Abstract
Certain epithelia secrete HCO3− to drive fluid secretion, to modify luminal pH and properties of secreted mucus, and to fulfill other functions of a given epithelium. Dysregulation of HCO3− secretion can lead to conditions such as malabsorption, acid/base disturbances, cystic fibrosis, biliary cirrhosis, peptic, and duodenal ulcers. In addition to the transport of HCO3− across the epithelium, epithelial cells also need to maintain intracellular pH, despite significant HCO3− extrusion and sometimes even despite exposure to external acid. In this chapter, we will introduce the main plasma membrane acid/base transporters and describe their role in general cellular homeostasis. The same transporters are also used in building the molecular machinery for vectorial HCO3− transport, i.e., bicarbonate secretion. We will highlight HCO3− secreting epithelia by examples from the digestive system (pancreas, salivary glands, hepatobiliary system, and duodenum), the renal collecting duct B-intercalated cell, as well as the choroid plexus epithelium of the brain. We seek an integrative approach to understand the HCO3− secretion processes by combining historical perspectives with molecular and genetic studies as well as studies of selected regulatory systems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
12.1.1 Overview
A number of epithelia in our body secrete significant amount of HCO3−, which is often accompanied by fluid secretion. One of the important early observations was made on the pancreas, which secretes pancreatic juice rich in HCO3− and poor in Cl−, a relation between two anions that together with later studies on isolated pancreatic duct epithelium became important steps for understanding general cellular models for HCO3− secretion (Fig. 12.1a and b). The purpose of epithelial HCO3− secretion is manifold, as presented by examples of epithelia chosen for this chapter. For example, HCO3− secretion can set extracellular pH, buffer and protect cells against acids produced and secreted by cells during digestive or metabolic processes, and solubilize proteins and other macromolecules. Dysregulation of these processes can lead to serious diseases such as cystic fibrosis, biliary cirrhosis, peptic, and duodenal ulcers. In addition to transporting significant amounts of HCO3− from interstitium to lumen, epithelia face another major challenge—they have to defend their intracellular pH (pHi). This fact is a challenge to scientists, as it is often difficult to study and separate the transepithelial acid/base transport as opposed to the transport across the single plasma cell membrane exerted for the purpose of pHi regulation. In the first part of the chapter, we will introduce the main H+/HCO3− transporters and describe their role in general cellular acid/base homeostasis. These “building blocks” will then be used to equip epithelial cells so that they can perform vectorial HCO3− transport, i.e., secretion. Other ion channels and transporters necessary for overall transepithelial HCO3− transport will be given in specific tissues/organs. We will focus on HCO3− secreting epithelia of the digestive system (pancreas, salivary glands, hepatobiliary system, and duodenum), choroid plexus epithelium of the brain, and renal collecting ducts. Combining the historical perspectives with molecular and genetic studies in this chapter, we hope to mark a more integrative approach that will help us to understand the challenges of HCO3− secretion.
(a) The classical electrolyte excretion curves showing the relationship between secretory rates and electrolyte concentrations in pancreatic juice collected from the dog pancreas stimulated with secretin. Reproduced with permission from (Bro-Rasmussen et al. 1956). Similar excretory curves were obtained for the cat pancreas (Case et al. 1969). Similar excretory curves are expected for the human pancreas. (b) The cellular model of ion transport in a pancreatic duct cell as established from electrophysiological studies of isolated perfused rat pancreatic ducts. Reproduced with permission from (Novak and Greger 1988b). (c) The relation between secretory rates and HCO3− concentrations in the pancreatic juice of various species. Secretion was stimulated with secretin and secretory rates were corrected for body weights. Reproduced with permission from (Novak et al. 2011)
12.1.2 Cellular Acid/Base Homeostasis
In secretory epithelial cells, as in most other cells, the intracellular pH is maintained within the range 7.1–7.4, as most cellular processes have a pH optimum within this range (Boron and Boulpaep 2017). The balance between production, consumption, and transmembrane movement of acid/base equivalents determines the intracellular pH (pHi). The cellular buffering capacity is not regulating the steady-state pHi, but determines the size and rate of the pH change inflicted by an acute acid or base challenge (Boron and Boulpaep 2017). The intrinsic buffering capacity is set by the cellular weak acid/base pairs such as phosphate, bicarbonate, and anionic proteins. In addition to the intrinsic buffers, the open buffer system of CO2/HCO3− enables very efficient buffering of pH. Virtually all cells express plasma membrane ion transporters that contribute to cellular pH homeostasis. Some of these exploit the inward gradient for Na+ to drive acid or base transport. Other transporters are dependent on, for example, the Cl− gradient, the HCO3− gradient, electrical gradient, or ATP hydrolysis to drive the transport. Also, some ion channels may contribute to acid/base transport. Most cells express acid/base transporters (and channels), depending on the function of the specific cell, and especially in HCO3−-secreting epithelia a great variety of such transporters are found.
12.1.2.1 Sodium Hydrogen Exchangers (NHEs, SLC9)
Most of the NHEs mediate the electroneutral exchange of intracellular H+ for extracellular Na+ given typical ionic distribution and intracellular pH. Of the nine members of the NHE gene family, only NHE1 (SLC9A1) seems ubiquitously expressed and is therefore regarded as the central cellular acid extruder (Orlowski and Grinstein 2004). Linked to this function, NHE1 plays a role in cell volume regulation, cell migration, and cell cycle regulation in various health cells, including cancer cells (Flinck et al. 2018). NHE2 and NHE3 (SLC9A2 and A3, respectively) are luminal proteins mainly found in Na+ absorptive epithelia. Nevertheless, both can be found alongside potent HCO3− secretory machinery in the alkaline secretory cells of the stomach surface, duodenal villus cells, and exocrine gland ducts. In these cases, NHE2 and NHE3 could potentially favor HCO3− absorption rather than secretion (Praetorius et al. 2000). The last plasma membrane SLC9 member, NHE4 (SLC9A4), is a basolateral alternative to NHE1 in specialized cells of the kidney, stomach, salivary glands, and liver. NHEs are inhibited by amiloride and its derivatives, as well as cariporide with the highest potency toward NHE1 (Scholz et al. 1995).
12.1.2.2 Sodium Bicarbonate Cotransporters (NBCs and NDCBEs, SLC4)
The electrogenic Na+-HCO3− cotransporter NBCe1 (SLC4A4) was the first Na+-HCO3− cotransporter to be identified at the molecular level (Romero et al. 1997). NBCe1 mediates electrogenic Na+-HCO3− cotransport with either 1:2 or 1:3 stoichiometry depending on the tissue and is localized to the basolateral surface in epithelial cells involved in vectorial HCO3− transport in the kidney, intestine and pancreatic ducts (Boron and Boulpaep 1983; Schmitt et al. 1999). The second electrogenic NBC, i.e., NBCe2 (SLC4A5), displays similar transport properties as NBCe1, also with varying Na+:HCO3− stoichiometries (Pushkin et al. 2000; Sassani et al. 2002; Virkki et al. 2002). NBCe2 expression pattern is more controversial; NBCe2 is described in epithelial tissues such as liver, testis, kidney, lung, and the choroid plexus, where it is localized to the luminal membrane (Abuladze et al. 2004; Pushkin et al. 2000; Virkki et al. 2002).
The Na+-dependent exchange of Cl− and HCO3− has been found in various tissues (Boron and Knakal 1992; Liu et al. 1990; Schlue and Deitmer 1988). Two Na+-dependent Cl− and HCO3− exchangers have been described NDCBE1 (SLC4A8) and NCBE (SLC4A10) (Grichtchenko et al. 2001; Virkki et al. 2003; Wang et al. 2000). These transporters were characterized as electroneutral, DIDS-sensitive, and work with an apparent stoichiometry of 1Na+:1Cl−:2HCO3−, where Na+ and HCO3− are normally imported and Cl− extruded from the cells. The Cl− dependence of NCBE has been challenged by compelling experiments in a study by Parker and colleagues (Parker et al. 2008). The only epithelial expression sites described thus far is the choroid plexus and connecting tubules for NCBE and hepatobiliary system for NDCBE1 (Grichtchenko et al. 2001; Wang et al. 2000;Strazzabosco et al. 1997; Banales et al. 2006b). At these sites, the transporters may well take part in transepithelial movement of both Na+ and HCO3−.
The electroneutral NBC, NBC3, or NBCn1 (Choi et al. 2000; Pushkin et al. 1999), also belongs to the SLC4 gene family (SLC4A7). As the name indicates, the apparent Na+:HCO3− stoichiometry is 1:1. This means that it is normally importing the two ions into cells. NBCn1 is expressed in the basolateral membranes in many epithelia including HCO3− secretory epithelia, such as the stomach surface cells, duodenum, colon, and choroid plexus. Except for epithelial variants of NBCn1, the NBCs and NDCBEs are inhibited by stilbene derivatives such as DIDS and SITS (Aalkjaer and Cragoe Jr. 1988; Boedtkjer et al. 2006; Bouzinova et al. 2005; Odgaard et al. 2004; Praetorius et al. 2001, 2004a). The drug S0859 seems to be a general inhibitor of Na+-HCO3− cotransporter (Larsen et al. 2012). For further details on NBCs see Chap. 4 of Vol. 3.
12.1.2.3 Classical Anion Exchangers (AE, SLC4)
AE1-3 are Na+-independent Cl−/HCO3− exchangers which are electroneutral and all belong to the SLC4 gene family (SLC4A1-3). AE1, or band-3 protein, was first demonstrated in red blood cells where it exports HCO3− (Lux et al. 1989). After entry into the red blood cells, CO2 is hydrated and carbonic anhydrases accelerate the subsequent formation of HCO3− and H+. The H+ is buffered by hemoglobin and HCO3− extrudes in exchange for Cl− (the chloride shift) by AE1. Type-A-intercalated cells of the renal collecting duct express an epithelial variant of AE1. This basolateral plasma membrane anion exchanger may play a supportive role for the apical acid secretion by extruding HCO3− to the blood side (Kollert-Jons et al. 1993). Another member, AE2 is expressed basolaterally in most epithelia, except for the hepatobiliary system, and is involved in the protection of the cells against alkalization (Alper 2006). AE2 deletion in mice results in a severe phenotype with growth retardation, gastrointestinal dysplasia, biliary cirrhosis, and death before weaning (Gawenis et al. 2004; Concepcion et al. 2013). AE3 is expressed mainly in excitable tissues, such as brain and heart, but is also found in gastrointestinal enterocytes (Yannoukakos et al. 1994). Human AE3 point mutations have been associated with seizures, most likely as a consequence of the impaired neuronal pHi regulation (Hentschke et al. 2006). The AE’s like NBC’s and NDCBE’s are inhibited by DIDS.
12.1.2.4 Promiscuous Anion Exchangers
A separate gene family of anion exchangers with a more promiscuous anion transport profile has taken a central position in understanding transepithelial HCO3− movement. The SLC26 genes give rise to 12 transporters, which are expressed in many different tissues and mediate very diverse functions, transporting anions, such as sulfate, oxalate, phosphate, chloride, bicarbonate, iodide, and formate to a variable extent (Alper and Sharma 2013; Mount and Romero 2004). Several of the gene family members encode HCO3− transporters: SLC26A3,-4,-6,-7, and -9 (Alper and Sharma 2013). The stoichiometry and thereby electrogenic properties of the HCO3− transport some of these proteins is debated (for detailed review (Alper and Sharma 2013; Cordat and Reithmeier 2014)). While DRA (Down-Regulated in Adenoma, SLC26A3) and Pendrin (SLC26A4) mediate electroneutral Cl−/HCO3− transport (Chernova et al. 2003; Shcheynikov et al. 2008), CFEX/PAT1 (SLC26A6), SUT2 (SLC26A7), and SLC26A9 has been described as both electrogenic and electroneutral Cl−/HCO3− exchangers, the latter two in some reports even as ion channels, probably depending on expression system and species (Chernova et al. 2005; Petrovic et al. 2004; Kim et al. 2005; Kosiek et al. 2007). Dysfunction of the intestinally expressed DRA produces congenital chlorodiarrhoea (Hoglund et al. 1996), which is caused by reduced luminal Cl−/HCO3− exchange in the intestinal tract (Melvin et al. 1999). Pendrin, SLC26A4, is defective in the Pendred syndrome, in which patients suffer from impaired hearing and thyroid function. The symptoms result from dysfunctional thyroid Cl−/I− exchange, defective Cl−/HCO3− exchange in the stria vascularis of the inner ear, and mice probably also decreased renal collecting duct HCO3− reabsorption (Masmoudi et al. 2000; Royaux et al. 2000, 2001). PAT-1 (or CFEX) also exchanges Cl− with HCO3− and seems necessary for normal pancreatic and duodenal bicarbonate secretion (Ko et al. 2002b; Shcheynikov et al. 2006). Finally, deletion of SLC26A9 has been shown to impair luminal alkalization in the gastric mucosa (Demitrack et al. 2010) and duodenal HCO3− secretion as well as worsening intestinal function and survival of CFTR-deficient mice (Liu et al. 2015)
12.1.2.5 Anion Channels
One of the long-lasting challenges in the bicarbonate transport field is the question of whether Cl−/anion channels can conduct HCO3− in physiological conditions. Many patch-clamp studies of the cystic fibrosis transmembrane regulator Cl− channel (CFTR) have shown that in physiological-like conditions, the permeability ratio PHCO3−/PCl− is 0.2–0.5, implying that secretion of Cl− would dominate. Several studies suggest that CFTR could become more HCO3− permeable if intracellular Cl− was reduced (Ishiguro et al. 2009; Park et al. 2010, 2012). It is proposed that some cell volume/Cl− regulatory mechanisms could be contributing to the regulation of HCO3− permeability and this will be discussed in relation to the pancreas (see Sect. 12.2.2.1). For additional information about CFTR, see Chaps. 15 and 16 of Vol. 3. Bicarbonate secreting epithelia also express Ca2+ activated Cl− channels, CaCC. The identity of CaCC channels has been difficult to pinpoint (see Duran et al. 2010). After suggestions of CCl-2 and bestrophins, the TMEM16/ANO family was discovered (Caputo et al. 2008; Schroeder et al. 2008; Yang et al. 2008). One of the members, TMEM16A/ANO1, is regarded as a good candidate for CaCC in epithelia and again relevance for HCO3− secretion has been raised. Modulation of the channel HCO3− permeability by Calmodulin, and not With No Lysine kinases (WNKs), has been tested and discussed (Jung et al. 2013; Yu and Chen 2015). Further information about TMEM16 can be found in Chap. 17 of Vol. 3. Recently, it was proposed that pore dilatation of CFTR, TMEM16A/ANO1, and glycine receptor increases PHCO3−/PCl− (Jun et al. 2016). Interestingly, Bestrophins (BEST1) have relatively high HCO3− permeability in HEK293 cells (Qu and Hartzell 2008; Kane Dickson et al. 2014). Bestrophin function is well documented in retinal diseases and in HCO3−-secreting epithelia, it is less clear (see below). The volume-regulated anion channels, VRACs, are ubiquitously expressed channels composed of LRRC8 heteromers (Jentsch 2016; Jentsch et al. 2016) (see Chap. 11 of this volume). Volume regulation is important in epithelia (Pedersen et al. 2013b), though the direct role of VRAC in ion/HCO3− secretion is difficult to unmask (Catalan et al. 2015).
As mentioned above, SLC26A9 also behaves as a Cl− channel, which is constitutively active and has a minimal conductance to HCO3−, but HCO3− can facilitate Cl− transport (Loriol et al. 2008). SLC16A9 is often co-expressed with CFTR and there may be direct physical interactions with CFTR mediated by PDZ proteins (Bertrand et al. 2017). Potential role of SLC16A9 channels in cystic fibrosis and other diseases is proposed, but the detailed role of SLC26A9 as a channel, anion exchanger, or modulator of other channels/transporters is yet to be elucidated in specific tissues (Balazs and Mall 2018; Liu et al. 2018).
12.1.2.6 Vacuolar H+-ATPase and H+/K+-ATPase
Vacuolar H+-ATPases, (V-ATPases), are expressed ubiquitously in the lysosomal system, but certain cells are known to express V-ATPases on the plasma membrane (Breton and Brown 2013; Cotter et al. 2015). The V-ATPases are large transmembrane protein complexes consisting of several subunits and resembles the mitochondrial ATP synthases. Only complexes with the certain specific composition of subunits can reside in the plasma membrane. The energy resulting from ATP hydrolysis is exploited to move H+ across the membrane without counterion transport. Hence, V-ATPases are electrogenic. Epithelial cells such as renal intercalated cells and epididymis use V-ATPase for transepithelial transport (Brown et al. 2009; Pastor-Soler et al. 2008). The V-ATPase is inhibited by bafilomycin and concanamycin (Huss and Wieczorek 2009). A separate group of P-type ATPases mediate the exchange of H+ and K+, i.e., the H+/K+-ATPases. The H+/K+-ATPases are classified in two families: gastric and non-gastric (also called colonic); and α-subunits are coded by ATP4A and ATP12A (ATP1AL1) genes, respectively (Modyanov et al. 1991, 1995; Sachs et al. 2007; Forte and Zhu 2010; Sangan et al. 2000). The pump complex consists of two α- and two β-subunits, whereby the gastric α assembles with gastric β subunit (ATP4B), while non-gastric α subunits can assemble with gastric or Na+/K+-ATPase β subunits. The gastric H+/K+-ATPase is best known from the gastric corpus/fundus glands, where it mediates potent H+ secretion for gastric acid, but is also expressed in kidney and cochlea; and the non-gastric form is expressed in colon, kidney, skin, and placenta (Pestov et al. 1998). Some HCO3− secreting epithelia also express these pumps (see below). Proton pump inhibitors such as omeprazole are potent inhibitors of gastric H+/K+-ATPases, while high concentrations of potassium-competitive acid blockers and ouabain probably inhibit the non-gastric type (Grishin and Caplan 1998; Grishin et al. 1996; Swarts et al. 2005).
12.1.2.7 Carbonic Anhydrases
HCO3− and H+ are the major biologically relevant base and acid, respectively. The most potent cellular pH homeostasis and base secretion relies on a steady supply of these ion species. The hydration of CO2 occurs spontaneously at a sufficient rate, while the uncatalyzed hydrolysis of H2CO3 is quite slow for biological purposes. Thus, the carbonic anhydrases, which catalyze the conversion of CO2 + H2O to HCO3− and H+, are of major importance both for pH homeostasis and bicarbonate secretion and there are 14 different CA isoenzymes: CA I–III and VII are cytosolic; IV, IX, and XII are membrane associated; V is mitochondrial and VI is secreted (Supuran 2008). The canonical and ubiquitously expressed form is CAII. This enzyme has one of the fastest turnovers in mammalian biology (Maren 1962) and is a soluble cytosolic protein. In recent years, it has become evident that other forms of carbonic anhydrases are resident in the plasma membrane, either with extracellular enzyme activity (GPI anchored) or with cytosolic sub-membrane activity. Acetazolamide has been used to block carbonic anhydrases for decades, and seem to block both cytosolic and membrane-associated enzyme forms. In any case, inhibition of HCO3− formation by this drug has a profound impact on epithelial bicarbonate secretion in several tissues. Interestingly, some investigators have reported the physical as well as functional interaction between carbonic anhydrases and bicarbonate transporters, such as AE2 and NBCe1 and such interactions could facilitate transport by securing the substrate to the HCO3− transporters (McMurtrie et al. 2004; Becker et al. 2014).
12.1.3 Vectorial Bicarbonate Transport
It is evident that bicarbonate secreting epithelia need to employ one of the abovementioned mechanisms to extrude HCO3− from the cytosol into the luminal/apical compartment. Epithelia with potent bicarbonate extrusion are generally equipped with anion channels, with promiscuous anion exchangers or with an electrogenic Na+-HCO3− cotransporter at the site of exit. However, to avoid cellular acidification, the cells must have just as effective means of getting rid of the H+ across the opposite plasma membrane to avoid damaging acidification and to sustain the production of new HCO3−. So, in the case of luminal HCO3− secretory epithelia, the cells must have sufficient acid extrusion mechanisms such as NHE1/4 or an H+-ATPase. Alternatively, the luminal HCO3− secretion can be supported by basolateral HCO3− entry through any of the Na+ driven HCO3−-transporters.
In the following sections, selected epithelia with distinct acid/base transport characteristics will be described: pancreatic ducts, salivary glands, hepatobiliary system, duodenum, collecting duct, and choroid plexus. While the first four organs have high to very high HCO3− output, the choroid plexus epithelium has an intermediate HCO3− output, and the terminal renal collecting ducts exemplifies epithelia with little or no transepithelial HCO3− movement, where a subset of specialized cells mediate HCO3− secretion depending on the acid/base status. Thus, similarities and differences in molecular machinery for HCO3− transport between these tissues may help establishing hypotheses regarding the functional roles of specific acid-base transporters and ion channels.
12.2 Pancreas
12.2.1 The Prototype of a Bicarbonate Secretor Is a Complex Gland: Integrated Function and Morphology
The pancreas and other exocrine glands are composed of at least two main types of epithelia—secretory acini/endpieces and excretory ducts. Thaysen and coworkers (Bro-Rasmussen et al. 1956) proposed the two-stage hypothesis of secretion for complex exocrine glands and this can still be used as a starting point to understand their integrated function. Basically, it says that acini/endpieces secrete fluid similar to that in plasma in their electrolyte composition, and they secrete macromolecules such as enzymes. The ducts may modify this secretion, and in the pancreas, they do so by adding a secretion of their own (Fig. 12.1a). Pancreatic ducts are generally regarded as leaky epithelia expressing aquaporins and they are able to secrete a fluid that is HCO3−-rich and alkaline (Bro-Rasmussen et al. 1956; Steward and Ishiguro 2009; Wilschanski and Novak 2013; Ishiguro et al. 1998; Fernandez-Salazar et al. 2004; Novak et al. 2011; Wang et al. 2015). In humans, the maximum HCO3− output in the secretin-stimulated gland is about 500 μmol/h/g pancreas tissue weight. This output would be at least five times higher if it is assumed that it arises from pancreatic ducts contributing around 20% to pancreas mass in humans.
The well-established function of pancreatic bicarbonate secretion is that it contributes to buffering of acid chyme entering duodenum; the other contributors are duodenal epithelium and bile duct epithelium (Ainsworth et al. 1992). Recently, it has been discussed whether the bicarbonate secretion has an additional function already within the pancreas (Hegyi et al. 2011; Wilschanski and Novak 2013; Novak et al. 2013). That is, there are some indications that the acinar secretion might be acidic and the function of adjoining ducts may be therefore to alkalinize this acinar secretion very early in its passage through the duct tree, and thus prevent premature activation of digestive enzymes and maintain the balance in exo-/endocytosis in acini (Freedman and Scheele 1994; Freedman et al. 2001; Behrendorff et al. 2010; Hegyi and Petersen 2013). The third possible function for bicarbonate secretion is to solubilize mucins, and although this has not been proven for the pancreas (Quinton 2008, 2010), it has been shown that the very early key symptom in cystic fibrosis is reduced HCO3− secretion and mucoviscidosis in the pancreas (Andersen 1938; Kopelman et al. 1985, 1988).
Pancreatic juice collected from the pancreas stimulated with the main “secretagogue” in many species, secretin, has electrolyte composition that depends on secretory rates (Fig. 12.1a). Basically, at high secretory rates, the pancreatic juice is rich in HCO3− and poor in Cl− and as secretion decreases HCO3− falls and Cl− increases in a mirror-like fashion. Na+ concentrations do not change with flow rate and are plasma-like. K+ concentrations are similar to or higher than in plasma.
Over many years, it has been regarded that some animals produce juice low in HCO3− concentrations (mice, rats, rabbits), while the pancreas of other species produces secretion with high HCO3− concentrations (man, dog, cat, pig, and guinea pig). Nevertheless, close analysis shows that HCO3− concentrations in pancreatic juice among different species may depend on the relative proportion of acinar to duct cells contributing to the final secretion. If corrected for this, it becomes apparent that secretion of all species, summarized in Fig. 12.1c, falls within one excretory curve, which implicates similar secretory mechanisms in all species. But why does HCO3− fall and Cl− increases with the falling secretory rate (Fig. 12.1a)? These curves are often pictured but are rarely elaborated. One explanation is provided by the ad-mixture hypothesis, which states that the final secretion is a mix of fluids with different compositions (acini and ducts) (Fig. 12.2a). Another theory is the exchange theory (implying exchange of HCO3− for Cl−). This is most apparent at low secretory rates, that is, when secretion from acini and proximal ducts is low, distal ducts are not overridden by incoming secreted fluid and thus they use their full capacity to simply exchange HCO3− for Cl−, a process referred to recently as “HCO3− salvaging”. This HCO3−/Cl− exchange was demonstrated many years ago on the cat main pancreatic duct (see below) that was perfused with various solutions and could carry out such an exchange (Case et al. 1969). Yet another, so far theoretical possibility is that pancreatic ducts can also secrete H+, a process most obvious in low secretory rates. These explanations might not be mutually exclusive and they implicate that the ductal tree is heterogeneous.
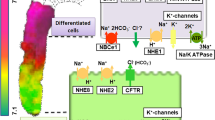
(a) Schematic diagram showing simplified pancreas with acini, proximal and distal ducts, and pancreatic juice with a typical range of HCO3− concentrations. Inserts show two types of cells with the cellular models for a HCO3− secreting cell (b) and a cell that is exchanging HCO3− for Cl− (c). Interstitial/plasma HCO3− concentration is 25 mmol/l, pancreatic juice contains 25–150 mmol/l HCO3− and depends on the secretory rate and stimulation (see Fig. 12.1). Molecular identities of ion transporters, channels, and receptors are discussed in the text; question marks indicate unclear identities, localization, or functions. Intracellular signaling is simplified, stimulatory (green) and inhibitory (red) pathways, and other interaction between cAMP and Ca2+ signaling (double-headed arrow) are discussed in Sect. 12.2.4.3. The ion transport model for pancreatic acinar cells is reviewed elsewhere (Heitzmann and Warth 2008) and is similar to Cl− secreting salivary acini (see Sect. 12.3)
The ductal tree comprises 5–20% of the pancreas tissue mass, depending on the species, and morphologically ducts are quite different—progressing from intercalated, small intralobular, larger intralobular, inter-/extralobular and eventually joining into main ducts that might join the bile duct in some species (Kodama 1983; Ashizawa et al. 1997; Githens 1988; Bouwens and Pipeleers 1998; Gmyr et al. 2004). The cell types progress from the flat small cells with large long primary cilia to cuboidal and later columnar cells with short primary cilia. Large ducts contain several cell types, including mucus-secreting cells and single endocrine cells. The centroacinar cells are very flat cells extending from intercalated cells into the acinar lumen and their physiological function is not established. Recently, pancreatic duct glands have been described as a potential progenitor niche (Yamaguchi et al. 2015).
Given the morphological heterogeneity of the ductal tree, one could expect some functional heterogeneity. However, functional studies are limited to ducts that can be isolated or micro-dissected from animals and to culture models, mostly pancreatic ductal adenocarcinoma cell models. Nevertheless, combined with data acquired from transgenic cell/animal models, immunohistochemistry, and many other techniques, coherent models can be proposed.
12.2.2 HCO3− and H+ Transporters in Pancreatic Ducts
12.2.2.1 CFTR and Cl−/HCO3− Exchangers
The first studies of cellular mechanisms for pancreatic duct HCO3− transport showed, surprisingly, that secretin/cAMP activated Cl− channels on the luminal membranes of isolated rat pancreatic ducts (Fig. 12.1b) (Novak and Greger 1988b; Gray et al. 1988). Almost in parallel, the cystic fibrosis transmembrane conductance regulator, CFTR, was discovered (Riordan et al. 1989; Kerem et al. 1989), and it was shown to have properties of a Cl− channel, also in the pancreatic ducts (Tabcharani et al. 1991; Gray et al. 1993) (Fig. 12.2b). Subsequently, CFTR was immunolocalized in human and rodent pancreas to intercalated and small intralobular ducts, which also express other key proteins in HCO3− secretion, aquaporins and carbonic anhydrases (Hyde et al. 1997; Marino et al. 1991; Kumpulainen and Jalovaara 1981; Burghardt et al. 2003). Since pancreatic HCO3− and fluid secretion is a defect in cystic fibrosis, and since the underlying signatures are mutations in CFTR, the channel has been considered as the key element in the pancreatic duct secretion (Wilschanski and Novak 2013). Nevertheless, the question whether and how CFTR Cl− channels could transport HCO3− has been a long-lasting challenge (see Sects. 12.1.2 and 12.2.4.3).
The first proposal for HCO3− exit pathway was the Cl−/HCO3− exchange mechanism coupled to luminal Cl− channels, thus allowing Cl− recirculation and net HCO3− secretion, though this would only account for 60–80 mM HCO3− in secretion (Novak and Greger 1988a, b). Through intensive efforts in molecular and cell biology, the following Cl−/HCO3− exchangers were identified. The anion exchanger SLC26A3, also known as DRA and SLC26A6, also known as PAT-1, was found expressed on the luminal membrane of large mouse and human pancreatic ducts and (Greeley et al. 2001; Lohi et al. 2000). These two exchangers have different stoichiometry showing Cl−:HCO3− of 2:1 for SLC26A3 and 1:2 for SLC26A6. It was proposed that SLC26A3 was expressed in more distal ducts. SLC26A6 was more proximal on the luminal membrane of intralobular ducts, and rarely on larger ones (Ko et al. 2002b, 2004). Theoretically, the Cl−/HCO3− exchange of 1:2 for SLC26A6 would be thermodynamically more favorable for HCO3− secretion, while SLC26A3 would favor HCO3− absorption (Fig. 12.2b and c). There is a functional coupling between SLC26A6 and CFTR, and this involves the R domain of CFTR and sulfate transporter anti-sigma (STAS) domains of SLC26A6 exchanger (Ko et al. 2004; Dorwart et al. 2008; Wang et al. 2006; Stewart et al. 2009). Nevertheless, studies using knockout strategy for SLC26A exchanger showed some interdependence between the two isoforms and varied effects on duct/pancreas secretion (Ishiguro et al. 2007; Song et al. 2012).
AE2 (SLC4A2), another anion exchanger from the SLC4 family, was demonstrated, usually on the basolateral membranes, in a number of pHi studies in pancreatic ducts (Stuenkel et al. 1988; Zhao et al. 1994; Rakonczay Jr. et al. 2006). However, immunohistochemical studies are not congruent as to which membrane the transporter is localized (Hyde et al. 1999; Roussa et al. 2001; Kulaksiz and Cetin 2002). Most likely, AE2 is more involved in pHi regulation rather than transepithelial HCO3− transport.
SLC26A9 is also weakly expressed in the pancreas (Liu et al. 2015), but whether and how it contributes to pancreatic HCO3− and fluid secretion remains to be explored in detail. Nevertheless, interesting speculations of whether SLC26A9 as a Cl− channel potentiates HCO3−/Cl− exchange, or is itself the exchanger and/or regulates CFTR (Balazs and Mall 2018; Liu et al. 2018).
12.2.2.2 Calcium-Activated Cl− channels
In addition to cyclic AMP regulated secretion, a number of studies show that agonists such as acetylcholine and extracellular nucleotides (see below) act via Ca2+ signaling to stimulate Ca2+-activated Cl− channels (CaCC) (see Chap. 17 of Vol. 3), and thus could support duct secretion (Gray et al. 1989, 1994; Pahl and Novak 1993; Hug et al. 1994; Winpenny et al. 1998; Szalmay et al. 2001; Pascua et al. 2009). In pancreatic ducts, studies on human duct cell lines show that they express TMEM16A/ANO1, which targets to the luminal membrane upon stimulation and gives rise to the secretory potential in polarized duct epithelia (Wang et al. 2013; Wang and Novak 2013). This channel could be relevant for pancreatic HCO3− secretion (see Sect. 12.1.2). There is also one immunohistological study on human pancreatic sections showing that the ANO1/DOG-1 antibody localizes to centroacinar and small ducts cells, and the channel is grossly over-expressed in pancreatic cancer and other gastrointestinal stromal tumors (Bergmann et al. 2011; Sauter et al. 2015). BEST1 is expressed in CFPAC-1 cells (Marsey and Winpenny 2009), but whether it plays an important role as CaCC in normal pancreatic ducts is not clear.
