Abstract
The chapter is devoted to amperometric biosensors for the determination of various mycotoxins that are contaminants of food, animal feed, and grain crops having toxic and carcinogenic properties. The analytical capabilities of various biosensors based on cholinesterase, tyrosinase, horseradish peroxidase, and aflatoxin oxidase were evaluated. Their advantages and disadvantages over other methods are noted. Immunochemical (immunoenzyme) methods of mycotoxin analysis and development of immunosensors with horseradish peroxidase and tyrosinase as labels are focused. Separate aptasensors for determining mycotoxins are considered. The influence of nanomaterials and composites based on these nanomaterials on the analytical characteristics of biosensors is shown. The advantages of using biosensors in comparison with the other methods of analysis, problems, and disadvantages of their application in practice are noted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Amperometric biosensor
- Immunosensor
- Aptasensor
- Mycotoxin
- Enzyme
- Cholinesterase
- Tyrosinase
- Horseradish peroxidase
- Aflatoxin oxidase
- Label
- Nanoparticles
- Nanostructured materials
- Carbon nanotubes
- Enzyme immunoassay
1 Introduction
The mycotoxins encompass a wide group of compounds that are very different in their chemical structure and, consequently , in their toxic effects. There are more than 250 species of microscopic fungi that can produce several hundred mycotoxins. Mycotoxins (from the Greek mykes (mushroom) and toxikon (poison)) are secondary metabolites of microscopic fungi that have pronounced toxic properties. Mycotoxins are produced mainly by fungi of the genera Aspergillus, Fusarium, and Penicillium. The most common mycotoxins that are distinguished by their toxic and carcinogenic properties, as well as foods that may be infected with mycotoxins, are shown in Table 12.1 (Haque et al. 2020).
Biosensors represent technology that can be applied to several sectors of the food industry for the storage of grains and raw materials, food production/processing, security and protection, and packaging of food. Diverse biosensors have emerged in the last decade as an alternative for analyzing microorganisms and toxins in food due to the capability for fast analysis, reproducibility, stability, and accuracy. A wide variety of transducers can be explored for mycotoxin and spoilage, and fungi detection, where optical (surface plasmon resonance – SPR and fluorescence), piezoelectric (quartz crystal microbalance – QCM), and electrochemical (impedimetric, potentiometric, and amperometric) spectroscopies stand out as main biosensing method (Oliveira et al. 2019).
Biosensors are one of the most effective tools to identify the fungi and mycotoxins. They are extremely sensitive and easy to operate, facilitating quick and reproducible analysis, followed by the advantage of low-cost tools, miniaturization, and the development of portable devices. The research work in this direction continues in different laboratories (Medyantseva et al. 2014; Varlamova et al. 2016).
Another widely used approach for the detection of mycotoxins is based on immunoassays, such as enzyme immunoassay (ELISA), which mainly examines the signal produced by chromogenic substrates after the formation of the antigen-antibody complex. Immunochemical tests are easy to use due to their simplicity, multiple reading of samples, and low cost. Oliveira et al. (2019) reviewed some types of bio- and immunosensors used for the detection of mycotoxins in detail. The aim of this chapter is to discuss the recent achievements in the development of amperometric biosensors for the determination of mycotoxins. Moreover, the role of modern nanomaterials and composites in changing the analytical capabilities of biosensors (immunosensors) has been also discussed.
1.1 Enzyme Sensors
Biosensors using enzymes, whole cells, and artificial receptors (molecular printed polymers, MIP), along with affine biosensors, provide high selectivity for binding mycotoxins. Among a sufficiently large number of enzymes known to date, only a limited number have found application in the development of biosensors. This is due to a number of reasons which mainly include the high cost of purified enzymes, as well as the difficulties associated with the choice of systems that record the result of biospecific interactions. The need for the electrochemical activity of the products of the enzymatic reaction or the substrates themselves is one of the conditions for the development of amperometric biosensors. These are the properties of compounds associated with the activity of esterases, hydrolases, and oxidoreductases. Therefore, enzymes of these classes are most often used for the development of amperometric biosensors.
1.1.1 Biosensors Based on Cholinesterase
Over the course of 9 years (from 2001 to 2010), as reviewed by Grieshaber et al. (2008), more than 100 research papers related to the creation of cholinesterase biosensors (CE biosensors) were published. At the same time, the possibilities of CE biosensors were considered by the example of determining irreversible inhibitors (organophosphorus insecticides and nerve poisons), pseudo-irreversible inhibitors (carbamic pesticides, insecticides, toxins), and reversible inhibitors, for example, aflatoxin B1 (AFB1).
