2010). A minimal toxicity of the nanoparticles was observed within 48 h of treatment. However, upon longer treatment, internalization of the nanoparticle was occurred while increased toxicity was observed. Mansfield et al. investigated the toxicity of TiO2 NPs in D. magna under natural light (Mansfield et al. 2015). The D. magna were exposed to anatase phase of the TiO2 NPs at concentrations of 20 and 200 ppm. The authors showed that under varying UV irradiation, the LC50 of the TiO2 NPs was 778 ppb in the 50% irradiation samples. Whereas in the 100% UV irradiation samples, the LC50 was 139 ppb (Mansfield et al. 2015).
9 TiO2 Toxicity in the Treatment of Bivalves
Moving into more complex organisms , studies have been performed on the toxicity of bivalve species using TiO2 NPs. The environment for the treatment of bivalves is more complex than algae and plankton/diatom treatments. The sediment introduces a secondary medium aside from the treatment of the organisms with suspended NPs in solution. There are transport questions on the movement of NPs through the sediments. However, ultimately oxidative stress appears to be one of the larger mechanisms involved in the interaction of TiO2 and bivalves. The effects of TiO2 on bivalve species are summarized in the following section. Marisa et al. investigated the effects of TiO2 NPs on clam Ruditapes philippinarum (Marisa et al. 2015). The authors investigated the effect of a mixture of anatase and rutile (70:30) with a focus on studying the hemocyte phagocytic activity. The authors showed that the effect on the hemocyte function was mediated by the internalization of the nanoparticles within the hemocytes. The TEM images taken of treated cells with the NPs were noted to be on the cell wall as well as inside the cell (Marisa et al. 2015). Along the same lines, Shi et al. investigated the effects of TiO2 NPs on a commercial clam Tegillarca granosa (Shi et al. 2017). The authors exposed the clams to environmentally relevant concentrations of TiO2 (no phase specified) at 10–100 ug/L. The authors also exposed the clams for 30 days where they studied the expression of genes, the immune-related molecules expressed in the clams after treatment. The clams showed a highly downregulated immune-related response, which indicates the TiO2 NPs suppressed the immune system of the clams. Furthermore, the gene expression suggested pattern recognition receptors may be receptors for NPs in marine invertebrates. The authors also showed data suggesting LMS reduction, phagocytosis degrease, and NO production increases and lysosome release, the reduction of ROS production, and increase in HM in other marine organisms after TiO2 treatment.
10 TiO2 Toxicity in the Treatment of Fish
The effects of TiO2 NPs on zebrafish have been investigated from different aspects, which include the toxicity, but also behavioral changes observed (Chakraborty et al. 2016). TiO2 NPs have been shown to induce pre-mature hatching and have been found to be toxic to zebrafish embryos, which was determined to be concentration dependent. Exposure of zebrafish embryos to TiO2 NPs has been shown to cause death (Chakraborty et al. 2016). Further studies with TiO2 NPs and zebra fish have shown neurotoxicity. In fish studies, the translocation of TiO2 NPs to different cell types and organs begins to be noticed. The treatment of zebrafish with TiO2 NPs shows the enhanced expression of particular genes and the suppression of other genes resulting in brain damage. Stressing of the zebra fish has also shown to reduce the reproductive rates of both male and female fish and embryo development (Chakraborty et al. 2016). Chen et al. investigated the effects of TiO2 NPs on the behavioral of zebrafish (Danio rerio) (Chen et al. 2011). The authors exposed embryos of zebrafish to P25 TiO2 NPs, which is a well-known photocatalytic nanomaterial and is a mixture of the anatase and rutile TiO2 phases. The TiO2 NP were found to range in size from 25 to 70 nm. The authors noted that there were no changes in the survivability, hatchability, or morphology in the larvae at low concentrations. However, the mobility and the percentage of time inactive were lower in the 0.1, 0.5, and 1.0 treatments but were unchanged in 5 and 10 mg/L treatments (Chen et al. 2011). However, it was not indicated that the changes in behavior were due to physiological damage from the exposure to the TiO2 NPs. It was also observed that the behavioral changes may be due to increase in antioxidant enzymes. The enzymes included in the discussion were superoxide dismutase, catalase, and peroxidase , which has been shown in other fish species exposed to TiO2 NPs (Chen et al. 2011). Lammel and Struve have investigated the toxicity of TiO2 NP on rainbow trout cell lines (Lammel and Sturve 2018). The authors investigated cell lines from liver and gills. In that work, TiO2 nanoparticles with 21 nm diameter were used in the P25 mixture, which as previously mentioned, it is a photocatalytic mixture of the anatase and rutile phases of TiO2. The cells were treated with NP concentrations from 1 to 100 ug/mL (or 1–100 ppm) for a 72 h period. The authors noted that there was a little cytotoxicity from the TiO2 NPs after the 72 h exposure (Lammel and Sturve 2018). However, the nanoparticles were present as agglomerates in the intracellular vesicles of the liver and gill cells. Furthermore, the TiO2 adsorbed to the plasma membrane were internalized in the cells (Marisa et al. 2015). Zhu et al. investigated the toxicity of TiO2 NPs on zebrafish (Zhu et al. 2010). The zebrafish was exposed to nanoscale non-stabilized TiO2 in the anatase phase with an average diameter of less than 20 nm. The studies were performed for 96 h using concentration of 1–500 mg/L. The zebrafish embryos had a hatching rate of approximately 100% even at concentrations of 500 mg/L. The TiO2 NPs did not appear to have adverse effects on the zebrafish in the study (Zhu et al. 2010). In another study, Yang et al. investigated the toxicity of TiO2 NPs on developing zebrafish (Yang et al. 2013). The authors examined the impact of humic acid with TiO2 NPs on the growth and development of zebrafish embryos under simulated sunlight (Yang et al. 2013). P25 TiO2 nanoparticles (3:1 anatase: rutile) with an average diameter of 21 nm were used in that study. The authors employed the TIO2 NPs as the control sample to investigate the effects of the HA/TiO2 NP treatment, which were TiO2 NPs with concentrations of 0–1000 mg/L and HA 0–30 mg/L. The authors showed significantly decreased survival rate at 5 dpf as the concentration of the TiO2 NPs and HA was increased as inherent toxicity without simulated sunlight (Yang et al. 2013). Similarly, in the presence of simulated sunlight , the survivability of the fish was reduced. However, the concentration showed to have a larger effect. Without simulated sunlight, there were approximately 50% of the embryos survived at 1000 ppm with HA and TiO2 treatment and approximately an 80% survival rate was observed with the TiO2 alone; however, under simulated sunlight , there were no survivors above 500 mg/L with and without the HA treatment. In developing zebrafish, the presence of the HA and TiO2 showed increased oxidative stress in the fish. It was determined through lipid peroxidation that increased oxidation damage and DNA damage was present in the fish with simulated sunlight (Yang et al. 2013). Jovanovic et al. investigated the effects of TiO2 on Fathead minnows (pimephales promelas) (Jovanovic et al. 2015). However, the authors investigated the stress with respect to the fish’s ability to respond to pathogens. The TiO2 NPs were observed to accumulate in the kidney, followed by the spleen, then the liver. The concentration in the liver was approximately equivalent to the concentration of NPs found in the whole fish. Additionally, It was observed that minnows treated at 2 ng/g and 10 ug/g (based on body weight) exposure to the TiO2 NPs followed by exposure to the pathogens either aeromonas hydrophila or Edwardsiella ictaluri showed inhibited response in the immune system and increased mortality. Further study of the minnows injected with the TiO2 NPs showed histopathology (Jovanovic et al. 2015). There appeared to be an interplay between the histopathology and the immune systems of organisms treated with NPs. Reeves et al. investigated the hydroxy radicals generated from TiO2 on cytotoxicity and oxidative damage to DNA in fish (Reeves et al. 2008). The authors used 5 nm anatase NPs at concentrations from 0.1 to 100 ug/mL (ppm) with and without UV irradiation using goldfish cell. The authors observed that all dose levels showed significant increase in oxidative damage to DNA (Reeves et al. 2008). The analysis of the data showed there was no dose-dependent response in the oxidative damage to the nanoparticles at low concentrations of NPs. For the goldfish cells exposed to the TiO2 NPs for 24 h in the absence of UV irradiation, a small decrease in cell viability was observed (~80% viability). When the cells were treated with 1000 ppm TiO2 nanoparticles and were subsequently co-exposed to UVA irradiation, the cell viability decreased to ~40%. The authors concluded that with and without photosensitization with UVA light, TiO2 NPs were potentially genotoxic to fish cells. In addition, the authors concluded that the major cause of toxicity was the generation of the hydroxyl radical (Reeves et al. 2008).
