Abstract
This overview describes and evaluates research methods used in the study of Neotropical social wasps. The emphasis is on field techniques; laboratory and museum procedures and detailed analytical methods can be found elsewhere. The first part of the chapter addresses techniques for working with collected materials. Topics include studies of biodiversity (wasp species richness and abundance), locating colonies in the field, collecting and preserving individuals and whole colonies, and a look at data obtainable from collected colonies. The second part covers procedures for working with live individuals and colonies in the field, including anesthetization, marking adults for individual recognition, uses of video recording, how to study foraging behavior and nesting behavior, and the bioassaying of pheromones. A section follows on manipulating active colonies for special purposes, which covers how to measure age polyethism, colony productivity, egg-to-adult development time, suggestions for working with epiponine swarms, and a look at the pros and cons of working with artificially housed colonies. The final section is a brief assessment of what to expect in the coming years by way of improvements in hardware and software relevant to the field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
19.1 Introduction
In many fields of science, major advances are often enabled by the application of new methods or approaches. This may come about either via new technological advances (e.g., the development of the electron microscope led to major advances in cell biology) or in the application of theory developed in one field to facilitate gains in another (e.g., application of economic theory to ecology). Although perhaps more modest in scope and scale than these examples, the same can be said for the study of the behavior and ecology of the social wasps. Technological innovations of many kinds have led to advances in our understanding of this group. The use of video recording and digital photography, for example, has enabled analysis of behavior at levels of accuracy and detail that are impossible with direct observation. The size and cost of camcorders continue to decline, while the quality of output has advanced to the point where high-definition recording is now the norm.
It is with this in mind that we believe a review of methods and technology used by wasp researchers will be of use, especially to students just entering the field. It is often the case that an innovation developed and applied by an author may remain obscure, despite having the potential to facilitate the work of others. Our aim here is to assemble in an organized way the techniques that have been found useful by those working with Neotropical social wasps. Some of these are high-tech, but others are simply shortcuts or practical solutions that we, and others, have found useful. Our hope is that such a compilation will not only serve as a useful reference, but will stimulate a more conscious attention to methods, which may in itself lead to new and useful techniques.
Although some earlier authors have described methods for taking nests (Rau 1933; Richards and Richards 1951), to our knowledge there has been no comprehensive review of research techniques useful in the study of tropical wasps. Spradbery (1973) and Edwards (1980) provide detailed methods applicable to the study of vespine biology, some of which are adaptable for use with polistines. Here we limit our coverage to methods useful in the study of tropical social wasps. Techniques used in studies of temperate-zone species are included if they may be of use for tropical species. Our focus is on field techniques; specialized laboratory methodology, such as chemical analyses and genomics and proteomics, which can be found elsewhere, are not included. The same goes for methods of controlling wasps as pests. We rely heavily on our own experience with what we have found to work, and readily acknowledge that others would likely put together a different account. Thus, we apologize in advance for any expectation that this compilation is complete.
The account below starts with conducting surveys, moves on to the collection of individuals and whole colonies, then to what can be learned from collected colonies. We next focus on how to work with active colonies in the field, including experimentation as well as observation. Finally, we discuss the manipulation of colonies to achieve special aims.
19.2 Survey Methods
19.2.1 Biodiversity Studies
Studies on social wasp diversity and abundance have appeared at an accelerating rate over recent decades, with 78 cited for Brazil alone from 1982 to 2015 (see Barbosa et al. 2016 for a meta analysis; see also Jacques et al. 2018). When the primary purpose is to determine the species occurring in a region or habitat, a combination of methods will lead to the most complete inventory (De Souza et al. 2011). These may include line censuses—visual searches for individuals and nests along forest edges, roadsides, trails, streams, or an artificial transect (Corbara et al. 2009), malaise trapping (Silveira 2002), light trapping (Aragão and Andena 2016), and sampling of foragers feeding at flowers (Silva-Pereira and Santos 2006), ripe fruit (Dvořák and Landolt 2006), or carrion (Silveira et al. 2005). Of the 11 methods used in such surveys (Barbosa et al. 2016), intensive active searching for individuals in all microhabitats typically yields the greatest number of species, with traps baited with sugar solutions coming in second (De Souza et al. 2011; Jacques et al. 2018). In some studies, malaise and bait traps have taken species not obtained by the other methods used (Barbosa et al. 2016; Jacques et al. 2018). Baited traps tend to be more effective at sampling swarm-founding than independent-founding species (Jacques et al. 2018). Sugary baits, especially sugarcane molasses, are more effective than fruit juices and much more effective than protein baits, with the latter attracting primarily the carrion-feeding species (Jacques et al. 2018). Baiting by spraying a solution of sugar and salt on vegetation and checking those sites frequently is a relatively easy means of enhancing the numbers caught by active searching (Noll and Gomes 2009). The nocturnal genus Apoica is best sampled with light traps (Aragão and Andena 2016). For as complete a list of species as possible, a combination of sampling techniques, including active search for nests, sweep netting of foragers, baiting and trapping (De Souza et al. 2011), and canopy sampling by fogging with a knockdown insecticide (Blüthgen and Stork 2007) is recommended. Not surprisingly, the number of species taken by sampling, as a fraction of the total species occurring in the sampled area, rises with the number of person-hours spent searching and the number of months of the year sampled (Somavilla et al. 2014; Barbosa et al. 2016).
A usual final step in reporting species-richness surveys is to apply some estimate of how close the sample comes to being a complete list of all the species occurring in the sampled area. The software EstimateS (Colwell 2013) has been used in a number of recent surveys of Neotropical wasp species diversity (e.g., Corbara et al. 2009; Barbosa et al. 2016). However, EstimateS is no longer being updated and the most recent version (9) does not run fully under current versions of Mac and Windows operating systems (Colwell 2013). More recently developed tools for estimating species richness include SpadeR and others that use the powerful R language (Chao and Colwell 2017). These are available for download from Anne Chao’s website (Chao 2019). Other estimators abound, and have been used by other authors (e.g., Silva-Pereira and Santos 2006; Gomes and Noll 2009; Melo et al. 2015; Jacques et al. 2018).
19.2.2 Avoiding Bias
If the goal is more than just a species list, but an accurate estimate of the relative abundance of each wasp species in a habitat or region, the potential for bias using each of the above methods must be carefully considered. Care must be taken to sample the range of habitats occurring in the region (e.g., ground level, understory, canopy, undisturbed vs. disturbed, primary vs. secondary forest, urban vs. natural). Sampling should be done over a wide area. Active searches for nests are likely to have a higher success rate with species whose nests are large and conspicuous than with those with small and/or cryptic nests. Searches and bait trapping close to large colonies may bias numbers in favor of those species.
19.3 Finding Colonies
Other than surveys, most studies of social wasps begin with locating colonies. If the goal is simply to find enough colonies of a target species to work with, several techniques are appropriate, varying from the haphazard to highly structured/organized searches. Active visual search in the species’ preferred habitat is the most practical method and can work for most species. Polybia occidentalis nests are readily found in low shrubs and trees in pastures and along fencerows and roadsides (O'Donnell and Jeanne 1992; Schueller et al. 2010). For species that utilize narrowly specific nesting substrates or sites, searches can focus on such places. For example, Angiopolybia pallens frequently nests under the broad leaves of low plants in the forest understory, and Polybia rejecta, Synoeca virginea, and Agelaia myrmecophila typically nest in close association with the large carton nests of the ant Azteca spp. (Jeanne 1991). For species that are specialists nesting on myrmecophilous plants, the most efficient approach is to search for those plants. Other species are synanthropic, nesting on human constructions (e.g., Jeanne 1972). Metapolybia, for example, nests on flat surfaces and can often be found on buildings.
For species that form large colonies but are rare, or nest in cavities or high in trees, the lining technique may be useful. Lining has long been used to locate colonies of honey bees (Visscher and Seeley 1989). To locate a colony using this technique, one or more bees foraging at flowers are first captured in a box and allowed to feed on a rich, anise-scented sugar syrup inside the box. As the bee exits, the bearing of its departure toward the nest—the “beeline”—is noted. Repeating this step after moving along the beeline can eventually lead to the nest. Triangulation—plotting (e.g., on a Google Earth map) the intersection of bee lines taken from two or more capture points—can help pinpoint the nest location. Applied to wasps, this technique can take advantage of the attraction of many species to fermenting fruit and to carrion (Fig. 19.1). If foragers feeding on a fruit patch depart to the north, some of the fruits, with foragers on them, can be moved some distance in that direction, perhaps in a box. After they depart, the bearing is adjusted based on the new flight path and the process is repeated. With luck, one or more such steps will bring the observer within sight of the nest. If the surroundings are in low light (e.g., forest understory), chilling the wasp on ice, then tying a few centimeters of white thread around the wasp’s petiole can extend the distance of visual contact. As the wasp regains mobility and flies toward the nest, the trailing thread provides a visual cue that can be seen for a greater distance than the wasp alone. It also reduces the wasp’s flight speed so that the observer can run along with it for a distance, thereby getting closer to the location of the colony. A modified version of this technique is traditionally used in Japan to locate vespine wasp nests (Saga 2019). Instead of flagging the wasp itself, thread is tied to pieces of carrion small enough to be carried by a forager. When a wasp picks up the flagged carrion, the thread provides the extra visual cue, as above. To our knowledge, this technique has not been tried for tropical wasps, but it could be useful for Agelaia, Angiopolybia, and Polybia that feed on carrion (Jeanne and Taylor 2009).
A useful strategy for locating colonies in urban areas is to place advertisements in local newspapers or online classifieds offering free removal of colonies from homeowners’ properties (Hastings et al. 1998). If a particular species is being sought, including a picture and description of the wasp and nest structure with the advertisement can improve success. However, the researcher must be ready to deal with a potentially large volume of responses and to take on the time and travel needed to follow up on each. It is good practice to provide the homeowners with an information sheet describing the methods they can use to remove a nest, in the event that their wasps are not the species needed.
19.4 Collecting Wasps
19.4.1 Collecting Individuals from the Nest
If only one or a few individuals from a nest are required for identification, foragers can be captured as they leave or approach the nest, provided enough nearby open space is available to swing a net. If not, the rim of the net can be placed gently against the nest until a wasp crawls onto it to inspect it. The net is then slowly pulled away from the nest and into an open area, and when the wasp eventually flies to return to the nest, it is swept into the net. It can be moved to the tip of the net by exploiting the tendency of a trapped wasp to move upward and toward light. Once there, it can be captured by forcing it into an open vial pushed up inside the net. When it is knocked into the vial, the vial is stoppered.
19.4.2 Collecting Whole Colonies
If the goal is to capture the entire adult population, the nest must be taken at night, when most or all of the foragers are inside (except, of course, for the nocturnal genus Apoica).
