Abstract
Cardiovascular calcification is prevalent among asymptomatic patients and those with prevalent cardiovascular disease. It is associated with increasing age and other cardiovascular disease risk factors, such as chronic kidney disease. Observational registries, small case series, and clinical trials have been the mainstay for clinical decision-making in patients with calcified coronary and peripheral vessels and mitral valve disease. These studies have provided insight into the prevalence of calcification in select patient populations and communities as well as demonstrated how noninvasive imaging and a blood test for calcification propensity can inform treatment options and outcomes. Early studies testing novel and repurposed pharmacological therapies to prevent or limit the progression of cardiovascular calcification are underway. For symptomatic patients that require interventions, evidence from clinical trials has guided percutaneous coronary interventions, coronary artery bypass grafting surgery, and mitral valve interventions. Pertinent clinical trials and studies in these areas are reviewed herein.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Clinical trials
- Coronary artery calcification
- Coronary artery bypass grafting surgery
- Peripheral arterial disease
- Mitral valve calcification
Cardiovascular calcification is a highly prevalent pathophenotype associated with risk factors for atherosclerotic disease, including normal aging, chronic kidney disease, and diabetes mellitus. In fact, in the Multi-Ethnic Study of Atherosclerosis (MESA) study, there was concordance between Framingham risk score and coronary artery calcium scores [1]. The prevalence of calcification in the coronary vessels increases with age and is present in more than 90% of men and 67% of women over age 70 years [2]. Calcium burden was similar between men and women but lower in African Americans compared to Caucasians. Coronary artery calcification was observed in 70.4%, 52.0%, 56.6%, and 59.2% of men of Caucasian, African American, Hispanic, and Chinese ethnicity, respectively [1].
The association between cardiovascular calcification and major adverse cardiac events are well documented. In the MESA study, individuals with a coronary calcium score of >300 had a 6.84 (95% CI: 2.93–15.99) increased risk of myocardial infarction: the risk was 3.89 (95% CI: 1.72–8.79) when the calcium score was 1–100 [3, 4]. Interestingly, approximately 18% of participants who did not have coronary calcification at the index study went on to develop coronary calcification during a 3-year follow-up period [5]. When examined in a meta-analysis of 30 studies that included 218,080 patients, the risk of cardiovascular mortality was markedly increased in patients with chronic kidney disease (OR = 6.22; 95% CI: 2.73, 14.14) or diabetes mellitus (OR = 2.27; 95% CI: 1.07–3.04) (Fig. 21.1) [6]. Taken together, the presence of a high burden of coronary artery calcification combined with the high prevalence in patients with diabetes mellitus or chronic kidney disease likely explains the high risk for cardiovascular events in these populations.
Vascular calcification , risk of adverse events, and risk associated with chronic kidney disease and diabetes mellitus. When present, vascular calcification is associated with an increased risk of adverse events, including all-cause mortality, cardiovascular (CV) mortality, coronary heart disease events, and stroke. Patients with chronic kidney disease (CKD) or diabetes mellitus are at increased risk of developing vascular calcification. (Adapted from Ref. [6])
Cardiovascular disease remains the leading cause of death throughout the world with ~15 million deaths in 2016 [7]. This has been associated with an ~$320 billion annual expenditure for healthcare related to cardiovascular disease in 2015, which is estimated to grow to $820 billion by 2030 [8]. Owing to the high risk of major adverse cardiovascular events associated with vascular calcification, efforts to reduce the burden of calcium in the hope of improving outcomes are underway. These efforts have focused mainly on two areas: drug development and repurposing, as there are currently no effective therapies that target cardiovascular calcification and selection of devices utilized during percutaneous coronary or peripheral interventions for symptomatic atherosclerotic disease. This chapter will focus on clinical trials that inform decision-making with respect to currently available and future methods to assess cardiovascular calcification, pharmacologic interventions, and the invasive diagnosis and treatment of symptomatic vascular disease in calcified vessels.
Clinical Studies of Methods to Assess Cardiovascular Calcification
Following large-scale population-based studies that revealed a relationship between coronary artery calcification and major adverse cardiovascular outcomes in asymptomatic individuals, noninvasive evaluation of coronary artery calcification, primarily via multidetector CT scanning, has emerged as a valuable tool for assessing cardiovascular risk [9,10,11,12,13]. Early clinical studies from single centers demonstrated that coronary artery calcification had predictive value and improved risk stratification of patients with intermediate Framingham risk scores [14]. This has been shown in both young and elderly individuals, diabetes mellitus, and smokers [15,16,17,18]. Population-based cohort studies, such as the MESA study, have confirmed the benefits of coronary CT assessments for risk prediction [4]. The Heinz Nixdorf Recall (Risk Factors, Evaluation of Coronary Calcium and Lifestyle) study included 4487 participants in its initial study and scheduled follow-up studies after 5 years. Here the prevalence of coronary artery calcification was found to be 82% in men (n = 1918) and 55% in women (n = 2148) [13]. The Rotterdam Study, which included 7893 individuals who were age 55 years or older, reported a median coronary artery calcium score of 98, which was similar to MESA and the Heinz Nixdorf Recall study [19]. The Framingham Heart Study added coronary artery calcium studies to the Offspring and Third Generation cohorts. After 7 years of follow-up, the investigators found that both the presence of calcium in the proximal dominant vessel and the number of vessels with calcium were associated with major adverse cardiovascular events [20].
Coronary artery calcium has also been measured in young adults aged 32–46 years in the Coronary Artery Risk Development in Young Adults (CARDIA) study. This prospective study demonstrated that coronary artery calcium scores >0 were not uncommon and that this predicted cardiovascular risk, beyond the traditional coronary heart disease risk factors [21]. The Jackson Heart Study reported similar findings in an African American population. In this group, coronary artery calcium scores also predicted risk beyond traditional risk factors and identified individuals for primary prevention [22, 23]. The Women’s Health Initiative reported the same in postmenopausal women [24].
Coronary artery plaque characteristics that are associated with adverse cardiovascular events were evaluated in the Scottish Computed Tomography of the HEART Trial (SCOT-HEART). In a post hoc analysis of this study, which evaluated patients with stable chest pain using coronary CT angiography, the investigators aimed to determine the association between clinical outcomes and adverse coronary plaque characteristics seen on the coronary CT angiogram. Of the 4146 patients enrolled in the study, 1778 underwent a coronary CT angiogram. As part of the analysis, 15 coronary segments per patient were examined for focal calcifications, which may be linked to plaque destabilization and rupture leading to adverse clinical events. Patients who had a higher calcium score were also found to have a higher cardiovascular risk score. In fact, compared to patients with nonobstructive disease, those patients with obstructive coronary artery disease had an eightfold higher calcium score (435 vs. 54 Agatston units, p < 0.001). Moreover, those patients with a coronary artery calcium score ≥1000 Agatston units were found to have a 13-fold increase in nonfatal myocardial infarction or death attributable to coronary heart disease. Thus, a higher burden of coronary plaque calcification portends increased risk of adverse cardiovascular events [25].
To date, the only risk score that incorporates coronary artery calcification in the model was derived from the MESA study. This model includes age, gender, race/ethnicity, diabetes, current smoking, family history, cholesterol, systolic blood pressure, the use of lipid lowering and hypertension medications, and coronary artery calcification and was created to predict the 10-year coronary heart disease risk [26]. Unsupervised machine learning has been applied to define characteristics associated with adverse events. Algorithms using chest and abdominal CT scans from 2924 asymptomatic participants in the Framingham Heart Study revealed that global calcification, defined as aortic, thoracic, coronary, and valvular calcification, was one of the components that conferred increased cardiovascular risk [23].
Despite the evidence that coronary artery calcification improves clinical risk assessment beyond traditional cardiovascular risk factors, clinical practice guidelines have not universally included coronary artery calcium score. The 2019 American College of Cardiology/American Heart Association guidelines for the primary prevention of cardiovascular disease now recognize that in adults at intermediate risk for coronary heart disease events, coronary artery calcium scores can be effective for reclassifying risk that would ultimately determine treatment. The guidelines now recommend that initiation of statin therapy is reasonable in patients with a coronary artery calcium score of ≥100 Agatston units. Individuals with a calcium score of 0 were considered low risk and didn’t warrant pharmacological intervention, unless they had diabetes mellitus, active tobacco use, a family history, or chronic inflammatory conditions. By contrast, for individuals with a score of 1–99 Agatston units , where risk reclassification was only modest, the consensus opinion was to discuss risk and repeat the scan in 5 years [27].
Blood Calcification Propensity and Cardiovascular Events
A novel methodology to assess blood calcification propensity has now been described. This method involves a blood test that examines an individual’s potential for ectopic calcification by measuring the time to form primary calciprotein particles in serum (Fig. 21.2). The timing of spontaneous formation of secondary calciprotein particles is, in part, dependent upon serum concentrations of calcification inhibitors, such as fetuin-A and pyrophosphate among others [28, 29]. If the transformation time (T50) is short, this indicates that there is rapid formation of calciprotein particles and a higher blood calcification propensity. This finding has been associated with an increased risk of adverse clinical outcomes. In patients with stage 3 or 4 chronic kidney disease, or following renal transplantation, the test was inversely correlated with all-cause mortality [29, 30].