11 TiO2 Toxicity in the Treatment of Mammals and Mammalian Cells
The treatment of mammals and cell lines show mixed results with TiO2 NP toxicity. Ultimately, the toxicity manifests itself largely at the level of oxidative stress affecting cellular viability, similar to the other organisms mentioned earlier treated with TiO2 NPs. Similar to the fish TiO2 NP toxicity studies, the potential for translocation of nanomaterials to different organs exists, which may cause multisystem toxicity within the organism. However, for the most part, the observed effects on the mammals, excluding cell lines, appear to have an initial effect, which reduces with the exposure time. Wu et al. have investigated the toxicity and penetration of TiO2 NPs in hairless mice and porcine skin after dermal exposure (Wu et al. 2009). The authors investigated Anatase NPs with sizes of 4 and 10 nm, rutile NPs with sizes of 25, 60, and 90 nm, and Degaus P25 (anatase/rutile mixture 70:30), a commercial photocatalytic material. The TiO2 NPs were tested for their ability to transport across porcine skin in vitro and in vivo. The in vitro studies showed not penetration of the TiO2 NPs inside the tissue. On the other hand, the in vivo studies indicated that TiO2 NPs were in the stratum corneum, stratum granulosum, prickle cell layer, and basal cell layer; however, there was no deep penetration into the dermis (Wu et al. 2009). The author’s study on the hairless mice showed that mice treated with the 10 nm, 25 nm, and P25 NPs had decreased weight after treatment. The liver and spleen coefficients were also significantly higher. The nanoparticles were found to accumulate in liver, spleen , and heart, but no transport was observed into the blood. In addition, the particles were observed in the liver; the bile ducts close to the nanoparticle aggregates were shown to be swollen indicating tissue damage (Wu et al. 2009). The effects of TiO2 NPs after intraperitoneal injection to mice were investigated by Chen et al. (Chen et al. 2009). The mice were injected with TiO2 NPS at concentrations ranging from 324 to 2592 mg/kg using the anatase phase of TiO2, which they were synthesized utilizing a sol gel method. The average grain size of the NPs was approximately 3.6 nm. TiO2 NPs were observed to be accumulated in the spleen after 24 h treatment. The authors observed a dose-dependent relationship as the concentration of the TiO2 NPs was increased the accumulation of the NPs in organs increased. The spleen concentrations of the TiO2 NPs decreased as time progressed. As the time increased, the lung, kidney, and liver showed the presence of TiO2 NPs. The authors concluded that some of the NPs were excreted from the kidney and the toxicity of the NPs to the liver than kidney. The study also showed that TiO2 NPs could transport to different tissues after injection. All the organs that contained TiO2 NPs showed adverse effects to some degree but were minor in the extent of damage (Chen et al. 2009). Warheit et al. investigated the effects of TiO2 NPs on pulmonary instillation in rats to determine if toxicity was a function of NP size (Warheit et al. 2006). The authors used rutile NPs with an average diameter of 300 nm, anatase nano-rods (up to 233 nm long), and nanodots which were in the anatase phase. The rats were treated intratracheally with TiO2 NPs dispersed in phosphate-buffered saline (PBS) solution. Exposure to the nanoparticles produced a trainset short-lived pulmonary inflammation response within the first 24 h of treatment. However, the NPs administered at a high dosage of 5 mg/kg did not induce any long-lasting effects in the rats (Warheit et al. 2006). Gerloff et al. have investigated the toxicity of TiO2 mixed anatase/rutile phase on Caco-2 cells from human intestine (Gerloff et al. 2012). The authors noted that TiO2 has been a common food additive for many years. The authors studied pure anatase, and a mixture of anatase/rutile from various vendors. The average crystallite size was determined to range from 6.7 nm up to 215 nm. It was concluded from the study that specific surface area and crystallinity of the NPs was important for the toxicity to the intestinal cells. However, it was noted that there was no correlation or evidence for the TiO2 nanoparticles playing a role in the ROS-mediated stress of the Caco-2 cells (Gerloff et al. 2012). Hsiao and Huan studied the effects of TiO2 on human lung epithelial cells (Hsiao and Huang 2011). The authors purchased phase pure anatase nanoparticles and anatase/rutile mixed phase nanoparticles and subjected to treatments of the TiO2 NPs from 50 to 1.56 μg/mL from time intervals of 12, 24, or 72 hrs. The authors followed the cell morphology and performed MMT assay for cell viability, followed the production of IL-8 for the concentration treatments. This exposure caused a decrease in toxicity as the average grain size of the nanoparticles increased for 12 and 72 h treatments. The amorphous particles induced more pro-inflammatory factor in human cells than the larger NPs. It was also noted that the anatase phase of the TiO2 NP induced a higher cytotoxicity to the cells. The authors also indicted that point of zero charge (PZC) plays a role in the transport of the nanoparticles across the cells and plays a role in the toxic effects of the nanoparticles (Gerloff et al. 2012). Jeng and Swanson, on the other hand, studied the toxicity of metal oxide NPs on mammalian cells from mouse (Jeng and Swanson 2006). The authors studied TiO2 among other metal oxide NPs with a mean size range from 50 to 70 nm. The authors used the MMT assay to investigate the cell viability of TiO2 where no effect was observed on cell viability. Furthermore, it was shown that TiO2 did not affect the mitochondrial function of the cells. The authors concluded that the TiO2 NPs were only lightly toxic to the cells (Jeng and Swanson 2006). Karlsson et al. exposed human epithelial cells to TiO2 NPs with an average diameter of 63 nm, which showed no reduced cell viability (Karlsson et al. 2008). These nanoparticles showed the ability to damage DNA. The TiO2 NPS showed an increase in the ROS species generated within the cells over the concentrations in the control cells (Karlsson et al. 2008).
12 ZnO NP Toxicity
ZnO NPs have been investigated for their toxic effects in different biological systems. Like TiO2 NPs ZnO NPs are a commercially important Type of NP. One of the common uses of ZnO has been traditionally in high SPF sunscreens. The application of ZnO in sunscreens especially with NPs raises several questions about the toxicity, considering the direct dermal application of sunscreen. Also, the high use of sunscreens in and around aquatic environments raises questions about the environmental transfer and potentially elevated concentrations of ZnO NPs. Unlike TiO2 NPS ZnO NPs can be dissolved at physiological pHs and the Zn2+ ions are able to cause similar toxic effects as the NPs. The toxicity of ZnO NPs becomes a question of the solubility and organismal response to dissolve Zn2+ ions in solution as well as the ZnO NPs effects. The most commonly observed toxicity effects of ZnO NPs on the different organisms are summarized in Table 5.3 and in the following sections.
13 ZnO Toxicity in the Treatment of Algae
The toxicity of ZnO NPs is similar to that of TiO2 NP in algal species, specifically with respect to the increase in ROS generation and decrease in cell viability. However, unlike TiO2, NPs ZnO NPs are soluble and can form ions. The formation of Zn2+ ions in solution complicates the study of ZnO toxicity. Zn2+ ions can be toxic in high enough concentration, and thus the question becomes: is the observed toxicity caused by the ZnO or the Zn2+ ion. Bhuvaneshwari et al. have investigated the cytotoxicity of ZnO NPs on Scenedesmus obliquus under low concentration, VU-light, as well as dark and visible light conditions (Bhuvaneshwari et al. 2015). The authors used ZnO NPs with sizes less than 100 nm and a second set of ZnO NPs with an average size of 40 nm. The authors used three concentrations of 0.25, 0.5, and 1.0 mg/L and used a 72 h treatment, with either UV-C, visible light, or dark conditions. Subsequent to treatment, the authors determined the following: oxidative stress, cellular membrane integrity, total organic carbon (TOC), and the surface interaction of the NPs with the cells. As well, the authors investigated the internalization and uptake of the NPs. The authors showed that cell viability had decreased for both NPs under all illumination techniques and increased with increasing concentration of the ZnO NPs. Under UV-C irradiation the amount of ROS species generated were increased, as well as the membrane integrity decreased with increasing concentration. The internalization of the NPs into the algal cells was also observed (Bhuvaneshwari et al. 2015). Ji et al. studied the toxicity of ZnO NPs on the green algae Chlorella sp. (Ji et al. 2011). The authors observed a nano-Zn particles-led inhibition of algal growth with a 6-day EC50 of 20 mg/L. The authors used 20 nm ZnO particles and showed that as the concentration of the NP increased, the toxicity was increased. It was also noted that the amount of dissolved Zn in solution increased with an increase in the concentration. The data suggested that the toxicity of the ZnO was caused by the NP dissolution (Bhuvaneshwari et al. 2015). Manzo et al. investigated the toxic effects of ZnO nanoparticles on a marine alga Dunaliella tertiolecta (Manzo et al. 2013). The authors used ZnO nanoparticles uncoated with an average diameter of 100 nm, as well the authors used ZnCl2 to assay the Zn2+ ion toxicity. It was reported that the dissolution of the ZnO was almost complete after 24 h of exposure. The data from the study showed an EC50 of 1.94 for the ZnO NPs, and EC50 of 3.57 for bulk ZnO NPs, and an EC50 of 0.65 for the ZnCl2. The authors concluded that the ZnO was more toxic than the ionic form (Ji et al. 2011). Peng et al. have investigated the toxicity of and the effect of morphology of ZnO NPs on marine algae (Peng et al. 2011). In that work, spherical NPs, nanoplatelets (2-D structures), and nanoneedles/nanorods were investigated (Peng et al. 2011). The algae T. pseudonana and P. tricornutum were exposed to 10-80 mg/mL of the ZnO NPs for up to 72 h. It was observed that the dissolution of the NPs occurred within hours of exposing the ZnO NPs to the growth medium releasing Zn2+ ions. The growth of both the T. pseudonana and C. gracilis occurred and was thought to be a result of the NP dissolution due to the acute toxicity of the Zn2+ to the organisms. However, the P. tricornutum growth was not as greatly affected by the Zn2+ ions and observed effects could be correlated to particle concertation and morphology (Peng et al. 2011). The 1-D structures showed greater toxicity than the 3-d morphologies. The study suggested that both the ZnO and Zn2+ were the cause of the toxicity in the algae(Peng et al. 2011). Miller et al. have studied the effects of ZnO nanoparticle on marine phytoplankton S. marinoi, T. pseudonana, D. tertiolecta, and I. galbana (Miller et al. 2010). The authors used zincite nanoparticles with an average diameter of 20-30 nm and exposure concentrations of 10–1000 ug/L for 96 h. The growth of the phytoplankton was significantly reduced after exposure to the ZnO NPs. More importantly, the ZnO NPs were quickly dissolved in the saltwater medium 32% of the NPs mass and was dissolved in the first 12 h and still observed to be dissolving at the 96 h time period.
14 ZnO Toxicity in the Treatment of Bivalves and Aquatic Organisms
Similar effects with respect to the toxicity have been observed within bivalve species that have been studied as have been observed in algal and plankton species. Similarities in toxicity have been observed even though bivalve spices are somewhat more complex than algal and plankton species. In addition, the route of exposure requiring the movement of the particle through a sediment is also different. Buffet et al. have studied the effects of ZnO NPs on clams and ragworms (Buffet et al. 2012). The authors exposed the organisms to 3 mg/kg concentrations based on the sediment, which they considered to be an environmental relevant concentration. The NPs were sized between 21 and 34 nm with a positive surface charge from diethylene glycol. The organisms were treated under three difference conditions, seawater alone, DEG alone, and ZnO NPs in sediment (3 mg/kg). It was concluded that there were no consistent changes in biochemical markers (Buffet et al. 2012). The effects appeared to be related to the DGE presence on the NPs while the behavior of burrowing for the DEG-treated and ZnO-treated NPs were not significantly different. The authors noted that ZnO NP toxicity was generally attributed to the presence of Zn2+ ions; however it was difficult to differentiate between the natural background Zn2+ and that accumulated from the NPs and NP dissolution (Buffet et al. 2012). Ali et al. observed the oxidative stress and genotoxic effect of ZnO NPs in a freshwater snail (Ali et al. 2012). The authors exposed the freshwater snails to ZnO NPs with an average diameter of 50 nm for 96 h at below lethal concentrations. It was reported that at 32 ppm, a reduction of glutathione was observed with increases in the malondialdehyde level and catalyze levels in the digestive glands. The authors also observed significant DNA damage with the organisms treated for 24 and 96 h. The authors concluded that the ZnO NPs induced genotoxicity in digestive gland due to induced oxidative stress (Ali et al. 2012).