For species nesting on leaves or twigs, it is often possible to collect the entire nest and its adults by enclosing it in a sturdy transparent plastic bag. If dense vegetation surrounds the nest, this can be clipped away a few hours in advance of collection to provide unhindered access. Without disturbing the colony, the opening of the plastic bag is brought up around the nest and sealed tightly around the supporting twig proximal to the nest with a wire tie. The twig is then clipped. Following collection, care must be taken to prevent the formation of water droplets due to condensation inside the bag, lest wasps be lost to drowning. A few sheets of crumpled paper toweling inside the bag will help. If the adults need to be killed, a wad of cotton soaked in ethyl acetate or chloroform can be dropped into the bag. Colonies so captured can be stored in a refrigerator and the adults will remain fresh enough for dissection for about a day. If the wasps need only be anesthetized, a plastic snap-cap vial with holes in the top and containing ether-soaked cotton can be placed in the bag (wasps are killed by direct contact with ether-soaked cotton).
Species nesting on solid substrates present a different challenge. If the nest carton is sturdy (Synoeca, Chartergus, Epipona), the entrance can be plugged with a tight wad of cotton, which is then soaked with chloroform or diethyl ether. After the buzzing stops, the bottom of the carton can be carefully opened and the anesthetized adults dropped into a plastic bag. For more fragile nests (Metapolybia, Clypearia), the opening of a plastic bag can be tacked tightly around the nest at night. A small amount of anesthetic is then injected via hypodermic through the bag and the nest carton to subdue the adults. (Extreme care must be taken when storing and working with these chemicals. Ether is flammable and the fumes are explosive. Inhaling chloroform can cause fatigue, dizziness, and headache; elevated doses may damage the liver and kidneys.)
Colonies nesting in cavities can be anesthetized by stuffing a wad of cotton tightly into the entrance at night, then soaking it with diethyl ether or chloroform. After activity ceases, the nest can be excavated.
Collecting nests high in trees requires ingenuity. One of us collected a large nest of Agelaia areata from a height of ~20 m in a tree in Mexico (Jeanne 1975b). The large branch bearing the nest was carefully cut during the day and left suspended from a rope. By late afternoon, the adults had settled down and were on or inside the nest. After dark, the branch was slowly lowered on the rope to a point 1 m above the ground, where the nest was collected in a plastic bag.
One of us used a specially rigged net to collect a nest of Chartergus artifex and its adults from a height of about 7 m (R. L. Jeanne, unpublished). The metal rim of a standard insect net was removed and replaced with an elastic band (a chain of sturdy rubber bands) that constricted the net opening to about 10 cm; 3-cm loops of string were tied around the elastic at four points around the net, and each was passed through a hole in the side of a 50-cm square frame made of light wood. Each loop was held in place by a nail, stretching the net open. At night, the frame, mounted on a bamboo pole, was brought up around the nest and the nails were simultaneously pulled via a lanyard, releasing the string loops and allowing the net to snap tight around the middle of the nest. The branch bearing the nest was cut the next morning and lowered to the ground.
19.4.3 Preservation
The preservation of collected material falls into several categories, depending on its end use. Wasps collected for museum specimens or taxonomic studies, such as those used to study morphological variation within a colony, can be collected and preserved according to standard entomological techniques (i.e., dry-pinning, or preservation in Kahle’s or Dietrich’s solution and storage in 70% alcohol). These methods are described in a variety of entomological texts (e.g., Arnett Jr. 2000; Johnson and Triplehorn 2004).
Preservation for molecular analyses requires special procedures in order to maintain the integrity of the molecules to be analyzed. Generally, this means storage at −20°C or lower for DNA analysis and −80°C for RNA and protein work. Roskens et al. (2010) studied the proteins and enzymatic activity of adult and larval wasp saliva and found that placing samples on dry ice and storing them at −80°C protected the integrity of their material. They also developed desiccation methods wherein the samples were dried via silica beads or Drierite (W.A. Hammond Drierite, Xenia, OH), with SDS or protease inhibitors added. They were able to successfully preserve the proteins and enzymatic activity of these desiccated samples for up to 3 weeks at 37°C (Roskens et al. 2010). These techniques facilitate the study of proteomics in non-model species, which typically are not kept in labs and must be collected in the field.
19.5 Working with Collected Colonies
In a series of landmark monographs, O. W. Richards (Fig. 19.2) showed how much could be learned about the biology of Neotropical wasps by quantitative analyses of the adults, brood, and nests of collected colonies (Richards 1945; Richards and Richards 1951; Richards 1978). These works generated questions that stimulated a great deal of new work over subsequent decades. They remain useful references today.
19.5.1 Adults
The most basic information that can be taken from a collected colony is the size of the adult population, broken down into males and females. If the colony is collected at night with no escapees, the totals are especially valuable. Dissection of all the females (or a large random sample) can yield a wide range of data, including the number of queens, their degree of ovary development, and the presence of endoparasites.
Dissection to detect insemination, a marker of queens, is most quickly done with fresh material. The terminal gastral segment is grasped with watchmaker’s forceps and pulled out, bringing the gut, ovaries, sting apparatus, and spermatheca with it. Care must be taken not to confuse the spermatheca with the venom sac; the spermathecal duct opens dorsally into the common oviduct, whereas the larger and visibly muscular venom sac ducts to the base of the sting. Under low magnification (dissection microscope or strong hand lens), an opaque, pearly mass in the center of the otherwise clear spermatheca indicates a sperm load; an empty spermatheca is clear throughout (Murakami et al. 2009). In preserved material, the tissues of the spermatheca become opaque and the sac must be crushed on a microscope slide and examined with a compound microscope to detect the presence of sperm, a more time-consuming process (Reed et al. 1988).
Degree of ovary development can be assessed with the same dissection. For the epiponines, if the colony is in an early stage of development, it is likely to have many queens, each with slight ovary development. Presence of one or more mature eggs (i.e., at or close to the size of a laid egg) is good evidence that the female is an active egg-layer. A number of authors have quantified the degree of ovary development among females; excellent recent examples include Murakami et al. (2009) and Desuo et al. (2011).
Unless the species is one with clear queen–worker dimorphism, queens will be reliably recognizable only through dissection to check for insemination and developed ovaries. In later stages of colony development, when the number of queens has been reduced, their greater ovary development may be recognizable by a gaster that is visibly swollen and, in at least some species (e.g., Polybia occidentalis, P. sericea), chocolate-brown in color as opposed to the jet black of workers and young queens. A caveat: a heavy load of gregarine gametocysts can swell the gaster of a worker to a deceptive, queen-like size (Bouwma et al. 2005); only dissection will determine whether queens can be reliably recognized externally.
Presence of endoparasites can also be revealed via dissection of the gaster. Gregarine parasites in the gametocyst stage are sometimes found in the gasters of infected adults, where they appear as one or more (sometimes >100) white spheres in the body cavity. Heavy infestations can reduce the productivity of a colony (Bouwma et al. 2005). The range of polistine wasp species subject to infection by these protozoan parasites is poorly known. Nothing is known about how the wasps become infected or how the parasites disperse from their hosts.
Body size is a descriptive species statistic of considerable value to ecological and sociobiological studies of social wasps, but has unfortunately been underappreciated. Among the measures used are full body length, various measures of the thorax, and length of the forewing or part of it. In the interest of facilitating cross-species comparisons, we advocate adoption of a standard measure. For the following reasons, we strongly recommend the full length of the forewing: (1) it is unambiguously measured, unlike full body length that can vary depending on the degree of flexure of the gaster in preserved specimens; (2) because it is a two-dimensional structure it is not subject to potential errors introduced when the two ends of a three-dimensional structure are in different planes of focus under a dissection microscope; (3) it is the longest nonarticulated part of the body, thereby minimizing measurement imprecision; (4) it is a part of the thorax, the tagma that varies least among female castes (Jeanne 2003); (5) it is the most widely used measure of body size in the social wasp literature (see chapter 2, this volume). Richards (1978), for example, reported full wing length, measured from the center of the tegula to the tip of the wing, for 86 Neotropical polistine species. In contrast, in a number of other studies, some subdivision of the wing is measured. We can think of no advantage of this over the full wing. While it is true that in older workers the wingtip may be frayed, in our experience this is a rare occurrence.
A number of morphometric studies of caste differences have appeared in recent years (e.g., Noll and Zucchi 2002). Most of these make use of ten or so measurements of the head, thorax, and abdomen, but often differ in the details. Again, for the sake of enabling cross-species comparisons, it would make sense to standardize these measures.
Body weight, fresh or dried, is an alternative measure of body size that is more useful in certain kinds of studies, such as measures of colony productivity (e.g., Bouwma et al. 2006).
19.5.2 Brood
To a greater extent than the adults , the brood in the nest embodies a record of the colony’s developmental history. The age of the colony in number of generations of brood, measured as the egg-to-adult development time in days, can be estimated from the distribution of brood stages in the combs (Richards and Richards 1951). In a recently founded nest, if the oldest comb has a central mass of pupae, with one or two central (oldest) cells having recently produced an adult, the nest is just over one developmental period (or “generation”) old. If the central mass of pupae is surrounded by open cells of eggs and larvae, and beyond these by a ring of pupae, the nest is just over two periods old (Fig. 19.3). By examining in this way the distribution pattern of eggs, larvae, and pupae across all the combs of the nest, it is possible to estimate age for up to about three periods. Beyond that, the distribution pattern becomes increasingly irregular, and therefore unreliable as an indicator of age, due to the accumulating effects of brood mortality and interindividual variation in development time. For such older colonies, careful inspection of the oldest cells in the nest can reveal how many times those cells have been used to rear an immature to the pupal stage. Prepupae spin a silken cocoon before they pupate, then void their hindgut contents (meconia) into the bottom of the cell. In some species the silk network at the bottom of the cell is substantial enough that longitudinal sections through the cell bottom will reveal, under magnification, how many layers of meconium-on-silk layers there are. Because meconia are often pasted off-center at the bottom of the cell and thus some may be missed by the cut through the cell, it is critical that a sample of cells be sectioned. An alternative approach is to peel away the cell’s carton from around the stacked meconia, then carefully tease them apart to determine their number. If ten cells are sampled and found to contain 6, 6, 6, 6, 6, 6, 6, 5, 5, 4 meconia, the nest is at least six brood generations old. Cells with fewer than six housed one or more brood that failed to survive to pupation.