Principle of blood calcification propensity measurement. A new serum assay is able to determine the propensity for blood calcification. In this test, a laser is used to determine light scatter in serum that is artificially supersaturated with calcium and phosphate. The addition of calcium and phosphate to serum results in the formation of primary calciprotein particles. These particles undergo spontaneous transformation to secondary particles that contain calcium phosphate in crystalline form and serum proteins. The time for 50% transformation from primary to secondary particles is indicative of an individual’s ability to inhibit calcification [28]
The relationship between calcification propensity evaluated by serum T50 and cardiovascular events was tested in the Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE) trial. In this study, baseline serum samples from 2785 eligible patients with chronic kidney disease on dialysis were evaluated for calcification propensity. Approximately 30% of the patients had diabetes mellitus, and 23% had established coronary artery disease. The primary composite endpoint included all-cause mortality, myocardial infarction, heart failure, unstable angina requiring hospitalization, or a peripheral vascular event. After a median follow-up period of 619 days, the primary composite endpoint was reached in 1350 patients. A lower T50 time was associated with increased risk of cardiovascular events with a HR = 1.38 (95% CI: 1.19, 1.60, p < 0.001) for myocardial infarction, HR = 1.22 (95% CI: 1.05, 1.42, p = 0.01) for peripheral vascular events, and HR = 1.10 (95% CI: 1.00, 1.20, p = 0.05) for cardiovascular death. Interestingly, the T50 time was not associated with unstable angina or heart failure [31]. While this study had several limitations, including use of only baseline samples and a retrospective study design, the findings are not surprising as it has long been recognized that hemodialysis patients have excess morbidity and mortality, mostly attributable to cardiovascular events [32].
More recently, calcification propensity was evaluated in 3404 patients enrolled in the longitudinal Chronic Renal Insufficiency Cohort Study. Mean follow-up time for participants was an average of 7.1 years. In this prospective cohort, a lower T50 time was associated with an increased risk of atherosclerotic cardiovascular disease (HR = 1.14; 95% CI: 1.09–1.24), which was not independent of renal function [33]. In a subgroup of 1274 patients who had coronary artery calcification measured by CT scan, approximately 65% had prevalent coronary calcification. In these patients, the T50 time was found to be associated with the severity of coronary calcification. At 3-year follow-up, 20% of patients who did not have coronary artery calcification at baseline developed incident coronary calcification, while 19% with calcification present at baseline demonstrated calcification progression. Here, a lower T50 time and hence a higher serum calcification propensity were associated with coronary artery calcification progression and severity [34]. Worsening calcification propensity over 24 months was associated with an increased hazard for cardiovascular mortality (HR = 2.15; 95% CI: 1.15–3.97, p = 0.02) [35]. To date, tests of calcification propensity have only been applied to patients with some degree of chronic kidney disease or on hemodialysis. Whether or not this test has applicability in broader community-based populations or in select patients at risk for or with established atherosclerotic cardiovascular disease remains to be determined.
Clinical Trials of Pharmacological Therapeutics
At present, there is no known pharmacologic therapy that has definitively been shown to prevent or regress established cardiovascular calcification in a double-blind randomized clinical trial and been incorporated into mainstream guidelines and practice. However, several therapies have shown promise in smaller studies or clinical trials. In many instances, early studies have been done in select patient subsets, such as chronic kidney disease that have a high propensity for cardiovascular calcification. Whether or not results from these studies have utility in other patient populations is unknown.
Vitamin K
Vitamin K is a fat-soluble vitamin that mediates blood coagulation as well as cardiovascular calcification through its regulation of the calcification inhibitor matrix Gla protein (MGP) . Vitamin K is necessary for the carboxylation of glutamic acid in activation of MGP. Vitamin K levels are maintained through the diet by intake of leafy green vegetables or ingestion of fermented foods, which are then processed by commensal bacteria; however, many patients are vitamin K deficient. This is well documented in patients with chronic kidney disease and may be a larger problem in the population as a whole [36,37,38]. A recent survey found that only 58–63% of diabetic women and 61–68% of women without diabetes mellitus reported daily intake of vegetables [39]. Furthermore, clinical studies of patients taking warfarin, a vitamin K antagonist, have demonstrated increased vascular calcification compared to patients who do not take this drug. A post hoc analysis of eight randomized trials that included serial intravascular ultrasound evaluated the effect of warfarin on calcification in patients with coronary artery disease. Of the 171 patients who were treated with warfarin, there was a significant annualized increase in calcification as compared to 4129 individuals who were not taking warfarin. Warfarin was found to be independently associated with increased calcification (OR, 1.2; 95% CI, 1.1–1.3, p = 0.003), and the annual increase in calcification occurred independent of changes in atheroma volume [40]. This has also been observed in the peripheral vasculature. A cohort analysis of 430 patients matched by age, sex, and diabetes status revealed that the prevalence of peripheral vascular calcification was significantly higher in warfarin-treated patients (30.2% vs 20.9%, p = 0.0023) [41]. To demonstrate that warfarin likely had a causal effect, a randomized trial of 66 patients with nonvalvular atrial fibrillation was conducted. Patients were randomized to warfarin or apixaban for 1 year, and coronary CT angiography was performed to evaluate calcium burden. Here, CT scans demonstrated lower levels of calcified plaque volume in apixaban-treated patients as compared to those randomized to warfarin [42].
The association between vitamin K deficiency and cardiovascular calcification has led to the strategy of vitamin K supplementation as a method to limit calcification and subsequent adverse events. A meta-analysis that included 12,888 patients enrolled in 13 controlled trials and 14 longitudinal studies found that vitamin K supplementation was associated with a reduction in decarboxylated MGP and a significant 9.1% decrease in vascular calcification [43]. Clinical trials are underway that will evaluate the efficacy of vitamin K supplementation on the progression of coronary artery calcification. Results from these trials will be critical to understand whether or not this will be a viable therapeutic option for patients. In a recently reported double-blind, placebo-controlled, randomized trial of vitamin K supplementation in patients with type 2 diabetes mellitus and established cardiovascular disease, calcification was measured after 6 months using 18F-NaF positron emission tomography. After 6 months, vitamin K supplementation was associated with an increase, and not a decrease, in femoral artery calcification [44]. Since this finding is in contradistinction to results from prior studies, additional data will be required prior to issuing recommendations pertaining to vitamin K supplementation to reduce cardiovascular calcification.
Sodium Thiosulfate
Sodium thiosulfate is an inorganic sodium salt with reducing properties and has clinical application in the treatment of cyanide poisoning [45]. Sodium thiosulfate to limit cardiovascular calcification has been trialed in hemodialysis patients. In one study of 87 patients with coronary calcium scores >300, patients received intravenous infusions twice weekly. After 4 months, vascular calcification increased in 63% of patients in the control group, but only 25% of patients received sodium thiosulfate (p = 0.03). This benefit, however, occurred at the expense of a decrease in bone mineral density, and patients who were treated with sodium thiosulfate experienced metabolic acidosis and persistent anorexia [46]. In a second pilot study, 22 hemodialysis patients were treated with sodium thiosulfate 3 times weekly. After 5 months, there was no difference in the mean annualized rate of calcification; however, 14 out of 22 patients did demonstrate progression of coronary artery calcification [47]. While these results appear promising, the side effects of this agent, namely, metabolic acidosis and bone toxicity, suggest that it is unlikely to have the acceptable long-term safety profile required to treat cardiovascular calcification.
Bisphosphonates
The bisphosphonates are used widely to prevent and treat osteoporosis. These drugs are analogs of pyrophosphate, which inhibits calcification by chelating calcium ions in hydroxyapatite. Early clinical studies with these agents reported conflicting results. One study evaluated alendronate in 56 patients with osteoporosis compared to matched controls and a referent cohort. After 2 years, there was no observable difference in coronary artery calcification between groups [48]. Another study performed in women age 55–80 years who were treated for 3 years with either oral or intravenous ibandronate found that there was no difference in the rate of aortic calcification at the end of the study between groups [49]. Similarly, a pilot randomized controlled trial of alendronate in 51 patients with stage 3–4 chronic kidney disease revealed that compared to placebo, at 18 months there was no difference in the progression of ectopic calcification in the aorta in alendronate-treated patients [50]. This is in contrast to several small studies in hemodialysis patients that reported a positive effect of bisphosphonates on cardiovascular calcification. In these studies, which each enrolled fewer than 25 patients, treatment with bisphosphonates was associated with either halting or reversing the progression of aortic calcification [51, 52].
A recent randomized trial of etidronate in patients with pseudoxanthoma elasticum was performed to test the hypothesis that a bisphosphonate could limit arterial calcification in this patient population with low pyrophosphate levels. A cohort of 74 patients was randomized to etidronate (20 mg/kg for 2 weeks every 12 weeks) or placebo. After 12 months of follow-up, arterial calcification assessed by CT scan was decreased by 4% in etidronate-treated patients but increased by 8% in control subjects. However, when quantified by 18F-sodium positron emission tomography activity, there was no difference observed between the active treatment and placebo group. Importantly, while patients assigned to etidronate were more likely to experience hyperphosphatemia, this was reversible, and there were no safety issues noted [53].
There have been some issues raised with respect to the long-term durability of these drugs to prevent or limit cardiovascular calcification owing to concerns related to bone density and cardiac arrhythmias. There has been a long-standing debate regarding the effect of bisphosphonates on skeletal toxicity. This has been attributed to the bisphosphonate drug selected, oral versus intravenous administration, dosage regimen with continuous dosing versus cyclical dosing, and the patient’s comorbidity profile [54]. Another issue was raised by the finding that patients treated with parenteral zoledronic acid annually for osteoporosis management had a higher signal of atrial fibrillation [55]. This has not been explored further, so it is unknown if bisphosphonate drugs played a causal role in arrhythmia generation. These agents continue to be investigated and these issues remain to be resolved.
