Abstract
Behavior in eusocial insects likely reflects a long history of selection imposed by parasites and pathogens because the conditions of group living often favor the transmission of infection among nestmates. Yet, relatively few studies have quantified the effects of parasites on both the level of individual colony members and of colony success, making it difficult to assess the relative importance of different parasites to the behavioral ecology of their social insect hosts. Colonies of Polybia occidentalis, a Neotropical social wasp, are commonly infected by gregarines (Phylum Apicomplexa; Order Eugregarinida) during the wet season in Guanacaste, Costa Rica. To determine the effect of gregarine infection on individual workers in P. occidentalis, we measured foraging rates of marked wasps from colonies comprising both infected and uninfected individuals. To assess the effect of gregarines on colony success, we measured productivity and adult mortality rates in colonies with different levels of infection prevalence (proportion of adults infected). Foraging rates in marked individuals were negatively correlated with the intensity of gregarine infection. Infected colonies with high gregarine prevalence constructed nests with fewer brood cells per capita, produced less brood biomass per capita, and, surprisingly, experienced lower adult mortality rates than did uninfected or lightly infected colonies. These data strongly suggest that gregarine infection lowers foraging rates, thus reducing risk to foragers and, consequently, reducing adult mortality rates, while at the same time lowering per-capita input of materials and colony productivity. In infected colonies, queen populations were infected with a lower prevalence than were workers. Intra-colony infection prevalence decreased dramatically in the P. occidentalis population during the wet season.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Parasites and pathogens are hypothesized to have significant evolutionary effects on their hosts, including the maintenance of sex (Hamilton 1980; Seger and Hamilton 1988; Lively 1992), female choice (Hamilton and Zuk 1982; Möller 1990), and promotion of speciation (Shoemaker et al. 1999; Bordenstein et al. 2001). Social insects face especially strong selection from parasites and pathogens (hereafter referred to as parasites), because the high intra-colony genetic relatedness, close proximity of nestmates, and high frequency of interactions associated with social behavior often favor horizontal transmission within the colony (reviewed by Schmid-Hempel 1998; Naug and Camazine 2002; Pie et al. 2004; but see Traniello et al. 2002). Therefore, an understanding of the relationships between social insects and their parasites may be helpful in elucidating the adaptive value of social behavior (Schmid-Hempel 1995, 1998, 2001; O'Donnell 1997; Naug and Camazine 2002; Pie et al. 2004 and references therein).
Determining the importance of parasite virulence in coevolution with social insect hosts is often a complex process. Because selection by parasites acts primarily at the level of the colony (Schmid-Hempel 1998), it is not sufficient to find effects of infection at the level of individual colony members, since only if there are measurable effects at the colony level is a parasite likely to affect fitness. Further, even if deleterious effects are not detectable at the colony level, examining multiple stages of the host's colony cycle may be necessary to rule out strong virulence for a particular host/parasite relationship, since expression of parasite virulence in hosts can be colony-stage-specific (Brown et al. 2000, 2003). Relatively few host/parasite systems have been investigated for colony-level effects of parasite virulence or for effects in multiple colony stages (reviewed by Schmid-Hempel 1998), although several recent studies have begun to close this gap (honey bees [Tarpy 2003], bumble bees [Imhoof and Schmid-Hempel 1999; Brown et al. 2000, 2003], wood ants [Wenseleers et al. 2002] and dampwood termites [Rosengaus et al. 1998; Pie et al. 2004]).
Polybia occidentalis (Olivier) is a common social wasp of the American tropics that has been the subject of intensive research at both the level of individual behavior (task performance, age polyethism, dominance) and the colony (productivity, colony foraging rates, adult mortality rates, regulation of nest construction) (Jeanne 1991a, b, 1996, 1999; Jeanne and Nordheim 1996; O'Donnell and Jeanne 1992, 1995; O'Donnell 2001; Bouwma et al. 2003a, b; Howard and Jeanne 2004, 2005). Parasites are common in this species (Richards and Richards 1951; Parfin 1958; Richards 1978; Dejean and Canard 1990; London and Jeanne 1998), but little is known about their effects on individual behavior or colony success. Recent studies report that infection in P. occidentalis by an undescribed species of gregarine (Phylum Apicomplexa; Subclass Gregarinasina; Order Eugregarinida; Suborder Aseptatina) is associated with slower brood development (Howard and Jeanne 2004) and an increased proportion of empty brood cells in young nests (Howard and Jeanne 2005).
Gregarines are common and diverse protozoans that are exclusively parasitic on invertebrates, especially arthropods (Levine 1988; Roberts and Janovy 2000). In solitary insects, gregarines may be harmless commensals (Weiser and Briggs 1971) or debilitating but non-lethal parasites (reviewed by Zuk 1987a, b, 1988; Thomas et al. 1999; Aliabadi and Juliano 2002). Gregarines have been found in all of the four major groups of social insects: termites (Huger and Lenz 1976), ants (Jouvenaz and Anthony 1979; Crosland 1988; Kleespies et al. 1997; Buschinger and Kleespies 1999), bees (Stejskal 1955; Liu et al. 1974; Lipa and Triggiani 1992, 1996; Durrer and Schmid-Hempel 1995), and wasps (Richards and Richards 1951; Richards 1978; Rodrigues and Moraes 1981; Howard and Jeanne 2004, 2005; Kudô et al. 2004).
The goal of this study was to quantify the effects of gregarines at both the individual and colony level in the wasp P. occidentalis, and this is the first study to do so for a social insect. We tested for an effect of infection on behavior in individual workers by measuring foraging rates of both infected and uninfected marked foragers during nest construction. We tested for an effect of infection on colonies by measuring per-capita productivity and adult mortality rates of colonies with different levels of infection prevalence. We also tested for differences in infection prevalence between queen and worker populations within infected colonies and for changes in infection prevalence in colonies at our field site throughout the wet season.
Methods
Location
We conducted the field work at Finca Las Pumas (adjacent to the property formerly known as Centro Ecológico La Pacífica), located 5 km west of Cañas, Guanacaste, Costa Rica (10°25′N, 85°7′W). Cañas is located in the tropical dry forest (moist province transition) life zone (Tosi 1969). This region is marked by strong wet–dry seasonality, with the wet season extending from May into November. Mean annual rainfall at the site is 1430 mm (1921–1999; W Hagnauer, personal communication). Native vegetation is tropical dry forest, but at our site this has been widely replaced with pasture and cropland. Colonies of P. occidentalis commonly nest in shrubs and trees in pastures, hedgerows, and along roadsides. We conducted the foraging-rate study from July through August of 2002, the productivity and mortality study from June through September of 1998, 1999, and 2000, and the intra-colony infection prevalence study from June through December of 1999 and 2000.