One of the major concerns is the contamination of food products, grains, and feed by mycotoxins, which are usually produced by various fungi, has forced researchers to turn to CE again. It was shown that cholinesterase BSs based on printed graphite electrodes have the necessary sensitivity in determining one of the most toxic mycotoxins – AFВ1. The detection of AFB1 is more profitable than the detection of insecticides by the CE biosensor, since reversible inhibition usually results in a complete recovery of the enzyme activity after measuring the inhibitory effect by simply washing the biosensor even with water.
The potentiometric biosensor based on AChE was developed for inhibition determination of AFB1, and a possibility in principle of the real samples analysis using the developed biosensor was shown. The working parameters of AChE biosensors for inhibition determination of AFB1 were studied and optimized. The bioselective membrane contained 1% AChE, and 4 mM AChCl was chosen as a working substrate concentration. Dynamic range of AFB1 determination was 0.2–40 μg/ml. The developed biosensor was characterized by sufficient signal reproducibility over 1 working day and could be stored for a month. An influence of the sample preparation on the biosensor operation and the matrix effect were also studied. A possibility to measure AFB1 in real samples by the biosensor developed is stated (Stepurska et al. 2015)
1.1.2 Biosensors Based on Horseradish Peroxidase (HRP)
Immobilized HRP biosensors can be used primarily to determine the peroxidase substrate –hydrogen peroxide. This task is very relevant: there is a need for analyses of biological fluids and other solutions for determining hydrogen peroxide due to its key role in various processes in the human body and in the environment.
Alonso-Lomillo et al. (2011) summarized the process of developing biosensors based on HRP for the determination of OTA. Biosensors were manufactured using a single screen-printing technology. Ink containing HRP was directly applied to carbon electrodes by screen printing, which ensures high speed and simplicity of the process of manufacturing biosensors for determining OTA. The change in the formal redox potential of the Fe (III)/Fe (II) pair was used to demonstrate the efficiency of the process of loading HRP as a fragment of ink. Chrono-amperometrically detected oxidation current associated with the concentration of OTA in various beer samples. Under optimal conditions, the range of working concentrations is 23.85–203.28 nmol/L. Reproducibility, expressed in relative standard deviation, was 10%. Regarding stability, the biosensor retained 30% of the initial sensitivity even after the third calibration. The LOD was 26.77 ± 3.61 nmol/L.
An amperometric biosensor based on HRP for determining the content of citrine mycotoxin in rice samples was described in Zachetti et al. (2013). The biosensor is based on the use of a carbon-paste electrode modified with carbon nanotubes included in mineral oil, HRP, and ferrocene as a redox mediator. The biosensor is coated externally with a dialysis membrane, which is fixed on the electrode. Reproducibility was 70%. The linear region of the determined contents is from 1 to 11.6 nmol/L, LOD 0.75 nmol/L. To confirm the results, a fluorimetric determination of citrine was carried out in the same rice samples. A very good correlation was obtained between electrochemical and spectrophotometric methods.
The combination of the two enzymes was very successful, which was reported in the study by Puiu et al. (2012). A kinetic approach is described for studying the interaction of AChE of electric eel and AFB1 or its protein conjugate (e.g., AFB1-HRP-) to develop a simple and sensitive method for the detection of these compounds. The dissociation constant Kd of the AChE/AFB1-HRP interaction (0.4 mM), obtained using surface plasma resonance, is very close to the inhibition constant obtained by amperometry (Ki = 0.35 mM). This proves that the conjugation of AFB1 with a carrier protein does not significantly affect the affinity of AFB1 for AChE. Thus, the AChE/AFB1-HRP pair can be used as a system simulating the binding of AChE to other AFB1 protein adducts and then can be used to develop biosensors for determining AFB1 bound to plasma proteins. The immobilization protocol suggested minimizing nonspecific adsorption on a self-organizing monolayer of the functionalized surface of the surface plasma resonance chip without additional hydrophilic components. At the same time, the immobilization process was developed in such a way as to prevent the possible occurrence of mass transfer restriction effects during the operation of the biosensor. The LOD was 0.008 mM for AFB1-HRP (2.5 ng/ml) and 0.94 ng/ml for AFB1 itself, which is lower than for spectrophotometric and amperometric analyses.
