Abstract
Mitral annular calcification (MAC) is a fibrous, degenerative calcification of the mitral valve supporting ring and is common in elderly patients. Patients with MAC are older, more likely to be female, and more likely to have higher systolic blood pressure and clinical heart valve disease. MAC is also common among dialysis patients. The calcified annular bar poses demanding technical problems for the surgeon attempting mitral valve surgery in these high risk patients. In operation, MAC is a contributing factor in cardiac rupture at the atrial ventricular junction and rupture of the left ventricular free wall. Since we do not have simple and reproducible technical resolutions of mitral valve surgery in patients with MAC, mitral valve surgery in this condition is always challenging to prevent left ventricular rupture and periprosthetic leakage. This article reviews the history, details of relevant surgical techniques, and results of mitral valve surgery associated with MAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
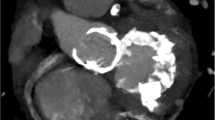
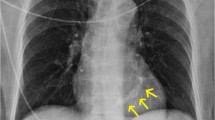
Mitral annular calcification (MAC) is a fibrous, degenerative calcification of the mitral valve supporting ring and is common in elderly patients. As the population ages, MAC will be encountered with increasing frequency. Prevalence of MAC is strongly associated with female gender and increasing age and is independently associated with the incidents of cardiovascular disease and cardiovascular death [1–3]. An extensive MAC prevents systolic contraction of the atrioventricular annulus. MAC may involve the basal portion of the valve leaflets and cause distortion of the posterior mitral leaflet limiting its movement. This may result in mitral regurgitation. MAC is also prevalent and often severe in patients with chronic kidney disease [4]. Jesri et al. [5] reported that there were two types of MAC (mobile & restricted) according to the mobility of the anterior mitral valve in patients with chronic kidney disease. In patients with “MAC restricted” group, kidney function was significantly lower than in patients with “MAC mobile” group. Therefore, mitral valve replacement for mitral stenosis may be required in this patients group. Extensive MAC is encountered either in elderly patients with fibroelastic deficiency (FED) of the mitral valve or in younger adults with a billowing mitral valve. The mitral valve procedure is largely divided into two groups which include mitral regurgitation with MAC and mitral stenosis with MAC. Extensive calcification of the mitral annulus may present a formidable surgical challenge during mitral valve surgery. The patient is at risk from such potentially fatal complications as intractable hemorrhage, atrioventricular disruption, and ventricular rupture. Therefore, it is not surprising that a variety of different surgical techniques has been used to approach this difficult problem with varying results. Figure 1 shows chest X-ray picture and plain computer tomography of 73 year-old female who developed congestive heart failure due to severe mitral regurgitation associated with atrial fibrillation and tricuspid regurgitation. Echocardiographic data are shown in Fig. 2. Which procedure for severe mitral regurgitation associated with MAC is recommended for this old lady?
Materials and methods
A search of PubMed database was conducted for articles from September 1977 to April 2012. Pertinent articles were selected on the basis of the following keywords: MAC, mitral valve replacement, mitral valve repair, and mitral annular reconstruction. Articles containing information on the pathophysiology and epidemiology of MAC were included.
Historical review of fatal complications of mitral valve replacement
In the early era of mitral valve replacement for mitral valve disease, Roberts [6] reported the causes of postoperative deaths. They noted left ventricular perforation in 2 patients. MacVaugh and associates [7] reported unusual complications during mitral valve replacement in the presence of calcification of the annulus in 1971. Of 253 mitral valve replacements between November 1961 and November 1969, 10 patients developed complications at the posterior interatrial groove, generally in association with dense calcification of the posterior leaflet and annulus. Three of the 10 patients had fatal acute posterior myocardial infarction, and 1 had a myocardial infarction with chronic aneurysm formation. Four patients developed operative hemorrhage from the left ventricle, and 1 had delayed hemorrhage from the ventricle. In one patient a subacute aneurysm of the left ventricle developed. The authors noted that all complications resulted either from too radical an excision of the calcified valve and annulus or from too deep a placement of the valve sutures. Spencer and associates [8] reported that debridement of calcified mitral annulus at the time of valve replacement developed left ventricular rupture in 3 cases. Excessive removal of a calcified annulus can easily create a perforation in the atrioventricular groove.
