Abstract
A pericardial effusion is the excess fluid between the heart and the pericardium, the sac surrounding the heart. Pericardiocentesis is a procedure performed to remove excess fluid from the pericardial sac. The potentially life-saving procedure is often performed in the setting of cardiac tamponade to correct hemodynamic instability caused by the extrinsic pressure the fluid places on the cardiac chambers, thus compromising cardiac output. The procedure can be performed quickly and safely as expediting time to the diagnosis of pericardial effusion and evacuation of the excess fluid is crucial in order to ensure successful outcomes in hemodynamically unstable patients. Point-of-care ultrasound (PoCUS) is one of the tools Advanced Practice Providers (APPs) have at their disposal to achieve this goal. Although pericardiocentesis has inherent risks, APPs can perform the procedure at the bedside using PoCUS to obtain continuous real-time image guidance. To perform PoCUS-guided pericardiocentesis, APPs can use several different approaches including subxiphoid/subcostal, apical, and parasternal. A thorough understanding of the indications, contraindications, and risks and benefits of the procedure is mandatory, as is an awareness of the common pitfalls and perils that can lead to complications when performing PoCUS-guided pericardiocentesis. A proper informed consent dialogue with patients and review of pertinent laboratory data, ensuring the appropriate equipment is available and ensuring patients receive appropriate hemodynamic monitoring during the procedure, are all keys to determining the etiology of the effusion and successful patient outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pericardiocentesis
- Pericardial effusion
- Pericardium
- Point-of-care ultrasound
- PoCUS
- Cardiac tamponade
- Advanced Practice Providers
- APP
- Physician assistant
- PA
- Advanced practice registered nurse
- APRN
Introduction
Pericardiocentesis is a therapeutic procedure usually performed to relieve a large, symptomatic pericardial effusion or pericardial tamponade and occasionally to identify the etiology of a pericardial effusion. Initial pericardial drainage dates back over 200 years to Romero in 1815 and the first “blind” pericardiocentesis performed by Schuh in 1840 with a trocar and cannula [1].
The etiology of most pericardial effusions correlates to the underlying clinical condition. In developed countries, up to 50% of pericardial effusions remain idiopathic despite diagnostic workup [2, 3]. Although the cause may be difficult to establish in many patients, for others it is often associated with an underlying disease [4, 5]. If clinical clues are absent, the most common etiologies of effusion are cancer (10–25%); pericarditis and infectious causes (15–30%), mainly tuberculosis (TB); iatrogenic (15–20%); and connective tissue disease (5–15%). In developing countries, >60% of effusions are related to TB [2, 6]. When the etiology is not apparent, sampling the effusion to aid has been shown to have a diagnostic yield of less than 40% [7].
Cardiac tamponade is a life-threatening compression of the heart that may occur in a slow or rapid fashion typically due to an increased volume of pericardial fluid due to inflammation or injury but may also be from clots, pus, blood, or gas [8, 9].
Clinical Features
The classic presentation of patients with pericardial tamponade includes Beck’s triad of jugular venous distention from elevated systemic venous pressure, distant heart sounds, and hypotension [10]. The sensitivity and specificity of Beck’s triad are limited, and there is a dearth of evidence demonstrating the diagnostic accuracy of the clinical examination for cardiac tamponade [7, 11, 12]. Although tamponade is frequently referred to as a “clinical” diagnosis, echocardiography has established itself as essential and routine in the diagnosis of pericardial effusion and the assessment of tamponade [2, 13]. Once the presence of pericardial effusion has been established, dyspnea, tachycardia, elevated jugular venous pressure, pulsus paradoxus, and cardiomegaly on chest radiograph are seen in over 70% of patients with tamponade [11].
Pathophysiology
The pericardium is a fibrous sac comprised of two layers, the visceral and parietal pericardium. A small amount of pericardial fluid, 20–60 mL, is normally present but is usually not noticed except occasionally in the atrioventricular and intraventricular sulcus. Pericardial fluid is an ultrafiltrate of plasma that originates from epicardial and parietal pericardial capillaries. Pericardial fluid is drained by the lymphatic system on the epicardial surface of the heart and in the parietal pericardium. An abnormal, excessive volume may develop with a variety of conditions related to increased production or impaired removal [14]. In addition to stabilizing the position, lubricating the moving surfaces, and isolating the heart from adjacent anatomic structures thereby preventing adhesions or extension of neoplasms, the pericardium functions to augment hemodynamics. By limiting heart dilatation during diastole, endomyocardial stress is reduced and negative intrathoracic pressure is preserved, which is essential for atrial filling, and the creation of a hydrostatic compensation system which ensures consistent end-diastolic pressure at all hydrostatic levels and the Frank–Starling mechanism remains functional [15].