1.1.3 Biosensors Based on Aflatoxin Oxidase
Li et al. (2011) first developed an amperometric biosensor for determining AFB1 based on immobilized aflatoxin oxidase incorporated in a sol-gel, which was deposited on a platinum electrode modified by multilayer carbon nanotubes (MLCNs). The covalent bond between aflatoxin oxidase and MLCNs made it possible to maintain enzyme activity and sensitive response to the oxidation of AFB1. The apparent Michaelis-Menten constant for AFB1 is 7.03 mmol/L. The sensor allowed us to obtain a linear dependence in the concentration ranges from 3.2 nmol/L to 721 nmol/L (from 1 ng/ml to 225 ng/ml) with a LOD of 1.6 nmol/L (signal-to-noise ratio is 3), average response time of 44 s (at a concentration of AFB1 greater than 45 ng/ml – in less than 30 s.), and a high sensitivity coefficient. The activation energy was 18.8 kJ/mol, which indicates a large value of the catalytic effect of aflatoxin oxidase during the oxidation of AFB1 for this biosensor. Aflatoxin oxidase covalently attached to multiwalled CNTs has been successfully used to detect AFB1, which turns into an oxidized form with oxygen consumption and the formation of H2O2.
Thus, amperometric biosensors are a very successful application for the analysis of objects infected with mycotoxins. It can be noted that today the process of sensitivity and selectivity of determinations can be controlled using different options for immobilizing enzymes on the surfaces of various detectors, including those modified with various nanomaterials. The combination of the two enzymes also contributes to the improvement of mycotoxin determination methods. And finally, the use of narrowly targeted enzymes (using aflatoxin oxidase as an example) improves the selectivity of determinations.
Medyantseva et al. (2012, 2014) proposed new amperometric biosensors for the determination of mycotoxins based on modified multiwalled carbon nanotubes (MLCNs), planar platinum electrodes, and immobilized enzymes: CE, cysteine desulfhydrase, alkaline phosphatase, and tyrosinase. Mycotoxins have shown to exhibit the properties of reversible inhibitors: OTA and ZEN-ChE, AFB1, OTA, and ZEN-alkaline phosphatase and CDG-patulin- alkaline phosphatase-ZEN-tyrosinase, which allows their determination using appropriate modified MLCN biosensors in the concentration range from n×10−(5-6) to n×10−(9-12) mol/L. The use of a modifier allows you to expand the range of detectable concentrations; reduce LOD, by no less than an order of magnitude; increase the sensitivity coefficient; improve the correlation coefficient; and obtain more reproducible results compared to the unmodified version of biosensors.
The kinetic parameters of the reactions of the enzymatic conversion of substrates in the presence of enzyme sensors and mycotoxins in the corresponding concentration ranges for each case are estimated. It would be necessary to specify. The proposed biosensors were tested in the analysis of grain crops (wheat, barley, corn) and animal feed (bran of cereal crops) in Russia and Vietnam, and food products (peanut kernels, buckwheat, apples, apple juice) allowed to detect mycotoxins at and below maximum allowable concentration (MAC) with relative standard deviation no more than 0.076.
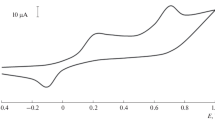
Amperometric biosensors based on planar platinum electrodes modified with multilayer carbon nanotubes, gold nanoparticles, and an immobilized enzyme (tyrosinases) are designed to detect patulin mycotoxin in the concentration range of 1×10−6–8×10−12 mol/L with an error of no more than 0.063. The results were used to control the content of patulin in foods (Fig. 12.1) (Varlamova et al. 2016).
Scheme of the action of a biosensor based on immobilized tyrosinase includes graphite screen-printed electrode with CNTs and AuNPs (1), with immobilized enzyme (tyrosinase) (2), with patulin in solution (3). E enzyme, CNTs carbon nanotubes, AuNPs gold nanoparticles, S substrate, P product of the enzymatic reaction
New amperometric biosensors have been proposed for the determination of AFM1 based on modified CNTs, graphene oxide (GO), and gold nanoparticles (Au NPs) in chitosan of planar platinum electrodes and an immobilized tyrosinase enzyme. It has been established for the first time that AFM1 exhibits the properties of a reversible tyrosinase inhibitor, which allows its determination using biosensors modified with nanomaterials in the concentration range of 1×10−6 –1×10−11 mol/L, with сн 5×10−12 mol/L. The kinetic parameters of the enzymatic conversion of phenol in the presence of a tyrosinase biosensor modified with CNT, GO, AuNPs, and mycotoxin M1 were estimated. The proposed enzyme sensors based on tyrosinase have been used to test methods for determining AFM1 in food products (Varlamova et al. 2019).
