Abstract
In complex societies, communication signals function as fine tools for regulating social structure and coordinating activities, while enabling groups to adjust their strategies flexibly in response to ecological conditions. Social wasps of the genus Polistes perform vibratory movements, which are expressed by dominant individuals mainly during adult-larva feeding interactions. Recent investigations have hypothesized that these signals may influence caste differentiation during larval development. We tested this hypothesis by conducting behavioural observations in the field, in three populations of social wasps (Polistes biglumis) differing in caste ratio: In some populations, foundresses produce workers and future reproductives; in others, workers are rarely produced. We observed that only foundresses produced vibratory signals, which were expressed during larval feeding sessions and only in the period before offspring emergence. Foundresses belonging to populations with workers spent more time producing vibratory signals than those from populations where workers are rare. In some populations, social parasites invaded colonies and subdued host foundresses. Subdued foundresses produced fewer vibratory signals than foundresses of unparasitized colonies. Our data suggest that the dominant status is necessary for the expression of vibratory signals and show that foundresses from different populations produced different numbers of vibratory signals. This difference can be explained well by the hypothesis that vibratory signals influence larval development and promote the production of workers. We suggest that these signals may have been the target of selective forces, in order to regulate caste ratio and maximize colony fitness under local conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding how phenotypic variation arises is a question that brings together all research fields in biology. In several cases, genetic information only accounts for a small part of the observed phenotypic variation. Both physical and social environments play a critical role during development, finely regulating the expression of genes and producing a wide range of phenotypic differences (Nijhout 2003; Monaghan 2008; Branchi 2009; Champagne 2010). Highly organized societies composed of closely related individuals, such as colonies of eusocial insects, provide excellent examples for the existence of such mechanisms.
In eusocial insects, adult individuals are differentiated into castes: Functionally sterile individuals (workers) work on their natal nest and help raise reproductive individuals (gynes) that will find new colonies in the following reproductive season. While future queens and workers differ both behaviourally and morphologically in “advanced eusocial” species, individuals are generally non-dimorphic and have a flexible caste expression in “primitively eusocial” species (O’Donnell 1998).
In a few cases, such as some genera of ants, caste differences are strongly determined by genetic diversity (Volny and Gordon 2002; Julian et al. 2002; Anderson et al. 2008); in most eusocial insects, individuals share similar genetic information and their phenotypic differences are, to a variable extent, determined by the effect of non-genetic factors (Schwander et al. 2010; Weiner and Toth 2012).
A widely investigated environmental factor influencing caste determination is larval nutrition. The current paradigm states that differential amounts and qualities of nutrients provided during larval growth can bias larval development into one of the caste-specific phenotypes (Wheeler 1986). In advanced eusocial species, caste determination through differential nutrition has been largely demonstrated (Kucharski et al. 2008). Yet, in primitively eusocial wasps, such as the wasps of the genus Polistes, differential nutrition alone may not be sufficient to explain the variable pattern of caste development, because the rate of nutrition, rate of development and adult size are not always linearly related (Hunt 2007; Jeanne and Suryanarayanan 2011). Recent studies suggest that special mechanical signals provided by adults to larvae may integrate or modulate the effects of differential nutrition on larval physiology and represent an additional non-genetic factor influencing caste determination in Polistes (Jeanne 2009; Suryanarayanan et al. 2011b).
Mechanical signals in Polistes wasps
Although chemical communication is considered to be the principal way of sharing information in social insects, mechanical signals also play an important role. Mechanical communication may play an active role in eliciting behavioural responses in insects (Kirchner 1997; Virant-Doberlet and Cokl 2004; Barbero et al. 2009; Hunt and Richard 2013) or even be responsible for physiological and organizational changes during larval development. For instance, mechanical cues were found to provoke changes in larval body weight (Hirashima et al. 1992, 1993) and changes in levels of juvenile hormone (JH) (Schneider et al. 2004) and biogenic amines (Davenport and Evans 1984; Woodring et al. 1988; Hirashima et al. 1992, 1993; Rauschenbach et al. 1993).
In social wasps, mechanical communication has been observed during dominance interactions (Pardi 1948; West-Eberhard 1986; Toth et al. 2014), defensive behaviours (Strassmann et al. 1990; Nascimento et al. 2005), feeding interactions (Ishay and Landau 1972; Ishay et al. 1974; Jeanne 1977) and recruitment of foragers (O’Donnell 2006). At least three variants of vibratory signals have been described in Polistes. Pratte and Jeanne (1984) first reported that adult wasps perform the “antennal drumming” behaviour when they return to the nest with a prey. This behaviour has by now been observed in at least 11 species and consists of a fast, vigorous drumming of the antennae against cell rims (28.96 strikes/s), just before feeding larvae (Pratte and Jeanne 1984; Suryanarayanan and Jeanne 2008). Savoyard et al. (1998) accurately described a second vibratory movement, called “lateral vibration”, involving adult wasps shaking their abdomen horizontally while patrolling their nests and inspecting cells. This behaviour is usually very intense, causing the movement of the whole body and producing a distinct sound. A variation of this behaviour is the “abdominal wagging”, which is less intense and performed more often than lateral vibration (Gamboa and Dew 1981; Brillet et al. 1999; Brennan 2007).