Important in predicting tamponade are the rate of rise of the volume of pericardial fluid along with pericardial compliance. The pericardium is acutely non-compliant; therefore, even a small increase in volume can lead to hemodynamic compromise if accumulated rapidly. If excessive pericardial fluid accumulates slowly, the pericardium can stretch avoiding hemodynamic compromise. This slow accumulation will eventually reach a limit where the pericardium is unable to stretch further leading to hemodynamic collapse [16]. Tamponade develops when intrapericardial pressure surpasses intracardiac pressure resulting in impaired ventricular filling, increased venous pressure, and reduction in stroke volume [17].
Diagnosis
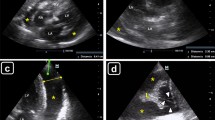
The primary means for confirming the presence, size, and hemodynamic effects of a pericardial effusion are via echocardiography [2]. Echocardiographic features of cardiac tamponade commonly used include diastolic collapse of the right atrium and right ventricle, ventricular shifting with respiration, and engorgement of the inferior vena cava [12] (Figs. 17.1 and 17.2). If time permits, the presence of a pericardial effusion should be evaluated by a formal echocardiogram. Although formal echocardiography remains the mainstay imaging modality, a growing consensus describes the role of point-of-care ultrasound (PoCUS) to diagnose and aid in management of pericardial effusions [16,17,18]. PoCUS has been demonstrated to have a high sensitivity and specificity for detection of pericardial effusion [19]. PoCUS provides diagnostic information relevant to immediate care of the critically ill patient in real time. PoCUS can identify pathologic processes and guide life-saving interventions [19].

Apical four chamber (A4C) echocardiogram with a pericardial effusion and collapse of the right atrium and right ventricle consistent with tamponade (https://doi.org/10.1007/000-2rr)

Short-axis echocardiogram with circumferential pericardial effusion (https://doi.org/10.1007/000-2rn)
All levels of PoCUS-trained clinicians (basic and advanced) should be able to assess for pericardial effusion and tamponade [20]. PoCUS use has expanded significantly as smaller, more portable, and affordable machines have become available. For advanced practice providers (APPs) which includes physician assistants (PAs) and advanced registered nurse practitioners (ARNPs), this invaluable tool can be used in a variety of clinical specialties including critical care [21]. PoCUS training has now become incorporated into many medical education programs including PA programs [22]. PAs have demonstrated competency in the use of PoCUS for diagnosis and management of pericardial effusions [23]. Even in inexperienced hands, PoCUS has been shown to be more sensitive and specific than physical exam for several conditions including pericardial effusion [24].
Fluid Analysis
Pericardiocentesis is not only therapeutic, but examination of it can aid in determining the etiology. Pericardial fluid can be categorized as transudative or exudative. Diagnosis of pericardial effusion is typically achieved by echocardiography; however, it cannot determine the etiology of the effusion. Normal pericardial fluid is clear and pale yellow. Bloody or turbid fluid suggests malignancy or infection with tuberculosis typically being bloody. A milky appearance may suggest chylopericardium.
Light’s criteria, established for distinguishing transudate from exudate for pleural fluid, have a sensitivity of 98% and a specificity of 72% for identifying exudates. Pericardial effusions, like pleural effusions, may be misclassified as exudates when applying Light’s criteria in the setting of diuretic therapy. Utilization of SEAG (serum-effusion albumin gradient) improves accuracy of pleural fluid analysis with diuretic therapy and may be applied to pericardial fluid analysis. Furthermore, pericardial effusion cholesterol concentrations ≥1.2 mmol/L improved diagnostic identification of exudates and demonstrated further accuracy when a pericardial fluid/serum cholesterol ratio was calculated [9]. Common laboratory tests on pericardial fluid include cytology, bacteriological smears and cultures, lactate dehydrogenase (LDH), protein, and cholesterol. Caution should be used when utilizing Light’s criteria to pericardial fluid as the physiologically normally found high protein and LDH could lead to mischaracterization as an exudate [25]. Finally, when pericardial fluid was demonstrated to have a concentration of >40 U/L of adenosine deaminase (ADA), the sensitivity and specificity for TB were over 80% [9]. Other pericardial fluid tests to consider are viral polymerase chain reaction (PCR) and carcinoembryonic antigen (CEA) [26].
