Abstract
Nanoparticles (NPs) are dangerous micropollutants that exhibit biotoxicity even in low (ng/L range) concentrations. Apart from direct toxicity to living organisms, NPs can absorb and transfer organic or inorganic toxicants as well as potentiate the toxicity of other micropollutants. Increasing use of NPs in industrial and domestic applications leads to their increased production and discharge into the environment giving rise to diverse risks for ecosystems. These risks are exacerbated by the resilience of NPs to biodegradation in natural ecosystems and traditional wastewater treatment plants. Efficient NP removal technologies are complex and expensive, so they cannot be affordably replicated in common wastewater treatment plants. Despite the risks associated with NPs, humanity will not abandon their use in the nearest future, since NPs are now at the foundation of many modern technologies. The biodestruction and biosorption of NPs using microalgae cultures and algal-bacterial consortia are considered promising approaches regarding environmental safety and the conservation of natural resources. However, the progress of this approach is hindered by the paucity and fragmentary nature of the information about the effects of NPs on microalgae cells and microbial communities. This review attempts to fill this gap, at least partially, by considering common industrial NP types based on metals and their oxides as well as carbon nanomaterials. The pathways of their entry into aquatic ecosystems, toxicity to living organisms, accumulation and biotransformation in cells, synergistic effects of NPs in combination with heavy metals and antibiotics, as well as methods for the bio-removal of NPs and nanomaterials from aquatic ecosystems using microalgae are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Pollutants exhibiting biotoxicity in low (on the order of ng/L) concentrations have received a special name: emerging/hazardous micropollutants (HM) [1]. They are represented by drugs, antiseptics, personal hygiene products, food additives, pesticides, plasticizers, natural and synthetic hormones, heavy metals, and nanoparticles (NPs). Metallic and metal oxide NPs of silver (Ag) [2], zinc oxide (ZnO) [3], and titanium dioxide (TiO2) are the most widely used [4]. Nano zero-valent iron (nZVI) [5], copper (Cu) and copper oxide (CuO) [6], aluminum (Al) and aluminum oxide (Al2O3) [7], and gold (Au) nanoparticles [8], as well as a number of other substances, are continuously mentioned in the literature [9].
NPs and nanomaterials are increasingly used for industrial and domestic purposes, which entails a steady increase in their production. The expected annual growth of the world market for NPs made of metal oxides and metalloids will amount to 7% in the period of 2020–2025 [10]. Annual global production of TiO2 and ZnO-based NPs exceeds hundreds of tons [11]. Accordingly, the emissions of NPs into the environment are growing and, at the same time, the risks of adverse effects on natural systems are increasing. The studies of the influence of NPs on aquatic and terrestrial ecosystems is attracting more and more attention. NPs can not only directly affect living organisms but also serve as carriers of organic and inorganic pollutants as well as enhance the toxic effects of other HM. Resistance to biodegradation in natural ecosystems and traditional treatment facilities exacerbates the problems associated with the accumulation of NPs in the environment. Nevertheless, despite the risks associated with NPs, humans will not stop using them in the near future since they are widely used in modern technologies.
Effective technologies for the removal of HM (including NPs) based on chemical sorption and oxidative destruction are complex and expensive; therefore, their widespread introduction into wastewater treatment plants is not yet possible. The products of HM oxidation reactions, which can be even more toxic, are also dangerous. On the other hand, biodegradation and biosorption (bioconcentration) using microalgae cultures and microalgal-bacterial consortia (MBC) is considered one of the most promising approaches from the point of view of environmental safety and the conservation of natural resources. However, the development of this approach is hindered by the lack and fragmentation of information on the effect of NPs on microalgae as well as including microbial communities in natural and artificial ecosystems. The data on the distribution, effects, and transformation of antibiotics and other pharmaceuticals in nature and in wastewater treatment plants is not yet possible. The products of HM oxidation reactions, which can be even more toxic are fairly well systematized, unlike the information on NPs. An analysis of recent reviews on the effect of metallic NPs on ecosystems, including aquatic ecosystems [12–15], indicates that, despite active research, there are gaps in our knowledge due to the lack of modeling and field research results. The main source of uncertainty is the lack of data on the concentrations of NPs in the environment and the dosimetry of NPs in general [13]. This review attempts to fill this gap, at least partially. The article discusses common industrial NP types based on metals and their oxides as well as carbon nanomaterials. The pathways of their entry into aquatic ecosystems, toxicity to living organisms, accumulation and biotransformation in cells, synergistic effects of NPs in combination with heavy metals and antibiotics, and methods for the bio-removal of NPs and nanomaterials from aquatic ecosystems using microalgae are discussed.
SOURCES OF NANOPARTICLE ENTRY INTO THE ENVIRONMENT
There are three possible scenarios for the entry of NPs into natural ecosystems, including aquatic ones: during production, during operation, and after the disposal of products containing NPs. Out of the nanomaterials produced, 63–91% eventually end up in landfill sites, 8–28% in soils, 0.4–0.7% in natural water bodies, and 0.1–1.5% in the atmosphere [16]. Thus, it was shown that 1 m2 of commercially available self-cleaning cement releases 18.7–33.5 mg TiO2 NPs after 168 h of leaching [17], and facade paints with TiO2 NPs under the influence of atmospheric conditions, release these NPs, which are then transported by sewage into water bodies [18]. The use of low-frequency TiO2 and ZnO in cosmetic products also leads to their entry into water bodies [19]. Thus, using electron microscopy, it was possible to identify TiO2 NPs from sun-protection creams in suspended matter of the Old Danube Lake (Vienna, Austria); their content increased in the summer season [20]. The popularity of textiles containing NPs with bactericidal properties, releasing these NPs, for example, during washing, is increasing [21]. Thus, microscopy showed that silver particles with a diameter of 10 to 500 nm are released into the washing water from socks containing nanosilver [22].
NPs formed as a result of natural phenomena, such as volcanic activity, and during industrial processes, such as cutting, grinding, melting, casting, welding, etc., also enter the aquatic environment [23]. For example, NPs with abnormally high concentrations of Cu, Zn, Ag, Cd, Sn, Sb, Hg, Pb, Tl, and Bi were found in road dust [24]. Such NPs can enter aquatic ecosystems with wastewater. Emissions of metallic NPs are also possible as the result of their use for the remediation of soils and groundwater, as in the case of zero-valent iron [25], as well as when plants are treated with NPs-based growth regulators and pesticides [26].