Polistes instabilis . The nest grows downward from its attachment at the top, so the age of the brood decreases downward. Thus, the cells in the larger mass of pupae and below contain brood of the first generation (i.e., first use of those cells for rearing brood). Cells above that contain brood of the second generation (second use). The few opens cells at the very top contain young brood of the third generation. Thus, this nest is slightly greater than two egg-to-adult developmental periods old. (On the twig to the left of the nest is a katydid (Ancistrocercus sp.), with the antennae of a second one visible top right. These tettigoniids presumably gain some protection from predators by roosting near wasp nests during the day.) (Photo: R. L. Jeanne. Costa Rica)
The number of larval instars characterizing a species can be determined by measuring the maximum width of the head capsule of a sample of larvae (e.g., Rocha and Giannotti 2016). For epiponines it is most convenient to do this under a dissection microscope with fresh-killed larvae still in their cells. If the sample is large enough, the number of peaks in the plotted size-frequency distribution will clearly indicate the number of instars.
For some studies it is useful to report the population of brood in a nest. The amount of brood can be quantified either by counts (numbers of eggs, larvae, pupae) or by biomass. For colony productivity studies, total dry mass of brood is the most direct measure of total colony output. All brood are removed from their cells, stored in a preservative, then dried and weighed (Jeanne and Nordheim 1996).
19.6 Working with Active Colonies
It is our experience that techniques developed for working with active colonies of one species often do not work well for others, even congeners, and have to be modified. It is our hope that the procedures described below will nevertheless provide useful starting points for those wishing to apply them to little-studied species.
19.6.1 Gaining Access
Other than mating behavior and work with foragers at food sources, investigations into the social behavior of wasps require close access to active colonies. If the nest is in an inconvenient location—too high, over water, etc.—it can often be moved. Over many years of working with Polybia occidentalis, we have routinely transplanted nests to shaded positions in low vegetation about 1.5 m above the ground, where they can be observed close-up at eye level by an observer sitting in a field chair (Fig. 19.4). The nest is prepared for moving by clipping vegetation around it, then the twig bearing the nest is clipped at night and carried (typically over several tens of meters) carefully to its new location and wired, zip-tied, or spring-clipped into place on a twig previously prepared to receive it. We have found for this species that enclosure in a plastic bag is unnecessary during the move, as long as care is taken not to excessively jar the nest or bash it against passing vegetation during transport (exception: if the nest is moved a long distance or in a vehicle, bagging it is prudent). Even gentle jarring of the nest may cause some adults to emerge onto the envelope and the supporting twig, but they will not fly at night (Schueller et al. 2010). However, because wasps will fly up a flashlight beam in the dark, artificial white light must be kept away from the nest until it is at its new site. Wasps do not see into the red portion of the spectrum (Peitsch et al. 1992), so a flashlight or headlamp that produces red light allows the collector to see, yet prevents the wasps from flying. After the nest is attached to the twig, Tanglefoot® can be applied to the base of the twig to prevent predation by ants (Schueller et al. 2010).
For nests that cannot be moved—e.g., Synoeca and others built high and/or on solid surfaces—the observer must be moved to the nest. Naumann’s use of free-standing scaffolding platforms allowed him to reach the high nests of Protopolybia acutiscutis that typically were constructed at the tips of flexible branches or palm fronds (Naumann 1970). One of us (RLJ) used the same system to reach similarly situated nests of Ropalidia romandi in Queensland, Australia.
Once close access to the nest is in place, care must be taken to avoid disturbances that might alarm the colony. Three stimuli cause alarm: mechanical disturbance, human breath, and alarm pheromone (a component of venom). Alarmed workers will fly at and attempt to sting nearby moving objects, especially dark-colored ones (Jeanne 1981a). Jarring the nest can be avoided by taking care to eliminate any contact by the observer with the substrate bearing the nest. The risk of the second is reduced by placing oneself downwind of the nest, or otherwise being careful not to exhale on it. The third can be a problem if one is working with alarm pheromones or handling wasps (e.g., for marking—see below) in ways that cause them to release venom. When a mishandled wasp returns to the nest, venom residue on its gaster may trigger an alarm response in nearby individuals. Depending on the aggressiveness of the species, protective clothing—a beekeeper’s suit with helmet and veil—may be prudent. In our experience, such protective gear is usually unnecessary when working with the relatively docile Polybia occidentalis. Nitrile gloves are effective in preventing the sting from penetrating to the skin. It goes without saying that use of mosquito repellent by the observer must be avoided.
19.6.2 Anesthetization
Anesthetization may be necessary in some cases in order to mark individuals or to collect whole, active colonies. Three methods are typically used to anesthetize: chilling on ice or in a refrigerator, exposure to diethyl ether, and exposure to carbon dioxide (CO2). Of these, chilling typically requires the greatest amount of time to make wasps immobile, and wasps typically recover most rapidly from it provided the ambient temperature is sufficient. It is likely that it is also the most benign of the three methods, but some studies on honey bees suggest that immobilization by chilling affects hoarding behavior (Mardan and Rinderer 1980). Chilling may also be used as a means of temporary immobilization of an entire colony before transferring it to a new container or observation nest box (see Sect. 19.8 below). Colonies of Vespula germanica have successfully been kept overnight in a ~4°C refrigerator with very little loss of individuals (Taylor and Jeanne 2018)
Diethyl ether is a useful anesthetic for handling whole, active colonies. Sealing the nest or other container holding the wasps maximizes its effectiveness as an anesthetizing agent. The duration of anesthesia is lessened when wasps are exposed to sunlight or other forms of UV radiation (Ishay et al. 1994). Repeated uses over a short time period also seem to lessen its effects (B. J. Taylor pers. obs.). Because ether fumes are heavier than air, anesthetization will be more effective if it is placed at a level above where most wasps are located. Care must also be taken when using ether, as prolonged exposure can be lethal to insects. In studies on Vespula germanica, Taylor (pers. obs.) found that the queen seemed especially susceptible to its effects. Diethyl ether’s effects on other aspects of the biology of wasps are not well-known. Plath (1924) found that ether anesthetization of bumble bees did not cause the bees to lose their memory of nest location. However, nest location may be a particularly well-learned memory and not as readily subject to loss as other memories, such as a recently learned food odor (Alloway 1972).
Carbon dioxide is a commonly used anesthetic for entomological studies. Unlike diethyl ether, the risk of wasp mortality when using CO2 seems to be low. Indeed, Owen (1962) found that CO2 never caused death when used to anesthetize Polistes fuscatus. Its effects on behavior and physiology have long been reported to be minor and short-lived (Nicolas and Sillans 1989). However, CO2-induced anesthesia has been found to accelerate age polyethism in Apis mellifera (Nicolas and Sillans 1989), reduce pollen gathering (Ribbands 1950), and modify hoarding behavior (Mardan and Rinderer 1980). Prolonged exposure also affects memory and foraging behavior (Nicolas and Sillans 1989). Even more extreme are reports that it increases brood cannibalism in ants (Sorensen et al. 1983) and ejection of larvae in bumble bees (Pomeroy and Plowright 1979). Warwick Kerr once informed the senior author that ether has less effect on the behavior of stingless bees than does CO2 (Jeanne 1972). Finally, CO2 has the added disadvantage of requiring that a portable CO2 container be carried into the field.
So, which method is best? All have their drawbacks, but it largely depends on the study being conducted. When rapid anesthetization is required, CO2 and ether are the best bet. Ether is easier to use if rapid anesthetization is required in the field, especially when collecting whole colonies. If rapid anesthetization is not required, chilling can be done with little fear that it will cause harm to the wasps. In contrast, ether has the potential to kill if exposure is too long. In addition, as mentioned under “Collecting whole colonies” (below), ether fumes are highly combustible. Ashburner and Thompson Jr (1978) reviewed all three methods and recommended both CO2 and chilling over ether .
19.6.3 Marking Individuals
Most studies of behavior require the marking of at least some of the adults of a colony for individual recognition. For small colonies, one can get away with a small number of unique color combinations. West-Eberhard was able to mark Polistes without capture or anesthetization by daubing one or more colors of paint haphazardly onto the wings and bodies of workers on the nest (West-Eberhard 1969). This is workable only for marking small numbers of wasps; beyond a few tens of individuals, relying on randomly placed marks becomes too cumbersome and prone to errors of identification.
For larger colonies, a code that translates to numbers is essential. Karl von Frisch, working with honey bees, was apparently the first to do this, beginning in the 1920s. Using a color- and position-coded system of paint dots on the thorax and abdomen, he was able to uniquely mark up to several thousand individuals (von Frisch 1967). The numbered disks (Opalithplättchen), developed later for use on honey bees, are the wrong size for most wasp species and have rarely been used by wasp researchers.
19.6.3.1 Color Codes
For many years, we have used a coding system combining five colors of paint with positions on the thorax (Fig. 19.5). The colors are arranged alphabetically and code for numbers as follows: blue = 1 and 6, green = 2 and 7, red = 3 and 8, white = 4 and 9, yellow = 5. Position on the thorax determines the range: left side = 1–5, right side = 6–9. Positions from front to back represent ones, tens, hundreds, thousands. Thus, blue in the upper-left quadrant of the mesoscutum = 1, lower-left = 10, right half of the postscutum = 600, and so on (Fig. 19.6). Although von Frisch applied paint spots to the gaster as well as the thorax of his bees, we found that spots on the gaster are obscured by the folded wings when the wasp is on the nest. Hence, our system uses the thorax exclusively.
Others have developed different systems. Gadagkar uses ten colors (plus a blank) in four positions (thorax, abdomen, left wing, right wing) to achieve over 14,000 unique combinations; adding a fifth (unspecified) position increases the number to over 161,000 (Gadagkar 2001 and personal communication). Unlike the system described above, each wasp is identified by its unique combination of the color and position of its spots, rather than with a number. Another option is to use ten colors to represent digits 1–10, and assigning three different positions on the body to represent ones, tens, and hundreds (Brenner and Patterson 1988). Other marking systems are reviewed by Southwood (1978) and Walker and Wineriter (1981).
We have found that for field studies, using more than five colors is unworkable. Under low-light field conditions (early morning/late afternoon; forest understory) it becomes impossible to distinguish gray from silver, or dark blue from light blue, for example. We advise against marking the wings, as it risks fouling the wing-folding mechanism. Furthermore, especially for smaller wasp species, a paint spot is a substantial addition to the mass of the wing and could potentially hinder flight by altering wingbeat frequency.
19.6.3.2 Media
A wide variety of substances have been used to mark insects for individual or group recognition (reviewed by Hagler and Jackson 2001). Paints are an inexpensive solution. A number of wasp workers have used model airplane dope (e.g., Testors™ enamel). A droplet is applied to the wasp with a toothpick, insect pin, paper clip, or fine brush. In our experience, these paints are less than satisfactory. The dope comes in small screw-cap bottles, which are hard to handle under field conditions. If the bottles are left open for quick access, the solvent evaporates and the paint thickens and forms a skin; thick paint applied to cuticle will fail to make firm contact upon drying and will be at risk of flaking off. While this can be corrected by adding thinner, if too much is added, the paint risks running by capillarity into the wing articulations, potentially hindering flight or even proving fatal (Owen 1962). Opening and closing the bottles at each use requires two hands, risks spillage, and interrupts behavioral observation. Over time, the threads on cap and bottle become clogged with dried paint and have to be scraped clean.