Therefore, a variety of surgical procedures has been proposed to avoid left ventricular rupture and periprosthetic leakage for patients who required mitral valve surgery for mitral valve disease associated with MAC.
Mitral valve replacement without annulus reconstruction
Cammack and associates [9] described 11 septuagenarians who had nonrheumatic bar calcification of the mitral annulus and open mitral valve operations. Five patients had mixed mitral stenosis and regurgitation, one had pure stenosis, and five had pure regurgitation. The bar of calcium was disturbed or debrided in 7 patients and partially debrided in 4 patients. Polyester (0) mattress sutures buttressed with Teflon felt pledgets were passed from the atrium over, through, and sometimes under the calcium bar to anchor the prosthetic valve or ring. Left ventricular rupture was the cause in 2 patients of the 4 hospital deaths. They noticed that the presence of bar calcification of the posterior mitral annulus was an independent risk factor of mitral valve operation in elderly patients.
Nataf and associates [10] proposed intraatrial insertion of a mitral prosthesis in a destroyed or calcified mitral annulus. Mitral valve replacement was performed in 16 patients with MAC. In cases of extensive annular calcification, leaflets were only removed. The intraatrial prosthesis was inserted with a series of 2-0 mattress sutures placed in the left atrial wall approximately 0.5 to 1.5 cm lateral to the mitral annulus. This implantation was secured by running 3-0 suture along the free edge of the Dacron collar. Among the 16 patients with MAC, 5 died in the perioperative period (23.8 %). Intractable bleeding resulting from tearing of the very thin and fragile left atrial wall was the cause of death in one patient. Perivalvular leak was found in one patient. Reoperation at late follow-up period showed fibrous ingrowth with complete endothelialization of the Dacron collar. Atoui also reported intra-atrial implantation of mitral valve prosthesis [11]. Cosseli described a technique that circumvents many of the problems associated with valve replacement under this condition by leaving the valvular apparatus intact and using a suture technique that incorporates the leaflet tissue to secure the prosthesis [12]. Di Stefano and associates described a new technique that allowed the intra-annular placement of the prosthesis, creating a new annulus by placating both mitral leaflets and the atrial wall [13]. They applied this new technique for 4 elderly females who required mitral valve surgery. After a mean follow-up of 13.5 months, all 4 patients were in NYHA functional class I and the prostheses were functioning normally without paravalvular leakage or dehiscence. The limitation of these techniques is the fact that one has to implant a prosthesis inside a calcified annulus. Therefore, given the reduction of the annular diameter, smaller sized prostheses are needed. They commented that this type of reconstruction can be performed safely when mitral valve replacement is impossible by means of conventional techniques because of severe MAC, and when it is feasible with minimum risk of technical complications. Fukada et al. [14] reported a case of collar-reinforced prosthetic valve for heavily calcified mitral annulus. They made 2-0 polyester pledgeted mattress sutures placed through the posterior leaflet from the ventricle without excision of the calcium bar. And the sewing cuff was reinforced by pericardial collar. Iida et al. [15] reported partial debridement of MAC and collar-reinforced mechanical valve replacement in two dialysis patients successfully. In these conditions, shape of calcium bar and size selection of a prosthesis are essential to avoid periprosthetic leak late after surgery.
Mitral valve procedure with annulus reconstruction
Vander Salm [16, 17] reported complete ultrasonic debridement of the calcification, reconstitution of the disassembled atrioventricular groove, and retention of the mitral valve leaflets. After ultrasonic debridement, the sutures are buttressed with Teflon felt pledgets and inserted as horizontal mattress sutures passing through the atrial wall, then through the full thickness of the ventricular wall and, finally, around the retained mitral valve. They reported 19 patients who required mitral valve surgery for mitral valve disease with MAC. The mean age of the patients was 73.4 years old. There were no patients who developed left ventricular rupture postoperatively. During the follow-up period (mean 3.4 years), 12 patients were NYHA class I and 2 were NYHA class II without valve-related events.