12.2.2.3 NBCs, NHEs, and Carbonic Anhydrases
HCO3− transport across the luminal membrane, whatever the mechanism is, relies on the provision of cellular HCO3−. One well-supported solution is the import of HCO3− across the basolateral membrane via a Na+ coupled process, i.e., Na+-HCO3− cotransporters, NBC. One NBC isoform was cloned from the pancreas, pNBC (NBCe1B, SLC4A4) and it transports 1 Na+: 2 HCO3− and putative inhibitor H2DIDS inhibits about 50% of duct secretion (Abuladze et al. 1998; Ishiguro et al. 1998; Choi et al. 1999). pNBC is found on the basolateral membranes of epithelial cells of a duct tree in the human pancreas, though in rat pancreas it was also acinar and duct labeling was occasionally on both membranes (Marino et al. 1999; Satoh et al. 2003). If pancreatic ducts were relying predominantly on this transporter, secretion would be highly dependent on the provision of HCO3− from interstitium/plasma rather than endogenous CO2 production, CA activity, and H+ extrusion mechanism. This was found to be the case for isolated cat pancreas and guinea pig ducts, though H2DIDS inhibited about 50% of secretions (Schulz 1971; Case et al. 1970; Ishiguro et al. 1998).
Another isoform, the electroneutral NBCn1 (NBC3, SLC4A7), is also expressed in pancreas (Damkier et al. 2006), though one study shows that in mouse ducts it interacts with CFTR, it is inhibited by cAMP and therefore should be placed on the luminal membrane and possibly regulate HCO3− salvage (Park et al. 2002b) (Fig. 12.2c).
An alternative or additional solution for cellular HCO3− (and H+) provision is carbonic anhydrase (CA) catalyzed hydration of CO2, provided from metabolism and/or HCO3−/CO2 buffer system. Isoforms CAII, IV, VI, IX, and XII are expressed in the human pancreas and cultured duct tumor cells (Kumpulainen and Jalovaara 1981; Nishimori et al. 1999; Nishimori and Onishi 2001). CAII and CAIV interact with H+/HCO3− transporters, however, localization of the CA isoforms do not always match the predicted localization of the transporters in pancreatic ducts. For example, CAII is found intracellularly and on the luminal membrane (Alvarez et al. 2001), and it seems to interact with NHE1 and NBC3 (Li et al. 2002; Loiselle et al. 2003). CAIV is expressed in the luminal membrane of the ductal tree (centroacinar cells and in intercalated, intralobular, and interlobular ductal cells) (Fanjul et al. 2004; Mahieu et al. 1994), but it interacts with NBCe1 in expression studies (Alvarez et al. 2003). CA IX and XII are expressed on the basolateral membranes of normal and pathological samples of the pancreas (Kivela et al. 2000; Juhasz et al. 2003). Carbonic anhydrases have been somewhat neglected in pancreatic duct studies in recent years. Nevertheless, CAs are key enzymes in pancreatic duct function, as their inhibition leads to marked effects on pHi and pancreatic secretion (Hollander and Birnbaum 1952; Case et al. 1979; Cheng et al. 1998; Steward et al. 2005; Rakonczay Jr. et al. 2006).
Intracellular H+, generated from CA activity or metabolism, can be extruded out of the cell by a Na+/H+ exchanger (NHE). Such exchanger was proposed based on the observation that pancreatic duct secretion could be maintained efficiently without HCO3− by a number of weak lipid-soluble acids, such as acetate (Schulz et al. 1971; Case et al. 1979). NHE, sensitive to amiloride and derivatives, has been detected in many studies monitoring secretion of the whole pancreas in different species and later on isolated pancreatic ducts (Wizemann and Schulz 1973; Veel et al. 1992; Novak and Greger 1988a; Ishiguro et al. 1998; de Ondarza and Hootman 1997; Fernandez-Salazar et al. 2004; Szucs et al. 2006). Nevertheless, amiloride type inhibitors could decrease secretion by about 20–50%. One of the NHE isoforms, the ubiquitous NHE1 (SLC9A1) is the major pHi regulator. In functional studies, it was revealed that NHE contributed significantly to pHi regulation in many duct preparations including pig, guinea pig, rat and mice ducts and human duct cell lines (Veel et al. 1992; Szucs et al. 2006; de Ondarza and Hootman 1997; Ishiguro et al. 2000; Novak and Christoffersen 2001; Lee et al. 2000; Demeter et al. 2009; Rakonczay Jr. et al. 2006; Olszewski et al. 2010). There is some molecular evidence for NHE1 expression is normal ducts and localization appears to be on the basolateral membrane (Lee et al. 2000; Roussa et al. 2001), thus function in secretion (and pHi regulation) could be supported. In addition, the NHE2 and 3 isoforms are expressed on the luminal membrane of main ducts and are proposed to interact with CFTR via PDZ domains (Lee et al. 2000; Ahn et al. 2001; Marteau et al. 1995). These exchangers would then not support secretion, but conduct HCO3− salvage (or pHi regulation) (Fig. 12.2c).
12.2.2.4 Proton Pumps
The above models do provide a number of answers, but still, we are left with the problem of how to explain high HCO3− concentrations and why inhibitors of NHE1, NBC, and CA are relatively ineffective in blocking secretion (Fernandez-Salazar et al. 2004; Grotmol et al. 1986). The above transporters and ion channels rely on gradients that are created by the Na+/K+-ATPase. Another solution to create HCO3−/H+ gradients would be to extrude H+ via the V-ATPase. In one early study V-ATPase on the basolateral membrane was proposed (Villanger et al. 1995), and V-ATPase was detected on basolateral membrane of intralobular ducts, although occasionally some cells had luminal staining (Roussa et al. 2001). A number of functional studies gave contradictory findings (Zhao et al. 1994; Ishiguro et al. 1996; de Ondarza and Hootman 1997; Cheng et al. 1998), perhaps depending on which parameters were measured. It seems that the contribution of the pump to pHi regulation is relatively small (compared to NHE1), but inhibition of secretion or short-circuit currents with V-ATPase blockers can be significant.
Recently, other types of H+ pumps have been detected in pancreatic ducts. Both rodent (and human) ducts express the gastric type H+/K+-ATPases (ATP4A and ATP4B) and non-gastric types H+/K+-ATPase (ATP12A) (Novak et al. 2011; Wang et al. 2015). Inhibition of these with proton pump inhibitors such as omeprazole and SCH28080 reduced pHi recovery in response to acid loads, and more importantly, they reduced secretion in isolated pancreatic ducts and in the whole pancreas tested in vivo (Novak et al. 2011; Wang et al. 2015). The immunohistochemical study showed that the H+/K+-ATPases (mainly colonic type) are localized to the basolateral membrane, and thus is consistent with HCO3−- secretion model. However, some H+/K+-ATPases were also localized to the luminal membrane, especially the gastric form (Novak et al. 2011). At present, the function of these pumps in pancreatic ducts is unclear, similar to other HCO3−-secreting epithelia such as the airway epithelia (Novak et al. 2013), but interestingly ATP12A is upregulated in CF airways (Shah et al. 2016; Scudieri et al. 2018). It is speculated that these luminal pumps are creating a buffer zone protecting cells against bulk secretion which is pH > 8. In addition, luminal H+/K+ pumps in distal ducts would by virtue of H+ secretion have more impact on pancreatic juice composition at low flow rates and minor at high flow rates, thus explaining excretory curves for HCO3− (Fig. 12.1). Furthermore, the luminal H+/K+ pumps would recirculate K+ extruded by the luminal K+ channels (Hayashi et al. 2012; Novak et al. 2013; Wang et al. 2015).
12.2.2.5 K+ Channels
In addition to HCO3−/H+ transporters, it K+ channels are important for pancreatic duct secretion. They maintain the resting potential, and during stimulation opening of K+ channels and hyperpolarization of the membrane potential maintains the driving force for Cl− or HCO3− exit (Novak and Greger 1988a, 1991). Conductance for K+ (GK) is both present on the basolateral and luminal membranes and equivalent-circuit analysis has shown that the luminal K+ channels contribute with at least with 10% to the total conductance in stimulated duct (Novak and Greger 1988a, 1991). Modeling in salivary glands confirms that such a ratio of luminal to basolateral K+ channels would optimize secretion without destroying the transepithelial potential and transport (Almassy et al. 2012; Cook and Young 1989). Another function of luminal K+ channels could be to contribute to secreted K+, as pancreatic juice contains 4–8 mM K+ (Sewell and Young 1975; Caflisch et al. 1979; Seow et al. 1991; Wang et al. 2015). The molecular identities and function of only some K+ channels are known (see Hayashi and Novak 2013). The best studied until now are the Ca2+-activated K+ channels. The KCa1.1 channels (maxi-K, BK, coded by KCNMA1) are present in pancreatic ducts (Hede et al. 2005; Venglovecz et al. 2011) (Fig. 12.2b). Earlier patch-clamp studies indicate that these channels are located basolaterally (Gray et al. 1990; Hede et al. 1999). However, recent studies indicate that these channels are expressed on the luminal membrane and activated by, e.g., low concentrations of bile acids (see below). Evidence for another K+ channel activated by extracellular ATP via purinergic P2 receptors was provided in studies of rat and dog duct epithelia (Hug et al. 1994; Nguyen et al. 1998) and later the intermediate conductance, KCa3.1 channel (IK, SK4, coded by KCNN4) was documented (Hede et al. 2005; Jung et al. 2006; Hayashi et al. 2012). Immunolocalization indicates that KCa3.1 is expressed on both luminal and basolateral membranes (Fig. 12.2b). The KCa3.1 channel activator EBIO enhanced secretion potential in Capan-1 monolayer indicating that these channels are important in pancreatic duct secretion (Hayashi et al. 2012; Wang et al. 2013). Recent studies on pancreatic ducts offer molecular identities of several other K+ channels, including KVLQT1, HERG, EAG2; Slick and Slack (Hayashi et al. 2012), and interestingly the pH sensors TASK-2 and TREK-1 (Fong et al. 2003; Sauter et al. 2016). Nevertheless, the function and regulation of these channels in pancreatic physiology need to be studied.
12.2.2.6 Aquaporins and NKCC1
Taking that pancreatic ducts are secretory, water follows paracellularly and transcellulary via aquaporins (AQP). AQP1 is expressed on centroacinar cells and luminal and basolateral membrane of intercalated ducts and AQP5 is expressed luminally and labeling decreases in larger ducts in the human pancreas and is more distal in rodent pancreas (Burghardt et al. 2003, 2006). Notably, AQPs are co-expressed with CFTR in the same cells.
Upon stimulation of secretion, there would be a significant reduction in cell volume due to solute transport followed by osmotically obliged water. Subsequently, the cell volume would need to be reinstituted and one of the most important transporters in that respect is the Na+-K+-2Cl− cotransporter (NKCC1, SLC12A2). This transporter is expressed in pancreatic ducts, however, it is not clear whether shrinkage-activation of NKCC1 occurs in pancreatic ducts, or first following withdrawal of stimuli, as is the case in salivary acinar cells (see Sect. 12.3.2). Additionally, NKCC1 could provide cellular Cl− for Cl−-driven fluid transport. In fact, diuretics such as bumetanide can inhibit duct/pancreas secretion, but the effect depends on the species (Fernandez-Salazar et al. 2004; Grotmol et al. 1986).
12.2.3 Integrating Ion Channels and Transporters to Pancreatic Ducts
Taking the above-described channels and ion transporters and placing them into one cell model becomes rather problematic—such cell would secrete and absorb at the same time. Therefore, it should be also considered where these transporters are localized within the pancreatic duct tree. Since there are only very few functional studies on native ducts from different regions of the ductal tree, we have to resort to taking into account heterogeneity in duct morphology and immunohistochemistry, and interaction between channels/transporters in expression studies. Studies summarized in the preceding sections have indicated that small proximal ducts express CFTR, CA, SLC26A, AE2, NBC1e, and AQP1. The larger, distal ducts express SLC26A3, and possibly CFTR, NBCn1, and NHE3, as well. It is not yet clear whether AE2, K+ channels, H+-pumps, and NKCC1 are differentially expressed.
For simplicity, if one inserts these transporters into two cells (Fig. 12.2b and c), it becomes apparent that the first cell has a potential to secrete, while the second has the possibility to exchange HCO3− for Cl−. It cannot be excluded that these two models are two different states of one cell at different times or conditions. However, a very likely scenario is that one cell represents small proximal ducts that are secreting HCO3−-rich fluid, and the other represents large interlobular/lobar ducts that are modifying incoming fluid but not contributing with a net fluid secretion. The simplest interpretation for these data obtained for large distal ducts is that they reabsorb HCO3− in exchange for Cl−—thus giving the rise to the excretory curve (Fig. 12.1). Nevertheless, with maximal stimulation and maximal secretory rates, pancreatic secretion is HCO3− rich. Potentially, one would expect acidic interstitial pH, which could favor pathogenic processes in pancreatitis and pancreatic cancer (Novak et al. 2013; Pedersen et al. 2017).
12.2.4 Regulation of Pancreatic Duct Secretion
The classical HCO3−-evoking secretagogue is secretin, though a number of other hormones and transmitters can also evoke and co-regulate HCO3− secretion. Even cholinergic stimulation and cholecystokinin (CCK) can evoke HCO3− secretion in some species, and they can potentiate the secretin effect on the volume of secretion (Hickson 1970; Holst 1993; Park et al. 1998; You et al. 1983; Evans et al. 1996; Chey and Chang 2001; Szalmay et al. 2001). Here, we will consider the novel paracrine and autocrine regulators of pancreatic ducts—those secreted by acini and ducts themselves (nucleotides) and those that are entering the duct via the retrograde route (bile acids). Subsequently, we will consider novel interaction between signaling pathways and ion transporters and how they can in an integrative way affect pancreatic duct secretion.
12.2.4.1 Purinergic Signaling
In physiological settings, the function of pancreatic acini and ducts is coordinated (Hegyi and Petersen 2013). It has become accepted that purinergic signaling contributes to integrating acinar and duct functions, in particular fine-tuning duct performance. Pancreatic acini release ATP, some of which is stored in zymogen granules and released upon hormonal and cholinergic stimulation (Sorensen and Novak 2001; Haanes and Novak 2010; Haanes et al. 2014) and small amounts of ATP can be also detected in pancreatic juice, though most is hydrolyzed by ecto-nucleotidases (Kordas et al. 2004; Yegutkin et al. 2006) (Fig. 12.2a). ATP is also most likely released from nerves, as well as from acini and duct cells in response to cell volume changes, mechanical and chemical stress. In contrast to acini, pancreatic ducts express a number of functional purinergic (P2Y2, P2Y4, P2Y11, P2X4, and P2X7) and adenosine (A2A and A2B) receptors that regulate various epithelial transporters (see Novak 2008, 2011) (Fig. 12.2b and c). For example, luminal ATP (or UTP) can increase anion and fluid secretion, and this involves the regulation of TMEM16A/ANO1 and CFTR, as well as KCa3.1 and Cl−/HCO3− exchange (Chan et al. 1996; Hug et al. 1994; Ishiguro et al. 1999; Namkung et al. 2003; Hede et al. 2005; Jung et al. 2006; Novak et al. 2010; Hayashi et al. 2012; Wang et al. 2013). In addition, luminal ATP stimulates P2X7 receptors and potentiates cholinergically evoked ductal secretion (Novak et al. 2010). Furthermore, ATP/UTP also potentiates cAMP-evoked mucin secretion (Jung et al. 2010). Ca2+ signaling and P2Y2 and P2X7 receptors in particular have been considered in these actions. Adenosine receptors via cAMP signaling regulate CFTR (Novak et al. 2008). From the basolateral side, ATP released by nerves and/or distended epithelium can also affect the secretion and some purinergic receptors are inhibitory to secretion (e.g., P2Y2 receptors inhibit KCa1.1 channels), while other P2 receptors, including P2Y11 receptors, may have positive effects on secretion (Hede et al. 1999, 2005; Ishiguro et al. 1999; Nguyen et al. 2001; Wang et al. 2013). A number of processes in purinergic signaling are pH sensitive, and it will be relevant to investigate those in the microenvironment of the duct epithelium (Novak et al. 2013; Kowal et al. 2015b). Due to the fact that nucleotides/side could stimulate a multitude of P2 and adenosine receptors acting via Ca2+ and cAMP signaling, interactions need to be considered. For further information about P2X receptors in epithelial transport, the reader is directed to Chap. 28 of Vol. 3.
12.2.4.2 Bile Acids
Systemic bile acids (primary or secondary) produced in the liver and by gut microbiota, are becoming regarded as important physiological regulators of a wide range of cells. In the exocrine pancreas, though, bile acids (BA) may exert additional effects, as they can enter pancreatic duct tree by reflux following outflow obstruction by gallstones, and apparently affect both duct and acinar cells. Biliary acute pancreatitis (AP), or gallstone obstruction-associated AP, account for a significant percentage of clinical cases of AP and animal and cellular models are important tools for understanding development of this disease (Wan et al. 2012). Many studies have been carried out on acinar cells, and it has been shown that at high concentrations (mM) of, for example, taurine-conjugated BA cause large increases in intracellular Ca2+, activation of intracellular trypsinogen and necrosis (Voronina et al. 2002, 2004; Gerasimenko et al. 2006; Kim et al. 2002). These BA effects are mediated by TGR5/Gpbar1 receptor, which is expressed on the apical surface of pancreatic acini in mice (Perides et al. 2010).
Only a few studies show that BA also have effects on pancreatic ducts, but these may be important since pancreatic ducts are pivotal for the maintenance of the physiological function of the whole pancreas. BA have a bimodal effect on pancreatic ducts inducing pancreatic fluid hypersecretion in the early stages of pancreatitis and hyposecretion during the onset of the disease (Czako et al. 1997). Studies on isolated ducts and duct epithelia show that this bimodal effect may be related to concentration of BA used. At high (>mM) concentrations BA have a detrimental effect. For example, in guinea pig ducts non-conjugated BA, chenodeoxycholate acid (CDCA) at 1 mM, caused large sustained increase in Ca2+, inhibited HCO3− transport, caused mitochondrial damage and increased permeability of duct cells, and caused mitochondrial damage (Venglovecz et al. 2008; Maleth et al. 2011). In bovine duct cell monolayer 5 mM taurodeoxycholic acid (TDCA) decreased epithelial resistance due to damage of the epithelial barrier (Alvarez et al. 1998). At lower concentrations, BAs have positive effects. For example, in epithelium derived from the dog main duct, TDCA increased luminal GCl and basolateral GK in Ca2+-dependent manner (Okolo et al. 2002). In other studies on guinea pig ducts and CFPAC-cells, it was shown that 0.1 mM CDCA increased Ca2+ via PLC and IP3 (inositol 1,4,5-trisphosphate) and stimulated HCO3− transport (i.e., pHi monitoring), though NBC, NHE, AE or CFTR or other Cl− channels seem not to be primary targets (Venglovecz et al. 2008; Ignath et al. 2009). Studies on guinea pig ducts show that a low dose CDCA activated maxi-K+ channels on the luminal membrane and thereby could initiate the secretory machinery (Venglovecz et al. 2011). Thus, it is proposed at high concentrations BA are damaging, but at low concentrations BA would be able to promote duct secretion, and thus wash out refluxed bile. It was not yet clear whether these physiological-like BA signals are mediated via TGR5/Gpbar1 receptors One study offers another explanation. CDAC can evoke ATP release from duct cells, which then stimulates purinergic receptors and thereby increases cellular Ca2+. TGR5 receptor is not involved in this process but can play a protective role at high Ca2+ conditions by stimulating Na+/Ca2+ exchanger (Kowal et al. 2015a).
12.2.4.3 Synergistic Intracellular Signaling: Calcium, cAMP, and Cell Volume
In pancreatic ducts, as in other biological systems, physiological regulation would involve stimulation of several types of receptors and coordination of several signaling pathways to stimulate relevant ion transporters on both luminal and basolateral membranes to achieve transcellular secretion of ions/fluid, as well balancing cell volume and pHi changes. Utilizing synergism of signaling pathways would ensure maximum effect without running each pathway at a maximum capacity, which could be detrimental to cell survival, as exemplified by Ca2+-mediated cellular toxicity (Berridge 2012). Here, we summarize the evidence for the interaction of Ca2+ and cAMP signaling pathways and their effect on pancreatic duct ion transport.
First of all, some agonists, such as ubiquitous nucleotides/sides signal via multiple receptors: coupled to Gq, Gs, Gi proteins (P2Y and adenosine receptors) and ligand-gated ion channels (P2X receptors) (Jacobson and Muller 2016; Burnstock 2017). Pancreatic ducts express G-protein coupled P2Y receptors, P2X receptors, and various nucleotidases, such that ATP would have multiple effects via cAMP and Ca2+ signaling (Novak 2008, 2011) (Fig. 12.2).
Another synergistic mechanism occurs at the ion channel level, where Ca2+-sensitive K+ channels can alter the driving force for anion secretion through cAMP/PKA regulated CFTR. Further potentially synergistic mechanism to increase secretory output is the parallel anion transport through CaCC channels and CFTR channels in pancreatic ducts. However, there is evidence that there is some interdependence between CFTR and CaCC, such that malfunctioning CFTR (CF models) down-regulates expression or function of CaCC in parallel to CFTR (Gray et al. 1994; Winpenny et al. 1995; Pascua et al. 2009; Wang et al. 2013).
The central channel in the pancreatic duct is CFTR, which is a part of signaling complex that includes scaffolds, adaptors, and many regulatory enzymes associated with cAMP/PKA signaling (Frizzell and Hanrahan 2012). Several studies show that there is cross-talk between Ca2+ signaling and CFTR activation. There are Ca2+ sensitive adenylate cyclases 1 and 8 (Namkung et al. 2010; Martin et al. 2009) and Ca2+-dependent activation of tyrosine kinases (Src2/Pyk complex), both of which could eventually alter the activity of CFTR, as shown for airway and intestinal epithelia (Billet and Hanrahan 2013; Billet et al. 2013). Another effect at the CFTR level would be priming of some PKC isoforms that enhance the activity of CFTR (see Billet and Hanrahan 2013).
Furthermore, synergy between Ca2+ and cAMP signaling could be exerted by the third messenger IRBIT (IP3 receptor-binding protein released with IP3). Agonists that couple to Gs, increase cAMP and via PKA phosphorylation of the IP3 receptor, and receptors that activate Gq increase level of IP3. Increased affinity of IP3R to IP3 facilitates the release of IRBIT from the apical pools, which then translocates and coordinates epithelial fluid and HCO3− secretion by stimulating NBCe1B and CFTR and SLC26A6 (Yang et al. 2009, 2011). This type of synergy is well studied in pancreatic ducts using genetic modifications of SLC26A6 and IRBIT (Park et al. 2013). Using cAMP/Ca2+ signaling agonist pairs such as forskolin/carbachol, secretin/carbachol, forskolin/carbachol—synergy in fluid secretion and HCO3− flux is revealed.
Lastly, the inhibitory pathways, which are downstream of Ca2+ and cAMP should be considered. Cell signaling pathways involving volume- and low Cl−-sensitive With No Lysine kinases (WNKs), acting via Ste20-like kinases, SPS-related proline/alanine-rich kinase (SPAK) and oxidative stress responsive kinase (OSR1), may be key factors in secretory epithelia, since they regulate NKCC1 and other transporters (Kahle et al. 2006; McCormick and Ellison 2011). Basically, these kinases are activated by hyperosmolarity (cell shrinkage) and low intracellular Cl−, and thus would restore cell volume. In relation to pancreas, WNK1 and 4 are expressed in lateral membranes of interlobular and main pancreatic ducts and they inhibit NKCC1 and SLC26A6 (Choate et al. 2003; Kahle et al. 2004). Other studies show that WNKs inhibit CFTR (Yang et al. 2007, 2011). For example, in mice ducts, it was shown that WNKs and SPAK reduced expression of CFTR and NBCe1—and duct secretion (Yang et al. 2011). IRBIT increases membrane surface expression of NBCe1B, CFTR, and SCL26A6 and thus overcomes antagonizing WNK/SPAK signaling, which otherwise reduces secretion (see above). It seemed somewhat surprising then that the WNK/OSR1/SPAK system stimulated by low intracellular Cl− could change the permeability of CFTR in favor of HCO3−, i.e., PHCO3−/PCl− increased from 0.24 to 1.09, and at the same time inhibited SLC26A6 and A3 (Park et al. 2010, 2012). It is proposed that WNK signaling for distal ducts and IRBIT signaling for proximal ducts could be a part of the mechanisms underlying overall pancreatic duct function (see Lee et al. 2012; Park and Lee 2012).
The above section indicates that cell volume regulation, e.g., via WNK/OSR1/SPAK system may be important for pancreatic ducts. Similarly, autocrine and paracrine signaling via volume-sensitive ATP release must be a key regulator in short- and long-term cell volume and ion transport in epithelia, including the pancreatic duct. Although cell volume regulation it is a cornerstone in epithelial physiology and pathophysiology we know very little about this process in in pancreatic ducts (Pedersen et al. 2013b).
12.3 Salivary Glands
12.3.1 Salivary Glands: Heterogenous Structures and Functions
Saliva is a complex mixture of fluid containing amylase, lipase, glycoproteins (e.g., mucins, vitamin B12 binding haptocorrin), proline-rich proteins and proteins regulating calcium phosphate and hydroxyapatite formation (e.g., statherins, histatins, and cystatins), growth factors (e.g., EGF), antibacterial agents (immunoglobulins, lysozyme, lactoferrin), water, and electrolytes (including varied concentrations of HCO3−) and minerals. The major function of the salivary glands is to protect the teeth and oral-oesophageal mucosa (by modulating re-/demineralization of teeth enamel, protecting gingiva, and antibacterial actions); initiate digestive processes; enhance taste perception and provide lubrication; and provide pH buffering capacity (bicarbonate, phosphate, proteins) (Humphrey and Williamson 2001; Matsuo 2000; Pedersen et al. 2002, 2013a). In some animals, salivary glands have additional functions, e.g., in grooming and evaporative cooling keeping oral cavity moist in panting, regulating salt homeostasis (e.g., in crocodiles). Major human salivary glands supply about 90% of the whole saliva and comprise of three pairs of exocrine glands: parotid glands (P), submandibular (submaxillary) (SM) glands and sublingual glands (SL). The rest of the secretion is provided by hundreds of minor glands spread throughout the oral cavity (Pedersen et al. 2013a). The largest glands in human, parotid glands, contain serous acinar cells and secrete amylase-rich secretions. The submandibular glands contain seromucous and serous acini and produce mucous saliva. The sublingual glands are the smallest glands and contain prevalently mucous acini and produce mucin-rich viscous secretion. Secretions that originate in acini are conducted through short intercalated ducts (that may be secretory) to striated ducts, characterized by many mitochondria and folds on the basal membrane, and to excretory (extralobular) ducts leading to the main excretory duct of Stensen (P), Wharton (SM) or series of excretory ducts (SL) (Pedersen et al. 2013a). In some glands/animals, e.g. male rodents, the striated ducts of SM contain granules and are referred to as granular ducts (Schneyer et al. 1972). Salivary glands are under the control of both branches of autonomic nervous systems, as well as higher brain centers and autocrine/paracrine regulation (Schneyer et al. 1972; Garrett 1987; Pedersen et al. 2002, 2013a; Proctor and Carpenter 2014).
The major component of saliva is water (99%) and electrolytes and there are special relationships between those and secretory rates (see Fig. 12.3). Based on these relationships Thaysen and coworkers proposed that saliva was formed in two stages—in acini and ducts (Thaysen et al. 1954). Similar hypotheses were proposed for the pancreas and other exocrine glands, though importantly, the function of ducts differs among various gland types (Bro-Rasmussen et al. 1956; Schwartz and Thaysen 1956). In the following paragraphs, the simplest scenario valid for most salivary glands will be outlined (Fig. 12.4). In the first stage, salivary acini generate primary saliva that is isotonic plasma-like fluid that is high in Na+ and Cl− concentrations, K+ concentrations are slightly above the plasma (Schneyer et al. 1972; Young et al. 1980). The anion-gap is most likely HCO3− secreted at about plasma-concentrations (see Sect. 12.3.4 for exceptions). In the second stage, the ducts reabsorb Na+ and Cl− and partially compensate electrolytes by secreting some K+ and HCO3−. The ductal epithelium is electrically tight and water impermeable (Young et al. 1980). In this respect, the salivary ducts are fundamentally different from the pancreatic ducts, the latter being a leaky and secretory epithelium (see Sect. 12.2.1). Due to hypertonic salt transport in salivary ducts, final saliva in many gland types and species is hypotonic. Importantly though, tonicity and electrolyte patterns depend on the secretory rate (acini) and saturation of various transporters on the downstream ducts. Above-described processes would give rise to the simplest excretory curves (Fig. 12.3 rat, rabbit, human glands).
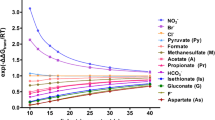
The relation between secretory rates and electrolyte concentrations in saliva collected from salivary glands of various species. Preparations were in vivo glands or ex vivo perfused glands stimulated with cholinergic agonist, e.g., pilocarpine or carbachol, (full lines). In some experiments on perfused glands, Cl− transport was inhibited, e.g., with furosemide (dot-dash line). Some glands were stimulated with β-adrenergic agonist, isoproterenol (dotted line). Secretory rates were corrected for gland weights and data were redrawn from publications on the rabbit submandibular gland (Case et al. 1980, 1984; Novak and Young 1986); the rat submandibular gland (Young and Martin 1971); the human parotid gland (Thaysen et al. 1954); the sheep parotid gland (Compton et al. 1980) and the kangaroo parotid gland (Beal 1984)
(a) Schematic diagram showing a simplified salivary gland with acini and excretory ducts and saliva with a typical range of HCO3− concentrations for most cholinergically stimulated salivary glands and special salivary glands and/or special circumstance (see Fig. 12.3). (b) Inserts show the cellular models for acinar cells that can secrete Cl− relatively independent of HCO3− and rely on NKCC1 and double exchange system (e.g., SM glands). Some acini rely primarily on HCO3− transport (e.g., parotid glands) and express NBCe1, marked with *. See also Fig. 12.3. (c) Cellular model of a duct cell that is absorbing Na+ and Cl− (via luminal ENaC-CFTR channels or double exchange system) and secreting K+ and HCO3−
A number of studies were conducted to verify the two-stage theory, including micropuncture studies that involve sampling and analyzing fluid at or close to acini/intercalated ducts and in downstream ducts, as well as studies on isolated salivary ducts. These are summarized in earlier reviews in this field (Martinez et al. 1966; Young and Schögel 1966; Young et al. 1980). In the current research on exocrine glands, many advanced techniques on cellular/genetic level are used, but it is still very valuable to take the integrative approach and return to the whole gland secretion and electrolyte patterns. Nonetheless, understanding of ion transport in salivary glands is particularly challenging as there are three different major glands (parotid, submandibular, and sublingual), there are large interspecies and even male/female variations in structure and regulation, and these may reflect very varied salivary gland functions.
In the following section, we will describe the basic ion transport mechanisms in acini and ducts of most common experimental animals (rat, mouse, rabbit) stimulated with cholinergic stimulation that evokes the largest fluid secretion rates (Figs. 12.3 and 12.4). Later (Sects. 12.3.4 and 12.3.5) we will consider other modes of stimulation, e.g., sympathetic, as well as specific glands/animals and experimental conditions that evoke saliva with unusual ion compositions. This holds in particular HCO3− secretion, which shows the most bewildering variety of excretion patterns (Fig. 12.3) and may originate in acini and/or ducts (Young et al. 1980; Novak 1993) (Fig. 12.3b). In humans, parotid glands can secrete up to 40–60 mM HCO3−, though mixed saliva from major and minor salivary glands rarely exceeds 20–25 mM HCO3− (Thaysen et al. 1954; Bardow et al. 2000). Human parotid glands have HCO3− output ranging from about 40 to 500 μmol/h/g gland weight. Rat and rabbit submandibular stimulated with cholinergic stimulus have similar HCO3− output ranging from about 40 to 400 μmol/h/g gland weight. In sheep and kangaroo parotid the output ranges from around 100 to 1500 μmol/h/g gland weight (see Fig. 12.3).
