Abstract
Aluminum (Al) is the third most abundant metal in the earth’s crust after oxygen and silicone. Geologically Al has existed as a complex compound with oxygen and carbon. In addition to natural Al in the soil, in the last century Al is used in various types of industrial products giving rise to excessive accumulation in the soil. When soil pH decreases under 5, complex Al dissolves into phytotoxic forms. Al3+, which is the most phytotoxic form, is absorbed by plant roots and has adverse effects on plant growth and development. Al toxicity is an important agricultural problem causing dramatic yield decrease and has been substantially investigated in plant systems. The mechanisms of Al toxicity and tolerance in plants have been described as morphological, physiological, and molecular perspectives; however, it has not yet been fully elucidated because of its complex chemistry.
It has been considered that metal toxicity has been controlled genetically. To study the molecular genetics of Al toxicity and tolerance are important issues in the field of plant growth and development. The development and application of Al-tolerant cultivars in fields is a better environmental solution to permit agricultural production in regions with acidic soils. Thus to clarify the signaling pathways and molecular markers for the fast and accurate diagnosis of Al toxicity symptoms may help to create strategies for strengthening Al tolerance in plants. This review summarizes the responses to Al and proposed molecular mechanisms of Al toxicity and tolerance in plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Aluminum (in American English, aluminium in British English) is the third most abundant element following oxygen and silicon, while its oxide is the fourth among the most common compounds in the earth’s crust. Aluminum (Al) is also the most abundant metal on the planet. Al is dense in the outer 16 km of earth’s cortex constituting about 8.1% by mass. Naturally, Al never occurs in the metallic form because of its chemical activity; it is found in chemical compounds with other elements like bauxite. To remove Al from natural ores, it must first be reduced. Al is considered as an active metal reacting with concentrated acids and alkalis (Sade et al. 2016; Li et al. 2016).
The trivalent Al has three oxidation states. The most common oxidation state of Al is +3 and it reacts rapidly with the oxygen in the moist air to form aluminum oxide (Al2O3-alumina). Al2O3 is the refractory oxide of Al existing in bauxite. Occasionally, the oxidation state of +2 and +1 exists as aluminum monoxide (AlO) and aluminum hydride (AlH3), respectively. The Al3+ ion can be stabilized by hydration, and the octahedral ion [Al(H2O)6]3+ occurs both in aqueous solution and in several salts (Roesky and Kumar 2005; Li et al. 2016).
It has been known that Al is also the most widely used metal in the industrial world after iron (Table 1). The large-scale (28%) use of Al is in the transportation industry. Packaging follows it by 23%. Because Al can be melted and reused, or recycled, it is ideal for foil, beer and soft drink cans, paint tubes, and containers for home products such as aerosol sprays. 14% of Al goes into building and construction such as windows and door frames, screens, roofing, and siding, as well as the construction of mobile homes and structural parts. The remaining 35% of Al is used in electrical wires and appliances due to being an excellent conductor, automobile engines, heating and cooling systems, bridges, vacuum cleaners, kitchen utensils, garden furniture, heavy machinery, and specialized chemical equipment (http://www.chemistryexplained.com/elements/A-C/Aluminum.html).
The widespread presence in earth crust and prevalent use of bioavailable Al may have immense and far-reaching implications for the health of humans and animals. In fact, much evidence shows that Al seems to be toxic to all forms of life on earth, and where it also appears in terrestrial biochemistry, it is invariably deleterious (Exley 2009; Shaw and Tomljenovic 2013).
2 Aluminum Toxicity in Plants
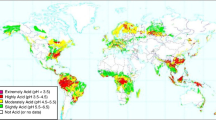
Considering the plants, Al is one of the abiotic stress factors. It is definite that anthropogenically released and/or naturally existing Al can solubilize and be absorbed by plants at low pH (acid) soils constituting one of the major plant growth-limiting factors. It has been known that potential farmable lands (approx. 67%) have acid soil worldwide (Abate et al. 2013; Ma et al. 2014). Al exists as a nontoxic complex in neutral or weakly acidic soils; however, when the complex Al is solubilized it turns to phytotoxic forms in acid soils. The most phytotoxic and dominant form is Al(H2O)63+ and dissolves to Al3+ which can be absorbed by plant roots (Matsumoto 2000; Vardar and Ünal 2007). It has been stated that solubilized Al presents in the range of 10–100 μM in acid soils affecting adversely the plant growth and development within a few minutes (Ciamporová 2002; Vitorello et al. 2005; Abate et al. 2013). Absorbed Al interacts with apoplasmic (cell wall), plasma membrane, and symplasmic (cytosol) targets. Al ions are penetrated from roots primarily and only a small proportion may be taken up through leaves (Kochian et al. 2005; Singh et al. 2017).