All these difficulties are much reduced with the use of paint pens. These are available in both oil-based paint and water-based acrylic. Both dry quickly and are opaque and waterproof, although oil-based paints were found to be more durable than water-based ones (Wineriter and Walker 1984). We have marked thousands of Polybia occidentalis individuals using a brand of oil-based paints (Fig. 19.7). The fine or extra-fine points make it quick and easy to apply spots precisely in the desired quadrant of the mesothorax (Fig. 19.8), despite the 1.6 mm width of this part of the body. Loss of spots due to flaking is rare. What works well for fieldwork is to drill five holes into the edge of a wood block, one for each color and sized to allow the paint pens to be inserted, cap down, tightly into them. With each pen resting lightly in its cap, it can quickly be picked up with one hand and used, with only a few seconds of exposure of the open pen before it is replaced loosely into its cap. RLJ fastens such a block inside the front wall of the plastic fishing tackle box he uses to carry forceps, mirrors, stopwatches, and other tools and supplies into the field (see Fig. 19.4).
Tests of the effects of these paints on the behavior and longevity of bees and wasps have shown mixed results. In a lab study, Testors PLA™ enamel applied to the heads of two species of halictine bee was shown to increase cooperative behavior in one and increase aggression and decrease cooperation in the other (Packer 2005). De Souza and coworkers tested Acrilex®, an acrylic paint, for its effect on behavior and survival of Polistes wasps (Souza et al. 2012). Wasps that had paint spots applied to them groomed at higher rates than controls for 5–7 min after marking; thereafter, the rate of grooming was indistinguishable from controls. There was no effect on longevity. The authors conclude that use of acrylics on social wasps is unlikely to influence social behavior in any measurable way. Although oil-based paints have not been tested on wasps, our use of them over many years gives us confidence that they have no effect on behavior or survival.
Another method is to paint the mesothorax with liquid-paper correction fluid and then write a number on it with indelible ink (McIntosh 1999). Different colors of correction fluid can be used as an additional variable. Our experience with this technique is that after a few days the correction fluid abrades and picks up stray dark streaks, making it increasingly hard to read the number as the wasp ages.
19.6.3.3 Handling Wasps for Marking
When it comes to paint marking, honey bees differ from many social vespids in at least two respects. Honey bee foragers can be marked without capture by applying the paint as they feed at a dish; pressure from the applicator, even if heavy enough to push their faces into their food solution, typically does not disturb them. Ropalidia marginata and Polistes spp., especially if newly eclosed, can be marked in this way on the nest (West-Eberhard 1969; Gadagkar 2001). In our experience this is not possible with vespines and many polistine wasps, which are extremely sensitive to movement nearby and will fly in response to contact with (or even to the approach of) a marking pen. Finally, smaller wasps, such as Polybia occidentalis, will not tolerate the kind of pressure from a paint applicator required to apply a spot.
The alternative is to capture the wasp on the nest and hold it for marking. Owen (1962) removed individual P. fuscatus from the nest with forceps, anesthetized them (with CO2), marked them, and then returned them to the nest as they recovered. Naumann (1970), working with the much smaller Protopolybia acutiscutis, used the same method. In our work with Polybia occidentalis, capture enables the precise placement of the multiple dots required by the coding system described above (Figs. 19.5 and 19.6). Over many years of working with colonies of this wasp, we have found that the best method is to remove the selected individual directly from the nest surface using curved-tip reverse-action forceps (e.g., from SRA Soldering Products). These open when squeezed; when released they grip the wasp with a constant pressure that can be adjusted by bending the two arms at the base so that it is just sufficient to hold the wasp without injuring it; larger species require more pressure than smaller ones. The wasp is grasped from above around the thorax or the waist by releasing pressure on the forceps. The bent tips allow the wasp to be approached unnoticed from behind (rather than from above, as with straight tips). The opened bent tips can also be more precisely positioned around the wasp before closing, requiring only minute finger movements rather than whole wrist. To position the wasp for marking, it is transferred to a second pair of reverse-action bent-tip forceps, closed dorsoventrally over the posterior of the thorax (Fig. 19.8). This holds the wings out of the way while paint spots are applied to the thorax. Released after the paint dries (a few seconds), the wasp typically flies off and then returns to the nest after a few minutes. There is no evidence that this treatment affects the subsequent behavior of the marked wasps; rates of injury, fatal or otherwise, are negligible.
If a large random sample of a colony’s adults must be marked, it may become expedient to capture them in bulk and chill them on ice to immobilize them before marking.
19.6.4 Uses of Video Recording
In the old days, 8- and 16-mm movie film was too expensive to be practical as a research tool, and was used primarily to document behavior for archiving, e.g., via Encyclopaedia Cinematographica. The advent of analog and now digital video has changed that. Digital camcorders have excellent macro-capabilities and are now cheap and compact enough to be used routinely in the field, and through repeated playbacks can enhance data collection in a variety of ways. The advantages are numerous: (1) obtaining precise counts and interarrival times of foragers in and out of a nest; (2) recording of interindividual interactions on the nest for detailed behavioral analysis; (3) analyzing the behavior of numerous simultaneously interacting individuals; (4) sharing of video with other scientists through the internet, as part of a publication, and/or through social media. In a recent study on gastral drumming in Vespula germanica, the use of recording made it possible to obtain rates of movement and trophallaxis for large samples of workers in a short amount of real time (Taylor and Jeanne 2018). Gathering such data would have been impossible without the use of video. Furthermore, by going backward through a video record, the behavior of an individual leading up to an event (e.g., its departure from the nest or from a swarm cluster) can be documented in detail (Sonnentag and Jeanne 2009) (see also Sect. 19.7.4)
On the downside, extracting data from videotapes can be very time-consuming. However, technology exists for streamlining the process. JWatcher, for example, is a free program that can be used as an event recorder to log the time at which keys are pressed while the observer follows the behavior of a focal insect as the video is played back (Blumstein and Daniel 2007). Its analysis routines can calculate time budgets, the duration of behavioral states, intervals between them, and run sequential analyses. Mignini and Lorenzi (2015) recently used the software in an analysis of the role of vibratory signals in Polistes biglumis. Image-based tracking technology can automate the collection of data on movement of individuals in video recordings (see Sect. 19.9).
19.6.5 Behavior-Sampling Techniques
Depending on the purpose of one’s study, one or more behavioral sampling techniques will come into play, including ad libitum, focal-animal, and all-occurrences of a behavior pattern of interest. Altmann (1974) provides an excellent guide to these and other observational methods. Other useful resources include Martin and Bateson (1993) and, for analyzing sequential data, Bakeman and Gottman (1986).
19.6.6 Foraging
19.6.6.1 Collecting Foraged Loads
Prey loads brought to the nest by foragers can be collected and weighed and/or examined to determine the species. Polistes and Mischocyttarus foragers macerate the prey in the field before returning to the nest, so the identification of what was taken must rely on determining taxa based on fragments of cuticle. In contrast, Polybia occidentalis, P. emaciata, and others bring virtually intact prey to the nest, making it easy to identify large numbers of prey quickly (Hernandez et al. 2009; Yeison et al. 2013). Using reverse-action bent-tip forceps makes it easy to grasp returned prey foragers from the nest and make them drop their loads into vials of preservative. The same technique can be used to collect pulp loads from foragers (Jeanne 1986).
Water loads can be quantified by similarly restraining newly returned foragers and touching the tip of a microcapillary tube to their mouthparts. Lightly squeezing the gaster can encourage complete emptying of the crop (Roskens et al. 2010). If a regurgitated load fills a 10 μl capillary to 75% of its length, the load is 7.5 mg. Lightly tapping the tip of a capillary against the mouthparts of a larva will stimulate the release of larval saliva into the tube, where its volume can similarly be measured. Crop capacity of a wasp can be determined by letting a forager imbibe a sugar solution from a graduated capillary tube (Jeanne 1986). The same technique can be applied to nectar foragers (Fig. 19.9).
19.6.6.2 Foraging Distance
Especially for studies involving the use of social wasps in the biological control of agricultural pests, it is useful to know the distance to which wasps of a particular species will forage for prey. One approach is to determine the rates of return to the nest of workers released at a range of distances from the nest. This assumes that homing ability is based on knowledge of the territory surrounding the nest, which in turn is based on foraging experience. Clearly, older individuals, those likely to have gained such experience, will be able to home over greater distances than younger ones that have not yet begun to forage (Prezoto and Gobbi 2005) (for the use of harmonic radar tracking, see Sect. 19.9).
19.6.6.3 Recruitment to Food
A first step in determining whether a species has the ability to recruit nestmates to a food source is to train foragers to an artificial feeder . This can be difficult to accomplish for some species. Setting out feeders at some distance from the nest may or may not attract foragers from a nearby focal colony. It worked well for carrion-feeding species of Agelaia (Jeanne et al. 1995). In contrast, Polybia occidentalis foragers will rarely come to a sugar solution placed a few meters from a nest, and will readily abandon a feeder in the initial stages of training. Training this species to an artificial feeder was best accomplished by placing a dish of highly concentrated sucrose solution directly against the nest envelope below the entrance (Schueller et al. 2010; Taylor et al. 2010) (Fig. 19.10). Before long, several wasps will crawl onto the dish and feed from it, then return to the nest. We often found that as soon as the dish was moved away from the nest so that workers had to fly to it, they stopped coming (Schueller et al. 2010). Repeating the process several times, however, eventually resulted in some wasps learning to fly to and from the dish. Once trained to the dish, they typically continued to feed from it for several days (Taylor et al. 2011).
Wasps can learn to associate scents with food (Jandt and Jeanne 2005; Schueller et al. 2010; Taylor et al. 2011; Schueller 2012). Some studies have shown that wasps tend to be attracted to anise extract (Schueller et al. 2010; Taylor et al. 2010). Thus, if simple training to a single food source is required for an experiment, anise may be the best choice. However, if the aim is to test associative learning of a scent, anise should be avoided as its attractiveness could skew the results (Schueller et al. 2010). A feeder may consist simply of an open dish placed atop a tripod (Hrncir et al. 2007; Schueller et al. 2010; Taylor et al. 2010) (Fig. 19.10), or it may be more elaborate. Hrncir et al. (2007) inverted a glass cup filled with sugar solution onto a small acrylic plate cut with radial grooves. As the liquid in the grooves is drained by feeding wasps, they are kept filled from the reservoir in the glass cup.