12.3.2 Ion Channels and Transporters in Salivary Gland Acini
Since many salivary glands commonly studied (e.g., rodent P and SM) can secrete very efficiently without exogenous HCO3− and/or with CA inhibitors, the ion transport models for acini are based predominantly on the transport of Cl−. Cl− is transported across the basolateral membrane via loop diuretic sensitive Na+-Cl− cotransporter, later identified as NKCC1 in several preparations including human parotid acini (Case et al. 1982, 1984; Martinez and Cassity 1983; Turner et al. 1986; Nauntofte and Poulsen 1986; Moore-Hoon and Turner 1998; Evans et al. 2000; Nakamoto et al. 2007) (Fig. 12.4b). An alternative mechanism for NaCl transport is the parallel transport via Na+/H+ and Cl−/HCO3− exchangers, as proposed from studies of isolated glands (Novak and Young 1986; Turner and George 1988). Interestingly, the Cl−/HCO3− exchanger, most likely AE2, is upregulated when NKCC1 is inhibited or genetically silenced (Evans et al. 2000). AE2 is expressed together with NHE1 on the basolateral membrane of acini (He et al. 1997; Lee et al. 1998; Park et al. 1999). In NHE1–/– mice, parotid acini express higher activity of AE2 and CAII, as determined by pHi measurements, indicating increased Cl− and HCO3− transport across the plasma membranes, though no data on salivary secretion are available (Gonzalez-Begne et al. 2007). There is also evidence for NBC1e expression on salivary gland acini (see Sect. 12.3.4), but in many glands transport of Cl− is sufficient to drive full fluid secretion.
Parasympathetic stimulation produces large saliva volumes (see Sect. 12.3.5), e.g., acetylcholine acting via muscarinic receptors, increases intracellular Ca2+ concentrations and Ca2+ signaling has been well-studied mode of stimulus-secretion coupling (Petersen 2014), though more recent studies show that similar to the pancreas there is a synergism between Ca2+ and cAMP signaling (see Jung and Lee 2014; Ahuja et al. 2014). In salivary acini, Ca2+ signals are essential in regulating Cl− efflux via the luminal channels. These CaCC properties are corresponding to recently identified Cl− channel TMEM16A/ANO1, and submandibular glands from TMEM16a-/- mice produce lower amount of saliva (Romanenko et al. 2010; Catalan et al. 2015). Some TMEM16A/ANO1 is also expressed on the luminal membrane of intercalated ducts, though another CaCC candidate Besthropin-2 may be relevant to duct function (Romanenko et al. 2010). Expression of TMEM16A/ANO1 is also found on the luminal membrane of human parotid acini and intercalated ducts (Chenevert et al. 2012) (Fig. 12.4b). Studies in HEK293 cells and SM gland acini indicate that TMEM16A/ANO1 anion selectivity is dynamically modulated by Ca2+/calmodulin, possibly increasing PHCO3−/PCl− (Jung et al. 2013). Regarding CFTR, the protein is expressed on the luminal membrane of ducts, but there are contradicting reports regarding the expression of CFTR in SM of rodent acini. Nevertheless, since in mice with ΔF508 mutation in CFTR or inhibition of CFTR had no significant effect on salivary secretion rate, other Cl− channel must have rescued secretion (He et al. 1997; Zeng et al. 1997; Catalan et al. 2010).
Sympathetic stimulation leading to β-adrenoceptor stimulation and cAMP signaling produces lower volumes of HCO3−- and protein-rich saliva (Case et al. 1980). One study shows that isoproterenol stimulates secretion in salivary glands of mice where TMEM16A and CFTR have been ablated, and inhibitor sensitivity profiles indicate VRAC channels may be involved (Catalan et al. 2015).
One of the most marked effects in salivary acini is the loss of intracellular K+ upon stimulation, as observed in initial studies on in vivo glands and isolated acini (Burgen 1956; Nauntofte 1992). Also, many electrophysiological studies on plasma membrane potentials in acini reported hyperpolarizing “secretory potentials”, which would be consistent with increased K+ conductance, and Ca2+-activated maxi-K+ channels were characterized in patch-clamp studies (Imai 1965; Petersen and Poulsen 1967; Maruyama et al. 1983; Petersen and Gallacher 1988). Ca2+-activated K+ channels (BK–KCa1.1 and IK–KCa3.1) have been identified on the basolateral membrane (Wegman et al. 1992; Park et al. 2001a; Nehrke et al. 2003; Begenisich et al. 2004; Romanenko et al. 2007) (Fig. 12.4b). It is now well accepted that the basolateral K+ channels serve for K+ recirculation necessary for the operation of the Na+/K+ pump and thus secretion. However, micropuncture studies and analysis of fluid close to acini, indicated that the primary secretion has K+ concentrations higher than the plasma, e.g., up to 10 mM K+ (Young and Schögel 1966; Mangos et al. 1966, 1973) (see Schneyer et al. 1972), indicating that there may be some secretory mechanisms for K+ on the luminal membrane. Hence, it was proposed that K+ channels are also present on the luminal membrane of salivary acini and various mathematical models verified that luminal K+ channels are necessary for creating the driving force for Cl− exit and account for at least 10–20% of total K+ conductance in acinar cells (Cook and Young 1989; Palk et al. 2010). Recent studies on mouse parotid acini using spatially localized manipulation of Ca2+ and whole cell patch clamp show that the very small area of the luminal membrane, e.g., approximately 3–8% of the overall plasma membrane (Poulsen and Bundgaard 1994), expresses high density of KCa1.1 and KCa3.1 channels (Almassy et al. 2012). These channels exhibit some interdependence/interaction (Thompson and Begenisich 2006, 2009). In submandibular acini, it seems that the apical Ca2+ signals stimulate CaCC, and only when signals spread to the basolateral membrane and/or the membrane is depolarized, then the basolateral K+ channels are activated. It also seems that either KCa1.1 or KCa3.1 can support full secretion in the mouse submandibular gland; and only a double knockout of these K+ channels reduces secretion significantly (Romanenko et al. 2007). Whether incongruence between parotid and submandibular acini is related to different Ca2+ signaling or patterns of K+ channel expression is not clear yet. Nevertheless, the most significant K+ secretion is contributed by the ducts (see Sect. 12.3.3) (Fig. 12.4c).
In normal salivary secretion water transport, occurring by transcellular and paracellular routes and the cell volume regulation is dependent on the expression of water channels, aquaporins. The most important aquaporin in salivary gland acini is AQP5, as determined in knockout studies on mice (Ma et al. 1999; Krane et al. 2001; Murakami et al. 2006; Kawedia et al. 2007). Cell volume regulation is important in many cellular functions, including epithelial transport (Pedersen et al. 2013b). In secreting epithelia, physiological stimulus leads to opening of luminal Cl− channels and basolateral/luminal K+ channels and osmotically driven water movement leads to shrinkage of secreting cells. Basolateral transporters and pHi regulating mechanisms need to be activated to provide ions for luminal exit. Nevertheless, in the secretory state these mechanisms are unable to maintain the cell volume of salivary secretory cells which remain shrunken, until the stimulus is terminated, after which, cell volume recovers (Dissing et al. 1990; Foskett 1990; Nakahari et al. 1990, 1991; Lee and Foskett 2010). This seems to be the case for the Ca2+ signaling pathways, as cAMP-mediated signaling leads to increased cell volume in salivary acini and VRAC may be involved (Catalan et al. 2015).
12.3.3 Ion Channels and Transporters in Salivary Gland Ducts
The cornerstone in salivary duct ion transport is NaCl absorption (and KHCO3 secretion), and it is apparent that Na+ and Cl− excretion curves are following each other (Fig. 12.3a and c). One possible mechanism for NaCl absorption is the electroneutral model—luminal Na+/H+ and Cl−/HCO3− exchangers. The alternative model is the parallel activity of epithelial Na+ channels (ENaC) and CFTR (Fig. 12.4c). There is evidence for both systems and it has been proposed that ducts of low-HCO3− secretors (mouse and rabbit SM) are dominated by Na+ and Cl− channels on the luminal membrane, while ducts of high HCO3− secretors (rat SM) are dominated by the double exchangers (Chaturapanich et al. 1997).
There is solid evidence for ENaC expression on the luminal membrane of salivary ducts (Fig. 12.4c), and ENaC is regulated by ubiquitin-protein ligase Nedd4 (Komwatana et al. 1996b; Dinudom et al. 1998, 2001; Cook et al. 2002). There are number of electrophysiological and inhibitors studies on isolated ducts and glands supporting the evidence for ENaC, e.g., low concentrations of amiloride leads to increased NaCl content in saliva (Bijman et al. 1981; Komwatana et al. 1996a). CFTR is expressed on the luminal membrane of salivary ducts and its inhibition by specific inhibitors and CFTR knockout leads to decreased NaCl absorption and increased salt excretion in saliva, as seen in murine models of CF (Dinudom et al. 1995; Zeng et al. 1997; Catalan et al. 2010). If CFTR is to transport Cl− from lumen to the cell, it requires markedly depolarized luminal membrane potential, which has not been measured, but quantitative modeling of salivary ion transporters strongly supports this model (Patterson et al. 2012). Exit pathway for Cl− on the basolateral membrane is not clear and proposals include a hyperpolarization-activated Cl− channel (Clcn2), KCl cotransporter (KCC1), or Cl−/HCO3− exchanger (AE4) (Romanenko et al. 2008; Roussa et al. 2002; Ko et al. 2002a). Note that the basolateral membrane needs to be more hyperpolarized than the luminal to permit Cl− exit out of the cell toward interstitium.
The molecular basis for the alternative electroneutral NaCl transport model is more difficult to pinpoint. Salivary ducts express NHE2 and NHE3 on the luminal membrane and their function is not clear, as knockout of either NHE isoform has no effect on salivary secretion in mice (Park et al. 2001b; Lee et al. 1998). Salivary ducts also express SLC26A4 and A6 (Shcheynikov et al. 2008) and although they differ in coupling Cl−: HCO3− (i.e., 1:1 versus 1:2), they could ensure Cl− influx into duct cells and HCO3− efflux, and it seems that either one can explain the excretory curves in a model simulation of salivary ducts (Patterson et al. 2012).
There are several other transporters that could contribute to duct HCO3− secretion (or HCO3− absorption). On the basolateral membrane, the means of HCO3− import into duct cells could be NBCe1 (e.g., guinea pig SM) or NBCn1 (e.g., rat SM ducts, human SM, and P) (Li et al. 2006; Gresz et al. 2002). Alternatively, or in addition, NHE1 on the basolateral membrane together with CAII could be a part of HCO3−/H+ system involved in secretion and/or pHi regulation (Park et al. 1999). Interestingly, some NBC transporters, NBC3 and NBCe1, are also expressed on the luminal membrane of several types of salivary ducts, and their proposed functions are to absorb (salvage) HCO3− (Park et al. 2002b; Li et al. 2006; Gresz et al. 2002). Presumably, HCO3− ductal absorption would occur if salivary acini were secreting primary fluid rich in HCO3− (see below).
Regarding H+ pumps, immunohistochemical studies show a presence of V-ATPases in intracellular compartments in SM granular and main ducts in rats with normal acid-base balance, but in the rat parotid gland, the pumps are close to the luminal membrane of the striated and excretory ducts (Roussa et al. 1998; Roussa and Thevenod 1998). The pump has not been studied functionally, though heterogenic distribution may indicate that the parotid glands have special acid/base challenges. The H+/K+ pump (not the putative passive H+/K+ exchanger, Fig. 12.4c) has not been seriously considered in salivary glands, except for one study where proton pump inhibitors (omeprazole and SCH28080) did not have effect on pHi on striated ducts from the rat parotid in a given experimental condition (Paulais et al. 1994).
In many species, the collected saliva has K+ concentrations several-fold higher than the plasma and it is inversely related to secretory rate, and often HCO3− excretion follows similar pattern (see exception Sect. 12.3.4). Therefore, it is not surprising that one of the original proposals to explain KHCO3 secretion (Knauf et al. 1982; Paulais et al. 1994) was a K+/H+ exchanger working in parallel with Na+/H+ exchange and Cl−/HCO3− exchange (Fig. 12.4c). Together these would perform a functional K+/HCO3− cotransport on luminal membrane and provide Na+ and Cl− for the basolateral exit. Such K+/H+ transporter has not been cloned and several studies show that K+ and HCO3− transport is not very tightly coupled (Nakamoto et al. 2008; Chaturapanich et al. 1997). Therefore, other K+ exit pathways have been considered, such as the luminal K+ channels. Striated and excretory SM ducts express KCa1.1 channels and knockout studies show that much decreased K+ secretion in whole glands, indicating that indeed these channels are important for ductal secretion (Nakamoto et al. 2008).
Using the above transport components for salivary glands ducts, and experimental values obtained from various studies and modeling, it has been possible to reproduce excretory curves similar to Fig. 12.3 for major ions in saliva, including HCO3− concentrations 35–45 mM and K+ of 20–60 mM (Patterson et al. 2012).
12.3.4 Salivary Glands Can Secrete Very High Bicarbonate and/or Potassium: Where and When?
Now that the basics of ion transport in salivary gland acini and ducts have been presented, we can consider three special circumstances in which some salivary glands secrete saliva with very high HCO3− concentrations, with/without accompanying K+ (Fig. 12.3b and d), and try to resolve how this happens.
First of all, as introduced above, ducts can secrete HCO3− (and K+), though without accompanying water fluxes and the lower the secretory rate of saliva is, the higher the concentrations of HCO3− and K+ are (Fig. 12.3b and d hyperbolic curves). These patterns can be particularly dominant with some forms of stimulation (see Sect. 12.3.5).
Salivary glands of some animals have a large capacity to secrete HCO3− and concentrations are almost as high as in the pancreas. This is the case for foregut fermenters such as sheep, cattle, camels, and kangaroos, where usually parotid glands supply well-buffered saliva to stabilize pH of the fermenting digesta and saliva can contain 110–120 mM HCO3− (and also 20–60 mM phosphate), though K+ is relatively low 5–15 mM (Kay 1960; Young and van Lennep 1979; Beal 1984) (Fig. 12.3). The analysis of the relationship between the salivary flow rate and electrolyte concentrations suggests that the primary secretion itself must be high in HCO3− concentration and that the duct contribution is relatively small. This conclusion is supported by micropuncture studies on sheep parotid, which showed that Cl− concentrations were about 50 mM (Compton et al. 1980). By inferences then, HCO3− must have been correspondingly high (due to very small volumes H+/HCO3− could not be measured) and that furosemide, and inhibitor of NKCC1, had a relatively small effect on saliva secretion (Wright et al. 1986). Further studies showed that the sheep parotid acini exhibited acetylcholine stimulated a Na+-dependent HCO3−-influx and a Na+-HCO3− cotransport was proposed (Poronnik et al. 1995; Steward et al. 1996). Subsequently, it was verified that bovine parotid acini express NBCe1B at high levels (on mRNA and protein levels) and show large electrogenic currents (Yamaguchi and Ishikawa 2005) (Fig. 12.4b). The transporter is localized on the basolateral membrane of acini and interacts with IRBIT (Yamaguchi and Ishikawa 2012).
The NBCe1 transporter is also expressed in other acini, such as rat and human parotid acini, and this is in accordance with observations that parotid glands can secrete saliva with relatively high HCO3− concentrations (Roussa et al. 1999; Park et al. 2002a; Bardow et al. 2000). Correspondingly, the excretory curve patterns are consistent with the acinar origin of HCO3− secretion (Fig. 12.3b). In submandibular glands, acinar secretion is not dependent on HCO3− driven transport normally, as the Cl− driven transport is dominating (Case et al. 1982, 1984). Nevertheless, if Cl− transport is inhibited, these glands can also produce saliva with concentrations >100 mM HCO3− (although secretion rate is diminished) (Fig. 12.3b rabbit SM). This indicates that, most likely, SM acini also have the machinery to transport and drive HCO3− transport (Novak and Young 1986). This is in fact revealed if glands are stimulated with β-adrenergic rather than muscarinic agonists (Case et al. 1980).
12.3.5 Regulation of Salivary Gland Secretion
The main regulation of saliva secretion is via the autonomic nervous system, and little if any by hormones (Proctor and Carpenter 2014). Parasympathetic and sympathetic stimulation leads to activation of muscarinic M1 and M3 receptors and α1 receptors on salivary gland cells, respectively, that via Gq/11 eventually leading to Ca2+ signaling. Furthermore, noradrenaline via β receptors and Gs and stimulates cAMP/PKA signaling pathway. Both parasympathetic and sympathetic stimulations result in approximately similar plasma-like primary secretion, as measured in, e.g., rat SM (Young and Martin 1971). However, parasympathetic stimulation causes 6–8 times higher fluid secretion compared to sympathetic one, the latter resulting in more concentrated protein secretion. Salivary ducts are also well innervated by autonomic nerves and they modify secretion mainly by stimulating K+ and HCO3− secretion (Young and Martin 1971; Martin and Young 1971). In particular, β-adrenergic receptors stimulation can result in disproportionately greater effect on K+ and HCO3− concentrations in small volumes of final saliva (Case et al. 1980) (Figs. 12.3b and d dotted lines). Furthermore, sympathetic stimulation via β-adrenergic receptors (at low concentrations) can modulate saliva composition by activation of CFTR, and thereby increasing the rate of Na+ absorption (Dinudom et al. 1995).
Effects of acetylcholine and noradrenaline are modulated by a number of non-adrenergic non-cholinergic (NCNA) co-transmitters, including VIP, substance P, neuropeptide Y, NO, ATP, and others (Pedersen et al. 2013a; Proctor and Carpenter 2014). Under physiological conditions, it may be expected that multiple transmitters would collaborate to induced salivary fluid and protein secretion. The synergistic effects of cAMP/PKA and Ca2+ signaling have been demonstrated in many studies and recent data indicate that IRBIT mediates synergism between these signaling pathways (Bruce et al. 2002; Ahuja et al. 2014; Jung and Lee 2014).
Extracellular nucleotides/sides are probably NANC and also autocrine/paracrine signaling molecules that have an important function in coordinating salivary gland functions including HCO3− secretion. The purinergic signaling has been pioneered in salivary glands (see Novak 2011). It is most likely that ATP is a cotransmitter with acetylcholine or noradrenaline and a number of P2 receptors are expressed and functional on salivary gland acini. In particular, P2X4 and P2X7 receptors have been described in early studies and it is clear that in physiological conditions they do not form permeable pores, but rather remain cation channels/receptor that co-regulate secretion, for example, by aiding Ca2+ signaling and stimulating K+ and Cl− channels. Recent studies show that in the rat parotid acini P2X4 receptors are located on the basolateral membrane, while P2X7 receptors are enriched on the luminal membrane, and they evoke spatially distinct Ca2+ signals and effects on protein exocytosis (Bhattacharya et al. 2012), and fluid secretion in mouse salivary glands (Nakamoto et al. 2009; Novak et al. 2010) (Fig. 12.4). Interestingly, the P2Y receptors are expressed transiently, e.g., during gland development (P2Y1) and stress (P2Y2). Furthermore, ATP is most likely also stored in secretory granules and it is secreted into duct lumen and ATP can be detected in the whole saliva (Ishibashi et al. 2008; Novak et al. 2010). In addition, the duct epithelium also releases ATP spontaneously or in response to, for example, mechanical stimulation (Shitara et al. 2009; Ryu et al. 2010). Here it acts via P2Y2 receptors to increase Cl− absorption by stimulating CFTR (Ishibashi et al. 2008). It is not clear how HCO3− transport is affected and how luminally expressed P2X7 receptors regulates salivary duct function.
12.4 Hepatobiliary System
12.4.1 Hepatobiliary System: Concerted Action of Several Types of Epithelial Cells
The hepatobiliary system shares many similarities with the pancreas. It secretes highly complex bile containing 95% water with electrolytes, bile salts and bilirubin, lipids, proteins, enzymes, peptides and amino acids, nucleotides, heavy metals, and vitamins (Boyer 2013). Most importantly, bile contains 15–55 mM HCO3−. The hepatobiliary system is composed of several types of epithelial cells: hepatocytes, cholangiocytes, and gallbladder epithelial cells; all of which contribute to the formation of bile in several stages. Hepatocytes, the major liver cell population (65%) secrete primary or canalicular bile, which is driven by bile salt-dependent transport and bile salt-independent transport of bicarbonate and reduced glutathione, and osmotically obliged water flux. Canalicular bile is delivered to the intrahepatic bile ducts, which are lined with cholangiocytes, forming about 3–5% of liver cell population, and these modify bile by secretion and absorption (Fig. 12.5a). The intrahepatic biliary duct system is the most important epithelium in the liver regarding the ability to secrete HCO3− containing fluid in response to secretin and other regulators. This secretion contributes to alkalization and fluidization of bile, which prevents protonation and absorption of weak lipophilic acids. In addition, distally it provides the buffer function in the duodenum—similar to the pancreatic duct system (Boyer 2013). In the last stage, gallbladder concentrates and stores bile, but also modifies it by secretion in some species, and lastly delivers it to the duodenum during feeding. Assuming that in a human the canalicular bile acid-independent flow and bile duct flow together are about 4 μl/min/kg body weight and final bile contains 15–55 mM HCO3− (Banales et al. 2006b; Boyer 2013) it can be approximated that HCO3− output is about 0.3–0.7 μmol/h/g liver weight. Considering the whole organ though, HCO3− output of 300–700 μmol/h is a significant output.
(a) Schematic diagram showing a simplified hepatobiliary system composed of hepatocytes and cholangiocytes. Hepatocytes secrete canalicular bile fluid driven by bile-salt dependent fraction and bile-salt independent fraction. Cholangiocytes secrete Cl−, HCO3−, and fluid and absorb bile acids, glucose, amino acids, and water. (b) Insert shows cellular model for bile-salt independent HCO3−—driven fluid secretion only. (c) Insert shows cellular model for ion transport and regulation in cholangiocytes with a primary cilium (expressing several receptors and channels). CFTR* indicates that CFTR is functional in large but not small cholangiocytes
12.4.2 Canalicular Bile Salt-Independent Flow Generated by Hepatocytes
Canalicular bile fluid is secreted by hepatocytes and secretion has two components (see above). The bile-salt dependent fraction is driven by a series of organic anionic transporters, BA transporters, multidrug resistance transporters (MRP) (Esteller 2008). The bile salt-independent fraction is driven by the transport of glutathione and HCO3−, each contributing about 50% to this fraction (Banales et al. 2006b; Boyer 2013; Esteller 2008). Glutathione is secreted via organic anion transporter MPR2/ABCC2. Bicarbonate is secreted via a DIDS-sensitive Na+-independent Cl−/HCO3− exchanger (AE2) expressed on the luminal membrane (Fig. 12.5b) (Meier et al. 1985; Martinez-Anso et al. 1994). Apparently, CFTR is not expressed in hepatocytes and it might be another Cl− channel that together with AE2 coordinates or performs HCO3− secretion, and water follows via AQP8 (Banales et al. 2006b). HCO3− loaders on the basolateral membrane of hepatocytes are most likely NHE1, and also NHE2 and 4 (Moseley et al. 1986; Pizzonia et al. 1998). In addition, NHE3 is expressed on the canalicular membrane and more importantly on the apical membrane of cholangiocytes, where it most likely participates in fluid absorption (Mennone et al. 2001). Electrogenic Na+-HCO3− cotransport was demonstrated in studies using plasma membrane vesicles and microlectrodes and subsequently, NBCe1/SLC4A4 and NBCe2/NBC4/SLC4A5 were identified (Fitz et al. 1989; Renner et al. 1989). However, the expression of NBCe1 is low while NBC4, especially NBC4c variant, is high in the liver (Pushkin et al. 2000; Abuladze et al. 2004).
12.4.3 Intrahepatic Biliary Duct System: Ion Transport in Cholangiocytes
Intrahepatic biliary duct system is a network of interconnecting bile ductules and ducts that extend from canals of Hering, partly lined with hepatocytes, to ductules (<15 μm) lined with cholangiocytes, and then converge to large bile ducts (>15 μm) of increasing diameter to interlobular, septal, segmental ducts and then hepatic and common hepatic duct (Tabibian et al. 2013). Along the duct system, cholangiocytes change in size, morphology, regulation, and function. Although they account only for 3–5% of the liver population, they are responsible for about 30–40% of bile volume, depending on species. The magnitude of the ductal contribution of basal bile flow depends on species and can vary from 10 to 30%. Primarily cholangiocytes secrete Cl−, HCO3−, and fluid, and they reabsorb bile acids, glucose, and amino acids. Secretin stimulates HCO3− secretion and this determines the pH and hydration of bile, and thereby decreases protonation of glycine-conjugated bile acids and thus their passive reabsorption (see Sect. 12.4.5), and lastly, it contributes to alkalization of duodenal contents (Boyer 2013; Beuers et al. 2012). In the following section, we will only focus on cholangiocyte ion channels and transporters involved in HCO3− transport.
Ductal bile secretion is a regulated process that is initiated by the transport of Cl− across the luminal membrane into the ductal lumen and coupling to AE2, as verified in isolated bile duct unit preparations (IBDU). CFTR has been identified and it is functional in large but not small IBDU (Fitz et al. 1993; Alpini et al. 1997; Cohn et al. 1993; Dutta et al. 2011). However, some studies have challenged the role of CFTR as the primary route for Cl− exit; rather they ascribed the protein a regulatory role in ATP release (see below). In response to mechanical stimulation (fluid flow and cell swelling) and ATP stimulation, cholangiocytes isolated from various species exhibit CaCC (Tabibian et al. 2013). The molecular identity of these channels was unknown until recent studies showed that TMEM16A/ANO1 is a good candidate (Dutta et al. 2011, 2013). It seems that CFTR is only expressed in larger bile ducts, while TMEM16A is expressed in both small and large bile ducts (Dutta et al. 2011). Interestingly, CaCC contribution to anion transport is several-fold higher than CFTR.
Similar to pancreatic ducts, Cl− channels are functionally coupled to Cl−/HCO3− exchanger and AE2 is indeed the main effector of both basal and stimulated Cl−/HCO3− exchange (Banales et al. 2006a, b; Strazzabosco et al. 1997; Aranda et al. 2004; Concepcion et al. 2013). Interestingly, AE2–/– mice develop biochemical, histological, and immunological alterations resembling primary biliary cirrhosis (Concepcion et al. 2013). The luminal membrane of cholangiocytes also expresses amiloride-insensitive NHE2/SLC9A2 and amiloride-sensitive NHE3/SCL9A3 and NHE4/SLC9A4 (Banales et al. 2006b). The function of these is not clear, though NHE3 knockout studies showed that the exchanger is important in fluid absorption in resting duct epithelium and also interacts with CFTR (Mennone et al. 2001). The Cl− secretory response is maintained by small conductance Ca2+-activated K+ channels, SK2 (KCa2.2, KCNN2) expressed on the luminal membrane (Feranchak et al. 2004).
NHE1 is expressed on the basolateral membrane of cholangiocytes and it has a multitude of functions, including regulation of pHi and cell volume, and transepithelial HCO3− transport in rat and human bile ducts (Spirli et al. 1998; Banales et al. 2006b). In addition, another acid extruder, V-ATPase was identified and functional in pig cholangiocytes (Villanger et al. 1993), though it seems not important in human cholangiocytes (Strazzabosco et al. 1997). CA is also expressed in bile ducts, though compared to other HCO3− secretory organs CA activity is rather low (Banales et al. 2006b).
Regarding the Na+ coupled HCO3− import mechanisms across the basolateral membrane, there seem to be some interspecies variations (Fig. 12.5c). Na+-dependent Cl−/HCO3− exchanger, NDCBE1/SLC4A8, which has been cloned in humans (Grichtchenko et al. 2001) is assumed to import HCO3− across the basolateral membrane of cholangiocytes (Strazzabosco et al. 1997; Banales et al. 2006b). In rat cholangiocytes, several HCO3− transport mechanisms are functional as revealed by pHi measurements (Strazzabosco et al. 1997). But apparently in the rat liver only the NBC4c variant of NBCe2/NBC4/SLC4A5 is expressed in cholangiocytes and hepatocytes, though in cholangiocytes it is expressed apically (Abuladze et al. 2004). In murine cholangiocytes, NBCe1/SLC4A4 is expressed on the luminal membrane (Uriarte et al. 2010), and in order to secrete HCO3− into the lumen, it would require coupling of several HCO3− to one Na+ and highly hyperpolarized membrane potential.
K+ channels are providing the driving force secretion and at least two types of channels are expressed on the basolateral membrane—KCa3.1 and SK2/KCa2.2 (KCNN2) (Dutta et al. 2009; Feranchak et al. 2004). NKCC1 and Na+/K+-pump are also expressed on the basolateral membranes. A number of AQP are expressed in cholangiocytes, in particular, AQP4 on the basolateral membrane and AQP1 on the luminal membrane, though there are species differences (Banales et al. 2006b; Tabibian et al. 2013).
There are additional transport systems in cholangiocytes in addition to those dealing with anion transport. Cholangiocytes take up bile salts via Na+-dependent transporter ABAT or ASBT (SLC10A2) and released them across the basolateral membrane through truncated isoform of the same carrier (Tabibian et al. 2013; Banales et al. 2006b). Glucose is taken up by sodium-glucose transporter SGLT1 (SLC5A1) and released by GLUT1 (SLC2A1) (Tabibian et al. 2013). Glutathione, the tripeptide that is one of the principal driving forces for canalicular bile salt-independent secretion, is degraded by γ-glutamyltranspeptidase, expressed in cholangiocyte apical membranes, and resulting glutamate, cysteine and glycine are reabsorbed by Na+-dependent transport in cholangiocytes (Tabibian et al. 2013).
12.4.4 Gallbladder Epithelium
The function of the gallbladder is to store bile and concentrate it during interdigestive phases by salt-dependent water reabsorption. Most studies on the rabbit and Necturus gallbladders showed that this transport was electroneutral (Na+/H+ and Cl−/HCO3− exchangers in parallel) and these epithelia have low resistance and are regarded as a leaky type (Petersen and Reuss 1983; Petersen et al. 1990). However, in studies on human and primate gallbladders it was discovered that these electrically silent absorptive organs can secrete, under the influence of secretin, HCO3−-containing fluid after meals (Igimi et al. 1992; Svanvik et al. 1984). This secretion seems to depend on CFTR-like cAMP activated channels that have relatively high permeability to HCO3− (Meyer et al. 2005). The importance of CFTR is highlighted in pig models of cystic fibrosis, where the disruption of CFTR leads to gallbladder and bile duct abnormalities (Rogers et al. 2008). Furthermore, prairie dog gallbladder shows forskolin-induced short-circuit current that was due to CFTR (Moser et al. 2007). This preparation is often used as an experimental model of human cholelithiasis, due to its unique propensity for developing gallstones on high-cholesterol chow. The basolateral HCO3− transport in gallbladder epithelium is carried out by pNBC1 and the driving force for secretion is maintained by cAMP stimulated K+ channels that are also sensitive to Ca2+ and pH, but their identity is not clear (Moser et al. 2007; Meyer et al. 2005).
12.4.5 Regulation of Bile Formation
The bile formation that occurs in at least three steps is also regulated at three levels. The first step is the canalicular bile fluid secretion by hepatocytes that occurs continuously and is relatively poorly regulated (Esteller 2008; Banales et al. 2006b). Interestingly, one regulator of canalicular HCO3− secretion is glucagon (Lenzen et al. 1997; Alvaro et al. 1995), which increases cAMP and stimulates the insertion of vesicles containing Cl−/HCO3− exchanger (AE2, SLC4A2) and AQP8 (Gradilone et al. 2003). In the last step, gallbladder secretion is regulated by secretin and gallbladder contraction is stimulated by CCK.