At the whole plant level, toxic Al affects adversely their anatomical and physiological structure such as chlorosis, reduction in leaf number, reduced photosynthesis, necrosis, and retardation of root growth. It has been widely known that roots are the first target of Al toxicity which have direct contact with rhizosphere. Al-induced root growth inhibition restrains the uptake of soil water and essential minerals leading to reduction in crop quality (Delhaize et al. 2004; Singh et al. 2017; Vardar et al. 2018). Root apex is the foremost region with regard to Al toxicity. As a first target, root apex plays a critical role in Al penetration and accumulation. This region absorbs more Al than the upper parts of root resulting in morphological alterations of root apices such as swelling, cracking, and appearing to be stubby and stiff (Matsumoto 2000; Vardar et al. 2006). It has also been visible that branching and root hair occurrence reduce significantly (Ciamporová 2002; Vardar et al. 2011). Several researches revealed that Al has detrimental effects reacting with different subcellular regions performed in different species and also varieties.
2.1 Cell Wall
Considering the cell structure, cell wall is the primary target of Al ions. It has been identified that Al binds and accumulates in the apoplasm in the range of 30–90% in root cortex cells (Rengel and Reid 1997; Vardar et al. 2011). Pectin matrix which has negatively charged carboxylic groups is the first Al-binding site in the cell wall (Chang et al. 1999; Singh et al. 2017). It has also been known that Al reacts with apoplasmic face of plasma membrane. After Al-cell wall interaction, Al translocates to plasma membrane and symplasm (Schmohl and Horst 2000). Researchers suggested that Al accumulation degree is in direct correlation with pectin content and dissociation of carboxylic and hydroxylic groups of the pectin (Godbold and Jentschke 1998; Ahn and Matsumoto 2006). Strong binding and accumulation of Al alter the structural and mechanical properties of cell wall causing reduction in mechanical extensibility causing cracked and unoriented root growth (Kochian et al. 2005).
Al accumulation in cell wall discomposes the stability of other cations such as Ca2+ which is responsible for the strength of cell wall. This disturbance causes callose (β-1,3-glucan) synthesis and accumulation between cell wall and plasma membrane being one of the significant markers of Al toxicity (Tabuchi and Matsumoto 2001; Vardar et al. 2011; Ünal et al. 2013). Although callose may collaborate root to cope with Al toxicity by blocking the plasmodesmata, it also blocks the movement of water and minerals causing reduction of nutrient uptake (Singh et al. 2017; Vardar et al. 2018).
Eventually, Al reaction in cell walls causes reduced extensibility, disrupted growth orientation, callose formation, and accordingly restriction of water and mineral nutrient uptake across the plasma membrane (Kochian et al. 2005).
2.2 Plasma Membrane
Plasma membrane is the external barrier of the cell and it regulates the ion traffic. Negatively charged membrane displays strong interaction with A13+ (Kinraide et al. 1998). Plasma membrane-Al reaction alters the structure and function of membrane causing disruption in the cellular homeostasis (Kochian et al. 2005). It has been revealed that A13+ may interact with both phospholipids and proteins leading to lipid peroxidation in plasma membrane. Researchers revealed that the severity of Al toxicity causes to break the plasma membrane integrity (Vitorello et al. 2005; Panda et al. 2009; Singh et al. 2017). Lipid peroxidation also causes highly toxic free radical generation and accumulation (Panda et al. 2009).
Al has greater affinity than other cations such as Ca2+ and Mg2+ during competing for the choline head of phosphatidylcholine. This situation culminates in Al-induced positively charged bridges between head groups of the phospholipid layer and displacement of other cations (Bhalerao and Prabhu 2013; Singh et al. 2017). The positively charged layer restricts cation motion, but increases anion movement altering membrane electrochemical potential (Nichol et al. 1993). As we have stated above Al-induced cation alteration, principally Ca2+ displacement , also triggers callose synthesis (Gupta et al. 2013). Callose also inhibits intercellular transport through plasmodesmatal plugs (Sivaguru et al. 2000). Alterations in cation uptake of essential ions such as Ca2+, K+, Mg2+, and NH4+ also cause nutrient imbalances (Pineros and Kochian 2001; Singh et al. 2017).