1.2 Enzyme-Linked Immunosorbent Assay (ELISA) for the Determination of Mycotoxins
For the determination of mycotoxins, various variants of the highly sensitive enzyme-linked immunosorbent assay (ELISA) are widely used. They allow you to get accurate results and use minimally purified and even untreated extracts. It should be noted that recently a number of review articles have appeared on the problems of mycotoxin ICA, which summarize the relevant literature on this issue (Gogin 2005), which is presented in this section.
A new amperometric immunosensor for supersensitive detection of ZEN based on mesoporous carbon (MC) and trimetallic nanograys (core/shell particles with moving cores enclosed in shells) is proposed. MC improves the sensitivity of the immunosensor due to its extremely large specific surface area, corresponding pore location, and good conductivity. Rattles are composed of an Au core and an imperfect (defective) AgPt-shell structure (Au/AgPt) and are retained in the MC by physical adsorption. The method of X-ray spectroscopy confirmed the composition of the synthesized nanopowders. Compared to monometallic and bimetallic nanoparticles (NPs), Au/AgPt nanopowders show a higher electron transfer rate due to the synergistic effect of NPs of Ag and Pt. Ab to ZEN was immobilized on nanorotations through Ag-NH2 and Pt-NH2 groups. An immunosensor based on cyclic and square-wave voltammetry makes it possible to determine ZEN with a LOD of 1.7 μg/ml and has a wide linear range of detectable concentrations from 0.005 to 15 ng/ml, as well as good stability, reproducibility, and selectivity of results. It is possible to use the sensor in clinical analysis (Liu et al. 2014).
An electrochemical immunosensor consisting of magnetic balls and disposable printed electrodes was used to determine the ZEN in baby food samples. The number of paramagnetic balls is limited by the surface of the modified printed electrode, where electrochemical detection is achieved by the participation of the substrate and the mediator for HRP LOD is 0.007 μg/L (Hervаs et al. 2010).
Another variant of immunoassay was developed by Mai (2013). Since AFB1 is one of the most dangerous toxins causing aflatoxicosis, an enzyme-linked immunosorbent sensor was developed to detect AFB1 using tyrosinase as an enzyme label. The range of working concentrations of the developed immunosensor for determining AFB1 was 1×10−6–1×10−12 mol/L. Low percent cross-reactivity for other mycotoxins indicates a high selectivity for the determination of AFB1. The binding constants of the formed immune complexes Ab-AFB1: Ка1 = (6.9±0.2)×1010 mol/L and Кa2 = (2.7 ±0.1)×109 mol/L. The developed immunosensors were used to control the content of AFB1 in food (nuts), which made it possible to determine this mycotoxin at and below the MAC (Fig. 12.2).
Scheme of the action of an immunosensor based on immobilized Ab against AFB1 and tyrosinase includes co-immobilized enzyme (tyrosinase) and Ab (1), Ag in solution - AFB1 (2), the formed immune complex and options for approaching the substrate to the active surface of the enzyme (3). T transducer, E enzyme, Ab antibody, Ag antigen, S substrate, P product of the enzymatic reaction
1.2.1 Immunosensors for Detecting Mycotoxins with Horseradish Peroxidase as a Label
Peroxidase is one of the most studied enzymes and is most often used as a label for one of the biocomponents in biosensors based on the principles of immunochemical recognition. The use of the enzyme significantly increases the sensitivity of the method. Due to the relatively low cost of peroxidase in comparison with fluorescent or radioactive labels, peroxidase biosensors are widely used.
Istamboulié et al. (2016) reported the development of an electrochemical immunosensor for detecting ultra-trace amounts of AFM1 in food products. The sensor was based on competitive immunoassay using HRP as a label. Ab-coated magnetic NPs were used to separate bound and unbound fractions. Samples containing AFM1 were incubated with a certain amount of Ab and HRP conjugate. The resulting mixture was applied to the surface of a planar graphite electrode, and, in the presence of an organic mediator [5-methylphenazinium methyl sulfate], the electrochemical response was evaluated chronoamperometrically. Such an immunosensor has a LOD of 0.01 ppb, which is below the maximum allowable concentration for milk.