Carbon nanostructures are nanoscale allotropic modifications of carbon, including representatives of zero- (quantum dots, fullerenes), one- (nanotubes), and two-dimensional (graphenes) NP types, are now widespread and actively produced. They are used in many branches of industry, agriculture, and medicine. Due to a wide variety of structures and unique physicochemical properties, carbon NPs are used to develop agents for targeted drug delivery; photo-, radio-, and gene therapy [27, 28]; antibacterial drugs [29]; biosensors [30]; sensors for monitoring pollution [31]; adsorbents for wastewater treatment [32]; etc. All this significantly increases the volume of these NPs entering the ecosystems. Carbon NPs can be transferred to the aquatic environment from aerosols formed as a result of forest and steppe fires, burns, volcanic eruptions, the burning of agricultural waste, and the use of hydrocarbon fuels at power plants. Natural oil and bitumen can act as sources of NPs [33]. Therefore, all living organisms experience their effect to one degree or another. In the last decade, NP sources included wastewater treatment plants [34] and industrial facilities where carbon nanostructures are synthesized or used [34]. Currently, there are no precise data on the concentrations of carbon NPs in the aquatic environment; however, the calculated concentrations of carbon nanotubes (CNTs) and graphene in natural environments are 0.001–1000 μg/L [35].
Thus, the increase in the emissions of NPs based on metals, their oxides, and carbon nanomaterials into the aquatic environment due to the widespread distribution of nanoindustry products, as well as due to natural and man-made processes, is a general trend that can lead to unpredictable environmental effects.
TOXIC EFFECTS OF NANOPARTICLES ON HUMANS AND ANIMALS
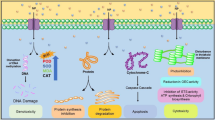
Metal and metal oxide NPs can affect the human body both when they are deliberately used in nanomedicine, taken with food or as part of personal hygiene products [36], and as a result of unintentional exposure during industrial, natural, and other processes, when NPs are used in agriculture, etc. [10]. The number of studies on the direct effect of metal and metal oxide NPs on human health is low. However, many studies devoted to the effect of NPs on human cells and experimental animals in in vitro and in vivo experiments indicate their potential danger to humans. Metal and metal oxide NPs are cyto- and genotoxic, causing oxidative damage to DNA and cell death [37]. In vivo research showed that various types of metallic NPs tend to form deposits in the liver, causing toxic effects [38]. In addition, NPs can accumulate in the digestive tract, lungs, heart, spleen, cardiac muscle, and kidneys [39, 40]. It was found that metal NPs can be transported to the central nervous system through a damaged blood-brain barrier and stimulate the activation of glial cells for the release of proinflammatory cytokines and the generation of reactive oxygen species (ROS) as well as the production of nitric oxide, which leads to neuroinflammation [41].
The biosecurity of carbon nanomaterials is also debatable. Due to their stability and mobility, these nanostructures are capable of bioconcentration and migration along food chains [42]. Both the general toxic effect and the selective toxicity of CNTs for the respiratory [43], digestive [44], and reproductive [45] systems of mammals have been confirmed many times. Graphene also has a toxic effect on mammals, since its NPs are capable of aggregating in tissues and causing oxidative stress damaging cells of the lungs, liver, spleen, kidneys [46], and the cornea [47].
Thus, metal and metal oxide NPs, as well as carbon nanomaterials, exhibit proven toxicity for mammals and, in some cases, for humans. In this regard, the search for means reducing the content of NPs in the environment is of particular relevance.
EFFECTS OF NANOPARTICLES ON AQUATIC ORGANISMS
The effect of NPs on aquatic organisms is determined by the chemical composition of water, including the content of dissolved organic matter (DOM), ionic strength, and pH [48] as well as illumination and temperature [49]. DOM can be adsorbed on the surface of NPs, forming thin films, changing their functional surface and increasing their aggregate stability [50]. A DOM coating can limit the release of ions from NPs into water [51], increase the ability of NPs to migrate and diffuse [52], and affect their toxicity [53]. The ionic strength and pH of water bodies can change the parameters of aqueous suspensions of NPs, which also affects the adsorption of DOM [49].
Temperature is an important determinant of the growth and productivity of primary producers, such as microalgae. It was shown that an increase in temperature increases the rate of NP suspension [54]. This can increase their toxicity to microalgae. In addition, some NPs (TiO2, ZnO) are semiconductors with photocatalytic and photodynamic properties. Such NPs can generate ROS, which have a toxic effect on microalgae cells when irradiated with ultraviolet radiation [55]. NPs, such as ZnO particles, can penetrate into the cytoplasm, damaging organelles and subcellular structures, including chloroplasts, vacuoles, endoplasmic reticulum, Golgi apparatus, and mitochondria, or altering their functionality [56].
There is information about the toxicity of carbon nanostructures for aquatic organisms. It is believed that NPs rarely exhibit acute toxicity towards microalgae; however, there is evidence of structural and negative functional disorders of microalgae cells (upon prolonged contact with NPs). Thus, reduced graphene oxide with attached ZrO2 induced a cytotoxic effect in microalgae Chlorella pyrenoidosa causing oxidative stress and functional changes in cell membranes [57]. Graphene oxide and reduced graphene oxide slowed down cell division, damaged cell membranes of C. pyrenoidosa, and also reduced the bioavailability of nutrients for microalgae due to adsorption on the surface of NPs [58]. Fullerenes in sublethal concentrations reduced chlorophyll content and Mg2+-ATPase activity, inhibiting cell division of Scenedesmus obliquus [59]. Double-walled CNTs already inhibited the cell division of Thalassiosira pseudonana diatoms and the growth of Tigriopus japonicus crustaceans at a concentration of 0.1 mg/L, while a 50% effective concentration (EC50) of NPs was only 1.86 mg/L [60]. A delay in growth and development was also observed in experiments with Pseudokirchneriella subcapitata (EC50 = 17.95 and 10.93 mg/L for pure and oxidized double-walled CNTs, respectively) [61]. Both direct damage to cellular structures and the impairment of trophism and photosynthesis due to adhesion of carbon nanostructures to the surface of microalgae cells are considered as a mechanism of the toxic effect on algae.
Growth and development retardation under the action of CNTs was also observed in Daphnia pulex (LC50 = 2.81 and 4.45 mg/L for pure and oxidized double-walled CNTs, respectively) [61]. Graphene, fullerene C60, single- and multiwalled CNTs at low concentrations stimulated the growth and reproduction of D. magna probably due to the adsorption of nutrients on the surface of NPs, which contributed to an increase in their absorption by daphnia. However, with an increase in NP concentration, an increase in toxicity was observed, manifested as the suppression of growth and reproduction [62]. Some studies did not reveal the acute toxicity of CNTs and fullerene C60 for D. magna but NPs remained in the intestines of daphnia even after 48 h [63], which increases the likelihood of the transfer of carbon nanostructures to the next trophic level of the food chain. Multilayer CNTs with 28 days of exposure at a concentration of 0.01–1 mg/L had a neurotoxic effect on Ruditapes philippinarum mollusks [64]. Graphene oxide at a concentration of 0.4–1 mg/mL caused significant embryonic mortality, delayed hatching, cardiotoxicity, and the development of cardiovascular defects in Danio rerio fish embryos [65]. Reduced TiO2-graphene oxide composite at a concentration of 30 μg/mL did not have a toxic effect on D. rerio embryos; however, with an increase in concentration to 1 mg/mL, teratogenic and cardiotoxic effects were observed [66]. Chronic graphene oxide exposure of adult D. rerio induced the generation of ROS in cells, damaging the gills and liver [67]. The toxic effects of graphene in D. rerio were observed in other studies [68]. Growth suppression was observed in Oryzias melastigma under the action of 10 mg/L of double-walled CNTs [60]. However, it should be noted that the toxicity of carbon nanostructures for fish is lower than for other aquatic organisms. Thus, the analysis of literature sources indicates the vulnerability of aquatic organisms to metallic, metal-oxide, and carbon NPs.