Foraging-rate study
We located gregarine-infected colonies with a sampling procedure in which we placed a plastic bag over the entrance of the nest, and then alarmed the colony by lightly tapping the supporting twig until approximately 50 wasps flew from the nest into the bag. We sacrificed these individuals with ethyl acetate and then dissected them under a dissecting microscope to determine infection status (see below). After allowing the sampled colonies at least 2 weeks to recover from the loss of adults, we moved four infected colonies (colony 02108 = total 302 adults, 02101=174 adults, 02102=424 adults, 02107=533 adults) after dark to observation stands in order to facilitate observation and manipulation (O'Donnell 2001). We gave colonies at least 1 day to acclimate to the new nest site before further manipulation.
A few minutes before sunrise (approximately 05:30 h), we enclosed the nest in a plastic bag containing ether-soaked cotton in a film canister with a screen cap. The film canister prevented the wasps from touching the ether. The ether alarmed the colonies and the individuals that exited in response became lightly etherized in the bag. We then removed the bag from the nest and transferred it to the laboratory, where we divided the captured wasps into groups of 20 and stored them in a refrigerator. We lightly etherized each group a second time and marked the individuals on the thorax for individual recognition (DecoColor Opaque Paint Markers) using a color-coded numbering system. While the wasps were recovering from etherization, we gently dropped them back into the nest entrance with forceps. Occasionally the wasps did not remain sufficiently anesthetized after marking for reintroduction into the nest, and in these cases we briefly chilled them in the refrigerator first. Marking each colony took 7–12 h to complete, but we returned all individuals to their nest before nightfall.
We removed the lower envelope from the nests of the observation colonies during the afternoon of the day after marking. Envelope removal usually induces nest repair in P. occidentalis, facilitating observation of nest construction (Jeanne 1996). However, two of the four colonies (colonies 02108 and 02101) did not repair their damaged envelopes during 2 days of observation. In order to compel these colonies to engage in nest construction, we induced them to form absconding swarms by dismantling their nests (Jeanne 1991a; Jeanne and Nordheim 1996; Bouwma et al. 2000, 2003a, b). We then followed the swarms to their new nest sites and observed them as they constructed their new nests. (Swarm-founding species such as P. occidentalis initiate colonies as a swarm of queens and workers and emigrate and renest as a swarm when their nest is destroyed [Jeanne 1991a].) Nest construction behavior by founding swarms resembles behavior during nest repair in nearly every discernable way (Jeanne 1996).
We observed the four focal colonies during the morning and the afternoon for 2–3 days. Morning sessions ranged from 45 min to 3 h, and afternoon sessions ranged from 1 to 2 h, depending on weather. We did not observe colonies in the rain. P. occidentalis forages for nest materials (pulp or water) and food (nectar or prey) (O'Donnell and Jeanne 1990). We recorded the type of foraging trip and the number of trips for all marked foragers. In order to increase the number of marked foragers for colony 02107, we marked eight unmarked foragers (without anesthetization) by removing them from the nest envelope with reverse-grip forceps between observation periods. We then discontinued observations for at least 1 h after marking. In the evening of the final day of observations, we collected each colony with its nest. Escapees and any foragers that spent the night away from the nest clustered at the nest site the next morning and we added them to the collection.
Colony-productivity/adult-mortality study
We selected colonies for this study while blind to infection status. We located most of them on Finca Las Pumas property, but moved some there from Rancho Rey, approximately 5 km WNW of our site. In order to reset the colony cycle to the founding stage, we induced colonies to form absconding swarms by dismantling their nests. Resetting the colony cycle allowed us to remove the potentially confounding effect of differences in colony ontogeny on productivity (Jeanne and Nordheim 1996) and thus focus on the potential effect of parasites. We dismantled nests approximately 2 h before sunset, which allowed enough time for all the wasps to evacuate the nest and form a cluster, but not enough time for them to select a new site and emigrate before dark. We then censused the swarms the next day at dawn (see Bouwma et al. 2003a and b for census methods and a description of swarm emigration). After the census, we monitored the swarms until they emigrated later in the day and began building their new nests. If swarms departed the old nest site before 14:00 h on the day of swarm emigration, we counted that day as day 1 of colony development. If swarms moved after 14:00 h, we counted the next day as day 1.
In 1998 (N=13) and 1999 (N=41) we allowed each swarm to construct a nest at the new site and rear brood undisturbed for 25 days. In 2000 (N=12), we used the swarms in a concurrent study requiring a slightly longer developmental period for some colonies (mean collection date = 27 days, range = 25–29). Since swarms of this species complete the nest within 1–3 weeks (Forsyth 1978; Jeanne and Bouwma 2004), we assumed there was no bias in brood-cell number due to the extra developmental time allotted colonies in 2000. Because brood growth does continue after day 25, however, we did not include data from 2000 in the analysis of brood weight.
On the evening of the final day of growth allotted each colony, we collected the colony with its nest. We collected escapees and any foragers that spent the night away from the nest from the cluster they formed at the nest site the next morning. We counted the combs and the cells in each comb and removed the immature stages (eggs, larvae, and pupae) from all brood cells and preserved them in Kahle's fixative. Because none of the colonies had pupae in the latest stage of development when collected, we are confident that no adult offspring had yet eclosed, and, therefore that the producers (adults) remained distinct from the results of their efforts (nest and brood). We later vacuum-filtered each colony's brood on pre-weighed, oven-dried filter paper over a Büchner funnel to remove excess liquid. We then desiccated the brood and filter paper to uniform dryness in a vacuum oven at 60 °C and weighed them to the nearest 0.1 mg. We were not able to obtain brood weight data for one of the colonies in 1999 because the heat from a nearby light bulb killed the brood 1 day before collection.
Gregarine infection
To determine infection status for the foraging-rate study, we dissected all of the marked adults (N=546) under a dissecting microscope while still fresh. We identified gregarine infections by searching for the large, opaque spheres (gametocysts) that occur exclusively in the hemocoel of the gaster of infected wasps (AMB, personal observation). To provide a measure of the intensity of infection (number of parasites per individual) we counted the gametocysts in infected individuals, but because counting all gametocysts was extremely time consuming, we stopped after reaching 100. In order to determine the naturally occurring upper limit to infection intensity in infected wasps, we counted all of the gametocysts from random samples of infected individuals from colonies 99211, 99213, 99224, and 99331 (colony-productivity/adult-mortality study) in 1999 and from colony 02107 (foraging-rate study) in 2002 (N=112 individuals).