2.3 Cell Signaling and Cytoskeleton
Several researchers stated that Al stress affected signal transduction pathway adversely mediated by secondary messengers due to imbalance of Ca2+ and pH homeostasis (Jones and Kochian 1997; Ma et al. 2002; Singh et al. 2017). In plasma membrane Al prefers to react with specific lipids which are important signaling molecules such as G proteins (guanine nucleotide-binding proteins) and a phosphatidylinositol-4,5-diphosphate (PIP2)-specific phospholipase C commonly (He et al. 2015). Besides, Al stress decreases inositol-1,4,5-triphosphate (IP3) amount in the plasma membrane (Rengel and Elliott 1992). After Al reaction, signaling pathways are interrupted in the cell.
Cell cytoskeleton including microtubules, microfilaments, and intermediate filaments is also one of the potential targets of Al ions. Al causes disruption in cytoskeletal dynamics which has a critical importance during cell-wall biosynthesis, cell growth, and cell division. It has been revealed that Al-induced disruption of microtubule and actin filament results in lateral cell swelling (Frantzios et al. 2001; Sivaguru et al. 2003). It has been suggested that Al disruption in cytoskeleton occurs either through direct interaction with cytoskeletal elements or through alteration in signaling pathway (Sivaguru et al. 1999). Protein phosphorylation-dephosphorylation and mitogen-activated protein kinase (MAPK) cascade which take charge during signal transduction are also reorganized by Al ions (Matsumoto 2000; Osawa and Matsumoto 2001; Singh et al. 2017). This interaction impairs the signal transduction pathway causing chaos in the cell.
2.4 Genotoxicity
Several researches reveal that Al has genotoxic impact and long-term Al exposure causes adverse effects on DNA composition and replication due to more rigid double-helix and chromatin structure (Vitorello et al. 2005; Panda et al. 2009; Gupta et al. 2013). It has been observed that Al ions decrease cell viability and mitotic index and increases chromosomal aberrations which are associated with Al-induced disturbance in tubulin polymerization-depolymerization . Tubulin disturbance limits the movement of chromosome on mitotic spindle causing chromosome laggards, bridges, micronuclei, and c-mitosis under Al stress (Frantzios et al. 2000; Vardar et al. 2011). It can also be considered that Al exposure may decrease the frequency of S-phase cells inducing delay in M phase (mitotic division) (Jaskowiak et al. 2018). Grabski and Schindler (1995) showed that Al has greater affinity to nucleoside triphosphates much more than Mg2+. Hence, Al prefers to interact with DNA than histone proteins at first. Besides, several researches revealed that Al exposure may cause double-strand DNA breaks even at 15 min (Vardar et al. 2015, 2016). Recent studies also revealed that Al ions cause DNA methylation and polymorphism of LTR retrotransposons (Guo et al. 2018; Taspinar et al. 2018).
2.5 Oxidative Stress and Programmed Cell Death
Al toxicity stimulates generation of reactive oxygen species (ROS) leading to oxidative stress in plants. Lower concentrations of ROS have a role as signaling molecules; however, higher concentrations regress the balance between antioxidant machinery and ROS detoxification. Overproduction of ROS (⋅O2−, .OH, HO−, H2O2) is generated in mitochondria, chloroplast, and peroxisomes causing imbalance of antioxidant enzyme, lipid peroxidation, protein denaturation, carbohydrate oxidation, pigment breakdown, and DNA damage (Sharma and Dubey 2007; Gupta et al. 2013; Vardar et al. 2018).
Phytotoxic levels of ROS also trigger programmed cell death (PCD) in plants. It has been suggested that ROS weakens the binding strength of cytochrome c (cyt c) through oxidation of cardiolipins in the inner mitochondrial membrane and reduces mitochondrial membrane potential (ΔΨm) inducing cytochrome c release to the cytoplasm (Williams et al. 2014). Besides cytochrome c release amplifies more ROS generation and triggers vacuolar processing enzyme (VPE) activity. Although there are some studies concerning Al toxicity and PCD (Table 2), more detailed studies are needed to clarify the Al-induced PCD mechanism.