Management
Medical Management
Medical management of pericardial effusions is based on hemodynamics and underlying condition. Some effusions such as uremic effusions will often resolve with renal replacement therapy. Medical management of effusions with tamponade physiology is limited. In the setting of tamponade with hypotensive hypovolemia, IV fluids may be of limited benefit, but this has not been demonstrated in normovolemic patients [27]. In some patient populations, diuretics, vasodilators, and mechanical ventilation should be avoided in the setting of tamponade [7, 28,29,30].
Procedural Management
Pericardial fluid may be evacuated from the pericardial space by either a surgical (pericardial window) or percutaneous procedure (pericardiocentesis). Both are effective; however, pericardiocentesis has been demonstrated to have a shorter length of stay and few complications [28].
Indications
According to the 2015 European Society of Cardiology (ESC) Guidelines for the diagnosis and management of pericardial diseases, Class I indications for pericardiocentesis include cardiac tamponade, symptomatic moderate to large pericardial effusions not responsive to medical therapy, and evaluation and evacuation of possible purulent pericardial effusions, to relieve symptoms and establish a diagnosis of malignancy [28]. Table 17.1 lists Class I, II, and III indications for pericardiocentesis.
In the critical care setting, hemodynamic instability from cardiac tamponade would be an indication for pericardiocentesis. Cardiac tamponade is characterized by the clinical signs of hypotension, tachycardia, elevated jugular venous pressure, muffled heart sounds, pulsus paradoxus, diminished voltage on electrocardiogram, electrical alternans of electrocardiogram, and enlarged cardiac silhouette on chest x-ray [29].
Contraindications
Absolute Contraindications
In emergency situations of cardiac tamponade and shock, when hemodynamic collapse is imminent, there are no absolute contraindications. Pericardiocentesis, in these cases, is often a life-saving intervention.
Relative Contraindications
-
1.
Coagulopathy – the risk of bleeding from pericardiocentesis is low; however, uncorrected coagulopathy is a relative contraindication.
-
2.
Aortic dissection – normally a contraindication if thoracic surgery capability is readily available; however, a small amount of pericardial effusion may be drained from these patients to temporize hemodynamics in emergent situations [31, 32].
-
3.
Small volume effusion – (<10 mm in diastole on echo) or when pericardial fluid is not free or when loculated in a lateral or posterior position.
-
4.
Asymptomatic – if the pericardial effusion is small and is resolving.
Risks/Benefits
The use of direct ultrasound guidance has led to a dramatic decrease in complications [5, 33, 34]. Although there are complications inherent to pericardiocentesis (listed below), each approach offers risks and benefits.
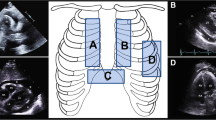
Apical Approach
Utilizing the apical approach, there is a higher risk of left ventricular puncture; however, the wall is thicker than the right atrium and right ventricle and more likely to self-seal [35]. The pleura are usually absent over the cardiac apex making pneumothorax less likely when ultrasound is employed [36].
Subxiphoid/Subcostal Approach
The safest approach for emergent unguided approach as risk for pneumothorax is low; however, the angle of approach carries an increased risk of right atrial puncture [36].
Parasternal Approach
This approach is often provides the shortest route to the effusion; however, this approach may carry a higher risk of injury to the left internal mammary artery or pneumothorax. A puncture site above the rib is required to avoid the intercostal neurovascular bundle [36, 37].
Patient Preparation
The patient should be prepared for a pericardiocentesis as follows:
-
Obtain informed consent only if time and patient condition allows.
-
Review relevant laboratory data to include coagulation profile.
-
To facilitate patient cooperation and ease anxiety, thoroughly explain and discuss all aspects of the procedure with the patient and family.