Although early studies argued that vibratory signals in Polistes may be important in nest defence (West-Eberhard 1969; Esch 1971), it has become evident that they are involved in communication between nest mates. Several authors suggested that vibratory signals may play a role in dominance interactions among adults (Strassmann 1981; West-Eberhard 1986; Brillet et al. 1999). However, the most recent evidence calls for a role of vibratory signals in adult-larva communication, often in association with food exchanges (reviewed in Jeanne 2009). In particular, the “mechanical switch hypothesis” proposed by Jeanne (2009) suggests that the vibratory signals produced by adult wasps may act on the biochemical pathways associated with larval nutrition and affect larval growing rates and the development of caste-specific traits. According to Jeanne and Suryanarayanan (2011), these signals may favour the production of worker phenotypes by inhibiting the synthesis of fat stores and storage proteins, which are essential to reproductive individuals for overwintering (Hunt and Amdam 2005). A recent study on Polistes fuscatus (Suryanarayanan et al. 2011b) reinforced this hypothesis, showing that larvae exposed to artificial vibrations of the same frequency as antennal drumming developed into adults with reduced fat bodies, a physiological trait typical of the worker caste (Eickwort 1969).
Polistes biglumis as the test species
Populations of P. biglumis living under different ecological conditions provide an exceptional natural model for investigating the link between mechanical signals and caste expression.
P. biglumis (Vespidae, Polistini) is a primitively eusocial wasp that lives in mountain areas (1600–2350 m above sea level—hereafter, m a.s.l.) in Southern Europe. It has solitary nest foundation (i.e. a single foundress initiates the colony and is the only adult individual on the nest until her offspring emerge 40–50 days later) and a short nesting season of 3–4 months, from May–June to early September (Lorenzi and Turillazzi 1986).
Populations of P. biglumis living in different ecological conditions show different social strategies. An investigation conducted on the same populations and in the same years as the present study (Fucini et al. 2009) showed that under low-temperature regimes, as occurs in the Alps, workers (females with no or scarce fat body) are rarely produced and the first adult offspring mainly consists of gynes (females with abundant fat bodies, which are able to overwinter and reproduce) and males. In contrast, populations living in the Apennines, where temperatures are generally higher, have a longer nesting season. Their colonies have a conspicuous production of workers, and gynes emerge at the end of the nesting season (a pattern of caste succession shared with most Polistes species, Reeve 1991).
P. biglumis colonies may be parasitized by Polistes atrimandibularis (Cervo et al. 1990; Lorenzi and Thompson 2011), an obligate social parasite which reduces host fitness by destroying host eggs and larvae and repressing host females’ egg-laying capacity (Lorenzi et al. 1992; Cervo and Lorenzi 1996). Parasite females invade host nests when the foundress is still the only adult individual on the colony; from that moment, they cohabit with their hosts throughout the season (Cervo et al. 1990). Parasite females also feed larvae in their host colony, plundering brood from surrounding P. biglumis nests (Cervo et al. 1990).
After nest invasion, the parasite starts displaying dominance behaviours, which involve a variety of tactile stimuli performed with antennae and mouthparts over the foundress’s head and body (Cervo et al. 1990). Gradually, the foundress loses her reproductive potential due to the suppression of her ovary development (Cervo and Lorenzi 1996) and becomes more active, intensively dedicating herself to colony activities (Fucini et al. 2014). Fucini et al. (2009) reported that differential caste expression also varies as a function of parasite prevalence, with colonies in highly parasitized populations producing fewer worker-like offspring than those in populations with low or no presence of social parasites (see also Lorenzi and Thompson 2011). Low-temperature regimes and high parasite prevalence may, therefore, favour the production of behaviourally and physiologically totipotent females over workers, which are regularly produced under less severe environmental conditions.
The proximate mechanisms favouring the variation in caste expression in P. biglumis have not been investigated; in the present work, we aim to examine how, and to which extent, vibratory signalling relates to this variation. We performed a long-term, observational field study in three geographically distinct populations, aiming to (1) describe the structure and occurrence of vibratory signals performed by P. biglumis; (2) investigate the possible link between the expression of vibratory signals and individual hierarchical status, taking advantage of the presence of social parasites which usurp the dominant position; and (3) check for correlations between the occurrence of vibratory signals and the differences in social structure between populations, in the attempt to test the mechanical switch hypothesis in a natural setting.
Methods
Field sites
We collected data on three populations of P. biglumis, two in the Alps (Montgenèvre, Hautes Alpes, France, 44° 55′ N, 6° 43′ E, 1860 m a.s.l.; Ferrere, Cuneo, Italy, 44° 22′ N, 6° 57′ E, 1900 m a.s.l.), and one in the Apennines (Monte Mare, Isernia, Italy, 41° 37′ N, 14° 00′ E, 1740 m a.s.l.). Because of the high elevation, the three sites are characterized by severe climate conditions, with short summer seasons and harsh winters. In contrast, the population in the Apennines is exposed to relatively milder temperature regimes (Fucini et al. 2009).
Data collection and colony surveys
Data were collected during two consecutive nesting seasons (May–September 2006 and 2007). At the beginning of each season, we surveyed the study areas and identified the newly founded colonies. We individually marked nest foundresses and noted the number and developmental stage of brood (egg, small larva, large larva or pupa). Subsequently, we checked colonies weekly, noting the presence of the foundress in each colony and the number of brood and marking newly emerged wasps and P. atrimandibularis females, if present, using unique colour combinations (enamel paint).
Behavioural pattern and sequence analysis
We performed a preliminary set of observations in Montgenèvre and Monte Mare by video recording eight foundresses from eight unparasitized colonies in the pre-emergence phase (i.e. in the period before the emergence of adult offspring, with a total recording time of 13 h and 45 min) in order to identify the type and frequency of body vibrations performed by P. biglumis. We used a Canon MV960 camcorder, placed at approximately 20 cm from the nests. We scored behaviours from the video recordings using JWatcher, a free behaviour analysis program (Blumstein and Daniel 2007). All scored behaviours were included in our ethogram and employed to perform a sequential analysis to investigate the behavioural context in which body vibrations occur.