For the colony-productivity/adult-mortality study, we counted all adults in each colony and then dissected a random sample of at least 10% to determine infection status and reproductive condition. In 1999 and 2000, we dissected still-fresh females, while in 1998 we preserved the specimens in Kahle's fixative and dissected them later. We defined queens as those individuals having at least one opaque egg (egg with yolk) per ovary and sperm in the spermatheca, and determined insemination by visually inspecting the spermatheca under a dissecting microscope. Because of the difficulty of finding the spermatheca in preserved specimens, we did not verify insemination in 1998. We used the proportion of infected individuals in the sample we dissected as an estimate of intra-colony infection prevalence, the proportion of the adults (queens and workers) in the colony with at least one gregarine gametocyst. We did not measure infection intensity for each individual because the often large numbers of gametocysts present (100+) made this too time consuming.
Seasonal effects
In addition to the colonies that were used in the colony-productivity/adult-mortality study, we destructively sampled colonies throughout the wet season in 1999 (total N=54) and 2000 (total N=29) to test for population-level changes in intra-colony gregarine prevalence during the wet season at Finca Las Pumas. We dissected a sample of the adults from these colonies to estimate intra-colony prevalence as described above. We did not attempt to measure inter-colony gregarine prevalence (the proportion of colonies in the population containing at least one infected individual); therefore, all references to gregarine prevalence refer to intra-colony prevalence. For this study, we excluded colonies that were moved to Finca Las Pumas from Rancho Rey (two in 1998, four in 1999, none in 2000), because no Rancho Rey colonies were found to be infected during the period of high prevalence at Finca Las Pumas, suggesting that the Rancho Rey population was uninfected.
Analysis
Foraging rates
The observation colonies were not always engaged in nest construction during observation periods, but for the analysis we included data only from observation periods during which there was nest construction (27 total hours). The data for each marked wasp comprised one dependent variable (number of foraging trips) and three independent variables (number of gametocysts, colony ID, and observation time). We used Poisson rate regression analysis to test the null hypothesis of no effect of gametocyst number on foraging rates using PROC GENMOD in SAS version 8.2. The Poisson regression model allows hypothesis-testing in skewed distributions of count data (McCullagh and Nelder 1983). Since trips for pulp and water (nest materials) take much less time than trips for nectar and prey (food materials), and foragers tend to specialize on one of these two classes of materials (O'Donnell and Jeanne 1990; AMB, personal observation), we analyzed foraging trips for nest materials separately from trips for food materials to determine if gregarines had different effects on foraging for these two classes of materials. We also pooled the data from both classes of foraging trips and analyzed them together.
We fitted the tallies of trips for nest materials (N; pulp or water), trips for food (F; nectar or prey), and all foraging trips (T; pulp, water, nectar, and prey) recorded for marked individuals to separate Poisson rate regression models. For each model, we included the number of gametocysts (G gam), a class variable for colony (C), and an offset of the log of hours of observation. For the N and F models, we also included a class variable for material specialization (S), with a value of 1 for individuals observed foraging at least once for the opposite class of materials, and 0 for all others. The offset of the log of observation time controlled for the different lengths of observation periods for different marked wasps. The colony variable controlled for the potential effect of colony differences on foraging rates. The material-specialization variable controlled for the possibility that individuals would be less likely to be observed foraging for one class of materials because they had specialized on the other class of materials. We determined the significance of independent variables using a backwards elimination procedure. Preliminary models indicated that the data were overdispersed for Poisson analysis, so we corrected for this by scaling the standard errors and hypothesis tests by the fitted deviance.
Colony productivity
We used the number of brood cells built by the swarm as a measure of nest size and the dry weight of brood as a measure of brood productivity (Michener 1964; Jeanne and Nordheim 1996). We divided the two response variables, cell number and dry brood weight (mg), by the total number of adults on day 25 to yield per-capita measures of nest size and brood productivity. Nine colonies from 2000 developed for more than 25 days. For these, we used the daily hazard rates measured over their entire growth period to estimate the slightly larger adult population on day 25 of their development in order to calculate cells per capita (see Bouwma et al. 2003b for methods). The collection-date variable was the number of days between 30 June, the date of the earliest collection for the 3 years, and each colony's collection date.
In a multiple weighted regression (weighted by A squared), using a backwards elimination procedure, we fitted cells per capita (C pc) on infection prevalence (G prev), total adults in the colony on day 25 (A), the proportion of queens in the colony (Q), infection prevalence in queens (Q prev), collection date (D), a class variable for year (Y) and interaction terms (G prev×Y, A×Y, Q×Y, Q prev×Y, and D×Y) (N=13 for 1998, 41 for 1999, and 12 for 2000). We weighted by A squared because inspection of residual plots indicated greater variance in C pc for small values of A (Chatterjee et al. 2000).
In a second multiple regression, we fitted brood weight (mg) per capita (B pc) on the aforementioned independent variables (N=13 for 1998, 40 for 1999, and 0 for 2000). We did not weight by A squared for this model because variance was constant. We used PROC GLM in SAS version 8.2 for all multiple regression analyses.
Adult mortality rates
An emigration and its concomitant mortality always followed our censuses (Bouwma et al. 2003a). Because we were interested in adult mortality rates beginning with the arrival of the swarm at its new nest site, we corrected swarm size for losses accrued during the emigration day using methods detailed in Bouwma et al. (2003b). We calculated daily hazard rate (H d; the average daily probability that a colony member will die) from the total losses of adults between the first census and the census at colony collection, less estimated losses during the day of emigration (see Bouwma et al. 2003b for methods).
We fitted daily hazard rate (H d) on infection prevalence (G prev), colony size after emigration (N 0), the proportion of infected queens in the colony (Q prev), collection date (D), a class variable for year (Y), and interaction terms for G prev×Y, N 0×Y, Q prev×Y, and D × Y in multiple regression using a backwards elimination procedure (N=13 for 1998, 41 for 1999, and 12 for 2000).