To determine OTA in wine samples, an electrochemical immunosensor was developed using a printed gold working electrode and the Ag/AgCl system as a pseudo-comparison electrode (Heurich et al. 2011). A competitive enzyme-linked immunosorbent assay was carried out with an immobilized OTA conjugate, which was achieved by passive adsorption or covalent immobilization using an amine bond with a carboxymethylated dextran hydrogel onto a gold working electrode. Electrochemical detection was carried out using TMB and Н2О2 and HRP as the enzyme label. Chronoamperometry at −150 mV was used to register the generated signal. As a result, the immunosensor for OTA reached a LOD of 0.5 μg/L with a linear range of detectable concentrations of 0.1–10 μg/L for passive adsorption of toxin conjugants, while for covalent immobilization on a gold electrode modified with a hydrogel of carboxymethylated dextran, the LOD was 0.05 mcg/l with a linear range of concentrations of 0.01–100 μg/L. The modified gold immunosensor was tested in the analysis and affinity-purified wine samples . LOD in buffer solutions amounted to 0.05 μg/L.
1.3 Aptasensors for Detecting Mycotoxins
Aptamers are short oligonucleotide sequences obtained by combinatorial chemistry and selected against the target analyte. They offer a wide range of functions, including an affinity for mycotoxins and modifications necessary for implementation in the assembly of biosensors, also called aptasensors (Evtugyn and Hianik 2020).
Yang et al. (2020) successfully constructed a novel electrochemical aptasensor for sensitive and selective detection of AFB1. The thiolated complementary strand (cDNA) of the AFB1 aptamer was immobilized on the surface of the GCE modified with AuNPs through Au-S bond. The aptamer was attached on the surface of GCE via by specific base pairing. In the presence of AFB1, the aptamer was detached from the electrode surface by specific recognition between the AFB1 and aptamer, forming aptamer-AFB1 conjugates in solution. The conjugates were digested by exonuclease I to trigger AFB1 recycling. DNA-AuNPs-HRP nanoprobes were bound with cDNA on the surface of electrode by specific base pairing. HRP could catalyze the oxidation of hydroquinone (HQ) to benzoquinone (BQ) by H2O2 for producing a strong electrochemical signal. The electrochemical signals increased with increasing concentrations of AFB1 in a range from 10−3 ng/ml−1 to 200 ng/ml−1 with a low detection limit of 3.3×10−4 ng/ml−1. The aptasensor has been applied for the determination of AFB1 in peanuts and corn samples and the recoveries were 88.5%–110.2%.
An electrochemical aptasensor based on competitive immunoassay for the determination of OTA was described by Bonel et al. (2011). The OTA-specific aptamer was functionalized with magnetic beads and competed with mycotoxin conjugated to HRP (HRP-OTA) and free OTA. After the separation and purification step for magnetic separation, the aptamers modified with paramagnetic beads were immobilized on disposable graphite-printed electrodes in a magnetic field, and the product of the enzymatic reaction with the substrate was detected by differential-pulse voltammetry. Magnetic separation results were previously tested, optimized, and compared with other competitive immunoassay schemes (direct/indirect with the aid of an aptasensor immobilized on the surface of disposable printed graphite electrodes or with the participation of an aptamer functionalized using gold nanoparticles). The magnetic immunosensor showed a linear response to OTA in the range of 0.78–8.74 ng/ml, with LOD of 0.07 ± 0.01 ng/ml, and was accurately applied to extracts of certified wheat ears with a relative standard deviation of at least 8%.
Prabhakar et al. (2011) developed a highly sensitive impedimetric aptasensor based on a Langmuir-Blodgett film (polyaniline-stearic acid) containing a DNA aptamer specific for OTA supported on the indium-tin-oxide electrode for determining OTA. It has been proven that the aptasensor operates in a linear concentration range from 0.0001 μg/ml (0.1 ng/ml) to 0.01 μg/ml (10 ng/ml) and 1 μg/ml–25 mg/ml with a LOD of 0.1 ng/ml. Analysis time does not exceed 15 min. The aptasensor can be reused about 13 times. The binding constant of the aptamers with OTA, calculated using the Langmuir adsorption isotherm, was 1.21×107 M−1.