-
Patients should be informed that the procedure may result in post-procedural discomfort.
-
Remind the patient and family that, despite relatively low complication rates in ultrasound-guided pericardiocentesis, complications are possible and can be serious.
-
Ensure continuous cardiac and hemodynamic monitoring. This includes blood pressure, heart rate, respiratory rate, electrocardiogram, and oxygen saturation. This is particularly important in critically ill patients.
-
Use ultrasound to pinpoint or confirm the proximity of the largest effusion pocket and underlying anatomical structures.
Procedure
In addition to formal echocardiographic guidance, PoCUS devices with appropriately trained clinicians have demonstrated the capability to provide image guidance when performing pericardiocentesis [38]. As the following technique documents only the vital aspects of the three PoCUS-guided pericardiocentesis approaches, the references contain documents providing more procedural details.
General Technique
-
Ensure that all necessary materials and personnel are readily available at the bedside before beginning the procedure. Clinical deterioration of the patient must be anticipated when the decision is made to proceed with a pericardiocentesis.
-
If the clinical situation allows, position the patient in a recumbent 30–45 degree angle to promote inferior and apical pooling bringing the effusion closer to the anterior chest wall.
-
In an anxious patient without signs of overt hemodynamic compromise, short-acting medications can be considered.
-
Every effort to maintain procedural sterility must be ensured. All individuals participating in the procedure must wear sterile gloves, hat, mask, and gown.
-
Ensure the ultrasound probe is placed in a sterile sheath.
-
Antiseptically prepare the skin from the chest to the abdomen using a chlorohexidine-based solution and then drape the site with sterile towels.
-
Anesthetize the skin at the selected site with a local anesthetic.
-
Use a local anesthetic and a small gauge needle to anesthetize along the anticipated trajectory. Use ultrasound guidance for this to avoid potential injury to underlying anatomical structures.
-
Using continuous ultrasound guidance, carefully advance a sheath-covered needle attached to a saline-filled syringe toward the pericardium, while using gentle continuous negative suction, until the pericardial sac is entered and fluid is obtained.
-
Once fluid is obtained, gently advance the sheath over the needle and withdraw the needle.
-
Confirm placement of the needle in the pericardial space by injecting agitated saline through the catheter under direct ultrasound visualization, observing for formed microbubbles in the pericardial sac (Figs. 17.3 and 17.4).
-
Gently advance guidewire through the sheath and then remove the sheath over the guidewire (Figs. 17.5 and 17.6).
-
Make a small “nick” incision at the wire insertion site and then gently introduce the dilator over the wire.
-
Remove the dilator over the guidewire and then insert the catheter over the guidewire (Figs. 17.7 and 17.8).
-
Inject agitated saline again into the pericardial sac to confirm placement of the catheter.
-
Drain the pericardial fluid using gentle syringe suction (Fig. 17.9).
-
Remove catheter and hold pressure at the site.
-
Obtain follow-up echocardiogram and CXR.
-
Provide low-dose analgesics if the patient is hemodynamically stable.

A4C view injecting agitated saline through the catheter under direct ultrasound visualization, observing for formed microbubbles in the pericardial sac. (https://doi.org/10.1007/000-2rp)

Short-axis injecting agitated saline through the catheter under direct ultrasound visualization, observing for formed microbubbles in the pericardial sac. (https://doi.org/10.1007/000-2rq)

Hybrid views to visualize the guidewire within the pericardial space (https://doi.org/10.1007/000-2rm)

Hybrid views to visualize the guidewire within the pericardial space (https://doi.org/10.1007/000-2rs)

Hybrid view demonstrating presence of catheter within the pericardial space (https://doi.org/10.1007/000-2rt)

A4C view of catheter in pericardial space (https://doi.org/10.1007/000-2rv)

A4C view demonstrating removal of 1 l of pericardial fluid (https://doi.org/10.1007/000-2rw)
Specific Techniques
PoCUS-Guided Subxiphoid/Subcostal Technique
Using a 30-degree angle, insert the sheathed needle into the skin below the xiphoid process and 1 cm to the left of the costoxiphoid angle (Fig. 17.10). Using continous ultrasound guidance, identify the site of the largest effusion and any underlying structures. With the transducer pointed under the xiphoid process and aimed cephalad, slowly advance the sheathed needle toward the left shoulder while maintaining gentle continuous negative suction. Once fluid is obtained and the catheter is in place, connect the syringe and catheter to a drainage bag via a three-way stopcock. Completely drain pericardial fluid by manual syringe suction. Continue to assess the patient for hemodynamic stability.