SYNERGISM OF DANGEROUS MICROPOLLUTANTS
Aquatic organisms are usually exposed to multicomponent mixtures of HMs, including various NPs. Metal and metal oxide NPs are an effective adsorbent for various HMs, including ions of other heavy metals and antibiotics [69]. The high sorption of HMs on metal NPs is due to the presence of coordination center particles on their surface (protrusions, edges, bends, or corner areas) [70]. Thus, the presence of TiO2 NPs at a concentration of 1 mg/L increased the toxicity of Zn2+ ions for cyanobacteria Anabaena sp.; however, with an increase in the concentration of TiO2 up to 10 mg/L, toxicity of the Zn2+/TiO2 system decreased due to the adsorption of a majority of the Zn2+ on the TiO2 surface [71–73]. Dichloro-dihydro-fluorescein diacetate assay (H2DCF-DA) showed an increase in the level of ROS in microalgae cells under the influence of the Zn2+/TiO2; with increase in Zn2+ concentration above 0.7 mg/L, cell destruction was observed [71]. Similar results were obtained in experiments on assessing the toxicity of arsenic in the presence of TiO2 NPs towards Ceriodaphnia dubiaс [72]. A decrease in the toxicity of heavy metals for green microalgae in the presence of TiO2 NPs has been shown [73]; however, NPs did not affect the absorption and toxicity of Cd2+ in an experiment with D. magna and Lumbriculus variegatus [74].
The analysis of the publications available to the authors of the review suggests that NPs, being an effective sorbent, can reduce the toxicity of heavy metals. The adsorption of antibiotics on Al2O3 [75] and TiO2 NPs [76] was demonstrated. However, in contrast to complexes of NPs with heavy metals, complexes of NPs with antibiotics are more toxic than antibiotics and NPs separately [77]. The action of antibiotics (azithromycin, cefotaxime, cefuroxime, phosphomycin, and chloramphenicol) against Escherichia coli increased in the presence of Ag NPs, but the antibacterial effect of Ag NPs with oxacillin and neomycin antibiotics against Staphylococcus aureus was significantly weaker than the effect of antibiotics alone [78]. The combinations “tetracycline + Ag NPs,” as well as “neomycin + Ag NPs,” more strongly inhibited the growth of Salmonella typhimurium DT104 in comparison with the effect of the antibiotic alone; at the same time, no enhancement of the antibiotic effect on this bacterial strain was observed in the combination of “penicillin + Ag NPs.” The potentiation of the antibiotic action was probably due to an increase in bacterial binding of Ag NPs under the action of tetracycline or neomycin but not penicillin [79]. The combination of carvacrol with ZnO NPs increased the antimicrobial effect against Campylobacter jejuni [80]. It was assumed that NPs promote the penetration of antibiotics into the cell, changing the membrane permeability, and then, together with them, destroy the cell wall [80]. Another mechanism of the toxic action of the NP + antibiotic complex can be mediated by the generation of ROS. The “NP + Ag-kanamycin” complex generated significantly higher amount of ROS compared to a system containing only an antibiotic or only NPs [81].
Unmodified carbon nanostructures can be safe for living organisms [82]; however, the surface functionalization or interaction with other pollutants can provide a synergistic effect, significantly increasing their bioavailability and toxicity [83]. At the same time, the behavior of nanostructures upon interaction with other pollutants is rather difficult to predict. For example, CNTs in an aqueous medium at a high rate adsorb Cd ions on their surface, tripling the toxicity of Cd for D. magna [84]. The herbicide diuron in the presence of various multilayer CNTs (industrial, purified, nonfunctionalized, and oxidized) was actively adsorbed on the CNT surface, remaining bioavailable for green microalgae C. vulgaris, which led to a fivefold increase in the toxicity of the herbicide [85].
The surface properties of functionalized multiwalled CNTs variously affected the toxicity of lead for D. magna [86]. Negatively charged carboxylate multiwalled CNTs markedly reduced lead toxicity (LC50 increased from 0.15 to 1.08 mg/L in the presence of 10 mg/L of multiwalled CNTs). In contrast, positively charged multilayer CNTs modified with polyethyleneimine had only a minor effect on the toxicity of lead (LC50 increased from 0.15 to 0.16 mg/L under the same conditions). The decrease in lead toxicity was associated with a decrease in the bioavailability of the free metal form (Pb2+) upon adsorption on the surface of multilayer CNTs [86].
Despite the fact that both CNTs and Cu NPs in an aquatic environment inhibited the growth of microalgae Skeletonema costatum, CNTs were capable of adsorbing Cu NPs, thereby reducing its toxicity [87].
Due to their high specific surface area and sorption capacity, carbon nanostructures, especially graphene and CNTs, are increasingly considered as sorbent materials with an antibacterial effect for wastewater treatment. Their ability to adsorb surfactants [88], heavy metals [89], organic substances and dyes [90], antibiotics [91], and radioactive waste [92] has been experimentally proved. Studies have shown that multiwalled CNTs can remove up to 99% of Zn2+ from an aqueous solution [93].
The analysis of the above publications allows us to conclude that NPs and nanomaterials are capable, depending on the properties of the NPs themselves, environmental conditions, and the presence of other substances, both to enhance or to weaken the toxic effect of other pollutants in the aquatic environment, such as heavy metals and antibiotics.
POTENTIAL OF MICROALGAE FOR THE REMOVAL OF NANOPARTICLES FROM WASTEWATER
The ability of algae to adsorb the NPs of metals and their oxides has been shown in a number of studies [94–96]. Mariano et al. detected internalized Ag NPs inside large vacuoles of C. vulgaris; these NPs were not released into the environment even after 1 week and were not biotransformed [97]. After a 4-h incubation of C. vulgaris with Ag NPs at a concentration of 2 mg/L, the content of these Ag NPs in microalgae cells reached 1200–3300 μg/g dry weight [98], and microalgae Raphidocelis subcapitata accumulated Ag NPs in the amount of 45 and 93.7 µg/g dry weight after 24 h incubation with these NPs at a concentration of 15 and 30 µg/L, respectively [99].