Magnesium
Magnesium is suggested to prevent cardiovascular calcification by regulating calcium influx, mediating pro-calcific enzyme activity, and stimulating activity of calcification inhibitory enzymes [56]. Magnesium also prevents the transformation of calcium phosphate nanocrystals to apatite [56]. In patients with chronic kidney disease, in dialysis patients, and in patients with prevalent cardiovascular calcification, increased cardiovascular mortality has been associated with low serum magnesium levels [57,58,59,60]. Small observational clinical studies have suggested that there may be some benefit to magnesium supplementation. A pilot study in seven hemodialysis patients found that after 18 months of supplementation with magnesium carbonate, there was no progression of coronary artery calcification by CT scan [61]. These findings were confirmed in a similarly small study that compared magnesium supplementation with magnesium carbonate and calcium acetate as a phosphate binder with calcium acetate alone. After 1 year, calcification assessed by X-ray was improved in 15% of the magnesium group compared to 0% in the calcium acetate group [62]. Another small study that included 34 patients with chronic kidney disease (stage 3, 4) and trialed short-term magnesium hydroxide for 8 weeks was shown to decrease calcification propensity measured in the serum [63]. Currently, there is a randomized clinical trial of 250 patients with chronic kidney disease that is underway that will test magnesium hydroxide versus placebo on coronary artery calcium score after 1 year of treatment [64].
SNF472
The investigational agent, SNF472, a myo-inositol hexaphosphate, is a hexasodium salt of phytate and is under development for the treatment of cardiovascular calcification. This agent may have utility in patients on hemodialysis as phytate is a low molecular mass and highly soluble crystallization inhibitor [65]. The safety and tolerability of SNF472 were demonstrated in healthy volunteers and dialysis patients. In a Phase I study, there were no clinically significant effects, and pharmacodynamic analyses showed that SNF472 decreased hydroxyapatite crystallization potential in dialysis patients [66]. A phase 2b double-blind, placebo-controlled study of SNF472 in hemodialysis patients randomized patients to one of two doses of SNF472 delivered three times per week with dialysis or placebo and evaluated calcification by CT scan after 1 year. After 1 year, the coronary artery calcium volume score was significantly lower in patients treated with SNF472 as compared to placebo (11% vs 20%, p = 0.016). There was also a decrease in the progression of calcification in the aortic valve (14% vs. 98%, p < 0.001). There was no difference between active treatment and placebo groups with respect to treatment-emergent adverse events. Thus, while SNF472 attenuated progression of coronary artery and aortic valve calcification in this patient population, it remains to be determined if this translates to an effect on cardiovascular outcomes and is translatable to other patient populations at high risk for cardiovascular calcification [67].
Denosumab
Denosumab is an FDA-approved human monoclonal antibody against the receptor activator of NF-kB ligand (RANKL) and is used primarily to treat bone tumors and osteoporosis. Theoretically, denosumab may limit cardiovascular calcification by preventing RANKL interaction with its receptor and preventing deposition of calcified minerals. A post hoc analysis of postmenopausal women at high risk for cardiovascular events examined lateral spine X-rays to evaluate for progression of aortic calcification. The frequency of aortic calcification progression and adverse cardiovascular events over 3 years was not different between the denosumab and placebo groups [68]. The phase 2 SALTIRE II trial is currently evaluating the effect of denosumab and alendronate as interventions for calcific aortic stenosis. Patients with calcific aortic stenosis assessed by echocardiography will be treated with denosumab, alendronate, or both, or placebo and calcification will be examined using standard CT calcium scoring and with 18F-fluoride positron emission tomography activity.
While many of the aforementioned agents have shown promise in the treatment of cardiovascular calcification, evidence to support routine use in broader patient populations remains limited. This is not surprising that the mechanism for cardiovascular calcification and, therefore, the appropriate pharmacological intervention, likely differs between patient subsets. Moreover, safety and efficacy profiles will need to be evaluated on a longer-term basis to understand optimal dosing regimens. Forthcoming results from randomized trials to resolve these questions are eagerly awaited.
Clinical Studies of the Invasive Diagnosis and Treatment of Cardiovascular Calcification
It has been recognized that conventional coronary angiography does not always demonstrate coronary calcification adequately leading to an underappreciation of the severity of calcification. In a series of 1155 target lesions in native coronary arteries, angiography detected calcification in 38% of the stenoses, and 26% were classified as moderately calcified, while 12% were severely calcified. These same stenoses were then evaluated with intravascular ultrasound. This imaging modality detected calcium deposits in 73% of lesions, indicating that coronary calcification is both common and frequently underestimated [69]. Optical coherence tomography, which has a higher resolution than intravascular ultrasound, can assess calcium arc and length in a coronary artery but has limited depth penetration and, therefore, is likely to miss deep intramural calcium [70]. This imaging modality can also provide an assessment of the degree of the calcific arch in the vessel cross section; calcium area, thickness, and length; and, calcium three-dimensional volume [71]. A head-to-head comparison of the two imaging modalities with angiography revealed that angiography detected calcium in only 40% of lesions, while intravascular ultrasound and optical coherence tomography detected calcium in 83% and 77% of stenoses, respectively [72].
In contemporary percutaneous coronary intervention (PCI), significant coronary calcification is present in approximately 20% of patients, and these patients are characterized by a demographic, clinical, and angiographic pathophenotype that differs from that observed in individuals with less severe calcification [73]. The introduction of next-generation DES hasn’t reduced the hazard associated with calcification, and calcified lesions are associated with a threefold increase in adverse events [74]. A large registry analysis of 16,001 patients from a single academic medical center revealed that patients with moderate or severe target lesion calcification tended to be older, Caucasian, have a higher body mass index, less likely to be a current smoker, and more likely to have renal dysfunction, anemia, and peripheral arterial disease. They had more complex target lesions (classified B2/C) with a higher SYNTAX score and had a higher rate of procedural complications. Within this group, moderate to severe calcification was associated with an increased risk of death, myocardial infarction, and/or target vessel revascularization [75]. These patients also have higher 30-day rates of major bleeding (11.2% vs. 7.2% vs. 5.9%, p = 0.0003) compared to patients with moderate or mild or no target lesion calcium [76]. Coronary lesion calcification also impacts chronic total occlusion procedures. In 1476 procedures performed across 11 centers in the United States between 2012 and 2016, moderate to severe calcification was identified in 58% of target lesions. Percutaneous revascularization of calcified chronic total occlusion lesions often required a change in procedural strategy with increased use of the retrograde approach, more contrast use, and increased radiation exposure as well as lower success rates and increased incidence of adverse cardiovascular events (3.7% vs. 1.8%, p = 0.033) compared to patients without calcified stenoses [77].
Percutaneous Coronary Intervention for Calcified Plaques and Coronary Arteries
Given the high prevalence of coronary calcification, patients who require PCI present a unique challenge. The optimal treatment for calcified plaque modification in complex coronary anatomies remains a decision point for coronary interventions that has been the subject of clinical trials. The standard method of using a balloon inflated to high pressure to release the coronary plaque can result in vessel dissection and perforation when calcified stenoses are present. The risk of not achieving adequate enlargement of a coronary lumen due to inability to release or “crack” a calcified stenosis is incomplete expansion or malapposition of a coronary stent, which predisposes to stent failure and adverse clinical outcomes [78]. At present, clinical trials to treat calcified coronary stenoses in the cardiac catheterization laboratory have focused on rotational atherectomy (Fig. 21.3), orbital atherectomy, excimer laser coronary atherectomy, or shockwave lithotripsy.
Atherectomy in a calcified coronary stenosis . (a) A left anterior descending artery has a significant calcified stenosis in the midsection of the vessel. (b) Attempts to dilate the stenosis with balloon angioplasty were unsuccessful as the balloon would not expand fully (dogbone shape) owing to the calcified stenosis. (c) Rotational atherectomy was used to debulk calcium from the vessel. The burr can be seen on the angioplasty wire. (d) After rotational atherectomy, the lesion was dilated with a scored angioplasty balloon and stented. The final result shows an enlarged and patent vessel
Rotational atherectomy relies on a diamond-encrusted elliptical burr that rotates at high speeds (140,000–180,000 rpm) and pulverizes calcified or inelastic tissue, while the relatively normal elastic arterial wall remains uninjured. The device ablates the calcified tissue to particles smaller than a red blood cell, which allows them to mix with blood and is ultimately taken up by macrophages [79,80,81,82]. Early clinical trials that compared rotational atherectomy with balloon angioplasty for de novo calcified coronary stenoses or for in-stent restenosis demonstrated equivocal results with respect to rotational atherectomy. In these studies, rotational atherectomy adequately increased the lumen diameter but was associated with high rates of restenosis. A meta-analysis that included 9222 patients enrolled in 16 early trials (1993–2002) revealed that the rate of periprocedural myocardial infarction was increased with rotational atherectomy compared to balloon angioplasty (OR = 1.83; 95% CI: 1.43–2.34) despite similar angiographic outcomes [83]. Similarly, a more recent review that included 3474 patients enrolled in 12 clinical trials (2002–2012) found that there was no difference between rotational atherectomy with adjunctive balloon angioplasty as compared to balloon angioplasty alone with respect to restenosis rates at 6 months or 1 year. There was also no difference in the risk of in-hospital major adverse cardiac events (RR; 1.27; 95% CI: 0.86–1.90) [84].