PoCUS-Guided Apical Technique
Palpate for the apex and use PoCUS to identify the site of the largest apical effusion and any underlying structures. Using continuous ultrasound guidance, with the transducer placed just inferior and lateral to the left nipple, insert the sheathed needle into the intercostal space below and 1 cm lateral to the apical beat (Fig. 17.11). Slowly advance the sheathed needle toward the right shoulder while maintaining gentle continuous negative suction until fluid is obtained.
PoCUS-Guided Parasternal Technique
Use PoCUS ultrasound to identify the site of the largest parasternal effusion and underlying structures. Using continuous ultrasound guidance, with the transducer left of the sternum in the third or fourth intercostal space, insert the sheathed needle perpendicularly into the fifth intercostal space 1 cm lateral to the sternal border (Fig. 17.12). Slowly advance the sheathed needle over the upper border of the rib while maintaining gentle continuous negative suction until fluid is obtained.
Equipment (Figs. 17.13, 17.14, and 17.15)
Pericardiocentesis Tray:
-
Skin antiseptic – (Chloraprep or povidone-iodine)
-
Sterile Transparent Fenestrated Drape
-
One 20–25G needle for local anesthesia infiltration
-
Local anesthetic (e.g., 1–2% lidocaine)
-
Scalpel – #11 Blade
-
4 × 4 Gauze
-
18-gauge Teflon-sheathed needle (with a length of 5–8 cm)
-
Syringes – 10, 20, and 50 mL
-
0.035 mm J-tipped guidewire of sufficient length
-
5F to 8F dilator or introducer sheath
Other Supplies:
-
Echocardiography with phased array probe (however, curvilinear and in some instances a linear probe may be used)
-
Sterile gown and gloves
-
Sterile mask and surgical cap/bouffant
-
Sterile isotonic saline for bubble confirmation and catheter flush
-
Sterile probe cover with sterile ultrasound gel
-
5F to 8F, 65 cm pigtail catheter with multiple side holes
Complications
Major complications for echo-guided or fluoroscopic-guided pericardiocentesis range from 0.3% to 3.9% with minor complications ranging from 0.4% to 20%. Major complications include death, laceration of the coronary arteries or intercostal vessels, injury of the cardiac chambers, ventricular arrhythmias, pneumopericardium, pneumothorax requiring chest tube placement, puncture of abdominal organs, and pericardial decompression syndrome [17, 35, 39, 40].
Pericardial decompression syndrome is a rare but potentially fatal syndrome with a 30% mortality that is characterized by hemodynamic deterioration and/or pulmonary edema after an uncomplicated pericardial drainage and is often associated with unexplained development of ventricular dysfunction with an onset of 1–2 days. The mechanism remains poorly understood but may be related to abrupt withdrawal of the entire effusion, and a proposed preventative measure is to initially remove enough pericardial fluid to relieve tamponade and then to prolong the drainage via a drainage catheter [41, 42].
Although this has become less common, electrocardiographic monitoring with an electrode attached to the needle for guidance (ST elevations seen when needle contacts myocardium) has fallen out of favor as the risk of current leak could induce ventricular fibrillation [43].
Minor complications include supraventricular arrhythmias, pneumothorax without hemodynamic sequelae, and temporary vasovagal hypotension [34].
Keys to Success, Perils, and Pitfalls
Intracardiac blood will clot, whereas blood that has transmigrated into the pericardial space will not as it is fibrin free [44]. Two common false positives which can be mistaken for a pericardial effusion by users of PoCUS include pleural effusion and pericardial fat pads. To distinguish a pleural effusion, the descending aorta may be used as a landmark in the parasternal long-axis view. A pericardial effusion will be anterior, whereas a pleural effusion will be inferior to this structure. Concerning a pericardial fat pad, pericardial fluid is typically anechoic, while a fat pad will appear echoic and may have a mottled appearance. Additionally, fat pads also move in concert with the myocardium without competing with the cardiac chambers for space within the pericardium [15] [45]. Finally, difficult pericardiocentesis should be anticipated in patients with prior median sternotomy, obesity, cardiac chamber enlargement/dilation, or loculated pericardial effusions [46].
