Abstract
Blunt traumatic aortic injury (BTAI) after trauma is an uncommon but lethal entity, with incidence of <1% (Arthurs et al., J Vasc Surg 49(4):988–994, 2009; Smith and Chang, Am J Surg 152(6):660–663, 1986). Information recovered at autopsy suggests that these injuries are actually the second-most frequent cause of mortality after blunt trauma (Clancy et al., J Trauma 51(2):346–51, 2001; Richens et al., Eur J Cardiothorac Surg 21(2):288–293, 2002) with the majority of patients expiring prior to arrival at the emergency department (Teixeira et al., J Trauma 70(1):197–202, 2011). This chapter explores the open and endovascular approach to BTAI.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Open
- Endovascular injury
- Blunt traumatic aortic injury (BTAI)
- Thoracic endovascular aortic repair (TEVAR)
- Aortic disruption
Introduction
Blunt traumatic aortic injury (BTAI) after trauma is an uncommon but lethal entity, with incidence of <1% [1, 2]. Information recovered at autopsy suggests that these injuries are actually the second-most frequent cause of mortality after blunt trauma [3, 4] with the majority of patients expiring prior to arrival at the emergency department [5]. Blunt thoracic aortic injury is imparted by significant blunt force trauma and is associated with an 85% prehospital mortality [6]. A more recent autopsy study of trauma victims identified these injuries as the primary cause of death in 1/3 of automobile collision mortalities, with 80% of these deaths occurring prior to arrival to an emergency department [5]. Motor vehicle collisions (>70%) appear to comprise the majority of mechanisms contributing to this specific injury, followed by motorcycle collisions, struck pedestrians, and falls [7, 8].
Numerous pathophysiologic mechanisms have been proposed as causative with regard to BTAI—including shear, torsion, pinch, stretch, and hydrostatic forces. A combination of such mechanical forces, however, is likely at play in the types of deceleration injury observed [4]. The location of the injury is consistent anatomically. More than 60% of blunt aortic injuries occur at the aortic isthmus where the fixed descending thoracic aorta meets the relatively mobile aortic arch [5]. This results in significant strain in the setting of abrupt deceleration. Blunt thoracic aortic injuries less commonly occur at other locations: the ascending aorta (8–27%), aortic arch (8–18%), and distal descending aorta (11–21%) [5, 6, 9,10,11,12].
Historically, the American Association of Surgery for Trauma (AAST) classified thoracic vascular injuries based on the type of artery and the extent of arterial circumference involved [13], but this system fails to adequately characterize these heterogeneous lesions. The current most commonly utilized grading system—proposed by Azzizadeh et al. [14] and later adopted by the Society for Vascular Surgery (SVS) in its clinical practice guidelines for BTAI management [15]—describes BTAI as a spectrum of lesions based on the anatomical layers of the aorta involved. These include intimal tear (grade I), intramural hematoma (grade II), pseudoaneurysm (III), and full-thickness injury resulting in frank rupture (IV) [14, 15].
The treatment of BTAI patients who survive to reach care has evolved considerably over the past several decades. Traditionally, early open repair was the mainstay of intervention. In recent years, however, initial medical management with delayed thoracic endovascular aortic repair (TEVAR) has emerged as the preferred treatment paradigm for patients with these injuries and appropriate anatomy and physiology conducive to this modality [7, 14,15,16,17,18,19,20,21,22,23,24,25,26,27,28].
Presentation and Workup
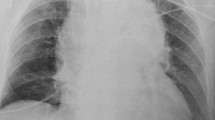
BTAI patients may be asymptomatic or may experience a range of symptoms including chest pain radiating to neck, back, or shoulder. Patients may be hemodynamically normal or present in obvious shock [29]. Anteroposterior chest X-ray is the initial diagnostic test of choice. A widened or otherwise abnormal-appearing mediastinum may be seen in up to 93% of patients with BTAI [30, 31]. Pathologic widening of the mediastinum is defined as a mediastinal silhouette of >8 cm at the level of the aortic knob or a width at the same level that exceeds 25% of the total chest width [30, 31] (Fig. 1). Other suggestive radiographic findings include left pleural effusion, first and second rib fractures, tracheal deviation, depressed left bronchus, an indistinct aortic knob, or apical capping [30, 31]. Patients with any of these abnormalities visualized on chest X-ray should undergo additional imaging, especially in the setting of a suspicious mechanism of injury. Importantly, a normal chest X-ray has a low sensitivity in the diagnosis of thoracic aortic injury and consequently should not be used to definitively exclude the diagnosis [32].