3 Al Tolerance Mechanisms
Al has the ability to make stable complexes with oxygen donor ligands; thus Al chelating with root exudates plays a critical role in the prevention of phytotoxic Al uptake by roots (Barceló and Poschenrieder 2002). It has been evidenced that Al chelating mechanism is performed by mucilage formation, organic anion efflux, phosphate secretion, and secondary metabolite production from tolerant root apices (Miyasaka and Hawes 2001; Ma et al. 2001; Ofei-Manu et al. 2001; Vardar and Ünal 2007; Singh et al. 2017). Whereas tolerant plants may use different types of Al exclusion strategies, organic anion efflux plays a central role in the exclusion of Al. Several genetic and molecular approaches concerning organic acid release were reported in different plant species (Ma et al. 2001). Al chelation by organic acids decreases or prevents its uptake through apoplasm and symplasm. Type of organic acids secreted by roots varies depending on Al-tolerant plant species. It has been reported that malate, citrate, and oxalate are the most commonly encountered organic secretions (Magalhães et al. 2007; Ryan et al. 2009). Researches revealed that organic acid exudation is activated by Al exposure rapidly suggesting a transporter located in the plasma membrane of tolerant roots (Kochian et al. 2005).
Whereas organic acid exclusion from roots and Al chelation in the rhizosphere appear to be the most common, several species tolerate Al toxicity by internal or symplastic detoxification after Al uptake into the root or shoot cells. This situation was first attained in Al-accumulating plant root, shoot, and leaf such as tea (Camelia sinensis), buckwheat (Fagopyrum esculentum), and Hydrangea (Hydrangea macrophylla). Internal detoxification consists of Al chelation with organic ligands in cytosol and their transfer to the vacuole for deposition (Kochian et al. 2004; Delhaize et al. 2012). Although most of the plants prefer only organic acid exudation or internal detoxification, some species such as Pinus taeda make use of both of the mechanisms to protect itself from Al toxicity (Nguyen et al. 2003; Nowak and Friend 2005).
4 Aluminum Tolerance Genes in Plants
Many plant species vary considerably in their ability to tolerate the toxic Al concentrations via efflux of organic anions such as malate, citrate, and oxalate from roots. Al tolerance has a strong correlation with genotype-dependent efflux capacity of organic anion and exclusion of Al once it enters cytosol (Kochian et al. 2004; Hiradate et al. 2007; Delhaize et al. 2012). Sasaki et al. (2004) isolated a gene controlling the Al-dependent efflux of malate from Triticum aestivum (wheat) named TaALMT1 (Triticum aestivum aluminum-activated malate transporter 1) . TaALMT1 (formerly named ALMT1) encodes a hydrophobic protein (anion channel) localizing in the plasma membrane of root cells (Yamaguchi et al. 2005; Ligaba et al. 2006). ALMT protein family has 5–7 membrane-spanning regions in the N-terminal half of the protein and a long C-terminal tail (Delhaize et al. 2004, 2012). Researchers revealed that TaALMT1 expression in Al-tolerant genotypes of wheat is 5- to 10-fold higher than in Al-sensitive genotypes (Sasaki et al. 2004; Raman et al. 2005). Subsequent analyses revealed that specific variations in diverse bread wheat genotypes could be classified into seven patterns, type I to type VII (Sasaki et al. 2006; Garcia-Oliveira et al. 2014). After the discovery of ALMT1 in wheat, Arabidopsis ALMT1 members were identified as AtALMT1, and similarly their homologs characterized in rape (BnALMT1 and BnALMT2), soybean (GmALMT1), and rye (ScALMT1). All of them share similar functional characteristics that induce malate exudation in Al tolerance (Hoekenga et al. 2006; Ligaba et al. 2006).
Further studies revealed that another gene responsible for citrate exudation in response to Al toxicity exists in barley (HvAACT1-Hordeum vulgare aluminum-activated citrate transporter 1) which belongs to MATE (multidrug and toxic compound extrusion) gene family (Furukawa et al. 2007). Besides, SbMATE gene was also identified in Sorghum bicolor responsible for citrate transporter in response to Al toxicity (Magalhães et al. 2007).
It has been known that tolerant genotypes within species have significantly much more organic acid expression than sensitive genotypes. The extra expression is due to a series of cis mutations in the promoter of TaALMT1 in wheat (Sasaki et al. 2006; Ryan et al. 2010). Raman et al. (2008) revealed that the promoter region is more polymorphic than coding region in TaALMT1 and several alleles have accurate tandem repeats (Ryan et al. 2010). Besides, several examples indicated that transposable elements are able to alter the level and localization of gene expression during enhancing Al tolerance (Morgante et al. 2007; Delhaize et al. 2012).