Carpentier and associates [18] reported their surgical experience of 68 patients (mean age 62 years old) who required mitral valve surgery for mitral regurgitation with MAC. The calcification process involved at least one-third of the posterior annulus circumferentially in 88 % of the cases and the whole posterior annulus in 10 %. Vertically, the calcification process was restricted to the annulus itself in 77 % of the cases. They noted that the calcium formation was encapsulated into a fibrous sheath quite distinct from the surrounding tissue. Fibrous encapsulation was not well delineated in the areas of myocardial infiltration by the calcium process. Their surgical technique comprises two steps: 1) en bloc decalcification and reconstruction of the annulus and 2) valve repair or replacement. Decalcification is begun using a knife to incise the atrial endothelium around the borders of the calcified bar. Then the base of the mural leaflet is incised at the edge of the calcium block and retracted. Keeping the edge of the dissecting knife blade against the calcium allows the attachment of the annulus to be sharply removed from the AV tissue. This dissection facilitated by the fact that the calcium formation is encapsulated within a fibrous sheath, allows an en bloc resection without calcium fragmentation. The dissection is most easily performed by developing a plane from one extremity of the calcium bar and then dissecting under the entire length of the block until it is removed. In most instances, the calcification process is limited to the annulus itself, and the en bloc decalcification leaves two edges of fibrous tissue delimiting the atrium superiorly and the ventricle inferiorly. The AV junction is reconstructed by a series of figure-of-eight braided sutures placed into the atrial and ventricular edges. These sutures are then brought out on the atrial side. Whenever calcium extends to the ventricular myocardium, the en bloc removal of the calcium bar leaves a large area of muscular fibers not covered by fibrous tissue. In such a case, the atrial edge is dissected free to mobilize the atrial flap, which is used to cover the decalcified area. If a valve repair cannot be successfully achieved after decalcification, a valve replacement can be done by following a double-layer suture technique. The first row (inner) is a series of 2-0 horizontal mattress sutures passed through the edges of the reconstructed annulus and then through the inner portion of the sewing ring. The second row (outer) consists of the figure-of-eight sutures used to reconstruct the annulus and brought out through the atrial edges. These sutures are passed through the outer portion of the sewing ring. Their mean cross-clamp time was 78 ± 37 min. In the group of the patients with valve repair, in-hospital mortality rate was 3.3 %. Actuarial survival rate up to 7 years was 93.1 ± 7.5 %.They noted that two reasons can explain these good results: [1] the removal of all abnormal tissue facilitates a good healing process and [2] the strength of the repair results from the three-tiered reconstruction: figure-of-eight sutures, leaflet remnant fixation to the reconstructed annulus, and prosthetic ring reinforcement.
Feindel and associates [19] reported their surgical experience of 54 patients (mean age 63 ± 14 years) with mitral regurgitation and extensive MAC. The calcium bar was excised and a new mitral annulus was created by suturing a strip of pericardium onto the endocardium of the left ventricle from the lateral to the medial fibrous trigones and to the endocardium of the left atrium [19, 20]. There were 5 operative deaths (9.3 %). They noted that the patch technique for annulus reconstruction has the advantage that it can be used when extensive repairs involving the posterior aspect as well the superior aspect of the annulus are necessary. Using this technique they have not seen any long-term failures such as aneurysm formation or dehiscence. They concluded that resection of the calcium bar and creation of a new annulus with pericardium provided good clinical results in patients with extensive MAC.
Ng et al. [21] described mitral valve repair in mitral regurgitation complicated by severe MAC. Their study population consisted of 37 patients with extensive MAC. Decalcification was performed as an en bloc resection to prevent calcium fragmentation. Sharp dissection was used to incise the atrial endothelium around the borders of the calcium bar. Meticulous attention with precise detachment and gentle handling of the tissue was taken to avoid disrupting the AV continuity. To reconstruct the annulus with “pericardial patch-extended endocardial annuloplasty”, a strip of 0.62 % glutaraldehyde-pretreated autologous pericardium was tailored and its margins were sutured to the ventricular inflow endocardium or to the fibrous skeleton of the heart, and to the posterior left atrial wall with continuous 5-0 or 6-0 ePTFE sutures. They also performed “sliding atrioplasty” in 9 patients. There were no operative deaths, perioperative complications or hospital mortality.