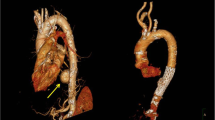
Computed tomographic angiography (CTA), with its high sensitivity (95–100%) and negative predictive value (99–100%) is the diagnostic test of choice for blunt thoracic aortic injury [9, 33,34,35] (Fig. 2). This imaging technology and improved trauma imaging protocols have supplanted traditional catheter-based angiography as the most efficient means of identifying BTAI. It is important to appreciate that false positives using this modality are not uncommon with low-grade injuries. There is evidence as well that CTA may have limitations—particularly in the detection of minor injuries. In one examination of patients undergoing formal angiography after CTA, Bruckner et al. found that CTA had a specificity of only 40% and a positive predictive value of only 15% [35]. This study is, however, now over a decade old; continued improvements in computed tomography imaging capabilities and trauma imaging protocols have improved the utility of modern CTA for the accurate detection and precise characterization of BTAI. Most subsequent studies have validated well the utilization of CTA after trauma due to the high sensitivity and ability to exclude BTAI with a very high negative predictive value [33,34,35].
If the diagnosis remains unclear after CTA, formal angiography and transesophageal echocardiogram [36] are both established methods of evaluating a patient for BTAI (Fig. 3). Intravascular ultrasound (IVUS), an emerging ultrasound capability, has also proven a reliable and sensitive tool in this setting, especially useful for the more precise characterization of minor injuries [37] (Fig. 4). While IVUS negates the need for the volume of intravascular contrast required by traditional angiography and decreases radiation exposure, it is predicated upon additional equipment, expertise, and placement of a slightly larger vascular sheath. This modality holds promise, as IVUS has demonstrated improved sensitivity over angiography [36, 37] for diagnosis of BTAI in several series.
Management: Medical
Immediate management of all BTAI injuries includes aggressive blood pressure control. Effective pharmacologic suppression of aortic pressure fluctuations is used to reduce impulse and stress on the injured aortic wall. This has been reported to decrease the risk of aortic rupture after BTAI from 12% to 1.5% [38]. SVS grade 1 injuries can be treated by medical management alone. For higher-grade injuries, SVS guidelines recommend medical management as a bridge to subsequent repair [15]. Some groups have reported successful nonoperative management of both grade I and grade II injuries finding that only 5% advanced [22]. Optimal blood pressure control regimen is not well established, with protocols varying by institution. Esmolol is often chosen due to its short half-life and titratability. For example, Fabian et al. reported the use of intravenous beta-blockers (esmolol or labetalol), titrated to a systolic blood pressure <100 mmHg and heart rate <100 bpm. Nitroprusside was added if adequate blood pressure control could not be obtained with beta-blockade alone [39].
Polytrauma patients with competing injury priorities may have contraindications to maintaining hemodynamics within these parameters. In the setting of traumatic brain and spinal cord injury, maximal neurologic recovery is predicated on maintenance of adequate vertebral and spinal cord perfusion pressures. This may necessitate use of vasopressors to maintain an elevated peripheral blood pressure, which is in direct contradistinction to medical management of BTAI. The heterogeneity of trauma patients and competing injury priorities requires close cooperation with trauma and neurosurgical providers in a multidisciplinary approach to determine if early endovascular repair for lower-grade BTAI is warranted.
Patients undergoing medical management of their BTAI must be closely monitored. Providers must be vigilant for the manifestation of obvious signs of malperfusion or possible early progression of injury among those selected for medical management of BTAI. With effective monitoring and in-patient imaging follow-up, however, progression of these injuries can be detected early and intervened upon safely. In the largest retrospective multicenter series of BTAI to date, the Aortic Trauma Foundation (ATF) reported that medical therapy alone was selected for 32.2% of BTAI patients. Only two in-hospital failures of medical therapy were noted in this population, both undergoing endovascular salvage without subsequent complication [16].
Upon diagnosis of BTAI, anti-impulse medications should be initiated in the absence of contraindication. Although SVS clinical practice guidelines recommend urgent (<24 h) repair [15], several large series suggest that delayed therapy is well tolerated and may be associated with improved outcomes. In one of the largest series on the topic, comparison of patients undergoing early endovascular (<24 h) and delayed endovascular (>24 h) repair showed a significantly lower mortality rate in the delayed group compared to those patients subjected to early repair (5.8% vs. 16.5%, p = 0.034) [19].
Open Repair
Early case reports of blunt traumatic aortic injury initially appeared in the literature in the 1960s. Early repairs were performed through left posterolateral thoracotomy with left atrium to femoral artery bypass [40] or via left posterolateral thoracotomy without bypass [41].
In the modern day, when open repair is required, a variety of approaches can be considered depending on the site and length of the descending thoracic aorta involved. A left posterolateral thoracotomy through the fourth interspace is usually the most expedient approach, as this provides excellent access to the area of the aortic isthmus where the majority of these injuries occur. This exposure is the most commonly described when employing the “clamp and sew” technique required by emergent repair or when extracorporeal support is not feasible or available. In the context of this approach, the chest is opened with the initial objective to rapidly acquire proximal and distal control around the area of injured thoracic aorta. The proximal clamp is typically applied between the left common carotid and left subclavian arteries, while the distal clamp is placed at some point on the distal descending thoracic aorta distal to the zone of injury.