For a sensitive determination of OTA, Barthelmebs et al. (2011) formed a new electrochemical aptasensor based on disposable printed electrodes. Two strategies for determining OTA were investigated: using indirect and direct competitive analysis based on the use of superparamagnetic nanoparticles. The characteristics of modified aptasensors, such as reproducibility, stability, sensitivity, and analysis time, were studied. The best characteristics were obtained by implementing a direct competitive analysis format. In this case, free OTA competed with labeled alkaline phosphatase OTA; in this case, magnetic beads were immobilized to bind OTA to the DNA aptamer. For electrochemical detection due to the conversion of the corresponding substrate for the alkaline phosphatase enzyme, differential pulse voltammetry was used. The developed aptasensor made it possible to achieve LOD of 0.11 ng/ml. The range of working concentrations is from 0.11 to 0.15 ng/ml.
2 Conclusion and Future Perspectives
The chapter is devoted to issues related to the analytical capabilities of some types of amperometric biosensors (immunosensors) for the determination of mycotoxins in various objects – food, animal feed, and cereals. The prevalence and high toxicity have led to the fact that the focus on the development of appropriate biosensors is given to the determination of aflatoxins (AFB1 and M1), ZEN, and ОТA. The most common transducers (primary converters) in such biosensors (immunosensors) are printed graphite electrodes, although GCE continues to be used successfully in these devices (biosensors).
For the development of amperometric biosensors, enzymes of the esterase, hydrolase, and oxidoreductase class are most often used, due to the electrochemical activity of the products of the enzymatic reaction or the substrates themselves. A separate section is devoted to the use of HRP as a label, which helps to reduce the LOD of mycotoxins.
Another technique that is currently widely used to improve analytical characteristics is the modification of the surface of electrodes with various carbon materials, metal nanoparticles, and composites based on them in combination with various matrix materials. All this contributes to an increase in the working area of the electrodes and, as a result, to an increase in the analytical signal. All this leads to an improvement in the sensitivity of determinations and determination of mycotoxins at and below the MAC.
Thus, modern analytical needs for the determination of mycotoxins can be successfully implemented using amperometric biosensors. The goal is to bring research results to consumers, i.e., in the commercialization of related developments. Despite the excellent analytical characteristics in many cases, it should be noted that the problem remains the preservation of immunological and catalytic properties of biocomponents in the composition of biosensors over time.
Abbreviations
- Ab:
-
Antibody
- AFB1 :
-
Aflatoxin В1
- АFМ1 :
-
Aflatoxin М1
- OTA :
-
Ochratoxin A
- ZEN :
-
Zearalenone
- T-2 toxin:
-
Trichothecenes T2
- CNTs:
-
Carbon nanotubes
- ELISA:
-
Enzyme immunoassay
- CE:
-
Cholinesterase
- HRP :
-
Horseradish peroxidase
- LOD:
-
Limit of detection
- MWCNTs:
-
Multiwalled carbon nanotubes
- GO:
-
Graphene oxide
- Au NPs:
-
Gold nanoparticles
- GCE:
-
Glassy-carbon electrodes
- MAC:
-
Maximum allowable concentration
References
Alonso-Lomillo MA, Domнnguez-Renedo O, Torno-de Romõn L, Arcos-Martnez MJ (2011) Horseradish peroxidase-screen printed biosensors for determination of Ochratoxin A. Anal Chim Acta 688:49–53
Barthelmebs L, Hayat A, Limiadi A, Marty J, Noguer T, Barthelmebs L (2011) Electrochemical DNA aptamer-based biosensor for OTA detection, using superparamagnetic nanoparticles. Sensors Actuators B 54:2180–2218
Bonel L, Vidal JC, Duato P, Castillo JR (2011) An electrochemical competitive biosensor for ochratoxin A based on a DNA biotinylated aptamer. Biosens Bioelectron 26:3254–3259
Evtugyn G, Hianik T (2020) Aptamer-based biosensors for mycotoxin detection. In: Nanomycotoxicology: treating mycotoxins in the nano way. Elsevier, London, pp 35–70
Gogin А (2005) Mycotoxins: effective control effective production. Compound Feed J 2:68–70