15 ZnO Toxicity in the Treatment of Fish
Similar to fish studies with TiO2 NPs, ZnO NPs have shown to be toxic. Again, the pertinent question is whether the observed toxicity is due to the interaction of NPs or Zn2+ ions. It is also observed in fish like algae, ZnO NPs caused oxidative stress as well as immune system impairment and affected viability. Zhu et al. investigated the effects of ZnO NPs on zebrafish (Zhu et al. 2008). The authors treated the fish with ZnO NPs (20 nm) and Bulk ZnO particles at concentrations from 1 to 50 for up to 96 h. At the 96th hour of treatment, the zebrafish survival rate dropped from approximately 98% in the controls to 0 in the 50-ppm treatment. Similar results were observed in the Bulk studies. As the ZnO concentrations were increased, the survivability of the zebrafish decreased. Similar results were observed for the hatching rate with a decrease of 0% for the 50 ppm treated fish. The EC50 for both the Bulk ZnO and NP ZnO were approximately 2. It was noted that the tissue ulceration when the hatched zebrafish embryos after 72 h treatment sand increased with increasing particle concentration (Zhu et al. 2008). Zhao et al. studied the acute effects of ZnO NPs exposure on the development of toxicity, oxidative stress, and DNA damage in zebrafish (Zhao et al. 2013). The authors suspended ZnO less than 100 nm NPs in zebrafish culture media with concertation variation from 1 to 100 ppm. The authors analyzed the concertation of free zinc in solution after treatment and prepared Zn solutions from ZnSO4 to determine the toxic effects of the Zn2+ ions. The results of the study showed the embryo hatch was significantly slow and malformation increased at 96 h of exposure (Zhao et al. 2013). The ZnO NPs also induced DNA damage through ROS generation. In addition, the activities of defense enzymes were changed while MDA concentration in the larvae were increased. It was also indicated that the Zn2+ ions were less responsible for the damage than the ZnO NPs. The data from the study also showed mRNA was damaged, which can be used to encode response enzymes and responses for oxidative stress (Zhao et al. 2013).
16 ZnO Toxicity in the Treatment of Mammals and Mammalian Cells
ZnO NPs have been shown to be toxic to mammals and mammal cells. However, the toxic effects of the ZnO NPs seem to correlate strongly to the concentration of free Zn2+ ions. In addition, in in vivo studies using mice or other mammalian models, one begins to see multisystem effects with the treatment of whole organisms with ZnO NPs. In addition, some studies begin to show secondary effects or causal effects due to direct effect on a different cellular system. Song et al. have investigated the role of Zn2+ in ZnO NP toxicity in mouse macrophage Ana-1 (Song et al. 2010). The authors focused on the toxicity from the generation and damage caused by ROS. The study focused on nanoparticles of four different sizes: one set of particles was on the order of microns in size, the second set was less than 100 nm, the third set were 30 nm, and the fourth set were 10–30 nm in diameter. The cells were treated with NP concentrations of 2.5–100 ppm for 24 h. In that work, a dose-dependent toxic effect was observed (Song et al. 2010). In addition, there was no effect observed due to the size of the nanoparticles. But a Zn2+ concentration of 10 ppm induced a 50% death which was close to the NP toxicity. The cellular viability and membrane integrity were observed to have high correlation with the concentrations of the Zn2+ in the supernatant of the culture. Finally, the authors concluded that ROS may be generated by the ZnO and dissolved Zn2+ but had a minor role in cytotoxicity but was a cytotoxic response to ZnO NP and Zn2+ (Song et al. 2010). Roy et al. investigated the therapeutic effects of ZnO NPs in balb/c mice (Roy et al. 2014). Concentrations of 1–12 mg/mL of the ZnO NPs with average diameters below 50 nm were used in the study with a treatment time of 30 days. The ZnO NPs effectively boosted the immune response leading to inflammatory response, which was determined by the expression of Cox2, MMP, and PGE2. The authors concluded that the ZnO NPs could be potentially utilized in therapeutics (Roy et al. 2014). Esmaeillou et al. investigated the oral toxicity of ZnO NPs in adult mice (Esmaeillou et al. 2013). The authors used 20–30 nm ZnO NPs and were given to the mice using oral gavage at a concentration of 333.33 mg/kg/day. Loss of appetite, ever lethargy, and vomiting in some mice were observed over the exposure period. Within 3 days of treatment , one mouse was deceased. The authors performed a series of biochemical assays and showed lower HDL and LDL in the treated mice but the triglycerides in the serum were increased. The results of the assays also showed potential liver and kidney damage in the mice. The authors also showed that lung damage was induced by the ZnO NPs. ZnO NPs (50-70 nm) have shown to be toxic in mammalian cells (Esmaeillou et al. 2013). Jeng and Swanson showed nanoparticle-exposed Neuro-2A cells exposed to doses of 100 ppm changed in morphology, and size, as well became detached (Hsiao and Huang 2011). Furthermore, the mitochondrial function degreased at treatments from 50 to 100 ppm. The authors also noted that the cellular membrane integrity was affected as indicated by LDL leakage. Apoptosis occurred at under ZnO NP treatment and cells became necrotic with the increase in concentrations. Buerki-Thurnherr et al. have investigated the mechanisms of ZnO NP toxicity using Jurkat cell or human T cells (Buerki-Thurnherr et al. 2013). The authors used four different ZnO materials in the study, which included commercially available 10 nm ZnO NP, a mandelic modified 18.3 nm ZnO, a 29 nm ZnO NP with Silica shell, and lab made methoxy-coated NPs 8 nm. In that study, it was determined the toxicity induced in the T cells by the NPs was due to the dissolution of the ZnO NPs. The presence of the Zn2+ ions in the growth media, which resulted in the storage of the zincosomes and the expression of MTs was upregulated. Above the threshold concentration, apoptosis occurred and eventually cellular death. Interestingly, the authors noted that ROS were not a major contributor to the studied mechanism (Buerki-Thurnherr et al. 2013). Hsiao and Huang have studied the toxicity of ZnO NPs on lung cells (Hsiao and Huang 2011). This study was designed to test a common premise that the physiochemical properties of NPs are critical players in the toxicity of NPs. The authors prepared rod-shaped, spherical, and sphere-like ZnO NPs. The sphere ZnO NPs had diameter of approximately 5 nm and lengths of 16-48 nm. Whereas the spherical NPs had diameters in the range of 5–10 nm. The sphere like NPS had sizes from 36 to 60 and 50 to 122 nm. The cells were treated with 1.56-50 ppm concentrations for times of 12, 24, and 72 hrs. The authors demonstrated convincingly that the nanorods were more toxic than the nanospheres with EC50 values of 8.5 and 12.1 ug/mL, respectively. The shape appeared to control the toxicity of the NPs when size and surface area were comparable. The authors concluded that both size and shape of the ZnO NPs influence the mitochondrial activity and chemokine productivity (Hsiao and Huang 2011). Karlsson exposed epithelial cells to 100 nm ZnO NPs which showed a decrease in the cell viability of 38% (Jeng and Swanson 2006). The ZnO showed almost no damage to the DNA of the cells. The ZnO NPs showed increase in the ROS generation in the cells after treatment compared to the control cells (Jeng and Swanson 2006).
17 CuO NP Toxicity
Numerous studies were reported on the toxicity of CuO NPs, which are perhaps the third most studied NPs for their toxicity. Copper oxide NPs find commercial use in products such as antifouling paints, antimicrobials, and cosmetics (Khan et al. 2019; Grigore et al. 2016; Adeleye et al. 2016; Khashan et al. 2016). These commercial and personal care applications have the potential to increase the presence of CuO NPs in the environment and human exposure. Like ZnO NPs, CuO NPs can readily go through dissolution at physiological pHs and cause increased copper concentrations in cells. Elevated copper concentrations in humans have been linked to several different health conditions. The copper-related health conditions due to elevated copper concentrations can be summarized as follows: liver damage, gastrointestinal symptoms, and at extremely high concentration death (Institute of Medicine 1998; National Research Council Committee on Copper in Drinking Water 2000). It should also be noted that copper ions are essential to the healthy growth and development of organisms. However, the toxicity of CuO NPs may be a combination of the dissolved copper species and the NPs, or caused by either, as in nano zinc treatments. Mixed results have been observed when authors attempt to determine CuO NP toxicity. The most commonly observed toxic effects observed across the different organisms are summarized in Table 5.4.