While the clamp and sew technique has been demonstrated to be a safe method of operative management of BTAI [42], depending on the clinical scenario and patient condition, a distal aortic perfusion strategy should be employed to improve tolerance of proximal aortic clamping. The most expeditious technique for establishing distal aortic perfusion is “left-sided bypass,” which can be performed through cannulation of the left inferior pulmonary vein and distal thoracic aorta. Rarely, the proximal clamp positioning is untenable. In this scenario, full cardiopulmonary bypass (CPB) is advisable, commonly through a femoral artery and a femoral vein cannulation. If total circulatory arrest is warranted, then careful venting of the left ventricle during times of cooling and rewarming is crucial.
In the setting of multiply injured trauma patient with competing injury priorities, the need for systemic anticoagulation with the use of bypass utilized in open repair is problematic. When left-sided bypass is performed, an activated clotting time >200 s is targeted; when full cardiopulmonary bypass is required, an activated clotting time >480 s is targeted [43]. Once the patient is on bypass and the perfusion circuit has appropriate flows, the periaortic hematoma is incised, the extent of injury is defined, and the aortic repair is undertaken. It is paramount that the aortic adventitia is incorporated into the subsequent suture lines, as this layer provides most of the tensile strength of the aorta. The patient is gradually rewarmed during the latter phase of the anastomosis to facilitate removal of the clamps at a moderate degree of hypothermia (32–34 °C). Once the clamps are off, the patient is weaned from bypass until adequate hemostasis is achieved and the wound is closed.
A significant consideration when undertaking open BTAI repair is spinal cord protection and the importance of distal aortic perfusion in minimizing the risk of postoperative paraplegia [44]. Several potential strategies to mitigate this risk have been described in the literature [45,46,47,48,49]. The most significant contributor to spinal cord ischemia as a result of aortic clamping is the occlusion of critical segmental spinal cord arterial branches originating from the distal thoracic aorta. Important factors in determining the incidence of immediate onset or delayed paraplegia following aortic repair include duration of cross-clamping, level and length of aortic segment excluded by clamping, duration of systemic hypotension, cerebrospinal fluid pressure, distal aortic pressure, and the number of intercostals ligated during repair [50]. Multiple adjuncts have helped lower the incidence of paraplegia following aortic repair for BTAI, including cerebrospinal fluid drainage, administration of steroids, generalized and localized hypothermia, and reimplantation of key intercostal arteries during the repair [47,48,49,50].
Management-Endovascular Repair
Endovascular repair has emerged as the mainstay of treatment of blunt thoracic aortic injuries. The first endovascular device, thoracic aortic graft (TAG) (W.L. Gore & Associates, Flagstaff, AZ), approved by the US Food and Drug Administration (FDA) for the treatment of thoracic aortic aneurysms was introduced in 2005. This device was utilized off-label for treatment of BTAI that same year [14]. The initial capabilities of this device limited TEVAR application to patients with a minimum aortic diameter of 23 mm. The subsequent approval of smaller diameter delivery systems, including the Talent (Medtronic, Santa Rosa, CA) and TX2 (Bloomington, IN) devices in 2008, made TEVAR feasible for a broader range of patients when employed in “off-label” fashion. The later introduction, and FDA approval for BTAI treatment, of two additional devices, CTAG (W.L. Gore, Flagstaff, AZ) and Valiant (Medtronic, Santa Rosa, CA), have further increased the tools available for definitive treatment of these injuries by effective endovascular means. In the largest multicenter retrospective BTAI examination to date conducted by the Aortic Trauma Foundation (ATF), TEVAR was utilized for 76.4% of the 382 BTAI patients studied, with only 23.6% requiring open repair during the study period [16].
A prospective, nonrandomized, multicenter trial utilizing Medtronic Valiant stents in 50 patients with blunt thoracic aortic injury (RESCUE trial) reported 100% successful device delivery and deployment, and 30-day all-cause mortality of 8.0%, which compares favorably to the previously reported 13% [51]. A recent American College of Surgeons National Trauma Databank review demonstrated a 196.8% increase in diagnosis of BTAI over the reviewed decade with a marked increase in TEVAR repair of BTAI, following FDA approval of capable endovascular devices (0.0 vs. 94.9% p < 0.001) overall [52].
The patient is prepped and draped such that both groins can be accessed and that the chest and abdomen may be rapidly entered if the procedure must convert to open. Femoral access is obtained and an arch aortogram is performed to confirm the location and characteristics of the injury. The cerebrovascular anatomy is evaluated with attention paid to determine if the take-off of the left subclavian artery will need to be covered in order to obtain an adequate proximal seal. IVUS can be utilized selectively based on the discretion of the attending surgeon and institutional capabilities to provide similar actionable information. Depending on the chosen device, most require femoral access with a sheath ranging in size from 20 to 24 F (6.67–8 mm) although lower profile devices (16 Fr) are now emerging on the market. These large-caliber devices can be challenging to place in the relatively small iliofemoral arteries of young trauma patients. The diameter of conduit vessels in this population is often less than 7 mm. Small iliac artery size has been demonstrated to be an independent risk factor for development of iliofemoral complications including dissection, rupture, and hematoma formation following TEVAR [53].