Some wasp species are attracted to resources by “local enhancement,” the visual cue of others feeding there (Raveret Richter 2000; Hrncir et al. 2007; Jeanne and Taylor 2009). To control for local enhancement, specialized feeders can eliminate its effect (Jandt and Jeanne 2005; Taylor et al. 2011). One type consists of a Syracuse dish covered with a metal tin with a rectangular hole cut into one side. A microscope slide inserted into the hole rests on the rim and slopes down into the sugar solution. To access the food, wasps have to walk down the slide and under the opaque cover. Thus, while feeding they are out of view of any wasps flying near the feeder.
In instances where nests are closely spaced, confirming the nest affiliation of wasps arriving at feeders can be accomplished in several ways. The most expedient is to mark all visitors to the feeder for individual recognition, then confirm their affiliation by observing each at the focal nest (Taylor et al. 2010). Another approach is to take advantage of the effect of colony odor. Schueller et al. (2010) marked foragers coming to the feeder, then presented each (held lightly in forceps) to wasps on the envelope of the focal colony or placed them inside the nest entrance. If the resident wasps attacked the introduced wasp, it was evidence that it did not belong to that colony. With this method, care must be taken to avoid disturbing the colony during the introduction. The greatest risk is that the handling of the wasp may cause it to release venom that could give a false negative with regard to membership. In addition, depending on the species , some colonies may accept non-nestmates (Sumner et al. 2007).
19.6.7 Testing Pheromones
19.6.7.1 Alarm
Although venom is the source of the alarm pheromone in all polistines so far tested, it is possible that in some species alarm substances are produced by other glands, either instead of the venom gland or in addition. Vespula squamosa, for example, produces an alarm pheromone in the head as well as in the venom (Landolt et al. 1999). The simplest approach to narrowing down the source(s) of alarm pheromone is to present crushed body parts—head, thorax, gaster, legs—of fresh-killed workers to other workers on the nest and scoring whether an alarm reaction is elicited. Once the tagma(ta) with activity are identified, the search can move to bioassaying specific glands in that body region by crushing and presenting them to nest workers in the same way. Because alarm pheromones are highly volatile and evaporate within seconds, each preparation must be presented to responders as soon after crushing as possible. Similarly, if the chemical components of a particular glandular secretion have been analyzed and likely active chemical components purchased or synthesized, they can be spotted on filter paper and tested in the same way.
For a more sophisticated approach to testing for alarm activity, it may be desired to quantify the responses of colony members to a candidate chemical. For example, Dani et al. (2000) showed that (2S,6R,8S)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane, a venom component of Polybia occidentalis, had alarm-releasing activity by spotting fixed amounts of it (in a solution of methanol) on filter paper and presenting it 1–3 cm upwind of an active nest. Behavioral responses were quantified and compared with controls (methanol only) in two ways. First, the number of wasps exiting the nest was determined from playbacks of video recordings of the nest. Second, the number of wasps attacking a nearby target (a plastic bottle wrapped in black paper) was determined from audible hits picked up by the camcorder’s external microphone hung inside the bottle .
19.6.7.2 Emigration Trail
At least some Epiponini spot chemical trails that guide swarm members to a new nest site. An exception is Apoica pallens, which instead uses in-flight chemical calling (Howard et al. 2002). Circumstantial evidence for scent-marking can be obtained by observing the comings and goings of scouts a few meters from the swarm to see if they appear to be applying a glandular secretion to leaves. Gaster-dragging is a common scent-marking behavior seen in many epiponines (see Landolt et al. 1998 for a review), but it is possible that some may use glands other than sternal glands. To confirm that a chemical trail is produced and followed requires an experimental demonstration that swarm members can be led down an artificial trail (Jeanne 1981b). It would be worthwhile to investigate whether any independent-founding polistines engage in such marking, even if only around the new nest, as has been reported for Mischocyttarus labiatus by Litte (1981).
19.6.8 Nesting Behavior
A relatively neglected aspect of the biology of the Epiponini, in particular, concerns the details of nesting behavior . Much has been written about the final product, the architecture of the nest (Jeanne 1975a; Wenzel 1991), but relatively little about the behavioral sequences and stigmergic cues involved in building it (but see Downing and Jeanne 1988). Learning more about such details across species by mapping them onto a cladogram could very well provide new insights into how various nest types evolved. Moreover, there are tantalizing mysteries about how some species accomplish certain feats. For example, Polybia occidentalis constructs each new curved envelope so as to exactly parallel the curved surface of the comb being covered, but 2 cm out. How they maintain that distance without physically measuring the gap has not been answered. Careful observation at the nest can lead to hypotheses that can be tested with well-designed experiments.
19.7 Manipulating Colonies for Special Purposes
19.7.1 Age Polyethism
Studies of age polyethism require knowing the age of marked individuals. Various attempts have been made to estimate the ages of adult wasps based on morphological indicators. For example, the degree of pigmentation of the transverse apodeme of the first gastral sternite increases with age (Forsyth 1981). Unfortunately, this measure can at best indicate relative, not absolute, age and so is not of much use.
A better procedure is to introduce individually marked one-day-old adults into the observation colony and track their behavioral repertories as they age. Because newly eclosed adults take about a day to acquire their colony’s odor from the nest, day-old individuals eclosing from colony A will be accepted and become part of the workforce of colony B. Jeanne and coworkers separated and incubated the combs of donor colonies of P. occidentalis (Jeanne et al. 1988). A source comb, kept dry and away from ants, will produce newly eclosing adults for a week or more. Each day the newly eclosed adult females were lightly anesthetized, marked for individual recognition (or merely for their day of eclosion), and dropped into the entrance of the observation nest . The tasks performed by these workers were then followed as they aged (Jeanne et al. 1988; O'Donnell and Jeanne 1992). In a slight variation, Simões and Zucchi (1980) used the lowermost of three combs of a nest of Protopolybia exigua as a daily source of newly eclosed individuals, which they marked and reintroduced to the same colony.
19.7.2 Colony Productivity
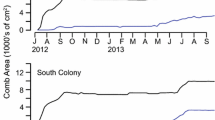
Michener (1964) argued that per-capita productivity decreases as colony size increases. Because of the wide range of size among founding swarms within a species, swarm-founders are ideal for testing the hypothesis. Including only founding swarms in the data set controls for the effect of stage of development on per-capita productivity. Colonies can be reset to the founding stage by dismantling the nest, then following the swarm to its new site (see next section). The colony is allowed to develop for n days, where n is just short of the egg-to-adult development time, and is then collected. All the adults will have been members of the founding swarm, and thus provide a measure of input, whereas the total brood biomass is a clean measure of output. Output/input gives a measure of per-capita productivity. Additional measures of output can include the number of brood cells and/or mass of the nest itself. Plotting per-capita productivity against colony size for a large sample of colonies provides a test of Michener’s hypothesis. Only two species have been tested so far (Polybia occidentalis, Parachartergus fraternus); neither supports the hypothesis of decreasing per-capita output with increasing swarm size (Jeanne and Nordheim 1996; Bouwma et al. 2005; Bouwma et al. 2006).
19.7.3 Egg-to-Adult Development Time
Newly founded colonies can also be used to determine the egg-to-adult (E-A) development time, a fundamental life-history trait. For independent founders on their exposed combs, the data are easily obtained by keeping accurate daily records of the stage of brood in each cell, through cell-maps, tracking each newly laid egg until it gives rise to an adult. On the other hand, the multiple covered combs of most swarm founders make this difficult, if not impossible, so it is not surprising that brood-development times are known for less than a handful of species of epiponines. The scant data available suggest that E-A of swarm founders is significantly less than for independent-founding polistines (Jeanne in press), so data for more species would be welcome. For species whose nests are a single comb it is possible to keep track of brood development by periodically opening a flap of the envelope, as West-Eberhard (1978) did for Metapolybia. For species with multiple combs, this is impractical because the oldest brood will be in the upper comb, which is likely to be inaccessible without seriously damaging the nest. The alternative is to follow swarms from founding, then collecting them after x days, where the starting value of x is close to E-A for known species, around 30 days (Jeanne in press). The age of the oldest brood in the nest is determined and x is adjusted accordingly for subsequent trials until x = E-A by successive approximation. If the first colony in the series is collected a few days ahead of 30—25, for example—and the oldest comb is found to contain pupae, the comb can be incubated until the first adult ecloses. Adding the number of days of incubation to 25 will give a close approximation to the value of E-A, making it possible to close in on the value of x more quickly with subsequent colonies. It is important to keep in mind a complicating factor: as swarm size increases within a species, development time decreases (Howard and Jeanne 2004).
19.7.4 Working with Swarms
Although reproductive swarming characterizes all of the 200+ species of Epiponini, the details of behavior during this dispersal phase have been little studied. For some studies it is useful to know the population of the founding swarm. Working with Polybia occidentalis, Bouwma et al. (2003b) developed a method of obtaining precise counts. The swarm, either naturally occurring or induced, is captured at night by enclosing it and its twig in a plastic bag and refrigerating it overnight. Before daylight the next morning, the wasps are transferred to a capped 2 L plastic soda bottle via the removed bottom; after all are in the bottle, the bottom is sealed. The bottle is then clamped in an upright position to a tripod in the field and a 5 × 22-mm slit in the cap is untaped. With daybreak, the wasps become active and move up in the bottle. A camcorder zoomed in on the cap records the wasps as they exit through the slit. On video playback, a counter is used to tally the total number in the swarm, with repeated playbacks to confirm accuracy if necessary. Within an hour or two after leaving the bottle, all the wasps will again coalesce into a single cluster on a nearby leaf or twig.
For most studies involving swarming it is necessary to locate the new nesting site of a swarm. The first step is to mark some in the group so it can be recognized when found. A single spot of one color on the thorax for 3–5% of the population is sufficient (Bouwma et al. 2003a). If the swarm is lost during emigration, but a young nest is found a day or more later, its identity can be confirmed by the presence of marked individuals.
With some experience, it is possible to follow swarms as they emigrate to a new site. P. occidentalis takes several hours to a day to select a new site and move to it. Close observation of the swarm cluster can reveal when the activity level of swarm members increases, often a precursor to departure. Frequent inspections of vegetation a few meters in all directions from the swarm will often reveal where scouts are scent-marking vegetation on the way to a site they have found suitable. As time goes on, the concentration of scent marking will converge on a single direction from the swarm, and at that point, searching farther in that direction will reveal the scent-marked line leading to the selected new site. In the case of P. occidentalis, we have often been able in this way to find the chosen site—indicated by heavy scent marking by 10–20 scouts—long before the majority of the wasps arrive.
Our understanding of the behavior of the swarm up to the time it begins to emigrate is rudimentary. The typically dense packing of wasps in the swarm cluster makes it impossible to see any behavior except what little occurs on the surface facing the observer. Sonnentag and Jeanne (2009) got around this limitation by coaxing swarms of P. occidentalis onto a vertically mounted rectangle of plywood (Fig. 19.11). On this surface the wasps dispersed into numerous small clusters, exposing much of the interaction among swarm members to view and to videotaping, making it possible to work out much of the behavior leading up to the collective decision to make the move.