Intra-colony infection prevalence in queens versus workers
We tested for a difference in infection prevalence between the queen and worker populations of infected colonies collected during a period of high gregarine prevalence (June through August of 1999 and 2000). We performed a paired analysis by calculating the difference in prevalence between the queens (Q prev) and workers (W prev) from each colony (N =59 colonies) and then performing a Wilcoxon sign-rank test (H 0: Q prev−W prev=0) using PROC UNIVARIATE in SAS version 8.2.
Seasonal effects
We used regression analysis to determine whether intra-colony infection prevalence in the population changed throughout the wet season. Collection-date variables (D) for each colony were as described above, except that we used a different day 0 for each of the 2 years (6 July for 1999 and 30 June for 2000). We fitted the proportion of infected adults in each colony (G prev) to regression models separately for 1999 and 2000, because the 2000 data appeared curvilinear on inspection of residual plots, while the 1999 data fit the assumptions of a simple linear model. We fitted the 1999 data to a multiple regression with D and a class variable for a putative outlier colony (O) (see Jeanne and Nordheim 1996 for a description of the outlier test), and the 2000 data to a multiple regression model with D and a quadratic term, D 2.
Results
Infection intensity
In the 112 infected wasps that we dissected and counted all gametocysts, the mean number of gametocysts was 48.4±48.5 SD and the range was 1–264 (Fig. 1). Wasps with approximately 70 gametocysts or more had noticeably distended gasters so that gametocysts were visible through the inter-segmental membranes (under a dissecting microscope).
Foraging rates
In colony 02108, 68 out of the 177 (0.38) marked individuals were infected with at least one gametocyst in the hemocoel, colony 02101, 8 out of 65 (0.12), colony 02102, 21 out of 78 (0.27), and colony 02107, 59 out of 226 (0.26). In the Poisson rate regression model for nest-material foraging, the rate of foraging for pulp and water correlated negatively with the number of gametocysts (G gam) (P<0.01) in marked individuals from these colonies (Table 1a; Fig. 2a). The significant class variable for colony (C) (P=0.04) allowed for different intercepts for different observation colonies. The significant class variable for material specialization (S) (P<0.01) allowed for a larger intercept for the group of individuals that had been observed foraging at least once for food materials. In a separate Poisson rate regression model for food foraging, the rate of foraging for nectar and prey also correlated negatively with the number of gametocysts (G gam) (P<0.001) (Table 1b; Fig. 2b). However, neither the colony variable (C) (P=0.12) nor the specialization variable (S) were significant (P=0.70). Not surprisingly, the Poisson rate regression model for the pooled data followed the same pattern as for N and F (Table 1c). Foraging rates for all materials (T) correlated negatively with gametocysts (G gam) (P<0.001), but there was not a significant colony effect (C) (P =0.08).
Mean foraging trips per hour made by marked P. occidentalis workers from four different colonies as a function of gregarine infection intensity (number of gregarine gametocysts in the gaster). 0=uninfected, 20=1−20 gametocysts, 40=21−40, 60=41−60, 80=61−80, 100=81−100+. Error bars indicate one standard error of the mean. Numbers above error bars indicate sample sizes (total N=546). a Mean pulp and water trips per hour. b Mean nectar and prey trips per hour
Colony productivity
Gregarine prevalence (G prev), year (Y) and collection date (D) were significant predictors of brood cells per capita and gave a model with an adjusted R 2 of 0.57 (Table 2a). All terms were significant at alpha=0.01, but infection prevalence explained most of the variance in brood cells per capita. The model included different intercepts, but the same slope for the 3 years. Gregarine prevalence (G prev), year (Y), a G prev×Y interaction, and collection date (D) were significant predictors of brood weight (mg) per capita, giving a model with an adjusted R 2 of 0.51 (Table 2b). As for cells per capita, all terms were significant at alpha =0.01, but infection prevalence explained most of the variance. The model included different intercepts and different slopes for the 2 years. For both models (brood cells and brood weight), gregarine infection and collection date reduced per-capita productivity.
Adult mortality rates
In multiple regression, the proportion of the colony infected by gregarines (G prev), year (Y), the total adults in the colony on day 1 (N 0), and collection date (D) were significant predictors of daily hazard rate and gave a model with an adjusted R 2 of 0.38 (Table 3). As with brood weight and cell number, while all terms were significant (alpha =0.05) gregarine infection explained most of the variance. The model included different intercepts, but the same slope for each of the 3 years. Gregarine infection prevalence, colony size, and collection date all reduced adult mortality rates.
Infection of queens versus workers
In infected colonies, the mean infection prevalence was higher for workers (0.68±0.24 SD) than for queens (0.18±0.31 SD). We rejected the null model of no difference between the paired infection prevalence of queens and workers within infected colonies (Wilcoxon sign-rank test; S=770, P<0.0001).
Seasonal effects
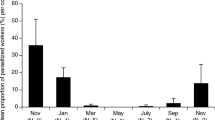
Intra-colony infection prevalence in 1999 declined from the early rainy season to the final sampling period in mid-November (late wet season) (first-order model, adjusted R 2 =0.79; Table 4a; Fig. 3a). Residual plots showed colony 99322 to be a potential outlier. We collected this colony on 9 November when it had a much higher gregarine prevalence (0.67) than predicted by the model. The outlier test was significant, yielding a P-value of 0.016 with Bonferroni correction for selection bias. Removing colony 99322 improved the adjusted R 2 from 0.73 to 0.79, but had no qualitative effect on the model. In 2000, intra-colony infection prevalence began the rainy season low, increased in mid-wet season, then declined in the late wet season (second-order model with D and D 2, adjusted R 2 =0.56; Table 4b; Fig. 3b).
a For 1999 gregarine infection prevalence in P. occidentalis colonies (N=54) as a function of their collection date (D; the number of days since the collection of the first colony from the sample, 6 July 1999). Colony 99322 was an outlier in the analysis and was excluded from the regression model. b For 2000 gregarine infection prevalence in P. occidentalis colonies (N=29) as a function of their collection date (D; the number of days since 30 June 2000) and collection date squared (D2)
Discussion
Foraging rates
Foraging rates by marked wasps were negatively correlated with the intensity of their gregarine infections, and the result was the same whether the data were pooled or analyzed separately for food trips and nest-material trips. Independent of the effect of gregarines, wasps from colony 02101 foraged at higher rates for nest materials than did wasps from colony 02107 (the reference colony in the Poisson model), while nest-material-foraging rates in colonies 02108 and 02102 were not significantly different from colony 02107. The cause of this difference is not known, but it may relate to colony 02101 having been engaged in the construction of a new nest while colony 02107 was repairing its nest. Individuals that had been observed making at least one trip for food materials, foraged for nest materials at higher rates than those that had not been observed foraging for food. This result was the opposite of what we anticipated, since O'Donnell and Jeanne (1990) observed very high rates of specialization by P. occidentalis foragers on one of these two classes of materials. However, switching between classes of materials does occur at low rates (O'Donnell and Jeanne 1990), and in our study marked wasps switched between classes of materials enough for the specialization term to be significant as a negative coefficient.