Expression of vibratory signals within and between populations
We performed on-site field behavioural observations on 181 colonies over the two nesting seasons (Ferrere: 33 colonies, 2 of which were parasitized by the obligate social parasite P. atrimandibularis; Montgenèvre: 49 colonies, 27 of which parasitized; Monte Mare: 99 colonies, 21 of which parasitized). Twenty-seven colonies were observed both in the pre- and in the post-emergence phases; the others were observed in only one phase. In detail, we observed pre-emergence colonies where at least one large larva was present (larvae at the third instar of development, or higher, were considered as large). We also observed post-emergence colonies (i.e. in the period after the emergence of the offspring) with three or more newly emerged offspring.
Overall, we collected behavioural observations on 175 nest foundresses and 50 parasite females. Additionally, we observed P. biglumis female adult offspring (overall, more than 400 individuals) that were on their nests during the observation of foundress and/or parasite females.
Behavioural observations were conducted between 9:30 a.m. and 3:30 p.m. and lasted between 90 and 120 min per colony, during which all wasps on the nests were observed simultaneously (the number of individuals per nest ranged between 1 and 13). During each observation, we noted the occurrence and time duration of vibratory signals.
Data analysis
Behavioural pattern and sequence analysis by video recording
Using JWatcher, we measured the average duration, frequency and intervals of occurrence of vibratory movements from the scored observations in order to establish what types of body vibrations were expressed in P. biglumis.
To test for the existence of recurring behavioural contexts involving vibratory signals (i.e. the existence of stereotyped, non-random temporal structures in a behavioural sequence), we performed a first-order Markovian sequence analysis. We exported all scored behaviours to SPSS and created two variables corresponding to the “preceding” and the “following” series of behaviours. We first ran a general log-linear analysis to obtain adjusted z-scores and determine the significance of the transition between two consecutive behaviours. Since our goal was to identify non-random temporal sequences among consecutive behaviours, we ignored the transitions involving the same, repeated behaviour. For this purpose, we assigned a structural “zero” value to all cases in which the preceding and following behaviours were the same (Bakeman and Quera 1995). Preceding and following variables were entered as categorical factors and the structural zero variable as cell structure. Using JWatcher, we then obtained a matrix of observed frequencies, which represents how often a given behaviour follows another behaviour, and a matrix of transitional probabilities, which provides the relative frequency of the transition between two consecutive behaviours, given the total occurrence of the first behaviour. We used these matrices to build a kinematic diagram, which visually represents the observed frequencies of each behaviour and the frequencies of the transitions between two consecutive behaviours (Chen et al. 2002; Nilsen et al. 2004; Egge et al. 2010). Adjusted z-score values larger than 1.96 were selected to identify the transitions that occurred more often than expected by chance at the 0.05 probability level. Only significant transitions were taken into account to build our diagram (cf. Table S2, Online Resource 1).
Within- and between-population comparisons
We ran a generalized linear mixed model (GLMM) with a binomial distribution and a logit link function to investigate the variation in the occurrence of vibratory signals within populations (i.e. in parasitized vs. unparasitized colonies) and between populations. The total time each wasp spent performing vibrations was fitted as the numerator, and the total time each wasp was active on the nest was fitted as the denominator. Populations (Ferrere, Montgenèvre and Monte Mare) and nest status (unparasitized or parasitized) were entered as fixed factors. The nest ID was entered as a random factor.
Pairwise comparisons of estimated means were also performed, to account for the difference in the expression of vibratory signals amongst populations (unlike observed means, estimated means take into account the effect on the given variable of all factors included in the model).
We used IBM SPSS 21.0 to perform this analysis.
Results
Occurrence and description of vibratory signals in P. biglumis
Only P. biglumis foundresses and P. atrimandibularis parasite females expressed vibratory signals (the behaviour was virtually absent in P. biglumis offspring).
P. biglumis foundresses performed body vibrations by standing or walking on the nest while oscillating the abdomen horizontally (as shown in the video clip of Online Resource 2). Similarly to what was observed in the closely related species Polistes dominula (Brillet et al. 1999; Brennan 2007), P. biglumis and P. atrimandibularis expressed one single form of vibratory signal, the abdominal wagging (hereafter “AW”). The intensity of this behaviour was variable amongst the individuals, ranging from a slow oscillation to a faster, more energetic swing, the latter being often accompanied by the shaking of the entire nest and the production of an audible sound. A quantitative analysis of frequency and amplitude of the body oscillations (Gamboa and Dew 1981; Savoyard et al. 1998; Brillet et al. 1999) is omitted, being beyond the purpose of our study. Nevertheless, the long duration (2.35 s ± 2.32) and the high frequency (121.92 AW/h) of the vibratory bouts (n = 1147 AW bouts recorded from eight foundresses in pre-emergence phase) were consistent with those quantified for the AW in other Polistes species (Gamboa and Dew 1981; Savoyard et al. 1998; Brillet et al. 1999).
AW bouts were temporally clustered: 60 % of AW bouts were separated by time intervals <5 s and 80 % by time intervals <10 s. In absolute terms, wasps performed cell inspection and AW more often than any other behaviour (30 and 31 % of all scored behaviours, respectively). All scored behaviours are listed and described in Table S1 (Online Resource 1). The occurrence of each scored behaviour can be found in Table S2 in the Online Resource 1.