19.8 Studying Behavior in Artificially Housed Colonies
Housing colonies in nest boxes can be helpful if lab colonies need to be kept, or if predation on the colony is a concern. Also, while some groups of wasps build nests with open combs that allow observers to see colony activities, others nest in cavities and/or build an envelope that encloses the combs of the nest. This makes the use of an observation box necessary in order to study the inner workings of a colony. The use of such boxes to keep captive colonies became prominent in the 1970s for work on vespines (Greene 1991) and has enabled research in areas such as worker–queen interactions, worker–larva interactions, competition during colony founding, colony demographics, task partitioning, polyethism, communication, and the organization of foraging. The majority of studies using captive colonies have been conducted on two groups, Polistes and the vespine wasps.
Nest box design varies. Polistes have long been reared in captive or semi-captive conditions. Colonies can be kept in simple enclosures such as cardboard boxes (Reeve 1991) and provisioned with honey, water, and a source of protein (e.g., waxworms). If visual observations need to be performed, screen or plexiglass panels can be included in the nest box design (Post and Jeanne 1982; Jandt and Toth 2015). Boxes used in the field have a weather-tight roof and sides with a bottom of coarse wire mesh that allows wasps to pass through, but restricts access by predators (Judd and Carpenter 1996; Jandt and Toth 2015). Young colonies can be transplanted into such boxes. In areas with high densities of Polistes, foundresses often start nests in them (Rossi and Hunt 1988; Judd and Carpenter 1996; Hunt et al. 2007). These can then be moved to the lab, if needed.
Two main types of nest boxes have been used for studying vespine wasps. One style is an upright “vertical design” (Loope 2015). Here, the diameter of each comb is restricted to a width of approximately 3.75 cm by panes of glass on both sides. Because the nest is so narrow, it allows the observer to see what is happening between the nest combs. Furthermore, it allows the wasps to vertically expand the nest by adding additional combs below the existing ones as they would in a typical field nest (Loope 2015). The other type of nest box is a “horizontal design” originally described by Akre et al. (1976). In this type, the combs are separated and laid out next to one another in a single plane within the box. This design allows the colony to expand combs horizontally, but prevents vertical expansion. This type of box has undergone several improvements since the original design. Later iterations found that if the height of the box was reduced to 3 cm, wasps were discouraged from building envelope between the nest and the glass bottom, which would otherwise obstruct the view of the observer (Jandt and Jeanne 2005). Other improvements have included changes in the materials of the nest box itself. Originally, the box was constructed of wood, but Taylor et al. (2010) replaced it with nylon plastic because wooden nest boxes exposed to rain or high humidity warped. Further, they added slots for feeding the colony different types of food (Taylor et al. 2010; Taylor et al. 2012). Later versions of this same design replaced the Masonite ceiling with Plexiglas, which allowed the colony to be viewed from above and below the combs (Taylor and Jeanne 2018).
There are two options for providing access to food for artificially housed colonies. One is to allow the colony to forage outside via a short tube leading through a wall or window. This is all but mandatory for large colonies (i.e., the Vespinae and some Epiponini). The other is to limit the colony to foraging entirely indoors, by providing food either in the box housing the nest or via access to an adjacent foraging arena where food resources are provided by the experimenter. Completely enclosing colonies has been used with considerable success for Polistes, and both techniques have been successfully used with Ropalidia marginata (Gadagkar 2001).
There are some disadvantages to working with captive colonies. For one, most of the studies have been conducted with independent-founding species (Polistes, Ropalidia, Vespula), which do not readily adapt to changed nesting location unless they have already invested a considerable amount in the brood in the nest. Very few, if any, studies have been conducted on swarm-founding wasps held in enclosures, and it is unknown if colonies would find nest boxes desirable enough to remain within them. Secondly, if foraging is constrained to an enclosed arena in the lab, the artificial provision of an adequate diet may be an issue. Thirdly, a nest box restricts nest growth, since the colony cannot expand beyond the size of the box (Greene 1991). In addition, for subterranean-nesting species (e.g., Vespula) the entire task of cavity excavation is eliminated and therefore cannot be studied. It is unclear what effect this may have in an artificially housed colony, but it undoubtedly skews the allocation of worker effort away from what is experienced in the natural environment. Finally, rearing indoors may have effects on the colony that do not allow the results to be extended to natural colonies. For example, Jandt and coworkers found that for Polistes reared in a lab setting, some traits were skewed to be more gyne-like, possibly due to excess food. However, gene-expression analysis revealed that more worker-like genes were expressed. In addition, nest construction and male production were affected by rearing location (Jandt and Toth 2015). These results advise caution when extrapolating the results from lab-reared colonies to those in the field.
Use of nest boxes may provide some advantages for the study of behavior of Neotropical polistines. Cavity-nesters , such as many species of Agelaia, may adapt well to the “cavity” of a horizontal nest box, making possible close-up and detailed observation of behavior on the combs. For many of the species that construct envelopes, a narrow vertical design that constricts the width of the combs enough that in-nest behavior can be seen may be the best solution.
19.9 The Future
We foresee several ways that the development of new technology and methodologies will increase our understanding of Neotropical social wasp behavior and ecology. For one, the tools to study animal behavior and ecology will continue to increase in sophistication, allowing for ever-higher-throughput monitoring of individual behavior. Radio-frequency identification (RFID) tags, for example, attached to each individual are automatically read each time they pass by an RFID reader, such as when entering or leaving the nest. The tags are already small enough to attach to Temnothorax ants, and thus are likely suitable for most, if not all species of social wasps, provided the weight does not impede flight. This technology has already been used for several years on ants, bees, and a small number of social wasps to study behavior such as house-hunting during swarming, foraging, nest-drifting, shifts from nest work to foraging, adult life span, and reproductive parasitism (Sumner et al. 2007; Robinson et al. 2009; Van Oystaeyen et al. 2013; Nunes-Silva et al. 2018; Santoro et al. 2019). Future developments will likely include further miniaturization and longer reading distances.
Harmonic radar tracking has been utilized to track insects, including bees, over large spatial scales, and it was recently used to track the flight paths of bumble bee (Bombus terrestris) workers over their entire lifetimes (Woodgate et al. 2016). As transponders are further reduced in size, they will become available for use on smaller species of social wasps. The use of this technology, along with Geographic Information System (GIS) mapping techniques could allow us to understand how wasps explore their environment, how far they forage from the nest, the ontogeny of foraging behavior, and how foraging behavior changes with spatial heterogeneity of the environment.
Advances have also occurred in the area of image-based tracking technology (Mersch et al. 2013; Dell et al. 2014; Crall et al. 2015; Wario et al. 2015). The software tracks movement in video recordings and extracts information related to spatial use, activity patterns, and, increasingly, the characterization of specific behavioral acts. If individual identification is necessary, tags with unique barcodes or quick-response (QR) codes can be attached to each individual (Crall et al. 2015).
The advantages of these technologies are many. Perhaps most obvious is the amount of data that can be collected. In pre-tech days, a trained ethologist would be limited to the individuals they could readily observe or record with a camcorder. Current technologies allow for constant monitoring of multiple individuals, sometimes for months, and during a timeframe that can encompass millions of interactions (Mersch et al. 2013; Wario et al. 2015). Furthermore, the effort required to extract data from videotapes by hand is lengthy and labor-intensive. Now, the collection and compiling of data sets on behavior requires much less time, and in some cases is instantaneous, giving researchers access to the data in a fraction of the time. The data collected is also highly accurate, avoids observer bias, and precludes the need to correct for individual biases when multiple observers are working on the same study.
The methods are not without limitations. For example, RFID technology can only register particular individuals when they are within a few millimeters of the RFID reader. Similarly, image-based tracking technology is limited by what can be recorded via video. This is especially relevant to Neotropical social wasps, largely because the technology of housing colonies in observation nest boxes for most species is not developed. While it is certainly possible to video-record individuals on the outside of the nest or at a foraging site, it leaves out the richness of the behavioral interactions inside the nest for all species whose nests are covered by an envelope. If most Neotropical social wasps cannot be housed in observation boxes, the use of image-based tracking techniques to record internal interactions may be limited. All of the methods mentioned above are limited by the sophistication of the software that can be used to recognize individuals, track them, and categorize behavior into particular types. They are also limited by the statistical techniques that can be used to mine the extensive data sets that are produced. Fortunately, programs for tracking individuals, such as Track-a-Forager, BEEtag, and ToxTrac (programmed in Java, MATLAB ®, or R) are available for all platforms, have come a long way toward solving these issues, and will continue to improve (Crall et al. 2015; Van Geystelen et al. 2016; Rodriguez et al. 2018).