The most extensive regulation of bile modification (secretion and absorption) occurs in bile ducts (Banales et al. 2006b; Tabibian et al. 2013). Firstly, there are regulatory factors modifying the basal secretion of cholangiocytes. The most important hormone is secretin that is released as a physiological response to meals; the other two are bombesin (gastrin-releasing peptide) and vasoactive intestinal peptide (also a neurotransmitter). Secondly, acetylcholine and noradrenaline potentiate secretin- stimulated HCO3− and fluid secretion. Thirdly, many factors such as somatostatin, gastrin, insulin, dopaminergic agonists, α2 adrenergic receptor agonists, endothelin, GABA, and cytokines (e.g., IL1, IL6, TNFα), inhibit basal and secretin-stimulated cholangiocyte secretion. Lastly, there a number of bile-borne factors (flow and osmolality, amino acids, glucose, nucleotides, bile acids), that regulate cholangiocyte function. Here, we will focus on regulation by nucleotides and bile acids, similar to the section above dealing with pancreatic ducts.
12.4.5.1 Purinergic Signaling
Both hepatocytes and cholangiocytes release adenosine nucleotides, they express a number of ecto-nucleotidases, and both cell types express a number of P2 and P1 receptors (Schlosser et al. 1996; Chari et al. 1996; Fausther and Sevigny 2011). Cholangiocytes exhibit shear/flow-sensitive and cell volume sensitive ATP is release, as well as release stimulated by forskolin and ionomycin, and it has been proposed that CFTR is regulating this ATP release (Schlosser et al. 1996; Chari et al. 1996; Fiorotto et al. 2007; Minagawa et al. 2007). Nevertheless, it seems that ATP release is larger in small upstream cholangiocytes compared to downstream ones, indicating that smaller ducts could signal to larger ones via paracrine ATP signal (Woo et al. 2008), and that other mechanism than CFTR are important in regulating ATP secretion. In fact, recent studies indicate that ATP is also released by vesicular exocytosis similar to the pancreas (Feranchak et al. 2010; Woo et al. 2010).
Both P2 and adenosine receptors regulate cholangiocytes by stimulating K+, Cl−, and HCO3− secretion. P2 receptors on the basolateral membrane of cholangiocytes, similar to acetylcholine evoked Ca2+ signaling, seem to have minimal effect on bile duct secretion (Nathanson et al. 1996), possibly due to rapid hydrolysis of nucleotides (Dranoff et al. 2001). The most prominent effects are relayed by the P2 receptors on the luminal membrane, many of which stimulate Ca2+ signaling or induce Ca2+ influx, which leads to stimulation of CaCC, short-circuit current and ductal alkalization (Woo et al. 2010; Dranoff et al. 2001). Mechanosensitive ATP release stimulates TMEM16A/ANO1 in human cholangiocytes from small and large biliary ducts (Dutta et al. 2011, 2013). There a number of receptors expressed on cholangiocytes, e.g., mouse and rat express P2X4 and P2Y2, as well as P2Y1, P2Y4, P2Y6, P2Y12, and P2Y13 receptors (Woo et al. 2010; Dranoff et al. 2001; Doctor et al. 2005). Interestingly, the P2Y12 receptor is expressed on the primary cilium and induces cAMP signaling (Masyuk et al. 2008).
12.4.5.2 Bile Acids
Bile acids are synthesized from cholesterol in the liver and excreted into bile and subsequently into the small intestine, where they facilitate digestion and absorption of dietary fats and fat-soluble vitamins. Bile acids are also signaling hormone-like molecules that act on many tissues/cells in our body and do so by activating several different receptors and sensing proteins including nuclear farnesoid X receptor (FXR), pregnane X receptor (PXR) and G-protein coupled receptor TGR5 (Gpbar-1) (Keitel and Haussinger 2013; Pols et al. 2011). FXR and PXR are strongly expressed in hepatocytes, while TGR5 is strongly expressed in cholangiocytes.
Cholangiocytes are exposed to millimolar concentrations of bile acids and conjugated salts. Such concentrations would be toxic to cholangiocytes and several defense mechanisms are at hand. One of these is the formation of micelles of phospholipids and bile salts. Another defense mechanism proposed is the biliary HCO3− secretion (van Niekerk et al. 2018). It has been suggested that BA profile/composition changes radially from the midstream to the apical membrane of cholangiocytes (Keitel and Haussinger 2013). Cholangiocytes express TGR5 in the primary cilia, the apical membrane, and sub-cellular (Keitel et al. 2010; Masyuk et al. 2013). In particular, the primary cilia, which are mechano-, chemo-, and osmosensors, could sense BA and via TGR5 receptors that trigger cAMP signaling pathways that stimulate CFTR and AE2 and thus HCO3− secretion. Bile acids also stimulate CaCC directly (Shimokura et al. 1995), or through CFTR regulated ATP release and P2 receptor-mediated stimulation of CaCC (Fiorotto et al. 2007). The outcome of these events would be the formation of unstirred HCO3− layer, so-called “HCO3− umbrella”, which together with dense glycocalyx creates a protective microenvironment at the apical membrane of cholangiocytes and prevents the protonation of glycine-conjugated bile acids dominating in human bile (Beuers et al. 2010; Keitel and Haussinger 2013; van Niekerk et al. 2018). Thereby, cholangiocytes would be protected from diffusion of these polar and pro-apototic acids. Relevance of this system is demonstrated in patients with primary biliary cirrhosis, in which AE2 expression in bile ducts is reduced, leading to defective HCO3− secretion and BA mediated cell injury (Keitel and Haussinger 2013).
12.5 Duodenum
The pH of the duodenal luminal contents is more or less dictated by the acid chyme arising from the stomach until the pH is neutralized by the pancreatic and hepatic outlets. In order to withstand the potentially damaging effect of low bulk pH, the duodenal epithelium creates a mucous layer. This layer is supplemented with HCO3− from the epithelial cells to create a protective barrier toward acid and contributes to pH neutralization of duodenal contents (Allen and Flemstrom 2005; Flemstrom and Kivilaakso 1983; Williams and Turnberg 1981; Ainsworth et al. 1990, 1992). In rabbit duodenum, HCO3− secretion amounts to 154 μEQ/cm/h, while other species have lower rates (Flemstrom et al. 1982). Duodenal ulcers result from an unbalance between protective factors such as mucus and HCO3−, and the aggressive factors gastric acid, pepsin, and the infestation with pathogenic Helicobacter pylori. The long-prevailing model for duodenal epithelial HCO3− secretion was inspired by studies of the exocrine pancreas given above. As mentioned, the functional coupling of a luminal surface Cl− channel and a Cl−/HCO3− exchanger is fuelled by intracellular conversion of CO2 and H2O to form HCO3− and H+ catalyzed by intracellular carbonic anhydrases. The intracellular pH is normalized by basolateral NHE mediated extrusion of protons. The essence of this model is still standing, but a number of recent investigations have greatly expanded and refined the understanding of the molecular machinery for duodenal bicarbonate secretion (Fig. 12.6).
Schematic diagram showing a current model for HCO3− secretion from duodenal enterocytes. In this model, secreted HCO3− does not arise from the blood side CO2 and intracellular conversion to HCO3− and H+. Instead, CO2 enters from the luminal side, which has high pCO2, and potentially counteracting the HCO3− secretion and challenging the cellular pH homeostasis. HCO3− for maintaining intracellular pH and for secretion may arise from the basolateral HCO3− import
The involvement of interstitial CO2, which enters the cells and CA and generates HCO3− for secretion, has been challenged. In the duodenum, the luminal pCO2 is higher than anywhere else in the mammalian body (Rune and Henriksen 1969), due to the massive neutralization of gastric acid mainly by the pancreatic bicarbonate and hepatobiliary outlets. CO2 crosses the luminal membrane to allow the enterocytes to sense and respond to luminal acid in a complex interplay with cytosolic carbonic anhydrase and a moderating effect of luminal carbonic anhydrases (Holm et al. 1998; Kaunitz and Akiba 2002, 2006a, b; Sjoblom et al. 2009). Beside the cytosolic CAII, the duodenal enterocytes express the membrane-bound luminal CAXIV and basolateral CAIX (Lonnerholm et al. 1989; Saarnio et al. 1998). As reviewed recently, it is most likely that the CA activity is centrally involved in acid sensing and signaling in the duodenal enterocytes rather than participating in HCO3− secretion directly (Sjoblom et al. 2009).
Isolated duodenal epithelial cells exhibit NHE activity (Ainsworth et al. 1996, 1998; Isenberg et al. 1993). The findings were first interpreted as basolateral NHE1 activity according to the general working model. However, a subsequent study of mouse duodenal enterocytes reported several distinct NHE activities and evidenced the molecular expression of NHE1 in the basolateral membrane, and NHE2 and NHE3 in the luminal membrane (Praetorius et al. 2000; Praetorius 2010). NHE1 seems expressed at low abundance as compared to NHE2 and NHE3 and is an unlikely mechanism for eliminating intracellularly produced protons from duodenal enterocytes. Interestingly, Na+-HCO3− cotransport may serve an important role in the duodenum as a supply for intracellular HCO3− as a defense against intracellular acid formed from CO2 entering from the lumen (Akiba et al. 2001a, b). The robust expression of the HCO3− loaders NBCe1 and NBCn1 in the basolateral membrane of duodenal epithelial cells spurred the hypothesis that HCO3− is transported from the interstitial space to the lumen via a transcellular route, where the two transporters import HCO3− in symport with Na+ from the interstitium to the cell (Praetorius et al. 2001).
Acidified rabbit enterocytes display potent Na+-dependent recovery of pHi in the presence of the CO2/HCO3− buffer system (Ainsworth et al. 1996; Isenberg et al. 1993). DIDS and H2DIDS only slightly affected the recovery rate from the acid load. A similar DIDS-insensitive Na+ dependent HCO3− import was observed in mouse (Praetorius et al. 2001) and rabbit duodenal enterocytes (Jacob et al. 2000b). Human duodenal enterocytes express both NBCn1 and NBCe1, although immunoreactivity for the latter is subtle compared to the renal and pancreatic staining (Damkier et al. 2007). Nevertheless, the DIDS-sensitive electrogenic NBCe1 has been put forward by several investigators as the main HCO3− import mechanism for transcellular HCO3− secretion, although both NBCn1 transport and the duodenal Na+-HCO3− import are relatively DIDS insensitive (Jacob et al. 2000a; Praetorius et al. 2001). Importantly, based on NBCn1 knockout studies in mice, it was recently shown that NBCn1 is the major contributor to Na+-dependent HCO3− transport as well as basal and acid-induced HCO3− secretion (Chen et al. 2012; Singh et al. 2013b).
The mechanism by which duodenal enterocytes extrude HCO3− across the luminal membrane has been a matter of intense debate. Many transporters and channels have been investigated, among which CFTR and the proteins DRA (SLC26A3), PAT1 (SLC26A6), and SLC26A9 have been the most prominent examples. First, it is firmly established that basal and agonist-induced duodenal HCO3− secretion strongly depends on CFTR expression (Clarke and Harline 1998; Hogan et al. 1997a, b; Seidler et al. 1997). It is still not clear how much CFTR conductance contributes to HCO3− secretion directly. First, HCO3− extrusion does not depend on CFTR, at least in isolated duodenal villus cells (Praetorius et al. 2002). Second, DRA mediates electroneutral Cl−/HCO3− exchanger (Alper et al. 2011), is involved in NaCl absorption throughout the leaky intestinal segments, and seems mainly expressed in the lower villus/crypt axis in the duodenum (Walker et al. 2009). The study indicates that DRA contributes to bicarbonate secretion mainly in the lower villus/crypt. PAT1 is mainly expressed in the proximal small intestine and is prevalent in the villus region where it also functions as the predominant anion exchanger (Simpson et al. 2007; Singh et al. 2013a; Walker et al. 2009). Knockout of PAT1 reduces basal duodenal HCO3− secretion by approximately 70%, while knockout of DRA only induced a 30% reduction (Simpson et al. 2007). However, only a minor role for PAT1 was found in acid-stimulated HCO3− secretion (Singh et al. 2013a). The relevance of this transport system was highlighted in a recent study. Here, H. pylori infestation decreased duodenal mucosal CFTR and SLC26A6 expression via increased serum transforming growth factor β (TGFβ) in both mice and a human duodenal cell line (Wen et al. 2018). The SLC26A9 gene product is mainly expressed in the proximal gastrointestinal tract and found in the crypt region of the duodenum (Liu et al. 2015). SLC26A9 is associated with anion conductance and the corresponding knockdown mice have reduced acid-induced duodenal HCO3− secretion and worsened intestinal function and survival of CFTR-deficient mice (Singh et al. 2013a; Walker et al. 2009; Liu et al. 2015). An integrative model of duodenal bicarbonate secretion is shown in Fig. 12.6.
Among the many stimulators of duodenal bicarbonate secretion, luminal acid is a physiological and effective agonist (Flemstrom and Kivilaakso 1983; Isenberg et al. 1986; Allen and Flemstrom 2005). The neurohumoral control of duodenal HCO3− transport is mediated either directly by cholinergic innervation acting on M3 receptors and indirectly via paracrine melatonin secretion from enterochromaffin cells, both using Ca2+ signaling to induce stimulation (Sjoblom and Flemstrom 2003). The same study shows that CCK also enhances HCO3− secretion through Ca2+ signaling. One of the first agonists to be discovered was PGE2, which acts via cAMP as well as Ca2+ signaling (Aoi et al. 2004), while the stimulatory effect of enterotoxin and guanylins occurs through cGMP increases (Guba et al. 1996; Joo et al. 1998). The intracellular signals are most likely to stimulate HCO3− secretion via regulation of the luminal membrane transporters and channels, with CFTR being the best documented.
12.6 Renal Intercalated Cells
The overall function of the kidneys is broadly to secure homeostasis and this is achieved by maintaining water and electrolyte balance, acid/base balance, blood pressure, and rid the organism of waste products (Boron and Boulpaep 2012, 2017). The kidneys filter the blood plasma in the renal corpuscles and then modify the filtrate to conserve appropriate amounts of water, nutrients, and salt by secretion and reabsorption processed in an intricate system of renal tubules. As an integral part of the process, the kidney helps to maintain normal acid/base balance by adjusting the secretion of acid/base equivalents. Among the many epithelial cell types in the renal tubular system, a single one has the capacity of secreting HCO3− which is the type-B intercalated cell (IC). These cells are restricted to the late distal convoluted tubules, the connecting tubules, and cortical collecting ducts, and are functionally mirror images of the acid-secreting type-A ICs (Kwon et al. 2012). In the mentioned renal cortical segments, the intercalated cells comprise almost 50% of the tubular cells. Most of the cortical ICs are type-B cells, whereas medullary collecting ducts exclusively contain type-A ICs. Interestingly, the numbers of intercalated cell types can vary in a regulated and dynamic fashion. The number of type-A ICs is increased in response to metabolic acidosis (Schwartz et al. 1985). It seems that the change in relative numbers relies on the ability of type-B ICs to differentiate into type-A ICs, given the proper stimulus (Schwartz et al. 1985). Hensin is a protein that is secreted by the epithelial cells and incorporated in the extracellular matrix and is necessary for cell differentiation (Gao et al. 2010; Al-Awqati 2013; Schwartz et al. 2002). It stimulated differentiation of type-B ICs to type-A ICs only in its polymeric forms induced by binding of certainly activated integrins and the activity of extracellular galectin 3 (Hikita et al. 2000; Schwaderer et al. 2006; Vijayakumar et al. 2008). Figures 12.7a and b depict the two types of cells and evidence for ion transporters is elaborated below.
The bicarbonate secreting type-B IC (or β-IC) was first described in the turtle bladder (Leslie et al. 1973; Stetson and Steinmetz 1985), which like similar cells in toad skin shares the functional significance for HCO3− secretion, as well as transport properties with mammalian renal type-B ICs (Schwartz et al. 1985). The studies showed that these cells were characterized by the luminal extrusion of HCO3− in exchange for Cl−, thereby affecting pH homeostasis in the opposite direction as the already described type-A ICs. The opposite function of the two cell types is accompanied by different polarization of V-ATPases and anion exchangers. The acid-secreting type-A ICs expresses the V-ATPase in the luminal membrane, whereas type-B ICs have basolateral V-ATPase (Brown et al. 1988a). It is now evident that the type-B ICs use the SLC26A4 gene product pendrin to extrude bicarbonate apically into the urine. Pendrin is an anion exchanger shown to function as a Cl−/HCO3− exchanger in the type-B ICs (Royaux et al. 2001). This was the first study to show that pendrin is expressed in the apical membrane of type-B ICs and is involved in HCO3− secretion when animals were challenged by alkalosis, but not under baseline conditions. Importantly, the renal tubules lose apical Cl−/HCO3− exchange and the capability to rid the body of excess base by the urine when the pendrin gene is disrupted (Amlal et al. 2010; Royaux et al. 2001). Type-A ICs expresses a renal specific variant of the anion exchanger AE1 (SLC4A1) in the basolateral membrane to extrude excess HCO3− (Alper et al. 1989). For both types of ICs, the source for acid/base extrusion is the intracellular conversion of CO2 and H2O to protons and HCO3−. The reaction rate is critically dependent on the intracellular carbonic anhydrase, CAII, and therefore inhibited by acetazolamide or CAII gene deletion (Breton et al. 1995; Sly et al. 1983). Pendrin function is sensitive to changes in local pH (Azroyan et al. 2011), whereas the expression level and function are reduced by metabolic acidosis and increased in alkalosis (Frische et al. 2003; Petrovic et al. 2003; Wagner et al. 2002). The key modulator of pendrin expression may not be systemic pH alone but include a concomitant change in circulating Cl− (Hafner et al. 2008; Vallet et al. 2006; Verlander et al. 2006). At the hormone level, there is an agreement that angiotensin II stimulates pendrin either directly or via activation of the V-ATPase (Wagner et al. 2011).
Thus, the acid/base transport models for ICs are quite simple and well established, but have nevertheless been challenged somewhat by more recent findings. First, antibodies against the human NBC3, also known as NBCn1 (SLC4A7) colocalized the protein with the V-ATPase in vesicles and the apical membrane of rat type-A ICs and to the basolateral membrane of type-B ICs (Kwon et al. 2000; Pushkin et al. 1999). The authors detected DIDS-sensitive NBC activity in the apical surface of type–A ICs. These findings were surprising, as NBC would function as an electroneutral alternative transporter for type-A ICs to acidify the urine. In the type-B ICs the function would be to acidify the interstitium in parallel to direct H+ transport by V-ATPase. However, antibodies against the corresponding rat protein, NBCn1, localized the protein basolaterally in rat type-A ICs (Vorum et al. 2000). This localization of NBC is counterintuitive, as a main task for that membrane is in fact base extrusion. It should be mentioned that neither antibody produced labeling of human or mouse renal intercalated cells, but was expressed elsewhere in the kidney in humans (Damkier et al. 2007). Thus, data on NBCn1 expression in the ICs are inconsistent and the lack of renal phenotype of SLC4A7 depleted mice indicates that its function may be much less important than the H+-ATPase function.
Second, recent discoveries have led to a change of the paradigm that intercalated cells are simply acid/base regulating cells, but participate also in Cl− transport. Previously, Cl− was expected to take a paracellular route depending solely on a transepithelial potential difference and Cl− permeable tight junctions such as the aldosterone regulated claudin-4 (Le et al. 2005). Pendrin was established as a transcellular route for connecting tubule and collecting duct Cl− reabsorption (Wall et al. 2004). Genetic deletion of pendrin was shown to eliminate collecting duct Cl− reabsorption in mineralocorticoid- and bicarbonate-treated mice. Thus, pendrin knockout mice are protected from mineralocorticoid-induced hypertension, while overexpression of pendrin leads to hypertension in mice on high-NaCl diet (Jacques et al. 2013; Verlander et al. 2003). The significance of HCO3− secretion by pendrin was also suggested to directly affect the function of the principal cell Na+ loader ENaC (Pech et al. 2010). Whereas single-gene knockout for the distal tubule NaCl reabsorption protein NCC and pendrin are surprisingly benign, mice with NCC-pendrin double knockout have a severe salt and volume wasting alkalotic phenotype, even under control conditions (Soleimani et al. 2012). Thus, according to the current model of collecting duct, Na+ reabsorption and Cl− reabsorption takes place in separate cells—principal cells and type-B ICs, respectively (Wall and Pech 2008). Pendrin now seems to take part in the electroneutral NaCl reabsorption mechanism in addition to its role in alkalization in metabolic alkalosis. It is not known how these roles are separated and regulated.
The SLC4A8 gene product NDCBE1 has been associated with electroneutral and thiazide sensitive NaCl reabsorption in the cortical collecting duct (Leviel et al. 2010). It may reside and function in the luminal membrane of the type-B ICs and in the proposed model (Fig. 12.7), NDCBE1 and pendrin work in concert with two turnovers of pendrin for each NDCBE1 turnover to yield electroneutral, pH neutral NaCl reabsorption through type-B ICs. The model is based on the observations that Na+ reabsorption in collecting ducts are not completely inhibited by amiloride, but contains a thiazide component under some experimental conditions. Also, the authors find luminal Cl− and CO2/HCO3− dependence of the thiazide sensitive Na+ transport in isolated cortical collecting ducts from Na+ depleted animals. Furthermore, they detect NDCBE1 protein expression and mRNA in the relevant tubular segments and demonstrate lack of such transport in tubules from NDCBE1 knockout mice (Leviel et al. 2010). In a separate study, the SLC4A9 gene product, usually named AE4, was suggested as the basolateral Na+ exit pathway in these cells (Chambrey et al. 2013). It was shown, that the AE4 gene deletion greatly reduced thiazide sensitive Na+ and Cl− reabsorption. Interestingly, AE4 is suggested to work as an electrogenic Na+-HCO3− extruder in their model. This would, however, render the total reabsorption process electrogenic, as extrusion of Na+ and HCO3− (depending on the electrochemical gradients) would usually be in a 1:3 stoichiometry and the model, only 1 Cl− follows through ClC channels in the basolateral membrane. As all these discoveries have a profound impact of our understanding of renal electrolyte handling, it is highly important to establish the electrochemical gradients of the type-B ICs in order to validate the model, which also suggests the V-ATPase as the creating mechanism for transmembrane gradients (as opposed to the Na+/K+-ATPase (Chambrey et al. 2013)). It is also necessary to localize the NDCBE1 protein in the apical membrane of type-B ICs, and directly evidence that thiazides target this protein e.g., by ligand binding assays. In Xenopus laevis oocytes, neither NDCBE1 nor pendrin seemed to be the site of action for thiazides, and carbonic anhydrase was only discharged as target in control experiments in untreated collecting ducts (Leviel et al. 2010). Furthermore, before NDCBE1 is recognized as important in renal Na+ and Cl− reabsorption and in blood pressure regulation, it is necessary to report normal urine electrolytes, pH, osmolarity as well as blood pressure values at baseline and in Na+ depleted NDCBE1 KO mice. Whereas principal cell-specific ENaC subunit knockout has a profound impact on baseline Na+ and K+ homeostasis, as well as urine output (Christensen et al. 2010), both pendrin KO and NDCBE1 KO phenotypes seem mild as far as they have been reported.
12.7 Choroid Plexus Epithelium
The choroid plexus epithelium (CPE) produces the majority of the intraventricular cerebrospinal fluid, CSF. Rougemont and colleagues directly demonstrated the involvement of the choroid plexus in CSF formation in 1960 (Rougemont et al. 1960), although this had already been suggested by J. Faivre and H. Cushing (1914) and Faivre (1854). Rougemont’s group established that the solute contents in the nascent CSF differed from a simple plasma ultrafiltrate. Taken together with later studies, the evidence that CSF cannot be a simple ultrafiltrate includes (1) the CSF is approximately 5 mOsM hypertonic compared to plasma (Davson and Purvis 1954); (2) the [Na+] and [HCO3−] are slightly higher, whereas [K+] and [Cl−] are lower than expected in the CSF at equilibrium (Ames III et al. 1964); and (3) there is a 5 mV lumen positive electrical potential difference across the choroid plexus epithelium. Therefore, the choroid plexus must sustain a secretory function, given that most intraventricular CSF arises from this structure. The CSF has many important functions in the central nervous system. CSF fills the internal and external fluid spaces and thereby cushions the central nervous system, keeps the intracranial pressure at controlled levels, and it stabilizes the composition of the brain extracellular fluid. Primarily, CPE secreted fluid contains NaHCO3, and therefore, can neutralize acid produced by neuronal activity. The choroid plexus epithelium also secretes growth factors and nutrients into the CSF that are crucial for the brain development and function (Damkier et al. 2013). Importantly, CSF also helps to remove, i.e., absorb, K+ produced by active neurons, as well as breakdown products of serotonin and dopamine. The composition of newly formed CSF is roughly 150 mM Na+, 3 mM K+, 130 mM Cl−, and 25 mM HCO3− (Davson and Segal 1996; Johanson and Murphy 1990). The choroid plexus produces up to 0.4 ml/g/min fluid, which gives approximately 60 μmol HCO3−/h/g tissue output.
While some mechanisms of CSF secretion are well established, other aspects of secretion are not fully understood. For example, the main transport mechanisms for Na+ into the choroid plexus epithelium from the blood side are not defined. Another central question is how HCO3− is extruded from the cells into the CSF. Clues into the main transport pathways are reflected by the wide profile of drugs inhibiting CSF secretion: ouabain, acetazolamide, amiloride, DIDS, bumetanide, and furosemide (Ames III et al. 1965; Davson and Segal 1970; Johanson and Murphy 1990; Melby et al. 1982; Wright 1972). The secretion rate of CSF is also influenced by the HCO3− concentration at the basolateral side (Mayer and Sanders-Bush 1993; Saito and Wright 1983). Thus, CO2/HCO3− metabolism seems important for CSF secretion. Before describing the putative HCO3− extrusion mechanisms, we will give the general transport machinery that creates and sustains the gradients permitting HCO3− secretion. Lastly, other acid/base transporters that may assist or modify HCO3− secretion are considered.
12.7.1 Basic Secretory Machinery
As opposed to most other epithelia, the choroid plexus Na+/K+-ATPase is localized to the luminal membrane. Ventricular application of ouabain blocks the transepithelial net Na+ flux as well as the CSF secretion (Ames III et al. 1965; Davson and Segal 1970; Wright 1978). The luminal Na+/K+-ATPase expression is confirmed by both immunoreactivity and ouabain binding studies (Ernst et al. 1986; Masuzawa et al. 1984; Praetorius and Nielsen 2006; Quinton et al. 1973; Siegel et al. 1984). The luminal Na+/K+-ATPase directly transports Na+ into the CSF and also maintains the Na+ and K+ gradients that drive most other transport processes in CSF secretion (Fig. 12.8). Na+/K+-ATPase complexes consisting of α1, and either β1 or β2 subunits, which are expressed in the choroid plexus together with the γ-subunit, phospholemman (FXYD1) (Feschenko et al. 2003; Klarr et al. 1997; Zlokovic et al. 1993). These subunits were confirmed in a recent proteomic study on FACS isolated mouse choroid plexus epithelial cells (Damkier et al. 2018). This study also revealed the expression of α2, α4, and β3 Na+/K+-ATPase subunits.
Schematic diagram showing a simplified model of the CSF and thereby HCO3− secretion process by the choroid plexus epithelium. Although HCO3− secretion greatly depends on carbonic anhydrase activity, it also fully relies on a DIDS-sensitive basolateral supply of Na+ and HCO3−. The luminal HCO3− extrusion is electrogenic and CSF pH increases are followed by an increase in CSF Na+ content, indicating a molecular or functional coupling of the extrusion of both ions. Interconversion between CO2 + H2O and H+ + HCO3− is catalyzed by carbonic anhydrases, i.e., luminal CAXII and intracellular CAII. Cl− conductances are characterized as a volume sensitive anion conductance (VRAC) and an inward rectifying Cl− conductance (Clir), whereas the K+ channels probably include Kv1.3, Kv1.1, and Kir7.1. KCCs are also a luminal K+ exit pathway
K+ channels expressed on the luminal membrane have several functions. Firstly, they are important for K+ recycling in connection with the Na+/K+-ATPase activity. Secondly, K+ channels are the main determinant for the membrane potential. This is important for the luminal HCO3− secretion, which is electrogenic. Thirdly, certain K+ channels (inward rectifiers) are also important for cellular uptake of K+ in hyperpolarized voltages in order to provide a K+ sink, e.g., removal of neuron derived K+. K+ channels were first identified in the choroid plexus by patch-clamping the luminal membrane of Necturus maculosa cells (Brown et al. 1988b; Christensen and Zeuthen 1987; Zeuthen and Wright 1981). Several K+ conductances have been identified: an inward-rectifying conductance (Kir7.1 channels), and outward-rectifying conductances (Kv1.1 and Kv1.3) (Doring et al. 1998; Kotera and Brown 1994; Speake and Brown 2004). KCNQ1/KCNE2 channels may also contribute to the outward-rectifying conductance (Roepke et al. 2011). All these K+ channel proteins are expressed in the luminal membrane of rat and mouse choroid plexus (Nakamura et al. 1999; Roepke et al. 2011; Speake and Brown 2004). Proteomic analysis confirmed the expression of Kir7.1 and voltage-gated potassium channel KCNE2, and detected potassium channel subfamily K+ member 1 KCNK1/TWIK-1 as well (Damkier et al. 2018).
The CPE cells express abundant NKCC1 in the luminal membrane (Keep et al. 1994; Plotkin et al. 1997). With the typical ionic distribution across the luminal membrane in CPE, NKCC1 is close to equilibrium (Keep et al. 1994). Thus, both inward and outward NKCC1 transport has been observed (Steffensen et al. 2018; Gregoriades et al. 2018). Measurements of the precise ionic gradients operating in vivo will hopefully soon settle this discrepancy. The K+-Cl−-cotransporters (KCCs) are electroneutral and transport of the ions out the cells is driven by the outward [K+] gradient across cell membranes. KCCs are like NKCC1 inhibited by furosemide (Russell 2000). KCCs were described as a furosemide-sensitive K+- and Cl−-dependent transport in the luminal membrane of bullfrog choroid plexus (Zeuthen 1991). KCC1 and KCC4 (SLC12A7) most likely mediate the observed transport in the luminal membrane, whereas KCC3a (SLC12A6) is expressed in the basolateral membrane (Karadsheh et al. 2004; Pearson et al. 2001). KCC4 may, therefore, contribute to the recycling of K+ across the luminal membrane helping to sustain the Na+/K+-ATPase activity (Fig. 12.8). Interestingly, the proteomic study mentioned above identified only NKCC1, KCC1, KCC4, and included a new candidate, CCC1/Cip1 (SLC12A9) in mouse choroid plexus epithelial cells (Damkier et al. 2018).
12.7.2 Luminal HCO3− Extrusion
A substantial Cl− and HCO3− efflux across the luminal membrane of the choroid plexus occurs via electrogenic pathways, mainly anion channels for Cl−. The mechanisms involved in HCO3− efflux are less well described, but may overlap to some extend with the Cl− extrusion. The majority of Cl− moves via a transcellular route, because luminal DIDS minimizes Cl− secretion (Deng and Johanson 1989). There are several potential candidates for Cl− channels. Inward-rectifying anion conductances (Clir) have been shown by patch-clamping in CPE cells from several species (Kajita et al. 2000; Kibble et al. 1996, 1997) and Clir channel activity is augmented by protein kinase A (Kibble et al. 1996), and inhibited by protein kinase C (Kajita et al. 2000). For the amphibian choroid plexus, Saito and Wright proposed that cAMP–regulated ion channels conducting Cl− and HCO3− form the main efflux pathway for the anions in the luminal membrane (Saito and Wright 1984). Inward rectifier Cl− (Clir) channels have high HCO3− permeability in mammals (Kibble et al. 1996). The molecular identity of the Clir channel remains to be determined. Apart from the intracellular chloride channels CLIC1, CLIC3-6, and CLCC1, only voltage-dependent anion-selective channel VDAC1, VDAC2, VDAC3 were detected at the molecular level (Damkier et al. 2018)
The CFTR does not seem to contribute to cAMP-regulated Cl− conductance in the choroid plexus (Kibble et al. 1996, 1997). ClC-2 channels are also unlikely to play a significant role in the choroid plexus transport, as Cl− conductance is unaffected in the choroid plexus epithelium from ClC-2 knockout mice (Speake et al. 2002). VRACs were also identified in the choroid plexus epithelium (Kibble et al. 1996, 1997). VRACs are most likely less important for CSF secretion, as their Cl− conductance at normal cell volumes is low (Kibble et al. 1996; Millar and Brown 2008). LRRC8A was the only subunit of volume-regulated anion channels detected in the recent mouse proteomic study (Damkier et al. 2018).