For the penetration into the microalgae cell, NPs should penetrate through the cell wall containing cellulose and other polysaccharides, as well as glycoproteins, and the cytoplasmic membrane. It was found by scanning electron microscopy that CuO NPs were attached to the surface of microalgal cells and interacted with the exopolysaccharide matrix, which promotes the adsorption of NPs by microalgal cells. Transmission electron microscopy revealed a fourfold thickening of the exopolysaccharide layer after exposure to NPs, which indicates a possible protective role of this layer of the cell wall of microalgae. However, despite the thickening of the exopolysaccharide layer, NPs penetrated through the cytoplasmic membrane by endocytosis and were deposited in the cell vacuoles [49, 56].
Inside the cells, metal NPs can undergo changes, including oxidation–reduction and complexation [100], or dissolve in the acidic medium of lysosomes [101]. The latter mechanism ensures the transport of toxic metal ions into cells. For microalgae, the entry of Ag NPs into the periplasmic space after 48 h of incubation with NPs has been described [102]. Using synchrotron X-ray absorption spectroscopy, it was established that Ag is present inside the cytoplasm in both crystalline and amorphous forms, identified as β-Ag2S and silver thiolates. These studies convincingly proved the ability of microalgal cells to internalize and biotransform Ag NPs.
Carbon nanostructures entering aquatic ecosystems are involved in various transformation processes, including homo- and heteroaggregation, agglomeration, sedimentation, oxidation, sulfidation, as well as biodegradation and biomodification processes, which affects their properties [103]. There is evidence of the bioaccumulation of carbon nanostructures by cells of microalgae, protozoa, mollusks, crustaceans, and fish [104]. The biodegradation of carbon nanostructures in the natural environment occurs using enzymatic catalysis [105], for example, with the involvement of peroxidases in the corresponding subcompartments of hydrobiont cells [105].
CONCLUSIONS
This review is an attempt to concisely systematize information on the most common types of NPs, associated risks, entry into the aquatic environment, and pathways of biotransformation in aquatic cells. Even such a brief examination shows large gaps in our knowledge about these processes. The need to fill these gaps becomes more acute the faster and more widely technologies using NPs and nanomaterials are distributed. At the same time, NPs are a “double-edged weapon” that can be both a dangerous toxicant and a powerful means of removing pollutants from wastewater and the environment. Special attention in the review is devoted to the potential of using unicellular oxygenic phototrophs (eukaryotic microalgae and cyanobacteria) as a basis for creating biotechnologies providing effective and economically affordable bioremediation of HMs. Potential pathways for the binding (bioconcentration) and elimination of NPs using microalgal cells include the uptake and accumulation of NPs in cell compartments, aggregation, sedimentation, chemical modification (oxidation, reduction, sulfidation, and complexation), and enzymatic biodegradation. In this case, important factors are the initial concentration of NPs, the pH of the medium, and the metabolic and physiological plasticity of microalgae cells. However, unlocking the potential of microalgae as components of biotechnology for reducing the risks associated with NPs requires a large amount of research for the elucidation of the mechanisms of NPs tolerance and search for effective strains performing bioconcentration and biodegradation of these particles.
REFERENCES
Nguyen, H.T., Yoon, Y., Ngo, H.H., and Jang, A., The application of microalgae in removing organic micropollutants in wastewater, Crit. Rev. Environ. Sci. Technol., 2021, vol. 51, no. 12, pp. 1187–1220.
Xu, L., Wang, Y.-Y., Huang, J., Chen, C.-Y., Wang, Z.-X., and Xie, H., Silver nanoparticles: synthesis, medical applications, and biosafety, Theranostics, 2020, vol. 10, no. 20, pp. 8996–9031.
Jiang, J., Pi, J., and Cai, J., The advancing of zinc oxide nanoparticles for biomedical applications, Bioinorg. Chem. Appl., 2018, vol. 2018, art. ID 1062562.
Ziental, D., Czarczynska-Goslinska, B., Mlynarczyk, D.T., Glowacka-Sobotta, A., Stanisz, B., Goslinski, T., and Sobotta, L., Titanium dioxide nanoparticles: prospects and applications in medicine, Nanomaterials, 2020, vol. 10, no. 2, art. ID 387.
Li, J., Li, C., Zhao, L., Pan, X., Cai, G., and Zhu, G., The application status, development and future trend of nano-iron materials in anaerobic digestion system, Chemosphere, 2021, vol. 269, art. ID 129389.
Bezza, F.A., Tichapondwa, S.M., and Chirwa, E.M.N., Fabrication of monodispersed copper oxide nanoparticles with potential application as antimicrobial agents, Sci. Rep., 2020, vol. 10, no. 1, art. ID 16680.
Hassanpour, P., Panahi, Y., Ebrahimi-Kalan, A., Akbarzadeh, A., Davaran, S., Nasibova, A., Khalilov, R., and Kavetskyy, T., Biomedical applications of aluminium oxide nanoparticles, Micro Nano Lett., 2018, vol. 13, no. 9, pp. 1227–1231.
Jahangirian, H., Kalantari, K., Izadiyan, Z., Rafiee-Moghaddam, R., Shameli, K., and Webster, T.J., A review of small molecules and drug delivery applications using gold and iron nanoparticles, Int. J. Nanomed., 2019, vol. 14, pp. 1633–1657.
Yonezawa, T., Preparation of metal nanoparticles and their application for materials, in Nanoparticle Technology Handbook, Naito, M., Yokoyama, T., Hosokawa, K., and Nogi, K., Eds., Amsterdam: Elsevier, 2018, pp. 829–837.
Soares, E.V. and Soares, H.M.V.M., Harmful effects of metal(loid) oxide nanoparticles, Appl. Microbiol. Biotechnol., 2021, vol. 105, pp. 1379–1394.
Piccinno, F., Gottschalk, F., Seeger, S., and Nowack, B., Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world, J. Nanopart. Res., 2012, vol. 14, no. 9, art. ID 1109.
Meyer, J.S., Lyons-Darden, T., Garman, E.R., Middleton, E.T., and Schlekat, C.E., Toxicity of nanoparticulate nickel to aquatic organisms: review and recommendations for improvement of toxicity tests, Environ. Toxicol. Chem., 2020, vol. 39, no. 10, pp. 1861–1883.
Lead, J.R., Batley, G.E., Alvarez, P.J.J., Croteau, M.-N., Handy, R.D., McLaughlin, M.J., Judy, J.D., and Schirmer, K., Nanomaterials in the environment: Behavior, fate, bioavailability, and effects—An updated review, Environ. Toxicol. Chem., 2018, vol. 37, no. 8, pp. 2029–2063.
Déniel, M., Errien, N., Daniel, P., Caruso, A., and Lagarde, F., Current methods to monitor microalgae-nanoparticle interaction and associated effects, Aquat. Toxicol., 2019, vol. 217, art. ID 105311.