The lower foraging rates observed for infected individuals coupled with the lower adult mortality rates in colonies with high gregarine prevalence suggest that gregarine infection disrupts the progression of individuals through the age polyethism (a process in which individuals switch from nest work to foraging as they age), so that infected wasps are less likely to become foragers, which reduces their risk of dying. An alternative explanation for the lack of foraging behavior in infected wasps is that it is an artifact of age polyethism rather than due to parasite infection. This would be possible if the younger individuals (not yet forager age) harbor gregarines and the older individuals, the foragers, lose them. However, this explanation is implausible since it is highly unlikely that any insect could rid itself of large gregarine gametocysts from the hemocoel, such as those observed in P. occidentalis (J. Janovy Jr, personal communication). It is not known whether the gregarine-infected adults that did not forage remained entirely idle or actively engaged in other tasks inside the nest.
The reduced foraging in infected wasps is clearly an example of a parasite-induced change in behavior, while the exact fitness consequences for the parasite and host are less clear. For any parasite-induced change in behavior there are three basic possibilities: (i) The change in behavior could provide a benefit to the parasite, (ii) it could be a defensive response and thus benefit the host, or (iii) it could be an incidental byproduct of pathology and benefit neither (Combes 2001; Moore 2002). In order to determine if altered foraging behavior in P. occidentalis benefits either the parasite or host, we need to identify the mode of parasite transmission, which is not known for wasp gregarines. Gregarines have direct life cycles and are not transmitted vertically (Roberts and Janovy 2000). Therefore, if foraging less and spending more time in the nest by P. occidentalis benefited the gregarines it would have to come through increased horizontal transmission. However, it is unlikely that transmission occurs from living wasps since the gametocysts are found exclusively in the hemocoel and thus can escape into the environment only through the death and dismemberment of the host (J. Janovy Jr, personal communication). Further, there is little evidence that P. occidentalis adults die in the nest (AMB, personal observation; RLJ, personal observation). While we will need to know the mode of gregarine transmission in order to fully understand this host/parasite relationship, the parsimonious explanation for the reduced foraging we observed in infected P. occidentalis workers is that it occurs as a byproduct of parasite-induced pathology and, while detrimental to host fitness, it neither benefits nor inhibits parasite transmission.
Colony productivity and adult mortality
Infection prevalence, year, and collection date all had significant effects on brood cells per capita constructed by swarms. Since low individual foraging rates for nest materials are correlated with gregarine infection, the negative effect of gregarines is likely due to colonies with high infection prevalence making fewer foraging trips per capita for nest materials during nest construction, and thus producing smaller nests (fewer brood cells per capita) relative to lightly infected and uninfected colonies. The result that foraging rates of marked individuals in the foraging-rate study were significantly correlated with the number of parasites per individual also suggests that, had we been able to collect those data, incorporating a measure of average infection intensity in colonies into the models for per-capita productivity and adult mortality could have reduced the unexplained variance. The significance of the year term indicates that, after accounting for the effect of gregarines, colonies built the smallest nests per capita in 1999 and the largest nests per capita in 1998. The negative collection date term indicates that colonies also constructed slightly smaller nests per capita later in the sampling period. The causes for the year and collection date effects are unknown, but it is clear that these effects were small relative to the effect of gregarines. The lack of a significant colony-size effect is at odds with Michener's (1964) finding for some social insects of decreasing per-capita productivity with increasing colony size, and with the positive colony-size effect observed by Jeanne and Nordheim (1996) for P. occidentalis.
The per-capita dry weight of the brood reared in nests constructed by the swarms showed a significant relationship with infection prevalence, year, infection prevalence × year interaction, and collection date. Infection prevalence was the most important factor affecting colony brood productivity, but the mechanism for lower per-capita brood productivity with increasing gregarine infection is unclear. Infected workers forage for nectar and prey (prey is fed to the larvae) at lower rates than uninfected workers, suggesting that reduced per-capita brood output in colonies with high infection prevalence was due to lower provisioning rates per larva. However, Howard and Jeanne (2005) found that colony-wide foraging rates per larva did not correlate with gregarine infection. This suggests that per-capita brood output in colonies with high gregarine prevalence was more constrained by the smaller nests they constructed in which to rear their brood, than by lower per-larva food foraging rates.
As was true for cells per capita, colonies reared greater brood biomass per capita in 1998 than in 1999. The significant interaction term indicates that the negative effect of infection prevalence on per-capita brood output was stronger in 1998 than 1999. Colonies also reared smaller brood populations per capita later in the field period. As for cells per capita, the causes of the year and collection date effects on brood weight are unknown, but may relate to year-to-year and seasonal variance in food availability. Just as for cells per capita, colony size had no effect on brood weight per capita, which does not support either the Michener (1964) pattern or Jeanne and Nordheim (1996) findings for P. occidentalis.
Colonies that had higher gregarine prevalence, larger colonies, and colonies collected later in the season, experienced the lowest adult mortality rates. The year effects for adult mortality were the same as for per-capita productivity: independent of gregarine infection, colonies collected in 1998 experienced the highest mortality rates and colonies collected in 1999 had the lowest mortality rates. The mechanisms for the colony-size, year, and collection-date effects on mortality rates are unknown. As discussed above, the unexpected result of lower mortality rates in colonies with more infected individuals, coupled with the lower foraging rates observed for infected individuals, strongly suggests that colonies with high gregarine prevalence simply make fewer foraging trips per capita. Because foraging is the riskiest task (O'Donnell and Jeanne 1992), this results in lower adult mortality rates relative to colonies with lower gregarine prevalence.