An alternative mechanism for luminal HCO3− extrusion to Cl− channels is the electrogenic cotransporter NBCe2 (or NBC4), which mediates the transport of 1 Na+ with 2–3 HCO3− ions (Sassani et al. 2002; Virkki et al. 2002). In rat choroid plexus, NBCe2 is situated in the luminal membrane (Bouzinova et al. 2005). In the mouse, it mediates the export of 1 Na+ for 3 HCO3− across the luminal membrane (Millar and Brown 2008). A significant increase in the CSF Na+ content without a reciprocal change in CSF K+ occurs in rodents exposed to 11% CO2 at the CSF side (Nattie 1980). This finding indicates that a Na+-dependent acid/base transporters, such as NBCe2, is involved in compensating CSF pH for the increased pCO2. Indeed, the composition of the CSF is changed in NBCe2 gene trap knockout mice with a significant decrease in [HCO3−] from 24 to 20 mM (Kao et al. 2011). Direct evidence for a role in CSF pH regulation was shown recently in another NBCe2 knockout mouse model. When HCl was injected into the ventricle cavity, only NBCe2 wildtype mice efficiently normalized the low CSF pH (Christensen et al. 2018). This effect was confirmed by NBCe2 siRNA injections. These findings strongly suggest that NBCe2 participates directly in the regulation of CSF pH by extruding HCO3−.
12.7.3 Other Acid/Base Transporters of Consequence for HCO3− Secretion
The V-ATPase does not seem to be expressed in the luminal plasma membrane of the choroid plexus epithelium. Among the many V-ATPase subunits only a few are associated with plasma membrane expression of the entire complex. The B1 subunit, which is involved in plasma membrane targeting, is not expressed at mRNA level in the choroid plexus, but B2 mRNA is detectable in these cells (Christensen, Damkier, and Praetorius, unpublished data). Thus, the V-ATPase complexes seem restricted to the lysosomal system in these cells. The rabbit choroid plexus expresses mRNA encoding the non-gastric H+/K+-ATPase (Lindvall-Axelsson et al. 1992; Pestov et al. 1998). Interestingly, luminally applied omeprazole inhibited CSF secretion in another study indicating the gastric H+/K+-ATPase activity gastric (Lindvall-Axelsson et al. 1992). Both V-ATPase and H+/K+-ATPase are expressed at the luminal membrane together with HCO3− transporters, thus, counteracting ongoing HCO3− secretion. Thus also in this epithelium, the role of H+-pumps is not clear.
Two NHE forms are expressed in the choroid plexus epithelium: NHE1 and NHE6. The mRNA encoding the NHE1 isoform was detected in the mouse choroid plexus (Damkier et al. 2009; Kalaria et al. 1998) and the Na+/H+ exchange activity of isolated epithelial cells from rats and mice is inhibited by EIPA (Bouzinova et al. 2005; Damkier et al. 2009). In most epithelia, NHE1 is localized to the basolateral membrane (Orlowski and Grinstein 2004), and this was also believed to be the case for the choroid plexus epithelium, as intravenous application of amiloride was reported to inhibit the rate of CSF secretion in vivo (Murphy and Johanson 1989). Nevertheless, NHE1 is located in the luminal membrane of both mouse and human choroid plexus (Damkier et al. 2009; Kao et al. 2011). NHE6 mRNA was recently detected in the mouse choroid plexus epithelium and the protein was mainly localized to the luminal membrane (Damkier et al. 2018). Functionally, NHE activity in the choroid plexus seems confined to the luminal plasma membrane (Damkier et al. 2009). The luminal membrane location of the NHE1 and NHE6 in CPE predicts that the protein mediates H+ extrusion from the epithelium to the CSF, Thus, NHE1 and/or NHE6 may serve roles in preventing increases in CSF pH, thereby counteracting efficient HCO3− secretion.
The SLC4A10 gene product NCBE or NBCn2 has been described as a DIDS-sensitive, electroneutral Na+-HCO3− cotransporter driven by the inward Na+ gradient (Damkier et al. 2010; Giffard et al. 2003; Wang et al. 2000). These authors found that the Na+ dependent HCO3− transport required intracellular Cl− in mammalian cell lines, and the transporter was therefore called NCBE for Na+-dependent Cl−/HCO3− exchanger. However, Parker et al. showed that the human SLC4A10 gene product did not extrude Cl− when expressed in Xenopus laevis oocytes, and protein was renamed the protein NBCn2 by these scientists (Parker et al. 2008). This controversy is not resolved and in this chapter, we use the rodent name NCBE for simplicity.
The NCBE is a basolateral plasma membrane protein in the choroid plexus (Praetorius and Nielsen 2006; Praetorius et al. 2004b), and it is believed to contribute to both Na+ and HCO3− uptake from the blood side. The involvement in cellular Na+ and HCO3− uptake is supported by a finding that reports a 70% decrease in the Na+-dependent HCO3− import into choroid plexus cells in an NCBE knockout mouse model (Jacobs et al. 2008). In the murine choroid plexus, NCBE activity can account for almost all of the DIDS-sensitive Na+-dependent HCO3− import (Damkier et al. 2009).
The electroneutral Na+-HCO3− cotransporter 1, NBCn1, is also expressed in the choroid plexus epithelium (Praetorius et al. 2004b). It is normally found in the basolateral membrane of mammalian choroid plexus epithelial cells, but it is localized to the luminal membrane in certain mouse strains and regionally in human choroid plexus (Damkier et al. 2009; Praetorius and Nielsen 2006). The epithelial NBCn1 form seems to be DIDS insensitive. In rat choroid plexus, a large fraction of the pHi recovery from acid load was mediated by DIDS insensitive Na+-HCO3− cotransport (Bouzinova et al. 2005). However, the NBC activity in choroid plexus from NBCn1 knockout mice is indistinguishable from that of wild type littermates (Damkier, unpublished results). Thus, NBCn1 protein is not an obvious candidate to participate in transcellular HCO3− transport.
AE2 mediates electroneutral uptake of Cl− in exchange for HCO3− and is the only anion exchanger from the SLC4 family in the choroid plexus. AE2 was first localized in the basolateral membrane of mouse choroid plexus epithelium (Lindsey et al. 1990) and later found at the same site in choroid plexus tissue from other mammals (Alper et al. 1994; Praetorius and Nielsen 2006). AE2 is the only known basolateral entry pathway for Cl− and may, therefore, be very important for CSF secretion. Two lines of evidence suggest the implication of basolateral AE in CSF formation. The inward chemical gradient for Cl− would favor AE2 mediated Cl− uptake across the basolateral membrane in the choroid plexus. Furthermore, DIDS applied from the blood side greatly reduce Cl− transport into the CSF (Deng and Johanson 1989; Frankel and Kazemi 1983).
12.7.4 Model for Bicarbonate Secretion by the Choroid Plexus
A significant body of evidence shows that HCO3− transport is an essential element of the CSF secretion and here we summarize the most important transporters (Fig. 12.8). CSF secretion is greatly decreased by carbonic anhydrase inhibition (Vogh et al. 1987; Ames III et al. 1965; Davson and Segal 1970; Welch 1963), and Na+/K+-ATPase activity is diminished in the absence of CO2/HCO3− in amphibian CPE (Saito and Wright 1983). In humans, the intracranial pressure is also reduced by the carbonic anhydrase inhibition in humans (Cowan and Whitelaw 1991). It appears that the majority of the ions secreted to the CSF take a transcellular route. Na+, Cl, HCO3−, and Ca2+ are secreted into the CSF, while K+ is reabsorbed. The transport of these ions is carefully regulated to provide a relatively constant CSF composition despite fluctuations in their plasma or brain extracellular fluid concentrations (Husted and Reed 1976; Jones and Keep 1988; Murphy et al. 1986). NCBE may be a good candidate for mediating HCO3− uptake from the blood side, while NBCn1 can only assist in cases where this protein is situated in the basolateral membrane. In the choroid plexus as elsewhere, cellular HCO3− is also generated from CO2 and H2O catalyzed by carbonic anhydrases, and inhibition of the carbonic anhydrases by acetazolamide reduced CSF secretion by approximately 50% (Vogh et al. 1987). The choroid plexus expresses the cytosolic carbonic anhydrase CAII as well as the membrane-associated isoforms CAXII and CAIX (Kallio et al. 2006). For the actual extrusion of HCO3− to the lumen, NBCe2 is identified as a key contributor for regulating CSF pH (Christensen et al. 2018). This does not rule out that the luminal anion conductances also provide HCO3− transport across the luminal membrane.
12.7.5 Regulation of CP Bicarbonate Secretion
The control and regulation of choroid plexus secretion of HCO3− and CSF is a topic of major clinical importance. Several life-threatening conditions resulting in an increased intracranial pressure, such as head trauma, tumors, stroke, and irradiation damage, can potentially be alleviated by acute reduction of CSF secretion. The relevance of the bicarbonate transporters was underscored in a recent study, where inhibition of NCBE was found efficient as a therapeutic target in post-hemorrhagic hydrocephalus in rat pups (Li et al. 2018). The CSF contains very little protein buffers and the appropriate pH level is most likely kept solely by the CO2/HCO3− buffer system. Adjustments of the HCO3− extrusion from the choroid plexus can potentially control the deviations of CSF pH from neutral values. However, virtually nothing is known about the potential acid/base sensing.
Arginine vasopressin (AVP, or antidiuretic hormone ADH) decreases the CSF secretion and reduces the blood flow to the choroid plexus (Johanson et al. 1999). The choroid plexus expresses V1a receptors (Ostrowski et al. 1992; Phillips et al. 1988). AVP is actually produced by CPE stimulated by local angiotensin II through AT1 receptor-mediated activation. Activation of V1a receptor in the luminal membrane decreases CSF secretion by the CPE (Szmydynger-Chodobska et al. 2004). Accordingly, Ca2+ increases transiently in the choroid plexus epithelium upon vasopressin administration (Battle et al. 2000), without affecting cAMP levels (Crook et al. 1984). A local system of renin, angiotensinogen as well as angiotensin-converting enzyme (ACE) and AT1 receptors are expressed in the choroid plexus epithelium (Chai et al. 1987a, b; Gehlert et al. 1986; Imboden et al. 1987; Inagami et al. 1980). However, the significance of this local RAS system in the choroid plexus is unclear apart from increasing AVP production. The mineralocorticoid and glucocorticoid receptors are expressed in the choroid plexus (de Kloet et al. 2000; Weber et al. 2003). Plasma glucocorticoid levels usually greatly exceed aldosterone levels, and in aldosterone sensitive cells, the specificity of MR receptor activation is assured by intracellular conversion of cortisol to inactive metabolites by enzyme 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2). Importantly, the choroid plexus expresses 11βHSD type 1 instead of 11βHSD2 (Sinclair et al. 2007) and it is therefore unlikely that aldosterone exerts specific MR mediated actions on the choroid plexus epithelium.
Regarding intracellular signaling, it is unknown how Ca2+ inhibits CSF secretion by the choroid plexus. There are substantial numbers of expressed transport mechanisms that are activated by cAMP and/or PKA. However, cAMP and/or PKA induction has only been shown for isoproterenol, prostaglandin, serotonin, and histamine (Crook et al. 1984). Finally, it is noted that the choroid plexus also expresses receptors for atrial natriuretic factor, endothelin-1, serotonin, bradykinin, and insulin, all of which could affect HCO3− secretion (Chodobski and Szmydynger-Chodobska 2001).
12.8 Conclusions and Perspectives
The importance of HCO3− secretion is clearly demonstrated by the large selection of ion transporters and channels involved in the process, and the fact that mutations or dysregulation of these can lead to a large spectrum of diseases. In this chapter, we have covered a wide selection of, but not all, epithelia that secrete HCO3−. In leaky epithelia, HCO3− secretion is accompanied by fluid secretion, while in tight epithelia (such as salivary gland ducts and CCDs), HCO3− is usually exchanged for another anion. One of the analyses made in this chapter is that there is a great spectrum of secreted HCO3− concentrations, secretion rates and therefore HCO3− outputs, which range from 1 to 1000 μmol/h/g tissue or organ. These values could be more revealing if corrected for the mass of cells actually performing HCO3− secretion in the given organ. Nevertheless, it should be pointed out that HCO3− secretion is usually only a part of a complex function any given epithelium performs, and it is regulated by specific neurotransmitters/hormones and locally generated agents or agents from neighboring organs or tissues. Therefore, it is not unexpected to see that there are many molecularly different transporters and constellations for bicarbonate secreting epithelia presented in this chapter. Nevertheless, we seek to deduce whether there are any common mechanisms for HCO3− secretion. It appears that the basolateral membranes of epithelia express one or more members of the NBC transporter family, and/or NHEs and intracellular CA generating H+ and HCO3− from CO2. The HCO3− exit on the luminal membrane is a little more controversial. It seems that Cl− channels in the combination of HCO3−/Cl− exchangers (SLC26 family), especially the electrogenic type of exchanger, are favored and suffice for many epithelia. Also, permeability of Cl− channels to HCO3− has been proposed. In a few epithelia, electrogenic NBC has been proposed, but functional evidence for general epithelial HCO3− secretion is required. Nevertheless, transport mechanism producing fluid with more than 100 mM HCO3− concentrations are still elusive and we are missing information about the electrochemical gradients (membrane voltages, intracellular anion concentrations), CO2 permeability, and the question still remains whether passive anion transporters are sufficient to explain HCO3− exit across the luminal membrane. Key components in the secretory models are also various Cl− and K+ channels, that can be stimulated by agonists to open and provide accompanying ions and appropriate membrane voltages.
The most potent HCO3− secretors, such as pancreas, some salivary glands, and duodenum could potentially affect body acid/base balance. Therefore, it is not surprising that these epithelia are very finely regulated, perform only periodically when stimulated, and at low secretion rates, HCO3− is reabsorbed or salvaged. This requires HCO3− reabsorbing mechanism, usually localized at the distal part of the complex structures of glands or gastrointestinal tract. These mechanisms could include certain isoforms of NBC transporters, or NHEs, and H+ transporters.
Since the 1990s research has led to molecular identification of many transport proteins involved in bicarbonate secretion. Future challenges are to pinpoint the most important pH and CO2 sensors, elucidate the effect of local regulating agents, and clarify which acid/base transporters are involved in pHi regulation as opposed to pHe regulation carried out by HCO3− secretion. Most importantly, studies of bicarbonate secretion and regulation on more integrated organ and whole-body settings could provide some important answers.
References
Aalkjaer C, Cragoe EJ Jr (1988) Intracellular pH regulation in resting and contracting segments of rat mesenteric resistance vessels. J Physiol 402:391–410
Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I (1998) Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem 273:17689–17695
Abuladze N, Pushkin A, Tatishchev S, Newman D, Sassani P, Kurtz I (2004) Expression and localization of rat NBC4c in liver and renal uroepithelium. Am J Physiol Cell Physiol 287:781–789
Ahn W, Kim KH, Lee JA, Kim JY, Choi JY, Moe OW, Milgram SL, Muallem S, Lee MG (2001) Regulatory interaction between the cystic fibrosis transmembrane conductance regulator and HCO3− salvage mechanism in model systems and the mouse pancreatic duct. J Biol Chem 276:17236–17243
Ahuja M, Jha A, Maleth J, Park S, Muallem S (2014) cAMP and Ca signaling in secretory epithelia: crosstalk and synergism. Cell Calcium 55:385–393
Ainsworth MA, Kjeldsen J, Olsen O, Christensen P, Schaffalitzky de Muckadell OB (1990) Duodenal disappearance rate of acid during inhibition of mucosal bicarbonate secretion. Digestion 47:121–129
Ainsworth MA, Svendsen P, Ladegaard L, Cantor P, Olsen O, Schaffalitzky de Muckadell OB (1992) Relative importance of pancreatic, hepatic, and mucosal bicarbonate in duodenal neutralization of acid in anaesthetized pigs. Scand J Gastroenterol 27:343–349
Ainsworth MA, Amelsberg M, Hogan DL, Isenberg JI (1996) Acid-base transport in isolated rabbit duodenal villus and crypt cells. Scand J Gastroenterol 31:1069–1077
Ainsworth MA, Hogan DL, Rapier RC, Amelsberg M, Dreilinger AD, Isenberg JI (1998) Acid/base transporters in human duodenal enterocytes. Scand J Gastroenterol 33:1039–1046
Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Kaunitz JD (2001a) Acute adaptive cellular base uptake in rat duodenal epithelium. Am J Physiol Gastrointest Liver Physiol 280:1083–1092
Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Sassani P, Dukkipatis R, Pushkin A, Kurtz I, Kaunitz JD (2001b) Cellular bicarbonate protects rat duodenal mucosa from acid-induced injury. J Clin Invest 108:1807–1816
Al-Awqati Q (2013) Cell biology of the intercalated cell in the kidney. FEBS Lett 587:1911–1914
Allen A, Flemstrom G (2005) Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol 288:1–19
Almassy J, Won JH, Begenisich TB, Yule DI (2012) Apical Ca2+-activated potassium channels in mouse parotid acinar cells. J Gen Physiol 139:121–133
Alper SL (2006) Molecular physiology of SLC4 anion exchangers. Exp Physiol 91:153–161
Alper SL, Sharma AK (2013) The SLC26 gene family of anion transporters and channels. Mol Aspects Med 34:494–515
Alper SL, Natale J, Gluck S, Lodish HF, Brown D (1989) Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci U S A 86:5429–5433
Alper SL, Stuart-Tilley A, Simmons CF, Brown D, Drenckhahn D (1994) The fodrin-ankyrin cytoskeleton of choroid plexus preferentially colocalizes with apical Na+K+-ATPase rather than with basolateral anion exchanger AE2. J Clin Invest 93:1430–1438
Alper SL, Stewart AK, Vandorpe DH, Clark JS, Horack RZ, Simpson JE, Walker NM, Clarke LL (2011) Native and recombinant Slc26a3 (downregulated in adenoma, Dra) do not exhibit properties of 2Cl-/1HCO3- exchange. Am J Physiol Cell Physiol 300:276–286
Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, LeSage GD (1997) Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol 272:1064–1074
Alvarez C, Fasano A, Bass BL (1998) Acute effects of bile acids on the pancreatic duct epithelium in vitro. J Surg Res 74:43–46
Alvarez L, Fanjul M, Carter N, Hollande E (2001) Carbonic anhydrase II associated with plasma membrane in a human pancreatic duct cell line (CAPAN-1). J Histochem Cytochem 49:1045–1053
Alvarez BV, Loiselle FB, Supuran CT, Schwartz GJ, Casey JR (2003) Direct extracellular interaction between carbonic anhydrase IV and the human NBC1 sodium/bicarbonate co-transporter. Biochemistry 42:12321–12329
Alvaro D, Della GP, Bini A, Gigliozzi A, Furfaro S, La RT, Piat C, Capocaccia L (1995) Effect of glucagon on intracellular pH regulation in isolated rat hepatocyte couplets. J Clin Invest 96:665–675
Ames A III, Sakanoue M, Endo S (1964) Na, K, Ca, Mg, and Cl concentrations in choroid plexus fluid and cisternal fluid compared with plasma ultrafiltrate. J Neurophysiol 27:672–681
Ames A III, Higashi K, Nesbett FB (1965) Effects of Pco2 acetazolamide and ouabain on volume and composition of choroid-plexus fluid. J Physiol 181:516–524
Amlal H, Petrovic S, Xu J, Wang Z, Sun X, Barone S, Soleimani M (2010) Deletion of the anion exchanger Slc26a4 (pendrin) decreases apical Cl−/HCO3− exchanger activity and impairs bicarbonate secretion in kidney collecting duct. Am J Physiol Cell Physiol 299:33–41
Andersen DH (1938) Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathological study. Am J Dis Child 56:344–399
Aoi M, Aihara E, Nakashima M, Takeuchi K (2004) Participation of prostaglandin E receptor EP4 subtype in duodenal bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol 287:96–103
Aranda V, Martinez I, Melero S, Lecanda J, Banales JM, Prieto J, Medina JF (2004) Shared apical sorting of anion exchanger isoforms AE2a, AE2b1, and AE2b2 in primary hepatocytes. Biochem Biophys Res Commun 319:1040–1046
Ashizawa N, Endoh H, Hidaka K, Watanabe M, Fukumoto S (1997) Three-dimensional structure of the rat pancreatic duct in normal and inflammated pancreas. Microsc Res Tech 37:543–556
Azroyan A, Laghmani K, Crambert G, Mordasini D, Doucet A, Edwards A (2011) Regulation of pendrin by pH: dependence on glycosylation. Biochem J 434:61–72
Balazs A, Mall MA (2018) Role of the SLC26A9 chloride channel as disease modifier and potential therapeutic target in cystic fibrosis. Front Pharmacol 9:1112
Banales JM, Arenas F, Rodriguez-Ortigosa CM, Saez E, Uriarte I, Doctor RB, Prieto J, Medina JF (2006a) Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology 43:266–275
Banales JM, Prieto J, Medina JF (2006b) Cholangiocyte anion exchange and biliary bicarbonate excretion. World J Gastroenterol 12:3496–3511
Bardow A, Madsen J, Nauntofte B (2000) The bicarbonate concentration in human saliva does not exceed the plasma level under normal physiological conditions. Clin Oral Investig 4:245–253
Battle T, Preisser L, Marteau V, Meduri G, Lambert M, Nitschke R, Brown PD, Corman B (2000) Vasopressin V1a receptor signaling in a rat choroid plexus cell line. Biochem Biophys Res Commun 275:322–327
Beal AM (1984) Electrolyte composition of parotid saliva from sodium-replete red kangaroos (Macropus rufus). J Exp Biol 111:225–237
Becker HM, Klier M, Deitmer JW (2014) Carbonic anhydrases and their interplay with acid/base-coupled membrane transporters. Subcell Biochem 75:105–134
Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE (2004) Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem 279:47681–47687
Behrendorff N, Floetenmeyer M, Schwiening C, Thorn P (2010) Protons released during pancreatic acinar cell secretion acidify the lumen and contribute to pancreatitis in mice. Gastroenterology 139:1711–1720
Bergmann F, Andrulis M, Hartwig W, Penzel R, Gaida MM, Herpel E, Schirmacher P, Mechtersheimer G (2011) Discovered on gastrointestinal stromal tumor 1 (DOG1) is expressed in pancreatic centroacinar cells and in solid-pseudopapillary neoplasms--novel evidence for a histogenetic relationship. Hum Pathol 42:817–823
Berridge MJ (2012) Calcium signalling remodelling and disease. Biochem Soc Trans 40:297–309
Bertrand CA, Mitra S, Mishra SK, Wang X, Zhao Y, Pilewski JM, Madden DR, Frizzell RA (2017) The CFTR trafficking mutation F508del inhibits the constitutive activity of SLC26A9. Am J Physiol Lung Cell Mol Physiol 312:912–925
Beuers U, Hohenester S, de Buy Wenniger LJ, Kremer AE, Jansen PL, Elferink RP (2010) The biliary HCO3− umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology 52:1489–1496
Beuers U, Maroni L, Elferink RO (2012) The biliary HCO3− umbrella: experimental evidence revisited. Curr Opin Gastroenterol 28:253–257
Bhattacharya S, Verrill DS, Carbone KM, Brown S, Yule DI, Giovannucci DR (2012) Distinct contributions by ionotropic purinoceptor subtypes to ATP-evoked calcium signals in mouse parotid acinar cells. J Physiol 590:2721–2737
Bijman J, Cook DI, van Os CH (1981) Effect of amiloride on electrolyte transport parameters of the main duct of the rabbit mandibular gland. Pflügers Arch 398:96–102
Billet A, Hanrahan JW (2013) The secret life of CFTR as a calcium-activated chloride channel. J Physiol 591(21):5273–5278
Billet A, Luo Y, Balghi H, Hanrahan JW (2013) Role of tyrosine phosphorylation in the muscarinic activation of the cystic fibrosis transmembrane conductance regulator (CFTR). J Biol Chem 288:21815–21823
Boedtkjer E, Praetorius J, Aalkjaer C (2006) NBCn1 (slc4a7) mediates the Na+-dependent bicarbonate transport important for regulation of intracellular pH in mouse vascular smooth muscle cells. Circ Res 98:515–523
Boron WF, Boulpaep EL (1983) Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3− transport. J Gen Physiol 81:53–94
Boron WF, Boulpaep EL (2012) Medical physiology. Elsevier, Philadelphia
Boron WF, Boulpaep EL (2017) Medical physiology. Elsevier, Philadelphia
Boron WF, Knakal RC (1992) Na(+)-dependent Cl-HCO3 exchange in the squid axon: dependence on extracellular pH. J Gen Physiol 99:817–837
Bouwens L, Pipeleers DG (1998) Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia 41:629–633
Bouzinova EV, Praetorius J, Virkki LV, Nielsen S, Boron WF, Aalkjaer C (2005) Na+-dependent HCO3-uptake into the rat choroid plexus epithelium is partially DIDS sensitive. Am J Physiol Cell Physiol 289:1448–1456
Boyer JL (2013) Bile formation and secretion. Compr Physiol 3:1035–1078
Breton S, Brown D (2013) Regulation of luminal acidification by the V-ATPase. Physiology (Bethesda ) 28:318–329
Breton S, Alper SL, Gluck SL, Sly WS, Barker JE, Brown D (1995) Depletion of intercalated cells from collecting ducts of carbonic anhydrase II-deficient (CAR2 null) mice. Am J Physiol Renal Physiol 269:761–774
Bro-Rasmussen F, Killmann SA, Thaysen JH (1956) The composition of pancreatic juice as compared to sweat, parotid saliva and tears. Acta Physiol Scand 37:97–113
Brown D, Hirsch S, Gluck S (1988a) An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature 331:622–624
Brown PD, Loo DD, Wright EM (1988b) Ca2+-activated K+ channels in the apical membrane of Necturus choroid plexus. J Membr Biol 105:207–219
Brown D, Paunescu TG, Breton S, Marshansky V (2009) Regulation of the V-ATPase in kidney epithelial cells: dual role in acid-base homeostasis and vesicle trafficking. J Exp Biol 212:1762–1772
Bruce JI, Shuttleworth TJ, Giovannucci DR, Yule DI (2002) Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells. A mechanism for the synergistic effects of cAMP on Ca2+ signaling. J Biol Chem 277:1340–1348
Burgen ASV (1956) The secretion of potassium in saliva. J Physiol (Lond) 132:20–39
Burghardt B, Elkaer ML, Kwon TH, Racz GZ, Varga G, Steward MC, Nielsen S (2003) Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut 52:1008–1016
Burghardt B, Nielsen S, Steward MC (2006) The role of aquaporin water channels in fluid secretion by the exocrine pancreas. J Membr Biol 210:143–153
Burnstock G (2017) Purinergic signalling: therapeutic developments. Front Pharmacol 8:661
Caflisch CR, Solomon S, Galey WR (1979) Exocrine ductal pCO2 in the rabbit pancreas. Pflugers Arch 380:121–125
Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322:590–594
Case RM, Harper AA, Scratcherd T (1969) The secretion of electrolytes and enzymes by the pancreas of the anaesthetized cat. J Physiol (Lond) 201:335–348
Case RM, Scratcherd T, Wynne RDA (1970) The origin and secretion of pancreatic juice bicarbonate. J Physiol (Lond) 210:1–15
Case RM, Hotz J, Hutson D, Scratcherd T, Wynne RDA (1979) Electrolyte secretion by the isolated cat pancreas during replacement of extracellular bicarbonate by organic anions and chloride by inorganic anions. J Physiol (Lond) 286:563–576
Case RM, Conigrave AD, Novak I, Young JA (1980) Electrolyte and protein secretion by the perfused rabbit mandibular gland stimulated with acetylcholine or catecholamines. J Physiol (Lond) 300:467–487
Case RM, Conigrave AD, Favaloro EJ, Novak I, Thompson CH, Young JA (1982) The role of buffer anions and protons in secretion by the rabbit mandibular salivary gland. J Physiol (Lond) 322:273–286
Case RM, Hunter M, Novak I, Young JA (1984) The anionic basis of fluid secretion by the rabbit mandibular gland. J Physiol (Lond) 349:619–630
Catalan MA, Nakamoto T, Gonzalez-Begne M, Camden JM, Wall SM, Clarke LL, Melvin JE (2010) Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol 588:713–724
Catalan MA, Kondo Y, Pena-Munzenmayer G, Jaramillo Y, Liu F, Choi S, Crandall E, Borok Z, Flodby P, Shull GE, Melvin JE (2015) A fluid secretion pathway unmasked by acinar-specific Tmem16A gene ablation in the adult mouse salivary gland. Proc Natl Acad Sci U S A 112:2263–2268
Chai SY, McKinley MJ, Mendelsohn FA (1987a) Distribution of angiotensin converting enzyme in sheep hypothalamus and medulla oblongata visualized by in vitro autoradiography. Clin Exp Hypertens A 9:449–460
Chai SY, Mendelsohn FA, Paxinos G (1987b) Angiotensin converting enzyme in rat brain visualized by quantitative in vitro autoradiography. Neuroscience 20:615–627
Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hubner CA, Eladari D (2013) Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci U S A 110:7928–7933
Chan HC, Cheung WT, Leung PY, Wu LJ, Chew SB, Ko WH, Wong PY (1996) Purinergic regulation of anion secretion by cystic fibrosis pancreatic duct cells. Am J Physiol 271:469–477
Chari RS, Schutz SM, Haebig JE, Shimokura GH, Cotton PB, Fitz JG, Meyers WC (1996) Adenosine nucleotides in bile. Am J Physiol 270:246–252
Chaturapanich G, Ishibashi H, Dinudom A, Young JA, Cook DI (1997) H+ transporters in the main excretory duct of the mouse mandibular salivary gland. J Physiol 503(Pt 3):583–598