CPT Coding
-
33010. Pericardiocentesis; initial
-
76930-26. Ultrasonic guidance for pericardiocentesis, imaging supervision, and interpretation; professional component
-
93308-26. Transthoracic echocardiogram; limited or follow-up
Summary
Pericardiocentesis is an important, potentially life-saving procedure that is no longer limited to cardiologists [47]. The diagnosis of tamponade or significant pericardial effusion should be established in a timely fashion. PoCUS, which includes cardiac ultrasound, can be expeditiously performed by APPs [48]. Effective management is essentially limited to pericardial fluid evacuation as the use of volume expansion may be of little benefit and potentially harmful [27]. Procedural guidance with ultrasound is often readily available, even in resource limited settings [49].
References
Kilpatrick ZM, Chapman CB. On pericardiocentesis. Am J Cardiol. 1965;16(5):722–8. https://doi.org/10.1016/0002-9149(65)90057-3.
Imazio M, Gaido L, Battaglia A, Gaita F. Contemporary management of pericardial effusion: practical aspects for clinical practice. Postgrad Med. 2017;129(2):178–86. https://doi.org/10.1080/00325481.2017.1285676.
Levy P-Y, Corey R, Berger P, et al. Etiologic diagnosis of 204 pericardial effusions. Medicine. 2003;82(6):385–91. https://doi.org/10.1097/01.md.0000101574.54295.73.
Sagristà-Sauleda J, Mercé J, Permanyer-Miralda G, Soler-Soler J. Clinical clues to the causes of large pericardial effusions. Am J Med. 2000;109(2):95–101. https://doi.org/10.1016/s0002-9343(00)00459-9.
Akyuz S, Zengin A, Arugaslan E, et al. Echo-guided pericardiocentesis in patients with clinically significant pericardial effusion. Outcomes over a 10-year period. Herz. 2015;40 Suppl 2:153–9. https://doi.org/10.1007/s00059-014-4187-x.
Manjusha M, Kumar BM, Rajaiah NV, Narayana P. Study of characteristic of pericardial effusion and to analyze pericardial fluid in various etiologies. IAIM. 2017;4(10):221–9.
Hoit BD. Pericardial effusion and cardiac tamponade in the new millennium. Curr Cardiol Rep. 2017;19(7):57. https://doi.org/10.1007/s11886-017-0867-5.
Spodick DH. Acute cardiac tamponade. N Engl J Med. 2003;349(7):684–90. https://doi.org/10.1056/NEJMra022643.
Kopcinovic LM, Culej J. Pleural, peritoneal and pericardial effusions – a biochemical approach. Biochem Med (Zagreb). 2014;24(1):123–37. https://doi.org/10.11613/BM.2014.014.
Beck CS. Two cardiac compression triads. JAMA. 1935;104(9):714. https://doi.org/10.1001/jama.1935.02760090018005.
Roy CL, Minor MA, Brookhart MA, Choudhry NK. Does this patient with a pericardial effusion have cardiac tamponade? JAMA. 2007;297(16):1810–8. https://doi.org/10.1001/jama.297.16.1810.
Fowler NO. Cardiac tamponade. A clinical or an echocardiographic diagnosis? Circulation. 1993;87(5):1738–41. https://doi.org/10.1161/01.cir.87.5.1738.
Argulian E, Messerli F. Misconceptions and facts about pericardial effusion and tamponade. Am J Med. 2013;126(10):858–61. https://doi.org/10.1016/j.amjmed.2013.03.022.
Rodriguez ER, Tan CD. Structure and anatomy of the human pericardium. Prog Cardiovasc Dis. 2017;59(4):327–40. https://doi.org/10.1016/j.pcad.2016.12.010.
Vogiatzidis K, Zarogiannis SG, Aidonidis I, et al. Physiology of pericardial fluid production and drainage. Front Physiol. 2015;6:62. https://doi.org/10.3389/fphys.2015.00062.