Seasonal effects
Gregarine intra-colony infection prevalence was not constant throughout the wet season in the Finca Las Pumas population of P. occidentalis during 1999 or 2000. In 1999, infection prevalence started high in July (day 0=6 July) and then decreased by November (late wet season) when some colonies yielded population samples without a single infected adult. We started sampling 6 days earlier in 2000 (day 0=30 June) and found infection prevalence to be low. Prevalence then increased to high levels by the end of July, similar to those observed in 1999, and again decreased substantially by late-November. The pattern of infection prevalence in the dry season (January–April) is not known.
Because the mode of transmission is not known, there are no data bearing directly on the mechanism for the observed change in infection prevalence over the wet season. The fact that in both 1999 and 2000 all colonies collected in August were infected, while by November infection prevalence was universally low, argues against the possibility that colonies with high gregarine prevalence are not surviving to the end of the wet season. Rather, the decline in infection prevalence with season appears to be an intra-colony phenomenon.
In Polybia paulista, Kudô et al. (2004) found that adults infected by gregarines were larger than uninfected adults, indicating that those wasps became infected as larvae. In P. occidentalis, we have observed gametocysts in pupae and newly emerged adults early in the wet season; however, dissection data suggest that larvae do not become infected during the mid-wet season (July and August; AMB, unpublished data). The observed decline in intra-colony prevalence could be produced if larvae became infected only at the end of the dry season and/or beginning of the wet season. As adults these individuals would be more likely to remain in the nest, thus reducing their risk of mortality due to predation relative to uninfected nestmates, and colony infection prevalence would decline during the wet season due to the slow attrition of these infected wasps without recruitment of new infections into the worker population.
Colony 99322 was an outlier in the regression model for 1999 with high infection prevalence (0.67) on 9 November when all of the other colonies collected had either low prevalence or were uninfected. The colony appeared to be close to failure, comprising only 23 workers, 4 queens, and almost no brood. Only scattered cells contained meconia (larval feces), indicating that relatively few adults had been produced. The colony probably remained with a high prevalence late in the wet season because its rate of production of adults (uninfected) was so low.
An interesting outcome of the seasonal decrease in gregarine prevalence is that colonies are infected at much lower levels when they reproduce at the end of the wet season (Forsyth 1978). However, heavy infections early in the season still must have a negative effect on fitness via smaller mature colony size as a consequence of reduced productivity and retarded growth. Such colonies would produce smaller and/or fewer reproductive swarms relative to colonies that remained uninfected or lightly infected through their growth cycle and thus achieved larger mature colony sizes.
Gregarine prevalence was significantly lower in queen populations than in their worker nestmates. The proximate cause for this difference is unknown, but one possibility is that infected queens are eliminated by the workers. In swarm-founding wasps the workers reduce queen number throughout the colony cycle by challenging queens, presumably selecting the least fecund and eliminating them (West-Eberhard 1978). If gregarines in queens cause reduced fecundity as do microsporidia in honey bee and fire ant queens (Bailey and Ball 1991; Williams et al. 1999) it is possible that infected queens are eliminated at a higher rate during this queen selection process. Alternatively, gregarine prevalence may differ between queens and workers simply because they are produced at different times of the year. Queens in colonies collected during the early to mid-wet season were mostly produced in the late wet season the previous year (Forsyth 1978), when gregarine prevalence is low. The workers have a 3- to 4-week lifespan (O'Donnell and Jeanne 1992); therefore those in our samples were produced much closer to the collection date than were queens.
Conclusions
In P. occidentalis, foraging rates in individual workers are negatively correlated with the intensity of their gregarine infections, and this likely induces the colony-level effects of reduced per-capita productivity and lower adult mortality rates observed in colonies with high infection prevalence. Howard and Jeanne (2005) showed that foraging rates at the colony level in young P. occidentalis colonies are tightly correlated with the number of larvae in the nest, but that foraging rates per larva did not correlate with gregarine prevalence. This suggests that even colonies with high infection prevalence are able to sufficiently provision the larvae that they produce. One possible scenario is that in response to demand from larvae, uninfected workers in parasitized colonies increase foraging rates, thus compensating for low foraging rates in their infected nestmates. If so, the negative effect of gregarines on per-capita brood production may be a more direct result of low foraging rates during initial nest construction than of low foraging rates per larva. One likely mechanism is that, by packing the gaster with gametocysts, gregarine infections reduce crop volumes of pulp and water foragers such that their water loads are small. Therefore, colonies with high gregarine prevalence may build smaller nests per capita during the initial nest construction because they have a lower proportion of competent foragers. Per-capita brood production would thus be limited from then on by the size of the nest. Through colony development, these colonies could maintain normal foraging rates per larva, but low foraging rates per capita, resulting in low adult mortality rates. Additionally, Howard and Jeanne (2005) found a greater proportion of empty brood cells in colonies with high gregarine prevalence, which suggests that gregarine infection may also reduce productivity by reducing oviposition rates and/or increasing the consumption of eggs by workers.
Notwithstanding the marked decrease in infection prevalence before colony reproduction, the reduction in colony productivity associated with high infection prevalence probably results in colonies' producing smaller and/or fewer reproductive swarms and having reduced fitness. To the best of our knowledge, this is the only social insect in which both significant individual- and colony-level effects from gregarine infection have been demonstrated. As has been hypothesized for the microsporidia in fire ants and honey bees (Schmid-Hempel 1998; Naug and Camazine 2002), trypanosomes in bumble bees (Shykoff and Schmid-Hempel 1991; Brown et al. 2000, 2003) and for entomopathogenic fungi in dampwood termites (Traniello et al. 2002), our data suggest that gregarines may be an important source of selection in the behavioral ecology and life history of P. occidentalis. Since gregarines have been observed in four other genera of social wasps (Richards and Richards 1951; Richards 1978; AMB, unpublished data), it is likely other social wasp species are similarly affected. Clearly, any future studies measuring foraging, mortality, colony productivity, or fitness in social wasps must consider the potential influence of gregarines.