References
Akre RD, Garnett WB, MacDonald JF, Greene A, Landolt PJ (1976) Behavior and colony development of Vespula pensylvanica and V. atropilosa. J Kansas Entomol Soc 49:63–84
Alloway TM (1972) Learning and memory in insects. Annu Rev Entomol 17:43–56
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267
Aragão M, Andena SR (2016) The social wasps (Hymenoptera: Vespidae: Polistinae) of a fragment of Atlantic Forest in southern Bahia, Brazil. J Nat Hist 50:1411–1426
Arnett RH Jr (2000) American insects: a handbook of the insects of America north of Mexico. CRC Press, Boca Raton, Florida
Ashburner M, Thompson JN Jr (1978) The laboratory culture of Drosophila. In: Ashburner M, Wright TRF (eds) Genetics and biology of Drosophila. Academic Press, London, pp 1–109
Bakeman R, Gottman JM (1986) Observing interaction: an introduction to sequential analysis. Cambridge University Press, Cambridge, UK
Barbosa BC, Detoni M, Maciel TT, Prezoto F (2016) Studies of social wasp diversity in Brazil: over 30 years of research, advancements and priorities. Sociobiology 63:858–880
Blumstein DT, Daniel JC (2007) Quantifying behavior the JWatcher way. Sinauer Associates Incorporated, Sunderland, Massachusetts
Blüthgen N, Stork NE (2007) Ant mosaics in a tropical rainforest in Australia and elsewhere: a critical review. Austral Ecol 32:93–104
Bouwma AM, Bouwma PE, Nordheim EV, Jeanne RL (2003a) Adult mortality rates in young colonies of a swarm-founding social wasp. J Zool 260:11–16
Bouwma AM, Bouwma PE, Nordheim EV, Jeanne RL (2003b) Founding swarms in a tropical social wasp: adult mortality, emigration distance, and swarm size. J Insect Behav 16:439–452
Bouwma AM, Howard KJ, Jeanne RL (2005) Parasitism in a social wasp: effect of gregarines on foraging behavior, colony productivity, and adult mortality. Behav Ecol Sociobiol 59:222–233
Bouwma AM, Nordheim EV, Jeanne RL (2006) Per-capita productivity in a social wasp: no evidence for a negative effect of colony size. Insect Soc 53:412–419
Brenner RJ, Patterson RS (1988) Efficiency of a new trapping and marking technique for peridomestic cockroaches (Dictyoptera: Blattaria). J Med Entomol 25:489–492
Chao A (2019) Anne Chao’s website. http://chao.stat.nthu.edu.tw/wordpress/software_download/softwarespader_online/
Chao A, Colwell RK (2017) Thirty years of progeny from Chao's inequality: estimating and comparing richness with incidence data and incomplete sampling. Sort-Statistics Operat Res Transact 41:3–54
Colwell RK (2013) EstimateS: statistical estimation of species richness and shared species from samples. Version 9 and earlier. User’s Guide and application. http://purl.oclc.org/estimates
Corbara B, Carpenter JM, Cereghino R, Leponce M, Gibernau M, Dejean A (2009) Diversity and nest site selection of social wasps along Guianese forest edges: assessing the influence of arboreal ants. C R Biol 332:470–479
Crall JD, Gravish N, Mountcastle AM, Combes SA (2015) BEEtag: a low-cost, image-based tracking system for the study of animal behavior and locomotion. PLoS One 10:1–13
Dani FR, Jeanne RL, Clarke SR, Jones GR, Morgan ED, Francke W, Turillazzi S (2000) Chemical characterization of the alarm pheromone in the venom of Polybia occidentalis and of volatiles from the venom of P. sericea. Physiol Entomol 25:363–369
De Souza AR, Venancio DFA, Zanuncio JC, Prezoto F (2011) Sampling methods for assessing social wasps species diversity in a eucalyptus plantation. J Econ Entomol 104:1120–1123
Dell AI, Bender JA, Branson K, Couzin ID, de Polavieja GG, Noldus LP, Pérez-Escudero A, Perona P, Straw AD, Wikelski M, Brose U (2014) Automated image-based tracking and its application in ecology. Trends Ecol Evol 29:417–428
Desuo IC, Souza-Galheico CB, Shima SN, Santos GMM, Cruz JD, Bichara CC, Dias CTS (2011) An adaptive view of caste differentiation in the Neotropical wasp Polybia (Trichothorax) sericea Olivier (Hymenoptera: Vespidae). Neotrop Entomol 40:653–660
Downing HA, Jeanne RL (1988) Nest construction by the paper wasp, Polistes: a test of stigmergy theory. Anim Behav 36:1729–1739
Dvořák L, Landolt PJ (2006) Social wasps trapped in the Czech Republic with syrup and fermented fruit and comparison with similar studies (Hymenoptera Vespidae). Bulletin of Insectology 59:115–120
Edwards R (1980) Social wasps. Their biology and control. Rentokil Unlimited, East Grinstead, UK
Forsyth AB (1981) Swarming activity of polybiine social wasps (Hymenoptera: Vespidae: Polybiini). Biotropica 13:93–99
Gadagkar R (2001) The social biology of Ropalidia marginata. Toward understanding the evolution of eusociality. Harvard University Press, Cambridge, Massachusetts
Gomes B, Noll FB (2009) Diversity of social wasps (Hymenoptera, Vespidae, Polistinae) in three fragments of semideciduous seasonal forest in the northwest of São Paulo state, Brazil. Revista Brasileira de Entomologia 53:428–431
Greene A (1991) Dolichovespula and Vespula. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, NY, pp 263–305
Hagler JR, Jackson CG (2001) Methods for marking insects: current techniques and future prospects. Annu Rev Entomol 46:511–543
Hastings MD, Queller DC, Eischen F, Strassmann JE (1998) Kin selection, relatedness, and worker control of reproduction in a large-colony epiponine wasp, Brachygastra mellifica. Behav Ecol 9:573–581
Hernandez J, Sarmiento CE, Fernandez C (2009) Foraging activity of Polybia occidentalis venezuelana (Hymenoptera, Vespidae). Revista Colombiana de Entomologia 35:230–234
Howard KJ, Jeanne RL (2004) Rates of brood development in a social wasp: effects of colony size and parasite infection. Insect Soc 51:179–185
Howard KJ, Smith AR, O'Donnell S, Jeanne RL (2002) Novel method of swarm emigration by the epiponine wasp, Apoica pallens (Hymenoptera Vespidae). Ethol Ecol Evolut 14:365–371
Hrncir M, Mateus S, Nascimento FS (2007) Exploitation of carbohydrate food sources in Polybia occidentalis: social cues influence foraging decisions in swarm-founding wasps. Behav Ecol Sociobiol 61:975–983
Hunt JH, Kensinger BJ, Kossuth JA, Henshaw MT, Norberg K, Wolschin F, Amdam GV (2007) A diapause pathway underlies the gyne phenotype in Polistes wasps, revealing an evolutionary route to caste-containing insect societies. Proc Natl Acad Sci U S A 104:14020–14025
Ishay JS, Pertsis V, Levtov E (1994) Duration of hornet sleep induced by ether anesthesia is curtailed by exposure to sun or UV irradiation. Experientia 50:737–741
Jacques GC, Pires E, Hermes MG, Faria LDB, Souza MM, Silveira LCP (2018) Evaluating the efficiency of different sampling methods to survey social wasps (Vespidae: Polistinae) in an anthropized environment. Sociobiology 65:515
Jandt JM, Jeanne RL (2005) German yellowjacket (Vespula germanica) foragers use odors inside the nest to find carbohydrate food sources. Ethology 111:641–651
Jandt JM, Toth AL (2015) Physiological and genomic mechanisms of social organization in wasps (family: Vespidae). Adv Insect Physiol 48:95–130
Jeanne RL (1972) Social biology of the Neotropical wasp Mischocyttarus drewseni. Bull Mus Comp Zool Harv Univ 144:63–150
Jeanne RL (1975a) The adaptiveness of social wasp nest architecture. Q Rev Biol 50:267–287
Jeanne RL (1975b) Social biology of Stelopolybia areata (Say) in Mexico (Hymenoptera: Vespidae). Insect Soc 22:27–34
Jeanne RL (1981a) Alarm recruitment, attack behavior, and the role of the alarm pheromone in Polybia occidentalis (Hymenoptera: Vespidae). Behav Ecol Sociobiol 9:143–148
Jeanne RL (1981b) Chemical communication during swarm emigration in the social wasp Polybia sericea (Olivier). Anim Behav 29:102–113
Jeanne RL (1986) The organization of work in Polybia occidentalis: costs and benefits of specialization in a social wasp. Behav Ecol Sociobiol 19:333–341
Jeanne RL (1991) The swarm-founding Polistinae. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, New York, pp 191–231
Jeanne RL (2003) Social complexity in the Hymenoptera, with special attention to the wasps. In: Kikuchi T, Azuma N, Higashi S (eds) Genes, behaviors and evolution of social insects. Hokkaido University Press, Sapporo, Japan, pp 81–130
Jeanne RL (in press) Swarm-founding wasps. In: Starr CK (ed) Encyclopedia of social insects. Springer, Cham, Switzerland
Jeanne RL, Downing H, Post DC (1988) Age polyethism and individual variation in Polybia occidentalis, an advanced eusocial wasp. In: Jeanne RL (ed) Interindividual behavioral variability in social insects. Westview Press, Boulder, Colorado, pp 323–357
Jeanne RL, Hunt JH, Keeping MG (1995) Foraging in social wasps: Agelaia lacks recruitment to food (Hymenoptera: Vespidae). J Kansas Entomol Soc 68:279–289
Jeanne RL, Nordheim EV (1996) Productivity in a social wasp: per capita output increases with swarm size. Behav Ecol 7:43–48
Jeanne RL, Taylor B (2009) Individual and social foraging in social wasps. In: Jarau S, Hrncir M (eds) Food exploitation by social insects: ecological, behavioral, and theoretical approaches. CRC Press, Boca Raton, Florida, pp 53–79
Johnson NF, Triplehorn CA (2004) Introduction to the study of insects. Thompson Brooks Cole, Belmont, California
Judd TM, Carpenter JM (1996) Polistes dominulus (Hymenoptera: Vespidae) found in Michigan. Great Lakes Entomol 29:45–46
Landolt P, Reed H, Heath R (1999) An alarm pheromone from heads of worker Vespula squamosa (Hymenoptera: Vespidae). Fla Entomol 82:356–359
Landolt PJ, Jeanne RL, Reed HC (1998) Chemical communication in social wasps. In: Meer RKV, Breed MD, Winston ML, Espelie KE (eds) Pheromone communication in social insects. Westview Press, Boulder, Colorado, pp 216–235
Litte M (1981) Social biology of the polistine wasp Mischocyttarus labiatus: survival in a Colombian rain forest. Smithsonian Contribut Zool 327:1–27
Loope KJ (2015) Queen killing is linked to high worker-worker relatedness in a social wasp. Curr Biol 25:2976–2979
Mardan M, Rinderer TE (1980) Effects of carbon dioxide and cold anaesthesia on the hoarding behaviour of the honeybee. J Apic Res 19:149–153
Martin P, Bateson P (1993) Measuring behaviour. Cambridge University Press, Cambridge, UK
McIntosh RL (1999) Technique for labeling individual Pissodes strobi (Coleptera: Curculonidae) for mark-recapture studies. Canadian Entomol Suppl 131:131–136
Melo AM, Barbosa BC, Castro MM, Santos GMM, Prezoto F (2015) The social wasp community (Hymenoptera, Vespidae) and new distribution record of Polybia ruficeps in an area of Caatinga biome, northeastern Brazil. Check List 11:1–5
Mersch DP, Crespi A, Keller L (2013) Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science 340:1090–1093
Michener CD (1964) Reproductive efficiency in relation to colony size in hymenopterous societies. Insect Soc 11:317–342