Chen M, Praetorius J, Zheng W, Xiao F, Riederer B, Singh AK, Stieger N, Wang J, Shull GE, Aalkjaer C, Seidler U (2012) The electroneutral Na(+):HCO(3)(-) cotransporter NBCn1 is a major pHi regulator in murine duodenum. J Physiol 590:3317–3333
Chenevert J, Duvvuri U, Chiosea S, Dacic S, Cieply K, Kim J, Shiwarski D, Seethala RR (2012) DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol 25:919–929
Cheng HS, Leung PY, Cheng Chew SB, Leung PS, Lam SY, Wong WS, Wang ZD, Chan HC (1998) Concurrent and independent HCO3− and Cl− secretion in a human pancreatic duct cell line (CAPAN-1). J Membr Biol 164:155–167
Chernova MN, Jiang L, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL (2003) Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol 549:3–19
Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL (2005) Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J Biol Chem 280:8564–8580
Chey WY, Chang T (2001) Neural hormonal regulation of exocrine pancreatic secretion. Pancreatology 1:320–335
Choate KA, Kahle KT, Wilson FH, Nelson-Williams C, Lifton RP (2003) WNK1, a kinase mutated in inherited hypertension with hyperkalemia, localizes to diverse Cl− - transporting epithelia. Proc Natl Acad Sci U S A 100:663–668
Chodobski A, Szmydynger-Chodobska J (2001) Choroid plexus: target for polypeptides and site of their synthesis. Microsc Res Tech 52:65–82
Choi I, Romero MF, Khandoudi N, Bril A, Boron WF (1999) Cloning and characterization of a human electrogenic Na+-HCO3− cotransporter isoform (hhNBC). Am J Physiol 276:576–584
Choi I, Aalkjaer C, Boulpaep EL, Boron WF (2000) An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405:571–575
Christensen O, Zeuthen T (1987) Maxi K+ channels in leaky epithelia are regulated by intracellular Ca2+, pH and membrane potential. Pflügers Arch 408:249–259
Christensen BM, Perrier R, Wang Q, Zuber AM, Maillard M, Mordasini D, Malsure S, Ronzaud C, Stehle JC, Rossier BC, Hummler E (2010) Sodium and potassium balance depends on alphaENaC expression in connecting tubule. J Am Soc Nephrol 21:1942–1951
Christensen HL, Barbuskaite D, Rojek A, Malte H, Christensen IB, Fuchtbauer AC, Fuchtbauer EM, Wang T, Praetorius J, Damkier HH (2018) The choroid plexus sodium-bicarbonate cotransporter NBCe2 regulates mouse cerebrospinal fluid pH. J Physiol 596:4709–4728
Clarke LL, Harline MC (1998) Dual role of CFTR in cAMP-stimulated HCO3− secretion across murine duodenum. Am J Physiol 274:718–726
Cohn JA, Strong TV, Picciotto MR, Nairn AC, Collins FS, Fitz JG (1993) Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology 105:1857–1864
Compton JS, Nelson J, Wright RD, Young JA (1980) A micropuncture investigation of electrolyte transport in the parotid glands of sodium-replete and sodium-deplete sheep. J Physiol (Lond) 309:429–446
Concepcion AR, Lopez M, Ardura-Fabregat A, Medina JF (2013) Role of AE2 for pH regulation in biliary epithelial cells. Front Physiol 4:413
Cook DI, Young JA (1989) Effect of K+ channels in the apical plasma membrane on epithelial secretion based on secondary active Cl− transport. J Membr Biol 110:139–146
Cook DI, Dinudom A, Komwatana P, Kumar S, Young JA (2002) Patch-clamp studies on epithelial sodium channels in salivary duct cells. Cell Biochem Biophys 36:105–113
Cordat E, Reithmeier RA (2014) Structure, function, and trafficking of SLC4 and SLC26 anion transporters. Curr Top Membr 73:1–67
Cotter K, Stransky L, McGuire C, Forgac M (2015) Recent insights into the structure, regulation, and function of the V-ATPases. Trends Biochem Sci 40:611–622
Cowan F, Whitelaw A (1991) Acute effects of acetazolamide on cerebral blood flow velocity and pCO2 in the newborn infant. Acta Paediatr Scand 80:22–27
Crook RB, Farber MB, Prusiner SB (1984) Hormones and neurotransmitters control cyclic AMP metabolism in choroid plexus epithelial cells. J Neurochem 42:340–350
Cushing H (1914) Studies on the cerebrospinal fluid. J Med Res 26:1–19
Czako L, Yamamoto M, Otsuki M (1997) Exocrine pancreatic function in rats after acute pancreatitis. Pancreas 15:83–90
Damkier HH, Nielsen S, Praetorius J (2006) An anti-NH2-terminal antibody localizes NBCn1 to heart endothelia and skeletal and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 290:172–180
Damkier HH, Nielsen S, Praetorius J (2007) Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol 293:2136–2146
Damkier HH, Prasad V, Hubner CA, Praetorius J (2009) Nhe1 is a luminal Na+/H+ exchanger in mouse choroid plexus and is targeted to the basolateral membrane in Ncbe/Nbcn2-null mice. Am J Physiol Cell Physiol 296:1291–1300
Damkier HH, Aalkjaer C, Praetorius J (2010) Na+-dependent HCO3− import by the slc4a10 gene product involves Cl− export. J Biol Chem 285:26998–27007
Damkier HH, Brown PD, Praetorius J (2013) Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev 93:1847–1892
Damkier HH, Christensen HL, Christensen IB, Wu Q, Fenton RA, Praetorius J (2018) The murine choroid plexus epithelium expresses the 2Cl−/H+ exchanger ClC-7 and Na+/H+ exchanger NHE6 in the luminal membrane domain. Am J Physiol Cell Physiol 314:439–448
Davson H, Purvis C (1954) Cryoscopic apparatus suitable for studies on aqueous humour and cerebro-spinal fluid. J Physiol 124:12–3P
Davson H, Segal MB (1970) The effects of some inhibitors and accelerators of sodium transport on the turnover of 22Na in the cerebrospinal fluid and the brain. J Physiol 209:131–153
Davson H, Segal MB (1996) Physiology of the CSF and blood-brain barriers. CRC Press, Boca Raton
de Kloet ER, Van Acker SA, Sibug RM, Oitzl MS, Meijer OC, Rahmouni K, de Jong JW (2000) Brain mineralocorticoid receptors and centrally regulated functions. Kidney Int 57:1329–1336
de Ondarza J, Hootman SR (1997) Confocal microscopic analysis of intracellular pH regulation in isolated guinea pig pancreatic ducts. Am J Physiol 272:124–134
Demeter I, Hegyesi O, Nagy AK, Case MR, Steward MC, Varga G, Burghardt B (2009) Bicarbonate transport by the human pancreatic ductal cell line HPAF. Pancreas 38:913–920
Demitrack ES, Soleimani M, Montrose MH (2010) Damage to the gastric epithelium activates cellular bicarbonate secretion via SLC26A9 Cl−/HCO3−. Am J Physiol Gastrointest Liver Physiol 299:255–264
Deng QS, Johanson CE (1989) Stilbenes inhibit exchange of chloride between blood, choroid plexus and cerebrospinal fluid. Brain Res 501:183–187
Dinudom A, Komwatana P, Young JA, Cook DI (1995) A forskolin-activated Cl− current in mouse mandibular duct cells. Am J Physiol 268:806–812
Dinudom A, Harvey KF, Komwatana P, Young JA, Kumar S, Cook DI (1998) Nedd4 mediates control of an epithelial Na+ channel in salivary duct cells by cytosolic Na+. Proc Natl Acad Sci U S A 95:7169–7173
Dinudom A, Harvey KF, Komwatana P, Jolliffe CN, Young JA, Kumar S, Cook DI (2001) Roles of the C termini of alpha-, beta-, and gamma- subunits of epithelial Na+ channels (ENaC) in regulating ENaC and mediating its inhibition by cytosolic Na+. J Biol Chem 276:13744–13749
Dissing S, Hansen HJ, Undén M, Nauntofte B (1990) Inhibitory effects of amitriptyline on the stimulation-induced Ca2+ increase in parotid acini. Eur J Pharmacol 177:43–54
Doctor RB, Matzakos T, McWilliams R, Johnson S, Feranchak AP, Fitz JG (2005) Purinergic regulation of cholangiocyte secretion: identification of a novel role for P2X receptors. Am J Physiol Gastrointest Liver Physiol 288:779–786
Doring F, Derst C, Wischmeyer E, Karschin C, Schneggenburger R, Daut J, Karschin A (1998) The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties. J Neurosci 18:8625–8636
Dorwart MR, Shcheynikov N, Yang D, Muallem S (2008) The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 23:104–114
Dranoff JA, Masyuk AI, Kruglov EA, LaRusso NF, Nathanson MH (2001) Polarized expression and function of P2Y ATP receptors in rat bile duct epithelia. Am J Physiol Gastrointest Liver Physiol 281:1059–1067
Duran C, Thompson CH, Xiao Q, Hartzell HC (2010) Chloride channels: often enigmatic, rarely predictable. Annu Rev Physiol 72:95–121
Dutta AK, Khimji AK, Sathe M, Kresge C, Parameswara V, Esser V, Rockey DC, Feranchak AP (2009) Identification and functional characterization of the intermediate-conductance Ca2+-activated K+ channel (IK-1) in biliary epithelium. Am J Physiol Gastrointest Liver Physiol 297:1009–1018
Dutta AK, Khimji AK, Kresge C, Bugde A, Dougherty M, Esser V, Ueno Y, Glaser SS, Alpini G, Rockey DC, Feranchak AP (2011) Identification and functional characterization of TMEM16A, a Ca2+-activated Cl- channel activated by extracellular nucleotides, in biliary epithelium. J Biol Chem 286:766–776
Dutta AK, Woo K, Khimji AK, Kresge C, Feranchak AP (2013) Mechanosensitive Cl− secretion in biliary epithelium mediated through TMEM16A. Am J Physiol Gastrointest Liver Physiol 304:87–98
Ernst SA, Palacios JR, Siegel GJ (1986) Immunocytochemical localization of Na+,K+-ATPase catalytic polypeptide in mouse choroid plexus. J Histochem Cytochem 34:189–195
Esteller A (2008) Physiology of bile secretion. World J Gastroenterol 14:5641–5649
Evans RL, Ashton N, Elliott AC, Green R, Argent BE (1996) Interaction between secretin and acetylcholine in the regulation of fluid secretion by isolated rat pancreatic ducts. J Physiol (Lond) 461:265–273
Evans RL, Park K, Turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE (2000) Severe impairment of salivation in Na+/K+/2Cl− cotransporter (NKCC1)-deficient mice. J Biol Chem 275:26720–26726
Faivre J (1854) Structure du conarium et des plexus chorode chez lhommes et des animaux. Gaz Med Paris 9:555–556
Fanjul M, Alvarez L, Salvador C, Gmyr V, Kerr-Conte J, Pattou F, Carter N, Hollande E (2004) Evidence for a membrane carbonic anhydrase IV anchored by its C-terminal peptide in normal human pancreatic ductal cells. Histochem Cell Biol 121:91–99
Fausther M, Sevigny J (2011) Extracellular nucleosides and nucleotides regulate liver functions via a complex system of membrane proteins. C R Biol 334:100–117
Feranchak AP, Doctor RB, Troetsch M, Brookman K, Johnson SM, Fitz JG (2004) Calcium-dependent regulation of secretion in biliary epithelial cells: the role of apamin-sensitive SK channels. Gastroenterology 127:903–913
Feranchak AP, Lewis MA, Kresge C, Sathe M, Bugde A, Luby-Phelps K, Antich PP, Fitz JG (2010) Initiation of purinergic signaling by exocytosis of ATP-containing vesicles in liver epithelium. J Biol Chem 285:8138–8147
Fernandez-Salazar MP, Pascua P, Calvo JJ, Lopez MA, Case RM, Steward MC, San Roman JI (2004) Basolateral anion transport mechanisms underlying fluid secretion by mouse, rat and guinea-pig pancreatic ducts. J Physiol (Lond) 556:415–428
Feschenko MS, Donnet C, Wetzel RK, Asinovski NK, Jones LR, Sweadner KJ (2003) Phospholemman, a single-span membrane protein, is an accessory protein of Na,K-ATPase in cerebellum and choroid plexus. J Neurosci 23:2161–2169
Fiorotto R, Spirli C, Fabris L, Cadamuro M, Okolicsanyi L, Strazzabosco M (2007) Ursodeoxycholic acid stimulates cholangiocyte fluid secretion in mice via CFTR-dependent ATP secretion. Gastroenterology 133:1603–1613
Fitz JG, Persico M, Scharschmidt BF (1989) Electrophysiological evidence for Na+-coupled bicarbonate transport in cultured rat hepatocytes. Am J Physiol 256:491–500
Fitz JG, Basavappa S, McGill J, Melhus O, Cohn JA (1993) Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest 91:319–328
Flemstrom G, Kivilaakso E (1983) Demonstration of a pH gradient at the luminal surface of rat duodenum in vivo and its dependence on mucosal alkaline secretion. Gastroenterology 84:787–794
Flemstrom G, Garner A, Nylander O, Hurst BC, Heylings JR (1982) Surface epithelial HCO3− transport by mammalian duodenum in vivo. Am J Physiol 243:348–358
Flinck M, Kramer SH, Pedersen SF (2018) Roles of pH in control of cell proliferation. Acta Physiol (Oxf) 223:e13068
Fong P, Argent BE, Guggino WB, Gray MA (2003) Characterization of vectorial chloride transport pathways in the human pancreatic duct adenocarcinoma cell line, HPAF. Am J Physiol Cell Physiol 285:433–445
Forte JG, Zhu L (2010) Apical recycling of the gastric parietal cell H,K-ATPase. Annu Rev Physiol 72:273–296
Foskett JK (1990) [Ca2+]i modulation of Cl− content controls cell volume in single salivary acinar cells during fluid secretion. Am J Physiol 259:998–1004
Frankel H, Kazemi H (1983) Regulation of CSF composition--blocking chloride-bicarbonate exchange. J Appl Physiol Respir Environ Exerc Physiol 55:177–182
Freedman SD, Scheele GA (1994) Acid-base interactions during exocrine pancreatic secretion. Primary role for ductal bicarbonate in acinar lumen function. Ann N Y Acad Sci 713:199–206
Freedman SD, Kern HF, Scheele GA (2001) Pancreatic acinar cell dysfunction in CFTR-/- mice is associated with impairments in luminal pH and endocytosis. Gastroenterology 121:950–957
Frische S, Kwon TH, Frokiaer J, Madsen KM, Nielsen S (2003) Regulated expression of pendrin in rat kidney in response to chronic NH4Cl or NaHCO3 loading. Am J Physiol Renal Physiol 284:584–593
Frizzell RA, Hanrahan JW (2012) Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med 2:a009563
Gao X, Eladari D, Leviel F, Tew BY, Miro-Julia C, Cheema FH, Miller L, Nelson R, Paunescu TG, McKee M, Brown D, Al-Awqati Q (2010) Deletion of hensin/DMBT1 blocks conversion of beta- to alpha-intercalated cells and induces distal renal tubular acidosis. Proc Natl Acad Sci U S A 107:21872–21877
Garrett JR (1987) The proper role of nerves in salivary secretion: a review. J Dent Res 66:387–397
Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE (2004) Mice with a targeted disruption of the AE2 Cl-/HCO3- exchanger are achlorhydric. J Biol Chem 279:30531–30539
Gehlert DR, Speth RC, Wamsley JK (1986) Distribution of [125I]angiotensin II binding sites in the rat brain: a quantitative autoradiographic study. Neuroscience 18:837–856
Gerasimenko JV, Flowerdew SE, Voronina SG, Sukhomlin TK, Tepikin AV, Petersen OH, Gerasimenko OV (2006) Bile acids induce Ca2+ release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors. J Biol Chem 281:40154–40163
Giffard RG, Lee YS, Ouyang YB, Murphy SL, Monyer H (2003) Two variants of the rat brain sodium-driven chloride bicarbonate exchanger (NCBE): developmental expression and addition of a PDZ motif. Eur J Neurosci 18:2935–2945
Githens S (1988) The pancreas duct cell: proliferative capabilities, specific characteristics, metaplasia, isolation, and culture. J Pediatr Gastroenterol Nutr 7:486–506
Gmyr V, Belaich S, Muharram G, Lukowiak B, Vandewalle B, Pattou F, Kerr-Conte J (2004) Rapid purification of human ductal cells from human pancreatic fractions with surface antibody CA19-9. Biochem Biophys Res Commun 320:27–33
Gonzalez-Begne M, Nakamoto T, Nguyen HV, Stewart AK, Alper SL, Melvin JE (2007) Enhanced formation of a HCO3− transport metabolon in exocrine cells of Nhe1-/- mice. J Biol Chem 282:35125–35132
Gradilone SA, Garcia F, Huebert RC, Tietz PS, Larocca MC, Kierbel A, Carreras FI, LaRusso NF, Marinelli RA (2003) Glucagon induces the plasma membrane insertion of functional aquaporin-8 water channels in isolated rat hepatocytes. Hepatology 37:1435–1441
Gray MA, Greenwell JR, Argent BE (1988) Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol 105:131–142
Gray MA, Harris A, Coleman L, Greenwell JR, Argent BE (1989) Two types of chloride channel on duct cells cultured from human fetal pancreas. Am J Physiol 257:240–251
Gray MA, Greenwell JR, Garton AJ, Argent BE (1990) Regulation of maxi-K+ channels on pancreatic duct cells by cyclic AMP-dependent phosphorylation. J Membr Biol 115:203–215
Gray MA, Plant S, Argent BE (1993) cAMP-regulated whole cell chloride currents in pancreatic duct cells. Am J Physiol Cell Physiol 264:591–602
Gray MA, Winpenny JP, Porteous DJ, Dorin JR, Argent BE (1994) CFTR and calcium-activated chloride currents in pancreatic duct cells of a transgenic CF mouse. Am J Physiol 266:213–221
Greeley T, Shumaker H, Wang Z, Schweinfest CW, Soleimani M (2001) Downregulated in adenoma and putative anion transporter are regulated by CFTR in cultured pancreatic duct cells. Am J Physiol Gastrointest Liver Physiol 281:G1301–G1308
Gregoriades JMC, Madaris A, Alvarez FJ, Alvarez-Leefmans FJ (2018) Genetic and pharmacologic inactivation of apical NKCC1 in choroid plexus epithelial cells reveals the physiological function of the cotransporter. Am J Physiol Cell Physiol 316(4):525–544
Gresz V, Kwon TH, Vorum H, Zelles T, Kurtz I, Steward MC, Aalkjaer C, Nielsen S (2002) Immunolocalization of electroneutral Na+-HCO3− cotransporters in human and rat salivary glands. Am J Physiol Gastrointest Liver Physiol 283:473–480
Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF (2001) Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. J Biol Chem 276:8358–8363
Grishin AV, Caplan MJ (1998) ATP1AL1, a member of the non-gastric H,K-ATPase family, functions as a sodium pump. J Biol Chem 273:27772–27778
Grishin AV, Bevensee MO, Modyanov NN, Rajendran V, Boron WF, Caplan MJ (1996) Functional expression of the cDNA encoded by the human ATP1AL1 gene. Am J Physiol 271:539–551
Grotmol T, Buanes T, Bros O, Raeder MG (1986) Lack of effect of amiloride, furosemide, bumetanide and triamterene on pancreatic NaHCO3 secretion in pigs. Acta Physiol Scand 126:593–600
Guba M, Kuhn M, Forssmann WG, Classen M, Gregor M, Seidler U (1996) Guanylin strongly stimulates rat duodenal HCO3− secretion: proposed mechanism and comparison with other secretagogues. Gastroenterology 111:1558–1568
Haanes KA, Novak I (2010) ATP storage and uptake by isolated pancreatic zymogen granules. Biochem J 429:303–311
Haanes KA, Kowal JM, Arpino G, Lange SC, Moriyama Y, Pedersen PA, Novak I (2014) Role of vesicular nucleotide transporter VNUT (SLC17A9) in release of ATP from AR42J cells and mouse pancreatic acinar cells. Purinergic Signal 10:431–440
Hafner P, Grimaldi R, Capuano P, Capasso G, Wagner CA (2008) Pendrin in the mouse kidney is primarily regulated by Cl− excretion but also by systemic metabolic acidosis. Am J Physiol Cell Physiol 295:1658–1667
Hayashi M, Novak I (2013) Molecular basis of potassium channels in pancreatic duct epithelial cells. Channels (Austin) 7:432–441
Hayashi M, Wang J, Hede SE, Novak I (2012) An intermediate-conductance Ca2+-activated K+ channel is important for secretion in pancreatic duct cells. Am J Physiol Cell Physiol 303:151–159
He X, Tse C-M, Danowitz M, Alper SL, Gabriel SE, Baum BJ (1997) Polarized distribution of key membrane transport proteins in the rat submandibular gland. Pflügers Arch 433:260–268
Hede SE, Amstrup J, Christoffersen BC, Novak I (1999) Purinoceptors evoke different electrophysiological responses in pancreatic ducts. P2Y inhibits K+ conductance, and P2X stimulates cation conductance. J Biol Chem 274:31784–31791
Hede SE, Amstrup J, Klaerke DA, Novak I (2005) P2Y2 and P2Y4 receptors regulate pancreatic Ca2+-activated K+ channels differently. Pflugers Arch 450:429–436
Hegyi P, Petersen OH (2013) The exocrine pancreas: the acinar-ductal tango in physiology and pathophysiology. Rev Physiol Biochem Pharmacol 165:1–30
Hegyi P, Maleth J, Venglovecz V, Rakonczay Z Jr (2011) Pancreatic ductal bicarbonate secretion: challenge of the acinar acid load. Front Physiol 2:36
Heitzmann D, Warth R (2008) Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol Rev 88:1119–1182
Hentschke M, Wiemann M, Hentschke S, Kurth I, Hermans-Borgmeyer I, Seidenbecher T, Jentsch TJ, Gal A, Hubner CA (2006) Mice with a targeted disruption of the Cl−/HCO3− exchanger AE3 display a reduced seizure threshold. Mol Cell Biol 26:183–191
Hickson JC (1970) The secretion of pancreatic juice in response to stimulation of the vagus nerves in the pig. J Physiol 206:275–297
Hikita C, Vijayakumar S, Takito J, Erdjument-Bromage H, Tempst P, Al-Awqati Q (2000) Induction of terminal differentiation in epithelial cells requires polymerization of hensin by galectin 3. J Cell Biol 151:1235–1246
Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA (1997a) Acid-stimulated duodenal bicarbonate secretion involves a CFTR-mediated transport pathway in mice. Gastroenterology 113:533–541
Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA (1997b) CFTR mediates cAMP- and Ca2+-activated duodenal epithelial HCO3- secretion. Am J Physiol 272:872–878
Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, Albert de la CA, Kere J (1996) Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 14:316–319
Hollander F, Birnbaum D (1952) The role of carbonic anhydrase in pancreatic secretion. Trans N Y Acad Sci 15:56–58
Holm M, Johansson B, Pettersson A, Fandriks L (1998) Carbon dioxide mediates duodenal mucosal alkaline secretion in response to luminal acidity in the anesthetized rat. Gastroenterology 115:680–685
Holst JJ (1993) Neural regulation of pancreatic exocrine function. In: VLW G, EP DM, Gardner JD, Lebenthal E, Reber HA, Scheele GA (eds) The pancreas: biology, pathobilogy, and disease. Raven Press, New York, pp 381–402
Hug M, Pahl C, Novak I (1994) Effect of ATP, carbachol and other agonists on intracellular calcium activity and membrane voltage of pancreatic ducts. Pflügers Arch 426:412–418
Humphrey SP, Williamson RT (2001) A review of saliva: normal composition, flow, and function. J Prosthet Dent 85:162–169
Huss M, Wieczorek H (2009) Inhibitors of V-ATPases: old and new players. J Exp Biol 212:341–346
Husted RF, Reed DJ (1976) Regulation of cerebrospinal fluid potassium by the cat choroid plexus. J Physiol 259:213–221
Hyde K, Reid CJ, Tebbutt SJ, Weide L, Hollingsworth MA, Harris A (1997) The cystic fibrosis transmembrane conductance regulator as a marker of human pancreatic duct development. Gastroenterology 113:914–919
Hyde K, Harrison D, Hollingsworth MA, Harris A (1999) Chloride-bicarbonate exchangers in the human fetal pancreas. Biochem Biophys Res Commun 263:315–321
Igimi H, Yamamoto F, Lee SP (1992) Gallbladder mucosal function: studies in absorption and secretion in humans and in dog gallbladder epithelium. Am J Physiol 263:69–74
Ignath I, Hegyi P, Venglovecz V, Szekely CA, Carr G, Hasegawa M, Inoue M, Takacs T, Argent BE, Gray MA, Rakonczay Z Jr (2009) CFTR expression but not Cl− transport is involved in the stimulatory effect of bile acids on apical Cl−/HCO3− excahnge activity in human pancreatic duct cells. Pancreas 38:921–929
Imai Y (1965) Study of the secretion mechanism of the submaxillary gland of dog. Part 2. Effects of exchanging ions in the perfusate on salivary secretion and secretory potentials, with special reference to the ionic distribution in the gland tissue. Jpn J Physiol 27:313–324
Imboden H, Harding JW, Hilgenfeldt U, Celio MR, Felix D (1987) Localization of angiotensinogen in multiple cell types of rat brain. Brain Res 410:74–77
Inagami T, Celio MR, Clemens DL, Lau D, Takii Y, Kasselberg AG, Hirose S (1980) Renin in rat and mouse brain: immunohistochemical identification and localization. Clin Sci (Lond) 59(Suppl 6):49s–51s
Isenberg JI, Hogan DL, Koss MA, Selling JA (1986) Human duodenal mucosal bicarbonate secretion. Evidence for basal secretion and stimulation by hydrochloric acid and a synthetic prostaglandin E1 analogue. Gastroenterology 91:370–378
Isenberg JI, Ljungstrom M, Safsten B, Flemstrom G (1993) Proximal duodenal enterocyte transport: evidence for Na+-H+ and Cl−-HCO3− exchange and NaHCO3 cotransport. Am J Physiol 265:677–685
Ishibashi K, Okamura K, Yamazaki J (2008) Involvement of apical P2Y2 receptor-regulated CFTR activity in muscarinic stimulation of Cl− reabsorption in rat submandibular gland. Am J Physiol Regul Integr Comp Physiol 294:1729–1736
Ishiguro H, Steward MC, Wilson RW, Case RM (1996) Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. J Physiol (Lond) 495(1):179–191
Ishiguro H, Naruse S, Steward MC, Kitagawa M, Ko SB, Hayakawa T, Case RM (1998) Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. J Physiol (Lond) 511:407–422
Ishiguro H, Naruse S, Kitagawa M, Hayakawa T, Case RM, Steward MC (1999) Luminal ATP stimulates fluid and HCO3− secretion in guinea-pig pancreatic duct. J Physiol (Lond) 519:551–558
Ishiguro H, Naruse S, Kitagawa M, Suzuki A, Yamamoto A, Hayakawa T, Case RM, Steward MC (2000) CO2 permeability and bicarbonate transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol 528(Pt 2):305–315
Ishiguro H, Namkung W, Yamamoto A, Wang Z, Worrell RT, Xu J, Lee MG, Soleimani M (2007) Effect of Slc26a6 deletion on apical Cl−/HCO3− exchanger activity and cAMP-stimulated bicarbonate secretion in pancreatic duct. Am J Physiol Gastrointest Liver Physiol 292:447–455
Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, Yamamoto A (2009) CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol 133:315–326
Jacob P, Christiani S, Rossmann H, Lamprecht G, Vieillard-Baron D, Muller R, Gregor M, Seidler U (2000a) Role of Na(+)HCO(3)(-) cotransporter NBC1, Na(+)/H(+) exchanger NHE1, and carbonic anhydrase in rabbit duodenal bicarbonate secretion. Gastroenterology 119:406–419
Jacob P, Christiani S, Rossmann H, Lamprecht G, Vieillard-Baron D, Muller R, Gregor M, Seidler U (2000b) Role of Na+-HCO3− cotransporter NBC1, Na+/H+ exchanger NHE1, and carbonic anhydrase in rabbit duodenal bicarbonate secretion. Gastroenterology 119:406–419
Jacobs S, Ruusuvuori E, Sipila ST, Haapanen A, Damkier HH, Kurth I, Hentschke M, Schweizer M, Rudhard Y, Laatikainen LM, Tyynela J, Praetorius J, Voipio J, Hubner CA (2008) Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc Natl Acad Sci U S A 105:311–316
Jacobson KA, Muller CE (2016) Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 104:31–49
Jacques T, Picard N, Miller RL, Riemondy KA, Houillier P, Sohet F, Ramakrishnan SK, Busst CJ, Jayat M, Corniere N, Hassan H, Aronson PS, Hennings JC, Hubner CA, Nelson RD, Chambrey R, Eladari D (2013) Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol 24:1104–1113
Jentsch TJ (2016) VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol 17:293–307
Jentsch TJ, Lutter D, Planells-Cases R, Ullrich F, Voss FK (2016) VRAC: molecular identification as LRRC8 heteromers with differential functions. Pflugers Arch 468:385–393
Johanson CE, Murphy VA (1990) Acetazolamide and insulin alter choroid plexus epithelial cell [Na+], pH, and volume. Am J Physiol 258:1538–1546
Johanson CE, Preston JE, Chodobski A, Stopa EG, Szmydynger-Chodobska J, McMillan PN (1999) AVP V1 receptor-mediated decrease in Cl− efflux and increase in dark cell number in choroid plexus epithelium. Am J Physiol Cell Physiol 276:82–90
Jones HC, Keep RF (1988) Brain fluid calcium concentration and response to acute hypercalcaemia during development in the rat. J Physiol 402:579–593
Joo NS, London RM, Kim HD, Forte LR, Clarke LL (1998) Regulation of intestinal Cl− and HCO3− secretion by uroguanylin. Am J Physiol 274:633–644
Juhasz M, Chen J, Lendeckel U, Kellner U, Kasper HU, Tulassay Z, Pastorekova S, Malfertheiner P, Ebert MP (2003) Expression of carbonic anhydrase IX in human pancreatic cancer. Aliment Pharmacol Ther 18:837–846
Jun I, Cheng MH, Sim E, Jung J, Suh BL, Kim Y, Son H, Park K, Kim CH, Yoon JH, Whitcomb DC, Bahar I, Lee MG (2016) Pore dilatation increases the bicarbonate permeability of CFTR, ANO1 and glycine receptor anion channels. J Physiol 594:2929–2955
Jung J, Lee MG (2014) Role of calcium signaling in epithelial bicarbonate secretion. Cell Calcium 55:376–384
Jung SR, Kim K, Hille B, Nguyen TD, Koh DS (2006) Pattern of Ca2+ increase determines the type of secretory mechanism activated in dog pancreatic duct epithelial cells. J Physiol 576:163–178
Jung SR, Hille B, Nguyen TD, Koh DS (2010) Cyclic AMP potentiates Ca2+-dependent exocytosis in pancreatic duct epithelial cells. J Gen Physiol 135:527–543
Jung J, Nam JH, Park HW, Oh U, Yoon JH, Lee MG (2013) Dynamic modulation of ANO1/TMEM16A. Proc Natl Acad Sci U S A 110:360–365
Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP (2004) WNK4 regulates apical and basolateral Cl− flux in extrarenal epithelia. Proc Natl Acad Sci U S A 101:2064–2069
Kahle KT, Rinehart J, Ring A, Gimenez I, Gamba G, Hebert SC, Lifton RP (2006) WNK protein kinases modulate cellular Cl- flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology (Bethesda) 21:326–335
Kajita H, Whitwell C, Brown PD (2000) Properties of the inward-rectifying Cl− channel in rat choroid plexus: regulation by intracellular messengers and inhibition by divalent cations. Pflugers Arch 440:933–940
Kalaria RN, Premkumar DR, Lin CW, Kroon SN, Bae JY, Sayre LM, LaManna JC (1998) Identification and expression of the Na+/H+ exchanger in mammalian cerebrovascular and choroidal tissues: characterization by amiloride-sensitive [3H]MIA binding and RT-PCR analysis. Brain Res Mol Brain Res 58:178–187
Kallio H, Pastorekova S, Pastorek J, Waheed A, Sly WS, Mannisto S, Heikinheimo M, Parkkila S (2006) Expression of carbonic anhydrases IX and XII during mouse embryonic development. BMC Dev Biol 6:22
Kane Dickson V, Pedi L, Long SB (2014) Structure and insights into the function of a Ca2+-activated Cl− channel. Nature 516:213–218
Kao L, Kurtz LM, Shao X, Papadopoulos MC, Liu L, Bok D, Nusinowitz S, Chen B, Stella SL, Andre M, Weinreb J, Luong SS, Piri N, Kwong JM, Newman D, Kurtz I (2011) Severe neurologic impairment in mice with targeted disruption of the electrogenic sodium bicarbonate cotransporter NBCe2 (Slc4a5 gene). J Biol Chem 286:32563–32574
Karadsheh MF, Byun N, Mount DB, Delpire E (2004) Localization of the KCC4 potassium-chloride cotransporter in the nervous system. Neuroscience 123:381–391