In these three documents, sharp dissection of calcium bar and reconstruction of the annulus with particular maneuvers were safe and reproducible without significant complications.
d’Alessandro et al. [22] documented determinants of repair feasibility in patients with MAC-associated mitral regurgitation. In 124 patients with MAC undergoing surgery, two entities were distinguished: Barlow disease (n = 60) and FED (n = 64). Their repair rate depended upon the extent of annulus calcification (p < 0.001) and the type of degenerative disease (95 % vs. 44 % in Barlow and FED, p < 0.001). In repair cases, excision of the calcium bar and reconstruction of the annulus were performed according to Carpentier’s procedure. In case of valve replacement, the prosthesis was fixed in the anatomical position and firmly anchored to the calcifications left intact. They did not have periprosthetic leakage or rupture of the left ventricle. Although late results were superior in the repair group, repair was possible in less than half of the cases.
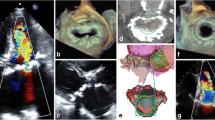
In our limited experience, we prefer a new annulus reconstruction with autologous pericardium after total decalcification [23]. Valve repair for severe MR due to Barlow disease associated with extensive MAC is shown in Fig. 3. Mitral valve replacement for mitral stenosis with MAC in a dialysis patient is also shown in Fig. 4. In both cases, meticulous and sharp dissection of the calcium bar as described in Carpentier’s technique is essential. Then, reconstruction of a new annulus mainly depends on the quality of the remaining fibrous capsule. In our experience, autologous pericardium is effective to reconstruct a new annulus without tension.
Mitral stenosis with MAC in a hemodialysis patient. a severely calcified mitral annulus and posterior leaflet, b MAC which extended left ventricular endocardium was sharply dissected, c left ventricular endocardium and annulus were reconstructed with autologous pericardium, d final configuration of annular reconstruction with autologous pericardium
Mitral valve repair without annulus reconstruction
One of the simple surgical managements for MR with MAC is edge-to-edge mitral repair without dissection of MAC or associated annuloplasty. Maisano et al. [24] described midterm results of edge-to-edge repair without annuloplasty in 81 patients including 32 patients with extensive MAC. A 4-0 polypropylene continuous suture without pledgets was used for leaflet approximation except when the leaflets were very thin. In these cases a 5-0 suture was preferred; pledgets were rarely used to reinforce the repair. Nine patients required reoperation. The cause of reoperation was recurrent severe regurgitation in all cases. Overall freedom from reoperation was 89 ± 4 % at 4 years. They noticed that more than trivial residual MR at intraoperative transesophageal color Doppler echocardiography and suboptimal water-testing results were associated with lower freedom from reoperation. Freedom from reoperation in patients with MAC (77 ± 22 %) was lower than those without MAC (95 ± 5 %).They concluded that the ringless edge-to-edge technique is not effective in cases of extensively calcified annulus, in which leaflet lesion can be effectively corrected using the techniques, but if the annulus is left untreated, a high risk of late failure can be expected.
Abe et al. [25] selected edge-to-edge repair for anterior leaflet prolapse with MAC in an 88 year-old female. Mitral regurgitation completely disappeared by Alfieri technique associated with paracommissural repair in this case. Cardiac arrest in this case was 33 min. Redundant anterior mitral leaflet might be enough to exclude residual regurgitation.
Dion commented that their experience of the first 3 patients with Alfieri technique for leaflet prolapse with MAC had early failure [24]. In the last 2 patients they tried leaflet augmentation of the posterior mitral leaflet with a pericardial patch. One of these patients underwent Alfieri technique after the leaflet augmentation.