Our current clinical evidence supporting the use of rotational atherectomy comes from the Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease (ROTAXUS) and the Comparison of Strategies to PREPARE Severely CALCified Coronary Lesions (PREPARE-CALC) trials. The ROTAXUS trial enrolled 240 patients with calcified coronary stenoses and randomized them to rotational atherectomy and stent implantation versus stent implantation alone (standard treatment). There was a higher rate of procedural success in the rotational atherectomy arm (92.5% vs. 83.3%) as well as a high crossover rate from the standard treatment group. At follow-up, there was no difference between the groups with respect to restenosis, target lesion revascularization, or adverse events. The trial, however, was criticized for missing follow-up angiography in 20% of patients, which likely affected the power of the study, as well as the use of a first-generation drug-eluting stent [85]. The PREPARE-CALC study, in contrast, randomized 200 patients to rotational atherectomy versus a cutting or scoring balloon prior to implantation of a third-generation drug-eluting stent. This trial also demonstrated a higher rate of procedural success for rotational atherectomy (98% vs. 81%). At 9-month follow-up, there were no differences between groups with respect to late lumen loss, target lesion revascularization, or adverse events. Taken together, these trials form the basis for the modern approach of selecting rotational atherectomy as a primary strategy for stenosis modification prior to stenting when severely calcified coronary lesions are present [86].
Orbital atherectomy , which is similar to rotational atherectomy, utilizes an eccentrically rotating 1.25 mm crown coated with diamonds on the front and back ends. The device has a low (80,000 rpm) and high (120,000 rpm) rotational speed, and the technique to ablate heavily calcified plaque is dependent upon contact time between the crown and the plaque. Safety and efficacy of this device was trialed in the ORBIT II trial, which enrolled 443 patients across the United States from 49 centers [87]. In this study, the primary safety endpoint, freedom from 30-day adverse cardiac events, and the primary efficacy endpoint (residual stenosis <50% after stenting and absence of in-hospital events) were met in 90% and 89% of participants, respectively. A real-world registry of 458 patients with complex comorbidities found that no reflow, dissection, or perforation associated with using this device occurred in <1% of cases with a 12.6% rate of major adverse cardiovascular and cerebrovascular events at 1 year [88].
Excimer laser has also shown utility for modification of calcified coronary plaques. The technique relies on photoablation of calcified plaque by photochemical, photothermal, and photomechanical methodologies. The laser can break molecular bonds, heat the plaque, or heat liquid near the plaque to create exploding bubbles that modify the plaque. This device is typically selected for coronary stenoses that can’t be dilated with procedural success rates of 93% reported [89]. However, more calcification in the plaque appears to decrease the laser efficacy, and intravascular imaging has shown that while laser increased the vessel cross-sectional area, this occurred without significant modification of the calcified areas [90, 91]. Evidence does support the use of laser to crack calcium behind stent struts in undilatable instent restenosis, especially when this occurred as a result of an underexpanded stent [92, 93].
A lithotripsy balloon has been developed as a device to treat deep intravascular calcium. The balloon emits pulsatile mechanical energy at a frequency of 1 Hz that cracks and fractures calcified plaques and calcium present in deeper layers of the vessel wall. In 43% of cases, this device was shown to fracture an arc of calcium in the vessel lumen and create multiple fractures in more than a quarter of the cases. Importantly, there was a higher rate of fracture when there was a greater burden of calcium [94]. The Disrupt Coronary Artery Disease II study evaluated safety and efficacy of lithotripsy in 120 patients with severe coronary artery calcification enrolled between 2018 and 2019 in 9 countries. There was 100% success in device delivery and use. In this study, myocardial infarction occurred in 5.8% of patients. A subset of patients underwent optical coherence tomography imaging, which demonstrated calcium factures in 78.7% of lesions [95]. Other limited studies have similarly reported no safety concerns, and larger-scale trials are currently underway.
The presence of calcified coronary stenoses as well as calcified coronary arteries is well recognized to increase the technical complexity of PCI. Calcification that limits plaque or lesion expansion predisposes to less than optimal stent expansion, which is associated with stent failure and major adverse cardiovascular events. Rotational atherectomy has been a mainstay as a therapeutic modality to treat calcified coronary stenoses, and other devices with adequate safety and efficacy profiles are emerging, some with niche indications. Intravascular imaging, which facilitates stenting procedures in the absence of coronary calcification, remains critical to define the calcium burden within a vessel and guide device selection for lesion modification. Device selection can be operator-dependent, but considerations include eccentricity versus concentricity of calcification, length of calcium, depth of calcium, and ability to deliver devices. Nonetheless, clinical trials have established that successful treatment of calcified stenoses for stent delivery and expansion is critical to limit procedural complications and adverse outcomes.
Coronary Artery Bypass Grafting Surgery in Patients with Calcified Coronary Arteries
In those instances where surgical coronary artery bypass grafting (CABG) is the preferred revascularization strategy, calcified coronary arteries represent a significant challenge for the surgeon. In high-risk patients, calcified coronary arteries and calcified stenoses have been associated with incomplete revascularization, atheroembolic complications, and worse clinical outcomes [96, 97]. Patients with calcified coronary arteries are also more likely to develop calcifications in saphenous vein bypass grafts, which predict both early and late graft failure [98]. It has been hypothesized that severe calcification impacts outcomes owing to loss of pulsatility in the vessels and an increase in ischemic events owing to endothelial dysfunction and atheroembolic events from calcified plaques despite bypassing significant stenoses [99]. Calcified vessels are also likely to increase the difficulty of creating vascular anastomoses and may affect the ability to achieve complete revascularization [100, 101]. Patients with significant aortic calcification also have restrictions on aortic cross-clamping during surgery owing to the high risk for cerebral embolization and stroke [102].
Patients with chronic kidney disease on hemodialysis have a high prevalence of vascular calcification and represent a high-risk patient population that frequently requires CABG for symptomatic coronary artery disease. Observational studies, including one that followed 1300 patients on hemodialysis, have shown that patients with chronic kidney disease on hemodialysis have higher operative mortality (7.8% vs. 2.1%) and 30-day mortality rates (4.8% vs 1.4%) [103]. These patients were also more likely to develop perioperative complications, such as mediastinitis and stroke [104]. A cohort of 21,981 hemodialysis patients from the US Renal Data System that underwent surgical or percutaneous revascularization revealed that CABG was associated with a lower composite of death or myocardial infarction (HR = 0.88; 95% CI: 0.86–0.91) and mortality (HR = 0.87; 95% CI: 0.84–0.90) compared to PCI at 5 years suggesting that, when appropriate, surgical revascularization may be preferred over PCI. Overall, it’s been reported that long-term outcomes are improved following CABG compared to optimal medical therapy [105, 106].
In patients without chronic kidney disease, post hoc analyses of randomized trials have shown that coronary calcification is associated with an increase in adverse events for patients that undergo CABG. In the Acute Catheterization and Urgent Intervention Triage StrategY (ACUITY) trial which examined heparin versus bivalirudin in patients with acute coronary syndromes, patients with severe coronary artery calcification that underwent CABG were older and more likely to have hypertension, chronic kidney disease, and a higher thrombolysis in myocardial infarction (TIMI) risk score than patients with mild or no calcification. The presence of severe calcification was associated with higher rates of myocardial infarction (22.5% vs. 13.8%, p = 0.03) and death (11.8% vs. 4.5%, p = 0.007) at 1 year following CABG [99, 107, 108]. In the Synergy between Percutaneous Coronary Intervention and Cardiac Surgery (SYNTAX) study, severe lesion calcification was present in 588 patients that underwent surgical revascularization. This cohort had a higher mortality rate (17.1% vs 9.9%, p < 0.001) at 5-year follow-up compared to patients without severe calcification. Furthermore, they had a higher rate of the composite endpoint of death and myocardial infarction (19.4% vs. 13.2%, p = 0.003) [109]. These 1-year mortality rates in patients with severe coronary calcification are substantially higher than those reported in other contemporary studies of CABG (3.5–4.2%), although patients with heavy calcification were more likely to be included in the registry arm of surgical trials, so the absolute mortality rate following CABG for this patient population may be higher.
Peripheral Arterial Disease and the Role of Calcification in Treatment Decision-Making
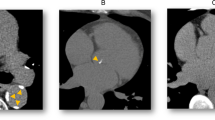
Vascular calcification is also prevalent in the peripheral vasculature although exact estimates vary (Fig. 21.4). In an unselected group of 650 patients that were asymptomatic and being evaluated for preventative care, 61% had evidence of atherosclerotic calcification in their coronary, carotid, aorta, or iliac vessels [110]. Calcification was associated with increased age, and in those patients that were ≥70 years, vascular calcification was present in all vascular beds. In a large cohort study that included 4291 individuals who were followed for a mean of 7.8 years, prevalence of calcification in the coronary, carotid, aorta, and iliac arteries ranged from 31% to 55%. Interestingly, while coronary artery calcification was associated with cardiovascular mortality, the presence of calcium in other major vascular beds, namely, the carotid, aorta, and iliac vessels, was associated with all-cause mortality [111].
Peripheral arterial calcification . Vascular calcification is highly prevalent in the peripheral arteries. Calcification can be seen with fluoroscopy (left panel). Following the injection of contrast, a significant calcified stenosis in the superficial femoral artery is seen (asterisk) (middle panel). After atherectomy, angioplasty, and stent placement, the vessel lumen is increased
Clinical decision-making for therapeutic interventions in patients with peripheral arterial is limited by the fact that many patients with severe calcification were excluded from clinical trials. Balloon angioplasty of calcified lesions doesn’t provide adequate lumen expansion with significant early elastic recoil, which contributes to observed poor outcomes [112]. There have been similar issues with nitinol stents in femoropopliteal vessels owing to high compressive forces exerted by rigid calcified plaques that prevent in full stent expansion [113]. However, newer nitinol stents with closed cell designs that allow for greater resistance to calcified plaque compression have been shown to have greater lumen preservation in severely calcified stenoses compared to traditional stents [114].