Heparin is administered using a weight-based protocol if there are no contraindications. In some situations a smaller dose of heparin (3000–5000 units) may be utilized. On occasion, as in patients with concomitant severe brain injury, anticoagulation use may represent a prohibitive risk. In these situations, thoughtful discussion with the other stakeholders of trauma patient care—including trauma surgeons and neurosurgical team members—must be undertaken in order to realistically balance the risk of thromboembolic complications for the limb that will occur without anticoagulation against the bleeding risk in the specific patient.
The thoracic device to be utilized for repair is selected, using CTA or angiographic images according to the manufacturer’s sizing recommendations. Most commonly measurements are made based on two-dimensional, thin-cut axial CT scans with IV contrast. Once selected, the device is delivered and deployed using standard techniques without any pharmacological adjunct; extension pieces may be deployed as indicated but are not routinely required for these injuries (Fig. 5). Aggressive distal extension of the TEVAR device is likely to significantly increase the risk for subsequent spinal cord ischemia.
The subclavian artery may be covered as needed to obtain a proximal landing zone or gain better apposition with the lesser curvature of the aortic arch. Although this coverage does represent a theoretical increase in risk for malperfusion of the arm and vertebrobasilar steal postoperatively, a wealth of data suggests that BTAI patients will routinely tolerate LSCA coverage [54]. Post-deployment balloon angioplasty is performed only very selectively when there is significant incomplete apposition of the graft at the proximal landing zone noted. Heparin is then reversed with protamine at the surgeon’s discretion. Postoperatively, patients are returned to the surgical-trauma intensive care unit and discharged following stabilization of their other injuries.
When Is Open Repair Indicated in the Age of TEVAR?
Clinical Practice Guidelines from the Society for Vascular Surgery state that “endovascular repair be performed preferentially over open surgical repair or non-operative management” [15].
A surgical repair is required if endovascular capabilities are unavailable or if a patient’s anatomy is unsuitable for TEVAR. In the modern era, open repair is indicated when the patient shows signs of hemodynamic compromise and the need for emergent intervention when at a facility that does not have the capability to mobilize endovascular resources in an expedient fashion; local factors such as hybrid rooms, equipment, and surgical expertise come into play. A critical determinant of the need for open repair is the absence of an adequate proximal landing zone to allow for proper “seal” of the site of injury by the device. Arch aortogram is generally performed at the beginning of the procedure to delineate cerebrovascular anatomy. As outlined, left subclavian coverage is a frequently employed maneuver in TEVAR for BTAI, required in approximately 40% of patients [16]. Left arm claudication and vertebrobasilar insufficiency are potential sequelae of left subclavian artery coverage that must be assessed postoperatively. Data suggests, however, that the majority of patients requiring left subclavian artery coverage will have good short- and mid-term outcomes with regard to physical and mental health, without the need for subsequent bypass [16, 54]. Hybrid approaches utilizing planned carotid-carotid or carotid-subclavian bypasses may be utilized to provide for even more proximal coverage when the anatomy of injury treatment requires it. Within the last several years, more advanced thoracic branched endoprosthetics have begun to be utilized for treatment of aortic arch aneurysms [55]. As the vascular community becomes more familiar with these novel devices, even more patients with BTAIs will become safe candidates for endovascular-alone management of more proximal lesions.
Current data suggest that in comparison to open repair, TEVAR reduces early death, paraplegia, renal insufficiency, transfusions, reoperation for bleeding, cardiac complications, pneumonia, and length of hospital stay [19, 20]. The common concerns for paralysis and stroke that have been associated with open repair appear to be mitigated with the use of TEVAR. In the Aortic Trauma Foundation multicenter study of 382 BTAI patients, only 1 paralysis following TEVAR was noted, occurring in an 81-year-old male requiring 20 cm device coverage of the thoracic aorta. Likewise, stroke was a very rare occurrence, identified in only 2 patients, ages 62 and 85. Both required coverage of the left subclavian artery to facilitate TEVAR. These findings support the safety profile for TEVAR in the setting of BTAI, but suggest that older patients with possible native atherosclerotic disease and BTAI patterns requiring more extensive endograft coverage may have increased risk for these ischemic complications [16].