Leaflet augmentation is one of the surgical solutions for moderate to severe mitral regurgitation with MAC as described by Dion. MAC may involve the basal portion of the valve leaflets and cause distortion of the posterior mitral leaflet with limited motion. The base of the posterior cusp is pushed up toward the atria, often stimulating a degree of mitral valve prolapse. This may result in insufficient leaflet coaptation area leading to regurgitation and congestive heart failure if untreated. In these conditions, anterior or posterior mitral leaflet augmentation with an autologous pericardium is effective to make a good coaptation area without touching MAC. We had a hemodialysis female patient who developed congestive heart failure due to severe aortic valve stenosis, mitral regurgitation with MAC, and severe functional tricuspid regurgitation. Anterior leaflet augmentation for severe mitral regurgitation with MAC, aortic valve replacement, and tricuspid annuloplasty were successfully performed (Fig. 5). As dissection of the calcium bar associated with valve repair or valve replacement was considered to be time consuming and a dangerous procedure for this particular patient, simple anterior mitral leaflet augmentation was selected. Another two patients successfully underwent leaflet augmentation for MR associated with heavy MAC in our experience. Leaflet augmentation was reported by Carpentier’s group in 1991 [26] and its long-term results were excellent in rheumatic mitral insufficiency, congenital mitral regurgitation, mitral regurgitation due to infective endocarditis, and lack of coaptation leaflet in other etiologies [27, 28].
The number of patients who require mitral valve procedure for mitral valve disease associated with MAC is increasing. Decalcification, annulus reconstruction, and valve repair are popular for patients with degenerative mitral regurgitation with MAC at experienced centers. In case of large mitral annulus with MAC, new annulus reconstruction using a mural leaflet can be an alternative procedure without decalcification. Although edge-to-edge technique is also considered to be an alternative procedure for selected patients, a large amount of coaptation surface of the leaflets is needed to avoid recurrent mitral regurgitation. In case of mitral stenosis with MAC in dialysis patients; valvectomy, decalcification, annulus reconstruction, and valve replacement are essential. Intraatrial implantation of a mitral prosthesis without decalcification of the annulus is another option in this condition.
A variety of surgical management methods of MAC is described in this review. Mitral valve repair is recommended following decalcification and reconstruction of the annulus, if possible. Mitral valve repair without decalcification (edge-to-edge technique, leaflet augmentation) may be applied for selected patients. Due to pliability and amount of leaflet tissue available for reconstruction, valve replacement with or without excision of the calcium bar may be the choice of procedure. As our experience is very limited in this particular condition, several procedures should be carefully considered before surgery.
References
Kanjanauthai S, Nasir K, Kats R, et al. Relationships of mitral annular calcification to cardiovascular risk factors: The multi-ethnic study of atherosclerosis. Atherosclerosis. 2010;213:558–62.
Fox CS, Vasan RS, Parise H, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality the framingham heart study. Circulation. 2003;107:1492–6.
Barasch E, Gottdiener JS, Larsen EKM, et al. Cardiovascular morbidity and mortality in community-dwelling elderly individuals with calcification of the fibrous skeleton of the base of the heart and aortosclerosis (cardiovascular health study). Am J Cardiol. 2006;97:1281–6.
Fox CS, Larson MG, Vasan RS, et al. Cross-sectional association of kidney function with valvular and annular calcification: the framingham heart study. J Am Soc Nephrol. 2006;17:521–7.
Jesri A, Braitman LE, Pressman GS. Severe mitral annular calcification predicts chronic kidney disease. Int J Cardiol. 2008;128:193–6.
Roberts WC, Morrow AG. Causes of early postoperative death following cardiac valve replacement. J Thorac Cardiovasc Surg. 1967;54:422–37.
MacVaugh H, Joyner CR, Johnson J. Unusual complications during mitral valve replacement in the presence of calcification of the annulus. Ann Surg. 1971;11:336–42.
Spencer FC, Galloway AC, Colvin SB. A clinical evaluation of the hypothesis that rupture of the left ventricle following mitral valve replacement can be prevented by preservation of the chordae of the mural leaflet. Ann Surg. 1985;202:673–80.
Cammack PL, Edie RN, Edmunds LH Jr. Bar calcification of the mitral annulus. A risk factor in mitral valve replacement. J Thorac Cardiovasc Surg. 1987;94:399–404.