In wheat the major Al tolerance locus was identified on chromosome 4DL (Luo and Dvořák 1996; Raman et al. 2008) and subsequently on chromosome 4BL responsible for phenotypic variation in citrate efflux (Ryan et al. 2009) suggesting that citrate is the secondary organic acid after malate in Al tolerance. Following molecular studies in different cultivars of wheat revealed that multiple genetic loci on the chromosome arms of 2DL, 3DL, 4BL, 4DL, 5AS, 6AL, 7AS, and 7D are very critical in Al tolerance mechanism (Aniol and Gustafson 1984; Aniol 1990; Papernik et al. 2001). However, it is still not clear that whether all of these loci are included in Al tolerance. Recently Al tolerance-related loci have been identified on different chromosomes in different plant species (Ryan et al. 2009; Boff et al. 2019).
Early studies suggested that Al resistance in wheat is driven by a single major genetic locus with different alleles inducing different degrees of Al tolerance (Campbell and Lafever 1981). Monogenic inheritance with multiple alleles was also identified in barley, maize, sorghum, pea, chickpea, and oat (Singh and Choudhary 2010; Singh and Raje 2011; Castilhos et al. 2011; Delhaize et al. 2012). However, subsequent microarray studies revealed the complexity of the genetic control of Al tolerance. Besides, most of the identified genes probably express response to Al stress rather than Al tolerance (Goodwin and Sutter 2009; Delhaize et al. 2012). According to the several researches in wheat root tips different genes expressed high amounts correlating with Al tolerance such as ALMT1, ent-kaurenoic, β-glucosidase, lectin, histidine kinase, pyruvate dehydrogenase, alternative oxidase, galactonolactone oxidase, and phosphoenolpyruvate carboxylate. These results suggest that Al tolerance can be co-regulated by multiple genes with diverse functions in plants in addition to ALMT1 (Guo et al. 2007; Houde and Oury 2008).
In conclusion, Al toxicity is a widespread problem in industrial regions and acidic soils limiting crop productivity in the world. It has been known that the severity of Al toxicity is due to plant genotype, cell/tissue type, types of chelators, concentrations of other cations, and pH (Kinraide and Parker 1987). Since Al toxicity and tolerance mechanism and also Al-detoxifying mechanisms need to be clarified with more detailed studies, in this chapter, we reviewed recent information concerning physiological and molecular effects of Al toxicity and Al tolerance mechanism. The intensive researches on gene-based mechanisms of Al toxicity and tolerance may help to develop Al-tolerant varieties or transgenic to enhance the crop quality under Al toxicity.
References
Abate E, Hussien S, Laing M et al (2013) Aluminum toxicity tolerance in cereals: Mechanisms, genetic control and breeding methods. Afr J Agric Res 8:711–722
Achary VMM, Panda BB (2009) Aluminium-induced DNA damage and adaptive response to genotoxic stress in plant cells are mediated through reactive oxygen intermediates. Mutagenesis 25:201–209
Achary VMM, Jena S, Panda KK et al (2008) Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicol Environ Saf 70:300–310
Achary VMM, Patnaik AR, Panda BB (2012) Oxidative biomarkers in leaf tissue of barley seedlings in response to aluminium stress. Ecotoxicol Environ Saf 75:16–26
Ahn SJ, Matsumoto H (2006) The role of the plasma membrane in the response of plant roots to aluminum toxicity. Plant Signal Behav 1:37–45
Aniol A (1990) Genetics of tolerance to aluminium in wheat (Triticum aestivum L. Thell). Plant Soil 123:223–227
Aniol A, Gustafson JP (1984) Chromosome location of genes controlling aluminum tolerance in wheat, rye, and triticale. Can J Genet Cytol 26:701–705
Aytürk Ö, Vardar F (2015) Aluminum induced caspase-like activities in some Gramineae species. Adv Food Sci 37:71–75
Barceló J, Poschenrieder C (2002) Fast root growth responses, root exudates and internal detoxification as clues to the mechanisms of aluminum toxicity and resistance: a review. Environ Exp Bot 48:75–92
Bhalerao SA, Prabhu DV (2013) Aluminium toxicity in plants—a review. J Appl Chem 2:447–474
Boff T, Espindula LF, Bücker-Neto L et al (2019) Inheritance of aluminum tolerance in the wheat cultivar Toropi and new findings about the introduction of this trait into the Brazilian wheat germplasm. Environ Exp Bot 157:91–99