Kaunitz JD, Akiba Y (2002) Luminal acid elicits a protective duodenal mucosal response. Keio J Med 51:29–35
Kaunitz JD, Akiba Y (2006a) Duodenal carbonic anhydrase: mucosal protection, luminal chemosensing, and gastric acid disposal. Keio J Med 55:96–106
Kaunitz JD, Akiba Y (2006b) Review article: duodenal bicarbonate - mucosal protection, luminal chemosensing and acid-base balance. Aliment Pharmacol Ther 24(Suppl 4):169–176
Kawedia JD, Nieman ML, Boivin GP, Melvin JE, Kikuchi K, Hand AR, Lorenz JN, Menon AG (2007) Interaction between transcellular and paracellular water transport pathways through Aquaporin 5 and the tight junction complex. Proc Natl Acad Sci U S A 104:3621–3626
Kay RNB (1960) The rate of flow and composition of various salivary secretions in sheep and calves. J Physiol (Lond) 150:515–537
Keep RF, Xiang J, Betz AL (1994) Potassium cotransport at the rat choroid plexus. Am J Physiol 267:1616–1622
Keitel V, Haussinger D (2013) TGR5 in cholangiocytes. Curr Opin Gastroenterol 29:299–304
Keitel V, Ullmer C, Haussinger D (2010) The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem 391:785–789
Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC (1989) Identification of the cystic fibrosis gene: genetic analysis. Science 245:1073–1080
Kibble JD, Trezise AE, Brown PD (1996) Properties of the cAMP-activated C1- current in choroid plexus epithelial cells isolated from the rat. J Physiol 496(Pt 1):69–80
Kibble JD, Garner C, Colledge WH, Brown S, Kajita H, Evans M, Brown PD (1997) Whole cell Cl− conductances in mouse choroid plexus epithelial cells do not require CFTR expression. Am J Physiol Cell Physiol 272:1899–1907
Kim JY, Kim KH, Lee JA, Namkung W, Sun AQ, Ananthanarayanan M, Suchy FJ, Shin DM, Muallem S, Lee MG (2002) Transporter-mediated bile acid uptake causes Ca2+-dependent cell death in rat pancreatic acinar cells. Gastroenterology 122:1941–1953
Kim KH, Shcheynikov N, Wang Y, Muallem S (2005) SLC26A7 is a Cl- channel regulated by intracellular pH. J Biol Chem 280:6463–6470
Kivela AJ, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK, Pastorekova S, Pastorek J, Waheed A, Sly WS, Rajaniemi H (2000) Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours. Histochem Cell Biol 114:197–204
Klarr SA, Ulanski LJ, Stummer W, Xiang J, Betz AL, Keep RF (1997) The effects of hypo- and hyperkalemia on choroid plexus potassium transport. Brain Res 758:39–44
Knauf H, Lubcke R, Kreutz W, Sachs G (1982) Interrelationships of ion transport in rat submaxillary duct epithelium. Am J Physiol 242:132–139
Ko SB, Luo X, Hager H, Rojek A, Choi JY, Licht C, Suzuki M, Muallem S, Nielsen S, Ishibashi K (2002a) AE4 is a DIDS-sensitive Cl−/HCO3− exchanger in the basolateral membrane of the renal CCD and the SMG duct. Am J Physiol Cell Physiol 283:1206–1218
Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S (2002b) A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J 21:5662–5672
Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S (2004) Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6:343–350
Kodama T (1983) A light and electron microscopic study on the pancreatic ductal system. Acta Pathol Jpn 33:297–321
Kollert-Jons A, Wagner S, Hubner S, Appelhans H, Drenckhahn D (1993) Anion exchanger 1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus. Am J Physiol 265:813–821
Komwatana P, Dinudom A, Young JA, Cook DI (1996a) Control of the amiloride-sensitive Na+ current in salivary duct cells by extracellular sodium. J Membr Biol 150:133–141
Komwatana P, Dinudom A, Young JA, Cook DI (1996b) Cytosolic Na+ controls and epithelial Na+ channel via the Go guanine nucleotide-binding regulatory protein. Proc Natl Acad Sci U S A 93:8107–8111
Kopelman H, Durie P, Gaskin K, Weizman Z, Forstner G (1985) Pancreatic fluid secretion and protein hyperconcentration in cystic fibrosis. N Engl J Med 312:329–334
Kopelman H, Corey M, Gaskin K, Durie P, Weizman Z, Forstner G (1988) Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology 95:349–355
Kordas KS, Sperlagh B, Tihanyi T, Topa L, Steward MC, Varga G, Kittel A (2004) ATP and ATPase secretion by exocrine pancreas in rat, Guinea pig, and human. Pancreas 29:53–60
Kosiek O, Busque SM, Foller M, Shcheynikov N, Kirchhoff P, Bleich M, Muallem S, Geibel JP (2007) SLC26A7 can function as a chloride-loading mechanism in parietal cells. Pflugers Arch 454:989–998
Kotera T, Brown PD (1994) Evidence for two types of potassium current in rat choroid plexus epithelial cells. Pflugers Arch 427:317–324
Kowal JM, Haanes KA, Christensen NM, Novak I (2015a) Bile acid effects are mediated by ATP release and purinergic signalling in exocrine pancreatic cells. Cell Commun Signal 13:28
Kowal JM, Yegutkin GG, Novak I (2015b) ATP release, generation and hydrolysis in exocrine pancreatic duct cells. Purinergic Signal 11:533–550
Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, Doetschman T, Menon AG (2001) Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem 276:23413–23420
Kulaksiz H, Cetin Y (2002) The electrolyte/fluid secretion stimulatory peptides guanylin and uroguanylin and their common functional coupling proteins in the rat pancreas: a correlative study of expression and cell-specific localization. Pancreas 25:170–175
Kumpulainen T, Jalovaara P (1981) Immunohistochemical localization of carbonic anhydrase isoenzymes in the human pancreas. Gastroenterology 80:796–799
Kwon TH, Pushkin A, Abuladze N, Nielsen S, Kurtz I (2000) Immunoelectron microscopic localization of NBC3 sodium-bicarbonate cotransporter in rat kidney. Am J Physiol Renal Physiol 278:327–336
Kwon TH, Fenton RA, Praetorius J, Nielsen S (2012) Section I: normal renal function: molecular, cellular, structural, and physiologic principles; chapter 2: anatomy and topography. In: Taal MW, Chertow GM, Marsden PA, Skoreckiand K, Yu ASL (eds) Brenner & Rector’s the kidney. Saunders, New York
Larsen AM, Krogsgaard-Larsen N, Lauritzen G, Olesen CW, Honore HS, Boedtkjer E, Pedersen SF, Bunch L (2012) Gram-scale solution-phase synthesis of selective sodium bicarbonate co-transport inhibitor S0859: in vitro efficacy studies in breast cancer cells. ChemMedChem 7:1808–1814
Le MC, Boulkroun S, Gonzalez-Nunez D, Dublineau I, Cluzeaud F, Fay M, Blot-Chabaud M, Farman N (2005) Aldosterone and tight junctions: modulation of claudin-4 phosphorylation in renal collecting duct cells. Am J Physiol Cell Physiol 289:1513–1521
Lee RJ, Foskett JK (2010) Mechanisms of Ca2+-stimulated fluid secretion by porcine bronchial submucosal gland serous acinar cells. Am J Physiol Lung Cell Mol Physiol 298:210–231
Lee MG, Schultheis PJ, Yan M, Shull GE, Bookstein C, Chang E, Tse M, Donowitz M, Park K, Muallem S (1998) Membrane-limited expression and regulation of Na+-H+ exchanger isoforms by P2 receptors in the rat submandibular gland duct. J Physiol 513:341–357
Lee MG, Ahn W, Choi JY, Luo X, Seo JT, Schultheis PJ, Shull GE, Kim KH, Muallem S (2000) Na+-dependent transporters mediate HCO3− salvage across the luminal membrane of the main pancreatic duct. J Clin Invest 105:1651–1658
Lee MG, Ohana E, Park HW, Yang D, Muallem S (2012) Molecular mechanism of pancreatic and salivary gland fluid and HCO3− secretion. Physiol Rev 92:39–74
Lenzen R, Elster J, Behrend C, Hampel KE, Bechstein WO, Neuhaus P (1997) Bile acid-independent bile flow is differently regulated by glucagon and secretin in humans after orthotopic liver transplantation. Hepatology 26:1272–1281
Leslie BR, Schwartz JH, Steinmetz PR (1973) Coupling between Cl- absorption and HCO3- secretion in turtle urinary bladder. Am J Physiol 225:610–617
Leviel F, Hubner CA, Houillier P, Morla L, El MS, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D (2010) The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120:1627–1635
Li X, Alvarez B, Casey JR, Reithmeier RA, Fliegel L (2002) Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J Biol Chem 277:36085–36091
Li J, Koo NY, Cho IH, Kwon TH, Choi SY, Lee SJ, Oh SB, Kim JS, Park K (2006) Expression of the Na+-HCO3− cotransporter and its role in pHi regulation in guinea pig salivary glands. Am J Physiol Gastrointest Liver Physiol 291:1031–1040
Li Q, Ding Y, Krafft P, Wan W, Yan F, Wu G, Zhang Y, Zhan Q, Zhang JH (2018) Targeting germinal matrix hemorrhage-induced overexpression of sodium-coupled bicarbonate exchanger reduces posthemorrhagic hydrocephalus formation in neonatal rats. J Am Heart Assoc 7:e007192
Lindsey AE, Schneider K, Simmons DM, Baron R, Lee BS, Kopito RR (1990) Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc Natl Acad Sci U S A 87:5278–5282
Lindvall-Axelsson M, Nilsson C, Owman C, Winbladh B (1992) Inhibition of cerebrospinal fluid formation by omeprazole. Exp Neurol 115:394–399
Liu S, Piwnica-Worms D, Lieberman M (1990) Intracellular pH regulation in cultured embryonic chick heart cells. Na+-dependent Cl−/HCO3− exchange. J Gen Physiol 96:1247–1269
Liu X, Li T, Riederer B, Lenzen H, Ludolph L, Yeruva S, Tuo B, Soleimani M, Seidler U (2015) Loss of Slc26a9 anion transporter alters intestinal electrolyte and HCO3− transport and reduces survival in CFTR-deficient mice. Pflugers Arch 467:1261–1275
Liu X, Li T, Tuo B (2018) Physiological and pathophysiological relevance of the anion transporter Slc26a9 in multiple organs. Front Physiol 9:1197
Lohi H, Kujala M, Kerkela E, Saarialho-Kere U, Kestila M, Kere J (2000) Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics 70:102–112
Loiselle FB, Morgan PE, Alvarez BV (2003) Regulation of the human NBC3 Na+/HCO3− co-transporter by carbonic anhydrase II and protein kinase A. Am J Physiol Cell Physiol 286:1423–1433
Lonnerholm G, Knutson L, Wistrand PJ, Flemstrom G (1989) Carbonic anhydrase in the normal rat stomach and duodenum and after treatment with omeprazole and ranitidine. Acta Physiol Scand 136:253–262
Loriol C, Dulong S, Avella M, Gabillat N, Boulukos K, Borgese F, Ehrenfeld J (2008) Characterization of SLC26A9, facilitation of Cl(-) transport by bicarbonate. Cell Physiol Biochem 22:15–30
Lux SE, John KM, Kopito RR, Lodish HF (1989) Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1). Proc Natl Acad Sci U S A 86:9089–9093
Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS (1999) Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem 274:20071–20074
Mahieu I, Becq F, Wolfensberger T, Gola M, Carter N, Hollande E (1994) The expression of carbonic anhydrases II and IV in the human pancreatic cancer cell line (Capan 1) is associated with bicarbonate ion channels. Biol Cell 81:131–141
Maleth J, Venglovecz V, Razga Z, Tiszlavicz L, Rakonczay Z Jr, Hegyi P (2011) Non-conjugated chenodeoxycholate induces severe mitochondrial damage and inhibits bicarbonate transport in pancreatic duct cells. Gut 60:136–138
Mangos JA, Braun G, Hamann KF (1966) Micropuncture study of sodium and potassium excretion in the rat parotid saliva. Pflügers Arch 291:99–106
Mangos JA, McSherry NR, Nousia-Arvanitakis S, Irwin K (1973) Secretion and transductal fluxes of ions in exocrine glands of the mouse. Am J Physiol 225:18–24
Maren TH (1962) The binding of inhibitors to carbonic anhydrase in vivo: drugs as markers for enzyme. Biochem Pharmacol 9:39–48
Marino CR, Matovcik LM, Gorelick FS, Cohn JA (1991) Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest 88:712–716
Marino CR, Jeanes V, Boron WF, Schmitt BM (1999) Expression and distribution of the Na+-HCO3− cotransporter in human pancreas. Am J Physiol 277:487–494
Marsey LL, Winpenny JP (2009) Bestrophin expression and function in the human pancreatic duct cell line, CFPAC-1. J Physiol 587:2211–2224
Marteau C, Silviani V, Ducroc R, Crotte C, Gerolami A (1995) Evidence for apical Na+/H+ exchanger in bovine main pancreatic duct. Dig Dis Sci 40:2336–2340
Martin CJ, Young JA (1971) A microperfusion investigation of the effects of a sympathomimetic and a parasympathomimetic drug on water and electrolyte fluxes in the main duct of the rat submaxillary gland. Pflügers Arch 327:303–323
Martin AC, Willoughby D, Ciruela A, Ayling LJ, Pagano M, Wachten S, Tengholm A, Cooper DM (2009) Capacitative Ca2+ entry via Orai1 and stromal interacting molecule 1 (STIM1) regulates adenylyl cyclase type 8. Mol Pharmacol 75:830–842
Martinez JR, Cassity N (1983) Effects of transport inhibitors on secretion by perfused rat submandibular gland. Am J Physiol 245:711–716
Martinez JR, Holzgreve H, Frick A (1966) Micropuncture study of submaxillary glands of adult rats. Pflügers Arch 290:124–133
Martinez-Anso E, Castillo JE, Diez J, Medina JF, Prieto J (1994) Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology 19:1400–1406
Maruyama Y, Gallacher DV, Petersen OH (1983) Voltage and Ca2+-activated K+ channel in baso-lateral acinar cell membranes of mammalian salivary glands. Nature 302:827–829
Masmoudi S, Charfedine I, Hmani M, Grati M, Ghorbel AM, Elgaied-Boulila A, Drira M, Hardelin JP, Ayadi H (2000) Pendred syndrome: phenotypic variability in two families carrying the same PDS missense mutation. Am J Med Genet 90:38–44
Masuzawa T, Ohta T, Kawamura M, Nakahara N, Sato F (1984) Immunohistochemical localization of Na+, K+-ATPase in the choroid plexus. Brain Res 302:357–362
Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, Stroope AJ, LaRusso NF (2008) Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol 295:725–734
Masyuk AI, Huang BQ, Radtke BN, Gajdos GB, Splinter PL, Masyuk TV, Gradilone SA, LaRusso NF (2013) Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Gastrointest Liver Physiol 304:1013–1024
Matsuo R (2000) Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med 11:216–229
Mayer SE, Sanders-Bush E (1993) Sodium-dependent antiporters in choroid plexus epithelial cultures from rabbit. J Neurochem 60:1308–1316
McCormick JA, Ellison DH (2011) The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev 91:177–219
McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, Johnson DE, Casey JR (2004) The bicarbonate transport metabolon. J Enzyme Inhib Med Chem 19:231–236
Meier PJ, Knickelbein R, Moseley RH, Dobbins JW, Boyer JL (1985) Evidence for carrier-mediated chloride/bicarbonate exchange in canalicular rat liver plasma membrane vesicles. J Clin Invest 75:1256–1263
Melby JM, Miner LC, Reed DJ (1982) Effect of acetazolamide and furosemide on the production and composition of cerebrospinal fluid from the cat choroid plexus. Can J Physiol Pharmacol 60:405–409
Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE (1999) Mouse down-regulated in adenoma (DRA) is an intestinal Cl−/HCO3− exchanger and is up-regulated in colon of mice lacking the NHE3 Na+/H+ exchanger. J Biol Chem 274:22855–22861
Mennone A, Biemesderfer D, Negoianu D, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Aronson PS, Boyer JL (2001) Role of sodium/hydrogen exchanger isoform NHE3 in fluid secretion and absorption in mouse and rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 280:247–254
Meyer G, Guizzardi F, Rodighiero S, Manfredi R, Saino S, Sironi C, Garavaglia ML, Bazzini C, Botta G, Portincasa P, Calamita G, Paulmichl M (2005) Ion transport across the gallbladder epithelium. Curr Drug Targets Immune Endocr Metabol Disord 5:143–151
Millar ID, Brown PD (2008) NBCe2 exhibits a 3 HCO3−:1 Na+ stoichiometry in mouse choroid plexus epithelial cells. Biochem Biophys Res Commun 373:550–554
Minagawa N, Nagata J, Shibao K, Masyuk AI, Gomes DA, Rodrigues MA, Lesage G, Akiba Y, Kaunitz JD, Ehrlich BE, LaRusso NF, Nathanson MH (2007) Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile. Gastroenterology 133:1592–1602
Modyanov NN, Petrukhin KE, Sverdlov VE, Grishin AV, Orlova MY, Kostina MB, Makarevich OI, Broude NE, Monastyrskaya GS, Sverdlov ED (1991) The family of human Na,K-ATPase genes. ATP1AL1 gene is transcriptionally competent and probably encodes the related ion transport ATPase. FEBS Lett 278:91–94
Modyanov NN, Mathews PM, Grishin AV, Beguin P, Beggah AT, Rossier BC, Horisberger JD, Geering K (1995) Human ATP1AL1 gene encodes a ouabain-sensitive H-K-ATPase. Am J Physiol 269:992–997
Moore-Hoon ML, Turner RJ (1998) Molecular and topological characterization of the rat parotid Na+-K+-2Cl− cotransporter1. Biochim Biophys Acta 1373:261–269
Moseley RH, Meier PJ, Aronson PS, Boyer JL (1986) Na-H exchange in rat liver basolateral but not canalicular plasma membrane vesicles. Am J Physiol 250:35–43
Moser AJ, Gangopadhyay A, Bradbury NA, Peters KW, Frizzell RA, Bridges RJ (2007) Electrogenic bicarbonate secretion by prairie dog gallbladder. Am J Physiol Gastrointest Liver Physiol 292:1683–1694
Mount DB, Romero MF (2004) The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch 447:710–721
Murakami M, Murdiastuti K, Hosoi K, Hill AE (2006) AQP and the control of fluid transport in a salivary gland. J Membr Biol 210:91–103
Murphy VA, Johanson CE (1989) Alteration of sodium transport by the choroid plexus with amiloride. Biochim Biophys Acta 979:187–192
Murphy VA, Smith QR, Rapoport SI (1986) Homeostasis of brain and cerebrospinal fluid calcium concentrations during chronic hypo- and hypercalcemia. J Neurochem 47:1735–1741
Nakahari T, Murakami M, Yoshida H, Miyamoto M, Sohma Y, Imai Y (1990) Decrease in rat submandibular acinar cell volume during ACh stimulation. Am J Physiol 258:878–886
Nakahari T, Murakami M, Sasaki Y, Kataoka T, Imai Y, Shiba Y, Kanno Y (1991) Dose effects of acetylcholine on the cell volume of rat mandibular salivary acini. Jpn J Physiol 41:153–168
Nakamoto T, Srivastava A, Romanenko VG, Ovitt CE, Perez-Cornejo P, Arreola J, Begenisich T, Melvin JE (2007) Functional and molecular characterization of the fluid secretion mechanism in human parotid acinar cells. Am J Physiol Regul Integr Comp Physiol 292:2380–2390
Nakamoto T, Romanenko VG, Takahashi A, Begenisich T, Melvin JE (2008) Apical maxi-K (KCa1.1) channels mediate K+ secretion by the mouse submandibular exocrine gland. Am J Physiol Cell Physiol 294:810–819
Nakamoto T, Brown DA, Catalan MA, Gonzalez-Begne M, Romanenko VG, Melvin JE (2009) Purinergic P2X7 receptors mediate ATP-induced saliva secretion by the mouse submandibular gland. J Biol Chem 284:4815–4822
Nakamura N, Suzuki Y, Sakuta H, Ookata K, Kawahara K, Hirose S (1999) Inwardly rectifying K+ channel Kir7.1 is highly expressed in thyroid follicular cells, intestinal epithelial cells and choroid plexus epithelial cells: implication for a functional coupling with Na+,K+-ATPase. Biochem J 342(Pt 2):329–336
Namkung W, Lee JA, Ahn W, Han W, Kwon SW, Ahn DS, Kim KH, Lee MG (2003) Ca2+ activates cystic fibrosis transmembrane conductance regulator- and Cl− -dependent HCO3 transport in pancreatic duct cells. J Biol Chem 278:200–207
Namkung W, Finkbeiner WE, Verkman AS (2010) CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol Biol Cell 21:2639–2648
Nathanson MH, Burgstahler AD, Mennone A, Boyer JL (1996) Characterization of cytosolic Ca2+ signaling in rat bile duct epithelia. Am J Physiol 271:86–96
Nattie EE (1980) Brain and cerebrospinal fluid ionic composition and ventilation in acute hypercapnia. Respir Physiol 40:309–322
Nauntofte B (1992) Regulation of electrolyte and fluid secretion in salivary acinar cells. Am J Physiol Gastrointest Liver Physiol 263:823–837
Nauntofte B, Poulsen JH (1986) Effects of Ca2+ and furosemide on Cl− transport on O2 uptake in rat parotid acini. Am J Physiol 251:175–185
Nehrke K, Quinn CC, Begenisich T (2003) Molecular identification of Ca2+-activated K+ channels in parotid acinar cells. Am J Physiol Cell Physiol 284:535–546
Nguyen TD, Moody MW, Savard CE, Lee SP (1998) Secretory effects of ATP on nontransformed dog pancreatic duct epithelial cells. Am J Physiol 275:104–113
Nguyen TD, Meichle S, Kim US, Wong T, Moody MW (2001) P2Y11, a purinergic receptor acting via cAMP, mediates secretion by pancreatic duct epithelial cells. Am J Physiol Gastrointest Liver Physiol 280:795–804
Nishimori I, Onishi S (2001) Carbonic anhydrase isozymes in the human pancreas. Dig Liver Dis 33:68–74
Nishimori I, FujikawaAdachi K, Onishi S, Hollingsworth MA (1999) Carbonic anhydrase in human pancreas: hypotheses for the pathophysiological roles of CA isozymes. Ann N Y Acad Sci 880:5–16
Novak I (1993) Cellular mechanism of salivary gland secretion. In: Gilles R (ed) Advances in comparative and environmental physiology, vol 15. Springer, Berlin, pp 1–43
Novak I (2008) Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal 4:237–253
Novak I (2011) Purinergic signalling in epithelial ion transport: regulation of secretion and absorption. Acta Physiol (Oxf) 202:501–522
Novak I, Christoffersen BC (2001) Secretin stimulates HCO3− and acetate efflux but not Na+/HCO3− uptake in rat pancreatic ducts. Pflügers Arch 441:761–771
Novak I, Greger R (1988a) Electrophysiological study of transport systems in isolated perfused pancreatic ducts: properties of the basolateral membrane. Pflügers Arch 411:58–68
Novak I, Greger R (1988b) Properties of the luminal membrane of isolated perfused rat pancreatic ducts: effect of cyclic AMP and blockers of chloride transport. Pflügers Arch 411:546–553
Novak I, Greger R (1991) Effect of bicarbonate on potassium conductance of isolated perfused rat pancreatic ducts. Pflügers Arch 419:76–83
Novak I, Young JA (1986) Two independent anion transport systems in rabbit mandibular salivary glands. Pflugers Arch 407:649–656
Novak I, Hede SE, Hansen MR (2008) Adenosine receptors in rat and human pancreatic ducts stimulate chloride transport. Pflugers Arch 456:437–447
Novak I, Jans IM, Wohlfahrt L (2010) Effect of P2X7 receptor knockout on exocrine secretion of pancreas, salivary glands and lacrimal glands. J Physiol (Lond) 588(18):3615–3627
Novak I, Wang J, Henriksen KL, Haanes KA, Krabbe S, Nitschke R, Hede SE (2011) Pancreatic bicarbonate secretion involves two proton pumps. J Biol Chem 286:280–289
Novak I, Haanes KA, Wang J (2013) Acid-base transport in pancreas-new challenges. Front Physiol 4:380. https://doi.org/10.3389/fphys.2013.00380
Odgaard E, Jakobsen JK, Frische S, Praetorius J, Nielsen S, Aalkjaer C, Leipziger J (2004) Basolateral Na+-dependent HCO3− transporter NBCn1-mediated HCO3− influx in rat medullary thick ascending limb. J Physiol 555:205–218
Okolo C, Wong T, Moody MW, Nguyen TD (2002) Effects of bile acids on dog pancreatic duct epithelial cell secretion and monolayer resistance. Am J Physiol Gastrointest Liver Physiol 283:1042–1050
Olszewski U, Hlozek M, Hamilton G (2010) Activation of Na+/H+ exchanger 1 by neurotensin signaling in pancreatic cancer cell lines. Biochem Biophys Res Commun 393:414–419
Orlowski J, Grinstein S (2004) Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch 447:549–565
Ostrowski NL, Lolait SJ, Bradley DJ, O'Carroll AM, Brownstein MJ, Young WS III (1992) Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinology 131:533–535
Pahl C, Novak I (1993) Effect of vasoactive intestinal peptide, carbachol and other agonists on cell membrane voltage of pancreatic duct cells. Pflügers Arch 424:315–320
Palk L, Sneyd J, Shuttleworth TJ, Yule DI, Crampin EJ (2010) A dynamic model of saliva secretion. J Theor Biol 266:625–640
Park HW, Lee MG (2012) Transepithelial bicarbonate secretion: lessons from the pancreas. Cold Spring Harb Perspect Med 2:155–170
Park HS, Lee YL, Kwon HY, Chey WY, Park HJ (1998) Significant cholinergic role in secretin-stimulated exocrine secretion in isolated rat pancreas. Am J Physiol 274:413–418
Park K, Olschowka JA, Richardson LA, Bookstein C, Chang EB, Melvin JE (1999) Expression of multiple Na+/H+ exchanger isoforms in rat parotid acinar and ductal cells. Am J Physiol 276:470–478
Park K, Case RM, Brown PD (2001a) Identification and regulation of K+ and Cl- channels in human parotid acinar cells. Arch Oral Biol 46:801–810
Park K, Evans RL, Watson GE, Nehrke K, Richardson L, Bell SM, Schultheis PJ, Hand AR, Shull GE, Melvin JE (2001b) Defective fluid secretion and NaCl absorption in the parotid glands of Na+/H+ exchanger-deficient mice. J Biol Chem 276:27042–27050
Park K, Hurley PT, Roussa E, Cooper GJ, Smith CP, Thevenod F, Steward MC, Case RM (2002a) Expression of a sodium bicarbonate cotransporter in human parotid salivary glands. Arch Oral Biol 47:1–9
Park M, Ko SB, Choi JY, Muallem G, Thomas PJ, Pushkin A, Lee MS, Kim JY, Lee MG, Muallem S, Kurtz I (2002b) The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO3− salvage transporter human Na+-HCO3− cotransport isoform 3. J Biol Chem 277:50503–50509
Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG (2010) Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology 139:620–631
Park S, Hong JH, Ohana E, Muallem S (2012) The WNK/SPAK and IRBIT/PP1 pathways in epithelial fluid and electrolyte transport. Physiology (Bethesda) 27:291–299
Park S, Shcheynikov N, Hong JH, Zheng C, Suh SH, Kawaai K, Ando H, Mizutani A, Abe T, Kiyonari H, Seki G, Yule D, Mikoshiba K, Muallem S (2013) Irbit mediates synergy between Ca2+ and cAMP signaling pathways during epithelial transport in mice. Gastroenterology 145:232–241
Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF (2008) Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl− self-exchange activity. J Biol Chem 283:12777–12788
Pascua P, Garcia M, Fernandez-Salazar MP, Hernandez-Lorenzo MP, Calvo JJ, Colledge WH, Case RM, Steward MC, San Roman JI (2009) Ducts isolated from the pancreas of CFTR-null mice secrete fluid. Pflugers Arch 459:203–214
Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S (2008) Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294:488–494
Patterson K, Catalan MA, Melvin JE, Yule DI, Crampin EJ, Sneyd J (2012) A quantitative analysis of electrolyte exchange in the salivary duct. Am J Physiol Gastrointest Liver Physiol 303:1153–1163
Paulais M, Cragoe EJ Jr, Turner RJ (1994) Ion transport mechanisms in rat parotid intralobular striated ducts. Am J Physiol 266:1594–1602
Pearson MM, Lu J, Mount DB, Delpire E (2001) Localization of the K+-Cl− cotransporter, KCC3, in the central and peripheral nervous systems: expression in the choroid plexus, large neurons and white matter tracts. Neuroscience 103:481–491
Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, Wall SM (2010) Pendrin modulates ENaC function by changing luminal HCO3−. J Am Soc Nephrol 21:1928–1941
Pedersen AM, Bardow A, Jensen SB, Nauntofte B (2002) Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis 8:117–129
Pedersen AML, Sørensen CE, Dynesen AW, Jensen SB (2013a) Salivary glands structure and function and regulation of saliva secretion in health and disease. In: Braxton L, Quinn L (eds) Salivary glands: anatomy, functions in digestion and rrole in disease. Nova Science, New York, pp 1–43
Pedersen SF, Hoffmann EK, Novak I (2013b) Cell volume regulation in epithelial physiology and cancer. Front Physiol 4:233
Pedersen SF, Novak I, Alves F, Schwab A, Pardo LA (2017) Alternating pH landscapes shape epithelial cancer initiation and progression: Focus on pancreatic cancer. Bioessays 39:1600253
Perides G, Laukkarinen JM, Vassileva G, Steer ML (2010) Biliary acute pancreatitis in mice is mediated by the G-protein-coupled cell surface bile acid receptor Gpbar1. Gastroenterology 138:715–725
Pestov NB, Romanova LG, Korneenko TV, Egorov MV, Kostina MB, Sverdlov VE, Askari A, Shakhparonov MI, Modyanov NN (1998) Ouabain-sensitive H,K-ATPase: tissue-specific expression of the mammalian genes encoding the catalytic alpha subunit. FEBS Lett 440:320–324
Petersen OH (2014) Calcium signalling and secretory epithelia. Cell Calcium 55:282–289
Petersen OH, Gallacher DV (1988) Electrophysiology of pancreatic and salivary acinar cells. Annu Rev Physiol 50:65–80
Petersen OH, Poulsen JH (1967) The effects of varying the extracellular potassium concentration on the secretory rate and on resting and secretory potentials in the perfused cat submandibular gland. Acta Physiol Scand 70:293–298
Petersen KU, Reuss L (1983) Cyclic AMP-induced chloride permeability in the apical membrane of Necturus gallbladder epithelium. J Gen Physiol 81:705–729
Petersen KU, Wehner F, Winterhager JM (1990) Transcellular bicarbonate transport in rabbit gallbladder epithelium: mechanisms and effects of cyclic AMP. Pflugers Arch 416:312–321
Petrovic S, Wang Z, Ma L, Soleimani M (2003) Regulation of the apical Cl−/HCO3− exchanger pendrin in rat cortical collecting duct in metabolic acidosis. Am J Physiol Renal Physiol 284:103–112
Petrovic S, Barone S, Xu J, Conforti L, Ma L, Kujala M, Kere J, Soleimani M (2004) SLC26A7: a basolateral Cl−/HCO3− exchanger specific to intercalated cells of the outer medullary collecting duct. Am J Physiol Renal Physiol 286:161–169
Phillips PA, Abrahams JM, Kelly J, Paxinos G, Grzonka Z, Mendelsohn FA, Johnston CI (1988) Localization of vasopressin binding sites in rat brain by in vitro autoradiography using a radioiodinated V1 receptor antagonist. Neuroscience 27:749–761
Pizzonia JH, Biemesderfer D, Abu-Alfa AK, Wu MS, Exner M, Isenring P, Igarashi P, Aronson PS (1998) Immunochemical characterization of Na+/H+ exchanger isoform NHE4. Am J Physiol 275:510–517
Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E (1997) Expression of the Na+-K+-2Cl− cotransporter BSC2 in the nervous system. Am J Physiol Cell Physiol 272:173–183
Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K (2011) The bile acid membrane receptor TGR5: a valuable metabolic target. Dig Dis 29:37–44
Poronnik P, Schumann SY, Cook DI (1995) HCO3−-dependent ACh-activated Na+ influx in sheep parotid secretory endpieces. Pflugers Arch 429:852–858