Access- and device-related complications also appear to be rare sequelae of TEVAR in contemporary practice. This improvement in safety profile has emerged as a benefit of increased experience and improvements in device technology. In the older 2008 report of the AAST BTAI study group, the investigators noted a significant rate of specific TEVAR-related complications. Demetriades and his group found that 18.4% of patients undergoing TEVAR had some form of stent graft-specific complication, most notably endoleak at 13.6% [20]. However, the Aortic Trauma Foundation study group has more recently reported a significantly lower rate of TEVAR-related complications. Of 382 BTAI patients, 6 malpositions of endograft at initial TEVAR occurred (3.0%) with a 2.5% post-TEVAR endoleak rate. Only one delayed stent migration was noted. Additionally, only two access site complications (one pseudoaneurysm, one bleeding requiring intervention) were identified. Among the six defined TEVAR treatment failures encountered in this large series, all underwent subsequent salvage with reintervention (two repeat TEVAR, four open repair).
The ATF study group identified only one patient treated with repeat TEVAR who suffered aortic-related mortality. No other mortalities were observed among the TEVAR failures [16]. Although increasingly rare in the modern era, it is important to note that complications during or after TEVAR remain possible in specific settings. Excessive oversizing or undersizing of endografts can lead to propagation of aortic injury or failure to achieve adequate seal. Patient-specific anatomical issues may also contribute, including the presence of a tight curvature of the aortic arch, native atherosclerotic disease, and the previously mentioned diameter limitations of iliac access vessels.
Is TEVAR the Answer for All Injuries?
The current SVS guidelines for BTAI management [15] published in 2011 were intended to provide an evidence-based, consensus-derived grading system and suggested course of treatment for BTAI. These guidelines still represent the most comprehensive effort in BTAI care optimization recommendations. The SVS guidelines do have some limitations and are specific to the aortic lesion alone. Under the SVS 2011 recommendations, grade I injuries are to be treated medically in patients without contraindication to the required anti-impulse blood pressure control. In a recent study by Osgood et al. [22], of 49 grade I and II injuries, investigators found that only 5% of these lesions advanced in grade on serial imaging. While there remains a need for additional studies to establish the relative risk of medical management verse TEVAR for these lower grade injuries, we have recently adopted a more selective approach to treatment of grade II injuries, managing some with medical therapy and serial imaging alone. In our present practice, grade II injuries deemed to require TEVAR and all grade III injuries are candidates for urgent repair via TEVAR. Grade IV injuries are transported expeditiously to the operating room for emergent repair, ideally by TEVAR [14].
Optimal management of patients with lower grade BTAI has recently emerged as a topic of considerable debate. Some group investigators have suggested that SVS grade I and II “minimal aortic injuries” do not universally require TEVAR [56,57,58]. The inclusion of SVS grade II injuries in this category conflicts with the present SVS clinical practice guidelines. In contrast, however, the findings from research conducted at several high-volume centers suggest that medical management with surveillance is a safe approach to BTAI treatment for patients in these categories [57, 58]. Ideal follow-up among patients selected for medical management and the natural history of SVS grade II injuries left untreated has not, however, been well established. Considering the small number of grade II injuries that present to each center on a yearly basis, properly addressing this issue will likely require a multi-institutional prospective study.
The ideal timing of BTAI treatment is another issue that requires further study. The results of the AAST Aortic Injury Study Group, reported in 2008, suggested that improved outcomes were associated with initial medical management including blood pressure and pulse pressure optimization. This group found that patients treated after a delayed (>24 h) period of optimization had improved survival compared to BTAI patients treated operatively within <24 h. However, there remains a need to adequately define whether there are specific risk factors associated with BTAI that represent a higher risk for early aortic rupture. If identified, these risk factors may inform considerations for more emergent timing of repair [19].
The long-term durability of these implanted endovascular devices for BTAI treatment also requires ongoing investigation. Improved conformability to aortic contour and various fixation element changes are attractive features of modern devices, but the impact of these design features on long-term integrity of the devices is not yet determined. Optimal graft sizing and graft utilization in patients with small aortic and iliac diameters are also inadequately studied issues. These challenges are exacerbated by the fact that optimal device indications and utilization have primarily been subjected only to industry-funded study, with associated inherent study bias potential. These issues require more objective investigation in a large, multicenter fashion.
The careful study of these issues also requires the foundation of a common vernacular to describe and categorize BTAI. The SVS grading system and associated guidelines for care are now widely utilized, but it is important to note that alternate algorithms have been proposed. Both the Vancouver simplified grading system [56] and the alternate classification scheme proposed by Starnes et al. [57, 58] have suggested that additional elements of imaging specific to BTAI may be of importance in guiding therapy. These groups have suggested that aortic lesion dimension measurements, which are not included in SVS criteria, are critical in determining the need for TEVAR. Initial work by investigators at the University of Maryland [23] has also demonstrated that associated secondary signs of injury are likely important for consideration. Specifically, this group has highlighted that the presence of extensive mediastinal hematoma and large left hemothorax may forecast impending aortic rupture. More recently, additional work by the Maryland group suggests that other markers of injury burden, including admission lactate, may also be predictive of early aortic adverse events [25].