18 CuO Toxicity in the Treatment of Algae
Similarly, effects with respect to the toxicity of ZnO NPs have been observed with CuO NPs. Ultimately, oxidative stress and cellular viability are the most common toxic effects of CuO NPs in algal species. Similar to ZnO NPs, CuO NPs are somewhat soluble in aqueous solution releasing Cu2+ ion, which are toxic to some algal species. In fact, CuSO4 is a well-known algicide, which generates free Cu2+ ions in solution. Thus, the study of CuO NP toxicity is more than likely a combination of the particles and the dissolved ions in solution. Melegari et al. have investigated the toxicity of CuO NPs on Chlamydomonas reinhardtii a green algal species (Melegari et al. 2013). The authors treated the algal species with 20–30 nm CuO NPs, which were approximately spherical in shape. The study used NP concentrations of 0.1–1000 ppm CuO NPs with treatment times up to 72 h. It was reported that the NPs induced an increase in the reactive species within the algae as well as lipid peroxidation of cellular membranes. The study also showed that the NPs were able to transport across the cell wall and were internalized within the cells. The toxic effects of the NPs were noticed after 72 h of treatment with a concentration of 0.1 mg/L. Aruoja et al. investigated the effects of CuO NPs Pseudokirchneriella subcapitata , a microalga (Aruoja et al. 2009). The authors used a CuO NP with an average size of 30 nm for a treatment period of 72 hrs., with concentration of 100 ppm. The authors determined that the EC50 for the CuO NPs was 0.71 mg/L, which was much more toxic than the comparable CuO bulk EC50 of 11.55 mg. The data showed a decrease in the cell growth and complete inhibition of growth at 6.4 mg Cu/L using the CuO NPs. In addition, it was concluded that the bioavailability of the CuO in the NPs was much higher than the bulk phase (Aruoja et al. 2009). Suppi et al. investigated CuO NP toxicity to algae (Suppi et al. 2015). The authors used CuO NPs with average diameters of 30 nm and treatment concentration of up to 100 ppm. The results showed minimum biocidal effects starting at CuO 10 ppm NP (Suppi et al. 2015). Cheloni et al. investigated the effects of CuO NPs on Chlamydomonas reinhardtii using different irradiation compositions (Cheloni et al. 2016). The authors studied natural light, UV B, and light from an incubator which is a mixture of UV A, UV B, and visible light. The microalgae were treated with CuO NPs with a size range of 30-50 nm for up to 24 hrs. at two concentrations of 8 ug/mL and 0.8 mg/mL. It was found that the hydrodynamic diameter of the NPS changes from 800 nm to 600 nm within 2 h of dispersing and remained constant thereafter. The light was observed to affect the algal the cells, under incubator light the cells showed a 1.7% growth inhibition at 0.8 ppm CuO, the 8 ug/L, the 0.8 mg/L treatment showed a 67.7% growth inhibition. As well under the natural sunlight, the 8 ug/L treatment showed a 2.3% growth inhibition whereas the 0.8 mg/L treatment showed approximately 6% growth inhibition. Under the UV B irradiation, the inhibition increased further for the 8 ug/L CuO NP treatment and showed approximately 82% inhibition of growth and the 0.8 mg/L treatment showed approximately 94% growth inhibition. Similarly, it was also noted that as the algae were treated with the CuO NPs with increasing concentration and changing light condition, the membrane permeability was altered with increasing Cu concentration especially under UV B. The authors concluded that the UV B in conjunction with the UV B irradiation had a synergistic effect on the algae growth and health (Cheloni et al. 2016). Sankar et al. have investigated the effect of larger particles 270 nm on Microcystis aeruginosa to investigate the growth inhibition of bloom formation (Sankar et al. 2014). The algal species were treated with CuO NP concentrations from 12.5 to 50 mg/L for up to 96 h. The authors showed that cell density in the growth media decreased with treatment time and with increasing concentration of the CuO NPs. At 50 ppm of the CuO NPs an inhibition rate of approximately 90% was observed. All NP treatments showed a decrease in the chlorophyll A and B as well as the total amount of carotenoids produced by the algae. The authors concluded that the CuO NPs were potentially a biocide to the algae to prevent the formation of algal blooms (Sankar et al. 2014).
19 CuO Toxicity in the Treatment of Plankton
Similar to algae species, plankton are affected by the toxicity of CuO NPs in solution. The major toxic effect observed in plankton species appears to be the generation of ROS species causing a decrease in the cellular viability. Heinlaan et al. investigated the effects of CuO NPs with an average dimeter of 30 nm on Daphnia magna and T. platyurus (Heinlaan et al. 2008). The authors treated the plankton and showed a EC50 of approximately 165 mg/L and 95 for D. magna and T. platyurus, respectively. The EC50 of the CuO NPs were much higher values than those observed for free Cu ions from CuSO4. The T. platyurus was more sensitive to the CuO NPs than the D. magna; the authors also showed that the NPs did not have to enter the cells of the organisms to cause toxicity (Heinlaan et al. 2008). In another study, Heinlaan et al. investigated the effects of CuO NPs with an average dimeter of 30–60 nm on Daphnia magna (Heinlaan et al. 2011). The authors investigated the changes to the midgut of the organism using TEM analysis. At 48 h of treatment, the authors showed an EC50 of 175 mg/L for the CuO NPs. The concertation of solubilized copper in solution from the CuO NPs was 0.05 mg/L and the determined EC50 was similar to that observed for free copper ions. The authors noted that in the presence of CuO NPs in the midgut lumen, internalization of the CuO NPs was not evident. In addition, the CuO nPs after 48 hrs were no longer in the peritrophic membrane but had moved to the midgut epithelium microvilli (Heinlaan et al. 2011). Mwaanga et al. investigated the effects of CuO NPs with an average dimeter of less than 50 nm on Daphnia magna (Mwaanga et al. 2014). The authors exposed 5-day-old D. magna for 72 h to sublethal concentrations of CuO NPs. The authors also investigated the effects of hard water and the presence of natural organic matter (NOM). From the treated organisms, the authors investigated several biochemical processes including glutathione activity, glutathione oxidation, thiol compounds, and metallothionein. The glutathione synthase enzyme was inactivated by the CuO NPs; the metallothionine was increased as well as the oxidation of the glutathione. The data suggests that the CuO NPs induced oxidative stress in the organisms. The authors also showed some dissolution of the CuO NPs in solution and concluded the toxicity observed was due to both the dissolved ion and the CuO NPs (Mwaanga et al. 2014). Similar results were observed by the authors when investigating the toxicity of CuO NPs on Thamnocephalus platyurus using CuO NPs with an average diameter of 30 nm (Mortimer et al. 2010). Mortimer et al. have investigated the effects of CuO NPs on Tetrahymena thermophila (Mortimer et al. 2010). The authors utilized CuO NPs with average diameters of 30 nm. The organisms were treated with Cu2+ ions, CuO NPs, and CuO in the bulk form. The concentrations used for the treatments for the CuO NPs ranged from 31.25 to 500 mg/L. The authors showed an EC50 of approximately 128 mg/L for the CuO NPs on the organisms. The toxic effects of the CuO NPs were not observed to be time dependent.
20 CuO Toxicity in the Treatment of Bivalves
CuO NP toxicity observed in bivalve species has been shown to be induced by the generation of ROS species inside the organisms as has been observed with other nanoparticles in bivalve species. However, the differentiation between Cu2+ ion toxicity and CuO NP toxicity is difficult, which is due to the similar effects. Buffet et al. also investigated the effects of CuO on Scrobicularia plana using 200 nm CuO particles (Buffet et al. 2011). In the same study, Buffet et al. studied the effects of CuO NPs on Hediste diversicolor treated with approximately 200 nm CuO NPs. The authors were attempting to observe the effects of CuO NPs on behavior and biochemical response to the CuO NPs. The authors treated both organisms with CuO NPs with concentrations that was equivalent to10 ug/L of dissolved Cu2+ ions. In addition, the authors also treated both organisms with dissolved Cu2+ ions for comparative purposes at a concentration of 10 ug/L. The defense mechanisms for both organisms being exposed to Cu2+ and CuO NPs were elevated compared to controls, which included CAT, GST, SOD, and MTLP. However, the LDH concentrations were only elevated in the N. diversicolor. The exposure to the Cu2+ ions had a negative effect on the burrowing behavior whereas the CuO NPs did not appear to affect the behavior (Mwaanga et al. 2014). Pradhan et al. investigated the effects of CuO NP with diameters ranging from 30 to 50 nm on Allogamus ligonifer (Pradhan et al. 2012). The insects were treated at the highest level of 75 mg/L using either contaminated food or through contaminated water. The treatment of the organisms showed a higher copper accumulation in the larvae bodies than the nontreated organisms. It was determined that the LC50 for the CuO NPs was 569 mg/L after 96 h of treatment. The insects treated with the CuO NPs showed decreased appetite and a decrease in the growth rate by approximately 46%. The author noted that the soluble fraction of Cu2+ was approximately 10% by body mass (Pradhan et al. 2012). The accumulation of copper oxide NPs in the digestive glands of Mytilus galloprovincialis was investigated by Gomes et al. (Gomes et al. 2012). The authors used CuO NPs with an average size distribution of 30 ± 10 nm. The bivalves were treated with 10 ug/L of dissolved Cu2+ ion or CuO NPs for a 15-day period. The authors noted that both forms of Cu were effective at causing oxidative stress in the digestive glands; the GPX activities from the exposure to both forms of copper were similar. However, an induction of metallothionein was observed only in the organisms exposed to Cu2+. The authors concluded that the digestive gland is susceptible to CuO NPs and the related oxidative stress and was the main tissue for their accumulation (Gomes et al. 2012). Along the same line, Hu et al. investigated the toxicity of CuO to Mytilus edulis (Hu et al. 2014). The authors used 100 nm CuO NPs and administered doses from 400 to 1000 ppb to the mussels. It was noted that copper was located in the gill tissue and small amounts in the digestive gland. The data from the study suggested decreased protein thiol and increased carbonylation (protein oxidation) (Hu et al. 2014). The toxic effects of CuO NPs ranging in size from 40 to 100 nm on Exaiptasia pallida was investigated by Siddiqui et al. (Siddiqui et al. 2015). The authors exposed the E. pallida to both Cu2+ and CuO NPs at concentrations ranges of 10–50 ppm and 50–100 mg/L, respectively. In addition, the treatment was performed over 21 days. The Cu2+-exposed organisms showed higher concentrations of copper accumulation and concentration increased with both the dosage and the time of the treatment. However, overall the organisms treated with the CuO NPs showed higher levels of oxidative stress. The oxidative stress was examined using standard enzyme tests for the determination of catalase, glutathione peroxidase, glutathione reductase, and carbonic anhydrase. The authors concluded that the behavior of the CuO NPs and Cu2+ had differences with respect to the magnitude of the effects on the organism (Siddiqui et al. 2015).