Sequence analysis
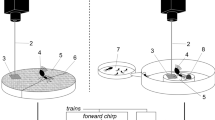
The graphic representation of the significant behavioural transitions shows that AW was significantly associated with larval feeding (Fig. 1a). Behaviours not involved in larval feeding were linked to each other but not to feeding-related behaviours or AW, resulting in a separated kinematic graph (Fig. 1b).
Diagram of behavioural transitions between consecutive behaviours, according to the analysis of the video recordings. Only significant transitions (z-score >1.96) are shown in the diagram. The size of circles represents the observed occurrence of each behaviour (n values are presented in Table S2, Online Resource 1); the width of the arrows represents the relative frequency of each transition (n values are presented in Table S3, Online Resource 1). No significant transitions occurred between the behaviours related to larval feeding (a) and the other scored behaviours (b). A description for each behaviour can be found in Table S1 (Online Resource 1)
We found that AW occurred most frequently after prey malaxation (the elaboration of freshly caught preys) and before the distribution of preys to larvae (Fig. 1a). Similarly to what Brillet et al. (1999) and Brennan (2007) observed in P. dominula, AW performed by P. biglumis was also associated (and often alternated) with cell inspection, as shown by the significant, highly frequent transitions linking the two behaviours (frequency of transition: AW → cell inspection, 78 %; cell inspection → AW, 65 %; Table S3 in the Online Resource 1).
Expression of abdominal wagging: within- and between-population comparisons
P. biglumis and P. atrimandibularis females performed AW exclusively during the pre-emergence phase (Fig. 2). Nearly all P. biglumis foundresses observed during the pre-emergence phase exhibited AW (107 out of 109 in unparasitized colonies; 31 out of 38 in parasitized colonies), whereas none of the 53 foundresses observed in post-emergence did (4 individuals performed AW on very rare occasions and at a negligible rate, as indicated by outlier stars in Fig. 2a). Similarly, almost all P. atrimandibularis parasite females in pre-emergence colonies performed AW (32 out of the 39), whereas none of the 18 parasite females observed during the post-emergence phase did. Therefore, the following analyses include data from pre-emergence colonies only.
There was a significant difference in the expression of AW amongst populations (GLMM, F 2,143 = 7.424, P = 0.001). The foundresses in the Alpine populations of Montgenèvre and Ferrere spent significantly less time performing AW than those in the Apennine population of Monte Mare (Monte Mare vs. Montgenèvre, t = 2.389, P = 0.018; Monte Mare vs. Ferrere, t = 3.855, P < 0.001). Furthermore, post hoc pairwise contrasts showed that there was no significant difference between the two Alpine populations (Montgenèvre vs. Ferrere, t = 1.271, P = 0.206; Fig. 3).
Foundresses performed AW both in unparasitized and in parasitized colonies. However, foundresses of parasitized colonies spent significantly less time performing AW than those of unparasitized colonies (F 1,143 = 22.016, P < 0.001, Fig. 4), and this difference was consistent across populations (the interaction between population and nest status, included as a fixed factor in a preliminary model, was not significant and was removed from the final model).
Discussion
Abdominal wagging is the only vibratory signal produced by P. biglumis wasps. It is exhibited exclusively by foundresses, is virtually absent in the offspring and can only be observed during the pre-emergence phase of the colony.
Abdominal wagging is expressed during adult-brood trophic exchanges
P. biglumis foundresses mostly perform AW in association with larval feeding; in our observations, AW most often followed prey malaxation and occurred before the distribution of liquid or solid food to larvae. AW in P. biglumis is also often performed in association with cell inspections. Brillet et al. (1999) reported a similar pattern in P. dominula, where nearly 90 % of AW bouts were observed just before a cell inspection. Wasps inspect nest cells mainly during feeding-related activities, when larvae are fed with liquid or solid food. This suggests a causal connection between AW and brood nutrition, yet some evidence contradicts this view. First, cell inspections often occurred without involving food exchanges between adults and larvae. In our study, 876 AW bouts were followed by cell inspections, but only 175 cell inspections resulted in an exchange of food. Second, about 20 % of the recorded AW bouts were not directly followed by cell inspection or feeding activities. Third, as noted by Brennan (2007), when wasps perform AWs, the contact point between their abdomen and the nest surface is one to two cells away from the inspected cell, hence relatively far from the larva potentially involved in food exchange. It should also be emphasized that every wasp species in which vibratory signals were observed builds nests made of paper, a rigid and elastic material that is extremely suitable as a propagation substrate (Gamboa and Dew 1981); when wasps perform AW, it is then likely that vibrations propagate throughout the nest and are perceived by all colony members.
Vibratory signals in Polistes are generally observed for the first time when at least one larva in the nest reaches the third developmental instar (Brillet et al. 1999; Suryanarayanan and Jeanne 2008). When larvae reach this instar, they start producing conspicuous amounts of very nutritious saliva, which is exploited by adults as a source of amino acids (Hunt et al. 1982; Hunt 2007). Several investigators hypothesized a link between the expression of vibratory signals and the release of larval saliva, but they often obtained contrasting results. Suryanarayanan and Jeanne (2008) suggested that vibratory signalling may not be used to solicit the release of larval saliva in P. fuscatus (but see Brennan 2005 on P. dominula), and showed that the removal of larvae does not cause a reduction in the expression of antennal drumming. In the same way, body vibrations are unlikely to inhibit the release of larval saliva during food distribution (as first hypothesized by Pratte and Jeanne 1984). In fact, a recent study showed that the rates of larval feeding do not match those of vibratory signalling (Suryanarayanan et al. 2011b). Whether there is a relationship between larval development and the expression of AW in P. biglumis is an issue not investigated in our study, but we would like to stress some considerations based on our observations. First, neither foundresses nor workers of P. biglumis performed AW during the post-emergence phase; yet larvae older than the third instar were still present in post-emergence colonies and food exchanges between larvae and adults continued throughout this phase. Second, larval saliva can be easily solicited with the tactile stimulation of larval mouthparts (Gamboa and Dew 1981; Hunt 2007). Consequently, it is unlikely that foundresses would need to enact energy-demanding body movements to stimulate the release of larval saliva; in the same way, it is also unlikely that foundresses would benefit from producing a signal which causes a collective release of saliva by larvae, because this would result in a large waste of nutrients.