Pulido-Reyes, G., Leganes, F., Fernández-Piñas, F., and Rosal, R., Bio-nano interface and environment: a critical review, Env. Toxicol. Chem., 2017, vol. 36, no. 12, pp. 3181–3193.
Keller, A.A., McFerran, S., Lazareva, A., and Suh, S., Global life cycle releases of engineered nanomaterials, J. Nanopart. Res., 2013, vol. 15, no. 6, art. ID 1692.
Bossa, N., Chaurand, P., Levard, C., Borschneck, D., Miche, H., Vicente, J., Geantet, C., Aguerre-Chariol, O., Michel, F.M., and Rose, J., Environmental exposure to TiO2 nanomaterials incorporated in building material, Environ. Pollut., 2017, vol. 220, pp. 1160–1170.
Kaegi, R., Ulrich, A., Sinnet, B., Vonbank, R., Wi-chser, A., Zuleeg, S., Simmler, H., Brunner, S., Vonmont, H., Burkhardt, M., and Boller, M., Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment, Environ. Pollut., 2008, vol. 156, no. 2, pp. 233–239.
Bundschuh, M., Filser, J., Lüderwald, S., McKee, M.S., Metreveli, G., Schaumann, G.E., Schulz, R., and Wagner, S., Nanoparticles in the environment: where do we come from, where do we go to?, Environ. Sci. Eur., 2018, vol. 30, no. 1, art. ID 6.
Gondikas, A.P., Kammer, F., Reed, R.B., Wagner, S., Ranville, J.F., and Hofmann, T., Release of TiO2 nanoparticles from sunscreens into surface waters: a one-year survey at the old Danube recreational lake, Environ. Sci. Technol., 2014, vol. 48, no. 10, pp. 5415–5422.
Andra, S., Balu, S.K., Jeevanandam, J., and Muthalagu, M., Emerging nanomaterials for antibacterial textile fabrication, Naunyn-Schmiedeberg’s Arch. Pharmacol., 2021, vol. 394, no. 7, pp. 1355–1382.
Benn, T.M. and Westerhoff, P., Nanoparticle silver released into water from commercially available sock fabrics, Environ. Sci. Technol., 2008, vol. 42, no. 18, pp. 7025–7026.
Sawicki, K., Czajka, M., Matysiak-Kucharek, M., Fal, B., Drop, B., Męczyńska-Wielgosz, S., Sikorska, K., Kruszewski, M., and Kapka-Skrzypczak, L., Toxicity of metallic nanoparticles in the central nervous system, Nanotechnol. Rev., 2019, vol. 8, no. 1, pp. 175–200.
Ermolin, M.S., Fedotov, P.S., Ivaneev, A.I., Karandashev, V.K., Fedyunina, N.N., and Eskina, V.V., Isolation and quantitative analysis of road dust nanoparticles, J. Anal. Chem., 2017, vol. 72, no. 5, pp. 520–532.
Galdames, A., Ruiz-Rubio, L., Orueta, M., Sánchez-Arzalluz, M., and Vilas-Vilela, J.L., Zero-valent iron nanoparticles for soil and groundwater remediation, Int. J. Environ. Res. Public Health, 2020, vol. 17, no. 16, art. ID 5817.
Pirzadah, B., Jan, A., Hakeem, K.R., and Bilal, T., Nanofertilizers: a way forward for green economy, in Nanobiotechnology in Agriculture: An Approach towards Sustainability, Hakeem, K.R. and Pirzadah, T.B., Eds., Cham: Springer-Verlag, pp. 99–112.
Rani, U.A., Ng, L.Y., Ng, C.Y., and Mahmoudi, E., A review of carbon quantum dots and their applications in wastewater treatment, Adv. Colloid Interface Sci., 2020, vol. 278, art. ID 102124.
Efimova, S.S., Khaleneva, D.A., Litasova, E.V., Piotrovskiy, L.B., and Ostroumova, O.S., The mechanisms of action of water-soluble aminohexanoic and malonic adducts of fullerene C60 with hexamethonium on model lipid membranes, Biochim. Biophys. Acta, Biomembr., 2020, vol. 1862, no. 11, art. ID 183433.
Moradlou, O., Rabiei, Z., and Delavari, N., Antibacterial effects of carbon quantum dots@hematite nanostructures deposited on titanium against Gram-positive and Gram-negative bacteria, J. Photochem. Photobiol., A, 2019, vol. 379, pp. 144–149.
Joshi, P., Mishra, R., and Narayan, R.J., Biosensing applications of carbon-based materials, Curr. Opin. Biomed. Eng., 2021, vol. 18, art. ID 100274.
Li, H.-Y., Li, D., Guo, Y., Yang, Y., Wei, W., and Xie, B., On-site chemosensing and quantification of Cr(VI) in industrial wastewater using one-step synthesized fluorescent carbon quantum dots, Sens. Actuators, 2018, vol. 277, pp. 30–38.
Fallah, Z., Zare, E.N., Ghomi, M., Ahmadijokani, F., Amini, M., Tajbakhsh, M., Arjmand, M., Sharma, G., Ali, H., Ahmad, A., Makvandi, P., Lichtfouse, E., Sillanpää, M., and Varma, R.S., Toxicity and remediation of pharmaceuticals and pesticides using metal oxides and carbon nanomaterials, Chemosphere, 2021, vol. 275, art. ID 130055.
Velasco-Santos, C., Martinez-Hernández, A.L., Consultchi, A., Rodriguez, R., and Castaño, V.M., Naturally produced carbon nanotubes, Chem. Phys. Lett., 2003, vol. 373, nos. 3–4, pp. 272–276.
Bäuerlein, P.S., Emke, E., Tromp, P., Hof-man, J.A.M.H., Carboni, A., Schooneman, F., de Voogt, P., and Wezel, A.P., Is there evidence for man-made nanoparticles in the Dutch environment?, Sci. Total Environ., 2017, vol. 576, pp. 273–283.
De Marchi, L., Pretti, C., Gabriel, B., Marques, P.A.A.P., Freitas, R., and Neto, V., An overview of graphene materials: properties, applications, and toxicity on aquatic environments, Sci. Total Environ., 2018, vols. 631–632, pp. 1440–1456.
Di Felice, G. and Colombo, P., Nanoparticle-allergen complexes for allergen immunotherapy, Int. J. Nanomed, 2017, vol. 12, pp. 4493–4504.
Singh, S.P., Chinde, S., Kamal, S.S.K., Rahman, M.F., Mahboob, M., and Grover, P., Genotoxic effects of chromium oxide nanoparticles and microparticles in Wistar rats after 28 days of repeated oral exposure, Environ. Sci. Pollut. Res. Int., 2016, vol. 23, no. 4, pp. 3914–3924.
Liu, F., Chang, X., Tian, M., Zhu, A., Zou, L., Han, A., Su, L., Li, S., and Sun, Y., Nano NiO induced liver toxicity via activating the NF-κB signaling pathway in rats, Toxicol. Res., 2017, vol. 6, no. 2, pp. 242–250.