Atherectomy has been trialed in the peripheral vasculature with studies performed with varying degrees of procedural and early success [114,115,116,117,118,119]. The DEFINITIVE Ca++ registry evaluated the safety and efficacy of directional atherectomy to treat moderate to severely calcified femoropopliteal vessels. This trial enrolled 133 patients and found that directional atherectomy was safe and resulted in adequate debulking (<50% residual diameter) in 92% of the lesions treated with a 33% mean residual diameter stenosis. There was a 52.3% increase in the number of patients who were asymptomatic at the 30-day follow-up visit with 88.5% of patients showed clinical improvement [120].
Significant peripheral vascular calcification has also been implicated as a barrier to the efficacy of drug-coated balloons. In 60 patients with severe claudication or rest pain, the presence of circumferential calcium in the vessels was associated with a 50% increase in loss of patency. Although the mechanism for reduced efficacy wasn’t clear, it was hypothesized that this occurred as a result of the calcium, which acted as a barrier to the drug reaching or penetrating the vessel wall [121]. To improve patency rates, other investigators trialed debulking of severe vascular calcification using atherectomy prior to drug-coated balloon intervention. In a small 30-patient trial with severe claudication or threatened limbs where severe calcification was identified, debulking was associated with a 90% patency rate at 1 year [122]. The more recent Directional Atherectomy Followed by a Paclitaxel-Coated Balloon to Inhibit Restenosis and Maintain Vessel Patency – A Pilot Study of Anti-Restenosis Treatment (DEFINITIVE-AR) trial randomized patients to directional atherectomy and drug-coated balloon or drug-coated balloon alone. In this study, technical success was higher for the arm with directional atherectomy (89.6% vs 64.2%, p = 0.004). There was no difference, however, in 1-year percent diameter stenosis assessed by angiography or target lesion revascularization between the groups [117].
The thoracic and abdominal aortas are prone to aneurysm formation with abdominal aortic aneurysms affecting 4.8% of individuals with a preponderance in men [123]. Among 356 patients that underwent infrarenal abdominal aortic aneurysm repair, aortoiliac calcification was associated with mortality with patients who had high calcium scores had lower probability of 5-year survival [124]. Thoracic aneurysms tend to be more heterogeneous with both genetic and atherosclerotic etiologies. Endovascular interventions have decreased the short-term risks related to the intervention; however, they have not mitigated longer-term risk of cardiovascular events [125]. In a study that included 196 patients with abdominal aortic aneurysms where 85 patients had an endovascular repair and 18 had a surgical repair, aortic calcium score was related significantly to all-cause mortality (OR = 2.25; 95% CI: 1.59–9.47, p < 0.001) and predictive of cardiac mortality (OR = 1.32; 95% CI: 1.08–2.76, p = 0.003). With respect to thoracic aneurysms, 123 patients were studied with 75 patients undergoing endovascular repair. Here calcium score was also related to all-cause mortality (OR = 6.44; 95% CI: 2.57–6.14, p < 0.001) and cardiac mortality (OR = 3.46; 95% CI: 1.97–4.34, p = 0.002) [126]. Given the relationship between calcification and stent failure, it is not surprising that calcification is a noted risk factor for graft-related complications after endovascular aneurysm repair. Studies of patients who underwent endovascular repair revealed that calcification in the neck of the aneurysm and common iliac vessels was associated with increased risk for graft-related failure [127].
Calcification of the Mitral Valve
Calcification of the mitral annulus is reported in 8–15% of individuals without known coronary artery disease but increases with age and risk factors, such as chronic kidney disease (Fig. 21.5) [128,129,130,131,132]. When present, mitral annular calcification is associated with an increased risk of cardiovascular disease and mortality [133]. The Framingham Heart Study reported that there was an increased risk of incident cardiovascular disease (HR = 1.5; 95%CI: 1.1, 2.0) and death attributable to cardiovascular disease (HR = 1.6; 95% CI: 1.1, 2.3) in patients with mitral annular calcification [133]. Mitral annular calcification has also been linked to an increased risk for stroke, possibly due to atrial fibrillation or other conduction system abnormalities, which were found to be prevalent in only 34% of control subjects but 70% of patients with mitral annular calcification [30, 134]. Patients with mitral annular calcification were also shown to have a higher rate of intraventricular conduction delays, bundle branch block, and atrioventricular block [135, 136]. Atrial fibrillation is prevalent in patients with mitral annular calcification, and the Framingham Heart Study and MESA reported a hazard ratio of 1.5–1.9 for atrial fibrillation in their respective community-based populations [137, 138].
Mitral annular calcification . Calcification of the mitral annulus occurs frequently and is often seen by fluoroscopy at the time of routine cardiac catheterization (red arrows, left). Mitral annular calcification is also seen on echocardiography. Calcification appears as bright white areas at the edges of the mitral valve leaflets (below red asterisks). LA left atrium, LV left ventricle
Mitral annular calcification has implications for percutaneous and surgical interventions that involve the mitral valve. Among patients referred for mitral valve surgeries, mitral annular calcification is associated with injury to the left circumflex coronary artery and cardiac rupture when the valve requires debridement [139, 140]. Surgical approaches that have tried to avoid disrupting the calcium ring have led to perivalvular leakage or high gradients [140, 141]. Owing to this, the current approach is to decalcify the annulus via dissection [139]. Although mitral annular calcification was initially considered a contraindication to the percutaneous MitraClip procedure, case series have demonstrated that it is feasible and was not associated with a decrease in procedural success or durability of repair [142, 143].
In patients with mitral valve stenosis, valve calcification is recognized as a predictor of poor immediate and long-term clinical outcomes [144,145,146,147]. In fact, the Abascal-Wilkins echocardiography score , which predicts the success of percutaneous balloon mitral valvuloplasty for patients with mitral stenosis, includes valvular calcification in the scoring system with a score = 1 indicating a single area of calcification while a score = 4 indicating calcification is present throughout most of the valve leaflets. For overall scores >8, based on assessment of leaflet mobility, valve thickness, subvalvular thickening, and valvular calcification, surgery is preferred [148]. Nonetheless, a contemporary analysis of 1024 patients who underwent percutaneous balloon mitral valvuloplasty for mitral stenosis compared outcomes in patients with and without calcified valves. Immediate result post-procedure was classified as good for 80% of 314 patients with calcification as compared to 93% of 710 patients without calcified valves. Patients with calcified valves were also less likely to achieve good functional results, defined as New York Heart Association Class I or II and survival without reintervention or cardiovascular death. Thus, percutaneous balloon mitral valvuloplasty remains a frontline therapy for patients with calcific mitral stenosis [149]. More recently, investigators have moved forward with transcatheter mitral valve implantation for inoperable severely calcified mitral valves. In a small report of 11 patients, the procedural success rate was 73% and without paravalvular leaks [150]. Therefore, it is likely that in well-selected patients, this procedure may have benefit, but larger series, clinical trial, and outcome data is needed.
Clinical studies and decision-making regarding aortic valve calcification is reviewed in Chap. 22.
Conclusion
Calcification in the cardiovascular system is highly prevalent and associated with worse clinical outcomes. Advances in imaging technologies have allowed for more precise detection of calcified vessels, and CT scans are increasingly employed as a diagnostic modality to assess patient risk. In addition, novel blood assays to assess calcification potential are proving useful in high-risk patient populations, such as patients with chronic kidney disease, but the broad applicability of the test to other patient populations remains to be determined. Pharmacological therapeutics to prevent and/or regress calcification are under development or moving forward in early phase clinical trials, suggesting that it is likely that these agents will soon have a role in the management of patients with calcified vessels. For patients with symptomatic vascular disease that require percutaneous or surgical revascularization, it is clear that the presence of calcification is associated with higher risk and higher rates of major adverse cardiovascular events despite the introduction of newer devices and techniques. Thus, it remains imperative that promising new therapies and interventions continue to be trialed in more patients with cardiovascular calcification in randomized clinical trials to aid clinical decision-making.
References
Okwuosa TM, Greenland P, Burke GL, Eng J, Cushman M, Michos ED, et al. Prediction of coronary artery calcium progression in individuals with low Framingham Risk Score: the Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2012;5(2):144–53.
Wong ND, Kouwabunpat D, Vo AN, Detrano RC, Eisenberg H, Goel M, et al. Coronary calcium and atherosclerosis by ultrafast computed tomography in asymptomatic men and women: relation to age and risk factors. Am Heart J. 1994;127(2):422–30.
Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–45.
Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111(10):1313–20.
DeFilippis AP, Blaha MJ, Ndumele CE, Budoff MJ, Lloyd-Jones DM, McClelland RL, et al. The association of Framingham and Reynolds risk scores with incidence and progression of coronary artery calcification in MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2011;58(20):2076–83.
Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185–97.
World Health Organization. Fact sheets cardiovascular diseases. who.int. 2020.
CDC Foundation. Heart disease and stroke cost America nearly $1 billion a day in medical costs, lost productivity. cdcfoundation.org. 2015.
Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81.
Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study). Am J Cardiol. 2008;102(9):1136–41, 41.e1.
Oei HH, Vliegenthart R, Hak AE, Iglesias del Sol A, Hofman A, Oudkerk M, et al. The association between coronary calcification assessed by electron beam computed tomography and measures of extracoronary atherosclerosis: the Rotterdam Coronary Calcification Study. J Am Coll Cardiol. 2002;39(11):1745–51.
Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434–47.
Schmermund A, Mohlenkamp S, Stang A, Gronemeyer D, Seibel R, Hirche H, et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Risk Factors, Evaluation of Coronary Calcium and Lifestyle. Am Heart J. 2002;144(2):212–8.
Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291(2):210–5.
Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary artery calcium to predict all-cause mortality in elderly men and women. J Am Coll Cardiol. 2008;52(1):17–23.
Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43(9):1663–9.
Shaw LJ, Raggi P, Berman DS, Callister TQ. Coronary artery calcium as a measure of biologic age. Atherosclerosis. 2006;188(1):112–9.
McEvoy JW, Blaha MJ, Nasir K, Blumenthal RS, Jones SR. Potential use of coronary artery calcium progression to guide the management of patients at risk for coronary artery disease events. Curr Treat Options Cardiovasc Med. 2012;14(1):69–80.
Yano Y, O’Donnell CJ, Kuller L, Kavousi M, Erbel R, Ning H, et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol. 2017;2(9):986–94.
Ferencik M, Pencina KM, Liu T, Ghemigian K, Baltrusaitis K, Massaro JM, et al. Coronary artery calcium distribution is an independent predictor of incident major coronary heart disease events: results from the Framingham Heart Study. Circ Cardiovasc Imaging. 2017;10(10):e006592.
Carr JJ, Jacobs DR Jr, Terry JG, Shay CM, Sidney S, Liu K, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol. 2017;2(4):391–9.
Sung JH, Yeboah J, Lee JE, Smith CL, Terry JG, Sims M, et al. Diagnostic value of coronary artery calcium score for cardiovascular disease in African Americans: the Jackson Heart Study. Br J Med Med Res. 2016;11(2). pii: BJMMR/2016/21449. Epub 2015 Sep 21.
Shah RV, Yeri AS, Murthy VL, Massaro JM, D’Agostino R Sr, Freedman JE, et al. Association of multiorgan computed tomographic phenomap with adverse cardiovascular health outcomes: the Framingham Heart Study. JAMA Cardiol. 2017;2(11):1236–46.
Manson JE, Allison MA, Carr JJ, Langer RD, Cochrane BB, Hendrix SL, et al. Calcium/vitamin D supplementation and coronary artery calcification in the Women’s Health Initiative. Menopause. 2010;17(4):683–91.
Williams MC, Moss AJ, Dweck M, Adamson PD, Alam S, Hunter A, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol. 2019;73(3):291–301.
McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66(15):1643–53.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140(11):e563–e95.
Pasch A, Farese S, Graber S, Wald J, Richtering W, Floege J, et al. Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol. 2012;23(10):1744–52.
Smith ER, Ford ML, Tomlinson LA, Bodenham E, McMahon LP, Farese S, et al. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J Am Soc Nephrol. 2014;25(2):339–48.
Keyzer CA, de Borst MH, van den Berg E, Jahnen-Dechent W, Arampatzis S, Farese S, et al. Calcification propensity and survival among renal transplant recipients. J Am Soc Nephrol. 2016;27(1):239–48.
Pasch A, Block GA, Bachtler M, Smith ER, Jahnen-Dechent W, Arampatzis S, et al. Blood calcification propensity, cardiovascular events, and survival in patients receiving hemodialysis in the EVOLVE trial. Clin J Am Soc Nephrol. 2017;12(2):315–22.
Thompson S, James M, Wiebe N, Hemmelgarn B, Manns B, Klarenbach S, et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26(10):2504–11.
Bundy JD, Cai X, Mehta RC, Scialla JJ, de Boer IH, Hsu CY, et al. Serum calcification propensity and clinical events in CKD. Clin J Am Soc Nephrol. 2019;14(11):1562–71.
Bundy JD, Cai X, Scialla JJ, Dobre MA, Chen J, Hsu CY, et al. Serum calcification propensity and coronary artery calcification among patients with CKD: the CRIC (chronic renal insufficiency cohort) study. Am J Kidney Dis. 2019;73(6):806–14.
Lorenz G, Steubl D, Kemmner S, Pasch A, Koch-Sembdner W, Pham D, et al. Worsening calcification propensity precedes all-cause and cardiovascular mortality in haemodialyzed patients. Sci Rep. 2017;7(1):13368.
Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, et al. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010;5(4):568–75.
Holden RM, Morton AR, Garland JS, Pavlov A, Day AG, Booth SL. Vitamins K and D status in stages 3–5 chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(4):590–7.
Nigwekar SU, Bloch DB, Nazarian RM, Vermeer C, Booth SL, Xu D, et al. Vitamin K-dependent carboxylation of matrix Gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28(6):1717–22.
Julius JK, Fernandez CK, Grafa AC, Rosa PM, Hartos JL. Daily fruit and vegetable consumption and diabetes status in middle-aged females in the general US population. SAGE Open Med. 2019;7:2050312119865116.
Andrews J, Psaltis PJ, Bayturan O, Shao M, Stegman B, Elshazly M, et al. Warfarin use is associated with progressive coronary arterial calcification: insights from serial intravascular ultrasound. JACC Cardiovasc Imaging. 2018;11(9):1315–23.
Han KH, O’Neill WC. Increased peripheral arterial calcification in patients receiving warfarin. J Am Heart Assoc. 2016;5(1). pii: e002665. https://doi.org/10.1161/JAHA.115.002665.
Win TT, Nakanishi R, Osawa K, Li D, Susaria SS, Jayawardena E, et al. Apixaban versus warfarin in evaluation of progression of atherosclerotic and calcified plaques (prospective randomized trial). Am Heart J. 2019;212:129–33.
Lees JS, Chapman FA, Witham MD, Jardine AG, Mark PB. Vitamin K status, supplementation and vascular disease: a systematic review and meta-analysis. Heart. 2019;105(12):938–45.
Zwakenberg SR, de Jong PA, Bartstra JW, van Asperen R, Westerink J, de Valk H, et al. The effect of menaquinone-7 supplementation on vascular calcification in patients with diabetes: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2019;110(4):883–90.
Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol. 1992;32(4):368–75.
Adirekkiat S, Sumethkul V, Ingsathit A, Domrongkitchaiporn S, Phakdeekitcharoen B, Kantachuvesiri S, et al. Sodium thiosulfate delays the progression of coronary artery calcification in haemodialysis patients. Nephrol Dial Transplant. 2010;25(6):1923–9.
Mathews SJ, de Las Fuentes L, Podaralla P, Cabellon A, Zheng S, Bierhals A, et al. Effects of sodium thiosulfate on vascular calcification in end-stage renal disease: a pilot study of feasibility, safety and efficacy. Am J Nephrol. 2011;33(2):131–8.
Hill JA, Goldin JG, Gjertson D, Emerick AM, Greaser LD, Yoon HC, et al. Progression of coronary artery calcification in patients taking alendronate for osteoporosis. Acad Radiol. 2002;9(10):1148–52.
Tanko LB, Qin G, Alexandersen P, Bagger YZ, Christiansen C. Effective doses of ibandronate do not influence the 3-year progression of aortic calcification in elderly osteoporotic women. Osteoporos Int. 2005;16(2):184–90.
Toussaint ND, Elder GJ, Kerr PG. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol. 2009;4(1):221–33.
Hashiba H, Aizawa S, Tamura K, Kogo H. Inhibition of the progression of aortic calcification by etidronate treatment in hemodialysis patients: long-term effects. Ther Apher Dial. 2006;10(1):59–64.
Ariyoshi T, Eishi K, Sakamoto I, Matsukuma S, Odate T. Effect of etidronic acid on arterial calcification in dialysis patients. Clin Drug Investig. 2006;26(4):215–22.
Kranenburg G, de Jong PA, Bartstra JW, Lagerweij SJ, Lam MG, Ossewaarde-van Norel J, et al. Etidronate for prevention of ectopic mineralization in patients with pseudoxanthoma elasticum. J Am Coll Cardiol. 2018;71(10):1117–26.
Schantl AE, Ivarsson ME, Leroux J-C. Investigational pharmacological treatments for vascular calcification. Adv Ther. 2019;2:1800094.
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22.
Massy ZA, Drueke TB. Magnesium and cardiovascular complications of chronic kidney disease. Nat Rev Nephrol. 2015;11(7):432–42.
Kanbay M, Yilmaz MI, Apetrii M, Saglam M, Yaman H, Unal HU, et al. Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am J Nephrol. 2012;36(3):228–37.
Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014;85(1):174–81.
Molnar AO, Biyani M, Hammond I, Harmon JP, Lavoie S, McCormick B, et al. Lower serum magnesium is associated with vascular calcification in peritoneal dialysis patients: a cross sectional study. BMC Nephrol. 2017;18(1):129.
Ishimura E, Okuno S, Yamakawa T, Inaba M, Nishizawa Y. Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnes Res. 2007;20(4):237–44.
Spiegel DM, Farmer B. Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: a pilot study. Hemodial Int. 2009;13(4):453–9.
Tzanakis IP, Stamataki EE, Papadaki AN, Giannakis N, Damianakis NE, Oreopoulos DG. Magnesium retards the progress of the arterial calcifications in hemodialysis patients: a pilot study. Int Urol Nephrol. 2014;46(11):2199–205.
Bressendorff I, Hansen D, Schou M, Silver B, Pasch A, Bouchelouche P, et al. Oral magnesium supplementation in chronic kidney disease stages 3 and 4: efficacy, safety, and effect on serum calcification propensity-a prospective randomized double-blinded placebo-controlled clinical trial. Kidney Int Rep. 2017;2(3):380–9.
Bressendorff I, Hansen D, Schou M, Kragelund C, Brandi L. The effect of magnesium supplementation on vascular calcification in chronic kidney disease-a randomised clinical trial (MAGiCAL-CKD): essential study design and rationale. BMJ Open. 2017;7(6):e016795.
Perello J, Gomez M, Ferrer MD, Rodriguez NY, Salcedo C, Buades JM, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31(2):287–96.