Grieshaber D, MacKenzie R, Voros J, Reimhult E (2008) Electrochemical biosensors – sensor principles and architectures. Sensors 8(3):1400–1458
Haque MA, Wang Y, Shen Z, Li X, He C (2020) Mycotoxin contamination and control strategy in human, domestic animal and poultry: a review. Microb Pathog 142:104095. https://doi.org/10.1016/j.micpath.2020.104095
Hervаs M, Lуpez АM, Escarpa A (2010) Simplified calibration and analysis on screen-printed disposable platforms for electrochemical magnetic bead-based inmunosensing of zearalenone in baby food samples. Biosens Bioelectron 25:1755–1760
Heurich M, Kadir M, Tothill IE (2011) An electrochemical sensor based on carboxymethylated dextran modified gold surface for ochratoxin A analysis. Sensors Actuators 156:162–116
Istamboulié G, Paniel N, Zara L, Granados LR, Barthelmebs L, Noguer T (2016) Development of an electrochemical biosensor for the detection of Aflatoxin M1 in milk. Talanta 146:464–469
Li S, Chen J, Cao H, Yao D, Liu D (2011) Amperometric biosensor for aflatoxin B1 based on aflatoxin-oxidase immobilized on multiwalled carbon nanotubes. Food Control 22:43–49
Liu LA, Chao Y, Cao W, Wang Y, Luo C, Pang X, Fan D, Wei Q (2014) Label-free amperometric immunosensor for detection of zearalenone based on trimetallic Au-core/AgPt-shell nanorattles and mesoporous carbon. Anal Chim Acta 847:29–36
Mai TTH (2013) Amperometric biosensors for the detection of some mycotoxins. PRhD dissertation. Kazan Federal University. Kazan:148
Medyantseva EP, Mai TTH, Varlamova RM, Tarasova EY, Sahapova GR, Budnikov HC (2012) Amperometric biosensors for determining ochratoxin A. Kazan Univ Ser Nat Sci 154:92–104
Medyantseva EP, Mai TTH, Tarasova EY, Sahapova GR, Nikolaeva OV, Budnikov HK (2014) Amperometric biosensors based on alkaline phosphatase and carbon nanotubes for the detection of certain mycotoxins Factory laboratory diagnostics of materials. 80:5–12
Oliveira IS, Junior AGS, Andrade CAS, Oliveira MDL (2019) Biosensors for early detection of fungi spoilage and toxigenic and mycotoxins in food. Curr Opin Food Sci 29:64–79
Prabhakar N, Matharu Z, Malhotra BD (2011) Polyaniline Langmuir–Blodgett film based aptasensor for ochratoxin A detection. Biosens Bioelectron 26:4006–4011
Puiu M, Istrate O, Rotariu L, Bala C (2012) Kinetic approach of aflatoxin B1–acetylcholinesterase interaction: a tool for developing surface plasmon resonance biosensors. Anal Biochem 421:587–594
Stepurska KV, Soldatkin OO, Arkhypova VM, Lagarde F, Jaffrezic-Renault N, Dzyadevych SV (2015) Development of novel enzyme potentiometric biosensor based on pH-sensitive field-effect transistors for aflatoxin B1 analysis in real samples. Talanta 144:1079–1084
Varlamova RM, Medyantseva EP, Hamidullina RR, Budnikov HC (2016) Determination of patulin by amperometric tyrosinase biosensors based on electrodes modified with carbon nanotubes and gold nanoparticles. Sci Notes Kazan Univ Ser Nat Sci 158:351–368
Varlamova RM, Medyantseva EP, Hamidullina RR, Budnikov HC (2019) Amperometric tyrosinase biosensors based on modified nanomaterials electrodes for the determination of aflatoxin M1. J Anal Chem 74:71–80
Yang H, Zhang Q, Liu X, Yang Y, Yang Y, Liu M, Li P, Zhou Y (2020) Antibody-biotin-streptavidin-horseradish peroxidase (HRP) sensor for rapid and ultra-sensitive detection of fumonisins. Food Chem 316:126356. https://doi.org/10.1016/j.foodchem.2020.126356
Zachetti VGL, Granero AM, Robledo SN, Zon MA, Fernández H (2013) Development of an amperometric biosensor based on peroxidases to quantify citrinin in rice samples. Bioelectrochemistry 91:37–43
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Medyantseva, E.P., Beylinson, R.M., Khaybullina, A.I., Budnikov, H.C. (2021). Variants of Amperometric Biosensors in the Determination of Some Mycotoxins: Analytical Capabilities. In: Rai, M., Reshetilov, A., Plekhanova, Y., Ingle, A.P. (eds) Macro, Micro, and Nano-Biosensors. Springer, Cham. https://doi.org/10.1007/978-3-030-55490-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-55490-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-55489-7
Online ISBN: 978-3-030-55490-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)