Mignini M, Lorenzi CM (2015) Vibratory signals predict rank and offspring caste ratio in a social insect. Behav Ecol Sociobiol 69:1739–1748
Murakami ASN, Shima SN, Desuo IC (2009) More than one inseminated female in colonies of the independent-founding wasp Mischocyttarus cassununga von Ihering (Hymenoptera, Vespidae). Revista Brasileira de Entomologia 53:653–662
Naumann MG (1970) The nesting behavior of Protopolybia pumila in Panama (Hymenoptera: Vespidae). Ph.D. thesis. University of Kansas, Lawrence, Kansas
Nicolas G, Sillans D (1989) Immediate and latent effects of carbon dioxide on insects. Annu Rev Entomol 34:97–116
Noll FB, Gomes B (2009) An improved bait method for collecting Hymenoptera, especially social wasps (Vespidae: Polistinae). Neotrop Entomol 38:477–481
Noll FB, Zucchi R (2002) Castes and the influence of the colony cycle in swarm-founding polistine wasps (Hymenoptera, Vespidae, Epiponini). Insect Soc 49:62–74
Nunes-Silva P, Hrncir M, Guimarães JTF, Arruda H, Costa L, Pessin G, Siqueira JO, de Souza P, Imperatriz-Fonseca VL (2018) Applications of RFID technology on the study of bees. Insect Soc 66:15–24
O'Donnell S, Jeanne RL (1992) Lifelong patterns of forager behavior in a tropical swarm-founding wasp - effects of specialization and activity level on longevity. Anim Behav 44:1021–1027
Owen J (1962) The behavior of a social wasp Polistes fuscatus (Vespidae) at the nest, with special reference to differences between individuals. Ph.D. thesis. University of Michigan, Ann Arbor
Packer L (2005) The influence of marking upon bee behaviour in circle tube experiments with a methodological comparison among studies. Insect Soc 52:139–146
Peitsch D, Fietz A, Hertel H, de Souza J, Ventura DF, Menzel R (1992) The spectral input systems of hymenopteran insects and their receptor-based colour vision. J Comp Physiol A 170
Plath OE (1924) Do anesthetized bees lose their memory? Am Nat 58:162–166
Pomeroy N, Plowright RC (1979) Larval ejection following CO2 narcosis of bumble bees (Hymenoptera: Apidae). J Kansas Entomol Soc 52:215–217
Post DC, Jeanne RL (1982) Recognition of former nestmates during colony founding by the social wasp Polistes fuscatus (Hymenoptera: Vespidae). Behav Ecol Sociobiol 11:283–285
Prezoto F, Gobbi N (2005) Flight range extension in Polistes simillimus Zikán, 1951 (Hymenoptera, Vespidae). Braz Arch Biol Technol 48:947–950
Rau P (1933) The jungle bees and wasps of Barro Colorado Island. Phil Rau, Kirkwood, Missouri
Raveret Richter M (2000) Social wasp (Hymenoptera: Vespidae) foraging behavior. Annu Rev Entomol 45:121–150
Reed HC, Gallego J, Nelson J (1988) Morphological evidence for polygyny in post-emergence colonies of the red paper wasp, Polistes perplexus Cresson (Hymenoptera: Vespidae). J Kansas Entomol Soc 61:453–463
Reeve HK (1991) Polistes. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, New York, pp 99–148
Ribbands CR (1950) Changes in the behaviour of honey-bees following their recovery from anaesthesia. J Exp Biol 27:302–310
Richards OW (1945) A revision of the genus Mischocyttarus de Saussure (Hymen., Vespidae). Transact Royal Entomol Soc London 95:295–462
Richards OW (1978) The social wasps of the Americas excluding the Vespinae. British Museum (Natural History), London
Richards OW, Richards MJ (1951) Observations on the social wasps of South America (Hymenoptera Vespidae). Transact Royal Entomol Soc London 102:1–170 + 174 plates
Robinson EJ, Richardson TO, Sendova-Franks AB, Feinerman O, Franks NR (2009) Radio tagging reveals the roles of corpulence, experience and social information in ant decision making. Behav Ecol Sociobiol 63:627–636
Rocha AA, Giannotti E (2016) External morphology of immatures during the post-embryonic development of Mischocyttarus nomurae Richards (Hymenoptera: Vespidae). Sociobiology 63:998–1004
Rodriguez A, Zhang H, Klaminder J, Brodin T, Andersson PL, Andersson M (2018) ToxTrac: a fast and robust software for tracking organisms. Methods Ecol Evol 9:460–464
Roskens VA, Carpenter JM, Pickett KM, Ballif BA (2010) Preservation of field samples for enzymatic and proteomic characterization: analysis of proteins from the trophallactic fluid of hornets and yellowjackets. J Proteome Res 9:5484–5491
Rossi AM, Hunt JH (1988) Honey supplementation and its developmental consequences: evidence for food limitation in a paper wasp, Polistes metricus. Ecol Entomol 13:437–442
Saga T (2019) Evaluation of the productivity of social wasp colonies (Vespinae) and an introduction to the traditional Japanese Vespula wasp hunting technique. J Vis Exp 151:e59044
Santoro D, Hartley S, Lester PJ (2019) Behaviourally specialized foragers are less efficient and live shorter lives than generalists in wasp colonies. Sci Rep 9:1–10
Schueller TI (2012) Aspects of integration in colonies of a highly eusocial wasp with emphasis on foraging, nest construction, and defense. Ph.D. thesis. The University of Wisconsin - Madison
Schueller TI, Nordheim EV, Taylor B, Jeanne RL (2010) The cues have it: nest-based, cue-mediated recruitment to carbohydrate resources in a swarm-founding social wasp. Naturwissenschaften 97:1017–1022
Silva-Pereira V d, Santos GMM (2006) Diversity in bee (Hymenopetra, Apoidea) and social wasp (Hymenoptera, Vespidae) community in Campos rupestres, Bahia, Brazil. Neotrop Entomol 35:165–174
Silveira OT (2002) Surveying neotropical social wasps: an evaluation of methods in the “Ferreira Penna” research station (ECFPn), in Caxiuanã, PA, Brazil (Hym., Vespidae, Polistinae). Papéis Avulsos de Zoologia 42:299–323
Silveira OT, Esposito MC, Santos JN d Jr, Gemaque FE Jr (2005) Social wasps and bees captured in carrion traps in a rainforest in Brazil. Entomol Sci 8:33–39
Simões D, Zucchi R (1980) Bionomics of Protopolybia exigua exigua (de Saussure) I – Age polyethism and life-table. (Hymenoptera, Vespidae, Polybiini). Naturalia 5:79–87
Somavilla A, de Oliveira ML, Silveira OT (2014) Diversity and aspects of the ecology of social wasps (Vespidae, Polistinae) in central Amazonian "terra firme" forest. Revista Brasileira de Entomologia 58:349–355
Sonnentag PJ, Jeanne RL (2009) Initiation of absconding-swarm emigration in the social wasp Polybia occidentalis. J Insect Sci 9:1–11
Sorensen AA, Busch TM, Vinson SB (1983) Factors affecting brood cannibalism in laboratory colonies of the imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae). J Kansas Entomol Soc 56:140–150
Southwood TRE (1978) Ecological methods. With particular reference to the study of insect populations. Chapman and Hall, New York
Souza AR d, Ribeiro B, Jose N, Prezoto F (2012) Paint marking social wasps: an evaluation of behavioral effects and toxicity. Entomol Exp Appl 144:244–247
Spradbery JP (1973) Wasps. An account of the biology and natural history of solitary and social wasps. University of Washington Press, Seattle, Washington
Sumner S, Lucas E, Barker J, Isaac N (2007) Radio-tagging technology reveals extreme nest-drifting behavior in a eusocial insect. Curr Biol 17:140–145
Taylor BJ, Jeanne RL (2018) Gastral drumming: a nest-based food-recruitment signal in a social wasp. Sci Nat 105
Taylor BJ, Nordheim EV, Jeanne RL (2012) Allocation of colony-level foraging effort in Vespula germanica in response to food resource quantity, quality, and associated olfactory cues. Ethology 118:594–605
Taylor BJ, Nordheim EV, Schueller TI, Jeanne RL (2011) Recruitment in swarm-founding wasps: Polybia occidentalis does not actively scent-mark carbohydrate food sources. Psyche 2011:7
Taylor BJ, Schalk DR, Jeanne RL (2010) Yellowjackets use nest-based cues to differentially exploit higher-quality resources. Naturwissenschaften 97:1041–1046
Van Geystelen A, Benaets K, de Graaf DC, Larmuseau MHD, Wenseleers T (2016) Track-a-forager: a program for the automated analysis of RFID tracking data to reconstruct foraging behaviour. Insect Soc 63:175–183
Van Oystaeyen A, Alves DA, Oliveira RC, do Nascimento DL, do Nascimento FS, Billen J, Wenseleers T (2013) Sneaky queens in Melipona bees selectively detect and infiltrate queenless colonies. Anim Behav 86:603–609
Visscher PK, Seeley TD (1989) Bee-lining as a research technique in ecological studies of honey bees. Am Bee J 129:536–539
von Frisch K (1967) The dance language and orientation of bees. Harvard University Press, Cambridge, Massachusetts
Walker TJ, Wineriter SA (1981) Marking techniques for recognizing individual insects. Fla Entomol 64:18–29
Wario F, Wild B, Couvillon MJ, Rojas R, Landgraf T (2015) Automatic methods for long-term tracking and the detection and decoding of communication dances in honeybees. Front Ecol Evol 3:103
Wenzel J (1991) Evolution of nest architecture. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, New York, pp 480–519
West-Eberhard MJ (1969) The social biology of polistine wasps. Miscellaneous Publications of the Museum of Zoology, University of Michigan 140:1–101
West-Eberhard MJ (1978) Temporary queens in Metapolybia wasps: nonreproductive helpers without altruism? Science 200:441–443
Wineriter SA, Walker TJ (1984) Insect marking techniques: durability of materials. Entomol News 95:117–123
Woodgate JL, Makinson JC, Lim KS, Reynolds AM, Chittka L (2016) Life-long radar tracking of bumblebees. PLoS One 11
Yeison LG, John HD, Caraball P (2013) Foraging activity of the social wasp Polybia emaciata (Hymenoptera: Vespidae: Polistinae). Revista Colombiana de Entomologia 39:250–255
Acknowledgments
The senior author acknowledges students, postdocs, and colleagues (including the junior author) who have worked with him in the tropics and, whether they know it or not, contributed in ways large and small to what is in this chapter: Roger Swain, David Post, Holly Downing, Eloy Castellon Bermudez, Michel Pratte, Monica Raveret Richter, Neal Williams, Sean O’Donnell, Larry Phelps, Jim Hunt, Malcolm Keeping, Karen London, Andy Bouwma, Kenneth Howard, Teresa Schueller, Peter Sonnentag, and Kevin Loope. We would also like to thank Kevin Mark for his assistance in the section on ether anesthesia. We also thank Priyantha Wijesinghe and Jim Carpenter for their assistance with the section on preservation for museum specimens and for molecular analysis.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jeanne, R.L., Taylor, B.J. (2021). Research Techniques Used in the Study of Social Wasps. In: Prezoto, F., Nascimento, F.S., Barbosa, B.C., Somavilla, A. (eds) Neotropical Social Wasps. Springer, Cham. https://doi.org/10.1007/978-3-030-53510-0_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-53510-0_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53509-4
Online ISBN: 978-3-030-53510-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)