21 CuO Toxicity in the Treatment of Fish and Fish Cell Lines
CuO nanoparticles have shown mixed results in the treatment of different fish species . Like the toxicity observed in different species with ZnO NPs, CuO NPs have the added complexity of the Cu ion toxicity. Oxidative stress appears to be common among different fish species treated with CuO NPs. There appears to be organ accumulation of copper in the liver and kidney was observed after treatment with CuO NPs; the form of the copper is generally not specified in the organs. Diano rerio (zebrafish) embryos were investigated by Ganesan et al. for the toxic effects of 51 nm CuO NPs (Ganesan et al. 2016). The authors treated the embryos with concentrations of CuO NPs from 4 to 80 ppm for a total time of 48 h. The authors noticed a 2% death in the embryos at 5 ppm and the LC50 for the CuO NPs was determined to be 64 ppm; there was almost a linear increase in mortality with increasing concentration of the NPs. Similarly, the rate of hatching was observed to decrease with nearing CuO NP concentration. Furthermore, the concentration of the CuO NP increased, the heart rate was decreased in the embryos. As the concentration of the CuO NPs increased, the malformation in the embryos increased. The total protein in the fish was observed to increase as the concentration of CuO NPs increased; however, the AcHe and ATPase concentrations were observed to decrease with increasing NP concentrations. Overall indicators of oxidative stress were increased with increasing CuO NP treatment, which included ROS, lipid peroxidation, PCC, and NO. The antioxidant responses to oxidative stress were observed to decrease with increasing CuO NP concentrations (Ganesan et al. 2016). Carassius auratus or goldfish were subjected to CuO NPs with an average diameter of 40 nm by Ates et al. to investigate the toxic effects through waterborne and dietary exposure (Ates et al. 2015). The fish were exposed to concentrations of 1 and 10 ppm in either the diet or in the water. The intestine showed the highest levels of copper from the NP treatment from dietary and waterborne exposure. The organ with the next highest levels were the gills followed by the liver tissue. However, the concentration of copper in the heart, brain, and muscle was almost the same as the control samples. The high doses for treatment showed increased malondialdehyde concentrations indicating oxidative stress (Ates et al. 2015). The authors concluded that the waterborne exposure exhibited much higher toxicity than observed through dietary exposure. Villarreal et al. studied the toxic effects of 100 nm CuO NPs on Oreochromis mossambicus , a species of Tilapia (Villarreal et al. 2014). The authors used flame synthesized CuO NPs and exposed the fish to concentrations from 0.5 to 5 mg/L. The fish were studied under two different environments: a constant supply of fresh water and an increasing salinity. The fish exposed to the CuO NPs in the saltwater increased in the opercular ventilation. However, the fish exposed to fresh water showed a much smaller response. In addition, the authors noted that the CuO NPs showed effects on the reduced and oxidized glutathione ratios, changes in metal response genes. Furthermore, the organs with increased Cu included the gills and liver, indicating osmotic stress was induced by the CuO NPs (Ates et al. 2015). Isani et al. investigated the effects of 51 nm CuO NPs on Oncorhynchus mykiss , commonly known as rainbow trout (Isani et al. 2013). The authors conducted a comparative study between Cu2+ ions and CuO NPs. The authors performed in vitro and in vivo studies. The in vitro experiment involved the treatment of red blood cells and treated at multiple CuO concentrations. The treatment concentrations used in the in vivo study were 1 ug/g body weight injected into the fish. Both the ionic form and NP form of the copper increased the hemolysis rate in a concentration-dependent manner for the in vitro studies. The hemolysis was more marked in the cells treated with the Cu2+ ions when compared to the CuO NP treatments. The in vivo studies showed high concentrations of the Cu in the gills, kidney, and liver. The data also showed the concentration found in the organs was significantly higher in the ion-treated fish when compared to the NP-treated fish. The toxic effects of 30-40 nm CuO NPs on Oreochromis niloticus , a species of tilapia, were investigated by Abdel-Khalek et al. (Abdel-Khalek et al. 2016). The authors exposed the fish to CuO NPs does at 1/10 and 1/20 the LC50 determined at 96 h of treatment. The fish were exposed to both CuO NPs and bulk. The Cu tissue accumulation of the following treatment was observed in decreasing order liver, kidney, gills, skin, muscle, which caused organ damage. The authors concluded that the CuO NPs were more toxic than the bulk phase. The NPs had a higher ability to be internalized into the cells compared to the bulk phase. As well the fish treated with the NPs showed intracellular osmotic disorders and increased in a determined blood parameter. Cells from Chinook salmon have been investigated for the toxic effects of CuO NPs with an average diameter of 50 nm, by Srikanth et al. (Srikanth et al. 2016). The authors exposed the CHES-214 cells from the Chinook salmon to CuO NPs at concentrations from 5 ppm to 15 ppm. The cells at all concentrations showed increased oxidative stress after treatment compared to the control cells. In addition, the protein carbonyl concentrations were increased, while the lipid peroxidation were increased. The reduced form of glutathione initially was observed to increase at the 5-ppm treatment and then decrease at 10 and 15 ppm, but all levels of glutathione were at increased levels compared to the control. Furthermore, the concentration of the oxidized glutathione was observed to be lower than the control samples in the study. Both glutathione peroxidase and glutathione sulfo-transferase were increased at all treatments compared to the control samples. As well, all the responses to oxidative stress were at increased levels compared to the control samples. The authors also showed cellular abnormalities with respect to morphological changes. The study concluded that CuO NPs were cytotoxic in a concertation-dependent manner.
22 CuO Toxicity in the Treatment of Mammalian Cells
In cell line studies, CuO NPs have been shown to reduce the cellular viability and induce oxidative stress. Karlsson et al. investigated the effects of CuO on human lung epithelial cell line A549 (Jeng and Swanson 2006). The authors studied the effects of 42 nm CuO nanoparticles at an 80-ppm concentration in solution for 18 h. The study showed a reduce viability of the cells of 96%. In addition, the CuO NPs showed high DNA damage within the cells. In addition, there was a significant increase in the ROS generation within the cells comparted to control treatments. The authors showed that the CuO NPs had an eightfold increase in the dose-response curve when compared to the treatment of the cells with the ionic form of Cu2+ from CuCl2.
23 Conclusion
The toxicity of metal oxide NPs can be generally summed up as an oxidative stress process in different species. However, it is not as simple as one mechanism being responsible for the toxicity. In some species such as diatoms, the molting process becomes inhibited causing imminent death. In addition, the behavior of different nanoparticles in solution also has an effect on the toxicity. For example, TiO2 is insoluble in water, but ZnO and CuO NPs are somewhat water soluble which generate the free metal ion in solution. In discussing the toxicity of ZnO and CuO NPs, the formation of the free ions in solution adds complicity to the question of NP toxicity and the causes. In addition, there does appear to be a link between the phases of the NPs that organisms are exposed to, which has been discussed in the case of TiO2 NP toxicity. For example, the toxicity of the anatase phase of TiO2 appears to be higher and has more effects than the rutile phase of TiO2. The difference in the effect of anatase and rutile may be due to the relatively higher structural stability of rutile when compared to anatase. It is noteworthy that many of the TiO2 NP toxicity studies have used the Degussa mixture of TiO2 (anatase and rutile phases in a ratio of about 3:1), which is a common photocatalytic commercial specimen of TiO2. In addition, the toxicity of NPs does appear to be aggravated in the presence of light. On perusal of literature, it appears that multiple levels of nanomaterial interactions contribute to specific toxicity phenotypes in an organism. In addition, toxic effects also arise from the stabilizing agents utilized in the synthesis and dispersion of NPs in solution. This review thus suggests using multiple levels of interaction studies to develop a full grasp of nanotoxicity.
References
Abdel-Khalek, A. A., Badran, S. R., & Marie, M. (2016). Toxicity evaluation of copper oxide bulk and nanoparticles in Nile tilapia, Oreochromis niloticus, using hematological, bioaccumulation and histological biomarkers. Fish Physiology and Biochemistry, 42, 1225–1236.
Abdel-Mohsen, A. M., Hrdina, R., Burgert, L., Abdel-Rahman, R. M., Hašová, M., Šmejkalová, D., Kolář, M., Pekar, M., & Aly, A. S. (2013). Antibacterial activity and cell viability of hyaluronan fiber with silver nanoparticles. Carbohydrate Polymers, 92, 1177–1187.
Absar, A., Satyajyoti, S., Islam, K. M., Sastry, K. R., & Murali, S. (2005). Extra-/intracellular biosynthesis of gold nanoparticles by an Alkalotolerant fungus, Trichothecium sp. Journal of Biomedical Nanotechnology, 1, 47–53.
Adeleye, A. S., Oranu, E. A., Tao, M., & Keller, A. A. (2016). Release and detection of nanosized copper from a commercial antifouling paint. Water Research, 102, 374–382.
Akhtar, M. J., Ahamed, M., Kumar, S., Majeed Khan, M. A., Ahmad, J., & Alrokayan, S. A. (2012). Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. International Journal of Nanomedicine, 7, 845–857.
Ali, D., Alarifi, S., Kumar, S., Ahamed, M., & Siddiqui, M. A. (2012). Oxidative stress and genotoxic effect of zinc oxide nanoparticles in freshwater snail Lymnaea luteola L. Aquatic Toxicology, 124–125, 83–90.
Arora, S., Sharma, P., Kumar, S., Nayan, R., Khanna, P. K., & Zaidi, M. G. H. (2012). Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regulation, 66, 303.
Aruoja, V., Dubourguier, H., Kasemets, K., & Kahru, A. (2009). Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Science Total Environment, 407, 1461–1468.
Asharani, P. V., Wu, Y. L., Gong, Z., & Valiyaveettil, S. (2008). Toxicity of silver nanoparticles in zebrafish models. Nanotechnology, 19, 255102.
Ates, M., Arslan, Z., Demir, V., Daniels, J., & Farah, I. O. (2015). Accumulation and toxicity of CuO and ZnO nanoparticles through waterborne and dietary exposure of goldfish (Carassius auratus). Environmental Toxicology, 30, 119–128.
Bhuvaneshwari, M., Iswarya, V., Archanaa, S., Madhu, G. M., Kumar, G. K. S., Nagaraj, R., Chandrasekaran, N., & Mukherjee, A. (2015). Cytotoxicity of ZnO NPs towards fresh water algae Scenedesmus obliquus at low exposure concentrations in UV-C, visible and dark conditions. Aquatic Toxicology, 162, 29–38.
Borghi, E. B., Morando, P. J., & Blesa, A. (1991). Dissolution of magnetite by Mercaptocarboxylic acids. Langmuir, 7, 1652–1659.
Bozich, J. S., Lohse, S. E., Torelli, M. D., Murphy, C. J., Hamers, R. J., & Klaper, R. D. (2014). Surface chemistry, charge and ligand type impact the toxicity of gold nanoparticles to Daphnia magna. Environmental Science. Nano, 1, 260–270.
Buerki-Thurnherr, T., Xiao, L., Diener, L., Arslan, O., Hirsch, C., Maeder-Althaus, X., Grieder, K., Wampfler, B., Mathur, S., Wick, P., & Krug, H. F. (2013). In-vitro mechanistic study towards a better understanding of ZnO nanoparticle toxicity. Nanotoxicology, 7, 402–416.
Buffet, P., Tankoua OF, Pan, J., Berhanu, D., Herrenknecht, C., Poirier, L., Amiard-Triquet, C., Amiard, J., Berard, J., Risso, C., Guibbolini, M., Romeo, M., Reip, P., Valsami-Jones, E., & Mouneyrac, C. (2011). Behavioural and biochemical responses of two marine invertebrates Scrobicularia plana and Hediste diversicolor to copper oxide nanoparticles. Chemosphere, 84, 166–174.