Nataf P, Pavie A, Jault F, et al. Intraatrial insertion of a mitral prosthesis in a destroyed or calcified mitral annulus. Ann Thorac Surg. 1994;58:163–7.
Coselli JS, Crawford ES. Calcified mitral valve annulus: prosthesis insertion. Ann Thorac Surg. 1988;46:584–6.
Stefano SD, Lopez J, Florez S, et al. Building a new annulus: a technique for mitral valve replacement in heavily calcified annulus. Ann Thorac Surg. 2009;87:1625–7.
Atoui R, Lash V, Mohammadi S, et al. Intra-atrial implantation of a mitral valve prosthesis in a heavily calcified mitral annulus. Eur J Cardiothorac Surg. 2009;36:776–8.
Fukada Y, Matsui Y, Sasaki S, et al. A case report of mitral valve replacement with a collar-reinforced prosthetic valve for heavily calcified mitral annulus. Ann Thorac Cardiovasc Surg. 2005;11:260–4.
Iida H, Mochizuki Y, Matsushita Y, et al. A valve replacement technique for heavily calcified mitral valve and annulus. J Heart Valve Dis. 2005;14:209–11.
Vander Salm TJ. Mitral annular calcification: a new technique for valve replacement. Ann Thorac Surg. 1989;48:437–9.
VanderSalm TJ. Mitral annular calcification: a new technique for valve replacement. Ann Thorac Surg. 1997;63:1819–20.
Carpentier AF, Pellerin M, Fuzellier JF, et al. Extensive calcification of the mitral valve: pathology and surgical management. J Thorac Cardiovasc Surg. 1996;111:718–30.
Feindel CM, Tufail Z, David TE, et al. Mitral valve surgery in patients with extensive calcification of the mitral annulus. J Thorac Cardiovasc Surg. 2003;126:777–81.
David TE, Feindel CM, Armstrong S, et al. Reconstruction of the mitral annulus: a ten years experience. J Thorac Cardiovasc Surg. 1995;110:1323–32.
Ng CK, Punzengruber C, Pachinger O, et al. Valve repair in mitral regurgitation complicated by severe annulus calcification. Ann Thorac Surg. 2000;70:53–8.
d’Alessandro C, Vistarini N, Aubert S, Acar C, et al. Mitral annulus calcification: determinants of repair feasibility, early and late surgical outcome. Eur J Cardio Thorac Surg. 2007;32:596–603.
Adachi K, Sato T, Okada Y, et al. A case report of mitral valve replacement for patients with severely calcified mitral annulus after long-term hemodialysis. Jpn J Cardiovasc Surg. 2003;32:293–6. (in Japanese).
Maisano F, Caldarola A, Alfieri O, et al. Midterm results of edge-to-edge mitral valve repair without annuloplasty. J Thorac Cardiovasc Surg. 2003;126:1987–97.
Abe T, Ito T, Sunada M, et al. Edge-to-edge mitral valve repair for anterior leaflet prolapse associated with extensive calcification of the mitral annulus: report a case. Surg Today. 2010;40:251–3.
Chauvaud S, Jebara V, Carpentier A, et al. Valve extension with glutaraldehyde-preserved autologous pericardium. Results in mitral valve repair. J Thorac Cardiovasc Surg. 1991;102:171–7.
Acar C, de Ibara JS, Lansac E. Anterior leaflet augmentation with autologous pericardium for mitral repair in rheumatic valve insufficiency. J Heart Valve Dis. 2004;13:741–6.
Romano MA, Patel HJ, Pagani FD, et al. Anterior leaflet repair with patch augmentation for mitral regurgitation. Ann Thorac Surg. 2005;79:1500–4.
Author information
Authors and Affiliations
Corresponding author
Additional information
The review was submitted at the invitation of the editorial committee.
Rights and permissions
About this article
Cite this article
Okada, Y. Surgical management of mitral annular calcification. Gen Thorac Cardiovasc Surg 61, 619–625 (2013). https://doi.org/10.1007/s11748-013-0207-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-013-0207-7