Shabbir, S., Kulyar, M.F., et al., Toxicological consequences of titanium dioxide nanoparticles (TiO2NPs) and their jeopardy to human population, BioNanoScience, 2021, vol. 11, no. 2, pp. 621–632.
Baranowska-Wójcik, E., Szwajgier, D., Oleszczuk, P., and Winiarska-Mieczan, A., Effects of titanium dioxide nanoparticles exposure on human health-a review, Biol. Trace Elem. Res., 2020, vol. 193, no. 1, pp. 118–129.
Wu, T. and Tang, M., The inflammatory response to silver and titanium dioxide nanoparticles in the central nervous system, Nanomedicine, 2018, vol. 13, no. 2, pp. 233–249.
Sarma, S.J., Bhattacharya, I., Brar, S.K., Tyagi, R.D., and Surampalli, R.Y., Carbon nanotube—Bioaccumulation and recent advances in environmental monitoring, Crit. Rev. Environ. Sci. Technol., 2015, vol. 45, no. 9, pp. 905–938.
Khaliullin, T.O., Yanamala, N., Newman, M.S., Kisin, E.R., Fatkhutdinova, L.M., and Shvedova, A.A., Comparative analysis of lung and blood transcriptomes in mice exposed to multi-walled carbon nanotubes, Toxicol. Appl. Pharmacol., 2020, vol. 390, art. ID 114898.
Adedara, I.A., Anao, O.O., Forcados, G.E., Awogbindin, I.O., Agbowo, A., Ola-Davies, O.E., Patlolla, A.K., Tchounwou, P.B., and Farombi, E.O., Low doses of multi-walled carbon nanotubes elicit hepatotoxicity in rats with markers of oxidative stress and induction of pro-inflammatory cytokines, Biochem. Biophys. Res. Commun., 2018, vol. 503, no. 4, pp. 3167–3173.
Liu, X., Liu, T., Song, J., Hai, Y., Luan, F., Zhang, H., Yuan, Y., Li, H., and Zhao, C., Understanding the interaction of single-walled carbon nanotube (SWCNT) on estrogen receptor: A combined molecular dynamics and experimental study, Ecotoxicol. Environ. Saf., 2019, vol. 172, pp. 373–379.
Sasidharan, A., Swaroop, S., Koduri, C.K., Girish, C.M., Chandran, P., Panchakarla, L.S., Somasundaram, V.H., Gowd, G.S., Nair, S., and Koyakutty, M., Comparative in vivo toxicity, organ biodistribution and immune response of pristine, carboxylated and PEGylated few-layer graphene sheets in Swiss albino mice: A three month study, Carbon, 2015, vol. 95, pp. 511–524.
An, W., Zhang, Y., Zhang, X., Li, K., Kang, Y., Akhtar, S., Sha, X., and Gao, L., Ocular toxicity of reduced graphene oxide or graphene oxide exposure in mouse eyes, Exp. Eye Res., 2018, vol. 174, pp. 59–69.
Cupi, D., Hartmann, N.B., and Baun, A., Influence of pH and media composition on suspension stability of silver, zinc oxide, and titanium dioxide nanoparticles and immobilization of Daphnia magna under guideline testing conditions, Ecotoxicol. Environ. Saf., 2016, vol. 127, pp. 144–152.
Wang, F., Guan, W., Xu, L., Ding, Z., Ma, H., Ma, A., and Terry, N., Effects of nanoparticles on algae: adsorption, distribution, ecotoxicity, and fate, Appl. Sci., 2019, vol. 9, no. 8, art. ID 1534.
Lee, S., Kim, K., Shon, H.K., Kim, S.D., and Cho, J., Biotoxicity of nanoparticles: effect of natural organic matter, J. Nanopart. Res., 2011, vol. 13, no. 7, pp. 3051–3061.
Levard, C., Hotze, E.M., Lowry, G.V., and Brown, G.E., Environmental transformations of silver nanoparticles: Impact on stability and toxicity, Environ. Sci. Technol., 2012, vol. 46, no. 13, pp. 6900–6914.
Wang, Z., Zhang, L., Zhao, J., and Xing, B., Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter, Environ. Sci.: Nano, 2016, vol. 3, no. 2, pp. 240–255.
Collin, B., Tsyusko, O.V., Starnes, D.L., and Unrine, J.M., Effect of natural organic matter on dissolution and toxicity of sulfidized silver nanoparticles to Caenorhabditis elegans, Environ. Sci.: Nano, 2016, vol. 3, no. 4, pp. 728–736.
Li, L., Fernández-Cruz, M.L., Connolly, M., Schuster, M., and Navas, J.M., Dissolution and aggregation of Cu nanoparticles in culture media: effects of incubation temperature and particles size, J. Nanopart. Res., 2015, vol. 17, art. ID 38.
Fu, L., Hamzeh, M., Dodard, S., Zhao, Y.H., and Sunahara, G.I., Effects of TiO2 nanoparticles on ROS production and growth inhibition using freshwater green algae pre-exposed to UV irradiation, Environ. Toxicol. Pharmacol., 2015, vol. 39, no. 3, pp. 1074–1080.
Zhao, J., Cao, X., Liu, X., Wang, Z., Zhang, C., White, J.C., and Xing, B., Interactions of CuO nanoparticles with the algae Chlorella pyrenoidosa: adhesion, uptake, and toxicity, Nanotoxicology, 2016, vol. 10, no. 9, pp. 1297–1305.
Wang, Z., Zhang, F., Vijver, M.G., and Peijnen- burg, W.J.G.M., Graphene nanoplatelets and reduced graphene oxide elevate the microalgal cytotoxicity of nano-zirconium oxide, Chemosphere, 2021, vol. 276, art. ID 130015.
Zhao, J., Cao, X., Wang, Z., Dai, Y., and Xing, B., Mechanistic understanding toward the toxicity of graphene-family materials to freshwater algae, Water Res., 2017, vol. 111, pp. 18–27.
Tao, X., Yu, Y., Fortner, J.D., He, Y., Chen, Y., and Hughes, J.B., Effects of aqueous stable fullerene nanocrystal (nC60) on Scenedesmus obliquus: Evaluation of the sub-lethal photosynthetic responses and inhibition mechanism, Chemosphere, 2015, vol. 122, pp. 162–167.
Kwok, K.W.H, Leung, K.M.Y., Flahaut, E., Cheng, J., and Cheng, S.H., Chronic toxicity of double-walled carbon nanotubes to three marine organisms: influence of different dispersion methods, Nanomedicine, 2010, vol. 5, no. 6, pp. 951–961.
Lukhele, L.P., Mamba, B.B., Musee, N., and Wepener, V., Acute toxicity of double-walled carbon nanotubes to three aquatic organisms, J. Nanomater., 2015, vol. 2015, pp. 1–19.