Campbell LG, Lafever HN (1981) Heritability of aluminum tolerance in wheat. Cereal Res Commun 9:281–287
Castilhos G, Farias JG, Schneider AB et al (2011) Aluminum-stress response in oat genotypes with monogenic tolerance. Environ Exp Bot 74:114–121
Chang YC, Yamamoto Y, Matsumoto H (1999) Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant Cell Environ 22:1009–1017
Ciamporová M (2002) Morphological and structural responses of plant roots to aluminum at organ, tissue and cellular levels. Biol Plant 45(2):161–171
Delhaize E, Ryan PR, Hebb DM et al (2004) Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc Natl Acad Sci U S A 101:15249–15254
Delhaize E, Ma JF, Ryan PR (2012) Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci 17(6):341–348
Exley C (2009) Darwin, natural selection and the biological essentiality of aluminium and silicon. Trends Biochem Sci 34:589–589
Frantzios G, Galatis B, Apostolakos P (2000) Aluminum effects on microtubule organization in dividing root tip cells of Triticum turgidum. I. Mitotic cells. New Phytol 145:211–224
Frantzios G, Galatis B, Apostolakos P (2001) Aluminum effects on microtubule organization in dividing root tip cells of Triticum turgidum. II Cytokinetic cells. J Plant Res 114:157–170
Furukawa J, Yamaji N, Wang H et al (2007) An aluminum-activated citrate transporter in barley. Plant Cell Physiol 48:081–1091
Garcia-Oliveira AL, Martins-Lopes P, Tolrá R et al (2014) Molecular characterization of the citrate transporter gene TaMATE1 and expression analysis of upstream genes involved in organic acid transport under Al stress in bread wheat (Triticum aestivum). Physiol Plant 152:441–452
Godbold DL, Jentschke G (1998) Aluminium accumulation in root cell walls coincides with inhibition of root growth but not with inhibition of magnesium uptake in Norway spruce. Physiol Plant 102:553–560
Goodwin SB, Sutter TR (2009) Microarray analysis of Arabidopsis genome response to aluminum stress. Biol Plant 53:85–99
Grabski S, Schindler M (1995) Aluminum induces rigor within the actin network of soybean cells. Plant Physiol 108(3):897–901
Guo P, Bai G, Carver B et al (2007) Transcriptional analysis between two wheat near-isogenic lines contrasting in aluminum tolerance under aluminum stress. Mol Gen Genomics 277:1–12
Guo P, Qi YP, Cai YT et al (2018) Aluminum effects on photosynthesis, reactive oxygen species and methylglyoxal detoxification in two Citrus species differing in aluminum tolerance. Tree Physiol 00:1–18
Gupta N, Gaurav SS, Kumar A (2013) Molecular basis of aluminium toxicity in plants: a review. Am J Plant Sci 4:21–37
He H, He L, Gu M (2015) Signal transduction during aluminum-induced secretion of organic acids in plants. Biol Plant 59:601–608
Hiradate S, Ma JF, Matsumoto H (2007) Strategies of plants to adapt to mineral stresses in problem soils. Adv Agron 96:65–132
Hoekenga OA, Maron LG, Piñeros MA et al (2006) AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci U S A 103(25):9738–9743
Houde M, Oury DAO (2008) Identification of genes and pathways associated with aluminium stress and tolerance using transcriptome profiling of wheat near-isogenic lines. BMC Genomics 9:400
Huang WJ, Oo TL, He HY et al (2014) Aluminum induced rapidly mitochondria dependent programmed cell death in Al sensitive peanut root tips. Bot Stud 55:67–78
Jaskowiak J, Tkaczyk O, Slota M et al (2018) Analysis of aluminum toxicity in Hordeum vulgare roots with an emphasis on DNA integrity and cell cycle. PLoS One 13:e0193156
Jones DL, Kochian LV (1997) Aluminum interaction with plasma membrane lipids and enzyme metal binding sites and its potential role in Al cytotoxicity. FEBS Lett 400:51–57
Kariya K, Demiral T, Sasaki T et al (2013) A novel mechanism of aluminium-induced cell death involving vacuolar processing enzyme and vacuolar collapse in tobacco cell line BY-2. J Inorg Biochem 128:196–201
Kariya K, Tsuchiya Y, Sasaki T et al (2018) Aluminium induced cell death requires upregulation of NtVPE1 gene coding vacuolar processing enzyme in tobacco (Nicotiana tabacum L.). J Inorg Biochem 181:152–161
Kinraide TB, Parker DR (1987) Cation amelioration of aluminum toxicity in wheat. Plant Physiol 83:546–551
Kinraide TB, Yermiyahu U, Rytwo G (1998) Computation of surface electrical potentials of plant cell membranes. Correspondence to published zeta potentials from diverse plant sources. Plant Physiol 118:505–512