All this evidence leads us to suggest that vibratory signals are probably not related to single food exchanges or to communication with a single larva, and salivary exchanges may only be marginally (if at all) linked to the expression of this behaviour. Although we found evidence that AW in P. biglumis is expressed mainly during brood nutrition, we still need a better understanding of the causal meaning of this relationship. Other factors, such as age-related hormonal changes, ovary condition and hierarchical status of the individuals, may also be important in finely tuning the expression of AW and could help us understanding its function.
Is the expression of AW a peculiarity of dominant individuals? Evidence from social parasites
A major finding of our study is that foundresses in colonies parasitized by P. atrimandibularis performed AW at a significantly lower rate than foundresses in unparasitized colonies. This result provides indirect support to evidence that vibratory behaviours in Polistes are associated with dominant status (Gamboa and Dew 1981; Strassmann 1981; Savoyard et al. 1998; Jeanne and Suryanarayanan 2011). Itô (1995) observed that lateral vibrations in Polistes canadensis were performed at higher rates by females with well-developed ovaries. Röseler and Röseler (1989) found that ovariectomized foundresses of Polistes gallicus (=P. dominula) lost their reproductive ability but were able to acquire and maintain social dominance, including the expression of dominance behaviours and AW.
P. atrimandibularis parasite females subdue P. biglumis foundresses and create a social context of dominance-subordinance interactions similar to those found in multi-foundress colonies of other Polistes wasps (Cervo et al. 1990; Fucini et al. 2014).
The physiological mechanisms through which dominance behaviours become effective in determining the hierarchical status of individuals are still poorly understood. Dominant females expressing these behaviours usually have distinct anatomical and physiological features, e.g. bigger corpora allata, higher level of JH in the haemolymph and larger ovaries than subordinate females (Röseler et al. 1984; Bloch et al. 1996, 2009). Biogenic amines, such as octopamine, serotonin and dopamine, play a major role in the stimulation of corpora allata activity and ovarian development (Bloch et al. 2009) and, in turn, the production of these amines is strongly influenced by social and physical stressors, including mechanical stimuli (Davenport and Evans 1984; Woodring et al. 1988; Hirashima et al. 1992, 1993; Rauschenbach et al. 1993).
We speculate that the dominance behaviours provided by P. atrimandibularis females to P. biglumis host foundresses may trigger a chain of physiological responses in the hosts, including changes in biogenic amine levels, which ultimately lead to diminished corpora allata activity and suppression of ovarian activation (parasites “castrate” host foundresses, Cervo and Lorenzi 1996). The subordinate status in which foundresses of parasitized colonies are constrained may then explain the reduced expression of AW, similarly to what is observed in subordinate females in multi-foundress colonies.
The proximate factors triggering the expression of vibratory signals in dominant individuals still have to be investigated. Suryanarayanan and Jeanne (2008), studying antennal drumming in P. fuscatus, suggested that chemical components in larval saliva may function as primers for the exhibition of vibratory behaviour. They noted that similar salivary glands in bees are important sources of primer pheromones modulating adult behaviour (Conte et al. 2006; Conte and Hefetz 2008). Yet, this hypothesis is still waiting for a test in social wasps.
Rates of AW predict offspring caste ratio in P. biglumis
To our knowledge, this the first study that documents variation in the rate of performance of a signal amongst populations of a social wasp. In both Alpine populations, where temperatures were low and workers were rarely produced (Fucini et al. 2009), foundresses performed AW at significantly lower rates than foundresses living in Monte Mare, where colonies produce a relatively high number of workers. This difference is consistent with the hypothesis that vibratory signals play a role in modulating caste plasticity, biasing pre-imaginal development towards the production of workers.
If this hypothesis is correct, we would also expect to observe these signals only before larvae destined to become reproductives start to differentiate. In Polistes species living in temperate zones, worker production normally continues for several weeks, with reproductives only emerging at the end of the season (Reeve 1991). Several studies reported that, in these species, vibratory signals are expressed for several weeks after the emergence of the first worker (Gamboa and Dew 1981; Brillet et al. 1999; Suryanarayanan et al. 2011a).
The low-temperature regimes constrain P. biglumis colonies to a short life. These colonies have a very short or absent worker phase. They maximize the production of reproductive individuals and present “almost none of the caste succession found in temperate species” (Lorenzi and Turillazzi 1986). This means that when the first workers emerge, about 45 days after foundation, larvae destined to become reproductives are already developing and would not benefit from receiving a “castrating” signal like AW. Consistently, and in accordance with our expectation, we did not observe AW in post-emergence colonies of P. biglumis.