References
Aliabadi BW, Juliano SA (2002) Escape from gregarine parasites affects the competitive interactions of an invasive mosquito. Biol Invasions 4:283–297
Bailey L, Ball BV (1991) Honey bee pathology, 2nd edn. Academic Press, London, UK
Bordenstein SR, O'Hara FP, Werren JH (2001) Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 409(6821):707–710
Bouwma PE, Bouwma AM, Jeanne RL (2000) Social wasp swarm emigration: males stay behind. Ethol Ecol Evol 12:35–42
Bouwma AM, Bouwma PE, Nordheim EV, Jeanne RL (2003a) Founding swarms in a tropical social wasp: adult mortality, emigration distance, and swarm size. J Insect Behav 16:439–452
Bouwma AM, Bouwma PE, Nordheim EV, Jeanne RL (2003b) Adult mortality rates in young colonies of a swarm-founding social wasp. J Zool 260:11–16
Brown MJF, Loosli R, Schmid-Hempel P (2000) Condition-dependent expression of virulence in a trypanosome infecting bumblebees. Oikos 91:421–427
Brown MJF, Schmid-Hempel R, Schmid-Hempel P (2003) Strong context-dependent virulence in a host–parasite system: reconciling genetic evidence with theory. J Anim Ecol 72:994–1002
Buschinger A, Kleespies RG (1999) Host range specificity of an ant-pathogenic gregarine parasite Mattesia geminata (Neogregarinida: Lipotrophidae). Entomol Gen 24:93–104
Chatterjee S, Hadi AS, Price B (2000) Regression analysis by example, 3rd edn. Wiley, New York, NY
Combes C (2001) Parasitism: the ecology and evolution of intimate interactions. University of Chicago Press, Chicago, IL
Crosland MWJ (1988) Effect of a gregarine parasite on the color of Myrmecia pilosula (Hymenoptera: Formicidae). Ann Entomol Soc Am 81:481–484
Dejean A, Canard M (1990) Reproductive behaviour of Trichoscelia santareni (Navas) (Neuroptera: Mantispidae) and parasitization of the colonies of Polybia diguetana R. du Buysson (Hymenoptera: Vespidae). Neuroptera Int 6:19–26
Durrer S, Schmid-Hempel P (1995) Parasites and the regional distribution of bumble bee species. Ecography 18:114–122
Forsyth AB (1978) Studies on the behavioral ecology of polygynous social wasps. PhD Dissertation, Harvard University, Cambridge, MA
Hamilton WD (1980) Sex vs. non-sex vs. parasite. Oikos 35:282–290
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Howard KJ, Jeanne RL (2004) Rates of brood development in a social wasp: effects of colony size and parasite infection. Insectes Soc 51:179–185
Howard KJ, Jeanne RL (2005) Shifting foraging strategies in colonies of the social wasp Polybia occidentalis (Hymenoptera: Vespidae). Behav Ecol Sociobiol 57:481–489
Huger AM, Lenz M (1976) Occurrence of a coelomic gregarine (Protozoa: Sporozoa) with the Australian termite Coptotermes acinaciformis (Isoptera: Rhinotermitidae). Zeitschrift für Angewandte Entomologie 81:252–258
Imhoof B, Schmid-Hempel P (1999) Colony success of the bumble bee, Bombus terrestris, in relation to infections by two protozoan parasites, Crithidia bombi and Nosema bombi. Insectes Soc 46:233–238
Jeanne RL (1991a) The swarm-founding Polistinae. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 191–231
Jeanne RL (1991b) Polyethism. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 389–425
Jeanne RL (1996) Regulation of nest construction behaviour in Polybia occidentalis. Anim Behav 52:473–488
Jeanne RL (1999) Group size, productivity, and information flow in social wasps. In: Detrain C, Pasteels JM, Deneubourg JL (eds) Information processing in social insects. Birkhäuser Verlag, Basel, pp 3–30
Jeanne RL, Nordheim EV (1996) Productivity in a social wasp: per capita output increases with swarm size. Behav Ecol 7:43–48
Jeanne RL, Bouwma AM (2004) Divergent patterns of nest construction in eusocial wasps. J Kan Ent Soc 77:429–447
Jouvenaz DP, Anthony DW (1979) Mattesia geminata sp. n. (Neogregarinida: Ophrocystidae) a parasite of the tropical fire ant, Solenopsis geminata (Fabricius). J Protozool 26:354–356
Kleespies RG, Huger AM, Buschinger A, Nähring S, Schumann RD (1997) Studies on the life history of a neogregarine parasite found in Leptothorax ants from North America. Biocont Sci Technol 7:117–129
Kudô K, Yamane So, Mateus S, Tsuchida K, Itô Y, Miyano S, Zucchi R (2004) Parasitism affects worker size in the Neotropical swarm-founding wasp, Polybia paulista (Hymenoptera, Vespidae). Insectes Soc 51:221–225
Levine ND (1988) The protozoan phylum of Apicomplexa, vol. I. CRC Press, Boca Raton, FL
Lipa JJ, Triggiani O (1992) A newly recorded neogregarine (Protozoa, Apicomplexa), parasite in honey bees (Apis mellifera) and bumble bees (Bombus spp.). Apidologie 23:533–536
Lipa JJ, Triggiani O (1996) Apicystis gen. nov. and Apicystis bombi (Liu, Macfarlane & Pengelly) comb. nov. (Protozoa: Neogregarinida), a cosmopolitan parasite of Bombus and Apis (Hymenoptera: Apidae). Apidologie 27:29–34
Liu HJ, Macfarlane RP, Pengelly DH (1974) Mattesia bombi n. sp. (Neogregarinida: Ophrocystidae), a parasite of Bombus (Hymenoptera: Apidae). J Invert Pathol 23:225–231
Lively CM (1992) Parthenogenesis in a freshwater snail: reproductive assurance versus parasitic release. Evolution 46:907–913
London KB, Jeanne RL (1998) Envelopes protect social wasps' nests from phorid infestation (Hymenoptera: Vespidae, Diptera: Phoridae). J Kan Ent Soc 71:175–182
McCullagh P, Nelder JA (1983) Generalized linear models. Chapman and Hall, London, UK
Michener CD (1964) Reproductive efficiency in relation to colony size in hymenopterous societies. Insectes Soc 11:317–342
Möller AP (1990) Effects of a hematophagous mite on the barn swallow (Hirundo rustica)—A test of the Hamilton and Zuk hypothesis. Evolution 44:771–784
Moore J (2002) Parasites and the behavior of animals. Oxford University Press, Oxford, UK
Naug D, Camazine S (2002) The role of colony organization on pathogen transmission in social insects. J Theor Biol 215:427–439
O'Donnell S, Jeanne RL (1990) Forager specialization and the control of nest repair in Polybia occidentalis Olivier (Hymenoptera: Vespidae). Behav Ecol Sociobiol 27:359–364
O'Donnell S, Jeanne RL (1992) Life-long patterns of forager behaviour in a tropical swarm-founding wasp: effects of specialization and activity level on longevity. Anim Behav 44:1021–1027