A recent review of prospective trauma registry data suggests that limited aortic injury (grade I and II) may successfully be managed medically with observed complete resolution in approximately 8 weeks [59]. The routine employment of the SVS algorithm which prescribes liberal TEVAR for treatment of grade II injuries [15] may, subsequently, contribute to the overtreatment a significant number of injuries.
Conclusion
The future challenge for additional study of BTAI is to determine the ability to reconcile alternative viewpoints with those of the existing SVS BTAI grading system and treatment guidelines. The development and implementation of a consensus grading system and treatment algorithm for the management of BTAI patients is a challenging enterprise given the relative rarity of the disease process and the complexity and heterogeneity of the patient population. It will require a multi-institutional cohort of professionals and improved data on the diagnosis, management, and long-term outcomes of BTAI. In the interim, the data suggests that initial medical management is appropriate for most lower-grade injuries and that, among those requiring repair, delayed TEVAR should be the intervention of choice.
References
Arthurs ZM, Starnes BW, Sohn VY, Singh N, Martin MJ, Andersen CA. Functional and survival outcomes in traumatic blunt thoracic aortic injuries: an analysis of the National Trauma Databank. J Vasc Surg. 2009;49(4):988–94.
Smith RS, Chang FC. Traumatic rupture of the aorta: still a lethal injury. Am J Surg. 1986;152(6):660–3.
Clancy TV, Gary Maxwell J, Covington DL, Brinker CC, Blackman D. A statewide analysis of level I and II trauma centers for patients with major injuries. J Trauma. 2001;51(2):346–51.
Richens D, Field M, Neale M, Oakley C. The mechanism of injury in blunt traumatic rupture of the aorta. Eur J Cardiothorac Surg. 2002;21(2):288–93.
Teixeira PG, Inaba K, Barmparas G, Georgiou C, Toms C, Noguchi TT, et al. Blunt thoracic aortic injuries: an autopsy study. J Trauma. 2011;70(1):197–202.
Parmley LF, Mattingly TW, Manion WC, Jahnke EJ Jr. Nonpenetrating traumatic injury of the aorta. Circulation. 1958;17(6):1086–101.
Estrera AL, Miller CC 3rd, Guajardo-Salinas G, Coogan S, Charlton-Ouw K, Safi HJ, et al. Update on blunt thoracic aortic injury: fifteen-year single-institution experience. J Thorac Cardiovasc Surg. 2013;145(3 Suppl):S154–8.
Fabian TC, Richardson JD, Croce MA, Smith JS Jr, Rodman G Jr, Kearney PA, et al. Prospective study of blunt aortic injury: multicenter trial of the American Association for the Surgery of Trauma. J Trauma. 1997;42(3):374–80; discussion 80–3.
Neschis DG, Scalea TM, Flinn WR, Griffith BP. Blunt aortic injury. N Engl J Med. 2008;359(16):1708–16.
Feczko JD, Lynch L, Pless JE, Clark MA, McClain J, Hawley DA. An autopsy case review of 142 nonpenetrating (blunt) injuries of the aorta. J Trauma. 1992;33(6):846–9.
Arajarvi E, Santavirta S, Tolonen J. Aortic ruptures in seat belt wearers. J Thorac Cardiovasc Surg. 1989;98(3):355–61.
Burkhart HM, Gomez GA, Jacobson LE, Pless JE, Broadie TA. Fatal blunt aortic injuries: a review of 242 autopsy cases. J Trauma. 2001;50(1):113–5.
Moore EE, Malangoni MA, Cogbill TH, Shackford SR, Champion HR, Jurkovich GJ, et al. Organ injury scaling. IV: thoracic vascular, lung, cardiac, and diaphragm. J Trauma. 1994;36(3):299–300.
Azizzadeh A, Keyhani K, Miller CC 3rd, Coogan SM, Safi HJ, Estrera AL. Blunt traumatic aortic injury: initial experience with endovascular repair. J Vasc Surg. 2009;49(6):1403–8.
Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53(1):187–92.
DuBose JJ, Leake SS, Brenner M, Pasley J, O’Callaghan T, Luo-Owen X, et al. Contemporary management and outcomes of blunt thoracic aortic injury: a multicenter retrospective study. J Trauma Acute Care Surg. 2015;78(2):360–9.
Murphy SL, Kochanek KD, Xu J, Heron M. Deaths: final data for 2012. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2015;63(9):1–117.
Azizzadeh A, Charlton-Ouw KM, Chen Z, Rahbar MH, Estrera AL, Amer H, et al. An outcome analysis of endovascular versus open repair of blunt traumatic aortic injuries. J Vasc Surg. 2013;57(1):108–14; discussion 15.
Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, et al. Blunt traumatic thoracic aortic injuries: early or delayed repair—results of an American Association for the Surgery of Trauma prospective study. J Trauma. 2009;66(4):967–73.
Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, et al. Operative repair or endovascular stent graft in blunt traumatic thoracic aortic injuries: results of an American Association for the Surgery of Trauma multicenter study. J Trauma. 2008;64(3):561–70; discussion 70–1.