With these new findings, we can then add new, although correlative, evidence in support of the mechanical switch hypothesis proposed by Jeanne (2009). Although we did not directly correlate the rate of expression of AW to the offspring caste ratio per colony in our study, we could benefit from the quantitative data on caste ratio obtained from a parallel, population-based study (Fucini et al. 2009, briefly presented in the “Introduction” section of this paper), which was performed on the same populations and in the same nesting seasons as the present study.
There are certainly several details that still beg for an explanation, which calls for targeted investigation. For example, one of the puzzling aspects of our observations is the fact that P. atrimandibularis females also performed AWs during larval feeding activities, with a pattern and rate of occurrence analogous to those of their hosts (M.M. personal observation) as shown in the video clip of Online Resource 3. Similarly to their hosts, parasites exhibited AW exclusively in the pre-emergence period. Being obligate social parasites, P. atrimandibularis gynes produce no workers and their ability to express AW may appear counterintuitive. However, the target of parasites’ vibrations is probably not to be found in the parasite larvae. P. atrimandibularis females invade host colonies towards the end of the pre-emergence period, when nests contain late-instar P. biglumis larvae (Cervo et al. 1990). Consequently, when parasite females express AW, vibrations are likely to target host larvae; parasite larvae are still too small and not yet sensitive to the vibratory signal performed by their mother, as they will reach their mature instars only later in the season. It is possible that the capacity to express AW of P. atrimandibularis was already present in its free-living ancestor and has been maintained as an additional mechanism of host exploitation, to enhance the production of host workers and consequently increase the reproductive success of the parasite female.
Conclusion
In primitively eusocial Polistes species, the use of mechanical signals seems to be an exclusive prerogative of dominant individuals. These signals can be involved both in the establishment of dominance hierarchies among adults (through local, tactile interactions) and in the manipulation of brood caste differentiation (through nest vibrations). In both contexts, as already noted by Jeanne (2009), vibrations are used to control the reproductive physiology of the receiver and relegate it to a subordinate status.
In our study, we provide new, correlative evidence that vibratory signals may play a role in larval development and favour the production of workers. Ecological factors such as parasite prevalence and temperature regimes may have driven the onset of mechanisms influencing the offspring caste ratio and maximizing foundress fitness under local conditions. Some signals, like AW, may have been the target of these forces and may have been finely tuned to be involved in the regulation of larval development.
Although further investigation is needed, we hypothesize that selection may have favoured P. biglumis foundresses that express low rates of vibratory signals under severe ecological conditions, thus increasing the chance of producing future foundresses instead of workers.
References
Anderson KE, Linksvayer TA, Smith CR (2008) The causes and consequences of genetic caste determination in ants (Hymenoptera: Formicidae). Myrmecol News 11:119–132
Bakeman R, Quera V (1995) Log-linear approaches to lag-sequential analysis when consecutive codes may and cannot repeat. Psychol Bull 118:272–284. doi:10.1037/0033-2909.118.2.272
Barbero F, Thomas JA, Bonelli S et al (2009) Queen ants make distinctive sounds that are mimicked by a butterfly social parasite. Science 323:782–785. doi:10.1126/science.1163583
Bloch G, Borst DW, Huang ZY et al (1996) Effects of social conditions on juvenile hormone mediated reproductive development in Bombus terrestris workers. Physiol Entomol 21:257–267
Bloch G, Shpigler H, Wheeler DE, Robinson GE (2009) Endocrine influences on the organization of insect societies. In: Pfaff DW, Arnold AP, Etgen AM et al (eds) Hormones, brain and behavior, 2nd edn. Academic Press, San Diego, pp 1027–1068
Blumstein DT, Daniel JC (2007) Quantifying behavior the JWatcher way. Sinauer Associates Incorporated
Branchi I (2009) The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Rev 33:551–559. doi:10.1016/j.neubiorev.2008.03.011
Brennan BJ (2005) Vibratory communication in the social paper wasp Polistes dominulus (Hymenoptera: Vespidae). Dissertation, Cornell University
Brennan BJ (2007) Abdominal wagging in the social paper wasp Polistes dominulus: behavior and substrate vibrations. Ethology 113:692–702. doi:10.1111/j.1439-0310.2007.01373.x
Brillet C, Tian-Chansky SS, Le Conte Y (1999) Abdominal waggings and variation of their rate of occurrence in the social wasp, Polistes dominulus Christ. I. Quantitative analysis. J Insect Behav 12:665–686
Cervo R, Lorenzi MC (1996) Inhibition of host queen reproductive capacity by the obligate social parasite Polistes atrimandibularis (Hymenoptera, Vespidae). Ethology 102:1042–1047