Buffet, P.-E., Amiard-Triquet, C., Dybowska, A., Risso-de Faverney, C., Guibbolini, M., Valsami-Jones, E., & Mouneyrac, C. (2012). Fate of isotopically labeled zinc oxide nanoparticles in sediment and effects on two endobenthic species, the clam Scrobicularia plana and the ragworm Hediste diversicolor. Ecotoxicology and Environmental Safety, 84, 191–198.
Calzolai, L., Gilliland, D., & Rossi, F. (2012). Measuring nanoparticles size distribution in food and consumer products: A review. Journal of Food Additives Contaminants A., 29, 1183–1193.
Casaletto, M. P., Longo, A., Martorana, A., Prestianni, A., & Venezia, A. M. (2006). XPS study of supported gold catalysts: The role of Au0 and Au+δ species as active sites. Surface and Iterface Analysis, 38, 215–218.
Chakraborty, C., Sharma, A. R., Sharma, G., & Lee, S.-S. (2016). Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. Journal of Nanbiotechnology, 14. https://doi.org/10.1186/s12951-016-0217-6.
Cheloni, G., Marti, E., & Slaveykova, V. I. (2016). Interactive effects of copper oxide nanoparticles and light to green alga Chlamydomonas reinhardtii. Aquatic Toxicology, 170, 120–128.
Chen, J., Dong, X., Zhao, J., & Tang, G. (2009). In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. Journal of Applied Toxicology, 29, 330–337.
Chen, L., Zhou, L., Liu, Y., Deng, S., Wu, H., & Wang, G. (2012). Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii. Ecotoxicology and Environmental Safety, 84, 155–162.
Chen, T.-H., Lin, C.-Y., & Tseng, M.-C. (2011). Behavioral effects of titanium dioxide nanoparticles on larval zebrafish (Danio rerio). Marine Pollution Bulletin, 63, 303–308.
Chwalibog, A., Sawosz, E., Hotowy, A., Szeliga, J., Mitura, S., Mitura, K., Grodzik, M., Orlowski, P., & Sokolowska, A. (2010). Visualization of interaction between inorganic nanoparticles and bacteria or fungi. International Journal of Nanomedicine, 5, 1085–1094.
Clément, L., Hurel, C., & Marmier, N. (2013). Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants – Effects of size and crystalline structure. Chemosphere, 90, 1083–1090.
Currie, H. A., & Silica in Plants, P. C. C. (2007). Biological, biochemical and chemical studies. Annals of Botany, 100, 1383–1389.
Dabrunz, A., Duester, L., Prasse, C., Seitz, F., Rosenfeldt, R., Schilde, C., Schaumann, G. E., & Schulz, R. (2011). Biological surface coating and molting inhibition as mechanisms of TiO2 nanoparticle toxicity in Daphnia magna. PLoS One, 6, e20112. https://doi.org/10.1371/journal.pone.0020112.
Dasari, T. P., Pathakoti, K., & Hwang, H.-M. (2013). Determination of the mechanism of photoinduced toxicity of selected metal oxide nanoparticles (ZnO, CuO, Co3O4 and TiO2) to E. coli bacteria. Journal of Environmental Sciences, 25, 882–888.
Deng, Q., Tang, H., Liu, G., Song, X., Kang, S., Wang, H., Ng, D. H. L., & Wang, G. (2015). Photocatalytic degradation of 2,4,40-trichlorobiphenyl into long-chain alkanes using Ag nanoparticle decorated flower-like ZnO microspheres. New Journal of Chemistry, 39, 7781.
Durán, N., Durán, M., Jesus, M. B., Seabra, A. B., Fávaro, W. J., & Nakazato, G. (2016). Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine: Nanotechnology, Biology and Medicine, 12, 789–799.
Durán, N., Marcato, P. D., De Cont, R., Alves, O. L., Costa, F. T. M., & Brocchi, M. (2010). Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. Journal of the Brazilian Chemical Society, 21, 949–959.
Esmaeillou, M., Moharamnejad, M., Hsankhani, R., Tehrani, A. A., & Maadi, H. (2013). Toxicity of ZnO nanoparticles in healthy adult mice. Environmental Toxicology and Pharmacology, 35, 67–71.
Fabrega, J., Fawcett, S. R., Renshaw, J. C., & Lead, J. R. (2009). Silver nanoparticle impact on bacterial growth: effect of pH, concentration, and organic matter. Environmental Science & Technology, 43, 7285–7290.
Fafarman, A. T., Koh, W.-K., Diroll, B. T., Kim, D. K., Ko, D.-K., Oh, S. J., Ye, X., Doan-Nguyen, V., Crump, M. R., Reifsnyder, D. C., Murray, C. B., & Kagan, C. R. (2011). Thiocyanate-capped nanocrystal colloids: Vibrational reporter of surface chemistry and solution-based route to enhanced coupling in nanocrystal solids. Journal of the American Chemical Society, 133, 15753–15761.
Fröhlich, E., & Roblegg, E. (2012). Models for oral uptake of nanoparticles in consumer products. Toxicology, 291, 10–17.
Fu, L., Hamzeh, M., Dodard, S., Zhao, Y. H., & Sunahara, G. I. (2015). Irradiation effects of TiO2 nanoparticles on ROS production and growth inhibition using freshwater green algae pre-exposed to UV irradiation. Environmental Toxicology and Pharmacology, 39, 1074–1080.
Ganesan, S., Thirumurthi, N. A., Raghunath, A., Vijayakumar, S., & Perumal, E. (2016). Acute and sub-lethal exposure to copper oxide nanoparticles causes oxidative stress and teratogenicity in zebrafish embryos. Journal of Applied Toxicology, 36, 554–567.
Gardea-Torresdey, J. L., Parsons, J. G., Gomez, E., Peralta-Videa, J., Troiani, H. E., Santiago, T. P., & Yacaman, M. J. (2002). Formation and growth of au nanoparticles inside live alfalfa plants. Nano Letters, 2, 397–401.
Gerloff, K., Fenoglio, I., Carella, E., Kolling, J., Albrecht, C., Boots, A. W., Förster, I., & Schins, R. P. F. (2012). Distinctive toxicity of TiO2 rutile/Anatase mixed phase nanoparticles on Caco-2 cells. Chemical Research in Toxicology, 25, 646–655.
Gomes, T., Pereira, C. G., Cardoso, C., Pinheiro, J. P., Cancio, I., & Bebianno, M. J. (2012). Accumulation and toxicity of copper oxide nanoparticles in the digestive gland of Mytilus galloprovincialis. Aquatic Toxicology, 118-119, 72–79.
Govindaraju, K., Khaleel, S., Vijayakumar, B., Kumar, G., & Singaravelu, G. (2008). Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. Journal of Materials Science, 43, 5115–5122.
Gregory, J. (2005). Particles in water: Properties and processes. CRC Press.
Greulich, C., Braun, D., Peetsch, A., Diendorf, J., Siebers, B., Epple, M., & Köller, M. (2012). The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Advances, 2, 6981–6987.
Griffin, S., Masood, M. I., Nasim, M. J., Sarfraz, M., Ebokaiwe, A. P., Schäfer, K. H., Keck, C. M., & Jacob, C. (2018). Natural nanoparticles: A particular matter inspired by nature. Antioxidants (Basel), 7, 3.
Grigore, M. E., Biscu, E. R., Holban, A. M., Gestal, M. C., & Grumezescu, A. M. (2016). Methods of Synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals (Basel), 9, 75.
Grzelczak, M., Pérez-Juste, J., Mulvaney, P., & Liz-Marzán, L. M. (2008). Shape control in gold nanoparticle synthesis. Chemical Society Reviews, 37, 1783–1791.
Hajipour, M. J., Fromm, K. M., Ashkarran, A. A., de Aberasturi, D. J., de Larramendi, I. R., Rojo, T., Serpooshan, V., Parak, W. J., & Mahmoudi, M. (2012). Antibacterial properties of nanoparticles. Trends in Biotechnology, 30, 499–511.
Haldorai, Y., Kim, B.-K., Jo, Y.-L., & Shim, J.-J. (2014). Ag@graphene oxide nanocomposite as an efficient visible-light plasmonic photocatalyst for the degradation of organic pollutants: A facile green synthetic approach. Materials Chemistry and Physics, 143, 1452–1461.
Heike, L. M., Norbert, T., Daniela, J., Vladimir, D., Baranov, I., & Kneipp, J. (2014). Trends in single-cell analysis by use of ICP-MS. Analytical and Bioanalytical Chemistry, 206, 6963.
Heinlaan, M., Ivask, A., Blinova, I., Dubourguier, H., & Kahru, A. (2008). Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere, 71, 1308–1316.
Heinlaan, M., Kahru, A., Kasemets, K., Arbeille, B., Prensier, G., & Dubourguier, H. (2011). Changes in the Daphnia magna midgut upon ingestion of copper oxide nanoparticles: A transmission electron microscopy study. Water Research, 45, 179–190.
Ho, C.-M., Yau, S. K.-W., Lok, C.-N., So, M.-H., & Che, C. M. (2010). Oxidative dissolution of silver nanoparticles by biologically relevant oxidants: A kinetic and mechanistic study. Chemistry, an Asian Journal, 5, 285–293.
Hough, R. M., Noble, R. R. P., Hitchen, G. J., Hart, R., Reddy, S. M., Saunders, M., Clode, P., Vaughan, D., Lowe, J., Gray, D. J., An, R. R., Butt, C. R. M., & Verrall, M. (2008). Naturally occurring gold nanoparticles and nanoplates. Geology, 36, 571–574.
Hsiao, I.-L., & Huang, Y.-J. (2011). Effects of various physicochemical characteristics on the toxicities of ZnO and TiO2 nanoparticles toward human lung epithelial cells. Science of the Total Environment, 409, 1219–1228.
Hu, W., Culloty, S., Darmody, G., Lynch, S., Davenport, J., Ramirez-Garcia, S., Dawson, K. A., Lynch, I., Blasco, J., & Sheehan, D. (2014). Toxicity of copper oxide nanoparticles in the blue mussel, Mytilus edulis: A redox proteomic investigation. Chemosphere, 108, 289–299.
Hu, Y., Xie, J., Tong, Y. W., & Wang, C.-H. (2007). Effect of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the HepG2 cells. Journal of Controlled Release, 118, 7–17.
Hurst, J. K., & Lymar, S. V. (1997). Toxicity of Peroxynitrite and related reactive nitrogen species toward Escherichia coli. Chemical Research in Toxicology, 10, 802–810.