Perello J, Joubert PH, Ferrer MD, Canals AZ, Sinha S, Salcedo C. First-time-in-human randomized clinical trial in healthy volunteers and haemodialysis patients with SNF472, a novel inhibitor of vascular calcification. Br J Clin Pharmacol. 2018;84(12):2867–76.
Raggi P, Bellasi A, Bushinsky D, Bover J, Rodriguez M, Ketteler M, et al. Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis: results of a randomized, phase 2b study. Circulation. 2020;141:728–39.
Samelson EJ, Miller PD, Christiansen C, Daizadeh NS, Grazette L, Anthony MS, et al. RANKL inhibition with denosumab does not influence 3-year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular risk. J Bone Miner Res. 2014;29(2):450–7.
Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91(7):1959–65.
Mehanna E, Bezerra HG, Prabhu D, Brandt E, Chamie D, Yamamoto H, et al. Volumetric characterization of human coronary calcification by frequency-domain optical coherence tomography. Circ J. 2013;77(9):2334–40.
De Maria GL, Scarsini R, Banning AP. Management of calcific coronary artery lesions: is it time to change our interventional therapeutic approach? JACC Cardiovasc Interv. 2019;12(15):1465–78.
Wang X, Matsumura M, Mintz GS, Lee T, Zhang W, Cao Y, et al. In vivo calcium detection by comparing optical coherence tomography, intravascular ultrasound, and angiography. JACC Cardiovasc Imaging. 2017;10(8):869–79.
Bourantas CV, Zhang YJ, Garg S, Iqbal J, Valgimigli M, Windecker S, et al. Prognostic implications of coronary calcification in patients with obstructive coronary artery disease treated by percutaneous coronary intervention: a patient-level pooled analysis of 7 contemporary stent trials. Heart. 2014;100(15):1158–64.
Onuma Y, Tanimoto S, Ruygrok P, Neuzner J, Piek JJ, Seth A, et al. Efficacy of everolimus eluting stent implantation in patients with calcified coronary culprit lesions: two-year angiographic and three-year clinical results from the SPIRIT II study. Catheter Cardiovasc Interv. 2010;76(5):634–42.
Copeland-Halperin RS, Baber U, Aquino M, Rajamanickam A, Roy S, Hasan C, et al. Prevalence, correlates, and impact of coronary calcification on adverse events following PCI with newer-generation DES: findings from a large multiethnic registry. Catheter Cardiovasc Interv. 2018;91(5):859–66.
Genereux P, Madhavan MV, Mintz GS, Maehara A, Kirtane AJ, Palmerini T, et al. Relation between coronary calcium and major bleeding after percutaneous coronary intervention in acute coronary syndromes (from the Acute Catheterization and Urgent Intervention Triage Strategy and Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction Trials). Am J Cardiol. 2014;113(6):930–5.
Karacsonyi J, Karmpaliotis D, Alaswad K, Jaffer FA, Yeh RW, Patel M, et al. Impact of calcium on chronic total occlusion percutaneous coronary interventions. Am J Cardiol. 2017;120(1):40–6.
Genereux P, Redfors B, Witzenbichler B, Arsenault MP, Weisz G, Stuckey TD, et al. Two-year outcomes after percutaneous coronary intervention of calcified lesions with drug-eluting stents. Int J Cardiol. 2017;231:61–7.
Shavadia JS, Vo MN, Bainey KR. Challenges with severe coronary artery calcification in percutaneous coronary intervention: a narrative review of therapeutic options. Can J Cardiol. 2018;34(12):1564–72.
Mintz GS, Potkin BN, Keren G, Satler LF, Pichard AD, Kent KM, et al. Intravascular ultrasound evaluation of the effect of rotational atherectomy in obstructive atherosclerotic coronary artery disease. Circulation. 1992;86(5):1383–93.
Gupta T, Weinreich M, Greenberg M, Colombo A, Latib A. Rotational atherectomy: a contemporary appraisal. Interv Cardiol. 2019;14(3):182–9.
Farb A, Roberts DK, Pichard AD, Kent KM, Virmani R. Coronary artery morphologic features after coronary rotational atherectomy: insights into mechanisms of lumen enlargement and embolization. Am Heart J. 1995;129(6):1058–67.
Bittl JA, Chew DP, Topol EJ, Kong DF, Califf RM. Meta-analysis of randomized trials of percutaneous transluminal coronary angioplasty versus atherectomy, cutting balloon atherotomy, or laser angioplasty. J Am Coll Cardiol. 2004;43(6):936–42.
Wasiak J, Law J, Watson P, Spinks A. Percutaneous transluminal rotational atherectomy for coronary artery disease. Cochrane Database Syst Rev. 2012;12:CD003334.
Abdel-Wahab M, Baev R, Dieker P, Kassner G, Khattab AA, Toelg R, et al. Long-term clinical outcome of rotational atherectomy followed by drug-eluting stent implantation in complex calcified coronary lesions. Catheter Cardiovasc Interv. 2013;81(2):285–91.
Abdel-Wahab M, Toelg R, Byrne RA, Geist V, El-Mawardy M, Allali A, et al. High-speed rotational atherectomy versus modified balloons prior to drug-eluting stent implantation in severely calcified coronary lesions. Circ Cardiovasc Interv. 2018;11(10):e007415.
Chambers JW, Feldman RL, Himmelstein SI, Bhatheja R, Villa AE, Strickman NE, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7(5):510–8.
Lee MS, Shlofmitz E, Park KW, Goldberg A, Jeremias A, Shlofmitz R. Orbital atherectomy of severely calcified unprotected left main coronary artery disease: one-year outcomes. J Invasive Cardiol. 2018;30(7):270–4.
Bilodeau L, Fretz EB, Taeymans Y, Koolen J, Taylor K, Hilton DJ. Novel use of a high-energy excimer laser catheter for calcified and complex coronary artery lesions. Catheter Cardiovasc Interv. 2004;62(2):155–61.
Bittl JA. Excimer laser angioplasty: focus on total occlusions. Am J Cardiol. 1996;78(7):823–4.
Mintz GS, Kovach JA, Javier SP, Pichard AD, Kent KM, Popma JJ, et al. Mechanisms of lumen enlargement after excimer laser coronary angioplasty. An intravascular ultrasound study. Circulation. 1995;92(12):3408–14.
Latib A, Takagi K, Chizzola G, Tobis J, Ambrosini V, Niccoli G, et al. Excimer Laser LEsion modification to expand non-dilatable stents: the ELLEMENT registry. Cardiovasc Revasc Med. 2014;15(1):8–12.
Lee T, Shlofmitz RA, Song L, Tsiamtsiouris T, Pappas T, Madrid A, et al. The effectiveness of excimer laser angioplasty to treat coronary in-stent restenosis with peri-stent calcium as assessed by optical coherence tomography. EuroIntervention. 2019;15(3):e279–e88.
Ali ZA, Brinton TJ, Hill JM, Maehara A, Matsumura M, Karimi Galougahi K, et al. Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions: first description. JACC Cardiovasc Imaging. 2017;10(8):897–906.
Ali ZA, Nef H, Escaned J, Werner N, Banning AP, Hill JM, et al. Safety and effectiveness of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: the disrupt CAD II study. Circ Cardiovasc Interv. 2019;12(10):e008434.
Osswald BR, Blackstone EH, Tochtermann U, Schweiger P, Thomas G, Vahl CF, et al. Does the completeness of revascularization affect early survival after coronary artery bypass grafting in elderly patients? Eur J Cardiothorac Surg. 2001;20(1):120–5; discussion 5–6.
Nakayama Y, Sakata R, Ura M, Miyamoto TA. Coronary artery bypass grafting in dialysis patients. Ann Thorac Surg. 1999;68(4):1257–61.
Castagna MT, Mintz GS, Ohlmann P, Kotani J, Maehara A, Gevorkian N, et al. Incidence, location, magnitude, and clinical correlates of saphenous vein graft calcification: an intravascular ultrasound and angiographic study. Circulation. 2005;111(9):1148–52.
Ertelt K, Genereux P, Mintz GS, Reiss GR, Kirtane AJ, Madhavan MV, et al. Impact of the severity of coronary artery calcification on clinical events in patients undergoing coronary artery bypass grafting (from the Acute Catheterization and Urgent Intervention Triage Strategy Trial). Am J Cardiol. 2013;112(11):1730–7.
Genereux P, Palmerini T, Caixeta A, Rosner G, Green P, Dressler O, et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol. 2012;59(24):2165–74.
Rosner GF, Kirtane AJ, Genereux P, Lansky AJ, Cristea E, Gersh BJ, et al. Impact of the presence and extent of incomplete angiographic revascularization after percutaneous coronary intervention in acute coronary syndromes: the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. Circulation. 2012;125(21):2613–20.
Yamaguchi A, Adachi H, Tanaka M, Ino T. Efficacy of intraoperative epiaortic ultrasound scanning for preventing stroke after coronary artery bypass surgery. Ann Thorac Cardiovasc Surg. 2009;15(2):98–104.
Yamauchi T, Miyata H, Sakaguchi T, Miyagawa S, Yoshikawa Y, Takeda K, et al. Coronary artery bypass grafting in hemodialysis-dependent patients: analysis of Japan Adult Cardiovascular Surgery Database. Circ J. 2012;76(5):1115–20.
Liu JY, Birkmeyer NJ, Sanders JH, Morton JR, Henriques HF, Lahey SJ, et al. Risks of morbidity and mortality in dialysis patients undergoing coronary artery bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 2000;102(24):2973–7.
Chang TI, Shilane D, Kazi DS, Montez-Rath ME, Hlatky MA, Winkelmayer WC. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol. 2012;23(12):2042–9.