Cervo R, Lorenzi MC, Turillazzi S (1990) Nonaggressive usurpation of the nest of Polistes biglumis bimaculatus by the social parasite Sulcopolistes atrimandibularis (Hymenoptera Vespidae). Insect Soc 37:333–347
Champagne FA (2010) Epigenetic influence of social experiences across the lifespan. Dev Psychobiol 52:299–311. doi:10.1002/dev.20436
Chen S, Lee AY, Bowens NM et al (2002) Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci 99:5664–5668. doi:10.1073/pnas.082102599
Conte YL, Hefetz A (2008) Primer pheromones in social Hymenoptera. Annu Rev Entomol 53:523–542. doi:10.1146/annurev.ento.52.110405.091434
Conte YL, Bécard J-M, Costagliola G et al (2006) Larval salivary glands are a source of primer and releaser pheromone in honey bee (Apis mellifera L.). Naturwissenschaften 93:237–241. doi:10.1007/s00114-006-0089-y
Davenport AP, Evans PD (1984) Stress-induced changes in the octopamine levels of insect haemolymph. Insect Biochem 14:135–143. doi:10.1016/0020-1790(84)90021-0
Egge AR, Brandt Y, Swallow JG (2010) Sequential analysis of aggressive interactions in the stalk-eyed fly Teleopsis dalmanni. Behav Ecol Sociobiol 65:369–379. doi:10.1007/s00265-010-1054-5
Eickwort K (1969) Separation of the castes of Polistes exclamans and notes on its biology (Hym.: Vespidae). Insect Soc 16:67–72. doi:10.1007/BF02224464
Esch H (1971) Wagging movements in the wasp Polistes versicolor vulgaris Bequaert. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 72:221–225
Fucini S, Di Bona V, Mola F et al (2009) Social wasps without workers: geographic variation of caste expression in the paper wasp Polistes biglumis. Insect Soc 56:347–358. doi:10.1007/s00040-009-0030-4
Fucini S, Uboni A, Lorenzi MC (2014) Cuckoo wasps manipulate foraging and resting activities in their hosts. Behav Ecol Sociobiol 68:1753–1759. doi:10.1007/s00265-014-1783-y
Gamboa GJ, Dew HE (1981) Intracolonial communication by body oscillations in the paper wasp, Polistes metricus. Insect Soc 28:13–26. doi:10.1007/BF02223618
Hirashima A, Ueno R, Eto M (1992) Effects of various stressors on larval growth and whole-body octopamine levels of Tribolium castaneum. Pestic Biochem Physiol 44:217–225. doi:10.1016/0048-3575(92)90092-E
Hirashima A, Nagano T, Eto M (1993) Stress-induced changes in the biogenic amine levels and larval growth of Tribolium castaneum herbst. Biosci Biotechnol Biochem 57:2085–2089
Hunt JH (2007) The evolution of social wasps. Oxford Univ. Press, Oxford
Hunt JH, Amdam GV (2005) Bivoltinism as an antecedent to eusociality in the paper wasp genus Polistes. Science 308:264–267. doi:10.1126/science.1109724
Hunt JH, Richard F-J (2013) Intracolony vibroacoustic communication in social insects. Insect Soc 60:403–417. doi:10.1007/s00040-013-0311-9
Hunt JH, Baker I, Baker HG (1982) Similarity of amino acids in nectar and larval saliva: the nutritional basis for trophallaxis in social wasps. Evolution 36:1318–1322. doi:10.2307/2408164
Ishay J, Landau EM (1972) Vespa larvae send out rhythmic hunger signals. Nature 237:286–287. doi:10.1038/237286a0
Ishay J, Motro A, Gitter S, Brown MB (1974) Rhythms in acoustical communication by the oriental hornet, Vespa orientalis. Anim Behav 22:741–744
Itô Y (1995) Notes on social behavior and ovarian condition in Polistes canadensis (Hymenoptera: Vespidae) in Panama. Sociobiology 26:247–257
Jeanne RL (1977) Behavior of the obligate social parasite Vespula arctica (Hymenoptera: Vespidae). J Kansas Entomol Soc 50:541–557
Jeanne RL (2009) Vibrational signals in social wasps: a role in caste determination? In: Gadau J, Fewell J (eds) Organization of insect societies: from genome to sociocomplexity. Harvard University Press, Cambridge, pp 243–265
Jeanne RL, Suryanarayanan S (2011) A new model for caste development in social wasps. Commun Integr Biol 4:373–377
Julian GE, Fewell JH, Gadau J et al (2002) Genetic determination of the queen caste in an ant hybrid zone. Proc Natl Acad Sci 99:8157–8160. doi:10.1073/pnas.112222099
Kirchner WH (1997) Acoustical communication in social insects. In: Lehrer DM (ed) Orientation and communication in arthropods. Birkhäuser Basel, pp 273–300
Kucharski R, Maleszka J, Foret S, Maleszka R (2008) Nutritional control of reproductive status in honeybees via DNA methylation. Science 319:1827–1830. doi:10.1126/science.1153069
Lorenzi MC, Thompson JN (2011) The geographic structure of selection on a coevolving interaction between social parasitic wasps and their hosts hampers social evolution. Evolution 65:3527–3542. doi:10.1111/j.1558-5646.2011.01403.x
Lorenzi MC, Turillazzi S (1986) Behavioural and ecological adaptations to the high mountain environment of Polistes biglumis bimaculatus. Ecol Entomol 11:199–204. doi:10.1111/j.1365-2311.1986.tb00295.x
Lorenzi MC, Cervo R, Turillazzi S (1992) Effects of social parasitism of Polistes atrimandibularis on the colony cycle and brood production of Polistes biglumis bimaculatus (Hymenoptera, Vespidae). B Zool 59:267–271. doi:10.1080/11250009209386681
Monaghan P (2008) Early growth conditions, phenotypic development and environmental change. Philos Trans R Soc Lond Ser B Biol Sci 363:1635–1645. doi:10.1098/rstb.2007.0011
Nascimento FS, Hrncir M, Tolfiski A, Zucchi R (2005) Scraping sounds produced by a social wasp (Asteloeca ujhelyii, Hymenoptera: Vespidae). Ethology 111:1116–1125. doi:10.1111/j.1439-0310.2005.01124.x