Hwang, E. T., Lee, J. H., Chae, Y. J., Kim, Y. S., Kim, B. C., & Sang, B.-I. (2008). Gu MB. Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent Bacteria. Small, 4, 746–750.
Institute of Medicine, Food and Nutrition Board. (1998). Dietary reference intakes: Thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press. Copper related health conditions.
Isani, G., Falcioni, M. L., Barucca, G., Sekar, D., Andreani, G., Carpene, E., & Falcioni, G. (2013). Comparative toxicity of CuO nanoparticles and CuSO4 in rainbow trout. Ecotoxicology and Environmental Safety, 97, 40–46.
Jansa, H., & Huo, Q. (2012). Gold nanoparticle-enabled biological and chemical detection and analysis. Chemical Society Reviews, 41, 2849–2866.
Jeevanandam, J., Barhoum, A., Chan, Y. S., Dufresne, A., & Danquah, M. K. (2018). Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein Journal of Nanotechnology, 9, 1050–1074.
Jeng, H. A., & Swanson, J. (2006). Toxicity of metal oxide nanoparticles in mammalian cells. Journal of Environmental Science and Health, Part A, 41, 2699–2711.
Ji, J., Longa, Z., & Lin, D. (2011). Toxicity of oxide nanoparticles to the green algae Chlorella sp. Chemical Engineering Journal, 170, 525–530.
Jovanovic, B., Whitley, E. M., Kimura, K., Crumpton, A., & Pali, D. (2015). Titanium dioxide nanoparticles enhance mortality of fish exposed to bacterial pathogens. Environmental Pollution, 203, 153e164.
Karlsson, H. L., Cronholm, P., Gustafsson, J., & Moller, L. (2008). Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chemical Research in Toxicology, 21, 1726–1732.
Kerkmann, M., Costa, L. T., Richter, C., Rothenfusser, S., Battiany, J., Hornung, V., Johnson, J., Engler, S., Ketterer, T., Heckl, W., Thalhammer, S., Endres, S., & Hartmann, G. (2004). Spontaneous formation of nucleic acid-based nanoparticles is responsible for high interferon-α induction by CpG-A in Plasmacytoid dendritic cells. The Journal of Biological Chemistry, 280, 8086–8093.
Khan, R., Inam, M. A., Park, D. R., Khan, S., Akram, M., & Yeom, I. T. (2019). The removal of CuO nanoparticles from water by conventional treatment C/F/S: The effect of pH and natural organic matter. Molecules, 24, 914.
Khashan, K. S., Sulaiman, G. M., & Abdulameer, F. A. (2016). Synthesis and antibacterial activity of CuO nanoparticles suspension induced by laser ablation in liquid. Arabian Journal for Science and Engineering, 41, 301–310.
Kimling, J., Maier, M., Okenve, B., Kotaidi, V., Ballot, H., & Plech, A. (2006). Turkevich method for gold nanoparticle synthesis revisited. The Journal of Physical Chemistry. B, 110, 15700–15707.
Kimura, H., Sawada, T., Oshima, S., Kozawa, K., Ishioka, T., & Kato, M. (2005). Toxicity and roles of reactive oxygen species. Current Drug Targets. Inflammation and Allergy, 4, 489–495.
Kitchens, C. L., McLeod, M. C., & Roberts, C. B. (2005). Chloride ion effects on synthesis and directed assembly of copper nanoparticles in liquid and compressed alkane microemulsions. Langmuir, 21, 5166–5173.
Kumar, A., Swarup, D., & Maji, K. (2014). Adhikary B. γ-Fe2O3 nanoparticles: An easily recoverable effective photo-catalyst for the degradation of rose bengal and methylene blue dyes in the waste-water treatment plant. Materials Research Bulletin, 49, 28–34.
Kumar, V., Guleria, P., Vinay, G., Sudesh, K., & Yadav, K. (2013). Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana. Science of Total Environment, 461–462, 462–468.
Lammel, T., & Sturve, J. (2018). Assessment of titanium dioxide nanoparticle toxicity in the rainbow trout (Onchorynchus mykiss) liver and gill cell lines RTL-W1 and RTgill-W1 under particular consideration of nanoparticle stability and interference with fluorometric assays. NanoImpact, 11, 1–19.
Le, T. T., Nguyen, K.-H., Jeon, J.-R., Francis, A. J., & Chang, Y.-S. (2015). Nano/bio treatment of polychlorinated biphenyls with evaluation of comparative toxicity. Journal of Hazardous Materials, 287, 335–341.
Lee, J., Lilly, G. D., Doty, R. C., Podsiadlo, P., & Kotov, N. A. (2009). In vitro toxicity testing of nanoparticles in 3D cell culture. Small, 5, 1213–1221.
Lee, W.-M., & An, Y.-J. (2013). Effects of zinc oxide and titanium dioxide nanoparticles on green algae under visible, UVA, and UVB irradiations: No evidence of enhanced algal toxicity under UV pre-irradiation. Chemosphere, 91, 536–544.
Li, F., Liang, Z., Zheng, X., Zhao, W., Wu, M., & Wang, Z. (2015). Toxicity of nano-TiO2 on algae and the site of reactive oxygen species production. Aquatic Toxicology, 158, 1–13.
Li, X., Liu, Y., Song, L., & Liu, J. (2003). Responses of antioxidant systems in the hepatocytes of common carp (Cyprinus carpio L.) to the toxicity of microcystin-LR. Toxicon, 42, 85–89.
Lim, B., Xiong, Y., & Xia, Y. (2007). A water-based synthesis of octahedral, decahedral, and icosahedral Pd nanocrystals. Angewandte Chemie, International Edition, 46, 9279–9282.
Love, S. A., Maurer-Jones, M. A., Thompson, J. W., Lin, Y.-S., & Haynes, C. L. (2012). Assessing nanoparticle toxicity. Annual Review of Analytical Chemistry, 5, 181–205.
Luis, I. P.-S., & Liz-Marzán, M. (2002). Synthesis of silver Nanoprisms in DMF. Nano Letters 2, 8, 903–905.
Mansfield, C. M., Alloy, M. M., Hamilton, J., Verbeck, G. F., Newton, K., Klaine, S. J., & Roberts, A. P. (2015). Photo-induced toxicity of titanium dioxide nanoparticles to Daphnia magna under natural sunlight. Chemosphere, 120, 206–210.
Manzo, S., Miglietta, M. L., Rametta, G., Buono, S., & Francia, G. D. (2013). Toxic effects of ZnO nanoparticles towards marine algae Dunaliella tertiolecta. Science of Total Environment, 445–446, 371–376.
Marisa, I., Marin, M. G., Caicci, F., Franceschinis, E., Martucci, A., & Matozzo, V. (2015). In vitro exposure of haemocytes of the clam Ruditapes philippinarum to titanium dioxide (TiO2) nanoparticles: Nanoparticle characterisation, effects on phagocytic activity and internalisation of nanoparticles into haemocytes. Marine Environment Research, 103, 11e17.
Marquis, B. J., Love, S. A., Braun, K. L., & Haynes, C. L. (2009). Analytical methods to assess nanoparticle toxicity. Analyst, 134, 425–439.
McDonogh, R. M., Fell, C. J. D., & Fane, A. G. (1984). Surface charge and permeability in the ultrafiltration of non-flocculating colloids. Journal of Membrane Science, 21, 285–294.
Melegari, S. P., Perreault, F., Ribeiro Costa, R. H., Popovic, R., & Matias, W. G. (2013). Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquatic Toxicology, 142, 431–440.
Merrifield, R. C., Stephan, C., & Lead, J. R. (2018). Quantification of Au nanoparticle biouptake and distribution to freshwater algae using single cell—ICP-MS. Environmental Science & Technology, 52, 2271–2277.
Metzler, D. M., Li, M., Erdem, A., & Huang, C. P. (2011). Responses of algae to photocatalytic nano-TiO2 particles with an emphasis on the effect of particle size. Chemical Engineering Journal, 170, 538–546.
Miller, R. J., Lenihan, H. S., Muller, E. B., Tseng, N., Hanna, S. K., & Keller, A. A. (2010). Impacts of metal oxide nanoparticles on marine phytoplankton. Environmental Science & Technology, 44, 7329–7334.
Misra, S. K., Dybowska, A., Berhanu, D., Luoma, S. N., & Valsami-Jones, E. (2012). The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Science of the Total Environment, 438, 225–232.
Mortimer, M., Kasemets, K., & Kahru, A. (2010). Toxicity of ZnO and CuO nanoparticles to ciliated protozoa Tetrahymena thermophila. Toxicology, 269, 182–189.
Mwaanga, P., Carraway, E. R., & van den Hurk, P. (2014). The induction of biochemical changes in Daphnia magna by CuO and ZnO nanoparticles. Aquatic Toxicology, 150, 201–209.
National Research Council Committee on Copper in Drinking Water. (2000). Copper in Drinking Water. Washington, DC: National Academies Press.
Nosaka, Y., & AY, N. (2017). Generation and detection of reactive oxygen species in photocatalysis. Chemical Reviews, 117(17), 11302–11336.
Parke, D. V., & Sapota, A. (1996). Chemical toxicity and reactive oxygen species. International Journal of Occupational Medicine Environmental Health, 9, 331–340.
Pauksch, L., Hartmann, S., Rohnke, M., Szalay, G., Alt, V., Schnettler, R., & Lips, K. S. (2014). Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomaterialia, 10, 439–449.
Peng, X., Palma, S., Fisher, N. S., & Wong, S. S. (2011). Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquatic Toxicology, 102, 186–196.
Personick, M. L., Langille, M. R., Zhang, J., Harris, N., Schatz, G. C., & Mirkin, C. A. (2011). Synthesis and isolation of {110}-faceted gold bipyramids and rhombic dodecahedra. Journal of the American Chemical Society, 133, 6170–6173.
Postma, D. (1993). The reactivity of iron oxides in sediments: A kinetic approach. Geochimica et Cosmochimica Acta, 57, 5027–5034.
Prabhu, S., & Poulose, E. K. (2012). Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. International Nano Letters, 2. https://doi.org/10.1186/2228-5326-2-32.
Pradhan, A., Seena, S., Pascoal, C., & Cássio, F. (2012). Copper oxide nanoparticles can induce toxicity to the freshwater shredder Allogamus ligonifer. Chemosphere, 89, 1142–1150.