Fan, W., Liu, Y., Xu, Z., Wang, X., Li, X., and Luo, S., The mechanism of chronic toxicity to Daphnia magna induced by graphene suspended in a water column, Environ. Sci.: Nano, 2016, vol. 3, no. 6, pp. 1405–1415.
Tervonen, K., Waissi, G., Petersen, E.J., Akkanen, J., and Kukkonen, J.V.K., Analysis of fullerene-C60 and kinetic measurements for its accumulation and depuration in Daphnia magna, Environ. Toxicol. Chem., 2010, vol. 29, no. 5, pp. 1072–1078.
De Marchi, L., Neto, V., Pretti, C., Figueira, E., Chiellini, F., Morelli, A., Soares, A.M.V.M., and Freitas, R., Toxic effects of multi-walled carbon nanotubes on bivalves: Comparison between functionalized and nonfunctionalized nanoparticles, Sci. Total Environ., 2018, vols. 622–623, pp. 1532–1542.
Bangeppagari, M., Park, S.H., Kundapur, R.R., and Lee, S.J., Graphene oxide induces cardiovascular defects in developing zebrafish (Danio rerio) embryo model: In-vivo toxicity assessment, Sci. Total Environ., 2019, vol. 673, pp. 810–820.
Prakash, J., Venkatesan, M., Sebastian Prakash, J.S., Bharath, G., Anwer, S., Veluswamy, P., Prema, D., Venkataprasanna, K.S., Venkatasubbu, G.D., Investigations on the in-vivo toxicity analysis of reduced graphene oxide/TiO2 nanocomposite in zebrafish embryo and larvae (Danio rerio), Appl. Surf. Sci., 2019, vol. 481, pp. 1360–1369.
Souza, J.P., Baretta, J.F., Santos, F., Paino, I.M.M., and Zucolotto, V., Toxicological effects of graphene oxide on adult zebrafish (Danio rerio), Aquat. Toxicol., 2017, vol. 186, pp. 11–18.
Audira, G., Lee, J.-S., Siregar, P., Malhotra, N., Rol-den, M.J.M., Huang, J.C., Chen, K.H.-C., Hsu, H.S., Hsu, Y., Ger, T.-R., and Hsiao, C.-D., Comparison of the chronic toxicities of graphene and graphene oxide toward adult zebrafish by using biochemical and phenomic approaches, Environ. Pollut., 2021, vol. 278, art. ID 116907.
Malakootian, M., Yaseri, M., and Faraji, M., Removal of antibiotics from aqueous solutions by nanoparticles: a systematic review and meta-analysis, Environ. Sci. Pollut. Res., 2019, vol. 26, no. 9, pp. 8444–8458.
Li, M., Liu, W., and Slaveykova, V.I., Effects of mixtures of engineered nanoparticles and metallic pollutants on aquatic organisms, Environments, 2020, vol. 7, no. 4, art. ID 27.
Tang, Y., Li, S., Qiao, J., Wang, H., and Li, L., Synergistic effects of nano-sized titanium dioxide and zinc on the photosynthetic capacity and survival of Anabaena sp., Int. J. Mol. Sci., 2013, vol. 14, no. 7, pp. 14395–14407.
Wang, D., Hu, J., Irons, D.R., and Wang, J., Synergistic toxic effect of nano-TiO2 and As(V) on Ceriodaphnia dubia, Sci. Total Environ., 2011, vol. 409, no. 7, pp. 1351–1356.
Yang, W.-W., Li, Y., Miao, A.-J., and Yang, L.-Y., Cd2+ toxicity as affected by bare TiO2 nanoparticles and their bulk counterpart, Ecotoxicol. Environ. Saf., 2012, vol. 85, pp. 44–51.
Hartmann, N.B., Legros, S., Kammer, F., Hofmann, T., and Baun, A., The potential of TiO2 nanoparticles as carriers for cadmium uptake in Lumbriculus variegatus and Daphnia magna, Aquat. Toxicol., 2012, vol. 118–119, pp. 1–8.
Peterson, J.W., Burkhart, R.S., Shaw, D.C., Schui-ling, A.B., Haserodt, M.J., and Seymour, M.D., Experimental determination of ampicillin adsorption to nanometer-size Al2O3 in water, Chemosphere, 2010, vol. 80, no. 11, pp. 1268–1273.
Wieren, E.M., Seymour, M.D., and Peterson, J.W., Interaction of the fluoroquinolone antibiotic, ofloxacin, with titanium oxide nanoparticles in water: Adsorption and breakdown, Sci. Total Environ., 2012, vol. 441, pp. 1–9.
Surwade, P., Ghildyal, C., Weikel, C., Luxton, T., Peloquin, D., Fan, X., and Shah, V., Augmented antibacterial activity of ampicillin with silver nanoparticles against methicillin-resistant Staphylococcus aureus (MRSA), J. Antibiot., 2019, vol. 72, no. 1, pp. 50–53.
Abo-Shama, U.H., El-Gendy, H., Mousa, W.S., Hamouda, R.A., Yousuf, W.E., Hetta, H.F., and Abdeen, E.E., Synergistic and antagonistic effects of metal nanoparticles in combination with antibiotics against some reference strains of pathogenic microorganisms, Infect. Drug. Resist., 2020, vol. 13, pp. 351–362.
McShan, D., Zhang, Y., Deng, H., Ray, P.C., and Yu, H., Synergistic antibacterial effect of silver nanoparticles combined with ineffective antibiotics on drug resistant Salmonella typhimurium DT104, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2015, vol. 33, no. 3, pp. 369–384.
Windiasti, G., Feng, J., Ma, L., Hu, Y., Hakeem, M.J., Amoako, K., Delaquis, P., and Lu, X., Investigating the synergistic antimicrobial effect of carvacrol and zinc oxide nanoparticles against Campylobacter jejuni, Food Control, 2019, vol. 96, pp. 39–46.
Hwang, I.-S., Hwang, J.H., Choi, H., Kim, K.-J., and Lee, D.G., Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved, J. Med. Microbiol., 2012, vol. 61, no. 12, pp. 1719–1726.
Sun, C., Li, W., Xu, Y., Hu, N., Ma, J., Cao, W., Sun, S., Hu, C., Zhao, Y., and Huang, Q., Effects of carbon nanotubes on the toxicities of copper, cadmium and zinc toward the freshwater microalgae Scenedesmus obliquus, Aquat. Toxicol., 2020, vol. 224, art. ID 105504.
Freixa, A., Acuña, V., Sanchís, J., Farré, M., Barceló, D., and Sabater, S., Ecotoxicological effects of carbon based nanomaterials in aquatic organisms, Sci. Total. Environ., 2018, vols. 619-620, pp. 328–337.