Alerhand S, Carter JM. What echocardiographic findings suggest a pericardial effusion is causing tamponade? Am J Emerg Med. 2019;37(2):321–6. https://doi.org/10.1016/j.ajem.2018.11.004.
Kern MJ, Society of Cardiac Angiography and Intervention. Pericardial disease interventions. In: SCAI interventional cardiology board review. 2nd ed. Philadelphia: Wolters Kluwer Health; 2014. p. 752–60.
Kimura BJ, Gilcrease GW, Showalter BK, Phan JN, Wolfson T. Diagnostic performance of a pocket-sized ultrasound device for quick-look cardiac imaging. Am J Emerg Med. 2012;30(1):32–6. https://doi.org/10.1016/j.ajem.2010.07.024.
Labovitz AJ, Noble VE, Bierig M, et al. Focused cardiac ultrasound in the emergent setting: a consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J Am Soc Echocardiogr. 2010;23(12):1225–30. https://doi.org/10.1016/j.echo.2010.10.005.
Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part II: cardiac ultrasonography. Crit Care Med. 2016;44(6):1206–27. https://doi.org/10.1097/CCM.0000000000001847.
Monti J. Revolution or evolution? A proposal for the integration of point-of-care ultrasound into physician assistant clinical practice. J Physician Assist Educ. 2017;28(1):27–32. https://doi.org/10.1097/JPA.0000000000000101.
Rizzolo D, Krackov RE. Integration of ultrasound into the physician assistant curriculum. J Physician Assist Educ. 2019;30(2):103–10. https://doi.org/10.1097/JPA.0000000000000251.
Daymude ML, Mehta S, Gruppo L. Use of emergency bedside ultrasound by emergency medicine physician assistants: a new training concept. J Physician Assist Educ. 2007;18(1):29–33. https://doi.org/10.1097/01367895-200718010-00005.
Liu RB, Donroe JH, McNamara RL, Forman HP, Moore CL. The practice and implications of finding fluid during point-of-care ultrasonography: a review. JAMA Intern Med. 2017;177(12):1818–25. https://doi.org/10.1001/jamainternmed.2017.5048.
Ben-Horin S, Shinfeld A, Kachel E, Chetrit A, Livneh A. The composition of normal pericardial fluid and its implications for diagnosing pericardial effusions. Am J Med. 2005;118(6):636–40. https://doi.org/10.1016/j.amjmed.2005.01.066.
Maisch B, Ristić AD, Seferović PM, Tsang TSM. Interventional pericardiology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. https://doi.org/10.1007/978-3-642-11335-2.
Sagristà-Sauleda J, Angel J, Sambola A, Permanyer-Miralda G. Hemodynamic effects of volume expansion in patients with cardiac tamponade. Circulation. 2008;117(12):1545–9. https://doi.org/10.1161/CIRCULATIONAHA.107.737841.
Adler Y, Charron P, Imazio M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC)endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921–64. https://doi.org/10.1093/eurheartj/ehv318.
Imazio M, Mayosi BM, Brucato A, et al. Triage and management of pericardial effusion. J Cardiovasc Med (Hagerstown). 2010;11(12):928–35. https://doi.org/10.2459/JCM.0b013e32833e5788.
Ristić AD, Imazio M, Adler Y, et al. Triage strategy for urgent management of cardiac tamponade: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2014;35(34):2279–84. https://doi.org/10.1093/eurheartj/ehu217.
Hayashi T, Tsukube T, Yamashita T, et al. Impact of controlled pericardial drainage on critical cardiac tamponade with acute type A aortic dissection. Circulation. 2012;126(11 Suppl 1):S97–S101. https://doi.org/10.1161/CIRCULATIONAHA.111.082685.
Cruz I, Stuart B, Caldeira D, et al. Controlled pericardiocentesis in patients with cardiac tamponade complicating aortic dissection: experience of a centre without cardiothoracic surgery. Eur Heart J Acute Cardiovasc Care. 2015;4(2):124–8. https://doi.org/10.1177/2048872614549737.
Lazaros G, Imazio M, Tousoulis D. Percutaneous pericardiocentesis: safety first! Cardiology. 2015;130(1):34–6. https://doi.org/10.1159/000368892.