Azizzadeh A, Ray HM, Dubose JJ, Charlton-Ouw KM, Miller CC, Coogan SM, et al. Outcomes of endovascular repair for patients with blunt traumatic aortic injury. J Trauma Acute Care Surg. 2014;76(2):510–6.
Osgood MJ, Heck JM, Rellinger EJ, Doran SL, Garrard CL 3rd, Guzman RJ, et al. Natural history of grade I-II blunt traumatic aortic injury. J Vasc Surg. 2014;59(2):334–41.
Rabin J, DuBose J, Sliker CW, O’Connor JV, Scalea TM, Griffith BP. Parameters for successful nonoperative management of traumatic aortic injury. J Thorac Cardiovasc Surg. 2014;147(1):143–9.
Martinelli O, Malaj A, Gossetti B, Bertoletti G, Bresadola L, Irace L. Outcomes in the emergency endovascular repair of blunt thoracic aortic injuries. J Vasc Surg. 2013;58(3):832–5.
Harris DG, Rabin J, Kufera JA, Taylor BS, Sarkar R, O’Connor JV, et al. A new aortic injury score predicts early rupture more accurately than clinical assessment. J Vasc Surg. 2015;61(2):332–8.
Hoffer EK, Karmy-Jones R, Bloch RD, Meissner MH, Borsa JJ, Nicholls SC, et al. Treatment of acute thoracic aortic injury with commercially available abdominal aortic stent-grafts. J Vasc Interv Radiol. 2002;13(10):1037–41.
Tang GL, Tehrani HY, Usman A, Katariya K, Otero C, Perez E, et al. Reduced mortality, paraplegia, and stroke with stent graft repair of blunt aortic transections: a modern meta-analysis. J Vasc Surg. 2008;47(3):671–5.
Xenos ES, Abedi NN, Davenport DL, Minion DJ, Hamdallah O, Sorial EE, et al. Meta-analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. J Vasc Surg. 2008;48(5):1343–51.
O’Conor CE. Diagnosing traumatic rupture of the thoracic aorta in the emergency department. Emerg Med J. 2004;21(4):414–9.
Woodring JH. The normal mediastinum in blunt traumatic rupture of the thoracic aorta and brachiocephalic arteries. J Emerg Med. 1990;8(4):467–76.
Mirvis SE, Bidwell JK, Buddemeyer EU, Diaconis JN, Pais SO, Whitley JE, et al. Value of chest radiography in excluding traumatic aortic rupture. Radiology. 1987;163(2):487–93.
Gutierrez A, Inaba K, Siboni S, Effron Z, Haltmeier T, Jaffray P, et al. The utility of chest X-ray as a screening tool for blunt thoracic aortic injury. Injury. 2016;47(1):32–6.
Gavant ML, Menke PG, Fabian T, Flick PA, Graney MJ, Gold RE. Blunt traumatic aortic rupture: detection with helical CT of the chest. Radiology. 1995;197(1):125–33.
Wicky S, Capasso P, Meuli R, Fischer A, von Segesser L, Schnyder P. Spiral CT aortography: an efficient technique for the diagnosis of traumatic aortic injury. Eur Radiol. 1998;8(5):828–33.
Bruckner BA, DiBardino DJ, Cumbie TC, Trinh C, Blackmon SH, Fisher RG, et al. Critical evaluation of chest computed tomography scans for blunt descending thoracic aortic injury. Ann Thorac Surg. 2006;81(4):1339–46.
Patel NH, Hahn D, Comess KA. Blunt chest trauma victims: role of intravascular ultrasound and transesophageal echocardiography in cases of abnormal thoracic aortogram. J Trauma. 2003;55(2):330–7.
Azizzadeh A, Valdes J, Miller CC 3rd, Nguyen LL, Estrera AL, Charlton-Ouw K, et al. The utility of intravascular ultrasound compared to angiography in the diagnosis of blunt traumatic aortic injury. J Vasc Surg. 2011;53(3):608–14.
Hemmila MR, Arbabi S, Rowe SA, Brandt MM, Wang SC, Taheri PA, et al. Delayed repair for blunt thoracic aortic injury: is it really equivalent to early repair? J Trauma. 2004;56(1):13–23.
Fabian TC, Davis KA, Gavant ML, Croce MA, Melton SM, Patton JH Jr, et al. Prospective study of blunt aortic injury: helical CT is diagnostic and antihypertensive therapy reduces rupture. Ann Surg. 1998;227(5):666–76; discussion 76–7.
McKnight JT, Meyer JA, Neville JF Jr. Nonpenetrating traumatic rupture of the thoracic aorta. Ann Surg. 1964;160:1069–72.
DeMuth WE Jr, Roe H, Hobbie W. Immediate repair of traumatic rupture of thoracic aorta. Arch Surg. 1965;91(4):602–3.