Nijhout HF (2003) Development and evolution of adaptive polyphenisms. Evol Dev 5:9–18. doi:10.1046/j.1525-142X.2003.03003.x
Nilsen SP, Chan Y-B, Huber R, Kravitz EA (2004) Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A 101:12342–12347
O’Donnell S (1998) Reproductive caste determination in eusocial wasps (Hymenoptera: Vespidae). Annu Rev Entomol 43:323–346
O’Donnell S (2006) Polybia wasp biting interactions recruit foragers following experimental worker removals. Anim Behav 71:709–715. doi:10.1016/j.anbehav.2005.07.013
Pardi L (1948) Dominance order in Polistes wasps. Physiol Zool 21:1–13
Pratte M, Jeanne RL (1984) Antennal drumming behavior in Polistes wasps (Hymenoptera: Vespidae). Z Tierpsychol 66:177–188
Rauschenbach IY, Serova LI, Timochina IS et al (1993) Analysis of differences in dopamine content between two lines of Drosophila virilis in response to heat stress. J Insect Physiol 39:761–767. doi:10.1016/0022-1910(93)90051-R
Reeve HK (1991) Polistes. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 99–148
Röseler P-F, Röseler I (1989) Dominance of ovariectomized foundresses of the paper wasp, Polistes gallicus. Insect Soc 36:219–234
Röseler P-F, Röseler I, Strambi A, Augier R (1984) Influence of insect hormones on the establishment of dominance hierarchies among foundresses of the paper wasp, Polistes gallicus. Behav Ecol Sociobiol 15:133–142
Savoyard JL, Gamboa GJ, Cummings DLD, Foster RL (1998) The communicative meaning of body oscillations in the social wasp, Polistes fuscatus (Hymenoptera, Vespidae). Insect Soc 45:215–230
Schneider SS, Lewis LA, Huang ZY (2004) The vibration signal and juvenile hormone titers in worker honeybees, Apis mellifera. Ethology 110:977–985
Schwander T, Lo N, Beekman M et al (2010) Nature versus nurture in social insect caste differentiation. Trends Ecol Evol 25:275–282. doi:10.1016/j.tree.2009.12.001
Strassmann JE (1981) Wasp reproduction and kin selection: reproductive competition and dominance hierarchies among Polistes annularis foundresses. Fla Entomol 64:74–88. doi:10.2307/3494602
Strassmann JE, Hughes CR, Queller DC (1990) Colony defense in the social wasp, Parachartergus colobopterus. Biotropica 22:324–327. doi:10.2307/2388546
Suryanarayanan S, Jeanne RL (2008) Antennal drumming, trophallaxis, and colony development in the social wasp Polistes fuscatus (Hymenoptera: Vespidae). Ethology 114:1201–1209. doi:10.1111/j.1439-0310.2008.01561.x
Suryanarayanan S, Hantschel AE, Torres CG, Jeanne RL (2011a) Changes in the temporal pattern of antennal drumming behavior across the Polistes fuscatus colony cycle (Hymenoptera, Vespidae). Insect Soc 58:97–106. doi:10.1007/s00040-010-0122-1
Suryanarayanan S, Hermanson JC, Jeanne RL (2011b) A mechanical signal biases caste development in a social wasp. Curr Biol 21:231–235. doi:10.1016/j.cub.2011.01.003
Toth AL, Tooker JF, Radhakrishnan S et al (2014) Shared genes related to aggression, rather than chemical communication, are associated with reproductive dominance in paper wasps (Polistes metricus). BMC Genomics 15:75. doi:10.1186/1471-2164-15-75
Virant-Doberlet M, Cokl A (2004) Vibrational communication in insects. Neotrop Entomol 33:121–134
Volny VP, Gordon DM (2002) Genetic basis for queen–worker dimorphism in a social insect. Proc Natl Acad Sci 99:6108–6111. doi:10.1073/pnas.092066699
Weiner SA, Toth AL (2012) Epigenetics in social insects: a new direction for understanding the evolution of castes. Genet Res Int 2012:1–11. doi:10.1155/2012/609810
West-Eberhard MJ (1969) The social biology of polistine wasps. Museum of Zoology. University of Michigan, Ann Arbor
West-Eberhard MJ (1986) Dominance relations in Polistes canadensis (l.), a tropical social wasp. Ital J Zool 20:263–281. doi:10.1080/00269786.1986.10736502
Wheeler DE (1986) Developmental and physiological determinants of caste in social Hymenoptera: evolutionary implications. Am Nat 128:13–34
Woodring JP, Meier OW, Rose R (1988) Effect of development, photoperiod, and stress on octopamine levels in the house cricket, Acheta domesticus. J Insect Physiol 34:759–765. doi:10.1016/0022-1910(88)90149-7
Acknowledgments
We are grateful to the several students that helped with data collection in the field. We are particularly thankful to Alessia Uboni and Riccardo Pansini for the constructive discussion and helpful comments on previous drafts of this manuscript. We are very grateful to Dr. Vittorio Ducoli, former Director of the Parco Nazionale d’Abruzzo, Lazio e Molise, and to Dr. Cinzia Sulli, Servizio Scientifico Ambientale of the Parco Nazionale d’Abruzzo, Lazio e Molise, for permission to work in the park. Funding was obtained from the Italian Ministry of Education, University and Research (MIUR) (ex 60 % to M. C. L.).
Compliance with ethical standards
MM declares that he has no conflict of interest. MCL declares that she has no conflict of interest. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. T. Wcislo
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 347 kb)
Online Resource 2
(WMV 8261 kb)
Online Resource 3
(WMV 4358 kb)
Rights and permissions
About this article
Cite this article
Mignini, M., Lorenzi, M.C. Vibratory signals predict rank and offspring caste ratio in a social insect. Behav Ecol Sociobiol 69, 1739–1748 (2015). https://doi.org/10.1007/s00265-015-1986-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1986-x