O'Donnell S, Jeanne RL (1995) Implications of senescence patterns for the evolution of age polyethism in eusocial insects. Behav Ecol 6:269–273
O'Donnell S (1997) How parasites can promote the expression of social behaviour in their hosts. Proc R Soc Lond B 264:689–694
O'Donnell S (2001) Worker biting interactions and task performance in a swarm-founding eusocial wasp (Polybia occidentalis, Hymenoptera: Vespidae). Behav Ecol 12:353–359
Parfin S (1958) Notes on the bionomics of the Mantispidae (Neuroptera: Planipennia). Entomol News 69:203–207
Pie MR, Rosengaus RB, Traniello JFA (2004) Nest architecture, activity pattern, worker density and the dynamics of disease transmission in social insects. J Theor Biol 226:45–51
Richards OW, Richards MJ (1951) Observations on the social wasps of South America (Hymenoptera: Vespidae). Trans R Entomol Soc Lond 102:1–170
Richards OW (1978) The social wasps of the Americas, excluding the Vespinae. British Museum of Natural History, London, UK
Roberts LS, Janovy JJ (2000) Gerald D. Schmidt & Larry S. Roberts' foundations of parasitology, 6th edn. McGraw-Hill, Boston, MA
Rodrigues VM, de Moraes RAO (1981) Social wasps—observations on Polybia (Apopolybia) jurinei de Saussure, 1854 (Polistinae – Polybiini). An Soc Entomol Brasil 10:3–7
Rosengaus RB, Maxmen AB, Coates LE, Traniello JFA (1998) Disease resistance: a benefit of sociality in the dampwood termite Zootermopsis angusticollis (Isoptera:Termopsidae). Behav Ecol Sociobiol 44:125–134
Schmid-Hempel P (1995) Parasites and social insects. Apidologie 26:255–271
Schmid-Hempel P (1998) Parasites in social insects. Princeton University Press, Princeton, NJ
Schmid-Hempel P (2001) On the evolutionary ecology of host-parasite interactions: addressing the question with regard to bumblebees and their parasites. Naturwissenschaften 88:147–158
Seger J, Hamilton WD (1988) Parasites and sex. In: Michod RE, Levin BR (eds) The evolution of sex. Sinauer, Sunderland, MA, pp 176–193
Shoemaker DD, Katju V, Jaenike J (1999) Wolbachia and the evolution of reproductive isolation between Drosophila recens and Drosophila subquinaria. Evolution 53:1157–1164
Shykoff JA, Schmid-Hempel P (1991) Parasites delay worker reproduction in the bumblebee: consequences for eusociality. Behav Ecol 2:242–248
Stejskal M (1955) Gregarines found in the honey bee (Apis mellifera Linnaeus) in Venezuela. J Protozool 2:185–188
Tarpy DR (2003) Genetic diversity within honeybee colonies prevents severe infections and promotes colony growth. Proc R Soc Lond B Bio 270:99–103
Thomas F, Oget E, Gente P, Desmots D, Renaud F (1999) Assortative pairing with respect to parasite load in the beetle Timarcha maritima (Chrysomelidae). J Evol Biol 12:385–390
Tosi JAJ (1969) Ecological map of Costa Rica. Tropical Science Center, San Jose, Costa Rica
Traniello JFA, Rosengaus RB, Savoie K (2002) Group living enhances immunity in a social insect. Proc Soc Natl Acad Sci USA 99:6838–6842
Weiser J, Briggs JD (1971) Identification of pathogens. In: Burges HD, Hussey NW (eds) Microbial control of insects and mites. Academic Press, London, pp 13–66
Wenseleers T, Sundstrom L, Billen J (2002) Deleterious Wolbachia in the ant Formica truncorum. Proc R Soc Lond B Bio 269:623–629
West-Eberhard MJ (1978) Temporary queens in Metapolybia wasps: non-reproductive helpers without altruism? Science 200:441–443
Williams DF, Oi DH, Knue GJ (1999) Infection of red imported fire ant (Hymenoptera: Formicidae) colonies with the entomopathogen Thelohania solenopsae (Microsporidia: Thelohaniidae) J Econ Entomol 92:830–836
Zuk M (1987a) The effects of gregarine parasites, body size, and time of day on spermatophore production and sexual selection in field crickets. Behav Ecol Sociobiol 21:65–72
Zuk M (1987b) The effects of gregarine parasites on longevity, weight loss, fecundity and developmental time in the field crickets Gryllus veletis and G. pennsylvanicus. Ecol Entomol 12:349–354
Zuk M (1988) Parasite load, body size, and age of wild-caught male field crickets (Orthoptera: Gryllidae): effects on sexual selection. Evolution 42:969–976
Acknowledgements
Our thanks go to Paul Hanson, curator of the Museo de Insectos of the Universidad de Costa Rica–San Pedro, for providing us with chemicals, for logistical support, and for the loan of specimens. We collected all specimens under the auspices of the Escuela de Biología and the Museo de Insectos of the Universidad de Costa Rica. We are grateful to the Hagnauer family in Costa Rica for permission to work on their property and for providing constant logistical support. Peter Bouwma, Andrew Carmichael, Kory Kramer and Miranda Moon provided invaluable assistance with data collection. Adam Smith shared methods for etherizing and marking wasps. Sean O'Donnell shared methods and provided valuable feedback on the ideas herein. Kevin Gross and Erik Nordheim provided statistical advice. James Traniello and two anonymous reviewers suggested ways to improve the manuscript. John Janovy Jr determined the gregarines as an undescribed species and was helpful and generous with his time in discussions of these results. This research was supported by National Science Foundation grant IBN-9514010 to RLJ, a Smithsonian Tropical Research Institute Short-Term Fellowship to AMB, University of Wisconsin–Madison Department of Zoology research grants to AMB and KJH, a National Science Foundation Pre-doctoral Fellowship to KJH, and by the College of Agricultural and Life Sciences, University of Wisconsin–Madison. The experiments performed comply with the current laws of Costa Rica.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. F. A. Traniello
An erratum to this article can be found at http://dx.doi.org/10.1007/s00265-006-0205-1
Rights and permissions
About this article
Cite this article
Bouwma, A.M., Howard, K.J. & Jeanne, R.L. Parasitism in a social wasp: effect of gregarines on foraging behavior, colony productivity, and adult mortality. Behav Ecol Sociobiol 59, 222–233 (2005). https://doi.org/10.1007/s00265-005-0028-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0028-5