Maggiolini S, Gentile G, Farina A, et al. Safety, efficacy, and complications of Pericardiocentesis by real-time Echo-monitored procedure. Am J Cardiol. 2016;117(8):1369–74. https://doi.org/10.1016/j.amjcard.2016.01.043.
Maggiolini S, De Carlini CC, Imazio M. Evolution of the pericardiocentesis technique. J Cardiovasc Med (Hagerstown). 2018;19(6):267–73. https://doi.org/10.2459/JCM.0000000000000649.
Gluer R, Murdoch D, Haqqani HM, Scalia GM, Walters DL. Pericardiocentesis – how to do it. Heart Lung Circ. 2015;24(6):621–5. https://doi.org/10.1016/j.hlc.2014.11.009.
Chetrit M, Lipes J, Mardigyan V. A practical approach to pericardiocentesis with periprocedural use of ultrasound training initiative. Can J Cardiol. 2018;34(9):1229–32. https://doi.org/10.1016/j.cjca.2018.06.004.
Osranek M. Hand-carried ultrasound-guided pericardiocentesis and thoracentesis. J Am Soc Echocardiogr. 2003;16(5):480–4. https://doi.org/10.1016/S0894-7317(03)00080-4.
Wakabayashi Y, Hayashi T, Mitsuhashi T, Fujita H. Tension pneumopericardium after pericardiocentesis: useful echocardiographic obscured heart sign and effective postural change during air aspiration. Heart Rhythm. 2018;15(7):1116. https://doi.org/10.1016/j.hrthm.2018.02.021.
Tsang TSM, Enriquez-Sarano M, Freeman WK, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77(5):429–36. https://doi.org/10.4065/77.5.429.
Imazio M. Pericardial decompression syndrome: a rare but potentially fatal complication of pericardial drainage to be recognized and prevented. Eur Heart J Acute Cardiovasc Care. 2015;4(2):121–3. https://doi.org/10.1177/2048872614557771.
Pradhan R, Okabe T, Yoshida K, Angouras DC, DeCaro MV, Marhefka GD. Patient characteristics and predictors of mortality associated with pericardial decompression syndrome: a comprehensive analysis of published cases. Eur Heart J Acute Cardiovasc Care. 2015;4(2):113–20. https://doi.org/10.1177/2048872614547975.
Shabetai R, Oh JK. Pericardial effusion and compressive disorders of the heart: influence of new technology on unraveling its pathophysiology and hemodynamics. Cardiol Clin. 2017;35(4):467–79. https://doi.org/10.1016/j.ccl.2017.07.001.
Hatch N, Wu TS, Barr L, Roque PJ. Advanced ultrasound procedures. Crit Care Clin. 2014;30(2):305–29, vi. https://doi.org/10.1016/j.ccc.2013.10.005.
Ceriani E, Cogliati C. Update on bedside ultrasound diagnosis of pericardial effusion. Intern Emerg Med. 2016;11(3):477–80. https://doi.org/10.1007/s11739-015-1372-8.
Narula J, Choudhury A, Sharma A. Pericardiocentesis can be nasty. Accidents do occur while “Rail-roading” Sheaths and pigtails! Ann Card Anaesth. 2018;21(3):290. https://doi.org/10.4103/aca.ACA_115_17.
Imazio M, De Ferrari GM. Editorial commentary: Pericardiocentesis: no more a subspecialty technique! Trends Cardiovasc Med. 2019;29(7):384–5. https://doi.org/10.1016/j.tcm.2019.01.010.
Baeten R, Masinelli S. A pilot study for physician assistant focused cardiac point of care ultrasound assessment-PA FOCUS. Chest. 2018;154(4):1121A. https://doi.org/10.1016/j.chest.2018.08.1014.
Loughborough W. Emergency pericardiocentesis under dynamic ultrasound guidance in the resource limited setting. Afr J Emerg Med. 2014;4(3):127–9.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Baeten, R.G., Alexander, D.L. (2021). Pericardiocentesis. In: Taylor, D.A., Sherry, S.P., Sing, R.F. (eds) Interventional Critical Care. Springer, Cham. https://doi.org/10.1007/978-3-030-64661-5_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-64661-5_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64660-8
Online ISBN: 978-3-030-64661-5
eBook Packages: MedicineMedicine (R0)