Kochian LV, Hoekenga OA, Piñeros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55:459–493
Kochian LV, Piñeros MA, Hoekenga OA (2005) The physiology, genetics and molecular biology of plant aluminium resistance and toxicity. Plant Soil 274:175–195
Li Z, Xing D (2011) Mechanistic study of mitochondria-dependent programmed cell death induced by aluminium phytotoxicity using fluorescence techniques. J Exp Bot 62:331–343
Li H, Yang LT, Qi YP et al (2016) Aluminum toxicity-induced alterations of leaf proteome in two Citrus species differing in aluminum tolerance. Int J Mol Sci 17:1180
Ligaba A, Katsuhara M, Ryan PR et al (2006) The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminium resistance of plant cells. Plant Physiol 142:1294–1303
Luo MC, Dvořák J (1996) Molecular mapping of an aluminum tolerance locus on chromosome 4D of Chinese Spring wheat. Euphytica 91:31–35
Ma JF, Ryan PR, Delhaize E (2001) Aluminum tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6:273–278
Ma QF, Rengel Z, Kuo J (2002) Aluminium toxicity in rye (Secale cereale): Root growth and dynamics of cytoplasmic Ca2+ in intact root tips. Ann Bot 89:241–244
Ma JF, Chen ZC, Shen RF (2014) Molecular mechanism of Al tolerance in Gramineous plants. Plant Soil 381(1–2):1–12
Magalhães JV, Liu J, Guimarães CT et al (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39:1156–1161
Matsumoto H (2000) Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 200(4):1–46
Miyasaka SC, Hawes C (2001) Possible role of root border cells in detection and avoidance of aluminum toxicity. Plant Physiol 125:1978–1987
Morgante M, De Paoli E, Radovic S (2007) Transposable elements and the plant pangenomes. Curr Opin Plant Biol 10:149–155
Nguyen NT, Nakabayashi K, Thompson J et al (2003) Role of exudation of organic acids and phosphate in aluminum tolerance of four tropical woody species. Tree Physiol 23:1041–1050
Nichol B, Oliveria LA, Glass ADM et al (1993) The effects of aluminum on the influx of calcium, potassium, ammonium, nitrate and phosphate in an aluminum-sensitive cultivar of barley (Hordeum vulgare L.). Plant Physiol 101:1263–1266
Nowak J, Friend AL (2005) Aluminum fractions in root tips of slash pine and loblolly pine families differing in Al resistance. Tree Physiol 25:245–250
Ofei-Manu P, Wagatsuma T, Ishikawa S et al (2001) The plasma membrane strength of the root tip cells and root phenolic compounds are correlated with Al tolerance in several common woody plants. Soil Sci Plant Nutr 47:359–375
Osawa H, Matsumoto H (2001) Possible involvement of protein phosphorylation in aluminum-responsive malate efflux from wheat root apex. Plant Physiol 126(1):411–420
Pan JW, Zhu MY, Chen H (2001) Aluminum-induced cell death in root tip cells of barley. Environ Exp Bot 46:71–79
Panda SK, Baluška F, Matsumoto H (2009) Aluminum stress signaling in plants. Plant Signal Behav 4:592–597
Papernik LA, Bethea AS, Singleton TE et al (2001) Physiological basis of reduced Al tolerance in ditelosomic lines of Chinese Spring wheat. Planta 212:829–834
Pineros MA, Kochian LV (2001) A patch–clamp study on the physiology of aluminum toxicity and aluminum tolerance in maize. Identification and characterization of Al3+-induced anion channels. Plant Physiol 125:292–305
Raman H, Zhang K, Cakir M et al (2005) Molecular characterization and mapping of ALMT1, the aluminium-tolerance gene of bread wheat (Triticum aestivum L.). Genome 48:781–791
Raman H, Ryan PR, Raman R et al (2008) Analysis of TaALMT1 traces the transmission of aluminum resistance in cultivated common wheat (Triticum aestivum L.). Theor Appl Genet 116:343–354
Rengel Z, Elliott DC (1992) Aluminum inhibits net Ca2+ uptake by Amaranthus protoplasts. Biochem Physiol Pflanz 188:177–186
Rengel Z, Reid RJ (1997) Uptake of Al across the plasma membrane of plant cells. Plant Soil 192:31–35
Roesky HW, Kumar SS (2005) Chemistry of Aluminium (I). Chem Commun 32:4027–4038
Ryan PR, Raman H, Gupta S et al (2009) A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol 149:340–351
Ryan PR, Raman H, Gupta S et al (2010) The multiple origins of aluminium resistance in hexaploid wheat include Aegilops tauschii and more recent cis mutations to TaALMT1. Plant J 64:446–455