Afsar B, Turkmen K, Covic A, Kanbay M. An update on coronary artery disease and chronic kidney disease. Int J Nephrol. 2014;2014:767424.
Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–72.
Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375–84.
Bourantas CV, Zhang YJ, Garg S, Mack M, Dawkins KD, Kappetein AP, et al. Prognostic implications of severe coronary calcification in patients undergoing coronary artery bypass surgery: an analysis of the SYNTAX study. Catheter Cardiovasc Interv. 2015;85(2):199–206.
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–654.
Rifkin DE, Ix JH, Wassel CL, Criqui MH, Allison MA. Renal artery calcification and mortality among clinically asymptomatic adults. J Am Coll Cardiol. 2012;60(12):1079–85.
Capek P, McLean GK, Berkowitz HD. Femoropopliteal angioplasty. Factors influencing long-term success. Circulation. 1991;83(2 Suppl):I70–80.
Bausback Y, Botsios S, Flux J, Werner M, Schuster J, Aithal J, et al. Outback catheter for femoropopliteal occlusions: immediate and long-term results. J Endovasc Ther. 2011;18(1):13–21.
Scheinert D, Grummt L, Piorkowski M, Sax J, Scheinert S, Ulrich M, et al. A novel self-expanding interwoven nitinol stent for complex femoropopliteal lesions: 24-month results of the SUPERA SFA registry. J Endovasc Ther. 2011;18(6):745–52.
Das T, Mustapha J, Indes J, Vorhies R, Beasley R, Doshi N, et al. Technique optimization of orbital atherectomy in calcified peripheral lesions of the lower extremities: the CONFIRM series, a prospective multicenter registry. Catheter Cardiovasc Interv. 2014;83(1):115–22.
Kim JH, Ahanchi SS, Panneton JM. Identifying the target lesions for 245 laser angioplasty cases and a review of the literature. Vasc Endovasc Surg. 2012;46(8):640–7.
Zeller T, Langhoff R, Rocha-Singh KJ, Jaff MR, Blessing E, Amann-Vesti B, et al. Directional atherectomy followed by a paclitaxel-coated balloon to inhibit restenosis and maintain vessel patency: twelve-month results of the DEFINITIVE AR study. Circ Cardiovasc Interv. 2017;10(9):e004848.
Shrikhande GV, McKinsey JF. Use and abuse of atherectomy: where should it be used? Semin Vasc Surg. 2008;21(4):204–9.
Todd KE Jr, Ahanchi SS, Maurer CA, Kim JH, Chipman CR, Panneton JM. Atherectomy offers no benefits over balloon angioplasty in tibial interventions for critical limb ischemia. J Vasc Surg. 2013;58(4):941–8.
Roberts D, Niazi K, Miller W, Krishnan P, Gammon R, Schreiber T, et al. Effective endovascular treatment of calcified femoropopliteal disease with directional atherectomy and distal embolic protection: final results of the DEFINITIVE Ca(+)(+) trial. Catheter Cardiovasc Interv. 2014;84(2):236–44.
Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv. 2014;83(6):E212–20.
Cioppa A, Stabile E, Popusoi G, Salemme L, Cota L, Pucciarelli A, et al. Combined treatment of heavy calcified femoro-popliteal lesions using directional atherectomy and a paclitaxel coated balloon: one-year single centre clinical results. Cardiovasc Revasc Med. 2012;13(4):219–23.
Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population--a meta-analysis. PLoS One. 2013;8(12):e81260.
TerBush MJ, Rasheed K, Young ZZ, Ellis JL, Glocker RJ, Doyle AJ, et al. Aortoiliac calcification correlates with 5-year survival after abdominal aortic aneurysm repair. J Vasc Surg. 2019;69(3):774–82.
Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT Jr, Matsumura JS, Kohler TR, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302(14):1535–42.
Chowdhury MM, Zielinski LP, Sun JJ, Lambracos S, Boyle JR, Harrison SC, et al. Editor’s choice – calcification of thoracic and abdominal aneurysms is associated with mortality and morbidity. Eur J Vasc Endovasc Surg. 2018;55(1):101–8.
Wyss TR, Dick F, Brown LC, Greenhalgh RM. The influence of thrombus, calcification, angulation, and tortuosity of attachment sites on the time to the first graft-related complication after endovascular aneurysm repair. J Vasc Surg. 2011;54(4):965–71.
Fertman MH, Wolff L. Calcification of the mitral valve. Am Heart J. 1946;31:580–9.
Allison MA, Cheung P, Criqui MH, Langer RD, Wright CM. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation. 2006;113(6):861–6.
Kanjanauthai S, Nasir K, Katz R, Rivera JJ, Takasu J, Blumenthal RS, et al. Relationships of mitral annular calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2010;213(2):558–62.
Asselbergs FW, Mozaffarian D, Katz R, Kestenbaum B, Fried LF, Gottdiener JS, et al. Association of renal function with cardiac calcifications in older adults: the cardiovascular health study. Nephrol Dial Transplant. 2009;24(3):834–40.
Foley PW, Hamaad A, El-Gendi H, Leyva F. Incidental cardiac findings on computed tomography imaging of the thorax. BMC Res Notes. 2010;3:326.
Fox CS, Vasan RS, Parise H, Levy D, O’Donnell CJ, D’Agostino RB, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107(11):1492–6.
Nair CK, Runco V, Everson GT, Boghairi A, Mooss AN, Mohiuddin SM, et al. Conduction defects and mitral annulus calcification. Br Heart J. 1980;44(2):162–7.
Nestico PF, Depace NL, Morganroth J, Kotler MN, Ross J. Mitral annular calcification: clinical, pathophysiology, and echocardiographic review. Am Heart J. 1984;107(5 Pt 1):989–96.
Fulkerson PK, Beaver BM, Auseon JC, Graber HL. Calcification of the mitral annulus: etiology, clinical associations, complications and therapy. Am J Med. 1979;66(6):967–77.
O’Neal WT, Efird JT, Nazarian S, Alonso A, Heckbert SR, Soliman EZ. Mitral annular calcification and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis. Europace. 2015;17(3):358–63.
Fox CS, Parise H, Vasan RS, Levy D, O’Donnell CJ, D’Agostino RB, et al. Mitral annular calcification is a predictor for incident atrial fibrillation. Atherosclerosis. 2004;173(2):291–4.
Carpentier AF, Pellerin M, Fuzellier JF, Relland JY. Extensive calcification of the mitral valve anulus: pathology and surgical management. J Thorac Cardiovasc Surg. 1996;111(4):718–29; discussion 29–30.
Okada Y. Surgical management of mitral annular calcification. Gen Thorac Cardiovasc Surg. 2013;61(11):619–25.
Maisano F, Caldarola A, Blasio A, De Bonis M, La Canna G, Alfieri O. Midterm results of edge-to-edge mitral valve repair without annuloplasty. J Thorac Cardiovasc Surg. 2003;126(6):1987–97.
Bilge M, Ali S, Alsancak Y, Yasar AS. Percutaneous mitral valve repair with the MitraClip system in mitral regurgitation due to mitral annular calcification. J Heart Valve Dis. 2015;24(3):316–9.
Cheng R, Tat E, Siegel RJ, Arsanjani R, Hussaini A, Makar M, et al. Mitral annular calcification is not associated with decreased procedural success, durability of repair, or left ventricular remodelling in percutaneous edge-to-edge repair of mitral regurgitation. EuroIntervention. 2016;12(9):1176–84.
Palacios IF, Block PC, Wilkins GT, Weyman AE. Follow-up of patients undergoing percutaneous mitral balloon valvotomy. Analysis of factors determining restenosis. Circulation. 1989;79(3):573–9.
Iung B, Cormier B, Ducimetiere P, Porte JM, Nallet O, Michel PL, et al. Immediate results of percutaneous mitral commissurotomy. A predictive model on a series of 1514 patients. Circulation. 1996;94(9):2124–30.
Meneveau N, Schiele F, Seronde MF, Breton V, Gupta S, Bernard Y, et al. Predictors of event-free survival after percutaneous mitral commissurotomy. Heart. 1998;80(4):359–64.
Bouleti C, Iung B, Laouenan C, Himbert D, Brochet E, Messika-Zeitoun D, et al. Late results of percutaneous mitral commissurotomy up to 20 years: development and validation of a risk score predicting late functional results from a series of 912 patients. Circulation. 2012;125(17):2119–27.
Abascal VM, Wilkins GT, O’Shea JP, Choong CY, Palacios IF, Thomas JD, et al. Prediction of successful outcome in 130 patients undergoing percutaneous balloon mitral valvotomy. Circulation. 1990;82(2):448–56.
Bouleti C, Iung B, Himbert D, Messika-Zeitoun D, Brochet E, Garbarz E, et al. Relationship between valve calcification and long-term results of percutaneous mitral commissurotomy for rheumatic mitral stenosis. Circ Cardiovasc Interv. 2014;7(3):381–9.
Puri R, Abdul-Jawad Altisent O, del Trigo M, Campelo-Parada F, Regueiro A, Barbosa Ribeiro H, et al. Transcatheter mitral valve implantation for inoperable severely calcified native mitral valve disease: a systematic review. Catheter Cardiovasc Interv. 2016;87(3):540–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Leopold, J.A. (2020). Clinical Trials and Calcification-Based Treatment Decisions. In: Aikawa, E., Hutcheson, J. (eds) Cardiovascular Calcification and Bone Mineralization. Contemporary Cardiology. Humana, Cham. https://doi.org/10.1007/978-3-030-46725-8_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-46725-8_21
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-46724-1
Online ISBN: 978-3-030-46725-8
eBook Packages: MedicineMedicine (R0)