Poulsen JH, Bundgaard M (1994) Quantitative estimation of the area of luminal and basolateral membranes of rat parotid acinar cells: some physiological applications. Pflugers Arch 429:240–244
Praetorius J (2010) Na+-coupled bicarbonate transporters in duodenum, collecting ducts and choroid plexus. J Nephrol 23(Suppl 16):35–42
Praetorius J, Nielsen S (2006) Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am J Physiol Cell Physiol 291:59–67
Praetorius J, Andreasen D, Jensen BL, Ainsworth MA, Friis UG, Johansen T (2000) NHE1, NHE2, and NHE3 contribute to regulation of intracellular pH in murine duodenal epithelial cells. Am J Physiol Gastrointest Liver Physiol 278:197–206
Praetorius J, Hager H, Nielsen S, Aalkjaer C, Friis UG, Ainsworth MA, Johansen T (2001) Molecular and functional evidence for electrogenic and electroneutral Na+-HCO3− cotransporters in murine duodenum. Am J Physiol Gastrointest Liver Physiol 280:332–343
Praetorius J, Friis UG, Ainsworth MA, Schaffalitzky de Muckadell OB, Johansen T (2002) The cystic fibrosis transmembrane conductance regulator is not a base transporter in isolated duodenal epithelial cells. Acta Physiol Scand 174:327–336
Praetorius J, Kim YH, Bouzinova EV, Frische S, Rojek A, Aalkjaer C, Nielsen S (2004a) NBCn1 is a basolateral Na+-HCO3− cotransporter in rat kidney inner medullary collecting ducts. Am J Physiol Renal Physiol 286:903–912
Praetorius J, Nejsum LN, Nielsen S (2004b) A SLC4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells. Am J Physiol Cell Physiol 286:601–610
Proctor GB, Carpenter GH (2014) Salivary secretion: mechanism and neural regulation. Monogr Oral Sci 24:14–29
Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I (1999) Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem 274:16569–16575
Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I (2000) Cloning, characterization and chromosomal assignment of NBC4, a new member of the sodium bicarbonate cotransporter family. Biochim Biophys Acta 1493:215–218
Qu Z, Hartzell HC (2008) Bestrophin Cl− channels are highly permeable to HCO3−. Am J Physiol Cell Physiol 294:1371–1377
Quinton PM (2008) Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 372:415–417
Quinton PM (2010) Role of epithelial HCO3− transport in mucin secretion: lessons from cystic fibrosis. Am J Physiol Cell Physiol 299:1222–1233
Quinton PM, Wright EM, Tormey JM (1973) Localization of sodium pumps in the choroid plexus epithelium. J Cell Biol 58:724–730
Rakonczay Z Jr, Fearn A, Hegyi P, Boros I, Gray MA, Argent BE (2006) Characterization of H+ and HCO3− transporters in CFPAC-1 human pancreatic duct cells. World J Gastroenterol 12:885–895
Renner EL, Lake JR, Scharschmidt BF, Zimmerli B, Meier PJ (1989) Rat hepatocytes exhibit basolateral Na+/HCO3− contransport. J Clin Invest 83:1225–1235
Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066–1073
Roepke TK, Kanda VA, Purtell K, King EC, Lerner DJ, Abbott GW (2011) KCNE2 forms potassium channels with KCNA3 and KCNQ1 in the choroid plexus epithelium. FASEB J 25:4264–4273
Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB Jr, Zabner J, Prather RS, Welsh MJ (2008) Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321:1837–1841
Romanenko VG, Nakamoto T, Srivastava A, Begenisich T, Melvin JE (2007) Regulation of membrane potential and fluid secretion by Ca2+-activated K+ channels in mouse submandibular glands. J Physiol 581:801–817
Romanenko VG, Nakamoto T, Catalan MA, Gonzalez-Begne M, Schwartz GJ, Jaramillo Y, Sepulveda FV, Figueroa CD, Melvin JE (2008) Clcn2 encodes the hyperpolarization-activated chloride channel in the ducts of mouse salivary glands. Am J Physiol Gastrointest Liver Physiol 295:1058–1067
Romanenko VG, Catalan MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE (2010) Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J Biol Chem 285:12990–13001
Romero MF, Hediger MA, Boulpaep EL, Boron WF (1997) Expression cloning and characterization of a renal electrogenic Na+/HCO3- cotransporter. Nature 387:409–413
Rougemont JD, Ames A III, Nesbett FB, Hofmann HF (1960) Fluid formed by choroid plexus; a technique for its collection and a comparison of its electrolyte composition with serum and cisternal fluids. J Neurophysiol 23:485–495
Roussa E, Thevenod F (1998) Distribution of V-ATPase in rat salivary glands. Eur J Morphol 36:147–152
Roussa E, Thevenod F, Sabolic I, Herak-Kramberger CM, Nastainczyk W, Bock R, Schulz I (1998) Immunolocalization of vacuolar-type H+-ATPase in rat submandibular gland and adaptive changes induced by acid-base disturbances. J Histochem Cytochem 46:91–100
Roussa E, Romero MF, Schmitt BM, Boron WF, Alper SL, Thevenod F (1999) Immunolocalization of anion exchanger AE2 and Na+-HCO3- cotransporter in rat parotid and submandibular glands. Am J Physiol 277:1288–1296
Roussa E, Alper SL, Thevenod F (2001) Immunolocalization of anion exchanger AE2, Na+/H+ exchangers NHE1 and NHE4, and vacuolar type H+-ATPase in rat pancreas. J Histochem Cytochem 49:463–474
Roussa E, Shmukler BE, Wilhelm S, Casula S, Stuart-Tilley AK, Thevenod F, Alper SL (2002) Immunolocalization of potassium-chloride cotransporter polypeptides in rat exocrine glands. Histochem Cell Biol 117:335–344
Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED (2000) Pendrin, the protein encoded by the pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology 141:839–845
Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED (2001) Pendrin, encoded by the pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A 98:4221–4226
Rune SJ, Henriksen FW (1969) Carbon dioxide tensions in the proximal part of the canine gastrointestinal tract. Gastroenterology 56:758–762
Russell JM (2000) Sodium-potassium-chloride cotransport. Physiol Rev 80:211–276
Ryu SY, Peixoto PM, Won JH, Yule DI, Kinnally KW (2010) Extracellular ATP and P2Y2 receptors mediate intercellular Ca2+ waves induced by mechanical stimulation in submandibular gland cells: role of mitochondrial regulation of store operated Ca2+ entry. Cell Calcium 47:65–76
Saarnio J, Parkkila S, Parkkila AK, Waheed A, Casey MC, Zhou XY, Pastorekova S, Pastorek J, Karttunen T, Haukipuro K, Kairaluoma MI, Sly WS (1998) Immunohistochemistry of carbonic anhydrase isozyme IX (MN/CA IX) in human gut reveals polarized expression in the epithelial cells with the highest proliferative capacity. J Histochem Cytochem 46:497–504
Sachs G, Shin JM, Vagin O, Lambrecht N, Yakubov I, Munson K (2007) The gastric H,K ATPase as a drug target: past, present, and future. J Clin Gastroenterol 41(Suppl 2):226–242
Saito Y, Wright EM (1983) Bicarbonate transport across the frog choroid plexus and its control by cyclic nucleotides. J Physiol 336:635–648
Saito Y, Wright EM (1984) Regulation of bicarbonate transport across the brush border membrane of the bull-frog choroid plexus. J Physiol 350:327–342
Sangan P, Thevananther S, Sangan S, Rajendran VM, Binder HJ (2000) Colonic H-K-ATPase alpha- and beta-subunits express ouabain-insensitive H-K-ATPase. Am J Physiol Cell Physiol 278:182–189
Sassani P, Pushkin A, Gross E, Gomer A, Abuladze N, Dukkipati R, Carpenito G, Kurtz I (2002) Functional characterization of NBC4: a new electrogenic sodium-bicarbonate cotransporter. Am J Physiol Cell Physiol 282:408–416
Satoh H, Moriyama N, Hara C, Yamada H, Horita S, Kunimi M, Tsukamoto K, Iso O, Inatomi J, Kawakami H, Kudo A, Endou H, Igarashi T, Goto A, Fujita T, Seki G (2003) Localization of Na+-HCO3− cotransporter (NBC-1) variants in rat and human pancreas. Am J Physiol Cell Physiol 284:729–737
Sauter DR, Novak I, Pedersen SF, Larsen EH, Hoffmann EK (2015) ANO1 (TMEM16A) in pancreatic ductal adenocarcinoma (PDAC). Pflugers Arch 467:1495–1508
Sauter DR, Sorensen CE, Rapedius M, Bruggemann A, Novak I (2016) pH-sensitive K+ channel TREK-1 is a novel target in pancreatic cancer. Biochim Biophys Acta 1862:1994–2003
Schlosser SF, Burgstahler AD, Nathason MH (1996) Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci USA 93:9948–9953
Schlue WR, Deitmer JW (1988) Ionic mechanisms of intracellular pH regulation in the nervous system. Ciba Found Symp 139:69
Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF (1999) Immunolocalization of the electrogenic Na+-HCO3− cotransporter in mammalian and amphibian kidney. Am J Physiol Renal Physiol 276:27–38
Schneyer LH, Young JA, Schneyer CA (1972) Salivary secretion of electrolytes. Physiol Rev 52:720–777
Scholz W, Albus U, Counillon L, Gogelein H, Lang HJ, Linz W, Weichert A, Scholkens BA (1995) Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc Res 29:260–268
Schroeder BC, Cheng T, Jan YN, Jan LY (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134:1019–1029
Schulz I (1971) Influence of bicarbonate-CO2 - and glycodiazine buffer on the secretion of the isolated cat’s pancreas. Pflugers Arch 329:283–306
Schulz I, Ströver F, Ullrich KJ (1971) Lipid soluble weak organic acid buffers as “substrate” for pancreatic secretion. Pflügers Arch 323:121–140
Schwaderer AL, Vijayakumar S, Al-Awqati Q, Schwartz GJ (2006) Galectin-3 expression is induced in renal beta-intercalated cells during metabolic acidosis. Am J Physiol Renal Physiol 290:148–158
Schwartz IL, Thaysen JH (1956) Excretion of sodium and potassium in human sweat. J Clin Invest 35:114–120
Schwartz GJ, Barasch J, Al-Awqati Q (1985) Plasticity of functional epithelial polarity. Nature 318:368–371
Schwartz GJ, Tsuruoka S, Vijayakumar S, Petrovic S, Mian A, Al-Awqati Q (2002) Acid incubation reverses the polarity of intercalated cell transporters, an effect mediated by hensin. J Clin Invest 109:89–99
Scudieri P, Musante I, Caci E, Venturini A, Morelli P, Walter C, Tosi D, Palleschi A, Martin-Vasallo P, Sermet-Gaudelus I, Planelles G, Crambert G, Galietta LJ (2018) Increased expression of ATP12A proton pump in cystic fibrosis airways. JCI Insight 3(20):123616
Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M (1997) A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca2+-dependent HCO3− secretion. J Physiol 505(Pt 2):411–423
Seow KTFP, Case RM, Young JA (1991) Pancreatic secretion by the anaesthetized rabbit in response to secretin, cholecystokinin, and carbachol. Pancreas 6:385–391
Sewell WA, Young JA (1975) Secretion of electrolytes by the pancreas of the anaesthetized rat. J Physiol (Lond) 252:379–396
Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou AM, Ernst SE, Karp PH, Wohlford-Lenane CL, Heilmann KP, Leidinger MR, Allen PD, Zabner J, McCray PB Jr, Ostedgaard LS, Stoltz DA, Randak CO, Welsh MJ (2016) Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 351:503–507
Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S (2006) Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J Gen Physiol 127:511–524
Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S (2008) The Slc26a4 transporter functions as an electroneutral Cl−/I−/HCO3− exchanger: role of Slc26a4 and Slc26a6 in I− and HCO3− secretion and in regulation of CFTR in the parotid duct. J Physiol 586:3813–3824
Shimokura GH, McGill JM, Schlenker T, Fitz JG (1995) Ursodeoxycholate increases cytosolic calcium concentration and activates Cl- currents in a biliary cell line. Gastroenterology 109:965–972
Shitara A, Tanimura A, Sato A, Tojyo Y (2009) Spontaneous oscillations in intracellular Ca2+ concentration via purinergic receptors elicit transient cell swelling in rat parotid ducts. Am J Physiol Gastrointest Liver Physiol 297:1198–1205
Siegel GJ, Holm C, Schreiber JH, Desmond T, Ernst SA (1984) Purification of mouse brain (Na++K+)-ATPase catalytic unit, characterization of antiserum, and immunocytochemical localization in cerebellum, choroid plexus, and kidney. J Histochem Cytochem 32:1309–1318
Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL (2007) PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292:1079–1088
Sinclair AJ, Onyimba CU, Khosla P, Vijapurapu N, Tomlinson JW, Burdon MA, Stewart PM, Murray PI, Walker EA, Rauz S (2007) Corticosteroids, 11beta-hydroxysteroid dehydrogenase isozymes and the rabbit choroid plexus. J Neuroendocrinol 19:614–620
Singh AK, Liu Y, Riederer B, Engelhardt R, Thakur BK, Soleimani M, Seidler U (2013a) Molecular transport machinery involved in orchestrating luminal acid-induced duodenal bicarbonate secretion in vivo. J Physiol 591:5377–5391
Singh AK, Xia W, Riederer B, Juric M, Li J, Zheng W, Cinar A, Xiao F, Bachmann O, Song P, Praetorius J, Aalkjaer C, Seidler U (2013b) Essential role of the electroneutral Na+-HCO3− cotransporter NBCn1 in murine duodenal acid-base balance and colonic mucus layer build-up in vivo. J Physiol 591:2189–2204
Sjoblom M, Flemstrom G (2003) Melatonin in the duodenal lumen is a potent stimulant of mucosal bicarbonate secretion. J Pineal Res 34:288–293
Sjoblom M, Singh AK, Zheng W, Wang J, Tuo BG, Krabbenhoft A, Riederer B, Gros G, Seidler U (2009) Duodenal acidity “sensing” but not epithelial. Proc Natl Acad Sci U S A 106:13094–13099
Sly WS, Hewett-Emmett D, Whyte MP, Yu YS, Tashian RE (1983) Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A 80:2752–2756
Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K, Amlal H (2012) Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci U S A 109:13368–13373
Song Y, Yamamoto A, Steward MC, Ko SB, Stewart AK, Soleimani M, Liu BC, Kondo T, Jin CX, Ishiguro H (2012) Deletion of Slc26a6 alters the stoichiometry of apical Cl−/HCO3− exchange in mouse pancreatic duct. Am J Physiol Cell Physiol 303:815–824
Sorensen CE, Novak I (2001) Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem 276:329525–329532
Speake T, Brown PD (2004) Ion channels in epithelial cells of the choroid plexus isolated from the lateral ventricle of rat brain. Brain Res 1005:60–66
Speake T, Kajita H, Smith CP, Brown PD (2002) Inward-rectifying anion channels are expressed in the epithelial cells of choroid plexus isolated from ClC-2 ‘knock-out’ mice. J Physiol 539:385–390
Spirli C, Granato A, Zsembery K, Anglani F, Okolicsanyi L, LaRusso NF, Crepaldi G, Strazzabosco M (1998) Functional polarity of Na+/H+ and Cl−/HCO3− excahngers in a rat choliangiocyte cell line. Am J Physiol 275:1236–1245
Steffensen AB, Oernbo EK, Stoica A, Gerkau NJ, Barbuskaite D, Tritsaris K, Rose CR, MacAulay N (2018) Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat Commun 9:2167
Stetson DL, Steinmetz PR (1985) Alpha and beta types of carbonic anhydrase-rich cells in turtle bladder. Am J Physiol 249:553–565
Steward MC, Ishiguro H (2009) Molecular and cellular regulation of pancreatic duct cell function. Curr Opin Gastroenterol 25:447–453
Steward MC, Poronnik P, Cook DI (1996) Bicarbonate transport in sheep parotid secretory cells. J Physiol 494(Pt 3):819–830
Steward MC, Ishiguro H, Case RM (2005) Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol 67:377–409
Stewart AK, Yamamoto A, Nakakuki M, Kondo T, Alper SL, Ishiguro H (2009) Functional coupling of apical Cl−/HCO3− exchange with CFTR in stimulated HCO3− secretion by guinea pig interlobular pancreatic duct. Am J Physiol Gastrointest Liver Physiol 296:1307–1317
Strazzabosco M, Joplin R, Zsembery A, Wallace L, Spirli C, Fabris L, Granato A, Rossanese A, Poci C, Neuberger JM, Okolicsanyi L, Crepaldi G (1997) Na+-dependent and -independent Cl−/HCO3− exchange mediate cellular HCO3− transport in cultured human intrahepatic bile duct cells. Hepatology 25:976–985
Stuenkel EL, Machen TE, Williams JA (1988) pH regulatory mechanism in rat pancreatic ductal cells. Am J Physiol 254:925–930
Supuran CT (2008) Carbonic anhydrases--an overview. Curr Pharm Des 14:603–614
Svanvik J, Allen B, Pellegrini C, Bernhoft R, Way L (1984) Variations in concentrating function of the gallbladder in the conscious monkey. Gastroenterology 86:919–925
Swarts HG, Koenderink JB, Willems PH, De Pont JJ (2005) The non-gastric H,K-ATPase is oligomycin-sensitive and can function as an H+,NH4+-ATPase. J Biol Chem 280:33115–33122
Szalmay G, Varga G, Kajiyama F, Yang XS, Lang TF, Case RM, Steward MC (2001) Bicarbonate and fluid secretion evoked by cholecystokinin, bombesin and acetylcholine in isolated guinea-pig pancreatic ducts. J Physiol (Lond) 535:795–807
Szmydynger-Chodobska J, Chung I, Kozniewska E, Tran B, Harrington FJ, Duncan JA, Chodobski A (2004) Increased expression of vasopressin v1a receptors after traumatic brain injury. J Neurotrauma 21:1090–1102
Szucs A, Demeter I, Burghardt B, Ovari G, Case RM, Steward MC, Varga G (2006) Vectorial bicarbonate transport by Capan-1 cells: a model for human pancreatic ductal secretion. Cell Physiol Biochem 18:253–264
Tabcharani JA, Chang X-B, Riordan JR, Hanrahan JW (1991) Phosphorylation-regulated Cl− channel in CHO cells stably expressing the cystic fibrosis gene. Nature 352:628–631
Tabibian JH, Masyuk AI, Masyuk TV, O’Hara SP, LaRusso NF (2013) Physiology of cholangiocytes. Compr Physiol 3:541–565
Thaysen JH, Thorn NA, Schwartz IL (1954) Excretion of sodium, potassium, chloride, and carbon dioxide in human parotid saliva. Am J Physiol 178:155–159
Thompson J, Begenisich T (2006) Membrane-delimited inhibition of maxi-K channel activity by the intermediate conductance Ca2+-activated K channel. J Gen Physiol 127:159–169
Thompson J, Begenisich T (2009) Mechanistic details of BK channel inhibition by the intermediate conductance, Ca2+-activated K channel. Channels (Austin) 3:194–204
Turner RJ, George JN (1988) Cl−-HCO3− exchange is present with Na+-K+-Cl− cotransport in rabbit parotid acinar basolateral membranes. Am J Physiol 254:391–396
Turner RJ, George JN, Baum BJ (1986) Evidence for a Na+/K+/Cl− cotransport system in basolateral membrane vesicles from the rabbit parotid. J Membr Biol 94:143–152
Uriarte I, Banales JM, Saez E, Arenas F, Oude Elferink RP, Prieto J, Medina JF (2010) Bicarbonate secretion of mouse cholangiocytes involves Na+-HCO3− cotransport in addition to Na+-independent Cl−/HCO3− exchange. Hepatology 51:891–902
Vallet M, Picard N, Loffing-Cueni D, Fysekidis M, Bloch-Faure M, Deschenes G, Breton S, Meneton P, Loffing J, Aronson PS, Chambrey R, Eladari D (2006) Pendrin regulation in mouse kidney primarily is chloride-dependent. J Am Soc Nephrol 17:2153–2163
van Niekerk J, Kersten R, Beuers U (2018) Role of bile acids and the biliary HCO3− umbrella in the pathogenesis of primary biliary cholangitis. Clin Liver Dis 22:457–479
Veel T, Villanger O, Holthe MR, Cragoe EJ, Raeder MG (1992) Na+-H+ exchange is not important for pancreatic HCO3− secretion in the pig. Acta Physiol Scand 144:239–246
Venglovecz V, Rakonczay Z Jr, Ozsvari B, Takacs T, Lonovics J, Varro A, Gray MA, Argent BE, Hegyi P (2008) Effects of bile acids on pancreatic ductal bicarbonate secretion in guinea pig. Gut 57:1102–1112
Venglovecz V, Hegyi P, Rakonczay Z Jr, Tiszlavicz L, Nardi A, Grunnet M, Gray MA (2011) Pathophysiological relevance of apical large-conductance Ca2+-activated potassium channels in pancreatic duct epithelial cells. Gut 60:361–369
Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM (2003) Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42:356–362
Verlander JW, Kim YH, Shin W, Pham TD, Hassell KA, Beierwaltes WH, Green ED, Everett L, Matthews SW, Wall SM (2006) Dietary Cl− restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol 291:833–839
Vijayakumar S, Erdjument-Bromage H, Tempst P, Al-Awqati Q (2008) Role of integrins in the assembly and function of hensin in intercalated cells. J Am Soc Nephrol 19:1079–1091
Villanger O, Veel T, Raeder MG (1993) Secretin causes H+ secretion from intrahepatic bile ductules by vacuolar-type H+-ATPase. Am J Physiol 265:719–724
Villanger O, Veel T, Ræder MG (1995) Secretin causes H+/HCO3− secretion from pig pancreatic ductules by vacuolar-type H+-adenosine triphosphatase. Gastroenterology 108:850–859
Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF (2002) Functional characterization of human NBC4 as an electrogenic Na+-HCO3− cotransporter (NBCe2). Am J Physiol Cell Physiol 282:1278–1289
Virkki LV, Choi I, Davis BA, Boron WF (2003) Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am J Physiol Cell Physiol 285:771–780
Vogh BP, Godman DR, Maren TH (1987) Effect of AlCl3 and other acids on cerebrospinal fluid production: a correction. J Pharmacol Exp Ther 243:35–39
Voronina S, Longbottom R, Sutton R, Petersen OH, Tepikin A (2002) Bile acids induce calcium signals in mouse pancreatic acinar cells: implications for bile-induced pancreatic pathology. J Physiol 540:49–55
Voronina SG, Barrow SL, Gerasimenko OV, Petersen OH, Tepikin AV (2004) Effects of secretagogues and bile acids on mitochondrial membrane potential of pancreatic acinar cells: comparison of different modes of evaluating DeltaPsim. J Biol Chem 279:27327–27338
Vorum H, Kwon TH, Fulton C, Simonsen B, Choi I, Boron W, Maunsbach AB, Nielsen S, Aalkjaer C (2000) Immunolocalization of electroneutral Na-HCO3− cotransporter in rat kidney. Am J Physiol Renal Physiol 279:901–909
Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, Geibel JP (2002) Regulation of the expression of the Cl-/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int 62:2109–2117
Wagner CA, Mohebbi N, Capasso G, Geibel JP (2011) The anion exchanger pendrin (SLC26A4) and renal acid-base homeostasis. Cell Physiol Biochem 28:497–504
Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL (2009) Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology 136:893–901
Wall SM, Pech V (2008) The interaction of pendrin and the epithelial sodium channel in blood pressure regulation. Curr Opin Nephrol Hypertens 17:18–24
Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW (2004) NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl− conservation. Hypertension 44:982–987
Wan MH, Huang W, Latawiec D, Jiang K, Booth DM, Elliott V, Mukherjee R, Xia Q (2012) Review of experimental animal models of biliary acute pancreatitis and recent advances in basic research. HPB (Oxford) 14:73–81
Wang J, Novak I (2013) Ion transport in human pancreatic duct epithelium, Capan-1 cells, is regulated by secretin, VIP, acetylcholine, and purinergic receptors. Pancreas 42:452–460
Wang CZ, Yano H, Nagashima K, Seino S (2000) The Na+-driven Cl−/HCO3− exchanger. Cloning, tissue distribution, and functional characterization. J Biol Chem 275:35486–35490
Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S (2006) Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3- secretion: relevance to cystic fibrosis. EMBO J 25:5049–5057
Wang J, Haanes KA, Novak I (2013) Purinergic regulation of CFTR and Ca2+-activated Cl− channels and K+ channels in human pancreatic duct epithelium. Am J Physiol Cell Physiol 304:673–684
Wang J, Barbuskaite D, Tozzi M, Giannuzzo A, Sorensen CE, Novak I (2015) Proton pump inhibitors inhibit pancreatic secretion: role of gastric and non-gastric H+/K+-ATPases. PLoS One 10:e0126432
Weber KT, Sun Y, Wodi LA, Munir A, Jahangir E, Ahokas RA, Gerling IC, Postlethwaite AE, Warrington KJ (2003) Toward a broader understanding of aldosterone in congestive heart failure. J Renin Angiotensin Aldosterone Syst 4:155–163
Wegman EA, Ishikawa T, Young JA, Cook DI (1992) Cation channels in basolateral membranes of sheep parotid secretory cells. Am J Physiol Gastrointest Liver Physiol 263:786–794
Welch K (1963) Secretion of cerebrospinal fluid by the choroid plexus of the rabbit. Am J Physiol 205:617–624
Wen G, Deng S, Song W, Jin H, Xu J, Liu X, Xie R, Song P, Tuo B (2018) Helicobacter pylori infection downregulates duodenal CFTR and SLC26A6 expressions through TGFbeta signaling pathway. BMC Microbiol 18:87
Williams SE, Turnberg LA (1981) Demonstration of a pH gradient across mucus adherent to rabbit gastric mucosa: evidence for a ‘mucus-bicarbonate’ barrier. Gut 22:94–96
Wilschanski M, Novak I (2013) The cystic fibrosis of exocrine pancreas. Cold Spring Harb Perspect Med 3:a009746. https://doi.org/10.1101/cshperspect.a009746
Winpenny JP, Verdon B, McAlroy HL, Colledge WH, Ratcliff R, Evans MJ, Gray MA, Argent BE (1995) Calcium-activated chloride conductance is not increased in pancreatic duct cells of CF mice. Pflügers Arch 430:26–33
Winpenny JP, Harris A, Hollingsworth MA, Argent BE, Gray MA (1998) Calcium-activated chloride conductance in a pancreatic adenocarcinoma cell line of ductal origin (HPAF) and in freshly isolated human pancreatic duct cells. Pflugers Arch 435:796–803
Wizemann V, Schulz I (1973) Influence of amphotericin, amiloride, ionophores, and 2,4- dinitrophenol on the secretion of the isolated cats pancreas. Pflügers Arch 339:317–338
Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP (2008) Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl- transport in biliary epithelial cells through a PKCzeta-dependent pathway. J Physiol 586:2779–2798
Woo K, Sathe M, Kresge C, Esser V, Ueno Y, Venter J, Glaser SS, Alpini G, Feranchak AP (2010) Adenosine triphosphate release and purinergic (P2) receptor-mediated secretion in small and large mouse cholangiocytes. Hepatology 52:1819–1828
Wright EM (1972) Mechanisms of ion transport across the choroid plexus. J Physiol 226:545–571
Wright EM (1978) Transport processes in the formation of the cerebrospinal fluid. Rev Physiol Biochem Pharmacol 83:3–34
Wright RD, Blair-West JR, Nelson JF (1986) Effects of ouabain, amiloride, monenesin, and other agents on ovine parotid secretion. Am J Physiol 250:503–510
Yamaguchi S, Ishikawa T (2005) Electrophysiological characterization of native Na+-HCO3− cotransporter current in bovine parotid acinar cells. J Physiol 568:181–197
Yamaguchi S, Ishikawa T (2012) IRBIT reduces the apparent affinity for intracellular Mg2+ in inhibition of the electrogenic Na+-HCO3− cotransporter NBCe1-B. Biochem Biophys Res Commun 424:433–438
Yamaguchi J, Liss AS, Sontheimer A, Mino-Kenudson M, Castillo CF, Warshaw AL, Thayer SP (2015) Pancreatic duct glands (PDGs) are a progenitor compartment responsible for pancreatic ductal epithelial repair. Stem Cell Res 15:190–202
Yang CL, Liu X, Paliege A, Zhu X, Bachmann S, Dawson DC, Ellison DH (2007) WNK1 and WNK4 modulate CFTR activity. Biochem Biophys Res Commun 353:535–540
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455:1210–1215
Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S (2009) IRBIT coordinates epithelial fluid and HCO3− secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest 119:193–202
Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S (2011) IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest 121:956–965
Yannoukakos D, Stuart-Tilley A, Fernandez HA, Fey P, Duyk G, Alper SL (1994) Molecular cloning, expression, and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circ Res 75:603–614
Yegutkin GG, Samburski SS, Jalkalen S, Novak I (2006) ATP-consuming and ATP-generating enzymes secreted by pancreas. J Biol Chem 281:29441–29447
You CH, Rominger JM, Chey WY (1983) Potentiation effect of cholecystokinin-octapeptide on pancreatic bicarbonate secretion stimulated by a physiologic dose of secretin in humans. Gastroenterology 85:40–45
Young JA, Martin CJ (1971) The effect of a sympatho- and a parasympathomimetic drug on the electrolyte concentrations of primary and final saliva of the rat submaxillary gland. Pflügers Arch 327:285–302
Young JA, Schögel E (1966) Micropuncture investigation of sodium and potassium excretion in rat submaxillary saliva. Pflügers Arch 291:85–98
Young JA, van Lennep EW (1979) Transport in salivary and salt glands. In: Giebisch G, Tosteson DC, Ussing HH (eds) Membrane transport in biology, vol IVB. Springer, Berlin, pp 563–692
Young JA, Case RM, Conigrave AD, Novak I (1980) Transport of bicarbonate and other anions in salivary secretion. Ann NY Acad Sci 341:172–190
Yu Y, Chen TY (2015) Purified human brain calmodulin does not alter the bicarbonate permeability of the ANO1/TMEM16A channel. J Gen Physiol 145:79–81
Zeng W, Lee MG, Yan M, Diaz J, Benjamin I, Marino CR, Kopito R, Freedman S, Cotton C, Muallem S, Thomas P (1997) Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol 273:442–455
Zeuthen T (1991) Secondary active transport of water across ventricular cell membrane of choroid plexus epithelium of Necturus maculosus. J Physiol 444:153–173
Zeuthen T, Wright EM (1981) Epithelial potassium transport: tracer and electrophysiological studies in choroid plexus. J Membr Biol 60:105–128
Zhao H, Star RA, Muallem S (1994) Membrane localization of H+ and HCO3− transporters in the rat pancreatic ducts. J Gen Physiol 104:57–85
Zlokovic BV, Mackic JB, Wang L, McComb JG, McDonough A (1993) Differential expression of Na,K-ATPase alpha and beta subunit isoforms at the blood-brain barrier and the choroid plexus. J Biol Chem 268:8019–8025
Acknowledgments
Research projects founding basis for this chapter were supported by The Danish Council for Independent Research | Natural Sciences, The Danish Council for Independent Research | Medical Sciences, The Lundbeck Foundation, The Novo Nordisk Foundation, and The Carlsberg Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The American Physiological Society
About this chapter
Cite this chapter
Novak, I., Praetorius, J. (2020). Fundamentals of Bicarbonate Secretion in Epithelia. In: Hamilton, K.L., Devor, D.C. (eds) Basic Epithelial Ion Transport Principles and Function. Physiology in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-52780-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-52780-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-52779-2
Online ISBN: 978-3-030-52780-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)