Raffi, M., Hussain, F., Bhatti, T. M., Akhter, J. I., Hameed, A., & Hasan, M. M. (2008). Antibacterial characterization of silver nanoparticles against E. Coli ATCC-15224. Journal of Materials Science and Technology, 24, 192–196.
Reeves, J. F., Davies, S. J., Dodd, N. J. F., & Jha, A. N. (2008). Hydroxyl radicals (•OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutation Research, 640, 113–122.
Rico, C. M., Lee, S. C., Rubenecia, R., Mukherjee, A., Hong, J., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2014). Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.). Journal of Agricultural and Food Chemistry, 62, 9669–9675.
Rodriguez, A., Bruno, C. A., Marie-José, C., Lecante, C. P., & Bradley, J. S. (1996). Synthesis and isolation of cuboctahedral and icosahedral platinum nanoparticles. Ligand-dependent structures. Chemistry of Materials, 8, 1978–1986.
Rogers, M. A. (2016). Naturally occurring nanoparticles in food. Current Opinion in Food Science, 7, 14–19.
Roy, R., Kumar, D., Sharma, A., Gupta, P., Chaudhari, B. P., Tripathi, A., Das, M., & Dwivedi, P. D. (2014). ZnO nanoparticles induced adjuvant effect via toll-like receptors and Src signaling in Balb/c mice. Toxicology Letters, 230, 421–433.
Sadiq, M., Dalai, S., Chandrasekaran, N., & Mukherjee, A. (2011). Ecotoxicity study of titania (TiO2) NPs on two microalgae species: Scenedesmus sp. and Chlorella sp. Ecotoxicology and Environmental Safety, 74, 1180–1187.
Saif, H. S., Singh, S., Parikh, R. Y., Dharne, M. S., Patole, M., Prasad, B. L. V., & Shouche, Y. S. (2008). Bacterial synthesis of copper/copper oxide nanoparticles. Journal of Nanoscience and Nanotechnology, 8, 3191–3196.
Sankar, R., Prasath, B. B., Nandakumar, R., Santhanam, P., Shivashangari, K. S., & Ravikumar, V. (2014). Growth inhibition of bloom forming cyanobacterium Microcystis aeruginosa by green route fabricated copper oxide nanoparticles. Environmental Science and Pollution Research, 21, 14232–14240.
Scarabelli, L., Grzelczak, M., & Liz-Marzán, L. M. (2013). Tuning gold nanorod synthesis through prereduction with salicylic acid. Chemistry of Materials, 25, 4232–4238.
Schnippering, M., Powell, H. V., Zhang, M., Macpherson, J. V., Unwin, P. R., Mazurenka, M., & Mackenzie, S. R. (2008). Surface assembly and redox dissolution of silver nanoparticles monitored by evanescent wave cavity ring-down spectroscopy. Journal of Physical Chemistry C, 112, 15274–15280.
Sharma, V. K., Sayes, C. M., Guo, B., Pillai, S., Parsons, J. G., Wang, C., Yan, B., & Ma, X. (2019). Interactions between silver nanoparticles and other metal nanoparticles under environmentally relevant conditions: A review. Science of Total Environment, 653, 1042–1051.
Shi, W., Han, Y., Guo, C., Zhao, X., Liu, S., Su, W., Zha, S., Wang, Y., & Liu, G. (2017). Immunotoxicity of nanoparticle nTiO2 to a commercial marine bivalve species, Tegillarca granosa. Fish & Shellfish Immunology, 66, 300–306.
Shibata, S., Miyajima, K., Kimura, Y., & Yano, T. (2004). Heat-induced precipitation and light-induced dissolution of metal (Ag & Au) nanoparticles in hybrid film. Journal of Sol-Gel Science and Technology, 31, 123–130.
Siddiqui, S., Goddard, R. H., & Bielmyer-Fraser, G. K. (2015). Comparative effects of dissolved copper and copper oxide nanoparticle exposure to the sea anemone, Exaiptasia pallida. Aquatic Toxicology, 160, 205–213.
Song, K. C., Lee, S. M., Park, T. S., & Lee, B. S. (2009). Preparation of colloidal silver nanoparticles by chemical reduction method. Korean Journal of Chemical Engineering, 26, 153.
Song, W., Zhang, J., Guo, J., Zhang, J., Ding, F., Li, L., & Sun, Z. (2010). Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicology Letters, 199, 389–397.
Srikanth, K., Pereira, E., Duarte, A. C., & Rao, J. V. (2016). Evaluation of cytotoxicity, morphological alterations and oxidative stress in Chinook salmon cells exposed to copper oxide nanoparticles. Protoplasma, 253, 873–884.
Strasser, P., Koh, S., Anniyev, T., Greeley, J., More, K., Yu, C., Liu, Z., Kaya, S., Nordlund, D., Ogasawara, H., Toney, M. F., & Nilsson, A. (2010). Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nature Chemistry, 2, 454–460.
Stumm, W., & Morgan, J. J. (1996). Aquatic chemistry: Chemical equilibria and rates in natural waters. New York: Wiley.
Sulzberger, B., & Laubscher, H. (1995). Reactivity of various types of iron(III) (hydr)oxides towards light-induced dissolution. Marine Chemistry, 50, 103–115.
Sun, R. W.-Y., Chen, R., Chung, N. P.-Y., Ho, C.-M., Lin, C.-L. S., & Che, C.-M. (2005). Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chemical Communications, 40, 5059–5061.
Suppi, S., Kasemets, K., Ivask, A., Kuennis-Beres, K., Sihtmaee, M., Kurvet, I., Aruoja, V., & Kahru, A. (2015). A novel method for comparison of biocidal properties of nanomaterials to bacteria, yeasts and algae. Journal of Hazardous Materials, 286, 75–84.
Tetard, L., Passian, A., Venmar, K. T., Lynch, R. M., Voy, B. H., Shekhawat, G., Dravid, V. P., & Thundat, T. (2008). Imaging nanoparticles in cells by nanomechanical holography. Nature Nanotechnology, 3, 501–505.
Tulve, N. S., Stefaniak, A. B., Vance, M. E., Rogers, K., Mwilu, S., LeBouf, R. L., Schwegler-Berry, D., Willis, R., Thomas, T. A., & Marr, L. C. (2015). Characterization of silver nanoparticles in selected consumer products and its relevance for predicting children's potential exposures. International Journal of Hygiene and Environmental Health, 218, 345–357.
Vance, M. E., Kuiken, T., Vejerano, E. P., McGinnis, S. P., Hochella, M. F., Jr., Rejeski, D., & Hull, M. S. (2015). Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein Journal of Nanotechnology, 6, 1769–1780.
Villarreal, F. D., Das, G. K., Abid, A., Kennedy, I. M., & Kultz, D. (2014). Sublethal effects of CuO nanoparticles on Mozambique tilapia (Oreochromis mossambicus) are modulated by environmental salinity. PLoS One, 9, 88723.
Wang, Z., von dem Bussche, A., Kabadi, P. K., Kane, A. B., & Hurt, R. H. (2013). Biological and environmental transformations of copper-based nanomaterials. ACS Nano, 7, 8715–8727.
Warheit, D. B., Hoke, R. A., Finlay, C., Donner, E. M., Reed, K. L., & Sayes, C. M. (2007). Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicology Letters, 171, 99–110.
Warheit, D. B., Webb, T. R., Sayes, C. M., Colvin, V. L., & Reed, K. L. (2006). Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: Toxicity is not dependent upon particle size and surface area. Toxicological Sciences, 91, 227–236. https://doi.org/10.1093/toxsci/kfj140.
Wu, J., Liu, W., Xue, C., Zhou, S., Lan, F., Bi, L., Xu, H., Yang, X., & Zeng, F.-D. (2009). Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicology Letters, 191, 1–8.
Wuithschick, M., Birnbaum, A., Witte, S., Sztuck, M., Vainio, U., Pinna, N., Rademann, K., Emmerling, F., Kraehnert, R., & Polte, J. (2015). Turkevich in New Robes: Key questions answered for the most common gold nanoparticle synthesis. ACS Nano, 9, 7052–7071.
Yang, S. P., Bar-Ilan, O., Peterson, R. E., Heideman, W., Hamers, R. J., & Pedersen, J. A. (2013). Influence of humic acid on titanium dioxide nanoparticle toxicity to developing zebrafish. Environmental Science & Technology, 47, 4718–4725.
Yasun, E., Chunmei Li, C., Barut, I., Janvier, D., Qiu, L., Cuia, C., & Tan, W. (2015). BSA modification to reduce CTAB induced nonspecificity and cytotoxicity of aptamer-conjugated gold nanorods. Nanoscale, 22, 10240–10248.
Zhang, S., Zhang, X., Jiang, G., Zhu, H., Guo, S., Su, D., Lu, G., & Sun, S. (2014). Tuning nanoparticle structure and surface strain for catalysis optimization. Journal of the American Chemical Society, 136, 7734–7739.
Zhao, X., Wang, S., Wu, Y., You, H., & Lv, L. (2013). Acute ZnO nanoparticles exposure induces developmental toxicity, oxidative stress and DNA damage in embryo-larval zebrafish. Aquatic Toxicology, 136–137, 49–59.
Zhong, Z., Patskovskyy, S., Bouvrette, P., Luong, J. H. T., & Gedanken, A. (2004). The surface chemistry of Au colloids and their interactions with functional amino acids. The Journal of Physical Chemistry. B, 108, 4046–4052.
Zhu, X., Chang, Y., & Chen, Y. (2010). Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere, 78, 209–215.
Zhu, X., Zhu, L., Duan, Z., Qi, R., Li, Y., & Lang, Y. (2008). Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to zebrafish (Danio rerio) early developmental stage. Journal of Environmental Science and Health, Part A, 43, 278–284.
Acknowledgments
J.G. Parsons is grateful for the generous support provided by a Departmental Grant from the Robert A. Welch Foundation (Grant No. BX-0048). M. Alcoutlabi would like to acknowledge the support from NSF PREM /award/ under grant No. DMR-1523577: UTRGV-UMN Partnership for Fostering Innovation by Bridging Excellence in Research and Student Success.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Parsons, J.G., Alcoutlabi, M., Dearth, R.K. (2021). Metal Oxide Nanoparticle Toxicity in Aquatic Organisms: An Overview of Methods and Mechanisms. In: Sharma, N., Sahi, S. (eds) Nanomaterial Biointeractions at the Cellular, Organismal and System Levels. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-65792-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-65792-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65791-8
Online ISBN: 978-3-030-65792-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)