Sade H, Meriga B, Surapu V et al (2016) Toxicity and tolerance of aluminum in plants: tailoring plants to suit to acid soils. Biometals 29(2):187–210
Sasaki T, Yamamoto Y, Ezaki B et al (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37:645–653
Sasaki T, Ryan PR, Delhaize E et al (2006) Sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminum resistance. Plant Cell Physiol 47:1343–1354
Schmohl N, Horst WJ (2000) Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cells grown in suspension culture. Plant Cell Environ 23:735–742
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep 26:2027–2038
Shaw CA, Tomljenovic L (2013) Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res 56(2–3):304–316
Singh S, Choudhary AK (2010) Inheritance pattern of aluminium tolerance in pea. Plant Breed 129:688–692
Singh D, Raje RS (2011) Genetics of aluminium tolerance in chickpea (Cicer arietinum). Plant Breed 130:563–568
Singh S, Tripathi DK, Singh S et al (2017) Toxicity of aluminium on various levels of plant cells and organism: a review. Environ Exp Bot 137:177–193
Sivaguru M, Baluska F, Volkmann D et al (1999) Impacts of aluminum on the cytoskeleton of the maize root apex. Short term effects on the distal part of the transition zone. Plant Physiol 119:1073–1082
Sivaguru M, Fujiwara T, Šamaj J et al (2000) Aluminum-induced 3-b-D-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiol 124(3):991–1006
Sivaguru M, Pike S, Gassmann W et al (2003) Aluminum rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane: Evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol 44:667–675
Tabuchi A, Matsumoto H (2001) Changes in cell wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiol Plant 112:353–358
Taspinar MS, Aydin M, Sigmaz B et al (2018) Aluminum-induced changes on DNA damage, DNA methylation and LTR retrotransposon polymorphism in maize. Arab J Sci Eng 43:123–131
Ünal M, Vardar F, Aytürk Ö (2013) Callose in plant sexual reproduction. In: Silva-Opps M (ed) Current progress in biological research. In Tech, Croatia, pp 319–343
Vardar F, Ünal M (2007) Aluminum toxicity and resistance in higher plants. Adv Mol Biol 1(1):1–12
Vardar F, Arıcan E, Gözükırmızı N (2006) Effects of aluminum on in vitro root growth and seed germination of tobacco (Nicotiana tabacum L.). Adv Food Sci 28:85–88
Vardar F, İsmailoğlu I, İnan D et al (2011) Determination of stress responses induced by aluminum in maize (Zea mays L. Karadeniz Yıldızı). Acta Biol Hung 62:156–170
Vardar F, Akgül N, Aytürk Ö et al (2015) Assessment of aluminum induced genotoxicity with comet assay in wheat, rye and triticale roots. Fresenius Environ Bull 37:3352–3358
Vardar F, Çabuk E, Aytürk Ö et al (2016) Determination of aluminum induced programmed cell death characterized by DNA fragmentation in Gramineae species. Caryologia 69:111–115
Vardar F, Yanık F, Çetinbaş-Genç A et al (2018) Aluminum-induced toxicity and programmed cell death in plants. In: Yüksel B, Karagül MS (eds) Advances in health and natural sciences. Nova Science Publishers Inc, Hauppauge, NY, pp 155–182
Vitorello VA, Capaldi FRC, Stefanuto VA (2005) Recent advances in aluminum toxicity and resistance in higher plants. Braz J Plant Physiol 17:129–143
Williams B, Verchot J, Dickman MB (2014) When supply does not meet demand-ER stress and plant programmed cell death. Front Plant Sci 5:211
Yamaguchi M, Sasaki T, Sivaguru M et al (2005) Evidence for the plasma membrane localization of Al-activated malate transporter (ALMT1). Plant Cell Physiol 46:812–816
Yao S, Huang W, Pan C et al (2016) Caspase-like proteases regulate aluminum-induced programmed cell death in peanut. Plant Cell Tiss Org 127:691–703
Zhan J, Li W, He HY et al (2014) Mitochondrial alterations during Al-induced PCD in peanut root tips. Plant Physiol Biochem 75:105–113. http://www.chemistryexplained.com/elements/A-C/Aluminum.html. Accessed 31 July 2019
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vardar, F. (2020). Recent Advances in Aluminum Phytotoxicity. In: Faisal, M., Saquib, Q., Alatar, A.A., Al-Khedhairy, A.A. (eds) Cellular and Molecular Phytotoxicity of Heavy Metals. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-45975-8_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-45975-8_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45974-1
Online ISBN: 978-3-030-45975-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)