Wang, X., Qu, R., Liu, J., Wei, Z., Wang, L., Yang, S., Huang, Q., and Wang, Z., Effect of different carbon nanotubes on cadmium toxicity to Daphnia magna: the role of catalyst impurities and adsorption capacity, Environ. Pollut., 2016, vol. 208, pp. 732–738.
Schwab, F., Bucheli, T.D., Camenzuli, L., Magrez, A., Knauer, K., Sigg, L., and Nowack, B., Diuron sorbed to carbon nanotubes exhibits enhanced toxicity to Chlorella vulgaris, Environ. Sci. Technol., 2013, vol. 47, no. 13, pp. 7012–7019.
Jang, M.-H. and Hwang, Y.S., Effects of functionalized multi-walled carbon nanotubes on toxicity and bioaccumulation of lead in Daphnia magna, PLoS One, 2018, vol. 13.
Zhang, C., Chen, X., Tan, L., and Wang, J., Combined toxicities of copper nanoparticles with carbon nanotubes on marine microalgae Skeletonema costatum, Environ. Sci. Pollut. Res., 2018, vol. 25, no. 13, pp. 13127–13133.
Song, B., Xu, P., Zeng, G., Gong, J., Wang, X., Yan, J., Wang, S., Zhang, P., Cao, W., and Ye, S., Modeling the transport of sodium dodecyl benzene sulfonate in riverine sediment in the presence of multi-walled carbon nanotubes, Water Res., 2018, vol. 129, pp. 20–28.
Sun, Y., Liu, X., Lv, X., Wang, T., and Xue, B., Synthesis of novel lignosulfonate-modified graphene hydrogel for ultrahigh adsorption capacity of Cr(VI) from wastewater, J. Clean. Prod., 2021, vol. 295, art. ID 126406.
Chenab, K.K., Sohrabi, B., Jafari, A., and Ramakrishna, S., Water treatment: functional nanomaterials and applications from adsorption to photodegradation, Mater. Today Chem., 2020, vol. 16, art. ID 100262.
Lucía, I., Campos-Mañas, M.C., Agüera, A., Leganés, F., Fernández-Piñas, F., and Rosal, R., Combined toxicity of graphene oxide and wastewater to the green alga Chlamydomonas reinhardtii, Environ. Sci.: Nano, 2018, vol. 5, no. 7, pp. 1729–1744.
Zhang, X. and Liu, Y., Nanomaterials for radioactive wastewater decontamination, Environ. Sci.: Nano, 2020, vol. 7, no. 4, pp. 1008–1040.
Mubarak, N.M., Sahu, J.N., Abdullah, E.C., Jayakumar, N.S., and Ganesan, P., Microwave-assisted synthesis of multi-walled carbon nanotubes for enhanced removal of Zn(II) from wastewater, Res. Chem. Intermed., 2016, vol. 42, no. 4, pp. 3257–3281.
Miao, A.-J., Luo, Z., Chen, C.-S., Chin, W.-C., Santschi, P.H., and Quigg, A., Intracellular uptake: a possible mechanism for silver engineered noparticle toxicity to a freshwater alga Ochromonas danica, PLoS One, 2010, vol. 5, no. 12, , art. ID e15196.
Mahana, A., Guliy, O.I., and Mehta, S.K., Accumulation and cellular toxicity of engineered metallic nanoparticle in freshwater microalgae: Current status and future challenges, Ecotoxicol. Environ. Saf., 2021, vol. 208, art. ID 111662.
Chen, J., Li, H., Han, X., and Wei, X., Transmission and accumulation of nano-TiO2 in a 2-step food chain (Scenedesmus obliquus to Daphnia magna), Bull. Environ. Contam. Toxicol., 2015, vol. 95, no. 2, pp. 145–149.
Mariano, S., Panzarini, E., Inverno, M.D., Voulvoulis, N., and Dini, L., Toxicity, bioaccumulation and biotransformation of glucose-capped silver nanoparticles in green microalgae Chlorella vulgaris, Nanomaterials, 2020, vol. 10, no. 7, art. ID 1377.
Kalman, J., Paul, K.B., Khan, F.R., Stone, V., and Fernandes, T.F., Characterisation of bioaccumulation dynamics of three differently coated silver nanoparticles and aqueous silver in a simple freshwater food chain, Environ. Chem., 2015, vol. 12, no. 6, pp. 662–672.
Ribeiro, F., Gallego-Urrea, J.A., Goodhead, R.M., Gestel, C.A., Moger, J., Soares, A.M., and Loureiro, S., Uptake and elimination kinetics of silver nanoparticles and silver nitrate by Raphidocelis subcapitata: the influence of silver behaviour in solution, Nanotoxicology, 2015, vol. 9, no. 6, pp. 686–695.
Chen, F., Xiao, Z., Yue, L., Wang, J., Feng, Y., Zhu, X., Wang, Z., and Xing, B., Algae response to engineered nanoparticles: current understanding, mechanisms, and implications, Environ. Sci.: Nano, 2019, vol. 6, no. 4, pp. 1026–1042.
Oh, N. and Park, J.-H., Endocytosis and exocytosis of nanoparticles in mammalian cells, Int. J. Nanomed., 2014, vol. 9, suppl. 1, pp. 51–63.
Wang, S., Lv, J., Ma, J., and Zhang, S., Cellular internalization and intracellular biotransformation of silver nanoparticles in Chlamydomonas reinhardtii, Nanotoxicology, 2016, vol. 10, no. 8, pp. 1129–1135.
Turan, N.B., Sari, H., Engin G.O., and Bilgili, M.S., Nanoparticles in the aquatic environment: Usage, properties, transformation, and toxicity—A review, Process Saf. Environ. Prot., 2019, vol. 130, pp. 238–249.
Mortimer, M., Petersen, E.J., Buchholz, B.A., Orias, E., and Holden, P.A., Bioaccumulation of multiwall carbon nanotubes in Tetrahymena thermophila by direct feeding or trophic transfer, Environ. Sci. Technol., 2016, vol. 50, no. 16, pp. 8876–8885.
Allen, B.L., Kichambare, P.D., Gou, P., Vlasova, I.I., Kapralov, A.A., Konduru, N., Kagan, V.E., and Star, A., Biodegradation of single-walled carbon nanotubes through enzymatic catalysis, Nano Lett., 2008, vol. 8, no. 11, pp. 3899–3903.
Funding
The research was funded by the Russian Science Foundation, project no. 21-74-20004 (the part on metal-oxide and carbon nanoparticles) and the Russian Foundation for Basic Research, project no. 20-34-90115 (the part on metal nanoparticles).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by V. Mittova
About this article
Cite this article
Gusev, A.A., Zakharova, O.V., Vasyukova, I.A. et al. Nanoparticles in the Aquatic Environment: The Risks Associated with Them and the Possibilities of Their Mitigation with Microalgae. Moscow Univ. Biol.Sci. Bull. 76, 165–174 (2021). https://doi.org/10.3103/S0096392521040039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0096392521040039