Mattox KL, Holzman M, Pickard LR, Beall AC Jr, DeBakey ME. Clamp/repair: a safe technique for treatment of blunt injury to the descending thoracic aorta. Ann Thorac Surg. 1985;40(5):456–63.
DuBose J, Azizzadeh A. Thoracic vascular trauma. In: Sidawy A, Bruce A, editors. Rutherford’s vascular surgery and endovascular therapy. 9th ed. Philadelphia, PA: Elsevier; 2019.
Moore WM Jr, Hollier LH. The influence of severity of spinal cord ischemia in the etiology of delayed-onset paraplegia. Ann Surg. 1991;213(5):427–31; discussion 31–2.
Adams HD, Van Geertruyden HH. Neurologic complications of aortic surgery. Ann Surg. 1956;144(4):574–610.
Gharagozloo F, Larson J, Dausmann MJ, Neville RF Jr, Gomes MN. Spinal cord protection during surgical procedures on the descending thoracic and thoracoabdominal aorta: review of current techniques. Chest. 1996;109(3):799–809.
Cambria RP, Davison JK, Zannetti S, L’Italien G, Brewster DC, Gertler JP, et al. Clinical experience with epidural cooling for spinal cord protection during thoracic and thoracoabdominal aneurysm repair. J Vasc Surg. 1997;25(2):234–41; discussion 41–3.
Kouchoukos NT, Daily BB, Rokkas CK, Murphy SF, Bauer S, Abboud N. Hypothermic bypass and circulatory arrest for operations on the descending thoracic and thoracoabdominal aorta. Ann Thorac Surg. 1995;60(1):67–76; discussion −7.
Fowl RJ, Patterson RB, Gewirtz RJ, Anderson DK. Protection against postischemic spinal cord injury using a new 21-aminosteroid. J Surg Res. 1990;48(6):597–600.
Safi HJ, Miller CC 3rd, Carr C, Iliopoulos DC, Dorsay DA, Baldwin JC. Importance of intercostal artery reattachment during thoracoabdominal aortic aneurysm repair. J Vasc Surg. 1998;27(1):58–66; discussion −8.
Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, et al. Diagnosis and treatment of blunt thoracic aortic injuries: changing perspectives. J Trauma. 2008;64(6):1415–8; discussion 8–9.
Scalea TM, Feliciano DV, DuBose JJ, Ottochian M, O’Connor JV, Morrison JJ. Blunt thoracic aortic injury: endovascular repair is now the standard. J Am Coll Surg. 2019;228(4):605–10.
Vandy FC, Girotti M, Williams DM, Eliason JL, Dasika NL, Michael Deeb G, et al. Iliofemoral complications associated with thoracic endovascular aortic repair: frequency, risk factors, and early and late outcomes. J Thorac Cardiovasc Surg. 2014;147(3):960–5.
McBride CL, Dubose JJ, Miller CC 3rd, Perlick AP, Charlton-Ouw KM, Estrera AL, et al. Intentional left subclavian artery coverage during thoracic endovascular aortic repair for traumatic aortic injury. J Vasc Surg. 2015;61(1):73–9.
Patel HJ, Dake MD, Bavaria JE, Singh MJ, Filinger M, Fischbein MP, et al. Branched endovascular therapy of the distal aortic arch: preliminary results of the feasibility multicenter trial of the Gore thoracic branch endoprosthesis. Ann Thorac Surg. 2016;102(4):1190–8.
Lamarche Y, Berger FH, Nicolaou S, Bilawich AM, Louis L, Inacio JR, et al. Vancouver simplified grading system with computed tomographic angiography for blunt aortic injury. J Thorac Cardiovasc Surg. 2012;144(2):347–54.e1.
Gunn ML, Lehnert BE, Lungren RS, Narparla CB, Mitsumori L, Gross JA, et al. Minimal aortic injury of the thoracic aorta: imaging appearances and outcome. Emerg Radiol. 2014;21(3):227–33.
Starnes BW, Lundgren RS, Gunn M, Quade S, Hatsukami TS, Tran NT, et al. A new classification scheme for treating blunt aortic injury. J Vasc Surg. 2012;55(1):47–54.
Sandhu HK, Leonard SD, Perlick A, Saqib NU, Miller CC 3rd, Charlton-Ouw KM, et al. Determinants and outcomes of nonoperative management for blunt traumatic aortic injuries. J Vasc Surg. 2018;67(2):389–98.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Romagnoli, A., Teixeira, P., Reva, V., DuBose, J. (2021). Blunt Thoracic Aortic Injury in Thoracic Surgery for the Acute Care Surgeon. In: Galante, J.M., Coimbra, R. (eds) Thoracic Surgery for the Acute Care Surgeon. Hot Topics in Acute Care Surgery and Trauma. Springer, Cham. https://doi.org/10.1007/978-3-030-48493-4_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-48493-4_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-48492-7
Online ISBN: 978-3-030-48493-4
eBook Packages: MedicineMedicine (R0)